Role of Estradiol in the Dynamic Control of Tanycyte Plasticity Mediated by Vascular Endothelial Cells in the Median Eminence (original) (raw)
Abstract
In the ever-changing physiological context of the neuroendocrine brain, the mechanisms by which cellular events involving neurons, astroglia, and vascular cells are coordinated to bring forth the appropriate neuronal signaling is not yet known but is amenable to examination. In the median eminence of the hypothalamus, endothelial cells are key players in the plasticity of tanycytes (specialized astroglia) and neuroendocrine synapse efficacy. Here we report that estradiol acts on both purified endothelial cells and isolated tanycytes to trigger endothelial-to-glial communication that leads to a sudden and massive retraction of tanycyte processes. The blockade of endothelial nitric oxide synthase by in vitro adenoviral-mediated gene transfer of a dominant-negative form of endothelial nitric oxide synthase abrogates the estradiol-induced tanycyte plasticity mediated by endothelial cells. In parallel, increases in prostaglandin-E2 (PGE2) due to changes in cyclooxygenase (COX)-1 and COX-2 expression induced by the exposure of tanycytes to estradiol promote acute tanycyte plasticity. We also demonstrate by electron microscopy that the administration of PGE2 to median eminence explants induces rapid neuroglial plasticity at the neurovascular junction of neurons that release GnRH (the neuropeptide controlling reproduction). Conversely, preventing local PGE2 synthesis in the median eminence of adult female rats with the COX inhibitor indomethacin impairs the ovarian cycle, a process that requires a pulsatile, coordinated delivery of GnRH into the hypothalamo-hypophyseal portal system. Taken together, our findings show that estradiol controls the dialog between endothelial cells and astroglia to regulate neuroglial plasticity in the neuroendocrine brain.
Estradiol controls the dialogue between endothelial cells and tanycytes to regulate neuroglial plasticity in the neuroendocrine brain, which is hypothesized to modulate GnRH secretion.
It is generally accepted that neurons, glia, and brain capillaries are organized into well-structured neuro-glio-vascular units, in which individual astroglial cells support the function of specific neuronal populations and territories and communicate with associated segments of the microvasculature (1,2). These microfunctional domains are likely to play an important role in maintaining a precisely regulated microenvironment for reliable neuronal signaling in an ever-changing physiological context. Gaining new insights into how cellular events that involve neurons, astroglia, and vascular cells are orchestrated is therefore fundamental to an improved understanding of brain function.
The median eminence of the hypothalamus, which constitutes the ventral border of the third ventricle, provides an excellent model in which to investigate the complex relationship between neurosecretion, function-related morphological plasticity involving neuronal-glial-endothelial interactions and the expression of key physiological functions. Over the past decade, it has been established that fluctuating physiological conditions during the ovarian cycle do indeed have the power to reversibly alter structural relationships among the various cell types of the median eminence that specifically interact with axon terminals containing GnRH (3,4), the neuropeptide that controls gonadotropin secretion and reproduction. Median eminence dynamics involve neurosecretory axons, tanycytes (specialized ependymoglial cells), and the basal lamina of the brain, the last of which secreted peptides must cross to enter the blood (5,6,7). During the ovarian cycle, under conditions of low gonadotropin output, GnRH-secreting axon terminals are completely surrounded or engulfed by tanycytes, which prevent direct access to the vascular wall and thus create a diffusion barrier impeding GnRH entry into the pituitary portal circulation (8). During the preovulatory surge, a structural remodeling of tanycytes occurs, resulting in the release of the engulfed axons and the establishment of direct neurovascular contacts between GnRH neurons and the endothelial Wall (8). Although the cell-cell signaling mechanisms underlying tanycyte plasticity have been investigated to some extent (9,10), it is not yet known how tanycytes differentially retract under the various physiological conditions mentioned above.
We have previously shown that vascular endothelial cells of the median eminence play a key role in modulating neuroglial remodeling via a signaling pathway mediated by nitric oxide (NO), thereby modifying neuroendocrine synapse efficacy at the GnRH neurovascular junction (9). NO, which travels readily across cellular membranes, mediates most of its effects by binding to the prosthetic heme group of the enzyme NO-sensitive guanylyl cyclase, resulting in increased production of cGMP (11,12,13). NO can also regulate the activity of cyclooxygenase (COX)-1 and -2, other heme-containing enzymes, and thus elicits prostaglandin release (14). Here we propose that local neuroglial plasticity in response to the ovarian cycle depends to a great extent on the activation of endothelial cells by locally delivered blood-borne estrogens. Our hypothesis is rooted in three observations: 1) cyclic fluctuations in estrogen levels have previously been shown to dramatically alter brain structure and function (15,16,17,18,19), 2) endothelial cells respond to a rise in the gonadal secretion of estrogens with an increase in NO efflux that stimulates GnRH release (20) and may thus constitute a key element of the process by which peripheral information is transferred from the microcirculation to the brain, and 3) NO-induced signaling in tanycytes triggers the release of bioactive compounds such as eicosanoids that mediate certain effects of estrogen on both brain plasticity and reproduction (9,21,22,23). The findings reported here point to a critical role for brain vascular endothelial cells in the dynamic control of brain communication with the periphery and raise the exciting possibility that endothelial cell-initiated signaling is capable of coordinating local neuroglial activity within specific functional microdomains.
Materials and Methods
Animals
Sprague Dawley rats were purchased from Janvier (Saint Berthevin, France). They were housed in a room with a controlled photoperiod (14 h of light and 10 h of darkness) and temperature (23–25 C). Ad libitum access to water and food pellets was provided. All experiments were carried out in accordance with the European Communities Council Directive of November 24, 1986 (86/609/EEC) regarding mammalian research and were approved by the Animal Use Committee of the University of Lille 2.
Reagents
17β-Estradiol, _N_ω-nitro-l-arginine methyl ester (L-NAME; a nitric oxide synthase inhibitor), l-arginine (the precursor of NO), sodium nitroprusside (SNP; a NO donor) were from Sigma (St. Louis, MO), and prostaglandin E2 (PGE2) and 8-bromo-cGMP (Br-cGMP; a cell permeable cGMP analog) were purchased from Calbiochem (Meudon, France). The in situ cell death detection terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) kit was from Roche (Mannheim, Germany).
Primary cell culture
Tanycytes and endothelial cells were isolated from the median eminence of the hypothalamus of 10-d-old rats, cultured, and characterized as described in our previous studies (9,10) and detailed in the Supplemental Methods published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org. Dissociated tanycytes and purified endothelial cells of the median eminence were cultured in DMEM/F12 (Invitrogen, Cergy Pontoise, France) supplemented with 10% (vol/vol) donor calf serum (Invitrogen) and in DMEM (Invitrogen) supplemented with 10% of fetal bovine serum (Invitrogen) and 1% l-glutamine (Invitrogen), respectively, under humid atmosphere of 5% CO2-95% air at 37 C.
Coculture of tanycytes with endothelial cells of the median eminence
Tanycyte cultures seeded onto 18-mm diameter coverslips coated with poly-l-lysine (Sigma) and purified endothelial cells placed in 12-well plates were grown until they reached 90% confluence. Tanycytes and endothelial cells were then placed in tanycyte/endothelial-defined medium (T/EDM; DMEM/F12 devoid of phenol red supplemented with 5 μg/ml insulin and 100 μm putrescine) for 48 h in the presence or absence of physiological levels of 17β-estradiol (5 nm) (21,24). After these 2 d of culture, the coverslips were placed above a confluent monolayer of vascular endothelial cells, with the tanycyte cell layer facing toward the endothelial cells and separated from them by glass chips. Cells were cultured for 30 min in fresh T/EDM containing estradiol or not, depending on the aforementioned pretreatment condition. The coverslips were then fixed in 4% paraformaldehyde. Tanycytes were stained with Alexa 588-X phalloidin (Molecular Probes, Eugene, OR) to visualize filamentous actin.
Determination of actin cytoskeleton remodeling
For morphological evaluation of the reorganization of the actin cytoskeleton in isolated tanycytes submitted to different treatments, cells were classified into three different phenotypic classes, defined as follows: class 1, cells with cortical actin, i.e. actin present around the edges of the cell, and that can be considered submembranous actin; class 2, cells bearing heavy stress fibers, parallel actin fibers present throughout the cytoplasm but no cortical actin; and class 3, retracted cells, i.e. processes-bearing cells with retracted and reduced cytoplasm. Alexa 588-X phalloidin-stained cultures were imaged using a fluorescent system (DMRB microscope, DC300FX camera, FW4000 software; Leica, Nussloch, Germany). Twelve fields per coverslip, chosen at random, were acquired at ×400 magnification. For each image, the total number of cells present in each field was counted. Then cells corresponding to each class were counted sequentially. With this method, each cell was counted only once and thus reported in only one group. The results obtained from sequential analysis of the same field were added and the percentage of cells belonging to each class calculated. At least four coverslips and an average of 479 ± 22 cells were analyzed per experimental condition. Each experiment was repeated at least twice using independent cultures. To avoid bias on the part of the observer, the quantification was repeated by an independent investigator blind to the experimental conditions.
Adenoviral gene transfer in endothelial cells of the median eminence
Purified vascular endothelial cells of the median eminence were infected with 1.5 × 109 plaque-forming units per milliliter of Ad-DN-eNOS, a replication-deficient recombinant adenoviral vector driving the expression of a truncated endothelial NO synthase (eNOS) under the constitutive control of the human cytomegalovirus promoter enhancer (25). DN-eNOS lacks catalytic activity yet retains the first 737 N-terminal amino acids required for myristoylation (26), i.e. for its anchorage to the plasma membrane (27). It acts as a dominant-negative (DN) inhibitor of wild-type eNOS activity by heterodimerization with the native protein to form nonfunctional dimers (28). To visualize the efficiency of infection in cultures, Ad-DN-eNOS was mixed with Ad-CMV-eGFP (2 × 109 plaque-forming units per milliliter), which expresses enhanced green fluorescent protein (eGFP). Endothelial cells were seeded onto 12-well plates, grown to 80% confluence, and infected for 1 h at 37 C with the adenoviral vectors in DMEM with 2% fetal bovine serum, 1% l-glutamine, and 1% penicillin/streptomycin. After infection, cells were cultured for 4 additional days in a culture medium containing 10% fetal bovine serum. Endothelial cells of the median eminence were then placed in T/EDM for 48 h and used for coculture experiments. Infected cells were visualized using a Leica DMIL inverted fluorescent microscope.
Immunoprecipitation and Western blotting
Protein extracts were prepared in lysis buffer, immunoprecipitated, size fractionated, and transferred to polyvinylidene difluoride membranes (Invitrogen, Carlsbad, CA), as described previously (15,29). The following antibodies were used: rabbit polyclonal anti-eNOS (C-ter) (sc-654; Santa Cruz Biotechnology, Santa Cruz, CA) and eNOS (600) (1:1000, no. 9572; Cell Signaling Technology, Beverly, MA) antibodies; rabbit polyclonal antibody to soluble guanylate cyclase (sGC; 1:1000, no. 371712; Calbiochem); polyclonal goat antibodies to COX-1 and COX-2 (1:500, sc-1752 and sc-1745, respectively; Santa Cruz); rabbit polyclonal antibody to estrogen receptor (ER)-α (1:500, no. 06-935; Upstate, Waltham, MA); and goat polyclonal antibody to estrogen receptor-β (1:100, sc-6822; Santa Cruz). Densitometric analyses of Western blot signals were performed using Image J analysis software (W.s. Rasband; National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov:ij/, 1997–2006).
Assessment of ultrastructural changes induced by PGE2 treatment in median eminence tissue
To determine whether PGE2 promotes morphological changes in the external zone of the median eminence, ex vivo experiments were performed using a protocol described previously (9) and detailed in the Supplemental Methods.
Intracerebral infusion of an inhibitor of COX activity
To determine the importance of functional COX activity within the median eminence for the central control of the reproductive cycle, in vivo experiments were performed. Indomethacin, an inhibitor of COX activity, was chronically infused into the median eminence using a protocol described previously (9) and detailed in the Supplemental Methods.
Statistics
Differences between several groups were analyzed by one-way ANOVA followed by the Student-Newman-Keuls multiple comparison test for unequal replications. The comparison of two groups was subjected to the unpaired t test. Data from animals within the same treatment group were subjected to one-way, repeated-measures ANOVA before being subjected to the Student-Newman-Keuls test. The threshold for significance was set at P < 0.05. To compare percentages, groups were subjected to arc-sine transformation before statistical analysis to convert them from a binomial to a normal distribution (30).
Results
Estradiol induces acute cell retraction in tanycytes cocultured with purified endothelial cells of the median eminence
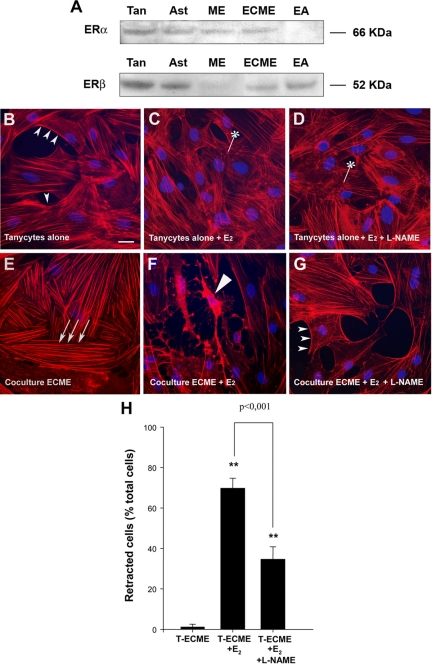
To explore the role of estradiol in modulating the signals conveyed by endothelial cells to tanycytes and determine what impact it has on the morphology of these ependymoglial cells, median eminence tissue from postnatal d 10 rats was used to generate primary cultures of tanycytes and to purify vascular endothelial cells by a sequential panning method. The putative expression of ER proteins in cultured glial and endothelial cells, as previously shown in the median eminence in vivo by others (31), was assessed by Western blots. Whereas ERα was the predominant form in protein extracts from the median eminence, both ERα and ERβ were present in cultured tanycytes and endothelial cells of the median eminence (ECMEs) (Fig. 1A). Tanycytes and ECMEs may thus be capable of sensing estrogens in vitro. Both cell types were exposed to physiological levels of estradiol (5 nm) for 48 h before coculture. The tanycytes were cultured as a monolayer on coverslips that were then placed on top of the ECME monolayer so that the astroglial cells faced the endothelial cells. After 30 min, the tanycytes were fixed and subjected to F-actin staining using Alexa 588-conjugated phalloidin. In control (nonestradiol treated) tanycytes cultured without ECMEs, 72 ± 8% had the features of class 1 tanycytes, i.e. cells with a substantial fraction of the actin filaments located adjacent to the cell membrane (cortical actin; Fig. 1B, small arrowheads). In contrast, in the presence of ECMEs, bundles of actin filaments formed heavy parallel stress fibers arranged along the entire longitudinal axis of the cell in 81 ± 9% of the tanycytes (class 2 tanycytes; Fig 1E). Strikingly, whereas 48 h estradiol treatment resulted in no major actin cytoskeleton remodeling in tanycytes cultured alone, instead increasing the proportion of cells harboring puncta of polymerized actin (Fig. 1C, asterisk, 28 ± 2% under estradiol treatment vs. 12 ± 4% in control conditions), it promoted the dramatic retraction of tanycytes exposed for 30 min to endothelial cells (class 3 tanycytes; Figs. 1, F, long arrowhead, and H).
Figure 1.
Ability of estradiol to influence endothelial cell-mediated plasticity in tanycytes. A, Western blot analysis of ERα and ERβ expression in primary cultures of tanycytes (Tan), cultured hypothalamic astrocytes (Ast), the median eminence (ME), immunopurified ECME, and the EAhy926 endothelial cell line (EA). Each well was loaded with 50 μg of protein. B–G, Tanycytes were cultured alone (B–D) or suspended above a conditioning layer of purified ECME (E–G) for 30 min and then stained with Alexa-conjugated phalloidin to visualize filamentous actin (red) and with Hoechst to stain nuclei (blue). Tanycytes alone (B–D) or both tanycytes and ECME (E–G) were cultured in the presence (C, D, F, and G) or absence (B and E) of 5 nm 17β-estradiol (E2) for 48 h (before coculture). Under basal conditions, when tanycytes were cultured alone (B), actin was localized adjacent to the cell membrane (cortical actin, arrowheads) and was also diffused throughout the cytoplasm. In contrast, tanycytes cultured with ECME for 30 min (E) exhibited bundles of actin filaments forming parallel stress fibers (arrows) running throughout the length of the cells but did not exhibit cortical actin. Note that although estradiol on its own had little effect on actin cytoskeleton remodeling in tanycytes (C), it promoted tanycyte retraction in coculture experiments (long arrowhead, F). Tanycyte retraction caused by endothelial cells in the presence of estradiol was prevented by pretreating endothelial cells with L-NAME (1 mm) (G), indicating that these plastic changes required endothelial NO secretion. *, Polymerized actin merged to puncta. Scale bar, 10 μm. H, Bar graph illustrating the percentage of tanycytes (T) that underwent retraction under various treatments. [ANOVA F(2,53) = 24.563, P < 0.001]. Error bars, sem. **, P < 0.001, as compared with untreated cocultures (T-ECME).
We have previously shown that endothelial cells of the median eminence promote an acute remodeling of the actin cytoskeleton in tanycytes via the release of the gaseous messenger NO (9). We thus tested the possibility that the retraction of estradiol-treated tanycytes induced by exposure to estradiol-treated ECMEs was caused by NO. The pretreatment of estradiol-treated endothelial cells with L-NAME (1 mm), an inhibitor of NO synthase activity before the introduction of tanycytes into the culture, significantly attenuated cell retraction in the latter (Fig. 1, G and H). These findings suggest that NO is necessary for endothelial cells to promote tanycyte plasticity in an environment containing nanomolar concentrations of estrogen.
Estradiol-induced acute cell retraction in cocultured tanycytes requires the functional integrity of eNOS signaling in endothelial cells
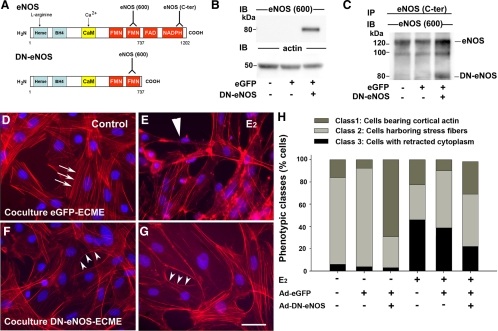
As in the median eminence in vivo (32), purified vascular endothelial cells in culture express eNOS (9), which we have previously shown to be a major source of NO production in the median eminence of the hypothalamus (8). To more specifically investigate the role of eNOS in estradiol-induced endothelial cell-mediated tanycyte plasticity, a DN eNOS protein (DN-eNOS) was expressed in ECMEs using replication-deficient adenoviral vectors (Ad-DN-eNOS) (25,33,34). This mutant eNOS contains the NH2-terminal sequence required for myristoylation, i.e. membrane anchoring, but lacks catalytic activity (Fig. 2A) (25). Cells were coinfected with an adenoviral vector driving endothelial GFP expression (Ad-eGFP). Western blot analyses showed that Ad-DN-eNOS-infected endothelial cells expressed an 80-kDa eNOS-immunoreactive species, but this truncated isoform of eNOS was not seen in cells infected with Ad-eGFP alone (Fig. 2B). To determine whether the truncated eNOS protein is capable of physically interacting with wild-type eNOS to form nonfunctional dimers in ECMEs (25), we next performed coimmunoprecipitation assays. Immunoprecipitation with antibodies raised against wild-type eNOS resulted in the coprecipitation of DN-eNOS in endothelial cells coinfected with Ad-DN-eNOS and Ad-eGFP (Fig. 2C) but not in uninfected cells or cells infected with Ad-eGFP alone (Fig. 2C).
Figure 2.
eNOS activity is required for endothelial cell to promote actin cytoskeleton remodeling and cell plasticity in tanycytes. A, Schematic representations of the wild-type isoform of eNOS naturally expressed in endothelial cells of the median eminence and the DN-eNOS, which is a truncated form lacking the C terminus and catalytic activity. Whereas eNOS (600) antibodies recognize both the wild-type and truncated isoforms, eNOS(C-ter) antibodies recognize only the wild-type isoform of eNOS. B, Western blot analyses show truncated eNOS expression in ECMEs infected with the adenoviral vector driving DN-eNOS-expression. DN-eNOS is expressed in neither control conditions nor ECMEs infected solely with an adenoviral vector driving the expression of eGFP. Actin was used as a loading control. C, DN-eNOS is physically associated with wild-type eNOS in cells infected with the adenoviral vector driving the expression of the truncated eNOS. Protein extracts were immunoprecipitated (IP) with eNOS (C-ter) antibodies, electrophoresed to size fractionate immunoprecipitated species, and immunoblotted (IB) with eNOS (600) antibodies. D–G, Effects of eGFP (D and E) and DN-eNOS (F and G) expression in ECMEs on the morphology of cocultured tanycytes in the presence (E and G) or absence (D and F) of estradiol treatment (E2, 5 nm, 48 h). Whereas eGFP expression in ECMEs had no significant effect on endothelial cell-induced stress fiber formation (arrows) (D) or cytoplasmic retraction (long arrowheads) in tanycytes (E), DN-eNOS expression prevented endothelial cells from promoting such plastic changes in tanycytes cultured in the absence (F) or presence (G) of estradiol, respectively. Tanycytes cocultured with DN-eNOS-expressing ECMEs displayed cortical actin (arrowheads; F and G) as in the control conditions illustrated in Fig. 1B. Scale bar, 10 μm. H, Quantitative analysis of the plastic changes elicited by estradiol (E2) in tanycytes cocultured with ECMEs that do or do not express eGFP (Ad-eGFP) and DN-eNOS (Ad-DN-eNOS). Changes are measured by calculating the percentage of cells belonging to each phenotypic class.
To determine whether functional eNOS is required for endothelial cells to promote plastic changes in the actin cytoskeleton and/or the shape of tanycytes, we cocultured these ependymoglial cells with ECMEs that were or were not infected with viral constructs, in the presence or absence of estradiol (5 nm, 48 h) (Fig. 2, D–G). Most tanycytes cocultured for 30 min with ECMEs treated with Ad-eGFP alone (Fig. 2D) or left uninfected (not shown) had the features of class 2 tanycytes [Fig. 2H (ANOVA, FClass 2[5, 17] = 8.826, P < 0.001), tanycytes cocultured with Ad-eGFP infected vs. uninfected endothelial cells P = 0.376]. In contrast, when tanycytes were cocultured with Ad-DN-eNOS-infected ECME (Fig. 2F), there was a significant increase of class 1 cells, i.e. cells showing mainly cortical actin [(ANOVA, FClass 1[5, 17] = 13.568, P < 0.001, P < 0.001) from cocultures with uninfected ECME] and a concomitant reduction in the number of class 2 cells (P = 0.004; Fig. 2I). Similarly, the expression of truncated eNOS in endothelial cells prevented the latter cells from triggering retraction, i.e. the formation of class 3 cells, in estrogen-treated tanycytes [Fig. 2, G and H (ANOVA, FClass 3[5, 17] = 25.275, P <0.001), P < 0.001 from estradiol-treated cocultures with uninfected ECME], whereas coculture with ECME infected with Ad-eGFP did not (Fig 2, E and H; P = 0.057). Taken together, these findings suggest that eNOS activity is required by endothelial cells both for the promotion of rapid cytoskeletal remodeling in tanycytes under control conditions and for acute cell retraction in the latter under the influence of estradiol.
Triggering endothelial NO secretion causes tanycyte retraction
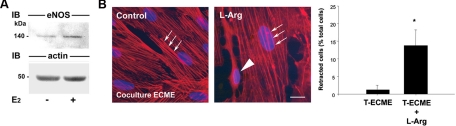
In previous studies, we have shown that estradiol treatment causes a dramatic increase in eNOS expression (35) and activity (20,36) within the median eminence of the hypothalamus. To determine whether estradiol exerts a similar stimulatory effect on eNOS expression in vitro, cultured ECMEs were treated with estradiol (5 nm, 48 h) and subjected to Western blot analysis. Estradiol increased the expression of eNOS in ECMEs by 50% (eNOS to actin signal ratio 0.681 ± 0.077 in controls vs. 1.240 ± 0.104 in estradiol treated ECMEs: t test, P = 0.005, n = 4; Fig. 3A). These findings, together with our data demonstrating that eNOS activity plays a key role in endothelial-to-ependymoglial communication processes (Fig. 2), suggest that endothelial cell-induced tanycyte retraction in the presence of estradiol may result from an increased production of NO by endothelial cells. To further substantiate this hypothesis, we triggered NO biosynthesis in nonestradiol-treated cocultures using l-arginine (500 μm), the precursor of NO and a well-established activator of eNOS activity in cultured endothelial cells (37). Whereas 500 μm l-arginine had no effect on actin cytoskeleton remodeling when it was applied to simple tanycyte cultures (data not shown), quantitative analysis revealed that 30 min of l-arginine treatment induced the occurrence of tanycytes with retracted cytoplasm and long, slender processes (Fig. 3B, arrowhead) in addition to cells containing thick stress fibers (Fig. 3B, arrows) when tanycytes were cocultured with ECMEs. However, because the incidence of cell retraction promoted by l-arginine in cocultures (∼15%, Fig. 3B) was much lower than that seen after estradiol application (∼50–70%, Figs. 1H and 2), an increase in eNOS expression and/or activity in ECMEs is unlikely to fully account for the endothelial cell-mediated tanycyte retraction induced by the effect of estradiol on both cell types.
Figure 3.
Increases in endothelial NO production causes cell retraction in tanycytes. A, Representative blot showing the effect of 5 nm estradiol treatment for 48 h on eNOS expression in ECMEs. Actin was used as a loading control. B, Left panel, triggering NO production in cocultures by the addition of its precursor, l-arginine (L-Arg; 500 μm), causes a significant subset of tanycytes to retract (long arrowhead). Scale bar, 10 μm. Right panel, Bar graph illustrating the percentage of tanycytes (T) that underwent retraction in cocultures with ECMEs, with or without treatment with l-arginine. Arrows, Actin stress fibers. *, P < 0.05, t test, n = 6 wells each. Error bars, sem.
Estradiol sensitizes tanycytes to NO
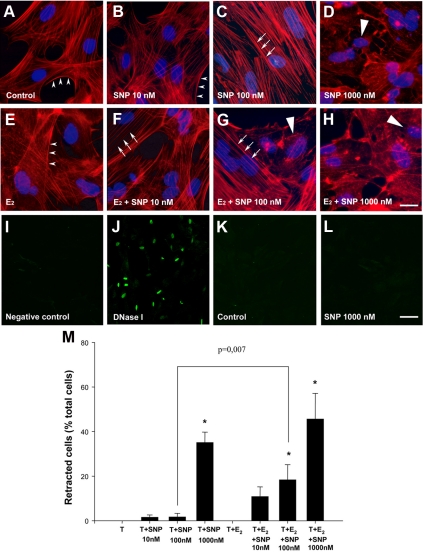
The application of the NO donor SNP (100 nm) to primary cultures of tanycytes for 30 min precisely mimicked the effects of coculture with ECME (Figs. 1B and 2E), causing a significant increase in the proportion of tanycytes exhibiting heavy stress fibers (class 2 phenotype) compared with controls or to tanycytes exposed to 10 nm SNP (Fig. 4, A–C). Increasing the concentration of SNP to 1000 nm caused cytoplasm retraction in tanycytes (Fig. 4, D and M). Control experiments using TUNEL assays in tanycytes cultured for an additional 24 h in tanycyte-defined medium after being exposed to SNP for 30 min showed that 1000 nm SNP do not promote cell death in tanycytes (Fig. 4, I–L). Strikingly, in the presence of 5 nm estradiol (48 h), SNP was able to promote stress fiber formation in tanycytes at a lower, previously ineffective dose of SNP (10 nm; Fig. 4F) and cell retraction at 100 nm (Fig. 4, G and M). These results strongly suggest that estrogens sensitize tanycytes to NO.
Figure 4.
Effect of estradiol on tanycyte sensitivity to NO. Estradiol-treated (E–H) and -untreated (A–D) tanycytes were exposed for 30 min to various concentrations of SNP, an NO donor. In the absence of estrogen, 10 nm SNP was insufficient to the disappearance of cortical actin (arrowheads) or other plastic changes (B), 100 nm SNP induced the disappearance of cortical actin and the formation of actin stress fibers (arrows), and 1000 nm SNP caused cell retraction (long arrowhead; D). In contrast, in tanycytes treated with 5 nm estradiol for 48 h, 10 nm (F), and 100 nm (G) of SNP were sufficient to cause significant actin cytoskeleton remodeling and cell retraction, respectively. I–L, Representative images of a TUNEL assay showing that 1000 nm SNP treatment for 30 min did not cause programmed cell death in tanycytes cultured for an additional day in tanycyte-defined medium (L) when compared with controls (K). Scale bars, 10 μm (A–H), 40 μm (I–L). M, Bar graph illustrating the percentage of tanycytes (T) that underwent retraction in the various treatment groups. [ANOVA F(7,111) = 15.248, P < 0.001]. Error bars, sem. *, P < 0.05, as compared with untreated tanycytes.
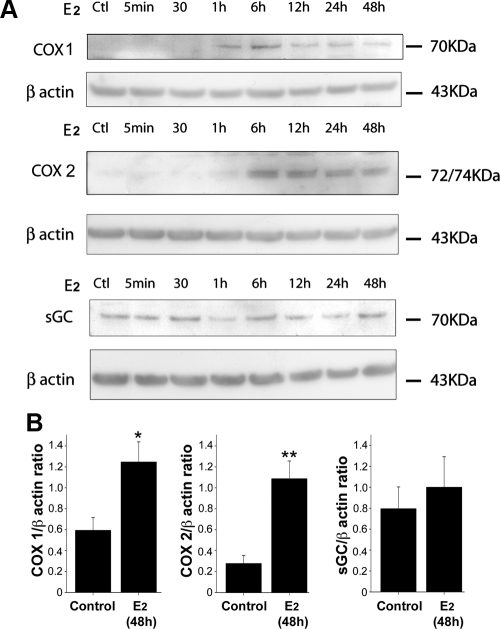
Estradiol increases the expression of both COX-1 and COX-2 but not sGC in tanycytes
Because the NO-induced actin cytoskeleton remodeling in tanycytes requires both sGC and COX activities (9), we performed experiments to determine whether changes in sGC and/or COX expression parallel the estradiol-induced sensitization of tanycytes to NO. Median eminence tanycyte cultures were subjected to 5 nm estradiol treatments for different periods of time, the cells collected, and protein levels analyzed by Western blotting. Figure 5A shows that whereas COX-1 and COX-2 are barely detectable under control conditions, estradiol induces a dramatic increase in the expression of both enzymes after treatment for 6 h. In contrast, estradiol did not promote any consistent change in sGC expression in tanycytes (Fig. 5A). These data thus raise the intriguing possibility that estrogen, by increasing COX protein synthesis although leaving sGC expression unchanged (Fig. 5B), displaces signaling pathways downstream of NO toward an increase in COX byproducts, i.e. increases the ratio of concentration of eicosanoids to cGMP.
Figure 5.
The effect of estradiol on tanycytes depends mainly on a COX product. A, Estradiol (5 nm) increased the expression of both COX 2 and 1 in tanycytes but left sGC expression unchanged. β-Actin was used as a loading control. B, Bar graph showing the quantitative analysis of COX 1, COX 2, and sGC protein expression levels in tanycytes treated or not with estradiol during 48 h. Levels are expressed as the ratio between the densitometric signal of the protein of interest and that obtained with constitutively expressed actin in each sample. Error bars, sem. **, P < 0.01; *, P < 0.05, t test, n = 4.
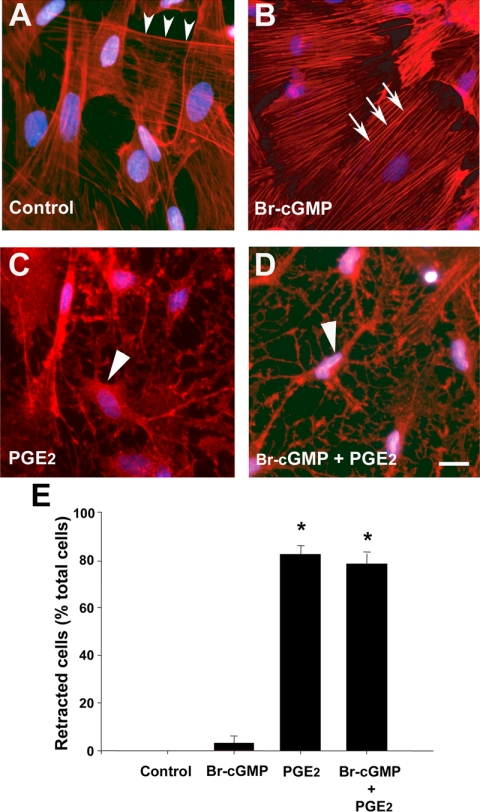
A COX product causes tanycyte retraction in vitro
We next asked whether sGC and/or COX products, i.e. cGMP and/or prostaglandins-thromboxanes, respectively, are capable of promoting tanycyte plasticity on their own (Fig. 6). Among the prostanoids synthesized by COX, PGE2 is one of the most biologically active, and its biosynthesis is specifically up-regulated by estrogen in the neuroendocrine hypothalamus (38). Because, in addition, tanycytes have previously been shown to produce PGE2 at nanomolar concentrations (10), we studied the effects of PGE2 in subsequent experiments. The application of PGE2 (280 nm) for 30 min closely mimicked the effects of NO in estradiol-treated cocultures, causing a significant increase in cell retraction in cultured tanycytes (Fig. 6, C and E). In contrast, the cell-membrane crossing cGMP analog, Br-cGMP (280 nm), promoted actin stress-fiber formation in tanycytes but had no effect on cell retraction (Fig. 6B). Similar effects were recorded when PGE2 was used at 1 μm (data not shown), at which concentration it is known to stimulate GnRH release in median eminence explants (39,40).
Figure 6.
PGE2, but not cGMP, causes cell retraction in tanycytes. A–D, Whereas a cell-permeable sGC product (Br-cGMP, 280 nm, 30 min) caused stress fiber formation in tanycytes (B), the addition of PGE2 (280 nm, 30 min), one of the most biologically active of COX products, caused acute tanycyte retraction (C). E, Bar graph illustrating the percentage of tanycytes that underwent retraction under the various treatments. ANOVA F(3,59) = 104.352, P < 0.001. Error bars, sem. *, P < 0.05, as compared with control.
PGE2 induces neuroglial plasticity in the median eminence in situ
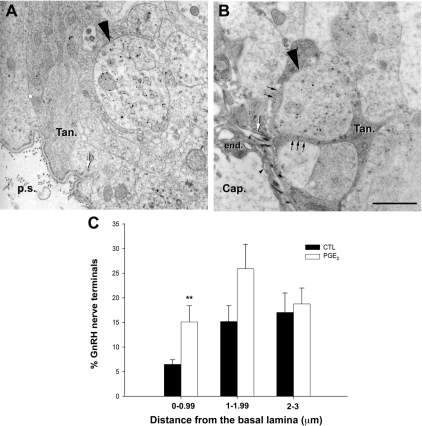
In view of the potent acute effect of PGE2 on tanycyte plasticity (Fig. 6), together with our previous in vitro study showing that PGE2 is one of the key downstream effectors involved in growth factor-mediated tanycyte plasticity (10), we next aimed to determine whether PGE2 promotes structural changes in tanycytes in situ and whether these putative changes have an impact on the anatomical relationship between GnRH axon terminals and tanycyte cell processes in the external zone of the median eminence. Hypothalamic explants containing the median eminence were subjected to 1 μm PGE2 treatment for 30 min, fixed, and processed for electron microscopy. Experiments were carried out on explants from rats killed on the day of diestrus, a stage of the estrous cycle at which GnRH nerve terminals remain separated from the pituitary portal vasculature by tanycyte endfeet (3,41). Using 15 nm gold particle labeling, we saw a striking transformation in GnRH nerve terminals as a function of the presence or absence of PGE2. Whereas neither the amount of gold particles, reflecting their load of neuropeptide, nor the size of the GnRH nerve endings varied among conditions (data not shown), the distance between GnRH nerve terminals and the basal lamina of the parenchyma of the brain (Fig 7, white arrow) appeared to shorten significantly in PGE2-treated explants (Fig. 7B) when compared with control explants (Fig. 7A). Quantitative analysis showed that whereas the total number of GnRH nerve terminals confined to a distance of 5 μm or less from the parenchymatous basal lamina, which delineates the pericapillary space did not vary significantly (182 ± 47 GnRH nerve terminals in control explants vs. 269 ± 78 GnRH nerve terminals in PGE2 treated explants; n = 4 hypothalamic explants; t test, P = 0.398), their distribution changed markedly (Fig. 7C). The fraction of GnRH nerve terminals located at less than 1 μm from the pericapillary space (Fig. 7) significantly increased in median eminence explants exposed to 1 μm PGE2 treatment for 30 min when compared with controls (Fig. 7C; n = 4 hypothalamic explants; t test, P = 0.004). Intriguingly, even though many GnRH nerve terminals were found to be in close proximity to the parenchymatous basal lamina in PGE2-treated explants, no direct contact between GnRH axons and the vascular wall was seen in either of the groups. However, in the PGE2-treated group, tanycyte processes engulfing GnRH nerve terminals were much less conspicuous in areas in which GnRH axon terminals were separated from the pericapillary space by only a few nanometers (Fig. 7B, small arrows), suggesting that tanycyte endfeet had undergone retraction precisely at these locations.
Figure 7.
The COX product PGE2 promotes neuroglial plasticity in hypothalamic explants containing the median eminence, causing the advancement of GnRH neurosecretory terminals toward the pericapillary space. A and B, Representative electron micrographs of GnRH immunoreactive axon terminals (15 nm gold particles, long black arrowhead) from female rat median eminence explants incubated for 30 min in the presence (B) or absence (A) of PGE2 (1 μm). A, Under basal unstimulated conditions, GnRH nerve endings (long black arrowhead) were maintained at a distance from the brain basal lamina (white arrow) delineating the pericapillary space (p.s.) by thick enclosing tanycyte endfeet (Tan.). B, PGE2 treatment caused the advancement of GnRH axon terminals (long arrowhead) toward the brain basal lamina (white arrow) and the apparent retraction of most of the astroglial sheath (black arrows) from those neurosecretory terminals that were separated from the fenestrated (small arrowhead) portal capillaries (cap.) by only a few nanometers. end., Endothelium. Scale bar, 1 μm. C, Quantitative analysis of the spatial distribution of GnRH nerve terminals in the external zone of the median eminence in explants treated with PGE2 and in controls (CTL). **, P = 0.004 (PGE2 vs. CTL), t test, n = 4 hypothalamic explants each. Error bars, sem.
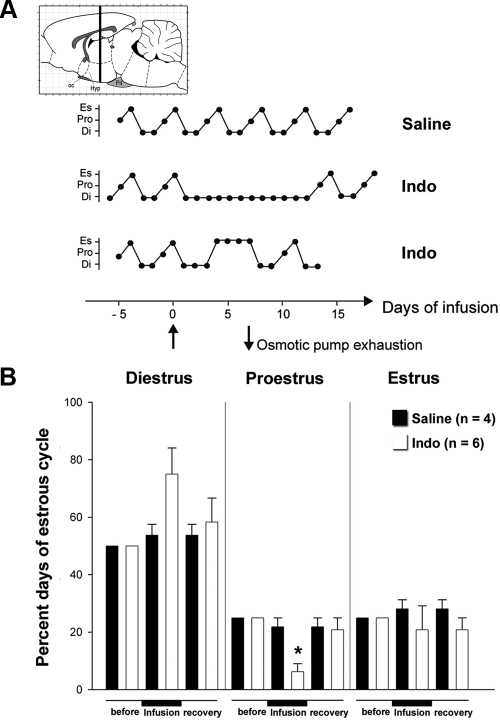
Infusion of a COX inhibitor into the median eminence disrupts the female reproductive cycle
It has been more than 30 yr since it was shown that the acute sc or intracranial injection of indomethacin, a well-known inhibitor of COX activity, alters the onset of the steroid-induced LH surge in ovariectomized female rats (42). Here, to determine whether indomethacin alters the estrous cycle in intact adult virgin female rats, stainless steel cannulas were stereotaxically implanted into the median eminence and connected to a sc placed osmotic pump. The pumps were set to deliver 100 ng/h of indomethacin or saline containing 1% ethanol at a rate of 0.5 μl/h for up to 7 d. Indomethacin markedly altered the estrous cycle after a few days of infusion (Fig. 8). Indomethacin-infused rats were unable to enter the proestrus phase of the cycle, in contrast to the saline group (Fig. 8B). After osmotic pump exhaustion, indomethacin-infused rats recovered their normal estrous cycle (Fig. 8B). Although these results indicate that COX activity, and thus prostaglandin-initiated signaling, is important for the correct progression of the phases of the estrous cycle, they also demonstrate that the central regulatory component that controls adult female reproductive function can fully, and expeditiously, recover from the inhibitory effect of indomethacin on termination of the treatment.
Figure 8.
Blockade of COX activity in the median eminence in vivo impairs adult reproductive function. A, Representative estrous cycle profiles showing disruption of ovarian cyclicity in young adult rats by the infusion of indomethacin (Indo) into the median eminence of the hypothalamus. The COX inhibitor indomethacin (560 μm) or saline containing 1% ethanol was delivered via a stereotaxically implanted stainless steel cannula connected to a sc placed osmotic pump with a delivery rate of 0.5 μl/h for 7 d. Infusion started at d 0 (upward arrow) and ended 7 d later (downward arrow) when pump content was exhausted. Animals were allowed to survive for an additional week (recovery) after pump exhaustion. Di, Diestrus; Pro, proestrus; Es, estrus; oc, optic chiasm; Hyp, hypothalamus, Pit, pituitary. B, Quantitative analysis of the alterations in estrous cyclicity caused by indomethacin infusion into the median eminence. *, P < 0.05 (comparison between before and after recovery time points for the same estrous stage and the same treatment), repeated-measures ANOVA. Error bars, sem.
Discussion
In this study, we show that brain vascular endothelial cells are centrally involved in the dynamic control exerted by estradiol on neuroglial plasticity in the neuroendocrine brain.
The first clue as to the involvement of endothelial cells in the control of tanycyte morphology was the observation that isolated tanycytes cocultured with purified endothelial cells of the median eminence promote acute actin cytoskeleton remodeling in these ependymoglial cells and that NO mediates these cytoarchitectonic changes (9). NO, which is a volume transmitter capable of coordinating neuronal activity in a restricted brain volume delimited by its half-life and diffusion constant (43,44,45,46), may thus represent an endothelial-dependent signaling system that allows endothelial cells to work as bridges between blood-borne molecules and specific functional brain microdomains in which endothelial and glial cells interact to regulate neuronal activity. This conclusion is now supported by the observation that estradiol enhances endothelial-to-glial communication by causing endothelial cells to promote the retraction of tanycyte processes. The transduction of endothelial cells with adenoviral vectors expressing a DN form of eNOS that inhibits endogenous eNOS activity via the formation of nonfunctional dimers (25), results in the impairment of both estradiol-dependent and estradiol-independent endothelial cell-induced morphological changes in tanycytes, thereby demonstrating that eNOS signaling in endothelial cells is key to this communication process. It also indicates that the alteration of a single endothelial signaling pathway function hampers the ability of estradiol to promote tanycyte plasticity. In accordance with previous findings in vivo (35), Western blot analysis demonstrates that estradiol stimulates the expression of eNOS in endothelial cells of the median eminence, suggesting that endothelial cell-induced tanycyte retraction in the presence of estrogen may result, at least in part, from an increased production of NO by the former. Previous studies showing that estrogen has both acute (36) and long-term (20) stimulatory effects on endothelial NO release from the median eminence further supports this interpretation. Thus, the primary action of estradiol on tanycytes to enable them to respond to basal levels of NO signaling from endothelial cells appears unlikely.
Increased NO production by endothelial cells elicited by the NO precursor l-arginine triggers some tanycyte retraction, even in the absence of estrogen treatment. This is in line with our previous findings showing that in median eminence explants incubated with l-arginine, the tanycyte processes surrounding GnRH nerve terminals undergo retraction, enabling GnRH neuroendocrine terminals to establish direct neurovascular junctions (9).
Several lines of evidence support the conclusion that the acute estradiol-induced tanycyte retraction mediated by NO depends mainly on the action of COX products: 1) inhibition of COX activity abrogates NO-mediated actin cytoskeleton remodeling in tanycytes (9), 2) isolated tanycytes secrete PGE2 both under control and stimulated conditions (10), 3) estradiol up-regulates COX-1 and COX-2 expression in tanycytes and leaves unchanged the expression of sGC, the other known target for NO in this cell type (9), 4) PGE2 mimics, in simple tanycyte cultures, the estrogen-induced acute cellular retraction of tanycytes seen in cocultures with endothelial cells. These results provide evidence for a major role for a COX product such as PGE2 in the estradiol-induced tanycyte retraction mediated by endothelial cells, although additional factors released by tanycytes and/or endothelial cells may also contribute to it.
Direct evidence for the ability of PGE2 to control neuroglial plasticity at the neuroendocrine synapse was obtained from the experiments in which we directly applied PGE2 to median eminence explants at concentrations known to stimulate GnRH release and observed the occurrence of structural remodeling in a matter of minutes. PGE2 treatment causes the advancement of GnRH neurosecretory terminals toward the pericapillary space, a phenomenon that probably results from the retraction of tanycyte endfeet. By analogy with the function-related plasticity documented in the neural lobe of the pituitary (47), it is tantalizing to suggest based on these results that PGE2-promoted plastic changes could increase the efficacy of the GnRH neuroendocrine synapse, i.e. that they could facilitate GnRH release by removing part of the diffusion barrier formed by the tanycyte processes that engulf GnRH nerve terminals. Intriguingly, PGE2 fails to promote direct neurovascular contacts between GnRH axons and the vascular wall. This is in contrast to the effects of l-arginine treatment mentioned earlier, and suggest that additional downstream signaling pathways, such as those involving cGMP, are required for NO to fully exert its effects on neuroglial plasticity. The recent demonstration of an involvement of NO-cGMP signaling in axonal elongation and/or growth cone orientation (9,48,49,50) supports this interpretation.
Results obtained in adult rats in vivo corroborate the role of PGE2 in the mechanism that controls neuroglial plasticity at the neuroendocrine synapse. After the intracerebral infusion of indomethacin, the same COX antagonist that blocks NO-mediated actin cytoskeleton remodeling in primary cultures (9), into the median eminence, there was a marked impairment of the ovarian cycle, which requires the coordinated delivery of GnRH into the hypothalamo-hypophyseal portal system. Local inhibition of PG synthesis arrests the ovarian cycle in either the diestrus or the estrus phase when GnRH release is low (51) and GnRH neuroendocrine terminals are enclosed by tanycyte endfeet (3,41), which form a diffusion barrier to GnRH entering the portal vasculature. These results highlighting the physiological importance of eicosanoids in the cell-cell communication processes regulating GnRH release are in full agreement with data obtained more than 25 yr ago, demonstrating that PGE2 synthesis increases within the hypothalamic region containing the median eminence during the onset of the first preovulatory surge at puberty (38) and that inhibitors of PG synthesis alter the onset of the steroid-induced LH surge in ovariectomized female rats (42).
COX products and PGE2 in particular have been shown to be key bioactive substances involved in the communication processes between glia and GnRH neurons set in motion by the paracrine activation of astroglial erbB receptors, which have important physiological implications both at the onset of puberty (40,52,53) and during adult reproductive function (54). Recent evidence suggests that TGFα-erbB1 signaling in tanycytes may also account for part of the plastic remodeling that takes place in the median eminence during the ovarian cycle and that PGE2 plays a pivotal role in this process as well (10). However, the kinetics of the response of tanycytes to the activation of the TGFα-erbB1 signaling pathway is different from that mediated by endothelial NO. Indeed, NO-mediated tanycyte retraction under the influence of estradiol occurs 30 min after they are brought into contact with endothelial cells, whereas the tanycyte retraction elicited by TGFα occurs more than 24 h after the onset of the treatment (10). Taken together, these data suggest that tanycyte plasticity could be under the control of two complementary mechanisms, one initiated by endothelial cells and the other by astroglial cells.
There is evidence to suggest that agents released during normal neural activity potentially influence both astrocytes and the endothelium (55,56), raising the interesting possibility that signaling linking brain endothelial cells and glia could occur physiologically (1,2,57,58). Here we show that there could be a physiological advantage in recruiting endothelial signaling to coordinate the activity of neuroglia at the neuroendocrine synapse to trigger functionally meaningful episodes of GnRH secretion every 4 d during the ovarian cycle. Endothelial cells are in position to sense peripheral information such as estradiol and provide a feedback mechanism that, through the release of NO, profoundly affects the communication between glial cells and neurons and the formation of specialized neuronal junctions. The machinery needed to coordinate the activity of neurons, astroglia, and endothelial cells in specific neurovascular microdomains of the central nervous system is thus available, and its deregulation may become particularly significant under pathological conditions. Based on current data, it is thus evident that vascular endothelial cells must be considered an integral part of the cellular mechanisms that regulate brain function in specific functional brain microdomains, and their role needs to be integrated into current models of brain communication with the periphery.
Supplementary Material
[Supplemental Data]
Footnotes
This work was supported by Institut National de la Santé et de la Recherche Médicale (France) Grants U816 and U837, the Fondation pour le Recherche Médicale (Equipe FRM), l’Agence Nationale de la Recherche, the Université de Lille 2, the imaging Core of IFR114 (to V.P.), National Institutes of Health Grants HD25123 and U54 HD18185 through cooperative agreement as part of the Specialized Cooperative Center’s Program in Reproduction and Infertility Research, and Grant RR00163 for the operation of the Oregon National Primate Research Center (to S.R.O.). S.d.S. and X.d.A.d.T. were PhD students supported by a fellowship from the French Ministère délégué à la Recherche et aux Nouvelles Technologies and Institut National de la Santé et de la Recherche Médicale/Nord Pas de Calais region, respectively.
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 4, 2010
Abbreviations: Br-cGMP, 8-Bromo-cGMP; COX, cyclooxygenase; DN, dominant negative; ECME, endothelial cells of the median eminence; eGFP, enhanced green fluorescent protein; eNOS, endothelial NO synthase; ER, estrogen receptor; L-NAME, _N_ω-nitro-l-arginine methyl ester; NO, nitric oxide; PGE2, prostaglandin E2; sGC, soluble guanylate cyclase; SNP, sodium nitroprusside; T/EDM, tanycyte/endothelial-defined medium; TUNEL, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling.
References
- Haydon PG, Carmignoto G 2006 Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 86:1009–1031 [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M 2007 Glial regulation of the cerebral microvasculature. Nat Neurosci 10:1369–1376 [DOI] [PubMed] [Google Scholar]
- Prevot V, Croix D, Bouret S, Dutoit S, Tramu G, Stefano GB, Beauvillain JC 1999 Definitive evidence for the existence of morphological plasticity in the external zone of the median eminence during the rat estrous cycle: implication of neuro-glio-endothelial interactions in gonadotropin-releasing hormone release. Neuroscience 94:809–819 [DOI] [PubMed] [Google Scholar]
- Yamamura T, Hirunagi K, Ebihara S, Yoshimura T 2004 Seasonal morphological changes in the neuro-glial interaction between gonadotropin-releasing hormone nerve terminals and glial endfeet in Japanese quail. Endocrinology 145:4264–4267 [DOI] [PubMed] [Google Scholar]
- Kozlowski GP, Coates PW 1985 Ependymoneuronal specializations between LHRH fibers and cells of the cerebroventricular system. Cell Tissue Res 242:301–311 [DOI] [PubMed] [Google Scholar]
- Meister B, Hökfelt T, Tsuruo Y, Hemmings H, Ouimet C, Greengard P, Goldstein M 1988 DARPP-32, a dopamine- and cyclic AMP-regulated phosphoprotein in tanycytes of the mediobasal hypothalamus: distribution and relation to dopamine and luteinizing hormone-releasing hormone neurons and other glial elements. Neuroscience 27:607–622 [DOI] [PubMed] [Google Scholar]
- King JC, Rubin BS 1994 Dynamic changes in LHRH neurovascular terminals with various endocrine conditions in adults. Horm Behav 28:349–356 [DOI] [PubMed] [Google Scholar]
- Prevot V, Dehouck B, Poulain P, Beauvillain JC, Buée-Scherrer V, Bouret S 2007 Neuronal-glial-endothelial interactions and cell plasticity in the postnatal hypothalamus: implications for the neuroendocrine control of reproduction. Psychoneuroendocrinology 32:S46–S51 [DOI] [PubMed] [Google Scholar]
- De Seranno S, Estrella C, Loyens A, Cornea A, Ojeda SR, Beauvillain JC, Prevot V 2004 Vascular endothelial cells promote acute plasticity in ependymoglial cells of the neuroendocrine brain. J Neurosci 24:10353–10363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevot V, Cornea A, Mungenast A, Smiley G, Ojeda SR 2003 Activation of erbB-1 signaling in tanycytes of the median eminence stimulates transforming growth factor β1 release via prostaglandin E2 production and induces cell plasticity. J Neurosci 23:10622–10632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS 2003 Nitric oxide signaling specificity—the heart of the problem. J Cell Sci 116:9–15 [DOI] [PubMed] [Google Scholar]
- Garthwaite J 2008 Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci 27:2783–2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koesling D, Friebe A 1999 Soluble guanylyl cyclase: structure and regulation. Rev Physiol Biochem Pharmacol 135:41–65 [DOI] [PubMed] [Google Scholar]
- Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P 1993 Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci USA 90:7240–7244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Campagne C, Dehouck B, Leroy D, Holstein GR, Beauvillain JC, Buée-Scherrer V, Prevot V 2007 Coupling of neuronal nitric oxide synthase to NMDA receptors via postsynaptic density-95 depends on estrogen and contributes to the central control of adult female reproduction. J Neurosci 27:6103–6114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi A, Ciana P, Belcredito S, Vegeto E 2004 Estrogens in the nervous system: mechanisms and nonreproductive functions. Annu Rev Physiol 66:291–313 [DOI] [PubMed] [Google Scholar]
- Woolley CS 2007 Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol 47:657–680 [DOI] [PubMed] [Google Scholar]
- Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts J, Gao XB, Mobbs C, Shulman GI, Diano S, Horvath TL 2007 Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med 13:89–94 [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Naftolin F, Hutchison JB, Azcoitia I, Chowen JA 1999 Role of astroglia in estrogen regulation of synaptic plasticity and brain repair. J Neurobiol 40:574–584 [PubMed] [Google Scholar]
- Knauf C, Prevot V, Stefano GB, Mortreux G, Beauvillain JC, Croix D 2001 Evidence for a spontaneous nitric oxide release from the rat median eminence: influence on gonadotropin-releasing hormone release. Endocrinology 142:2343–2350 [DOI] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM 2002 A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J Neurosci 22:8586–8596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Lomniczi A, Mastronardi C, Heger S, Roth C, Parent AS, Matagne V, Mungenast AE 2006 Minireview: the neuroendocrine regulation of puberty: is the time ripe for a systems biology approach? Endocrinology 147:1166–1174 [DOI] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM 2004 Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci 7:643–650 [DOI] [PubMed] [Google Scholar]
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM 2004 Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology 145:2906–2917 [DOI] [PubMed] [Google Scholar]
- Kantor DB, Lanzrein M, Stary SJ, Sandoval GM, Smith WB, Sullivan BM, Davidson N, Schuman EM 1996 A role for endothelial NO synthase in LTP revealed by adenovirus-mediated inhibition and rescue. Science 274:1744–1748 [DOI] [PubMed] [Google Scholar]
- Liu J, Sessa WC 1994 Identification of covalently bound amino-terminal myristic acid in endothelial nitric oxide synthase. J Biol Chem 269:11691–11694 [PubMed] [Google Scholar]
- Busconi L, Michel T 1993 Endothelial nitric oxide synthase. N-terminal myristoylation determines subcellular localization. J Biol Chem 268:8410–8413 [PubMed] [Google Scholar]
- Lee CM, Robinson LJ, Michel T 1995 Oligomerization of endothelial nitric oxide synthase. Evidence for a dominant negative effect of truncation mutants. J Biol Chem 270:27403–27406 [DOI] [PubMed] [Google Scholar]
- Sharif A, Duhem-Tonnelle V, Allet C, Baroncini M, Loyens A, Kerr-Conte J, Collier F, Blond S, Ojeda SR, Junier MP, Prevot V 2009 Differential erbB signaling in astrocytes from the cerebral cortex and the hypothalamus of the human brain. Glia 57:362–379 [DOI] [PubMed] [Google Scholar]
- Zar JH 1984 Biostatistical analysis. Englewood Cliffs, NJ: Prentice Hall [Google Scholar]
- Langub Jr MC, Watson Jr RE 1992 Estrogen receptor-immunoreactive glia, endothelia, and ependyma in guinea pig preoptic area and median eminence: electron microscopy. Endocrinology 130:364–372 [DOI] [PubMed] [Google Scholar]
- Prevot V, Bouret S, Stefano GB, Beauvillain J 2000 Median eminence nitric oxide signaling. Brain Res Brain Res Rev 34:27–41 [DOI] [PubMed] [Google Scholar]
- Wong LF, Polson JW, Murphy D, Paton JF, Kasparov S 2002 Genetic and pharmacological dissection of pathways involved in the angiotensin II-mediated depression of baroreflex function. FASEB J 16:1595–1601 [DOI] [PubMed] [Google Scholar]
- Paton JF, Deuchars J, Ahmad Z, Wong LF, Murphy D, Kasparov S 2001 Adenoviral vector demonstrates that angiotensin II-induced depression of the cardiac baroreflex is mediated by endothelial nitric oxide synthase in the nucleus tractus solitarii of the rat. J Physiol 531:445–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf C, Ferreira S, Hamdane M, Mailliot C, Prevot V, Beauvillain JC, Croix D 2001 Variation of endothelial nitric oxide synthase synthesis in the median eminence during the rat estrous cycle: an additional argument for the implication of vascular blood vessel in the control of GnRH release. Endocrinology 142:4288–4294 [DOI] [PubMed] [Google Scholar]
- Prevot V, Croix D, Rialas CM, Poulain P, Fricchione GL, Stefano GB, Beauvillain JC 1999 Estradiol coupling to endothelial nitric oxide stimulates gonadotropin-releasing hormone release from rat median eminence via a membrane receptor. Endocrinology 140:652–659 [DOI] [PubMed] [Google Scholar]
- Hardy TA, May JM 2002 Coordinate regulation of l-arginine uptake and nitric oxide synthase activity in cultured endothelial cells. Free Radic Biol Med 32:122–131 [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Campbell WB 1982 An increase in hypothalamic capacity to synthesize prostaglandin E2 precedes the first preovulatory surge of gonadotropins. Endocrinology 111:1031–1037 [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Negro-Vilar A 1985 Prostaglandin E2-induced luteinizing hormone-releasing hormone release involves mobilization of intracellular Ca+2. Endocrinology 116:1763–1770 [DOI] [PubMed] [Google Scholar]
- Prevot V, Rio C, Cho GJ, Lomniczi A, Heger S, Neville CM, Rosenthal NA, Ojeda SR, Corfas G 2003 Normal female sexual development requires neuregulin-erbB receptor signaling in hypothalamic astrocytes. J Neurosci 23:230–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevot V, Dutoit S, Croix D, Tramu G, Beauvillain JC 1998 Semi-quantitative ultrastructural analysis of the localization and neuropeptide content of gonadotropin releasing hormone nerve terminals in the median eminence throughout the estrous cycle of the rat. Neuroscience 84:177–191 [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Harms PG, McCann SM 1975 Effect of inhibitors of prostaglandin synthesis on gonadotropin release in the rat. Endocrinology 97:843–854 [DOI] [PubMed] [Google Scholar]
- Clasadonte J, Poulain P, Beauvillain JC, Prevot V 2008 Activation of neuronal nitric oxide release inhibits spontaneous firing in adult gonadotropin-releasing hormone neurons: a possible local synchronizing signal. Endocrinology 149:587–596 [DOI] [PubMed] [Google Scholar]
- Prast H, Philippu A 2001 Nitric oxide as modulator of neuronal function. Prog Neurobiol 64:51–68 [DOI] [PubMed] [Google Scholar]
- Steinert JR, Kopp-Scheinpflug C, Baker C, Challiss RA, Mistry R, Haustein MD, Griffin SJ, Tong H, Graham BP, Forsythe ID 2008 Nitric oxide is a volume transmitter regulating postsynaptic excitability at a glutamatergic synapse. Neuron 60:642–656 [DOI] [PubMed] [Google Scholar]
- Agnati LF, Zoli M, Strömberg I, Fuxe K 1995 Intercellular communication in the brain: wiring versus volume transmission. Neuroscience 69:711–726 [DOI] [PubMed] [Google Scholar]
- Hatton GI 1997 Function-related plasticity in hypothalamus. Annu Rev Neurosci 20:375–397 [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Hoshino A, Tsai L, Henley JR, Goshima Y, Tessier-Lavigne M, Poo MM, Hong K 2003 Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature 423:990–995 [DOI] [PubMed] [Google Scholar]
- Seidel C, Bicker G 2000 Nitric oxide and cGMP influence axonogenesis of antennal pioneer neurons. Development 127:4541–4549 [DOI] [PubMed] [Google Scholar]
- Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M 1998 Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science 281:1515–1518 [DOI] [PubMed] [Google Scholar]
- Levine JE, Ramirez VD 1982 Luteinizing hormone-releasing hormone release during the rat estrous cycle and after ovariectomy, as estimated with push-pull cannulae. Endocrinology 111:1439–1448 [DOI] [PubMed] [Google Scholar]
- Ma YJ, Hill DF, Creswick KE, Costa ME, Cornea A, Lioubin MN, Plowman GD, Ojeda SR 1999 Neuregulins signaling via a glial erbB-2-erbB-4 receptor complex contribute to the neuroendocrine control of mammalian sexual development. J Neurosci 19:9913–9927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Urbanski HF, Costa ME, Hill DF, Moholt-Siebert M 1990 Involvement of transforming growth factor α in the release of luteinizing hormone-releasing hormone from the developing female hypothalamus. Proc Natl Acad Sci USA 87:9698–9702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevot V, Lomniczi A, Corfas G, Ojeda SR 2005 erbB-1 and erbB-4 receptors act in concert to facilitate female sexual development and mature reproductive function. Endocrinology 146:1465–1472 [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G 2003 Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci 6:43–50 [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M 2006 Astrocyte-mediated control of cerebral blood flow. Nat Neurosci 9:260–267 [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Rönnbäck L, Hansson E 2006 Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7:41–53 [DOI] [PubMed] [Google Scholar]
- Paton JF, Kasparov S, Paterson DJ 2002 Nitric oxide and autonomic control of heart rate: a question of specificity. Trends Neurosci 25:626–631 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Data]