Predominant Role for Directly Transfected Dendritic Cells in Antigen Presentation to CD8+ T Cells after Gene Gun Immunization (original) (raw)
Abstract
Cutaneous gene (DNA) bombardment results in substantial expression of the encoded antigen in the epidermal layer as well as detectable expression in dendritic cells (DC) in draining lymph nodes (LNs). Under these conditions, two possible modes of DC antigen presentation to naive CD8+ T cells might exist: (a) presentation directly by gene-transfected DC trafficking to local lymph nodes, and (b) cross-presentation by untransfected DC of antigen released from or associated with transfected epidermal cells. The relative contributions of these distinct modes of antigen presentation to priming for cytotoxic T cell (CTL) responses have not been clearly established. Here we show that LN cells directly expressing the DNA-encoded antigen are rare; 24 h after five abdominal skin bombardments, the number of these cells does not exceed 50–100 cells in an individual draining LN. However, over this same time period, the total number of CD11c+ DC increases more than twofold, by an average of 20,000–30,000 DC per major draining node. This augmentation is due to gold bombardment and is independent of the presence of plasmid DNA. Most antigen-bearing cells in the LNs draining the site of DNA delivery appear to be DC and can be depleted by antibodies to an intact surface protein encoded by cotransfected DNA. This finding of predominant antigen presentation by directly transfected cells is also consistent with data from studies on cotransfection with antigen and CD86-encoding DNA, showing that priming of anti-mutant influenza nucleoprotein CTLs with a single immunization is dependent upon coexpression of the DNAs encoding nucleoprotein and B7.2 in the same cells. These observations provide insight into the relative roles of direct gene expression and cross-presentation in CD8+ T cell priming using gene gun immunization, and indicate that augmentation of direct DC gene expression may enhance such priming.
Keywords: dendritic cells, DNA immunization, cytotoxic T lymphocytes, gene gun, antigen presentation
Since the discovery that plasmid DNA encoding a protein antigen can serve as an effective immunogen, DNA-based vaccination has become an attractive alternative to recombinant live vector immunization strategies. By direct injection, both skeletal muscles and skin have demonstrated the ability to take up and express DNA-encoded sequences without a special delivery system (1, 2). Alternatively, gold particles coated with DNA can be physically delivered into living tissues using a helium-powered gene delivery device, the “gene gun” (3). All of these routes of DNA delivery have been shown to successfully induce both cellular and humoral immunity in several antigen systems (4–7). Such plasmid DNA immunization can evoke immune responses dependent on both MHC class I and class II molecule–mediated antigen presentation to CD8+ and CD4+ T cells, respectively. The addition of DNAs encoding various cytokines or costimulatory molecules admixed with DNA encoding antigens serves to enhance the magnitude and type of desired immune responses (8). Using this technology, protective immunity in several virus and cancer disease models has been demonstrated in animal systems.
The exact mechanism by which injected or particle-coated DNA leads to antigen presentation capable of eliciting a T cell immune response has yet to be fully defined. CD8+ CTL responses induced by either gene gun or intramuscular inoculations appear to be initiated by bone marrow–derived APCs, as shown in recent experiments performed in parent-into-F1 bone marrow–reconstituted mice (9–12). Condon et al. have demonstrated that some skin-derived dendritic cells (DC)1 that subsequently localize in the draining LNs are directly transfected after cutaneous genetic immunization with the gene gun (13). However, after gene gun bombardment of the abdominal epidermis, keratinocytes are the main cell type found to be expressing the DNA-encoded proteins (14). Similarly, striated muscle cells are the predominant transfected, antigen-positive cells after intramuscular injection (15).
It remains unclear whether priming of naive CD8+ T cells depends on antigen produced by the transfected myocytes or keratinocytes that is taken up, processed, and presented by professional APCs migrating to the draining lymphoid tissue, by the direct transfection of and antigen production and presentation by professional APCs, or by both mechanisms. Surgical ablation experiments have suggested that there may be a different contribution of local tissue antigen expression, depending on the route of immunization (16). For example, the muscle bundle can be removed within 10 min of DNA injection without any effect on the longevity and magnitude of the humoral and CTL responses (16). On the other hand, excision of the epidermal site 24 h after gene gun bombardment abrogated the induction of CTL responses, suggesting an immune dependence on the transfected skin cells (16). However, Klinman et al. showed that gene gun immunization is independent of cells in the bombarded tissue site with regard to induction of memory T cell responses (17). Casares et al. have recently demonstrated that the direct uptake of DNA by draining LN-derived DC, but not B cells, resulted in effective presentation to MHC class II–restricted T cells (18). Antigen released from cultured transfected muscle cells can also generate ligands able to activate MHC class II–restricted T cells when supernatants are pulsed onto DC (18).
Here we further explore the cellular mechanisms responsible for the priming of CD8+ T cells after epidermal DNA immunization. First, we determined the number of directly transfected cells found in the draining LNs after immunization as well as the total number of LN-migrating DC induced by this procedure. Second, we evaluated MHC class I–dependent presentation of DNA-encoded determinants by directly transfected versus nontransfected migrating DC. Third, we used a mutant form of the nucleoprotein (NP) influenza antigen that requires coadministration of DNA encoding CD86 to examine whether the same cells required coexpression of the antigen and costimulatory molecule to prime naive CD8+ T cells. These studies all suggest that the predominant contribution to priming for CD8+ CTL responses after DNA immunization using the gene gun approach involves the small number of directly transfected, migrating DC rather than the much larger number of migrating DC that could potentially present antigen via a cross-priming mechanism after antigen expression by epidermal cells.
Materials and Methods
Animals.
Female BALB/c mice were obtained at 6–8 wk of age (Charles River Laboratories, Quebec, Canada), and female C57BL/6 mice were obtained at 6–10 wk of age (Animal Production Colonies, Frederick Cancer Research Facility, National Institutes of Health, Frederick, MD). All procedures with animals were carried out in accordance with institutionally approved protocols.
Cell Lines.
The P815 cell line is a murine mastocytoma obtained from the American Type Culture Collection, Rockville, MD (TIB 64), cultured in RPMI 1640 medium supplemented with 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine.
Plasmids and Peptides.
A plasmid encoding the Escherichia coli LacZ gene under the control of the human CMV intermediate-early promoter, designated pCMV/β-gal, was provided by J. Haynes (Agracetus Inc., Middleton, WI [5]). T4TM is a plasmid encoding human CD4 under the control of the CMV promoter in a CDM8 plasmid. Plasmid DNA was amplified in the E. coli DH5α bacterial strain and purified through large scale plasmid preparations, using the cesium chloride purification method. The isolation, by reverse transcriptase PCR amplification, and cloning of a nonimmunogenic mutant of influenza nucleoprotein (NPo) and mouse CD86 (B7.2) used in the immunization constructs have been previously described (19). The NPo/B7.2 colinear expression cassette contains NPo and B7.2 in tandem, each gene separately driven by its own CMV promoter and terminated by a bovine growth hormone polyA signal (19). This plasmid DNA was amplified in the JM109 bacterial strain and purified through large scale plasmid preparations, using endotoxin-free Mega Prep columns (QIAGEN, Inc., Chatsworth, CA). The H-2Kd–restricted peptide NP147-155 (TYQRTRALV) from the NP of influenza virus A/PR/8/34 was synthesized and purified commercially (Alberta Peptide Institute, Alberta, Canada).
Gene Gun Delivery of DNA.
For experiments involving β-gal, sodium pentobarbital–anesthetized C57BL/6 mice received one to five nonoverlapping abdominal deliveries of DNA-coated gold beads (2 μm) using the Accell gene gun (Agracetus, Inc.) at a helium discharge pressure of 350–400 psi. Plasmid DNA was coated onto gold particles as described (5). 0.5 mg of gold beads was coated with 1 μg of pCMV/β-gal or control plasmid (per one shot); when cocoating pCMV/β-gal and T4TM (human [h]CD4) DNAs, 0.5 μg of each plasmid were admixed.
For experiments involving induction of anti-NPo CTLs, Avertin-anesthetized BALB/c mice received six abdominal deliveries of DNA-coated gold beads (1 μm) using a gene gun (Bio-Rad Laboratories, Mississauga, Ontario, Canada) at a helium discharge pressure of 400–450 psi. Plasmid DNA was coated onto gold particles as described (20). Groups of mice received the following gene gun bombardments: the NPo plus B7.2 (colinear) group received six nonoverlapping shots of 0.5 mg of gold beads coated with 0.125 μg of colinear NPo/B7.2 construct; the NPo and B7.2 (cocoat) group received six shots of 0.5 mg gold beads cocoated with 0.125 μg each of B7.2 and NPo; the NPo plus B7.2 (overlap) group was given six shots at three separate abdominal sites, with two shots per site, one consisting of 0.5 mg gold beads coated with 0.125 μg of B7.2, and another with 0.5 mg gold beads coated with 0.125 μg of NPo. Mice were assayed 3–4 wk later for CTL priming using an in vitro restimulation assay (see below).
Collagenase Treatment of LNs and DC Enrichment.
Collagenase-digested LNs were prepared as described previously (21), except that in all steps after collagenase treatment, an EDTA-containing buffer was used to prevent reassociation of T cells with DC (22). For DC enrichment, suspended LN cells were layered over a metrizamide gradient column, centrifuged, and low density cells (DC-enriched) and high density cells (DC-depleted) were collected (23).
Flow Cytometry.
LN cells were incubated with an antibody that blocks Ig binding to FcγII and III receptors and then incubated with N418 supernatant for 30 min at 4°C, washed with PBS/5% FCS/0.1% sodium azide, and then incubated with PE- or FITC-goat F(ab′)2 anti–hamster IgG, mouse/rat adsorbed (Caltag Laboratories, Inc., Burlingame, CA) for 30 min at 4°C. The cells were then washed and resuspended in the same medium plus propidium iodide to allow exclusion of dead cells during analysis. Stained cells were analyzed using a FACScan® flow cytometer (Becton Dickinson, Bedford, MA). Between 100,000 and 150,000 events were monitored per sample.
Staining for β-gal Activity.
Draining LNs were snap frozen in liquid nitrogen and stored at −70°C. Frozen sections (10 μM) were cut; every other section was mounted on glass slides to minimize counting the same stained cell twice, and the slides were stored at −70°C. For β-galactosidase staining, frozen sections or cells in 24-well plates were fixed in PBS/2% formaldehyde/0.2% glutaraldehyde at 4°C for 7 min, washed in PBS, and incubated with X-Gal substrate (1 mg/ml X-Gal in PBS/5 mM potassium ferricyanide/5 mM potassium ferrocyanide/2 mM magnesium chloride) at 37°C until positive cells (blue) appeared. Slides and 24-well plates were screened for positive cells using a Labophot microscope and a TMS inverted microscope (both from Nikon Inc., Melville, NY), respectively.
Depletion of Cell Subpopulations.
Suspended LN cells were incubated for 30 min with the following mAbs (in supernatant form): B21-2 (anti–I-Ab,d, TIB-229; American Type Culture Collection), NLDC-145 (anti–DEC-205 antigen [24]), F4/80 (anti–mouse macrophage, HB-198; American Type Culture Collection), and RA3-3A1/6.1 (anti-B cell surface protein B220, TIB-146; American Type Culture Collection). After incubation, cells were washed, and antibody-coated cells were removed by sheep anti–rat IgG–coupled magnetic beads (Dynabeads; Dynal A.S., Oslo, Norway). The beads and cells were mixed at a ratio of 10:1 and incubated for 30 min with continuous slow rotation at 4°C, diluted, and beads/bead-bound cells were removed using a magnet (Dynal A.S.). mAb supernatants and washing buffer contained 5 mM EDTA (22). For depletion of human CD4+ LN cells or DC-enriched LN cells, cells were treated as above except that the mAb was OKT4 (anti–human CD4, CRL-8002; American Type Culture Collection) and the beads were coupled to sheep anti–mouse IgG.
Stimulation of β-gal–specific T Cell Clone.
A β-gal–specific T cell line that recognizes the Kb-restricted sequence DAPIYTNV corresponding to β-gal 96–103 was provided by W.W. Overwijk (National Institutes of Health, Bethesda, MD [25]). T cells (105/ well) were incubated at different ratios with LN cells or DC-enriched LN cells in U-bottomed 96-well plates for 18 h, after which IFN-γ levels in the supernatant were measured using an ELISA kit (Endogen, Inc., Woburn, MA).
CTL Lysis Assay.
4 wk after gene gun immunization, spleens were harvested, and single cell suspensions were prepared. Spleen cells from individual mice in each DNA-immunized group were cultured for 7 d at 5 × 106/ml in the presence of 2.5 × 106/ml syngeneic irradiated spleen cell stimulators prepulsed for 1 h with the H-2Kd–restricted epitope NP(147-155) at 0.1 μg/μl. CTLs specific for NP(147-155) used in the target lysis experiments were obtained from splenocytes of influenza-infected mice, which were restimulated in vitro as above. Antigen-specific cell-mediated cytotoxicity was assayed using P815 cells that had been labeled by incubation for 1 h at 37°C with 100 μCi of Na51CrO4 and concomitantly pulsed with 10 μM of the NP(147-155) peptide. Washed target cells at 104/well were incubated for 4 h in triplicate at 37°C with serial dilutions of effector cells. Maximum and spontaneous release were determined from wells containing 2% Triton X-100 or medium alone, respectively. Specific lysis was calculated as (experimental 51Cr release − spontaneous 51Cr release)/(maximum 51Cr release − spontaneous 51Cr release) × 100%. Nonspecific lysis was evaluated using unpulsed labeled P815 cells. Previous experiments have shown that such unpulsed cells give the same background lysis (<5%) as P815 cells pulsed with an irrelevant peptide (A. Iwasaki, unpublished results).
Results and Discussion
The Total Number of DC in Draining LNs Doubles after Gene Gun Particle Bombardment.
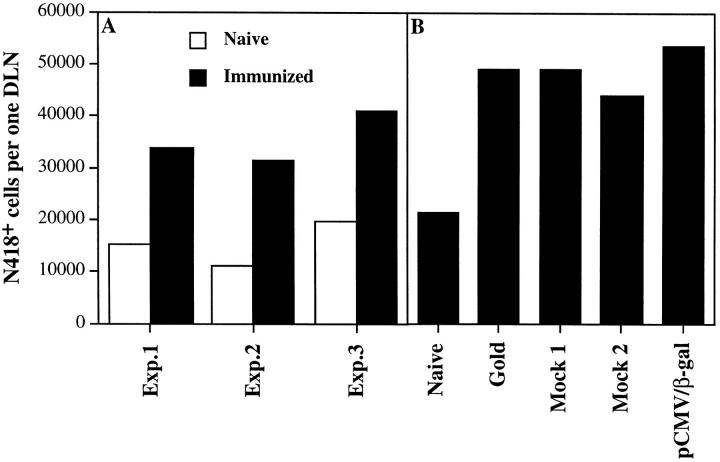
We first examined the number of DC in LNs draining the site(s) of gene gun bombardment compared with naive LNs. This was done 24 h after gene bombardment, as the number of DC and antigen-carrying DC in draining LNs peaks between 24 and 48 h after skin contact with antigen (23, 26). After abdominal gene gun delivery (five shots), draining inguinal LNs contained twice the number of total nucleated cells as naive LNs. LN cells were isolated using the collagenase method to insure release of interdigitating DC and stained with the N418 mAb against CD11c, then the number of N418+ DC in the gate of large forward and side scatter was determined. The total number of N418+ DC in draining inguinal LNs was 2–2.5-fold more than their number in naive inguinal LNs (Fig. 1 A). A smaller increase in nucleated and dendritic cell number was seen in axillary and brachial LNs, which also drain the abdominal skin (23), but not in spleen (data not shown). The class II and CD80 (B7.1)/CD86 (B7.2) costimulatory phenotypes of the N418+ DC in LNs draining the site(s) of bombardment compared with those present in naive LNs were similar (data not shown).
Figure 1.
Number of N418+ DC recruited to the draining LNs after gene gun immunization. Groups of C57BL/6 mice (n = 3–6) received five abdominal deliveries of gold beads coated with different plasmids. 24 h after bombardment, nucleated LN cells from draining inguinal nodes of immunized or naive mice were isolated using the collagenase method and pooled. FcγII and III receptors were blocked, and cells were stained with N418 followed by PE-goat anti–hamster antibodies (adsorbed on mouse and rat Ig). The number of N418+ cells was monitored by FACS® (>105 total cells/sample) and calculated per one draining LN (DLN) based on the number of nucleated cells in the pooled LNs and the number of LNs in each group. (A) Three different experiments (Exp.) comparing DC number in inguinal LNs between naive mice and mice bombarded with pCMV/β-gal. (B) Comparison of DC number in inguinal LNs among naive mice and mice bombarded either with gold beads alone or with gold beads coated with pCMV/β-gal or control plasmids (Mock 1, Mock 2). Mock 1, pCDNA3, which contains the ampicillin resistance gene. Mock 2, Wrg7077, which contains the kanamycin resistance gene. Similar results were obtained in another experiment.
The enlargement of the LNs as well as the increase in the number of DC was due to the bombardment process itself, as delivery of gold particles lacking plasmid DNA resulted in similar findings (Fig. 1 B). Thus, it is likely that cellular (presumably inflammatory cytokine/chemokine) responses to the physical insult of gold particle bombardment induces the response in draining LNs. Consistent with this interpretation, the cell number increase was proportional to the number of shots (data not shown). The DC that appear in the draining LNs are probably derived in large measure from the bombarded skin area, given the proportionality between their number and the skin surface subjected to particle exposure, as well as the evidence for such migration from skin painted with fluorescent sensitizers (13, 23). However, additional recruitment from circulating cells or expansion from blood or local precursors cannot be ruled out, especially given the overall increase in nucleated cells that presumably arises from such sources.
Draining LNs Contain a Small Number of Directly Transfected Cells after Biolistic Delivery of DNA.
Condon et al. have reported that gene bombardment of skin results in directly transfected cells in the draining LNs, and that these cells are skin-derived DC, although the number of such cells was not determined, nor was their possible role in T cell priming (13). Such directly transfected DC that migrate to the draining LNs might produce the antigen internally and process it very efficiently via the conventional class I pathway for presentation to CD8+ T cells. We further explored this issue by attempting to quantitate the number of such directly transfected cells and their functional significance. Mice received nonoverlapping abdominal deliveries (one, two, and five shots) of gold beads coated with a β-gal–encoding plasmid. 24 h after immunization, draining LNs were harvested and sectioned for analysis. Frozen sections of LN were stained for β-gal activity. To assure accurate counting of β-gal–positive cells, we analyzed between 100 and 200 alternate sections (10 μm cuts) per experimental group, thus screening a total volume of one to two LNs. Because β-gal is a cytoplasmic enzyme, only cells directly transfected with the gene and producing the enzyme endogenously have enough β-gal to yield a positive staining signal under these conditions. Fig. 2 A shows that there is a linear correlation between the number of nonoverlapping abdominal deliveries and the number of β-gal–positive cells in the draining inguinal LNs. Overall, the numbers are very low and average ∼15 β-gal–positive cells in each inguinal LN after one abdominal exposure to DNA-coated gold particles. No β-gal–positive cells were observed in draining LNs from unimmunized mice or mice bombarded with a control plasmid. Inguinal LNs are the major draining LNs after abdominal skin contact with antigen, but cells presenting the antigen are also found in other skin-draining LNs (23). The average of such positive cells in axillary and brachial LNs for one delivery of DNA was less than in the inguinal LNs (Fig. 2 B). In contrast to the few transfected cells in the draining LNs, the epidermal layer in skin sections at the delivery site was practically all β-gal positive (data not shown).
Figure 2.
Number of directly transfected (β-gal–positive) cells in draining LNs. Groups of C57BL/6 mice (n = 3) received nonoverlapping abdominal deliveries (one, two, or five shots) of gold beads coated with pCMV/β-gal. 24 h after bombardment, draining LNs were harvested, pooled, and sectioned for analysis (A), or nucleated cells from the LNs were pooled and plated, 106/well, into 24-well plates (B and C). Frozen sections and 24-well plates were stained for β-gal activity. To assure accurate enumeration of β-gal–positive cells, we (a) screened between 100 and 200 alternate serial sections (10 μm cuts) per each experimental group to evaluate a tissue mass equal to one to two LNs (A) and (b) screened wells containing cells from a total of two to three pooled LNs (B). No cells showing strong uniform staining were observed in draining LNs from unimmunized mice or mice bombarded with a control plasmid. The results in A and B/C are from two different experiments. Similar results were obtained in another experiment.
On average, the total number of directly transfected cells in the skin-draining LNs after abdominal delivery did not exceed 200 cells per mouse, as assessed by expression of a sufficient amount of active β-gal to yield a highly visible amount of reacted substrate. Some directly transfected cells might make a small amount of protein below the detection limit of this assay but sufficient for presentation and T cell priming. However, positive cells showed strong staining with very few if any faint positives, suggesting that the catalytic activity of even a small number of β-gal molecules in the cytoplasm of a cell is sufficient for detection. This makes it unlikely that our estimate of directly transfected cells is substantially below the actual number able to serve as functional APCs. Additional evidence that the small number of cells containing β-gal in the draining LNs express this protein as a result of direct transfection comes from PCR detection of plasmid DNA in LN cell suspensions (data not shown). Although direct characterization of these few cells by FACS® or immunohistochemical costaining has not been successful, studies by other investigators of directly transfected cells appearing in draining LNs after gene gun delivery of green fluorescent protein (GFP)-encoding DNA or after skin painting with fluorescent sensitizers have demonstrated these migrating cells to be skin-derived DC (13). Given the likelihood that such cells expressing the plasmid-encoded antigen would be especially effective at processing and presenting antigenic peptides in association with MHC class I molecules, the question arose of whether these cells or the larger number of migrating DC that might present antigen via class I by processing of exogenous antigen from transfected epidermal cells were responsible for sensitization of CD8+ CTL precursors under these immunization conditions.
APCs Involved in Class I–restricted Presentation after Biolistic Delivery of β-gal DNA Are Predominantly Directly Transfected DC.
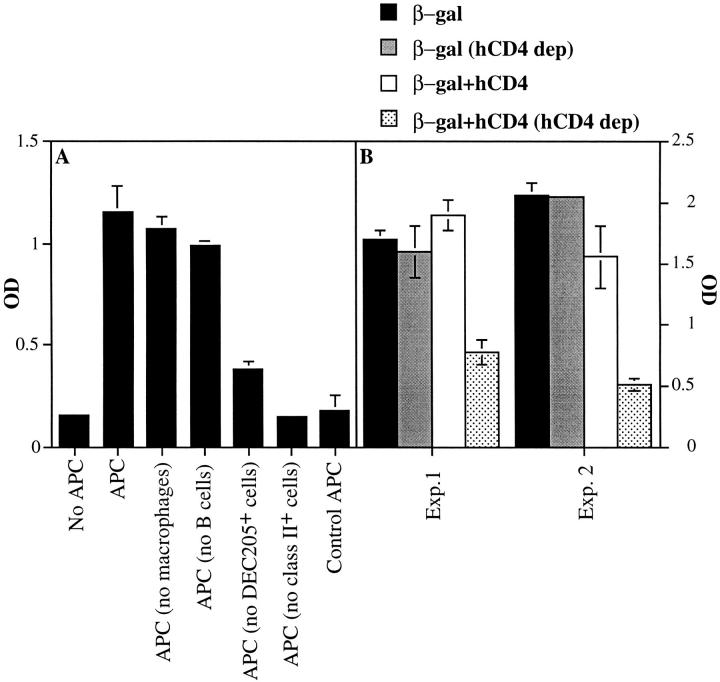
We first tested what type of APCs in draining LNs 24 h after gene gun delivery of pCMV/β-gal into C57BL/6 mice presents a class I–restricted determinant. LN cells were isolated using the collagenase method that allows recovery of CD8+/DEC-205+ interdigitating DC and were subjected to depletion of different cell subpopulations using mAbs and magnetic beads. These cells were then incubated with CD8+ T cells from a CTL line specific for a β-gal– derived determinant presented by Kb, and IFN-γ secretion by the T cells was measured (Fig. 3 A). Class II–positive cells were essential for this presentation, but B cells and macrophages were not. Depletion of DEC-205+ cells eliminated up to 70% of the presentation activity. As only ∼50% of CD11c+ DC in the inguinal LNs express even moderate levels of cell surface DEC-205 (data not shown, and reference 27), these data suggest that DEC-205–surface positive DC are especially effective at presentation of antigen in association with MHC class I molecules after DNA vaccination by gene gun. These data are consistent with the conclusion that DC in the draining LNs are the primary cell type bearing stimulatory levels of the β-gal determinant associated with Kb after gene gun delivery of β-gal– encoding plasmid.
Figure 3.
Analysis of APCs in draining LNs presenting the class I–restricted epitope. Groups of C57BL/6 mice (n = 4–5) received five abdominal deliveries of gold beads coated with different plasmids. 24 h after bombardment, inguinal LN cells were harvested using the collagenase method and pooled. (A) APCs and control APCs are from mice immunized with pCMV/β-gal– and mock plasmid–coated particles, respectively. APCs (106) were depleted of either macrophages, B cells, DEC205+ cells, or class II+ cells using specific mAbs and magnetic beads as described in Materials and Methods. Cells then were incubated with 105 T cells specific for a Kb-restricted β-gal epitope for 18 h in U-bottomed 96-well plates. IFN-γ levels in the supernatants were measured using an ELISA kit, and results are presented as OD ± SE. (B) APCs are from mice immunized with pCMV/β-gal–coated gold beads (β-gal) or from mice immunized with pCMV/β-gal and T4TM (hCD4 plasmid)–cocoated gold beads (β-gal+hCD4). APCs (experiment [_Exp._] 1, DC-enriched LN cells, 105; experiment 2, LN cells, 106) were either mock-depleted (i.e., treated only with magnetic beads) or depleted of cells expressing cell surface hCD4 protein as described in Materials and Methods (hCD4 dep). Cells were then incubated with T cells, and IFN-γ levels were measured as described above. Background OD, obtained from T cells without APCs (0.15– 0.19), was subtracted from the results in B. Bars, ±SE. Experiments were repeated three times with similar results.
We next analyzed whether the DC capable of this presentation acquired antigen from other transfected cells or by direct gene expression. Gold particles were cocoated with plasmid DNA encoding the human CD4 cell surface molecule and with pCMV/β-gal DNA. Thus, directly transfected DC will express human CD4 as a membrane protein and should also present directly processed β-gal determinants. C57BL/6 mice were immunized with five abdominal deliveries of these cocoated beads, and 24 h later LN cells or DC-enriched LN cells were specifically depleted of human CD4+ cells using OKT4 and anti–mouse IgG–coated magnetic beads, or mock depleted using anti– mouse IgG–coated beads alone. Responses of the Kb–β-gal peptide–specific T cell line to these APC populations were then measured. Mock-depleted total or DC-enriched cells from mice immunized with either cocoated beads or beads singly coated with pCMV/β-gal alone, as well as cells from mice immunized with beads coated with pCMV/β-gal alone and treated to deplete human CD4-expressing cells, all retained their full functional activity in stimulating the CD8+ T cells (Fig. 3 B, and data not shown). In contrast, depletion of cells expressing human CD4 from total or DC-enriched cell populations obtained from the LNs of mice immunized with beads cocoated with pCMV/β-gal and human CD4 plasmids reproducibly reduced presentation by 60–70%.
Experiments involving in vitro gene gun cotransfection into B16 melanoma cells with plasmids for GFP and human CD4 demonstrated coexpression in ∼70% of the cells expressing GFP (data not shown). Although these in vitro data do not provide a direct measure of the extent of effective in vivo gene coexpression in DC under these DNA delivery conditions, they suggest that the coexpression/depletion method will likely underestimate the fraction of cells expressing an intracellular antigen due to direct transfection. Although B cells are also depleted by the anti– mouse IgG–coated magnetic beads used to remove hCD4+ cells, B cells are not the presenting cells as deduced from Fig. 3 A and from the observation that depletion only with anti–mouse IgG–coated magnetic beads or depletion of LN cells from mice immunized only with β-gal did not affect presentation (Fig. 3 B). Together, these data imply that directly transfected DC play a predominant role in presentation of antigen via MHC class I molecules after gene gun DNA delivery.
Coexpression of Antigen and DNA-encoded B7.2 Is Essential for CTL Induction in Conditions that Minimize the Effect of Direct Presentation by Transfected Skin Cells.
To further examine the importance of direct gene transduction of APCs for in vivo CD8+ CTL priming, we turned to a previously described model system involving a nonimmunogenic mutant of influenza nucleoprotein (NPo). The NPo construct is unable to induce a CTL response on its own due to three nonconservative mutations near the COOH terminus of the molecule (F304L, N370S, and R441G). This same mutant protein can elicit CD8+ CTL responses when produced after intramuscular injection of a plasmid also encoding B7.2 (19). However, these previous studies did not determine whether expression of transfected B7.2 by antigen-presenting muscle cells, antigen-presenting LN cells, or both was responsible for the observed enhancement of CTL responses. We revisited this issue by using immunization conditions that minimize the effect of direct presentation by transfected tissue cells. Intramuscular delivery of DNA results in a relatively consistent expression of the transfected gene in muscle cells. In contrast, gene bombardment of skin with plasmid DNA results in transient expression of transfected gene in the skin, peaking at 24 h after inoculation and largely lost by 3 d after inoculation (16). Also, antigen-specific T cells stimulated in the draining LNs are initially retained in the LNs, with efflux of antigen-stimulated T cells from the LNs beginning after 3–4 d (28). Hence, after one gene gun immunization of the skin, encounters between antigen-specific naive T cells stimulated in the draining LNs and directly transfected skin cells are rare. This permitted the design of experiments that compared the effect on CTL priming of coexpression of transfected DNA sequences for NPo and B7.2 in individual cells with that of separate but simultaneous expression of the two proteins.
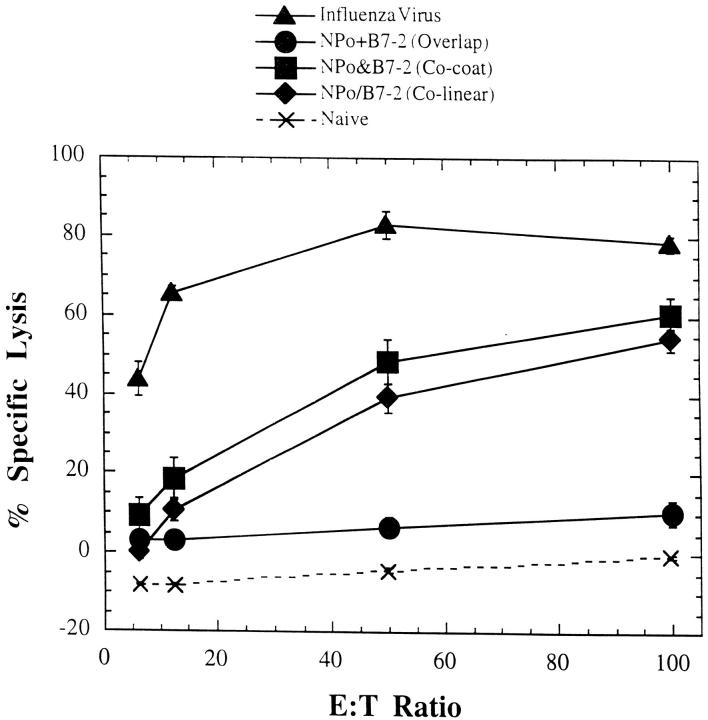
Gold beads were coated with either the NPo or the B7.2 plasmids or were cocoated with both plasmids. Mice were immunized once with overlapping abdominal deliveries of either the cocoated beads (six shots) or the NPo- and B7.2-coated beads separately (three shots each). The amount of gold and the amount of DNA/gene/mouse were standardized so that each mouse received 0.75 μg of each gene with a total of 3.0 mg of gold beads. The cocoated beads should allow most individual cells (>70%, based on the in vitro data) to synthesize both the antigen and the B7.2 costimulatory molecule, whereas separate delivery of the two plasmids on distinct particles is unlikely to introduce both DNAs into more than a very small fraction of the transfected cell pool. Fig. 4 shows that overlapping deliveries with NPo-coated beads and B7.2-coated beads did not prime for NP-specific CTLs. In contrast, immunization with the cocoated beads primed for CTLs that specifically lysed P815 cells pulsed with the NP(147-155) peptide. These CTLs were as efficient as the CTLs induced by immunization with beads coated with colinear vector encoding both NPo and B7.2 (Fig. 4). These data support the results of the in vitro presentation studies described above in indicating a predominant role for directly transfected cells in priming CD8+ T cell responses in draining LNs after gene gun DNA delivery.
Figure 4.
CTL responses induced by gene gun delivery of NPo and B7.2 plasmids. Groups of BALB/c mice were immunized once by gene gun with a total of six shots, with gold particles either coated with the colinear construct NPo/B7.2 or cocoated with NPo and B7.2 constructs. Another set of mice received overlapping shots of gold separately coated with NPo or B7.2 as described in Materials and Methods. 4 wk after immunization, spleens were harvested and restimulated as described in Materials and Methods. Influenza virus–infected mice were used as positive controls. Lysis of P815 cells not pulsed with peptide was <5%. Each point corresponds to the mean ± SE obtained from two mice in each group. The experiment was repeated twice with similar results.
This conclusion is consistent with the study by Klinman et al. noted above (17). In addition, Ciernik et al. demonstrated that gene gun inoculation with minigenes encoding class I MHC–restricted T cell epitopes resulted in efficient induction of CTLs (29). The minigenes encoded cytoplasmically expressed CTL epitopes that are not likely to be secreted and transferred to another cell for class I MHC presentation, although these results do not exclude presentation by DC after apoptotic cell uptake (30). However, our data and these previous results contrast with the observations of Torres et al., who have recently reported that 3 d are required before skin resection at the site of gene gun bombardment is without effect on priming (16). At present, these discrepant results cannot be fully explained, but may depend on the nature of the plasmid construct, the encoded antigen, and the method used to evaluate the response. It also might reflect DC efflux rate from bombarded skin. These considerations bear on the issue of whether under circumstances other than those studied here acquisition of antigen from transfected nonhematopoietic cells may play a more important role in CD8+ T cell priming. The phenomenon of cross-priming is now recognized as arising from hematopoietic cell (DC) class I MHC molecule presentation of peptides derived from proteins synthesized by other cell types. This pathway has been shown to play a major role in priming for CTL responses to protein-coated spleen cells (31), minor H antigen–bearing spleen cells (32), tumor-associated antigens (33), and responses to foreign antigens expressed after transfection of myoblasts, studied as an analogue of intramuscular DNA delivery (34). However, in the last case, the possibility of direct antigen expression in DC due to transfer of episomal plasmid DNA retained by the transfected myoblasts has not been ruled out. Overall, the preponderance of evidence indicates that biologically effective presentation of exogenously acquired foreign protein antigens by DC can occur, perhaps by the apoptotic cell uptake pathway reported recently (30). Nevertheless, this pathway was not found here to make a substantial contribution to CD8+ T cell priming using gene gun DNA delivery. A similar conclusion about the importance of endogenous synthesis of foreign antigen by DC was also reached in a different model involving expression of β-gal by recombinant vaccinia viruses (35).
Previous studies have demonstrated the potent T cell priming capacity of small numbers of antigen-bearing DC introduced into naive animals (for reviews, see references 36 and 37). The present findings are in agreement with and amplify these previous results, strongly implying that a few directly transfected DC are critical for effective CD8+ T cell priming after gene gun DNA immunization. Given these data, it would seem prudent to explore methods for enhancing the number of directly transfected DC during gene gun DNA vaccination, and/or for improving gene expression within any such transfected DC, as a means of optimizing the potency of DNA vaccines.
Acknowledgments
We are grateful to Dr. W.W. Overwijk for supplying the β-gal–specific T cell line.
Abbreviations used in this paper
DC
dendritic cell(s)
GFP
green fluorescent protein
NP
nucleoprotein
Footnotes
A. Porgador and K.R. Irvine contributed equally to this paper.
References
- 1.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 2.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen MD, Silverman GJ, Lotz M, Carson DA, Raz E. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273:352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 3.Williams RS, Johnston SA, Riedy M, DeVit MJ, McElligott SG, Sanford JC. Introduction of foreign genes into tissues of living mice by DNA-coated microprojectiles. Proc Natl Acad Sci USA. 1991;88:2726–2730. doi: 10.1073/pnas.88.7.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowski SH, Deck RR, DeWitt CM, Friedman A, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 5.Irvine KR, Rao JB, Rosenberg SA, Restifo NP. Cytokine enhancement of DNA immunization leads to effective treatment of established pulmonary metastases. J Immunol. 1996;156:238–245. [PMC free article] [PubMed] [Google Scholar]
- 6.Doolan DL, Sedegah M, Hedstrom RC, Hobart P, Charoenvit Y, Hoffman SL. Circumventing genetic restriction of protection against malaria with multigene DNA immunization: CD8+ cell–, interferon gamma–, and nitric oxide–dependent immunity. J Exp Med. 1996;183:1739–1746. doi: 10.1084/jem.183.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulmer JB, Sadoff JC, Liu MA. DNA vaccines. Curr Opin Immunol. 1996;8:531–536. doi: 10.1016/s0952-7915(96)80042-2. [DOI] [PubMed] [Google Scholar]
- 8.Xiang Z, Ertl HC. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity. 1995;2:129–135. doi: 10.1016/s1074-7613(95)80001-8. [DOI] [PubMed] [Google Scholar]
- 9.Doe B, Selby M, Barnett S, Baenziger J, Walker CM. Induction of cytotoxic T lymphocytes by intramuscular immunization with plasmid DNA is facilitated by bone marrow-derived cells. Proc Natl Acad Sci USA. 1996;93:8578–8583. doi: 10.1073/pnas.93.16.8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corr M, Lee DJ, Carson DA, Tighe H. Gene vaccination with naked plasmid DNA: mechanism of CTL priming. J Exp Med. 1996;184:1555–1560. doi: 10.1084/jem.184.4.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu T, Ulmer J, Caulfield M, Deck R, Friedman A, Wang S, Liu X, Donnelly J, Liu M. Priming of cytotoxic T lymphocytes by DNA vaccines: requirement for professional antigen presenting cells and evidence for antigen transfer from myocytes. Mol Med. 1997;3:362–371. [PMC free article] [PubMed] [Google Scholar]
- 12.Iwasaki A, Torres CA, Ohashi PS, Robinson HL, Barber BH. The dominant role of bone marrow-derived cells in CTL induction following plasmid DNA immunization at different sites. J Immunol. 1997;159:11–14. [PubMed] [Google Scholar]
- 13.Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD., Jr DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 14.Lu B, Scott G, Goldsmith LA. A model for keratinocyte gene therapy: preclinical and therapeutic considerations. Proc Assoc Am Physicians. 1996;108:165–172. [PubMed] [Google Scholar]
- 15.Danko I, Williams P, Herweijer H, Zhang G, Latendresse JS, Bock I, Wolff JA. High expression of naked plasmid DNA in muscles of young rodents. Hum Mol Genet. 1997;6:1435–1443. doi: 10.1093/hmg/6.9.1435. [DOI] [PubMed] [Google Scholar]
- 16.Torres CAT, Iwasaki A, Barber BH, Robinson HL. Differential dependence on target site tissue for gene gun and intramuscular DNA immunizations. J Immunol. 1997;158:4529–4532. [PubMed] [Google Scholar]
- 17.Klinman DM, Sechler JMG, Conover J, Gu M, Rosenberg AS. Contribution of cells at the site of DNA vaccination to the generation of antigen-specific immunity and memory. J Immunol. 1998;160:2388–2392. [PubMed] [Google Scholar]
- 18.Casares S, Inaba K, Brumeanu TD, Steinman RM, Bona CA. Antigen presentation by dendritic cells after immunization with DNA encoding a major histocompatibility complex class II–restricted viral epitope. J Exp Med. 1997;186:1481–1486. doi: 10.1084/jem.186.9.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwasaki A, Stiernholm BJ, Chan AK, Berinstein NL, Barber BH. Enhanced CTL responses mediated by plasmid DNA immunogens encoding costimulatory molecules and cytokines. J Immunol. 1997;158:4591–4601. [PubMed] [Google Scholar]
- 20.Eisenbraun MD, Fuller DJ, Haynes JR. Examination of parameters affecting the elicitation of humoral immune response by particle bombardment-mediated genetic immunization. DNA Cell Biol. 1993;12:791–797. doi: 10.1089/dna.1993.12.791. [DOI] [PubMed] [Google Scholar]
- 21.Inaba, K., W.J. Swiggard, R.M. Steinman, N. Romani, and G. Schuler. 1998. Enrichment of dendritic cells by plastic adherence and EA rosetting. In Current Protocols in Immunology. J.E. Coligan, A.M. Kruisbeek, D.H. Margulies, E.M. Shevach, and W. Strober, editors. John Wiley & Sons, Inc., New York. 3.7.1–3.7.15.
- 22.Vremec D, Shortman K. Dendritic cell subtypes in mouse lymphoid organs: cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J Immunol. 1997;159:565–573. [PubMed] [Google Scholar]
- 23.Macatonia SE, Knight SC, Edwards AJ, Griffiths S, Fryer P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J Exp Med. 1987;166:1654–1667. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, Nussenzweig MC. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–155. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 25.Overwijk WW, Surman DR, Tsung K, Restifo NP. Identification of a Kb-restricted CTL epitope of beta-galactosidase: potential use in development of immunization protocols for “self” antigens. Methods. 1997;12:117–123. doi: 10.1006/meth.1997.0461. [DOI] [PubMed] [Google Scholar]
- 26.van Wilsem EJ, Breve J, Kleijmeer M, Kraal G. Antigen-bearing Langerhans cells in skin draining lymph nodes: phenotype and kinetics of migration. J Investig Dermatol. 1994;103:217–220. doi: 10.1111/1523-1747.ep12393088. [DOI] [PubMed] [Google Scholar]
- 27.Salomon B, Cohen JL, Masurier C, Klatzman D. Three populations of mouse lymph node dendritic cells with different origins and dynamics. J Immunol. 1998;160:708–717. [PubMed] [Google Scholar]
- 28.Abbas, A.K., A.H. Lichtman, and J.S. Pober. 1994. Functional anatomy of local and systemic immune responses. In Cellular and Molecular Immunology, 2nd ed. W.B. Saunders Company, Philadelphia. 222–236.
- 29.Ciernik IF, Berzofsky JA, Carbone DP. Induction of cytotoxic T lymphocytes and antitumor immunity with DNA vaccines expressing single T cell epitopes. J Immunol. 1996;156:2369–2375. [PubMed] [Google Scholar]
- 30.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 31.Carbone FR, Bevan MJ. Class I–restricted processing and presentation of exogenous cell-associated antigen in vivo. J Exp Med. 1990;171:377–387. doi: 10.1084/jem.171.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bevan MJ. Minor H antigens introduced on H-2 different stimulating cells cross-react at the cytotoxic T cell level during in vivo priming. J Immunol. 1976;117:2233–2238. [PubMed] [Google Scholar]
- 33.Huang AYC, Golumbek P, Ahmadzadeh M, Jaffee EM, Pardoll DM, Levitsky HI. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–964. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 34.Ulmer JB, Deck RR, Dewitt CM, Donnhly JI, Liu MA. Generation of MHC class I-restricted cytotoxic T lymphocytes by expression of a viral protein in muscle cells: antigen presentation by non-muscle cells. Immunology. 1996;89:59–67. doi: 10.1046/j.1365-2567.1996.d01-718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bronte V, Carroll MW, Goletz TJ, Wang M, Overwijk WW, Marincola F, Rosenberg SA, Moss B, Restifo NP. Antigen expression by dendritic cells correlates with the therapeutic effectiveness of a model recombinant poxvirus tumor vaccine. Proc Natl Acad Sci USA. 1997;94:3183–3188. doi: 10.1073/pnas.94.7.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inaba K, Metlay JP, Crowley MT, Witmer-Pack M, Steinman RM. Dendritic cells as antigen presenting cells in vivo. Int Rev Immunol. 1990;6:197–206. doi: 10.3109/08830189009056630. [DOI] [PubMed] [Google Scholar]
- 37.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]