Mitochondrial Structural and Functional Dynamics in Huntington’s Disease (original) (raw)
. Author manuscript; available in PMC: 2010 Jun 1.
Abstract
Huntington’s disease (HD) is an autosomal, dominantly inherited neurodegenerative disorder, characterized by chorea, involuntary movements, and cognitive impairments. Tremendous progress has made since the discovery of HD gene in 1993, in terms of developing animal models to study the disease process, unraveling the expression and function of wild-type and mutant huntingtin (Htt) proteins in the central and peripheral nervous systems, and understanding expanded CAG repeat containing mutant Htt protein interactions with CNS proteins in the disease process. HD progression has been found to involve several pathomechanisms, including expanded CAG repeat protein interaction with other CNS proteins, transcriptional dysregulation, calcium dyshomeostasis, abnormal vesicle trafficking, and defective mitochondrial bioenergetics. Recent studies have found that mutant Htt is associated with mitochondria and causes mitochondrial structural changes, decreases mitochondrial trafficking, and impairs mitochondrial dynamics in the neurons affected by HD. This article discusses recent developments in HD research, with a particular focus on intracellular and intramitochondrial calcium influx, mitochondrial DNA defects, and mitochondrial structural and functional abnormalities in HD development and progression. Further, this article outlines the current status of mitochondrial therapeutics with a special reference to Dimebon.
1. Introduction
Huntington’s disease (HD) is an autosomal, dominantly inherited neurodegenerative disease, characterized by chorea, seizures, involuntary movements, dystonia, cognitive decline, intellectual impairment, and emotional disturbances (Vonsattel et al., 1985; Folstein, 1990; Bates, 2005; Lin and Beal 2006, Montoya et al., 2006). HD occurs in 4 to 10 per 100,000 persons mainly of Caucasian origin. HD is a midlife disease with some exceptional cases of early onset as early as 2 years and of late onset in the mid 80s (Kremer, 2002). Typically, HD patients survive for about 15–20 years from the date of disease onset.
In patients with HD, selective medium spiny neuronal loss has been observed in the caudate and putamen of the striatum of basal ganglia, in pyramidal neurons of the cerebral cortex and, to lesser extent, in hippocampal and subthalamus neurons (Byers et al., 1973; Vonsattel et al., 1985, Spargo et al., 1993). The neuronal loss has been found up to 80% in patients with severe HD (Vansettal et al., 1985). Reactive astrogliosis has also been observed in the affected brain regions of HD patients. In addition, mutant huntingtin (Htt) protein aggregates or intra-neuronal inclusions have been found in pathological sites in HD postmortem brain specimens and brain specimens from HD mouse models (Mangiarini et al., 1996; DiFiglia et al., 1997; Davies et al., 1997; Reddy et al., 1998, 1999; Schilling et al., 1999; Hodgson et al., 1999; Levine et al., 1999; Wheeler et al., 1999; Yamamoto et al., 2000).
In the last 15–20 years, tremendous progress has been made in HD research in terms of: 1) discovering HD gene, 2) understanding the expanded polyglutamine repeat containing the mutant Htt protein, 3) developing HD cell, animal models, which now include HD fly, worm, mouse, and non-human primate models (Mangiarini et al., 1996; Reddy et al., 1998; Jackson et al., 1998; Schilling et al., 1999; Kim et al., 1999; Hodgson et al., 1999; Levine et al., 1999; Yamamoto et al., 2000; Wheeler et al., 1999; Faber et al., 1999; Marsh et al., 2000; Romero et al., 2008; Lin et al., 2001; Laforet et al., 2001; Yang et al., 2008), 4) developments in decreasing the expression of the expanded polyglutamine repeat allele that has been found to damage or kill medium spiny neurons in HD patients (Harper et al., 2005; DiFiglia et al., 2007; Van Bilen et al., 2008; Zhang et al., 2009; Boudreau et al., 2009), and 5) developing therapeutics to reduce symptoms of HD in animal models and HD patients. However, the causal factors that selectively target medium spiny neurons in HD patients are still unclear. Further, the precise link between chorea and neuronal damage in HD progression is not completely understood. This article briefly reviews HD gene, the role of mutant Htt in HD progression, and mechanisms that are involved in HD pathogenesis. This article also discusses the latest developments in mitochondrial structural and functional abnormalities in relation to mutant Htt in HD pathogenesis.
2. HD gene and mutant huntingtin in HD progression
HD is a purely genetic disease unlike Alzheimer’s and Parkinson’s (Reddy, 2007, 2008). In 1983, HD gene was mapped to the p arm of chromosome 16 (Gusella et al., 1983), and after 10 years of intense search with state-of-the-art molecular biology techniques and collaborations among several labs across the world, in 1993 the HD gene was identified (The Collaborative Research Group, 1993). The discovery of the HD gene (‘CAG repeat or polyglutamine repeat expansion as a mutation’) opened the door for the identification of mutant genes associated with another eight brain diseases with expanded polyglutamine repeats (see Table 1 identifying these diseases).
Table 1.
Summary of Polyglutamine Repeat Diseases in Humans
| Polyglutamine Disease | Normal CAG Repeat length | Affected CAG Repeat Length | Affected Brain Region | Clinical Symptoms | Protein Size & Localization |
|---|---|---|---|---|---|
| Huntington’s Disease | 6–35 | 39–120 | Striatum, cerebral cortex, thalamus | Chorea, involuntary movements, dementia, inteclluctual impairments, seizures | 3144aa; Cytoplasm, nucleus, mitochondria, ER |
| Dentatorubral- palliolysian atrophy | 6–35 | 48–88 | Globus pallidus, dentatorubral and subthalamus | Choreathetosis, ataxia, and dementia | 1190aa; Nucleus |
| Spinobulbar muscular atrophy | 1–34 | 40–62 | Loss of bulbar neurons | Muscle weakness and atrophy | 906aa; Cytoplasm and nucleus |
| Spinocerebellar ataxia 1 | 6–39 | 41–81 | Cerebellum, dentate nucleus, brain-stem | Ataxia, cognitive impairments, spasticity | 8166aa; Nucleus and cytoplasm |
| Spinocerebellar ataxia 2 | 15–29 | 35–59 | Cerebellum, pontine nucleus and substantia niagra | Ataxia and dystharthia | 1313aa; Cytoplasm and nucleus |
| Spinocerebellar ataxia 3 | 13–36 | 62–84 | Substantia niagra, globus pallidus, pontine nucleus and caudate nucleus | Ataxia, dystonea, opthalmoplegia | 354aa; Nucleus and cytoplasm |
| Spinocerebellar ataxia 6 | 4–16 | 21–27 | Cerebellum and mild brain-stem atrophy | Ataxia, dystharthia, vibratory sensory loss | 2502aa; Cytoplasm and nucleus |
| Spinocerebellar ataxia 7 | 7–17 | 38–130 | Cerebral cortex, basis pontis, inferior olive and retinal ganglial cells | Ataxia, dementia, blindness, cardiac failure infantile form | 892aa; Cytoplasm and nucleus |
| Spinocerebellar ataxia 17 | 25–42 | 47–63 | Cerebellum, brain-stem and cerebral cortex | Ataxia, cognitive impairments, psychiatric problems | 339aa; Cytoplasm and nucleus |
HD is caused by a genetic mutation that results in an expanded polyglutamine encoding repeat, within exon 1 of the HD gene. In persons affected with HD, the number of polyglutamine repeats ranges from 36–120, whereas in unaffected persons, it ranges from only 6–35 (Reddy et al., 1999). Polyglutamine repeats are highly polymorphic in general, and their length increase in every generation when expanded polyglutamine repeats inherited through males, this phenomenon referred to as genetic anticipation (Reddy et al., 1999). Available epidemiological and genetic data suggest that the onset of HD inversely correlates with the length of the polyglutamine repeats in the HD gene.
Htt, the product of the HD gene, is a 350 kDa protein, ubiquitously expressed in the brain and peripheral tissues (see reviews of Reddy et al., 1999; Li and Li, 2005; Bates, 2005; Orr and Zoghbi, 2007). In the brain, Htt is primarily localized in the cytoplasm of neurons (see reviews of Reddy and Tagle, 1999; Li and Li, 2005; Bates, 2005; Orr and Zoghbi, 2007). However, several recent studies have reported a small portion of mutant polyglutamine Htt in several subcellular organelles, including the nucleus, plasma membrane, mitochondria, lysosomes, endoplasmic reticulum, and that the translocated Htt impairs organelle function (Kegel et al., 2002, 2005; Panov et al., 2002; Choo et al., 2004; Truant et al., 2006; Strehlow et al., 2007; Atwal et al., 2007; Orr et al., 2008). Mutant Htt interacts with a large number of brain proteins, with the extent of this interaction dependent on the number of expanded polyglutamine repeats (see review of Borell-Pages et., 2006; Charles et al., 2000). This mutant protein interaction ultimately, leading to the gain of function of mutant Htt in the progression of HD.
Although both mutant and wild-type Htt are expressed ubiquitously in the brain, the selective and premature death of striatal projection neurons has been reported in HD patients and HD transgenic mice (Vonsattel et al., 1985; Folstein, 1990; Reddy et al., 1998; Reddy and Tagle, 1999; Hodgson et al., 1999; Schilling et al., 1999; Van Raamsdonk et al., 2007). Causes of this selective neuronal loss are not completely understood, and how the mutant Htt causes HD progression is also unclear. Several mechanisms and pathways have been proposed to explain causes of HD progression, including: transcriptional dysregulation, expanded polyglutamine repeat protein interaction with other proteins in the central nervous system (see review of Borell-Pages, 2006), caspase activation (Rigmonte et al., 2000; Jana et al., 2001; Hermel et al., 2004; Zhang et al., 2006; Majumdar et al., 2007; Warby et al., 2008), NMDAR activation (Sun et al 2001; Centoze et al., 2001; Lee and Chang, 2004; Fernandes et al., 2007), calcium dyshomeostasis (Panov et al., 2002, 2005; Oliveira et al., 2006, Milakovic et al., 2006; Oliveira et al., 2007; Rochabrand et al., 2007; Fernandes et al., 2007; Gellerich et al., 2008; Lim et al., 2008; Oliveira and Goncalves, 2009) and abnormal mitochondrial bioenergetics and axonal trafficking (Browne and Beal, 2004, 2006;Trushina et al., 2004; Li and Li, 2006; Chang et al., 2006; Orr et al., 2008).
3. Abnormal transcriptional dysregulation and HD
Abnormal transcriptional regulation of nuclear-encoded mitochondrial genes may be involved in HD pathogenesis. Indeed, mutant Htt has been found to bind to several transcription factors, including TATA binding proteins (Huang et al., 1998; Perez et al., 1998), Sp1 (Shimohata et al., 2000), and the nuclear scaffold protein NAKAP (Sayer et al., 2005). Mutant Htt interaction may interfere with the gene expression, activity, and transcriptional regulation of HD neurons. This possibility is supported by recent studies of PGC1α (potent suppressor of reactive oxygen species [ROS]) in HD (Cui et al., 2006; St-Pierre et al., 2006; Weydt et al., 2006). PGC1α was found decreased in HD postmortem brains, in cell lines expressing mutant Htt, and in HD mouse models, suggesting that the mutant Htt promotes the increased production of ROS; this increase in ROS may promote the interaction of Htt with the outer membrane of mitochondria, ultimately leading to decreased levels of PGC1α in mutant HD neurons (Cui et al., 2006; St-Pierre et al., 2006; Weydt et al., 2006).
PGC-1α is a transcription coactivator that interacts with a range of transcription factors involved in a wide variety of biological responses, including adaptive thermogenesis and mitochondrial biogenesis of several tissues, including brain tissues (Liang and Ward, 2006). Recently, using brain tissues from HD mice, postmortem brain tissues from HD patients, several researchers independently studied the connection between PGC-1α and HD mitochondrial bioenergetics (Cui et al., 2006; St-Pierre et al., 2006; Weydt et al., 2006). Cui and colleagues (2006) studied striatal neurons from postmortem brain tissues from HD patients, brain tissues from an HD knock-in mouse model that over-expresses mutant Htt, and cultured striatal neuronal cells from a knockin mice expressing 111 polyglutamines. They found a decrease in mRNA expression of PGC-1α in all 3 sources of striatal neurons, suggesting that mutant Htt interferes with the formation of the CREB/TAF4 complex that regulates transcription of the gene encoding PGC-1α. Using HD mice lines, Weydt and colleagues (2006) studied the connection between PGC-1α and adaptive thermogenesis in HD (Weydt et al., 2006) and found marked hypothermia at baseline temperatures, following cold exposure in two truncated HD mouse models. St. Pierre and colleagues (2006) found an increased expression of genes encoding ROS defense enzymes, including copper/zinc superoxide dismutase (SOD1), manganese SOD (SOD2), catalase, and glutathione peroxidase. In a study of PGC-1α-deficient mice, they also found that the basal expression of SOD1, SOD2, and catalase was considerably lower in the heart and brain of PGC-1α-deficient mice, regions known to be sensitive to oxidative stress, suggesting that the activation of PGC-1α protects HD neurons from mitochondrial toxicity caused by mutant Htt.
In another study of PGC1α, Lee et al. tested the hypothesis that mutant Htt influences the mitochondria via the interaction of polyglutamine repeats or the decrease in PGC-1α expression (Lee et al., 2007). They compared gene expression changes due to mutant Htt expressed in STHdh(Q111/Q111) cells with changes in gene expression produced by 3-NP treatment of wild-type striatal cells. In general, the HD mutation did not mimic 3-NP features, although both changes in gene expression produced a state of energy collapse that was mildly alleviated by the PGC-1α-coregulated nuclear respiratory factor 1. Moreover, unlike 3-NP, the HD polyglutamine repeat did not significantly alter mitochondrial pathways in STHdh(Q111/Q111) cells, despite a decrease in Ppargc1α gene expression. Instead, the HD mutation enriched for processes linked to the normal functioning of huntingtin and of NFκ-B signaling. Thus, rather than directly impacting mitochondria, the HD polyglutamine repeats protein may modulate some aspect of Htt’s activity in metabolizing extra-mitochondrial energy.
Findings from these studies suggest that in HD pathogenesis, PGC-1α may play a significant role in protecting neurons against mitochondrial toxicity and oxidative damage by increasing PGC-1α transcription and interaction with several transcription factors in HD neurons.
4. Mitochondrial abnormalities and HD
Several lines of evidence suggest that abnormal mitochondrial bioenergetics is involved in HD progression.
- Body weight loss is a major factor in the progression of HD that is reported in patients with HD and mouse models of HD (Kirkwood et al., 2001; Mahant et al., 2003; Hamilton et al., 2004; Phan et al., 2009; Aziz et al., 2008; Browne, 2008; Boss-Wetzel et al., 2008).
- Studies using magnetic resonance imaging of postmortem brains of HD patients revealed a progressive atrophy of the striatum compared to brain images of age-matched control subjects (Bamford et al., 1989; Aylward et al., 1994). Several other studies found atrophy in the caudate nucleus, putamen, globus pallidus and thalamus (Jernigan et al., 1991; Harris et al., 1996, Aylward et al., 1997; Fenna-Notestine et al., 2004). Reduced volume of the frontal and temporal cortical lobes in patients with HD has also been reported, and this reduction in volume may reflect white-matter loss (Backman et al., 1997; Aylward et al., 1998; Dierks et al., 1999). Findings from these imaging studies suggest that neuronal loss occurs in the striatum and cortical regions known to be affected in HD progression, and that white matter loss is involved in HD progression.
- Using positron emission tomography in functional studies of the brains of HD patients and control subjects, researchers found a marked decrease in glucose utilization in the striatum of patients (Kuhl et al., 1982; Young et al., 1986; Hayden et al., 1986; Kuwert et al., 1990; Berent et al., 1998; Powers et al., 2007). Decreased glucose metabolism was shown to correlate with several performance tasks in HD patients, including immediate recall memory, verbal associative learning, and executive functions, suggesting that cerebral glucose metabolism is defective in HD patients.
- Biochemical studies of mitochondria in striatal neurons from late-stage HD patients revealed reduced activity of several components of oxidative phosphorylation, including complexes II, III, and IV of the electron transport chain (Browne et al., 1997; Tabrizin et al., 1999; Senatorov et al., 2003; Browne and Beal, 2004; Fukui and Moraes, 2007). Further, in studies of HD transgenic and knockin mice and in experimental HD rodent models, decreases in enzyme activities of complexes I, II, III, and IV were found in brain tissues (Pandey et al., 2008), suggesting that mitochondria are involved in HD pathogenesis.
Recently, Fukui and Moreas investigated mitochondrial respiratory function using human osteosarcoma 143B cells expressing mutant Htt in an inducible manner (Fukui and Moreas, 2007). They found that cells expressing mutant Htt but not wild-type Htt exhibited a reduced activity of complex III and an increased activity of complex IV. In these studies, they also conversely found that pharmacological treatments inhibited complex III activity and significantly promoted the formation of Htt aggregates. This complex III-mediated modulation of Htt aggregates was observed in a neuronal progenitor RN33B cell line transduced by lentivirus carrying a mutant Htt. This effect of complex III inhibition on Htt aggregates appeared to be mediated by the inhibition of proteasome activity, but not by the depletion of ATP) or the production of ROS. Accordingly, complex III mutant cells also showed a decrease in proteasome activity. These results suggest a feedback system in the HD brain, connecting the mitochondrial respiratory complex III and Htt aggregates.
Recently, Solans et al. investigated mitochondrial respiration activity in yeast cells using an expanded polyglutamine repeat protein that they labeled with a green fluorescence protein (Solans et al., 2006). They found that in yeast cells expressing 103 polyglutamine repeats, cell respiration progressively reduced after 4–6 h of induction with galactose, and after 10 h of induction, it further reduced to 50% of the control. They also found cell respiration defects when the function and amount of mitochondrial respiratory chain complex II+III were altered with HD progression in congruency to data obtained from postmortem brain of HD patients and from toxin models. The production of ROS was also found significantly enhanced in yeast cells expressing 103 polyglutamines. The quenching of ROS with resveratrol partially prevented defects in cell respiration. Mitochondrial morphology and distribution were also altered in cells expressing 103 polyglutamines; this may have resulted from the interaction of aggregates and portions of the mitochondrial web and from a progressive disruption of the actin cytoskeleton. Interactions of misfolded aggregated polyglutamine domains with the mitochondrial and actin networks led to disturbances in mitochondrial distribution and function and to a increase in ROS production. Oxidative damage could preferentially affect the stability and function of enzymes containing iron-sulfur clusters such as complexes II and III. Findings from this yeast mitochondrial study further support that mitochondrial dysfunction may be involved in HD.
- Recent studies of HD knock-in striatal cells and lymphoblasts from HD patients revealed that expanded polyglutamine repeats are associated with low mitochondrial ATP and decreased mitochondrial ADP-uptake, suggesting that HD mutation is associated with mitochondrial functional defects (Seong et al., 2005).
- Biochemical studies of HD mice and HD cell lines revealed that calcium-induced mitochondrial permeability is a major factor in HD pathogenesis (Panov et al., 2002, 2005). This evidence is the strongest among all pathomechanisms reported in HD pathogenesis thus far and has been replicated in a large number of studies (Milakovic et al., 2006; Oliveira et al., 2006; Lim et al. 2008, Fernandes et al., 2007; Oliveira et al., 2007; Rochabrand et al., 2007; Gellerich et al., 2008; Oliveira and Goncalves, 2009). Details are given below.
- Recent studies of mitochondrial trafficking in HD cortical neurons revealed that mutant Htt aggregates impair mitochondrial movement (Trushina et al., 2004; Chang et al., 2006; Orr et al., 2008).
5. Mutant Htt and mitochondrial trafficking abnormalities
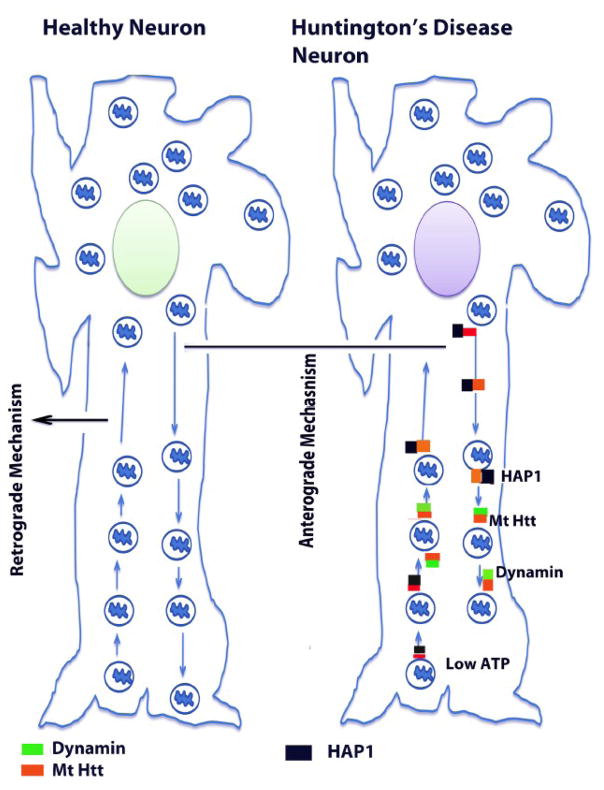
Recently, several studies reported that mutant Htt in association with mitochondria and microtubules impair the axonal transport of mitochondria to nerve terminals (Trushina et al., 2004; Chang et al., 2006; Orr et al., 2008) (see Fig. 1). This defective mitochondrial transport ultimately impairs neural transmission, and results in synaptic damage and selective neuronal damage or loss.
Figure 1.
Abnormal axonal transport of mitochondria in neurons affected by HD. Mitochondria are synthesized in the cell body, travel along the axons and dendrites to supply energy to nerve terminals, and then travel back to the cell body via mitochondrial trafficking. They are transported from the cell body to nerve terminals via an anterograde mechanism and from nerve terminals back to the cell body via a retrograde mechanism. In functionally active neurons, anterograde and retrograde transport of mitochondria are equal and active. Wild-type Htt regulates anterograde and retrograde transport of mitochondria and other endocytic vesicles in the neurons by interacting with several key trafficking proteins such as Huntington associated protein 1, dynamin, and clathryn. The interaction between wild-type Htt and trafficking proteins regulates microtubule mediated mitochondrial and vesicle transport that lead to the mitochondria moving along the axons. In medium spiny neurons in persons with HD, both anterograde and retrograde transport of mitochondria are slow because 1) mutant Htt interacts heavily with trafficking proteins, which may block/derail mitochondrial movement in the axons, 2) mutant Htt aggregates themselves may block mitochondrial movement, 3) a large number of defective mitochondria accumulate due to excessive mitochondrial fragmentation in HD neurons, and these defective mitochondria may not change their shape and size, may not travel along the axons to the nerve terminals, and 4) mutant Htt may create an imbalance between mitochondrial fission and fusion, leading to a decrease in overall mitochondrial dynamics in HD neurons. All these events may be responsible for low ATP production, mitochondrial dysfunction, and damaged medium spiny protection neurons in HD.
In studies of HD pathogenesis, Trushina et al. investigated mutant Htt involvement in impairment of fast axonal trafficking (Trishna et al., 2004). The expression of full-length mutant Htt was found to impair vesicular and mitochondrial trafficking in mammalian neurons in vitro and in whole animals in vivo. Mitochondria, in particular, became progressively immobilized and stopped more frequently in neurons from HD animal models. These defects occurred early in the development of disease, before the onset of measurable mitochondrial abnormalities. Consistent with a progressive loss of function, wild-type Htt, trafficking motors, and mitochondrial components were selectively sequestered by muant Htt in postmortem brain specimens from HD patients. Findings indicated that loss of Htt function may cause toxicity; mutant Htt-mediated aggregation sequestered Htt and components of trafficking machinery, leading to a loss of mitochondrial mobility and eventually to mitochondrial dysfunction (Trishna et al., 2004).
Chang et al. investigated whether mutant Htt alters mitochondrial trafficking and morphology in primary cortical neurons (Chang et al., 2006). They demonstrate that full-length mutant Htt was more effective than N-terminal mutant Htt in blocking mitochondrial movement, an effect that correlated with the heightened expression of full-length Htt in the cytosolic compartment. Aggregates impaired the passage of mitochondria along neuronal processes, causing mitochondria to accumulate adjacent to aggregates and to become immobilized. Further, mitochondrial trafficking was reduced specifically at sites of aggregates while remaining unaltered in regions lacking aggregates. Chang and colleagues suggested that in cortical neurons, an early event in HD pathophysiology may be the aberrant mobility and trafficking of mitochondria caused by cytosolic Htt aggregates (Chang et al., 2006).
Very recently, using HD knockin mice expressing N-terminal 150 polyglutamine repeats, Orr and colleagues investigated the association of N-terminal mutant Htt fragments with mitochondria. They found the N-terminal expanded polyglutamine repeat protein associated with mitochondria and that this association increased with age in knockin mice (Orr et al., 2008). The interaction between soluble N-terminal mutant Htt and mitochondria interfered with the in vitro association of microtubule-based transport of proteins and mitochondria. Mutant Htt reduced the distribution and transport rate of mitochondria in the processes of cultured neuronal cells. Reduced ATP levels were also found in the synaptosomal fraction isolated from mutant knock-in mouse brains. These findings suggest that before aggregates form, N-terminal mutant Htt fragments can impair mitochondrial function directly (Orr et al., 2008).
Findings from these elegant studies suggest that both N-terminal and full-length mutant Htt are associated with mitochondria and impair axonal transport, mitochondrial movements specially in neurons affected by HD. These studies also indicated that abnormal axonal transport and mitochondrial trafficking are early events in HD progression.
6. Abnormal mitochondrial dynamics and HD
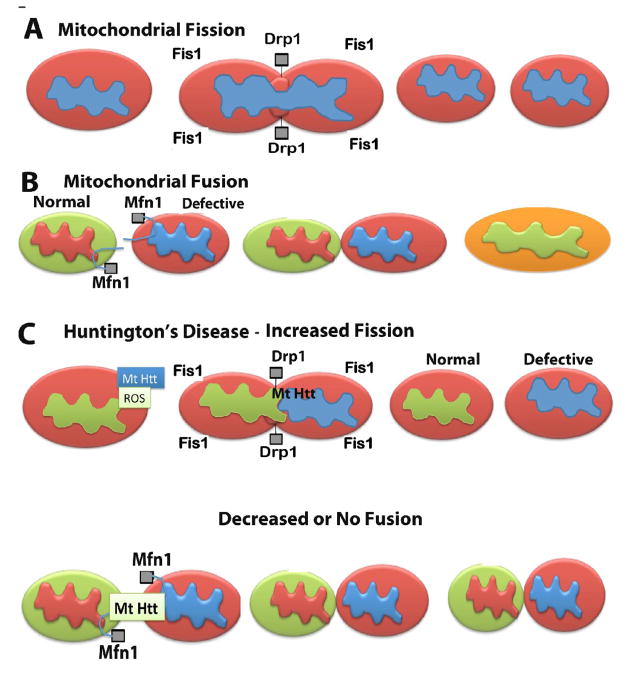
Mitochondrial shape and structure are maintained by 2 opposing forces: mitochondrial fission and mitochondrial fission (Chan et al., 2006; Reddy, 2007; Reddy, 2009). In a healthy neuron, fission and fusion mechanisms balance equally. Mitochondria alter their shape and size to move, through mitochondrial trafficking, from the cell body to the axons, dendrites, and synapses, and back to the cell body. Fission and fusion are controlled by evolutionary conserved, large GTPases belonging to the family of dynamin. Fission is controlled and regulated by the dynamin-related protein (Drp1) and mitochondrial fission 1 (Fis1), the latter of which is localized to the outer membrane of mitochondria (Reddy, 2007). Most of the Drp1 protein is localized in the cytoplasm, but a small part punctures the outer membrane, which promotes mitochondrial fragmentation. The increase in mitochondrial free radicals activates Fis1, which is also critical for mitochondrial fission.
Mitochondrial fusion is controlled by 3 GTPase proteins: 2 outer-membrane localized proteins Mfn1 and Mfn2, and 1 inner-membrane localized protein Opa (Reddy, 2007). The C-terminal part of Mfn1 mediates oligomerization between Mfn molecules of adjacent mitochondria and facilitates mitochondrial fusion. In HD neurons, mitochondrially generated free radicals activate Fis1 and promote an increase in mitochondrial fragmentation, which in turn produces defective mitochondria that ultimately damage neurons (see Fig 2). Mitochondrial fusion protects cells from the toxic effects of mitochondrial DNA, and mitochondrial mutant Htt by allowing functional complementation of mitochondrial DNA, proteins and metabolites. Cell hybrids resulting by fusing the parental cells carrying pathogenic mutations have been found to restore mitochondrial electron transport chain activity (Ono et al., 2001). It is possible that increased mitochondrial fission due to the association of mutant Htt in HD neurons may decrease mitochondrial fusion activity and may subsequently damage HD neurons.
Figure 2.
Increased mitochondrial fragmentation and decreased mitochondrial fusion in HD neurons. Mitochondrial shape and structure are maintained by 2 opposing forces: mitochondrial fission (A) and mitochondrial fission (B). In a healthy neuron, fission and fusion mechanisms balance equally. In HD neurons, an increase in mutant Htt may promote increased production of free radicals, ultimately activating Fis1 and Drp1. Increased Fis1 and Drp1 fragment mitochondria excessively in HD neurons (C). It is possible that mitochondrial fusion is decreased or absent in mitochondria from HD neurons because of mutant Htt interaction with fusion proteins (Mfn1, Mfn2 and Opa1).
There is limited evidence to suggest that, in HD, the balance between mitochondrial fission and fusion is abnormal. Recently, Wang et al. studied mitochondrial properties of HeLa cells that expressed green fluorescent protein or FLAG-tagged N-terminal portions of the Htt protein containing either, 17, 28, 74 or 138 polyglutamine repeats (Wang et al., 2009). Immunofluorescence staining using antibodies against Tom20, a mitochondrion localized protein, revealed that cells expressing Htt proteins with 74 or 138 CAG repeats were more sensitive to oxidative stress-induced mitochondria fragmentation and had reduced ATP levels than to cells expressing Htt proteins with 17 or 28 polyglutamine repeats. By measuring changes in fluorescence of a photoactivated GFP protein targeted to mitochondria, they found that cells expressing red fluorescent protein-tagged Htt protein containing 74 polyglutamine repeats had mitochondria that displayed reduced movement and reduced fusion compared to cells expressing RFP-Htt protein with 28 polyglutamine repeats. The overexpression of Drp-1(K38A), a dominant-negative mitochondria-fission mutant, and of Mfn2, a protein that promotes mitochondria fusion, suppressed polyglutamine-induced mitochondria fragmentation, reduced ATP levels, and damaged neurons. These results suggest that the increase in cytotoxicity induced by Htt proteins containing expanded polyglutamine tracts is likely mediated by an alteration in normal mitochondrial dynamics, which results in increased mitochondrial fragmentation (Wang et al., 2009).
Based on findings, we propose a model of mitochondrial dynamics in HD neurons that involves an increase of mitochondrial fragmentation, decrease the number of functionally active mitochondria, which ultimately imbalances mitochondrial dynamics (increased fission and decreased fission) in neurons from HD patients (Fig. 2).
7. Mitochondrial DNA defects and HD
Age-dependent mitochondrial DNA (mtDNA) damage is hypothesized to play a role in HD pathogenesis.
Acevedo-Torres and colleagues investigated mitochondrial DNA defects in two HD mouse models: the chemically induced 3-nitropropionic acid model and the HD transgenic mouse model of the R6/2 strain containing 115–150 polyglutamine repeats in the HD gene (Acevedo-Torres et al., 2009). They found that mitochondrial toxin 3-NPA inhibits complex II of the ETC and causes neurodegeneration that resembles HD in the striatum of postmortem brain specimens from HD patients and the HD mice. They measured nuclear and mtDNA damage by quantitative PCR in the striatum of 5- and 24-month-old untreated and 3-NPA-treated C57BL/6 mice. They found an increase in damage in both nuclear and mitochondrial genomes in the untreated 24-month-old mice. 3-NPA induced 4–6 times more damage in the mtDNA than in the nuclear DNA in the 5-month-old mice; mtDNA damage was repaired by 48 h after 3-NPA treatment. In the 24-month-old mice, 3NPA caused equal amounts of nuclear and mitochondrial damage that persistent in both genomes for 48 h. QPCR analysis showed a progressive increase in the levels of mtDNA damage in the striatum and cerebral cortex of 7–12 week-old R6/2 mice. Striatum exhibited eight-fold more damage to the mtDNA than to the nuclear gene. These data suggest that mtDNA damage is an early biomarker for HD-associated neurodegeneration, and they support the hypothesis that mtDNA lesions may contribute to the pathogenesis observed in HD (Acevedo-Torres et al., 2007).
Chen et al. investigated whether pathological changes in HD brains may also be present in peripheral tissues (Chen et al., 2007). Leukocyte 8-hydroxydeoxyguanosine and plasma malondialdehyde were elevated, and activities of erythrocyte Cu/Zn-superoxide dismutase and glutathione peroxidase were reduced in 16 HD patients when compared to 36 age- and gender-matched controls. Deleted and total mtDNA copy numbers were increased, whereas the mRNA expression levels of mtDNA-encoded mitochondrial enzymes were not elevated in the HD leukocytes compared to the leukocytes from normal controls. Plasma malondialdehyde levels also significantly correlated with HD disease severity. These results indicate that means to suppress oxidative damage may be beneficial in restoring mitochondrial function in HD patients.
Banoei et al. investigated 4 mtDNA deletions based on the size of deletion: 9 kb, 7.5 kb, 7 kb, and 5 kb in the mitochondrial DNA of HD patients (Banoei et al. 2007). Studying a group of 60 Iranian patients clinically diagnosed with HD and 70 healthy age-matched Controls, they found that 41 of the 60 HD patients exhibited polyglutamine expansion (Banoei et al., 2007). The 19 HD patients who did not show expansion exhibited clinical symptoms of HD. One of the four mtDNA deletions were in at least 90% of the samples from HD patients. Multiple deletions were also observed in 63% of the HD patients. None of the normal controls showed mtDNA deletions. The sizes and locations of the deletions did not correlate with expanded polyglutamine repeats or subject age. The study presented evidence that HD patients had higher frequencies of mtDNA deletions in lymphocytes compared to the controls. Overall, this study suggests that mutant Htt and instability in polyglutamine repeats may cause mtDNA damage in neurons affected by HD.
Findings from these studies suggest that mutant Htt cause mitochondrial DNA defects in HD brains and peripheral tissues from HD patients.
8. Calcium dyshomeostasis and HD
Several lines of evidence have recently suggested that abnormal Ca2+ uptake capacity is involved in HD neurons.
Oliveira and Goncalves investigated the buffering capacity of mitochondrial Ca2+ in cortical and striatal neuron-astrocyte co-cultures (Oliveira and Goncalves, 2009). They found that mitochondria not only in neurons but also in astrocytes from striatal origin exhibited a decrease in mitochondrial Ca2+ buffering capacity when compared with cortical counterparts. The decrease in this buffering capacity did not stem from variations in mitochondrial concentration or in the rate of intracellular Ca2+ elevation, but was mechanistically linked to an increased propensity of the mitochondria to undergo cyclosporin A-sensitive permeability transition. Indeed, 1 μM cyclosporin A selectively was found to increase the mitochondrial Ca2+ buffering capacity of striatal astrocytes, without modifying the neurons or cortical astrocytes. Neither the thapsigargin nor FK506 modified mitochondrial Ca2+ buffering in between cell types, excluding a predominant contribution of endoplasmic reticulum or calcineurin. These results provided additional evidence into the mechanisms of striatal vulnerability, showing an increase in Ca2+ vulnerability of striatal versus cortical mitochondria, in both intact neurons and astrocytes, thus positioning the striatum at greater risk for disturbed neuron-astrocyte interactions (Oliveira and Goncalves, 2009).
Rockabrand et al studied the mutant Htt subcellular localization, aggregation and intracellular Ca2+ dynamics in PC12 cells expressing various domains of mutant Htt (Rochabrand et al., 2007). They found that sub-cellular localization is most strongly influenced by the first 17 amino acids, with this sequence critically controlling Htt exon1 region mitochondrial localization and also promoting association with the endoplasmic reticulum and Golgi. This domain also enhances the formation of visible aggregates and together with the expanded polyglutamine repeats acutely disrupts intracellular Ca2+ levels in glutamate-challenged PC12 cells. Isolated cortical mitochondria incubated with Htt exon 1region resulted in uncoupling and depolarization of these organelles, further supporting the idea that Htt exon1-dependent mitochondrial dysfunction could be instrumental in promoting acute Ca2+ dyshomeostasis (Rochabrand et al., 2007).
Milakovic et al. elucidated the effects of Ca2+ on mitochondria from the wild type (STHdhQ7/Q7) and mutant (STHdhQ111/Q111) Htt-expressing cells of striatal origin (Milakovic et al., 2006). When treated with increasing Ca2+ concentrations, mitochondria from mutant Htt-expressing cells showed increased sensitivity to Ca2+, since mitochondria from mutant Htt-expressing cells were more sensitive to Ca2+ -induced decreases in state 3 respiration and DeltaPsim, than were mitochondria from wild-type cells. Further, mutant Htt-expressing cells had a reduced mitochondrial Ca2+ uptake capacity in comparison with the capacity of wild type cells. Decreases in state 3 respiration were associated with increased mitochondrial membrane permeability. The DeltaPsim defect was attenuated in the presence of ADP, and the decreases in Ca2+ uptake capacity were abolished in the presence of mitochondrial permeability transition pore inhibitors. These findings indicate that mutant Htt-expressing cells have mitochondrial Ca2+ handling defects that result in respiratory deficits and that the increased sensitivity of mutant Htt to Ca2+ induced mitochondrial permeabilization may be a contributing mechanism to mitochondrial dysfunction in HD.
Lim et al. investigated dysfunctions of Ca2+ homeostasis in mitochondria, in striatal neurons from postmortem brains of HD patients (Lim et al., 2008). They found mitochondria in mutant striatal neurons behaved normally, but are unable to handle large Ca2+ loads, may due to the increased sensitivity of Ca2+ to the permeability transition pore opening, which dissipates the membrane potential, prompting the release of accumulated Ca2+. Harmful ROS, produced by defective mitochondria and possibly stressing them, increases in mutant cells, particularly if the damage to mitochondria is artificially exacerbated with, for example, complex II inhibitors. Mitochondria in mutant cells are thus peculiarly vulnerable to stresses induced by Ca2+ and ROS. The observed decrease of cell Ca2+ could be a compensatory attempt to prevent Ca2+ stress that would irreversibly damage mitochondria and eventually lead to cell death.
Gellerich et al. studied brain mitochondria of transgenic HD rats with 51 glutamine repeats, which modeled the adult form of HD (Gellerich et al. 2008). Ca(free)(2+) up to 2 μM activated state 3 respiration of wild type mitochondria with glutamate/malate or pyruvate/malate as substrates. Ca(free)(2+) above 2 mum inhibited respiration via cyclosporin A-dependent permeability transition. Ruthenium red, an inhibitor of the mitochondrial Ca2+ uniporter, did not affect the Ca2+-dependent activation of respiration but reduced the Ca2+-induced inhibition. Thus, Ca2+ activation was mediated exclusively by extramitochondrial Ca2+, whereas Ca2+ inhibition was promoted by intramitochondrial Ca2+. In contrast, Htt(51Q) mitochondria showed a deficient state 3 respiration, a lower sensitivity to Ca2+ activation, and a higher susceptibility to Ca2+ dependent inhibition. Htt(51Q) mitochondria exhibited a diminished membrane potential stability in response to Ca2+, lower capacities and rates of Ca2+ accumulation, and a decreased Ca2+ threshold for permeability transition in a substrate-independent but cyclosporin A-sensitive manner. Compared with wild type, Ca2+ induced inhibition of respiration of Htt(51Q) mitochondria was less sensitive to ruthenium red, indicating the involvement of extra-mitochondrial Ca2+. This study concluded that interactions between Htt(51Q) and distinct targets such as aralar and/or the permeability transition pore may underlie mitochondrial dysregulation, leading to energetic depression, cell death, and tissue atrophy in HD.
Fernandes et al. investigated the connection between NMDA receptors and Ca2+ in full-length YAC HD transgenic mice expressing 128 polyglutamine repeats (Fernandes et al., 2007). NMDA-induced apoptosis were found to be enhanced in YAC128 medium spiny neurons in this mouse model. However, initial steps in the death-signaling pathway, including NMDA receptor current and cytosolic Ca2+ loading, were similar to those observed in wild-type medium spiny neurons. They also found that the NMDA receptor -mediated Ca2+ load triggered a strikingly enhanced loss of mitochondrial membrane potential in YAC128 medium spiny neurons, suggesting that NMDAR signaling via the mitochondrial apoptotic pathway is altered. This effect was accompanied by impaired cytosolic Ca2+ clearance after removal of NMDA, a difference that was not apparent after high potassium-evoked depolarization-mediated Ca2+ entry. Inhibition of the mitochondrial permeability transition reduced peak cytosolic Ca2+ and mitochondrial depolarization evoked by NMDA in YAC128 medium spiny neurons but not wild-type medium spiny neurons. These results suggest that the polyglutamine repeat length influences the mechanism by which mutant Htt enhances NMDA receptor-mediated excitotoxicity (Fernandes et al., 2007).
Oliveira et al. investigated bioenergetic behavior of mitochondria isolated from the from fore brains of R6/2 mice, YAC128 mice, and Hdh150 knock-in mice and wild-type littermates using in situ respiratory parameters in intact HD striatal neurons (Oliveira et al., 2007). They assessed the Ca2+ loading capacity of isolated mitochondria by steadily infusing Ca2+. Mitochondria from 12–13 weeks old R6/2 mice and 12 months old YAC128 mice, but not homozygous or heterozygous Hdh150 knock-in mice (15–17 weeks), exhibit increased Ca2+loading capacity when compared to non-transgenic, control mice. In situ mitochondria in intact striatal neurons show high respiratory control. Moreover, moderate expression of full-length mutant Htt does not significantly impair mitochondrial respiration in unstimulated neurons. However, when challenged with energy-demanding stimuli, Hdh150 neurons are more vulnerable to Ca2+ deregulation than neurons from nontransgenic, wild-type mice. These findings suggest to assess the HD mitochondrial function in the cellular context(Oliveira et al., 2007).
Using HD mouse models and real-time functional imaging of intracellular Ca2+ and mitochondrial membrane potential, Oliveira and colleagues studied the relationship between mitochondria and Ca2+ handling in intact HD striatal neurons (Oliveira et al., 2006). They treated HD striatal neurons with histone deacetylase inhibitors, which are known to protect neurons. This treatment reduced cell death in the HD models, but its effects on cellular function are unknown. Using use real-time functional imaging of intracellular Ca2+ and mitochondrial membrane potential, they explored the role of in situ HD mitochondria in Ca2+ handling. Immortalized striatal cells and striatal neurons from transgenic mice expressing full-length mutant Htt were used to model HD. They found that active glycolysis in HD striatal neurons occludes the mitochondrial role in Ca2+handling as well as the effects of mitochondrial inhibitors, HD striatal neurons and striatal neurons in the absence of glycolysis are critically dependent on oxidative phosphorylation for energy-dependent Ca2+handling, expression of full-length mutant Htt is associated with deficits in mitochondrial-dependent Ca2+handling that can be ameliorated by treatment with histone deacetylase inhibitors, and neurons with different response patterns to NMDA receptor activation exhibit different average somatic areas and are differentially affected by treatment with histone deacetylase inhibitors, suggesting subpopulation or functional state specificity. These findings indicate that neuroprotection induced by histone deacetylase inhibitors involves more efficient Ca2+ handling, thus improving the neuronal survival.
A closer examination of in vitro and in vivo studies of Ca2+ influx, mutant Htt and mitochondria reveal the following: 1) when treated with increasing Ca2+ concentrations, mitochondria from mutant Htt-expressing cells showed increased sensitivity to Ca2+, and decreased mitochondrial Ca2+ uptake capacity compared to wild type Htt expressing cells, 2) mutant Htt induce intracellular Ca2+ in HD neurons, 3) mutant Htt induced intracellular Ca2+ increases with polyglutamine repeat length in HD neurons, and 4) increased intracellular Ca2+ enter mitochondria and promote the opening of mitochondrial permeability transition pores, and damage HD neurons.
However, contrary to the above, in a recent study by Oliveira and colleagues found increased Ca2+ uptake capacity in forebrain mitochondria from 2 transgenic mice lines (R6/2 and YAC128) but not in HD knockin mice expressing 150 polyglutamine repeats. These authors used in situ hybridization techniques for the first time and assessed Ca2+ uptake capacity, and in situ hybridization technique is more reliable in assessing mitochondrial Ca2+ uptake capacity. Further research is needed to resolve the conflicting findings reported by Oliveira and colleagues using in situ hybridization technique by other groups.
Overall, overwhelming evidence clearly suggests that mutant Htt induce intracellular Ca2+ in neurons affected by HD and increased intracellular Ca2+ excessively enter mitochondria and induce to open the mitochondrial permeability transition pores, leading to decreased mitochondrial ATP, neuronal death, and ultimate tissue atrophy in HD brain.
9. Mitochondrial therapeutics, Dimebon and HD
As discussed above, recent studies suggest that mitochondrial dysfunction and calcium dyshomeostasis are keys players in HD progression and pathogenesis. To reduce mitochondrial toxicity and intracellular Ca2+ influx in neurons affected by HD, drugs that protect mitochondria need to be tested, in addition to agents that boost PGC1α expression and drugs that stabilize mitochondria and inhibit mitochondrial pore opening (Reddy, 2008; Reddy and Beal, 2008).
For last 5 years, several mitochondrial drugs have been tested in experimental animal models of HD and also clinical trials. Table 2 summarizes preclinical studies of several mitochondria drugs. Several mitochondrial drugs that act to protect neurons from mitochondrial damage, including Creatine, CoQ10, and resveratrol – have shown beneficial effects in transgenic animal models and/or neurons from HD mice. As summarized in Table 2, Creatine and CoQ10 have shown deceased HD pathology and abnormal phenotypic behavior in 2 mouse models (R6/2 and N171-82Q) of HD. These lines of mice express human exon 1 with expanded polyglutamine repeats, and findings from these studies suggest that Creatine and CoQ10 boost ATP levels and increase mitochondrial function (Andreasson et al., 2001; Ferrante et al., 2000; Schilling et al., 2001).
Table 2.
Summary of Experimental Mitochondrial Therapeutics in Huntington’s Disease
| Name of the Drug | Properties of the Drug | Mouse model tested | Outcome | Reference | |
|---|---|---|---|---|---|
| Behavior | Pathology | ||||
| Creatine | Natural occurring compund that provides a cellular reserve of high- energy phosphates to the muscle and brain cells. Creatine increases in strength in people with a variety of neurodegenerative diseases | R6/2 mice (Huntington’s transgenic mice) N171-82Q HD mice | 2% Creatine in the diet extended 18% survival of R6/2 mice. Body weight and motor performance were significantly improvedDietary supplementation of 2% creatine significantly improved survival, slowed the development of motor symptoms, and delayed the onset of weight loss. | Htt aggregate formation was delayed and Htt aggregates were reduced in mice treated with Creatine | Ferrante et al 2000 Andreasson et al., 2001 |
| CoQ10 | Is a essential biological co-factor of mitochondrial electron transport chain. It directly scavanges free radicals in the innermembrane of mitochondria | R6/2 mice N171-82Q HD mice | High dose of CoQ10 increase the survival of and improves phenotypic behaviorImproved motor performance but there was no survival extension | Decrease brain atrophyNo change in Htt aggregate formation in HD mice treated with CoQ10 | Smith et al., 2006 |
| Dimebon | Anti-histamine drug that may inhibits mitochondrial permeability pore opening and preserve mitochondrial structure and function | Used striatal cells from YAC 128 HD mice | Striatal cells from YAC 128 HD mice treated with Dimebon showed beneficial effects of NMDA receptors and voltage- gated calcium channels | Not applicable | Wu et al., 2008 |
| Resveratrol | Anti-aging and antioxidant, | Tested in nematode model of HD | Rescued neuronal dysfunction induced by polyglutamine repeats protein | Rescued polyglutamine-specific cell death in neuronal cells derived from HdhQ111 knock-in mice | Parker et al., 2005 |
An anti-aging agent, resveratrol also has shown beneficial effects in the worm model of HD, and further striatal neurons from knockin mice of HD have shown increased survival when HD striatal treated with resveratrol, suggesting that resveratrol protect against age-dependent mutant Htt toxicity in HD (Parker et al., 2005; Anekonda and Reddy, 2006).
Dimebon (or Dimebolin hydrochloride) is an antihistamine drug that has been used clinically in Russia to reduce cognitive deficits in AD patients. Its molecular formula is C21H25N3, and its molecular weight is 319.433. It has been proposed that Dimebon may inhibit mitochondrial permeability transition pore and protect neuronal mitochondria from mutant proteins such as Aβ, mutant Htt and other mitochondrial toxic insults (Bachurin et al., 2003). Recent studies suggest that Dimebon may have cognition-enhancing effects in healthy individuals (Bachrin et al., 2001).
Based on cell culture experiments indicating that Dimebon may be an effective treatment in AD patients, Bachurin and colleagues proposed Dimebon may inhibit the opening of mitochondrial permeability transition pore and protect neuronal mitochondria from Aβ and other mitochondrial toxic insults (Bachurin et al., 2003). In a recent study of clinical trials of AD patients from Russia, Doody and colleagues found that Dimebon was safe, well tolerated, and significantly improved the clinical course of patients with mild-to-moderate AD (Doody et al., 2008). Recently, Medivation, Inc., has completed phase II clinical trial of Dimebon in HD patients, and the outcome of this initial clinical trial will be useful to the families of HD patients, and also to the researchers of mitochondrial and HD fields.
Recently, to determine the neuroprotective effects of Dimebon, Wu et al. investigated the effects of Dimebon in primary striatal neuronal cultures from wild type mice and YAC128 HD transgenic mice (Wu et al., 2008). They found that Dimebon acts as an inhibitor of NMDA receptors and voltage-gated calcium channels in neurons from wild-type mice and YAC128 mice. They also found that the application of 50 μM Dimebon stabilized glutamate-induced Ca2+ signals in YAC128 medium spiny neurons and protected cultured YAC128 medium spiny neurons from glutamate-induced apoptosis. Lower concentrations of Dimebon (5 μM and 10 μM) did not stabilize glutamate-induced Ca2+ signals and did not exert neuroprotective effects in experiments with YAC128 medium spiny neurons. Evaluation of Dimebon against a set of biochemical targets indicated that Dimebon inhibits alpha-Adrenergic receptors, Histamine H1 and H2 receptors, and Serotonin 5-HT2c, 5-HT5A, 5-HT6 receptors with high affinity. Dimebon also had significant effects on a number of additional receptors. Findings of this study suggests that Dimebon may have beneficial effects in HD neurons through its capacity to neurons by altering NMDA receptors and voltage-gated calcium channels.
Currently, several laboratories across the world are actively involved to investigate the mode of neuroprotective action of Dimebon in neurodegenerative diseases by investigating cell and mouse models of neurodegenerative diseases, including Huntington’s and Alzheimer’s.
However, further research is still needed to test the efficacy of Dimebon and other molecules that reduce the induction of intracellular Ca2+ and entry of excessive Ca2+ to the mitochondria, and ultimately inhibit mitochondrial pore opening and in transgenic mouse models of neurodegenerative diseases, including HD, AD, PD and ALS. It is also important to understand the mechanisms by which Dimebon protect neuronal mitochondria and neurons in HD and other neurodegenerative diseases and also other mitochondrial diseases.
10. Conclusions and future directions
Since the discovery of the HD gene in 1993, tremendous progress has made in developing animal models of HD, unraveling the expression and function of wild-type and mutant Htt in the brain and peripheral tissues of HD patients, and understanding expanded polyglutamine repeats containing mutant Htt protein interactions with CNS proteins in HD progression. HD progression appears to involve several pathomechanisms, including the interaction of expanded polyglutamine repeat proteins with other CNS proteins, transcriptional dysregulation, Ca2+dyshomeostasis, abnormal vesicle trafficking, and mitochondrial bioenergetics. Recent findings have revealed that mutant Htt is associated with mitochondria and, in neurons affected by HD, causes mitochondrial structural changes, a decrease in mitochondrial trafficking, and an impairment of mitochondrial dynamics.
Despite such advances in HD research, we still do not fully understand the mechanism by which mutant Htt causes damage selectively to medium spiny neurons and cortical neurons in patients with HD. Recent research has focused on selectively blocking or reducing the expression of mutant Htt allele in HD patients. Further research is also needed to better understand how mutant Htt induces intracellular Ca2+ and how excessive Ca2+ accumulates in the mitochondria and promotes the opening of mitochondrial permeable pores. Such clarification can inform the development and testing of agents to reduce the entry of intracellular Ca2+ into mitochondria.
Methods to target the expression of the mutant polyglutamine allele in HD mice and HD patients are also needed to block or reduce the expression of mutant Htt selectively in neurons affected by HD, which will ultimately reduce the accumulation of mutant Htt in subcellular organelles, including mitochondria, nucleus, endoplasmic reticulum, and plasma membrane. This may further reduce mutant Htt oligomerization, which may, in turn, prevent or reduce the induction of intracellular Ca2+ and decrease intramitochondrial Ca2+ levels, ultimately stabilizing neuronal mitochondria and preserving neuronal function.
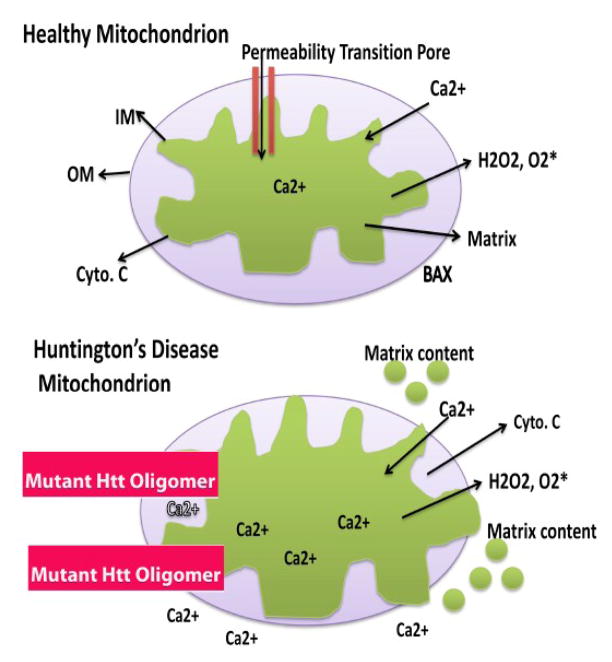
Figure 3.
Excess mitochondrial Ca2+ may open the mitochondrial permeability transition pore in mitochondria from HD patients. In healthy mitochondria, the inner mitochondrial membrane provides a highly efficient barrier to ionic flow and protects mitochondria from toxic insults. However, in neurons from patients with HD, an age-dependent accumulation of mutant Htt oligomers may induce a massive entry of Ca2+ into neurons and may promote mitochondrial Ca2+ overload. Excess mitochondrial Ca2+ may promote the opening of the mitochondrial permeability transition pore, facilitate to send matrix content out of mitochondria, and may destroy the mitochondria and neuron by apoptotic cell death.
Acknowledgments
The research for this article was supported by grants from National Institutes of Health (AG028072 and AG026051).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acevedo-Torres K, Berríos L, Rosario N, Dufault V, Skatchkov S, Eaton MJ, Torres-Ramos CA, Ayala-Torres S. Mitochondrial DNA damage is a hallmark of chemically induced and the R6/2 transgenic model of Huntington’s disease. DNA Repair (Amst) 2009;8:126–136. doi: 10.1016/j.dnarep.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen OA, Dedeoglu A, Ferrante RJ, Jenkins BG, Ferrante KL, Thomas M, Friedlich A, Browne SE, Schilling G, Borchelt DR, Hersch SM, Ross CA, Beal MF. Creatine increase survival and delays motor symptoms in a transgenic animal model of Huntington’s disease. Neurobiol Dis. 2001;8:479–491. doi: 10.1006/nbdi.2001.0406. [DOI] [PubMed] [Google Scholar]
- Anekonda TS, Reddy PH. Neuronal protection by sirtuins in Alzheimer’s disease. J Neurochem. 2006;96:305–13. doi: 10.1111/j.1471-4159.2005.03492.x. [DOI] [PubMed] [Google Scholar]
- Atwal RS, Xia J, Pinchev D, Taylor J, Epand RM, Truant R. Huntingtin has a membrane association signal that can modulate huntingtin aggregation, nuclear entry and toxicity. Hum Mol Genet. 2007;16:2600–2615. doi: 10.1093/hmg/ddm217. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Anderson NB, Bylsma FW, et al. Frontal lobe volume in patients with Huntington’s disease. Neurology. 1998;50:252–258. doi: 10.1212/wnl.50.1.252. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Brandt J, Codori AM, et al. Reduced basal ganglia volume associated with the gene for Huntington’s disease in asymptomatic at-risk persons. Neurology. 1994;44:823–828. doi: 10.1212/wnl.44.5.823. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Li Q, Stine O, et al. Longitudinal change in basal ganglia volume in patients with Huntington’s disease. Neurology. 1997;48:394–399. doi: 10.1212/wnl.48.2.394. [DOI] [PubMed] [Google Scholar]
- Aziz NA, van der Burg JM, Landwehrmeyer GB, Brundin P, Stijnen T, Roos RA EHDI Study Group. Weight loss in Huntington disease increases with higher CAG repeat number. Neurology. 2008;71:1506–1513. doi: 10.1212/01.wnl.0000334276.09729.0e. [DOI] [PubMed] [Google Scholar]
- Bachurin S, Bukatina E, Lermontova N, Tkachenko S, Afanasiev A, Grigoriev V, Grigorieva I, Ivanov Y, Sabli S, Zefirov N. Antihistamine agent Dimebon as a novel neuroprotector and a cognition enhancer. Ann N Y Acad Sci. 2001;939:425–435. doi: 10.1111/j.1749-6632.2001.tb03654.x. [DOI] [PubMed] [Google Scholar]
- Bachurin SO, Shevtsova EP, Kireeva EG, Oxenkrug GF, Sablin SO. Mitochondria as a target for neurotoxins and neuroprotective agents. Ann N Y Acad Sci. 2003;993:334–344. doi: 10.1111/j.1749-6632.2003.tb07541.x. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Robins-Wahlin TB, Lundin A, et al. Cognitive deficits in Huntington’s disease are predicted by dopaminergic PET markers and brain volumes. Brain. 1997;120:2207–2217. doi: 10.1093/brain/120.12.2207. [DOI] [PubMed] [Google Scholar]
- Bamford KA, Caine ED, Kido DK, et al. Clinical-pathologic correlation in Huntington’s disease: a neuropsychological and computed tomography study. Neurology. 1989;39:796–801. doi: 10.1212/wnl.39.6.796. [DOI] [PubMed] [Google Scholar]
- Banoei MM, Houshmand M, Panahi MS, Shariati P, Rostami M, Manshadi MD, Majidizadeh T. Huntington’s disease and mitochondrial DNA deletions: event or regular mechanism for mutant huntingtin protein and CAG repeats expansion? Cell Mol Neurobiol. 2007;27:867–875. doi: 10.1007/s10571-007-9206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates GP. History of genetic disease: the molecular genetics of Huntington disease - a history. Nat Rev Genet. 2005;6:766–773. doi: 10.1038/nrg1686. [DOI] [PubMed] [Google Scholar]
- Berent S, Giordani B, Lehtinen S, et al. Positron emission tomographic scan investigations of Huntington’s disease: cerebral metabolic correlates of cognitive function. Ann Neurol. 1998;23:541–546. doi: 10.1002/ana.410230603. [DOI] [PubMed] [Google Scholar]
- Boudreau RL, McBride JL, Martins I, Shen S, Xing Y, Carter BJ, Davidson BL. Nonallele-specific Silencing of Mutant and Wild-type Huntingtin Demonstrates Therapeutic Efficacy in Huntington’s Disease Mice. Mol Ther. 2009 doi: 10.1038/mt.2009.17. 2009 Feb 24. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell-Pagès M, Zala D, Humbert S, Saudou F. Huntington’s disease: from huntingtin function and dysfunction to therapeutic strategies. Cell Mol Life Sci. 2006;63:2642–2660. doi: 10.1007/s00018-006-6242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Petrilli A, Knott AB. Mutant huntingtin and mitochondrial dysfunction. Trends Neurosci. 2008;31:609–616. doi: 10.1016/j.tins.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne SE. Mitochondria and Huntington’s disease pathogenesis: insight from genetic and chemical models. Ann N Y Acad Sci. 2008;1147:358–382. doi: 10.1196/annals.1427.018. [DOI] [PubMed] [Google Scholar]
- Browne SE, Beal MF. The energetics of Huntington’s disease. Neurochem Res. 2004;29:531–546. doi: 10.1023/b:nere.0000014824.04728.dd. [DOI] [PubMed] [Google Scholar]
- Browne SE, Beal MF. Oxidative damage in Huntington’s disease pathogenesis. Antioxid Redox Signal. 2006;8:2061–2073. doi: 10.1089/ars.2006.8.2061. [DOI] [PubMed] [Google Scholar]
- Browne SE, Bowling AC, MacGarvey U, Baik MJ, Berger SC, Muqit MM, Bird ED, Beal MF. Oxidative damage and metabolic dysfunction in Huntington’s disease: selective vulnerability of the basal ganglia. Ann Neurol. 1997;41:646–653. doi: 10.1002/ana.410410514. [DOI] [PubMed] [Google Scholar]
- Byers RK, Gilles FH, Fung C. Huntington’s disease in children. Neuropathologic study of four cases. Neurology. 1973;23:561–569. doi: 10.1212/wnl.23.6.561. [DOI] [PubMed] [Google Scholar]
- Centonze D, Gubellini P, Picconi B, Saulle E, Tolu M, Bonsi P, Giacomini P, Calabresi P. An abnormal striatal synaptic plasticity may account for the selective neuronal vulnerability in Huntington’s disease. Neurol Sci. 2001;22:61–62. doi: 10.1007/s100720170047. [DOI] [PubMed] [Google Scholar]
- Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- Chang DT, Rintoul GL, Pandipati S, Reynolds IJ. Mutant huntingtin aggregates impair mitochondrial movement and trafficking in cortical neurons. Neurobiol Dis. 2006;22:388–400. doi: 10.1016/j.nbd.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Charles V, Mezey E, Reddy PH, Dehejia A, Young TA, Polymeropoulos MH, Brownstein MJ, Tagle DA. Alpha-synuclein immunoreactivity of huntingtin polyglutamine aggregates in striatum and cortex of Huntington’s disease patients and transgenic mouse models. Neurosci Lett. 2000;289:29–32. doi: 10.1016/s0304-3940(00)01247-7. [DOI] [PubMed] [Google Scholar]
- Chen CM, Wu YR, Cheng ML, Liu JL, Lee YM, Lee PW, Soong BW, Chiu DT. Increased oxidative damage and mitochondrial abnormalities in the peripheral blood of Huntington’s disease patients. Biochem Biophys Res Commun. 2007;359:335–340. doi: 10.1016/j.bbrc.2007.05.093. [DOI] [PubMed] [Google Scholar]
- Choo YS, Johnson GV, MacDonald M, Detloff PJ, Lesort M. Mutant huntingtin directly increases susceptibility of mitochondria to the calcium-induced permeability transition and cytochrome c release. Hum Mol Genet. 2004;13:1407–1420. doi: 10.1093/hmg/ddh162. [DOI] [PubMed] [Google Scholar]
- Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, Scherzinger E, Wanker EE, Mangiarini L, Bates GP. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90:537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- Dierks T, Linden DE, Hertel A, et al. Multimodal imaging of residual function and compensatory resource allocation in cortical atrophy: a case study of parietal lobe function in a patient with Huntington’s disease. Psychiatry Res. 199;90:67–75. [PubMed] [Google Scholar]
- DiFiglia M, Sena-Esteves M, Chase K, Sapp E, Pfister E, Sass M, Yoder J, Reeves P, Pandey RK, Rajeev KG, Manoharan M, Sah DW, Zamore PD, Aronin N. Therapeutic silencing of mutant huntingtin with siRNA attenuates striatal and cortical neuropathology and behavioral deficits. Proc Natl Acad Sci U S A. 2007;104:17204–17209. doi: 10.1073/pnas.0708285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, Aronin N. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS, Bachurin SO, Seely L, Hung D dimebo investigators. Effect of dimebon on cognition, activities of daily living, behaviour, and global functionin patients with mild-to-moderate Alzheimer’s disease: a randomised, double-blind, placebo-controlled study. Lancet. 2008;372:207–215. doi: 10.1016/S0140-6736(08)61074-0. [DOI] [PubMed] [Google Scholar]
- Faber PW, Alter JR, MacDonald ME, Hart AC. Polyglutamine-mediated dysfunction and apoptotic death of a Caenorhabditis elegans sensory neuron. Proc Natl Acad Sci U S A. 1999;96:179–84. doi: 10.1073/pnas.96.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes HB, Baimbridge KG, Church J, Hayden MR, Raymond LA. Mitochondrial sensitivity and altered calcium handling underlie enhanced NMDA induced apoptosis in YAC128 model of Huntington’s disease. J Neurosci. 2007;27:13614–13623. doi: 10.1523/JNEUROSCI.3455-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante RJ, Andreassen OA, Jenkins BG, Dedeoglu A, Kuemmerle S, Kubilus JK, Kaddurah-Daouk R, Hersch SM, Beal MF. Neuroprotective effects of creatine in a transgenic mouse model of Huntington’s disease. J Neurosci. 2000;20:4389–4397. doi: 10.1523/JNEUROSCI.20-12-04389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein SE. Huntington’s Disease. Johns Hopkins University Press; 1990. [Google Scholar]
- Fukui H, Moraes CT. Extended polyglutamine repeats trigger a feedback loop involving the mitochondrial complex III, the proteasome and huntingtin aggregates. Hum Mol Genet. 2007;16:783–797. doi: 10.1093/hmg/ddm023. [DOI] [PubMed] [Google Scholar]
- Gellerich FN, Gizatullina Z, Nguyen HP, Trumbeckaite S, Vielhaber S, Seppet E, Zierz S, Landwehrmeyer B, Riess O, von Hörsten S, Striggow F. Impaired regulation of brain mitochondria by extramitochondrial Ca2+ in transgenic Huntington disease rats. J Biol Chem. 2008;283:30715–30724. doi: 10.1074/jbc.M709555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusella JF, Wexler NS, Conneally PM, Naylor SL, Anderson MA, Tanzi RE, Watkins PC, Ottina K, Wallace MR, Sakaguchi AY, et al. A polymorphic DNA marker genetically linked to Huntington’s disease. Nature. 1983;306:234–238. doi: 10.1038/306234a0. [DOI] [PubMed] [Google Scholar]
- Hamilton JM, Wolfson T, Peavy GM, Jacobson MW, Corey-Bloom J Huntington Study Group. Rate and correlates of weight change in Huntington’s disease. J Neurol Neurosurg Psychiatry. 2004;75:209–212. doi: 10.1136/jnnp.2003.017822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JM, Wolfson T, Peavy GM, Jacobson MW, Corey-Bloom J, Harris GJ, Aylward EH, Peyser CE, et al. Huntington Study Group. Single photon emission computed tomographic blood flow and magnetic resonance volume imaging of basal ganglia in Huntington’s disease. Arch Neurol. 1996;53:316–324. doi: 10.1001/archneur.1996.00550040044013. [DOI] [PubMed] [Google Scholar]
- Harper SQ, Staber PD, He X, Eliason SL, Martins IH, Mao Q, Yang L, Kotin RM, Paulson HL, Davidson BL. RNA interference improves motor and neuropathological abnormalities in a Huntington’s disease mouse model. Proc Natl Acad Sci U S A. 2005;102:5820–5805. doi: 10.1073/pnas.0501507102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MR, Martin WR, Stoessl AJ, et al. Positron emission tomography in the early diagnosis of Huntington’s disease. Neurology. 1986;36:888–894. doi: 10.1212/wnl.36.7.888. [DOI] [PubMed] [Google Scholar]
- Hermel E, Gafni J, Propp SS, Leavitt BR, Wellington CL, Young JE, Hackam AS, Logvinova AV, Peel AL, Chen SF, Hook V, Singaraja R, Krajewski S, Goldsmith PC, Ellerby HM, Hayden MR, Bredesen DE, Ellerby LM. Specific caspase interactions and amplification are involved in selective neuronal vulnerability in Huntington’s disease. Cell Death Differ. 2004;11:424–438. doi: 10.1038/sj.cdd.4401358. [DOI] [PubMed] [Google Scholar]
- Hodgson JG, Agopyan N, Gutekunst CA, Leavitt BR, LePiane F, Singaraja R, Smith DJ, Bissada N, McCutcheon K, Nasir J, Jamot L, Li XJ, Stevens ME, Rosemond E, Roder JC, Phillips AG, Rubin EM, Hersch SM, Hayden MR. A YAC mouse model for Huntington’s disease with full-length mutant huntingtin, cytoplasmic toxicity, and selective striatal neurodegeneration. Neuron. 1999;23:181–192. doi: 10.1016/s0896-6273(00)80764-3. [DOI] [PubMed] [Google Scholar]
- Huang CC, Faber PW, Persichetti F, Mittal V, Vonsattel JP, MacDonald ME, Gusella JF. Amyloid formation by mutant huntingtin: threshold, progressivity and recruitment of normal polyglutamine proteins. Somat Cell Mol Genet. 1998;24:217–233. doi: 10.1023/b:scam.0000007124.19463.e5. [DOI] [PubMed] [Google Scholar]
- Jackson GR, Salecker I, Dong X, Yao X, Arnheim N, Faber PW, MacDonald ME, Zipursky SL. Polyglutamine-expanded human huntingtin transgenes induce degeneration of Drosophila photoreceptor neurons. Neuron. 1998;21:633–642. doi: 10.1016/s0896-6273(00)80573-5. [DOI] [PubMed] [Google Scholar]
- Jana NR, Zemskov EA, Wang GH, Nukina N. Altered proteasomal function due to the expression of polyglutamine-expanded truncated N-terminal huntingtin induces apoptosis by caspase activation through mitochondrial cytochrome c release. Hum Mol Genet. 2001;10:1049–1059. doi: 10.1093/hmg/10.10.1049. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Salmon DP, Butters N, et al. Cerebral structure on MRI, part II: specific changes in Alzheimer’s and Huntington’s diseases. Biol Psychiatry. 1991;29:68–81. doi: 10.1016/0006-3223(91)90211-4. [DOI] [PubMed] [Google Scholar]
- Kegel KB, Meloni AR, Yi Y, Kim YJ, Doyle E, Cuiffo BG, Sapp E, Wang Y, Qin ZH, Chen JD, Nevins JR, Aronin N, DiFiglia M. Huntingtin is present in the nucleus, interacts with the transcriptional corepressor Cterminal binding protein, and represses transcription. J Biol Chem. 2002;277:7466–7476. doi: 10.1074/jbc.M103946200. [DOI] [PubMed] [Google Scholar]
- Kegel KB, Sapp E, Yoder J, Cuiffo B, Sobin L, Kim YJ, Qin ZH, Hayden MR, Aronin N, Scott DL, Isenberg G, Goldmann WH, DiFiglia M. Huntingtin associates with acidic phospholipids at the plasma membrane. J Biol Chem. 2005;280:36464–36473. doi: 10.1074/jbc.M503672200. [DOI] [PubMed] [Google Scholar]
- Kim M, Lee HS, LaForet G, McIntyre C, Martin EJ, Chang P, Kim TW, Williams M, Reddy PH, Tagle D, Boyce FM, Won L, Heller A, Aronin N, DiFiglia M. Mutant huntingtin expression in clonal striatal cells: dissociation of inclusion formation and neuronal survival by caspase inhibition. J Neurosci. 1999;19:964–973. doi: 10.1523/JNEUROSCI.19-03-00964.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood SC, Su JL, Conneally P, Foroud T. Progression of symptoms in the early and middle stages of Huntington disease. Arch Neurol. 58:273–278. doi: 10.1001/archneur.58.2.273. [DOI] [PubMed] [Google Scholar]
- Kremer B. Clinical neurology of Huntington’s disease. In: Bates Gillian, Harper Peter, Jones Lesley., editors. Huntington’s Disease. 3 2002. [Google Scholar]
- Kuhl DE, Phelps ME, Markham CH, et al. Cerebral metabolism and atrophy in Huntington’s disease determined by 18FDG and computed tomographic scan. Ann Neurol. 1982;12:425–434. doi: 10.1002/ana.410120504. [DOI] [PubMed] [Google Scholar]
- Kuwert T, Lange HW, Langen KJ, et al. Cortical and subcortical glucose consumption measured by PET in patients with Huntington’s disease. Brain. 1990;113:1405–23. doi: 10.1093/brain/113.5.1405. [DOI] [PubMed] [Google Scholar]
- Laforet GA, Sapp E, Chase K, McIntyre C, Boyce FM, Campbell M, Cadigan BA, Warzecki L, Tagle DA, Reddy PH, Cepeda C, Calvert CR, Jokel ES, Klapstein GJ, Ariano MA, Levine MS, DiFiglia M, Aronin N. Changes in cortical and striatal neurons predict behavioral and electrophysiological abnormalities in a transgenic murine model of Huntington’s disease. J Neurosci. 2001;21:9112–9123. doi: 10.1523/JNEUROSCI.21-23-09112.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Ivanova EV, Seong IS, Cashorali T, Kohane I, Gusella JF, MacDonald ME. Unbiased gene expression analysis implicates the huntingtin polyglutamine tract in extra-mitochondrial energy metabolism. PLoS Genet. 2007;3(8):e135. doi: 10.1371/journal.pgen.0030135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WT, Chang C. Magnetic resonance imaging and spectroscopy in assessing 3-nitropropionic acid-induced brain lesions: an animal model of Huntington’s disease. Prog Neurobiol. 2004;72:87–110. doi: 10.1016/j.pneurobio.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Levine MS, Klapstein GJ, Koppel A, Gruen E, Cepeda C, Vargas ME, Jokel ES, Carpenter EM, Zanjani H, Hurst RS, Efstratiadis A, Zeitlin S, Chesselet MF. Enhanced sensitivity to N-methyl-D-aspartate receptor activation in transgenic and knockin mouse models of Huntington’s disease. J Neurosci Res. 1999;58:515–532. [PubMed] [Google Scholar]
- Li S, Li XJ. Multiple pathways contribute to the pathogenesis of Huntington disease. Mol Neurodegener. 2006;1:19. doi: 10.1186/1750-1326-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D, Fedrizzi L, Tartari M, Zuccato C, Cattaneo E, Brini M, Carafoli E. Calcium homeostasis and mitochondrial dysfunction in striatal neurons of Huntington disease. J Biol Chem. 2008;283:5780–5789. doi: 10.1074/jbc.M704704200. [DOI] [PubMed] [Google Scholar]
- Liang H, Ward WF. PGC-1alpha: a key regulator of energy metabolism. Adv Physiol Educ. 2006;30:145–151. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- Lin CH, Tallaksen-Greene S, Chien WM, Cearley JA, Jackson WS, Crouse AB, Ren S, Li XJ, Albin RL, Detloff PJ. Neurological abnormalities in a knock-in mouse model of Huntington’s disease. Hum Mol Genet. 2001 Jan 15;10(2):137–44. doi: 10.1093/hmg/10.2.137. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Mahant N, McCusker EA, Byth K, Graham S Huntington Study Group. Huntington’s disease: clinical correlates of disability and progression. Neurology. 2003;61:1085–1092. doi: 10.1212/01.wnl.0000086373.32347.16. [DOI] [PubMed] [Google Scholar]
- Majumder P, Raychaudhuri S, Chattopadhyay B, Bhattacharyya NP. Increased caspase-2, calpain activations and decreased mitochondrial complex II activity in cells expressing exogenous huntingtin exon 1 containing CAG repeat in the pathogenic range. Cell Mol Neurobiol. 2007;27:1127–1145. doi: 10.1007/s10571-007-9220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- Marsh JL, Walker H, Theisen H, Zhu YZ, Fielder T, Purcell J, Thompson LM. Expanded polyglutamine peptides alone are intrinsically cytotoxic and cause neurodegeneration in Drosophila. Hum Mol Genet. 2000;9:13–25. doi: 10.1093/hmg/9.1.13. [DOI] [PubMed] [Google Scholar]
- Milakovic T, Quintanilla RA, Johnson GV. Mutant huntingtin expression induces mitochondrial calcium handling defects in clonal striatal cells: functional consequences. J Biol Chem. 2006;281:34785–34795. doi: 10.1074/jbc.M603845200. [DOI] [PubMed] [Google Scholar]
- Montoya A, Price BH, Menear M, Lepage M. Brain imaging and cognitive dysfunctions in Huntington’s disease. J Psychiatry Neurosci. 2006;31:21–29. [PMC free article] [PubMed] [Google Scholar]
- Oliveira JM, Chen S, Almeida S, Riley R, Gonçalves J, Oliveira CR, Hayden MR, Nicholls DG, Ellerby LM, Rego AC. Mitochondrial-dependent Ca2+ handling in Huntington’s disease striatal cells: effect of histone deacetylase inhibitors. J Neurosci. 2006;26:11174–11186. doi: 10.1523/JNEUROSCI.3004-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira JM, Gonçalves J. In situ mitochondrial Ca2+ buffering differences of intact neurons and astrocytes from cortex and striatum. J Biol Chem. 2009;284:5010–5020. doi: 10.1074/jbc.M807459200. [DOI] [PubMed] [Google Scholar]
- Oliveira JM, Jekabsons MB, Chen S, Lin A, Rego AC, Gonçalves J, Ellerby LM, Nicholls DG. Mitochondrial dysfunction in Huntington’s disease: the bioenergetics of isolated and in situ mitochondria from transgenic mice. J Neurochem. 2007;101:241–249. doi: 10.1111/j.1471-4159.2006.04361.x. [DOI] [PubMed] [Google Scholar]
- Orr AL, Li S, Wang CE, Li H, Wang J, Rong J, Xu X, Mastroberardino PG, Greenamyre JT, Li XJ. N-terminal mutant huntingtin associates with mitochondria and impairs mitochondrial trafficking. J Neurosci. 2008;28:2783–2792. doi: 10.1523/JNEUROSCI.0106-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Ann Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- Pandey M, Varghese M, Sindhu KM, Sreetama S, Navneet AK, Mohanakumar KP, Usha R. Mitochondrial NAD+-linked State 3 respiration and complex-I activity are compromised in the cerebral cortex of 3- nitropropionic acid-induced rat model of Huntington’s disease. J Neurochem. 2008;104:420–434. doi: 10.1111/j.1471-4159.2007.04996.x. [DOI] [PubMed] [Google Scholar]
- Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT. Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nat Neurosci. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- Panov AV, Lund S, Greenamyre JT. Ca2+-induced permeability transition in human lymphoblastoid cell mitochondria from normal and Huntington’s disease individuals. Mol Cell Biochem. 2005;269:143–52. doi: 10.1007/s11010-005-3454-9. [DOI] [PubMed] [Google Scholar]
- Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, Néri C. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet. 2005;37:349–350. doi: 10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- Perez MK, Paulson HL, Pendse SJ, Saionz SJ, Bonini NM, Pittman RN. Recruitment and the role of nuclear localization in polyglutamine-mediated aggregation. J Cell Biol. 1998;143:1457–1470. doi: 10.1083/jcb.143.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan J, Hickey MA, Zhang P, Chesselet MF, Reue K. Adipose tissue dysfunction tracks disease progression in two Huntington’s disease mouse models. Hum Mol Genet. 2009 Mar 15;18(6):1006–16. doi: 10.1093/hmg/ddn428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers WJ, Videen TO, Markham J, McGee-Minnich L, Antenor-Dorsey JV, Hershey T, Perlmutter JS. Selective defect of in vivo glycolysis in early Huntington’s disease striatum. Proc Natl Acad Sci U S A. 2007;104:2945–2949. doi: 10.1073/pnas.0609833104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH. Mitochondrial dysfunction in aging and Alzheimer’s disease: strategies to protect neurons. Antioxidants & Redox Signaling. 2007;9:1647–1658. doi: 10.1089/ars.2007.1754. [DOI] [PubMed] [Google Scholar]
- Reddy PH. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromolecular Med. 2008;10:291–315. doi: 10.1007/s12017-008-8044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH. Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer’s disease. Experimental Neurology. 2009 doi: 10.1016/j.expneurol.2009.03.042. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Charles V, Williams M, Miller G, Whetsell WO, Jr, Tagle DA. Transgenic mice expressing mutated full-length HD cDNA: a paradigm for locomotor changes and selective neuronal loss in Huntington’s disease. Philos Trans R Soc Lond B Biol Sci. 1999;354:1035–1045. doi: 10.1098/rstb.1999.0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Tagle DA. Biology of trinucleotide repeat disorders. Genetic Aberrancies and Neurodegenerative Disorders. In: Mattson Mark P., editor. Advances in cell aging and gerontology. Vol. 3. Jai press Inc; Stanford and Connecticut: 1999. [Google Scholar]
- Reddy PH, Williams M, Tagle DA. Recent advances in understanding the pathogenesis of Huntington’s disease. Trends Neurosci. 1999;22:248–255. doi: 10.1016/s0166-2236(99)01415-0. [DOI] [PubMed] [Google Scholar]
- Reddy PH, Williams M, Charles V, Garrett L, Pike-Buchanan L, Whetsell WO, Jr, Miller G, Tagle DA. Behavioural abnormalities and selective neuronal loss in HD transgenic mice expressing mutated full-length HD cDNA. Nat Genet. 1998;20:198–202. doi: 10.1038/2510. [DOI] [PubMed] [Google Scholar]
- Rigamonti D, Bauer JH, De-Fraja C, Conti L, Sipione S, Sciorati C, Clementi E, Hackam A, Hayden MR, Li Y, Cooper JK, Ross CA, Govoni S, Vincenz C, Cattaneo E. Wild-type huntingtin protects from apoptosis upstream of caspase-3. J Neurosci. 2000;20:3705–3713. doi: 10.1523/JNEUROSCI.20-10-03705.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockabrand E, Slepko N, Pantalone A, Nukala VN, Kazantsev A, Marsh JL, Sullivan PG, Steffan JS, Sensi SL, Thompson LM. The first 17 amino acids of Huntingtin modulate its sub-cellular localization, aggregation and effects on calcium homeostasis. Hum Mol Genet. 16:61–77. doi: 10.1093/hmg/ddl440. [DOI] [PubMed] [Google Scholar]
- Romero E, Cha GH, Verstreken P, Ly CV, Hughes RE, Bellen HJ, Botas J. Suppression of neurodegeneration and increased neurotransmission caused by expanded full-length huntingtin accumulating in the cytoplasm. Neuron. 2008;57:27–40. doi: 10.1016/j.neuron.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayer JA, Manczak M, Akileswaran L, Reddy PH, Coghlan VM. Interaction of the nuclear matrix protein NAKAP with HypA and huntingtin: implications for nuclear toxicity in Huntington’s disease pathogenesis. Neuromolecular Med. 2005;7:297–310. doi: 10.1385/NMM:7:4:297. [DOI] [PubMed] [Google Scholar]
- Schilling G, Coonfield ML, Ross CA, Borchelt DR. Coenzyme Q10 and remacemide hydrochloride ameliorate motor deficits in a Huntington’s disease transgenic mouse model. Neurosci Lett. 2001;315:149–153. doi: 10.1016/s0304-3940(01)02326-6. [DOI] [PubMed] [Google Scholar]
- Schilling G, Becher MW, Sharp AH, et al. Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin. Hum Mol Genet. 1999;8:397–407. doi: 10.1093/hmg/8.3.397. [DOI] [PubMed] [Google Scholar]
- Senatorov VV, Charles V, Reddy PH, Tagle DA, Chuang DM. Overexpression and nuclear accumulation of glyceraldehyde-3-phosphate dehydrogenase in a transgenic mouse model of Huntington’s disease. Mol Cell Neurosci. 2003;22:285–297. doi: 10.1016/s1044-7431(02)00013-1. [DOI] [PubMed] [Google Scholar]
- Seong IS, Ivanova E, Lee JM, et al. HD CAG repeat implicates a dominant property of huntingtin in mitochondrial energy metabolism. Hum Mol Genet. 14:2871–2880. doi: 10.1093/hmg/ddi319. [DOI] [PubMed] [Google Scholar]
- Shimohata T, Nakajima T, Yamada M, et al. Expanded polyglutamine stretches interact with TAFII130, interfering with CREB-dependent transcription. Nat Genet. 2000;26:29–36. doi: 10.1038/79139. [DOI] [PubMed] [Google Scholar]
- Smith KM, Matson S, Matson WR, Cormier K, Del Signore SJ, Hagerty SW, Stack EC, Ryu H, Ferrante RJ. Dose ranging and efficacy study of high-dose coenzyme Q10 formulations in Huntington’s disease mice. Biochim Biophys Acta. 2006 Jun;1762(6):616–26. doi: 10.1016/j.bbadis.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Solans A, Zambrano A, Rodríguez M, Barrientos A. Cytotoxicity of a mutant huntingtin fragment in yeast involves early alterations in mitochondrial OXPHOS complexes II and III. Hum Mol Genet. 2006 Oct 15;15(20):3063–81. doi: 10.1093/hmg/ddl248. [DOI] [PubMed] [Google Scholar]
- Spargo E, Everall IP, Lantos PL. Neuronal loss in the hippocampus in Huntington’s disease: a comparison with HIV infection. J Neurol Neurosurg Psychiatry. 1993 May;56(5):487–91. doi: 10.1136/jnnp.56.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Strehlow AN, Li JZ, Myers RM. Wild-type huntingtin participates in protein trafficking between the Golgi and the extracellular space. Hum Mol Genet. 2007 Feb 15;16(4):391–409. doi: 10.1093/hmg/ddl467. [DOI] [PubMed] [Google Scholar]
- Sun Y, Savanenin A, Reddy PH, Liu YF. Polyglutamine-expanded huntingtin promotes sensitization of N-methyl-D-aspartate receptors via post-synaptic density 95. J Biol Chem. 2001;276:24713–24728. doi: 10.1074/jbc.M103501200. [DOI] [PubMed] [Google Scholar]
- Tabrizin SJ, Cleeter MW, Xuereb J, Taanman JW, Cooper JM, Schapira AH. Biochemical abnormalities and excitotoxicity in Huntington’s disease brain. Ann Neurol. 1999;45:25–32. doi: 10.1002/1531-8249(199901)45:1<25::aid-art6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- The Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Truant R, Atwal R, Burtnik A. Hypothesis: Huntingtin may function in membrane association and vesicular trafficking. Biochem Cell Biol. 2006 Dec;84(6):912–7. doi: 10.1139/o06-181. [DOI] [PubMed] [Google Scholar]
- Trushina E, Dyer RB, Badger JD, 2nd, Ure D, Eide L, Tran DD, Vrieze BT, Legendre-Guillemin V, McPherson PS, Mandavilli BS, Van Houten B, Zeitlin S, McNiven M, Aebersold R, Hayden M, Parisi JE, Seeberg E, Dragatsis I, Doyle K, Bender A, Chacko C, McMurray CT. Mutant huntingtin impairs axonal trafficking in mammalian neurons in vivo and in vitro. Mol Cell Biol. 2004;24:8195–8209. doi: 10.1128/MCB.24.18.8195-8209.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bilsen PH, Jaspers L, Lombardi MS, Odekerken JC, Burright EN, Kaemmerer WF. Identification and allele-specific silencing of the mutant huntingtin allele in Huntington’s disease patient-derived fibroblasts. Hum Gene Ther. 2008;19:710–719. doi: 10.1089/hum.2007.116. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Metzler M, Slow E, Pearson J, Schwab C, Carroll J, Graham RK, Leavitt BR, Hayden MR. Phenotypic abnormalities in the YAC128 mouse model of Huntington disease are penetrant on multiple genetic backgrounds and modulated by strain. Neurobiol Dis. 2007;26:189–200. doi: 10.1016/j.nbd.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Wang H, Lim PJ, Karbowski M, Monteiro MJ. Effects of overexpression of huntingtin proteins on mitochondrial integrity. Hum Mol Genet. 2009;18:737–752. doi: 10.1093/hmg/ddn404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warby SC, Doty CN, Graham RK, Carroll JB, Yang YZ, Singaraja RR, Overall CM, Hayden MR. Activated caspase-6 and caspase-6-cleaved fragments of huntingtin specifically colocalize in the nucleus. Hum Mol Genet. 2008;17:2390–2404. doi: 10.1093/hmg/ddn139. [DOI] [PubMed] [Google Scholar]
- Weydt P, Pineda VV, Torrence AE, et al. Thermoregulatory and metabolic defects in Huntington’s disease transgenic mice implicate PGC-1alpha in Huntington’s disease neurodegeneration. Cell Metab. 2006;4:349–362. doi: 10.1016/j.cmet.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Wheeler VC, Auerbach W, White JK, Srinidhi J, Auerbach A, Ryan A, Duyao MP, Vrbanac V, Weaver M, Gusella JF, Joyner AL, MacDonald ME. Length-dependent gametic CAG repeat instability in the Huntington’s disease knock-in mouse. Hum Mol Genet. 1999;8:115–122. doi: 10.1093/hmg/8.1.115. [DOI] [PubMed] [Google Scholar]
- Wu J, Li Q, Bezprozvanny I. Evaluation of Dimebon in cellular model of Huntington’s disease. Mol Neurodegener. 2008;3:15. doi: 10.1186/1750-1326-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington’s disease. Cell. 2000;101:57–66. doi: 10.1016/S0092-8674(00)80623-6. [DOI] [PubMed] [Google Scholar]
- Yang SH, Cheng PH, Banta H, Piotrowska-Nitsche K, Yang JJ, Cheng EC, Snyder B, Larkin K, Liu J, Orkin J, Fang ZH, Smith Y, Bachevalier J, Zola SM, Li SH, Li XJ, Chan AW. Towards a transgenic model of Huntington’s disease in a non-human primate. Nature. 2008;453:921–924. doi: 10.1038/nature06975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AB, Penney JB, Starosta-Rubinstein S, et al. PET scan investigations of Huntington’s disease: cerebral metabolic correlates of neurological features and functional decline. Ann Neurol. 1986;20:296–303. doi: 10.1002/ana.410200305. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Engelman J, Friedlander RM. Allele-specific silencing of mutant Huntington’s disease gene. J Neurochem. 2009;108:82–90. doi: 10.1111/j.1471-4159.2008.05734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Leavitt BR, van Raamsdonk JM, Dragatsis I, Goldowitz D, MacDonald ME, Hayden MR, Friedlander RM. Huntingtin inhibits caspase-3 activation. EMBO J. 2006;25:5896–5906. doi: 10.1038/sj.emboj.7601445. [DOI] [PMC free article] [PubMed] [Google Scholar]