Human Immunodeficiency Virus Type 1 Mediates Global Disruption of Innate Antiviral Signaling and Immune Defenses within Infected Cells (original) (raw)
Abstract
Interferon regulatory factor 3 (IRF-3) is essential for innate intracellular immune defenses that limit virus replication, but these defenses fail to suppress human immunodeficiency virus (HIV) infection, which can ultimately associate with opportunistic coinfections and the progression to AIDS. Here, we examined antiviral defenses in CD4+ cells during virus infection and coinfection, revealing that HIV type 1 (HIV-1) directs a global disruption of innate immune signaling and supports a coinfection model through suppression of IRF-3. T cells responded to paramyxovirus infection to activate IRF-3 and interferon-stimulated gene expression, but they failed to mount a response against HIV-1. The lack of response associated with a marked depletion of IRF-3 but not IRF-7 in HIV-1-infected cells, which supported robust viral replication, whereas ectopic expression of active IRF-3 suppressed HIV-1 infection. IRF-3 depletion was dependent on a productive HIV-1 replication cycle and caused the specific disruption of Toll-like receptor and RIG-I-like receptor innate immune signaling that rendered cells permissive to secondary virus infection. IRF-3 levels were reduced in vivo within CD4+ T cells from patients with acute HIV-1 infection but not from long-term nonprogressors. Our results indicate that viral suppression of IRF-3 promotes HIV-1 infection by disrupting IRF-3-dependent signaling pathways and innate antiviral defenses of the host cell. IRF-3 may direct an innate antiviral response that regulates HIV-1 replication and viral set point while governing susceptibility to opportunistic virus coinfections.
Immune evasion and dysregulation of the immune response to infection are major features that support human immunodeficiency virus type 1 (HIV-1) infection and pathogenesis. Acute exposure to HIV-1 through direct mucosal contact initiates infection in resident CD4+ cells, in which cell-intrinsic innate antiviral defenses impose the first level of immunity and restriction against infection (18, 23). Innate immune host factors induced by type I interferons (IFN), including members of the apolipoprotein B mRNA editing catalytic enzyme (APOBEC) and TRIM families, and products of certain IFN-stimulated genes (ISGs), such as ISG15 and ISG20, have been defined as HIV restriction factors because their effector actions can limit HIV infection (9, 10, 31, 34). However, innate antiviral defenses are overall largely ineffective at suppressing acute HIV-1 infection in vivo, and the virus most often progresses to a chronic infection after acute exposure. This inability to control HIV-1 infection has in part been attributed to properties of the virus that inhibit specific host defense factors, but the overall impact of HIV-1 on global intracellular innate immune programs has not been defined (9, 34).
Innate antiviral immune defenses are triggered during virus infection through the recognition of viral products by host cell pathogen recognition receptors (PRRs). RIG-I-like receptors (RLRs) and Toll-like receptors (TLRs) are PRR families that recognize microbial ligands known as pathogen-associated molecular patterns to initiate intracellular signaling cascades in the infected cell that induce IFN expression and production to direct a cellular antiviral state mediated by ISGs. ISG products, including IFN-induced proinflammatory cytokines, have antiviral and/or immunomodulatory functions that serve to suppress virus replication and enhance adaptive immunity, thus mediating a response that controls the viral “set point” and limits virus dissemination to peripheral sites (27, 35). A central feature of PRR signaling involves the activation of IFN regulatory factors (IRFs) and NF-κB. Among the IRF gene family, IRF-3, IRF-7, and IRF-9 play critical roles in inducing IFN and ISG expression. Whereas IRF-3 is widely expressed and highly abundant in most tissues, including T cells and macrophages, IRF-7 expression is more restricted. While IRF-7 is constitutively expressed in plasmacytoid dendritic cells (pDCs) and certain hematopoietic cells, it is typically induced by IFN in most tissues, where it serves to amplify the innate response (27). IRF-9 is widely expressed at a low level and is induced by IFN to play a pivotal role in mediating IFN signaling of ISG expression through its interactions with signal transducer and activator of transcription 1 (STAT-1) and STAT-2 (27). In this respect, IRF-7 and IRF-9 lie downstream of IRF-3 in a variety of cell types. RLRs signal innate defenses through the activation of IRF-3, and signaling bifurcates to trigger the additional activation of NF-κB, thereby directing the expression of both IRF-3 and NF-κB target genes (7). Moreover, TLR3 and TLR4 signal innate defenses and IFN production through the TRIF or TRAM adaptor proteins that activate IRF-3 and also converge on the NF-κB activation pathway (33). Thus, processes that regulate the signaling outcome of the RLRs, TLR3, or TLR4 globally impact innate immune gene expression.
Many pathogenic viruses direct strategies to antagonize innate defenses and IRF activation in order to support viral replication (27), and control of IRF-3 has been defined as a major determinant of evasion from innate antiviral defenses. Recent observations suggest that HIV-1 may antagonize IRF-3 (32), but its effects on mucosal infection and PRR pathway function and its impact on IRF-3 within CD4+ T cells from HIV-1-infected patients have not been defined. Moreover, various studies have implicated HIV-1 or the related simian immunodeficiency virus in the regulation of IFN defenses, but the mechanisms underlying such regulation are not known (20, 29, 40, 44).
In the present study, we examined IRF and PRR signaling during HIV infection. We observed the suppression of RLR signaling and TRIF/TRAM-dependent TLR signaling in HIV-1-infected cells, which was attributed to viral suppression of IRF-3 and which supported acute infection in mucosal tissue. Our studies demonstrate that IRF-3 can direct a robust innate antiviral response that controls HIV-1 replication but that HIV-1 suppression of IRF-3 abrogates these defenses, lending support for chronic infection and increasing the potential for opportunistic virus coinfection. These data validate observations of innate immune regulation by HIV and extend to implicate immune regulation at the virus/IRF-3 interface as a critical feature that contributes to the direction of HIV-1 infection outcome through immune evasion and support of opportunistic coinfections.
MATERIALS AND METHODS
Cell culture and transfections.
All cells were grown under standard conditions. SupT1 cells, THP-1 cells, and peripheral blood mononuclear cells (PBMCs) were cultured in RPMI medium supplemented with 10% fetal bovine serum (FBS), l-glutamine, and antibiotics. PBMCs were additionally activated with human interleukin-2 (Roche) and phytohemagglutinin (Sigma) prior to use. HEK293, 293T, 293-TLR3, HeLa CD4+, and Tzm-bl cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS, l-glutamine, and antibiotics. The 293-TLR3 cells (Invivogen) were additionally supplemented with 10 μg/ml blasticidin to maintain expression of TLR3. PBMCs were obtained from random screened blood packs from the American Red Cross (Portland, OR) and purified using standard procedures with Ficoll gradients, except for the experiments with results shown in Fig. 6 (see below). Transfection of adherent cells was performed using the calcium phosphate method. SupT1 cells were transfected using Fugene6 transfection reagent (Roche) according to the manufacturer's suggested protocol. RNA transfections were performed using TransMessenger (Qiagen) according to the manufacturer's instructions.
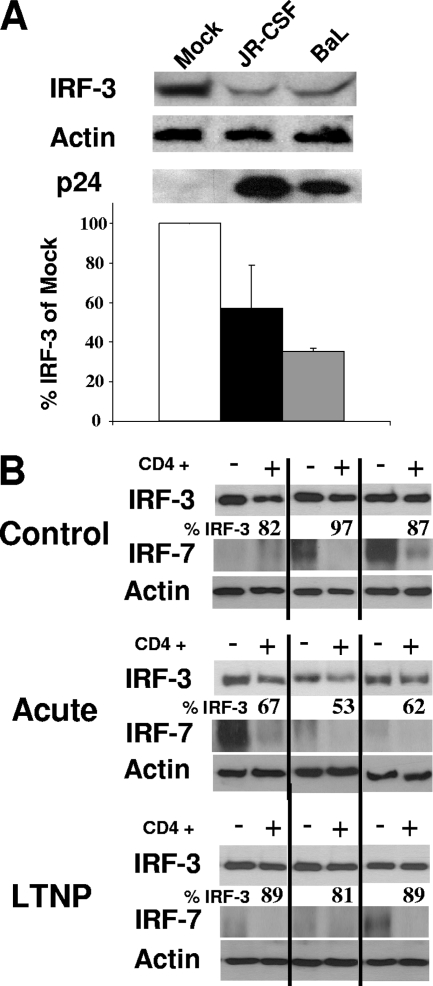
FIG. 6.
HIV-1 depletion of IRF-3 ex vivo and in vivo. (A) IRF-3, HIV p24, and actin protein levels in vaginal mucosal T cells infected ex vivo with HIV-1BaL or HIV-1JR-CSF (200 ng p24/ml). Virus was added to cultures and allowed to infect for 48 h, after which extracts were prepared and subjected to immunoblot analysis. The relative level of IRF-3 in each culture compared to that for mock-infected cells is presented, and results represent three independent analyses. (B) IRF-3, IRF-7, and actin protein levels in CD4+ or CD4− cells from HIV-1-infected patients. PBMCs from healthy donors or HIV-1 patients were thawed and allowed to rest overnight before the CD4+ and CD4− cells were separated by cell sorting. Extracts from each cell population were analyzed by immunoblotting. Three samples from each group are shown. A total of five samples from seronegative donors (control), five samples from LTNP, and six samples from patients with acute HIV-1 infection were screened. Values represent the percentages of IRF-3 compared to that from the CD4− cell population assessed from the same patient.
HIV study population and cell isolation.
We evaluated persons with newly diagnosed HIV-1 infection (subjects with acute infection), with long-term nonprogressive HIV disease (long-term nonprogressors [LTNP]), and with no evidence of HIV-1 infection (control subjects). Subjects with acute infection, enrolled based on previously defined criteria and followed longitudinally at the University of Washington Primary Infection Clinic (6), were selected based upon the availability of cryopreserved PBMCs from leukapheresis performed during primary infection. Primary infection was documented by signs and symptoms consistent with those from mucosa obtained following an acute retroviral syndrome (6, 38, 39). LTNP (defined as HIV infected for >10 years, with a viral load of <10,000 copies/ml and sustained CD4+ counts of ≥500 cells/μl in the absence of antiretroviral therapy) and seronegative subjects were enrolled and followed at the Seattle HIV Vaccine Trials Unit. The appropriate institutional review board approved the studies, and volunteers provided written consent.
PBMCs were isolated and cryopreserved as described previously (7). Cryopreserved PBMCs were thawed and rested overnight at 37°C and 5% CO2 prior to isolation of CD4+ and CD4− T-cell subsets. CD4+ and CD4− T-cell subsets were isolated using a RoboSep automated cell separator and CD4 T-cell enrichment kits according to the manufacturer's protocol (Stem Cell Technologies, Vancouver, BC, Canada). The purity of CD4+ T-cell fractions was assessed by flow cytometry, with an average purity of CD4+ T-cell fractions of >95% (data not shown). Isolation of T cells from healthy vaginal mucosa obtained from patients undergoing vaginal-repair surgeries was carried out as described in detail previously (16, 17) and followed the UW/FHCRC Institutional Review Board's approved protocol with written subject consent.
Cell treatments.
Cycloheximide (CHX) (Sigma) and IFN-β (Toray) were used in the experiments. CHX was used at 75 μg/ml, and IFN-β was used at 50 U/ml. poly(I:C) (Sigma) was used at various concentrations.
Viral stocks and infection.
HIV-1LAI was propagated using standard procedures with CEM-SS cells grown in RPMI supplemented with 10% FBS and antibiotics. HIV-1 strains pNL4-3, JR-CSF, and BaL were obtained as proviral clones and transfected into 293T cells, as described previously, to generate infectious virus (16, 17). Titers for all HIV-1 strains were determined with Tzm-bl cells to determine concentrations of infectious virus, unless otherwise noted. Sendai virus (SenV) strain Cantell was obtained from Charles River Laboratories.
Immunoblot analysis and immunofluorescent imaging.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis, immunoblot analysis, and immunofluorescence imaging were performed using standard procedures as described previously (26). The following antibodies were used in the study: rabbit (Rb) anti-ISG15 (A. Haas), Rb anti-ISG56 (G. Sen), mouse anti-p24, goat anti-β-actin (Santa Cruz), Rb total anti-IRF-3 (a gift from Michael David), Rb IRF-3-p (Cell Signaling), Rb IRF-7 (Santa Cruz), and goat anti-SenV (Charles River Laboratories). For immunoblot applications, the appropriate horseradish peroxidase-conjugated secondary antibody was used (Jackson Immunoresearch Laboratories), followed by treatment of the membrane with ECL-Plus reagent (Roche) and imaging on X-ray film. Densitometry was performed using ImageJ (NIH) on unsaturated blots. For immunofluorescence imaging, appropriate Alexa Fluor secondary antibodies (Invitrogen) were used, along with DAPI (4′,6-diamidino-2-phenylindole) during secondary staining for each slide. All images were photographed with a Nikon TE2000-E microscope and processed with Nikon EIS-Elements software.
qPCR.
Quantitative reverse transcriptase PCR (qPCR) using SYBR green technology has been described previously (37). IRF-3-, IFN-β-, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-specific primers are commercially available (SuperArray). GAPDH was used for normalization in all cases. RNA was extracted using an RNeasy mini kit (Qiagen) according to the manufacturer's instructions.
Dual luciferase assays.
Dual luciferase assays (Promega) were performed according to the manufacturer's specifications. The IFN-β, ISG56, IFN-stimulated response element (ISRE), PRDII, and NF-κB promoter plasmids have all been described previously (11, 12). Transfections were performed at a ratio of 3.75:1:0.25 for provirus/virus construct to promoter luciferase to internal control (Renilla) for all experiments.
Statistical analysis.
Differences between groups were analyzed for statistical significance by the Student t test.
The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: Tzm-bl cells from John C. Kappes, Xiaoyun Wu, and Tranzyme, Inc.; monoclonal antibody to HIV-1 p24 (AG3.0) from Jonathan Allan; pNL4-3 from Malcolm Martin; CEM-SS from Peter L. Nara; pcDNA-HVif from Stephan Bour and Klaus Strebel; pEGFP-Vpr from Warner C. Greene; darunavir from Tibotec, Inc.; and indinavir sulfate.
RESULTS
IRF-3 levels decrease during acute HIV-1 infection.
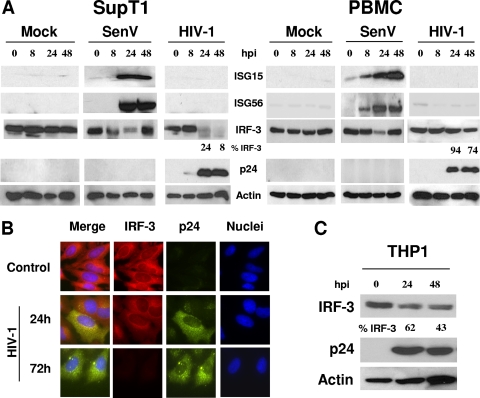
To determine how HIV-1 impacts the host cell innate antiviral response, we examined PRR signaling and ISG expression during acute virus infection in vitro. Infection of CD4+ SupT1 T cells with SenV, a model paramyxovirus, triggered the expression of IRF-3 target genes, including ISG15 and ISG56 (13). Expression of both ISG15 and ISG56 in SenV-infected cells occurred concomitantly with the activation of IRF-3, the latter observed as a transient reduction of IRF-3 coupled with the appearance of the slower-migrating IRF-3 species representing the phosphorylated, active IRF-3 isoform (Fig. 1A) (2, 19). In contrast, ISG expression was not induced in SupT1 cells infected with HIV-1 even under conditions of high multiplicity of infection (MOI). An MOI of 10 was used to ensure that we would not miss any low-level ISG induction. The lack of ISG expression in HIV-1-infected cells associated with a marked depletion of IRF-3 levels coincident with the appearance and accumulation of viral proteins (Fig. 1A). Similar results were obtained when Jurkat, H9, CEM174, and CEM-SS cells were infected with HIV-1 (data not shown). We performed the same experiments using cultures of purified PBMCs as targets of HIV-1 infection. As shown in Fig. 1A, SenV infection of PBMCs induced a robust expression of ISG15 and ISG56 in association with the transient activation of IRF-3, but ISGs were not expressed in HIV-1-infected PBMCs. We observed a relative reduction of IRF-3 protein levels in PBMC cultures that occurred in association with viral protein accumulation (Fig. 1A, right). The IRF-3 decrease in PBMCs was less extensive and took place with delayed kinetics compared to that for HIV-1-infected SupT1 cells, probably corresponding to differences in infection frequency and viral replication kinetics between cultures. These results confirm that HIV-1 infection associates with a reduction of IRF-3 protein in infected cells and link the reduction in IRF-3 with a lack of ISG expression during acute HIV-1 infection.
FIG. 1.
IRF-3 levels are reduced in response to HIV-1 infection. (A) SupT1 cells or PBMCs were infected with HIV-1LAI (MOI of 10). Cells were harvested at the indicated times postinfection and probed by immunoblotting to determine expression levels of ISG15, ISG56, p24, and IRF-3. Actin levels were monitored to control for protein leading. Where indicated, cells were infected with 50 hemagglutinating units/ml of SenV. (B) HeLa CD4+ cells were cultured in chamber slides and infected as described above. Cells were fixed and triple stained by using antibodies against IRF-3 and HIV p24 and by staining nuclei with DAPI. Cells were visualized by immunofluorescence microscopy. IRF-3 is shown in red, p24 is shown in green, and nuclei are shown in blue. (C) THP1 cells were infected with HIV-1JR-CSF at an MOI of 1, and infection was allowed to progress for the indicated amounts of time. Cells were harvested, and extracts were subjected to immunoblot analysis to measure the levels of IRF-3, HIV p24, and actin. Values represent the percentages of IRF-3 compared to that at time zero. Data shown are representative of three independent experiments. hpi, hours postinfection.
To further assess the impact of HIV-1 on IRF-3 levels, we examined IRF-3 in CD4+ HeLa cells infected with HIV-1. Cells were immunostained with antibodies specific to IRF-3 or HIV-1 p24 Gag protein and were visualized by fluorescence microscopy at various time points postinfection. As shown in Fig. 1B, IRF-3 was highly abundant in the cytoplasm of noninfected control CD4+ HeLa cells, but within 24 h postinfection the intracellular levels of IRF-3 were reduced concomitant with HIV-1 protein expression, and by 72 h postinfection IRF-3 levels were completely ablated. We also examined IRF-3 levels in THP1 cells, a human monocyte/macrophage-like cell line, as macrophages are an important reservoir of HIV-1 in vivo (42). As seen in Fig. 1C, the THP1 cells exhibited a high abundance of IRF-3 but levels were markedly reduced as HIV-1 infection progressed over a 48-h period. We found similar results using the U937 monocyte line (data not shown). Importantly, these experiments were performed with HIV-1JR-CSF, an R5-tropic HIV-1 strain, in contrast to the X4-tropic virus (HIV-1LAI) used for experiments with results shown in Fig. 1A and B. Collectively, these results demonstrate that HIV-1 infection associates with a rapid depletion of IRF-3 within CD4+ cells in a cell type-unrestricted manner and that this depletion is a general property of HIV-1 virus strains, independent of coreceptor usage.
HIV-1 promotes the specific decay of IRF-3 protein levels independently of mRNA expression.
To determine if IRF-3 ablation by HIV-1 is specific or part of a host metabolic shutdown of innate antiviral pathways, we examined IRF-3 and IRF-7 levels in HIV-1-infected cultures. Within HIV-1-infected SupT1 cells, IRF-3 levels started to decrease 24 h postinfection, with less than 20% remaining by 48 h, whereas IRF-7 levels remained relatively unchanged over the infection time course compared to the level for mock-infected cells (Fig. 2A). While IRF-3 protein levels were specifically suppressed during HIV-1 infection, we found that IRF-3 mRNA levels remained constant and relatively unchanged compared to the level for mock-infected control cells (Fig. 2B). Thus, the observed decrease in IRF-3 protein levels was not due to reduced IRF-3 mRNA expression.
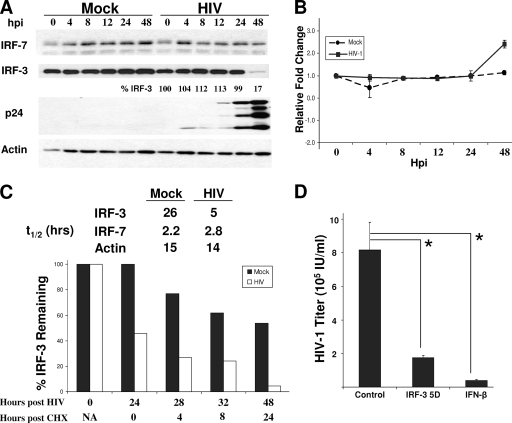
FIG. 2.
IRF-3 degradation by HIV-1 supports evasion of innate defenses that limit infection. (A) SupT1 cells were mock infected or infected with HIV-1LAI at an MOI of 1. Cells were harvested at the indicated times for immunoblot analysis of IRF-7, IRF-3, HIV p24, and actin levels. Values represent the percentages of IRF-3 compared to that at time zero of infection. (B) IRF-3 mRNA levels were determined by qPCR analysis. SupT1 cells were infected as described above. Total RNA was recovered, and qPCR was performed using specific primers for IRF-3 and GAPDH mRNA (for normalization) (n = 3). (C) SupT1 cells were mock infected or infected with HIV-1LAI. Cells were harvested 24 h later, or CHX was added to the cultures and the cells were harvested 28, 32, or 48 h after HIV-1 infection (corresponding to 4, 8, or 24 h of CHX treatment, respectively). IRF-3, IRF-7, and actin levels were measured by immunoblot assay. Densitometry was used to quantify the amount of each protein species at each time postinfection and posttreatment, from which the half-life for each molecule was determined. The graph shows the relative percentage of IRF-3 remaining at each time point for mock- or HIV-1-infected cultures compared to that at time zero for mock-infected cells. (D) SupT1 cells were transfected with a plasmid expressing IRF-3(5D) or a control vector or treated with 50 U/ml of IFN-β. Sixteen hours later, the cells were washed, infected with HIV-1LAI at an MOI of 1 for 3 h, and further washed, and fresh medium was added. Forty-eight hours later, culture supernatant was collected and infectious HIV-1 production was determined by virus titration assay (n = 3). *, P < 0.001. hpi, hours postinfection; _t_1/2, half-life; NA, not applicable. n, number of samples.
We therefore assessed the levels of stability of the IRF-3 and IRF-7 proteins during HIV-1 infection in a side-by-side comparison. We infected SupT1 cells with HIV-1 for 24 h before stopping protein biosynthesis by the addition of CHX to the cell culture. Cells were then harvested at sequential time points, and IRF-3 and IRF-7 protein levels were measured by immunoblot analysis to determine the half-life of each. In mock-infected cells, we observed only a slow decay of IRF-3 protein levels, with a calculated 26-h half-life (Fig. 2C), consistent with previous studies of other cell types (24). In HIV-1-infected cells, we found a significant decrease in IRF-3 protein levels within 24 h of infection and prior to CHX treatment. This decrease was followed by a rapid turnover of IRF-3 during CHX treatment, with a calculated half-life reduced to 5 h, revealing a fivefold increase in the rate of IRF-3 protein decay. In contrast, IRF-7 protein levels remained relatively unchanged between mock-infected and HIV-1-infected cells, with a half-life of <3 h (Fig. 2C). Thus, HIV-1 infection specifically decreases IRF-3 protein stability.
Active IRF-3 is deleterious to HIV-1 infection.
IRF-3 activation imparts a cellular response that limits virus replication and spread (27), suggesting that intact and active IRF-3 could have a deleterious effect on HIV-1 infection. To determine how IRF-3 signaling may impact HIV-1 infection, we examined virus replication in SupT1 cells ectopically expressing the constitutively active IRF-3(5D) mutant (25). Virus production was significantly suppressed in cells expressing IRF-3(5D) (P < 0.001) compared to that in control cells, and the suppression was similar to the suppression of HIV-1 production observed after pretreatment of cells with exogenous IFN-β (Fig. 2D). Thus, SupT1 cells are competent to mount an IRF-3-dependent antiviral response against HIV-1. These results demonstrate that HIV-1 is highly sensitive to the innate antiviral response induced by IRF-3 in T cells, wherein active IRF-3 exerts a marked deleterious effect on cellular HIV-1 infection.
HIV-1 replication is necessary for viral depletion of IRF-3.
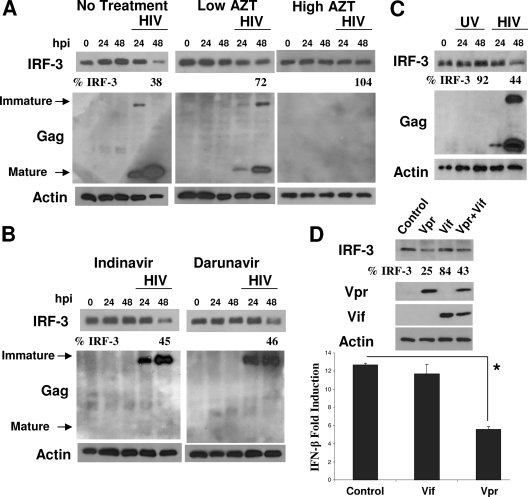
To assess the requirements for IRF-3 protein depletion by HIV-1, we pretreated SupT1 cells with azidothymidine (AZT) or either of two HIV-1 protease inhibitors (indinavir or darunavir) 3 h prior to HIV-1 challenge. HIV-1 replication was delayed with low-dose AZT treatment or was stopped altogether in a concentration-dependent manner after high-dose AZT treatment (as seen by the quantity of Gag protein species) (Fig. 3A). The depletion of IRF-3 by HIV-1 was partially or completely circumvented by low- or high-dose AZT treatment, respectively (Fig. 3A). We observed a complete block of mature Gag p24 production in cells treated with either protease inhibitor, demonstrating the functional inhibition of HIV-1 protease (Fig. 3B). In contrast to treatment with AZT, treatment with the protease inhibitors did not prevent IRF-3 protein depletion, which was reduced within 48 h of HIV-1 infection. Taken together, these data suggest that IRF-3 depletion requires replication and the initiation of a productive virus life cycle but occurs independently of HIV-1 protease function or full maturation of new virions. This conclusion is corroborated by our finding that UV-inactivated virus also failed to induce a decrease in IRF-3 protein levels (Fig. 3C).
FIG. 3.
IRF-3 depletion requires HIV-1 replication but occurs independently of viral protease function. (A) SupT1 cells were pretreated with 5 μM AZT (Low AZT) or 10 μM AZT (High AZT) and were then mock infected or infected with HIV-1LAI at an MOI of 1. Infection was allowed to continue in the presence of the drug, and the cells were harvested at the indicated times postinfection. Cell lysates were analyzed by immunoblotting to determine the levels of IRF-3, HIV-1 p24, and actin. (B) Cells were treated and infected with HIV-1 as described above, except with 100 nM of the HIV-1 protease inhibitor compound indinavir or darunavir. (C) SupT1 cells were infected with a common aliquot of HIV-1LAI (MOI of 1) that had been divided and left untreated or subjected to UV light inactivation for 15 min prior to addition to SupT1 cells. Values represent the percentages of IRF-3 remaining compared to that at time zero of infection. (D) HEK293 cells were transfected with 100 ng of Flag-IRF-3 and 900 ng of HIV-1 Vif, Vpr, control plasmid, or a mixture of both Vif and Vpr (450 ng each). (Top) Immunoblot of IRF-3, Vpr, Vif, and actin levels in the transfected cells. (Bottom) The bars show the levels of SenV-induced IFN-β promoter activity after coexpression of the same HIV-1 virus constructs with the IFN-β promoter luciferase construct and an internal control. SenV-induced IFN-β promoter activity was determined by infecting each culture of cells with 50 hemagglutinating units of SenV and assessing promoter activity 24 h later (n = 3). *, P < 0.01. hpi, hours postinfection. n, number of samples.
A previous report of IRF-3 dysregulation by HIV-1 suggested that the HIV-1 accessory proteins Vif and Vpr may mediate the viral suppression of IRF-3 levels (32). We tested these HIV-1 proteins for the control of IFN-β promoter signaling by using a similar transfection/expression approach. As shown in Fig. 3D, expression of Vpr but not Vif resulted in an attenuation of IFN-β promoter induction triggered upon SenV infection of transfected cells. This attenuation was associated with a similar reduction in the steady-state level of Flag-tagged IRF-3 when coexpressed with Vpr but not Vif in the transfected cells (Fig. 3D), suggesting that the HIV-1 Vpr protein may be dominant for IRF-3 regulation (32). In support of this notion, when Vif was coexpressed with Vpr, we did not observe an enhancement of IRF-3 depletion over that associated with Vpr expression alone (Fig. 3D).
Disruption of PRR signaling of innate immunity by HIV-1.
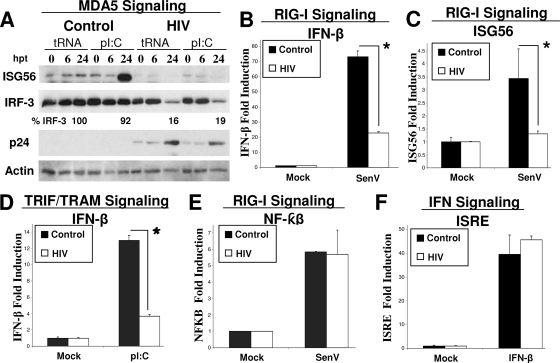
IRF-3 is an essential downstream factor of PRR signaling, and to determine to what extent HIV-1 disrupts IRF-3-dependent PRR signaling in HIV-1-infected cells, we examined RLR and specific TLR signaling in the context of HIV-1 infection. MDA5 and RIG-I are RLRs that recognize transfected/cytoplasmic, long, double-stranded RNA (dsRNA) and SenV infection, respectively (27, 36). To test MDA5 signaling, we infected SupT1 cells with HIV-1 for 24 h and then delivered poly(I:C) synthetic dsRNA directly into the cytoplasm of the infected cells via transfection. Treatment of cells in this manner specifically induces IRF-3-dependent MDA5 signaling of ISG expression (21). Accordingly, poly(I:C) transfection of noninfected cells induced high-level ISG56 expression within 24 h of treatment. However, this response was abrogated in HIV-1-infected cells in association with IRF-3 depletion (Fig. 4A). To determine how HIV-1 impacts RIG-I signaling, we measured IFN-β and ISG56 promoter activity in HEK293 cells transfected with HIV-1 provirus DNA. Forty-eight hours after HIV-1 provirus transfection, we challenged the cells with SenV, a RIG-I-specific stimulus (26), and promoter activity was determined after an additional 24 h. As seen in Fig. 4B and C, the cells harboring HIV-1 showed a significant decrease in IRF-3-dependent promoter induction signaled by RIG-I. These results demonstrate that depletion of IRF-3 by HIV-1 effectively disrupts RLR signaling.
FIG. 4.
Disruption of IRF-3-dependent PRR signaling by HIV-1. (A) poly(I:C) or tRNA was transfected into SupT1 cells that were mock infected or infected with HIV-1LAI (MOI of 1) for 24 h prior to transfection. Cells were harvested at the indicated times posttransfection and subjected to immunoblot analysis of ISG56, IRF-3, HIV p24, and actin protein levels. Values represent the percentages of IRF-3 compared to that at time zero of infection. Data shown are representative of three independent experiments. (B to F) Cells were cotransfected with HIV-1pNL4-3 provirus DNA or control plasmid and the indicated promoter-luciferase plasmid construct. Forty-eight hours posttransfection, the cells were infected with 50 hemagglutinating units/ml of SenV or treated as indicated to induce the indicated PRR signaling, and promoter-luciferase activity was measured 18 h later (n ≥ 3). (B) SenV-induced IFN-β promoter activity in HEK293 cells. *, P < 0.005. (C) SenV-induced ISG56 promoter activity in HEK293 cells. *, P < 0.02. (D) HEK293-TLR3 cells were transfected as described for panel B. Forty-eight hours posttransfection, the cells were treated with 100 μg/ml poly(I:C) added to the culture medium, and IFN-β promoter activity was measured 18 h later. *, P < 0.005. (E) SenV-induced NF-κB promoter activity in HEK293 cells. (F) IFN-β-induced ISRE promoter activity in HEK293 cells. hpt, hours posttransfection; pI:C, poly(I:C). n, number of samples.
To determine if TLR signaling through TRIF/TRAM-dependent pathways that activate IRF-3 was also ablated in HIV-1-infected cells, we similarly cotransfected HEK293 cells expressing TLR3 with HIV-1 proviral DNA and an IFN-β promoter-luciferase construct. Forty-eight hours after transfection, the cells were treated with extracellular poly(I:C) to specifically induce signaling through TLR3. Figure 4D shows that TLR-mediated TRIF/TRAM-dependent signaling of IFN-β promoter expression was significantly reduced in HIV-1-infected cells expressing the HIV-1 provirus. To affirm the specificity and breadth of these signaling defects, we examined RIG-I signaling to an NF-κB-responsive promoter construct and IFN signaling of an ISRE promoter construct in HEK293 cells that were cotransfected with the individual promoter constructs and the HIV-1 provirus DNA. SenV infection of cells triggered robust activity of the NF-κB-dependent promoter regardless of HIV-1 provirus expression (Fig. 4E). Similar results were obtained with cells cotransfected with HIV-1 provirus and the NF-κB-dependent PRDII promoter construct (data not shown). IFN signaling is dependent on IRF-9 and is mediated by the Jak-STAT pathway (27). HIV-1 provirus had no significant effect on signaling to the ISRE in IFN-β-treated cells (Fig. 4F). Taken together, these data demonstrate that HIV-1 infection leaves RIG-I signaling of NF-κB activation and IRF-9-dependent signaling of the Jak-STAT pathway intact while disrupting IRF-3-dependent PRR signaling programs through the specific depletion of IRF-3.
HIV-1 disruption of IRF-3-dependent PRR signaling impairs the innate immune response to secondary virus infection.
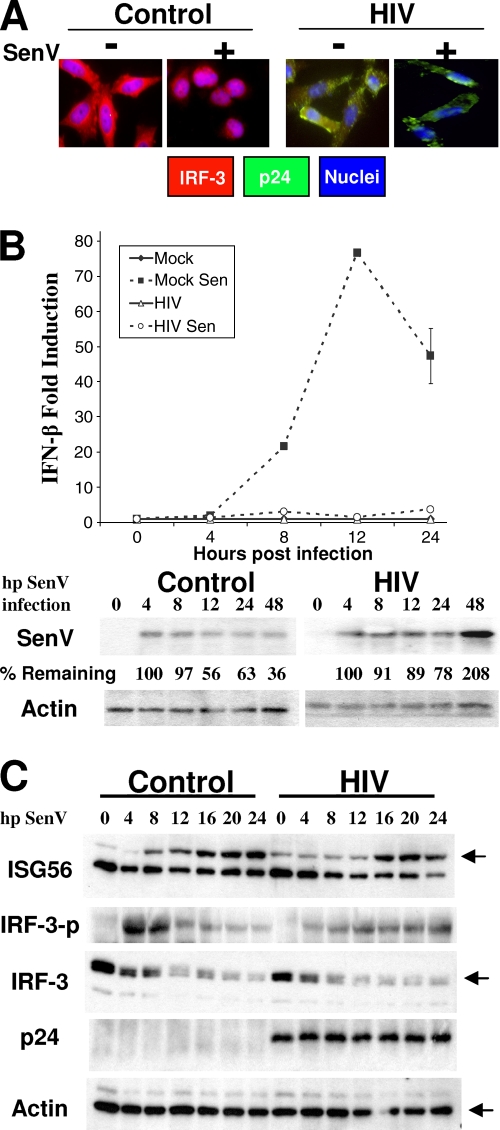
Disruption of PRR signaling programs of innate antiviral defense might influence the ability of HIV-1-infected cells to mount an effective response and resistance to other viral pathogens for opportunistic superinfection. To determine how HIV-1 depletion of IRF-3 affected susceptibility to secondary infection of cells, we utilized a model system consisting of a paramyxovirus (SenV) challenge in HIV-1-susceptible cells, which normally causes a robust activation of IRF-3. First, CD4+ HeLa cells were mock infected or infected with HIV-1 for 24 h, followed by challenge with SenV. Twenty-four hours later, cellular IRF-3 and HIV-1 Gag protein abundance was assessed by immunostaining of cells and examination by fluorescence microscopy. Non-HIV-infected control cells alone harbored abundant cytoplasmic IRF-3 that upon SenV challenge was redistributed to the cell nucleus, consistent with RIG-I signaling of IRF-3 activation. In contrast, HIV-1-infected cells exhibited only very weak IRF-3 staining concomitant with strong staining of p24 Gag, reflecting the general depletion of IRF-3 by HIV-1 (Fig. 5A). IFN-β mRNA levels were next examined over a secondary-infection time course in SupT1 cells. SenV infection alone triggered a robust induction of IFN-β message, but this response was suppressed in cells that were first infected with HIV-1 or in cells infected with HIV-1 alone (Fig. 5B, top). HIV-1 suppression of SenV-induced PRR signaling associated with increased and sustained levels of SenV protein expression (Fig. 5B, bottom) that reflected an overall increased permissiveness for superinfection with SenV. In PBMCs, SenV stimulated PRR signaling of IRF-3 phosphorylation/activation and ISG56 expression over an infection time course. However, this response was delayed and attenuated in those cultures infected with HIV-1 prior to SenV challenge. These results demonstrate that HIV-1 infection imparts defects in the kinetics and magnitude of IRF-3-dependent PRR signaling of the innate immune response to a secondary virus infection, thus conferring increased susceptibility to virus superinfection.
FIG. 5.
HIV-1 depletion of IRF-3 compromises innate antiviral immunity to enhance cellular permissiveness for secondary virus infection. (A) HeLa CD4+ cells were mock infected or treated with HIV-1LAI (MOI of 10) for 30 h before the addition (+) of 50 hemagglutinating units/ml of SenV. Eighteen hours later, cells were immunostained with antibodies against IRF-3 and HIV p24. Cell nuclei were stained with DAPI. Cells were visualized by fluorescence microscopy. Merged-color images are shown. (B) SupT1 cells were cultured alone or were infected with HIV-1LAI. Twenty-four hours later, the cells were mock treated or were infected with SenV. Cells were harvested at the indicated times (hours) after SenV infection (hp SenV infection), and total RNA was isolated for qPCR analysis of IFN-β mRNA levels (n = 3). Cells were treated in parallel, and SenV protein and actin levels were analyzed by immunoblotting. Values represent the percentages of SenV protein compared to input. (C) PBMCs were treated as described for panels A and B but were mock infected or infected with HIV-1 for 48 h prior to SenV infection. Cells were harvested and subjected to immunoblot analysis for determination of protein abundance. Data are representative of three independent experiments. n, number of samples.
Reduced IRF-3 levels in CD4+ cells ex vivo from mucosa and in vivo from patients with acute HIV-1 infection.
To examine the relevance of IRF-3 depletion for HIV-1 infection in vivo, we assessed whether T cells collected from a typical mucosal HIV transmission site demonstrated altered IRF-3 levels upon ex vivo infection. Healthy vaginal tissue was obtained and infected ex vivo with two different strains, HIV-1JR-CSF and HIV-1BaL. After 48 h of infection, the mucosal T cells were isolated from the tissue as previously described (16, 17), and IRF-3 levels were determined by immunoblotting and densitometric analysis (Fig. 6A). IRF-3 levels were markedly decreased compared to those for HIV-1-uninfected cells, with less than 60% of IRF-3 remaining in the HIV-1JR-CSF-infected cells and less than 40% of IRF-3 remaining in the HIV-1BaL-infected samples.
For in vivo confirmation of the HIV-1-dependent IRF-3 depletion, we obtained PBMCs from six individuals with acute HIV-1 infection (range, 32 to 106 days postinfection) and from five HIV-1-infected LTNP (CD4+ count of >400; >16 years HIV-1 seropositive). We sorted PBMCs from these individuals, as well those as from five healthy, uninfected donors, into CD4+ and CD4− cells and subjected the cell populations to immunoblot analysis of IRF-3 and IRF-7. Levels were compared to amounts of total protein, while actin levels were used as an internal control for overall protein content. As seen in Fig. 6B, little variation in IRF-3 levels was observed between the CD4+ and CD4− cell populations from HIV-1-seronegative subjects (n = 5). However, of the six patients with acute HIV-1 infection screened, we found three patients whose CD4+ cell populations showed decreased IRF-3 levels compared to those of the CD4− cell populations, with one patient showing a nearly 50% decrease in total IRF-3 levels. No such differences were observed for the five LTNP. We also examined IRF-7 in these same 16 subjects and observed widely varying protein levels. Surprisingly, we observed an overall differential pattern of IRF-7 levels between CD4+ and CD4− cells, with a trend of IRF-7 segregating to the CD4− cell population of all individuals regardless of HIV-1 serostatus. These data highlight the importance of IRF-3 in the CD4+ cell population as a central component of the PRR signaling pathways and provide evidence for viral depletion of IRF-3 in vivo during acute HIV-1 infection.
DISCUSSION
Our studies reveal a specific depletion of IRF-3 in HIV-1-infected cells that results in a loss of PRR signaling of innate antiviral defenses. The decline in IRF-3 levels occurred with the accumulation of viral proteins and was dependent on HIV-1 replication initiation. That HIV-1 mediates the targeted depletion of IRF-3 is supported by our observations that neither IRF-7 levels nor IRF-9-dependent signaling was impacted by HIV-1. In addition to the depletion of IRF-3 during infection within the CD4+ cell lines and primary cells, we observed IRF-3 dysregulation by HIV-1 in HEK293 cells expressing transfected HIV-1 provirus DNA. Thus, IRF-3 antagonism by HIV-1 is not restricted to a specific cell type. Our data show that IRF-3 depletion is a general property shared by R5- and X4-tropic viruses and is a corresponding early event of acute HIV-1 infection. Our studies indicate that, when activated, IRF-3 directs an intracellular innate antiviral response that can potently suppress HIV infection. Thus, reduction of IRF-3 levels provides a strategy for HIV-1 to evade the host innate immune response and to promote host cell permissiveness for infection.
Our results confirm previous work suggesting that HIV-1 targets IRF-3 for protein depletion, in which the authors concluded that HIV accessory proteins mediated early postentry depletion of IRF-3 by stimulating its ubiquitination and targeting to the proteosome (32). This conclusion was in part based on an observation that IRF-3 depletion by HIV-1 was insensitive to preinfection treatment of cells with AZT and was blocked by treatment of cells with proteosome inhibitors (32). Conversely, we found that similar treatment of cells with AZT actually prevented IRF-3 depletion concomitant with the ablation of provirus production, whereas provirus expression was necessary and sufficient for IRF-3 depletion and occurred independently of HIV-1 protease function. We also found that proteosome inhibitor treatment of cells could partially restore IRF-3 levels during HIV-1 infection (B. P. Doehle and M. Gale, Jr., unpublished results). While these differences between studies might be attributed to variation among the HIV strains and experimental systems used by each group, we note that proteosome inhibitors are deleterious to HIV-1 infection and that viral replication is suppressed in treated cells (32). Thus, we conclude that HIV-1 replication and provirus expression are required for IRF-3 suppression wherein one or more viral proteins, including possibly virion-associated proteins, direct the rapid destabilization and degradation of IRF-3. We found that the active, nuclear isoform of IRF-3 did not accumulate within HIV-1-infected cells even prior to the large reduction of IRF-3 levels early in acute infection. These observations may implicate a direct or indirect viral protein interaction with IRF-3 that interferes with its activation and may serve to catalyze protein degradation. Expression of the HIV-1 Vpr protein had a modest effect to attenuate virus-induced IFN-β promoter expression (Fig. 3D), which we attribute to an associated reduction in the level of coexpressed IRF-3 (Fig. 3D), further supporting a possible role for Vpr in IRF-3 regulation (32). However, Vif did not associate with reduced IFN-β promoter signaling or IRF-3 levels in our coexpression experiments. Thus, Vpr could have a dominant role among these viral accessory proteins in directing the suppression of IRF-3, or Vif may require additional viral factors to further influence IRF-3 levels, possibly explaining the differences between our results and the previous report (32). These possibilities are being explored.
HIV-1 infection is typically initiated at mucosal sites of transmission, where innate antiviral defenses of resident mucosal CD4+ T cells and macrophages provide the first level of immune protection against infection (18). Importantly, we found that IRF-3 is rapidly depleted within vaginal mucosal CD4+ T cells upon HIV-1 infection in an ex vivo tissue model. Our studies clearly demonstrate that PBMCs and T-cell lines can mediate RLR signaling in response to specific ligand treatment or SenV infection of cells. Disruption of RLR signaling compromises IFN and ISG expression to enhance susceptibility to RNA virus infection (27, 36). In contrast, TLR3 and TLR4 signal through TRIF/TRAM-dependent pathways, conferring cellular responsiveness to the dsRNA ligand and bacterial lipopolysaccharide, respectively (33, 43). The disruption of signaling by these PRRs has been shown to attenuate both innate and adaptive antimicrobial immunity (33, 43). Thus, the depletion of IRF-3 in mucosal cells of infection overall serves to compromise the integrity of mucosal antiviral defenses by limiting IFN production and secretion from infected resident macrophages and abrogating PRR signaling of the antiviral state, including signaling by RIG-I, MDA5, TLR3, and TLR4, in infected mucosal T cells. The HIV-1-mediated depletion of IRF-3 within a short time frame during initial acute infection therefore allows for unchallenged virus amplification and dissemination from the mucosa to peripheral sites of infection. We found that IRF-3 levels were not uniformly reduced in CD4+ T cells across all HIV-1-infected patients examined in our study, with differential levels observed among patients undergoing acute infection and normal IRF-3 levels observed among LTNP. While further studies of larger patient cohorts are required in order to understand these differences, it is possible that viral polymorphisms and/or host factor distinctions could impart the differential regulation of IRF-3 and innate defenses to impact the virologic features of HIV-1 infection. These differences in IRF-3 levels may also be due to the impact of viral load and could be subject to regulation by antiretroviral therapy, possibly leading to a rebound in IRF-3 levels. Such possibilities are being investigated.
In addition to decreased IRF-3 levels in CD4+ cells, our data reveal overall low levels of IRF-7 in CD4+ cell populations in general. These observations implicate IRF-3 as a central transcription factor of PRR signaling in CD4+ T cells and further affirm the importance of IRF-3-dependent innate response pathways in pathogen sensing and control. IRF-7 in T cells appears to have a less prominent role in infection than IRF-7 in dendritic cells, which have high constitutive IRF-7 levels. This difference may have played a role in the evolution of HIV-1 countermeasures against T-cell innate immune programs. Indeed, our data suggest that a strong evolutionary pressure to block these host defenses exists, as we observed that IRF-3 activation can severely limit HIV-1 infection.
HIV-1 is not unique in targeting IRF-3 as a countermeasure to host innate defenses, as other viruses have been shown to antagonize IRF-3 function through disruption of upstream signaling programs (27) and degradation of IRF-3 during infection (3, 4, 15). While HIV-1 targeting of IRF-3 is particularly effective in disrupting several host PRR pathways, we found that IRF-9-dependent IFN signaling and PRR signaling of NF-κB remain intact in HIV-1-infected cells. These observations underscore the specificity of IRF-3 regulation by HIV-1 and likely reflect the requirement for NF-κB function in viral mRNA transcription from the HIV-1 provirus (1, 8). In particular, RLR signaling and the actions of TLR3 and TLR4 drive the activation of IRF-3 and NF-κB concomitantly through adaptor protein signaling bifurcation (33). By specifically targeting IRF-3, HIV-1 therefore ensures the attenuation of IRF-3-dependent immunity while preserving pathways of NF-κB activation that might assist virus replication (1, 8). IRF-3 is central to the stimulation of IFN production in many cell types, and a decrease in IRF-3 levels functionally disrupts IFN production and the ISG response normally triggered by a variety of microbial pathogens through processes of PRR signaling (27, 33, 41). Overall, IRF-3 depletion in T cells and macrophages could provide a framework for opportunistic pathogens to invade mucosal sites of HIV-1 infection. A similar effect on secondary infection has recently been demonstrated with a chronic lymphocytic choriomeningitis virus infection model (45). Our studies of the SenV paramyxovirus model provide direct evidence that HIV-1 depletion of IRF-3 may support polymicrobial opportunistic infection.
Innate immune suppression through IRF-3 depletion could be expected to overall slow the kinetics of the immune response against HIV to support a systemic chronic infection. In support of this idea, studies of herpes simplex virus infection have demonstrated that immune suppression in the local infection environment can delay pDC infiltration to the mucosal site of acute infection and associates with increased viral production (28). IRF-3 depletion may have an analogous effect in HIV-1 infection, increasing peak viral load and therefore contributing to the viral set point and disease progression to AIDS. TLR7 and TLR9 recognition of HIV-1 has previously been demonstrated with pDCs and leads to IFN production in an IRF-7-dependent fashion (5, 14, 30). This recognition is important for the induction of the adaptive immune response that initially controls acute viremia in HIV-1 infection (22). Recent work with simian immunodeficiency virus-infected nonhuman primates has suggested that chronic pDC activation through TLR7 and TLR9 may contribute to AIDS pathogenesis (29). We propose that a higher HIV set point caused by IRF-3 depletion in infected cells could support a larger pool of chronically infected cells and hence provide an increased number of TLR ligands to trigger and sustain chronic immune activation.
Our results demonstrate that active IRF-3 directs a robust and lethal antiviral hit to HIV-1 that can suppress de novo virus production. This inhibition of HIV-1 in the face of activated IRF-3 unveils an additional avenue for both therapeutics and vaccine design to control HIV-1 replication and dissemination. IRF-3 activation or the rescue of IRF-3 levels may benefit infected patients by inducing antiviral defenses that suppress HIV-1 infection and replication while serving to enhance overall immunity to infection.
Acknowledgments
We thank Kristina Chang and Nadezda Andreeva for technical assistance, Mehul Suthar and Dan Stetson for helpful conversation and review, and Michael Katze for reagents and assistance.
This work was supported by grants from the U.S. Public Health Service, including grants AI057005 and AI047086 in support of the Seattle Primary Infection Program and grant M01-RR-00037 for leukapheresis. The work was also supported by funds from the State of Washington; by NIH grants DA024563 (M.G.), AI078768 (B.P.D.), and HD51455 (F.H.); and by The Burroughs-Wellcome Fund (M.G.).
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
▿
Published ahead of print on 12 August 2009.
REFERENCES
- 1.Alcami, J., T. L. Delera, L. Folgueira, M. A. Pedraza, J. M. Jacque, F. Bachelerie, A. R. Noriega, R. T. Hay, D. Harrich, R. B. Gaynor, J. L. Virelizier, and F. Arenzanaseisdedos. 1995. Absolute dependence on kappa-B responsive elements for initiation and Tat-mediated amplification of HIV transcription in blood CD4 T-lymphocytes. EMBO J. 14**:**1552-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Au, W. C., P. A. Moore, W. Lowther, Y. T. Juang, and P. M. Pitha. 1995. Identification of a member of the interferon regulatory factor family that binds to the interferon-stimulated response element and activates expression of interferon-induced genes. Proc. Natl. Acad. Sci. USA 92**:**11657-11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barro, M., and J. T. Patton. 2005. Rotavirus nonstructural protein 1 subverts innate immune response by inducing degradation of IFN regulatory factor 3. Proc. Natl. Acad. Sci. USA 102**:**4114-4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barro, M., and J. T. Patton. 2007. Rotavirus NSP1 inhibits expression of type I interferon by antagonizing the function of interferon regulatory factors IRF3, IRF5, and IRF7. J. Virol. 81**:**4473-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beignon, A. S., K. McKenna, M. Skoberne, O. Manches, I. DaSilva, D. G. Kavanagh, M. Larsson, R. J. Gorelick, J. D. Lifson, and N. Bhardwaj. 2005. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J. Clin. Investig. 115**:**3265-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berrey, M. M., T. Schacker, A. C. Collier, T. Shea, S. J. Brodie, D. Mayers, R. Coombs, J. Krieger, T. W. Chun, A. Fauci, S. G. Self, and L. Corey. 2001. Treatment of primary human immunodeficiency virus type 1 infection with potent antiretroviral therapy reduces frequency of rapid progression to AIDS. J. Infect. Dis. 183**:**1466-1475. [DOI] [PubMed] [Google Scholar]
- 7.Bull, M., D. Lee, J. Stucky, Y. L. Chiu, A. Rubin, H. Horton, and M. J. McElrath. 2007. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J. Immunol. Methods 322**:**57-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, B. K., M. B. Feinberg, and D. Baltimore. 1997. The κB sites in the human immunodeficiency virus type 1 long terminal repeat enhance virus replication yet are not absolutely required for viral growth. J. Virol. 71**:**5495-5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cullen, B. R. 2006. Role and mechanism of action of the APOBEC3 family of antiretroviral resistance factors. J. Virol. 80**:**1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espert, L., G. Degols, Y. L. Lin, T. Vincent, M. Benkirane, and N. Mechti. 2005. Interferon-induced exonuclease ISG20 exhibits an antiviral activity against human immunodeficiency virus type 1. J. Gen. Virol. 86**:**2221-2229. [DOI] [PubMed] [Google Scholar]
- 11.Foy, E., K. Li, R. Sumpter, Y. M. Loo, C. L. Johnson, C. F. Wang, P. M. Fish, M. Yoneyama, T. Fujita, S. M. Lemon, and M. Gale. 2005. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc. Natl. Acad. Sci. USA 102**:**2986-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foy, E., K. Li, C. F. Wang, R. Sumpter, M. Ikeda, S. M. Lemon, and M. Gale. 2003. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science 300**:**1145-1148. [DOI] [PubMed] [Google Scholar]
- 13.Grandvaux, N., M. J. Servant, B. Tenoever, G. C. Sen, S. Balachandran, G. N. Barber, R. T. Lin, and J. Hiscott. 2002. Transcriptional profiling of interferon regulatory factor 3 target genes: direct involvement in the regulation of interferon-stimulated genes. J. Virol. 76**:**5532-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303**:**1526-1529. [DOI] [PubMed] [Google Scholar]
- 15.Hilton, L., K. Moganeradj, G. Zhang, Y. H. Chen, R. E. Randall, J. W. McCauley, and S. Goodbourn. 2006. The NPro product of bovine viral diarrhea virus inhibits DNA binding by interferon regulatory factor 3 and targets it for proteasomal degradation. J. Virol. 80**:**11723-11732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hladik, F., G. Lentz, R. E. Akridge, G. Peterson, H. Kelley, A. McElroy, and M. J. McElrath. 1999. Dendritic cell-T-cell interactions support coreceptor-independent human immunodeficiency virus type 1 transmission in the human genital tract. J. Virol. 73**:**5833-5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hladik, F., G. Lentz, E. Delpit, A. McElroy, and M. J. McElrath. 1999. Coexpression of CCR5 and IL-2 in human genital but not blood T cells: implications for the ontogeny of the CCR5(+) Th1 phenotype. J. Immunol. 163**:**2306-2313. [PubMed] [Google Scholar]
- 18.Hladik, F., and M. J. McElrath. 2008. Setting the stage: host invasion by HIV. Nat. Rev. Immunol. 8**:**447-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juang, Y. T., W. Lowther, M. Kellum, W. C. Au, R. Lin, J. Hiscott, and P. M. Pitha. 1998. Primary activation of interferon A and interferon B gene transcription by interferon regulatory factor 3. Proc. Natl. Acad. Sci. USA 95**:**9837-9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamga, I., S. Kahi, L. Develioglu, M. Lichtner, C. Maranon, C. Deveau, L. Meyer, C. Goujard, P. Lebon, M. Sinet, and A. Hosmalin. 2005. Type I interferon production is profoundly and transiently impaired in primary HIV-1 infection. J. Infect. Dis. 192**:**303-310. [DOI] [PubMed] [Google Scholar]
- 21.Kato, H., O. Takeuchi, E. Mikamo-Satoh, R. Hirai, T. Kawai, K. Matsushita, A. Hiiragi, T. S. Dermody, T. Fujita, and S. Akira. 2008. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J. Exp. Med. 205**:**1601-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koup, R. A., J. T. Safrit, Y. Z. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68**:**4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy, J. A., L. Scott, and C. Mackewicz. 2003. Protection from HIV/AIDS: the importance of innate immunity. Clin. Immunol. 108**:**167-174. [DOI] [PubMed] [Google Scholar]
- 24.Lin, R. T., C. Heylbroeck, P. M. Pitha, and J. Hiscott. 1998. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell. Biol. 18**:**2986-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, R. T., Y. Mamane, and J. Hiscott. 1999. Structural and functional analysis of interferon regulatory factor 3: localization of the transactivation and autoinhibitory domains. Mol. Cell. Biol. 19**:**2465-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loo, Y.-M., J. Fornek, N. Crochet, G. Bajwa, O. Perwitasari, L. Martinez-Sobrido, S. Akira, M. A. Gill, A. García-Sastre, M. G. Katze, and M. Gale, Jr. 2008. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J. Virol. 82**:**335-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loo, Y. M., and M. Gale, Jr. 2007. Viral regulation and evasion of the host response. Curr. Top. Microbiol. Immunol. 316**:**295-313. [DOI] [PubMed] [Google Scholar]
- 28.Lund, J. M., L. Hsing, T. T. Pham, and A. Y. Rudensky. 2008. Coordination of early protective immunity to viral infection by regulatory T cells. Science 320**:**1220-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandl, J. N., A. P. Barry, T. H. Vanderford, N. Kozyr, R. Chavan, S. Mucking, F. J. Barrat, R. L. Coffman, S. I. Staprans, and M. B. Feinberg. 2008. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat. Med. 14**:**1077-1087. [DOI] [PubMed] [Google Scholar]
- 30.Meier, A., G. Alter, N. Frahm, H. Sidhu, B. Li, A. Bayhi, N. Teigen, H. Streeck, H. J. Stellbrink, J. Hellman, J. van Lunzen, and M. Altfeld. 2007. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. J. Virol. 81**:**8180-8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okumura, A., G. S. Lu, I. Pitha-Rowe, and P. M. Pitha. 2006. Innate antiviral response targets HIV-1 release by the induction of ubiquitin-like protein ISG15. Proc. Natl. Acad. Sci. USA 103**:**1440-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okumura, A., T. Alce, B. Lubyova, H. Ezelle, K. Strebel, and P. M. Pitha. 2008. HIV-1 accessory proteins VPR and Vif modulate antiviral response by targeting IRF-3 for degradation. Virology 373**:**85-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Neill, L. A. J., and A. G. Bowie. 2007. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 7**:**353-364. [DOI] [PubMed] [Google Scholar]
- 34.Ozato, K., D. M. Shin, T. H. Chang, and H. C. Morse. 2008. TRIM family proteins and their emerging roles in innate immunity. Nat. Rev. Immunol. 8**:**849-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sadler, A. J., and B. R. G. Williams. 2008. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 8**:**559-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saito, T., and M. Gale. 2007. Principles of intracellular viral recognition. Curr. Opin. Immunol. 19**:**17-23. [DOI] [PubMed] [Google Scholar]
- 37.Saito, T., D. M. Owen, F. G. Jiang, J. Marcotrigiano, and M. Gale. 2008. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 454**:**523-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schacker, T. 1997. Primary HIV infection. Early diagnosis and treatment are critical to outcome. Postgrad. Med. 102**:**143-151. [DOI] [PubMed] [Google Scholar]
- 39.Schacker, T., A. C. Collier, J. Hughes, T. Shea, and L. Corey. 1996. Clinical and epidemiologic features of primary HIV infection. Ann. Intern. Med. 125:257-264. [DOI] [PubMed] [Google Scholar]
- 40.Soumelis, V., I. Scott, F. Gheyas, D. Bouhour, G. Cozon, L. Cotte, L. Huang, J. A. Levy, and Y. J. Liu. 2001. Depletion of circulating natural type I interferon-producing cells in HIV-infected AIDS patients. Blood 98**:**906-912. [DOI] [PubMed] [Google Scholar]
- 41.Stetson, D. B., and R. Medzhitov. 2006. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity 24**:**93-103. [DOI] [PubMed] [Google Scholar]
- 42.Stevenson, M. 2003. HIV-1 pathogenesis. Nat. Med. 9**:**853-860. [DOI] [PubMed] [Google Scholar]
- 43.Trinchieri, G., and A. Sher. 2007. Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 7**:**179-190. [DOI] [PubMed] [Google Scholar]
- 44.Woelk, C. H., F. Ottones, C. R. Plotkin, P. Y. Du, C. D. Royer, S. E. Rought, J. Lozach, R. Sasik, R. S. Kornbluth, D. D. Richman, and J. Corbeil. 2004. Interferon gene expression following HIV type 1 infection of monocyte-derived macrophages. AIDS Res. Hum. Retrovir. 20**:**1210-1222. [DOI] [PubMed] [Google Scholar]
- 45.Zuniga, E. I., L. Y. Liou, L. Mack, M. Mendoza, and M. B. A. Oldstone. 2008. Persistent virus infection inhibits type I interferon production by plasmacytoid dendritic cells to facilitate opportunistic infections. Cell Host Microbe 4**:**374-386. [DOI] [PMC free article] [PubMed] [Google Scholar]