Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals (original) (raw)
Abstract
Cytomegalovirus (CMV) infects most individuals and elicits a strong CMV-specific immune response. We have studied the influence of CMV-seropositivity on the size of lymphoid subsets in healthy donors and demonstrate that the virus substantially modulates the peripheral lymphoid pool. CD8+ T cell numbers are increased in all CMV-seropositive individuals because of a striking 60% increment in the CD8+ T cell memory pool. The CD45RA+ resting memory pool is doubled after CMV infection and increases further with age. The magnitude of the naïve CD8+ T cell pool is dramatically reduced in CMV-seropositive individuals at all ages, and this accelerates the physiological decline by approximately 40 years. The number of CD4+ effector memory T cells is increased in CMV-seropositive individuals and is differentially accommodated by a reduction in the number of naïve and central memory CD4+ T cells in young and elderly donors respectively. CMV-seropositivity also increases the total number of B cells in older donors and suppresses the number of CD5+ B cells. These data reveal that CMV has a profound influence on the immune system of all healthy individuals and add to growing concern regarding the clinical and immunomodulatory significance of CMV infection in healthy donors.
Keywords: cytomegalovirus, immunocompetent, lymphoid subsets, memory cells, NK cells
Introduction
Cytomegalovirus (CMV) is one of the eight human herpesviruses and seroprevalence ranges from 60 to > 95% in different populations. In western countries infection rates increase gradually with age, such that approximately 70% of individuals over the age of 60 years are CMV-seropositive. In common with all herpesviruses, CMV undergoes latency following infection and the virus is very difficult to isolate from healthy donors. Despite this, there remains controversy as to the mechanisms by which latency is maintained within the host. A discrete transcriptional latency profile has been difficult to demonstrate, although the presence of unique transcripts within monocytes suggests that this pathway is of potential importance [1]. However, sustained immune control of lytic viral replication is also likely to be very important in maintenance of latency. Selective depletion of cellular subsets in mice [2], and clinical experience with immunosuppressed donors [3], indicates that the virus can reactivate promptly following periods of immunosuppression and provides support for the view that a substantial proportion of the immune repertoire may be required to control viral replication. The last few years has seen considerable interest in the study of CMV-specific T cell immune responses and this has revealed that CMV is arguably the most immunodominant antigen to be encountered by the human immune system [4]. The CD8+ T cell response to CMV typically constitutes a sizeable percentage of the CD8+ T cell repertoire in CMV-seropositive individuals [5,6]. In addition, the magnitude of this response appears to increase with age both in man and mouse [7–9], which is in direct contrast to the pattern of immunity to other pathogens that is seen with advancing age [10].
In view of the substantial component of the T cell repertoire that is committed to CMV-specific T cell immunity, it has been proposed that carriage of CMV may compromise the ability to respond to heterologous infection. Indeed, the magnitude of the T cell response to Epstein–Barr virus is suppressed markedly in patients with simultaneous carriage of CMV [6]. These data have led to a reappraisal of the clinical significance of CMV-seropositivity in immunocompetent individuals with an increasing realization that viral carriage may contribute directly to the development of immune senescence [7,11]. This suspicion has been strengthened greatly by epidemiological studies of elderly Scandinavian individuals in which CMV-seropositivity was associated with the development of an ‘immune risk phenotype’ which was predictive of early mortality [12]. Study of how CMV-seropositivity impacts on the human immune system is thus a compelling health concern and will need to address both the direct CMV-specific immune component as well as the indirect consequences of this large investment of immune function on other aspects of the immune response.
Cytomegalovirus-seropositivity has been associated with changes in the absolute lymphocyte count of elderly donors, suggesting that the accumulation of the CMV-specific immune response may influence the peripheral lymphoid pool [13]. Here we have performed a large cross-sectional study of healthy donors in a variety of age categories. CMV-seropositivity had a direct influence on the magnitude and proportion of the T cell, natural killer (NK) cell and B cell subsets. The findings indicate that CMV has a profound influence on the human lymphocyte repertoire and that this is likely to have major implications for immune function in CMV-seropositive donors of all ages.
Methods
Subjects
Blood samples were obtained from 132 healthy donors after obtaining informed consent. Sixty-two individuals were acquired through the National Blood Service, Birmingham and 70 donors aged over 60 years were recruited from the ‘Thousand Elders’ cohort at the Centre of Applied Gerontology, Selly Oak Hospital. Peripheral blood (20 ml) was collected into tubes containing sodium heparin or ethylenediamine tetraacetic acid. Ethical approval was granted through the South Birmingham Regional Ethics committee. The absolute lymphocyte count was determined using an automated cell counter on the day of collection.
Isolation of peripheral blood mononuclear cells
Peripheral blood mononuclear cells were isolated from heparinized blood using Ficoll density gradient centrifugation (Lymphoprep; Axis Shield, Kimbolton, UK). Cells were washed twice using RPMI media (RPMI-1640; Gibco brl, Invitrogen, Paisley, UK) and resuspended in 1 ml freezing media (10% dimethylsulphoxide, 90% fetal calf serum). Aliquots of cells were frozen down in 1 ml cryotubes at concentrations of 5 × 106 cells/ml. Cryotubes were placed in a Nalgene Cryo Freezing container and stored at −80°C.
Cell staining and fluorescence-activated cell sorter analysis
Frozen cells were thawed and washed twice in fluorescence-activated cell sorter buffer prior to incubation at 4°C for 25 min using a panel of antibodies. Anti-CD8-Tricolor (Caltag, Bucks, UK) was used in combination with anti-CD11a[lymphocyte function-associated antigen-1 (LFA-1)]-fluorescein isothiocyanate (FITC) (Pharmingen, Oxford, UK) and anti-CD45RA-phycoerythrin (PE) (Pharmingen). The antibodies used for analysis of CD4+ subpopulations were anti-CD4-Tricolor (Caltag), anti-CCR7-FITC (Becton-Dickinson, Oxford, UK) and anti-CD45RA-PE (Pharmingen). Regulatory cells were determined by CD4 in combination with anti-CD25-PE (Beckman Coulter, High Wycombe, UK) and anti-CD45RO-FITC (Dako, Ely, UK). NK cells were phenotyped by CD3-PC5 (Coulter) in combination with anti-CD56-PE and anti-CD16-FITC (both Dako). B cells were stained with anti-CD19-Tricolor (Caltag), CD27-FITC (Beckman Coulter) and CD5-PE (Beckman Coulter). Cell populations were analysed subsequently using Win MDI 2·8 following data collection using a Coulter XL flow cytometer.
Statistical analysis
Data analysis involved mathematical modelling using r stats and mass software packages. Age was used as a continuous variable and log-transformed (using the Box–Cox application). The Shapiro–Wilk normality test was used to ensure that data in all models was of Gaussian distribution. Multiple T tests were undertaken after transformation and significance regarded as P < 0·01 (to account for multiple testing corrections). Multiple sequential analyses of variance were performed such that the variance attributed to each variable was assessed and statistical significance regarded as P < 0·05.
Results
Cytomegalovirus-seropositivity increases the lymphocyte count in donors aged over 40 years
The lymphocyte count within peripheral blood is at its highest in young (< 40 years) donors and then declines in middle age before recovering in donors aged over 60 years (Table 1). CMV infection is associated with an increase in the lymphocyte count in the middle-aged and older donors because of an increase in the T cell population within these cohorts. The T cell count in elderly donors is 21% higher within CMV-seropositive individuals compared with the uninfected group and this is predominantly a reflection of the increase in the CD8 T cell pool (see below). These findings have implications for the CD4 : CD8 ratio. In CMV-uninfected individuals this ratio increases slightly with age, whereas in CMV-seropositive donors there is a decrease in the CD4 : CD8 ratio in elderly donors.
Table 1.
The effect of donor age and cytomegalovirus (CMV) serostatus on lymphocyte count and CD4 : CD8 ratio.
| Age (years) | CMV status | n = (M/F) | Lymphocyte count (×106/ml) blood (±s.d.) | T lymphocytes µl blood (±s.d.) | CD4+/CD8+ ratio (±s.d.) |
|---|---|---|---|---|---|
| 20–40 | CMV+ | 5 (3/2) | 1·98 ± 0·79 | 1767 ± 242 | 1·14 ± 0·31 |
| CMV− | 12 (8/4) | 2·17 ± 0·42 | 1626 ± 91 | 1·55 ± 0·25 | |
| 40–60 | CMV+ | 7 (5/2) | 1·91 ± 0·35 | 1393 ± 97 | 2·13 ± 0·4 |
| CMV− | 10 (4/6) | 1·55 ± 0·30 | 1075 ± 84 | 1·73 ± 0·28 | |
| 60+ | CMV+ | 24 (8/16) | 1·91 ± 0·67 | 1387 ± 103 | 1·42 ± 0·18 |
| CMV− | 31 (7/24) | 1·76 ± 0·81 | 1145 ± 96 | 1·91 ± 0·24 |
Cytomegalovirus-seropositivity has a major effect on the CD8+ T cell repertoire
The clearest effects of CMV infection on the lymphoid system were observed within the CD8 T cell population (Table 2). Age, CMV and gender did not have a statistically significant effect on the absolute CD8 T cell count. CD8 T cells are at their highest level in younger donors and fall in middle age, before increasing again within the older cohort. These changes reflect a marked decline in the number of naive CD8+ T cells during ageing which is not compensated in middle age by a comparable increase in the memory CD8+ T cell pool. However, this memory pool increases in older donors leading to a recovery in the total CD8 T cell count.
Table 2.
The effect of donor age and cytomegalovirus (CMV) serostatus on CD8+ T cells subsets within peripheral blood.
| Age (years) | Group | n = (M/F) | CD8+ lymphocytes ± s.d. | CD8+ LFA-1low (naïve) CD8 T cells ± s.d. | CD8+ LFA-1hi (memory) CD8 T cells ± s.d. | CD45RA+ LFA-1hi (revertant memory) CD8 T cells ± s.d. |
|---|---|---|---|---|---|---|
| 20–40 | CMV+ | 8 (4/4) | 788 ± 162 | 205 ± 43 | 584 ± 142 | 164 ± 43 |
| CMV− | 18 (9/9) | 711 ± 63 | 344 ± 44 | 367 ± 33 | 79 ± 16 | |
| 40–60 | CMV+ | 12 (6/6) | 553 ± 84 | 106 ± 13 | 448 ± 82 | 160 ± 37 |
| CMV− | 10 (7/3) | 417 ± 38 | 173 ± 15 | 243 ± 29 | 54 ± 7·7 | |
| 60+ | CMV+ | 47 (12/35) | 614 ± 78 | 70 ± 7·3 | 544 ± 76 | 181 ± 25 |
| CMV− | 29 (10/19) | 437 ± 40 | 106 ± 14 | 332 ± 33 | 73 ± 9·5 |
The effect of CMV-seropositivity is to increase the CD8 T cell count at all ages, although the absolute CD8 T cell count decreases with advancing age. This effect is particularly marked with age and CMV infection is associated with a 33% increase in the CD8+ T cell count in middle age, while in donors aged over 60 years this increase is 41%. This increase associated with CMV appears to be due entirely to a large increase in the CD8+ memory T cell pool within CMV-seropositive individuals. CMV infection increases this subset by over 60% in all age groups, with a maximum increment of 84% in the cohort aged 40–60 years. The effect of CMV on CD8 T cells was statistically significant, whereas age and gender had no effect on memory CD8 T cells (Supplementary material, Figs S1–S3).
The CD8+ memory pool was further analysed with respect to expression of the CD45RA isoform of CD45. CD45RO+CD45RA− CD8+ T cells are considered to comprise central and effector memory populations, whereas reversion to CD45RA+ expression is seen in a population of resting memory cells with a characteristic cytokine profile [14]. The most significant expansion of the memory CD8+ T cell pool following CMV infection was observed in the CD45RA+ resting memory subset where the virus was responsible with the majority of this CD8 subset. In CMV-seronegative individuals this CD45RA+ CD8 population comprised approximately 20% of the memory CD8+ T cell pool and this number remains stable at all ages (Fig. 1). Statistically significant associations with age and gender were not found. CMV-seropositive status was, however, associated with a significant increase in the CD45RA+ CD8+, pool which more than doubled at all age groups and showed a gradual accumulation with age (Table 2). These data reveal that CMV infection is the predominant stimulus for the generation of resting memory CD45RA+ CD8+ T cells and that this population increases with age.
Fig. 1.
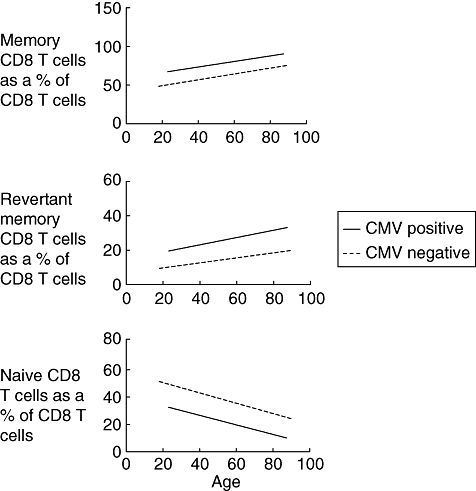
These graphs show the line of best fit where memory, revertant memory and naive CD8 T cells are expressed as a percentage of the absolute CD8 T cell count against age and cytomegalovirus status.
Lymphocyte functionassociated antigen-1 (CD11a) expression was then examined on CD8+ T cells in order to distinguish accurately between naive and antigen-experienced CD8+ T cell subsets. Naive CD8+ T cells express LFA-1 at low levels, whereas expression is increased on all memory T cell subsets [15]. Age and CMV had statistically significant associations with respect to naive CD8 T cells, whereas gender did not have an effect. A steady decline in the number of naive CD8+ T cells within peripheral blood was observed in association with ageing and reflects the decrease in thymic T cell output that occurs with age [16]. However, CMV-seropositivity is associated with a dramatic decrease in the naive CD8+ T cell repertoire in all ages [17]. Indeed, the naive CD8 T cell count was reduced by 40% in all age groups. The effect of CMV infection is therefore to accelerate the physiological decline in naive CD8+ T cells, and the proportion of naive T cells within the CD8+ pool was decreased by the equivalent of 40 years in CMV-seropositive donors.
Cytomegalovirus has a differential effect on the CD4+ T cell repertoire depending on the age of the donor
In contrast to the influence of CMV infection on the CD8+ T cell repertoire, a very different effect was observed when CD4+ T cell subsets were examined in CMV-seropositive donors at different ages (Table 3). Within CMV-seronegative individuals the total number of CD4+ T cells decreases during middle age and then increases within the elderly cohort, in a comparable fashion to the pattern observed for CD8+ T cells (Table 2). CMV infection was not associated with a major alteration in the size of the CD4+ T cell pool, but clear effects were observed on the composition of the memory subsets and naive pool. CCR7 and CD45RA co-expression is used to differentiate between CD4+ T cell subsets as CD4+ naive cells have a characteristic CD45RA+CCR7+ phenotype, while central memory cells lose expression of CD45RA and effector memory cells are CCR7-negative.
Table 3.
The effect of age and cytomegalovirus (CMV) on CD4+ T cell number µl/blood.
| Age | CMV status | n = M/F | CD4 cells (±s.d.) | CD4 naive (±s.d.) | CD4 central memory (±s.d.) | CD4 effector memory (±s.d.) | CD4 memory cells (±s.d.) | T regulatory cells (±s.d.) |
|---|---|---|---|---|---|---|---|---|
| 20–40 | CMV+ | 5 (3/2) | 849·6 ± 84·0 | 139·8 ± 18·8 | 351·6 ± 33·6 | 358·2 ± 80·0 | 709·8 ± 79·6 | 46·13 ± 4·0 |
| CMV− | 15 (10/5) | 911·6 ± 69·9 | 338·3 ± 52·4 | 357·6 ± 30·5 | 215·7 ± 25·9 | 573·2 ± 42·8 | 42·87 ± 5·4 | |
| 40–60 | CMV+ | 12 (4/8) | 861·5 ± 69·5 | 261·2 ± 47·4 | 315·5 ± 34·6 | 284·8 ± 29·4 | 600·2 ± 56·1 | 42·91 ± 6·7 |
| CMV− | 9 (6/3) | 660 ± 62·3 | 172·0 ± 34·3 | 295·5 ± 51·3 | 192·5 ± 34·6 | 488 ± 58·1 | 52·01 ± 9·6 | |
| 60+ | CMV+ | 32 (8/24) | 714·5 ± 47·8 | 210·5 ± 28·2 | 261·1 ± 22·3 | 242·9 ± 29·5 | 504 ± 36·9 | 51 ± 5·1 |
| CMV− | 26 (8/18) | 737·8 ± 80·7 | 231·8 ± 36·4 | 342·3 ± 45·4 | 161·7 ± 25·3 | 504 ± 54·1 | 62·90 ± 12·3 |
Age, gender and CMV did not have significant effects on absolute CD4 T cell counts, naive CD4 T cells or central memory CD4 T cells. However, CMV infection does have a significant influence on the magnitude of the CD4+ effector memory pool which increases in all groups studied (Table 3). CMV-seropositivity is associated with a 24% increase in the total (central and effector) CD4+ memory pool in younger donors and a 12% increase within the middle-aged cohort. In elderly donors it appears that the total memory CD4+ pool is not influenced by CMV infection. The increase in CD4+ effector memory cells is accommodated within the CD4+ repertoire by a decrease in the naive pool in young donors and a decrease in central memory cells in CMV-seropositive elderly donors. CD45RA+CCR7+ naive CD4+ T cell populations show a remarkably stable pattern with ageing, and in the CMV-seronegative group no major decline of the CD4+ naive T cell subset is seen with time. Indeed, an increase is seen between the middle-aged and elderly cohorts.
Cytomegalovirus infection suppresses an increase in CD4+CD25+ regulatory T cells with advancing age
CD4+ CD25high regulatory T cells play a critical role in immune homeostasis, and both ageing and chronic infection have been shown to influence their magnitude. Age, CMV and gender did not have significant effects on T regulatory cell counts. Interestingly, while T regulatory cell counts appear to increase with age, positive CMV serostatus is associated with a modest suppression of this increase with advancing age. A 43% increase in the CD4+ CD25high subset was observed with association with ageing in the CMV-seronegative donors, which is in line with previous observations (Table 3) [18].
Cytomegalovirus infection increases CD56dim NK cells in those over 60 years of age
The proportion of NK cells within the peripheral lymphoid pool increases with age from 9% in younger donors to 11% in the over 60 group (Table 4a). CMV infection was associated with a reduced number of NK cells in young donors, but this increased gradually with ageing, such that it represented 12·4% of the lymphoid pool in elderly CMV-seropositive donors compared with 8·8% within the CMV-uninfected group. This increase in NK cell numbers was due to an increase in the cytotoxic CD56dim population in the CMV-seropositive group > 60 years of age. Age, CMV and gender did not have a statistically significant effect on absolute NK cell counts or CD56dim NK cells. There was, however, a statistically significant decrease in CD56bright NK cells with advancing age (Table 4). CMV and gender did not have an effect on this subset.
Table 4.
(a)The effect of age and cytomegalovirus (CMV) on natural killer (NK) cell numbers.
| Age | Group | n = (M/F) | NK cells (µl/blood) ± s.d. |
|---|---|---|---|
| 20–40 | CMV+ | 8 (4/4) | 166·4 ± 88·5 |
| CMV− | 18 (9/9) | 206 ± 156·8 | |
| 40–60 | CMV+ | 12 (6/6) | 190·6 ± 150·1 |
| CMV− | 10 (6/4) | 183·7 ± 144·8 | |
| 60+ | CMV+ | 48 (12/36) | 236·8 ± 207·4 |
| CMV− | 29 (9/20) | 153·7 ± 122·8 |
Table 4.
(b)The effect of age and cytomegalovirus (CMV) on CD56bright and CD56dim natural killer (NK) cell subsets.
| Age | Group | n = M/F | CD56bright(µl/blood) ± s.d. | CD56dim(µl/blood) ± s.d. |
|---|---|---|---|---|
| 20–40 | CMV+ | 8 (4/4) | 14·74 ± 8·1 | 151·7 ± 87·8 |
| CMV− | 18 (9/9) | 16·00 ± 14·4 | 190 ± 150·6 | |
| 40–60 | CMV+ | 12 (6/6) | 14·79 ± 14·7 | 175·8 ± 137·2 |
| CMV− | 10 (6/4) | 10·23 ± 7·0 | 173·5 ± 139·5 | |
| 60+ | CMV+ | 48 (12/36) | 8·794 ± 7·4 | 228·0 ± 203·8 |
| CMV− | 29 (9/20) | 7·021 ± 8·7 | 146·7 ± 118·8 |
Age, gender and CMV infection have a differential effect on B cells
Finally, the influence of CMV infection was studied in relation to B cell numbers (Table 5). The total B cell pool showed a profile comparable to that of T cells, in that there was a decrease from the young to middle-aged groups followed by a slight increase in the elderly donors. Although there was significant interaction between age, CMV and gender with total B cell counts and CD5+ B cells, only the individual effect of CMV on B cells is statistically significant. A marked effect of CMV infection was noted in relation to CD5+ B cell numbers, where CMV-seronegative donors showed a sevenfold increase in cell numbers between the middle-aged and elderly groups. Our analysis showed that an association between age and CD5+ B cell numbers was statistically significant in the male CMV-seropositive cohort, whereby CD5+ B cells increase with advancing age. An overall CMV effect on CD5+ B cells, however, was not significant.
Table 5.
The effect of age and cytomegalovirus (CMV) on B cells µl/blood.
| Age | Group | n = M/F | B cells | CD5+ B cells µl/blood | CD27− B cells | CD27+ B cells |
|---|---|---|---|---|---|---|
| 20–40 | CMV+ | 6 (2/4) | 151·4 (±51·7) | 0·911 (±0·567) | 145·2 (±51·4) | 6·2 (±2·03) |
| CMV− | 14 (8/6) | 247 (±123·0) | 3·07 (±7·64) | 225·3 (±100·9) | 21·7 (±25·4) | |
| 40–60 | CMV+ | 9 (4/5) | 154·4 (±70·1) | 3·1 (±7·70) | 143·5 (±62·6) | 11 (±7·6) |
| CMV− | 8 (6/2) | 149·6 (±87·4) | 1·09 (±0·888) | 138·3 (±77·6) | 11·3 (±9·9) | |
| 60+ | CMV+ | 39 (10/29) | 171·8 (±95·5) | 1·93 (±2·25) | 155·6 (±78·1) | 16·2 (±20·0) |
| CMV− | 29 (10/19) | 148·4 (±79·5) | 8·34 (±21·2) | 137·0 (±68·6) | 11·4 (±11·5) |
CD27 was used as a B cell marker where CD27+ B cells are memory cells. No significant associations were due to the effect of age, CMV or gender on memory B cells, which remain a stable population. Naive B cells (CD19+CD27−) decrease with advancing age and are statistically significant, whereas no associations exist because of CMV and gender. Memory B cells decrease in CMV-seropositive young donors because of suppression of the naive B cell pool, but this profile recovers during ageing.
Discussion
Cytomegalovirus is a ubiquitous herpesvirus and infection is so common that studies of lymphocyte phenotype and function are commonly undertaken in predominantly CMV-seropositive cohorts. For this reason, the significance of CMV infection as a confounding factor in studies of immune function has generally been neglected. Chronic infection leads to accumulation of a very large CMV-specific immune response and the virus is arguably the most immunodominant antigen to which the human immune system is exposed [19,20]. In this report we have provided the first comprehensive quantitative study of the influence of CMV infection on all major lymphoid subsets in immunocompetent individuals at different ages (Table 6).
Table 6.
Summary of significant results (overall effect of each variable through sequential analysis of variance where P < 0·05).
| Advancing age | CMV positive | Gender | |
|---|---|---|---|
| Total T cells | – | – | – |
| CD4/8 ratio | – | – | – |
| CD8 T cells | Decrease | – | – |
| Naive CD8 T cells | Decrease | Lower | – |
| Memory CD8 T cells | – | Higher | – |
| Revertant memory CD8 T cells | – | Higher | – |
| CD4 naive T cells | – | – | – |
| CD4 central memory CD4 T cells | – | – | – |
| CD4 effector memory CD4 T cells | – | Higher | – |
| CD4 memory T cells | – | – | – |
| CD56bright NK cells | Decrease | – | – |
| CD56dim NK cells | – | – | – |
| CD5+ B cells | – | – | – |
| B cells | – | Higher | – |
| Naive (CD27−CD19+) B cells | – | – | – |
| Memory (CD27+ CD19+) B cells | Decrease | – | – |
We found that in CMV-seronegative donors the lymphocyte count falls naturally from young donors into middle age before recovering in donors aged over 60 years. CMV infection increases the lymphocyte count in donors aged over 40 years primarily because of accumulation of CD8+ T cells following viral infection. CMV infection caused a significant decrease in the CD4 : CD8 ratio in elderly individuals, because of a combination of increased CD8 T cell count and a reduction in CD4+ T cell numbers. A decline in the CD4 : CD8 ratio has been associated frequently with immunological dysfunction and a decline below unity is associated with a hazard ratio of 1·5 for early mortality [21].
Almanzar et al. reported that the relative proportion of CD8+ memory subsets was modulated by CMV infection, but were unable to determine the absolute size of these populations [17]. Our data reveal that CMV infection results in a profound alteration of the CD8+ T cell repertoire, where it increases the CD8 T cell count markedly at all ages. Indeed, the total CD8 T cell population is increased by > 30% in all donors aged over 40 years of age. Not surprisingly, this effect is a result of the dramatic increase in the CD8+ memory T cell pool within CMV-seropositive individuals, which is increased by > 60%. Strikingly, this dramatic increment in the size of the memory of CD8+ T cell pool was seen at all age groups, and there was no evidence of a further increase in the CD8 memory T cell response in the older CMV-seropositive age group. These data reveal, therefore, that the memory CD8 T cell pool in response to CMV is established relatively early following infection and remains stable for many decades.
The memory T cell compartment can be subdivided according to membrane phenotype and physiological function. Surface expression of isoforms of the tyrosine phosphatase CD45 is used widely to differentiate CD45RO+ central and effector memory cells from a subpopulation of memory cells which ‘revert’ to re-express the long CD45RA isoform [22]. Although considered previously as terminal differentiated [23], it is now appreciated that the CD45RA+ subpopulation is a resting memory pool that reverts rapidly to CD45RO expression following exposure to antigen [24]. This revertant memory CD8 population represents approximately 20% of the memory CD8+ T cell pool in donors who have never been infected with CMV. However, CMV infection was associated with a dramatic increase in the revertant memory CD8 pool, which more than doubled at all age groups, and showed a gradual increase with age. These data are comparable to findings in a paediatric cohort [25] and indicate that CMV infection is the predominant stimulus for the generation of CD45RA+ memory CD8+ T cells in adults and that this population increases with age. Our data therefore imply that memory T cells are exposed to viral antigen less frequently within elderly individuals, which is somewhat surprising in the light of evidence suggesting more frequent viral activation within this cohort.
The increase in the memory CD8+ T cell population in CMV-seropositive individuals appears to have a dramatic influence on the naive CD8+ T cell repertoire. In our study we used lack of LFA-1 expression as the marker of naive CD8+ T cell populations, as this has been shown to be a highly reliable phenotype for this subset. LFA-1 expression is increased following priming of a naive T cell but, more importantly for phenotypic analysis, expression is also retained on all memory subsets thereafter [15]. T cells which lack LFA-1 expression can therefore be considered as truly naive. In this regard CD45RA and CD28 expression, either alone or in combination, are inadequate as markers to identify naive populations reliably [15]. A decline in the proportion of CD45RA+CD28+ T cells has been observed in elderly CMV-seropositive individuals [17]. A physiological decline in naive CD8+ T cells with age was observed in CMV-seronegative donors with a half-life of approximately 20 years. The rate of this decline was not influenced by CMV infection, but the effect of CMV-seropositivity was to reduce the naive CD8 T cell count by 40% in all age groups and effectively to accelerate the decline in the proportion of naive CD8 T cells by approximately 40 years.
The most likely explanation for this effect is competition for physiological ‘space’ within the peripheral lymphoid system secondary to the dramatic increase in memory T cell populations following CMV infection. A direct effect of CMV infection on thymic CD8+ T cell output cannot be ruled out at this stage and analysis of the influence of CMV infection on T cell receptor excision circle (Trec) levels would be valuable in this regard. If the number of naive CD8 T cells within the peripheral lymphoid system is an important determinant of immune efficacy, then CMV infection is likely to be associated with a premature decline in immune responsiveness at all age groups.
The influence of CMV infection on the CD4+ T cell repertoire is very different from that seen on CD8+ T cells. The naive CD4+ T cell count remains relatively stable during ageing, in line with previous studies [26]. CMV-seronegative individuals exhibit a decrease in the number of CD4+ T cells during middle age which parallels patterns within CD8+ cells and determines the modest increase in the CD4 : CD8 ratio with increasing age. CMV infection increases the CD4+ memory count in donors aged less than 60 years, but in elderly donors this value is not changed. CMV infection is known to elicit a strong CMV-specific CD4+ immune response in elderly donors, and the implication of this finding is that this population must survive at the expense of memory populations against other pathogens. Support for this proposal comes from analysis of the composition of the CD4+ memory T cell pool. CMV-specific CD4+ T cells typically display an effector memory phenotype [19], and this subset is indeed increased in elderly CMV-seropositive donors at the expense of the central memory subset. The implication of these findings is that the increase in the CMV-specific CD4+ T cell pool leads to a decrease in the memory response against heterologous antigens and provides a further explanation for the potential immunosuppressive effect of CMV infection. CMV infection has an interesting differential effect on the CD4+ naive population, depending upon the donor age. Young donors showed a dramatic reduction in CD4+ naive T cells, but this recovers by middle age. Naive CD4+ T cells are more sensitive than memory cells to Fas-mediated apoptosis and a differential sensitivity of such cells in relation to age may explain this observation [27].
Thus, a very different pattern emerges for the influence of CMV infection on CD4+ T cell subsets in comparison with the CD8+ pool. The accommodation of the CMV-specific CD4+ memory T cell pool is made at the expense of a reduction in the naive CD4+ T cell population in younger donors and the memory pool against other pathogens in older subjects.
CD4+ CD25high regulatory T cells are an important subset for regulation of immune function. This population has a number of important properties and is now characterized typically by expression of phenotypic markers such as low CD123 expression and forkhead box P3 (FoxP3) positivity. Our analysis was performed prior to availability of these reagents, but the number of CD4+CD25 high and CD4+FoxP3+ T cells is correlated strongly. Within the total cohort of donors a 25% increase in the number of regulatory CD4+ CD25+ T cells was noticed during ageing which supports the concept that regulatory cells can expand in the peripheral lymphoid system as well as being generated through a distinct thymic pathway [18]. Interestingly, this increment was even more marked within the CMV-seronegative group, with a 43% increase over time. Evidence is accumulating that regulatory T cells can be derived from highly proliferative memory cells and it has been proposed that conditions associated with an increase in the memory pool might lead therefore to accumulation of an increased regulatory pool [28]. However, CMV associated was in fact associated with a modest suppression in regulatory T cell numbers.
Natural killer cells represent a considerable component of the lymphoid pool and we observed an age-related increase from 9% in donors aged less than 40 years to more than 11% in the elderly group [29]. The effect of CMV infection on the total NK cell subset was again dependent upon donor age, with a decrease in cell numbers being seen in young donors but a considerable increase to > 12·4% of the lymphoid pool in elderly CMV positive donors. This was a 41% increase compared with the CMV uninfected group and reinforces the observation that the influence of CMV infection on the lymphoid pool is dependent upon donor age. NK cells can be divided into CD56dim and CD56bright subsets, which differ quite broadly in their functional and phenotypic properties [30]. This increase in NK cell numbers in association with CMV was due almost entirely to an increase in the CD56dim population and reflects the age-associated decline in the CD56bright subset [31].
The significance of CMV infection on the B cell repertoire has been poorly studied, although primary infection has been associated with autoimmune phenomena suggesting that activation of the B cell pool can occur. Total B cell numbers decrease during middle age with a similar profile to that observed for T cell subsets. CD27 expression can be used to delineate naive from memory B cells [32], and a differential influence of CMV infection was again observed depending upon the age of the donor. A dramatic suppression of B cell numbers was observed in CMV-seropositive young donors with a 36% and 71% reduction in naive and memory populations respectively. However, these populations recovered with time such that no differences were observed in middle-aged and elderly individuals. It is unclear why B cell populations from young donors are particularly susceptible to CMV infection, but B cells from young donors are more sensitive to apoptosis that those from elderly individuals [33]. It is conceivable that the dramatic increase in CD8+ memory T cells that occurs following CMV infection, many of which will express FasL [34], leads to death of Fas-positive B cells in young donors. CD5+ B cells represent a unique lymphoid pool which is associated with the production of polyspecific low-affinity antibodies with the capacity to bind a range of self-antigens. These cells have been implicated in the generation of autoimmune disease, and within the CMV-seronegative group a sevenfold increase in the number of CD5+ B cells was noticed between the middle-aged and elderly groups. Strikingly, however, CMV-seropositivity was associated with the suppression of this expansion to only 23% of the numbers found in CMV-uninfected individuals. A reduction in the CD5+ B cell pool has been observed in elderly donors in cohorts which are likely to include predominantly CMV-seropositive individuals [35]. The implications of this observation need further study with regard to the potential impact of CMV-seropositivity on the incidence of autoimmune disease in elderly individuals.
Overall, these observations add considerable evidence to the growing appreciation that CMV infection has very significant effects on the composition of the human lymphoid pool. Our observations, all within immunocompetent donors, show that CMV has a significant influence on all major lymphoid pools and that, perhaps surprisingly, these observations are seen within all age groups. Almost all studies of human lymphoid pools in relation to health and ageing have been confounded by the influence of CMV-seropositivity which is rarely taken into account. Our data provide important control values for healthy CMV-seropositive and -seronegative individuals which will assist interpretation of lymphoid populations in disease states.
The influence of CMV infection on healthy immunocompetent individuals has been relatively poorly studied. However, within an elderly population CMV seropositivity has been associated with significant changes in immune parameters that are correlated with increased risk of mortality. Our observations reveal that the influence of CMV infection on the human lymphoid system extends to all age groups and indicate that more attention should be given into how this important viral infection may affect the immunocompetence of healthy seropositive donors.
Acknowledgments
This work was supported by the Medical Research Council, UK. The participation of donors in the study is gratefully appreciated. S. C. and A. McL. performed the laboratory work. Consent and collection of donors was organized by N. K., N. S. and L. N. The experimental plan was devised by N. K. and P. M. The manuscript was written by S. C. and P. M. W. W. performed the mathematical modelling and statistical analysis of data. None of the authors have any competing financial interests.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Naive CD8 T cells. These scatter-plots show the relationship between log naive CD8 cell counts and age. The four slopes are not significantly different from each other (P of _F_-tests for interactions > 0·5). Three of the four slopes are significantly different from zero (P = 0·000237 for Mneg, 0·0128 for Mpos, 2·01e-05 for Fneg and 1·6e-05 for Fpos). Age (P of F-test = 1·32e-17) and CMV (P of _F_-test = 4·01e- 05) have significant effects on naive CD8 cell counts, while gender (P of _F_-test = 0·62) does not. Interaction between age, gender and CMV was non-significant.
Fig. S2. Revertant memory CD8 T cells. The scatter-plots show the relationship between log-revertant CD8 cell counts and age. The four slopes are not significantly different from each other (P of F-tests for interactions > 0·1). The four slopes are not significantly different from 0 according to _t_-test (P = 0·163 for Mneg, 0·816 for Mpos, 0·431 for Fneg and 0·217 for Fpos). Cytomegalovirus (CMV) (P of _F_-test = 4·16e-08) has a significant effect on revertant CD8 cell counts, while age (P of _F_-test = 0·029) and gender (P of _F_-test = 0·16) does not. Interaction between age, gender and CMV was non-significant.
Fig. S3. Effector memory CD4 T cells. The scatter-plot shows the relationship between log effector memory CD4 cell counts and age. They are not significantly different from each other (P of _F_-tests for interactions > 0·2). The four slopes are not significantly different from 0 according to _t_-test (P = 0·242 for Mneg, 0·381 for Mpos, 0·382 for Fneg and 0·0564 for Fpos). Cytomegalovirus (CMV) (P of _F_-test = 0·0016) has a significant effect on effector memory CD4 cell counts, while age (P of _F_-test = 0·07) and gender (P of _F_-test = 0·69) does not. Interaction between age, gender and CMV was non-significant.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Bolovan-Fritts CA, Mocarski ES, Wiedeman JA. Peripheral blood CD14(+) cells from healthy subjects carry a circular conformation of latent cytomegalovirus genome. Blood. 1999;93:394–8. [PubMed] [Google Scholar]
- 2.Polic B, Hengel H, Krmpotic A, et al. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J Exp Med. 1998;188:1047–54. doi: 10.1084/jem.188.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandherr M, Einsele H, Hebart H, et al. Antiviral prophylaxis in patients with haematological malignancies and solid tumours: guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society for Hematology and Oncology (DGHO) Ann Oncol. 2006;17:1051–9. doi: 10.1093/annonc/mdj132. [DOI] [PubMed] [Google Scholar]
- 4.Sylwester AW, Mitchell BL, Edgar JB, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–85. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kern F, Surel IP, Faulhaber N, et al. Target structures of the CD8(+)-T-cell response to human cytomegalovirus: the 72-kilodalton major immediate-early protein revisited. J Virol. 1999;73:8179–84. doi: 10.1128/jvi.73.10.8179-8184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan N, Hislop A, Gudgeon N, et al. CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J Immunol. 2004;173:7481–9. doi: 10.4049/jimmunol.173.12.7481. [DOI] [PubMed] [Google Scholar]
- 7.Khan N, Shariff N, Cobbold M, et al. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169:1984–92. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 8.Karrer U, Sierro S, Wagner M, et al. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol. 2003;170:2022–9. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- 9.Ouyang Q, Wagner WM, Zheng W, Wikby A, Remarque EJ, Pawelec G. Dysfunctional CMV-specific CD8(+) T cells accumulate in the elderly. Exp Gerontol. 2004;39:607–13. doi: 10.1016/j.exger.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Targonski PV, Jacobson RM, Poland GA. Immunosenescence: role and measurement in influenza vaccine response among the elderly. Vaccine. 2007;25:3066–9. doi: 10.1016/j.vaccine.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 11.Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, Wikby A. Human immunosenescence: is it infectious? Immunol Rev. 2005;205:257–68. doi: 10.1111/j.0105-2896.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 12.Strindhall J, Nilsson BO, Lofgren S, et al. No immune risk profile among individuals who reach 100 years of age: findings from the Swedish NONA immune longitudinal study. Exp Gerontol. 2007;42:753–61. doi: 10.1016/j.exger.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Looney RJ, Falsey A, Campbell D, et al. Role of cytomegalovirus in the T cell changes seen in elderly individuals. Clin Immunol. 1999;90:213–19. doi: 10.1006/clim.1998.4638. [DOI] [PubMed] [Google Scholar]
- 14.Appay V, Dunbar PR, Callan M, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–85. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 15.Faint JM, Annels NE, Curnow SJ, et al. Memory T cells constitute a subset of the human CD8+CD45RA+ pool with distinct phenotypic and migratory characteristics. J Immunol. 2001;167:212–20. doi: 10.4049/jimmunol.167.1.212. [DOI] [PubMed] [Google Scholar]
- 16.Goronzy JJ, Lee WW, Weyand CM. Aging and T-cell diversity. Exp Gerontol. 2007;42:400–6. doi: 10.1016/j.exger.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almanzar G, Schwaiger S, Jenewein B, et al. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol. 2005;79:3675–83. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregg R, Smith CM, Clark FJ, et al. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin Exp Immunol. 2005;140:540–6. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fletcher JM, Vukmanovic-Stejic M, Dunne PJ, et al. CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J Immunol. 2005;175:8218–25. doi: 10.4049/jimmunol.175.12.8218. [DOI] [PubMed] [Google Scholar]
- 20.Pourgheysari B, Khan N, Best D, Bruton R, Nayak L, Moss PA. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J Virol. 2007;81:7759–65. doi: 10.1128/JVI.01262-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huppert FA, Solomou W, O'Connor S, Morgan K, Sussams P, Brayne C. Aging and lymphocyte subpopulations: whole-blood analysis of immune markers in a large population sample of healthy elderly individuals. Exp Gerontol. 1998;33:593–600. doi: 10.1016/s0531-5565(98)00033-3. [DOI] [PubMed] [Google Scholar]
- 22.Michie CA, McLean A, Alcock C, Beverley PC. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992;360:264–5. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- 23.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260–6. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 24.Carrasco J, Godelaine D, Van Pel A, Boon T, van der Bruggen P. CD45RA on human CD8 T cells is sensitive to the time elapsed since the last antigenic stimulation. Blood. 2006;108:2897–905. doi: 10.1182/blood-2005-11-007237. [DOI] [PubMed] [Google Scholar]
- 25.Kuijpers TW, Vossen MT, Gent MR, et al. Frequencies of circulating cytolytic, CD45RA+CD27-, CD8+ T lymphocytes depend on infection with CMV. J Immunol. 2003;170:4342–8. doi: 10.4049/jimmunol.170.8.4342. [DOI] [PubMed] [Google Scholar]
- 26.Fagnoni FF, Vescovini R, Passeri G, et al. Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood. 2000;95:2860–8. [PubMed] [Google Scholar]
- 27.Desbarats J, Wade T, Wade WF, Newell MK. Dichotomy between naive and memory CD4(+) T cell responses to Fas engagement. Proc Natl Acad Sci USA. 1999;96:8104–9. doi: 10.1073/pnas.96.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akbar AN, Vukmanovic-Stejic M, Taams LS, Macallan DC. The dynamic co-evolution of memory and regulatory CD4+ T cells in the periphery. Nat Rev Immunol. 2007;7:231–7. doi: 10.1038/nri2037. [DOI] [PubMed] [Google Scholar]
- 29.Miyaji C, Watanabe H, Minagawa M, et al. Numerical and functional characteristics of lymphocyte subsets in centenarians. J Clin Immunol. 1997;17:420–9. doi: 10.1023/a:1027324626199. [DOI] [PubMed] [Google Scholar]
- 30.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 31.Chidrawar SM, Khan N, Chan YL, Nayak L, Moss PA. Ageing is associated with a decline in peripheral blood CD56bright NK cells. Immun Ageing. 2006;3:10. doi: 10.1186/1742-4933-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–89. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chong Y, Ikematsu H, Yamaji K, et al. CD27(+) (memory) B cell decrease and apoptosis-resistant CD27(-) (naive) B cell increase in aged humans: implications for age-related peripheral B cell developmental disturbances. Int Immunol. 2005;17:383–90. doi: 10.1093/intimm/dxh218. [DOI] [PubMed] [Google Scholar]
- 34.Esser MT, Dinglasan RD, Krishnamurthy B, Gullo CA, Graham MB, Braciale VL. IL-2 induces Fas ligand/Fas (CD95L/CD95) cytotoxicity in CD8+ and CD4+ T lymphocyte clones. J Immunol. 1997;158:5612–18. [PubMed] [Google Scholar]
- 35.Colonna-Romano G, Bulati M, Aquino A, et al. B cells in the aged: CD27, CD5, and CD40 expression. Mech Ageing Dev. 2003;124:389–93. doi: 10.1016/s0047-6374(03)00013-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.