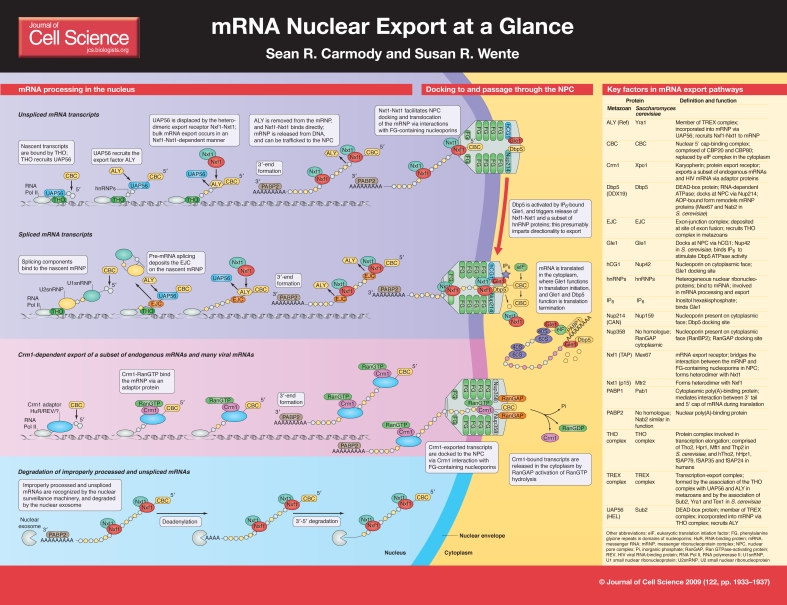
mRNA nuclear export at a glance (original) (raw)
Eukaryotic gene expression is controlled by multiple mechanisms, and its regulation is central for physiological responses to extracellular and intracellular signals. An essential step in this process involves the movement of mRNA transcripts from the site of synthesis in the nucleus to the cytoplasm, where they can be translated into proteins. The nuclear export of mRNA transcripts can be broken down into distinct stages: first, pre-mRNA is transcribed in the nucleus, where it is processed and packaged into messenger ribonucleoprotein (mRNP) complexes; second, the mRNPs are targeted to and translocate through nuclear pore complexes (NPCs) that are embedded in the nuclear envelope; and third, the mRNPs are directionally released into the cytoplasm for translation. Recent work has revealed that there is extensive mechanistic coupling between each of these steps (Kohler and Hurt, 2007). Moreover, it has been shown that perturbations in the factors that are essential for mRNA nuclear export have surprising links to different disease states. In this article and its accompanying poster, we provide an overview of the mRNA nuclear export pathway.
Figure 1.
Early mRNA processing events: assembly of mRNPs
The formation of an export-competent mRNP begins at transcription. During transcriptional elongation, the nascent mRNA transcript is bound by a number of factors, some of which are part of the family of heterogeneous nuclear ribonucleoproteins (hnRNPs). hnRNPs are highly abundant nuclear RNA-binding proteins that are essential for various steps in the mRNA life cycle, including packaging, export and translation. There are ∼30 different hnRNPs in humans, and about ten in Saccharomyces cerevisiae (Dreyfuss et al., 2002). Although some of these proteins have minimal sequence conservation from yeast to humans, several core functions are conserved. For example, human cells harbor a nuclear poly(A)-binding protein, PABP2 (Dreyfuss et al., 2002), and a cytoplasmic poly(A)-binding protein, PABP1 (Burd et al., 1991). In S. cerevisiae, Pab1 is a homologue of human PABP1 (Caponigro and Parker, 1995) but the nuclear poly(A)-binding protein, Nab2, is not structurally similar to human PABP2 (Anderson et al., 1993). Different hnRNP proteins associate with the mRNP at distinct steps in the export pathway (Dreyfuss et al., 2002). Notably, some hnRNPs harbor nuclear-retention signals and are removed prior to the export of the mRNP, whereas others are retained during export and released in the cytoplasm, and then are shuttled back into the nucleus.
Key steps in mRNA processing
There are four main processing events that occur during the formation of a mature mRNA transcript: 5′ capping, splicing, 3′-end cleavage and polyadenylation. Each of these modifications impacts export in two ways. First, if an mRNA is not properly processed, it will not be exported and instead will be targeted for degradation. Second, the processing events serve as triggers to recruit protein factors that are necessary for export.
The first change that a nascent pre-mRNA transcript undergoes is 5′ capping. When a transcript reaches about 20-30 nucleotides in length, a 7-methylguanosine cap is added to the 5′ end, which protects the nascent pre-mRNA from degradation (Shatkin and Manley, 2000). In microinjected Xenopus oocytes, uncapped mRNA is either poorly exported from the nucleus, or not exported at all (Cheng et al., 2006). The 5′ cap is bound by the cap-binding complex (CBC; composed of the proteins CBP20 and CBP80) (Izaurralde et al., 1995). Next, a transcript undergoes splicing, and a set of proteins is simultaneously deposited at the site of exon fusion. These proteins are defined as the exon-junction complex (EJC). Capping and splicing are both important for the recruitment of the transcription-export (TREX) complex (Cheng et al., 2006;Masuda et al., 2005). The TREX complex is highly conserved and is essential for mRNA export. It consists of the THO complex (which is made up of several components; see poster and table) and a set of export factors that include Sub2 (an ATP-dependent DEAD-box RNA helicase), Yra1 and Tex1 in S. cerevisiae and UAP56 (also known as HEL) and ALY (also known as REF) in human cells (Masuda et al., 2005;Piruat and Aguilera, 1998;Strasser et al., 2002). In_S. cerevisiae_, the THO complex associates with the nascent mRNA during transcription, and participates in both transcription elongation and mRNA export. Yeast strains that have mutations in the genes that encode any of the four THO-complex members have defects in mRNA export and transcription elongation and show an accumulation of transcripts in foci at or near their sites of transcription (Jimeno et al., 2002; Strasser et al., 2002). Once it is associated with the mRNA, the THO complex then recruits the remaining TREX-complex components (Strasser et al., 2002). Interestingly, in higher eukaryotes, the TREX complex is poorly recruited to transcripts that lack either the 5′ cap or the EJC, indicating that its mechanism of recruitment is linked to splicing and/or capping and not to transcription (Cheng et al., 2006; Zhou et al., 2000).
The final pre-mRNA processing events are 3′-end cleavage and polyadenylation. A polyadenylation site is recognized in the 3′-untranslated region (UTR), resulting in pre-mRNA cleavage immediately downstream. The poly(A) tail is added by a poly(A) polymerase and bound by a poly(A)-binding protein (Proudfoot, 2004). Notably, studies of Sub2 or THO-complex mutants have demonstrated that the 3′ end of genes is a site for the assembly of novel protein-DNA complexes that include NPC components (Rougemaille et al., 2008). This suggests that THO and Sub2 act at a step after 3′-end processing.
Recruitment of mRNA export factors, nuclear quality control and targeting to the NPC
The trafficking of most cargos that move between the nucleus and the cytoplasm involves karyopherin-mediated receptors, and transport directionality is determined by a gradient of the GTP-bound state of the small GTPase Ran (Madrid and Weis, 2006). In this regard, mRNA export is atypical in that it occurs by a mechanism that is distinct from that of proteins, tRNA or microRNA. Bulk mRNA is exported via the non-karyopherin heterodimer of Nxf1 (metazoan; also known as TAP; Mex67 in S. cerevisiae) and Nxt1 (metazoan; also known as p15; Mtr2 in S. cerevisiae), and does not rely on the RanGTP gradient (Herold et al., 2000;Segref et al., 1997). The Nxf1-Nxt1 heterodimer is recruited to the mRNP via the TREX component ALY. Early work in S. cerevisiae showed that Mex67-Mtr2 and Sub2 bind to the same domain in Yra1. As such, Sub2 could recruit Yra1 to the mRNP and then be displaced by Mex67-Mtr2 (Strasser and Hurt, 2001). Furthermore, recent work in vertebrates shows that the binding of the Yra1 homologue, ALY, to mRNA is stimulated by the presence of the ATP-bound form of the Sub2 homologue UAP56. This binding increases the ATPase activity of UAP56 (Taniguchi and Ohno, 2008). Moreover, Nxf1 binds mRNA-associated ALY, forming a ternary complex, and the RNA-binding affinity of Nxf1 is increased in the presence of ALY. Cells that express an altered form of Nxf1 that binds to ALY but not to mRNA have defective mRNA nuclear export (Hautbergue et al., 2008). Taken together, these data suggest a model whereby ATP-bound UAP56 (S. cerevisiae Sub2) recruits ALY (S. cerevisiae Yra1) to the mRNP. ATP hydrolysis by UAP56 triggers the transfer of the mRNA to ALY. Next, Nxf1-Nxt1 (S. cerevisiae Mex67-Mtr2) binds to ALY, which causes another transfer event and results in an mRNP with bound export receptor. It is unknown how many receptors must bind a single mRNA for efficient export to occur.
Notably, whereas bulk mRNA export occurs via the Nxf1 pathway, a subset of endogenous transcripts is exported via the karyopherin Crm1 (Xpo1 in S. cerevisiae). Crm1 also mediates the export of unspliced, or partially spliced, HIV mRNA via a virally encoded adaptor protein, Rev (Cullen, 2003;Fischer et al., 1995). Crm1 is not an RNA-binding protein, and thus must use an adaptor for the export of endogenous mRNAs, as it does for HIV transcripts. Some possible adaptors have been reported, including HuR for the export of Cd83 and Fos mRNAs, and eukaryotic translation initiation factor 4E (eIF4e) for cyclin D1 mRNA in human cells (Brennan et al., 2000; Culjkovic et al., 2006; Prechtel et al., 2006). However, adaptors for other potential Crm1-exported transcripts have yet to be discovered.
Therefore, correct nuclear processing and recruitment of an export factor targets an mRNA for export from the nucleus. However, if a transcript is not properly processed, it can be recognized by the nuclear surveillance machinery, retained in the nucleus and degraded by the nuclear exosome. This has been documented in elegant studies of mutants that are defective in mRNA splicing, export and polyadenylation (Brodsky and Silver, 2000;Hilleren et al., 2001;Lei and Silver, 2002;Libri et al., 2002;Zenklusen et al., 2002).
Docking to and passage through the NPC
Following completion of proper nuclear processing and the recruitment of an export receptor, an mRNP is considered to be export competent. This export-competent mRNP is specifically targeted to the NPC via its export receptor. For some transcribed genes, the positioning of the respective chromatin region near the NPC might facilitate export by physically linking the processes (reviewed by Akhtar and Gasser, 2007). Such a mechanism is described in an early gene-gating model (Blobel, 1985).
The export receptor docks at the NPC by interacting with a discrete class of NPC proteins known as the FG-Nups, which have been thus designated based on the presence of distinct domains containing multiple repeats of the amino acids phenylalanine (F) and glycine (G), separated by characteristic spacer sequences (Alber et al., 2007;Denning et al., 2003). Specific subtypes of FG repeats include FxFG and GLFG (L, leucine). Approximately one third of the 30 proteins that make up the NPC are FG-Nups. These assemble into peripheral substructures on the cytoplasmic and nuclear NPC faces and throughout the NPC central channel (Alber et al., 2007). Both the karyopherin receptors and Nxf1-Nxt1 mRNA export receptor directly bind to the FG repeats in domains that are distinct from their respective cargo or mRNP-binding domains (reviewed by Stewart, 2007). Thus, the export receptor serves to bridge the interaction of the mRNP and the NPC.
Binding of the export receptor to the FG-Nups is required for NPC docking and translocation of the mRNP. Studies of the Balbiani ring mRNP in_Chironomus tentans_ showed that the 5′ end of the mRNP docks first to the NPC nuclear face, with extrusion through the NPC proceeding with the 5′ end leading (Visa et al., 1996). Whether this `5′ first' mechanism is utilized by smaller mRNPs is unknown [the Balbiani ring mRNP measures ∼50 nm in diameter (Mehlin et al., 1992)]. The binding of transport receptors to the FG-Nups is thought to mediate the movement of the mRNP through the NPC by some type of facilitated diffusion mechanism (Weis, 2007). It is commonly agreed that the FG-Nups themselves do not provide directionality to translocation. Interestingly, however, work has revealed that a specific subset of the FG-Nups is required for Mex67-Mtr2-mediated mRNA export in S. cerevisiae (Terry and Wente, 2007). Crucial FG-Nup-binding sites for mRNA export are found on the nucleoplasmic face and in the central channel of the NPC. Moreover, Mex67-Mtr2 apparently utilizes a set of FG-Nups that are distinct from those used by several key karyopherins. Notably, the docking of transcripts that are exported in a Crm1-dependent manner is mediated by the interaction between Crm1 and FG-Nups.
Release into the cytoplasm and links to translation
The final step of mRNP translocation through the NPC involves directional release into the cytoplasm. Because mRNA export mediated by Nxf1-Nxt1 is not dependent on the RanGTP gradient, an alternative mechanism must determine directionality. Recent work in S. cerevisiae provides compelling evidence that the directionality of cytoplasmic release is determined by the function of two conserved, essential mRNA export factors, Dbp5 and Gle1, and soluble inositol hexakisphosphate (IP6) (Alcazar-Roman et al., 2006;Weirich et al., 2006). Dbp5 is an RNA-dependent ATPase of the DEAD-box protein family, and binds to the NPC cytoplasmic face by interacting with the NPC protein Nup214 (also known as CAN; Nup159 in S. cerevisiae) (Schmitt et al., 1999;Snay-Hodge et al., 1998;Tseng et al., 1998;Weirich et al., 2004). Gle1 specifically binds to IP6 and docks to a neighboring NPC protein hCG1 (Nup42 in S. cerevisiae) (Alcazar-Roman et al., 2006;Kendirgi et al., 2005;Murphy and Wente, 1996;Strahm et al., 1999). As an mRNP reaches the cytoplasmic side of the NPC, it associates with Gle1 and Dbp5. It is possible that Dbp5 is also cotranscriptionally recruited to the mRNP (Zhao et al., 2002). IP6-bound Gle1 stimulates the ATPase activity of Dbp5, thereby converting Dbp5 from an ATP- to ADP-bound state (Tran et al., 2007). It is thought that a conformational change induced by the Dbp5-ATP to Dbp5-ADP switch triggers the removal of a subset of proteins from the mRNP, including the export receptor Mex67 and the poly(A)-binding protein Nab2 (von Moeller et al., 2009). This changes the protein composition of the mRNP (Lund and Guthrie, 2005;Tran and Wente, 2006;Tran et al., 2007). As such, spatially controlled remodeling of the mRNP being exported confers the export directionality with removal of the export receptor. The number of Dbp5 ATP hydrolysis cycles that occur per mRNP transported, and the specificity mechanism for selective remodeling, are unknown. In contrast to mRNA export mediated by Nxt1-Nxf1 (Mex67-Mtr2), the release of Crm1-bound transcripts into the cytoplasm occurs with the hydrolysis of RanGTP.
The proteins that are removed by Dbp5 are recycled via import into the nucleus for another round of mRNA export. In addition, as the mRNP enters the cytoplasm, specific cytoplasmic mRNA-binding proteins are incorporated. Studies of Balbiani ring mRNP export find that the binding of cytoplasmic factors occurs immediately with entry of the 5′ end of the transcript into the cytoplasm; the CBC is replaced with eIF4e, ribosomes bind and translation begins before the entire mRNP has been extruded from the NPC (Daneholt, 2001). Interestingly, Dbp5, Gle1 and IP6 also have roles in translation (Bolger et al., 2008;Gross et al., 2007). Assembly of the termination complex on the mRNA might require Gle1-IP6-dependent stimulation of Dbp5, and Gle1 also has a distinct role in translation initiation. These links to translation further show the inherent connections between steps in gene expression.
Links to disease and development
Based on the essential roles of mRNA export in proper gene expression, an increasing number of connections to human disease and development are being discovered. Distinct pathophysiological states are correlated with defective mRNA export mechanisms. This includes perturbations resulting from mutations in genes encoding export factors or mRNA-binding proteins, from mutations in genes that cause inhibition of proper export of their own transcripts, and from downregulation or hijacking of the endogenous mRNA export machinery by viruses to allow specific viral gene expression. Examples of each are discussed below.
Recent work shows that LCCS1, a fetal motor neuron disease, is linked to mutations in human GLE1 (Nousiainen et al., 2008). The molecular mechanism for the Gle1 defect and the basis for the neuron-specific defects await analysis. In addition, the fragile X mental retardation protein, FMRP, interacts with an Nxf1 homologue, Nxf2, and destabilizes Nxf1 mRNA in neurons, presumably leading to a decrease in the protein levels of this mRNA export factor (Zhang et al., 2007). Such downregulation of the mRNA export receptor Nxf1 could potentially alter the pool of exported transcripts if Nxf1 and Nxf2 target different subsets of mRNAs, or if the two proteins export some transcripts more efficiently than others. Mutations of genes encoding specific export factors have also been shown to play a role in vertebrate development. Nxf2 is required for cardiac development in zebrafish (Huang et al., 2005), although the precise mechanism for this cardiac-specific phenotype is not fully known. Another disease that is associated with aberrant mRNA export is osteogenesis imperfecta type I (OI) (Hurt and Silver, 2008). In patients with OI, the mutation of one of the two genes encoding collagen, COL1A1 or COL1A2, results in bone fragility. Whereas multiple mutations in the coding region of collagen genes are linked to OI, a specific splice-site mutation in one cohort of patients has been reported to result in defective splicing and nuclear retention of collagen mRNA, thus lowering collagen expression (Johnson et al., 2000;Stover et al., 1993). Taken together, these data support the idea that defects in the mRNA nuclear export pathway can have direct consequences on human health and development.
Nucleocytoplasmic transport and mRNA nuclear export are essential for the proliferation of viruses that depend on nuclear replication (Fontoura et al., 2005;Greber and Fornerod, 2005). This includes DNA tumor viruses, RNA viruses, DNA retroviruses, and RNA retrotransposons, retroviruses and some negative-sense viruses. In regard to viral mRNA export, there are several distinct mechanisms that target the endogenous mRNA export machinery (Cullen, 2003; Fontoura et al., 2005). For example, HIV-encoded mRNA is exported via the karyopherin Crm1 (Cullen, 2003; Fischer et al., 1995; Greber and Fornerod, 2005), but also potentially utilizes the endogenous DEAD-box protein DDX3 for efficient mRNA export (Yedavalli et al., 2004). By contrast, to efficiently export its mRNA, influenza virus expresses the NS1 protein that forms inhibitory complexes with essential export factors, including Nxf1-Nxt1, and blocks the export of endogenous mRNA (Satterly et al., 2007). In this manner, the influenza virus ensures that its transcripts are preferentially exported. More defined and continued analyses of how viruses utilize and target the endogenous mRNA export machinery could potentially yield targets for future drug development.
Conclusion
Many outstanding questions remain in the field of mRNA nuclear export. It is currently unclear whether the transport of every mRNA occurs via the paradigm outlined above. The precise biochemical determinants of an export-competent mRNP, and whether they are the same for every mRNA, have not been fully defined. Genetic and biochemical studies in S. cerevisiae have hinted that there are distinct, differential requirements for export competency that are defined by the presence of different mRNA-associated hnRNPs (Duncan et al., 2000;Guisbert et al., 2005). Another outstanding issue in the field is whether mRNA export depends on the same core set of export factors and occurs in precisely the same manner in every organism, or in every cell type. Although the export factors discussed here are highly conserved, studies of different model organisms have provided potentially conflicting reports of their absolute necessity. For example, Mex67 is essential for mRNA export in the budding yeast S. cerevisiae (Segref et al., 1997), but not in the fission yeast Schizosaccharomyces pombe (Yoon et al., 2000); Yra1 is essential for export in S. cerevisiae (Strasser and Hurt, 2000), but not in Drosophila or Caenorhabditis elegans (Gatfield and Izaurralde, 2002; Longman et al., 2003). A thorough understanding of mRNA export will require analysis of these species-specific differences. Future studies offer the promise of insights into the mRNA export mechanism that will impact studies of overall gene expression regulation in both normal human development and pathophysiology.
This work was supported by NIH grant (R01-GM051219) to S.R.W. and NRSA NIH position (5T32-GM08554) to S.R.C. Deposited in PMC for release after 12 months.
References
- Akhtar, A. and Gasser, S. M. (2007). The nuclear envelope and transcriptional control. Nat. Rev. Genet. 8, 507-517. [DOI] [PubMed] [Google Scholar]
- Alber, F., Dokudovskaya, S., Veenhoff, L. M., Zhang, W., Kipper, J., Devos, D., Suprapto, A., Karni-Schmidt, O., Williams, R., Chait, B. T. et al. (2007). The molecular architecture of the nuclear pore complex. Nature 450, 695-701. [DOI] [PubMed] [Google Scholar]
- Alcazar-Roman, A. R., Tran, E. J., Guo, S. and Wente, S. R. (2006). Inositol hexakisphosphate and Gle1 activate the DEAD-box protein Dbp5 for nuclear mRNA export. Nat. Cell Biol. 8, 711-716. [DOI] [PubMed] [Google Scholar]
- Anderson, J. T., Wilson, S. M., Datar, K. V. and Swanson, M. S. (1993). NAB2: a yeast nuclear polyadenylated RNA-binding protein essential for cell viability. Mol. Cell. Biol. 13, 2730-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel, G. (1985). Gene gating: a hypothesis. Proc. Natl. Acad. Sci. USA 82, 8527-8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger, T. A., Folkmann, A. W., Tran, E. J. and Wente, S. R. (2008). The mRNA export factor Gle1 and inositol hexakisphosphate regulate distinct stages of translation. Cell 134, 624-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan, C. M., Gallouzi, I. E. and Steitz, J. A. (2000). Protein ligands to HuR modulate its interaction with target mRNAs in vivo. J. Cell Biol. 151, 1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky, A. S. and Silver, P. A. (2000). Pre-mRNA processing factors are required for nuclear export. RNA 6, 1737-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd, C. G., Matunis, E. L. and Dreyfuss, G. (1991). The multiple RNA-binding domains of the mRNA poly(A)-binding protein have different RNA-binding activities. Mol. Cell. Biol. 11, 3419-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caponigro, G. and Parker, R. (1995). Multiple functions for the poly(A)-binding protein in mRNA decapping and deadenylation in yeast. Genes Dev. 9, 2421-2432. [DOI] [PubMed] [Google Scholar]
- Cheng, H., Dufu, K., Lee, C. S., Hsu, J. L., Dias, A. and Reed, R. (2006). Human mRNA export machinery recruited to the 5′ end of mRNA. Cell 127, 1389-1400. [DOI] [PubMed] [Google Scholar]
- Culjkovic, B., Topisirovic, I., Skrabanek, L., Ruiz-Gutierrez, M. and Borden, K. L. (2006). eIF4E is a central node of an RNA regulon that governs cellular proliferation. J. Cell Biol. 175, 415-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen, B. R. (2003). Nuclear mRNA export: insights from virology. Trends Biochem. Sci. 28, 419-424. [DOI] [PubMed] [Google Scholar]
- Daneholt, B. (2001). Assembly and transport of a premessenger RNP particle. Proc. Natl. Acad. Sci. USA 98, 7012-7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning, D. P., Patel, S. S., Uversky, V., Fink, A. L. and Rexach, M. (2003). Disorder in the nuclear pore complex: the FG repeat regions of nucleoporins are natively unfolded. Proc. Natl. Acad. Sci. USA 100, 2450-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss, G., Kim, V. N. and Kataoka, N. (2002). Messenger-RNA-binding proteins and the messages they carry. Nat. Rev. Mol. Cell. Biol. 3, 195-205. [DOI] [PubMed] [Google Scholar]
- Duncan, K., Umen, J. G. and Guthrie, C. (2000). A putative ubiquitin ligase required for efficient mRNA export differentially affects hnRNP transport. Curr. Biol. 10, 687-696. [DOI] [PubMed] [Google Scholar]
- Fischer, U., Huber, J., Boelens, W. C., Mattaj, I. W. and Luhrmann, R. (1995). The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell 82, 475-483. [DOI] [PubMed] [Google Scholar]
- Fontoura, B. M., Faria, P. A. and Nussenzveig, D. R. (2005). Viral interactions with the nuclear transport machinery: discovering and disrupting pathways. IUBMB Life 57, 65-72. [DOI] [PubMed] [Google Scholar]
- Gatfield, D. and Izaurralde, E. (2002). REF1/Aly and the additional exon junction complex proteins are dispensable for nuclear mRNA export. J. Cell Biol. 159, 579-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber, U. F. and Fornerod, M. (2005). Nuclear import in viral infections. Curr. Top. Microbiol. Immunol. 285, 109-138. [DOI] [PubMed] [Google Scholar]
- Gross, T., Siepmann, A., Sturm, D., Windgassen, M., Scarcelli, J. J., Seedorf, M., Cole, C. N. and Krebber, H. (2007). The DEAD-box RNA helicase Dbp5 functions in translation termination. Science 315, 646-649. [DOI] [PubMed] [Google Scholar]
- Guisbert, K. K., Duncan, K., Li, H. and Guthrie, C. (2005). Functional specificity of shuttling hnRNPs revealed by genome-wide analysis of their RNA binding profiles. RNA 11, 383-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautbergue, G. M., Hung, M. L., Golovanov, A. P., Lian, L. Y. and Wilson, S. A. (2008). Mutually exclusive interactions drive handover of mRNA from export adaptors to TAP. Proc. Natl. Acad. Sci. USA 105, 5154-5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold, A., Suyama, M., Rodrigues, J. P., Braun, I. C., Kutay, U., Carmo-Fonseca, M., Bork, P. and Izaurralde, E. (2000). TAP (NXF1) belongs to a multigene family of putative RNA export factors with a conserved modular architecture. Mol. Cell. Biol. 20, 8996-9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilleren, P., McCarthy, T., Rosbash, M., Parker, R. and Jensen, T. H. (2001). Quality control of mRNA 3′-end processing is linked to the nuclear exosome. Nature 413, 538-542. [DOI] [PubMed] [Google Scholar]
- Huang, H., Zhang, B., Hartenstein, P. A., Chen, J. N. and Lin, S. (2005). NXT2 is required for embryonic heart development in zebrafish. BMC Dev. Biol. 5, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt, J. A. and Silver, P. A. (2008). mRNA nuclear export and human disease. Dis. Model. Mech. 1, 103-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaurralde, E., Lewis, J., Gamberi, C., Jarmolowski, A., McGuigan, C. and Mattaj, I. W. (1995). A cap-binding protein complex mediating U snRNA export. Nature 376, 709-712. [DOI] [PubMed] [Google Scholar]
- Jimeno, S., Rondon, A. G., Luna, R. and Aguilera, A. (2002). The yeast THO complex and mRNA export factors link RNA metabolism with transcription and genome instability. EMBO J. 21, 3526-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, C., Primorac, D., McKinstry, M., McNeil, J., Rowe, D. and Lawrence, J. B. (2000). Tracking COL1A1 RNA in osteogenesis imperfecta. Splice-defective transcripts initiate transport from the gene but are retained within the SC35 domain. J. Cell Biol. 150, 417-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendirgi, F., Rexer, D. J., Alcazar-Roman, A. R., Onishko, H. M. and Wente, S. R. (2005). Interaction between the shuttling mRNA export factor Gle1 and the nucleoporin hCG1: a conserved mechanism in the export of Hsp70 mRNA. Mol. Biol. Cell 16, 4304-4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler, A. and Hurt, E. (2007). Exporting RNA from the nucleus to the cytoplasm. Nat. Rev. Mol. Cell. Biol. 8, 761-773. [DOI] [PubMed] [Google Scholar]
- Lei, E. P. and Silver, P. A. (2002). Intron status and 3′-end formation control cotranscriptional export of mRNA. Genes Dev. 16, 2761-2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libri, D., Dower, K., Boulay, J., Thomsen, R., Rosbash, M. and Jensen, T. H. (2002). Interactions between mRNA export commitment, 3′-end quality control, and nuclear degradation. Mol. Cell. Biol. 22, 8254-8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longman, D., Johnstone, I. L. and Caceres, J. F. (2003). The Ref/Aly proteins are dispensable for mRNA export and development in Caenorhabditis elegans. RNA 9, 881-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund, M. K. and Guthrie, C. (2005). The DEAD-box protein Dbp5p is required to dissociate Mex67p from exported mRNPs at the nuclear rim. Mol. Cell 20, 645-651. [DOI] [PubMed] [Google Scholar]
- Madrid, A. S. and Weis, K. (2006). Nuclear transport is becoming crystal clear. Chromosoma 115, 98-109. [DOI] [PubMed] [Google Scholar]
- Masuda, S., Das, R., Cheng, H., Hurt, E., Dorman, N. and Reed, R. (2005). Recruitment of the human TREX complex to mRNA during splicing. Genes Dev. 19, 1512-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlin, H., Daneholt, B. and Skoglund, U. (1992). Translocation of a specific premessenger ribonucleoprotein particle through the nuclear pore studied with electron microscope tomography. Cell 69, 605-613. [DOI] [PubMed] [Google Scholar]
- Murphy, R. and Wente, S. R. (1996). An RNA-export mediator with an essential nuclear export signal. Nature 383, 357-360. [DOI] [PubMed] [Google Scholar]
- Nousiainen, H. O., Kestila, M., Pakkasjarvi, N., Honkala, H., Kuure, S., Tallila, J., Vuopala, K., Ignatius, J., Herva, R. and Peltonen, L. (2008). Mutations in mRNA export mediator GLE1 result in a fetal motoneuron disease. Nat. Genet. 40, 155-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piruat, J. I. and Aguilera, A. (1998). A novel yeast gene, THO2, is involved in RNA pol II transcription and provides new evidence for transcriptional elongation-associated recombination. EMBO J. 17, 4859-4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prechtel, A. T., Chemnitz, J., Schirmer, S., Ehlers, C., Langbein-Detsch, I., Stulke, J., Dabauvalle, M. C., Kehlenbach, R. H. and Hauber, J. (2006). Expression of CD83 is regulated by HuR via a novel cis-active coding region RNA element. J. Biol. Chem. 281, 10912-10925. [DOI] [PubMed] [Google Scholar]
- Proudfoot, N. (2004). New perspectives on connecting messenger RNA 3′ end formation to transcription. Curr. Opin. Cell Biol. 16, 272-278. [DOI] [PubMed] [Google Scholar]
- Rougemaille, M., Dieppois, G., Kisseleva-Romanova, E., Gudipati, R. K., Lemoine, S., Blugeon, C., Boulay, J., Jensen, T. H., Stutz, F., Devaux, F. et al. (2008). THO/Sub2p functions to coordinate 3′-end processing with gene-nuclear pore association. Cell 135, 308-321. [DOI] [PubMed] [Google Scholar]
- Satterly, N., Tsai, P. L., van Deursen, J., Nussenzveig, D. R., Wang, Y., Faria, P. A., Levay, A., Levy, D. E. and Fontoura, B. M. (2007). Influenza virus targets the mRNA export machinery and the nuclear pore complex. Proc. Natl. Acad. Sci. USA 104, 1853-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt, C., von Kobbe, C., Bachi, A., Pante, N., Rodrigues, J. P., Boscheron, C., Rigaut, G., Wilm, M., Seraphin, B., Carmo-Fonseca, M. et al. (1999). Dbp5, a DEAD-box protein required for mRNA export, is recruited to the cytoplasmic fibrils of nuclear pore complex via a conserved interaction with CAN/Nup159p. EMBO J. 18, 4332-4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segref, A., Sharma, K., Doye, V., Hellwig, A., Huber, J., Luhrmann, R. and Hurt, E. (1997). Mex67p, a novel factor for nuclear mRNA export, binds to both poly(A)+ RNA and nuclear pores. EMBO J. 16, 3256-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin, A. J. and Manley, J. L. (2000). The ends of the affair: capping and polyadenylation. Nat. Struct. Biol. 7, 838-842. [DOI] [PubMed] [Google Scholar]
- Snay-Hodge, C. A., Colot, H. V., Goldstein, A. L. and Cole, C. N. (1998). Dbp5p/Rat8p is a yeast nuclear pore-associated DEAD-box protein essential for RNA export. EMBO J. 17, 2663-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, M. (2007). Ratcheting mRNA out of the nucleus. Mol. Cell 25, 327-330. [DOI] [PubMed] [Google Scholar]
- Stover, M. L., Primorac, D., Liu, S. C., McKinstry, M. B. and Rowe, D. W. (1993). Defective splicing of mRNA from one COL1A1 allele of type I collagen in nondeforming (type I) osteogenesis imperfecta. J. Clin. Invest. 92, 1994-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahm, Y., Fahrenkrog, B., Zenklusen, D., Rychner, E., Kantor, J., Rosbach, M. and Stutz, F. (1999). The RNA export factor Gle1p is located on the cytoplasmic fibrils of the NPC and physically interacts with the FG-nucleoporin Rip1p, the DEAD-box protein Rat8p/Dbp5p and a new protein Ymr 255p. EMBO J. 18, 5761-5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser, K. and Hurt, E. (2000). Yra1p, a conserved nuclear RNA-binding protein, interacts directly with Mex67p and is required for mRNA export. EMBO J. 19, 410-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser, K. and Hurt, E. (2001). Splicing factor Sub2p is required for nuclear mRNA export through its interaction with Yra1p. Nature 413, 648-652. [DOI] [PubMed] [Google Scholar]
- Strasser, K., Masuda, S., Mason, P., Pfannstiel, J., Oppizzi, M., Rodriguez-Navarro, S., Rondon, A. G., Aguilera, A., Struhl, K., Reed, R. et al. (2002). TREX is a conserved complex coupling transcription with messenger RNA export. Nature 417, 304-308. [DOI] [PubMed] [Google Scholar]
- Taniguchi, I. and Ohno, M. (2008). ATP-dependent recruitment of export factor Aly/REF onto intronless mRNAs by RNA helicase UAP56. Mol. Cell. Biol. 28, 601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry, L. J. and Wente, S. R. (2007). Nuclear mRNA export requires specific FG nucleoporins for translocation through the nuclear pore complex. J. Cell Biol. 178, 1121-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, E. J. and Wente, S. R. (2006). Dynamic nuclear pore complexes: life on the edge. Cell 125, 1041-1053. [DOI] [PubMed] [Google Scholar]
- Tran, E. J., Zhou, Y., Corbett, A. H. and Wente, S. R. (2007). The DEAD-box protein Dbp5 controls mRNA export by triggering specific RNA:protein remodeling events. Mol. Cell 28, 850-859. [DOI] [PubMed] [Google Scholar]
- Tseng, S. S., Weaver, P. L., Liu, Y., Hitomi, M., Tartakoff, A. M. and Chang, T. H. (1998). Dbp5p, a cytosolic RNA helicase, is required for poly(A)+ RNA export. EMBO J. 17, 2651-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visa, N., Izaurralde, E., Ferreira, J., Daneholt, B. and Mattaj, I. W. (1996). A nuclear cap-binding complex binds Balbiani ring pre-mRNA cotranscriptionally and accompanies the ribonucleoprotein particle during nuclear export. J. Cell Biol. 133, 5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Moeller, H., Basquin, C. and Conti, E. (2009). The mRNA export protein DBP5 binds RNA and the cytoplasmic nucleoporin NUP214 in a mutually exclusive manner. Nat. Struct. Mol. Biol. 16, 247-254. [DOI] [PubMed] [Google Scholar]
- Weirich, C. S., Erzberger, J. P., Berger, J. M. and Weis, K. (2004). The N-terminal domain of Nup159 forms a beta-propeller that functions in mRNA export by tethering the helicase Dbp5 to the nuclear pore. Mol. Cell 16, 749-760. [DOI] [PubMed] [Google Scholar]
- Weirich, C. S., Erzberger, J. P., Flick, J. S., Berger, J. M., Thorner, J. and Weis, K. (2006). Activation of the DExD/H-box protein Dbp5 by the nuclear-pore protein Gle1 and its coactivator InsP6 is required for mRNA export. Nat. Cell Biol. 8, 668-676. [DOI] [PubMed] [Google Scholar]
- Weis, K. (2007). The nuclear pore complex: oily spaghetti or gummy bear? Cell 130, 405-407. [DOI] [PubMed] [Google Scholar]
- Yedavalli, V. S., Neuveut, C., Chi, Y. H., Kleiman, L. and Jeang, K. T. (2004). Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell 119, 381-392. [DOI] [PubMed] [Google Scholar]
- Yoon, J. H., Love, D. C., Guhathakurta, A., Hanover, J. A. and Dhar, R. (2000). Mex67p of Schizosaccharomyces pombe interacts with Rae1p in mediating mRNA export. Mol. Cell. Biol. 20, 8767-8782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenklusen, D., Vinciguerra, P., Wyss, J. C. and Stutz, F. (2002). Stable mRNP formation and export require cotranscriptional recruitment of the mRNA export factors Yra1p and Sub2p by Hpr1p. Mol. Cell. Biol. 22, 8241-8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M., Wang, Q. and Huang, Y. (2007). Fragile X mental retardation protein FMRP and the RNA export factor NXF2 associate with and destabilize Nxf1 mRNA in neuronal cells. Proc. Natl. Acad. Sci. USA 104, 10057-10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J., Jin, S. B., Bjorkroth, B., Wieslander, L. and Daneholt, B. (2002). The mRNA export factor Dbp5 is associated with Balbiani ring mRNP from gene to cytoplasm. EMBO J. 21, 1177-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Z., Luo, M. J., Straesser, K., Katahira, J., Hurt, E. and Reed, R. (2000). The protein Aly links premessenger-RNA splicing to nuclear export in metazoans. Nature 407, 401-405. [DOI] [PubMed] [Google Scholar]