Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis (original) (raw)
Abstract
Posttranscriptional gene regulation by microRNAs (miRNAs) is important for many aspects of development, homeostasis, and disease. Here, we show that reduction of endothelial miRNAs by cell-specific inactivation of Dicer, the terminal endonuclease responsible for the generation of miRNAs, reduces postnatal angiogenic response to a variety of stimuli, including exogenous VEGF, tumors, limb ischemia, and wound healing. Furthermore, VEGF regulated the expression of several miRNAs, including the up-regulation of components of the c-Myc oncogenic cluster miR-17-92. Transfection of endothelial cells with components of the miR-17-92 cluster, induced by VEGF treatment, rescued the induced expression of thrombospondin-1 and the defect in endothelial cell proliferation and morphogenesis initiated by the loss of Dicer. Thus, endothelial miRNAs regulate postnatal angiogenesis and VEGF induces the expression of miRNAs implicated in the regulation of an integrated angiogenic response.
Keywords: endothelium, VEGF
MicroRNAs (miRNAs) are short (≈22 nt) noncoding RNAs derived from long primary transcripts through sequential processing by the enzymes Drosha and Dicer. Dicer-generated miRNAs are incorporated into the RNA-induced silencing complex that mediates miRNA-dependent translational suppression or in some instances cleavage of respective mRNA targets or translational activation (1, 2). The significance of miRNAs in mammalian biology has been dissected by Dicer gene disruption in mice. Mutant and disrupted Dicer alleles caused embryonic lethality associated with a loss of pluripotent stem cells (3) and defective blood vessel formation (4). Tissue-specific inactivation of Dicer has led to the conclusion that Dicer is essential for several processes, for example, limb, lung, and skin morphogenesis, the maintenance of hair follicles, T cell development/ differentiation, and neuronal survival (5–11).
The growth of blood vessels is essential for organ growth and tissue repair. During adulthood, most blood vessels remain quiescent to fulfill their main function of conducting nutritive blood flow to organs; however, during pathological events such as tissue ischemia, inflammation, and tumor progression, endothelial cells (ECs) become activated and angiogenesis ensues to provide conduits for blood flow (12). An imbalance in the growth of blood vessels contributes to the pathogenesis of numerous disorders (13), and the growth of vessels is a complex process, requiring a finely tuned balance between numerous stimulatory and inhibitory signals (14). VEGF has been identified as a central mediator of angiogenesis (15). We (16) and others (17) have recently shown that reduction of miRNA levels via Dicer silencing strongly impacts EC functions in vitro, suggesting a critical role for miRNAs in angiogenesis. The role of Dicer-regulated miRNAs in ovarian angiogenesis is suggested by data obtained in mice expressing a global hypomorphic Dicer1 allele, where female mice are infertile because of corpus luteum insufficiency and defective ovarian angiogenesis (18). However, the importance of endothelial-specific miRNAs in postnatal angiogenesis has not been specifically addressed.
Results and Discussion
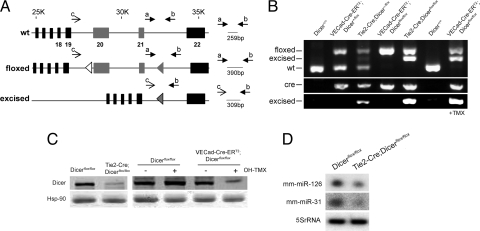
To address this question, we generated two mouse models that were homozygous for the conditional floxed Dicer allele (9) and expressed Cre-recombinase under the regulation of Tie2 promoter/enhancer (19) or Tamoxifen (TMX)-inducible expressed Cre-recombinase (Cre-ERT2) under the regulation of vascular endothelial cadherin promoter (VEcad) (20) to achieve specific inactivation of Dicer in EC [supporting information (SI) Fig. S1]. Both Tie2-Cre;Dicerflox/flox and VECad-Cre-ERT2;Dicer_flox/flox_ were identified by genotyping of tail biopsy DNA (Fig. 1 A and B). Mouse tail is highly vascularized, allowing the detection of the recombination of the floxed allele in both Tie2-Cre;Dicer_flox_/+ and Tie2-Cre;Dicer_flox/flox_ mice, heterozygous and homozygous for the floxed allele, respectively. As expected for the inducible model, the recombination of the floxed allele in VECad-Cre-ERT2;Dicer_flox/flox_ was detected only after induction of Cre by treatment with TMX. In all cases, the detection of the excised allele correlated with the presence of the transgene (Fig. 1B). The specific efficiency of the inactivation of Dicer was achieved by immunoblotting cell lysates of ECs isolated from the lung (see Fig. S2_A_, for purity). Dicer levels were reduced in Tie2-Cre;Dicer_flox/flox_ ECs (Fig. 1C) and after Cre induction in EC from VECad-Cre-ERT2;Dicer_flox/flox_ mice (Fig. 1C). In agreement with the reduced levels of Dicer expression, the levels of two abundant microRNAs in EC (i.e., miR-126 and miR-31) (16, 17) were also reduced in ECs isolated from 3-week-old Tie2-Cre;Dicer_flox/flox_ (Fig. 1D). Consistent with the presence of residual Dicer protein levels found in Tie2-Cre;Dicer_flox/flox_ EC, quantitative RT-PCR (qRT-PCR) analysis of Dicer expression showed detection of Dicer mRNA (Fig. S2_B_) that correlated with the steady detection of the floxed allele in these cells (Fig. S2_C_), reflecting an incomplete excision of the allele. Thus, these mice were hypomorphic for Dicer in ECs and Tie2-Cre;Dicer_flox/flox_ newborn litters were overtly normal and indistinguishable from their littermate controls. Thus, Tie2-Cre;Dicer_flox/flox_ and EC TMX-inducible Cre models allowed us to investigate the relevance of endothelial miRNAs in postnatal angiogenic paradigms.
Fig. 1.
Conditional inactivation of Dicer in ECs. (A) Gene targeting strategy for endothelial-specific deletion of Dicer. PCR primers sets and fragment size expected are shown. (B) PCR genotyping analysis. (Top) Detection of floxed, excised, and WT fragments with primers a, b, and c. (Middle) Detection of Cre. (Bottom) Detection of excised fragment with primers b and c. (C) (Left) Dicer protein levels from ECs, isolated from 3-week-old Dicer_flox/flox_ and Tie2-Cre;Dicer_flox/flox_ mice. (Right) Dicer protein levels from EC isolated from 3-week-old Dicer_flox/flox_ and VECad-Cre-ERT2;Dicer_flox/flox_ mice. ECs were treated with OH-TMX or vehicle. (D) Reduction in miR-126 and -31 in ECs isolated from Tie2-Cre;Dicer_flox/flox_ mice.
VEGF-A is a well characterized angiogenic cytokine that is a potent mitogen, chemotactic factor, and survival factor for ECs (15). To examine whether Dicer-dependent miRNAs were necessary for VEGF-induced angiogenesis, Dicer_flox/flox_ and Tie2-Cre;Dicer_flox/flox_ mice were injected intradermally into the ear with an adenovirus expressing murine VEGF 164 (Ad VEGF) and angiogenesis was assessed after 5 days. As expected, VEGF increased the number of angiogenic structures [quantified by platelet/EC adhesion molecule-1 (PECAM-1)-positive vascular structures] in Dicer_flox/flox_ mice, whereas injection of a control virus expressing eGFP (Ad EGFP) did not. However, the angiogenic response to VEGF was reduced in Tie2-Cre; Dicer_flox/flox_ mice (Fig. 2 A and B). This finding was corroborated in VECad-Cre-ERT2;Dicer_flox/flox_ mice (Fig. S3_A_), where postnatal reduction of miRNAs levels by TMX-inducible inactivation of Dicer also reduced the angiogenic response to injected Ad-VEGF. Similar results were obtained when isolectin B4 (Fig. S3_B_) or VE-cadherin (not shown) was used as a marker for ECs. Collectively, these results show that endothelial Dicer-dependent miRNAs are necessary for the direct angiogenic actions of VEGF.
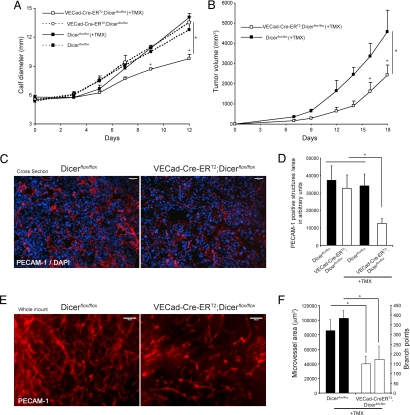
Fig. 2.
Endothelial Dicer inactivation reduces VEGF-induced angiogenesis. Ad-VEGF or Ad-eGFP was injected intradermally into the left and right ears, respectively, of 8-week-old mice. Frozen sections were stained for PECAM-1 (red) and counterstained with DAPI (blue). (A) Representative PECAM-1 staining showing reduced VEGF-induced angiogenesis in Tie2-Cre;Dicer_flox/flox_ mice compared with Dicer_flox/flox_ control mice. (B) Quantification of PECAM-1-positive structures (n = 6 per group). In B, for each animal, two to four images from five sections were quantified. Values are means ± SEM. *, P < 0.05, compared with Dicer_flox/flox_ by Student's t test. (Scale bars: 100 μm.)
Next, we examined the importance of endothelial miRNAs in tumor angiogenesis. Mice were treated with TMX for 5 consecutive days and Lewis lung carcinoma (LLC) cells were implanted in two different anatomical locations (intramuscularly in the calves or s.c. in the dorsal flank). As shown in Fig. 3A, LLC tumor growth was reduced in VECad-Cre-ERT2;Dicer_flox/flox_ mice (pretreated with TMX) compared with control mice (Dicer_flox/flox_, Dicer_flox/flox_ treated with TMX, or VECad-Cre-ERT2;Dicer_flox/flox_ not pretreated with TMX). When LLC cells were implanted s.c. into the dorsal flanks of TMX-pretreated Dicer_flox/flox_ (control) and VECad-Cre-ERT2;Dicer_flox/flox_ mice (Fig. 3B) similar results were obtained, and tumor growth was reduced only when implanted in mice where Dicer was previously inactivated in ECs. In agreement with the diminished tumor growth, a reduction of PECAM-1-positive structures (in cross-sections; Fig. 3C and quantified in Fig. 3D) and microvascular area and vascular branch points (from whole-mount images; Fig. 3E and quantified in Fig. 3F) in the tumor-associated vasculature was observed in VECad-Cre-ERT2;Dicer_flox/flox_ mice (TMX-induced Dicer inactivation in ECs), showing the importance of endothelial miRNAs for tumor-induced growth and angiogenesis.
Fig. 3.
Endothelial Dicer inactivation reduces tumor angiogenesis and progression. (A and B) LLC cells were implanted intramuscularly into the calves of 8-week-old Dicer_flox/flox_ or VECad-Cre-ERT2;Dicer_flox/flox_ mice (A) or implanted s.c. in the dorsal flank of Dicer_flox/flox_ and TMX-treated VECad-Cre-ERT2;DicerΔ/Δ mice (B), and tumor growth was assessed. n = 4 per group. P < 0.05 using a two-way ANOVA, followed by post hoc Bonferroni test. (C) Representative PECAM-1 staining (red) of cross-sections from tumors showing reduced tumor angiogenesis in VECad-Cre-ERT2;Dicer_flox/flox_ mice. Tissues were counterstained with DAPI (blue). (D) Quantification of PECAM-1-positive structures in the tumors of the indicated groups of mice. For each tumor, two to four images from three sections from two different tumor pieces were quantified. (E) Representative whole-mount PECAM-1 staining from tumors isolated from dorsal flank showing reduced tumor-induced angiogenesis in VECad-Cre-ERT2;Dicer_flox/flox_ mice. (F) Quantification of microvessel area and branch points, left and right bars for each group, respectively; data are from three to five images per sample. *, P < 0.05, compared with Dicer_flox/flox_ by Student's t test. All data are mean ± SEM. (Scale bars: C and E, 100 μm.)
To examine a broader role of Dicer-dependent endothelial miRNAs in additional models of angiogenesis, we investigated whether postnatal angiogenesis was altered in responses to limb ischemia and wound healing. In the first model, arteriectomy of the femoral artery induces arterialization of collateral vessels (arteriogenesis) of the upper limb and increases capillary to skeletal muscle fiber ratio (angiogenesis) in the lower limb (21, 22). As shown in Fig. S4_A_, baseline capillaries densities were very similar in Tie2-Cre;Dicer_flox/flox_ and control Dicer_flox/flox_ mice, assessed in the gastrocnemius muscle by quantifying capillary density per muscle fiber. After ischemia, control mice showed increased capillary density, whereas Tie2-Cre;Dicer_flox/flox_ mice exhibited reduced angiogenesis (Fig. S4_A_ and quantified in Fig. S4_B_). The reduced angiogenesis was associated with a reduction in blood flow recovery (Fig. S4_C_) and severity of damage scores (Fig. S4 D and E) that correlated with more fibrosis (Fig. S4_F_). In the second model, full thickness wounds were created in Tie2-Cre;Dicer_flox/flox_ and control Dicer_flox/flox_ mice, and the time course of wound healing was examined. The wound-healing process was significantly delayed in Tie2-Cre;Dicer_flox/flox_ as indicated by the wound area (Fig. S5 A and B) and a worse clinical score (Fig. S5_C_). Histologically, Tie2-Cre;Dicer_flox/flox_ showed larger areas of granulation tissue devoid of hair follicles, less granulation tissue deposition, and collagen accumulation. Similar effects were seen in VECad-Cre-ERT2;Dicer_flox/flox_ mice (Fig. S5 D–F). These results suggest that the impaired wound healing response was caused by a delayed angiogenic response.
Collectively, the above data show the importance of Dicer-dependent miRNAs for postnatal angiogenesis in four distinct models. These results are in agreement with previous in vitro data showing that Dicer-dependent miRNAs provide a posttranscriptional mechanism that complements transcriptional regulation to alter the balance of proangiogenic and antiangiogenic factors in ECs (16, 17, 23), culminating in an antiangiogenic phenotype.
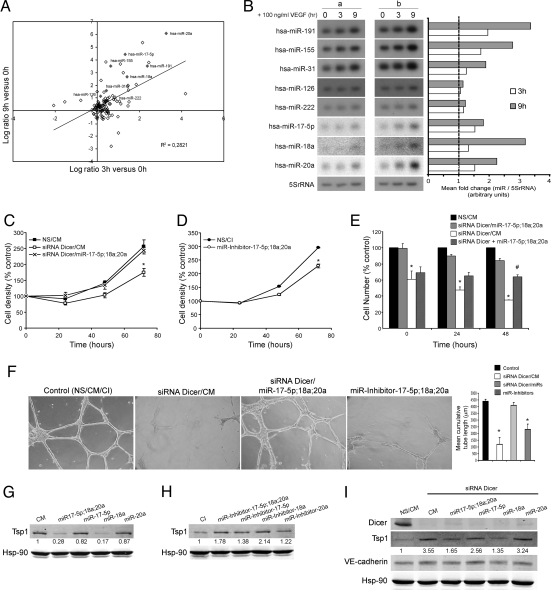
ECs are both active participants and regulators of the angiogenic process, that, in turn, require coordination of numerous, complex signaling pathways. There is growing evidence that miRNA activity and/or expression is regulated by extracellular signals (i.e., cytokines, hormones, stress) (24–26). Given that the direct angiogenic response to Ad-VEGF is reduced after the loss of Dicer in ECs, we wanted to explore the possibility that VEGF may modulate the expression levels of miRNAs that, in turn, may regulate aspects of the integrated angiogenic response. To test this hypothesis, human vascular ECs were treated with VEGF (100 ng/ml) for 0, 3, and 9 h (to induce signaling, but not cell division), and the expression of miRNAs was quantified by using miRNA microarrays. VEGF regulated the levels of several miRNAs (Fig. 4A and Table S1) and a positive correlation occurred between 3 and 9 h post-VEGF treatment, indicating that miRNAs induced after 3 h were further elevated after 9 h of VEGF treatment. VEGF induced the time-dependent expression of hsa-miR-191, hsa-miR-155, hsa-miR-31, hsa-miR-17-5p, hsa-miR-18a, and hsa-miR-20a (confirmed by Northern blotting and qRT-PCR; Fig. 4B and Fig. S6) with little change in hsa-miR-126 and hsa-miR-222. Interestingly, we noticed that VEGF increased the expression of a set of miRNAs commonly overexpressed in human tumors (hsa-miR-155, hsa-miR-191, hsa-miR-21, hsa-miR-18a, hsa-miR-17-5p, and hsa-miR-20a) that have been implicated in the control of the tumor growth, survival, and angiogenesis (27–30). Transcription factors c-myc and E2F control the expression of the miR-17-92 cluster (30), including hsa-17-5p, hsa-miR-18a, and hsa-miR-20a) and target the expression levels of E2F1 and proteins containing thrombospondin type 1 repeats such as thrombospondin-1 (Tsp1), CTGF, and SPARC (30–32). Interestingly, these miRNAs show low basal levels of expression in human umbilical vein endothelial cells (HUVECs) but are highly expressed after VEGF stimulation, suggesting that these miRNAs may regulate the angiogenic actions of VEGF functions. Because deletion of Dicer in various cell lineages has been shown to impair cell proliferation and survival (5, 9, 33), and the knockdown of Dicer in EC also impairs proliferation (16, 17), we tested whether these VEGF-regulated miRNAs (hsa-miR-18a, hsa-miR-17-5p, and hsa-miR-20a) could restore EC proliferation when Dicer is absent. As seen in Fig. 4C, knockdown of Dicer in human ECs reduced proliferation (16), and transfection of cells with the VEGF-induced miRNAs (miR-18a, miR-17-5p, and miR-20a) reversed this phenotype. Conversely, treatment of ECs with miRNA inhibitors for these miRNAs attenuated cell growth (Fig. 4D). More importantly, when the proliferation was assessed in the VEGF-treated state (Fig. 4E), cell number was not increased after VEGF stimulation in cells where Dicer was previously silenced (white bars), whereas proliferation approached control levels in cells where Dicer was silenced in the presence of the components of the miR-17-92 cluster (black and gray bars). Interestingly, when ECs (pretreated with Dicer siRNA) were transfected with these miRNAs at the time of VEGF stimulation (Fig. 4E, dashed bars), a partial rescue of the proliferation was observed. Furthermore, knockdown of Dicer in human ECs reduced cord formation in vitro (16), and transfection of cells with the above-mentioned VEGF-induced miRNAs, also reversed this phenotype (Fig. 4F). Conversely, treatment of HUVECs with the corresponding miRNA inhibitors reduced cord formation. Collectively, these data show that in the absence of miRNA processing (Dicer inactivation), VEGF-induced proliferation and morphogenesis are mediated, in part, by miR-17-92 activation.
Fig. 4.
VEGF regulation of miRNA expression in ECs. (A) Data are the Log2 ratio of miRNA expression 3 and 9 h after VEGF treatment. hsa-miR-191, hsa-miR-155, hsa-miR-31, hsa-miR-126, hsa-miR-222, hsa-miR-7-5p, hsa-miR-18a, and hsa-miR-20a are highlighted in grey. (B) Confirmation and quantification of miRNA expression by Northern blotting. 5S rRNA served as a loading control. a and b denote samples from two different isolations of HUVECs. (C) Dicer knockdown decreases EC growth, an effect rescued by hsa-miR-17-5p, hsa-miR-18a, and hsa-miR-20a. EC (EA.hy.926 cells) were transfected with control nonsilencing and control mimic sequences (NS/CM) or Dicer siRNA in the presence of CM (siRNA Dicer/CM) or Dicer siRNA in the presence of a mix of specific mimics. (D) Targeting components of the miR-17-92 cluster reduces EC growth. ECs were transfected as above with control inhibitor sequence (CI) or a mixture of specific miRNA inhibitors and cell density was quantified. Data are expressed as percentage of control at day 0. (E). ECs were transfected as described in C for 48 h. Cells were incubated in medium without serum for 12 h (time = 0 h) and subsequently incubated in the presence of VEGF for 24 or 48 h. siRNA Dicer + miR-17-5p;18a;20a indicates cells that were initially transfected with Dicer siRNA for 48 h then transfected with miRNA mimics at the time of VEGF treatment. At the indicated time points, cell number was quantified. Data are expressed as percentage of control (NS/CM). (F) HUVECs were transfected as indicated in C. After 60 h, cells were seeded on a growth factor-reduced Matrigel in the presence of FBS (0.5%) and VEGF (100 ng/ml). Cumulative sprout length of capillary-like structures was quantified after 16 h. Data are from n = 3 experiments. (Magnification: ×10.) (G and H) Western blot analysis of Tsp1 levels in ECs transfected with CM, a mix of specific mimics, or individual specific mimics as indicated in G or transfected with CI, a mix of specific miRNA inhibitors, or individual specific miRNA inhibitors as indicated in H. Hsp-90 was used as loading control. (I) Western blot analysis of Dicer and Tsp1 expression levels in ECs transfected as indicated in C and in the presence of Dicer siRNA. VE-cadherin served as an EC marker and Hsp-90 served as loading control. Data are reported as the mean ± SEM. * and #, P < 0.05, by Student's t test.
The above results and recent data suggest that miR-17-92 is important for EC morphogenesis (18) and tumor angiogenesis (30), specifically by targeting TIMP1 (tissue inhibitor for metalloproteinase 1) and the antiangiogenic protein Tsp1, respectively. In this regard, transfection of ECs with miRNA mimics (miR-17-5p, miR-18a, miR-20a) reduced Tsp1 levels (Fig. 4G), an effect that can be recapitulated by transfecting miR-18a alone, demonstrating specificity of miR-18a toward Tsp1 as a target (30). The converse effects were obtained when ECs were transfected with the miRNA inhibitors (Fig. 4H). Interestingly, Dicer silencing increased the levels of Tsp1 (Fig. 4I), which may explain, in part, the antiangiogenic phenotypes observed in vivo. Transfection of miRNA mimics (specifically the miR-18a mimic alone or in combination) into ECs with reduced Dicer levels restored Tsp1 expression back to control levels. The effects of the miR-18a were not shared by miR-20a or miR-17-5p, and the effect on Tsp1 was specific because the levels of VE-cadherin (a specific EC marker) and Hsp90 were not affected. Furthermore, it has been shown very recently that miR-17-92 miRNA suppressed expression of the tumor suppressor gene PTEN and the proapoptotic protein Bim, contributing to the lymphoproliferative disease in miR-17-92 transgenic mice and contributing to lymphoma development in patients with amplifications of the miR-17-92 coding region (34). Consistent with the importance of the miR-17-92 cluster, deletion of this locus resulted in smaller embryos and immediate postnatal death of all animals (35). Collectively, our data support the idea that VEGF modulation of miRNAs, specifically components of the miR-17-92 cluster, may participate in the control of angiogenic phenotypes such as proliferation, survival, and organization of ECs. There is growing evidence that cancer therapy will comprise a combination of antiangiogenic agents (VEGF inhibitors) and cytotoxic chemotherapy. Therefore, the identification of miRNAs as regulators of both angiogenesis and tumor cell survival (26–29) is an interesting approach for the therapy of cancer. The present findings provide another mechanism for the antiangionic actions of VEGF inhibitors (i.e., the inhibitor may reduce the expression of miRNAs that promote angiogenesis).
The above data show that reduction of endothelial miRNAs by conditional inactivation of Dicer reduces postnatal angiogenic responses to a variety of stimuli, including exogenous VEGF, tumors, limb ischemia, and wound healing and that endothelial miRNAs are required for an appropriate angiogenic response in vivo. Previous work showing the collective silencing of miRNAs by conditional Dicer inactivation has provided important phenotypic information regarding the role of miRNAs in various integrated cellular processes (5–11). Moreover, our study documents that the potent angiogenic cytokine, VEGF, induces the expression of miRNAs important for different aspects of the angiogenic process. These data support the intriguing idea that growth factors or cytokines may regulate miRNA expression levels (24) as a common mechanism to modulate the physiological levels of gene expression. This idea is supported by recent data showing that TGF-β increases the expression of a subset of miRNAs that promote vascular smooth muscle cell differentiation (25). However, additional work is needed to dissect which exact combinations of VEGF-regulated miRNAs are linked to the regulation of the angiogenic response. Antagonism or mimicry of key miRNAs regulating the angiogenic response in vivo would be a novel approach for the treatment of diseases associated with aberrant pathological angiogenesis (cancer or macular degeneration) or defective angiogenesis (myocardial ischemia or peripheral vascular disease), respectively.
Materials and Methods
Generation of Tie2-Cre;Dicer_flox/flox_ and VECad-Cre;Dicer_flox/flox_ Mice.
EC-specific or inducible inactivation of Dicer was achieved by cross-breeding Tie2-Cre (19) or VECad-Cre-ERT2 (20) transgenic mice with mice harboring loxP sites flanking an RNaseIII domain (exons 20 and 21) of Dicer (Dicer_flox/flox_) (9). From the progeny, heterozygous Tie2-Cre;Dicer_flox_/+ or VECad-Cre-ERT2;Dicer_flox_/+ mice were bred with Tie2-Cre;Dicer_flox_/+ or VECad-Cre-ERT2;Dicer_flox_/+ to obtain Tie2-Cre;Dicer_flox/flox_ and VECad-Cre-ERT2;Dicer_flox/flox_ mice, respectively. VECad-Cre-ERT2;Dicer_flox/flox_ or Tie2-Cre;Dicer_flox/flox_ were mated with Dicer_flox/flox_ mice. For the endothelial-specific and -inducible deletion of Dicer (Fig. S1), VECad-Cre-ERT2;Dicer_flox/flox_ and their littermate controls Dicer_flox/flox_ were injected daily (i.p.) with 1 mg TMX as described (20). In all experiments, sex- and age-matched Dicer_flox/flox_ littermates were used. For PCR genotyping genomic DNA was extracted from the tail and the following three primers were used: a, AGTGTAGCCTTAGCCATTTGC; b, CTGGTGGCTTGAGGACAAGAC, and c, AGTAATGTGAGCAATAGTCCCAG for the detection of WT, floxed, and excised alleles. The presence of the transgene was detected by using Cre primers (CGATGCAACGAATGAGG and CGCATAACCAGTAAACAGC). Animal procedures were approved by the Yale Animal Care Committee.
Mouse Lung EC (MLEC) Isolation.
MLECs were isolated by immunoselection as described (21) from 3-week-old Dicer_flox/flox_, Tie2-Cre;Dicer_flox/flox_ and VECad-Cre-ERT2;Dicer_flox/flox_. To induce deletion of Dicer in VECad-Cre-ERT2;Dicer_flox/flox_ MLECs were treated with 100 nM hydroxy-TMX (OH-TMX) as described (20).
Western Blot Analysis.
Proteins from tissue and cell lysates were resolved by SDS/PAGE and immunoblotting as described (16, 36). Primary antibodies used include the following: Dicer p-Ab (Abcam), β-actin m-Ab (Abcam), VE-Cadherin-pAb and Tsp1-pAb (Santa Cruz). Secondary antibodies were fluorophore-conjugated antibodies (LI-COR Biotechnology). Bands were visualized by using the Odyssey Infrared Imaging System (LI-COR Biotechnology).
Northern Blot Analysis of miRNAs.
miRNA expression was assessed by Northern blot as described (16). Oligonucleotides complementary to miRNAs (as described in the Sanger microRNA registry) were used as probes to detect their respective miRNAs.
qRT-PCR for miRNA.
qRT-PCR was performed with a _mir_Vana qRT-PCR miRNA detection kit (Ambion) following the manufacturer's instructions and as described (16).
Ear Angiogenesis.
Adenoviruses encoding murine VEGF-A164 (Ad5CMV VEGF164) (Gene Transfer Vector Core, University of Iowa, Iowa City) (2 × 108 viral particles) were injected intradermally as described (21) into the left ears of 8-week-old Dicer_flox/flox_ and Tie2-Cre;Dicer_flox/flox_ or Dicer_flox/flox_ and VECad-Cre-ERT2; Dicer_flox/flox_ mice previously pretreated with TMX as indicated above to induce Dicer Cre-dependent excision. The right ears were injected with a control virus expressing eGFP (Ad5CMV eGFP) (Gene Transfer Vector Core, University of Iowa). After 5 days, animals were euthanized, and the ears were removed and finally embedded and frozen in OCT compound (Tissue-Tek).
Tumor Implantation and Growth in Vivo.
LLC cells (106) in 100 μl of HBSS (Sigma) were injected in two different anatomical locations intramuscularly into the calf or s.c. in the dorsal flank of 8-week-old males as described (37, 38) and detailed in SI Text.
Mouse Hindlimb Ischemic Model.
Mouse ischemic hindlimb model was performed as described (21, 22) and detailed in SI Text.
Wound Healing Model.
Excision wound healing experiments were performed essentially as described (39) and detailed in SI Text.
Immunohistochemistry and Whole-Mount Analysis.
Stainings were carried out on 6- or 5-μm frozen or paraffin-embedded sections, respectively and whole-mount histology was performed as described (37). For detailed information see SI Text.
Quantitative Real-Time PCR.
RNA extraction and cDNA synthesis was performed as described (16) and detailed in SI Text. Specific primers for VEGFA, VEGF receptor 2, Tie-2, platelet-derived growth factor PDGF-b, MCP-1, IL-1β, and 18S ribosomal RNA have been described (22).
Flow Cytometry Analyses.
Cell surface expression of PECAM-1 was assayed by flow cytometry as described (16, 40). Immunostained cells were then washed twice with cold PBS and analyzed on a FACSort Flow Cytometry (Becton Dickinson) by using CellQuest analysis software collecting 10,000 gated cells per sample.
HUVECs.
HUVECs were isolated from discarded umbilical veins by collagenase digestion, under protocols approved by the Yale Human Investigation Committee and cultured as described (16, 40).
RNAi.
siRNA was used to silence Dicer in ECs (HUVEC and EA.hy.926 cells) as described (16). All of the siRNA sequences (16) were purchased from Qiagen. In some experiments, cells were cotransfected with mi_RIDIAN_ miRNA mimics or mi_RIDIAN_ miRNA inhibitors (Dharmacon), 15 nM when used in combination and 30 nM when used separately. miRIDIAN microRNA mimics are dsRNA oligonucleotides that mimic endogenous, mature miRNAs, and miRIDIAN microRNA inhibitors are single-stranded, chemically enhanced oligonucleotides that compete with endogenous miRNA for mRNA committed to the RISC complex. In all of these experiments control samples were treated with an equal concentration of a control nontargeting mimics sequence (CM) or inhibitor negative control sequence (CI), for use as controls for nonsequence-specific effects in miRNA experiments. Control sequences are based on Caenorhabditis elegans miRNAs (1, cel-miR-67; 2, cel-miR-239b).
Cell Density and Number Assessment.
Cell density was estimated at the indicated times after treatments as reported (16). ECs were also collected, and cell number was assessed after treatments by using a hemocytometer.
Cord Formation Assay.
After 60 h of Dicer silencing, cells (70 × 103) were cultured in a 24-well plate coated with 200 μl of Growth Factor Reduced Matrigel (BD Biosciences) as described (16).
miRNA Array Analysis.
RNA was isolated with TRIzol reagent from HUVECs that were treated or not for 3 or 9 h with VEGF (100 ng/ml). Cells were previously quiesced by overnight incubation media without serum. Duplicate samples of two different isolations of HUVECs and each isolation consisted of ECs from three different cords pooled together as described (16). RNA pooled reference and samples were tagged for labeling with Cy3 and Cy5 dyes, respectively, by using the miRCURY Labeling Kit (Exiqon) for use with Exiqon miRCURY LNA Arrays. Data were analyzed with GeneSpring GX 7.3. Background-subtracted median signals for both Cy5 and Cy3 channels were used for data analysis. Data were normalized by using spike-in controls. Student's t test was applied to identify statistically significant microRNAs between two comparison groups. The ratio for treated (3 or 9 h) vs. untreated (0 h) was calculated based on the average ratio of Cy5/Cy3 of replicates from two of two different isolations of HUVEC. For comparisons, data were also expressed as the Log2 ratio of average treated (3 or 9 h) versus average untreated (0 h). miRNA arrays were carried out by the Penn Microarray Facility, University of Pennsylvania, Philadelphia and the Keck Facility at Yale University.
Statistics.
Data are expressed as means ± SEM. Significance was tested by Student's two-tail t test_s_ or two-way ANOVA with Bonferroni correction for multiple comparisons when appropriate.
Supplementary Material
Supporting Information
Acknowledgments.
This work was supported in part by National Institute of Health Grants R01 HL64793, RO1 HL61371, R01 HL57665, and PO1 HL70295 (to W.C.S.), National Heart, Lung, and Blood Institute–Yale Proteomics Contract N01-HV-28186 (to W.C.S.), Program 3 + 3 Fellowship from the Centro Nacional de Investigaciones Cardiovasculares (to Y.S.), and an award from the American Heart Association (to Y.S.). C.F.-H. was supported by a fellowship from Phillip Morris USA and an award from the American Heart Association.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.He L, Hannon GJ. MicroRNAs: Small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 2.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein E, et al. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 4.Yang WJ, et al. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem. 2005;280:9330–9335. doi: 10.1074/jbc.M413394200. [DOI] [PubMed] [Google Scholar]
- 5.Harfe BD, McManus MT, Mansfield JH, Hornstein E, Tabin CJ. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc Natl Acad Sci USA. 2005;102:10898–10903. doi: 10.1073/pnas.0504834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris KS, Zhang Z, McManus MT, Harfe BD, Sun X. Dicer function is essential for lung epithelium morphogenesis. Proc Natl Acad Sci USA. 2006;103:2208–2213. doi: 10.1073/pnas.0510839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yi R, et al. Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nat Genet. 2006;38:356–362. doi: 10.1038/ng1744. [DOI] [PubMed] [Google Scholar]
- 8.Andl T, et al. The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles. Curr Biol. 2006;16:1041–1049. doi: 10.1016/j.cub.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cobb BS, et al. T cell lineage choice and differentiation in the absence of the RNase III enzyme Dicer. J Exp Med. 2005;201:1367–1373. doi: 10.1084/jem.20050572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cobb BS, et al. A role for Dicer in immune regulation. J Exp Med. 2006;203:2519–2527. doi: 10.1084/jem.20061692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, et al. A microRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317:1220–1224. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet P. Angiogenesis in life, disease, and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 14.Yancopoulos GD, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: Therapeutic implications. Semin Oncol. 2002;29:10–14. doi: 10.1053/sonc.2002.37264. [DOI] [PubMed] [Google Scholar]
- 16.Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer-dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100:1164–1173. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 17.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101:59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 18.Otsuka M, et al. Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J Clin Invest. 2008;118:1944–1954. doi: 10.1172/JCI33680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kisanuki YY, et al. Tie2-Cre transgenic mice: A new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 20.Monvoisin A, et al. VE-cadherin-CreERT2 transgenic mouse: A model for inducible recombination in the endothelium. Dev Dyn. 2006;235:3413–3422. doi: 10.1002/dvdy.20982. [DOI] [PubMed] [Google Scholar]
- 21.Ackah E, et al. Akt1/protein kinase Bα is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu J, et al. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci USA. 2005;102:10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poliseno L, et al. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108:3068–3071. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen IM, et al. Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature. 2007;449:919–922. doi: 10.1038/nature06205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Rooij E, et al. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 27.Volinia S, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng AM, Byrom MW, Shelton J, Ford LP. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res. 2005;33:1290–1297. doi: 10.1093/nar/gki200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 30.Dews M, et al. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006;38:1060–1065. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 32.Woods K, Thomson JM, Hammond SM. Direct regulation of an oncogenic micro-RNA cluster by E2F transcription factors. J Biol Chem. 2007;282:2130–2134. doi: 10.1074/jbc.C600252200. [DOI] [PubMed] [Google Scholar]
- 33.Murchison EP, Partridge JF, Tam OH, Cheloufi S, Hannon GJ. Characterization of Dicer-deficient murine embryonic stem cells. Proc Natl Acad Sci USA. 2005;102:12135–12140. doi: 10.1073/pnas.0505479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao C, et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17–92 expression in lymphocytes. Nat Immunol. 2008;9:405–414. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ventura A, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez-Hernando C, et al. Loss of akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metab. 2007;6:446–457. doi: 10.1016/j.cmet.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerber SA, et al. Mechanism of IL-12 mediated alterations in tumor blood vessel morphology: Analysis using whole-tissue mounts. Br J Cancer. 2003;88:1453–1461. doi: 10.1038/sj.bjc.6600907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin MI, Yu J, Murata T, Sessa WC. Caveolin-1-deficient mice have increased tumor microvascular permeability, angiogenesis, and growth. Cancer Res. 2007;67:2849–2856. doi: 10.1158/0008-5472.CAN-06-4082. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Z, et al. Impaired angiogenesis, delayed wound healing, and retarded tumor growth in perlecan heparan sulfate-deficient mice. Cancer Res. 2004;64:4699–4702. doi: 10.1158/0008-5472.CAN-04-0810. [DOI] [PubMed] [Google Scholar]
- 40.Suarez Y, Shepherd BR, Rao DA, Pober JS. Alloimmunity to human endothelial cells derived from cord blood progenitors. J Immunol. 2007;179:7488–7496. doi: 10.4049/jimmunol.179.11.7488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information