RAD18 transmits DNA damage signaling to elicit homologous recombination repair (original) (raw)
. Author manuscript; available in PMC: 2009 Nov 1.
Published in final edited form as: Nat Cell Biol. 2009 Apr 26;11(5):592–603. doi: 10.1038/ncb1865
Abstract
To maintain genome stability, cells respond to DNA damage by activating signaling pathways that govern cell cycle checkpoints and initiate DNA repair. Cell cycle checkpoint controls should somehow connect with DNA repair processes, however, exactly how such coordination occurs in vivo is largely unknown. Here we revealed a novel role of RAD18 as the integral component that translates the damage response signal to orchestrate homologous recombination (HR) repair. We show that RAD18 promotes HR in a manner strictly dependent upon its ability to be recruited to the sites of DNA breaks and this recruitment relies on a well-defined DNA damage-signaling pathway mediated by another E3 ligase RNF8. We further demonstrate that RAD18 functions as an adaptor to facilitate HR via a direct interaction with RAD51C. Together, our data uncovers RAD18 as a key factor that orchestrates HR repair via surveillance of the DNA damage signal.
DNA double-strand breaks (DSBs) are highly cytotoxic lesions that can cause cell death, mutations and chromosomal instability, which eventually lead to tumorigensis 1–5. Cells cope with DSBs by rapidly recruiting a host of proteins to chromatin regions surrounding DSBs. The concentration of these DNA damage and/or repair proteins at or near DSBs allows for the visualization of these proteins as ionizing radiation-induced foci (IRIF) 6. Accumulating evidence suggest that the ATM-dependent phosphorylation of histone variant H2AX is the initial signal for subsequent accumulation of various checkpoint and repair proteins to the sites of DNA breaks 7,8. Phosphorylated H2AX binds directly to the BRCT domains of MDC1, which allows for the recruitment of an E3 ligase RNF8. RNF8, together with an E2 ubiquitin conjugase UBC13, promotes protein ubiquitination at DNA damage sites, which facilitates the buildup of a number of additional mediator proteins such as BRCA1 and 53BP1, that culminates into proper checkpoint activation 9–14.
Similar to the advances in elucidation of checkpoint mechanisms, the last decade has also provided much detail in how damaged DNA is repaired in cells. There are at least two major repair pathways, the non-homologous end-joining (NHEJ) pathway and the homologous recombination (HR) pathway 1,2,15–17. DNA repair via HR requires the recombinase RAD51, and in vertebrates, five RAD51 paralogs 18,19. These Rad51 paralogs form two distinct protein complexes in vivo, a RAD51C/XRCC3 heterodimer and a RAD51B/ RAD51C/RAD51D/XRCC2 heterotetramer 20,21. Mutation of anyone of these five paralogs reduces the subnuclear assembly of RAD51 and renders cells hypersensitive to DSBs, highlighting the importance of these RAD51 paralogs in HR repair.
An emerging concept in the field is the continuous cross-talks between DNA repair and DNA damage checkpoint pathways, however, little is known about how such coordination is achieved in the cell. Here we provide evidence suggesting that RAD18 channels the DNA damage signal from a well-defined pathway involving ATM, MDC1, and RNF8 to orchestrate DSB repair via homologous recombination through a direct interaction with RAD51C. These data suggest a crucial role of RAD18 in coordinating DNA damage checkpoint response with DNA repair.
RESULTS
RAD18 localizes to sites of DNA double-strand breaks
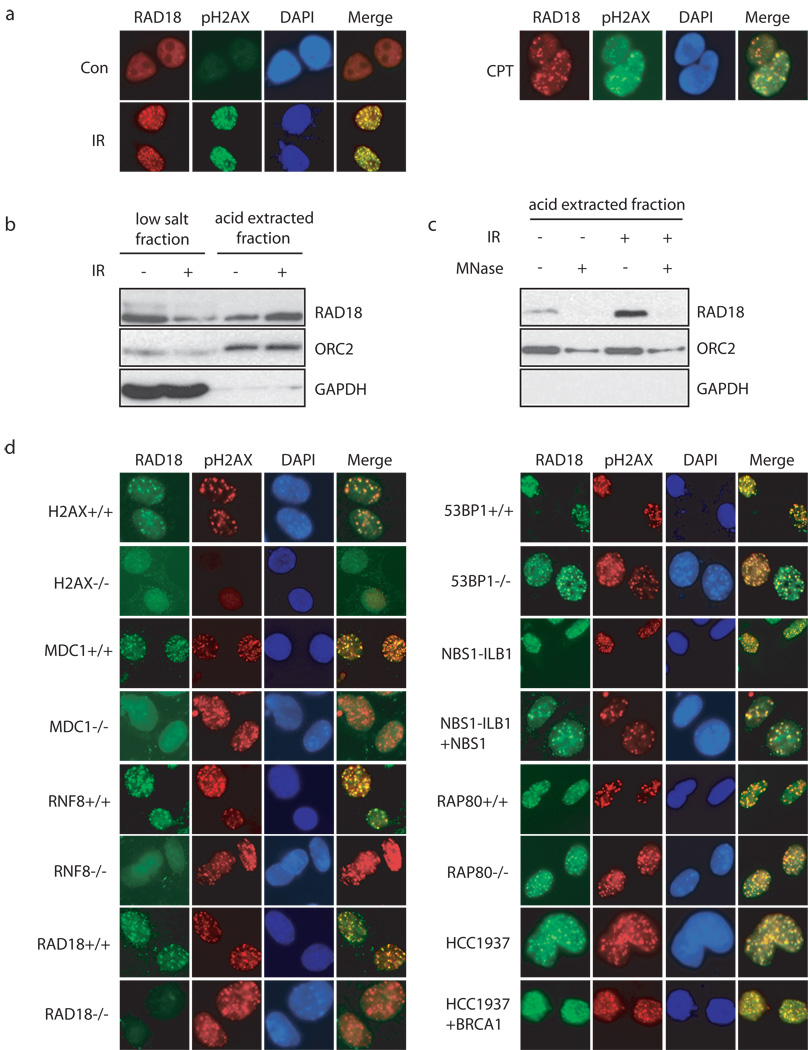
RAD18 is well-known for its function in DNA damage bypass and postreplication repair in yeast and vertebrates through promoting monoubiquitination of proliferating cell nuclear antigen (PCNA) at stalled replication forks 22–25. Although RAD18 has also been implied in HR repair 26,27, exactly how RAD18 participates in this process remains elusive. Many proteins involved in DSB-induced checkpoint control and DNA repair physically localize to DNA damage sites. As shown in Fig. 1a, RAD18 IRIF was readily detected following ionizing radiation or camptothecin (CPT) treatment, indicating a possible role of RAD18 in DSB response.
Figure 1. RAD18 forms DNA double-strand break-induced foci.
(a) Localization of RAD18 in response to IR or CPT. HeLa cells were treated with 10 Gy of ionizing radiation or 1 µM CPT, fixed and immunostained with anti-RAD18 and pH2AX antibodies. (b, c) RAD18 relocalizes to chromatin fraction after IR treatment (b), which is reversible following micrococcal nuclease treatment (c). Experiments were carried out as described in the Experimental Procedures and immunoblotting experiments were performed using indicated antibodies. (d) Genetic dependence of RAD18 relocalization following IR treatment. Indicated deficient cells and their respective wild-type counterparts were irradiated, fixed and immunostained with anti-RAD18 and pH2AX antibodies.
Generally, the damage-induced focus formation reflects the assembly of proteins at the vicinity of DNA breaks and hence these proteins become chromatin bound. Biochemical fractionation experiments show that a significant portion of RAD18 shifted from the low salt extractable fraction (soluble fraction) to the acid extracted fraction (chromatin fraction) following IR (Fig. 1b). Moreover, the chromatin fraction of RAD18 can be easily released upon nuclease treatment (Fig. 1c). These data suggest that RAD18 accumulates onto chromatin after DNA damage.
Damage-induced RAD18 foci formation requires RNF8 E3 ubiquitin ligase
To determine where RAD18 fits in the established DNA damage-signaling cascade, we examined its IRIF formation in an exhaustive panel of cell lines with known genetic defects in DNA damage checkpoint components. In sharp contrast to their respective wild-type counterparts, we failed to detect IR-induced RAD18 focus formation in H2AX, MDC1 or RNF8 deficient cells (Fig. 1d). However, RAD18 relocalization to γ-H2AX containing foci was not noticeably affected in cells with BRCA1, 53BP1, NBS1 or Rap80 deficiency (Fig. 1d). These data suggest that RAD18 acts downstream of H2AX, MDC1 and RNF8 in the known DNA damage signal transduction pathway.
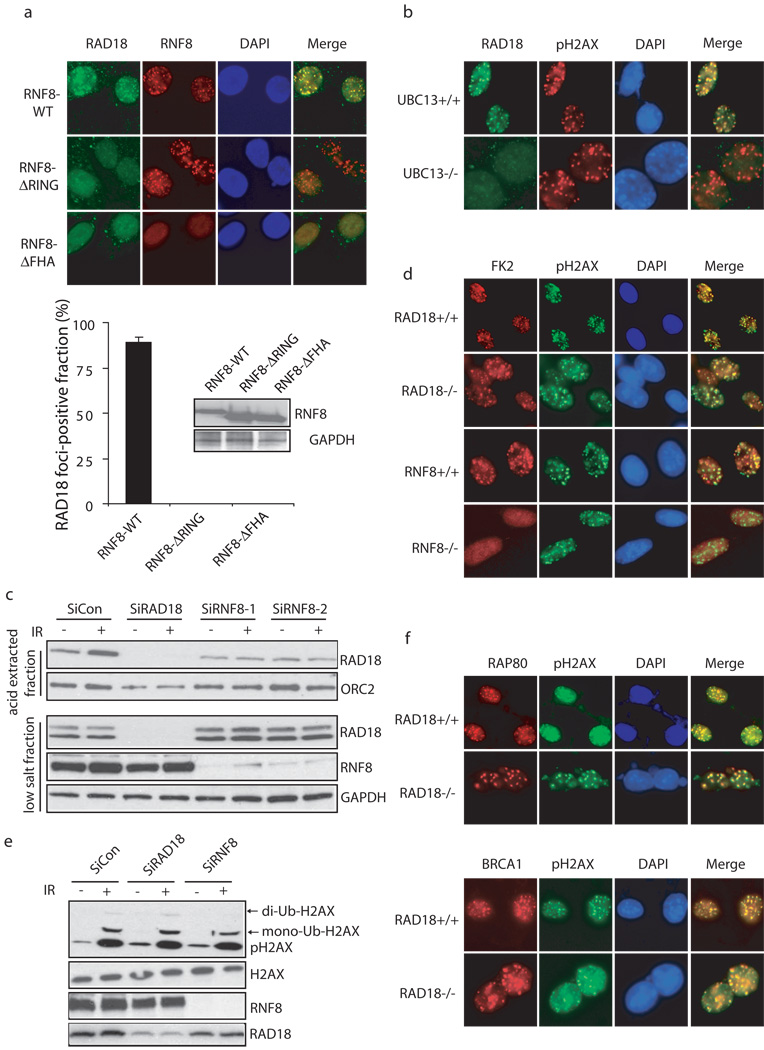
To further explore the role of RNF8 in targeting RAD18 to IRIF, we established mouse RNF8−/− MEFs stably expressing wild-type, the FHA or RING domain deletion mutants of human RNF8. Work from our lab and others have demonstrated while the FHA domain of RNF8 is required for its own recruitment via its interaction with MDC1, its E3 ligase activity is required for the accumulation of subsequent checkpoint and repair proteins to DSBs 10,12,13. Indeed, only reconstitution with wild-type RNF8 restored RAD18 IRIF (Fig. 2a), suggesting that RAD18 also requires RNF8 E3 ligase activity to be recruited to DSB sites. Likewise, UBC13, the E2 ubiquitin conjugating enzyme that works with RNF8, was also critical for RAD18 foci formation (Fig. 2b). Corroborating with its role in targeting RAD18 to DNA damage-induced foci, RNF8 depletion also significantly reduced the accumulation of RAD18 to chromatin fraction after IR (Fig. 2c).
Figure 2. RNF8/UBC13 is required for DSB-induced RAD18 recruitment.
(a) Both the FHA and RING domain of RNF8 are required for RAD18 relocalization. RNF8 deficient cells were reconstituted with wild-type or different internal deletion mutants of RNF8. The expression of wild-type and mutant RNF8 was confirmed by Western blotting (lower panel). Following irradiation, cells were fixed and immunostained using anti-RAD18 and anti-Flag antibodies. Representative RAD18 foci were shown in the upper panel. Results were the average of three independent experiments in which more than one hundred cells were counted per experiment and were presented as mean±SEM (lower panel). (b) UBC13 is required for RAD18 relocalization. Wild-type or UBC13 deficient cells were irradiated, fixed and immunostained with anti-RAD18 and anti-pH2AX antibodies. (c) RNF8 is required for IR-induced RAD18 chromatin localization. HeLa cells depleted of endogenous RAD18 or RNF8 were treated with 10 Gy IR or left untreated. Six hours later, cells were collected and chromatin fractions were isolated according to Experimental Procedures. Immunoblotting experiments were performed using indicated antibodies. (d) RAD18 is not required for IR-induced ubiquitin conjugate formation. Wild-type, RAD18 or RNF8 deficient cells were irradiated, fixed and immunostained with anti-FK2 and pH2AX antibodies. (e) RAD18 is not required for H2AX ubiquitination following DNA damage. HeLa cells transfected with control siRNA, RAD18 siRNA or RNF8 siRNA were treated with 10 Gy IR or left untreated. Cells were harvested one hour post-irradiation and cell lysates were immunoblotted with indicated antibodies. (f) RAD18 is not required for Rap80 and BRCA1 foci formation following IR. Wild-type or RAD18 deficient cells were irradiated, fixed and immunostained with anti-Rap80 or BRCA1 antibodies.
RNF8 is critical for the formation of IR-induced ubiquitin conjugates at DSBs 10–14,28, which can be detected by the use of anti-ubiquitin antibody FK2 29,30. These FK2 foci can be impaired by the depletion of free nuclear ubiquitin achieved by a short treatment of cells with proteasome inhibitor MG132 before irradiation. Consistently, the accumulation of RAD18 at DNA damage sites was abrogated when cell were pretreated with MG132 (Supplementary information, Fig. S1). Given that RAD18 also exhibits ubiquitin ligase activity, we further tested whether RAD18 itself would contribute to FK2 foci formation. In contrast to RNF8, RAD18 was not required for IR-induced FK2 foci formation and H2AX ubiquitination (Fig. 2d, e). Furthermore, RAD18 was not required for Rap80 or BRCA1 foci formation (Fig. 2f).
The Zinc Finger domain of RAD18 targets RAD18 to the sites of DNA breaks
Next, we sought to identify the region(s) within RAD18 that are important for its translocation to IRIF. As shown in Fig. 3a, b, only the Zinc Finger (ZNF) domain deletion mutant (ΔZNF) of RAD18 totally lost its focus forming ability, while wild-type, the RING domain point mutant (C28F mutant; Cys28 changed to Phe), the RING domain deletion mutant (ΔRING), the SAP (SAF-A/B, Acinus, and PIAS) domain deletion mutant (ΔSAP) and the RAD6 binding-domain deletion mutant (ΔR6) 25 all could form IRIF. These observations suggest the RAD18 ZNF domain, but not its E3 ligase activity, is essential for targeting RAD18 to IRIF.
Figure 3. The ZNF domain of RAD18 is required for RAD18 localization to the sites of DNA damage.
(a) Schematic representation of human RAD18 and its deletion/point mutants used in this study. (b) The ZNF domain of RAD18 targets it to IR-induced foci. 293T cells expressing Flag-tagged wild-type or mutants of RAD18 were irradiated, fixed and immunostained with anti-Flag and pH2AX antibodies. (c) The ZNF domain alone is sufficient for RAD18 IRIF. 293T cells expressing indicated Flag-tagged proteins were irradiated and immunostained as described in (b). (d) Genetic dependence of the ZNF domain relocalization following ionizing radiation. Immunostaining experiments were performed as described in Figure 1d. (e, f) The ZNF domain of RAD18 is essential and sufficient for binding to ubiquitin in vitro. GST or Ubi-GST fusion proteins were incubated with cell lysates containing exogenously expressed Flag-tagged wild-type or various internal deletion mutants of RAD18 (e) or NLS-ZNF or NLS-ZNF-C207F (f). After extensive washing, bound RAD18 fragments or fusion proteins were analyzed by immunoblotting with anti-Flag antibody.
To further clarify whether the ZNF domain alone is sufficient to target RAD18 to sites of DNA damage, we employed a RAD18 ZNF domain (residues 186–240) that harbors an N-terminal NLS (NLS-ZNF) and a ZNF point-mutation that would disrupt the zinc finger domain structure (NLS-ZNF-C207F) (mutation of Cys207 to Phe207). As shown in Fig. 3c, while the NLS-ZNF fusion protein could form foci, the NLS-ZNF-C207F mutant and the SAP domain of RAD18 that also harbors an N-terminal NLS (NLS-SAP; residues 241–290) failed to do so, suggesting that the ZNF domain alone fulfills the role of targeting RAD18 to DSBs. Moreover, similar to the full-length protein, the foci formation of the NLS-ZNF fusion protein also depends on RNF8, MDC1 and H2AX (Fig. 3d).
The ZNF domain of RAD18 binds directly to ubiquitin in vitro
RNF8 and UBC13 are known to generate polyubiquitin chains that further recruit ubiquitin-binding proteins to DNA damage sites 31–34. Recent studies have revealed that some ZNF domains are capable of binding to ubiquitin and have been renamed as ubiquitin binding ZNF (UBZ) domains. Here, we examined whether the ZNF domain of RAD18 would bind to ubiquitin in vitro. Using an ubiquitin-glutathione S-transferase fusion protein (Ubi-GST), we showed that Ubi-GST specifically bound to wild-type and the SAP domain deletion mutant (ΔSAP) of RAD18, but not to a RAD18 mutant that lacks the ZNF domain (ΔZNF) (Fig. 3e). In addition, Ubi-GST pulled down the NLS-ZNF fusion protein, but not NLS-ZNF-C207F mutant in vitro (Fig. 3f). This ubiquitin-binding activity of RAD18 in vitro is entirely consistent with its ability to localize to damage-induced foci in vivo, suggesting that RAD18, similar to Rap80, might associate with certain RNF8/UBC13-catalysed ubiquitylated protein(s) at DSBs.
Notably, the RAD18 ZNF domain, but not the Rap80 ZNF domain, has an affinity for different polyubiquitin chains in vitro (Supplementary Fig. S2a), implying the ability to bind ubiquitin is specific for the RAD18 ZNF domain. Moreover, GST-RAD18 ZNF domain binds to K48 or K63-linked ubiquitin chains with similar affinities, 36 ± 10 nM for the K63-linked chains, and 17 ± 4 nM for the K48-linked chains (Supplementary Fig. S2b, c). The significance of this finding is not yet clear.
RAD18 promotes homologous recombination in RNF8-dependent manner
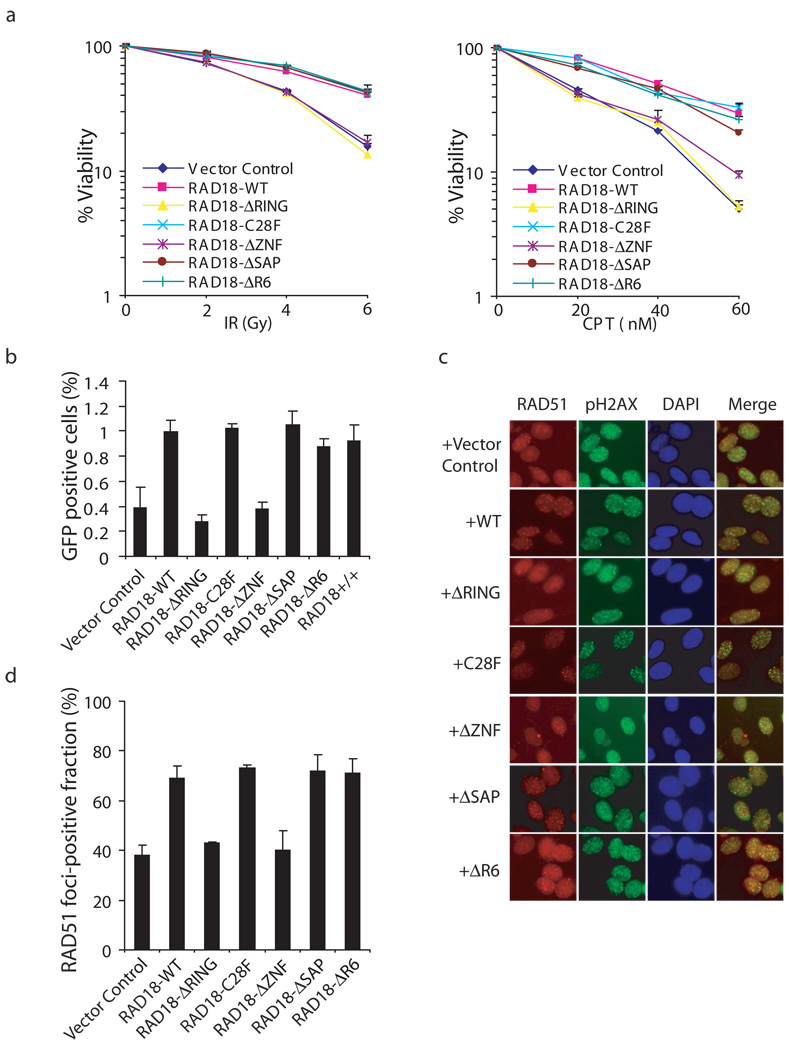
Since RAD18 localization appears to be regulated in response to DSBs, we determined whether RAD18 is required for cell survival following this type of DNA damage. We obtained mouse RAD18−/− MEF cells 23 and established derivative cell lines stably expressing either wild-type or the various deletion/point mutants of human RAD18. Cells deficient in RAD18 were sensitivity to IR or CPT treatment (Fig. 4a). Furthermore, cells reconstituted with wild-type or the ΔSAP mutant of RAD18, but not those with the RAD18 ZNF or its RING domain deletion mutants, restored cellular resistance to DNA breaks (Fig. 4a). Unexpectedly, the E3 ligase inactivating mutants C28F and ΔR6 still could restore cell survival following IR or CPT treatment (Fig. 4a). These results suggest that RAD18 is required for cell survival following DNA double-strand breaks and that both the ZNF and RING domains, but not its E3 ligase activity, are critical for this function of RAD18.
Figure 4. RAD18 promotes homologous recombination.
(a) The RAD18 ZNF and RING domains, but not its E3 ligase activity, are required for restoring cellular resistance to DSBs. Colony formation assays were performed according to the Experimental Procedures. Results were averages of three independent experiments and were presented as mean±SEM. (b) RAD18 promotes homologous recombination. RAD18-deficient cells were reconstituted with wild-type or different RAD18 mutants as indicated. Gene conversion assays were performed as described in the Experimental Procedures. Results (mean±SEM) were the average of three independent experiments. (c, d) RAD18 is required for efficient RAD51 foci formation. RAD18-deficient or reconstituted cells were irradiated, fixed and immunostained with anti-RAD51 and pH2AX antibodies. Representative RAD51 foci were shown (c). Data shown in (d) are from three independent experiments in which more than one hundred cells were counted in each experiment. Results were the average of three independent experiments and were presented as mean±SEM.
Since CPT-induced replication-associated DSBs are usually repaired by HR repair 35, the observed requirement of RAD18 in DSB repair may suggest that RAD18 is involved in HR. Indeed, this possibility was raised earlier by studies using chicken DT40 cells 26,27,36,37. To confirm the role of RAD18 in HR, we performed a gene conversion assay to examine HR efficiency using the DR-GFP reporter system 38. Significantly, re-introduction of wild-type RAD18 or its SAP domain deletion mutant restored HR repair in RAD18−/− cells, whereas the ZNF or RING domain deletion mutants are defective in this assay (Fig. 4b, Supplementary Fig. S3a). The E3 ligase inactivating mutants C28F and ΔR6 could also restore HR repair (Fig. 4b, Supplementary Fig. S3a), indicating that the E3 ligase activity of RAD18 is not required for its HR function. Consistently, depletion of RAD6A has no effect on gene conversion (Supplementary Fig. S4).
RAD18 deficient chicken DT40 cells are hypersensitive to CPT and this hypersensitivity can be reversed by additional inactivation of NHEJ 27, indicating that RAD18 in chicken cells may regulate NHEJ pathway and thus influence CPT sensitivity. To address whether this is the case in mammalian cells, we performed clonogenic survival assays using RAD18+/+ and RAD18−/− cells exposed to IR or camptothecin alone or in combination with the NHEJ inhibitor NU7441. NU7441 treatment increased the sensitivity in both RAD18 wild-type and deficient MEFs (Supplementary Fig. S5a), suggesting that unlike the situation in DT40 cells, RAD18 does not appear to participate in NHEJ and it may directly facilitate HR repair in mammalian cells. As a control, we showed that wild-type and RAD18 deficient cells have comparable cell cycle profiles (Supplementary Fig. S6a). The fact that RAD18 expression increases in S and G2/M phases (see Supplementary Fig. S6b) is also consistent with its role in HR repair since HR pathway mainly operates in S and G2 phases of the cell cycle.
The recombination protein RAD51 is the key component of the homologous recombination repair machinery and the formation of Rad51 foci can be used as an indicator of HR repair. Indeed, IR-induced Rad51 foci formation was reduced in RAD18−/− MEF cells (Fig. 4c, d, Supplementary Fig. S3b). We also obtained similar data using human HCT116 and HCT116-RAD18−/− cell lines (data not shown). In agreement with the results from our clonogenic and gene conversion assays, RAD51 foci formation can be restored by reconstitution of RAD18−/− cells with wild-type, the SAP deletion mutant and E3 ligase inactivating mutants C28F and ΔR6, but not with the ZNF or RING domain deletion mutants (Fig. 4c, d).
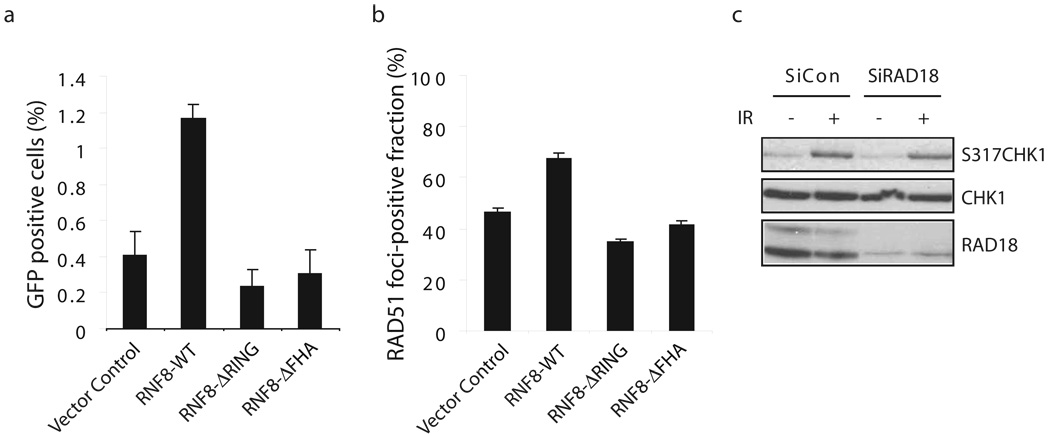
The striking correlation between the ZNF domain of RAD18 required for its recruitment to DSBs and also for HR repair led us to postulate that RNF8 might be the possible upstream signaling molecule that facilitates RAD18 function in HR. As expected, HR repair and IR-induced Rad51 foci formation were noticeably impaired in RNF8 −/− MEFs (Fig. 5a, b, Supplementary Fig. S3c). Furthermore, while wild-type RNF8 could restore this HR deficiency, the FHA domain deletion or the RING domain deletion mutants of RNF8 failed to do so (Fig. 5a, Supplementary Fig. S3c).
Figure 5. RNF8 participates in homologous recombination.
(a) RNF8 promotes homologous recombination. RNF8-deficient cells were reconstituted with wild-type or different mutants of RNF8 as indicated in Figure 2a. Gene Conversion assay were performed similar to those described in Figure 4b. (b) RNF8 is required for efficient RAD51 foci formation. (c) RAD18 is not required for CHK1 activation following DNA damage. Control or RAD18 siRNA-transfected HeLa cells were mock treated or exposed to 10 Gy of ionizing radiation. Cells were harvested 2 hours later and lystes were immunoblotted with indicated antibodies.
Previously report suggests that Chk1 promotes HR repair through directly interacting with and phosphorylating RAD51 39, raising the possibility that RAD18 might regulate HR repair by influencing Chk1 activation. As shown in Fig. 5c, RAD18 was not required for Chk1 activation following ionizing radiation, thus indicating that RAD18 may control HR repair via a novel mechanism.
RAD18 interacts with RAD51C
To explore how RAD18 participates in HR repair, we generated a 293T derivative cell line stably expressing a triple-tagged RAD18 for the identification of potential RAD18-interacting proteins. Following a tandem affinity purification (TAP) scheme, proteins associated with RAD18 were identified by mass spectrometry analysis (Supplementary Table 1). Results revealed a number of known RAD18-associated proteins, including PCNA, ubiquitin and RAD6A/UBE2A. Interestingly, one of these RAD18-associated proteins is RAD51C, a key component that exists in both Rad51 paralog complexes in human cells 20,21,40–44.
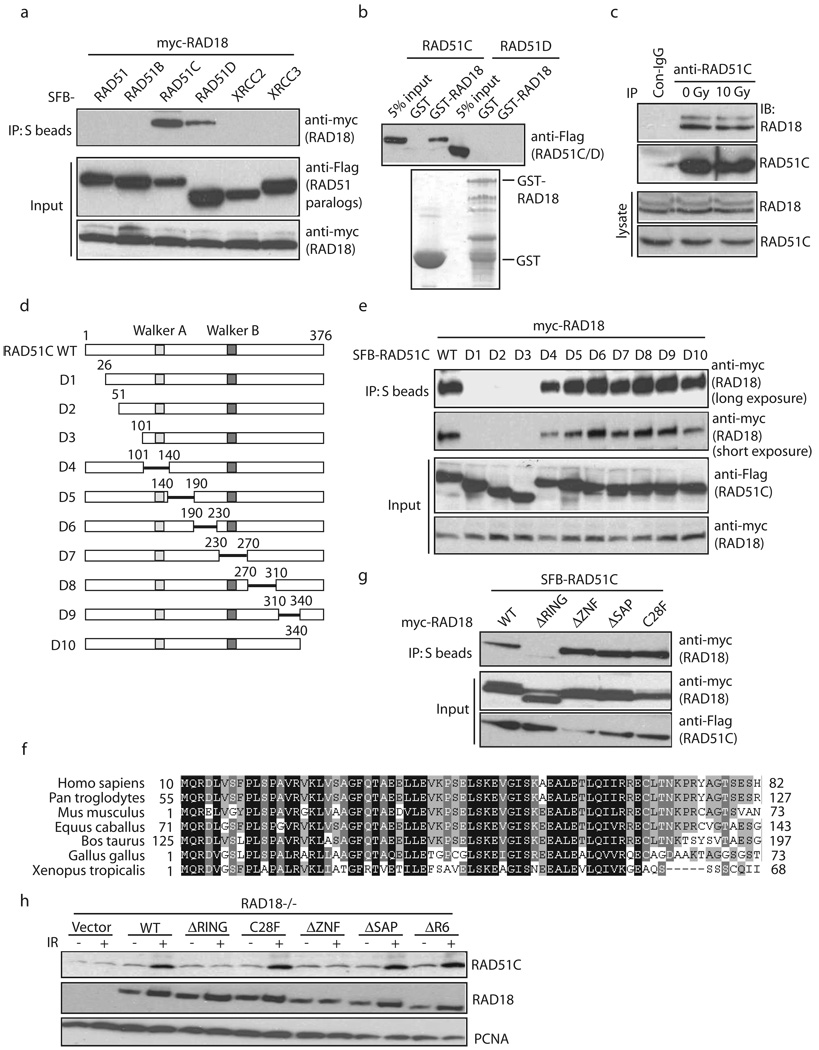
We first confirmed the interaction between RAD18 and RAD51C. As shown in Fig. 6a, RAD18 interacted with RAD51C, weakly with RAD51D. In addition, RAD51C specifically interacted with GST-RAD18 but not GST alone (Fig. 6b). This interaction was also detected in vivo between endogenous proteins before or after irradiation (Fig. 6c).
Figure 6. RAD18 interacts with RAD51C.
(a) Ectopically expressed RAD18 interacts with RAD51C. 293T cells were transfected with plasmids encoding Myc-tagged RAD18 together with plasmids encoding SFB-tagged RAD51 or RAD51 paralogs. Cells were collected 24 hour after transfection. Immunoprecipitation (IP) reactions were performed using S beads and then subjected to immunoblotting using antibodies as indicated. (b) RAD18 directly interacts with RAD51C. GST or GST-RAD18 immobilized on sepharose beads were incubated with cell lysates containing exogenously expressed Flag-tagged RAD51C or RAD51D. Bound RAD51C was analyzed by anti-Flag immunoblotting. (c) Endogenous RAD18 and RAD51C form a complex in vivo. 293T cells were mock treated or treated with IR (10 Gy). Control or anti-RAD51C immunoprecipitates were immunoblotted with RAD18 and RAD51C antibodies (upper panels). The expression levels of the endogenous proteins were detected by immunoblotting using RAD18 and RAD51C antibodies (lower panels). (d) Schematic representation of human RAD51C and its deletion mutants used in this study. (e, g) Mapping of the corresponding regions required for RAD18/RAD51C interaction. Immunoprecipitation (IP) reactions were performed using S beads and then subjected to immunoblotting using antibodies as indicated. (f) Alignment of RAD51C N-terminal sequences from different species. (h) RAD18 is required for IR-induced RAD51C chromatin localization. RAD18-deficient cells were reconstituted with wild-type or different RAD18 mutants as indicated. Cells were irradiated and chromatin fractions were isolated according to Experimental Procedures. Immunoblotting experiments were performed using indicated antibodies.
Next we sought to identify the region on RAD51C responsible for its interaction with RAD18 (Fig. 6d). While neither the ATPase Walker A/Walker B motifs nor the Linker domain in between were required for this interaction, we found that the very N-terminus of RAD51C was necessary for its binding to RAD18 (Fig. 6e). Interestingly, the N-terminus of RAD51C is highly conserved throughout evolution (Fig. 6f), suggesting that it may carry out an important function of RAD51C.
Conversely, we analyzed a series of internal deletion/point mutations of RAD18 and found that the RING domain of RAD18 is critical for its interaction with RAD51C (Fig. 6g). Moreover, although the interaction between RAD51C and RAD18 depends on the RING domain of RAD18, the conserved Cysteine mutants C28F, C25F and C46F retained the ability to interact with RAD51C, indicating that the RAD18 E3 ligase activity and the intact structure of its RING domain may not be required for this interaction (Fig. 6g, Supplementary Fig. S7). More importantly, the requirement of RAD18 RING domain, but not its E3 ligase activity, for the retention of RAD51C to damaged chromatin (Fig. 6h) further indicates that RAD18 facilitates the accumulation of RAD51C via a direct protein-protein interaction. Together, these data suggest that a physical interaction between RAD18 and RAD51C may play a significant role in regulating HR repair and explain why the RING domain, but not its endowed E3 ligase activity, is required for its function in HR repair and cell survival following DSBs.
The ability of RAD51C to function in HR correlates with its association with RAD18
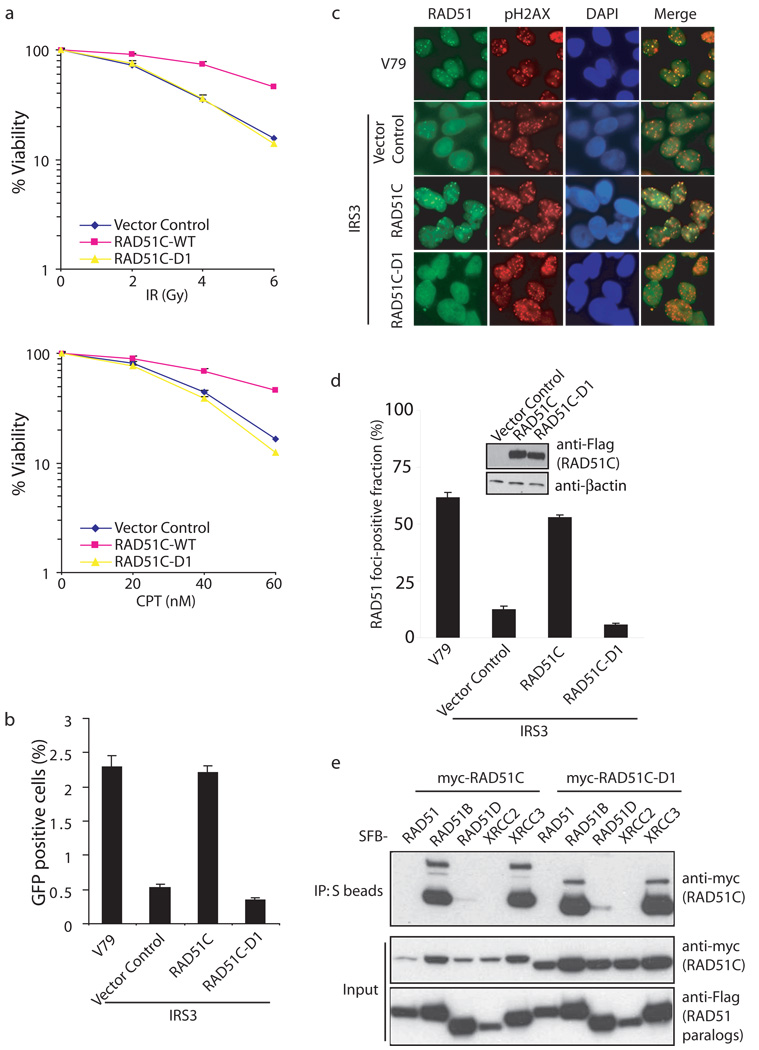
RAD51C-deficient cells display HR defects and exhibit hypersensitivity to agents that would induce DNA double-strand breaks 45–47. Previous studies have also reported a significant reduction of RAD51 foci formation in RAD51C-deficient or depleted cells 48,49. To explore the physiological relevance of the highly conserved N-terminus of RAD51C, which is required for its binding to RAD18, we used the RAD51C-D1 mutant that lacks this conserved region. Clonogenic assays indicated that only reconstitution with wild-type RAD51C, but not with the RAD51C-D1 mutant, in RAD51C-deficient IRS3 cells restored cell survival following DSBs (Fig. 7a). In addition, re-introduction of wild-type RAD51C restored HR repair efficiency to a level comparable with those observed in wild-type CHO-V79 cells, whereas the RAD51C-D1 mutant was defective in this assay (Fig. 7b, Supplementary Fig. S3d). Moreover, we also observed a significant reduction of RAD51 foci formation in RAD51C-D1 reconstituted IRS3 cells when compared with cells reconstituted with wild-type RAD51C (Fig. 7c, d).
Figure 7. RAD18-binding is critical for RAD51C function in homologous recombination.
(a) D1 mutant of RAD51C is defective in restoring cell survival following IR or CPT treatment. RAD51C-deficient IRS3 cells were transduced with control virus or viruses expressing HA-Flag-tagged wild-type RAD51C (WT) or the very N-terminal deletion mutant (D1), which does not bind to RAD18. Wild-type or RAD51C-D1 expression was confirmed by immunoblotting as indicated in (d). Clonogenic assays were performed according to the Experimental Procedures and results were averages of three independent experiments and presented as mean±SEM. (b) RAD18-binding is required for RAD51C function in homologous recombination. RAD51C-deficient IRS3 cells were reconstituted with wild-type or D1 mutant of RAD51C. Gene conversion assays were performed as described in Experimental Procedures. Results were the average of three independent experiments and were presented as mean±SEM. (c, d) Interaction with RAD18 is important for RAD51C function in promoting RAD51 foci formation. Cells as indicated were irradiated, fixed and immunostained with anti-RAD51 and pH2AX antibodies. Representative RAD51 foci were shown in (c). Results were the averages of three independent experiments in which more than one hundred cells were counted in every experiment and were presented as mean±SEM (d). (e) The N terminal region of RAD51C is not required for RAD51 paralogs-complexes formation. 293T cells were transfected with plasmids encoding Myc-tagged RAD51C or RAD51C-D1 together with plasmids encoding SFB-tagged RAD51 or RAD51 paralogs. Immunoprecipitation (IP) reactions were performed using S beads and then subjected to immunoblotting using indicated antibodies.
To rule out the possibility that the phenotypes observed in RAD51C-D1 reconstituted IRS3 cells may be due to a failure of forming RAD51 paralog complexes, we performed co-IP experiments. As shown in Fig. 7e, the interaction between RAD51B or XRCC3 with RAD51C-D1 is similar to that with wild-type RAD51C. These results suggest that the specific interaction between RAD18 and the N-terminus of RAD51C is likely to be important for RAD51C function in HR repair.
RAD18 participates in two independent DNA damage repair pathways
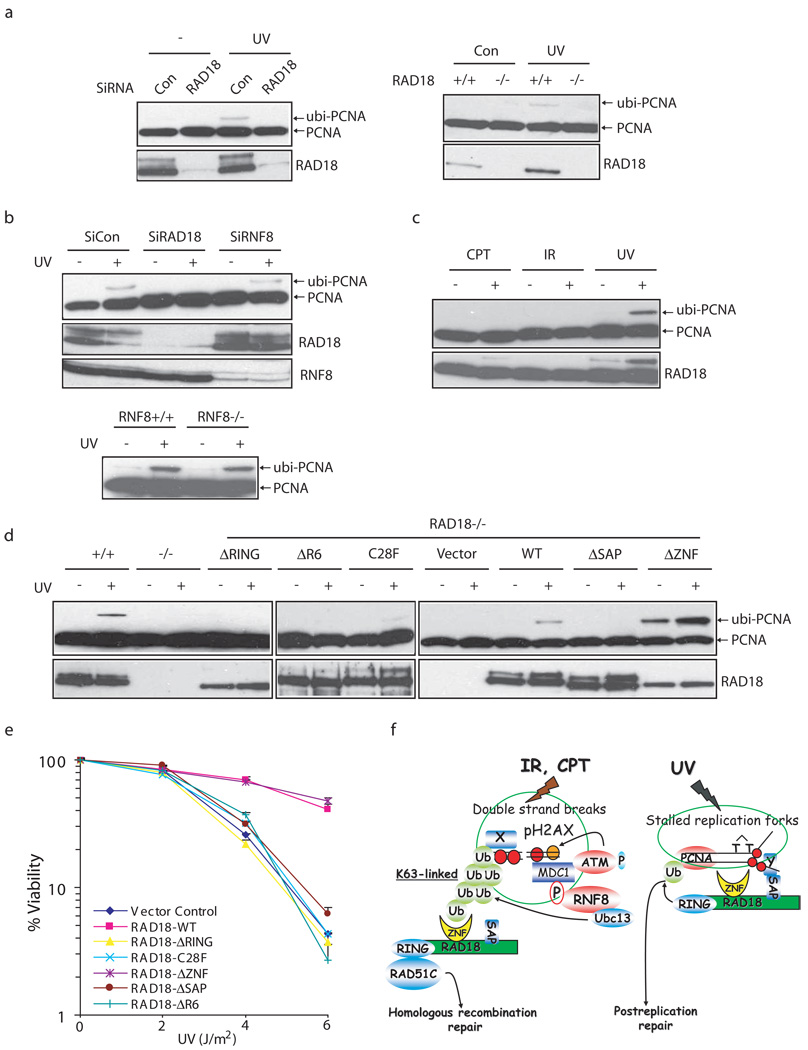
The known function of RAD18 in vivo is to facilitate PCNA monoubiquitination following DNA damage, especially in response to UV-induced lesions. We first confirmed the role of RAD18 in promoting UV-induced PCNA monoubiquitination (Fig. 8a). Since RNF8 is required for RAD18 relocalization following DSBs, we tested whether RNF8 would also be required for RAD18 function following UV damage. Interestingly, UV-induced PCNA monoubiquitination was readily detected in RNF8 depleted HeLa cells or RNF8 deficient MEFs (Fig. 8b), indicating that RNF8 is exclusively involved in RAD18 function following DNA double-strand breaks. Indeed, while PCNA monoubiquitination was easily detected upon UV irradiation, the monoubiquitination of PCNA was either absent or very weak in cells treated with CPT or ionizing radiation (Fig. 8c). These results indicate that RAD18 may participate in two independent repair processes. We further examined which domains of RAD18 would be required for its function in promoting PCNA monoubiquitination. Intriguingly, while UV-induced PCNA monoubiquitination was largely abrogated in RAD18 deficient cells ectopically expressing either the RAD18 E3 ligase inactivating mutants including ΔRING, C28F and ΔR6 or the SAP domain deletion mutant, PCNA monoubiquitination was readily observed in cells expressing wild-type or the RAD18 ZNF domain deletion mutant (Fig. 8d). These results suggest that both the SAP domain and a functional E3 ligase activity, but not the ZNF domain of RAD18 are required for PCNA monoubiquitination. In accordance with tolerance to UV-induced DNA damage involves damage-induced PCNA monoubiquitination, the UV sensitivity of RAD18−/− cells stably expressing RAD18 ZNF deletion mutant was similar to that observed in cells expressing wild-type RAD18. In contrast, RAD18−/− cells expressing the E3 ligase inactivating mutants (ΔRING, C28F or ΔR6) or the SAP domain deletion mutants were more sensitive to UV damage (Fig. 8e). Thus, different domains of RAD18 are required for cell survival following UV damage or DNA double-strand breaks, indicating that RAD18 participates in these repair pathways via distinct mechanisms (Fig. 8f).
Figure 8. RAD18 participates in two distinct DNA repair pathways.
(a) RAD18 is required for PCNA monoubiquitination following UV damage. Control cells, HeLa cells depleted of endogenous RAD18 (left panel) or RAD18 deficient MEF cells (right panel) were treated with 60 J/m2 of UV irradiation or left untreated. Chromatin fractions were isolated as described in the Experimental Procedures and immunoblotted with indicated antibodies. (b) RNF8 is not required for UV-induced PCNA monoubiquitination. Experiments were carried out similar to that described in (a). (c) Monoubiquitination of PCNA in cells following UV light, X-ray or CPT treatment. HeLa cells were irradiated with UV light (60 J/m2), X-rays (10 Gy) or treated with CPT (50 nM) and were allowed to recover for four, six and sixteen hours, respectively. Chromatin fractions were isolated as described in Experimental Procedures and immunoblotted with indicated antibodies. (d) Both the SAP domain and E3 ligase activity of RAD18 are required for PCNA monoubiquitination. UV-induced PCNA monoubiquitination were analyzed by Western blotting in control or RAD18−/− cells reconstituted with wild-type or various deletion/point mutants of RAD18. The experiments were carried out similar to that described in (a). (e) The zinc finger domain of RAD18 is not required for cell survival after UV treatment. Clonogenic assays were performed according to the Experimental Procedures. Results were averages of three independent experiments and presented as mean±SEM. (f) A model for RAD18 function in mediating PRR and HR repair pathways. See “Discussion” for details.
Discussion
Protein ubiquitination is emerging as an important form of covalent modification that regulates various biological processes including DNA damage signaling and repair pathways. In this study, we demonstrated that RAD18 can be recruited to damaged chromatin through its Zinc finger domain in a RNF8/UBC13-dependent manner. Because RAD18 ZNF domain directly binds to ubiquitin in vitro, we speculate that one or several ubiquitinated proteins on chromatin might interact with RAD18 and recruit RAD18 to the sites of DNA breaks. To date, the nature of these ubiquitinated proteins at sites of DNA breaks remains to be elusive, although the chromatin components such as H2A/H2B or H2AX are the likely candidates 10,12,13,28. This scenario is reminiscent of the damage-induced recruitment of Rap80, a recently identified ubiquitin-interacting domain (UIM)-containing protein that serves as an adaptor for BRCA1 accumulation at sites of DNA breaks 31–33. Through its ability of binding to ubiquitin (via its UIM domains), Rap80 can be tethered to certain FK2-reacting ubiquitinated proteins in a way similar to that of the ZNF domain of RAD18. All these data support a model wherein RNF8-dependent ubiquitin chains formed at DNA damage sites are used as docking sites for specific downstream complexes. Should this be a general phenomenon, RAD18 and Rap80 may represent a new class of DNA damage repair proteins that use ubiquitin-interacting domains as part of their recruitment to DSBs.
To achieve maximal efficiency for DNA damage response, cells need to synchronize the activation of DNA damage checkpoints with DNA repair processes. However, the precise mechanisms for this coordination are not well understood. In this study, we provide one mechanism, e.g. by the use of RAD18 as a molecular linker between checkpoint signaling and DNA damage repair. We show that RAD18’s function in DNA repair is strictly congruent with its recruitment to damaged chromatin. Deletion of the RAD18 zinc finger domain abolished the loading of RAD18 to DSB site and therefore eliminates its role in homologous recombination. Moreover, the fact that double knock-down of RNF8 and RAD18 did not lead to increased IR or CPT sensitivity when compared with RNF8 single knock-down further supports the notion that RAD18 activates HR repair downstream of RNF8 (Supplementary Fig. S5b). UBC13 is an E2 ubiquitin conjugating enzyme that works in concert with RNF8 in the assembly of checkpoint proteins at the damage site 10,12,13. Interestingly, UBC13 has also been shown to play a role in HR repair 28. All these data together support a cross-talk between cell cycle checkpoint regulation and DNA repair via a functional link between RNF8/UBC13 and RAD18.
We do not yet know exactly how RAD18 controls HR repair. One possibility is that RAD18 participates in HR by facilitating the accumulation of RAD51C at damaged chromatin (Fig. 6h). Since mammalian RAD51C is required for both the early and the late stages of HR repair, through respectively its abilities to facilitate the loading of RAD51 to DNA damage site and the dissolution of Holliday junctions 41,44,49, it is not surprising that we observed a severe reduction in gene conversion assays but only a moderate effect on RAD51 foci formation in RAD18 deficient cells (Fig. 4).
We believe that RAD18 carries out distinct functions in response to DSBs versus UV lesions (Fig. 8f). While the main function of RAD18 in UV repair seems to be mediated by its role in promoting PCNA monoubiquitination, increasing evidence suggest that the monoubiquitinated PCNA is dispensable for RAD18 function in HR repair: (1) RAD18 promotes PCNA monoubiquitination and HR repair using separate domains; (2) the E3 ligase activity of RAD18 is not required for RAD18-mediated HR repair; (3) DSBs can not efficiently induce PCNA monoubiquitnation; (4) RNF8 is required for RAD18 loading to DSB sites but not for UV-induced PCNA monoubiquitination; (5) RAD51 foci are induced normally following IR in the PCNA K164R mutant DT40 cells 28; (6) DT40 cells carrying the PCNA K164R mutation displayed only a modest increase in sensitivity to CPT 27. Similar to RAD18, the E2 enzyme UBC13 also acts both in DSB repair and UV-induced post-replication repair (PRR) in high eukaryotes 28. The dual roles of RAD18 and UBC13 are in marked contrast to their assigned function in PRR in yeast. It is possible that the functions of these regulatory molecules for PRR pathway may have changed during metazoan evolution to help cope with the more complex tasks of ensuring genome stability in vertebrate cells.
METHODS
Antibodies
RAD18 monoclonal and polyclonal antibodies were obtained form Novus and Bethyl respectively. Antibodies against the myc epitope, H2AX, γ-H2AX, ubiquitin, RNF8, Rap80, BRCA1 and RAD51 were described previously 10,50. The anti-FK2 and anti-ORC2 antibodies were purchased from Upstate Cell Signaling. Anti-CHK1 and anti-PCNA antibodies were obtained from Santa Cruz. Anti-Flag (M2), anti-RAD51C, anti-GAPDH and anti-p-CHK1 S317 antibodies were purchased from Sigma, Novus, Calbiochem and Cell Signaling, respectively.
Constructs
The full-length and deletion/point mutants of human RAD18, RAD51 and RAD51 paralogs were generated by PCR and subcloned into the pDONR201 vector using Gateway Technology (Invitrogen). For transient expression, the corresponding fragments in entry vectors were transferred into a Gateway compatible destination vector, which harbors an N terminal triple-epitope tag (S protein tag, Flag epitope tag and Streptavidin binding peptide tag) or Myc epitope tag. DNA fragments corresponding to residues 495–585 of Rap80 and residues 186–240 of RAD18 were also subcloned into the pDONR201 vector, transferred into a Gateway compatible destination vector to generate constructs for the expression of GST-Rap80-ZNF or GST-RAD18-ZNF in E. coli. Constructs containing full-length or deletion mutants of human RNF8 were previously described 10. Ubi-GST construct was kindly provided by Dr. Bruce Horazdovsky from the Mayo Clinic (Minnesota, USA).
Cell Cultures
For the generation of RAP80−/− MEFs, an ES cell line RRN158 was purchased from Bay Genomics. In this cell line, RAP80 gene was disrupted by a neo gene selection cassette inserted between exon 1 (with ATG in it) and exon 2 of RAP80. Similarly, an ES cell line RRR260 was also obtained from Bay Genomics for the generation of RNF8-deficient MEFs. In this case, RNF8 gene was disrupted by a neo gene selection cassette inserted between transcripted exon 4 to exon 5 of RNF8. The exact insertion sites were mapped by genomic PCR and DNA sequencing. These ES cells were injected into C57BL/6 blastocysts to generate chimeric mice, which were crossed back with C57BL/6 mice to obtain RAP80+/− or RNF8 +/− mice. The heterozygotes were intercrossed to generate RAP80−/− or RNF8−/− mice. The RAP80 or RNF8 deficient MEFs were generated from their corresponding E13.5 embryos. The full description of these mice will be published in separate manuscripts.
H2AX−/−, MDC1−/−, 53BP1−/− and wild-type mouse embryonic fibroblast (MEF) cells, NBS-deficient fibroblast cells (NBS-ILB1) and cells reconstituted with wild-type NBS1, HCC1937 and HCC1937-BRCA1 were previously reported 10,51. HCT116 Rad18−/− cells, RAD18−/− MEFs, UBC13 deficient cells, IRS3 and V79 cells were generous gifts from Tadahiro Shiomi at National Institute of Radiological Sciences (Chiba, Japan), Masaru Yamaizumi at Kumamoto University (Kumamoto, Japan), Shizuo Akira at Osaka University (Osaka, Japan) and Hatsumi Nagasawa at Colorado state university, respectively. U2OS cells with DR-GFP integration were kindly provided by Maria Jasin at Memorial Sloan-Kettering Cancer Center (New York).
GST pull-down assay
The GST fusion proteins were expressed in Escherichia coli and purified as previously described 52. 2 µg of GST-fusion protein or GST alone was immobilized on the glutathione-Sepharose 4B beads and incubated with lysates prepared from cells that were transiently transfected with plasmids encoding indicated proteins.
The establishment of stable cell lines and Affinity Purification of S-Flag-SBP(SFB)-tagged protein complexes
293T cells were transfected with plasmids encoding SFB-tagged proteins. Cell lines stably expressing tagged proteins were selected by culturing in the medium containing puromycin (2 µg/ml) and confirmed by immunoblotting and immunostaining. For affinity purification, 293T cells stably expressing tagged proteins were lysed with NETN buffer for twenty minutes. Crude lysates were cleared by centrifugation at 14,000 rpm at 4 °C for ten minutes, and supernatants were incubated with 300 µl streptavidin-conjugated beads (Amersham). The immunocomplexes were washed three times with NETN buffer and then bead-bound proteins were eluted with 1 ml NETN buffer containing 1 mg/ml biotin (Sigma). The eluted supernatant was incubated with 80 µl S protein beads (Novagen). The immunocomplexes were washed three times with NETN buffer and subjected to SDS-PAGE. Protein bands were excised, digested and the peptides were analyzed by mass spectrometry.
Gene conversion assay
1×106 cells were electroporated with 12 µg of DR-GFP plasmid together with 12 µg of pCBASce plasmid at 270V, 975uF using a BioRad genepulsar II. Cells were plated onto 10 cm dishes and incubated in culture media for forty-eight hours prior to FACS analyses. Results were the averages of data obtained from three independent experiments.
Immuofluorescence staining
To visualize IRIF, cells cultured on coverslips were treated with 10 Gy of gamma irradiation (1 Gy = 100 Rads) or 1 µM CPT followed by recovery for six hours or two hours, respectively. Cells were then pre-extracted with buffer containing 0.5% triton X-100 for five minutes and fixed using 3% paraformaldehyde solution for ten minutes at room temperature. Samples were blocked with 5% goat serum and incubated with primary antibody for thirty minutes. Samples were washed and incubated with secondary antibody for thirty minutes. Cells were then stained with DAPI to visualize nuclear DNA.
SiRNA
All siRNA duplexes were purchased from Dharmacon Research (Lafayette, CO). The sequences of RAD18 SiRNA, RNF8 SiRNA1 and SiRNA2 are ACUCAGUGUCCAACUUGCUdTdT, CAGAGAAGCUUACAGAUGUUU and AGA AUGAGCUCCAAUGUAUUU, respectively. The sequence of control SiRNA is UUCAAUAAAUUCUUGAGGUUU.
Retrovirus production and infection
pDONR201 derivative constructs containing full-length or mutants of RAD18 or RAD51C were transferred into a gateway compatible pEF1A-HA-FLAG retroviral vector. Virus supernatant was collected forty-eight hours after the co-transfection of pEF1A vectors and pcl-ampho into BOSC23 cells. MEF or IRS3 cells were infected with viral supernatant in the presence of polybrene (8 µg/ml). Cells were then selected in growth media containing 2 µg/ml or 5 µg/ml puromycin respectively.
Cell survival assays
1×103 cells were seeded onto 60 mm dish in triplicates. Twenty-four hours after seeding, cells were treated with CPT or irradiated with IR or UV. Medium was replaced twenty-four hours later and cells were then incubated for fourteen days. Resulting colonies were fixed and stained with Coomassie blue. Numbers of colonies were counted using a GelDoc with Quantity One software (BIORAD). Results were the averages of data obtained from three independent experiments.
Chromatin fractionation
Preparation of chromatin fractions were described previously with modifications 10,53. Briefly, cells were collected six hours after treatment with 10 Gy of ionizing radiation, four hours after treatment with 60 J/m2 of UV or sixteen hours after treatment with 50 nM of CPT and washed once with PBS. Cell pellets were subsequently resuspended in low salt permeabilization buffer (10 mM HEPES pH7.4, 10 mM KCl, 0.05% NP-40 and protease inhibitors) and incubated on ice for twenty minutes. Thereafter, nuclei were recovered and resuspended in 0.2 M HCl. The soluble fraction was neutralized with 1 M Tris-HCl pH 8.0 for further analysis. For microccocal nuclease (MNase) treatment, nuclei recovered after low salt extraction was washed and resuspended in nuclease reaction buffer (10 mM HEPES pH 7.4, 10 mM KCl, 0.5 mM MgCl2, 2 mM CaCl2). 20 Unit of nuclease was added and incubated for thirty minutes on ice. Thereafter, the insoluble fraction was treated essential as above to isolate the chromatin-bound proteins.
Supplementary Material
1
ACKNOWLEDGEMENTS
We thank Tadahiro Shiomi at National Institute of Radiological Sciences (Chiba, Japan) for HCT116 RAD18−/− cells, Masaru Yamaizumi at Kumamoto University (Kumamoto, Japan) for RAD18−/− MEFs, Shizuo Akira at Osaka University (Osaka, Japan) for UBC13 deficient cells, Hatsumi Nagasawa at Colorado state university for IRS3 and V79 cells, Maria Jasin at Memorial Sloan-Kettering Cancer Center (New York) for the U2OS cells with DR-GFP integration, DR-GFP, pCBASce plasmids and Benjamin P. Chen at University of Texas Southwestern Medical Center (Dallas) for NU7441. We would like to thank Jody Groenendyk at University of Alberta for helping with the BIAcore system. Jun would like to thank all colleagues in Chen’s laboratory for insightful discussion and technical assistance. Jun also thanks Jamie Wood for proofreading the paper. This work was supported by grants from the National Institutes of Health (to J.C.). M.S.Y.H is supported by the Anna Fuller Fund Fellowship. J.C is a recipient of an Era of Hope Scholar award from the Department of Defense and a member of the Mayo Clinic Breast SPORE program.
References
- 1.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 2.Friedberg EC. DNA damage and repair. Nature. 2003;421:436–440. doi: 10.1038/nature01408. [DOI] [PubMed] [Google Scholar]
- 3.Bartkova J, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 4.Gorgoulis VG, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 5.Bartek J, Lukas J. DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol. 2007;19:238–245. doi: 10.1016/j.ceb.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: the histone guardian of the genome. DNA Repair (Amst) 2004;3:959–967. doi: 10.1016/j.dnarep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Bassing CH, et al. Histone H2AX: a dosage-dependent suppressor of oncogenic translocations and tumors. Cell. 2003;114:359–370. doi: 10.1016/s0092-8674(03)00566-x. [DOI] [PubMed] [Google Scholar]
- 8.Celeste A, et al. H2AX haploinsufficiency modifies genomic stability and tumor susceptibility. Cell. 2003;114:371–383. doi: 10.1016/s0092-8674(03)00567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H, Huang J, Chen J. CCDC98 is a BRCA1-BRCT domain-binding protein involved in the DNA damage response. Nat Struct Mol Biol. 2007;14:710–715. doi: 10.1038/nsmb1277. [DOI] [PubMed] [Google Scholar]
- 10.Huen MS, et al. RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell. 2007;131:901–914. doi: 10.1016/j.cell.2007.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang B, Elledge SJ. Ubc13/Rnf8 ubiquitin ligases control foci formation of the Rap80/Abraxas/Brca1/Brcc36 complex in response to DNA damage. Proc Natl Acad Sci U S A. 2007;104:20759–20763. doi: 10.1073/pnas.0710061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mailand N, et al. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell. 2007;131:887–900. doi: 10.1016/j.cell.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 13.Kolas NK, et al. Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science. 2007;318:1637–1640. doi: 10.1126/science.1150034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plans V, et al. The RING finger protein RNF8 recruits UBC13 for lysine 63-based self polyubiquitylation. J Cell Biochem. 2006;97:572–582. doi: 10.1002/jcb.20587. [DOI] [PubMed] [Google Scholar]
- 15.Weinstock DM, Richardson CA, Elliott B, Jasin M. Modeling oncogenic translocations: distinct roles for double-strand break repair pathways in translocation formation in mammalian cells. DNA Repair (Amst) 2006;5:1065–1074. doi: 10.1016/j.dnarep.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 16.Lukas J, Bartek J. Watching the DNA repair ensemble dance. Cell. 2004;118:666–668. doi: 10.1016/j.cell.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy RD, D'Andreaz AD. DNA repair pathways in clinical practice: lessons from pediatric cancer susceptibility syndromes. J Clin Oncol. 2006;24:3799–3808. doi: 10.1200/JCO.2005.05.4171. [DOI] [PubMed] [Google Scholar]
- 18.West SC. Molecular views of recombination proteins and their control. Nat Rev Mol Cell Biol. 2003;4:435–445. doi: 10.1038/nrm1127. [DOI] [PubMed] [Google Scholar]
- 19.Thacker J. The RAD51 gene family, genetic instability and cancer. Cancer Lett. 2005;219:125–135. doi: 10.1016/j.canlet.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Masson JY, et al. Identification and purification of two distinct complexes containing the five RAD51 paralogs. Genes Dev. 2001;15:3296–3307. doi: 10.1101/gad.947001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sigurdsson S, et al. Mediator function of the human Rad51B-Rad51C complex in Rad51/RPA-catalyzed DNA strand exchange. Genes Dev. 2001;15:3308–3318. doi: 10.1101/gad.935501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tateishi S, Sakuraba Y, Masuyama S, Inoue H, Yamaizumi M. Dysfunction of human Rad18 results in defective postreplication repair and hypersensitivity to multiple mutagens. Proc Natl Acad Sci U S A. 2000;97:7927–7932. doi: 10.1073/pnas.97.14.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tateishi S, et al. Enhanced genomic instability and defective postreplication repair in RAD18 knockout mouse embryonic stem cells. Mol Cell Biol. 2003;23:474–481. doi: 10.1128/MCB.23.2.474-481.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe K, et al. Rad18 guides poleta to replication stalling sites through physical interaction and PCNA monoubiquitination. Embo J. 2004;23:3886–3896. doi: 10.1038/sj.emboj.7600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szuts D, Simpson LJ, Kabani S, Yamazoe M, Sale JE. Role for RAD18 in homologous recombination in DT40 cells. Mol Cell Biol. 2006;26:8032–8041. doi: 10.1128/MCB.01291-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saberi A, et al. RAD18 and poly(ADP-ribose) polymerase independently suppress the access of nonhomologous end joining to double-strand breaks and facilitate homologous recombination-mediated repair. Mol Cell Biol. 2007;27:2562–2571. doi: 10.1128/MCB.01243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao GY, et al. A critical role for the ubiquitin-conjugating enzyme Ubc13 in initiating homologous recombination. Mol Cell. 2007;25:663–675. doi: 10.1016/j.molcel.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 29.Morris JR, Solomon E. BRCA1 : BARD1 induces the formation of conjugated ubiquitin structures, dependent on K6 of ubiquitin, in cells during DNA replication and repair. Hum Mol Genet. 2004;13:807–817. doi: 10.1093/hmg/ddh095. [DOI] [PubMed] [Google Scholar]
- 30.Polanowska J, Martin JS, Garcia-Muse T, Petalcorin MI, Boulton SJ. A conserved pathway to activate BRCA1-dependent ubiquitylation at DNA damage sites. Embo J. 2006;25:2178–2188. doi: 10.1038/sj.emboj.7601102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim H, Chen J, Yu X. Ubiquitin-binding protein RAP80 mediates BRCA1-dependent DNA damage response. Science. 2007;316:1202–1205. doi: 10.1126/science.1139621. [DOI] [PubMed] [Google Scholar]
- 32.Sobhian B, et al. RAP80 targets BRCA1 to specific ubiquitin structures at DNA damage sites. Science. 2007;316:1198–1202. doi: 10.1126/science.1139516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang B, et al. Abraxas and RAP80 form a BRCA1 protein complex required for the DNA damage response. Science. 2007;316:1194–1198. doi: 10.1126/science.1139476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrini JH. Cell signaling. A touching response to damage. Science. 2007;316:1138–1139. doi: 10.1126/science.1143700. [DOI] [PubMed] [Google Scholar]
- 35.Sartori AA, et al. Human CtIP promotes DNA end resection. Nature. 2007;450:509–514. doi: 10.1038/nature06337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamashita YM, et al. RAD18 and RAD54 cooperatively contribute to maintenance of genomic stability in vertebrate cells. Embo J. 2002;21:5558–5566. doi: 10.1093/emboj/cdf534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshimura A, et al. A novel Rad18 function involved in protection of the vertebrate genome after exposure to camptothecin. DNA Repair (Amst) 2006;5:1307–1316. doi: 10.1016/j.dnarep.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 38.Weinstock DM, Nakanishi K, Helgadottir HR, Jasin M. Assaying double-strand break repair pathway choice in mammalian cells using a targeted endonuclease or the RAG recombinase. Methods Enzymol. 2006;409:524–540. doi: 10.1016/S0076-6879(05)09031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorensen CS, et al. The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat Cell Biol. 2005;7:195–201. doi: 10.1038/ncb1212. [DOI] [PubMed] [Google Scholar]
- 40.French CA, Tambini CE, Thacker J. Identification of functional domains in the RAD51L2 (RAD51C) protein and its requirement for gene conversion. J Biol Chem. 2003;278:45445–45450. doi: 10.1074/jbc.M308621200. [DOI] [PubMed] [Google Scholar]
- 41.Kuznetsov S, et al. RAD51C deficiency in mice results in early prophase I arrest in males and sister chromatid separation at metaphase II in females. J Cell Biol. 2007;176:581–592. doi: 10.1083/jcb.200608130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Tarsounas M, O'Regan P, West SC. Role of RAD51C and XRCC3 in genetic recombination and DNA repair. J Biol Chem. 2007;282:1973–1979. doi: 10.1074/jbc.M609066200. [DOI] [PubMed] [Google Scholar]
- 43.Reynolds J. Resolving a Holliday romance. Nat Cell Biol. 2004;6:184. doi: 10.1038/ncb0304-184a. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Masson JY, Shah R, O'Regan P, West SC. RAD51C is required for Holliday junction processing in mammalian cells. Science. 2004;303:243–246. doi: 10.1126/science.1093037. [DOI] [PubMed] [Google Scholar]
- 45.French CA, et al. Role of mammalian RAD51L2 (RAD51C) in recombination and genetic stability. J Biol Chem. 2002;277:19322–19330. doi: 10.1074/jbc.M201402200. [DOI] [PubMed] [Google Scholar]
- 46.Godthelp BC, et al. Mammalian Rad51C contributes to DNA cross-link resistance, sister chromatid cohesion and genomic stability. Nucleic Acids Res. 2002;30:2172–2182. doi: 10.1093/nar/30.10.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hinz JM, Helleday T, Meuth M. Reduced apoptotic response to camptothecin in CHO cells deficient in XRCC3. Carcinogenesis. 2003;24:249–253. doi: 10.1093/carcin/24.2.249. [DOI] [PubMed] [Google Scholar]
- 48.Takata M, et al. Chromosome instability and defective recombinational repair in knockout mutants of the five Rad51 paralogs. Mol Cell Biol. 2001;21:2858–2866. doi: 10.1128/MCB.21.8.2858-2866.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodrigue A, et al. Interplay between human DNA repair proteins at a unique double-strand break in vivo. Embo J. 2006;25:222–231. doi: 10.1038/sj.emboj.7600914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen J, et al. Stable interaction between the products of the BRCA1 and BRCA2 tumor suppressor genes in mitotic and meiotic cells. Mol Cell. 1998;2:317–328. doi: 10.1016/s1097-2765(00)80276-2. [DOI] [PubMed] [Google Scholar]
- 51.Maser RS, Zinkel R, Petrini JH. An alternative mode of translation permits production of a variant NBS1 protein from the common Nijmegen breakage syndrome allele. Nat Genet. 2001;27:417–421. doi: 10.1038/86920. [DOI] [PubMed] [Google Scholar]
- 52.Hofer B, Backhaus S, Timmis KN. The biphenyl/polychlorinated biphenyl-degradation locus (bph) of Pseudomonas sp. LB400 encodes four additional metabolic enzymes. Gene. 1994;144:9–16. doi: 10.1016/0378-1119(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 53.Yu X, Fu S, Lai M, Baer R, Chen J. BRCA1 ubiquitinates its phosphorylation-dependent binding partner CtIP. Genes Dev. 2006;20:1721–1726. doi: 10.1101/gad.1431006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1