PD-L1 regulates the development, maintenance, and function of induced regulatory T cells (original) (raw)
Abstract
Both the programmed death (PD) 1–PD-ligand (PD-L) pathway and regulatory T (T reg) cells are instrumental to the maintenance of peripheral tolerance. We demonstrate that PD-L1 has a pivotal role in regulating induced T reg (iT reg) cell development and sustaining iT reg cell function. PD-L1−/− antigen-presenting cells minimally convert naive CD4 T cells to iT reg cells, showing the essential role of PD-L1 for iT reg cell induction. PD-L1–coated beads induce iT reg cells in vitro, indicating that PD-L1 itself regulates iT reg cell development. Furthermore, PD-L1 enhances and sustains Foxp3 expression and the suppressive function of iT reg cells. The obligatory role for PD-L1 in controlling iT reg cell development and function in vivo is illustrated by a marked reduction in iT reg cell conversion and rapid onset of a fatal inflammatory phenotype in PD-L1−/−PD-L2−/− Rag−/− recipients of naive CD4 T cells. PD-L1 iT reg cell development is mediated through the down-regulation of phospho-Akt, mTOR, S6, and ERK2 and concomitant with the up-regulation of PTEN, all key signaling molecules which are critical for iT reg cell development. Thus, PD-L1 can inhibit T cell responses by promoting both the induction and maintenance of iT reg cells. These studies define a novel mechanism for iT reg cell development and function, as well as a new strategy for controlling T reg cell plasticity.
Regulatory T (T reg) cells are key mediators of peripheral tolerance that actively suppress effector T (T eff) cells and inhibit immune-mediated tissue damage (Kronenberg and Rudensky, 2005; Sakaguchi et al., 2008; Tang and Bluestone, 2008). T reg cells can be divided into naturally occurring T reg (nT reg) and induced T reg (iT reg) cells. nT reg cells develop in the thymus, express the cardinal transcription factor forkhead box p3 (Foxp3; Fontenot et al., 2003; Hori et al., 2003), have high levels of CD25, and have a TCR repertoire biased toward self-antigens. In contrast, iT reg cells develop in the periphery. In the presence of TGF-β, naive CD4+ T cells are induced to express Foxp3 and are converted toward an iT reg cell fate (Chen et al., 2003; Fantini et al., 2004; Coombes et al., 2007; Rubtsov and Rudensky, 2007). These TGF-β–induced T reg cells, like nT reg cells, express high levels of CD25, CTLA-4, and GITR (glucocorticoid-induced TNF receptor), require prior stimulation for T reg cell activity, and potently suppress T eff cells (Lohr et al., 2006). Programmed death (PD) 1 receptor (CD279) and its ligand PD–ligand (PD-L) 1 (B7-H1; CD274) are also highly expressed on T reg cells, but whether the PD-1–PD-L pathway plays a role in expansion or function of T reg cells is not yet clear (Baecher-Allan et al., 2003; Krupnick et al., 2005).
The pathway consisting of the receptor PD-1 and its ligands, PD-L1 and PD-L2 (B7-DC; CD273), is a newer pathway in the B7-CD28 family that regulates the balance between stimulatory and inhibitory signals needed for effective immunity and the maintenance of self-tolerance (Keir et al., 2007a, 2008). PD-1 is up-regulated on T cells upon activation, and its ligands have distinct expression patterns, with PD-L1 being expressed much more broadly than PD-L2. PD-L1 is constitutively expressed on mouse APCs (DCs, macrophages, and B cells) and T cells and is further up-regulated upon activation. PD-L1 is also expressed on a wide variety of nonhematopoietic cell types, including vascular endothelial cells, pancreatic islet cells, and at sites of immune privilege including the placenta, testes, and eye (Keir et al., 2008). In contrast, PD-L2 is inducibly expressed primarily on DCs and macrophages.
PD-1–PD-L interactions regulate peripheral CD4 and CD8 T cell tolerance at multiple checkpoints. The PD-1–PD-L1 pathway exerts its effects during the initial phase of activation and expansion of autoreactive T cells by attenuating self-reactive T cell responses during presentation of self-antigen by DCs. For example, loss of PD-1 enhances the responses of naive self-reactive CD8 T cells upon encounter with DCs bearing self-antigen (Probst et al., 2005; Keir et al., 2007b). In addition, PD-L1 has a novel role in inhibiting self-reactive T eff cell function. Bone marrow chimera studies have shown that PD-L1 on nonhematopoietic cells mediates tissue tolerance, controlling the intensity of T eff cell responses in nonlymphoid organs and shielding tissues from potentially pathogenic effects of self-reactive T cells and immune-mediated tissue damage (Keir et al., 2006).
T reg cells are also essential for the maintenance of peripheral tolerance, and roles for B7-CD28 family members during T reg cell development are emerging. CD28 is a critical regulator of T reg cell homeostasis and function (Tang et al., 2003; Liang et al., 2005). We demonstrate key roles for PD-L1 in promoting iT reg cell development and function. Significantly, PD-L1 can promote differentiation and maintain the function of induced T reg cells by sustaining and enhancing Foxp3 expression in iT reg cells. PD-L1 induces iT reg cells by inhibiting the Akt/mTOR signaling cascade, thereby flipping the “molecular switch” in a naive CD4+ T cell toward T reg cell development. The novel role for PD-L1 in the maintenance, as well as induction of iT reg cells, identifies PD-L1 as an attractive therapeutic target for controlling T reg cell plasticity.
RESULTS
PD-L1 synergizes with TGF-β to promote iT reg cell conversion
To investigate whether PD-L1 influences the development of iT reg cells, we cultured freshly isolated WT or PD-L1−/− APCs with naive CD4+CD62LhiFoxp3.GFP− T cells from Foxp3.GFP reporter mice in the presence of TGF-β and anti-CD3. WT APCs induced naive T cell conversion into Foxp3+ iT reg cells over a range of TGF-β concentrations (0.5, 1, 2, 4, and 8 ng/ml; 39.8–42.9% CD4+Foxp3+; Fig. 1 A). In contrast, when naive T cells were cultured with PD-L1−/− APCs, anti-CD3, and the same range of TGF-β concentrations, we observed a profound defect in conversion to Foxp3+ iT reg cells (17.4–22.1% CD4+Foxp3+; Fig. 1 A), suggesting a critical role for PD-L1 in regulating T reg cell induction.
Figure 1.
PD-L1 mediates Foxp3+ iT reg cell development. (A and B) Development of Foxp3+ iT reg cells was assessed by flow cytometric analysis of Foxp3-GFP expression after co-culture of naive CD4+CD62L+Foxp3.GFP− T cells with anti-CD3 and irradiated WT or PD-L1−/− APCs plus the indicated range of TGF-β concentrations for 3 d (A) or PD-L1–Ig or control Ig (human IgG1)–coupled beads (B). One representative experiment of at least three similar experiments is shown. (C) Analysis of Foxp3-GFP expression after culture of naive CD4+CD62L+Foxp3.GFP− T cells with PD-L1–Ig beads and over the indicated range of TGF-β concentrations. *, P < 0.001 for PD-L1 bead comparing 0 ng/ml TGF-β versus 0.5–8 ng/ml; **, P < 0.001 comparing PD-L1 bead versus control bead at 0.5 ng/ml TGF-β. Data represent the mean ± SD and are representative of at least four independent experiments. (D) Naive CD4 T cells were cultured in the presence of control Ig or PD-L1–Ig beads (with increasing amounts of PD-L1) in the presence of low levels of TGF-β. *, P = 0.05; **, P = 0.0006; ***, P < 0.0001. Data are representative of three similar experiments. (E) Analysis of CFSE dye dilution of naive CD4+CD62L+CD25− CD44low 2D2 Tg T cells cultured with increasing quantities of PD-L1–Ig (PD-L1 bead is coated with anti-CD3, anti-CD28, and 20, 40, or 60% PD-L1–Ig, respectively) or control Ig in the presence of TGF-β. After 3 d of co-culture, cells were stained for Foxp3 expression. 20 U/ml of exogenous IL-2 was added to cultures shown on the bottom (green). Data are representative of three similar experiments. (F) Quantitative analysis of Foxp3+ T cell conversion in the presence or absence of IL-2 and increasing quantities of immobilized PD-L1. *, P < 0.0001 for all concentrations of PD-L1–Ig versus control Ig in the absence of IL-2; *, P = 0.005 and **, P = 0.0021 for 40 and 60% PD-L1–Ig, respectively, versus control Ig, in the presence of IL-2. Data are representative of three similar experiments. (G) Analysis of Foxp3 expression on a per cell basis, quantified via MFI. *, P = 0.0003 and **, P < 0.001, PD-L1–Ig 20% and PD-L1–Ig 40% versus control Ig, in the absence of IL-2; *, P = 0.03, PD-L1–Ig 60 versus control Ig, in presence of IL-2. Data represent the mean ± SD and are representative of more than five independent experiments with n = 3 mice per treatment condition.
To further interrogate the role of PD-L1 in iT reg cell development and circumvent potentially confounding factors (such as surface molecules or soluble factors) that might be differentially expressed by WT and PD-L1−/− APCs, we conducted further studies using epoxy beads covalently coupled with anti-CD3, anti-CD28, and either PD-L1–Ig or control Ig as artificial APCs (henceforth referred to as PD-L1–Ig beads or control Ig beads). Co-culture of PD-L1–Ig beads, but not control Ig beads, with naive CD4+CD62LhiFoxp3− T cells significantly enhanced iT reg cell development in the presence of TGF-β (58.9 vs. 32.5%; Fig. 1 B).
Because TGF-β has been shown to be necessary for iT reg cell generation (Chen et al., 2003; Fantini et al., 2004; Marie et al., 2005; Pyzik and Piccirillo, 2007), we questioned whether PD-L1 alone could drive iT reg cell development and override the need for TGF-β. We cultured naive T cells with PD-L1–Ig beads or control Ig beads in the absence of exogenous TGF-β. PD-L1 beads alone could induce the conversion of naive T cells to Foxp3+ iT reg cells without TGF-β (2.67 vs. 0.64% for control Ig bead). Moreover, even with increasing amounts of TGF-β, we could not augment iT reg cell development with control Ig beads to the extent observed with PD-L1–Ig beads (Fig. 1 C). Very low amounts of TGF-β (0.033–0.33 ng/ml) were sufficient for significant conversion of naive CD4+ T cells into iT reg cells by PD-L1–Ig-coated beads (Fig. 1 D).
To determine the contribution of PD-L1 to iT reg cell development, we titered the amount of PD-L1–Ig on the surface of the beads and found a quantitative relationship between the amount of PD-L1 and the frequency of induced Foxp3+ CD4 T cells. Greater numbers of Foxp3+ CD4 T cells could be induced with increasing amounts of PD-L1, particularly in the absence of exogenous IL-2 (Fig. 1, E and F); however, at the highest concentration, PD-L1 strongly inhibited overall CD4 T cell expansion and survival. IL-2 was critical for overcoming high-dose PD-L1–mediated inhibition of T cell expansion and rescued iT reg cell development at high concentrations of PD-L1 (PD-L1–Ig 60; Fig. 1, E–G). Using CFSE dye dilution in these assays, we found that PD-L1 simultaneously promoted the development of T reg cells and suppressed the development and activation of T eff cells. Furthermore, PD-L1 induced greater levels of Foxp3 expression per cell as indicated by the mean fluorescence intensity (MFI) of GFP expression (Fig. 1 G). Thus, PD-L1 and TGF-β have synergistic roles in regulating Foxp3+ iT reg cell development.
PD-L1–induced CD4+Foxp3+ T reg cells suppress CD4+ T eff cells
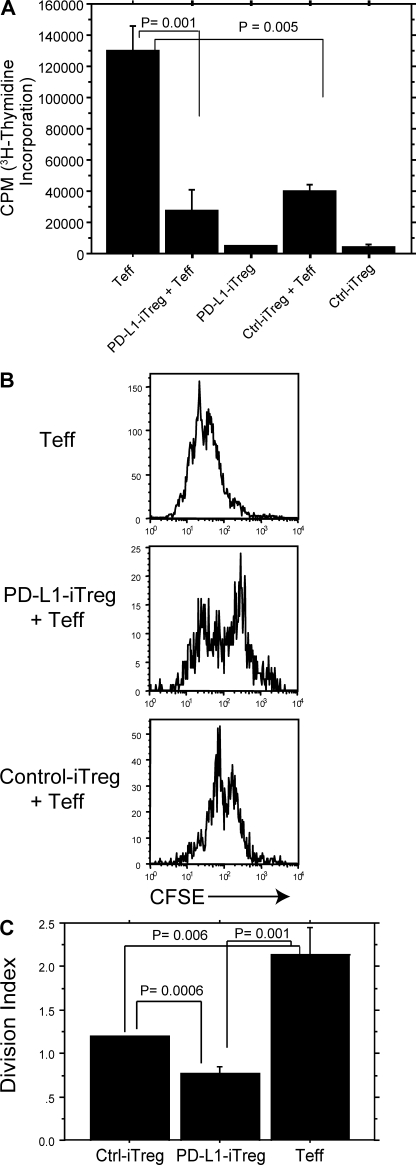
To assess whether PD-L1–induced iT reg cells not only express Foxp3 but also function as suppressor T cells, we treated naive T cells with TGF-β plus control Ig or PD-L1–Ig beads and sorted Foxp3.GFP+ iT reg cells after 3 d of culture. Sorted iT reg cells were then cultured in a standard suppression assay with CD4+CD25− responder T cells and bead-bound anti-CD3/anti-CD28 plus PD-L1–Ig for another 3 d. Both PD-L1 iT reg cells and control iT reg cells could suppress the proliferation of WT T eff cells to a similar extent as that measured by [3H]thymidine incorporation (Fig. 2 A). To evaluate whether PD-L1 iT reg cells or control iT reg cells affect the suppression of T eff cell proliferation on a per cell basis, we performed additional suppression assays measuring CFSE dilution of T eff cells. We cultured either CD45.1− PD-L1 iT reg cells or control iT reg cells with CD45.1+ T eff cells plus bead-bound anti-CD3/anti-CD28 and PD-L1–Ig for 3 d and analyzed CFSE dilution in CD45.1+ T eff cells by flow cytometry (Fig. 2 B). PD-L1 iT reg cells reduced T eff cell expansion at the single cell level to a greater extent than control iT reg cells (1.5-fold greater, P = 0.006), as measured by the division index (Fig. 2 C). These studies demonstrate that PD-L1 has a significant role in inducing the development of functional Foxp3+ iT reg cells.
Figure 2.
PD-L1–induced CD4+ Foxp3+ T reg cells suppress CD4+ T eff cells in vitro. (A) PD-L1 iT reg cell function was assessed by [3H]thymidine incorporation of naive CD4+CD25− T eff cells after 3 d of co-culture at a 1:1 T reg/T eff cell ratio plus PD-L1 beads (5:1 bead/T eff cell ratio). Data represent the mean ± SD and are representative of at least two independent experiments. (B) PD-L1 iT reg cell function was assessed by CFSE dilution of naive CD4+CD25− T eff cells after 3 d of incubation with 1:1 T reg/T eff cell ratio and PD-L1 beads (5:1 bead/T eff cell ratio). Data represent the mean ± SD and are representative of three similar experiments. (C) Quantification of T eff cell proliferation in B, analyzing the division index of gated CD4+CD45.1+ (the number of divisions a single cell has divided) by FlowJo software. Data represent the mean ± SD.
PD-L1 enhances and maintains Foxp3 expression on iT reg cell and augments suppression at low T reg/T eff cell ratios
Our studies thus far could not discriminate whether PD-L1 only controls iT reg cell development or also has a role in maintaining iT reg cell function. Recent studies indicate that continued Foxp3 expression is necessary for sustaining T reg cell function (Kim et al., 2007; Williams and Rudensky, 2007). Therefore, we investigated whether PD-L1 influences the maintenance of Foxp3 expression and iT reg cell suppressive function. We induced T reg cell development by culturing naive CD45.1− T cells with either PD-L1–Ig beads or control Ig beads plus TGF-β. After 3 d of culture, PD-L1 iT reg cells or control iT reg cells were sorted by Foxp3.GFP+ expression and used in secondary cultures to evaluate the maintenance of Foxp3 expression and iT reg cell suppressive capacity. The sorted PD-L1 or control iT reg cell were cultured with CD4+CD25−CD45.1+ T eff cells, in the presence of either PD-L1–Ig beads or control Ig beads for 3 d (Fig. 3 A). Foxp3.GFP expression was then assessed by flow cytometry. When iT reg cells were stimulated with control Ig beads, Foxp3 was better maintained in iT reg cells originally induced with PD-L1–Ig beads as compared with control Ig beads (24.2% positive vs. 7.07% positive; Fig. 3 B, top). In addition, stimulation of control iT reg cells or PD-L1 iT reg cells with PD-L1–Ig beads significantly enhanced the percentage of iT reg cells maintaining Foxp3 expression (16.1% for control-iT reg cells vs. 38% for PD-L1 iT reg cells; Fig. 3 B, bottom). Interestingly, iT reg cells that were both induced and restimulated in the presence of PD-L1–Ig maintained the greatest percentage of Foxp3-expressing cells (38%; Fig. 3, B and C). These studies demonstrate that PD-L1 strategically regulates Foxp3 expression in CD4 T cells at two stages: (1) during the induction phase of naive T cell conversion to iT reg cells and (2) during the effector phase of iT reg cell–mediated suppression. These findings point to a novel role for PD-L1 in regulating the stability of iT reg cells in the periphery.
Figure 3.
PD-L1 maintains Foxp3 expression by iT reg cells during suppression of effector cell function. (A) Schematic depiction of experiment. Naive CD4+CD62LhiFoxp3.GFP− T cells were induced toward T reg cell differentiation for 3 d in the presence of TGF-β plus IL-2 and either control or PD-L1 beads. Foxp3.GFP+CD45.1− cells were then sorted and co-cultured with sorted CD4+CD25−CD45.1+ in the presence of either control or PD-L1 beads during the 3-d suppression assay. (B) 72 h after co-culture, CD4+CD45.1− cells were gated and analyzed for GFP expression. (C) Quantification of experiment depicted in B. Data represent the mean ± SD and are representative of at least two independent experiments.
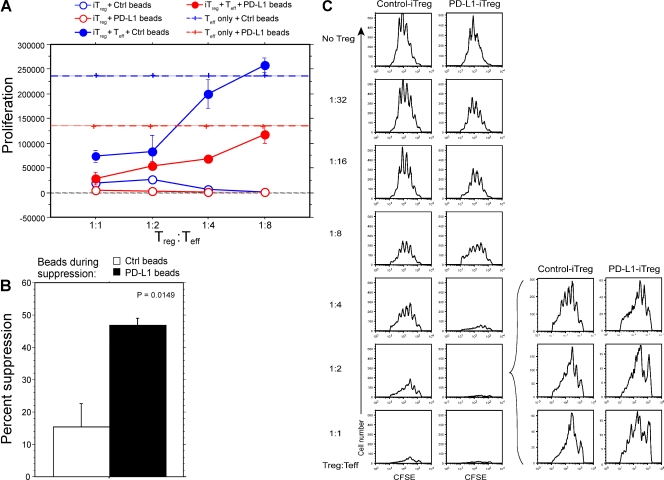
We next tested whether the presence of PD-L1 could influence the efficiency of suppression by iT reg cells. Foxp3.GFP+ iT reg cells were sorted and cultured with naive CD4+CD25−CD45.1+ T eff cells plus either PD-L1–Ig or control Ig beads at a variety of iT reg/T eff cell ratios, as graphically depicted in Fig. 3 A (right). PD-L1–Ig enhanced iT reg cell suppressive function at a low T reg/T eff cell ratio of 1:4 (46% suppression using PD-L1 bead vs. 3% suppression using control bead, P = 0.0149; Fig. 4, A and B). The effect was less pronounced when T reg cells were more numerous, but PD-L1–Ig iT reg cells still showed improved suppressive capacity (Fig. 4 A). Because T eff cells may be directly inhibited by the presence of PD-L1 on beads during the suppression assay, we tested the suppressive capacity of PD-L1–Ig iT reg cells or control iT reg cells in the presence of TCR stimulation alone (i.e., control Ig beads) over a range of T reg/T eff cell ratios (Fig. 4 C). PD-L1 iT reg cells are far more effective than control iT reg cells at limiting the proliferative capacity of T eff cells at very low T reg/T eff cell ratios. Collectively, these results show that PD-L1 enhances the efficiency of suppression by iT reg cells.
Figure 4.
PD-L1 enhances the efficiency of iT reg cell–mediated suppression of T eff cells. (A) Foxp3.GFP+ iT reg cells were sorted and co-cultured with naive CD4+CD25−CD45.1+ T eff cells plus either PD-L1–Ig beads or control Ig beads (at various T reg/T eff cell ratios). 72 h later, cultures were pulsed with [3H]thymidine for 12–14 h. P < 0.0009 at a 1:4 ratio cultured with PD-L1 beads (comparing T eff + iT reg vs. T eff cells). Data represent the mean proliferation ± SD and are representative of at least four independent experiments. (B) Quantification of suppression at 1:4 ratio of T reg/T eff cells. P = 0.0149. Data represent the mean ± SD and are representative of at least four independent experiments. (C) PD-L1 iT reg cells suppress T eff cells more effectively than control iT reg cells. CD4+CD62L+ FoxP3.GFP− naive T cells were induced with PD-L1 or control beads in the presence of TGF-β. GFP+ iT reg cells were sorted and co-cultured at the indicated T reg/T eff cell ratios with CFSE-labeled CD4+CD25− Thy1.1 T eff cells and beads coated with anti-CD3 and anti-CD28 (in the absence of PD-L1) for 3 d. Graphs are representative of three experimental replicates and data are representative of three independent experiments.
PD-L1 deficiency leads to impaired T reg cell conversion in vivo
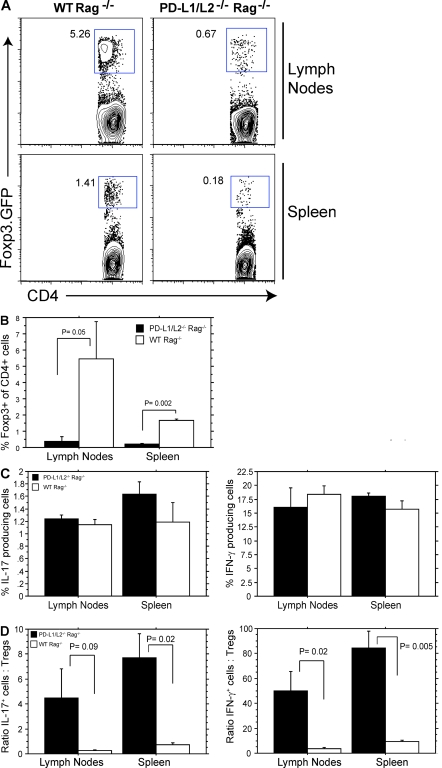
To investigate the role of PD-L1 in iT reg cell development and function in vivo, we compared iT reg cell conversion and function in Rag−/− mice because iT reg cells can spontaneously develop from naive T cells in a lymphopenic environment (Bloom et al., 2008; Calzascia et al., 2008; Winstead et al., 2008). We adoptively transferred naive CD4+CD62LhiFoxp3.GFP− T cells into PD-L1−/−PD-L2−/−Rag−/− or WT Rag−/− mice and analyzed the CD4+ T cells for Foxp3 expression at days 14–17 after transfer by flow cytometry. It should be noted that PD-L1−/− versus PD-L1−/−PD-L2−/− APCs are similarly deficient in their ability to induce Foxp3+ iT reg cells in the presence of TGF-β and anti-CD3 (unpublished data). There were ∼10-fold fewer Foxp3.GFP+ cells in PD-L1−/−PD-L2−/−Rag−/− recipients compared with WT Rag−/− recipients (Fig. 5, A and B). These data demonstrate the critical role of PD-L in regulating the de novo generation and/or maintenance of Foxp3+ iT reg cell in vivo. We next compared Foxp3−CD4+ T eff cell responses in PD-L1−/−PD-L2−/−Rag−/− and WT Rag−/− recipients by measuring cytokine production by Foxp3-CD4+ cells 14–17 d after naive T cell transfer. There were comparable numbers of IL-17 and IFN-γ–producing cells generated in PD-L1−/−PD-L2−/−Rag−/− and WT Rag−/− recipients (Fig. 5 C). However, IL-17– and IFN-γ–producing T eff cells highly outnumbered the iT reg cells in PD-L1−/−PD-L2−/−Rag−/− recipients as a result of the paucity of iT reg cells in these mice (Fig. 5 D), resulting in a dramatic increase in the T eff/T reg cell ratio.
Figure 5.
Attenuated iT reg cell development in PD-L1−/−PD-L2−/− mice in vivo. (A) CD4+CD62LhiFoxp3.GFP− cells were adoptively transferred i.v. into the tail veins of WT Rag−/− or PD-L1−/−PD-L2−/−Rag−/− mice. Spleens and lymph nodes were analyzed for Foxp3.GFP expression 14–17 d after transfer. (B) Quantitation of Foxp3.GFP expression from independent mice depicted in A. Data represent the mean ± SE of five independent mice. (C and D) Analysis of IL-17+ and IFN-γ+ T eff cells by intracellular cytokine staining (C) and ratios of IL-17–producing T eff/T reg cells and IFN-γ–producing T eff/T reg cells from WT Rag−/− or PD-L1−/−PD-L2−/−Rag−/− mice 14–17 d after transfer (D). Data represent the mean ± SD of n = 5 mice per group and are representative of two independent experiments.
PD-L1−/−PD-L2−/− Rag−/− mice develop fatal immune-mediated pulmonary damage after transfer of naive CD4+Foxp3− T cells
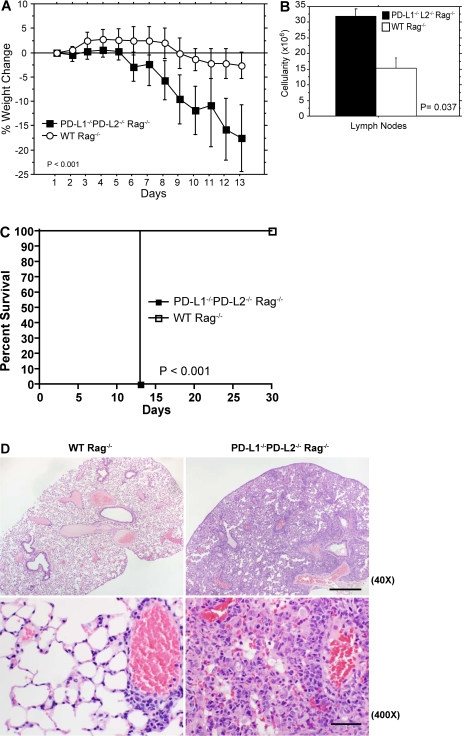
To evaluate the in vivo consequences of the skewed T reg/T eff cell ratio in PD-L1−/−PD-L2−/−Rag−/− recipients, we monitored PD-L1−/−PD-L2−/−Rag−/− and WT Rag−/− mice after transfer of CD4+CD62LhiFoxp3.GFP− T cells (Fig. 6 A). PD-L1−/−PD-L2−/−Rag−/− recipients exhibited rapid and dramatic weight loss beginning on day 6 after transfer, with a reduction of 17.6 ± 5.7% (P ≤ 0.0001) of the pretransfer body weight within 2 wk, compared with 2.65 ± 2.7% (P = 0.100) weight loss in WT Rag−/− recipients. There was a marked increase in cellularity of the lymph nodes and spleen in PD-L1−/−PD-L2−/−Rag−/− compared with WT Rag−/− adoptive transfer recipients on days 14–17 after transfer (Fig. 6 B).
Figure 6.
Dramatic weight loss, severe pulmonary inflammation, and fatal inflammatory disorder develop in PD-L1−/−PD-L2−/− Rag−/− mice after adoptive transfer of naive CD4+CD62LhiFoxp3.GFP− T cells. Sorted CD4+CD62LhiFoxp3.GFP− cells were adoptively transferred i.v. into WT Rag−/− or PD-L1−/−PD-L2−/− Rag−/− mice. (A and B) Clinical manifestations shown are the following: percentage of weight loss of mice after adoptive transfer of CD4+CD62LhiFoxp3.GFP− cells into WT Rag−/− or PD-L1−/−PD-L2−/−Rag−/− (P < 0.001; n = 5 mice per group; A) and quantified lymph node (axillary, brachial, and inguinal) cellularity (B). Data represent the mean ± SD and represent two independent experiments. (C) Survival of mice after adoptive transfer of naive CD4+CD62LhiFoxp3.GFP− T cells was monitored for 30 d. PD-L1−/−PD-L2−/− Rag−/−, n = 12; WT Rag−/−, n = 8. (D) Hematoxylin and eosin–stained paraffin sections of lung tissue obtained on days 14–17 after transfer of naive CD4+CD62LhiFoxp3− T cells. Bars: (top, 40×) 500 µm; (bottom, 400×) 50 µm. PD-L1−/−PD-L2−/− Rag−/−, n = 9; WT Rag−/−, n = 10. Data represent more than four independent experiments.
Strikingly, all PD-L1−/−PD-L2−/−Rag−/− recipients were moribund within 17 d after transfer of naive T cells (n = 12) in marked contrast to the survival of all WT Rag−/− recipients (n = 8; Fig. 6 C). In a separate experiment, we examined PD-L1−/−PD-L2−/−Rag−/− and WT Rag−/− recipients histologically on days 14–17 after transfer (Fig. 6 D). The lungs of PD-L1−/−PD-L2−/−Rag−/− recipients showed widespread severe perivascular, peribronchial, and interstitial infiltrates consisting predominantly of mononuclear cells and some neutrophils. Alveolar walls were markedly thickened by the inflammation, and there was severe alveolar consolidation and edema. This potentially reduced gas exchange in these mice. In contrast, all the WT Rag−/− recipients displayed a minimal degree of inflammation and absence of alveolar consolidation in the lungs. Both groups also showed varying degrees of mild colitis on days 14–17 after adoptive transfer. Analysis of brain, heart, pancreas, kidney, esophagus, stomach, small intestine, and skin revealed only mild scattered inflammation with no significant differences between WT and PD-L1−/−PD-L2−/− Rag−/− recipients (unpublished data).
To ascertain the critical role for PD-L1 in vivo, we transferred naive CD4 T cells to Rag−/− recipients treated with anti–PD-L1 blocking antibody (Fig. S1) and monitored the mice for 3–4 wk. Mice were sacrificed to assess T reg cell development and immunopathology. A significant defect in de novo iT reg cell development was observed in Rag−/− mice given anti–PD-L1 mAb compared with isotype control in both the spleen (isotype = 6.35% vs. anti–PD-L1 = 2.73%, P = 0.0318) and mesenteric lymph nodes (isotype = 30.2% vs. anti–PD-L1 = 18%, P = 0.0219). The lungs of Rag−/− mice treated with anti–PD-L1 mAb showed moderate to severe perivascular, peribronchial, and interstitial inflammation, consisting of mononuclear cells and a few scattered neutrophils. Thus, similar to the PD-L1−/−PD-L2−/−Rag−/−recipients of naive CD4 T cells, WT Rag−/− mice given anti–PD-L1 mAb exhibited defects in iT reg cell generation and developed pulmonary pathology. Collectively, these studies demonstrate a key role for PD-L1 in iT reg cell development in vivo.
PD-L1 antagonizes the Akt–mTOR signaling cascade during the induction of iT reg cells
Recent studies have shown notable differences in signaling pathways used by CD4+ T eff cells compared with T reg cells. In particular, Akt signaling is essential for naive T cell activation and proliferation but dispensible for T reg cell development and function (Battaglia et al., 2006; Coenen et al., 2007; Gao et al., 2007; Qu et al., 2007; Strauss et al., 2007; Haxhinasto et al., 2008; Sauer et al., 2008). These findings led us to hypothesize that PD-L1 may mediate T reg cell conversion by antagonizing the Akt signaling pathway. To test this, we cultured naive T cells in the presence of PD-L1–Ig or control Ig beads for 18 h and then measured phosphorylation of Akt, mTOR, and S6. Intracellular staining for phospho-Akt and phospho-mTOR revealed significantly diminished levels of Akt and mTOR phosphorylation when naive T cells were cultured in the presence of increasing quantities of PD-L1 relative to control Ig (MFIs of phospho-Akt and phospho-mTOR were significantly down-regulated; Fig. 7, A–D). As a downstream target of the mTOR-regulated p70 S6 kinase, phosphorylation of S6 ribosomal protein reflects the sustained activation of the Akt–mTOR pathway. Upon culture of naive CD4 T cells with increasing amounts of PD-L1, we observed a marked decrease in phospho-S6 as compared with control (Fig. 7, E and F). Furthermore, PD-L1 up-regulated the expression of PTEN (phosphatase and tensin homologue deleted on chromosome 10), a phosphoinositol 3,4,5-triphosphatase important for antagonizing PI3K signaling, demonstrating that PD-L1 antagonizes the Akt pathway during T reg cell differentiating conditions (Fig. 7, G and H). Western blots assessing the specific down-regulation of phospho-Akt, phospho-mTOR, and phospho-S6 and up-regulation of PTEN confirmed phospho-flow cytometry data (unpublished data).
Figure 7.
PD-L1 regulates T reg cell development by antagonizing the Akt–mTOR signaling cascade. (A, C, F, and G) Phospho-Akt, phospho-mTOR, PTEN, and phospho-S6 analysis at 18 h after culture with control Ig bead (hIgG = 60% of bead surface, with remaining surface coated with anti-CD3 and anti-CD28) or various titers of PD-L1–Ig bead (PD-L1–Ig 20, 40, 60 = 20, 40, and 60% of bead surface coated with PD-L1–Ig, with remaining surface coated with anti-CD3 and anti-CD28 plus control Ig). (B, D, E, and G) MFI analysis of phospho-Akt (B; *, P = 0.001; **, P = 0.003; and ***, P = 0.0008, at 20, 40, and 60% PD-L1, respectively, compared with control Ig), phospho-mTOR (D; *, P = 0.0064; and **, P = 0.0001, at 20 and 60% PD-L1, respectively, compared with control Ig), phospho-S6 (E; P = 0.0012, P = 0.0007, and P = 0.0002, at 20, 40, and 60% PD-L1, respectively, compared with control Ig), and PTEN (H; *, P = 0.0378, PD-L1–Ig 40 compared with control Ig) at 18 h. n = 3 mice per experiment, representative of three experiments. Data are representative of the MFI ± SD and are representative of three experiments.
Down-regulation of the MAP kinase signaling cascade has also been implicated in TGF-β–mediated T reg cell development (Luo et al., 2008). We questioned whether PD-L1 might regulate T reg cell differentiation by modulating the MAP kinase pathway. Stimulation of naive T cells with increasing amounts of PD-L1 Ig attenuated the phosphorylation of ERK2/p42 (Fig. S2). These data further substantiate our hypothesis that the PD-L1–PD-1 pathway truncates signaling cascades downstream of TCR signaling, preferentially converting naive T cells toward a Foxp3+ T reg cell lineage.
DISCUSSION
In this paper, we demonstrate a novel function for PD-L1 in promoting the development and sustaining the function of iT reg cells. There was a profound defect in conversion of naive CD4 T cells into Foxp3+ iT reg cells in the absence of PD-L1. Consistent with this observation, PD-L1 presented on beads (along with anti-CD3 and anti-CD28) could induce the development of functional Foxp3+ iT reg cells, demonstrating that PD-L1 is responsible for promoting iT reg cell development. Although TGF-β signaling is important for the conversion of naive CD4+ T cells toward Foxp3-expressing cells with suppressive capacity (Chen et al., 2003; Fantini et al., 2004), PD-L1 could induce T reg cells in the absence of exogenous TGF-β, suggesting that PD-L1 signaling alone can serve to promote iT reg cell development. Our signaling studies support the conclusion that PD-L1 reduces signaling of the Akt–mTOR pathway in naive T cells, which is critical for their conversion into iT reg cells. Furthermore, we show that PD-L1 has a novel role in sustaining expression of Foxp3 in iT reg cells and in enhancing iT reg cell suppressive function. Thus, these studies reveal a new mechanism by which PD-L1 mediates T cell tolerance. PD-L1 can inhibit self-reactive T cell responses by promoting iT reg cell development and maintaining iT reg cell function.
Where does PD-L1 exert its critical effects on iT reg cell development and function? PD-L1 is widely expressed on hematopoietic and nonhematopoietic cells (Keir et al., 2008). There may be a critical interaction between PD-L1 expressing DC and T cells during the induction of T reg cell development from naive T cells (Brown et al., 2003). PD-L1 is also expressed on T cells; however, WT T cells transferred into PD-L1−/−PD-L2−/− Rag−/− recipients could not convert to iT reg cells, suggesting that T cell–T cell interaction via PD-L1 is not sufficient to drive naive T cell conversion. Instead an interaction with the host environment is crucial for T reg cell conversion.
Our bone marrow chimera studies have demonstrated an important role for PD-L1 on nonhematopoietic cells in mediating tissue tolerance (Keir et al., 2006). Our findings of critical roles for PD-L1 in the development and maintenance of iT reg cell function suggest that PD-L1 may protect tissues from the potentially pathogenic self-reactive T eff cells not only by inhibiting the function of T eff cells but also by increasing the frequency and function of T reg cells in the target tissue. By promoting de novo generation of iT reg cells in situ, PD-L1 may play a role in mediating immune privilege, especially in environments where TGF-β is present (e.g., placenta and eye).
PD-L1 may also exert its inhibitory effects on anti-tumor and anti-microbial immunity, at least in part by inducing T reg cell development and sustaining iT reg cell function. PD-L1 is expressed on a wide variety of tumors, and high levels of PD-L1 expression strongly correlate with unfavorable prognosis in several cancers (Dong et al., 2002; Iwai et al., 2002; Strome et al., 2003; Konishi et al., 2004; Thompson et al., 2004; Blank et al., 2005; Hirano et al., 2005; Ohigashi et al., 2005; Dorfman et al., 2006; Wu et al., 2006; Inman et al., 2007; Nakanishi et al., 2007; Nomi et al., 2007; Zhang et al., 2008). The numbers of Foxp3+ T cells within tumors also correlates with a poor prognosis. Our work provides a mechanism by which high PD-L1 expression can lead to increased numbers of Foxp3+ T reg cells and thus, poor prognosis. Increased PD-L1 expression by tumor cells may induce and maintain iT reg cells in the periphery, thereby augmenting the suppression of anti-tumor T cell responses and allowing tumor progression.
Increased T reg cells are also seen during chronic infections (Belkaid, 2008), correlating with a lack of sterilizing immunity. During persistent infection by Helicobacter pylori, PD-L1 is up-regulated on gastric epithelial cells (Das et al., 2006). Blocking PD-L1 on gastric epithelial cells enhances CD4 T eff cell function and prevents the generation of CD4+CD25hiFoxp3+ T reg cells in vitro (Beswick et al., 2007). During chronic viral infections, PD-L1 has a key role in limiting the function of exhausted CD8 T cells (Barber et al., 2006; Sharpe et al., 2007). PD-L1 blockade reinvigorates the function of these T cells and enhances viral clearance. Thus, PD-L1 may exert inhibitory effects in chronic infections by promoting iT reg cell development and maintaining iT reg cell function, as well as by inhibiting anti-microbial T eff cell function.
PD-L1−/−PD-L2−/−Rag−/− recipients of Foxp3− naive T cells resemble scurfy mice, which have a deficit in Foxp3 and die by 3 wk of age as a result of multiorgan infiltration of CD4+CD8− T cells (Clark et al., 1999; Brunkow et al., 2001; Schubert et al., 2001). PD-L1−/−PD-L2−/−Rag−/− recipients had a marked deficit in Foxp3+ T reg cell development, altering the T reg/T eff cell ratio. These findings illustrate the important role of PD-L1 in regulating the dynamic balance between T eff and T reg cells in vivo. PD-L1 may do the following: (a) control T reg cell development in lymphoid organs, which is important for immune homeostasis; (b) promote T reg cell development at target tissues, protecting against immune-mediated tissue damage, and (c) sustain and enhance T reg cell function within an inflammatory microenvironment, effectively counterbalancing the pathogenic T eff cells.
Foxp3 is a transcription factor only expressed in the T reg cell lineage (Fontenot et al., 2003; Hori et al., 2003; Vignali et al., 2008). Along with contributing a distinct genetic signature to T reg cells, Foxp3 conveys regulatory activity to nT reg cells, iT reg cells, and, upon ectopic expression, in conventional T cells (Schubert et al., 2001; Fontenot et al. 2003, 2005; Hori et al., 2003; Gavin et al., 2007; Hill et al., 2007). PD-L1 can induce and maintain the expression of Foxp3 in iT reg cells, suggesting that PD-L1 may function to stabilize and sustain T reg cell function in the periphery, similar to effects reported for TGF-β (Marie et al., 2005; Pyzik and Piccirillo, 2007). This maintenance of Foxp3 expression may explain both the increased efficiency of suppression seen for T reg cells cultured with PD-L1 and the lack of T reg cell conversion in the PD-L1−/−PD-L2−/−Rag−/− adoptive transfer recipients. It has been suggested that although Foxp3 expression is a salient feature of the regulatory cell signature, it is not the master regulator of T reg cell development (Ramsdell, 2003; Collison et al., 2007; Hill et al., 2007). In fact, it is increasingly clear that other factors (such as TGF-β and IL-2; Ramsdell, 2003; Setoguchi et al., 2005) induce key changes within the regulatory cell transcriptome that contribute to the identity of T reg cells separate from, and in addition to, the effects attributed to Foxp3 (Lin et al., 2007). In vivo, TGF-β production is maintained at a basal level and up-regulated with inflammation. Interestingly, endogenous TGF-β was not sufficient to induce and/or maintain Foxp3+ iT reg cells in PD-L1−/−PD-L2−/− Rag−/− recipients. In complementary studies, PD-L1 blockade similarly impaired iT reg cell development in vivo, showing that loss of PD-L1 can diminish the effect of TGF-β, which is critical for iT reg cell identity and function. This indicates that PD-L1 contributes unique and essential signals that drive iT reg cell development and function.
We find that PD-L1 attenuates the Akt signaling pathway during the conversion of naive T cells to T reg cells by reducing the phosphorylation of Akt and its downstream substrates mTOR and S6 while simultaneously augmenting PTEN. Previous work has shown that truncation of TCR signaling and inhibition of the Akt–mTOR signaling axis is critical for T reg cell development (Qu et al., 2007; Strauss et al., 2007; Haxhinasto et al., 2008; Long and Buckner, 2008; Sauer et al., 2008). In these studies, drug inhibitors (FK506, rapamycin) or retrovirally modified Akt were used to demonstrate the role of Akt and mTOR in T reg cell development. We provide the first demonstration of a naturally occurring protein that can inhibit the Akt–mTOR cascade and regulate the development of T reg cells.
Similar to the Akt–mTOR pathway, recent data indicate an important role for the MAP kinase cascade in iT reg cell development (Adler et al., 2007; Huber et al., 2008; Luo et al., 2008). We found that PD-L1 attenuated the phosphorylation of p42/ERK2, suggesting that PD-L1 may mediate the induction of T reg cells by modulating ERK2 activity and, hence, the MAP kinase signaling cascade. No discernable effects of PD-L1–Ig on p38 were detected (unpublished data).
There is great interest in generating T reg cells ex vivo as a therapy for autoimmune diseases and transplant rejection (Roncarolo and Battaglia, 2007). However, recent studies indicate that T reg cells exhibit functional plasticity and can produce proinflammatory cytokines at the site of inflammation (Joetham et al., 2008; Yang et al., 2008). Thus, in order for T reg cell therapy to be a viable approach, it is critical to find ways to maintain and enhance the suppressive function of T reg cells. Our work suggests that administration of PD-L1–Ig or PD-1 agonists may harness the therapeutic potential of iT reg cells by providing a novel means of sustaining and enhancing their function in vivo while concomitantly suppressing the expansion and functions of activated T eff cells.
MATERIALS AND METHODS
Mice.
6–8-wk-old WT C57BL/6 and CD45.1 (B6.SJL-Ptprca Pepcb/BoyJ) mice were purchased from The Jackson Laboratory. PD-L1−/−PD-L2−/− (Keir et al., 2006) and PD-L1−/− (Latchman et al., 2004) mice were generated in our laboratory. 2D2 TCR Tg mice Foxp3-IRES-GFP knockin mice (Foxp3.GFP; Bettelli et al., 2006) were generated in our laboratory by crossing 2D2 TCR Tg mice (Bettelli et al., 2003) with Foxp3.GFP reporter mice. PD-L1−/−PD-L2−/− Rag 2−/− mice were generated by breeding PD-L1−/−PD-L2−/− with Rag 2−/− mice (B6.129S6-Rag2tm1Fwa N12; Taconic). Genotypes were verified by PCR and flow cytometry. Harvard Medical School is accredited by the American Association of Accreditation of Laboratory Animal Care. Mice were maintained in a pathogen-free facility and used according to the Harvard Medical School Standing Committee on Animals and National Institutes of Health Animal Care Guidelines. Animal protocols were approved by the Harvard Medical School Standing Committee on Animals.
Reagents.
The following anti–mouse antibodies were used in cell surface staining, intracellular cytokine staining, and epoxy bead conjugation: anti-CD16/CD32 (Fc Block), CD4 PerCP-Cy5.5 (clone RM4-5), CD62L PE (clone MEL-14), IL-2 APC (clone JES6-5H4; eBioscience), CD45.1 APC (clone A20), IL-17 PE (clone TC11-18H10), and IFN-γ PE (clone XMG1.2; BD). Anti-CD3 (clone 2C11) plus anti-CD28 (clone 37.51) were used for bead conjugation and were purchased from Bio X Cell. Cells were sorted on a FACSAria cell sorter (BD). Cell surface staining was performed at 4°C in FACS Buffer (1% FCS, PBS, 2 mM EDTA; Invitrogen). CFSE was purchased from Invitrogen.
Cell purification.
Naive CD4+CD62L+Foxp3.GFP− T cells were isolated from the spleen and lymph nodes (axillary, brachial, and inguinal) of male C57BL/6 Foxp3.GFP or 2D2 Foxp3.GFP reporter mice. Single cell suspensions were made by mechanical dissociation. After red blood cell lysis with ACK buffer (Invitrogen), cells were washed and isolated by incubation with CD4 microbeads, positively selected through LS columns (Miltenyi Biotec), and stained with anti-CD4 PerCP-Cy5.5 (clone RM4-5; eBioscience) and anti-CD62L APC (clone MEL-14; eBioscience) before cell sorting on a FACSAria cell sorter. Naive CD4+CD62L+Foxp3.GFP− T cells were always >99.2% pure.
In vitro iT reg cell development.
Anti-CD3 (clone 2C11; Bio X Cell) plus anti-CD28 (clone 37.51; Bio X Cell) were covalently attached to Dynabeads M450 glycidyl ether beads according to the manufacturer's directions (Invitrogen). We ensured equal loading of proteins during preparation by keeping constant the total amount of protein (antibodies and fusion proteins) at 5 µg per 107 beads as previously described (Broeren et al., 2000; Riley et al., 2002). In general, 107 beads were coated with 1 µg of anti-CD3 (20% of total protein), 1 µg of anti-CD28, and either 60% control human IgG1 (referred to control Ig beads; Bio X Cell) or 40% PD-L1-hIgG1 Fc fusion protein (referred to as PD-L1–Ig beads 40; R& D Systems) plus 20% control human IgG1 Fc. In some experiments, increasing amounts of PD-L1–Ig was used to coat the epoxy beads (20, 40, and 60% of total protein per 107 beads = 1, 2, and 3 µg of PD-L1–Ig per 107 beads). In these cases, the remaining surface of the beads were coated with control human IgG1. Covalent attachment of the proteins to the beads was performed in NaPO4 buffer for 24 h at room temperature on a Nutating Mixer (Lab-Tech Incorporated). Beads were then washed three times in PBS over a magnetic column and resuspended in complete media before use.
CD4+CD62L+Foxp3− naive T cells were cultured with beads at a fixed ratio of 1:5 (T cells/beads). In brief, 1–2 × 106 T cells were plated at 106/ml in a 24-well flat-bottom tissue culture plate with beads in complete media consisting of RPMI-1640 with L-glutamine (Invitrogen) supplemented with 10% FCS (Sigma-Aldrich), penicillin-streptomycin (100 U penicillin and 100 µg streptomycin; Invitrogen), 12 mM HEPES (Invitrogen), and 50 µM β-mercaptoethanol (Sigma-Aldrich) plus 2 ng/ml TGF-β (R&D Systems) and 20 U/ml rh-IL2 (R&D Systems) for 3 d at 37°C with 5% CO2. In some experiments, sorted CD4+CD25−CD62L+CD44low naive T cells were labeled with 1 µM CFSE before culture with beads and TGF-β for 3 d at 37°C with 5% CO2. T cells were then stained for Foxp3 expression (eBioscience). To further evaluate the effect of IL-2 on PD-L1–mediated T reg cell development, some experiments were conducted in the absence of exogenous rh-IL2 (as noted in the Fig. 1 legend). To analyze the effect of TGF-β on PD-L1–mediated T reg cell development, we performed some experiments using a range of TGF-β concentrations (0.033–8 ng/ml).
In some experiments, APCs were isolated from WT or PD-L1-/- mice. In brief, T cells were depleted from spleens of WT or PD-L1−/− mice with CD4 and CD8 microbeads (Miltenyi Biotec). Remaining splenocytes were irradiated and co-cultured with naïve CD4+CD62L+Foxp3.GFP− T cells plus anti-CD3 and a range of TGF-β concentrations (0.5–8 ng/ml).
In vitro suppression assays.
Naive T cells were induced toward T reg cell development in vitro using PD-L1 or control beads in the presence of 20 U/ml TGF-β and IL-2 (R&D Systems) for 3 d, at which time Foxp3.GFP+ T cells were sorted on a FACSAria (BD) cell sorter. Foxp3.GFP+ iT reg cells were then co-cultured with sorted CD4+CD25−CD45.1+ naive T eff cells and stimulated with PD-L1 beads (containing anti-CD3 (20%), anti-CD28 (20%), PD-L1–Ig (40%), and control Ig (20%) for 3 d. Proliferation of T cells was determined by incorporation of [3H]thymidine (1 µCi/well) for 12–14 h. Suppression assays were performed using a constant number of T eff cells (105) and by addition of decreasing numbers of Foxp3.GFP+ iT reg cells plus a 5:1 ratio of beads to T eff cells. The percentage of suppression of effector cell proliferation was calculated based on the proliferation of T eff cells with control versus PD-L1-beads in the absence of T reg cells.
For CFSE dilution experiments, CD4+CD25−CD45.1+ naive T eff cells were labeled with 1 mM CFSE for 10 min in RPMI-1640 (serum free) and washed twice with 100% FBS and twice with complete media before culture. 105 T eff cells were cultured with 105 iT reg cells and PD-L1–Ig beads (5:1 bead/T eff cell ratio) in 96-well flat-bottom plates (BD). 72 h later, CD4+CD45.1+ T cells were gated and analyzed for CFSE dilution. Division index (defined as the mean number of divisions that a cell has undergone) was calculated using FlowJo Proliferation analysis software (Tree Star, Inc.). For CFSE dilution experiments interrogating the suppressive capacity of PD-L1-iT reg cells versus control iT reg cells, naive T cells were induced toward T reg cell development in vitro using PD-L1 or control beads in the presence of TGF-β and IL-2 for 3 d, as described in the previous section, Foxp3.GFP+ T cells were sorted on a FACSAria cell sorter. Control and PD-L Foxp3.GFP+ iT reg cells were then co-cultured with CFSE-labeled CD4+CD25−Thy1.1+ T eff cells and stimulated with control beads (containing anti-CD3, anti-CD28, and control Ig). 105 CD4+CD25−Thy1.1+ T eff cells were cultured with varying numbers (3 × 103–105) of PD-L1 iT reg cells or control iT reg cells plus control Ig beads (5:1 bead/T eff cell ratio) in 96-well flat-bottom plates (BD). 4 d later, CD4+Thy1.1+ T cells were gated and analyzed for CFSE dilution.
In vivo adoptive transfer.
Naive CD4+ T cells were isolated from spleens and lymph nodes of C57BL/6 mice. CD4+CD62LhiFoxp3.GFP− cells were sorted on a FACSAria as described in the previous section, and 1–1.5 × 106 CD4+CD62LhiFoxp3.GFP− were i.v. injected into the tail veins of PD-L1−/−PD-L2−/− Rag−/− or WT Rag−/− mice. Mice were monitored and weighed for 14–17 d and euthanized for histological and cellular analysis. In some experiments, surviving mice were monitored for 30 d after T cell transfer. Organs were fixed in formalin, dehydrated, and embedded in paraffin. 5-µm sections stained with hematoxylin and eosin were independently evaluated by two pathologists in a blinded fashion. Digital photomicrographs were acquired using DP Controller software (Olympus) driving a DP71 camera (Olympus) mounted on a BH-2 light microscope (Olympus). Image sizes were reduced using Photoshop CS3 software (Adobe).
Intracellular cytokine staining.
Spleen and lymph node (axillary, brachial, inguinal, and mesenteric) cells were isolated and restimulated with 50 ng/ml PMA and 500 ng/ml ionomycin (Sigma-Aldrich) for 4 h with GolgiStop (BD) being added during the last 3 h of stimulation. After Fc block, cells were stained with anti-CD4 PCP-Cy5.5, fixed with 4% paraformaldehyde, and permeabilized with Cytofix/Cytoperm solution (BD). Intracellular staining with IL-17 PE, IFN-γ PE, or APC and IL-2 APC was performed in Cytoperm buffer (BD) according to the manufacturer's protocol. Cells were washed twice in Cytoperm buffer and twice in FACS buffer before data acquisition on a FACSCalibur (BD) and analysis by FlowJo software.
Phospho–flow cytometry.
Naive CD4+ T cells were sorted from 2D2 Foxp3.GFP reporter mice and cultured with either PD-L1–Ig beads or control beads in the presence of 2 ng/ml TGF-β and 20 U/ml IL-2 for 18 h. Signaling molecules were assessed with antibodies against phospho-Akt Ser473 Alexa Fluor 647 (clone D9E), phospho-mTOR Ser24448 (clone 49F9), phospho-S6 Ser235/236 Alexa Fluor 647 (clone D57.2.2E), and PTEN Alexa Fluor 647 (clone 138G6; Cell Signaling Technology). Isotype control staining was performed using rabbit IgG isotype mAb Alexa Fluor 647 (DA1E; Cell Signaling Technology). p-mTOR was detected with anti–rabbit Alexa Fluor 647 secondary (Invitrogen). Intracellular staining was performed as described in the manufacturer's protocol. In brief, T cells were collected and washed thoroughly with PBS in 96-well V-bottom plates. Cells were then fixed with 2% paraformaldehyde for 10 min at 37°C. After fixation, plates were prechilled on ice for 1 min before permeabilization by slowly adding ice-cold methanol to a final concentration of 90% methanol. Cells were then incubated on ice for 30 min for permeabilization before being washed with 1% FCS/PBS (incubation buffer). Cells were blocked with 10% FCS/PBS for 10 min at room temperature and subsequently stained with the antibodies listed in this section for 1 h at room temperature. After incubation, cells were washed four times with incubation buffer and brought up in PBS before analysis.
Statistical analysis.
Statistical analysis of Foxp3+ T reg cell development, T eff cell proliferation, intracellular cytokine production, and phospho–flow cytometry was performed using Student's t tests in StatView (SAS Institute Inc). PD-L1–Ig titration, TGF-β titration, and percentage of weight loss were analyzed by ANOVA using StatView. Prism (GraphPad Software, Inc.) was used for Log-rank tests comparing WT Rag−/− and PD-L1−/−PD-L2−/− Rag−/− survival after transfer of naive T cells. P-values <0.05 were considered statistically significant.
Online supplemental material.
Fig. S1 shows that blockade of PD-L1 attenuates T reg cell development in vivo. Fig. S2 shows that PD-L1–Ig attenuates the MAPK signaling pathway during iT reg cell differentiation. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20090847/DC1.
Acknowledgments
The authors thank Yin Wu and Robert Ortega for technical support, Dr. Sun J. Lee for western blotting assistance, Peter T. Sage for critical reading of the manuscript, and Dr. Manish J. Butte for technical advice, statistical analysis, and critical reading of the manuscript.
This work was supported by grants from the National Institutes of Health (AI38310 and AI40614 to A.H. Sharpe; PO1 56299 to A.H. Sharpe, V.K. Kuchroo, and G.J. Freeman) and The National Multiple Sclerosis Society (L.M. Francisco).
G.J. Freeman draws royalties from patents regarding PD-L1. The authors declare that they have no other conflicts of interest.
Footnotes
Abbreviations used:
Foxp3
forkhead box p3
iT reg
induced T reg
MFI
mean fluorescence intensity
nT reg
naturally occurring T reg
PD
programmed death
PD-L
PD-ligand
T eff
effector T
T reg
regulatory T
References
- Adler H.S., Kubsch S., Graulich E., Ludwig S., Knop J., Steinbrink K. 2007. Activation of MAP kinase p38 is critical for the cell-cycle-controlled suppressor function of regulatory T cells. Blood. 109:4351–4359 10.1182/blood-2006-09-047563 [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C., Brown J.A., Freeman G.J., Hafler D.A. 2003. CD4+CD25+ regulatory cells from human peripheral blood express very high levels of CD25 ex vivo. Novartis Found. Symp. 252:67–88, discussion :88–91: 106–114 10.1002/0470871628.ch6 [DOI] [PubMed] [Google Scholar]
- Barber D.L., Wherry E.J., Masopust D., Zhu B., Allison J.P., Sharpe A.H., Freeman G.J., Ahmed R. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 439:682–687 10.1038/nature04444 [DOI] [PubMed] [Google Scholar]
- Battaglia M., Stabilini A., Migliavacca B., Horejs-Hoeck J., Kaupper T., Roncarolo M.G. 2006. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J. Immunol. 177:8338–8347 [DOI] [PubMed] [Google Scholar]
- Belkaid Y. 2008. Role of Foxp3-positive regulatory T cells during infection. Eur. J. Immunol. 38:918–921 10.1002/eji.200738120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beswick E.J., Pinchuk I.V., Das S., Powell D.W., Reyes V.E. 2007. Expression of the programmed death ligand 1, B7-H1, on gastric epithelial cells after Helicobacter pylori exposure promotes development of CD4+ CD25+ FoxP3+ regulatory T cells. Infect. Immun. 75:4334–4341 10.1128/IAI.00553-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E., Pagany M., Weiner H.L., Linington C., Sobel R.A., Kuchroo V.K. 2003. Myelin oligodendrocyte glycoprotein–specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J. Exp. Med. 197:1073–1081 10.1084/jem.20021603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- Blank C., Gajewski T.F., Mackensen A. 2005. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol. Immunother. 54:307–314 10.1007/s00262-004-0593-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom D.D., Chang Z., Fechner J.H., Dar W., Polster S.P., Pascual J., Turka L.A., Knechtle S.J. 2008. CD4+ CD25+ FOXP3+ regulatory T cells increase de novo in kidney transplant patients after immunodepletion with Campath-1H. Am. J. Transplant. 8:793–802 10.1111/j.1600-6143.2007.02134.x [DOI] [PubMed] [Google Scholar]
- Broeren C.P., Gray G.S., Carreno B.M., June C.H. 2000. Costimulation light: activation of CD4+ T cells with CD80 or CD86 rather than anti-CD28 leads to a Th2 cytokine profile. J. Immunol. 165:6908–6914 [DOI] [PubMed] [Google Scholar]
- Brown J.A., Dorfman D.M., Ma F.R., Sullivan E.L., Munoz O., Wood C.R., Greenfield E.A., Freeman G.J. 2003. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J. Immunol. 170:1257–1266 [DOI] [PubMed] [Google Scholar]
- Brunkow M.E., Jeffery E.W., Hjerrild K.A., Paeper B., Clark L.B., Yasayko S.A., Wilkinson J.E., Galas D., Ziegler S.F., Ramsdell F. 2001. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 27:68–73 10.1038/83784 [DOI] [PubMed] [Google Scholar]
- Calzascia T., Pellegrini M., Lin A., Garza K.M., Elford A.R., Shahinian A., Ohashi P.S., Mak T.W. 2008. CD4 T cells, lymphopenia, and IL-7 in a multistep pathway to autoimmunity. Proc. Natl. Acad. Sci. USA. 105:2999–3004 10.1073/pnas.0712135105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Jin W., Hardegen N., Lei K.J., Li L., Marinos N., McGrady G., Wahl S.M. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198:1875–1886 10.1084/jem.20030152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L.B., Appleby M.W., Brunkow M.E., Wilkinson J.E., Ziegler S.F., Ramsdell F. 1999. Cellular and molecular characterization of the scurfy mouse mutant. J. Immunol. 162:2546–2554 [PubMed] [Google Scholar]
- Coenen J.J., Koenen H.J., van Rijssen E., Kasran A., Boon L., Hilbrands L.B., Joosten I. 2007. Rapamycin, not cyclosporine, permits thymic generation and peripheral preservation of CD4+ CD25+ FoxP3+ T cells. Bone Marrow Transplant. 39:537–545 10.1038/sj.bmt.1705628 [DOI] [PubMed] [Google Scholar]
- Collison L.W., Workman C.J., Kuo T.T., Boyd K., Wang Y., Vignali K.M., Cross R., Sehy D., Blumberg R.S., Vignali D.A. 2007. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 450:566–569 10.1038/nature06306 [DOI] [PubMed] [Google Scholar]
- Coombes J.L., Siddiqui K.R., Arancibia-Cárcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β– and retinoic acid–dependent mechanism. J. Exp. Med. 204:1757–1764 10.1084/jem.20070590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Suarez G., Beswick E.J., Sierra J.C., Graham D.Y., Reyes V.E. 2006. Expression of B7-H1 on gastric epithelial cells: its potential role in regulating T cells during Helicobacter pylori infection. J. Immunol. 176:3000–3009 [DOI] [PubMed] [Google Scholar]
- Dong H., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B., Roche P.C., Lu J., Zhu G., Tamada K., et al. 2002. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8:793–800 [DOI] [PubMed] [Google Scholar]
- Dorfman D.M., Brown J.A., Shahsafaei A., Freeman G.J. 2006. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am. J. Surg. Pathol. 30:802–810 10.1097/01.pas.0000209855.28282.ce [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini M.C., Becker C., Monteleone G., Pallone F., Galle P.R., Neurath M.F. 2004. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 172:5149–5153 [DOI] [PubMed] [Google Scholar]
- Fontenot J.D., Gavin M.A., Rudensky A.Y. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330–336 10.1038/ni904 [DOI] [PubMed] [Google Scholar]
- Fontenot J.D., Rasmussen J.P., Williams L.M., Dooley J.L., Farr A.G., Rudensky A.Y. 2005. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 22:329–341 10.1016/j.immuni.2005.01.016 [DOI] [PubMed] [Google Scholar]
- Gao W., Lu Y., El Essawy B., Oukka M., Kuchroo V.K., Strom T.B. 2007. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am. J. Transplant. 7:1722–1732 10.1111/j.1600-6143.2007.01842.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin M.A., Rasmussen J.P., Fontenot J.D., Vasta V., Manganiello V.C., Beavo J.A., Rudensky A.Y. 2007. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 445:771–775 10.1038/nature05543 [DOI] [PubMed] [Google Scholar]
- Haxhinasto S., Mathis D., Benoist C. 2008. The AKT–mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 205:565–574 10.1084/jem.20071477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J.A., Feuerer M., Tash K., Haxhinasto S., Perez J., Melamed R., Mathis D., Benoist C. 2007. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 27:786–800 10.1016/j.immuni.2007.09.010 [DOI] [PubMed] [Google Scholar]
- Hirano F., Kaneko K., Tamura H., Dong H., Wang S., Ichikawa M., Rietz C., Flies D.B., Lau J.S., Zhu G., et al. 2005. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 65:1089–1096 [PubMed] [Google Scholar]
- Hori S., Nomura T., Sakaguchi S. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299:1057–1061 10.1126/science.1079490 [DOI] [PubMed] [Google Scholar]
- Huber S., Schrader J., Fritz G., Presser K., Schmitt S., Waisman A., Lüth S., Blessing M., Herkel J., Schramm C. 2008. P38 MAP kinase signaling is required for the conversion of CD4+CD25- T cells into iTreg. PLoS One. 3:e3302 10.1371/journal.pone.0003302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman B.A., Sebo T.J., Frigola X., Dong H., Bergstralh E.J., Frank I., Fradet Y., Lacombe L., Kwon E.D. 2007. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 109:1499–1505 10.1002/cncr.22588 [DOI] [PubMed] [Google Scholar]
- Iwai Y., Ishida M., Tanaka Y., Okazaki T., Honjo T., Minato N. 2002. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA. 99:12293–12297 10.1073/pnas.192461099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joetham A., Matsubara S., Okamoto M., Takeda K., Miyahara N., Dakhama A., Gelfand E.W. 2008. Plasticity of regulatory T cells: subversion of suppressive function and conversion to enhancement of lung allergic responses. J. Immunol. 180:7117–7124 [DOI] [PubMed] [Google Scholar]
- Keir M.E., Liang S.C., Guleria I., Latchman Y.E., Qipo A., Albacker L.A., Koulmanda M., Freeman G.J., Sayegh M.H., Sharpe A.H. 2006. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 203:883–895 10.1084/jem.20051776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir M.E., Francisco L.M., Sharpe A.H. 2007a. PD-1 and its ligands in T-cell immunity. Curr. Opin. Immunol. 19:309–314 10.1016/j.coi.2007.04.012 [DOI] [PubMed] [Google Scholar]
- Keir M.E., Freeman G.J., Sharpe A.H. 2007b. PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J. Immunol. 179:5064–5070 [DOI] [PubMed] [Google Scholar]
- Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. 2008. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26:677–704 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.M., Rasmussen J.P., Rudensky A.Y. 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8:191–197 10.1038/ni1428 [DOI] [PubMed] [Google Scholar]
- Konishi J., Yamazaki K., Azuma M., Kinoshita I., Dosaka-Akita H., Nishimura M. 2004. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin. Cancer Res. 10:5094–5100 10.1158/1078-0432.CCR-04-0428 [DOI] [PubMed] [Google Scholar]
- Kronenberg M., Rudensky A. 2005. Regulation of immunity by self-reactive T cells. Nature. 435:598–604 10.1038/nature03725 [DOI] [PubMed] [Google Scholar]
- Krupnick A.S., Gelman A.E., Barchet W., Richardson S., Kreisel F.H., Turka L.A., Colonna M., Patterson G.A., Kreisel D. 2005. Murine vascular endothelium activates and induces the generation of allogeneic CD4+25+Foxp3+ regulatory T cells. J. Immunol. 175:6265–6270 [DOI] [PubMed] [Google Scholar]
- Latchman Y.E., Liang S.C., Wu Y., Chernova T., Sobel R.A., Klemm M., Kuchroo V.K., Freeman G.J., Sharpe A.H. 2004. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc. Natl. Acad. Sci. USA. 101:10691–10696 10.1073/pnas.0307252101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S., Alard P., Zhao Y., Parnell S., Clark S.L., Kosiewicz M.M. 2005. Conversion of CD4+ CD25− cells into CD4+ CD25+ regulatory T cells in vivo requires B7 costimulation, but not the thymus. J. Exp. Med. 201:127–137 10.1084/jem.20041201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Haribhai D., Relland L.M., Truong N., Carlson M.R., Williams C.B., Chatila T.A. 2007. Regulatory T cell development in the absence of functional Foxp3. Nat. Immunol. 8:359–368 10.1038/ni1445 [DOI] [PubMed] [Google Scholar]
- Lohr J., Knoechel B., Abbas A.K. 2006. Regulatory T cells in the periphery. Immunol. Rev. 212:149–162 10.1111/j.0105-2896.2006.00414.x [DOI] [PubMed] [Google Scholar]
- Long S.A., Buckner J.H. 2008. Combination of rapamycin and IL-2 increases de novo induction of human CD4(+)CD25(+)FOXP3(+) T cells. J. Autoimmun. 30:293–302 10.1016/j.jaut.2007.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X., Zhang Q., Liu V., Xia Z., Pothoven K.L., Lee C. 2008. Cutting edge: TGF-beta-induced expression of Foxp3 in T cells is mediated through inactivation of ERK. J. Immunol. 180:2757–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie J.C., Letterio J.J., Gavin M., Rudensky A.Y. 2005. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J. Exp. Med. 201:1061–1067 10.1084/jem.20042276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi J., Wada Y., Matsumoto K., Azuma M., Kikuchi K., Ueda S. 2007. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol. Immunother. 56:1173–1182 10.1007/s00262-006-0266-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi T., Sho M., Akahori T., Hamada K., Kubo A., Kanehiro H., Nakamura S., Enomoto K., Yagita H., Azuma M., Nakajima Y. 2007. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin. Cancer Res. 13:2151–2157 10.1158/1078-0432.CCR-06-2746 [DOI] [PubMed] [Google Scholar]
- Ohigashi Y., Sho M., Yamada Y., Tsurui Y., Hamada K., Ikeda N., Mizuno T., Yoriki R., Kashizuka H., Yane K., et al. 2005. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin. Cancer Res. 11:2947–2953 10.1158/1078-0432.CCR-04-1469 [DOI] [PubMed] [Google Scholar]
- Probst H.C., McCoy K., Okazaki T., Honjo T., van den Broek M. 2005. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat. Immunol. 6:280–286 10.1038/ni1165 [DOI] [PubMed] [Google Scholar]
- Pyzik M., Piccirillo C.A. 2007. TGF-beta1 modulates Foxp3 expression and regulatory activity in distinct CD4+ T cell subsets. J. Leukoc. Biol. 82:335–346 10.1189/jlb.1006644 [DOI] [PubMed] [Google Scholar]
- Qu Y., Zhang B., Zhao L., Liu G., Ma H., Rao E., Zeng C., Zhao Y. 2007. The effect of immunosuppressive drug rapamycin on regulatory CD4+CD25+Foxp3+T cells in mice. Transpl. Immunol. 17:153–161 10.1016/j.trim.2007.01.002 [DOI] [PubMed] [Google Scholar]
- Ramsdell F. 2003. Foxp3 and natural regulatory T cells: key to a cell lineage? Immunity. 19:165–168 10.1016/S1074-7613(03)00207-3 [DOI] [PubMed] [Google Scholar]
- Riley J.L., Mao M., Kobayashi S., Biery M., Burchard J., Cavet G., Gregson B.P., June C.H., Linsley P.S. 2002. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc. Natl. Acad. Sci. USA. 99:11790–11795 10.1073/pnas.162359999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarolo M.G., Battaglia M. 2007. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat. Rev. Immunol. 7:585–598 10.1038/nri2138 [DOI] [PubMed] [Google Scholar]
- Rubtsov Y.P., Rudensky A.Y. 2007. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat. Rev. Immunol. 7:443–453 10.1038/nri2095 [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Yamaguchi T., Nomura T., Ono M. 2008. Regulatory T cells and immune tolerance. Cell. 133:775–787 10.1016/j.cell.2008.05.009 [DOI] [PubMed] [Google Scholar]
- Sauer S., Bruno L., Hertweck A., Finlay D., Leleu M., Spivakov M., Knight Z.A., Cobb B.S., Cantrell D., O'Connor E., et al. 2008. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl. Acad. Sci. USA. 105:7797–7802 10.1073/pnas.0800928105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert L.A., Jeffery E., Zhang Y., Ramsdell F., Ziegler S.F. 2001. Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activation. J. Biol. Chem. 276:37672–37679 10.1074/jbc.M104521200 [DOI] [PubMed] [Google Scholar]
- Setoguchi R., Hori S., Takahashi T., Sakaguchi S. 2005. Homeostatic maintenance of natural Foxp3+ CD25+ CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J. Exp. Med. 201:723–735 10.1084/jem.20041982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe A.H., Wherry E.J., Ahmed R., Freeman G.J. 2007. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 8:239–245 10.1038/ni1443 [DOI] [PubMed] [Google Scholar]
- Strauss L., Whiteside T.L., Knights A., Bergmann C., Knuth A., Zippelius A. 2007. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J. Immunol. 178:320–329 [DOI] [PubMed] [Google Scholar]
- Strome S.E., Dong H., Tamura H., Voss S.G., Flies D.B., Tamada K., Salomao D., Cheville J., Hirano F., Lin W., et al. 2003. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 63:6501–6505 [PubMed] [Google Scholar]
- Tang Q., Bluestone J.A. 2008. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat. Immunol. 9:239–244 10.1038/ni1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q., Henriksen K.J., Boden E.K., Tooley A.J., Ye J., Subudhi S.K., Zheng X.X., Strom T.B., Bluestone J.A. 2003. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J. Immunol. 171:3348–3352 [DOI] [PubMed] [Google Scholar]
- Thompson R.H., Gillett M.D., Cheville J.C., Lohse C.M., Dong H., Webster W.S., Krejci K.G., Lobo J.R., Sengupta S., Chen L., et al. 2004. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc. Natl. Acad. Sci. USA. 101:17174–17179 10.1073/pnas.0406351101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali D.A., Collison L.W., Workman C.J. 2008. How regulatory T cells work. Nat. Rev. Immunol. 8:523–532 10.1038/nri2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L.M., Rudensky A.Y. 2007. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat. Immunol. 8:277–284 10.1038/ni1437 [DOI] [PubMed] [Google Scholar]
- Winstead C.J., Fraser J.M., Khoruts A. 2008. Regulatory CD4+CD25+Foxp3+ T cells selectively inhibit the spontaneous form of lymphopenia-induced proliferation of naive T cells. J. Immunol. 180:7305–7317 [DOI] [PubMed] [Google Scholar]
- Wu C., Zhu Y., Jiang J., Zhao J., Zhang X.G., Xu N. 2006. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 108:19–24 10.1016/j.acthis.2006.01.003 [DOI] [PubMed] [Google Scholar]
- Yang X.O., Nurieva R., Martinez G.J., Kang H.S., Chung Y., Pappu B.P., Shah B., Chang S.H., Schluns K.S., Watowich S.S., et al. 2008. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 29:44–56 10.1016/j.immuni.2008.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Su D.M., Liang M., Fu J. 2008. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol. Immunol. 45:1470–1476 10.1016/j.molimm.2007.08.013 [DOI] [PubMed] [Google Scholar]