CRD-BP protects the coding region of βTrCP1 mRNA from miR-183-mediated degradation (original) (raw)
. Author manuscript; available in PMC: 2010 Jul 31.
Abstract
miRNAs are largely known to base-pair with the 3′UTR of target mRNAs, downregulating their stability and translation. mRNA of βTrCP1 ubiquitin ligase is very unstable, but unlike the majority of mRNAs where 3′UTR determines the rate of mRNA turnover, βTrCP1 mRNA contains _cis_-acting destabilizing elements within its coding region. Here we show that degradation of mRNA of βTrCP1 is miRNA-dependent, and identified miR-183 as a microRNA that interacts with the coding region of βTrCP1 mRNA. Argonaute2 interacts with the same region of βTrCP1 mRNA in miR-183-dependent manner. Inhibition of mir-183 function or disruption of mir-183-binding site stabilizes βTrCP1 mRNA and elevates βTrCP1 levels, resulting in activation of the SCFβTrCP E3 ubiquitin ligase. We have previously shown that RNA-binding protein, CRD-BP, binds to the coding region of βTrCP1 mRNA and stabilizes it. In this report we demonstrate that CRD-BP prevents degradation of βTrCP1 mRNA by attenuating its miR-183-dependent interaction with Ago2.
Introduction
miRNAs are a class of genome-encoded small regulatory RNAs, which, as a rule, suppress gene expression via post-transcriptional mechanisms including mRNA degradation or translational repression. Maturation of miRNAs starts with processing of long primary transcripts (pri-miRNAs) into hairpin intermediates, precursor miRNAs (pre-miRNAs) by RNase III Drosha-containing complex in the nucleus. Double-stranded 60–70 nucleotide (nt) pre-miRNAs are subsequently transported to the cytoplasm, where they undergo further cleavage by the RNAaseIII endonuclease Dicer. The resulting ~22 nt single-stranded miRNAs are incorporated into Ago2-containing ribonucleoprotein (RNP) effector complex, known as RNA-induced silencing complex (RISC) (Hutvagner and Simard, 2008). Perfect or nearly perfect base-paring of miRNA with the target mRNA sequence results in direct endonucleolytic cleavage of a duplex by Ago2, the catalytic center of RISC (Meister et al., 2004). Imperfect miRNA-mRNA interaction generally causes translational repression or mRNA destabilization. In mammalian cells, miRNAs are thought to attenuate the expression of approximately 30% of animal genes by forming imperfect duplexes at 3′UTR of mRNAs (reviewed in (Bushati and Cohen, 2007; Filipowicz et al., 2008)). However, little is known about the role of miRNA-mRNA interaction within the coding regions of mammalian mRNAs.
Beta-transducin repeats-containing protein 1 (βTrCP1) serves as the substrate recognition subunit for the SCFβTrCP E3 ubiquitin ligases. SCFβTrCP E3 ligases mediate ubiquitination and proteasomal degradation of phosphorylated substrates that play a key role in signal transduction including inhibitors of nuclear factor-κB (IκB), and β-catenin. Endogenous βTrCP are expressed at low levels and their abundance plays an important role in the regulation of activities of SCFβTrCP E3 ubiquitin ligases (reviewed in (Fuchs et al., 2004)). mRNA of βTrCP1 is very unstable and modulation of mRNA turnover plays a central role in the regulation of βTrCP1 expression (Noubissi et al., 2006; Spiegelman et al., 2000; Spiegelman et al., 2001). We have previously shown that mRNA-binding protein, CRD-BP (also known as IMP-1 or IGF2BP1), binds to the coding region of βTrCP1 mRNA and stabilizes it. This results in elevated levels of βTrCP1, increased activity of SCFβTrCP1 E3 ubiquitin ligase, and accelerated degradation of its substrates, including I_κ_Bα and β-catenin (Noubissi et al., 2006). However, the mechanisms responsible for the regulation of turnover of βTrCP1 mRNA by CRD-BP were unknown. In this report, we identified miR-183 as a microRNA that interacts with the coding region of βTrCP1 mRNA and destabilizes it via recruitment of Ago2-containing RISC complex. We have also shown that CRD-BP stabilizes βTrCP1 mRNA by attenuating its miR-183-dependent interaction with Ago2.
Result and Discussion
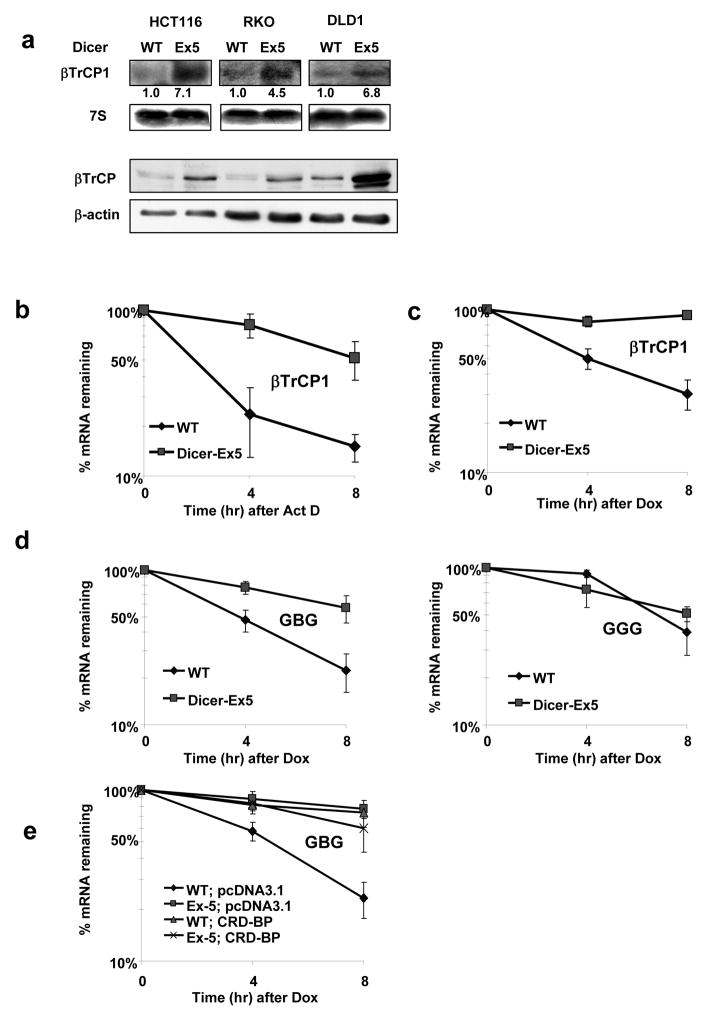
Given that instability determinants of mRNA of βTrCP1 are located within its coding region (Noubissi et al., 2006), we investigated whether degradation of this mRNA depends on miRNAs by using cells defective in Dicer1 function and thus miRNA maturation (Cummins et al., 2006). Steady-state levels and the half-life of βTrCP1 mRNA were dramatically increased in all three Dicer1Ex5/Ex5 cell lines examined (Figure 1a-c). Given that the chimeric construct containing the 577–1182 nt segment of the coding region of βTrCP1 mRNA inserted in-frame into the β-globin coding sequence (“GBG”, described in (Noubissi et al., 2006)) was also stabilized in Dicer1 hypomorphic cells (Figure 1d), it is likely that the structural miRNA-responding elements are located within, this segment. We have previously reported that elevated levels of RNA-binding protein, CRD-BP, increased the half-life of βTrCP1 mRNA (Noubissi et al., 2006). However, the stabilization of βTrCP1 mRNA in Dicer1Ex5/Ex5 cells appears to be independent of CRD-BP, since its levels were not increased in these cells (Figure S1a), and over-expression of CRD-BP did not affect the half-life of GBG mRNA (Figure 1e). These data suggest that the 577–1182 nt fragment of the coding region of βTrCP1 mRNA contains miRNA-binding site(s) that may be responsible for RISC-mediated degradation of mRNA of βTrCP1.
Figure 1. βTrCP1 mRNA is stabilized in DicerEx5/Ex5colorectal cancer cell lines.
a, Northern blot analysis of endogenous levels of βTrCP1 mRNA in HCT116, RKO, DLD1 Dicerwt (WT) and _Dicer_Ex5/Ex5 (Ex5) colorectal cancer cell lines. Immunoblot analysis of βTrCP in corresponding cell lines presented in the lower panel.
b, The stability of endogenous βTrCP1 mRNA in HCT116 cells, Dicer wild-type (WT) and DicerEx5/Ex5 (Ex5), was examined with real-time RT-PCR, by measuring _βTrCP1_mRNA levels at indicated time points after actinomycin D treatment (ActD, 10 μg/ml).
c, Dicerwt and DicerEx5/Ex5 DLD1 cells were co-transfected with Tet–off and p-BIG- βTrCP1 plasmids. Transcription was stopped by treatment with doxycycline (DOX) for the indicated durations. The stability of βTrCP1 transcripts was analyzed by measuring βTrCP1 mRNA levels with real-time RT-PCR.
d, Fusion transcripts GBG and GGG were expressed in HCT116 cells, Dicerwt (WT) and DicerEx5/Ex5 (Ex5) under the control of Tet-off system. The stability of indicated mRNAs was analyzed as in 1c.
e, Stability of GBG transcript expressed in DLD1wt and DLD1 DicerEx5/Ex5 cells transfected with the indicated constructs was analyzed as in 1c.
Data are represented as mean ±s.d..
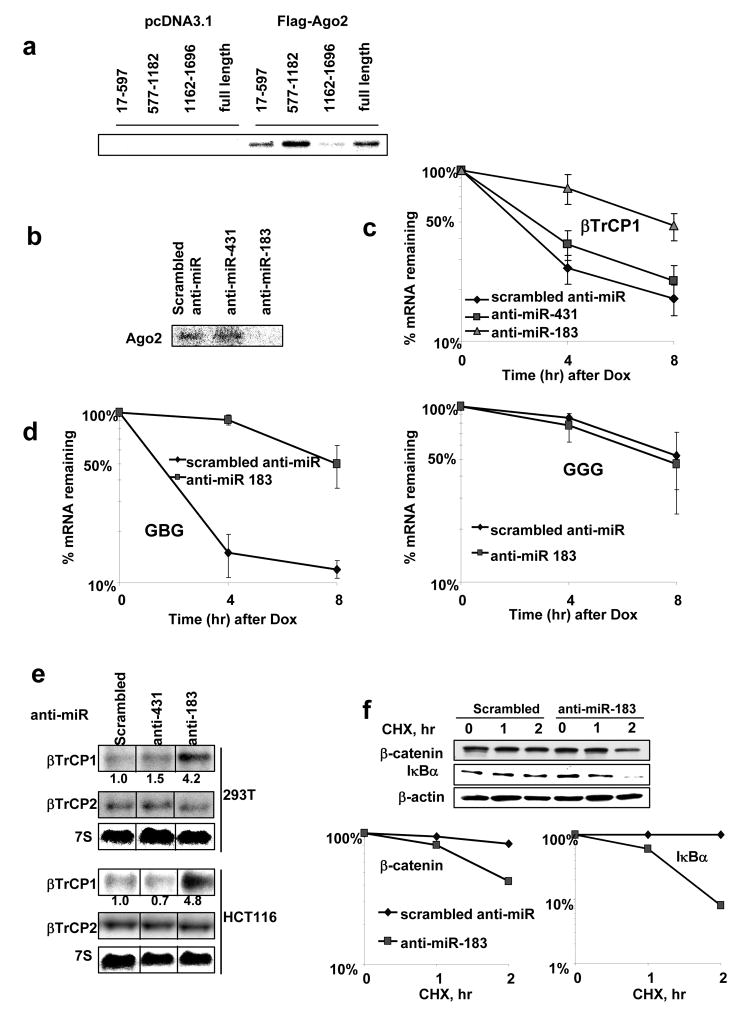
Ago2 mediates miRNA-dependent interaction of RISC complex with target mRNA(Meister et al., 2004). Data presented in Figure 2a show that Ago2 interacts with the 577–1182 nt fragment of coding region of βTrCP1 mRNA. Although Figure 2a shows some binding of Ago2 to 17–597 and 1162–1696 segments of βTrCP1 coding region, this interaction is comparable with the binding observed with control RNA (Figure S1b, d) and is considered non-specific. In comparison to Ago2, other members of Argonaute family (Ago1, Ago3, and Ago4) exhibit significantly weaker binding to 577–1182 nt fragment of βTrCP1 mRNA (Figure S1c). In order to identify miRNA-binding site(s), we performed detailed analysis of deletion mutants of the 577–1182 nt fragment and found that the major determinant of instability lies within 654–921 nt of the coding region (Figure S2a-c). This segment contains potential binding sites for two human miRNAs, miR-183 and miR-431 (identified with MIRANDA software http://www.microrna.org/). Interestingly, miR-183 is shown to be over-expressed in colorectal tumors and cell lines including HCT116, RKO, DLD1 used in this study (Bandres et al., 2006). Inhibition of miR-183, (but not miR-431) function attenuated the interaction of βTrCP1 mRNA with Ago2 protein (Figure 2b). In contrast to 577–1182 nt region, 17–597 and 1162–1696 nt regions exhibit weak interaction with Ago2 and this interaction is not affected by inhibition of miR-183 function (Figure S1d). Both full-length βTrCP1 mRNA and chimeric GBG mRNA were dramatically stabilized by anti-miR-183 (Figure 2c-d), suggesting that miR-183 is responsible for the coding region-dependent degradation of βTrCP1 mRNA. Stabilization of βTrCP1 mRNA by anti-miR-183 resulted in increased levels of endogenous βTrCP1 (but not its homologue, βTrCP2) mRNA in several cell lines tested (Figure 2e). Anti-miR-183 also elevated the expression of βTrCP protein in a dose-dependent manner (Figure S1e). Levels of βTrCP determine the stability of its substrates under the conditions of constitutive phosphorylation (Fuchs et al., 2004). Indeed, elevated levels of βTrCP1, achieved by inhibition of miR-183 function, accelerated the degradation of known βTrCP1 substrates such as β-catenin and IκBα (Figure 2f), inhibition of β-catenin-driven transcriptional activity and decreased expression of β-catenin/Tcf target genes (Figure S3a–c).
Figure 2. Inhibition of binding of miR-183 with the coding region of βTrCP1 mRNA stabilizes it and induces βTrCP1 expression and activity.
a, FLAG immunoprecipitation of UV–crosslinked RNP complexes. Protein extracts from 293T cells, transfected as indicated, were incubated with internally [32P]-labeled RNA transcripts of three fragments of βTrCP1 mRNA coding region and βTrCP1 mRNA full-length. RNP complexes were precipitated with anti-FLAG antibodies and analyzed on PAGE.
b, FLAG immunoprecipitation of UV–crosslinked RNP complexes were analyzed as above. 577–1182 nt. fragment of βTrCP1 mRNA coding region was incubated with protein extracts from 293T cells with the presence of inhibitors of miR-scrambled, miR-431 and miR-183.
c, 293T cells were transfected with Tet–off and p-BIG- βTrCP1 plasmids along with modified ssRNA oligos, treated with doxycycline (DOX) and harvested at indicated time points. Stability of full-length βTrCP1 mRNA transcripts in the presence of indicated anti-miRs was estimated by real-time RT-PCR.
d, Stability of fusion GBG and GGG transcripts in HCT116wtcells co-transfected with the indicated anti-miRs was analyzed with real-time PCR as described above.
e, Northern blot analysis of βTrCP1 and βTrCP2 expression in the cells transfected with indicated anti-miR.
f, Stability of β-catenin and IκBα proteins was analyzed by immunoblot of protein extracts isolated from 293T cells transfected as indicated and treated with cyclohexemide (40 μg/ml) for the indicated times and graphed.
Data are represented as mean ±s.d..
To further confirm the role of miR-183 in the turnover of βTrCP1 mRNA, we mutated 6 nucleotides in the mir-183-binding region of βTrCP1 mRNA without affecting its amino acid sequence. This mutation was predicted to disrupt the formation of a nearly perfect duplex between miR-183 and βTrCP1 mRNA (Figure 3a). Indeed, miR-183-mutant of the βTrCP1 577–1182 nt fragment, βTrCP1δmiR-183, failed to interact with Ago2 (Figure 3b). Our results show that Ago2 does not bind wild type βTrCP1 mRNA in cells with inhibited function of mir-183 (Figure S4a) further confirming the role of miR-183 in the interaction of Ago2 with the βTrCP1 577–1182 nt fragment. The Ago2 binding to βTrCP1 mRNA was also diminished in Dicer-1 hypomorphic HCT116 cells as compared to parental HCT116 (Figure S4b).
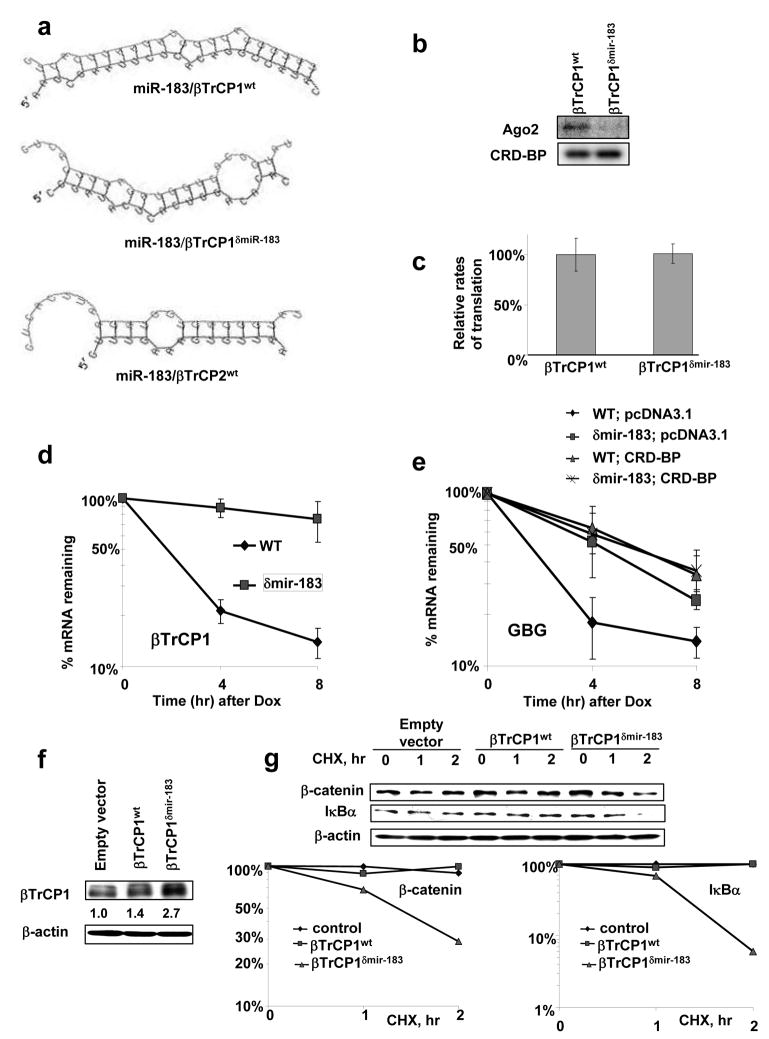
Figure 3. Disruption of miR-183 binding site in the coding region of βTrCP1 mRNA stabilizes it and induces βTrCP1 expression and activity.
a, Prediction of hybrids of βTrCP1 and βTrCP2 mRNAs (red) with the corresponding miRNA (green) were performed using RNAhybrid software (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/) (Rehmsmeier et al., 2004). Mutation in miR-183-binding site of βTrCP1 coding region does not affect the amino acid sequence.
b, FLAG immunoprecipitation of UV–crosslinked RNP complexes were analyzed as in 2a. Protein extracts from 293T cells were incubated with 577–1182 nt fragment of coding region of βTrCP1wt or βTrCP1-mir-183
c, Efficiency of translation of wild type and δmiR-183 mutant of βTrCP1 measured as a ratio of the amount de novo translated protein to the corresponding levels of mRNA.
d, The stability of RNA transcripts βTrCP1wt and βTrCP1δmiR-183 mutant was analyzed in 293T cells with real time RT-PCR as described in Figure 1c..
e, 293T cells were co-transfected with GBG, GBGδmiR-183 mutant, pcDNA3.1 and CRD-BP as indicated. The stability of mRNA transcripts was analyzed as described above.
f, youImmunoblot analysis of βTrCP1 expression in the 293T cells transfected with indicated constructs.
g, Stability of β-catenin and IκBα proteins was analyzed by immunoblot of protein extracts isolated from 293T cells transfected as indicated and treated with cyclohexemide (40 μg/ml) for the indicated times and graphed.
Data are represented as mean ±s.d..
Although we did not detect significant difference in the efficiency of translation between βTrCP1δmiR-183 and βTrCP1wt (Figure 3c), both full-length βTrCP1δmiR-183 and chimeric GBGδmiR-183 transcripts were more stable than their corresponding wild-type mRNAs (Figure 3d, e). Transfection of 293T cells with βTrCP1δmiR-183 resulted in higher levels of expression of βTrCP1 mRNA and protein, as compared with the wild-type (Figure 3f). When expressed, βTrCP1δmiR-183 promoted the degradation of SCFβTrCP substrates, β-catenin and IκBα, more efficiently than the wild-type protein (Figure 3g). Together, these results suggest that miR-183 regulates βTrCP1 levels and activity by directing the RISC complex to the coding region of βTrCP1 mRNA and triggering mRNA degradation. 5′ RACE analysis of in vivo degradation of βTrCP1wt mRNA showed scattered degradation products around the miR-183 binding site (Figure S5a). This suggests that βTrCP1 mRNA turnover, regulated by mir-183, is independent of endonucleolytic cleaveage and may occur through general exonucleolytic mechanisms of mRNA degradation. These data are in line with other reports showing that imperfect base paring of miRNA with mRNA results in destabilization and acceleration of mRNA degradation primarily through 5′ to 3′ mRNA decay (Giraldez et al., 2006; Wu et al., 2006).
Although the majority of reported miRNA binding sites lie within 3′UTRs, identification of a functional miRNA-interacting motif within the coding region of human mRNA of βTrCP1 suggests that the miRNAs may not be restricted to targeting only 3′UTRs of mammalian mRNAs. These data are in line with the bioinformatics prediction that target sites for miRNA can be identified in ORFs and 5′UTRs (Forman et al., 2008; Lewis et al., 2005; Lim et al., 2005), and with the recent data that miRNAs might target the coding regions of several mRNAs and downregulate the gene expression predominantly through inhibition of translation (Duursma et al., 2008; Forman et al., 2008; Lal et al., 2008; Tay et al., 2008). Exogenous reporter constructs containing target sites for miRNAs in 5′UTR or in a coding region were also shown to be silenced in zebrafish (Kloosterman et al., 2004) and in HeLa cells (Lytle et al., 2007). In this manuscript we characterized functional mRNA-destabilizing miRNA-binding site in the coding region of endogenous mRNA (mRNA of βTrCP1). Future studies will determine the relative frequency of naturally occurring miRNAs targets within the different regions of eukaryotic mRNAs (3′UTRs versus coding regions versus 5′UTRs).
CRD-BP is a multifunctional mRNA binding protein, modulating the stability, localization, and translation of several RNAs (c-myc, IGF-II, H19, CD4, MDR-1, etc) and has been shown to shield mRNAs of c-myc and MDR-1 from endoribonucleilytic (Sparanese and Lee, 2007; Tafech et al., 2007) attack. We have previously reported that RNA-binding protein CRD-BP binds to the 577–1182 nt fragment of the coding region of βTrCP1 mRNA and stabilizes it (Noubissi et al., 2006). In the current study, we sought to investigate whether CRD-BP protects mRNA of βTrCP1 from miRNA-directed degradation. Over-expression of CRD-BP was effective in stabilizing chimeric GBG mRNA in Dicer1wt cells, but in Dicer1 hypomorphic cells mRNA was already stabilized independent of CRD-BP over-expression (Figure 1e), suggesting that the destabilizing effect of mature microRNA is required to be counteracted by CRD-BP. Similarly, half-life of GBG mutant, lacking miR-183 binding site, was not affected by CRD-BP, as compared to GBG containing the wild-type 577–1182 nt fragment of βTrCP1 (Figure 3e). Knockdown of CRD-BP reduced expression of βTrCP1, but failed to do so when the function of miR-183 was inhibited (Figure 4a). These data imply that CRD-BP protects mRNA of βTrCP1 from miR-183-directed turnover. We have previously shown that the Wnt/β-catenin signaling pathway elevates the levels of βTrCP1 via induction of CRD-BP that, in turn, stabilizes mRNA of βTrCP1 (Noubissi et al., 2006). When mir-183 function was already inhibited, β-catenin did not further increase the levels of βTrCP1 mRNA, suggesting that Wnt pathway counteracts miR-183 in regulating βTrCP1 mRNA stability (Figure 4b, c).
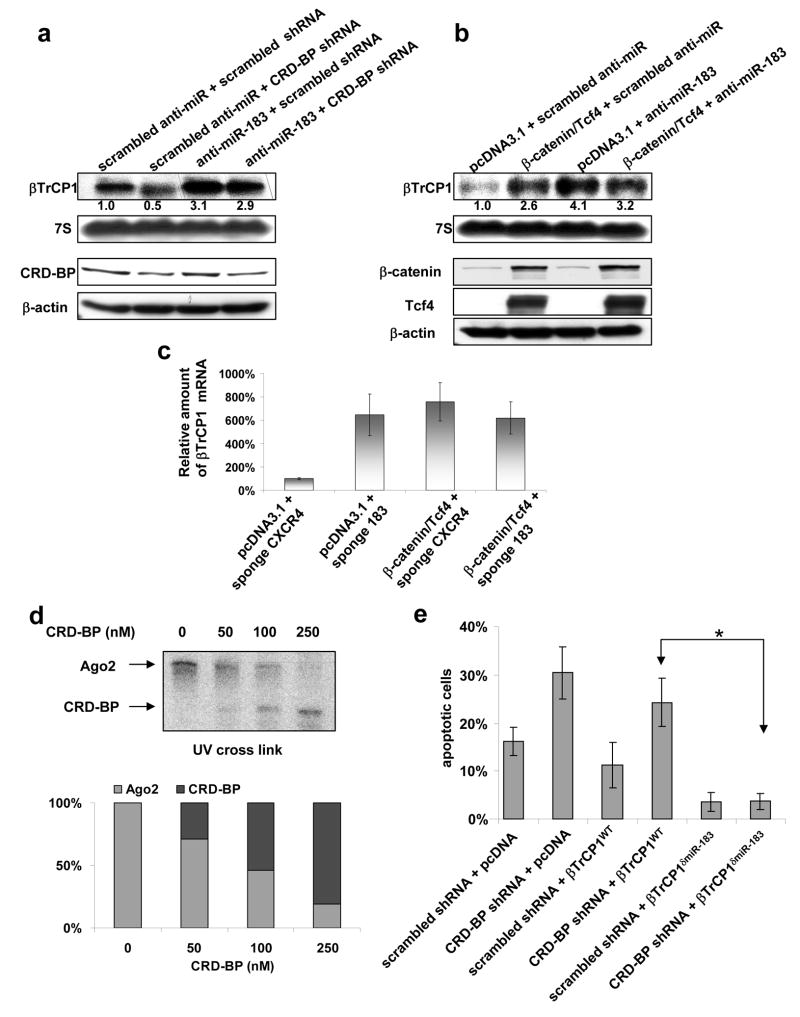
Figure 4. CRD-BP prevents binding Ago2 with the coding region of βTrCP1 mRNA.
a, b, Northern blot (upper panel) and immunoblot (lower panel) analyses of endogenous levels of βTrCP1 mRNA and specified proteins in 293T cells transfected as indicated.
c, The levels of endogenous _βTrCP1_mRNA in 293T cells, transfected with indicated expression vectors and miR-sponge constructs, were estimated by real-time RT-PCR.
d, FLAG immunoprecipitation of UV–crosslinked RNP complexes were analyzed as in 2a. 577–1182 nt fragment of βTrCP1 mRNA coding region was incubated with protein extracts from 293T cells with the presence of recombinant CRD-BP protein in indicated concentrations. Relative amount of RNA-bound Ago2 and CRD-BP was quantified by densitometry and graphed.
e, HCT116 cells were transfected with indicated constructs. Apoptosis was detected by staining with Annexin V-Cy3 apoptosis detection kit over 48 hr after transfection. * P<0.01 in the Student’s **t**-test.
Data are represented as mean ±s.d..
Recombinant CRD-BP disrupted the interaction of Ago2 with the wild-type 577–1182 nt fragment of βTrCP1 mRNA in a dose-dependent manner (Figure 4d), suggesting that CRD-BP directly interferes with Ago2 binding to βTrCP1 mRNA. Together, these data provide evidence that CRD-BP disrupts miRNA-dependent interaction of Ago2 with the coding region of βTrCP1 mRNA, resulting in the stabilization of βTrCP1 mRNA. In addition, over-expression of miR-183 mimic attenuates binding of CRD-BP to 577–1182 nt region of βTrCP1 mRNA in vitro (Figure S4c) further confirming competition between CRD-BP and miR-183-driven RISC for the interaction with this region of βTrCP1 mRNA. A miR-183 mutant of the 577–1182 nt fragment of βTrCP1 mRNA, that failed to interact with Ago2, could still efficiently bind to CRD-BP (Figure 3b). These results suggest that the binding of CRD-BP to the coding region of βTrCP1 mRNA is independent of micro-RNA and Ago2. Interestingly, the 577–1182 nt fragment of βTrCP1 mRNA contains five UUUAY motifs that were reported to interact with CRD-BP/IMP1 ortholog in Drosophila melanogaster (Munro et al., 2006), and these IMP-binding elements do not overlap with miR-183 interacting sequence.
Knockdown of CRD-BP leads to the inhibition of NF-κB activity, induction of apoptosis, and suppression of colony formation of HCT116 colorectal cancer cells (Noubissi et al., 2006). Mutant βTrCP1 lacking the mir183 binding site (but not the wild-type βTrCP1) rescued HCT116 human colorectal cancer cells from the induction of apoptosis induced by the knock down of CRD-BP (Figure 4e). Given that CRD-BP is a multifunctional RNA-binding protein that affects mRNA localization, translation, and/or stability, depending on the RNA to which it is bound (Hansen et al., 2004; Nielsen et al., 2001; Prokipcak et al., 1994; Tessier et al., 2004), our data suggest that some of the effects of CRD-BP might be attributed to its interference with microRNA machinery.
Supplementary Material
01
Acknowledgments
We thank Drs. K. Kinzler, P. Sharp, J. Ross, T. Tuschl, and B. Vogelstein for their generous gifts of reagents, Dr. S. Fuchs for critical reading of the manuscript, Dr. K. Spiegelman and Mrs. I. Larsen for help with the manuscript preparation. This work was supported by NCI grant CA121851 (to V.S.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bandres E, Cubedo E, Agirre X, Malumbres R, Zarate R, Ramirez N, Abajo A, Navarro A, Moreno I, Monzo M, Garcia-Foncillas J. Identification by Real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumoral tissues. Mol Cancer. 2006;5:29. doi: 10.1186/1476-4598-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushati N, Cohen SM. microRNA Functions. Annu Rev Cell Dev Biol. 2007 doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- Cummins JM, He Y, Leary RJ, Pagliarini R, Diaz LA, Jr, Sjoblom T, Barad O, Bentwich Z, Szafranska AE, Labourier E, et al. The colorectal microRNAome. Proc Natl Acad Sci U S A. 2006;103:3687–3692. doi: 10.1073/pnas.0511155103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duursma AM, Kedde M, Schrier M, le Sage C, Agami R. miR-148 targets human DNMT3b protein coding region. RNA. 2008;14:872–877. doi: 10.1261/rna.972008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- Forman JJ, Legesse-Miller A, Coller HA. A search for conserved sequences in coding regions reveals that the let-7 microRNA targets Dicer within its coding sequence. Proc Natl Acad Sci U S A. 2008;105:14879–14884. doi: 10.1073/pnas.0803230105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs SY, Spiegelman VS, Kumar KG. The many faces of beta-βTrCP E3 ubiquitin ligases: reflections in the magic mirror of cancer. Oncogene. 2004;23:2028–2036. doi: 10.1038/sj.onc.1207389. [DOI] [PubMed] [Google Scholar]
- Giraldez AJ, Mishima Y, Rihel J, Grocock RJ, Van Dongen S, Inoue K, Enright AJ, Schier AF. Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science. 2006;312:75–79. doi: 10.1126/science.1122689. [DOI] [PubMed] [Google Scholar]
- Hansen TV, Hammer NA, Nielsen J, Madsen M, Dalbaeck C, Wewer UM, Christiansen J, Nielsen FC. Dwarfism and impaired gut development in insulin-like growth factor II mRNA-binding protein 1-deficient mice. Mol Cell Biol. 2004;24:4448–4464. doi: 10.1128/MCB.24.10.4448-4464.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutvagner G, Simard MJ. Argonaute proteins: key players in RNA silencing. Nat Rev Mol Cell Biol. 2008;9:22–32. doi: 10.1038/nrm2321. [DOI] [PubMed] [Google Scholar]
- Kloosterman WP, Wienholds E, Ketting RF, Plasterk RH. Substrate requirements for let-7 function in the developing zebrafish embryo. Nucleic Acids Res. 2004;32:6284–6291. doi: 10.1093/nar/gkh968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal A, Kim HH, Abdelmohsen K, Kuwano Y, Pullmann R, Jr, Srikantan S, Subrahmanyam R, Martindale JL, Yang X, Ahmed F, et al. p16(INK4a) translation suppressed by miR-24. PLoS ONE. 2008;3:e1864. doi: 10.1371/journal.pone.0001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Lytle JR, Yario TA, Steitz JA. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5′ UTR as in the 3′ UTR. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0703820104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell. 2004;15:185–197. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Munro TP, Kwon S, Schnapp BJ, St Johnston D. A repeated IMP-binding motif controls oskar mRNA translation and anchoring independently of Drosophila melanogaster IMP. J Cell Biol. 2006;172:577–588. doi: 10.1083/jcb.200510044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen FC, Nielsen J, Christiansen J. A family of IGF-II mRNA binding proteins (IMP) involved in RNA trafficking. Scand J Clin Lab Invest Suppl. 2001;234:93–99. [PubMed] [Google Scholar]
- Noubissi FK, Elcheva I, Bhatia N, Shakoori A, Ougolkov A, Liu J, Minamoto T, Ross J, Fuchs SY, Spiegelman VS. CRD-BP mediates stabilization of betaβTrCP1 and c-myc mRNA in response to beta-catenin signalling. Nature. 2006;441:898–901. doi: 10.1038/nature04839. [DOI] [PubMed] [Google Scholar]
- Prokipcak RD, Herrick DJ, Ross J. Purification and properties of a protein that binds to the C-terminal coding region of human c-myc mRNA. J Biol Chem. 1994;269:9261–9269. [PubMed] [Google Scholar]
- Sparanese D, Lee CH. CRD-BP shields c-myc and MDR-1 RNA from endonucleolytic attack by a mammalian endoribonuclease. Nucleic Acids Res. 2007;35:1209–1221. doi: 10.1093/nar/gkl1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman VS, Slaga TJ, Pagano M, Minamoto T, Ronai Z, Fuchs SY. Wnt/beta-catenin signaling induces the expression and activity of betaβTrCP ubiquitin ligase receptor. Mol Cell. 2000;5:877–882. doi: 10.1016/s1097-2765(00)80327-5. [DOI] [PubMed] [Google Scholar]
- Spiegelman VS, Stavropoulos P, Latres E, Pagano M, Ronai Z, Slaga TJ, Fuchs SY. Induction of beta-transducin repeat-containing protein by JNK signaling and its role in the activation of NF-kappaB. J Biol Chem. 2001;276:27152–27158. doi: 10.1074/jbc.M100031200. [DOI] [PubMed] [Google Scholar]
- Tafech A, Bennett WR, Mills F, Lee CH. Identification of c-myc coding region determinant RNA sequences and structures cleaved by an RNase1-like endoribonuclease. Biochim Biophys Acta. 2007;1769:49–60. doi: 10.1016/j.bbaexp.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Tay Y, Zhang J, Thomson AM, Lim B, Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. 2008 doi: 10.1038/nature07299. [DOI] [PubMed] [Google Scholar]
- Tessier CR, Doyle GA, Clark BA, Pitot HC, Ross J. Mammary tumor induction in transgenic mice expressing an RNA-binding protein. Cancer Res. 2004;64:209–214. doi: 10.1158/0008-5472.can-03-2927. [DOI] [PubMed] [Google Scholar]
- Wu L, Fan J, Belasco JG. MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci U S A. 2006;103:4034–4039. doi: 10.1073/pnas.0510928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01