SNO–Hemoglobin Not Essential for Red Blood Cell Dependent Hypoxic Vasodilation (original) (raw)
. Author manuscript; available in PMC: 2009 Jul 1.
Published in final edited form as: Nat Med. 2008 May 30;14(7):773–777. doi: 10.1038/nm1771
Introductory Paragraph
The coupling of hemoglobin sensing of physiological oxygen gradients to stimulation of nitric oxide (NO) bioactivity is an established principle of hypoxic blood flow. One mechanism proposed to explain this O2 sensing/NO bioactivity linkage postulates an essential role for the conserved hemoglobin β93Cys residue and, specifically, for S–nitrosation of β93Cys to form S–nitrosohemoglobin (SNO–Hb)1. The SNO–Hb hypothesis, which conceptually linked hemoglobin and NO biology, has been debated intensely in recent years2,3. This debate has precluded a consensus on physiological mechanisms and on assessment of the potential role of SNO–Hb in pathology. Here we describe novel mouse models that express exclusively either human wild type hemoglobin or human hemoglobin in which the β93cys residue is replaced with alanine to assess the role of SNO–Hb in red cell mediated hypoxic vasodilation. Substitution of this residue, precluding hemoglobin S–nitrosation, did not change total red cell S–nitrosothiol levels but shifted S–nitrosothiol distribution to lower MWt species, consistent with the loss of SNO–Hb. Loss of β93cys resulted in no deficits in systemic nor pulmonary hemodynamics under basal conditions and, importantly, did not affect isolated red cell dependent hypoxic vasodilation. These results demonstrate that SNO–Hb is not essential for the physiologic coupling of erythrocyte deoxygenation with increased NO–bioactivity in vivo.
In addition to hemoglobin oxygen affinity, blood flow is a key component of the processes that match oxygen delivery to demand. Increased blood flow in response to hypoxia is a critical physiological response which does not correlate with dissolved oxygen tensions but does correlate with hemoglobin oxygen fractional saturation4. These observations have led to the concept that the red blood cell (RBC) itself is a regulator of flow and to the general paradigm that RBC/hemoglobin deoxygenation is coupled to the stimulation of vasodilation1,5,6. Three mechanisms for this coupling have been proposed (illustrated in supplementary Figure 1) and involve either i) ATP–release and subsequent activation of endothelial nitric oxide synthase7, ii) nitrite reduction to NO by deoxyhemoglobin5 and iii) S–nitrosohemoglobin (SNO–Hb) dependent pathways1. In the latter proposal, hemoglobin becomes S–nitros(yl)ated on a specific and conserved cysteine residue on the β–chain (β93Cys) as RBCs become oxygenated in the lungs (forming SNO–Hb). In the R–state, SNO–Hb remains relatively unreactive but upon deoxygenation, T–state SNO–Hb can rapidly react with thiols [GSH or anion exchanger–1 (AE–1) thiols] and transmit a vasodilatory signal out of the RBC (suppl Fig 1)8–12. This hypothesis has been extended recently to suggest that dysfunction in this pathway leads to a variety of systemic vascular and pulmonary diseases and contributes to pathologic effects associated with diabetes, congestive heart failure and with transfusion of banked blood13–17. These pioneering studies also began to resolve a long standing question in hemoglobin biology, namely the role of the highly conserved β93Cys residue. However, the function of SNO–Hb in vivo and the potential role in disease has been debated intensely over the past few years. Many aspects of the SNO–Hb hypothesis have been disputed including the measurements of SNO–Hb concentrations and the mechanisms of SNO formation and bioactivity (suppl Figure 1).
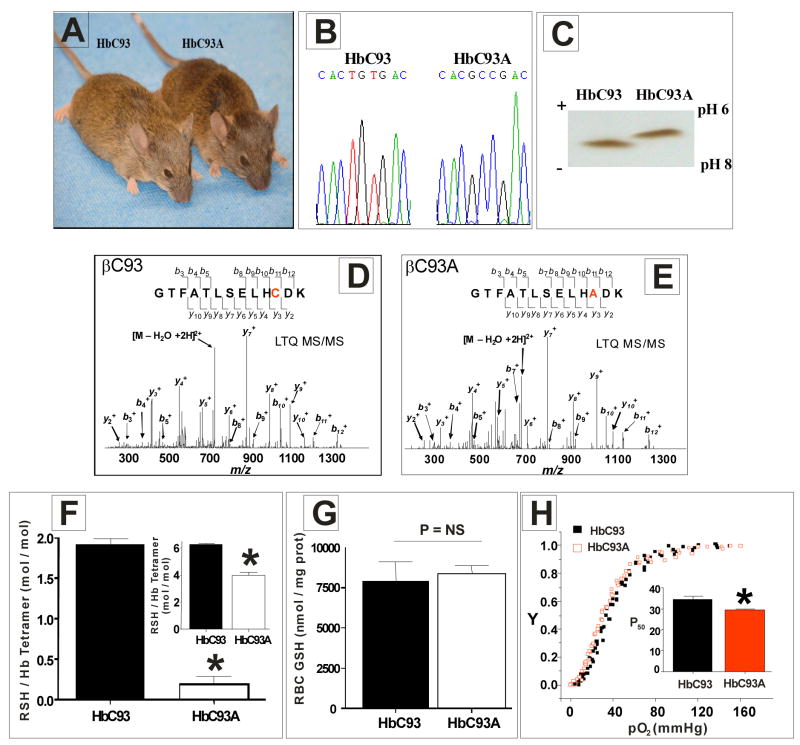
To directly address this issue and explore the role of the conserved β93cys residue, we created knockin mouse models in which RBCs contained exclusively wild–type (WT) human hemoglobin (HbC93) or exclusively human hemoglobin in which the β93cys residue was replaced with Ala (HbC93A) (Fig. 1a) (see suppl Fig. 2a, b for knockin scheme). Sequencing of mouse genomic DNA (Fig. 1b), isoelectric focusing (IEF) of hemolysates (Fig. 1c), β–globin protein sequencing by mass spectrometry (Fig. 1d, e) and a decrease of 2 reduced thiols per hemoglobin tetramer (Fig. 1f and inset) demonstrate unequivocally that β93cys was changed to alanine. No change in total glutathione levels (Fig. 1g) was observed suggesting no compensatory up–regulation of the other major thiol pool in RBC. Finally, replacement of β93cys with Ala resulted in a modest, but significant increase in RBC oxygen affinity (Fig. 1h and inset) consistent with cell–free recombinant hemoglobin studies18.
Figure 1. Genotypic, phenotypic and biochemical characterization of HbC93 and HbC93A mice.
a) HbC93 and HbC93A knockin mice. The animals are phenotypically indistinguishable. b) Genomic DNA sequence analysis of the β–globin gene in HbC93 and HbC93A knockin mice. The animals are homozygous for human βC93 or βC93A genes. c) Isoelectric focusing gel analysis of hemolysates from HbC93 and HbC93A mice. The animals synthesize a single, human adult hemoglobin consistent with HbC93 and HbC93A, respectively. d) and e) Mass spectrometry analysis (LTQ MS/MS) of βC93 and βC93A polypeptides respectively. Peptide sequence analysis unequivocally demonstrates the C93A substitution. f) Hemoglobin was isolated from HbC93 and HbC93A RBC, and surface accessible and total reduced thiols (inset) measured as described in methods using the DTNB assay. Consistent with 2 β93cys residues per hemoglobin tetramer, surface and total hemoglobin thiol decreased by 2 in HbC93A mice. Data show mean ± SEM (n= 3–4), * P = < 0.0002 relative to HbC93 hemoglobin by unpaired t–test. g) RBC concentrations of total GSH (reduced + oxidized) are not different between HbC93 and HbC93A RBC, Data shown mean ± SEM, P = 0.72, by unpaired t–test. NS= not significant. h) Hemoglobin oxygen affinity was measured in intact HbC93 or HbC93A RBC suspensions (0.3%Hct) in PBS, 37°C. Oxygen binding curves represent cumulative data points obtained from RBC isolated from 4 mice per group. Inset shows P50 values determined by fitting the oxygen binding curves to the Hill Equation. HbC93A RBC have lower P50 (increased oxygen affinity) compared to HbC93 RBC. Data shown mean ± SEM (n = 4), * P = < 0.006 by unpaired t–test.
Supplementary Table 1 shows hematological indices and urine concentrations in mice. Small increases in hemoglobin levels, red cell distribution widths, mean corpuscular volumes and mean corpuscular hemoglobin were observed in HbC93A compared to HbC93 mice. However, reticulocyte levels, which are the most sensitive indicator of anemia in mice, were equivalent in HbC93A and HbC93 mice. No differences in phenotypic evaluations (weight and visual inspection of whole organs) or systematic evaluation [hematoxylin and eosin stained tissues sections, (suppl table 1 and suppl Fig. 2–3)] of HbC93 and HbC93A mice were observed. Finally, embryonic development (suppl Fig. 2) and the size of litters derived from each group were not significantly different. These results suggest that the HbC93A mice develop normally.
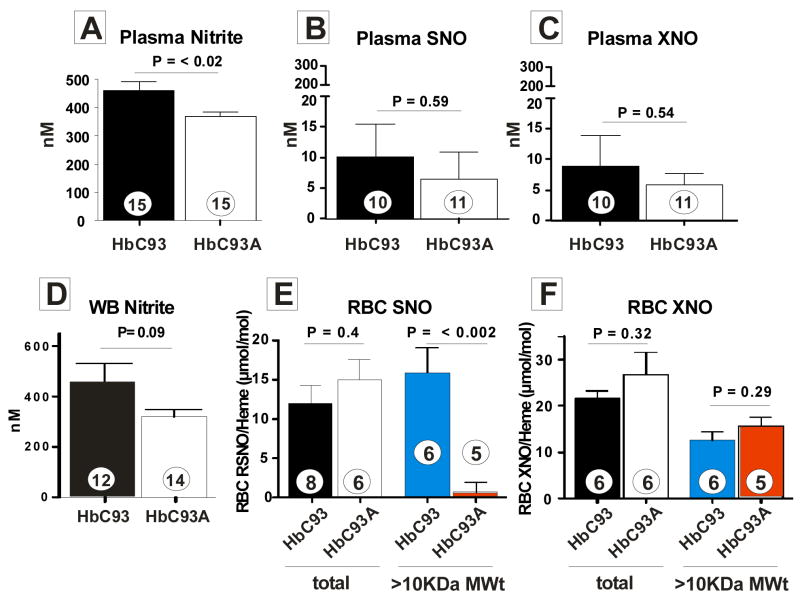
To evaluate the role of β93cys S–nitrosation in RBC–dependent modulation of NO–bioactivity, we first characterized basal nitric oxide metabolism in the circulation by measuring NO–metabolites nitrate, nitrite, S–nitrosothiols and C– or N–nitroso compounds (referred to as XNO) in the plasma and RBC. Total NO production (Fig. 2) in HbC93A and HbC93 mice is similar (nitrate levels were not different; 33.6 ± 10.6 μM in HbC93 mice and 29.5 ± 1.2 μM in HbC93A mice, n=3 per group). Interestingly, no differences in circulating NO–metabolites were observed except for plasma nitrite which was lower in HbC93A mice. A trend towards lower whole blood nitrite levels was also observed. Suppl Fig. 4 shows lower nitrite levels are not associated with different levels of eNOS expression nor activity. Recent studies show that loss of the β93cys residue increases the nitrite reductase activity of deoxyhemoglobin19,20, a result also observed with HbC93A RBC (not shown) and suggesting that this activity accounts for observed lower steady–state nitrite levels. Collectively, Fig. 2 suggests that loss of β93cys does not result in adaptive increases in endogenous NO formation. RBC levels of S–nitrosothiols (Fig. 2e) were not significantly different in HbC93A and HbC93 mice. Interestingly however, the distribution of S–nitrosothiols was altered. In HbC93 RBC, 78.2 ± 14.3% of the SNO’s were on the high Mwt fraction consistent with a predominantly hemoglobin localization. In the HbC93A RBCs, however, high MWt SNO’s were undetectable. More than 99% of SNO’s were in the low MWt fraction, most likely S–nitrosoglutathione (GSNO) underscoring a dynamic transnitrosation equilibrium between SNO–Hb and low Mwt thiols (as previously indicated)8,21 which in the absence of the β93cys is shifted to GSNO. This conclusion is further supported by the absence of any effect ofβ93cys substitution on XNO distribution in RBC (Fig 1f). In summary, the data in Fig. 1–2 demonstrate no functionally significant phenotypic or biochemical differences between HbC93 and HbC93A mice, except for a relatively small (~20%) decrease in circulating nitrite levels and re–distribution of RBC SNO’s from β93cys to a non–hemoglobin fraction.
Figure 2. Circulating nitric oxide metabolite profiles in HbC93 and HbC93A mice.
Panel A–F show different NO–metabolites in plasma, whole blood (WB) or RBC as indicated. Shown P–values were calculated by unpaired t–test with ‘n’ values indicated in each data bar and referring to number of mice. All values represent mean ± SEM. For SNO and XNO in RBC, samples lyzed and collected in stabilization solutions were divided into two aliquots, one of which was analyzed for total RBC SNO/XNO and the other fractionated on a gel–filtration column (Sephadex, G–25, using PBS + DTPA (10μM) as the eluant). The high MWt fraction (> 10KDa, predominantly hemoglobin) was collected and SNO/XNO measured and normalized to heme. RBC SNO concentrations (in packed RBC) were 214.6 ± 37nM and 147.9 ± 28.6 nM for HbC93A and HbC93 mice respectively, n=6). NO–metabolites were measured in plasma and RBC compartments as described in methods.
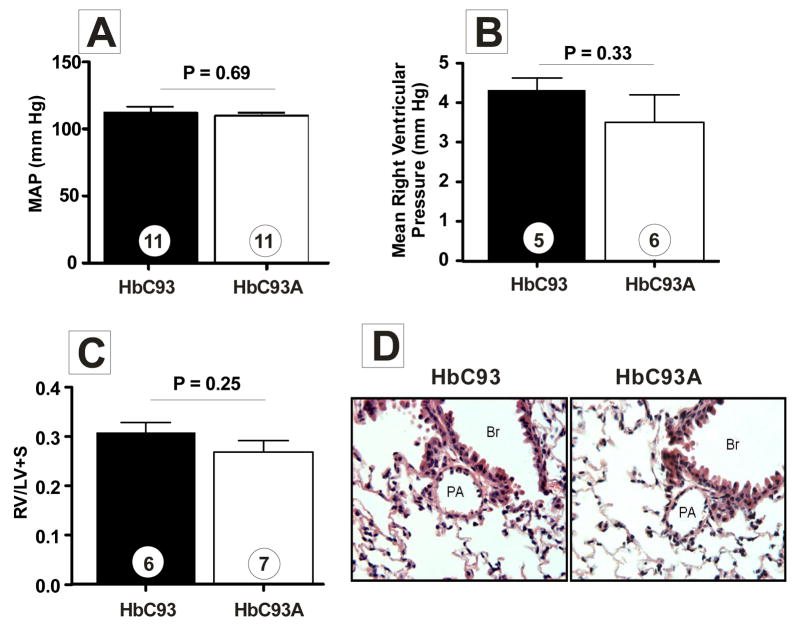
To evaluate potential hemodynamic effects of the altered RBC SNO profile, mean arterial pressure (Fig. 3a and suppl Fig. 5a), systolic pressure (suppl Fig. 5b), diastolic pressure (suppl Fig. 5c), and heart rate (suppl Fig. 5d) were measured. No differences between HbC93 and HbC93A mice were observed suggesting that SNO–Hb is not essential for physiologic modulation of blood pressure.
Figure 3. Loss of β93Cys affects neither systemic nor pulmonary hemodynamics.
A) Mean arterial blood pressure (MAP) was measured by tail–cuff on conscious mice. Note, similar MAP values were obtained in non–restrained mice via carotid artery catheters (suppl Fig B) shows mean right ventricular pressures in anesthetized mice. C) shows Fulton index (right ventricle (RV): left ventricle (LV) + septum (S) weight ratio) for assessing right ventricular hypertrophy secondary to pulmonary hypertension.. D) shows representative micrographs of H&E stained pulmonary arteries from HbC93 and HbC93A mice. No changes in vascular structure were observed (magnification = 40X). PA = pulmonary artery, Br = bronchus. Unpaired t–tests were used to evaluate significance with P–values and ‘n’ values shown on figure. In all cases data represent mean ± SEM
Recent studies suggest that depletion of SNO–Hb leads to pulmonary hypertension16. However, no differences in right ventricular pressures between HbC93 and HbC93A mice were observed (Fig. 3b). Moreover, assessment of right ventricular hypertrophy (Fig. 3c), or pulmonary arterial wall thickness (Fig. 3d), which are diagnostic features for pulmonary hypertension, showed no differences. Collectively these data indicate that loss of β93Cys and associated loss of SNO–Hb does not adversely impact systemic nor pulmonary hemodynamics. These data do not rule out a role for other RBC SNO’s22, but indicate that allosteric regulation of SNO–Hb bioactivity is not an essential component of physiologic mechanisms that control pulmonary blood flow.
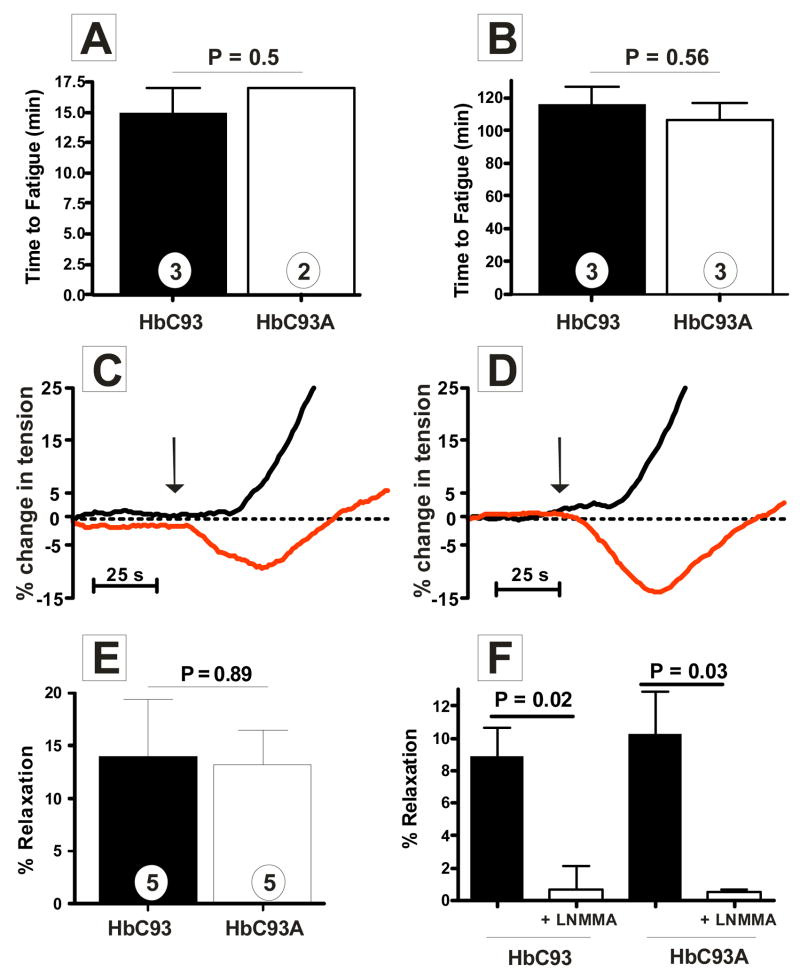
Given the multiple studies documenting a critical role for SNO–Hb, we were surprised by a lack of phenotype in β93cys deficient mice under physiological conditions. Since hypoxia is a critical element in the SNO–Hb hypothesis, we first considered that a role for this residue may only be evident under conditions of hypoxic stress. To test this, HbC93 and HbC93A mice were exposed to two types of exercise stress that model acute (analogous to sprinting) and chronic (analogous to long–distance running) exercise resulting in varying degrees of hypoxic stress. Figs. 4a, b demonstrate that HbC93 and HbC93A mice perform equally well at both exercise regimes suggesting that SNO–Hb is not essential for global exercise capacity. Adaptive responses of the vasculature, such as differences in capillary densities, could compensate in the absence of SNO–Hb; however no changes in capillary density in the heart were observed (suppl Fig. 6).
Figure 4. Effects ofβ93Cys on exercise tolerance and RBC dependent modulation of hypoxic vasodilation.
A) Mice were exposed to a graded exercise test and time to fatigue measured. Mice were allowed an initial warm up for 5 min at 10m/min and then speed increased by 3 meters every 3 minutes to a maximum speed of 30m/min. B) Mice were exposed to an endurance exercise test and time to fatigue determined. Mice were allowed to warm up for 10 minutes at 10m/min and speed increased to 15m/min for 30 minutes. Thereafter speed was increased by 3 meters every 30 minutes to a final of 27m/min. For A) and B) time to fatigue was recorded when mice could no longer maintain a running pace despite external stimuli (see methods). P–values shown were calculated by unpaired t–test and data represent mean ± SEM where shown with indicated ‘n’ values. Panels C) and D) are representative traces showing changes in tension of isolated rabbit pulmonary arteries exposed to HbC93 or HbC93A RBC respectively at 21% oxygen ( — ) or 0% oxygen ( — ). Pulmonary arteries were equilibrated at each oxygen tension for 5 min then pre treated with indomethacin (5μM) and pre–contracted with PE (200nM). Upon reaching a stable contractile tone RBC were added for a final 0.3 Hct in the baths (indicated by arrow). RBC from either HbC93 or HbC93A mice were collected by cardiac puncture, washed once with air equilibrated PBS (2000× g, 90s, 22°C) and then added to vessel bioassay chambers resulting in average times between blood collection and RBC addition of <5 mins. E) Average hypoxic vasodilation stimulated by either HbC93 or HbC93A RBC. F) Hypoxic vasodilation stimulated by HbC93 or HbC93A RBC (0.3%Hct) was measured in the absence or presence of LNMMA (1mM). Data show mean ± SEM (n=3). P–values calculated by unpaired t–test.
Finally, since multiple mechanisms contribute to regulation of hypoxic blood flow in vivo, which could be up–regulated to compensate for loss of SNO–Hb bioactivity, we tested the ability of isolated RBCs to stimulate hypoxic vasodilation responses using rabbit pulmonary arteries in vessel bioassay experiments. Previous studies have shown that addition of RBCs under oxygenated (21% oxygen) conditions constricts vessels (consistent with scavenging of endogenous NO) whereas RBC addition to vessels pre–equilibrated at ≤1% O2 (tensions which result in RBC deoxygenation) elicits a transient vasodilation response which is followed by vasoconstriction11. These data were pivotal in the generation of the SNO–Hb hypothesis. Fig. 4c demonstrates that addition of RBCs from HbC93 mice reproduce these data, with vasoconstriction observed at 21% oxygen but a transient vasodilation followed by vasoconstriction observed at 0% oxygen. Fig. 4d illustrates similar effects with HbC93A RBCs; the hypoxic vasodilation response with HbC93A and HbC93 RBCs are essentially identical (Fig. 4e) demonstrating that the β93Cys and hence SNO–Hb are not required for RBC–dependent hypoxic vasodilation. We have reported previously that under nitrite–free conditions, this dilation response, which has been ascribed to RBC SNO–Hb, is most likely due to ATP release from hypoxic RBCs19. In this model, ATP is released from hypoxic RBC, binds to endothelial purinergic receptors (P2Y) and thereby stimulates NO production by eNOS resulting in vasodilation7. Consistent with ATP release as the mediator of vasodilation with HbC93 and HbC93A RBCs, hypoxic vasodilation was inhibited by >90% in the presence of the eNOS inhibitor, LNMMA (1 mM) (Fig. 4f). It should be pointed out that hypoxic vasodilation responses ascribed to SNO–Hb have also been stated to occur with denuded vessels15,23. Denudation removes the endothelium, and hence eNOS, conceivably precluding ATP–dependent pathways. However, both HbC93 and HbC93A RBCs dilated denuded vessels to similar extents at low oxygen tensions (Suppl Fig. 7). Moreover, ATP also dilated denuded vessels (suppl. Fig 7). This response is most likely due to metabolism of ATP to adenosine, an endothelium independent vasodilator. A role for adenosine is indicated by significant inhibition of hypoxic RBC dependent dilation of denuded vessels by the adenosine receptor antagonist theophylline; this inhibition is equivalent with RBCs from HbC93 and HbC93A animals (suppl Fig. 7). We present this data i) to demonstrate that dilation of denuded vessels by hypoxic RBCs does not directly exclude ATP–dependent effects and that SNO–Hb is not required for vasodilation in this assay; and ii) to underscore the potential for SNO–Hb independent mechanisms for RBC–induced vasodilation. Further studies are required to determine how ATP–released from hypoxic RBC can be metabolized to adenosine and mediate dilation of denuded vessels.
In summary, our data demonstrate that i) β93cys is not essential for normal development or physiology under basal or exercise–stress conditions, ii) lack of SNO–Hb does not result in pulmonary hypertension under normal conditions and iii) SNO–Hb is not required for RBCs to stimulate hypoxic vasodilation ex–vivo. Although it is possible that the use of a humanized mouse model may mask a physiological effect of β93cys–deletion our studies strongly suggest that SNO–Hb does not play an essential role as a physiological regulator. We hypothesize that the conserved β93Cys residue plays a functional role in diseases characterized by vascular oxidative or nitrosative stress24–26. The use of HbC93 and HbC93A mouse models should provide important insights into the role of β93cys and SNO–Hb in these disorders.
Methods Summary
Detailed methods are provided in on–line supplementary material.
Production of knockin mice
The replacement of mouse alpha– and beta–globin genes with human alpha– and beta–globin genes was performed essentially as described27. See supplementary data for knockin scheme. All studies using animals were performed according to institutionally (IACUC) approved protocols.
Red Blood Cell Thiol Measurements
RBC glutathione, hemoglobin total and surface accessible reduced thiol were measured as described in supplementary information.
Hematology
Hematology profiles were determined as previously described27.
RBC–Hemoglobin Oxygen Affinity
Hemoglobin oxygen affinity in intact RBC was determined as previously described19.
Hemodynamics
Hemodynamic parameters were measured non–invasively using a specialized differential pressure transducer tail cuff (Kent Scientific). Right ventricular pressures and measurement of Fulton index to assess pulmonary hypertension were determined as previously described28.
Vessel Bioassays
Vessel studies were performed using female rabbit pulmonary arteries preconstricted with phenylephrine in Krebs Heinseleit buffer as previously described19. Whole blood from either HbC93 of HbC93A mice was collected and the RBC pellet washed once with PBS. A 0.3% final Hct of either HbC93 or HbC93A RBC was then immediately added to the vessel baths perfused with either 21% or 0% oxygen. Vasodilatory effects were determined by measuring the change in tension and expressing this as a percent relaxation with respect to the maximal PE constriction. Vessel bioassay experiments were performed in decreased lighting to protect photo–labile RSNO.
Measurement of Nitric Oxide Metabolites
NO–metabolites were measured as previously described29. For RBC measurements, samples were also passed through Sephadex G–25 gel–filtration column (pre–equilibrated with PBS + 10 μM DTPA) to assess S–nitrosothiol distribution between high Mwt (>10 kDa, hemoglobin containing) and low Mwt (<10kDa) fractions.
Exercise Stress Tests
Male mice were exposed to either graded or endurance exercise tests on a treadmill (Exer6M, Columbus Instruments) and in each case time to fatigue recorded when mice could no longer maintain a running pace despite external stimuli.
LC–MS/MS of globin polypeptides
See supplementary information for details.
Capillary Density Determination
Capillary density in the heart was determined as previously described30.
Supplementary Material
Supp data
Acknowledgments
This study was supported by grants from the US National Institutes of Health (HL057619) to T. Townes and from the American Heart Association, Southeast Affiliate (AHA 0655312B) to R. Patel and the Alabama Neuroscience Blueprint Core Center NS 057098. TS. Isbell was supported by a Cardiovascular Pathophysiology Training Fellowship. We also thank T. Lowder and M. Hewitt for technical assistance in exercise studies.
Footnotes
Author Contributions
TSI, CWS, LCW, XT, DAV and KMP were responsible for performing experiments. TSI, CWS, DAV, RPP and TMT were responsible for planning all experiments, analyzing data and writing manuscript. MBR contributed to mass spectrometry assays, LS was responsible for exercise related studies, CGK and BGB for capillary density measurements, NP and JW contributed to blood pressure measurements and NA for assessment of pulmonary hemodynamics. JR did the ES cell injections to generate the chimeras.
Author Information
Reprints and permissions information is available at npg.nature.com/reprints and permissions. Correspondence and requests for materials should be addressed to Tim Townes, ttownes@uab.edu
References
- 1.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S–nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 2.Crawford JH, Chacko BK, Kevil CG, Patel RP. The red blood cell and vascular function in health and disease. Antioxid Redox Signal. 2004;6:992–9. doi: 10.1089/ars.2004.6.992. [DOI] [PubMed] [Google Scholar]
- 3.Gladwin MT, Lancaster JR, Jr, Freeman BA, Schechter AN. Nitric oxide’s reactions with hemoglobin: a view through the SNO–storm. Nat Med. 2003;9:496–500. doi: 10.1038/nm0503-496. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez–Alonso J, Richardson RS, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol. 2001;530:331–41. doi: 10.1111/j.1469-7793.2001.0331l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gladwin MT, et al. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol Heart Circ Physiol. 2006;291:H2026–35. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 6.Ellsworth ML. The red blood cell as an oxygen sensor: what is the evidence? Acta Physiol Scand. 2000;168:551–9. doi: 10.1046/j.1365-201x.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 7.Sprague RS, Stephenson AH, Ellsworth ML. Red not dead: signaling in and from erythrocytes. Trends Endocrinol Metab. 2007;18:350–5. doi: 10.1016/j.tem.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Jia L, Bonaventura C, Bonaventura J, Stamler JS. S–nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–6. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 9.Stamler JS, et al. Blood flow regulation by S–nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–7. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 10.Pawloski JR, Hess DT, Stamler JS. Export by red blood cells of nitric oxide bioactivity. Nature. 2001;409:622–6. doi: 10.1038/35054560. [DOI] [PubMed] [Google Scholar]
- 11.McMahon TJ, et al. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8:711–7. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 12.Gow AJ, Stamler JS. Reactions between nitric oxide and haemoglobin under physiological conditions. Nature. 1998;391:169–73. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]
- 13.Datta B, et al. Red blood cell nitric oxide as an endocrine vasoregulator: a potential role in congestive heart failure. Circulation. 2004;109:1339–42. doi: 10.1161/01.CIR.0000124450.07016.1D. [DOI] [PubMed] [Google Scholar]
- 14.James PE, Lang D, Tufnell–Barret T, Milsom AB, Frenneaux MP. Vasorelaxation by red blood cells and impairment in diabetes: reduced nitric oxide and oxygen delivery by glycated hemoglobin. Circ Res. 2004;94:976–83. doi: 10.1161/01.RES.0000122044.21787.01. [DOI] [PubMed] [Google Scholar]
- 15.Bennett–Guerrero E, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104:17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMahon TJ, et al. A nitric oxide processing defect of red blood cells created by hypoxia: deficiency of S–nitrosohemoglobin in pulmonary hypertension. Proc Natl Acad Sci U S A. 2005;102:14801–6. doi: 10.1073/pnas.0506957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds JD, et al. S–nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci U S A. 2007;104:17058–62. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng Y, Shen TJ, Simplaceanu V, Ho C. Ligand binding properties and structural studies of recombinant and chemically modified hemoglobins altered at beta 93 cysteine. Biochemistry. 2002;41:11901–13. doi: 10.1021/bi0202880. [DOI] [PubMed] [Google Scholar]
- 19.Crawford JH, et al. Hypoxia, red blood cells, and nitrite regulate NO–dependent hypoxic vasodilation. Blood. 2006;107:566–74. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Z, et al. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115:2099–107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel RP, et al. Biochemical characterization of human S–nitrosohemoglobin. Effects on oxygen binding and transnitrosation. J Biol Chem. 1999;274:15487–92. doi: 10.1074/jbc.274.22.15487. [DOI] [PubMed] [Google Scholar]
- 22.Palmer LA, et al. S–nitrosothiols signal hypoxia–mimetic vascular pathology. J Clin Invest. 2007;117:2592–601. doi: 10.1172/JCI29444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pawloski JR, Hess DT, Stamler JS. Impaired vasodilation by red blood cells in sickle cell disease. Proc Natl Acad Sci U S A. 2005;102:2531–6. doi: 10.1073/pnas.0409876102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L, et al. Essential roles of S–nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–28. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 25.Crawford JH, et al. Transduction of NO–bioactivity by the red blood cell in sepsis: novel mechanisms of vasodilation during acute inflammatory disease. Blood. 2004;104:1375–82. doi: 10.1182/blood-2004-03-0880. [DOI] [PubMed] [Google Scholar]
- 26.Doctor A, et al. Hemoglobin conformation couples erythrocyte S–nitrosothiol content to O2 gradients. Proc Natl Acad Sci U S A. 2005;102:5709–14. doi: 10.1073/pnas.0407490102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu LC, et al. Correction of sickle cell disease by homologous recombination in embryonic stem cells. Blood. 2006;108:1183–8. doi: 10.1182/blood-2006-02-004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen YF, et al. Atrial natriuretic peptide–dependent modulation of hypoxia–induced pulmonary vascular remodeling. Life Sci. 2006;79:1357–65. doi: 10.1016/j.lfs.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 29.Lang JD, et al. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest. 2007;117:2583–2591. doi: 10.1172/JCI31892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chidlow JH, Jr, et al. Am J Pathol. 2006;169:2014–30. doi: 10.2353/ajpath.2006.051021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp data