Signaling through ShcA Is Required for Transforming Growth Factor β- and Neu/ErbB-2-Induced Breast Cancer Cell Motility and Invasion (original) (raw)
Abstract
Cooperation between the Neu/ErbB-2 and transforming growth factor β (TGF-β) signaling pathways enhances the invasive and metastatic capabilities of breast cancer cells; however, the underlying mechanisms mediating this synergy have yet to be fully explained. We demonstrate that TGF-β induces the migration and invasion of mammary tumor explants expressing an activated Neu/ErbB-2 receptor, which requires signaling from autophosphorylation sites located in the C terminus. A systematic analysis of mammary tumor explants expressing Neu/ErbB-2 add-back receptors that couple to distinct signaling molecules has mapped the synergistic effect of TGF-β-induced motility and invasion to signals emanating from tyrosine residues 1226/1227 and 1253 of Neu/ErbB-2. Given that the ShcA adaptor protein is known to interact with Neu/ErbB-2 through these residues, we investigated the importance of this signaling molecule in TGF-β-induced cell motility and invasion. The reduction of ShcA expression rendered cells expressing activated Neu/ErbB-2, or add-back receptors signaling specifically through tyrosines 1226/1227 or 1253, unresponsive to TGF-β-induced motility and invasion. In addition, a dominant-negative form of ShcA, lacking its three known tyrosine phosphorylation sites, completely abrogates the TGF-β-induced migration and invasion of breast cancer cells expressing activated Neu/ErbB-2. Our results implicate signaling through the ShcA adaptor as a key component in the synergistic interaction between these pathways.
Breast cancer is a heterogeneous disease, as evidenced by the diverse nature of histopathological features ascribed to breast tumors, their varied responses to therapy, and the identification of distinct molecular profiles that allow breast cancers to be classified into subgroups with unique clinical outcomes (39, 58-61). One such classification is the ErbB-2-positive subtype, the tumors of which are characterized by elevated ErbB-2 levels, which primarily are the result of gene amplification and often are associated with poor patient prognosis (1, 54, 55). These clinical correlations have been extended by the establishment of Neu/ErbB-2-dependent transgenic models of breast cancer, which clearly demonstrate a causal relationship between Neu/ErbB-2 overexpression/activation and the development of metastatic breast tumors (10, 22, 35, 50, 52). Clinical trials with herceptin, a neutralizing monoclonal ErbB-2 antibody, have demonstrated clear survival benefits when it is used in combination with chemotherapy for the treatment of patients with either advanced or early-stage breast cancer (26, 43, 56). Based on these observations, much effort has been focused on understanding the signaling pathways engaged by Neu/ErbB-2 that promote breast cancer progression. Following its overexpression, the tyrosine kinase activity of ErbB-2 is activated in breast tumors through its ability to dimerize with the epidermal growth factor receptor or, more frequently, with ErbB-3, which leads to the transphosphorylation of tyrosine residues located in the cytoplasmic tails of these receptors (24, 52). Once phosphorylated, these tyrosine residues serve as docking sites for numerous signaling molecules, including those with enzymatic activities and others that serve as adaptor proteins. These include Src homology 2 (SH2) and phosphotyrosine binding (PTB) domain-containing proteins such as PLCγ1, Src, Ras-GAP, Grb-2, Grb-7, and ShcA, among others (30). Indeed, high-throughput analyses have verified the interaction of known targets with specific autophosphorylation sites but also have identified many more potential Neu/ErbB-2-interacting proteins/substrates (27, 47). The contribution of several autophosphorylation sites to Neu/ErbB-2-mediated transformation and mammary tumorigenesis has been investigated by systematic mutational analysis (16). These studies indicate that the Neu/ErbB-2 receptor recruits both overlapping and nonredundant signaling molecules that transform the mammary gland and result in unique mammary tumor phenotypes in vivo (15, 16, 46).
Transgenic mouse models also have clearly demonstrated that signaling pathways engaged directly by Neu/ErbB-2 can be influenced by interactions with other signaling pathways to control numerous stages of Neu/ErbB-2-induced mammary tumorigenesis (63). In particular, the transforming growth factor β (TGF-β) pathway has emerged as an important modifier of mammary tumorigenesis and progression to metastasis in cancers initiated by different oncogenes, including the Neu/ErbB-2 receptor oncogene. Three TGF-β isoforms (TGF-β1, TGF-β2, and TGF-β3) initiate signaling by inducing the formation of an activated TGF-β receptor complex composed of a ligand binding type II receptor (alone or in conjunction with a TGF-β type III receptor), which then associates with and activates the type I receptor (32). The active TGF-β type I receptor constitutes the primary signaling component of the complex and transduces signals via both Smad-dependent (49) and -independent pathways (34). In a variety of normal cells, including mammary epithelial cells, TGF-β elicits strong antiproliferative responses and can function as a tumor suppressor pathway in certain tissues. However, in many cancers, TGF-β induces prosurvival, promigratory, and proinvasive responses that increase their aggressiveness and metastatic behavior (9, 51). The importance of endogenous TGF-β signaling for promoting the metastasis of Neu/ErbB-2-induced tumors to the lung was first observed in bigenic mice expressing the wild-type Neu receptor along with a soluble ligand trap composed of the extracellular domain of the TGF-β type II receptor fused to the Fc portion of human immunoglobulin G (IgG) (Fc-TβRII). The expression of Fc-TβRII did not alter the kinetics of Neu/ErbB-2-induced primary tumor formation but resulted in a significant decrease in the percentage of mice developing lung metastases (71). Subsequently, it was shown that the overexpression of constitutively active forms of TGF-β1 or the TGF-β type I receptor could promote the formation of lung metastases in transgenic mice developing Neu/ErbB-2-induced mammary tumors (36, 37, 53). Cell-based models also reveal collaboration between Neu/ErbB-2 and TGF-β in promoting prometastatic responses in Neu-ErbB-2-transformed mammary epithelial cells. Both TGF-β1 and TGF-β3 were isolated as factors that promoted the motility of MCF-10A human mammary epithelial cells expressing an inducibly activated form of Neu/ErbB-2 and subsequently were shown to induce their invasion (48). Similarly, MCF-10A cells engineered to express ErbB-2 display elevated motility and invasion in response to TGF-β stimulation (62). While these studies clearly demonstrate that TGF-β can enhance the aggressiveness of Neu/ErbB-2-expressing tumors, the mechanisms underlying the synergy between these pathways are largely unknown and are just beginning to be deciphered (18, 65, 66).
To systematically investigate this question, we have transformed an immortalized mammary epithelial cell line (NMuMG) with a panel of activated Neu/ErbB-2 receptors that couple to distinct signaling pathways (16). Using mammary tumor explants from these cell populations, we demonstrate that the synergistic effect of TGF-β on Neu/ErbB-2-induced motility and invasion requires the retention of signaling pathways downstream of Y1226/1227 or Y1253 on Neu/ErbB-2. We further demonstrate that TGF-β-stimulated breast cancer cell motility and invasion rely on ErbB-2-mediated signaling through the ShcA adaptor protein.
MATERIALS AND METHODS
DNA constructs.
Expression constructs were generated by inserting neu/erbB-2 cDNAs, each containing an activating mutation in the region encoding the transmembrane domain as well as mutations in codons that specify the tyrosine autophosphorylation sites, into pMSCVpuro (Clontech). Briefly, the neu/erbB-2 cDNAs were released from pcDNA3.1 (16) by digestion with HindIII, incubation of the digested plasmid DNA with Klenow, and then digestion with EcoRI. The pMSCVpuro vector was prepared by first digesting the vector with XhoI, incubating the digested plasmid DNA with Klenow, and then digesting the vector with EcoRI. The cDNAs were inserted into pMSCVpuro as blunt/EcoRI fragments.
Cell culture and transfections.
The NMuMG normal murine mammary cell line was obtained from the American Type Culture Collection (Manassas, VA). All NMuMG-derived pooled cell populations and tumor explants were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS), 10 mM HEPES, 1 mM sodium pyruvate, 1 mM l-glutamine, 10 μg/ml insulin, penicillin-streptomycin, and amphotericin B (Fungizone). Pooled stable NMuMG populations were generated by transfecting the pMSCVpuro-based expression vectors into the parental NMuMG cell line using Lipofectamine 2000 (Invitrogen) reagent. Stable populations were maintained under 1 μg/ml of puromycin antibiotic selection.
Primary mammary tumor outgrowth and metastasis assays.
NMuMG-derived explant cultures were established by injecting 1 × 106 cells of each pooled stable cell population into the number four mammary fat pads of athymic mice (National Cancer Institute, Frederick, MD). Mammary tumors were excised and explanted back into culture in the presence of 1 μg/ml puromycin. In the case of the NMuMG empty vector explant, no visible mammary tumors grew during the course of the experiment; however, one explant was made from cells that persisted at the injection site (empty vector). To assess the primary tumor outgrowth and spontaneous metastasis of each explant, 1 × 105 cells were resuspended in a 50:50 solution of 1× phosphate-buffered saline (PBS) and Matrigel (BD Biosciences) and injected into the number four mammary fat pads of nude mice. Tumor volumes were monitored by caliper measurements and were calculated using the formula π_LW_2/6, where L is the length and W is the width of the tumor. For spontaneous metastasis assays, mice were sacrificed at an 8-week common end point or mammary tumors were resected once they reached volumes ranging from 150 to 200 mm3, and the animals were monitored for outward signs of distress prior to sacrifice. Experimental metastasis assays were performed by injecting 2 × 105 cells directly into the lateral tail veins of athymic mice. All mice were sacrificed 8 weeks after tail vein injection to determine the extent of lung metastasis. In all cases, lungs were removed, embedded in paraffin, and subjected to hematoxylin/eosin staining to evaluate the metastatic burden. For scoring the presence of lung metastases, four step sections of the entire lung were taken, with 100 μm between each step.
Mice were housed in facilities managed by the McGill University Animal Resources Centre. All animal experiments were conducted under a McGill University-approved animal use protocol in accordance with guidelines established by the Canadian Council on Animal Care.
Northern blotting.
Total RNA was isolated from NMuMG-derived tumor explants, which were left untreated or stimulated for the indicated times with TGF-β1 (R&D Systems), using RNeasy minikits and QIAshredder columns (Qiagen). Ten micrograms of purified RNA was separated on a 1% agarose gel, and Northern blottings were performed as previously described (40) with the following modifications. The membranes were hybridized in Express-Hyb (BD Biosciences) at 65°C with a 32P-labeled probe to either E-Cadherin (mouse cDNA; nucleotides 590 to 1266; GenBank accession number NM_009864) or Fibronectin (mouse cDNA; nucleotides 7313 to 7618; GenBank accession number NM_010233). The membranes then were stripped with boiling 0.5% sodium dodecyl sulfate (SDS) and subsequently reprobed for GAPDH (full-length rat cDNA; GenBank accession number X02231) as a loading control. After exposure to X-ray films, the membranes were exposed to phosphorimager screens and the signal intensity was quantified with a Storm imaging system (GE Healthcare) and ImageQuant software.
Immunoblotting and immunoprecipitation.
Cells grown to 70 to 90% confluence were incubated for various times in the absence or presence of TGF-β1, washed in PBS, and lysed in TNE (Triton, NP-40, and EDTA) lysis buffer as previously described (52). Protein concentrations were determined by the Bradford assay (Bio-Rad), and 20 to 40 μg of total protein lysates was separated by 7.5 or 12% SDS-polyacrylamide gel electrophoresis (PAGE) and electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked in 5% milk and incubated for 1 h with the following primary antibodies: Smad2/3 (1:1,000 dilution; catalog no. 610842; BD Biosciences), phospho-Smad2 (Ser465/467) (1:1,000 dilution; catalog no. 3101; Cell Signaling), fibronectin (1:4,000 dilution; catalog no. F3648; Sigma), uvomorulin (E-cadherin; clone Decma-1; 1:2,000 dilution; catalog no. U3254; Sigma), α-smooth muscle actin (1:2,000 dilution; catalog no. A2547; Sigma), α-tubulin (1:5,000 dilution; catalog no. T9026; Sigma), and Neu/ErbB-2 (C-18) (1:1,000 dilution; catalog no. sc-284-G; Santa Cruz). The blots then were incubated with horseradish peroxidase-conjugated anti-IgG secondary antibodies (anti-mouse, catalog no. 715-035-150; anti-rabbit, catalog no. 711-035-152; Jackson ImmunoResearch Laboratories) and visualized with an enhanced chemiluminescence system (catalog no. 34080; Pierce).
Total cell lysates (1 mg) in PLC lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM EGTA, 1.5 mM MgCl2, 10 mM sodium orthovanadate, and 10 μg/ml of both aprotinin and leupeptin) from either cultured tumor explants or frozen tumor material were mixed with 30 μl of a 50/50 protein G-Sepharose 4 Fast Flow (catalog no. 17-0618-02; GE Healthcare)-PLC lysis buffer solution. Total Neu/ErbB-2 protein then was immunoprecipitated with 1 μg/ml c-Neu (Ab-4) antibody (catalog no. OP16; Oncogene Science) at 4°C overnight. After centrifugation, the beads were washed three times with PLC lysis buffer. The pellet then was resuspended in 2× SDS sample loading buffer, and the immunoprecipitate was resolved by SDS-PAGE, transferred to PVDF, and immunoblotted with antibodies against phosphotyrosine (4G10) (1:1,000 dilution; catalog no. 05-321; Millipore/Upstate). Duplicate immunoprecipitates were resolved by SDS-PAGE, transferred to PVDF, and immunoblotted with antibodies against Neu/ErbB-2 (C-18) (1:1,000 dilution; catalog no. sc-284-G; Santa Cruz).
Migration and invasion assays.
Migration and invasion assays were performed as previously described (41), with the following modifications. Cells were pretreated with or without TGF-β1 for 24 h, resuspended in serum-free medium, and plated in the top chamber of transwell inserts (catalog no. 353097; Falcon). In the experiments shown in Fig. 2, 3, and 7, 60,000 cells were plated for migration assays and 100,000 cells were plated for the invasion assays. For the experiments depicted in Fig. S5 in the supplemental material, the plating density was increased to 100,000 cells for the migration assays and 160,000 cells for the invasion assays. Cells were allowed to migrate through a membrane (8-μm pores) for 24 h toward medium containing 10% FBS in the bottom chamber. For invasion experiments, cells were plated onto a 5% Matrigel that was layered over the membrane. Cells first were scraped from the top surface of the membrane; the remaining cells were fixed in 10% formalin and stained with a crystal violet solution (Sigma-Aldrich Corporation). The migration and invasion data are representative of the average pixel count from five independent images that were quantitated using Scion Image software (Scion Corporation). Two independent inserts were quantified for each explant, in the absence or presence of TGF-β1, and the data represent the averages from three to four independent experiments.
FIG. 2.
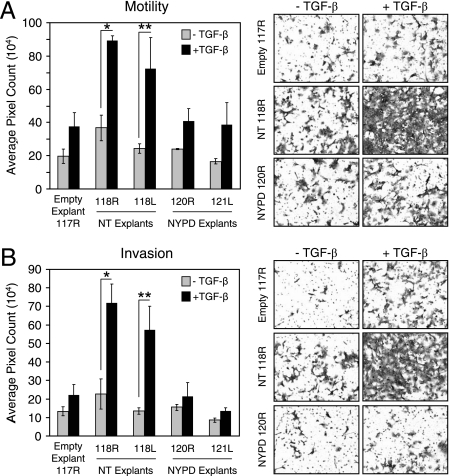
TGF-β stimulates the motility and invasion of Neu-NT-expressing mammary tumor explants. A single explant population of control NMuMG cells harboring the empty vector (Empty Explant 117R) and two tumor explants from Neu-NT-expressing (0118R and 0118L) and Neu-NYPD-expressing (0120R and 0121L) tumors were left untreated or were treated with TGF-β for 24 h, harvested, and plated for motility (A) and invasion assays (B) as described in Materials and Methods. The data represent results from three independent experiments for each cell population, with duplicate wells counted for each condition. Five images from each filter were quantified using Scion Image software. Representative images from one set of explant populations harboring each construct are shown (right) for both motility (A) and invasion (B). Significant differences in cell motility (A) (*, P < 0.05; **, P < 0.03) and invasion (B) (*, P < 0.002; **, P < 0.01) were observed only in TGF-β-treated Neu-NT-expressing tumor explants.
FIG. 3.
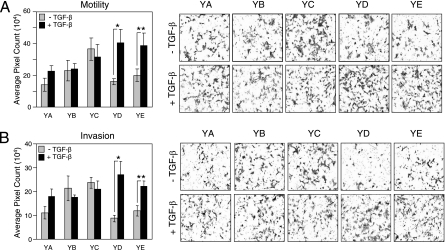
Signaling downstream of tyrosine residues 1226/1227 (YD) and 1253 (YE) in Neu/ErbB-2 synergize with TGF-β to promote cell motility and invasion. Explant cultures from mammary tumors expressing Neu/ErbB-2 add-back mutants were subjected to motility (A) and invasion (B) assays as described in Materials and Methods. Representative images from explant cultures expressing each Neu/ErbB-2 add-back receptor are shown (right) for both motility (A) and invasion (B). Significant differences in cell motility (A) (*, P < 0.02; **, P < 0.04) and invasion (B) (*, P < 0.02; **, P < 0.03) were observed in Neu-YD and Neu-YE tumor explants following TGF-β stimulation.
FIG. 7.
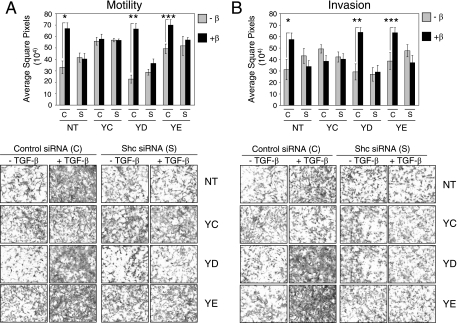
Loss of ShcA expression renders Neu-NT-, Neu-YD-, and Neu-YE-expressing NMuMG breast cancer cells refractory to TGF-β-induced motility and invasion. Explant cultures from mammary tumors expressing Neu-NT, Neu-YC, Neu-YD, and Neu-YE were subjected to motility (A) and invasion (B) assays as described in Materials and Methods. For each explant, cells were transfected with a scrambled control siRNA (C) or a pool of siRNAs targeting ShcA (S) prior to a 24-h incubation in the absence or presence of TGF-β. Representative images from control and ShcA siRNA-treated explant cultures, in the absence or presence of TGF-β, are shown (lower) for both motility (A) and invasion (B). Significant differences in cell motility (A) (*, P < 0.00097; **, P < 0.00005; ***, P < 0.01134) and invasion (B) (*, P < 0.02673; **, P < 0.00256; ***, P < 0.00946) were observed in Neu-NT, Neu-YD, and Neu-YE tumor explants following TGF-β stimulation.
BrdU incorporation assays.
For bromodeoxyuridine (BrdU) incorporation assays, cells were plated in chamber slides and grown to 60 to 70% confluence for 24 h. The cells subsequently were treated with or without TGF-β1 for a further 24 h. The cells were incubated in medium containing 10 μM BrdU (catalog no. 550891; BD Biosciences) for 2 h, fixed in 2% formalin, and immunostained with a BrdU in situ detection kit (catalog no. 550803; BD Biosciences) by following the manufacturer's instructions. Five images from each tumor explant cell population, untreated or treated with TGF-β, were captured with the ×40 objective of an Axioskop 40 microscope fitted with an Axiocam MRc digital camera (Zeiss). BrdU positivity was determined by manually counting both BrdU-positive and -negative cells using the cell counter feature of Image J software (NIH). The data shown represent the average percentages of BrdU-positive cells from three independent experiments.
GTPase activity assays.
Cells stimulated with or without TGF-β for 24 h were lysed in 20 mM HEPES (pH 7.5), 120 mM NaCl, 2 mM EDTA, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. Active GTP-bound Rac1 or RhoA was isolated from 1 mg of total cell lysate from the indicated explant cultures using equivalent amounts of recombinant glutathione _S_-transferase-CRIB (GST-CRIB) (for the active Rac1 binding domain of PAK) or GST-RBD (the active Rho binding domain of ROCK), respectively. The GST fusion proteins were kindly provided by Nathalie Lamarche-Vane, McGill University. Pull-down experiments were performed using glutathione-Sepharose 4B beads (catalog no. 17-0756-01; GE Healthcare) for 3 h on a rotator at 4°C, followed by three washes with buffer containing 0.5% NP-40 and 1 mM dithiothreitol. The beads were resuspended in 2× SDS sample loading buffer, boiled, and separated by SDS-PAGE through 12% gels. The contents of the pull-down experiments were analyzed by immunoblotting as described above using antibodies to Rac1 (C-11) (1:200; catalog no. sc-95; Santa Cruz) and RhoA (26C4) (1:200; catalog no. sc-418; Santa Cruz).
Immunofluorescence.
For immunofluorescence assays, cells were plated on glass coverslips and grown to subconfluence before treatment with or without TGF-β1 for 24 h. Cells then were fixed to the coverslips for 20 min with 2% paraformaldehyde in PBS. Following fixation, cells were permeabilized with PBS containing 0.2% Triton X-100 for 10 min and then washed three times with 100 mM glycine in PBS. To block the cells, a 30-min incubation in PBS containing 0.2% Triton X-100, 0.05% Tween-20, and 2% bovine serum albumin was performed. Cells were stained with FAK (1:400 dilution; catalog no. 06-543; Millipore/Upstate) or vinculin (1:1,000; catalog no. V 9131; Sigma) antibody for 1 h, followed by incubation with Alexa Fluor 555-conjugated anti-rabbit (catalog no. A-21429; Molecular Probes) or Alexa Fluor 555-conjugated anti-mouse (catalog no. A-21424; Molecular Probes) secondary antibody and Alexa Fluor 488 phalloidin (1:200 dilution; catalog no. A12379; Molecular Probes) to stain for actin filaments. Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI), and images were taken using a Zeiss Confocor2 LSM 510 Meta system on an Axiovert 200 M inverted microscope. The average number of focal adhesions was quantified using Volocity scientific imaging software from Improvision. Focal adhesions were distinguished by employing a fixed range of fluorescence intensity and a size exclusion of areas of less than 1 μm2 across all images. The total number of focal adhesions in each image was divided by the number of DAPI-stained nuclei to determine the number of focal adhesions per cell. Data shown are the averages from five images for each cell line derived from three independent experiments.
siRNA transfections.
For short interfering RNA (siRNA) transfection experiments, cells were transfected with a mixture of three dicer substrate duplex siRNAs targeting mouse ShcA (TriFECTa kit NM_011368; Integrated DNA Technologies) or a scrambled dicer substrate duplex siRNA using Lipofectamine 2000 reagent (catalog no. 11668-019; Invitrogen). siRNAs were used at a final concentration of 60 nM per transfection (20 nM for each individual ShcA siRNA). Cells were serially transfected a total of three times: once in the morning following cell plating, once in the evening of the same day, and once in the morning of the following day. At the time of each transfection, the cells were lightly trypsinized. Six hours after the last transfection, cells were passaged, treated with TGF-β for 24 h, and subjected to immunofluorescence, motility, and invasion assays as described above. A portion of the siRNA-transfected cells were maintained and lysed at different time points following the last transfection to monitor the extent and duration of the transient knockdowns. Whole-cell lysates were immunoblotted with an antibody against ShcA (1:1,000; catalog no. 610081; BD Biosciences).
Statistical analysis.
Statistical significance values (P values) for tumor growth, migration, invasion, and BrdU proliferation assays were obtained by performing a two-sample unequal-variance Student's t test.
RESULTS
Generation and characterization of Neu/ErbB-2-transformed NMuMG cells.
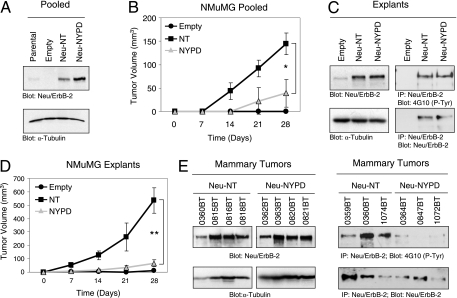
To examine the signaling pathways downstream of the Neu/ErbB-2 receptor that synergize with TGF-β to promote cancer progression, we established a Neu/ErbB-2-induced mammary tumor model. To achieve this, we transfected an immortalized but nontransformed mouse mammary epithelial cell line (NMuMG) (38) with expression constructs carrying two activated forms of the Neu/ErbB-2 receptor (16). The first possesses a V664E point mutation that constitutively activates the receptor (5, 6) and retains five major phosphorylation sites within the cytoplasmic tail (Neu-NT) (17, 23). The second mutant receptor harbors the same activating mutation in the transmembrane domain but possesses tyrosine-to-phenylalanine alterations of these five autophosphorylation sites (Neu-NYPD) (16), which include Y1028 (A site), Y1144 (B site), Y1201 (C site), Y1226/1227 (D site), and Y1253 (E site). Pooled stables were generated after the transfection of Neu-NT and Neu-NYPD, along with the empty vector as a control, into parental NMuMG cells. Immunoblot analysis confirmed that the pooled Neu-NT and Neu-NYPD populations expressed elevated levels of the activated receptors compared to the empty vector or parental NMuMG cell populations (Fig. 1A). To generate Neu/ErbB-2-transformed NMuMG cells capable of forming mammary tumors in vivo, we injected the pooled empty vector, Neu-NT, and Neu-NYPD cells into the mammary fat pads of athymic mice. Mammary tumor outgrowth was monitored by weekly caliper measurements (Fig. 1B), and tumor cells were explanted back into culture from three or four independent Neu-NT and Neu-NYPD mammary tumors. We did not observe progressively growing tumors in mice injected with NMuMG cells containing the empty vector control; however, at sacrifice, one animal possessed residual cells that persisted at the injection site, which we used to establish a nontransformed explant culture (empty). Immunoblot analysis revealed that Neu-NT- and Neu-NYPD-derived explant cultures retained elevated levels of Neu/ErbB-2 relative to that retained by the empty vector explant, and these receptors were tyrosine phosphorylated to similar extents in both explants (Fig. 1C). It should be noted that the Neu-NYPD receptor still can be phosphorylated on additional tyrosine residues located within the kinase domain and C terminus, and in cultured cells it has been shown to be phosphorylated to similar levels (16, 28). To characterize the relative tumorigenic abilities of Neu-NT- and Neu-NYPD-expressing tumor explants, we reinjected them into the mammary fat pads of nude mice and monitored tumor outgrowth by weekly caliper measurements. Reminiscent of the behavior of the pooled populations (Fig. 1B), the Neu-NT-expressing explants were much more aggressive at forming mammary tumors than the Neu-NYPD explants (Fig. 1D). Immunoblot analysis confirmed that primary mammary tumors retained the expression of tyrosine-phosphorylated Neu-NT and Neu-NYPD receptors in vivo (Fig. 1E). In contrast to the results seen in the mammary tumor explant cultures, these tumor lysates revealed that the degree of Neu-NYPD phosphorylation is reduced relative to that observed with Neu-NT-expressing mammary tumors (Fig. 1E). This observation has been made previously for mammary tumors arising in mouse mammary tumor virus (MMTV)/Neu-NYPD transgenic mice (46).
FIG. 1.
Neu/ErbB-2-transformed NMuMG cells form mammary tumors in vivo. (A) Immunoblot analysis on pooled stable populations of NMuMG cells expressing either Neu-NT, Neu-NYPD, or the empty vector control using antibodies against Neu/ErbB-2 and α-tubulin. (B) Mammary tumor outgrowth was measured by weekly caliper measurements following the injection of pooled stables into the fourth mammary fat pad. The average tumor volumes (± standard deviations) from four independent tumors expressing Neu-NT or Neu-NYPD are plotted (*, P < 0.0013), whereas NMuMG empty vector control cells did not form palpable tumors following injection. (C) Immunoblot analyses were performed on whole-cell lysates from the indicated explant cultures with both Neu/ErbB-2- and α-tubulin-specific antibodies (left). Neu/ErbB-2 immunoprecipitations also were performed, and the immunoprecipitates were split and blotted for phosphotyrosine and Neu/ErbB-2 (right). (D) Explant cultures were reinjected into the fourth mammary fat pad, and mammary tumor outgrowth was measured by weekly caliper measurement (**, P < 0.0001). (E) Neu/ErbB-2- and α-tubulin-specific immunoblot analyses were performed on protein lysates prepared from Neu-NT- and Neu-NYPD-expressing mammary tumors (numbers refer to mouse ear tag numbers) (left). Neu/ErbB-2 was immunoprecipitated from Neu-NT- and Neu-NYPD-expressing mammary tumor lysates in duplicate; one set of immunoprecipitates was blotted for phosphotyrosine, and the other was blotted for Neu/ErbB-2 (right). IP, immunoprecipitation; P-Tyr, phosphotyrosine.
Neu-NT-transformed mammary tumor cells are more metastatic to the lung than Neu-NYPD-expressing cells.
To determine the metastatic potential of Neu-NT- or Neu-NYPD-expressing tumor cells, lungs first were harvested from athymic mice 28 days following mammary fat pad injection, regardless of the tumor volume at necropsy. While 100% of the Neu-NT tumor-bearing mice developed lung lesions, none of the animals that developed Neu-NYPD-induced tumors had any detectable metastases (Table 1). This difference may reflect the fact that at necropsy the mammary tumor volume of the Neu-NT-expressing tumors was four- to sevenfold larger than that of the Neu-NYPD-expressing tumors. To control for this, we allowed Neu/ErbB-2-expressing tumors to reach a volume of 150 to 200 mm3, at which point the primary tumors were removed and the mice were monitored for evidence of lung metastases. Despite the fact that the Neu-NYPD-expressing tumors remained in the mice for a considerably longer length of time (Neu-NYPD tumors, 38 to 67 days; Neu-NT tumors, 7 to 25 days), 80% of mice bearing Neu-NT-expressing tumors developed lung metastases, whereas only 25% of the mice bearing Neu-NYPD-induced tumors developed lung metastases (Table 1). This suggests that Neu-NYPD tumor cells have a lower metastatic potential than Neu-NT-expressing mammary tumor cells. To verify this, we performed an experimental metastasis assay in which mice were injected with an equal number of Neu-NT- and Neu-NYPD-expressing NMuMG cells into the lateral tail vein and necropsied 8 weeks later. Again, 100% of the mice injected with Neu-NT-expressing cells developed multiple lung metastases, whereas no metastatic lesions were visible in the lungs of mice injected with Neu-NYPD-expressing cells (Table 1). Taken together, these results clearly indicate that Neu-NT-expressing NMuMG tumor explants are more aggressively metastatic to the lung than Neu-NYPD-expressing tumors.
TABLE 1.
Metastatic potential of Neu-expressing NMuMG-derived cell populations
| NMuMG explant | Metastasis results according to injection route | |||||
|---|---|---|---|---|---|---|
| Mammary fat pada (tumors not removed) | Mammary fat padb (tumors resected, 150-200 mm3) | Tail veinc | ||||
| % (no.) of mice with lung metastases | No. of lesions/lungd | % (no.) of mice with lung metastases | No. of lesions/lungd | % (no.) of mice with lung metastases | No. of lesions/lungd | |
| Neu-NT | 100 (4/4) | 2-5 | 80 (4/5) | 2-11 | 100 (4/4) | 8-18 |
| Neu-NYPD | 0 (0/4) | 0 | 25 (2/8) | 1-2 | 0 (0/5) | 0 |
TGF-β specifically enhances the motility and invasion of Neu-NT-expressing mammary tumor explants.
Signaling pathways downstream of Neu/ErbB-2 cooperate with TGF-β to augment breast cancer cell motility or invasion (48, 62). To assess the importance of known Neu/ErbB-2 autophosphorylation sites in these biological responses, we compared the basal and TGF-β-inducible motility and invasion of empty vector control cells with Neu-NT- or Neu-NYPD-expressing mammary tumor explants. Our initial experiments revealed that none of the explanted cells, when stimulated with TGF-β during the course of the assays, demonstrated enhanced motility or invasion (data not shown). However, the incubation of empty vector, Neu-NT, and Neu-NYPD explant populations in the absence or presence of TGF-β for 24 h prior to being plated on transwell filters revealed a TGF-β-inducible, synergistic increase in the level of motility and invasion in Neu-NT tumor explants (Fig. 2). Interestingly, TGF-β induced less than twofold increases in motility and invasion in explants harboring the empty vector or those expressing the Neu-NYPD receptor, values that failed to reach statistical significance (Fig. 2). Indeed, the TGF-β stimulation of two independent Neu-NT mammary tumor explants resulted in a 2.5- to 3.0-fold increase in motility and a 3.0- to 5.0-fold increase in invasion compared to that of untreated cultures.
To verify that the empty vector and Neu-NYPD-expressing explants remained responsive to TGF-β, we examined TGF-β-induced signaling in empty vector, Neu-NT, and Neu-NYPD mammary tumor explants that were left untreated or were stimulated with TGF-β for 30 min. Indeed, TGF-β stimulation induced Smad2 phosphorylation to a similar extent in empty vector, Neu-NT-expressing explant cultures, and Neu-NYPD-expressing explant cultures (see Fig. S1A in the supplemental material). We next performed BrdU incorporation assays to determine whether empty vector, Neu-NT, and Neu-NYPD cells remained sensitive to the antiproliferative effects of TGF-β. These analyses revealed that the percentage of BrdU-positive cells present in all three explant populations following TGF-β treatment was approximately half that observed in untreated controls (see Fig. S1B in the supplemental material), indicating that the expression of an activated Neu/ErbB-2 receptor in NMuMG cells is not sufficient to render them resistant to TGF-β cytostatic effects. NMuMG cells are well documented to undergo an epithelial-to-mesenchymal transition (EMT) in response to TGF-β (3, 33), and such a transition has been associated with enhanced cell migration and invasion (25). To determine if the NMuMG explants we have established retain this response, we examined the expression of epithelial and fibroblast-associated markers. All three explant cultures undergo a clear morphology change 24 h after TGF-β treatment (J. J. Northey and P. M. Siegel, unpublished observations), which is reflected by a repression of E-Cadherin and the induction of Fibronectin transcripts in all three explants during the same time period (see Fig. S1C in the supplemental material). Furthermore, immunoblot analysis revealed that E-cadherin protein levels became undetectable after 4 days of TGF-β treatment in empty vector, Neu-NT, and Neu-NYPD explant cultures, whereas fibronectin levels were induced after 1 day of TGF-β treatment and continued to rise until day 4 of TGF-β stimulation (see Fig. S1D in the supplemental material). Furthermore, smooth-muscle actin expression was induced transiently after 24 h of TGF-β treatment in all explant cultures, reduced to baseline levels by 48 h, and reinduced at 72 h following fresh TGF-β addition at 48 h (see Fig. S1D in the supplemental material). Taken together, these results conclusively demonstrate that numerous TGF-β-induced responses, including Smad signaling, growth inhibition, and EMT, remain intact in all three NMuMG explant cultures.
Activation of Rho and Rac GTPases does not account for the observed differences in TGF-β-induced increases in motility and invasion of Neu-NT breast cancer explants.
TGF-β has been shown to stimulate RhoA activity in NMuMG cells (3) and to enhance Rac1 activation in MCF10A cells overexpressing ErbB-2 (65). Immunoblot analysis of Neu-NT-expressing NMuMG mammary tumor explants revealed detectable levels of RhoA and Rac1 but very low levels of expression of Cdc42 (Northey and Siegel, unpublished). As previously reported, RhoA activation was observed in NMuMG (empty vector) cells in response to 24 h of TGF-β treatment (see the upper portion of Fig. S2A in the supplemental material). The steady-state levels of active RhoA were higher in Neu-NT- and Neu-NYPD-expressing mammary tumors cells than in empty vector control cells (without TGF-β); however, no increase in RhoA activity was observed in response to TGF-β stimulation (see the upper portion of Fig. S2A in the supplemental material). In the case of Rac1, TGF-β stimulation of empty vector- and Neu-NT-expressing cells resulted in an increase in GTP-bound Rac1, which failed to occur in Neu-NYPD-expressing cells (see the upper portion of Fig. S2B in the supplemental material). It is clear from these results that the synergistic activation of motility and invasion induced by TGF-β in Neu-NT-expressing NMuMG mammary tumor explants cannot be accounted for by a unique or strong activation of RhoA or Rac1 in these cells compared to that of the empty vector or Neu-NYPD-expressing explants.
TGF-β synergizes with signals downstream of tyrosine residues 1226/1227 (YD) and 1253 (YE) to induce motility and invasion.
To determine if distinct signaling pathways downstream of Neu/ErbB-2 contributed to TGF-β-induced motility and invasion, we generated NMuMG mammary tumor explants expressing a panel of Neu/ErbB-2 add-back receptors (16). These receptors all contain the activating transmembrane point mutation and have individual tyrosine residues reconstituted in the context of the Neu-NYPD backbone. Thus, each add-back receptor contains a single tyrosine autophosphorylation site; the following nomenclature was used: YA (tyrosine at position 1028), YB (tyrosine at 1144), YC (tyrosine at 1201), YD (tyrosines at 1226/1227), and YE (tyrosine at 1253) (16). We first generated pooled NMuMG populations that individually express the Neu/ErbB-2 add-back receptors and injected them into the mammary fat pads of nude mice. Mammary tumor explant cultures were established, each expressing Neu/ErbB-2 to comparable or slightly higher levels than those seen in Neu-NT- and Neu-NYPD-expressing mammary tumor cells (see Fig. S3A in the supplemental material). Upon reinjection, all NMuMG tumor explants expressing the Neu/ErbB-2 add-back receptors were able to form mammary tumors (see Fig. S3B in the supplemental material) that retained expression of the receptors (see Fig. S3C in the supplemental material). The least aggressive behavior was observed with Neu-YA-expressing NMuMG cells, which is not surprising given that tyrosine 1028 has been characterized as a negative regulatory site in fibroblasts and has been shown to impair Neu-NT-induced transformation in vitro (16).
We utilized these Neu/ErbB-2 add-back receptor-expressing tumor cells to identify signaling pathways that synergize with TGF-β to promote breast cancer cell motility and invasion. In the absence of TGF-β stimulation, the motilities of NMuMG mammary tumor explants expressing Neu-YA, Neu-YB, Neu-YD, and Neu-YE were similar to that observed in empty vector- and Neu-NYPD-expressing cells, whereas Neu-YC-expressing cells displayed the highest degree of baseline motility (Fig. 2A and 3A). Interestingly, only Neu-YD- and Neu-YE-expressing tumor explants displayed a twofold increase in cell motility in response to TGF-β stimulation (Fig. 3A), which is comparable to the 2.5- to 3-fold induction observed in Neu-NT-expressing cells (Fig. 2A), while Neu-YA, Neu-YB, and Neu-YC tumor cells failed to achieve statistically significant responses to this cytokine (Fig. 3A).
The Neu/ErbB-2 add-back receptor-expressing tumor explants also were assessed for their ability to invade through a Matrigel barrier in the absence of TGF-β stimulation or following 24 h of TGF-β pretreatment. The invasive abilities of Neu-YA-, Neu-YD-, and Neu-YE-expressing explants were similar to those observed in empty vector- and Neu-NYPD-expressing cells, whereas Neu-YB- and Neu-YC-expressing tumor cells exhibited a phenotype of higher basal invasiveness (Fig. 2B and 3B). Similar to the effects on motility, pretreatment with TGF-β specifically enhanced the invasive properties of NMuMG tumor explants expressing Neu-YD and Neu-YE by 2.0- to 2.5-fold (Fig. 3B), which was less than the 4.0- to 5.0-fold increase observed in Neu-NT cells pretreated with TGF-β (Fig. 2B). This suggests that signals emanating from tyrosines 1226/1227 or tyrosine 1253 of Neu/ErbB-2 are sufficient to mediate TGF-β-induced motility and contribute, in part, to the synergistic effects observed with TGF-β on breast cancer cell invasion.
To ensure that all of the NMuMG mammary tumor explants expressing the Neu/ErbB-2 add-back receptors remained responsive to TGF-β, we also characterized these cultures for TGF-β-induced Smad2 phosphorylation, growth inhibition, and EMT. All of the Neu/ErbB-2 explants expressing the individual add-back mutants displayed Smad2 phosphorylation (see Fig. S4A in the supplemental material) and were growth inhibited (see Fig. S4B in the supplemental material) following TGF-β treatment. Finally, all Neu/ErbB-2 add-back explants underwent a TGF-β-induced EMT as indicated by a clear morphology change (data not shown), the loss of E-cadherin (see Fig. S4C in the supplemental material), and the induction of both smooth-muscle actin (see Fig. S4D in the supplemental material) and fibronectin (see Fig. S4E in the supplemental material) expression. Taken together, these data verify that the TGF-β pathway is active in all Neu/ErbB-2 add-back receptor-expressing tumor explants; however, only signaling pathways downstream of tyrosines 1226/1227 and 1253 of Neu/ErbB-2 synergize with TGF-β to stimulate breast cancer motility and invasion.
The number and size of mature focal adhesions are reduced in mammary tumor explants with high baseline or TGF-β-induced motility.
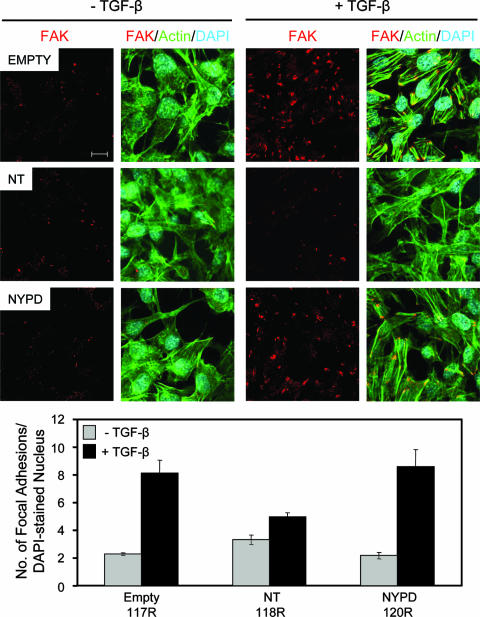
The ability of cells to migrate depends on their ability to coordinately regulate the establishment and turnover of focal adhesions (11). To further examine TGF-β effects on the motility of Neu-NT-expressing cells (Fig. 2), we determined the nature of the focal adhesions that were induced in these cells in response to TGF-β. Immunofluorescence staining with FAK-specific antibodies revealed similar numbers and sizes of large focal adhesions (>1 μm2) that formed in empty vector, Neu-NT-, and Neu-NYPD-expressing cells in the absence of TGF-β stimulation (Fig. 4). In contrast, TGF-β treatment was able to significantly increase the number and size of focal adhesions in empty vector and Neu-NYPD cells, but it had no such effect in Neu-NT-expressing cells (Fig. 4). A quantitative analysis revealed that empty vector- and Neu-NYPD-expressing explants possessed, on average, two to three large focal adhesions per cell prior to TGF-β stimulation, which increased to eight to nine large focal adhesions per cell following 24 h of TGF-β treatment. In contrast, Neu-NT cells possessed approximately three to four focal adhesions per cell in the absence of TGF-β, which only increased to four to five focal adhesions per cell following the addition of cytokine (Fig. 4).
FIG. 4.
Neu-NT-expressing mammary tumor explants form fewer and smaller focal adhesions in response to TGF-β treatment than empty vector- or Neu-NYPD-expressing explants. Immunofluorescence staining was performed for FAK (red) and actin (green) on all three explant populations in the absence or presence of TGF-β (24 h). DAPI staining (blue) was performed to visualize nuclei. The number of focal adhesions formed under each condition was quantified using Volocity software. Quantified data are expressed as the number of focal adhesions per DAPI-stained nucleus and were obtained from five fields for each condition from three independent experiments. The scale bar represents 10 μm and applies to all panels.
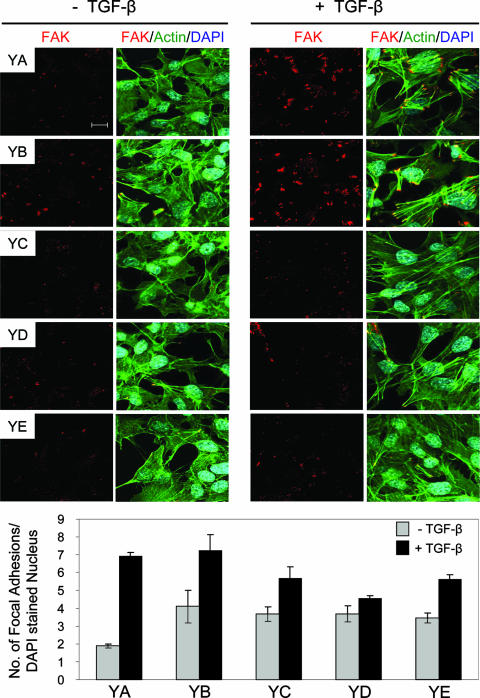
We also extended these analyses to NMuMG tumor explant cultures individually expressing the Neu/ErbB-2 add-back receptors. Indeed, Neu-YA and Neu-YB tumor cells demonstrated a TGF-β-inducible increase in the number and size of focal adhesion formation that was similar to that observed in empty vector- and Neu-NYPD-expressing cells (Fig. 4 and 5). In contrast, Neu-YC- and Neu-YE-expressing explants displayed an intermediate degree of TGF-β-stimulated focal adhesion formation, whereas the focal adhesions observed in tumor explants expressing Neu-YD were most similar to those observed in Neu-NT-expressing cells following TGF-β stimulation (Fig. 5). These data suggest that Neu-NT- and Neu-YD-expressing cells form fewer and smaller focal complexes than the numerous, larger, and more mature focal adhesions that develop in the empty vector-, Neu-NYPD-, Neu-YA-, and Neu-YB-expressing NMuMG explants in response to TGF-β. Thus, the appearance of larger and more stable focal adhesions in the latter group may limit their migratory ability in response to TGF-β.
FIG. 5.
Neu-YD-expressing explants display the lowest level of density of mature focal adhesions, which is associated with enhanced cell motility and invasion, in response to TGF-β stimulation. Immunofluorescence staining was performed for FAK (red) and actin (green) on all Neu add-back populations (Neu-YA, Neu-YB, Neu-YC, Neu-YD, and Neu-YE) in the absence or presence of TGF-β (24 h). DAPI staining (blue) was performed to visualize nuclei. The number of focal adhesions formed under each condition was quantified using Volocity software. Quantified data are expressed as the number of focal adhesions per DAPI-stained nucleus and were obtained from five fields for each condition from three independent experiments. The scale bar represents 10 μm and applies to all panels.
ShcA expression and phosphotyrosine-dependent signaling are required for TGF-β-induced motility and invasion of Neu-NT-expressing breast cancer cells.
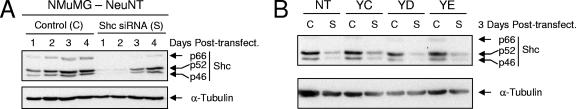
Previous studies have mapped the binding of the ShcA adaptor protein to tyrosines 1226/1227 (YD site) of the Neu/ErbB-2 receptor, with tyrosine 1227 representing the critical tyrosine for Shc association (14, 15). Additional studies, based on high-throughput associations with phosphorylated peptides, suggest that ShcA interacts with tyrosines 1201 (YC), 1227 (YD), and 1253 (YE) (27, 47, 57). Our results indicate that signals transmitted from the YD and YE sites are sufficient to synergize with TGF-β for the induction of breast cancer motility and invasion. To test the involvement of ShcA in these TGF-β-induced tumor cell responses, we have established conditions for a transient ShcA knock-down in Neu-NT-expressing NMuMG mammary tumor cells. Using a pool of siRNAs targeting ShcA, we achieved a clear and robust diminishment of ShcA levels up to 2 days posttransfection (Fig. 6A), representing the time at which cells were plated for motility and invasion assays. A partial recovery of ShcA expression was observed after 3 days, which represents the end point of motility and invasion assays, reaching normal levels at 4 days posttransfection (Fig. 6A). Importantly, a scrambled control siRNA had no effect on endogenous ShcA expression (Fig. 6A). We confirmed that these conditions also were effective at reducing ShcA expression in Neu-YC-, Neu-YD-, and Neu-YE-expressing NMuMG mammary tumor explants at 3 days posttransfection (Fig. 6B).
FIG. 6.
Transient siRNA-mediated knockdown of the ShcA adaptor protein in Neu/ErbB-2-expressing breast cancer explants. (A) Immunoblot analysis of Neu-NT-expressing NMuMG breast cancer cells treated with a scrambled control siRNA (C) or a mixture of three siRNAs targeting ShcA (S). Lysates were prepared 1, 2, 3, and 4 days following the transfection (Post-transfect.) of the siRNAs. (B) The effectiveness of the ShcA knockdowns was confirmed 3 days following transfection in Neu-NT-, Neu-YC-, Neu-YD-, and Neu-YE-expressing NMuMG breast cancer cells.
We utilized these knockdown conditions to perform motility and invasion assays with Neu-NT-, Neu-YC-, Neu-YD-, and Neu-YE-expressing mammary tumor cells. Neu-NT-, Neu-YD-, and Neu-YE-expressing cells, treated with the scrambled control siRNA, displayed a TGF-β-induced increase in both motility (Fig. 7A) and invasion (Fig. 7B), whereas Neu-YC-expressing cells were unresponsive to TGF-β. Interestingly, reducing the levels of ShcA expression was sufficient to completely abrogate the TGF-β-induced enhancement of motility and invasion in Neu-NT-, Neu-YD-, and Neu-YE-expressing mammary tumor explants but had no effect on Neu-YC-expressing mammary tumor cells (Fig. 7).
In support of these results, we have utilized NMuMG-Neu-NT tumor explants that overexpress a dominant-negative form of Shc (Shc-3F). This ShcA protein lacks three tyrosine residues (Y239, Y240, and Y313 in mouse ShcA) important for ShcA signaling (see Fig. S5A in the supplemental material) and has been shown to function in a dominant-negative manner to block epidermal growth factor-induced cell proliferation and migration (13, 20). A pool of three stable cell lines, expressing similar levels of ShcA-3F, was used for these studies. While the baseline motility and invasion of Neu-NT/ShcA-3F cells are elevated compared to those for parental Neu-NT-expressing cells, TGF-β fails to enhance the migratory and invasive characteristics of Neu-NT explants coexpressing the ShcA-3F mutant (see Fig. S5B and C in the supplemental material). Taken together with the ShcA knockdown data, these results strongly suggest that Neu/ErbB-2-initiated signals that are transmitted by the ShcA adaptor are critical for TGF-β-induced breast cancer motility and invasion in this breast cancer system.
Enlarged focal adhesion formation is observed in cells with reduced ShcA signaling following TGF-β stimulation.
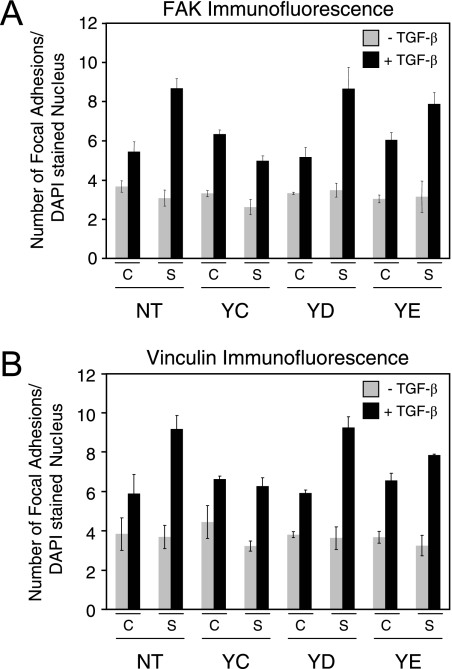
We next assessed whether the effects of diminished ShcA signaling on reduced motility and invasion correlated with increased mature focal adhesion formation. FAK immunofluorescence staining of cells treated with ShcA siRNAs revealed a clear increase in the number and size of mature focal adhesions in Neu-NT-, Neu-YD-, and Neu-YE-expressing breast cancer cells in response to TGF-β stimulation (Fig. 8A; also see Fig. S6 in the supplemental material), which is reminiscent of levels achieved in empty vector- or Neu-NYPD-expressing cells (Fig. 4). In contrast, reduced ShcA expression affected neither TGF-β-induced motility and invasion nor focal adhesion formation in Neu-YC-expressing mammary tumor explants (Fig. 8A; also see Fig. S6 in the supplemental material). Importantly, the treatment of all explant cultures with a scrambled control siRNA had no effect on TGF-β-stimulated focal adhesion formation (Fig. 8A; also see Fig. S6 in the supplemental material). These results were reinforced by the observation that Neu-NT mammary tumor explants coexpressing ShcA-3F also displayed an increase in the numbers of mature focal adhesions in response to TGF-β treatment (see Fig. S5D in the supplemental material).
FIG. 8.
Reduced ShcA expression results in the formation of increased numbers of large focal adhesions in Neu-NT-, Neu-YD-, and Neu-YE-expressing mammary tumor cells following TGF-β stimulation. Immunofluorescence staining was performed for FAK (A) and vinculin (B) on Neu-NT-, Neu-YC-, Neu-YD-, and Neu-YE-expressing cells that were treated with a scrambled control siRNA (C) or a cocktail of three siRNAs targeting ShcA. The cells were incubated in the absence (−) or presence (+) of TGF-β for 24 h. The number of FAK-stained (A) or vinculin-stained (B) focal adhesions formed under each condition was quantified using Volocity software. Quantified data are expressed as the number of focal adhesions per DAPI-stained nuclei and were obtained from five fields for each condition from three independent experiments.
Vinculin is another component of focal adhesion complexes, and it functions to reduce cell motility by contributing to the formation of large, stable focal adhesions with enhanced adhesive strength (73). Vinculin immunofluorescence staining of cells treated with the ShcA siRNAs revealed a very clear increase in the number and size of focal adhesions in Neu-NT-, Neu-YD-, and Neu-YE-expressing NMuMG explants in response to TGF-β treatment that was not observed in Neu-YC-expressing mammary tumor cells (Fig. 8B; also see Fig. S7 in the supplemental material). No differences were observed in the number or size of vinculin-positive adhesions in cells treated with a scrambled control siRNA following TGF-β stimulation (Fig. 8B; also see Fig. S7 in the supplemental material). Taken together, these results demonstrate that Neu/ErbB-2 signaling through the ShcA adaptor protein synergizes with TGF-β to favor the formation of small focal contacts that promote cell migration.
DISCUSSION
We have demonstrated that a constitutively active form of the Neu/ErbB-2 receptor tyrosine kinase cooperates with TGF-β to promote the motility and invasion of breast cancer cells and that the removal of five autophosphorylation sites in the C terminus of Neu/ErbB-2 completely abrogates the synergistic interaction of these two pathways. Furthermore, through the use of a panel of Neu/ErbB-2 add-back mutants, we have demonstrated that specific signals emanating from tyrosines 1226/1227 (YD) and 1253 (YE) of Neu/ErbB-2 are critical for TGF-β-induced motility and invasion. This enhanced motility of TGF-β-treated Neu-NT and Neu-YD breast tumor explants is associated with the formation of smaller, less mature focal adhesions. The ShcA adaptor protein is known to bind the Neu/ErbB-2 receptor through tyrosines 1226/1227 (YD) and potentially through tyrosines 1201 (YC) and 1253 (YE) (14, 15, 27, 47, 57). We demonstrate that the reduction of ShcA levels or the expression of a dominant-negative ShcA mutant (ShcA-3F) blocked TGF-β-induced motility and the invasion of Neu/ErbB-2-expressing breast cancer cells. Collectively, these observations demonstrate a central role for the ShcA adaptor protein in mediating the synergy between the ErbB-2 and TGF-β pathways.
The Neu/ErbB-2-expressing NMuMG tumor explant models that we have generated very closely resemble the phenotypes induced by these add-back receptors when they are expressed in the mammary epithelium of transgenic mice. In our system, Neu-NT-expressing explants displayed the most aggressive tumor outgrowth phenotype, and the longest latency was observed with Neu-NYPD cells. While intermediate effects on mammary tumor outgrowth were observed with the add-back mutants, Neu-YA, Neu-YB, and Neu-NYPD explants were less aggressive than Neu-YC-, Neu-YD-, and Neu-YE-transformed NMuMG cells. This is reflective of the results observed with the MMTV-based transgenic models, in which the onsets of mammary tumor formation were quite similar among Neu-YD-, Neu-YE-, and Neu-YC-expressing mice, followed by later onset in MMTV/Neu-YB transgenics and, finally, the longest latency in MMTV/Neu-NYPD animals (15, 46). We did observe that NMuMG cells expressing Neu-YA receptors were capable of forming mammary tumors, even though this tyrosine has been characterized as a negative regulatory site that suppressed the focus-forming ability of fibroblasts (16). Transgenic mice expressing an MMTV/Neu-YA transgene have not been derived; thus, it is unknown whether this mutant receptor is indeed capable of transforming normal mammary epithelial cells in vivo. It is conceivable that the negative regulator of transformation that binds to tyrosine 1028 (YA) of Neu/ErbB-2 in fibroblast cells is not expressed in NMuMG epithelial cells.
Our NMuMG cell-based mammary tumor explants also recapitulate features of the metastatic phenotypes induced by specific Neu/ErbB-2 add-back receptors. For instance, MMTV/Neu-YB animals display a more aggressive lung metastatic phenotype than MMTV/Neu-YD transgenic mice (15, 46). In our NMuMG mammary tumor explants, we consistently observe higher baseline invasion of the Neu-YB explants compared to that of cells expressing the Neu-YD receptor. Interestingly, only Neu-YD and Neu-YE explant cultures displayed TGF-β-induced motility and invasion. The observation that Neu-YB-expressing NMuMG explants possess high baseline invasiveness that is not TGF-β inducible, whereas Neu-YD cells display lower baseline invasiveness that is enhanced by TGF-β stimulation, also is reminiscent of our earlier transgenic studies. Indeed, we demonstrated that the overexpression of TβRI(AAD) in the mammary gland increased the number of extravascular lung metastases in MMTV/Neu-YD mice but could not enhance the aggressive lung metastatic phenotype exhibited by MMTV/Neu-YB transgenic animals (53).
To better understand the underlying basis for Neu/ErbB-2 and TGF-β cooperativity in enhancing cell motility, we examined focal adhesion formation in each of the NMuMG explant cultures. Several types of focal adhesions have been described, including focal complexes or contacts, focal adhesions, and fibrillar adhesions (72). Focal complexes are small (<0.25 μm2), short-lived adhesions that form close to the leading edge of cells, whereas focal adhesions are larger (1 to 10 μm2) and persist for longer periods of time. It has been postulated that the small, nascent focal complexes apply strong propulsive traction that facilitates cell migration, whereas larger, more mature focal adhesions promote a passive anchorage function (7). Other groups argue that small focal contacts (less than 1 μm2) exert negligible traction forces, whereas the tractional forces generated by larger adhesions are proportional to their areas (4). One possible resolution to this apparent contradiction is that small, closely spaced focal complexes at the leading edge of a cell are functionally interconnected, and the resulting integration of multiple small contacts produces greater tractional forces than individual, widely spaced large adhesions (29). When we examined the nature of focal adhesion formation by FAK immunofluorescence (with adhesions greater than 1 μm2 in size), we observed a TGF-β-mediated increase in the formation of large, mature focal adhesions only in those explants that did not demonstrate a TGF-β-inducible increase in migration (empty vector cells and Neu-NYPD, Neu-YA, and Neu-YB explants). In contrast, cells with a high-baseline migratory phenotype (Neu-YC explants), or those that displayed enhanced motility in response to TGF-β stimulation (Neu-NT, Neu-YD, and Neu-YE explants), had smaller and less numerous mature focal adhesions.
The receptor-like protein tyrosine phosphatase kappa (PTPRK) recently has been implicated as an important TGF-β target, the expression of which is required for TGF-β-induced cell adhesion, motility, and focal adhesion formation in ErbB-2-expressing MCF-10A cells (66). We did not observe TGF-β-induced PTPRK mRNA expression at 24 h after cytokine treatment (Northey and Siegel, unpublished), and PTPRK does not appear to be a TGF-β-inducible target in parental NMuMG cells (68). Focal adhesion formation and cytoskeletal changes associated with cell motility are regulated by small GTPases, such as Rho, Rac, and Cdc42. The TGF-β-induced activations of RhoA and Rac1 have been shown to be important for EMT and cell migration in NMuMG cells (2, 8). Furthermore, Rac1 activity is elevated in MCF-10A cells transformed by ErbB-2, which is further induced by TGF-β stimulation (62, 65). However, in our NMuMG system, we did not observe a unique or more robust induction of RhoA or Rac1 activation in Neu-NT-expressing cells in response to TGF-β that would explain the observed synergy in motility and invasion.
Our results implicate the ShcA adaptor protein as an important mediator of TGF-β-inducible focal adhesion turnover. Our observation that ShcA null breast cancer cells possess numerous, large vinculin-positive focal adhesions and are impaired in TGF-β-induced motility correlates well with studies demonstrating that vinculin overexpression leads to the formation of large focal adhesions that possess enhanced adhesive strength in fibroblast cells (19, 42). Conversely, it has been shown that vinculin-deficient fibroblasts are less adherent, form fewer and smaller focal adhesions, and are more motile than wild-type cells (12, 45, 69, 70). Thus, it is possible that TGF-β signaling enhances focal adhesion turnover in Neu-NT, Neu-YD, and Neu-YE explants in a ShcA-dependent fashion and prevent them from becoming larger, more stable adhesions. In contrast, cells with a less motile phenotype form large, mature adhesions that anchor them and limit their movement. This hypothesis will require further investigation using live-imaging techniques to quantify focal adhesion turnover.
The transient knockdown of ShcA in Neu-NT, Neu-YD, and Neu-YE tumor explants clearly is associated with reduced cell motility and invasion following TGF-β stimulation. Likewise, the stable expression of a dominant-negative ShcA-3F molecule impaired TGF-β-induced motility and the invasion of Neu-NT-transformed cells, while it paradoxically rendered them more motile and invasive in the absence of TGF-β. This is unlikely to be a clonal effect, since the NeuNT/ShcA-3F cells are a pool of three independent ShcA-3F expressors. Unlike the transient but complete knockdown of ShcA expression, the ShcA-3F dominant mutant still retains intact PTB and SH2 domains and thus is capable of mediating phosphotyrosine-independent adaptor functions. Thus, it is conceivable that ShcA-3F expression in NMuMG cells sequesters a factor(s) that normally impairs basal, but not TGF-β-inducible, cell motility and invasion. In addition, stable ShcA-3F expression, but not acute ShcA loss, may allow for the selection of adaptive responses that favor enhanced motility and invasion in these cells.
The use of the Neu/ErbB-2 add-back mutants indicates that signaling pathways initiating from tyrosines 1226/1227 (YD) and 1253 (YE) are responsible for the observed synergy with TGF-β. The ShcA adaptor molecule has been shown to bind directly to site D (Y1227) (14, 16), while others have shown that ShcA also may bind to tyrosines 1201 (YC) and 1253 (YE) (27, 47, 57). Our results indicate that the ShcA adaptor protein plays an essential role in the TGF-β-induced migration and invasion of Neu-NT-, Neu-YD-, and Neu-YE-expressing breast cancer cells. Numerous studies have indicated an important role for ShcA in promoting cancer cell motility and metastasis. While transgenic mice expressing the polyomavirus middle T oncogene (PyV mT) in the mammary gland develop aggressive mammary tumors that metastasize to the lung (21), the mutation of the ShcA binding site on PyV mT diminished mammary tumor formation (67). Interestingly, 7% of the mammary tumors and 36% of lung metastases displayed point mutations or in-frame deletions within PyV mT that re-created a functional ShcA binding site (67). These observations argue strongly for an important role for the ShcA adaptor in promoting mammary tumorigenesis and metastasis. More recent studies have demonstrated that the expression of activated Neu-NT, Neu-YC, Neu-YD, or Neu-YE receptors promotes the scattering and enhanced motility of MDCK cells (28). It is interesting that Neu-YC-expressing explants display a higher baseline motility than the other add-back receptor-expressing cells, which is not further induced by TGF-β stimulation. Moreover, the knockdown of ShcA has no effect on the motility of Neu-YC-expressing cells in either the absence or presence of TGF-β. Thus, the Neu-YC receptor signals enhanced cell motility through a distinct pathway that neither requires ShcA nor synergizes with TGF-β. Interestingly, it has been shown by phosphopeptide interactions that YC may be a docking site for Crk or CrkL (27), two adaptor proteins that have been shown to be important for cell motility and invasion (41).
ShcA can recruit Grb2 through interactions with tyrosine residues 239 and 313 (317 in human ShcA) (44, 64). While it is conceivable that Grb2 bound to ShcA is important for TGF-β-induced motility and invasion, it should be noted that tumor explants expressing Neu-YB, which directly binds Grb2, did not synergize with TGF-β to enhance breast cancer migration and invasion. This suggests that ShcA interacts with other proteins to promote these TGF-β responses. Indeed, ShcA has been shown to be important for heregulin-induced motility of ErbB-2-expressing T47D breast cancer cells via the recruitment of Memo to tyrosine residue 1227 of ErbB-2 (31). In this study, Memo was implicated as an important mediator of ErbB-2-induced motility by virtue of its role in microtubule formation. Whether Memo plays a role in TGF-β-induced motility and invasion of Neu/ErbB-2-expressing cells awaits further investigation; however, antitubulin staining did not reveal any discernible differences in microtubule outgrowth between Neu-NT- and Neu-NYPD-expressing mammary tumor explants (Northey and Siegel, unpublished). Future studies will address potential mechanisms downstream of ShcA that are responsible for Neu/ErbB-2 and TGF-β synergy in breast cancer motility and invasion.
Supplementary Material
[Supplemental material]
Acknowledgments
We thank members of the Siegel laboratory for helpful discussions and J. Ursini-Siegel and N. Lamarche-Vane for providing reagents for this study. We are grateful to William Hardy and Tony Pawson for the ShcA-3F cDNA. We thank J. Ursini-Siegel for her critical comments on the manuscript. We acknowledge the Centre for Bone and Periodontal Research (McGill University) for routine histological services.
J.J.N. is supported by a studentship from the Research Institute of the McGill University Health Centre, M.G.A. is a recipient of a postdoctoral fellowship from the Canadian Institutes of Health Research, W.J.M. is a Canada Research Chair in Molecular Oncology, and P.M.S. is a research scientist of the National Cancer Institute of Canada (supported by the Canadian Cancer Society). This work was supported solely by a grant from the Cancer Research Society.
Footnotes
▿
Published ahead of print on 10 March 2008.
REFERENCES
- 1.Andrulis, I. L., S. B. Bull, M. E. Blackstein, D. Sutherland, C. Mak, S. Sidlofsky, K. P. Pritzker, R. W. Hartwick, W. Hanna, L. Lickley, R. Wilkinson, A. Qizilbash, U. Ambus, M. Lipa, H. Weizel, A. Katz, M. Baida, S. Mariz, G. Stoik, P. Dacamara, D. Strongitharm, W. Geddie, D. McCready, et al. 1998. neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer. J. Clin. Oncol. 161340-1349. [DOI] [PubMed] [Google Scholar]
- 2.Bakin, A. V., C. Rinehart, A. K. Tomlinson, and C. L. Arteaga. 2002. p38 mitogen-activated protein kinase is required for TGFβ-mediated fibroblastic transdifferentiation and cell migration. J. Cell Sci. 1153193-3206. [DOI] [PubMed] [Google Scholar]
- 3.Bakin, A. V., A. K. Tomlinson, N. A. Bhowmick, H. L. Moses, and C. L. Arteaga. 2000. Phosphatidylinositol 3-kinase function is required for transforming growth factor β-mediated epithelial to mesenchymal transition and cell migration. J. Biol. Chem. 27536803-36810. [DOI] [PubMed] [Google Scholar]
- 4.Balaban, N. Q., U. S. Schwarz, D. Riveline, P. Goichberg, G. Tzur, I. Sabanay, D. Mahalu, S. Safran, A. Bershadsky, L. Addadi, and B. Geiger. 2001. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3466-472. [DOI] [PubMed] [Google Scholar]
- 5.Bargmann, C. I., M. C. Hung, and R. A. Weinberg. 1986. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell 45649-657. [DOI] [PubMed] [Google Scholar]
- 6.Bargmann, C. I., and R. A. Weinberg. 1988. Oncogenic activation of the neu-encoded receptor protein by point mutation and deletion. EMBO J. 72043-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beningo, K. A., M. Dembo, I. Kaverina, J. V. Small, and Y. L. Wang. 2001. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J. Cell Biol. 153881-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhowmick, N. A., M. Ghiassi, A. Bakin, M. Aakre, C. A. Lundquist, M. E. Engel, C. L. Arteaga, and H. L. Moses. 2001. Transforming growth factor-β1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol. Biol. Cell 1227-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bierie, B., and H. L. Moses. 2006. Tumour microenvironment: TGFβ: the molecular Jekyll and Hyde of cancer. Nat. Rev. Cancer 6506-520. [DOI] [PubMed] [Google Scholar]
- 10.Bouchard, L., L. Lamarre, P. J. Tremblay, and P. Jolicoeur. 1989. Stochastic appearance of mammary tumors in transgenic mice carrying the MMTV/c-neu oncogene. Cell 57931-936. [DOI] [PubMed] [Google Scholar]
- 11.Carragher, N. O., and M. C. Frame. 2004. Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol. 14241-249. [DOI] [PubMed] [Google Scholar]
- 12.Coll, J. L., A. Ben-Ze'ev, R. M. Ezzell, J. L. Rodriguez Fernandez, H. Baribault, R. G. Oshima, and E. D. Adamson. 1995. Targeted disruption of vinculin genes in F9 and embryonic stem cells changes cell morphology, adhesion, and locomotion. Proc. Natl. Acad. Sci. USA 929161-9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins, L. R., W. A. Ricketts, L. Yeh, and D. Cheresh. 1999. Bifurcation of cell migratory and proliferative signaling by the adaptor protein Shc. J. Cell Biol. 1471561-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dankort, D., N. Jeyabalan, N. Jones, D. J. Dumont, and W. J. Muller. 2001. Multiple ErbB-2/Neu phosphorylation sites mediate transformation through distinct effector proteins. J. Biol. Chem. 27638921-38928. [DOI] [PubMed] [Google Scholar]
- 15.Dankort, D., B. Maslikowski, N. Warner, N. Kanno, H. Kim, Z. Wang, M. F. Moran, R. G. Oshima, R. D. Cardiff, and W. J. Muller. 2001. Grb2 and Shc adapter proteins play distinct roles in Neu (ErbB-2)-induced mammary tumorigenesis: implications for human breast cancer. Mol. Cell. Biol. 211540-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dankort, D. L., Z. Wang, V. Blackmore, M. F. Moran, and W. J. Muller. 1997. Distinct tyrosine autophosphorylation sites negatively and positively modulate _neu_-mediated transformation. Mol. Cell. Biol. 175410-5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Fiore, P. P., O. Segatto, F. Lonardo, F. Fazioli, J. H. Pierce, and S. A. Aaronson. 1990. The carboxy-terminal domains of erbB-2 and epidermal growth factor receptor exert different regulatory effects on intrinsic receptor tyrosine kinase function and transforming activity. Mol. Cell. Biol. 102749-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman, D. B., S. E. Wang, C. W. Whitwell, R. M. Caprioli, and C. L. Arteaga. 2007. Multivariable difference gel electrophoresis and mass spectrometry: a case study on transforming growth factor-β and ERBB2 signaling. Mol. Cell. Proteomics 6150-169. [DOI] [PubMed] [Google Scholar]
- 19.Gallant, N. D., K. E. Michael, and A. J. Garcia. 2005. Cell adhesion strengthening: contributions of adhesive area, integrin binding, and focal adhesion assembly. Mol. Biol. Cell 164329-4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gotoh, N., M. Toyoda, and M. Shibuya. 1997. Tyrosine phosphorylation sites at amino acids 239 and 240 of Shc are involved in epidermal growth factor-induced mitogenic signaling that is distinct from Ras/mitogen-activated protein kinase activation. Mol. Cell. Biol. 171824-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guy, C. T., R. D. Cardiff, and W. J. Muller. 1992. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol. Cell. Biol. 12954-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guy, C. T., M. A. Webster, M. Schaller, T. J. Parsons, R. D. Cardiff, and W. J. Muller. 1992. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc. Natl. Acad. Sci. USA 8910578-10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hazan, R., B. Margolis, M. Dombalagian, A. Ullrich, A. Zilberstein, and J. Schlessinger. 1990. Identification of autophosphorylation sites of HER2/neu. Cell Growth Differ. 13-7. [PubMed] [Google Scholar]
- 24.Holbro, T., R. R. Beerli, F. Maurer, M. Koziczak, C. F. Barbas III, and N. E. Hynes. 2003. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc. Natl. Acad. Sci. USA 1008933-8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber, M. A., N. Kraut, and H. Beug. 2005. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 17548-558. [DOI] [PubMed] [Google Scholar]
- 26.Hudis, C. A. 2007. Trastuzumab—mechanism of action and use in clinical practice. N. Engl. J. Med. 35739-51. [DOI] [PubMed] [Google Scholar]
- 27.Jones, R. B., A. Gordus, J. A. Krall, and G. MacBeath. 2006. A quantitative protein interaction network for the ErbB receptors using protein microarrays. Nature 439168-174. [DOI] [PubMed] [Google Scholar]
- 28.Khoury, H., D. L. Dankort, S. Sadekova, M. A. Naujokas, W. J. Muller, and M. Park. 2001. Distinct tyrosine autophosphorylation sites mediate induction of epithelial mesenchymal like transition by an activated ErbB-2/Neu receptor. Oncogene 20788-799. [DOI] [PubMed] [Google Scholar]
- 29.Lehnert, D., B. Wehrle-Haller, C. David, U. Weiland, C. Ballestrem, B. A. Imhof, and M. Bastmeyer. 2004. Cell behaviour on micropatterned substrata: limits of extracellular matrix geometry for spreading and adhesion. J. Cell Sci. 11741-52. [DOI] [PubMed] [Google Scholar]
- 30.Marmor, M. D., K. B. Skaria, and Y. Yarden. 2004. Signal transduction and oncogenesis by ErbB/HER receptors. Int. J. Radiat. Oncol. Biol. Phys. 58903-913. [DOI] [PubMed] [Google Scholar]
- 31.Marone, R., D. Hess, D. Dankort, W. J. Muller, N. E. Hynes, and A. Badache. 2004. Memo mediates ErbB2-driven cell motility. Nat. Cell Biol. 6515-522. [DOI] [PubMed] [Google Scholar]
- 32.Mehra, A., and J. L. Wrana. 2002. TGF-β and the Smad signal transduction pathway. Biochem. Cell Biol. 80605-622. [DOI] [PubMed] [Google Scholar]
- 33.Miettinen, P. J., R. Ebner, A. R. Lopez, and R. Derynck. 1994. TGF-β-induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J. Cell Biol. 1272021-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moustakas, A., and C. H. Heldin. 2005. Non-Smad TGF-β signals. J. Cell Sci. 1183573-3584. [DOI] [PubMed] [Google Scholar]
- 35.Muller, W. J., E. Sinn, P. K. Pattengale, R. Wallace, and P. Leder. 1988. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell 54105-115. [DOI] [PubMed] [Google Scholar]
- 36.Muraoka, R. S., Y. Koh, L. R. Roebuck, M. E. Sanders, D. Brantley-Sieders, A. E. Gorska, H. L. Moses, and C. L. Arteaga. 2003. Increased malignancy of Neu-induced mammary tumors overexpressing active transforming growth factor β1. Mol. Cell. Biol. 238691-8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muraoka-Cook, R. S., I. Shin, J. Y. Yi, E. Easterly, M. H. Barcellos-Hoff, J. M. Yingling, R. Zent, and C. L. Arteaga. 2006. Activated type I TGFβ receptor kinase enhances the survival of mammary epithelial cells and accelerates tumor progression. Oncogene 253408-3423. [DOI] [PubMed] [Google Scholar]
- 38.Owens, R. B., H. S. Smith, and A. J. Hackett. 1974. Epithelial cell cultures from normal glandular tissue of mice. J. Natl. Cancer Inst. 53261-269. [DOI] [PubMed] [Google Scholar]
- 39.Perou, C. M., T. Sorlie, M. B. Eisen, M. van de Rijn, S. S. Jeffrey, C. A. Rees, J. R. Pollack, D. T. Ross, H. Johnsen, L. A. Akslen, O. Fluge, A. Pergamenschikov, C. Williams, S. X. Zhu, P. E. Lonning, A. L. Borresen-Dale, P. O. Brown, and D. Botstein. 2000. Molecular portraits of human breast tumours. Nature 406747-752. [DOI] [PubMed] [Google Scholar]
- 40.Rauh, M. J., V. Blackmore, E. R. Andrechek, C. G. Tortorice, R. Daly, V. K. Lai, T. Pawson, R. D. Cardiff, P. M. Siegel, and W. J. Muller. 1999. Accelerated mammary tumor development in mutant polyomavirus middle T transgenic mice expressing elevated levels of either the Shc or Grb2 adapter protein. Mol. Cell. Biol. 198169-8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodrigues, S. P., K. E. Fathers, G. Chan, D. Zuo, F. Halwani, S. Meterissian, and M. Park. 2005. CrkI and CrkII function as key signaling integrators for migration and invasion of cancer cells. Mol. Cancer Res. 3183-194. [DOI] [PubMed] [Google Scholar]
- 42.Rodríguez Fernández, J. L., B. Geiger, D. Salomon, and A. Ben-Ze'ev. 1992. Overexpression of vinculin suppresses cell motility in BALB/c 3T3 cells. Cell Motil. Cytoskeleton 22127-134. [DOI] [PubMed] [Google Scholar]
- 43.Romond, E. H., E. A. Perez, J. Bryant, V. J. Suman, C. E. Geyer, Jr., N. E. Davidson, E. Tan-Chiu, S. Martino, S. Paik, P. A. Kaufman, S. M. Swain, T. M. Pisansky, L. Fehrenbacher, L. A. Kutteh, V. G. Vogel, D. W. Visscher, G. Yothers, R. B. Jenkins, A. M. Brown, S. R. Dakhil, E. P. Mamounas, W. L. Lingle, P. M. Klein, J. N. Ingle, and N. Wolmark. 2005. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 3531673-1684. [DOI] [PubMed] [Google Scholar]
- 44.Salcini, A. E., J. McGlade, G. Pelicci, I. Nicoletti, T. Pawson, and P. G. Pelicci. 1994. Formation of Shc-Grb2 complexes is necessary to induce neoplastic transformation by overexpression of Shc proteins. Oncogene 92827-2836. [PubMed] [Google Scholar]
- 45.Saunders, R. M., M. R. Holt, L. Jennings, D. H. Sutton, I. L. Barsukov, A. Bobkov, R. C. Liddington, E. A. Adamson, G. A. Dunn, and D. R. Critchley. 2006. Role of vinculin in regulating focal adhesion turnover. Eur. J. Cell Biol. 85487-500. [DOI] [PubMed] [Google Scholar]
- 46.Schade, B., S. H. Lam, D. Cernea, V. Sanguin-Gendreau, R. D. Cardiff, B. L. Jung, M. Hallett, and W. J. Muller. 2007. Distinct ErbB-2 coupled signaling pathways promote mammary tumors with unique pathologic and transcriptional profiles. Cancer Res. 677579-7588. [DOI] [PubMed] [Google Scholar]
- 47.Schulze, W. X., L. Deng, and M. Mann. 2005. Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol. Syst. Biol. 12005.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seton-Rogers, S. E., Y. Lu, L. M. Hines, M. Koundinya, J. LaBaer, S. K. Muthuswamy, and J. S. Brugge. 2004. Cooperation of the ErbB2 receptor and transforming growth factor β in induction of migration and invasion in mammary epithelial cells. Proc. Natl. Acad. Sci. USA 1011257-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi, Y., and J. Massague. 2003. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113685-700. [DOI] [PubMed] [Google Scholar]
- 50.Siegel, P. M., D. L. Dankort, W. R. Hardy, and W. J. Muller. 1994. Novel activating mutations in the neu proto-oncogene involved in induction of mammary tumors. Mol. Cell. Biol. 147068-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siegel, P. M., and J. Massague. 2003. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nat. Rev. Cancer 3807-821. [DOI] [PubMed] [Google Scholar]
- 52.Siegel, P. M., E. D. Ryan, R. D. Cardiff, and W. J. Muller. 1999. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. EMBO J. 182149-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siegel, P. M., W. Shu, R. D. Cardiff, W. J. Muller, and J. Massague. 2003. Transforming growth factor β signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc. Natl. Acad. Sci. USA 1008430-8435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slamon, D. J., G. M. Clark, S. G. Wong, W. J. Levin, A. Ullrich, and W. L. McGuire. 1987. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235177-182. [DOI] [PubMed] [Google Scholar]
- 55.Slamon, D. J., W. Godolphin, L. A. Jones, J. A. Holt, S. G. Wong, D. E. Keith, W. J. Levin, S. G. Stuart, J. Udove, A. Ullrich, et al. 1989. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244707-712. [DOI] [PubMed] [Google Scholar]
- 56.Slamon, D. J., B. Leyland-Jones, S. Shak, H. Fuchs, V. Paton, A. Bajamonde, T. Fleming, W. Eiermann, J. Wolter, M. Pegram, J. Baselga, and L. Norton. 2001. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344783-792. [DOI] [PubMed] [Google Scholar]
- 57.Smith, M. J., W. R. Hardy, J. M. Murphy, N. Jones, and T. Pawson. 2006. Screening for PTB domain binding partners and ligand specificity using proteome-derived NPXY peptide arrays. Mol. Cell. Biol. 268461-8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sørlie, T., C. M. Perou, R. Tibshirani, T. Aas, S. Geisler, H. Johnsen, T. Hastie, M. B. Eisen, M. van de Rijn, S. S. Jeffrey, T. Thorsen, H. Quist, J. C. Matese, P. O. Brown, D. Botstein, P. Eystein Lonning, and A. L. Borresen-Dale. 2001. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 9810869-10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sorlie, T., R. Tibshirani, J. Parker, T. Hastie, J. S. Marron, A. Nobel, S. Deng, H. Johnsen, R. Pesich, S. Geisler, J. Demeter, C. M. Perou, P. E. Lonning, P. O. Brown, A. L. Borresen-Dale, and D. Botstein. 2003. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA 1008418-8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sotiriou, C., S. Y. Neo, L. M. McShane, E. L. Korn, P. M. Long, A. Jazaeri, P. Martiat, S. B. Fox, A. L. Harris, and E. T. Liu. 2003. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc. Natl. Acad. Sci. USA 10010393-10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sotiriou, C., and M. J. Piccart. 2007. Taking gene-expression profiling to the clinic: when will molecular signatures become relevant to patient care? Nat. Rev. Cancer 7545-553. [DOI] [PubMed] [Google Scholar]
- 62.Ueda, Y., S. Wang, N. Dumont, J. Y. Yi, Y. Koh, and C. L. Arteaga. 2004. Overexpression of HER2 (erbB2) in human breast epithelial cells unmasks transforming growth factor β-induced cell motility. J. Biol. Chem. 27924505-24513. [DOI] [PubMed] [Google Scholar]
- 63.Ursini-Siegel, J., B. Schade, R. D. Cardiff, and W. J. Muller. 2007. Insights from transgenic mouse models of ERBB2-induced breast cancer. Nat. Rev. Cancer 7389-397. [DOI] [PubMed] [Google Scholar]
- 64.van der Geer, P., S. Wiley, G. D. Gish, and T. Pawson. 1996. The Shc adaptor protein is highly phosphorylated at conserved, twin tyrosine residues (Y239/240) that mediate protein-protein interactions. Curr. Biol. 61435-1444. [DOI] [PubMed] [Google Scholar]
- 65.Wang, S. E., I. Shin, F. Y. Wu, D. B. Friedman, and C. L. Arteaga. 2006. HER2/Neu (ErbB2) signaling to Rac1-Pak1 is temporally and spatially modulated by transforming growth factor β. Cancer Res. 669591-9600. [DOI] [PubMed] [Google Scholar]
- 66.Wang, S. E., F. Y. Wu, I. Shin, S. Qu, and C. L. Arteaga. 2005. Transforming growth factor β (TGF-β)-Smad target gene protein tyrosine phosphatase receptor type kappa is required for TGF-β function. Mol. Cell. Biol. 254703-4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Webster, M. A., J. N. Hutchinson, M. J. Rauh, S. K. Muthuswamy, M. Anton, C. G. Tortorice, R. D. Cardiff, F. L. Graham, J. A. Hassell, and W. J. Muller. 1998. Requirement for both Shc and phosphatidylinositol 3′ kinase signaling pathways in polyomavirus middle T-mediated mammary tumorigenesis. Mol. Cell. Biol. 182344-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xie, L., B. K. Law, M. E. Aakre, M. Edgerton, Y. Shyr, N. A. Bhowmick, and H. L. Moses. 2003. Transforming growth factor β-regulated gene expression in a mouse mammary gland epithelial cell line. Breast Cancer Res. 5R187-R198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu, W., H. Baribault, and E. D. Adamson. 1998. Vinculin knockout results in heart and brain defects during embryonic development. Development 125327-337. [DOI] [PubMed] [Google Scholar]
- 70.Xu, W., J. L. Coll, and E. D. Adamson. 1998. Rescue of the mutant phenotype by reexpression of full-length vinculin in null F9 cells; effects on cell locomotion by domain deleted vinculin. J. Cell Sci. 1111535-1544. [DOI] [PubMed] [Google Scholar]
- 71.Yang, Y. A., O. Dukhanina, B. Tang, M. Mamura, J. J. Letterio, J. MacGregor, S. C. Patel, S. Khozin, Z. Y. Liu, J. Green, M. R. Anver, G. Merlino, and L. M. Wakefield. 2002. Lifetime exposure to a soluble TGF-β antagonist protects mice against metastasis without adverse side effects. J. Clin. Investig. 1091607-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zaidel-Bar, R., M. Cohen, L. Addadi, and B. Geiger. 2004. Hierarchical assembly of cell-matrix adhesion complexes. Biochem. Soc. Trans. 32416-420. [DOI] [PubMed] [Google Scholar]
- 73.Ziegler, W. H., R. C. Liddington, and D. R. Critchley. 2006. The structure and regulation of vinculin. Trends Cell Biol. 16453-460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental material]