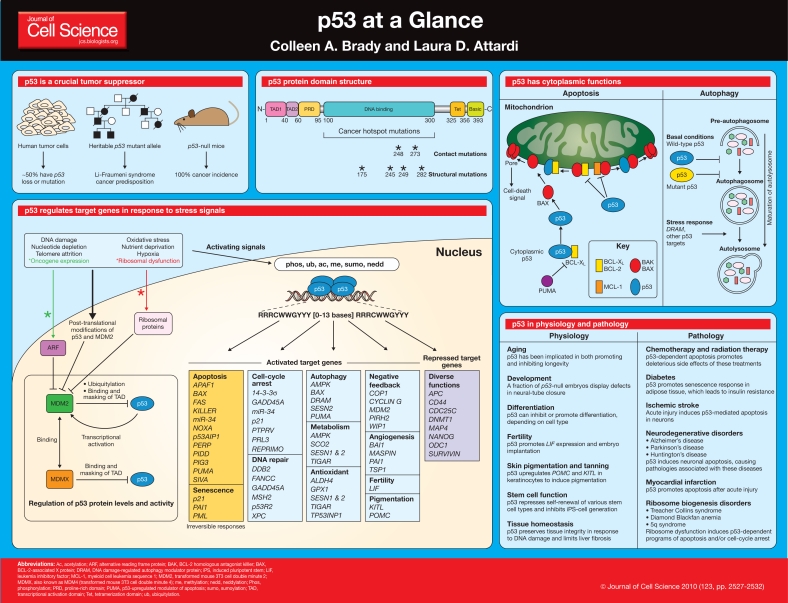
p53 at a glance (original) (raw)
Since its discovery in 1979, the role of the p53 protein in cancer has been studied intensively (Levine and Oren, 2009). p53 is a crucial tumor suppressor, long-recognized to suppress cancer through the induction of cell-cycle-arrest or apoptosis programs in response to a plethora of different cellular stress signals. Although this model paints a seemingly simple picture, the longer that p53 is studied, the more it confronts researchers with new questions about its functions and mechanisms of action. The tumor-suppressor function of p53 extends beyond the capacity to trigger cell-cycle arrest and apoptosis, and novel activities that impact tumor suppression are perpetually emerging, including the regulation of metabolism, autophagy and the oxidative status of the cell. Moreover, because it acts as a crucial node downstream of diverse stress signals, it is perhaps not surprising that p53 has been recently discovered to have broader roles in physiology and pathology. In this article and its accompanying poster, we outline the basic molecular and cellular functions of p53, describe the intricate network of p53 mediators, and provide insight into the roles of p53 in normal physiology and human disease.
p53 has a crucial role in tumor suppression
Analysis of human cancers reveals a fundamental role for p53 in tumor suppression. More than half of human cancers, of a wide variety of types, harbor p53 (TP53) mutations, and inheritance of a mutant p53 allele predisposes humans to the Li-Fraumeni cancer syndrome (Olivier et al., 2010). Unequivocal confirmation of the crucial role for p53 in tumor suppression was demonstrated by the completely penetrant cancer phenotype of _p53_-null mice (Kenzelmann Broz and Attardi, 2010). p53 restricts tumor development by serving as a sensor of cellular stress, responding to diverse signals, including DNA damage, hypoxia, oncogene expression, nutrient deprivation and ribosome dysfunction, and limiting the propagation of cells under these adverse conditions (Vousden and Prives, 2009). In metazoans, an ancestral function of p53 is thought to be its ability to trigger programmed cell death (also known as apoptosis) in response to DNA damage, as a measure to preserve the integrity of the germ line (Junttila and Evan, 2009; Lu and Abrams, 2006; Lu et al., 2009).
Subsequently, with the evolution of longer-living organisms, p53 is thought to have acquired the ability to respond to oncogenic signals, promoting apoptosis or senescence – a permanent cell-cycle-arrest response – as a safeguard against neoplasia. Thus, in cells exposed to potent stress signals, p53 drives irreversible programs of apoptosis or senescence to cull irreparably damaged or malignant cells, for the ultimate benefit of the organism (Vousden and Prives, 2009). Alternatively, under conditions of low-level stress, p53 elicits protective, pro-survival responses, such as temporary cell-cycle arrest, DNA repair and antioxidant protein production, to maintain genome integrity and viability in cells that sustain limited, reparable damage. These various responses rely on the ability of p53 to function as a transcriptional activator of a panoply of target genes, although transactivation-independent activities ascribed to p53 can also contribute to p53-mediated responses, as highlighted below.
p53 has protein domains typical of transcriptional activators
Similarly to other transcription factors, p53 has a modular protein domain structure (Joerger and Fersht, 2007; Vousden and Prives, 2009). The N-terminus of p53 comprises two transcriptional activation domains (TADs), TAD1 and TAD2, which span amino acid residues 1-40 and 40-60, respectively. These domains can independently enhance transcription of p53 target genes by recruiting histone-modifying enzymes, components of the basal transcriptional machinery and coactivator complexes, such as STAGA and Mediator (Gamper and Roeder, 2008; Joerger and Fersht, 2007). C-terminal to the transactivation domains, between residues 60-95, lies the proline-rich domain (PRD), which was originally proposed to participate in protein-protein interactions on the basis of the presence of PxxP motifs that resemble Src homology 3 (SH3)-domain-binding regions (Toledo et al., 2006; Toledo et al., 2007). However, a structural function might be the main role of this domain because knock-in mice carrying proline-to-alanine point mutations in the putative protein-protein interaction sites of this domain appear normal, whereas complete domain deletion disrupts p53 tumor-suppressor function. The central core of p53, which spans residues 100-300, comprises the DNA-binding domain that is responsible for sequence-specific binding of the protein to p53 response elements in DNA. Most cancer-associated p53 mutations are missense mutations in this domain and incapacitate DNA binding, illuminating the key importance of DNA binding for p53-mediated tumor suppression. Tumor-derived p53 mutations either alter residues that are essential for direct contact with p53 response elements (contact mutants) or impair proper folding of the domain (structural mutants). The six most common p53 amino acid residues altered in cancer – known as ‘hotspots’ – are R175, G245, R248, R249, R273 and R282 (Brosh and Rotter, 2009). In addition to disrupting DNA binding, these mutations can confer gain-of-function capabilities on p53, and have been linked to increased invasiveness and metastasis of tumors (Lang et al., 2004; Muller et al., 2009; Olive et al., 2004). p53 binds to its response elements as a tetramer, the formation of which relies on a discrete tetramerization domain (Tet) comprising residues 325-356. Finally, p53 contains a basic, lysine-rich domain at the extreme C-terminus, between residues 363-393. This basic region binds DNA in a non-sequence-specific manner and promotes linear diffusion that helps p53 hone in on its response elements, as well as undergoing extensive post-translational modifications that modulate p53 stabilization and sequence-specific DNA binding (Kruse and Gu, 2009; Laptenko and Prives, 2006; Liu and Kulesz-Martin, 2006; Murray-Zmijewski et al., 2008). Interestingly, p537KR knock-in mice, in which all seven C-terminal lysines are replaced with arginines, are largely phenotypically normal, suggesting a complexity in the role of these modifications that warrants further investigation (Krummel et al., 2005).
p53 induces a host of transcriptional programs involved in different responses
In response to myriad cellular stress signals, p53 is activated both through protein stabilization (discussed below) and post-translational modifications (for reviews, see Kruse and Gu, 2009; Murray-Zmijewski et al., 2008) that allow full p53 transactivation potential. p53 tetramers bind as dimers of dimers to sequence-specific p53 response elements, which are classically defined as two DNA half sites of RRRCWWGYYY (where R is a purine, W is adenine or thymine and Y is a pyrimidine) with a spacer of 0-13 base pairs between half sites (Menendez et al., 2009; Riley et al., 2008). These sites are frequently found either in the promoters or first introns of p53 target genes. Once active p53 is bound to DNA, it can stimulate the transcription of many protein-coding and non-protein-coding genes [e.g. microRNAs or large intergenic non-coding RNAs (lincRNAs)], which is a function of fundamental importance for all p53-mediated responses (Guttman et al., 2009; He et al., 2007; Menendez et al., 2009; Riley et al., 2008).
The exact cell fate specified by p53 activation is dictated by cell type, environmental milieu and the nature of the stress. In response to sustained or severe stress signals, p53 drives irreversible apoptosis or senescence programs. p53-triggered apoptosis involves the transcriptional induction of components of both the extrinsic and intrinsic death pathways, including BAX, FAS, NOXA and PUMA, among others, which collaboratively promote cell death (Riley et al., 2008; Zilfou and Lowe, 2009). In other cases, p53 responds to potent stress by inducing cellular senescence through transcriptional activation of target genes such as p21, PAI1 and PML (Kortlever et al., 2006; Riley et al., 2008). Under conditions of lower levels of stress, when repair is possible, p53 engages a temporary program of cell-cycle arrest and DNA repair to allow cells to pause and repair any damage incurred, thereby limiting the propagation of oncogenic mutations. This role for p53 as ‘guardian of the genome’ extends further to the maintenance of genomic stability at the chromosomal level, by limiting the accrual of aneuploid cells. Another protective, pro-survival mechanism is the capacity of p53 to upregulate the expression of antioxidant genes, such as sestrins 1 and 2 (SESN1 and SESN2, respectively), GPX1 and TIGAR, which suppress the accumulation of reactive oxygen species, thereby maintaining genomic integrity (Liu et al., 2008; Sablina et al., 2005).
Additional transcriptional programs controlled by p53 continue to be uncovered, and their roles in tumor suppression are being unraveled. For example, p53 inhibits glycolysis (through TIGAR) and promotes oxidative phosphorylation (through SCO2) to protect cells from metabolic reprogramming – known as the ‘Warburg effect’ – which is thought to be fundamental for malignant transformation (Vousden and Ryan, 2009). p53 can also limit tumorigenesis through autophagy, or ‘self-eating’, which can provoke cell death through the activation of genes such as AMPK, DRAM, SESN1 and SESN2. However, the role of autophagy in tumor suppression is complex because it might also have pro-survival effects by promoting ATP generation when nutrients are limiting. Interestingly, p53 can also exert non-cell-autonomous effects that are pivotal to tumor suppression; these functions are highlighted by the ability of p53 to impede angiogenesis through inducing the production of gene products such as thrombospondin-1 (TSP-1) and to inhibit tumor growth and metastasis by stimulating signaling from the fibroblast compartment of tumors (Teodoro et al., 2007; Bar et al., 2009).
Beyond its key roles in tumor suppression, p53 has other physiological functions, such as promoting embryo implantation through inducing the expression of leukemia inhibitory factor (LIF). This observation suggests a mechanism whereby p53 regulates reproduction in mammals, which is reminiscent of its ancestral function in germ-cell protection (Hu et al., 2007). It seems that each p53-driven response requires the activation of a select cohort of target genes, and that new players involved in each response will continue to be discovered. For a fairly comprehensive list of validated human p53 target genes and analysis of their p53 response elements, see the review by Riley et al. (Riley et al., 2008).
In addition to activating transcription, p53 can repress gene expression. p53-dependent repression can occur through direct binding of p53 to specific p53 response elements and recruitment of co-repressors such as Sin3a and histone deacetylases (Ho and Benchimol, 2003; Riley et al., 2008). p53 can also repress target genes by occluding binding sites for other transcriptional activators that are important for the expression of those genes. Additionally, a major component of repression probably occurs through indirect mechanisms, such as by p53-dependent activation of microRNAs or lincRNAs that repress gene expression (Guttman et al., 2009; He et al., 2007; Menendez et al., 2009; Riley et al., 2008).
The ability of p53 to dictate different cellular outcomes in a context-dependent manner implies an intricate level of control of this protein. The mechanisms by which p53 can promote distinct transcriptional patterns and cell fates is an area of intense investigation, and the mechanism used seems to depend on many factors, including the expression level and post-translational-modification status of p53, cofactor recruitment, and promoter architecture of the target gene. This topic has been detailed in recent reviews, and it is certain that future research will further unveil the complexity underlying the ability of p53 to specify different responses in different settings (Murray-Zmijewski et al., 2008; Vousden and Prives, 2009).
p53 has cytoplasmic roles in apoptosis and autophagy
In addition to its clear role as a transcription factor, it has been proposed that p53 has additional functions, a notion stemming from the observation that p53 can promote apoptosis in the presence of transcription inhibitors (Caelles et al., 1994). Accordingly, p53 has been implicated in other nuclear functions, such as regulating homologous recombination and microRNA processing (Romanova et al., 2004; Suzuki et al., 2009), as well as in several cytoplasmic processes. Most notably, p53 can directly promote mitochondrial outer-membrane permeabilization (MOMP) to trigger apoptosis (Green and Kroemer, 2009; Vaseva and Moll, 2009). Several mechanisms have been proposed to explain p53-induced MOMP, but the models converge on the concept that p53 protein modulates the BCL-2 family of apoptosis regulators. This family comprises three categories of proteins: the pro-apoptotic effectors BAK and BAX that can oligomerize to create pores in the mitochondrial outer membrane to induce apoptosis; the BH3 (BCL-2 homology region 3)-only proteins that stimulate BAX- or BAK-mediated pore formation; and the anti-apoptotic proteins that bind to BAK and BAX to impede their oligomerization, including BCL-2, BCL-XL and MCL-1 (Vaseva and Moll, 2009). One model suggests that p53 acts as a BH3-only protein at mitochondria to induce apoptosis either through the binding and sequestration of BCL-2 and BCL-XL to derepress BAK and BAX or through direct interaction with BAK to release it from MCL-1-mediated inhibition (Leu et al., 2004; Mihara et al., 2003). These protein-protein interactions rely on the DNA-binding domain of p53, and cancer-derived p53 mutants lack the ability to promote mitochondrial apoptosis. An alternate model for cytoplasmic p53-induced apoptosis proposes that basal levels of p53 protein are bound to free, cytoplasmic BCL-XL, rendering p53 inactive. Under conditions of stress, p53 activates the transcription of PUMA, the encoded protein of which in turn binds to BCL-XL and liberates p53 to activate BAX in a proposed ‘hit-and-run’ manner (Chipuk et al., 2005). Interestingly, both models highlight the interconnection between p53 cytoplasmic and nuclear activities, because the BAX and PUMA mitochondrial pathway components are also direct p53 target genes.
p53 also has a complex role in regulating autophagy. Autophagy allows a cell to catabolize macromolecules for reuse, either as a survival strategy under stress conditions or as a means to remove harmful, damaged structures (Tasdemir et al., 2008a). Autophagy can also promote cell death, and has hence been linked to tumor suppression. During this process, pre-autophagosome complexes mature to form autophagosomes, which are double-membrane vesicles that envelop organelles. Next, autophagosomes fuse with lysosomes to allow degradation of the enclosed material (Ravikumar et al., 2009). As mentioned above, activated nuclear p53 induces the expression of pro-autophagy target genes (Vousden and Ryan, 2009). In the cytoplasm, basal levels of wild-type p53 can also impede autophagosome formation, although the exact mechanism of inhibition of cytoplasmic autophagy remains unknown (Tasdemir et al., 2008b). Many tumor-derived p53 mutants can also suppress autophagy, which might contribute to tumor progression by enhancing cell survival (Morselli et al., 2008; Tasdemir et al., 2008b). This complex, dual role for p53 in autophagy reflects another example of how context-specific cues can promote different p53-triggered responses, and further studies of this system will better clarify these opposing roles.
The MDM2 family controls p53 protein levels and activity
Inappropriate p53 activity can be detrimental to cell and organism viability, and there are therefore numerous mechanisms to keep p53 in check. Several E3 ubiquitin ligases – mainly MDM2 but also others, including PIRH2, COP1 and ARF-BP1 – negatively regulate p53 protein levels, keeping levels low when p53 activity is not required (Brooks and Gu, 2006). MDM2 binds to the TAD of p53 and not only regulates p53 stability but also inhibits its transactivation function by blocking the recruitment of essential transcriptional machinery components. The crucial importance of MDM2 in regulating p53 was demonstrated using _Mdm2_-null mice, which display very early embryonic lethality (Jones et al., 1995; Montes de Oca Luna et al., 1995). This phenotype results from unrestrained p53 activity, as demonstrated by the complete rescue of lethality in Mdm2_−/−;p53_−/− mice. In response to stress signals, p53 is mobilized by inhibition of MDM2, through any of several mechanisms: stress-induced post-translational modifications of both MDM2 and p53, which disrupt the MDM2-p53 interaction; oncogene-induced sequestration of MDM2 by the tumor suppressor ARF; and nucleolar-stress-triggered ribosomal protein binding and inhibition of MDM2-mediated ubiquitylation of p53 (Marine et al., 2006; Wade et al., 2010; Zhang and Lu, 2009). p53 itself also controls MDM2 function by activating transcription of the MDM2 gene, providing a negative-feedback loop to attenuate p53 signaling at the appropriate moment.
The MDM2-family member MDMX (also known as MDM4) does not display E3-ligase activity but is nonetheless key for regulating p53 transcriptional activity, both through binding to and inhibiting the p53 TAD and through regulating MDM2 (Marine et al., 2007; Wade et al., 2010). Mdmx−/− mice exhibit p53-dependent embryonic lethality later during embryogenesis than do Mdm2−/− mice, underscoring the importance of this regulator but distinguishing its activity from that of MDM2 (Finch et al., 2002; Migliorini et al., 2002; Parant et al., 2001). MDMX can interact with MDM2, leading to enhanced MDM2 stabilization and p53 degradation, but the full importance of the MDMX-MDM2 interaction is unclear (Marine et al., 2006; Wade et al., 2010). A heterodimer of MDM2 and MDMX might act as an optimal E3 ubiquitin ligase, an idea that is supported by data on the structure of the heterodimer (Linke et al., 2008). To further complicate the situation, p53, MDM2 and MDMX can be deubiquitylated and stabilized by enzymes such as HAUSP and dephosphorylated by the p53-inducible phosphatase WIP1, which facilitates MDM2- and MDMX-mediated p53 destruction (Kruse and Gu, 2009; Wade et al., 2010).
The role of p53 as a cellular stress sensor extends to numerous physiological and pathological processes
Although research on p53 has focused on cancer for many years, it is now appreciated that p53 has a much more pleiotropic role as a stress sensor, and that p53 has functions in various physiological and pathological processes (Vousden and Lane, 2007; Vousden and Ryan, 2009). An initial indication that p53 has functions that extend beyond tumor suppression came from the observation that a fraction of p53−/− mouse embryos display developmental aberrations, including prominent defects in neural-tube closure and consequent exencephaly (Armstrong et al., 1995; Sah et al., 1995). Moreover, p53 provides protection against exposure to teratogens during embryogenesis by eliminating damaged embryos and ensuring that only healthy individuals survive (Donehower and Lozano, 2009). Further examination of p53−/− adult mice recently revealed a role for p53 in embryo implantation, indicated by the dramatically low fertility rates observed in female p53−/− mice (Hu et al., 2007). p53 is probably also important for the implantation of human embryos because individuals carrying p53 polymorphisms that decrease the function of the protein are overrepresented at fertility clinics (Kang et al., 2009). Whereas adult _p53_−/− mice all develop cancer at an early age, which impedes longevity analysis, p53 deficiency in both flies and worms increases lifespan. Again, however, controversy obfuscates the picture because both pro-aging and anti-aging roles have been reported in disparate mouse models that express elevated levels of p53 (Arum and Johnson, 2007; Bauer et al., 2005; Matheu et al., 2008). Specifically, mice with extra copies of both the Arf and p53 genes exhibit increased longevity, whereas mice expressing p53 truncation mutants that lead to aberrant p53 activation display reduced longevity and signs of premature aging (Maier et al., 2004; Matheu et al., 2007; Tyner et al., 2002).
In addition to its roles in development and aging, recent studies have elaborated newly identified functions of p53 in several other physiological contexts. p53 is involved in restricting the self-renewal of several types of stem cells, including neural stem cells and hematopoietic stem cells (Cicalese et al., 2009; Liu et al., 2009; Meletis et al., 2006). Furthermore, several groups have reported enhanced efficiency of cellular reprogramming of somatic cells to induced pluripotent stem (iPS) cells in the absence of p53, implicating p53 in the maintenance of a differentiated state (Krizhanovsky and Lowe, 2009). This idea is in alignment with studies showing that p53 has a role in promoting differentiation of certain cell types, such as skeletal-muscle-committed cells (Molchadsky et al., 2008). In contrast to these scenarios, p53 can also suppress differentiation, as exemplified by its ability to inhibit osteoblast differentiation and bone development in mice (Lengner et al., 2006; Wang et al., 2006). Even proper tissue homeostasis in the face of stress signals seems to rely on p53 in some organs (Begus-Nahrmann et al., 2009; Krizhanovsky et al., 2008; Ruzankina et al., 2009). Finally, activation of p53 in keratinocytes in response to ribosome dysfunction or ultraviolet light promotes pigmentation and protective suntanning responses, respectively, in mice (Cui et al., 2007; McGowan et al., 2008).
In contrast to the beneficial role of p53 in incipient tumors, activation of the p53 apoptotic or senescence programs by stress signals in other contexts can have detrimental consequences. For example, p53-induced apoptosis in radiosensitive tissues can account for the damaging side effects associated with genotoxic chemotherapies or radiation therapies (Gudkov and Komarova, 2003). In addition, ischemia at different organ sites can provoke p53-dependent apoptosis that contributes to the resultant pathologies, as in the cases of stroke and myocardial infarction (Liu et al., 2006; Luo et al., 2009; Morrison et al., 2003). Furthermore, in several neurological disorders, including Alzheimer's, Parkinson's and Huntington's diseases, p53-mediated neuronal apoptosis also contributes to the associated pathologies (Bae et al., 2005; Bretaud et al., 2007; Culmsee and Mattson, 2005; Duan et al., 2002). Finally, disorders associated with defective ribosomal biogenesis have also been linked to aberrant p53 activation. In mouse models of Diamond Blackfan anemia, Treacher Collins syndrome craniofacial abnormalities and 5q syndrome macrocytic anemia, p53 activity is induced in response to mutations that cause ribosome dysfunction, precipitating either apoptotic or cell-cycle-arrest responses that result in the manifestations of the disease (Barlow et al., 2010; Jones et al., 2008; McGowan et al., 2008). In contrast to the aforementioned phenotypes, which mainly rely on apoptosis, studies of diabetes in obese mice have uncovered a p53-dependent senescence response in fat cells; this response ultimately engendered insulin resistance in these mice (Minamino et al., 2009). Mouse models have been instrumental in revealing roles for p53 in these diverse pathologies because inhibition of p53 activity with a small-molecule antagonist of p53, pifithrin-α, or by genetic ablation effectively diminished many of the symptoms in models of these p53-dependent diseases (Bae et al., 2005; Barlow et al., 2010; Bretaud et al., 2007; Culmsee and Mattson, 2005; Duan et al., 2002; Jones et al., 2008; Liu et al., 2006; Luo et al., 2009; McGowan et al., 2008; Minamino et al., 2009; Morrison et al., 2003). Together, these findings underscore the importance of p53 in diverse pathological states and indicate that p53 inhibitors might be promising therapeutics for these diseases.
Perspectives
Research on p53 over the last 30 years has uncovered a wide spectrum of roles for p53 as a cellular stress sentinel, both in normal physiology and in disease states. Here, we have presented a general overview of these aspects of p53 biology and of the vast networks through which p53 acts. However, capturing the complete complexity of p53 function is beyond the scope of this article. For example, in addition to an intricate constellation of post-translational modifications that control p53 activity, polymorphisms in p53 itself or in components of the pathways in which it is involved can alter p53 expression or activity, and p53 has been shown to exist in numerous different protein isoforms, the significance of which remain to be elucidated (Levine and Oren, 2009). Furthermore, p53 is a member of a multi-protein family that includes p63 and p73; all of these proteins can bind to the same DNA recognition elements, and there might be significant functional interplay between family members (Lu et al., 2009). In terms of clinical applications, both the beneficial and deleterious effects of p53 activation make p53 a promising target for therapeutic intervention in a broad range of human diseases. Indeed, exciting recent findings have shown that reactivation of p53 expression in mouse models induces tumor regression (Martins et al., 2006; Ventura et al., 2007; Xue et al., 2007). Future investigations will continue to decipher the mechanisms of p53 action and its roles in diverse settings. These discoveries will help to pave the road to future advances in p53-based therapeutics for cancer and other diseases.
References
- Armstrong J. F., Kaufman M. H., Harrison D. J., Clarke A. R. (1995). High-frequency developmental abnormalities in p53-deficient mice. Curr. Biol. 5, 931-936 [DOI] [PubMed] [Google Scholar]
- Arum O., Johnson T. E. (2007). Reduced expression of the Caenorhabditis elegans p53 ortholog cep-1 results in increased longevity. J. Gerontol. A Biol. Sci. Med. Sci. 62, 951-959 [DOI] [PubMed] [Google Scholar]
- Bae B. I., Xu H., Igarashi S., Fujimuro M., Agrawal N., Taya Y., Hayward S. D., Moran T. H., Montell C., Ross C. A., et al. (2005). p53 mediates cellular dysfunction and behavioral abnormalities in Huntington's disease. Neuron 47, 29-41 [DOI] [PubMed] [Google Scholar]
- Bar J., Moskovits J., Oren M. (2009). Involvement of stromal p53 in tumor-stroma interactions. Semin. Cell Dev. Biol. 21, 47-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow J. L., Drynan L. F., Hewett D. R., Holmes L. R., Lorenzo-Abalde S., Lane A. L., Jolin H. E., Pannell R., Middleton A. J., Wong S. H., et al. (2010). A p53-dependent mechanism underlies macrocytic anemia in a mouse model of human 5q-syndrome. Nat. Med. 16, 59-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J. H., Poon P. C., Glatt-Deeley H., Abrams J. M., Helfand S. L. (2005). Neuronal expression of p53 dominant-negative proteins in adult Drosophila melanogaster extends life span. Curr. Biol. 15, 2063-2068 [DOI] [PubMed] [Google Scholar]
- Begus-Nahrmann Y., Lechel A., Obenauf A. C., Nalapareddy K., Peit E., Hoffmann E., Schlaudraff F., Liss B., Schirmacher P., Kestler H., et al. (2009). p53 deletion impairs clearance of chromosomal-instable stem cells in aging telomere-dysfunctional mice. Nat. Genet. 41, 1138-1143 [DOI] [PubMed] [Google Scholar]
- Bretaud S., Allen C., Ingham P. W., Bandmann O. (2007). p53-dependent neuronal cell death in a DJ-1-deficient zebrafish model of Parkinson's disease. J. Neurochem. 100, 1626-1635 [DOI] [PubMed] [Google Scholar]
- Brooks C. L., Gu W. (2006). p53 ubiquitination: Mdm2 and beyond. Mol. Cell 21, 307-315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosh R., Rotter V. (2009). When mutants gain new powers: news from the mutant p53 field. Nat. Rev. Cancer 9, 701-713 [DOI] [PubMed] [Google Scholar]
- Caelles C., Helmberg A., Karin M. (1994). p53-dependent apoptosis in the absence of transcriptional activation of p53-target genes. Nature 370, 220-223 [DOI] [PubMed] [Google Scholar]
- Chipuk J. E., Bouchier-Hayes L., Kuwana T., Newmeyer D. D., Green D. R. (2005). PUMA couples the nuclear and cytoplasmic proapoptotic function of p53. Science 309, 1732-1735 [DOI] [PubMed] [Google Scholar]
- Cicalese A., Bonizzi G., Pasi C. E., Faretta M., Ronzoni S., Giulini B., Brisken C., Minucci S., Di Fiore P. P., Pelicci P. G. (2009). The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell 138, 1083-1095 [DOI] [PubMed] [Google Scholar]
- Cui R., Widlund H. R., Feige E., Lin J. Y., Wilensky D. L., Igras V. E., D'Orazio J., Fung C. Y., Schanbacher C. F., Granter S. R., et al. (2007). Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell 128, 853-864 [DOI] [PubMed] [Google Scholar]
- Culmsee C., Mattson M. P. (2005). p53 in neuronal apoptosis. Biochem. Biophys. Res. Commun. 331, 761-777 [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Lozano G. (2009). 20 years studying p53 functions in genetically engineered mice. Nat. Rev. Cancer 9, 831-841 [DOI] [PubMed] [Google Scholar]
- Duan W., Zhu X., Ladenheim B., Yu Q. S., Guo Z., Oyler J., Cutler R. G., Cadet J. L., Greig N. H., Mattson M. P. (2002). p53 inhibitors preserve dopamine neurons and motor function in experimental parkinsonism. Ann. Neurol. 52, 597-606 [DOI] [PubMed] [Google Scholar]
- Finch R. A., Donoviel D. B., Potter D., Shi M., Fan A., Freed D. D., Wang C. Y., Zambrowicz B. P., Ramirez-Solis R., Sands A. T., et al. (2002). mdmx is a negative regulator of p53 activity in vivo. Cancer Res. 62, 3221-3225 [PubMed] [Google Scholar]
- Gamper A. M., Roeder R. G. (2008). Multivalent binding of p53 to the STAGA complex mediates coactivator recruitment after UV damage. Mol. Cell. Biol. 28, 2517-2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. R., Kroemer G. (2009). Cytoplasmic functions of the tumour suppressor p53. Nature 458, 1127-1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudkov A. V., Komarova E. A. (2003). The role of p53 in determining sensitivity to radiotherapy. Nat. Rev. Cancer 3, 117-129 [DOI] [PubMed] [Google Scholar]
- Guttman M., Amit I., Garber M., French C., Lin M. F., Feldser D., Huarte M., Zuk O., Carey B. W., Cassady J. P., et al. (2009). Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 458, 223-227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., He X., Lowe S. W., Hannon G. J. (2007). microRNAs join the p53 network-another piece in the tumour-suppression puzzle. Nat. Rev. Cancer 7, 819-822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J., Benchimol S. (2003). Transcriptional repression mediated by the p53 tumour suppressor. Cell Death Differ. 10, 404-408 [DOI] [PubMed] [Google Scholar]
- Hu W., Feng Z., Teresky A. K., Levine A. J. (2007). p53 regulates maternal reproduction through LIF. Nature 450, 721-724 [DOI] [PubMed] [Google Scholar]
- Joerger A. C., Fersht A. R. (2007). Structural biology of the tumor suppressor p53 and cancer-associated mutants. Adv. Cancer Res. 97, 1-23 [DOI] [PubMed] [Google Scholar]
- Jones N. C., Lynn M. L., Gaudenz K., Sakai D., Aoto K., Rey J. P., Glynn E. F., Ellington L., Du C., Dixon J., et al. (2008). Prevention of the neurocristopathy Treacher Collins syndrome through inhibition of p53 function. Nat. Med. 14, 125-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. N., Roe A. E., Donehower L. A., Bradley A. (1995). Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature 378, 206-208 [DOI] [PubMed] [Google Scholar]
- Junttila M. R., Evan G. I. (2009). p53-a Jack of all trades but master of none. Nat. Rev. Cancer 9, 821-829 [DOI] [PubMed] [Google Scholar]
- Kang H. J., Feng Z., Sun Y., Atwal G., Murphy M. E., Rebbeck T. R., Rosenwaks Z., Levine A. J., Hu W. (2009). Single-nucleotide polymorphisms in the p53 pathway regulate fertility in humans. Proc. Natl. Acad. Sci. USA 106, 9761-9766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenzelmann Broz D., Attardi L. D. (2010). In vivo analysis of p53 tumor suppressor function using genetically engineered mouse models. Carcinogenesis Epub ahead of print PMID:20097732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortlever R. M., Higgins P. J., Bernards R. (2006). Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat. Cell Biol. 8, 877-884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizhanovsky V., Lowe S. W. (2009). Stem cells: The promises and perils of p53. Nature 460, 1085-1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizhanovsky V., Yon M., Dickins R. A., Hearn S., Simon J., Miething C., Yee H., Zender L., Lowe S. W. (2008). Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657-667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel K. A., Lee C. J., Toledo F., Wahl G. M. (2005). The C-terminal lysines fine-tune P53 stress responses in a mouse model but are not required for stability control or transactivation. Proc. Natl. Acad. Sci. USA 102, 10188-10193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse J. P., Gu W. (2009). Modes of p53 regulation. Cell 137, 609-622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang G. A., Iwakuma T., Suh Y. A., Liu G., Rao V. A., Parant J. M., Valentin-Vega Y. A., Terzian T., Caldwell L. C., Strong L. C., et al. (2004). Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell 119, 861-872 [DOI] [PubMed] [Google Scholar]
- Laptenko O., Prives C. (2006). Transcriptional regulation by p53: one protein, many possibilities. Cell Death Differ. 13, 951-961 [DOI] [PubMed] [Google Scholar]
- Lengner C. J., Steinman H. A., Gagnon J., Smith T. W., Henderson J. E., Kream B. E., Stein G. S., Lian J. B., Jones S. N. (2006). Osteoblast differentiation and skeletal development are regulated by Mdm2-p53 signaling. J. Cell Biol. 172, 909-921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu J. I., Dumont P., Hafey M., Murphy M. E., George D. L. (2004). Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat. Cell Biol. 6, 443-450 [DOI] [PubMed] [Google Scholar]
- Levine A. J., Oren M. (2009). The first 30 years of p53: growing ever more complex. Nat. Rev. Cancer 9, 749-758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke K., Mace P. D., Smith C. A., Vaux D, L., Silke J., Day C. L. (2008).Structure of the MDM2/MDMX RING domain heterodimer reveals dimerization is required for their ubiquitylation in trans. Cell Death Differ. 5, 841-848 [DOI] [PubMed] [Google Scholar]
- Liu B., Chen Y., St Clair D. K. (2008). ROS and p53: a versatile partnership. Free Radic. Biol. Med. 44, 1529-1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Xu B., Cavalieri T. A., Hock C. E. (2006). Pifithrin-alpha attenuates p53-mediated apoptosis and improves cardiac function in response to myocardial ischemia/reperfusion in aged rats. Shock 26, 608-614 [DOI] [PubMed] [Google Scholar]
- Liu Y., Kulesz-Martin M. F. (2006). Sliding into home: facilitated p53 search for targets by the basic DNA binding domain. Cell Death Differ. 13, 881-884 [DOI] [PubMed] [Google Scholar]
- Liu Y., Elf S. E., Miyata Y., Sashida G., Huang G., Di Giandomenico S., Lee J. M., Deblasio A., Menendez S., Antipin J., et al. (2009). p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell 4, 37-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W. J., Abrams J. M. (2006). Lessons from p53 in non-mammalian models. Cell Death Differ. 13, 909-912 [DOI] [PubMed] [Google Scholar]
- Lu W. J., Amatruda J. F., Abrams J. M. (2009). p53 ancestry: gazing through an evolutionary lens. Nat. Rev. Cancer 9, 758-762 [DOI] [PubMed] [Google Scholar]
- Luo Y., Kuo C. C., Shen H., Chou J., Greig N. H., Hoffer B. J., Wang Y. (2009). Delayed treatment with a p53 inhibitor enhances recovery in stroke brain. Ann. Neurol. 65, 520-530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier B., Gluba W., Bernier B., Turner T., Mohammad K., Guise T., Sutherland A., Thorner M., Scrable H. (2004). Modulation of mammalian life span by the short isoform of p53. Genes Dev. 18, 306-319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marine J. C., Francoz S., Maetens M., Wahl G., Toledo F., Lozano G. (2006). Keeping p53 in check: essential and synergistic functions of Mdm2 and Mdm4. Cell Death Differ. 13, 927-934 [DOI] [PubMed] [Google Scholar]
- Marine J. C., Dyer M. A., Jochemsen A. G. (2007). MDMX: from bench to bedside. J. Cell Sci. 120, 371-378 [DOI] [PubMed] [Google Scholar]
- Martins C. P., Brown-Swigart L., Evan G. I. (2006). Modeling the therapeutic efficacy of p53 restoration in tumors. Cell 127, 1323-1334 [DOI] [PubMed] [Google Scholar]
- Matheu A., Maraver A., Klatt P., Flores I., Garcia-Cao I., Borras C., Flores J. M., Vina J., Blasco M. A., Serrano M. (2007). Delayed ageing through damage protection by the Arf/p53 pathway. Nature 448, 375-379 [DOI] [PubMed] [Google Scholar]
- Matheu A., Maraver A., Serrano M. (2008). The Arf/p53 pathway in cancer and aging. Cancer Res. 68, 6031-6034 [DOI] [PubMed] [Google Scholar]
- McGowan K. A., Li J. Z., Park C. Y., Beaudry V., Tabor H. K., Sabnis A. J., Zhang W., Fuchs H., de Angelis M. H., Myers R. M., et al. (2008). Ribosomal mutations cause p53-mediated dark skin and pleiotropic effects. Nat. Genet. 40, 963-970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meletis K., Wirta V., Hede S. M., Nister M., Lundeberg J., Frisen J. (2006). p53 suppresses the self-renewal of adult neural stem cells. Development 133, 363-369 [DOI] [PubMed] [Google Scholar]
- Menendez D., Inga A., Resnick M. A. (2009). The expanding universe of p53 targets. Nat. Rev. Cancer 9, 724-737 [DOI] [PubMed] [Google Scholar]
- Migliorini D., Lazzerini Denchi E., Danovi D., Jochemsen A., Capillo M., Gobbi A., Helin K., Pelicci P. G., Marine J. C. (2002). Mdm4 (Mdmx) regulates p53-induced growth arrest and neuronal cell death during early embryonic mouse development. Mol. Cell. Biol. 22, 5527-5538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara M., Erster S., Zaika A., Petrenko O., Chittenden T., Pancoska P., Moll U. M. (2003). p53 has a direct apoptogenic role at the mitochondria. Mol. Cell 11, 577-590 [DOI] [PubMed] [Google Scholar]
- Minamino T., Orimo M., Shimizu I., Kunieda T., Yokoyama M., Ito T., Nojima A., Nabetani A., Oike Y., Matsubara H., et al. (2009). A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med. 15, 1082-1087 [DOI] [PubMed] [Google Scholar]
- Molchadsky A., Shats I., Goldfinger N., Pevsner-Fischer M., Olson M., Rinon A., Tzahor E., Lozano G., Zipori D., Sarig R., et al. (2008). p53 plays a role in mesenchymal differentiation programs, in a cell fate dependent manner. PLoS ONE 3, e3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes de Oca Luna R., Wagner D. S., Lozano G. (1995). Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 378, 203-206 [DOI] [PubMed] [Google Scholar]
- Morrison R. S., Kinoshita Y., Johnson M. D., Guo W., Garden G. A. (2003). p53-dependent cell death signaling in neurons. Neurochem. Res. 28, 15-27 [DOI] [PubMed] [Google Scholar]
- Morselli E., Tasdemir E., Maiuri M. C., Galluzzi L., Kepp O., Criollo A., Vicencio J. M., Soussi T., Kroemer G. (2008). Mutant p53 protein localized in the cytoplasm inhibits autophagy. Cell Cycle 7, 3056-3061 [DOI] [PubMed] [Google Scholar]
- Muller P. A., Caswell P. T., Doyle B., Iwanicki M. P., Tan E. H., Karim S., Lukashchuk N., Gillespie D. A., Ludwig R. L., Gosselin P., et al. (2009). Mutant p53 drives invasion by promoting integrin recycling. Cell 139, 1327-1341 [DOI] [PubMed] [Google Scholar]
- Murray-Zmijewski F., Slee E. A., Lu X. (2008). A complex barcode underlies the heterogeneous response of p53 to stress. Nat. Rev. Mol. Cell Biol. 9, 702-712 [DOI] [PubMed] [Google Scholar]
- Olive K. P., Tuveson D. A., Ruhe Z. C., Yin B., Willis N. A., Bronson R. T., Crowley D., Jacks T. (2004). Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell 119, 847-860 [DOI] [PubMed] [Google Scholar]
- Olivier M., Hollstein M., Hainaut P. (2010). TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harbor Perspect. Biol. 2, a001008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parant J., Chavez-Reyes A., Little N. A., Yan W., Reinke V., Jochemsen A. G., Lozano G. (2001). Rescue of embryonic lethality in Mdm4-null mice by loss of Trp53 suggests a nonoverlapping pathway with MDM2 to regulate p53. Nat. Genet. 29, 92-95 [DOI] [PubMed] [Google Scholar]
- Ravikumar B., Futter M., Jahreiss L., Korolchuk V. I., Lichtenberg M., Luo S., Massey D. C., Menzies F. M., Narayanan U., Renna M., et al. (2009). Mammalian macroautophagy at a glance. J. Cell Sci. 122, 1707-1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley T., Sontag E., Chen P., Levine A. (2008). Transcriptional control of human p53-regulated genes. Nat. Rev. Mol. Cell Biol. 9, 402-412 [DOI] [PubMed] [Google Scholar]
- Romanova L. Y., Willers H., Blagosklonny M. V., Powell S. N. (2004). The interaction of p53 with replication protein A mediates suppression of homologous recombination. Oncogene 23, 9025-9033 [DOI] [PubMed] [Google Scholar]
- Ruzankina Y., Schoppy D. W., Asare A., Clark C. E., Vonderheide R. H., Brown E. J. (2009). Tissue regenerative delays and synthetic lethality in adult mice after combined deletion of Atr and Trp53. Nat. Genet. 41, 1144-1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablina A. A., Budanov A. V., Ilyinskaya G. V., Agapova L. S., Kravchenko J. E., Chumakov P. M. (2005). The antioxidant function of the p53 tumor suppressor. Nat. Med. 11, 1306-1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah V. P., Attardi L. D., Mulligan G. J., Williams B. O., Bronson R. T., Jacks T. (1995). A subset of p53-deficient embryos exhibit exencephaly. Nat. Genet. 10, 175-180 [DOI] [PubMed] [Google Scholar]
- Suzuki H. I., Yamagata K., Sugimoto K., Iwamoto T., Kato S., Miyazono K. (2009). Modulation of microRNA processing by p53. Nature 460, 529-533 [DOI] [PubMed] [Google Scholar]
- Tasdemir E., Chiara Maiuri M., Morselli E., Criollo A., D'Amelio M., Djavaheri-Mergny M., Cecconi F., Tavernarakis N., Kroemer G. (2008a). A dual role of p53 in the control of autophagy. Autophagy 4, 810-814 [DOI] [PubMed] [Google Scholar]
- Tasdemir E., Maiuri M. C., Galluzzi L., Vitale I., Djavaheri-Mergny M., D'Amelio M., Criollo A., Morselli E., Zhu C., Harper F., et al. (2008b). Regulation of autophagy by cytoplasmic p53. Nat. Cell Biol. 10, 676-687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodoro J. G., Evans S. K., Green M. R. (2007). Inhibition of tumor angiogenesis by p53: a new role for the guardian of the genome. J. Mol. Med. 85, 1175-1186 [DOI] [PubMed] [Google Scholar]
- Toledo F., Krummel K. A., Lee C. J., Liu C. W., Rodewald L. W., Tang M., Wahl G. M. (2006). A mouse p53 mutant lacking the proline-rich domain rescues Mdm4 deficiency and provides insight into the Mdm2-Mdm4-p53 regulatory network. Cancer Cell 9, 273-285 [DOI] [PubMed] [Google Scholar]
- Toledo F., Lee C. J., Krummel K. A., Rodewald L. W., Liu C. W., Wahl G. M. (2007). Mouse mutants reveal that putative protein interaction sites in the p53 proline-rich domain are dispensable for tumor suppression. Mol. Cell. Biol. 27, 1425-1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyner S. D., Venkatachalam S., Choi J., Jones S., Ghebranious N., Igelmann H., Lu X., Soron G., Cooper B., Brayton C., et al. (2002). p53 mutant mice that display early ageing-associated phenotypes. Nature 415, 45-53 [DOI] [PubMed] [Google Scholar]
- Vaseva A. V., Moll U. M. (2009). The mitochondrial p53 pathway. Biochim. Biophys. Acta 1787, 414-420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A., Kirsch D. G., McLaughlin M. E., Tuveson D. A., Grimm J., Lintault L., Newman J., Reczek E. E., Weissleder R., Jacks T. (2007). Restoration of p53 function leads to tumour regression in vivo. Nature 445, 661-665 [DOI] [PubMed] [Google Scholar]
- Vousden K. H., Lane D. P. (2007). p53 in health and disease. Nat. Rev. Mol. Cell Biol. 8, 275-283 [DOI] [PubMed] [Google Scholar]
- Vousden K. H., Prives C. (2009). Blinded by the light: the growing complexity of p53. Cell 137, 413-431 [DOI] [PubMed] [Google Scholar]
- Vousden K. H., Ryan K. M. (2009). p53 and metabolism. Nat. Rev. Cancer 9, 691-700 [DOI] [PubMed] [Google Scholar]
- Wade M., Wang Y. V., Wahl G. M. (2010). The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell. Biol. 20, 299-309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Kua H. Y., Hu Y., Guo K., Zeng Q., Wu Q., Ng H. H., Karsenty G., de Crombrugghe B., Yeh J., et al. (2006). p53 functions as a negative regulator of osteoblastogenesis, osteoblast-dependent osteoclastogenesis, and bone remodeling. J. Cell Biol. 172, 115-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W., Zender L., Miething C., Dickins R. A., Hernando E., Krizhanovsky V., Cordon-Cardo C., Lowe S. W. (2007). Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656-660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lu H. (2009). Signaling to p53: ribosomal proteins find their way. Cancer Cell 16, 369-377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilfou J. T., Lowe S. W. (2009). Tumor suppressive functions of p53. Cold Spring Harbor Perspect. Biol. 1, a001883 [DOI] [PMC free article] [PubMed] [Google Scholar]