CCR7 Signals Are Essential for Cortex–Medulla Migration of Developing Thymocytes (original) (raw)
Abstract
Upon TCR-mediated positive selection, developing thymocytes relocate within the thymus from the cortex to the medulla for further differentiation and selection. However, it is unknown how this cortex–medulla migration of thymocytes is controlled and how it controls T cell development. Here we show that in mice deficient for CCR7 or its ligands mature single-positive thymocytes are arrested in the cortex and do not accumulate in the medulla. These mutant mice are defective in forming the medullary region of the thymus. Thymic export of T cells in these mice is compromised during the neonatal period but not in adulthood. Thymocytes in these mice show no defects in maturation, survival, and negative selection to ubiquitous antigens. TCR engagement of immature cortical thymocytes elevates the cell surface expression of CCR7. These results indicate that CCR7 signals are essential for the migration of positively selected thymocytes from the cortex to the medulla. CCR7-dependent cortex–medulla migration of thymocytes plays a crucial role in medulla formation and neonatal T cell export but is not essential for maturation, survival, negative selection, and adult export of thymocytes.
Keywords: thymus, medulla, migration, CCR7, positive and negative selection
Introduction
During development within the thymus, maturing thymocytes migrate from the cortex to the medulla (1–3). This cortex–medulla migration coincides with the differentiation of CD4+CD8+ (double-positive; DP) immature thymocytes into CD4+CD8− (CD4 single positive; 4SP) and CD4−CD8+ (CD8 single positive; 8SP) mature thymocytes, during which positive and negative selection shapes the central framework of the T cell repertoire. Many studies have been conducted to determine how TCR signals regulate the survival and death of developing thymocytes. However, the mechanisms regulating the intrathymic migration of positively selected thymocytes remain largely unknown.
It has been shown recently that chemokines and chemokine receptors are differentially expressed by subsets of thymic stromal cells and thymocytes (4–6), suggesting that chemokines may be involved in directing the intrathymic localization of developing thymocytes. Indeed, CXCR4 has been shown to facilitate the localization of early lymphoid progenitors to the cortical region in the thymus (7). In addition, the in vivo treatment of mouse with pertussis toxin, a G protein inhibitor, caused the arrest of mature single-positive (SP) thymocytes in the cortex, suggesting that G protein–coupled receptor signals, e.g., the signals via chemokine receptors, may be involved in the accumulation of mature thymocytes in the medulla (8). The impaired accumulation of SP thymocytes in the medulla of mouse deficient for DOCK2, a Rac regulator downstream of surface receptors including the chemokine receptors, further suggests the involvement of chemokines in the cortex–medulla migration of developing thymocytes (9). However, none of the chemokines or chemokine receptors that are involved in the regulation of the cortex–medulla migration of thymocytes have been identified so far.
Using mice deficient for CCR7 or its ligands (CCR7L; i.e., CCL19 and CCL21), we show that CCR7 and CCR7L are essential for the intrathymic migration of positively selected thymocytes from the cortex to the medulla. Our results also indicate that CCR7 signals critically regulate the formation of the medullary region in the thymus and the export of T cells from newborn mouse thymus. The data further show that the cortex–medulla migration of thymocytes is not essential for their maturation, survival, negative selection, and adult T cell export.
Materials and Methods
Mice.
CCR7−/− mice and plt/plt mice were backcrossed to C57BL/6 mice for six generations. plt/plt mice of BALB/c background were also used. AND-TCR transgenic mice (10), HY-TCR transgenic mice (11), 2C-TCR transgenic mice (12), and B6.SJL-Ptprca congenic mice (CD45.1+CD45.2−) were provided by S. Hedrick (University of California, San Diego, La Jolla, CA), H. von Boehmer (Harvard Medical School, Boston, MA), D. Loh (Nippon Research Center, Kamakura, Japan), and H. Nakauchi (University of Tokyo, Tokyo, Japan), respectively. C57BL/6 (B6) mice (CD45.1−CD45.2+) were obtained from SLC. MHC class I/class II double-deficient mice were bred in our animal facility by crossing MHC class I–deficient B6 background mice (β2 microglobulin gene targeted mice from The Jackson Laboratory) and MHC class II–deficient B6 background mice (Aβ gene targeted mice provided by D. Mathis [Harvard Medical School, Boston, MA]; reference 13). Mice were bred and maintained under specific pathogen-free conditions in our animal facility, and experiments were performed with consent from the Animal Experimentation Committee of the University of Tokushima.
Irradiation of Mice.
Adult mice at 6–8 wk old were exposed to 4 Gy irradiation with an x-ray irradiation system (MBR-1520R-3; Hitachi Medico Technology Corporation). Thymuses were analyzed 24 h after irradiation.
Bone Marrow Chimeras.
Bone marrow cells prepared from femurs and tibias of CCR7-deficient mice (CD45.2) and B6 mice (CD45.2) and B6.SJL-Ptprca mice (CD45.1) were MACS depleted of T cells by using FITC-labeled anti-CD3, anti-CD4, and anti-CD8 antibodies and anti-FITC magnetic beads (Miltenyi Biotec). B6.SJL-Ptprca mice (CD45.1) were lethally irradiated (9.25 Gy) and reconstituted with T cell–depleted bone marrow cells (5 × 106 CD45.2 donor cells and 5 × 106 CD45.1 donor cells) 24 h after the irradiation. Mice were analyzed 5 wk after the reconstitution.
Analysis of Recent Thymus Emigrants in Adult Mice.
The intrathymic labeling technique was described previously (14). Briefly, 10 μl of 600 μg/ml freshly prepared FITC solution in PBS was injected directly into the thymuses of anesthetized mice. Spleen and lymph nodes were removed 24 and 48 h after the injection, and FITC+CD4+ T cells were measured by flow cytometry. Because the distribution of T cells was unequal between control and CCR7 signal–deficient mice, the numbers of systemically supplied CD4+ recent thymic emigrants (CD4 RTE) were estimated as described previously (15) using the following formula: (number of CD4 RTE = number of FITC+CD4 T cells in the spleen + 2 × [total number of FITC+CD4 T cells in mesenteric, inguinal, and axillary lymph nodes]).
Emigration of T cells from Newborn Thymus Organ Culture.
Organ culture of newborn thymus lobes from 5-d-old mice was performed as described previously (16, 17). After 16 h of culture, cells within and outside the thymus lobes were counted and analyzed by flow cytometry.
Detection of Apoptotic Cells.
Freshly isolated thymocytes were three-color stained with FITC–annexin V (Bender MedSystems), PE-labeled anti-CD8 antibody, and APC-labeled anti-CD4 antibody. Apoptotic cells were detected with annexin V staining of the cells that excluded propidium iodide.
Thymocyte Stimulation.
Thymocytes were stimulated with concanavalin A (2.5 μg/ml) in a 24-well plate for 48 h and were stained for CD25. Thymocytes from MHC class I/class II double-deficient mice were stimulated with plate-bound anti-TCRβ monoclonal antibody (H57-597, 10 μg/ml) or the combination of 0.2 ng/ml PMA and 200 ng/ml ionomycin in a 12-well plate for 24–48 h, and were measured for cell surface CCR7 expression.
Flow Cytometric Analysis.
Single cell suspensions were washed with PBS containing 0.2% BSA and 0.1% NaN3. Cells were first incubated with 2.4G2 anti-FcγR monoclonal antibody (reference 18; provided by Dr. J. Unkeless, Leiden University, Leiden, Netherlands) to block the binding of labeled antibodies to FcγR. Then, the cells were stained with FITC-labeled, PE-labeled, APC-labeled, and/or biotinylated antibody. Biotinylated antibodies were detected by PE- or APC-conjugated streptavidin (Molecular Probes). Labeled monoclonal antibodies and normal IgG controls were obtained from BD Biosciences and eBiosciences. For the detection of cell surface CCR7 expression, cells were stained with the recombinant CCL19-Ig chimeric protein (10 μg/ml) (19), followed by biotin-labeled goat anti–human Ig and PE-conjugated streptavidin. Multicolor flow cytometric analysis was performed using two-laser FACS®-Calibur (Becton Dickinson). Data were obtained with Cell Quest software for viable cells that were determined based on measurements of forward light scatter intensity and propidium iodide exclusion.
Hematoxylin and Eosin Staining of Thymus Sections.
Frozen thymuses embedded in OTC compound (Sakura Finetek) were sliced into 5-μm-thick sections and stained with hematoxylin and eosin. Serial sections of entire thymuses were observed with an Eclipse E1000 microscope (Nikon). The areas of the cortex and the medulla were measured by using NIH Image software, version 1.62. Three-dimensional volumes of the cortical and medullary regions were calculated as described previously (20).
Multicolor Fluorescence Analysis of Thymus Sections.
Frozen sections (5 μm) were fixed with acetone and stained with Alexa 488–conjugated anti-CD4 (GK1.5), Alexa 546–conjugated anti-CD8 (53-6-72), and Alexa 633–conjugated UEA1 (Vector Laboratories). Where indicated, sections were stained with FITC-conjugated anti-CD45.1 (clone A20) or biotinylated antibodies specific for CD45.2 (clone 104), CD31 (clone 390), CCL19 (DAKO Corporation), and CCL21 (R&D Systems), followed by Alexa 546– or Alexa 633–conjugated streptavidin (Molecular Probes). Staining with CDR1 (21), G8.8 (22), ER-TR4, and ER-TR5 (reference 23; provided by Dr. W. van Ewijk, Mount Sinai School of Medicine, New York, NY) was visualized with Alexa 488–conjugated anti–rat Ig (Molecular Probes). Staining with anti–keratin 5 (Covance) and anti–keratin 8 (Progen Biotechnik) was visualized with FITC-conjugated anti–rabbit Ig and Alexa 633–conjugated anti–mouse Ig, respectively. The images were acquired with a TCS SP2 confocal laser-scanning microscope (Leica) equipped with Ar and He-Ne lasers (excitations at 488, 546, and 633 nm) and analyzed using Leica confocal software, version 2.0.
RT-PCR Analysis of mRNA Transcripts.
Total cellular RNA from the thymus and the peripheral lymph nodes was reverse transcribed using Superscript II reverse transcriptase (Life Technologies) and oligo-dT oligonucleotide. cDNA was PCR amplified with Taq polymerase (Takara) for CCR7 (5′-TGTACGTCAGTATCACCAGC-3′ and 5′-TTTTCCAGGTGTGCTTCTGC-3′) and hypoxanthine phosphoribosyltransferase (HPRT) (5′-CACAGGACTAGAACACCTGC-3′ and 5′-GCTGGTGAAA-AGGACCTCT-3′). The primers for CCL19, CCL21-ser, and CCL21-leu were as described previously (24). Amplified signals were confirmed to be single bands by gel electrophoresis and were normalized to the housekeeping gene, HPRT. PCR products were electrophoresed on 1.5% agarose gel and visualized by ethidium bromide staining.
Results
Defective T Cell Supply in Newborn CCR7- or CCR7L-deficient Mice.
We have reported previously that the thymus-dependent supply of circulating T cells is defective in newborn CCR7-deficient mice (25). It was also found that peripheral T cell numbers are reduced only in the neonatal period and are restored to the normal level in adult CCR7-deficient mice (25, 26). In the present study, we further investigated how the central supply of T cells is affected by the lack of CCR7. To examine the contribution of CCR7 ligands (CCR7L) to CCR7 signals, we used plt/plt mice deficient for CCR7L expression (27) in addition to CCR7-deficient mice. To minimize the variability of genetic background among individual mice, we used CCR7-deficient mice and plt/plt mice that were both backcrossed to C57BL/6 genetic background for six generations. We confirmed that the CCR7-deficient mice and the plt/plt mice expressed undetectable levels of CCR7 and CCR7L (CCL19, CCL21-ser, and CCL21-leu), respectively, in the thymus and the peripheral lymph nodes (Fig. 1 A, PLN).
Figure 1.
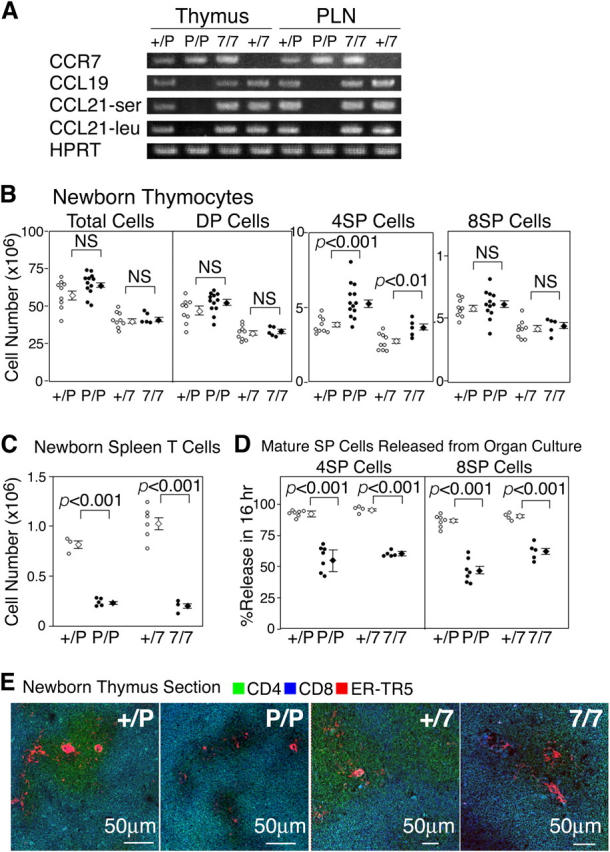
Characterization of thymuses of newborn CCR7- or CCR7L-deficient mice. (A) RT-PCR analysis of lymphoid organs in CCR7- or CCR7L-deficient mice. Total cellular cDNA of thymuses and peripheral lymph nodes (PLN) was isolated from +/plt (+/P), plt/plt (P/P), CCR7+/− (+/7), and CCR7−/− (7/7) mice and was PCR amplified for CCR7, CCL19, CCL21-ser, CCL21-leu, and HPRT. (B) Thymocytes from indicated newborn mice at 5 d old were stained for CD4, CD8, and TCRβ and analyzed by flow cytometry. DP, 4SP, and 8SP represent CD4+CD8+, CD4+CD8−TCRβhigh, and CD4−CD8+TCRβhigh, respectively. Data from individual mice and means ± SE are indicated. (C) CD3highTCRβhigh T cells in the spleen were measured in 5-d-old mice. (D) Newborn mouse thymus lobes were cultured for 16 h, and cells within and outside the thymus lobes were stained for CD4 and CD8. Cells that were released from the thymus lobes were measured for CD4+CD8− and CD4−CD8+ populations. (E) Three-color immunofluorescence analysis of newborn mouse thymus sections on the day of birth for CD4, CD8, and ER-TR5. Cells in cyan color (merging of green and blue) indicate CD4+CD8+ cells expressing both CD4 (in green) and CD8 (in blue). p values were calculated by the Student's t test. NS, not significant.
Both plt/plt mice and CCR7-deficient mice at 5-d-old generated unreduced numbers of thymocytes, including CD4+CD8+ (DP), CD4+CD8− (4SP), and CD4−CD8+ (8SP) cells, compared with control mice (Fig. 1 B). The numbers of 4SP thymocytes in those mutant mice were slightly but significantly larger than those in control mice (Fig. 1 B). In contrast, those mutant mice showed significantly smaller numbers of T cells in the spleen (Fig. 1 C). Unlike the thymus lobes from newborn control mice, which released nearly all of the 4SP and 8SP thymocytes in a 16-h organ culture, the thymus lobes from newborn plt/plt and CCR7-deficient mice exhibited significantly lower release of 4SP and 8SP cells (Fig. 1 D). These results indicate that mice deficient for either CCR7 or CCR7L are defective in the thymus-dependent supply of T cells in the newborn period.
Defective Medulla Formation in the Thymuses of CCR7- or CCR7L-deficient Mice.
To better understand how CCR7 signals contribute to T cell supply by newborn mouse thymus, we next examined the microanatomical architecture of thymuses in newborn mice deficient for CCR7 or CCR7L. As shown in Fig. 1 E, the thymuses from newborn control mice consisted of two distinct regions of the cortex and the medulla; the cortical region was filled with DP immature thymocytes (in cyan by merging green and blue), and the medullary region was identified by the concomitant presence of 4SP (in green; majority) and 8SP (in blue; minority) mature thymocytes amongst ER-TR5+ medullary epithelial cells (23) (in red). On the other hand, the thymuses from newborn plt/plt mice and newborn CCR7-deficient mice exhibited abnormal architectures. Most remarkable was that the medullary regions identified by the presence of ER-TR5+ cells appeared dark and empty, with no colocalization of thymocytes that expressed either CD4 or CD8 (Fig. 1 E). The absolute numbers and frequencies of 4SP and 8SP thymocytes were not reduced in those mutant neonates (Fig. 1 B). Together, the results indicate that mature SP thymocytes generated in the newborn mutant mouse thymus fail to accumulate in the medulla because of the lack of CCR7 signals. This may, in turn, contribute to the defect in T cell export from the newborn thymus.
The architecture of the thymic medullas of plt/plt and CCR7-deficient mice was abnormal even in adulthood. Histological analysis of the thymus by using the medullary–epithelial cell–specific monoclonal antibody, ER-TR5 (Fig. 2 A), and by hematoxylin and eosin staining (Fig. 2 B), revealed that the medullary regions in CCR7- or CCR7L-deficient mice were smaller than those in control mice. Individual medullary regions were small and sparsely distributed throughout the thymuses of the mutant mice, whereas control mouse thymus contained large interconnected medullas (Fig. 2, A and B). Calculation of the three-dimensional volumes of the cortical and medullary regions in the thymuses (20) indicated that the medulla volume of the mutant mouse thymus was ∼50% of that of the control mouse thymus, whereas the cortex volume of the mutant mouse thymus was comparable to that of control mouse thymus (Fig. 2 C). Not only was the medulla volume significantly reduced, the medullary region in the adult mutant mouse thymus showed poor accumulation of 4SP and 8SP thymocytes as well (Fig. 2 D). On the other hand, the CD4/CD8 profiles, including the ratios of the DP, 4SP, and 8SP populations and the TCRβ expression profiles of adult thymocytes were unaffected in the mutant mice (Fig. 2, E and F). These results indicate that even in adult mice mature thymocytes are poorly localized to the thymic medullas in the absence of CCR7 signals.
Figure 2.
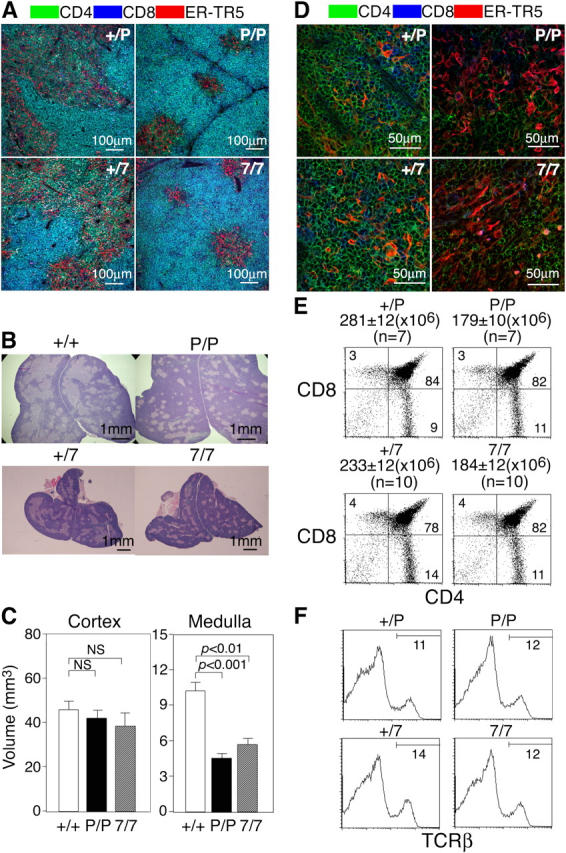
Characterization of thymuses of adult CCR7- or CCR7L-deficient mice. (A) Three-color immunofluorescence analysis for CD4 (green), CD8 (blue), and ER-TR5 (red). (B) Hematoxylin and eosin staining. (C) Three-dimensional analysis of the volumes (in cubic millimeters; means ± SEs) of cortical and medullary regions. (D) High magnification images of the immunofluorescence analysis for CD4, CD8, and ER-TR5. ER-TR5+ medullary regions are shown. (E and F) Flow cytometric analysis of thymocytes. Means ± SEs of total thymocytes (numbers of mice examined) are indicated. Numbers within each area indicate the frequency of cells within that area.
CCR7L proteins in the thymus, which were predominantly produced by medullary epithelial cells (25), were specifically detected in the medullary regions of control mice but not in the thymuses of plt/plt mice (Fig. 3 A), reinforcing the possibility that CCR7L expressed in the medulla is involved in the accumulation of CCR7-expressing mature thymocytes in the medulla. It should be noted also that unlike the thymic medullary region in control mouse, the thymic medullas in plt/plt and CCR7-deficient mice were defective in forming Hassall's corpuscle–like clusters of UEA1+ and G8.8+ epithelial cells (22, 28) (Fig. 3, B and C, arrows). On the other hand, epithelial cells in the cortical region identified by CDR1, ER-TR4, and keratin-8 (K8) were normally organized (21, 29) (Fig. 3, A, D, and E). These results indicate that because of the lack of CCR7 signals the thymic medullary region is small in volume and defective in forming an optimal architecture.
Figure 3.
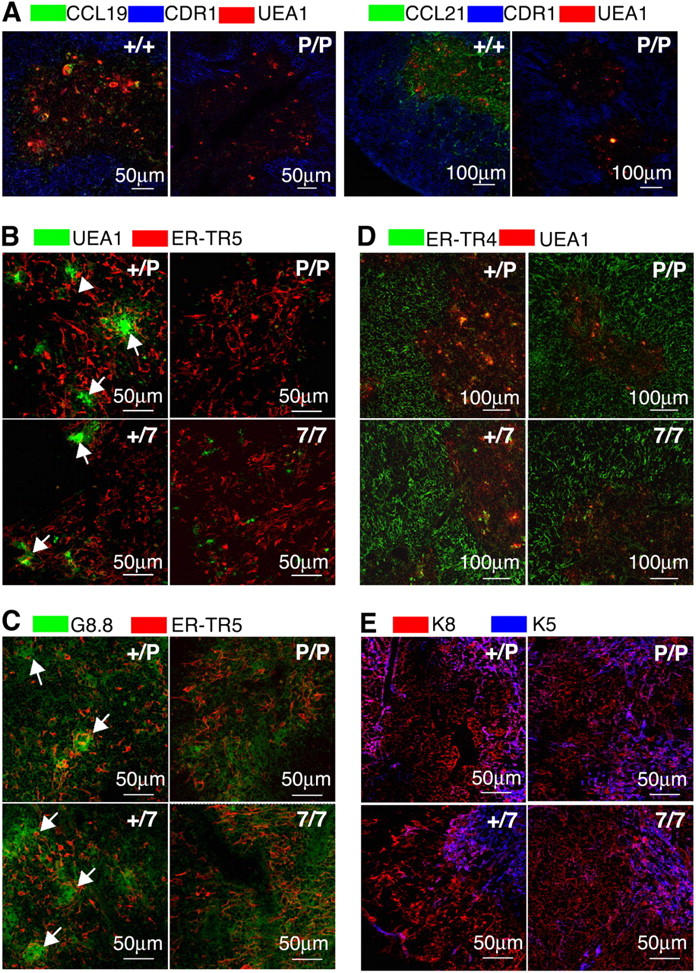
Thymic stromal cells in adult CCR7- or CCR7L-deficient mice. (A) Three-color immunofluorescence analysis for cortical epithelial cell specific CDR1 (blue), medullary epithelial cell specific UEA1 (red), and either CCL19 or CCL21 (green). (B) Medullary regions were stained for UEA1 (green) and ER-TR5 (red). (C) Medullary regions were stained for G8.8 (green) and ER-TR5 (red). (D) Staining for cortical epithelial cell–specific ER-TR4 (green) and medullary epithelial cell–specific UEA1 (red). (E) Staining for cortical epithelial cell–specific keratin-8 (red) and medullary epithelial cell–specific keratin-5 (blue). Arrows in B and C indicate clusters of highly condensed UEA1+ and G8.8+ cells in the medulla.
Arrest of SP Thymocytes in the Cortices of CCR7- or CCR7L-deficient Mice.
The results described above indicate that the lack of CCR7 signals results in the poor accumulation of mature thymocytes in the thymic medulla. Whereas SP thymocytes were sparsely populated in the thymic medullas of plt/plt and CCR7-deficient mice, the generation of SP thymocytes was not perturbed in those mice. We then examined where mature thymocytes were localized in the thymuses of CCR7- or CCR7L-deficient mice. Initial attempts at analyzing the thymus sections from untreated mice led to the detection of SP thymocytes in the cortices of plt/plt and CCR7-deficient mice but not of control mice (Fig. 4 A, arrows). However, an overwhelming number of DP thymocytes that were tightly packed in the cortices hampered the clear distinction between DP and SP thymocytes in the cortex of untreated mice by multicolor immunofluorescence analysis. Therefore, we used the following three approaches to better identify the intrathymic localization of SP thymocytes in CCR7- or CCR7L-deficient mice.
Figure 4.
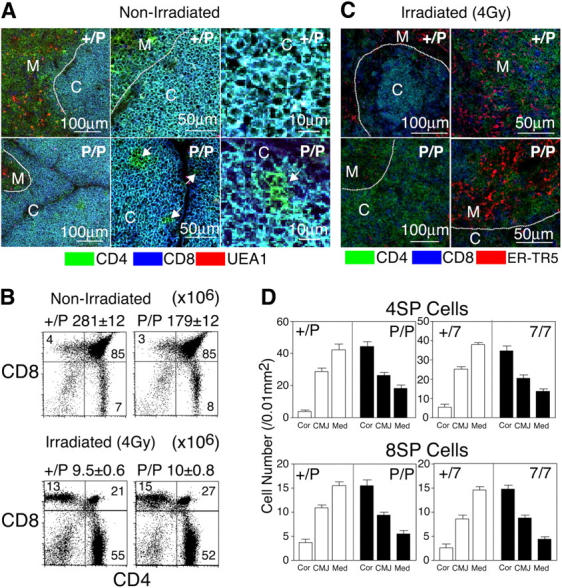
Distribution of SP thymocytes in the thymuses of sublethally irradiated adult CCR7- or CCR7L-deficient mice. (A and C) Three-color immunofluorescence analysis of the thymuses from nonirradiated (A) and 4 Gy–irradiated (C) mice. Dashed lines indicate junctions between the cortex and the medulla. C, cortex; M, medulla. Arrows in A indicate CD4+CD8− cells (in green) in the cortical region. (B) Flow cytometric analysis of thymocytes from nonirradiated and irradiated mice. Means ± SEs of total thymocytes are also indicated. (D) Means ± SEs of the numbers of CD4+CD8− (4SP) and CD4−CD8+ (8SP) cells per unit area (0.01 mm2) of indicated regions of thymus sections are indicated. Cor, cortex; CMJ, cortico-medullary junction; Med, medulla.
In the first set of experiments, mice were sublethally irradiated in order to deplete the majority of immature DP thymocytes. It has been shown that sublethal irradiation induces the apoptosis of immature cortical DP thymocytes rather than that of mature medullary SP thymocytes (30). Indeed, in both plt/plt mice and control mice the thymuses that were isolated from 4 Gy–irradiated mice contained <10% thymocytes compared with those from nonirradiated mice, with the loss of DP thymocytes being the most drastic (Fig. 4 B). We found that unlike the thymic cortices of irradiated control mice, which contained a small number of DP thymocytes, the thymic cortices of irradiated plt/plt and CCR7-deficient mice contained a considerable number of 4SP and 8SP thymocytes (Fig. 4, C and D). The majority of radiation-resistant 4SP and 8SP thymocytes were found in the thymic cortices rather than the medullas of those mutant mice (Fig. 4 D). However, it should be noted that small numbers of SP thymocytes were found in the medulla even in CCR7- or CCR7L-deficient mice. These results indicate that radiation-resistant mature SP thymocytes are mostly arrested in the thymic cortices of CCR7- and CCR7L-deficient mice.
Second, thymus sections from untreated AND-TCR transgenic mice were examined. It has been reported that class II MHC–restricted AND-TCR transgenic thymocytes are efficiently positively selected in H-2b mice, and up to 30–50% of the thymocytes become 4SP in those mice (reference 10 and Fig. 5 A). Thus, it was reasoned that the localization of 4SP thymocytes could be better visualized even in untreated mice in the presence of DP thymocytes. Accordingly, we found that in AND-TCR transgenic mice that were plt/plt or CCR7−/−, the medullary regions were poorly formed, and many CD4SP thymocytes were detected within the cortices (Fig. 5, B and C). These results indicate that positively selected mature SP thymocytes are poorly exported from the cortex because of the lack of CCR7 signals.
Figure 5.
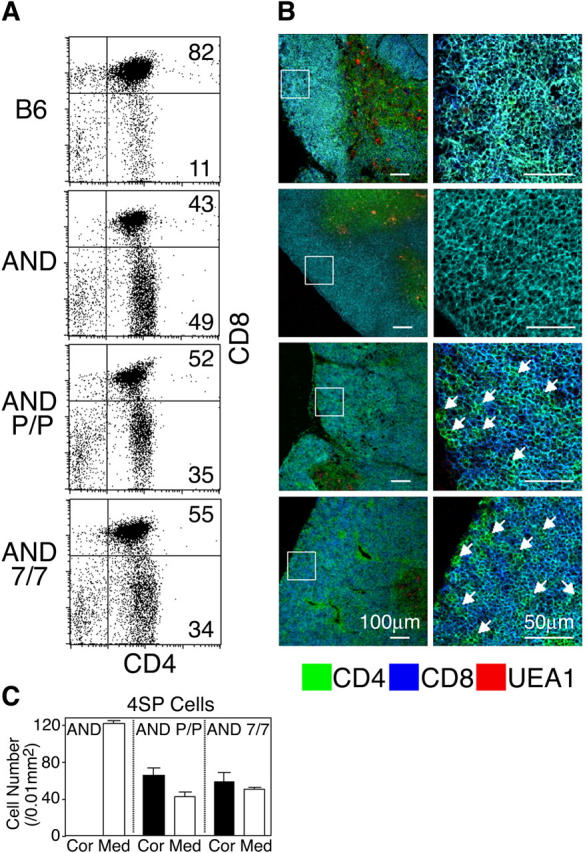
Distribution of CD4 SP thymocytes in adult CCR7- or CCR7L-deficient AND-TCR transgenic mice. (A) Flow cytometric analysis of thymocytes from indicated mice. Numbers within each area indicate the frequency of cells within that area. (B) Three-color immunofluorescence analysis of the thymus sections for CD4, CD8, and UEA1. Higher magnification images of boxes in the left panels are shown on the right. Arrows indicate CD4+CD8− cells (in green) in the cortex. (C) Means ± SEs of the numbers of CD4+CD8− (4SP) cells per unit area (0.01 mm2) of indicated regions of thymus sections are indicated.
Third, lethally irradiated mice (CD45.1) were reconstituted with a mixture of T cell–depleted bone marrow cells from wild-type (CD45.1) and CCR7−/− (CD45.2) mice (Fig. 6 A). In this experimental setting, CCR7−/− thymocytes (KO donor) or control thymocytes (WT donor) were expected to develop in the environment of normal thymic stromal cells in the presence of normal developing thymocytes. The staining with CD45.1- and CD45.2-specific monoclonal antibodies enabled us to distinguish between CD45.2+ thymocytes (derived from KO donor or WT donor bone marrow cells) and CD45.1+ thymocytes (derived from codeveloping normal thymocytes and radiation-resistant normal host cells). The thymuses analyzed at 5 wk after the bone marrow reconstitution showed comparable development of CD45.1+ and CD45.2+ thymocytes between the KO donor group and the WT donor group (Fig. 6 B). The inefficient repopulation of the CCR7-KO T cells in the lymph nodes (Fig. 6 B) was consistent with the previously reported role of CCR7 in the migration of T cells into the lymph nodes (26). Immunofluorescence analysis of the thymus sections indicated that both cortex and medulla were generated in the KO donor–reconstituted group and in the WT donor–reconstituted group (Fig. 6 C), consistent with the normal development and normal cortex–medulla distribution of wild-type bone marrow–derived CD45.1+ thymocytes (Fig. 6, B and C). Most importantly in this experiment, KO donor–derived CD45.2+ thymocytes were found only in the cortex and not in the medulla, whereas WT donor–derived CD45.2+ thymocytes were distributed in both the cortex and the medulla (Fig. 6, C and D). Thus, even in the normal thymus environment in which the medullary region is normally generated, CCR7-deficient thymocytes are defective in the migration from the cortex to the medulla. Together, these results indicate that because of the lack of CCR7 or CCR7L mature SP thymocytes are arrested within the cortex, being defective in the migration to the medulla.
Figure 6.
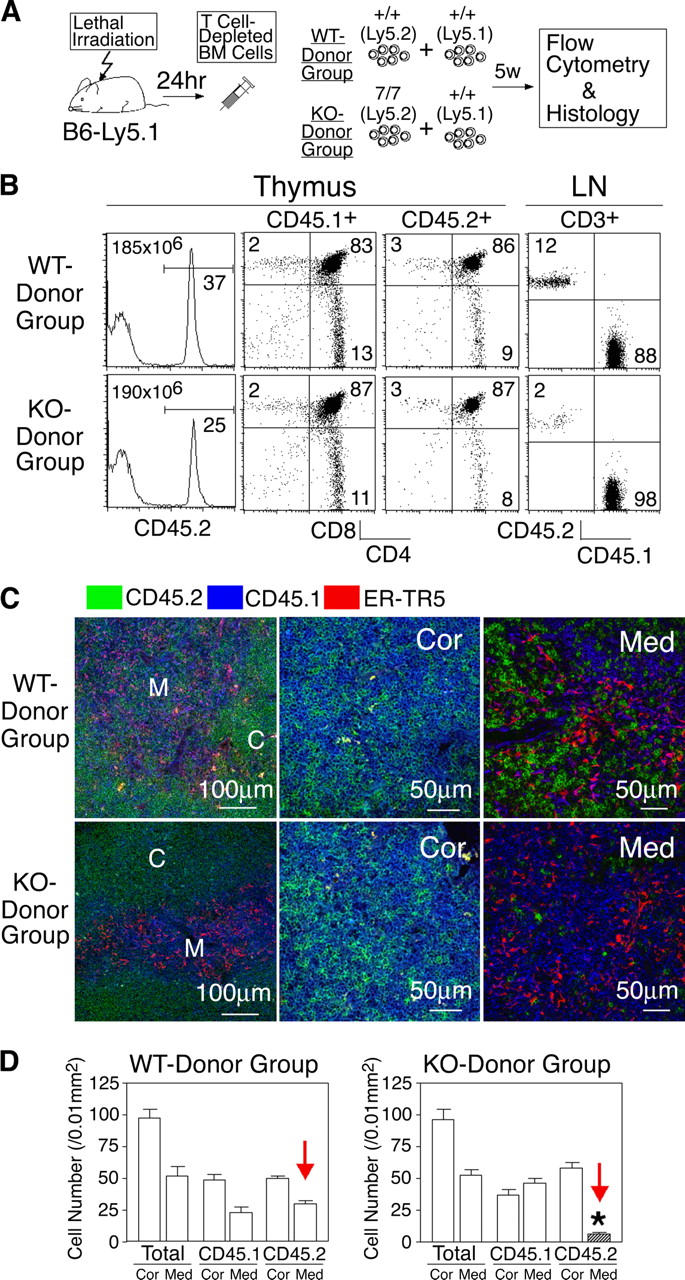
Intrathymic distribution of CCR7-deficient thymocytes in mixed bone marrow chimeras. (A) Scheme of the experimental protocol. (B) Flow cytometric analysis of thymocytes and lymph node (LN) cells. Where indicated, cells were gated for CD45.1+, CD45.2+, and CD3+ populations. Means of total thymocytes are also shown in histogram panels. (C) Three-color immunofluorescence analysis of thymus sections for CD45.2 (green), CD45.1 (blue), and ER-TR5 (red). (D) Means ± SEs of the numbers of total, CD45.1+, and CD45.2+ cells per unit area (0.01 mm2) of indicated regions of thymus sections are indicated. C and Cor, cortex; M and Med, medulla. *, P < 0.001.
Developmental Profiles of SP Thymocytes in CCR7- or CCR7L-deficient Mice.
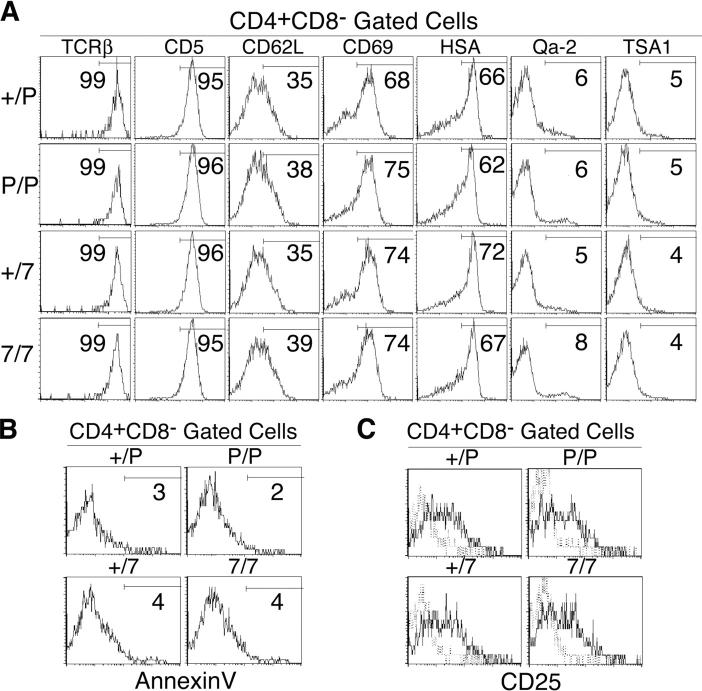
The results above indicated that SP thymocytes in CCR7- or CCR7L-deficient mice were defective in the cortex–medulla migration. Thus, it was interesting to examine whether SP thymocytes generated in CCR7- or CCR7L-deficient mice might be developmentally altered by the defective migration to the medulla. Flow cytometric analysis of 4SP thymocytes revealed no remarkable differences in the expression profiles of TCRβ, CD5, CD62L, CD69, HSA, Qa-2, and TSA1 between the mutant mice and the control mice (Fig. 7 A). In addition, 4SP thymocytes from those mutant mice showed neither signs of enhanced apoptosis (Fig. 7 B) nor alterations in receptor-induced activation, such as CD25 up-regulation upon concanavalin A stimulation (Fig. 7 C). Similar to 4SP thymocytes, 8SP thymocytes in CCR7- or CCR7L-deficient mice showed no abnormality in these criteria (unpublished data). Thus, in CCR7- or CCR7L-deficient mice, we found that there were no defects in the SP thymocytes based on the profiles of maturation, survival, and responsiveness, even though those cells were defective in the migration from the cortex to the medulla.
Figure 7.
Flow cytometric analysis of SP thymocytes in adult CCR7- or CCR7L-deficient mice. (A) Thymocytes from indicated mice were three-color stained for CD4, CD8, and indicated molecules. Shown are the profiles of CD4+CD8− cells. (B) Annexin V staining profiles of CD4+CD8− thymocytes from indicated mice. (C) Concanavalin A–stimulated thymocytes (solid lines) and control nonstimulated thymocytes (dashed lines) from indicated mice were stained for CD4, CD8, and CD25.
Positive selection and negative selection of TCR transgenic thymocytes appeared undisturbed in CCR7L-deficient plt/plt mice that were crossed to HY-TCR transgenic mice or 2C-TCR transgenic mice (Fig. 8 A). Positive selection of thymocytes was not disturbed in AND-TCR transgenic mice that were crossed to either plt/plt or CCR7−/− mice (Fig. 5 A). Thus, positive and negative selection of these TCR transgenic thymocytes could occur in the cortex without normal migration to the medulla. Vβ3+ and Vβ5+ T cells in CD4SP and CD8SP thymocytes were deleted in BALB/c–plt/plt mice, similar to normal BALB/c mice (Fig. 8, B and C), indicating that mtv superantigen–mediated negative selection may occur in the thymus lacking CCR7L.
Figure 8.
Selection and export of thymocytes in adult CCR7L- or CCR7-deficient mice, and CCR7 surface expression in thymocytes. (A) Thymocytes isolated from indicated mice were three-color stained for CD4, CD8, and transgenic TCR (T3.70 for HY-TCR and 1B2 for 2C-TCR). Shown are the dot plot profiles of transgenic TCR-expressing cells. Means ± SEs of total thymocytes are also indicated. (B and C) Thymocytes isolated from wild-type (wt) and plt/plt (plt) mice of C57BL/6 and BALB/c backgrounds were three-color stained for CD4, CD8, and indicated TCR-Vβs. Monoclonal antibodies specific for Cβ, Vβ3, Vβ4, Vβ5, Vβ6, and Vβ8 used were H57-597, KJ25, KT4, MR9-4, RR4-7, and F23.1, respectively. Shown are the frequencies of indicated Vβ+ cells within CD4SP (B) and CD8SP (C) populations. Means ± SEs of data from three individual mice are indicated. (D) Recent thymus emigrants within peripheral CD4+CD8− T cells were measured in adult mice at 24 and 48 h after the intrathymic FITC labeling. NS, not significant. (E) Thymocytes from adult B6 mice (top) or CCR7-deficient mice (bottom) were stained for CCL19-Ig. Dashed lines show control staining profiles. (F) CCL19-Ig staining profiles (solid lines) and control staining profiles (dashed lines) of indicated fractions of adult B6 thymocytes. (G) CCL19-Ig staining profiles (solid lines) and control staining profiles (dashed lines) of DP thymocytes from MHC-deficient mice. Thymocytes were cultured in suspension for indicated hours in the absence or presence of indicated reagents and were stained for CD4, CD8, and CCL19-Ig. (H) CCL19-Ig staining profiles of DP thymocytes from 2C-TCR transgenic mice of positively selecting H-2k/b background (kb, bold line) and null selecting H-2k/k background (kk, thin line). Dashed line indicates control staining profile.
We also examined whether the thymus-dependent supply of T cells was affected in adult mice deficient for CCR7 or CCR7L. As shown in Fig. 8 D, we detected no defects in the supply of intrathymically labeled T cells to the peripheral circulation in adult plt/plt or CCR7−/− mice by the recent thymus emigrant assay (14, 15). Thus, unlike newborn mouse thymocytes, adult mouse thymocytes could be exported from the thymus to the circulation in the absence of CCR7 signals or CCR7-dependent accumulation of mature thymocytes in the medulla.
TCR-mediated Elevation of CCR7 Expression on the Surface of Developing Thymocytes.
Finally, we examined how CCR7 was expressed on the surface of developing thymocytes. To do so, we used CCL19–Ig fusion protein that specifically detects CCR7 expression on the surface of control thymocytes and not CCR7-deficient thymocytes (Fig. 8 E). In agreement with previously reported mRNA levels of thymocyte subpopulations (4, 5), the surface expression of CCR7 was detected in a majority of 4SP and 8SP thymocytes (Fig. 8 F). The CCR7 surface expression was not detected in most immature DN and DP thymocytes, although low levels of CCR7 expression were detected in a small fraction of DN thymocytes (Fig. 8 F). To examine whether TCR signals could directly increase CCR7 expression on the surface of immature cortical DP thymocytes, preselected DP thymocytes isolated from MHC-deficient mice were stimulated with anti-TCRβ antibody or PMA plus ionomycin in suspension culture. Because of the lack of class I and class II MHC molecules, DP thymocytes in MHC-deficient mice do not experience TCR engagement in vivo and are arrested in the cortex at the preselected stage (31, 32). As shown in Fig. 8 G, TCR-stimulated or PMA/ionomycin-stimulated DP thymocytes from MHC-deficient mice exhibited an increase in the surface expression of CCR7 within 48 h, indicating that TCR engagement directly increases CCR7 expression on the surface of cortical DP thymocytes. 2C-TCR transgenic DP thymocytes expressed higher levels of CCR7 in positively selecting H-2k/b background than in null selecting H-2k/k background (Fig. 8 H) in agreement with a previous result showing that the functional responsiveness to CCR7 ligands is higher in CD69+ DP thymocytes than in CD69− DP thymocytes (5). These results suggest that positively selecting TCR signals up-regulate the surface expression of CCR7 in immature thymocytes.
Discussion
The present results indicate that in mice deficient for CCR7 or CCR7L, mature SP thymocytes are poorly localized to the thymic medulla. The arrest of SP thymocytes in the cortex of plt/plt or CCR7−/− mice was demonstrated by (a) careful examination of the cortex of untreated mutant mice, (b) determination of the distribution of radiation-resistant SP thymocytes, (c) detection of positively selected AND-TCR transgenic SP thymocytes, and (d) analysis of thymus sections in mixed bone marrow chimeras. All the results of these experiments indicate that in the absence of CCR7 signals, mature SP thymocytes are arrested within the cortex and that CCR7 signals are essential for developing thymocytes to migrate from the cortex to the medulla. Our results also show that TCR engagement of DP thymocytes elevates the cell surface expression of CCR7, suggesting the following scenario of intrathymic positive selection: DP thymocytes are newly generated in the thymic cortex, encounter positively selecting TCR ligands in the cortex, undergo a series of TCR signal–mediated maturation programs including CCR7 expression, and migrate to the medulla via chemoattraction to CCR7L produced by medullary epithelial cells. Consequently, the positively selected thymocytes in control mice migrate to the medulla upon the completion of the maturation program to become SP, whereas in mice deficient for CCR7 or CCR7L, the positively selected thymocytes remain in the cortex even after the differentiation to become SP.
Poor accumulation of mature SP thymocytes in the medullas of plt/plt or CCR7−/− mice was found in the newborn period and in adulthood. As suggested previously by the defective supply of circulating T cells (25) and as confirmed by the measurement of thymocyte release in the present study, the emigration of T cells from the thymus is defective in newborn mice lacking CCR7 signals. Even though the small numbers of peripheral T cells in newborn mutant mice may also be due to poor survival of circulating T cells in the absence of CCR7, the present results show that CCR7 is essential for the migration of SP thymocytes to the medulla and the optimal emigration of T cells from the thymus in newborn mice. The defective T cell export from the thymus in newborn CCR7 signal–deficient mice may be due to the defective accumulation of mature SP thymocytes in the medullas. Thus, the CCR7-dependent migration of mature thymocytes to the medullary regions may be involved in the export of T cells from the thymuses of the newborn mice.
On the other hand, the present results indicate that the thymuses of adult plt/plt or CCR7−/− mice are capable of normally exporting T cells even though their medullary regions exhibit poor accumulation of mature SP thymocytes. Thus, in contrast to the case of newborn mouse thymus, T cells generated in the adult mouse thymus can be exported in the absence of CCR7 signals and in the absence of CCR7-dependent accumulation of mature thymocytes in the medulla. It is possible that the accumulation of mature thymocytes in the medulla has a minor involvement in T cell emigration in the adult mouse, whereas CCR7-dependent migration to the medulla may be essential for newborn mouse thymic emigration. Indeed, it was reported that the thymuses in adult _relB_-deficient mice lacking thymic medullas can export fully functional T cells (33, 34), consistent with the possibility that T cell emigration from adult mouse thymus can occur without the thymic medulla.
Even though T cell emigration from the adult thymus occurs in the absence of CCR7 signals, T cell export from the adult thymus is dependent on G protein signals (35). It was shown recently that T cell egress from adult thymus is dependent on sphingosine-1–phosphate receptor and that the sphingosine-1–phosphate–mediated chemotaxis of mature thymocytes is dependent on G protein signals (36, 37). Thus, T cell export from the thymus is regulated by CCR7 signals and sphingosine-1–phosphate receptor signals, and the dependence on those two molecular mechanisms may be switched during ontogeny.
Our results indicate that because of the lack of CCR7 signals, the thymic medullary region is defective not only in accumulating SP thymocytes but also in forming an optimal architecture, including Hassall's corpuscle–like clusters of medullary epithelial cells. These results are consistent with the possibility of a lympho-epithelial cross talk between thymic epithelial cells and developing thymocytes (38). Poor accumulation of mature thymocytes in the medulla may perturb the full maturation of medullary epithelial cells, which is essential for the formation of an optimal architecture.
One recent report has shown that adult mice deficient for lymphotoxin β receptor exhibit defective maturation of thymic medullary epithelial cells and defective export of mature thymocytes (39). On the other hand, our results show that adult mice deficient for CCR7 signals exhibit defective maturation of medullary epithelial cells and unaffected export of mature thymocytes, indicating that there is a difference between the signals regulating the maturation of medullary structures and the signals regulating the export of mature thymocytes. Unlike CCR7 signals, lymphotoxin β receptor signals may control the development of thymic stromal components that are essential for both T cell export and the formation of medullary structures.
Finally, the present results revealed no defects in the maturation profiles of thymocytes in CCR7- or CCR7L-deficient mice, even though the thymocytes were defective in the migration from the cortex to the medulla. In the TCR transgenic mice used in the present study, both positive selection and negative selection were normally detected in the absence of CCR7 signals without accumulation to the medulla. Mtv superantigen–mediated negative selection in the thymus was also detected in the absence of CCR7 signals. Thus, it is conceivable that the accumulation to the medullary environment is not essential for T cell development including the processes of positive and negative selection and that T cell development and selection can occur only within the thymic cortex. However, it has been shown in _relB_-deficient mice that negative selection is impaired in the absence of the medulla, as evidenced by autoreactive proliferative responses (34). In addition, thymic medullary epithelial cells have been shown to express AIRE, which regulates the tolerance of T cells to organ-specific self-antigens (40, 41). Thus, negative selection in the thymus may occur in the cortex and the medulla depending on ubiquitous and organ-specific self-antigens that are expressed in the cortical and medullary environments, respectively.
In conclusion, the present results reveal that CCR7 and CCR7L are essential for the migration of developing thymocytes from the cortex to the medulla. CCR7 signals play a major role in intrathymic T cell migration during thymocyte development in addition to their previously appreciated role in T cell migration to secondary lymphoid organs in immune responses.
Acknowledgments
We thank Drs. S. Hedrick, H. von Boehmer, and D. Loh for TCR transgenic mice; Drs. M. Malin, U. Hopken, G. Hollander, and O. Yoshie for discussion; and Drs. N. Iwanami, S. Tomita, and T. Nitta for critical reading of the article.
This work was supported by the grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
The authors have no conflicting financial interests.
Abbreviations used in this paper: DP, double positive; HPRT, hypoxanthine phosphoribosyltransferase; 4SP, CD4 single positive; 8SP, CD8 single positive; SP, single positive.
References
- 1.van Ewijk, W. 1991. T-cell differentiation is influenced by thymic microenvironments. Annu. Rev. Immunol. 9:591–615. [DOI] [PubMed] [Google Scholar]
- 2.Gill, J., M. Malin, J. Sutherland, D. Gray, G. Hollander, and R. Boyd. 2003. Thymic generation and regeneration. Immunol. Rev. 195:28–50. [DOI] [PubMed] [Google Scholar]
- 3.Petrie, H.T. 2003. Cell migration and the control of post-natal T cell lymphopoiesis in the thymus. Nat. Rev. Immunol. 3:859–866. [DOI] [PubMed] [Google Scholar]
- 4.Kim, C.H., L.M. Pelus, J.R. White, and H.E. Broxmeyer. 1998. Differential chemotactic behavior of developing T cells in response to thymic chemokines. Blood. 91:4434–4443. [PubMed] [Google Scholar]
- 5.Campbell, J.J., J. Pan, and E.C. Butcher. 1999. Developmental switches in chemokine responses during T cell maturation. J. Immunol. 163:2353–2357. [PubMed] [Google Scholar]
- 6.Norment, A.M., and M.J. Bevan. 2000. Role of chemokines in thymocyte development. Semin. Immunol. 12:445-455. [DOI] [PubMed] [Google Scholar]
- 7.Plotkin, J., S.E. Prockop, A. Lepique, and H.T. Petrie. 2003. Critical role for CXCR4 signaling in progenitor localization and T cell differentiation in the postnatal thymus. J. Immunol. 171:4521–4527. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki, G., H. Sawa, Y. Kobayashi, Y. Nakata, K. Nakagawa, A. Uzawa, H. Sakiyama, S. Kakinuma, K. Iwabuchi, and K. Nagashima. 1999. Pertussis toxin-sensitive signal controls the trafficking of thymocytes across the corticomedullary junction in the thymus. J. Immunol. 162:5981–5985. [PubMed] [Google Scholar]
- 9.Fukui, Y., O. Hashimoto, T. Sanui, T. Oono, H. Koga, M. Abe, A. Inayoshi, M. Noda, M. Oike, T. Shirai, et al. 2001. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature. 412:826–831. [DOI] [PubMed] [Google Scholar]
- 10.Kaye, J., M.L. Hsu, M.E. Sauron, S.C. Jameson, N.R.J. Gascoigne, and S.M. Hedrick. 1989. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature. 341:746–749. [DOI] [PubMed] [Google Scholar]
- 11.Teh, H.S., H. Kishi, B. Scott, and H. von Boehmer. 1989. Deletion of autospecific T cells in T cell receptor (TCR) transgenic mice spares cells with normal TCR levels and low levels of CD8 molecules. J. Exp. Med. 169:795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sha, W.C., C.A. Nelson, R.D. Newberry, D.M. Kranz, J.H. Russell, and D.Y. Loh. 1988. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 336:73–76. [DOI] [PubMed] [Google Scholar]
- 13.Cosgrove, D., D. Gray, A. Dierich, J. Kaufman, M. Lemeur, C. Benoist, and D. Mathis. 1991. Mice lacking MHC class II molecules. Cell. 66:1051–1066. [DOI] [PubMed] [Google Scholar]
- 14.Scollay, R., E.C. Butcher, and I.L. Weissman. 1980. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur. J. Immunol. 10:210–218. [DOI] [PubMed] [Google Scholar]
- 15.Berzins, S.P., R.L. Boyd, and J.F.A.P. Miller. 1998. The role of the thymus and recent thymic migrants in the maintenance of the adult peripheral lymphocyte pool. J. Exp. Med. 187:1839–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahama, Y. 2000. Differentiation of murine thymocytes in fetal thymus organ culture. Methods Mol. Biol. 134:37–46. [DOI] [PubMed] [Google Scholar]
- 17.Lee, C., K. Kim, L.A. Welniak, W.J. Murphy, K. Muegge, and S.K. Durum. 2001. Thymic emigrants isolated by a new method possess unique phenotypic and functional properties. Blood. 97:1360–1369. [DOI] [PubMed] [Google Scholar]
- 18.Unkeless, J.C. 1979. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptor. J. Exp. Med. 150:580–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manjunath, N., P. Shankar, J. Wan, W. Weninger, M.A. Crowley, K. Hieshima, T.A. Springer, X. Fan, H. Shen, J. Liebenman, et al. 2001. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J. Clin. Invest. 108:871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nasreen, M., T. Ueno, F. Saito, and Y. Takahama. 2003. In vivo treatment of class II MHC-deficient mice with anti-TCR antibody restores the generation of circulating CD4 T cells and optimal architecture of thymic medulla. J. Immunol. 171:3394–3400. [DOI] [PubMed] [Google Scholar]
- 21.Rouse, R.V., L.M. Bolin, J.R. Bender, and B.A. Kyewski. 1988. Monoclonal antibodies reactive with subsets of mouse and human thymic epithelial cells. J. Histochem. Cytochem. 36:1511–1517. [DOI] [PubMed] [Google Scholar]
- 22.Farr, A., A. Nelson, J. Truex, and S. Hosier. 1991. Epithelial heterogeneity in the murine thymus: a cell surface glycoprotein expressed by subcapsular and medullary epithelium. J. Histochem. Cytochem. 39:645–653. [DOI] [PubMed] [Google Scholar]
- 23.van Vilet, E., M. Melis, and W. van Ewijk. 1984. Monoclonal antibodies to stromal cell types of the mouse thymus. Eur. J. Immunol. 14:524–529. [DOI] [PubMed] [Google Scholar]
- 24.Luther, S.A., H.L. Tang, P.L. Hyman, A.G. Farr, and J.G. Cyster. 2000. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc. Natl. Acad. Sci. USA. 97:12694–12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ueno, T., K. Hara, M. Swope Willis, M.A. Malin, U.E. Höpken, D.H.D. Gray, K. Matsushima, M. Lipp, T.A. Springer, R.L. Boyd, et al. 2002. Role for CCR7 ligands in the emigration of newly generated T lymphocytes from the neonatal thymus. Immunity. 16:205–218. [DOI] [PubMed] [Google Scholar]
- 26.Forster, R., A. Schubel, D. Breitfeld, E. Kremmer, I. Renner-Muller, E. Wolf, and M. Lipp. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 99:23–33. [DOI] [PubMed] [Google Scholar]
- 27.Nakano, H., S. Mori, H. Yonekawa, H. Nariuchi, A. Matsuzawa, and T. Kakiuchi. 1998. A novel mutant gene involved in T-lymphocyte-specific homing into peripheral lymphoid organs on mouse chromosome 4. Blood. 91:2886–2895. [PubMed] [Google Scholar]
- 28.Boyd, R.L., C.L. Tucek, D.I. Godfrey, D.J. Izon, T.J. Wilson, N.J. Davidson, A.G. Bean, H.M. Ladyman, M.A. Ritter, and P. Hugo. 1993. The thymic microenvironment. Immunol. Today. 14:445–459. [DOI] [PubMed] [Google Scholar]
- 29.Klug, D.B., C. Carter, E. Crouch, D. Roop, C.J. Conti, and E.R. Richie. 1998. Interdependence of cortical thymic epithelial cell differentiation and T-lineage commitment. Proc. Natl. Acad. Sci. USA. 95:11822–11827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huiskamp, R., and W. van Ewijk. 1985. Repopulation of the mouse thymus after sublethal fission neutron irradiation. J. Immunol. 134:2161–2169. [PubMed] [Google Scholar]
- 31.Grusby, M. J., H. Auchincloss Jr., L. Lee, R.S. Johnson, J.P. Spencer, M. Zijlstra, R. Jaenisch, V.E. Papaioannou, and L.H. Glimcher. 1993. Mice lacking major histocompatibility complex class I and class II molecules. Proc. Natl. Acad. Sci. USA. 90:3913–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zerrahn, J., W. Held, and D.H. Raulet. 1997. The MHC reactivity of the T cell repertoire prior to positive and negative selection. Cell. 88:627–636. [DOI] [PubMed] [Google Scholar]
- 33.Burkly, L., C. Hession, L. Ogata, C. Reilly, L.A. Marconi, D. Olson, R. Tizard, R. Cate, and D. Lo. 1995. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 373:531–536. [DOI] [PubMed] [Google Scholar]
- 34.DeKoning, J., L. DiMolfetto, C. Reilly, Q. Wei, W.L. Havran, and D. Lo. 1997. Thymic cortical epithelium is sufficient for the development of mature T cells in relB-deficient mice. J. Immunol. 158:2558–2566. [PubMed] [Google Scholar]
- 35.Chaffin, K.E., and R.M. Perlmutter. 1991. A pertussis toxin-sensitive process controls thymocyte emigration. Eur. J. Immunol. 21:2565–2573. [DOI] [PubMed] [Google Scholar]
- 36.Yagi, H., R. Kamba, K. Chiba, H. Soga, K. Yaguchi, M. Nakamura, and T. Itoh. 2000. Immunosuppressant FTY720 inhibits thymocyte emigration. Eur. J. Immunol. 30:1435–1444. [DOI] [PubMed] [Google Scholar]
- 37.Matloubian, M., C.G. Lo, G. Cinamon, M.J. Lesneski, Y. Xu, V. Brinkman, M.L. Allende, R.L. Proia, and J.G. Cyster. 2004. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 427:355–360. [DOI] [PubMed] [Google Scholar]
- 38.van Ewijk, W., E.W. Shores, and A. Singer. 1994. Crosstalk in the mouse thymus. Immunol. Today. 15:214–217. [DOI] [PubMed] [Google Scholar]
- 39.Boehm, T., S. Scheu, K. Pfeffer, and C.C. Bleul. 2003. Thymic medullary epithelial cells differentiation, thymocyte emigration, and the control of autoimmunity require lymph-epithelial cross talk via LTβR. J. Exp. Med. 198:757–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson, M.S., E.S. Venanzi, L. Klein, Z. Chen, S.P. Berzins, S.J. Turley, H. von Boehmer, R. Bronson, A. Dierich, C. Benoist, and D. Mathis. 2002. Projection of an immunological self shadow within the thymus by the aire protein. Science. 298:1395–1401. [DOI] [PubMed] [Google Scholar]
- 41.Liston, A., S. Lesage, J. Wilson, L. Peltonen, and C.C. Goodnow. 2003. Aire regulates negative selection of organ-specific T cells. Nat. Immunol. 4:350–354. [DOI] [PubMed] [Google Scholar]