Association of Dishevelled with the Clathrin AP-2 Adaptor Is Required for Frizzled Endocytosis and Planar Cell Polarity Signaling (original) (raw)
. Author manuscript; available in PMC: 2010 Mar 3.
SUMMARY
Upon activation by Wnt, the Frizzled receptor is internalized in a process that requires the recruitment of Dishevelled. We describe a novel interaction between Dishevelled2 (Dvl2) and μ2-adaptin, a subunit of the clathrin adaptor AP-2; this interaction is required to engage activated Frizzled4 with the endocytic machinery and for its internalization. The interaction of Dvl2 with AP-2 requires simultaneous association of the DEP domain and a peptide YHEL motif within Dvl2 with the C terminus of μ2. Dvl2 mutants in the YHEL motif fail to associate with μ2 and AP-2, and prevent Frizzled4 internalization. Corresponding Xenopus Dishevelled mutants show compromised ability to interfere with gastrulation mediated by the planar cell polarity (PCP) pathway. Conversely, a Dvl2 mutant in its DEP domain impaired in PCP signaling exhibits defective AP-2 interaction and prevents the internalization of Frizzled4. We suggest that the direct interaction of Dvl2 with AP-2 is important for Frizzled internalization and Frizzled/PCP signaling.
INTRODUCTION
Dishevelled is a component of the conserved Wnt-Wingless signaling pathway, which regulates major developmental events, including cell-fate specification and planar cell polarity (PCP) (Logan and Nusse, 2004; Veeman et al., 2003; Wallingford and Habas, 2005). Signaling is initiated by binding of Wnt ligands, a family of secreted glyco- and lipoproteins, to different members of the Frizzled family on the surface of target cells. In turn, activated Frizzled, a seven transmembrane-domain protein receptor, recruits the cytosolic protein, Dishevelled. The Frizzled/Dishevelled complex, in association with the low-density lipoprotein (LDL) receptor-related protein (LRP), induces a signaling cascade mediated by the so-called “canonical” pathway, which, in turn, regulates transcription of specific genes (He et al., 2004; Tamai et al., 2000; Wehrli et al., 2000). The Frizzled/Dishevelled complex also induces a signaling cascade mediated by the noncanonical pathway that involves activation of the small GTPases Rho and Rac (Boutros and Mlodzik, 1999; Eaton et al., 1996; Fanto et al., 2000; Habas et al., 2001, 2003; Strutt et al., 1997; Wallingford and Habas, 2005). Several lines of evidence indicate that membrane traffic, endocytosis, and intracellular localization of the Wnt ligands and its receptors are important for Wnt signaling. One form of general control involves the establishment of an extracellular spatial gradient of Wnt, achieved by balancing the secretion level of Wnt in one set of cells and its endocytosis and degradation in an adjacent set of cells (Dubois et al., 2001; Pfeiffer et al., 2002; Strigini and Cohen, 2000; Zecca et al., 1996). A second form of control involves regulation in the number of cell-surface Frizzled receptors available for activation, as well as their targeting, together with signaling components, to specific endosomal compartments (Blitzer and Nusse, 2006; Dubois et al., 2001; Piddini et al., 2005; Rives et al., 2006; Seto and Bellen, 2006). Endosomal targeting facilitates signaling and ultimate receptor downmodulation by sequestering Frizzled from signaling components and/or by facilitating its degradation.
Internalization of Frizzled activated by Wnt requires Dishevelled, and is likely to proceed by clathrin-mediated endocytosis (Chen et al., 2003). Recruitment of cargo in this pathway requires one or more clathrin “adaptor” proteins (APs) (reviewed by Kirchhausen, 1999 and Owen et al., 2004). There are several types of adaptors, with different levels of functional and structural complexity. AP-1, AP-2, and AP-3 are heterotetramers with four distinct subunits (adaptins)—two large chains, of molecular weights ~100 kDa, and two smaller chains, of ~50 and ~20 kDa, respectively; APs interact with clathrin, cargo, and regulatory proteins, and probably have pivotal roles in coat assembly (Kirchhausen, 1999; Owen et al., 2004). AP-1 and AP-3 participate in membrane vesicular traffic between endosomes and the trans-Golgi network, while AP-2 participates in endocytic traffic from the plasma membrane to endosomes. Adaptors with simpler structures include GGA1 and GGA3 (monomers), Eps15 (homotetramer), and β-arrestin2 (monomer) (reviewed by Bonifacino, 2004 and Owen et al., 2004). These adaptors interact with cargo proteins and clathrin, and also with APs (reviewed by Kirchhausen, 1999 and Owen et al., 2004). For example, β-arrestin2 is the adaptor for the 7TM β2-adrenergic receptor (Luttrell and Lefkowitz, 2002), and some evidence also links it to Frizzled/Dishevelled downregulation (Chen et al., 2003).
The AP complexes have an ~200 kDa core and two “ears” or “appendages”. The core contains the N-terminal regions of the two larger adaptins and the entirety of the two smaller adaptins (Collins et al., 2002; Heldwein et al., 2004). The ear domains interact with peptide motifs found in a large number of proteins, including β-arrestin2 (Edeling et al., 2006; Laporte et al., 2002; Owen et al., 1999, 2000). In contrast, relatively few motifs are known to interact with the significantly larger core. The C-terminal domain of μ-adaptins, which forms an elongated “platform” on one surface of the core, recognizes tyrosine-based sorting motifs of the form YxxØ (where x tends to be polar or charged, and Ø is a hydrophobic residue with large neutral side chain), normally found in the cytosolic “tail” of membrane cargo proteins (Boll et al., 1996; Ohno et al., 1996, 1995; Owen and Evans, 1998). The core also recognizes dileucine-based sorting motifs, certain phosphoinositides, and Arf1, a regulatory small GTPase (Dittié et al., 1996; Heldwein et al., 2004; Rapoport et al., 1998). The paucity of other known partners for the AP core is surprising, given its relatively large size and evolutionarily conserved sequence and structure.
In an effort to identify additional partners for the proteins of the AP core, we took advantage of the observation that the C-terminal region of μ2-adaptin (i.e., the 50 kDa chain from AP-2) recognizes YxxØ endocytic sorting motifs (Ohno et al., 1995) and folds correctly in the absence of the remaining N-terminal portion of μ2 and of the other core components (Collins et al., 2002; Owen and Evans, 1998). We therefore used the C-terminal region of μ chain from Caenorhabditis elegans APs (Boehm and Bonifacino, 2001) as bait in a large-scale, yeast two-hybrid screen to detect interacting partners encoded in a library containing most of the open reading frames of C. elegans (Reboul et al., 2003). We report here that the nematode Dsh2, a member of the Dishevelled family of proteins, binds tightly to μ2-adaptin, an interaction that we also find with their corresponding mammalian orthologs, including Dvl2. The interaction is dual, and involves the so-called DEP (Dishevelled, EGL-10, Pleckstrin) domain of Dvl2, together with a YHEL motif C-terminal to it. A well-known lysine-to-methionine (K-to-M) mutation (the _dsh_1 allele) in the DEP domain of Drosophila Dsh and murine Dvl2 leads to strong PCP phenotypes in flies and mice (Axelrod et al., 1998; Boutros et al., 1998; Park et al., 2005; Perrimon and Mahowald, 1987; Wang et al., 2006); we find that this mutation interferes with Dvl2 binding to AP-2. Likewise, point mutations in the YHEL motif that prevent association with μ2 also block the interaction of Dvl2 with AP-2. Importantly, both sets of Dvl2 mutations impair the endocytosis of Wnt-activated Frizzled4. Moreover, we show that the same point mutations in the YHEL motif of Xdsh, the Xenopus ortholog of Dvl2, compromise the activity of Xdsh in PCP signaling, but not in the canonical Wnt pathway. Our data show that both parts of the two-pronged interaction between Dishevelled and μ2 are important for endocytosis of Wnt-activated Frizzled and for signaling in the noncanonical Wnt pathway.
RESULTS
Yeast Two-Hybrid Screen Identifies Dishevelled Proteins as Interactors with the C-terminal Domain of μ-Adaptins
We carried out a genome-wide yeast two-hybrid screen for potential new protein partners of μ-adaptins using a prey library (Reboul et al., 2003) containing at least 70% of the predicted open reading frames of C. elegans. The C-terminal domains of the C. elegans μ-adaptins (μ1A, μ1B, μ2A, μ2B, and μ3) were selected as baits under the assumption that, like their mammalian μ1 and μ2 counterparts, they would also fold independently of the other adaptor subunits (Collins et al., 2002; Heldwein et al., 2004; Owen and Evans, 1998).
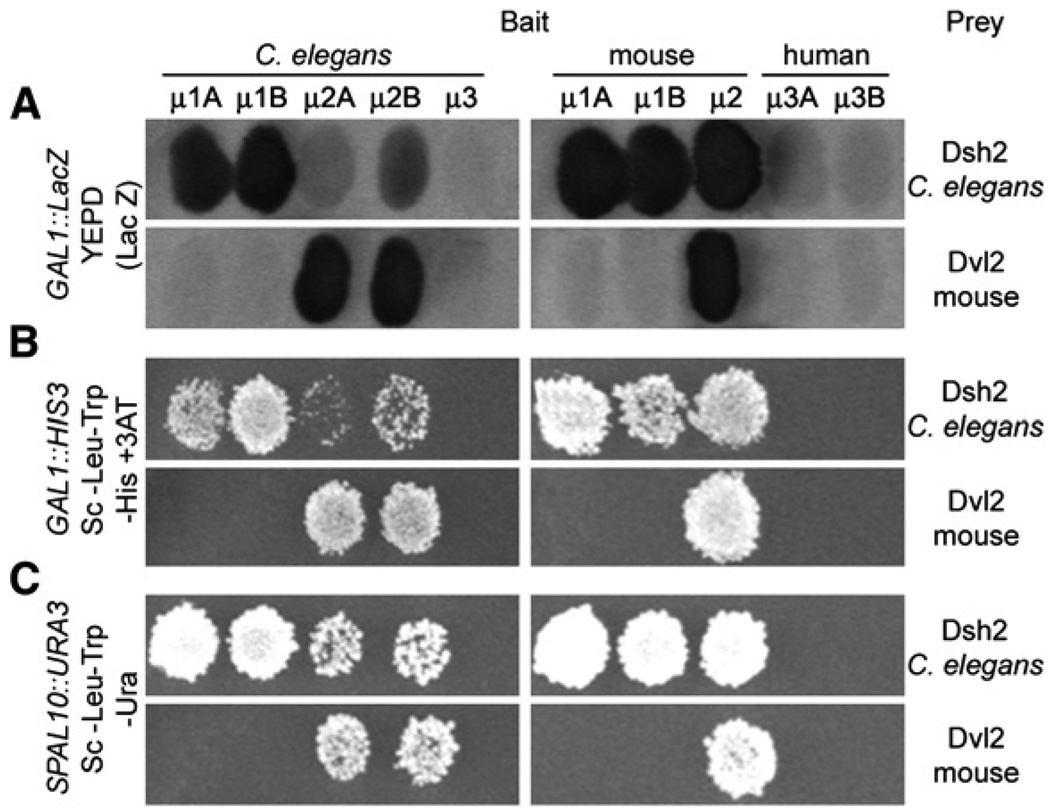
We identified 85 interactions that scored positive for at least two of the three transcriptional reporter activities (GAL1::lacZ, GAL1::HIS3, and SPAL10::URA3) used during the initial phase of the screen (Walhout and Vidal, 2001). This set did not contain proteins containing the canonical endocytic YxxØ motif, presumably because the interaction mediated by this motif with μ-adaptins is very weak and we filter out proteins with poor association. A subset (37) was selected for 5′-end DNA sequencing, and 12 were identified as unique interactors, 1 for μ1A, 5 for μ1B, 6 for μ2A and μ2B, and none for μ3. Most of them had no obvious connections to proteins known to be involved in membrane traffic, or had no significant sequence identity with proteins present in mammalian genomes (BLASTP search with e-value < 1 e–05) and, therefore, were not studied further. We focused our attention on C. elegans Dishevelled2 (Dsh2), which interacted strongly with μ-adaptins, particularly with μ1A and μ1B and less so with μ2A and μ2B (Figure 1, left panels); Dsh2 has substantial sequence identity with members of the mammalian family of Dishevelled (Dvl) proteins, which are involved in intracellular traffic of Frizzled and regulation of the Wnt signaling pathway (Boutros and Mlodzik, 1999; Chen et al., 2003).
Figure 1. Specific Interaction of Dishevelled with the C-Terminal Domain of μ-Adaptins of Clathrin Adaptors.
Yeast cells were cotransformed with AD-Dest plasmids (prey) encoding the nematode Dishevelled (Dsh2) or its mouse ortholog (Dvl2) (right) and DB-Dest plasmids (bait) encoding C-terminal domains for the nematode and mammalian μ-adaptins (top). The two-hybrid analysis was done by replica plating cells that grew in Sc-Leu-Trp agar plate transferred into the following plates: YEPD covered with nitrocellulose membrane (A), SD-Leu,−Trp,−His,+3AT (panel B), or SD-Leu,−Trp,−Ura (C). Cellson the nitrocellulose membrane (A) were lifted and processed for the β-galactosidase colorimetric assay; growth (B and C) is an indication of interaction.
C. elegans Dsh2 also interacts with the C-terminal domains of murine μ1A, μ1B, and μ2-adaptins, but not with human μ3 (Figure 1, right panels). Likewise, mouse Dvl2 interacts strongly with μ2-adaptin of C. elegans (Figure 1, left) and mouse (Figure 1, right); interaction with other μ-adaptins was below the limit of detection. The conservation of protein-protein interactions between Dishevelled and μ-adaptins suggests that this contact might also occur in the context of a completely assembled and functional clathrin adaptor complex.
Mammalian Dvl2 Interacts with AP-2
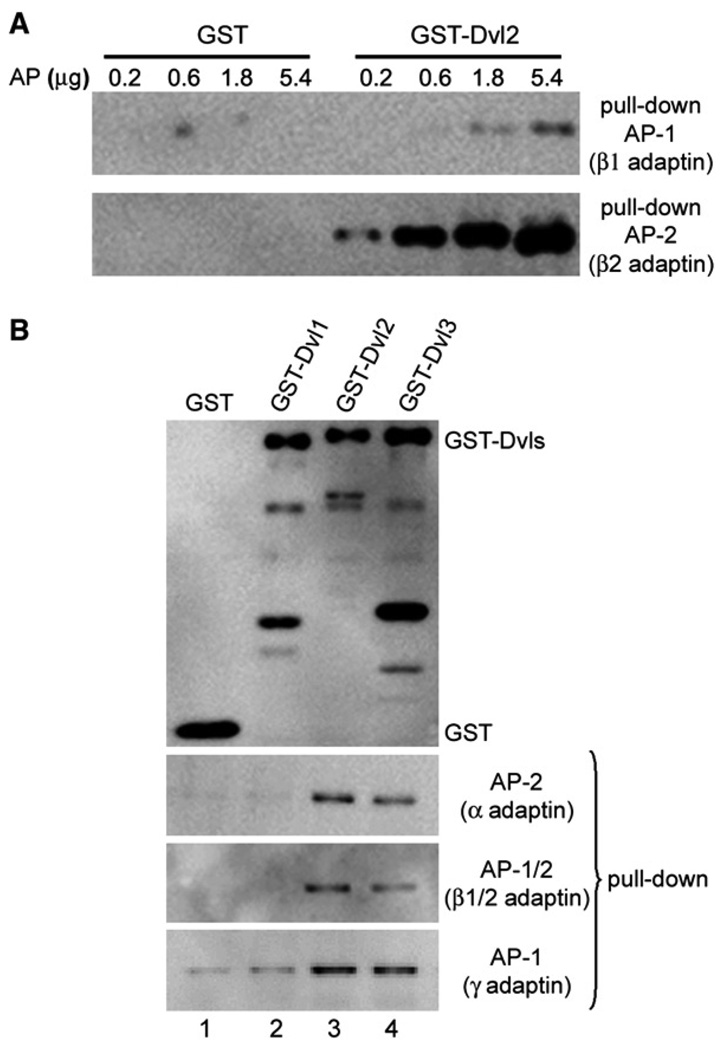
We showed that mouse Dvl2 binds AP-2 adaptor complexes by using two pull-down approaches. In the first approach, we showed that mouse Dvl2 expressed in Escherichia coli as a fusion protein with glutathione S-transferase (GST-Dvl2) can associate in vitro with clathrin adaptor AP-1 (endosomal) or AP-2 (endocytic) complexes purified from calf-brain coated vesicles (Figure 2A). GST-Dvl2 binds to AP-2 (containing μ2-adaptin) more effectively than to AP-1 (containing μ1-adaptin), while GST alone does not interact with either. In the second approach, we expressed in monkey COS7 cells mammalian Dvl1, Dvl2, and Dvl3 fused to GST (GST-Dvl1, GST-Dvl2, or GST-Dvl3) or GST alone, and showed that GST-Dvl2 and GST-Dvl3, but not GST-Dvl1, associate with endogenous AP-1 and AP-2 (Figure 2B). In principle, Dvl2 might interact indirectly with AP-2, as Dvl2 interacts with β-arrestin2 (Chen et al., 2001; Chen et al., 2003), which, in turn, can bind AP-2 β-adaptin (Laporte et al., 1999, 2002), but COS7 cells lack endogenous β-arrestin2 (Menard et al., 1997). In separate experiments based on tandem-affinity purification followed by mass spectrometry sequence analysis of proteins associated to Dvl2 ectopically expressed in HeLa cells, we identified β1, β2, and α and γ adaptins of AP-1 and AP-2, but not β-arrestin2, among partners of Dvl2 (X. Chen and X.H., unpublished data). From these observations, together with the results from the yeast two-hybrid assays, we propose that Dvl2 (and probably also Dvl3) associates with AP complexes through direct contact with μ-adaptins.
Figure 2. Association of Mammalian Dishevelled Proteins with Heterotetrameric Clathrin Adaptor Complexes.
(A) Pull-down assay of increasing amounts of clathrin adaptor proteins isolated from calf-brain coated vesicles that bound to GST alone or to GST fused to mouse Dishevelled2 (GST-Dvl2). The figure shows the western blot analysis using an antibody specific for β1/2-adaptins of AP-1 and AP-2. The results are representative of two experiments.
(B) Pull-down assay of endogenous clathrin adaptors present in COS7 cells transiently expressing GST alone or GST fused to mammalian Dishevelled1 (GST-Dvl1), Dishevelled2 (GST-Dvl2), or Dishevelled3 (GST-Dvl3). Bound clathrin adaptors were detected by western blot analysis with antibodies specific for α-adaptin of AP-2, β1/2-adaptins of AP-1 and AP-2, and γ-adaptin of AP-1. The results are representative of two experiments.
We expressed enhanced green fluorescent protein (EGFP)-Dvl fusion proteins (EGFP-Dvl1, EGFP-Dvl2, or EGFP-Dvl3) in COS7 cells, to follow association with membrane-bound AP-1 or AP-2. At low levels of expression, we could not detect any significant colocalization in agreement with absence of detectable colocalization between HA-tagged Dvl2 and AP-2 (Schwarz-Romond et al., 2005). At this low-expression level, the punctate distribution of AP-1 or AP-2 was not affected. In contrast, higher expression levels of EGFP-Dvl proteins led to the formation of distinct intracellular aggregates, some colocalizing with AP-1 and AP-2 (see Figure S1A and S1B in the Supplemental Data available with this article online). The imaging observations under conditions of overexpression provide further support for the conclusion, from biochemical data, that Dvl2 and Dvl3 can interact with AP-1 and AP-2 adaptors, but they did not allow us to determine whether Dishevelled proteins are recruited by adaptor complexes within coated pits and vesicles.
Two Regions in Dvl2 Mediate the Interaction with μ2-Adaptin
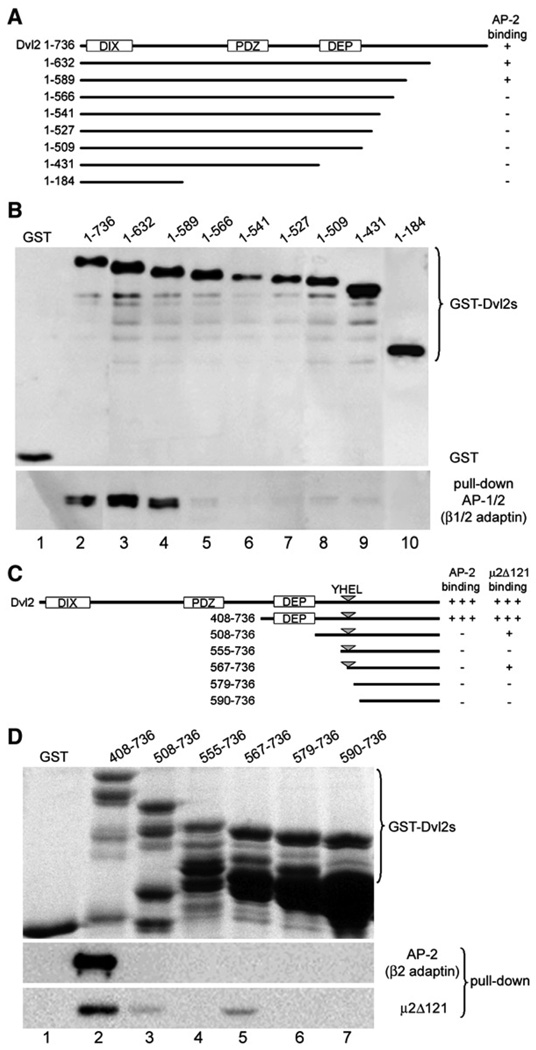
To define the region of Dvl2 that interacts with clathrin adaptors, we tested the association of C-terminal truncation mutants of Dvl2 fused to GST expressed in COS7 cells with endogenous AP-1 and AP-2 (Figure 3A and 3B). The results show that full-length Dvl2 and the two partial truncations up to Dvl2 1–589 bind AP-1 and AP-2 equally well, whereas Dvl2 1–566 (or shorter constructs) does not, suggesting that residues 566–589 are important for this association. To test the complementary set of N-terminal nested deletions (Figure 3C),we used GST-fusion proteins expressed in E. coli, because it was not possible to express them in COS7 cells at the levels required for the pull-down assay. We analyzed whether the GST-fusion proteins could interact with either the C-terminal fragment of μ2-adaptin or with brain AP-2 (Figure 3D). GST-Dvl2 (408–736) associates with both AP-2 and μ2-adaptin, whereas shorter fragments starting with position 508 interact weakly or not at all. We suggest that the interaction with AP-2 is two pronged, and is contributed by contacts provided by two regions of Dvl2, one between residues 408 and 507, and another between residues 566 and 589. The former segment contains the DEP domain, an independently folded structure (Wong et al., 2000).
Figure 3. Mapping of the Region within Dvl2 Required to Associate with AP-2.
(A) Domain organization of mouse Dishevelled2 (Dvl2) and different fragments expressed in COS7 cells used for the pull-down experiments. It highlights the position of its DIX, PDZ, and DEP domains. The amino acid residue boundaries and their ability to bind to AP-2 are indicated.
(B) Pull-down assay of endogenous clathrin adaptors present in COS7 cells transiently expressing GST alone or GST fused to the constructs outlined in (A). Bound proteins were detected by western blot analysis with antibodies specific for GST (top panel; 10% input) and for β1/2-adaptins of AP-1 and AP-2 (bottom panel; 25% input). The results are representative of two experiments.
(C) Schematic representation of the domain organization of mouse Dishevelled2 (Dvl2) and different fragments expressed in E. coli used for the pull-down experiments. It highlights the position of its DIX, PDZ, and DEP domains, and the location of the YHEL motif. The amino acid residue boundaries and their abilities to bind to AP-2 and the C-terminal fragment of μ2 (μ2Δ121) are indicated.
(D) Pull-down assay of clathrin adaptors purified from calf-brain coated vesicles or of recombinant μ2 (μ2Δ121) that bound to the bacterially expressed GST alone or GST fused to the constructs outlined in (C). Bound GST-containing proteins were detected by SDS-PAGE followed by Coomassie Blue staining (100% input). Bound clathrin adaptors and μ2Δ121 were detected from a second sample processed identically by western blot analysis with the antibody specific for β1/2-adaptins of AP-1 and AP-2 and the antibody specific for the His tag for μ2Δ121 (20% input). The results are representative of two experiments.
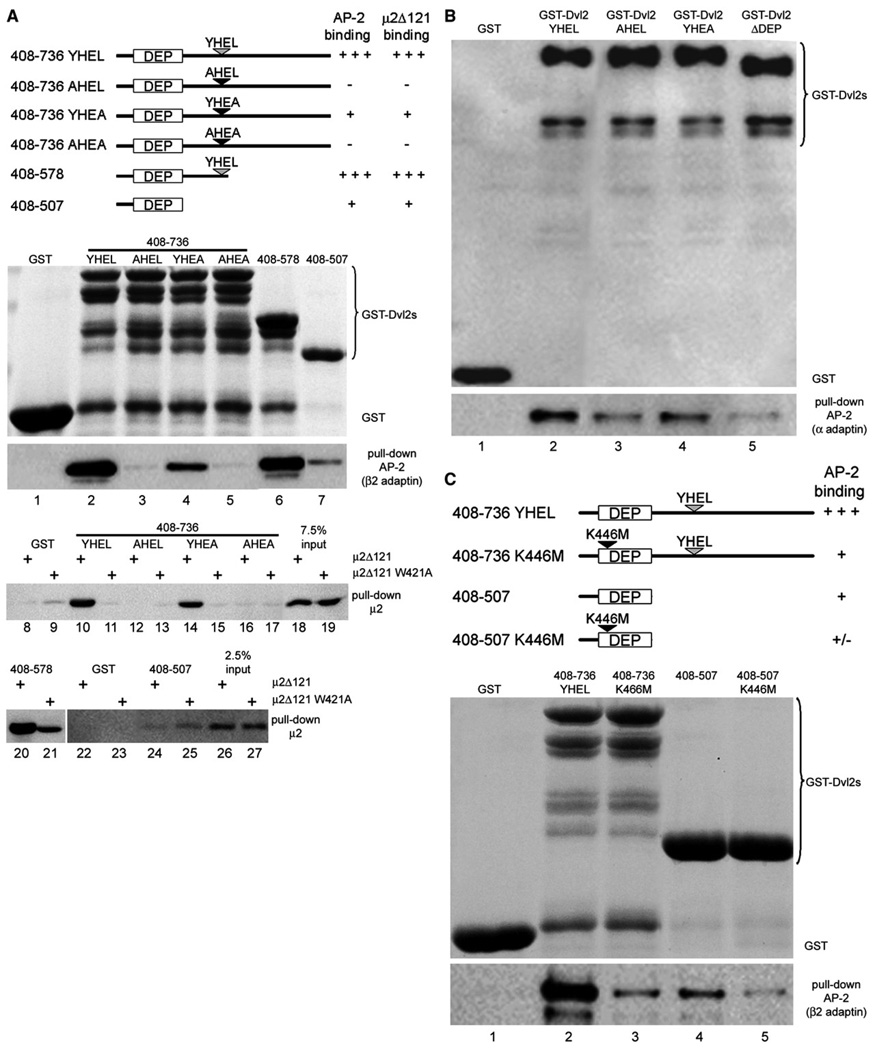
We further explored the nature of this dual interaction as follows. First, we confirmed that GST-Dvl2 (408–736) and GST-Dvl2 (408–578) associate to a similar extent with brain AP-2 (Figure 4A, lanes 2 and 6). In contrast, the interaction of brain AP-2 with GST-Dvl2 (408–507), which includes only the DEP domain, was weaker (Figure 4A, lane 7; see also Figure 4C, lane 4), but significantly higher than with GST alone (Figure 4A, lane 1, and Figure 4C, lane 1). The region spanning residues 566–589 of Dvl2 contains a tetrapeptide motif Y568HEL (Figure S2) reminiscent of the tyrosine-based YxxØ sorting motif. GST-Dvl2 (408–736), which includes both the DEP domain and the Y568HEL sequence, binds well to AP-2 purified from calf brain (Figure 4A, lane 2), while full-length GST-Dvl2 binds to endogenous AP-2 (Figure 4B, lane 2); in contrast, variants with point mutations in the tetrapeptide motif (AHEL, YHEA, or AHEA) known to prevent the interaction of the tyrosine motif with μ2 or AP-2 (Boll et al., 1996; Ohno et al., 1996, 1998) bound poorly (Figure 4A, lanes 3–5, and Figure 4B, lanes 3 and 4). We further showed loss of interaction between the C terminus of μ2-adaptin and GST-Dvl2 (Dvl2 408–736 YHEL) modified by the same point mutations (Dvl2 408–736 AHEL, Dvl2 408–736 YHEA, or Dvl2 408–736 AHEA; Figure 4A, compare lane 10 with lanes 12, 14, and 16). Likewise, a C-terminal μ2-adaptin fragment containing a point mutation (μ2Δ121 W421A) that hinders its ability to bind tyrosine-based motifs (Boll et al., 2002; Nesterov et al., 1999) fails to recognize GST-Dvl2 containing the YHEL motif (Figure 4A, lane 11).
Figure 4. The DEP Domain and the YHEL Motif of Dvl2 Are Required for its Efficient Association with AP-2.
(A) Schematic representation of different fragments of mouse Dvl2 expressed in E. coli used for the pull-down experiments. It highlights the role of the YHEL motif for the association with AP-2 purified from calf-brain coated vesicles or with recombinant μ2Δ121. The pull-down assay was done using GST alone or GST fused to the Dvl2 fragments. Bound GST-containing proteins were detected by SDS-PAGE followed by Coomassie Blue staining (100% input). Bound clathrin adaptors and wild-type μ2Δ121 or its mutant μ2Δ121 W421A (that cannot recognize tyrosine-based motifs) were detected from a second sample processed identically, followed by western blot analysis with the antibody specific for β1/2-adaptins of AP-1 and AP-2 and the antibody specific for the His tag for μ2Δ121 or μ2Δ121 W421A (20% input). Lanes 18 and 19 and 26–27 are included as calibration of bound μ2Δ121 and μ2Δ121 W421A, and represent 7.5% and 2.5% input. The results are representative of two experiments.
(B) Pull-down assay of endogenous clathrin adaptors present in COS7 cells that bound to transiently expressed GST alone or GST fused to full-length Dishevelled2 containing wild-type (GST-Dvl2 YHEL) or point mutations in its YHEL motif (GST-Dvl2 AHEL or GST-Dvl2 YHEA) and to the full-length Dishevelled2 lacking its DEP domain (GST-Dvl2 ΔDEP). Bound proteins were detected by western blot analysis with antibodies specific for GST (top panel; 10% input) and for β1/2-adaptins of AP-1and AP-2 (bottom panel; 25% input). The results are representative of two experiments.
(C) Schematic representation of different fragments of mouse Dvl2 expressed in E. coli used for the pull-down experiments. It highlights the role of the DEP domain for the association with AP-2 purified from calf-brain coated vesicles. The pull-down assay was done using GST alone or GST fused to the Dvl2 fragments. Bound GST-containing proteins were detected by SDS-PAGE followed by Coomassie Blue staining (100% input). Bound clathrin adaptors were detected from a second sample processed identically by western blot analysis with the antibody specific for β1/2-adaptins of AP-1 and AP-2. The results are representative of two experiments.
The weak interaction between the DEP domain (which does not contain tyrosine-based motifs) and μ2 does not require the region of μ2 involved in tyrosine-motif recognition, since the DEP domain (residues 408–507) binds equally well to μ2Δ121 and μ2Δ121 W421A (Figure 4A, lanes 24 and 25). Lys446 (equivalent to Lys417 in Drosophila Dsh) (Axelrod et al., 1998; Wong et al., 2000), an evolutionarily conserved residue within the DEP domain, has a role in Dishevelled signaling, as demonstrated by the effects of the K417M mutation in the Drosophila dsh1 allele and K446M in mouse Dvl2 on the noncanonical PCP signaling pathway (Axelrod et al., 1998; Boutros et al., 1998; Habas et al., 2003; Moriguchi et al., 1999; Park et al., 2005; Perrimon and Mahowald, 1987; Wang et al., 2006). This residue is important for the association of Dvl2 with purified AP-2, since the K446M mutation decreased AP-2 binding of GST-Dvl2 (408–736), which contains the DEP domain and the YHEL-motif (Figure 4C, lanes 2 and 3). The K446M mutation in the DEP domain alone also reduces the (weaker) interaction with AP-2 (Figure 4C, compare lanes 4 and 5).
We suggest that two regions in Dvl2 engage in the interaction with μ2-adaptin, one containing the DEP domain and another containing the tetrapeptide YHEL motif. Each one, on its own, has weak affinity for AP-2, but, when presented jointly, they cooperatively stabilize the Dvl2/AP-2 interaction.
Association of Dvl2 with AP-2 Is Required for the Wnt-5A-Dependent Internalization of Frizzled4
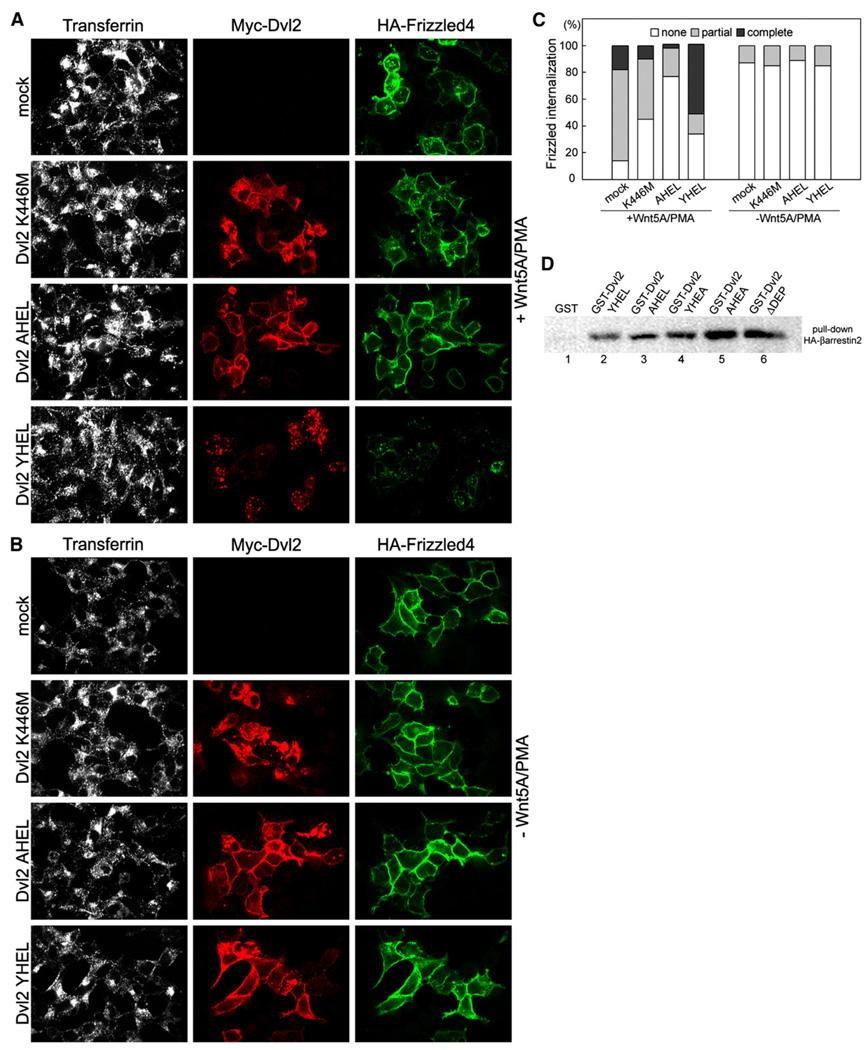
When activated by Wnt5A, Frizzled4-EGFP is rapidly internalized in cells simultaneously treated with the PKC activator, PMA(Chen et al., 2003). We confirmed this observation by using a Frizzled4 construct containing an extracellular N-terminal HA-tag (HA-Frizzled4) in HEK293T cells transiently expressing HA-Frizzled4 and Myc-tagged Dvl2; internalization and degradation of HA-Frizzled4 was monitored by following uptake of an anti-HA antibody added to the media immediately prior to Wnt and PMA treatment (Figure 5A).
Figure 5. Expression of Dvl2 AHEL or of Dvl2 K446M Prevents the Internalization of Activated Frizzled4.
(A and B) HEK293T cells were cotransfected with plasmids encoding an empty Myc-vector (mock), Myc-tagged Dishevelled2 containing the K-to-M mutation in the DEP domain (Myc-Dvl2 K446M, red), Myc-tagged Dishevelled2 containing AHEL instead of the YHEL motif (Myc-Dvl2 AHEL, red), or Myc-tagged wild-type mouse Dishevelled2 (Myc-Dvl2 YHEL, red), together with HA-Frizzled4 (green). After 24 hr, cells were incubated for 10 min and 37°C with an antibody specific for the HA-epitope (green); at this point, the cells were incubated with fresh media in the presence (A) or absence (B) of Wnt5A/PMA for another 30 min at 37°C. Alexa647-conjugated transferrin (white) was added during the last 5 min of incubation. Cells were fixed, permeabilized, and processed for immunofluorescence. Representative examples are shown.
(C) Analysis of images from fields corresponding to the experiments depicted in (A) and (B). The degree of Frizzled4 internalization was scored in cells coexpressing Dvl2 and Frizzled4 according to the following criteria: (1) HA-frizzled4 signal at the cell surface (none); (2) a mixture of HA-Frizzled4 signals at the cell surface together with a punctate intracellular pattern (partial); and (3) no discernable HA-Frizzled4 signal at the cell surface, together with a weak intracellular punctate pattern (complete). About 15 independent fields containing approximately 300 cells were analyzed for each one of the experimental conditions.
(D) Pull-down assay to verify the interaction between HA-β-arrestin2 and wild-type or several mutants of Dishevelled2. HEK293T cells stably expressing Frizzled4-EGFP were cotransfected with plasmids encoding HA-β-arrestin2 together with either GST alone or with GST fused to wild-type Dishevelled2, the indicated mutants in the YHEL motif or lacking the DEP domain.
As expected, addition of Wnt5A/PMA for 30 min stimulates the uptake and degradation of Frizzled4, most prominently observed in cells also expressing Dvl2. Internalization was detected by following the mobilization into an intracellular punctate pattern of a large fraction of the relatively uniform membrane signal observed in the absence of activation (Figures 5A and 5B; quantitation in Figure 5C). There was, however, no significant colocalization of internalized Frizzled4 and Myc-Dvl2 (Figure 5A), indicating that Myc-Dvl2 either remained at the surface or dissociated from Frizzled4 before internalization. The extensive degradation of activated Frizzled4 is reflected by the significant loss in its fluorescence signal (Figures 5A and 5C). Expression of Myc-Dvl2 AHEL or Myc-Dvl2 K446M prevented the stimulated uptake of Frizzled4 and its degradation, without affecting transferrin uptake (Figure 5A). Similar results were obtained by expression of Myc-Dvl2 AHEA (data not shown). None of the conditions used here affect the clathrin and AP-2-dependent internalization of transferrin, followed by a 5 min pulse of Alexa647-transferrin added to the medium immediately before the end of the experiment. We conclude that the association of Dvl2 with AP-2 is required for the Wnt-5A-dependent internalization and degradation of Frizzled4.
It has been proposed that β-arrestin2 links Dvl2 and AP-2 and thereby mediates internalization of activated Frizzled4 (Chen et al., 2001, 2003; Laporte et al., 1999, 2002). Failure to internalize activated Frizzled4 by expression of Myc-Dvl2 AHEL could be explained in this model by a decrease in the interaction between Myc-Dvl2 AHEL and β-arrestin2. We found, however, that the association between GST-Dvl2 and HA-β-arrestin2 coexpressed in HEK293T cells remains the same regardless of whether Dvl2 is wild-type, lacks a functional YHEL motif, or does not contain its DEP domain (Figure 5D). Thus, the internalization of activated Frizzled4 appears to require a direct interaction between Dvl2 and AP-2, and the significant reduction in its uptake, observed in the presence of Myc-Dvl2 AHEL or Myc-Dvl2 K446M, is explained by reduction in the association between Dvl2 and AP-2, and not by a lack of association of Dvl2 with β-arrestin2.
Effect of Xdsh Mutants in Wnt and PCP Signaling during Xenopus Development
To explore whether there is a connection between the mutations in Dvl2 that affect the intracellular traffic of Frizzled4 and Wnt signaling in the context of embryonic development, we studied the effect of different point mutations in the YHEL motif in Xenopus Dishevelled (Xdsh) (Figure S2), the ortholog of Dvl2, on cell fate linked to dorsal-ventral axis formation and on cell movements during gastrulation.
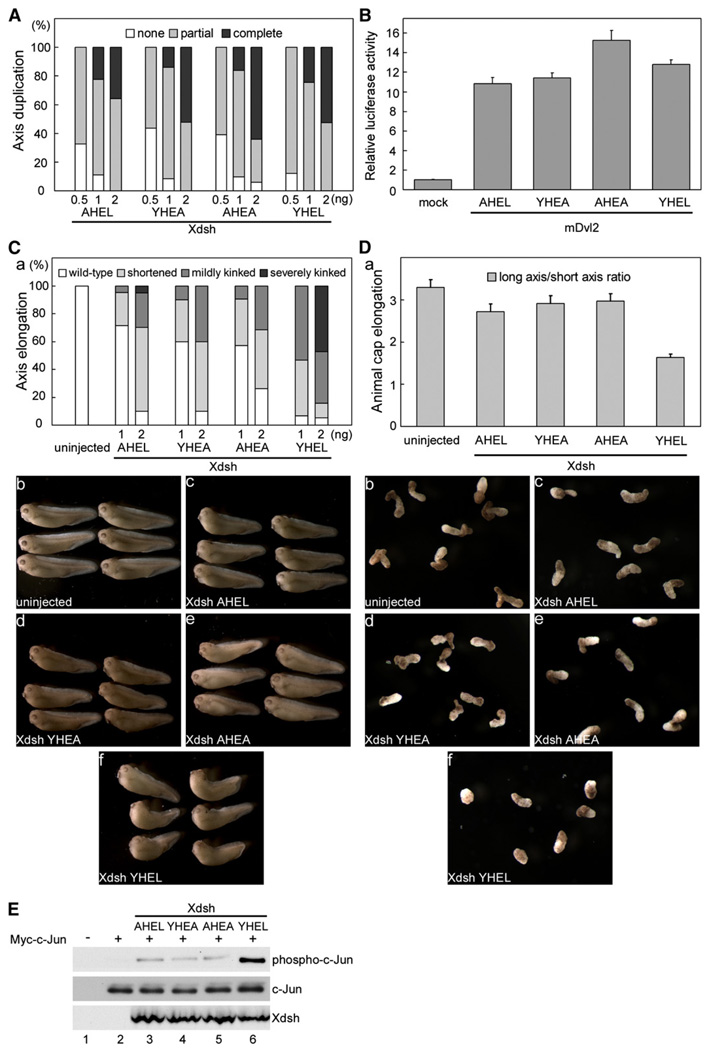
Dorsal-ventral axis formation involves preferential localization of Xdsh to the future dorsal side of the one-cell stage embryo and activation of the β-catenin signaling pathway (Heasman et al., 1994; Kelly et al., 1995; Larabell et al., 1997; Miller et al., 1999). Ectopic expression of Xdsh by injection of Xdsh mRNA at the ventral side of Xenopus embryos results in stabilization of β-catenin and axis duplication (Sokol et al., 1995). We found that injection of increasing amounts of RNA encoding wild-type Xdsh or Xdsh with mutations in the YHEL motif activated the β-catenin pathway to about the same extent in the axis duplication assay (Figure 6A), indicating that they are all active and well folded. Comparable expression of Dvl2 and its YHEL mutants equally activate TOPFlash (a TCF/β-catenin-responsive luciferase transcriptional activation reporter) in HEK293T mammalian cells (Korinek et al., 1997; van de Wetering et al., 1997) (Figure 6B). Thus, the YHEL motif and the presumed binding to AP-2 adaptor may not be critical for activation of the canonical β-catenin pathway by Xdsh or by Dvl2 in Xenopus and mammalian cells, respectively.
Figure 6. The YHEL Motif in Xdsh Is Involved in PCP Signaling and Xenopus Gastrulation.
(A) Axis duplication assay. Synthetic mRNAs were injected into both ventral blastomeres of four cell-stage embryos using the indicated doses per embryo. Induction of a secondary body axis was scored at stage 35 as follows: complete axis duplication containing a head, including the cement gland and/or eyes (black); partial axis duplication containing duplicated trunk but no head (gray). Data reflect the results from ~40 injected embryos for each mRNA dose.
(B) TOPFlash luciferase assay. Extracts of HEK293T cells transiently expressing wild-type mDvl2 or its YHEL mutants were assayed for relative luciferase activities. The data (mean ± SEM) derives from three independent experiments.
(C) Gastrulation movements assay. Synthetic mRNAs were injected into both dorsal blastomeres of four cell-stage embryos using the indicated doses per embryo. Induction of a kinked axis phenotype was scored according to the severity of the effect as follows: no effect (white), shortened but with no kink (light gray); mildly kinked (dark gray); and severely kinked anteroposterior axis (black). (a) Data from ~20 injected embryos scored at stage 35 for each RNA dose. (b–f) Representative images of stage-35 tadpoles corresponding to the various conditions used (at 1ng RNA/embryo).
(D) Animal cap-explant elongation assay. Two-cell-stage embryos were injected into the animal region with 2 ng RNA. Animal pole explants were dissected at stage 9 and treated with 10 ng/ml activin for 15 hr, and then scored for elongation by measuring the length versus width ratio. (a) This measurement (mean ± SEM) reflects morphogenetic elongation movements of activin-induced mesoderm (dorsal type) from ~20 caps for each condition. The caps injected with wild-type Xdsh were significantly less elongated compared with uninjected control caps (p < 0.01), but there was no significant difference between those injected with the Xdsh mutants and control caps (p > 0.05). (b–f) Representative images of the animal pole explants indicated in (a).
(E) JNK activation assay. Two-cell-stage embryos were injected with Xdsh wild-type or with Xdsh YHEL mutants together with c-Jun; the extent of c-Jun phosphorylation at stage 11.5 (top row) and the expression levels of c-Jun (middle row) and Xdsh (bottom row) are shown. The data are representative of results from three independent experiments.
Overexpression of wild-type Xdsh at the dorsal-marginal zone induces a kinked axis, a phenotype associated with the perturbation of the noncanonical PCP pathway (Sokol, 1996; Wallingford and Harland, 2001; Wallingford et al., 2000). Each Xdsh bearing modifications in the YHEL motif was markedly less efficient in inducing this phenotype (Figure 6C), suggesting that the YHEL motif might be required for Xdsh to efficiently activate the PCP pathway. We examined further the role of the YHEL motif of Xdsh in the PCP pathway by an animal pole-explant elongation assay (Figure 6D). In this assay, animal pole explants treated with activin become dorsal-type mesodermal tissues and elongate via convergent extension movements under control of the PCP pathway (Ariizumi et al., 1991; Sokol, 1996; Tada and Smith, 2000; Wallingford et al., 2000). As expected, overexpression of wild-type Xdsh in animal pole explants perturbed elongation, likely due to elevated PCP signaling (Tada and Smith, 2000; Wallingford et al., 2000), but Xdsh containing AHEL, YHEA, or AHEA had significantly smaller effects (Figure 6D). Dishevelled-mediated PCP signaling involves the activation of JNK (c-Jun N-terminal kinase) (Boutros et al., 1998; Habas et al., 2003; Li et al., 1999; Moriguchi et al., 1999). Wild-type Xdsh induced JNK activation in Xenopus embryos (Figure 6E, lane 6). In contrast, expression of similar amounts of Xdsh containing AHEL, YHEA, or AHEA failed to induce JNK activation and, hence, did not activate the PCP pathway (Figure 6E). These embryological and molecular assays suggest that Dvl2/Xdsh binding to AP-2 is required for PCP signaling.
DISCUSSION
We describe here a two-pronged protein-protein interaction between the C-terminal domain of μ-adaptin and members of the Dishevelled family. One part of this contact involves the tyrosine-based endocytic motif present in Dvl2 proteins (Figure S2); the other is a distinct and previously unsuspected interface of μ2 with the Dvl DEP domain. This two-part interaction is conserved in evolution, and its function is important for the endocytosis of activated Frizzled and for the regulation of the noncanonical Wnt/PCP signaling pathway in developing Xenopus embryos.
A small patch on the surface of the C-domain of μ-adaptins makes the key contacts with the YxxØ sorting motif (Owen and Evans, 1998) required to mediate the interaction between the cytosolic tails of many membrane proteins and AP complexes. Although relatively weak, this interaction is sufficient to support entrapment into coated pits and vesicles (Boll et al., 1996; Ohno et al., 1996, 1998). A number of cytosolic proteins, such as clathrin, β-arrestin2, epsinR, and Eps15, can associate with APs through contacts with the C-terminal domains of the large adaptin subunits (α, β, and γ) (Kirchhausen, 1999; Owen et al., 2004), but no interactions between cytosolic proteins and the μ-adaptin subunits have been described prior to this study. Having used the yeast two-hybrid assay to identify a significant interaction between family members of Dishevelled and μ-adaptins, first with C. elegans and then with mammalian proteins, we chose Dvl2, μ2-adaptin, and endocytic AP-2 complexes as our focus for the studies described here. We reached the following principal conclusions: (1) the interaction of μ2-adaptin or AP-2 with mouse Dvl2 is substantially stronger than the interaction of μ2 or AP-2 with protein fragments containing tyrosine-based sorting motifs (Boll et al., 1996; Ohno et al., 1996); (2) two distinct regions within Dvl2 are required for this strong interaction, one involving its DEP domain, and the other a conventional tyrosine-based tetrapeptide YHEL motif about 60 residues C-terminal to the DEP domain; (3) both regions are required for the tight association, and a perturbation in either results in Dvl2 variants having only very weak interactions with μ2 or AP-2; (4) the YHEL motif of Dvl2 interacts with the surface on μ2-adaptin responsible for the recognition of tyrosine-based motifs, while the DEP domain interacts elsewhere.
The YHEL motif is present in mouse and human Dvl2 and in Xenopus Xdsh, while the related sequence, FPEL, is found in mouse and human Dvl3. The motif is absent in human Dvl1, Drosophila Dsh and C. elegans Dsh2. It is possible that, in these cases, the DEP domain is sufficient to support the functional interaction of Dishevelled with μ-adaptin; alternatively, another protein element in Dishevelled (or within an additional protein, perhaps β-arrestin2 [Chen et al., 2001, 2003; Laporte et al., 1999, 2002]) might have evolved to bind other regions on β-adaptin or on one of the other AP subunits.
Based on four independent lines of evidence, we propose that a tight association between Dishevelled and AP-2 is important for at least some of the known biological functions of Dishevelled. One involves the observation that Frizzled4 is rapidly internalized upon its activation by Wnt, a process that requires Dvl2 (Chen et al., 2003). We found that this rapid and efficient uptake is coupled to Frizzled degradation, presumably in lysosomes, and both processes are greatly hindered in cells expressing variants of Dvl2 that fail to interact with AP-2 by virtue of selected point mutations in the YHEL motif or the DEP domain. We suggest that proper engagement of Dvl2 with AP-2 is a key step for Frizzled4 endocytosis and its eventual degradation. It is possible that, under certain conditions, Dvl2 engages productively with the endocytic machinery by associating with β-arrestin2, which in turn can bind to clathrin and AP-2, as shown by failure to internalize Frizzled4 in cells depleted of β-arrestin2 by siRNA treatment (Chen et al., 2003). It seems, however, that the interaction of Dvl2 and β-arrestin2 can be superseded, because we observe a block in Frizzled4 endocytosis upon expression of Dvl2 mutants in the tyrosine motif that, according to a pull-down assay, bind β-arrestin2 perfectly well (Figure 5D).
The second line of evidence involves Wnt signaling during frog embryonic development. Frog Xdsh has important regulatory roles in the canonical β-catenin and the noncanonical PCP pathways (Miller et al., 1999; Park et al., 2005; Sokol, 1996; Sokol et al., 1995; Wallingford and Harland, 2001; Wallingford et al., 2000). Our experiments, carried out in developing embryos, show that Xdsh with single-point mutations in its YHEL motif induces dorsal axis duplication as well as does the wild-type Xdsh, indicating that the mutations have little or no effect on the function of Xdsh in regulating the canonical β-catenin pathway. In contrast, presence of the YHEL motif is required for proper regulation of the noncanonical PCP pathway. This conclusion is based on the observation that overexpression of the wild-type Xdsh interferes with gastrulation in embryos and with elongation in the animal cap assay, whereas these processes are largely normal with any one of the YHEL mutant forms of Xdsh expressed at similar levels.
The third and fourth lines of corroborating evidence were obtained by following the effects of the Xdsh/Dvl2 mutants on two independent molecular signaling assays, one based on the activation of JNK in frog embryos, one of the hallmarks of PCP signaling, and the other based on stimulation of the TOPFlash reporter assay in mammalian cells, an indication of signaling through the canonical pathway. Xdsh, but none of the YHEL mutants, stimulated JNK, reflecting their failure to activate the noncanonical pathway; in contrast, both wild-type and Dvl2 mutants stimulated equally the TOPFlash assay, reflecting their comparable signaling through the canonical pathway. A possible caveat to the interpretation of these results is the fact that they involved gain of function effects by overexpression of mutant Dishevelled, rather than strict replacement of endogenous Dishevelled with the mutant forms. The latter experiment is currently not feasible, given the functional redundancy among different members of the Dishevelled family.
Others have shown defective PCP signaling in flies (Axelrod et al., 1998; Boutros et al., 1998) expressing the dsh1 mutation, or in mouse (Wang et al., 2006) expressing Dvl2 with the same K-to-M mutation in the DEP domain found here to prevent the interaction between mouse Dvl2 and AP-2. Assuming that, like its mammalian counterparts, Xdsh also interacts with AP-2 in the frog, we suggest that proper signaling activity through the noncanonical PCP pathway is linked to endocytosis by engagement of Xdsh with AP-2. Consistent with this proposal is the observation that Xenopus gastrulation movements are blocked by expression of S45N dynamin, a dominant-negative mutant form that prevents AP-2- and clathrin-mediated endocytosis (Jarrett et al., 2002). Finally, our biochemical data also indicate that Dishevelled family members can interact with AP-1, the adaptor involved in vesicular traffic between endosomes and the trans-Golgi network. Future studies will help establish whether this association has physiological relevance.
We have not yet determined the association constant between μ2-adaptin or AP-2 and Dvl2, but preliminary observations indicate that it is strong and that point mutations or complete removal of the YHEL motif in Dvl2 results in an interaction that is as weak as that observed with proteins containing just the tyrosine-based motif (A.Y. and T.K., unpublished data). Thus, modulation of the interaction between Dvl2 and AP-2 could be achieved by regulating access of the YHEL and/or the DEP domain to μ2-adaptin, perhaps by phosphorylation. Phosphorylation of Dvl1 or Dvl2, at one or more presently undetermined sites, increases its ability to interact with β-arrestin1 and β-arrestin2 (Chen et al., 2001, 2003), and perhaps a similar modification might regulate binding to AP-2.
To conclude, by using a combination of biochemical, cell biological, and embryological approaches, we have revealed a previously undetected interaction between members of the Dishevelled family and clathrin adaptor complexes. We suggest that this association is important during early stages of embryo development, and we highlight the importance of intracellular trafficking for the outcome of the Frizzled/Dishevelled-mediated PCP signaling pathway.
EXPERIMENTAL PROCEDURES
In Vitro Pull-Down Assay
E. coli BL21 cells expressing GST-tagged proteins were lysed with a solution containing 20% glycerol, 1% Triton X-100, 0.2 mM EDTA, 100 mM KCl, 20 mM Hepes (pH 7.3), and complete protease inhibitor (Roche Diagnostics). His-tagged μ2-adaptin μ2Δ121 and μ2Δ121 W421A were expressed in E. coli BL21 cells and purified with histidine-binding TALON beads (Clontech), as previously described (Boll et al., 2002). Calf brain AP-1 and AP-2 complexes were purified as previously described (Boll et al., 1996; Gallusser and Kirchhausen, 1993). Purified proteins (APs, μ2-adaptin) at final concentrations of 2.5 and 15 µg/ml were incubated in 200 µl with GST-tagged proteins immobilized on glutathione Sepharose 4B beads for 1 hr at 4°C. The beads were washed three times with buffer containing 100 mM MES (pH 7.0), 150 mM NaCl, 1 mM EDTA, 0.5 mM dithiothreitol, and 1% Triton X-100. Bound proteins were analyzed by SDS-PAGE and western blotting. The data represent the results obtained from two independent experiments.
In Vivo Pull-Down Assay
HEK293T cells (1.5 × 106) stably expressing Frizzle4-EGFP or COS7 cells (5 × 105) were cultured in full Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum, seeded overnight in six well plates, and then transfected with GST-tagged Dvl2 constructs alone or together with HA-tagged β-arrestin2 with Lipofect-AMINE2000 (Invitrogen). Two days after transfection, the cells were lysed on ice with 150 µl of a solution containing 10% glycerol, 1% Triton X-100, 1 mM sodium orthovanadate, 50 mM Hepes (pH 7.3), and complete protease inhibitor (Roche Diagnostics), span at 14,000 rpm, 15 min, 4°C. The supernatants were incubated with 20 µl glutathione 4B sepharose beads for 2 hr at 4°C, and then washed three times with lysis solution. Proteins retained in the beads were analyzed by SDS-PAGE and western blotting. The data represent the results obtained from two independent experiments.
Internalization Assay
HEK293T cells (1.5 × 106) cultured in full DMEM were seeded overnight in 6-well plates, transfected with plasmids encoding HA-Frizzled4 and Myc-Dvl2 for 6 hr with LipofectAMINE2000 (Invitrogen), and then reseeded into 12-well plates containing glass cover slips for 16 hr. The cells were then washed with fresh DMEM without serum, and the coverslips transferred to a parafilm-covered glass surface inside a humidified Petri dish kept at 37°C. The cells were exposed to anti-HA antibody dissolved in 100 µl DMEM for 10 min at 37°C. Cells were then washed two to three times with fresh DMEM and incubated with control (no PMA) or Wnt5A-conditioned medium in the presence of 1 µM PMA for another 30 min (Chen et al., 2003). Alexa647-transferrin (10 µg/ml) dissolved in control or Wnt5A–conditioned medium was added during the last 5 min of this incubation period. The cells were fixed at room temperature, permeabilized, and incubated, first with anti-Myc antibody and then with appropriate secondary antibodies. Images (Figure 5) were acquired with an inverted microscope (200M, Zeiss Co.) attached to a spinning-disk confocal head (1.4 NA, 63× lens) under control of SlideBook 4.0 (Intelligent Imaging Innovations). Single focal planes are shown approximately centered in the middle of the cell. Representative images correspond to data obtained from two independent experiments and more than 300 cells.
Embryo Manipulations and Animal Cap-Explant Assay
The axis duplication assay was done using capped RNAs generated by in vitro transcription (mMessagemMachine; Ambion) injected at the four cell stage into both ventral blastomeres (Kato et al., 1999; Sokol et al., 1995). Effects on gastrulation were analyzed following RNA injection into both dorsal blastomeres of four cell-stage embryos (Sokol, 1996) scored at stage 35 (Nieuwkoop and Faber, 1994). Three independent experiments were performed with similar results. The animal cap-explant assays were performed using 10 ng/ml activin (Habas et al., 2001). The length:width ratio was determined for each explant. Statistical analysis of variance and p values were determined using Tukey’s methods. Two independent experiments were performed with similar results.
JNK Activity Assay
Activation of JNK was determined by monitoring c-Jun phosphorylation; expression level of ectopic Xenopus Myc-tagged c-Jun (Myc-c-Jun/pCS2+) was established by western blot analysis. Wild-type or YHEL mutants of Xdsh mRNA (5 ng) and Myc-tagged c-Jun mRNA (200 pg) were coinjected into two-cell stage embryos. At stage 11.5, 20 injected embryos were solubilized with 800 µl of 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 2 mM EDTA, 1% (w/v) Triton X-100, 10% (w/v) glycerol, 50mM NaF, 1mM Na3VO4, and protease inhibitor cocktail. The lysates were centrifuged (18,400 × g, 5 min) and supernatants incubated with anti-Myc antibody (clone 9E10; Santa Cruz Biotechnology) at 4°C for 1 hr, followed by incubation with GammaBind G Sepharose at 4°C for 1 hr. The beads were washed three times with the lysis solution and subjected to western blot analysis with anti-phospho-c-Jun (Ser63; Cell Signaling) and anti-c-Jun (Santa Cruz Biotechnology) antibodies. The expression levels of wild-type and mutant Xdsh, established using antibodies specific for Dvl2 (Semenov and Snyder, 1997) were largely indistinguishable from each other.
TOPFlash Luciferase Assay
HEK 293T cells growing in 12-well plates were cotransfected with plasmids encoding mDvl2 (0.4 µg), TOPFLASH-luciferase (0.4 µg), and Renilla luciferase pRL-CMV (4 ng) by the calcium phosphate method. After 48 hr, cell extracts were prepared and used in the Dual-luciferase reporter assay system (Promega).
Supplementary Material
yu
ACKNOWLEDGMENTS
We thank Drs. W. Boll and I. Rapoport for supplying us with clathrin adaptors, and the members of our laboratories for helpful discussions. We also thank Dr. M. Asashima for the Xenopus pCS2+ vector encoding c-Jun, and Dr. M. Semenov for the antibodies specific for Dvl2. The data presented in Figure 6 were generated by K. Tamai and Y. Harada. This work was supported by National Institutes of Health grants GM036548 (T.K) and GM074241 (X.H.). X.H. is a W.M. Keck Foundation Distinguished Young Scholar and a Lymphoma and Leukemia Society Scholar.
Footnotes
REFERENCES
- Ariizumi T, Sawamura K, Uchiyama H, Asashima M. Dose and time-dependent mesoderm induction and outgrowth formation by activin A in Xenopus laevis. Int. J. Dev. Biol. 1991;35:407–414. [PubMed] [Google Scholar]
- Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer JT, Nusse R. A critical role for endocytosis in Wnt signaling. BMC Cell Biol. 2006;7:28. doi: 10.1186/1471-2121-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm M, Bonifacino JS. Adaptins: the final recount. Mol. Biol. Cell. 2001;12:2907–2920. doi: 10.1091/mbc.12.10.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll W, Ohno H, Songyang Z, Rapoport I, Cantley LC, Bonifacino JS, Kirchhausen T. Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. EMBO J. 1996;15:5789–5795. [PMC free article] [PubMed] [Google Scholar]
- Boll W, Rapoport I, Brunner C, Modis Y, Prehn S, Kirchhausen T. The mu2 subunit of the clathrin adaptor AP-2 binds to FDNPVY and YppØ sorting signals at distinct sites. Traffic. 2002;3:590–600. doi: 10.1034/j.1600-0854.2002.30808.x. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS. The GGA proteins: adaptors on the move. Nat. Rev. Mol. Cell Biol. 2004;5:23–32. doi: 10.1038/nrm1279. [DOI] [PubMed] [Google Scholar]
- Boutros M, Mlodzik M. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech. Dev. 1999;83:27–37. doi: 10.1016/s0925-4773(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- Chen W, Hu LA, Semenov MV, Yanagawa S, Kikuchi A, Lefkowitz RJ, Miller WE. beta-Arrestin1 modulates lymphoid enhancer factor transcriptional activity through interaction with phos-phorylated dishevelled proteins. Proc. Natl. Acad. Sci. USA. 2001;98:14889–14894. doi: 10.1073/pnas.211572798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, Caron MG, Barak LS, Nusse R, Lefkowitz RJ. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301:1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- Collins BM, McCoy AJ, Kent HM, Evans PR, Owen DJ. Molecular architecture and functional model of the endocytic AP2 complex. Cell. 2002;109:523–535. doi: 10.1016/s0092-8674(02)00735-3. [DOI] [PubMed] [Google Scholar]
- Dittié AS, Hajibagheri N, Tooze SA. The AP-1 adaptor complex binds to immature secretory granules from PC12 cells, and is regulated by ADP-ribosylation factor. J. Cell Biol. 1996;132:523–536. doi: 10.1083/jcb.132.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois L, Lecourtois M, Alexandre C, Hirst E, Vincent JP. Regulated endocytic routing modulates wingless signaling in Drosophila embryos. Cell. 2001;105:613–624. doi: 10.1016/s0092-8674(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Eaton S, Wepf R, Simons K. Roles for Rac1 and Cdc42 in planar polarization and hair outgrowth in the wing of Drosophila. J. Cell Biol. 1996;135:1277–1289. doi: 10.1083/jcb.135.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeling MA, Mishra SK, Keyel PA, Steinhauser AL, Collins BM, Roth R, Heuser JE, Owen DJ, Traub LM. Molecular switches involving the AP-2 beta2 appendage regulate endocytic cargo selection and clathrin coat assembly. Dev. Cell. 2006;10:329–342. doi: 10.1016/j.devcel.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Fanto M, Weber U, Strutt DI, Mlodzik M. Nuclear signaling by Rac and Rho GTPases is required in the establishment of epithelial planar polarity in the Drosophila eye. Curr. Biol. 2000;10:979–988. doi: 10.1016/s0960-9822(00)00645-x. [DOI] [PubMed] [Google Scholar]
- Gallusser A, Kirchhausen T. The beta 1 and beta 2 subunits of the AP complexes are the clathrin coat assembly components. EMBO J. 1993;12:5237–5244. doi: 10.1002/j.1460-2075.1993.tb06219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C. Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Heldwein EE, Macia E, Wang J, Yin HL, Kirchhausen T, Harrison SC. Crystal structure of the clathrin adaptor protein 1 core. Proc. Natl. Acad. Sci. U.S.A. 2004;101:14108–14113. doi: 10.1073/pnas.0406102101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett O, Stow JL, Yap AS, Key B. Dynamin-dependent endocytosis is necessary for convergent-extension movements in Xenopus animal cap explants. Int. J. Dev. Biol. 2002;46:467–473. [PubMed] [Google Scholar]
- Kato Y, Shi Y, He X. Neuralization of the Xenopus embryo by inhibition of p300/CREB-binding protein function. J. Neurosci. 1999;19:9364–9373. doi: 10.1523/JNEUROSCI.19-21-09364.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly GM, Erezyilmaz DF, Moon RT. Induction of a secondary embryonic axis in zebrafish occurs following the overexpression of beta-catenin. Mech. Dev. 1995;53:261–273. doi: 10.1016/0925-4773(95)00442-4. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. Adaptors for clathrin mediated traffic. Annu. Rev. Cell Dev. Biol. 1999;15:705–732. doi: 10.1146/annurev.cellbio.15.1.705. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Laporte SA, Miller WE, Kim KM, Caron MG. beta-Arrestin/AP-2 interaction in G protein-coupled receptor internalization: identification of a beta-arrestin binging site in beta 2-adaptin. J. Biol. Chem. 2002;277:9247–9254. doi: 10.1074/jbc.M108490200. [DOI] [PubMed] [Google Scholar]
- Laporte SA, Oakley RH, Zhang J, Holt JA, Ferguson SSG, Caron MG, Barak LS. The beta(2)-adrenergic receptor/beta arrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc. Natl. Acad. Sci. USA. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larabell CA, Torres M, Rowning BA, Yost C, Miller JR, Wu M, Kimelman D, Moon RT. Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in beta-catenin that are modulated by the Wnt signaling pathway. J. Cell Biol. 1997;136:1123–1136. doi: 10.1083/jcb.136.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yuan H, Xie W, Mao J, Caruso AM, McMahon A, Sussman DJ, Wu D. Dishevelled proteins lead to two signaling pathways: regulation of LEF-1 and c-Jun N-terminal kinase in mammalian cells. J. Biol. Chem. 1999;274:129–134. doi: 10.1074/jbc.274.1.129. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- Menard L, Ferguson SS, Zhang J, Lin FT, Lefkowitz RJ, Caron MG, Barak LS. Synergistic regulation of beta2-adrenergic receptor sequestration: intracellular complement of beta-adrenergic receptor kinase and beta-arrestin determine kinetics of internalization. Mol. Pharmacol. 1997;51:800–808. [PubMed] [Google Scholar]
- Miller JR, Rowning BA, Larabell CA, Yang-Snyder JA, Bates RL, Moon RT. Establishment of the dorsal-ventral axis in Xenopus embryos coincides with the dorsal enrichment of dishevelled that is dependent on cortical rotation. J. Cell Biol. 1999;146:427–437. doi: 10.1083/jcb.146.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T, Kawachi K, Kamakura S, Masuyama N, Yamanaka H, Matsumoto K, Kikuchi A, Nishida E. Distinct domains of mouse dishevelled are responsible for the c-Jun N-terminal kinase/stress-activated protein kinase activation and the axis formation in vertebrates. J. Biol. Chem. 1999;274:30957–30962. doi: 10.1074/jbc.274.43.30957. [DOI] [PubMed] [Google Scholar]
- Nesterov A, Carter RE, Sorkina T, Gill GN, Sorkin A. Inhibition of the receptor-binding function of clathrin adaptor protein AP-2 by dominant-negative mutant μ2 subunit and its effects on endocytosis. EMBO J. 1999;18:2489–2499. doi: 10.1093/emboj/18.9.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus Laevis (Daudin) Third Edition. New York: Garland Publishing, Inc.; 1994. [Google Scholar]
- Ohno H, Aguilar RC, Yeh D, Taura D, Saito T, Bonifacino JS. The medium subunits of adaptor complexes recognize distinct but overlapping sets of tyrosine-based sorting signals. J. Biol. Chem. 1998;273:25915–25921. doi: 10.1074/jbc.273.40.25915. [DOI] [PubMed] [Google Scholar]
- Ohno H, Fournier M-C, Poy G, Bonifacino JS. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J. Biol. Chem. 1996;271:29009–29015. doi: 10.1074/jbc.271.46.29009. [DOI] [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Owen DJ, Collins BM, Evans PR. Adaptors for clathrin coats: structure and function. Annu. Rev. Cell Dev. Biol. 2004;20:153–191. doi: 10.1146/annurev.cellbio.20.010403.104543. [DOI] [PubMed] [Google Scholar]
- Owen DJ, Evans PR. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science. 1998;282:1327–1332. doi: 10.1126/science.282.5392.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DJ, Vallis Y, Noble MEM, Hunter JB, Dafforn TR, Evans PR, McMahon HT. A structural explanation for the binding of multiple ligands by the alpha-adaptin appendage domain. Cell. 1999;97:805–815. doi: 10.1016/s0092-8674(00)80791-6. [DOI] [PubMed] [Google Scholar]
- Owen DJ, Vallis Y, Pearse BMF, McMahon HT, Evans PR. The structure and function of the beta 2-adaptin appendage domain. EMBO J. 2000;19:4216–4227. doi: 10.1093/emboj/19.16.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Gray RS, Sato A, Habas R, Wallingford JB. Subcellular localization and signaling properties of dishevelled in developing vertebrate embryos. Curr. Biol. 2005;15:1039–1044. doi: 10.1016/j.cub.2005.04.062. [DOI] [PubMed] [Google Scholar]
- Perrimon N, Mahowald AP. Multiple functions of segment polarity genes in Drosophila. Dev. Biol. 1987;119:587–600. doi: 10.1016/0012-1606(87)90061-3. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S, Ricardo S, Manneville JB, Alexandre C, Vincent JP. Producing cells retain and recycle Wingless in Drosophila embryos. Curr. Biol. 2002;12:957–962. doi: 10.1016/s0960-9822(02)00867-9. [DOI] [PubMed] [Google Scholar]
- Piddini E, Marshall F, Dubois L, Hirst E, Vincent JP. Arrow (LRP6) and Frizzled2 cooperate to degrade Wingless in Drosophila imaginal discs. Development. 2005;132:5479–5489. doi: 10.1242/dev.02145. [DOI] [PubMed] [Google Scholar]
- Rapoport I, Chen YC, Cupers P, Shoelson SE, Kirchhausen T. Dileucine-based sorting signals bind to the beta chain of AP-1 at a site distinct and regulated differently from the tyrosine-based motif-binding site. EMBO J. 1998;17:2148–2155. doi: 10.1093/emboj/17.8.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboul J, Vaglio P, Rual JF, Lamesch P, Martinez M, Armstrong CM, Li S, Jacotot L, Bertin N, Janky R, et al. C. elegans ORFeome version 1.1: experimental verification of the genome annotation and resource for proteome-scale protein expression. Nat. Genet. 2003;34:35–41. doi: 10.1038/ng1140. [DOI] [PubMed] [Google Scholar]
- Rives AF, Rochlin KM, Wehrli M, Schwartz SL, DiNardo S. Endocytic trafficking of Wingless and its receptors, Arrow and DFrizzled-2, in the Drosophila wing. Dev. Biol. 2006;293:268–283. doi: 10.1016/j.ydbio.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Romond T, Merrifield C, Nichols BJ, Bienz M. The Wnt signalling effector Dishevelled forms dynamic protein assemblies rather than stable associations with cytoplasmic vesicles. J. Cell Sci. 2005;118:5269–5277. doi: 10.1242/jcs.02646. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Snyder M. Human dishevelled genes constitute a DHR-containing multigene family. Genomics. 1997;42:302–310. doi: 10.1006/geno.1997.4713. [DOI] [PubMed] [Google Scholar]
- Seto ES, Bellen HJ. Internalization is required for proper Wingless signaling in Drosophila melanogaster. J. Cell Biol. 2006;173:95–106. doi: 10.1083/jcb.200510123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol SY. Analysis of Dishevelled signalling pathways during Xenopus development. Curr. Biol. 1996;6:1456–1467. doi: 10.1016/s0960-9822(96)00750-6. [DOI] [PubMed] [Google Scholar]
- Sokol SY, Klingensmith J, Perrimon N, Itoh K. Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homolog of dishevelled. Development. 1995;121:1637–1647. doi: 10.1242/dev.121.6.1637. [DOI] [PubMed] [Google Scholar]
- Strigini M, Cohen SM. Wingless gradient formation in the Drosophila wing. Curr. Biol. 2000;10:293–300. doi: 10.1016/s0960-9822(00)00378-x. [DOI] [PubMed] [Google Scholar]
- Strutt DI, Weber U, Mlodzik M. The role of RhoA in tissue polarity and Frizzled signalling. Nature. 1997;387:292–295. doi: 10.1038/387292a0. [DOI] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon: functions and mechanisms of beta-catenin-independent Wnt signaling. Dev. Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Walhout AJ, Vidal M. High-throughput yeast two-hybrid assays for large-scale protein interaction mapping. Methods. 2001;24:297–306. doi: 10.1006/meth.2001.1190. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM. Xenopus Dishevelled signaling regulates both neural and mesodermal convergent extension: parallel forces elongating the body axis. Development. 2001;128:2581–2592. doi: 10.1242/dev.128.13.2581. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, Fraser SE, Chen P, Wallingford JB, Wynshaw-Boris A. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- Wong HC, Mao J, Nguyen JT, Srinivas S, Zhang W, Liu B, Li L, Wu D, Zheng J. Structural basis of the recognition of the dishevelled DEP domain in the Wnt signaling pathway. Nat. Struct. Biol. 2000;7:1178–1184. doi: 10.1038/82047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G. Direct and long-range action of a wingless morphogen gradient. Cell. 1996;87:833–844. doi: 10.1016/s0092-8674(00)81991-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
yu