Tumor masses support naive T cell infiltration, activation, and differentiation into effectors (original) (raw)
Abstract
Studies of T cell responses to tumors have focused on the draining lymph node (LN) as the site of activation. We examined the tumor mass as a potential site of activation after adoptive transfer of naive tumor-specific CD8 T cells. Activated CD8 T cells were present in tumors within 24 h of adoptive transfer and proliferation of these cells was also evident 4–5 d later in mice treated with FTY720 to prevent infiltration of cells activated in LNs. To confirm that activation of these T cells occurred in the tumor and not the tumor-draining LNs, we used mice lacking LNs. Activated and proliferating tumor-infiltrating lymphocytes were evident in these mice 24 h and 4 d after naive cell transfer. T cells activated within tumors acquired effector function that was evident both ex vivo and in vivo. Both cross-presenting antigen presenting cells within the tumor and tumor cells directly presenting antigen activated these functional CD8 effectors. We conclude that tumors support the infiltration, activation, and effector differentiation of naive CD8 T cells, despite the presence of immunosuppressive mechanisms. Thus, targeting of T cell activation to tumors may present a tool in the development of cancer immunotherapy.
CD8 T cells are a crucial component of the adaptive immune response to malignancies. Antigen-experienced CD8 T cells specific for tumor antigens can be recovered from the blood, lymphoid organs, and tumors of both cancer patients and tumor-bearing mice. In multiple mouse tumor models, CD8 T cells are required for the immune control or rejection of the tumor (Dunn et al., 2004). In addition, recent studies have shown that the presence of tumor-infiltrating lymphocytes (TIL) is a positive prognostic factor for patients with colorectal, ovarian, esophageal, and pancreatic cancer, as well as for melanoma and glioma patients (Galon et al., 2006; Pagès et al., 2010). Multiple groups, including our own, have shown robust activation of CD8 T cells in the tumor-draining LNs over the course of tumor outgrowth (Marzo et al., 1999; Bai et al., 2001; Wolkers et al., 2001; Spiotto et al., 2002; Hargadon et al., 2006). Although two previous studies attempted to address the tumor as a potential site of naive T cell activation, they relied on temporal separation of the appearance of activated T cells in the draining LN and tumor (Bai et al., 2001) or used tumors at an early stage of growth (Shrikant and Mescher, 1999) that may not accurately reflect the structure, environment, and activating potential of an established tumor (Schreiber et al., 2006). Thus, the potential for the tumor to serve as a site of naive T cell activation remains largely unexplored.
The tumor mass is an attractive site of T cell priming, as it provides a large depot of antigen and contains multiple cell types that could present that antigen (Balkwill and Mantovani, 2001). Cross-presentation of tumor antigen by DCs in the tumor-draining LN (Nelson et al., 2001; Wolkers et al., 2001; Nguyen et al., 2002; Spiotto et al., 2002; Nowak et al., 2003; Hargadon et al., 2006), as well as direct presentation by tumor cells that have migrated to the tumor-draining LN (Wolkers et al., 2001; Hargadon et al., 2006), have been demonstrated. In addition, BM-derived stromal cells within the tumor have been shown to present antigen to differentiated effectors (Zhang et al., 2007) and activated CD8 T cells have been shown to proliferate further within the microenvironment of brain tumors (Masson et al., 2007). However, it is not known whether intratumoral DCs/myeloid cells or tumor cells can activate naive CD8 T cells within the tumor mass.
Naive T cells follow a restricted migratory pattern through blood, LN, and efferent lymph because of their high expression of CD62L and CCR7 and their low expression of tissue-specific adhesion molecules and chemokine receptors (von Andrian and Mackay, 2000). Therefore, a central question is whether naive T cells could access tumor masses in nonlymphoid tissues. However, multiple studies have also demonstrated that naive T cell infiltrate peripheral tissues as part of their normal migratory activity (Westermann et al., 1996; Zippelius et al., 2004; Cose et al., 2006; Galkina et al., 2006; Staton et al., 2006). Most recently, T cells with a naive phenotype have been detected in skin, lung, liver, lamina propria, and brain (Cose et al., 2006). Although tumors manipulated to express LIGHT (Yu et al., 2004) or lymphotoxin α (Schrama et al., 2001; Kim et al., 2004) can attract naive T cells, the ability of naive T cells to infiltrate unmanipulated tumors has not been addressed.
A final consideration is whether the tumor microenvironment will support or suppress the activation of naive T cells. Tumors contain a diverse array of immune cells and proinflammatory cytokines (Balkwill and Mantovani, 2001; Coussens and Werb, 2002). Tumor invasion into normal tissue, hypoxia, and necrosis can augment this inflammatory response. However, tumors are also considered to be immunosuppressive. Myeloid-derived suppressor cells, T reg cells, IDO, and TGF-β have all been associated with the presence of dysfunctional CD4 and CD8 TILs (Zou, 2005; Rabinovich et al., 2007). If naive T cells access the antigen presentation within tumors, the presence of immunosuppression might limit their potential for activation and differentiation.
In this study, we directly address whether naive T cells can infiltrate normal tumors and become activated there. We separated T cell activation in the tumor-draining LN from that potentially occurring in the tumor mass using FTY720, a drug that prevents the egress of T cells from LNs, and using mice lacking LNs. These methods also allowed us to track the outcomes of T cell activation in each location and examine the effector differentiation and functional activity of T cells activated within tumors. Our work demonstrates that naive CD8 T cells can infiltrate tumors, become activated there, and differentiate into functional effectors.
RESULTS
Naive and activated T cells are found in B16 melanoma tumors 24 h after transfer
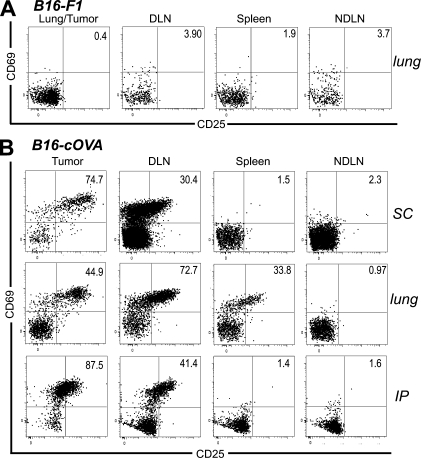
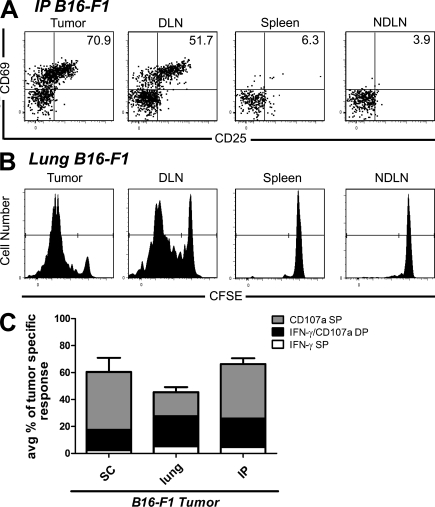
To explore the potential for naive T cells to infiltrate tumors and become activated there, we used B16-F1 melanoma cells stably transfected with cytoplasmically expressed chicken OVA (B16-cOVA). These cells present the OVA257 epitope in the context of H2-Kb, which is recognized by OT-I TCR transgenic T cells. Lung, s.c., or i.p. B16-F1 or B16-cOVA tumors were established in C57BL/6 mice. Naive OT-I T cells were then transferred into these mice and LN, spleen, and tumor were evaluated 24 h later. Naive OT-I cells were present in B16-F1 tumors growing in all locations, indicating that they could infiltrate tumors in the absence of antigen. (Fig. 1 A). Host-derived CD8 T cells with a naive surface phenotype (CD44lo) were also present among the TIL (Fig. S1 A), suggesting that naive endogenous CD8 T cells also infiltrate tumors. OT-I cells were also present in B16-cOVA tumors 24 h after transfer, but a majority displayed an activated phenotype based on expression of CD69 and CD25 (Fig. 1 B). The concurrent presence of naive and activated OT-I cells in B16-cOVA tumors 24 h after transfer suggests that activation occurs within the tumor when cognate antigen is present.
Figure 1.
Naive and activated OT-I cells are found within B16 melanoma tumors 24 h after transfer into mice. C57BL/6 mice bearing established s.c. (17–21-d-old), lung (17–21-d-old), or i.p. (10–15-d-old) B16-F1 (A) or B16-cOVA (B) tumors received Thy1-mismatched OT-I T cells. Axes on plots are displayed in Logicle scale. 24 h later, lymphocytes from tumors, LN, and spleen were harvested and stained for flow cytometry. Plots are gated on Thy1.1+ CD8+ lymphocytes, and numbers indicate the percentage of OT-I cells that are single positive for CD69 or CD25, or positive for both markers. Tumor location is noted to the right of dot plots. Results are representative of at least three independent experiments, with five or more mice each per tumor location.
OT-I cells found in tumors 24 h after transfer were activated in situ and are localized within tumor parenchyma
T cells activated 24 h after transfer were found not only in the B16-cOVA tumors growing in different locations but also in the tumor-draining LN and in the case of lung tumors, the spleen (Fig. 1 B). The absence of activated T cells in nondraining LN (Fig. 1 B) at this time suggested that they had not moved from their initial site of activation in appreciable numbers. To address this possibility more directly, we evaluated whether OT-I cells activated in a lymphoid organ accumulate within 24 h in established B16-F1 tumors, which cannot serve a site of their activation. 17–21 d after intravenous injection of B16-F1 to create lung tumors, mice were injected with B16-cOVA cells via the same route to target tumor-draining lymphoid organs (Hargadon et al., 2006). Naive OT-I cells were transferred 24 h later. Activated OT-I cells were found in lung tumor-draining mediastinal LN and spleen, but not in the B16-F1 tumor (Fig. 2 A). This strongly suggests that activated T cells do not move from the draining lymphoid organs within 24 h.
Figure 2.
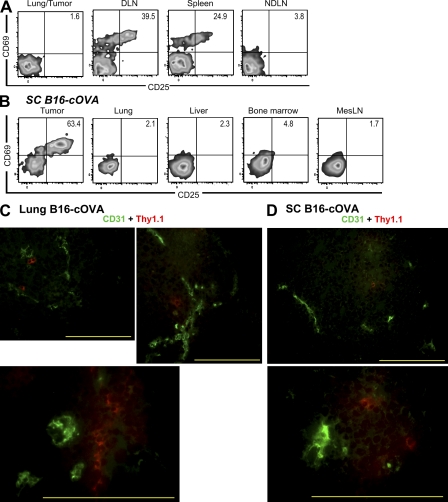
OT-I cells found in tumors 24 h after transfer were activated in situ and are localized within tumor parenchyma. (A) C57BL/6 mice bearing established B16-F1 lung tumors were challenged with B16-cOVA via the lateral tail vein, followed 24 h later by Thy1-mismatched OT-I cells. Lungs/tumors, LN, and spleen were harvested 24 h later, and lymphocytes were stained for flow cytometry. Results represent three independent experiments. n = 3. (B) C57BL/6 mice lacking LN after in utero ablation were splenectomized and injected s.c. with B16-cOVA 3 wk later. After tumors were established, mice were injected with Thy1.1+ OT-I cells. Tumors and indicated tissues were harvested 24 h later, and lymphocytes were stained for flow cytometry. Results are an example of n = 4 animals in 2 independent experiments. All plots in (A and B) are gated on Thy1.1+ CD8+ OT-I lymphocytes, and numbers indicate the percentage of OT- I cells that are single positive for CD69 or CD25, or positive for both markers. Axes on plots are displayed in Logicle scale. (C and D) Naive OT-I T cells were transferred into mice C57BL/6 mice bearing established lung (C) or s.c. (D) B16-cOVA tumors. Localization of OT-I cells within tumors was visualized via their Thy1.1 staining and CD31 staining on tumor vasculature. Images are representative of multiple fields and magnifications of three lung and s.c. tumors in three independent experiments. Bars, 200 µm.
A possible limitation of the above experiment is that specific antigens might be required to retain OT-I cells in the tumor. To directly demonstrate that OT-I cells could be activated within the tumor, we used in utero administration of lymphotoxin–β receptor–Ig and TNF receptor 55–Ig fusion proteins to block the formation of all LNs except for a small residual mesenteric LN (Rennert et al., 1998). At 8 wk of age, these mice were splenectomized, inoculated with B16-cOVA to form subcutaneous tumors, and injected with OT-I cells when the tumors were established. 24 h after T cell transfer, OT-I cells had infiltrated the residual mesenteric LN, liver, lung, and BM. However, these cells remained uniformly naive (Fig. 2 B). In contrast, robustly activated OT-I cells were found in tumors at this time. Similar results were observed in animals with established lung tumors (Fig. S2). These results demonstrate that naive CD8 T cells infiltrate established tumors and become activated there.
As it is not technically feasible to flush the tumor vasculature of passenger leukocytes, we sought to confirm that OT-I cells were actually located within tumors, and not merely in tumor-associated vasculature. Using immunofluoresence and anti–Thy-1.1 antibody, OT-I cells were observed both deep within tumor parenchyma and in a perivascular configuration (Fig. 2, C and D). Although we expect that some naive OT-I cells do remain in the bloodstream, our observations indicate that the OT-I cells are located intratumorally, consistent with their having encountered antigen in that location.
Characteristics of T cells infiltrating tumors
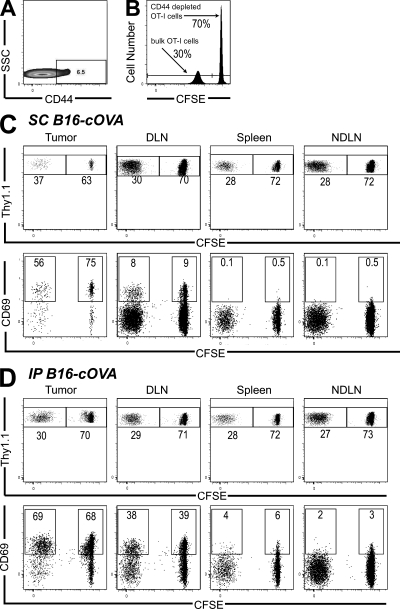
We hypothesized that some naive OT-I cells might express chemokine receptors or adhesion molecules that could enable them to infiltrate tumors. Although the vast majority of freshly isolated naive CD8 T cells expressed CD62L and CCR7, ∼5–10% of the naive OT-I cells transferred in the aforementioned experiments were CD44hi (Fig. 3 A). To determine whether these “memory-like” cells might be the rapid tumor-infiltrating cells, we evaluated the relative infiltration of unlabeled bulk OT-I cells and CFSE-labeled, CD44-depleted OT-I cells. 24 h after cotransfer into mice bearing s.c. or i.p. B16-cOVA tumors, the ratio of these two populations in the tumor was equivalent to the input ratio and to that observed in all evaluated lymphoid compartments (Fig. 3, B–D). In addition, activation occurred in both the bulk and CD44-depleted OT-I populations within the tumor. CD44+ OT-I cells were also not enriched in the tumor as compared with the tumor-draining LN 24 h after bulk OT-I transfer (Fig. S3 A). These results indicate that the presence of naive and activated T cells within tumors does not depend on CD44hi cells in the transferred population.
Figure 3.
Naive T cell tumor infiltration and intratumoral activation does not require the presence of CD44hi cells. (A) Expression of CD44 on CD8 cells from pooled LN and spleen of an OT-I Thy1.1+ mouse. (B) A 70:30 mixture of CFSE-labeled, CD44-depleted, and unlabeled bulk OT-I cells was transferred into Thy1-mismatched mice bearing established s.c. (C) or i.p. (D) tumors. 24 h after transfer, tumors, LN, and spleen were harvested and lymphocytes were stained for flow cytometry. Plots are gated on Thy1.1+ CD8+ lymphocytes. Numbers in the top row indicate the percentage of either CD44-depleted or bulk CD8 cells. Axes on plots are displayed in Logicle scale. Numbers in the bottom row indicate the percentage of each population that is CD69+. Results represent n = 4 mice per tumor location in 2 independent experiments.
Small populations of naive OT-I cells also expressed CD11a and/or α4. 24 h after transfer into s.c. B16-cOVA tumor-bearing animals, OT-I cells expressing α4 or CD11a were highly enriched among cells infiltrating the tumor as compared with the draining LN (Fig. S3 A). These two molecules were only partially coexpressed (unpublished data). In addition, α4+ and CD11a+ cells also expressed higher levels of those molecules as compared with their α4+ and CD11a+ counterparts in the tumor-draining LN (Fig. S3 B). This suggests that naive OT-Is expressing higher levels of α4 and/or CD11a preferentially enter the tumor over the draining LN.
Endogenous lymphocytes are not required for naive T cell infiltration into tumors or their in situ activation
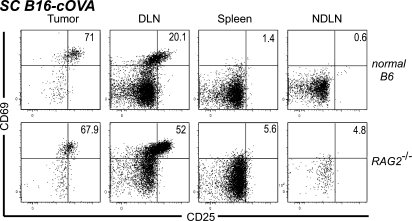
To determine whether endogenous lymphocytes that have accumulated in the tumor over the course of its development are required to support naive OT-I infiltration or intratumoral activation, we evaluated RAG2−/− mice. After transfer into RAG2−/− mice bearing established s.c. B16-cOVA tumors, OT-I cells were present in the tumor, and a large fraction displayed an activated phenotype in both the tumor and the draining LN (Fig. 4). The representation of activated cells in the tumor was comparable to that seen in control animals. These results demonstrate that endogenous B and T cells are not required to support the infiltration of naive OT-I cells into B16 tumors or their local activation there. Furthermore, these results show that the initiation of the OT-I response can occur within the tumor microenvironment, without prior endogenous cell recruitment.
Figure 4.
Endogenous lymphocytes are not required for naive T cell infiltration into tumors or their in situ activation. C57BL/6 mice or RAG2−/− mice bearing s.c. tumors received Thy1-mismatched OT-I T cells. 24 h later, lymphocytes from tumors, LN, and spleen were harvested and stained for flow cytometry. Plots are gated on Thy1.1+ CD8+ lymphocytes, and numbers indicate the percentage of OT-I cells that are single positive for CD69 or CD25, or positive for both markers. Axes on plots are displayed in Logicle scale. Results represent two independent experiments with three mice each per group.
Naive T cell infiltration and activation occurs in different tumor types and with T cells of different avidities
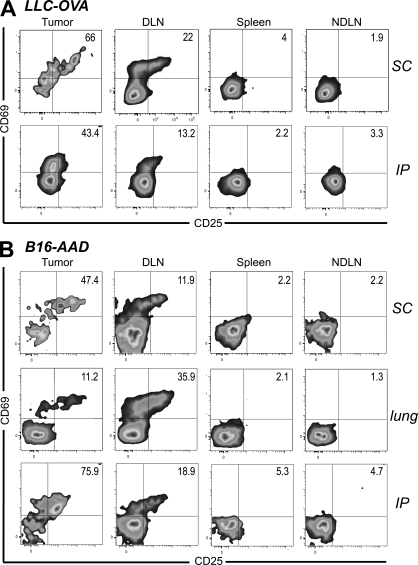
To determine whether tumors other than B16 could support naive T cell infiltration and activation, we used the Lewis lung carcinoma (LLC) transfected with OVA (LLC-OVA). 24 h after naive cell transfer, activated OT-I cells were present in established LLC-OVA tumors growing in different anatomical locations (Fig. 5 A). We also evaluated lower avidity FH TCR transgenic T cells, which recognize the endogenous tyrosinase-derived epitope Tyr369 presented on B16-F1 melanoma cells transfected with the recombinant class I MHC molecule, AAD (B16-AAD). Again, activated FH T cells were also present 24 h after naive cell transfer into AAD+ albino nice bearing established B16-AAD tumors (Fig. 5 B). In combination, these results indicate that infiltration of naive T cells into tumors and their subsequent activation is generalizable to different tumor types and T cell avidities.
Figure 5.
Naive T cell infiltration and activation within tumors extends to different tumor types and T cell avidities. C57BL/6 mice bearing established s.c. or i.p. LLC-OVA tumors (A) or AAD+ mice bearing established s.c., lung, or i.p. B16-AAD tumors (B) received Thy1-mismatched OT-I or FH T cells, respectively. 24 h later, tumors, LN, and spleen were harvested and stained for flow cytometry. Plots are gated on Thy1.1+ CD8+ OT-I cells (A) or Thy1.2+ CD8+ FH cells (B). Tumor location is indicated to the right of dot plots. Numbers indicate the percentage of OT-I or FH cells that are single positive for CD69 or CD25, or positive for both markers. Axes on plots are displayed in Logicle scale. Results represent at least two independent experiments with three or more mice each for each tumor type and location.
Naive T cells that infiltrate tumors proliferate in situ
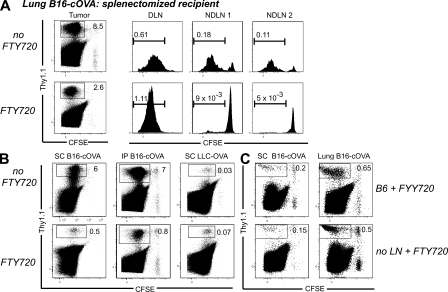
Because the tumor microenvironment is generally considered to be immunosuppressive, we next determined the fate of T cells activated there. 4 d after transfer, we observed divided OT-I cells in the tumor (Fig. 6 A). To eliminate the draining LN as a potential source of these divided TIL, we first used the sphingosine 1-phosphate analogue FTY720, which prevents T cell egress from LNs (Brinkmann et al., 2002; Brinkmann and Lynch, 2002). As observed previously (Sheasley-O’Neill et al., 2007), FTY720 treatment blocked the redistribution of divided OT-I cells from the draining LN to nondraining LN, which is normally seen by 4 d (Fig. 6 A and Fig. S4, A–C). However, significant numbers of divided tumor-specific T cells were still present in FTY720-treated animals bearing lung, s.c., or i.p. B16-cOVA, LLC-OVA, and B16-AAD tumors (Fig. 6, A and C, and not depicted). In the case of lung tumors, the spleen is also an initial priming site (Fig. 1 A). As it is controversial whether FTY720 causes retention of T cells in the spleen, splenectomized mice were used as lung tumor recipients (Fig. 6 A). The decrease in representation of divided OT-I cells in nondraining LN relative to untreated controls was significantly larger than the corresponding decrease in the tumor (Fig. S4, A–C), suggesting that the divided OT-I cells present in the tumor did not arise from the draining LN. We confirmed this using mice lacking LNs. Divided OT-I cells were found 4 d after transfer into mice bearing established s.c. or lung B16-cOVA tumors whose LNs had been ablated in utero (Fig. 6 C). Furthermore, the size of the tumor-infiltrating OT-I population in these LN-free mice was the same as the population in FTY720-treated animals with LNs in the same experiment. The lack of difference between these two populations further supports the conclusion that naive OT-I cells proliferate within tumors after being initially activated there. These results demonstrate that tumor masses, without any contribution from a draining LN, support the initial activation and proliferation of CD8 T cells.
Figure 6.
Naive T cells that infiltrate tumors proliferate in situ. (A and B) C57BL/6, ATA, or splenectomized C57BL/6 mice bearing established B16-cOVA, B16-AAD, or LLC-OVA tumors in the locations indicated received CFSE-labeled Thy1-mismatched OT-I or FH cells. FTY720 treatment was initiated at the time of T cell transfer and continued for the duration of the experiment. 4 d after T cell transfer, tumors and lymphoid organs were harvested, and lymphocytes were stained for flow cytometry. Only tumors are shown in B. Dot plots are gated on total lymphocytes. Numbers on dot plots indicate the percentage of tumor-specific T cells out of total lymphocytes. Axes on plots are displayed in Logicle scale. Histograms are gated on CD8+ OT-I or FH cells. Gates indicate all cells that have undergone at least one division. Results represent at least two independent experiments per tumor type, with at least three mice each. (C) Normal C57BL/6 mice or C56BL/6 mice lacking LN after in utero ablation were splenectomized. s.c. or lung tumors were established, and the mice received CFSE-labeled Thy 1.1+ OT-I cells. FTY720 treatment was initiated at the time of T cell transfer, and continued for the duration of the experiment. 4 d after T cell transfer, tumors were harvested and stained for flow cytometry. Plots are gated on total lymphocytes. Numbers on dot plots indicate the percentage of tumor-specific T cells out of total lymphocytes. Axes on plots are displayed in Logicle scale. Results represent n = 4 animals per tumor location in 2 independent experiments.
FTY720 treatment reduced the size of the B16 melanoma-infiltrating T cell population originating from the adoptively transferred OT-I cells, although it had no impact on endogenous T cells that had already infiltrated the tumor before treatment. However, although intratumorally primed T cells were always evident in tumors of FTY720-treated and LN-ablated mice, the size of this population depended on both tumor type and location. i.p. and s.c. tumors of FTY720-treated animals contained the smallest populations relative to the corresponding tumors in untreated animals (15 ± 2% [n = 9] and 10 ± 2% [n = 11], respectively; Fig. 6, A and B). However, the intratumorally primed population was a much larger fraction of the total TIL in lung tumors (40% ± 12%; n = 10). Interestingly, although the size of the TIL population in untreated mice bearing LLC-OVA tumors was much smaller than for B16, there was no diminution in the size of this population in FTY720-treated animals (Fig. 6 C). This identifies intratumorally primed T cells as the most significant source of TIL in that tumor type.
Activation of naive intratumoral T calls occurs at multiple T cell precursor frequencies
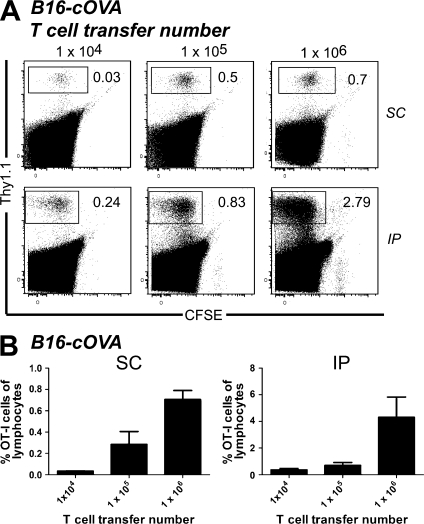
Because the aforementioned experiments were done using 106 adoptively transferred cells, it was of interest to know whether the T cells activated within the tumor represented a significant source of TIL at more physiological precursor frequencies. We titrated the OT-I adoptive transfer number from 104 to 106 in FTY720-treated, s.c., and i.p. B16-cOVA tumor-bearing animals. We observed divided T cells in the tumors of these animals at all precursor frequencies (Fig. 7). Although their representation among total lymphocytes decreased with decreasing transfer number, it was higher than expected given the differences in the number of transferred cells. This could be the result of more efficient infiltration of naive T cells into tumors or of more efficient expansion of intratumorally activated T cells at low adoptive transfer number. Regardless, these results suggest that activation of naive intratumoral T cells could contribute to TIL in an endogenous situation.
Figure 7.
Activation of naive intratumoral T calls occurs at multiple T cell precursor frequencies. C57BL/6 bearing established s.c. or i.p. B16-cOVA tumors received 104, 105, or 106 CFSE-labeled Thy1-mismatched OT-I cells. FTY720 treatment was initiated at the time of T cell transfer and continued for the duration of the experiment. 5 d after T cell transfer, tumors and lymphoid organs were harvested and lymphocytes were stained for flow cytometry. Tumor location is indicated to the right of dot plots. Dot plots in A are gated on total lymphocytes. Numbers in dot plots indicate the percentage of OT-I cells out of total lymphocytes in the tumor. Axes on plots are displayed in Logicle scale. Results represent 2 independent experiments, with n = 3–4 mice each for each tumor location and are summarized in B.
CD8 T cells activated within tumors acquire effector function
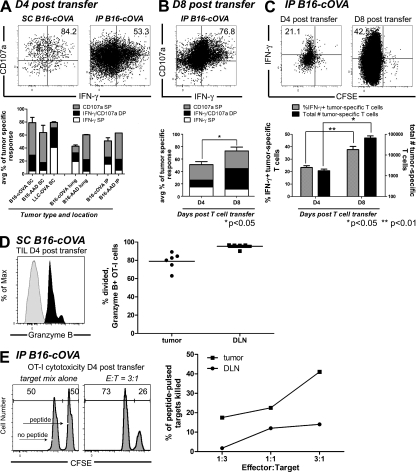
We next asked whether activation of naive CD8 T cells within tumors generates functional effectors. When OT-I cells isolated 4 d after transfer from B16-cOVA tumors of FTY720-treated mice were stimulated ex vivo with OVA257 peptide-pulsed stimulators, a significant fraction secreted IFN-γ and most also expressed CD107a, a proxy for cytotoxic activity (Fig. 8 A). These effector functions were also acquired by T cells activated in different tumor types, by different tumor antigens, and in different anatomical locations. The vast majority of intratumorally activated T cells also expressed Granzyme B (Fig. 8 D). Although a slightly lower percentage of OT-I cells in the tumor were Granzyme B+ as compared with cells in the draining LN of the same animal, this is consistent with extensive degranulation of these tumor-infiltrating cells in vivo as they continually encounter tumor cells. When T cells were isolated 8 d after transfer, an even higher overall percentage of OT-I cells expressed these effector functions, and the representation of dual functional (IFN-γ+ and CD107a+) effectors more than doubled (Fig. 8 B). The percentage of intratumorally activated T cells expressing effector functions was equivalent to or greater than that of T cells activated in the draining LN of the same animal (Fig. S5, A–C). The expression of effector function by intratumorally activated T cells was also comparable to that of T cells activated by peptide-pulsed, CD40L-activated BM-derived DCs (20–45% IFN-γ+ OT-I cells; Hargadon et al., 2006; Sheasley-O’Neill et al., 2007) or vaccinia virus expressing OVA (40–70% IFN-γ+, 70% Granzyme B+ OT-I cells; Fig. S6).
Figure 8.
CD8 T cells activated within tumors acquire effector function. (A and B) Ex vivo analysis of tumor-specific T cell function. Mice bearing established B16-cOVA, B16-AAD, or LLC-OVA in the indicated locations received CFSE-labeled Thy1-mismatched OT-I or FH cells. FTY720 treatment was initiated at the time of T cell transfer and continued for the duration of the experiment. 4 (A) or 8 (B) d after T cell transfer, tumors were harvested and stained for flow cytometry after a brief ex vivo restimulation. Plots are gated on tumor-specific Thy1.1+ CD8+ OT-I cells. Numbers on plots indicate the total percentage of OT-I cells that are single positive for IFN-γ or CD107a or double positive for both markers. Axes on plots are displayed in Logicle scale. Graphs summarize the composition of the antitumor effector response (A) and compare the antitumor response at 4 and 8 d after T cell transfer (B). Results represent at least two independent experiments with at least three mice per tumor type. (C) Analysis of in vivo IFN-γ production by tumor-specific T cells. C57BL/6 mice bearing established i.p. B16-cOVA tumors were given CFSE-labeled OT-I cells. FTY720 treatment was initiated at the time of T cell transfer and continued for the duration of the experiment 4 or 8 d after T cell transfer, mice were treated with BFA, and 4 h later tumors were harvested and stained for flow cytometry. Dot plots are gated on Thy1.1+ CD8+ OT-I cells. Numbers on plots indicate the percentage of OT-I cells that are divided (CFSE negative) and IFN-γ+. Axes on plots are displayed in Logicle scale. Graph summarizes in vivo IFN-γ production and the number of OT-I cells in the tumor. Results represent 2 independent experiments, with n = 3 mice at each time point. Statistical analyses in (A and B) were performed using a two-tailed Student’s T test (Prism version 5.0; GraphPad Software, Inc.). (D) Granzyme B expression. Lymphocytes were isolated from s.c. tumors and tumor-draining LN of FTY720-treated animals 4 d after initial naive cell transfer. Gray histogram indicates isotype control staining and black histogram indicates staining on OT-I T cells. Histograms are gated on Thy1.1+ CD8+ lymphocytes. Axes on plots are displayed in Logicle scale. Graph summarizes the percentage of divided OT-I that express Granzyme B when activated in the tumor or the draining LN. Staining was performed with n = 6 mice in 1 experiment. (E) In vitro cytotoxicity of intratumorally primed OT-I cells. Thy1.1+ OT-I cells were isolated from i.p. tumors and tumor-draining LN of FTY720-treated animals 4 d after initial naive cell transfer, and then incubated with a mix of CFSE-labeled peptide-pulsed and unpulsed targets. Histograms are gated on CFSE-labeled target cells. Numbers on histograms represent the percentage of target cells that are CFSElo or CFSEhi. Axes on plots are displayed in Logicle scale. Results represent pooling of OT-I cells from eight tumors and eight tumor-draining LN in each of three independent experiments.
A potential concern with the aforementioned experiment is that CD8 T cells could be suppressed in vivo, but might recover rapidly during an ex vivo stimulation. Therefore, we administered Brefeldin A (BFA) to FTY720-treated B16-cOVA-bearing mice to cause the accumulation of any IFN-γ produced by OT-I cells while they still remain exposed to both antigen and potential suppressive factors in the tumor. When OT-I cells were isolated and analyzed 4 h after BFA treatment without additional ex vivo stimulation, one third to one half of the cells had accumulated IFN-γ (Fig. 8 C). Again there was an evident increase in the fraction of functional effectors and their absolute number between 4 and 8 d of tumor residence. Importantly, intratumorally activated T cells were able to kill peptide-pulsed target cells in vitro, indicating that the other markers of effector function we observed correlated with actual cytotoxic ability (Fig. 8 E). These results demonstrate that tumors can support the activation, expansion, and in vivo effector activity of initially naive intratumoral CD8 T cells. In addition, they show that intratumorally activated effectors retain their functionality in vivo, despite prolonged exposure to potentially suppressive factors in the tumor microenvironment.
Both tumor cells and cross-presenting APCs induce the activation, proliferation, and effector differentiation of naive intratumoral CD8 T cells
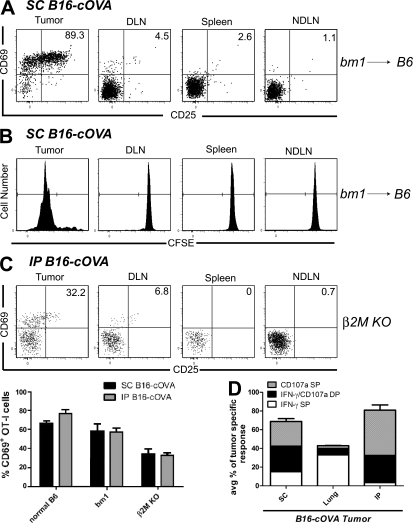
We sought to determine which cells within the tumor mediated the activation of intratumoral CD8 T cells. To evaluate the importance of cross-presenting APCs, we eliminated direct antigen presentation by tumor cells. B16-F1 expresses tyrosinase but lacks the AAD MHC I molecule necessary to present the Tyr369 epitope. AAD+ mice bearing established B16-F1 tumors received Tyr369-specific FH T cells, and in long-term experiments, repeated administration of FTY720. Intratumoral FH T cells up-regulated CD69 and CD25 24 h after transfer, proliferated, and acquired the ability to secret IFN-γ and degranulate 4 d later (Fig. 9, A–C). These results demonstrate the presence of functional cross-presenting APCs in the tumor.
Figure 9.
Intratumoral cross-presenting APCs are sufficient for the activation and effector differentiation of naive CD8 T cells. (A and B) ATA bearing established s.c., lung, or i.p. B16-F1 tumors received Thy1-mismatched unlabeled (A) or CFSE-labeled FH T cells. 24 h (A) or 4 d (B) after T cell transfer, tumors and lymphoid organs were harvested and stained for flow cytometry. In B, FTY720 treatment was initiated at the time of T cell transfer and continued for the duration of the experiment. All plots are gated on Thy1.2+ CD8+ FH lymphocytes. Numbers in A indicate the percentage of FH cells are single positive for CD69 or CD25, or positive for both markers. Gates on plots in B indicate FH cells that have undergone at least one division. Axes on plots are displayed in Logicle scale. (C) Ex vivo effector analysis. FH cells from tumors harvested 4 d after T cell transfer were also briefly restimulated ex vivo and stained for IFN-γ and CD107a. The composition of the antitumor effector response in each tumor location is summarized in C. Results are represent n = 6 mice per tumor location and time point in 4 independent experiments, 2 with s.c. tumors and 1 each with i.p. and lung tumors.
To determine whether direct presentation by the tumor could activate naive CD8 T cells, we eliminated cross-presentation of the OVA257 epitope using bm1→C57BL/6 chimeras bearing B16-cOVA. The BM-derived compartment of these mice is unable to present tumor antigen to OT-I T cells. OT-I activation and proliferation was drastically reduced in the draining LN of tumor-bearing chimeras, demonstrating that cross-presentation had been effectively eliminated. However, activation, proliferation, and acquisition of effector function by intratumoral OT-I cells in chimeras was comparable to that seen in normal C57BL/6 animals (Fig. 10, A, B, and D). To eliminate the possibility of continued cross-presentation by a radioresistant cell in the tumors of these chimeras, β2M−/− mice lacking MHC class I expression were challenged with B16-cOVA. Activation of transferred naive OT-I cells remained apparent in the tumors of these mice, although the percentage of CD69+ OT-I cells was somewhat decreased compared with that in tumors of bm1 chimeras. This is consistent with the possibility that a radioresistant cell may also cross-present antigen in the tumor. Although we cannot rule out the possibility of “cross-dressing” of bm1 or β2M−/− mice APCs with MHC–OVA257 peptide complexes derived from the tumor (Dolan et al., 2006), we consider that direct presentation by the tumor cells themselves accounts for the majority of OT-I activation in these animals. We conclude that APCs cross-presenting tumor antigen and tumor cells directly presenting antigen are each sufficient to direct the effector differentiation of naive intratumoral T cells.
Figure 10.
Tumor cells directly presenting antigen are sufficient for the activation and effector differentiation of naive CD8 T cells. Bm1 → B6 BM chimeras (A and B) bearing established s.c., lung, or i.p. B16-cOVA tumors or β2M−/− mice bearing established s.c. or i.p. tumors (C) received Thy1-mismatched unlabeled (A and C) or CFSE-labeled (B) OT-I T cells. 24 h (A and C) or 4 d (B) after T cell transfer, tumors and lymphoid organs were harvested and lymphocytes were stained for flow cytometry. Graph in C summarizes the initial activation of OT-I cells in control mice, bm1 chimeras, and β2M−/− mice In (B) FTY720 treatment was initiated at the time of T cell transfer and continued for the duration of the experiment. All plots are gated on Thy1.1+ CD8+ OT-I lymphocytes. Numbers in (A and C) indicate the percentage of OT-I cells that are single positive for CD69 or CD25, or positive for both markers. Gates on plots in (B) indicate OT-I cells that have undergone at least one division. Axes on plots are displayed in Logicle scale. (D) Ex vivo effector analysis. OT-I cells from tumors harvested 4 d after T cell transfer were also briefly restimulated ex vivo and stained for IFN-γ and CD107a. The composition of the antitumor effector response in each tumor location is summarized in (C). Results (A, B, and D) represent n = 6 mice per tumor location and time point in 4 independent experiments, 2 with s.c. tumors and 1 each with i.p. and lung tumors. Results in C represent 3 s.c. and 3 i.p. tumor-bearing animals in 2 independent experiments.
DISCUSSION
In this study, we investigated the capacity of tumors to support the entry and activation of naive CD8 T cells. Naive T cells were found in tumors lacking relevant antigen and significant numbers of activated T cells were found within 24 h in tumors that did express the relevant antigen. The presence of these activated T cells was independent of the presence of a draining LNs or endogenous B and T cells. These early infiltrating cells subsequently proliferated and acquired multiple markers of effector function. We observed similar processes of activation in different tumor types and with tumor-specific T cells of different avidities. Both cross-presenting APCs in the tumor and tumor cells themselves were capable of mediating naive T cell activation and differentiation. Our results show that naive T cells can infiltrate tumors regardless of the presence of antigen, and can become fully differentiated effectors when cognate antigen is present. Although the precise contribution of intratumorally activated T cells to overall antitumor immunity remains to be determined, our results clearly demonstrate that these cells possess the potential for antitumor activity while residing in the immunosuppressive tumor microenvironment.
The observation that both tumor-specific and nonspecific naive T cells can infiltrate multiple tumor types runs counter to the widely held view that naive T cells cannot access peripheral tissues. Naive T cell trafficking is highly skewed toward lymphoid organs (von Andrian and Mackay, 2000). However, multiple studies have demonstrated that naive T cells infiltrate peripheral tissues as part of their normal migratory activity (Westermann et al., 1996; Zippelius et al., 2004; Cose et al., 2006, Cose et al., 2007; Galkina et al., 2006; Staton et al., 2006). Robust naive T cell recruitment to inflamed peripheral tissues has also been reported in the setting of infection, autoimmune disease, and graft rejection (Kobayashi et al., 2004; Drayton et al., 2006; Lee et al., 2006; Nasr et al., 2007). Naive T cells infiltrate tumors engineered to express LIGHT or lymphotoxin α (Schrama et al., 2001; Kim et al., 2004; Yu et al., 2004), or tumors injected with DCs genetically modified to express CCL21 (Kirk et al., 2001). Our work extends this body of literature and demonstrates that recruitment of naive T cells to tumors occurs in the absence of any artificially introduced inflammatory stimulus or manipulation.
The infiltration of naive T cells into tumors may result from normal, stochastic migration of these cells through nonlymphoid sites or their specific attraction to an inflamed nonlymphoid site, a phenomenon that often correlates with the presence of tertiary lymphoid organs (TLOs; Drayton et al., 2003; Weninger et al., 2003; Kobayashi et al., 2004; Drayton et al., 2006; Lee et al., 2006). Some human tumors have been shown to contain lymphoid aggregates resembling TLO (Bell et al., 1999; Coronella et al., 2002; Miyagawa et al., 2004; Ladányi et al., 2007). A different B16 tumor than the one used in our study has also been reported to express CCL21, a chemokine which could attract naive T cells, and to develop a lymphoidlike stroma, whereas traditional TLO were not observed (Shields et al., 2010). It is an attractive possibility that the chronic inflammatory environment within tumors could drive the development of TLO that might promote the recruitment of naive CD8 T cells to the tumor. Development of TLO has been shown to be independent of lymphoid tissue-inducer cells, but dependent on the presence of CD4 T cells and DCs (Marinkovic et al., 2006; GeurtsvanKessel et al., 2009; Halle et al., 2009). Our observation of continued naive T cell infiltration of tumors and in situ activation in RAG−/− animals suggests that TLO are not absolutely required for these processes. Multiple pathways may exist for the infiltration of naive T cells into tumors, and TLO could represent one of these. Although not required, TLO and endogenous lymphocytes might enhance naive T cell infiltration and could qualitatively alter the tumor microenvironment by producing chemokines and other inflammatory mediators that could support activation of naive T cells. Further work is needed to understand the requirements for naive T cell infiltration into tumors and whether tumor vasculature resembles that of normal tissue, or recapitulates the vasculature and microenvironment of an inflamed tissue.
Our finding that intratumoral APCs cross-presenting tumor antigen can activate naive T cells is consistent with previous studies demonstrating similar functionality for APCs resident in tumor-draining LNs (Nelson et al., 2001; Spiotto et al., 2002; Hargadon et al., 2006). However, the observation that tumor cells directly drive naive T cell activation and effector differentiation is unexpected. Most tumors do not express the co-stimulatory molecules CD80 and CD86 and would be considered nonprofessional APCs. The differences in the percentage of naive OT-I cells that became activated in bm1 chimeras versus β2M−/− mice suggested that a radioresistant cell within tumors may also cross-present antigen. However, as our chimeras successfully eliminate >95% of professional APCs, this cell is likely to also be a nonprofessional APC, and the same set of questions regarding the ability of this cell type to activate naive T cells is relevant. TCR stimulation alone is usually insufficient for induction of a productive T cell response (Shahinian et al., 1993; Acuto and Michel, 2003; Smith-Garvin et al., 2009). However, responses to skin allografts (Kawai et al., 1996) and viruses with high replicative potential (Shahinian et al., 1993) can proceed in mice deficient in CD28, suggesting that high TCR occupancy can provide the signal amplification normally provided through CD28 co-stimulation (Acuto and Michel, 2003). Increased duration of TCR signaling can also overcome the necessity of CD28 co-stimulation (Kündig et al., 1996). The large number of tumor cells present in an established tumor may also provide a continuous, high level of antigen that bypasses CD28 signaling. Alternatively, tumor cells might express another molecule that serves a co-stimulatory function. Both CD24 and CD70 are expressed by certain tumors and have been shown to provide co-stimulation for naive T cells (Wang et al., 1995; Watts, 2005; Boursalian et al., 2009). CD24 is expressed by B16, the melanoma used in our study (Niers et al., 2009). In addition, a wide range of human and mouse tumors, including B16 (Morimoto et al., 2008), express ligands for DNAM-1, which has been shown to aid T cell recognition of antigen displayed by nonprofessional APCs (Gilfillan et al., 2008). Thus, the combination of high levels of antigen and potential expression of other molecules with co-stimulatory function may compensate for the lack of classical co-stimulatory molecules when tumor cells or other nonprofessional APCs are the source of antigen for naive T cells.
The extranodal activation of T cells within tumors identifies a previously unappreciated source of TIL. Although it is difficult to estimate the proportion of TIL that may arise from activation within the draining LN versus the tumor itself, intratumorally activated T cells clearly do contribute to the overall CD8 T cell response present in tumors. Interestingly, the contribution of the LN and the tumor as sources of TIL varies by tumor type and location. Although the LN draining B16 tumors contributes most of the TIL in those tumors, lung B16 in particular can generate a substantial percentage of the total population. In contrast, the representation of tumor-specific T cells in LLC tumors after FTY720 treatment is equal to or greater than that seen in untreated tumor-bearing animals. This suggests that LLC tumors produce a larger fraction of their own TIL than their draining LNs. These differences between tumors could reflect variation at several points: efficiency of priming in the draining LN, migration of cells from the draining LN to the tumor, initial infiltration of naive T cells into different tumors, or the ability of tumors in different locations to support the proliferation or survival of intratumorally primed T cells.
Beyond the initial activation and proliferation of T cells within tumors, our work also demonstrates high levels of in vivo functionality among intratumorally primed CD8 T cells. Other groups have also described functional TIL within both B16 and LLC masses (Prévost-Blondel et al., 1998; Nelson et al., 2001), but the tumors inevitably grow out in their hosts despite the presence of these functional tumor-specific CD8 effectors. Thus, although functional T cells can be observed within tumors, they are typically unable to control tumor growth. Several factors could contribute to this lack of control: an insufficient number of functional T cells might be present, T cells might become exhausted in the face of high levels of chronic antigen (Shin and Wherry, 2007; Mueller and Ahmed, 2009), or T cells might be actively suppressed within the tumor microenvironment (Zou, 2005; Rabinovich et al., 2007). Several immunosuppressive mechanisms including T reg cells (Ghiringhelli et al., 2005), STAT-3 activation (Wang et al., 2004; Kortylewski et al., 2005), myeloid-derived suppressor cell (Gabrilovich et al., 2001; Sica and Bronte, 2007), and IDO (Friberg et al., 2002; Munn et al., 2004) have been described for either B16 or LLC. B16 has also been suggested to foster a tolerogenic environment via a CCL21-driven development of lymphoidlike stroma (Shields et al., 2010). Although CCL21 could therefore both recruit naive T cells and support their suppression, a direct impact on newly tumor-infiltrating naive T cells was not evaluated. Immunosuppression could limit the infiltration of naive T cells into tumors, the number of functional cells that can be generated (Kortylewski et al., 2005; Fridlender et al., 2009), or could curtail T cell function over time. However, our work clearly demonstrates that immunosuppression within B16 and LLC tumors is not sufficient to prevent the initial activation and differentiation of naive CD8 T cells nor to eliminate their in vivo functionality. The finding that tumors support naive T cell differentiation suggests that the tumor itself could be exploited therapeutically as a vaccination site (Jackaman et al., 2008) for the in situ development of functional T cells. In situ activation of T cells in the tumor could address several potential culprits for the failure of the antitumor response. Our results show that the fraction of naive T cells that are activated in the tumor is much larger than that in the draining LN, likely caused by the large depot of antigen available in the tumor mass. Although the efficiency of naive T cell infiltration into tumors clearly limits the contribution of intratumorally cells to the total TIL population, intratumoral injection of chemoattractants (Kirk et al., 2001; Furumoto et al., 2004; Wong et al., 2008) or naive T cells themselves could overcome this limitation. This also provides the potential for repetitive injection, leading to a constant source of functional cells as long as antigen remains available.
In conclusion, this study demonstrates that tumors support the infiltration, activation, and effector differentiation of naive T cells. These T cells express this function in the tumor for prolonged periods of time. Although endogenous T cell responses often fail to control tumors, our results identify a therapeutic opportunity to harness intratumoral antigen presentation to enhance antitumor immunity in situ.
MATERIALS AND METHODS
Mice.
C57BL/6 and CD45.1 congenic (NCI-Frederick Animal Production Program), OT-I RAG1−/−, and RAG2−/− mice (Taconic), Thy1.1 congenic, bm1, and β2M−/− mice (Jackson Immunoresearch Laboratories) were maintained under specific pathogen–free conditions. C57BL/6 albino mice with a deletion of the tyrosinase locus and expressing AAD, a MHC class I molecule composed of the α1 and α2 domains of HLA-A*0201, and the α3 domain of the H2-Dd have been previously described (Colella et al., 2000). FH TCR transgenic mice expressing a TCR-specific for the Tyr369 epitope in the context of AAD and have been previously described (Nichols et al., 2007). Protocols were approved by the University of Virginia Institutional Animal Care and Use Committee.
In utero ablation of LN.
Pregnant C57BL/6 females were injected i.v. on gestational d 8, 11, and 14 with 100 µg of lymphotoxin-β receptor-Ig and TNF receptor 55-Ig fusions proteins in 200 µl sterile saline as previously described (Rennert et al., 1998). Absence of LN was confirmed by extensive dissection at the time of necropsy. Protocol was adapted from previously described methods.
Splenectomy.
8-wk-old C57BL/6 or Thy-1.1+ AAD+ albino mice were splenectomized and allowed to recover for at least 2–3 wk before tumor challenge or other manipulation.
BM chimeras.
C57BL/6 CD45.1 congenic mice were lethally irradiated (650 rad × 2) and reconstituted with 4 × 106 bm1 BM cells that had been depleted of CD4+ and CD8+ cells (Miltenyi Biotec). Chimerism of all mice was confirmed 8 wk after irradiation by sampling peripheral blood via the lateral tail vein. On average, >95% of all CD45+ cells and of all cell subsets with high potential for antigen presentation (CD11c, CD11b, and Gr-1) were of donor origin (CD45.2+).
Tumor cells and injections.
B16-F1 mouse melanoma cells were obtained from American Type Culture Collection. B16-cOVA (Hargadon et al., 2006) and B16-AAD (Mullins et al., 2001) have been previously described. LLC transfected to express cOVA was a gift of E. Podack (University of Miami, Miami, FL). 4 × 105 tumor cells were injected i.v. via the lateral tail vein, s.c. in the nape of the neck, or i.p. to establish tumors in the lungs, subcutaneous space, or i.p. cavity, respectively. Tumors in the peritoneum grow as a solid mass. Tumors were allowed to establish in the animal (10–15 d for i.p.; 17–21 d for i.v. or s.c.), before any other manipulations, unless otherwise noted.
Adoptive transfer of TCR transgenic T cells.
In experiments evaluating infiltration and activation at 24 h, 4 × 106 T cells from spleen and pooled LN of OT-I Thy1.1 or FH TCR transgenic mice were injected. Except where indicated, in experiments evaluating division and effector function 4 or 8 d after transfer, 106 cells were injected that had been labeled before injection with 5 µM CFSE for 15 min at 37°C.
CD44+ cell depletion.
Single-cell suspensions from pooled LN and spleen of OT-I Thy1.1 mice were enriched for CD8 T cells using a negative selection kit (Miltenyi Biotec). Enriched CD8 T cells were then incubated with PE-labeled anti-CD44 antibody and anti-PE microbeads (Miltenyi Biotec) to remove CD44+ CD8 T cells from the bulk CD8 population.
FTY720 treatment.
Mice were injected i.p. at the time of T cell transfer with 1 mg/kg FTY720 (gift from V. Brinkmann, Novartis Pharma AG, Basel, Switzerland) in sterile saline and maintained on drinking water containing 2 µg/ml FTY720 for the duration of the experiment.
Processing of tissues and tumors.
To isolate lymphocytes from the lungs and liver, the organs were flushed with 1 ml PBS via the right ventricle or the portal vein, as appropriate, and removed. Nonlymphoid tissues and tumors were minced and incubated in HBSS with 0.1 mg/ml collagenase A (Roche) and 60 U/ml DNase I (Sigma-Aldrich) for 30 min at 37°C. When possible, large tumor nodules were dissected away from normal tissue. All tissues and tumors were then homogenized, filtered through nylon mesh, and lymphocytes were isolated on Lympholyte-M (Cedarlane). When BM was taken, cells were harvested by repeatedly flushing the femurs and tibias from each mouse with PBS.
Flow cytometry.
Single-cell suspensions from LN or spleen and lymphocytes isolated from lungs, liver, or tumors were incubated with CD16/32 (2.4G2; BioXCell) to block Fc receptors, and then with one or more of the following: APC-Alexa Fluor 750 anti-CD8α (53–6.7), PerCP-Cy5.5 anti-Thy1.1 (HIS51), FITC anti-CD69 (H1.2F3), PE anti-CD25 (PC61.5), PE anti-CD44 (IM7), PE anti-CD107a (1D4B), APC anti-IFN-γ (XMG1.2), APC-Alexa Fluor 750 anti-CD45.1 (A20), PerCP-Cy5.5 anti-CD45.2 (104), FITC anti-CD11c (N418), PE anti-CD11b (M1/70), and APC anti-Ly-6G,C (Gr-1; all from eBioscience); APC-Cy7 anti CD4 (GK1.5; BD); and PerCP anti-Thy1.2 (30-H12; BioLegend). Samples were run on a FACSCanto (BD) and analyzed using FlowJo software (Tree Star, Inc.).
Immunofluorescence microscopy.
LNs, s.c. tumors, and tumor-bearing lung lobes were flash frozen in liquid nitrogen and subsequently cut into 6-µM slices in the Research Histology Core at the University of Virginia School of Medicine. Tissue sections were fixed with acetone and incubated with CD16/32 (2.4G2; BioXCell) to block Fc receptors. Sections were blocked with a Biotin-Avidin Blocking kit (Vector Laboratories) and stained with FITC anti-CD31(390) and biotinylated anti-Thy1.1(HIS51); followed by DyLight-488 anti-FITC (Jackson ImmunoResearch Laboratories) and Texas red streptavidin (SouthernBiotech). Slides were mounted in Vectashield with DAPI (Vector Laboratories). Sample slices were counterstained with hematoxylin and eosin by the Research Histology Core (University of Virginia School of Medicine) to examine LN and tumor structure; this was done in combination with DAPI, which was used to distinguish normal lung tissue from tumor nodules.
Ex vivo analysis of tumor-specific T cell function.
OT-I and FH cells isolated from tissues and tumors were incubated with LB15.13 cells pulsed with 10 µM OVA257 or AAD-transfected C1R lymphoblastoid cells pulsed with 10 µM Tyr369, respectively, for 5 h at 37°C. Media was supplemented with 50 U/ml IL-2 (Chiron), 10 µg/ml monensin (BD), and 10 µg/ml brefeldin-A (Sigma-Aldrich). CD16/32 was used to block Fc receptors for 15 min before PE anti-CD107a antibody was added for the duration of the 5-h stimulation. Cells were then stained for surface molecule expression, fixed, and permeabilized using Cytofix/Cytoperm (BD) and stained for intracellular IFN-γ.
In vivo analysis of tumor-specific T cell production of IFN-γ.
Tumor-bearing mice were injected i.p. with 250 ng of Brefeldin-A (Sigma-Aldrich). 4 h later, TILs were immediately isolated and stained for surface molecule expression, and then fixed, permeabilized, and stained for intracellular IFN-γ.
Ex vivo analysis of tumor-specific T cell cytotoxicity.
Lymphocytes isolated from tumors were incubated with anti-Thy1.1 microbeads (Miltenyi Biotec). Thy1.1+ OT-I cells were positively selected using an AutoMACS Pro Separator (Miltenyi Biotec). OT-I cells were then co-cultured for 5 h at 37°C at fixed ratios with a 1:1 mix of LB15.13 cells pulsed with 10 µM OVA257 and stained with 1 µM CFSE and unpulsed LB15.13 cells stained with 0.1 µM CFSE. Media was supplemented with 50 U/ml IL-2 (Chiron). Percent killing was assessed by evaluating percent loss of the peptide-pulsed population relative to the unpulsed population.
Online supplemental material.
Fig. S1 shows gating of transferred tumor-specific T cells and CD69 and CD25 expression on endogenous TIL. Fig. S2 shows that naive T cells become activated within lung tumors in mice lacking a tumor DLN. Fig. S3 shows enrichment of α4 and CD11a hi OT-I within tumors early after transfer. Fig. S4 shows the impact of FTY720 on the presence of divided OT-I cells in nondraining lymphoid compartments and in the tumor 4 d after transfer. Fig. S5 shows the effector function of CD8 T cells activated in the tumor-draining LN. Fig. S6 shows representative effector function of CD8 T cells after activation with vaccinia-OVA. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20092454/DC1.
Acknowledgments
We thank Dr. E. Podack for providing LLC-OVA and the Research Histology Core at the University of Virginia School of Medicine for tissue section preparation. We also thank Janet Gorman and Holly Davis for animal husbandry, Dr. Kara Cummings for laboratory support, and the rest of the Engelhard laboratory for insightful discussions and advice.
This work was supported by United States Public Health Service (USPHS) grant CA78400 to V.H. Engelhard. E.D. Thompson was supported by USPHS training grants GM007267 and AI7496. H.L. Enriquez was supported by USPHS training grant AI7496.
The authors declare that they have no competing financial interests.
Footnotes
Abbreviations used:
BFA
Brefeldin A
LLC
Lewis lung carcinoma
TIL
tumor-infiltrating lymphocyte
TLO
tertiary lymphoid organ
References
- Acuto O., Michel F. 2003. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat. Rev. Immunol. 3:939–951 10.1038/nri1248 [DOI] [PubMed] [Google Scholar]
- Bai X.F., Gao J.X., Liu J., Wen J., Zheng P., Liu Y. 2001. On the site and mode of antigen presentation for the initiation of clonal expansion of CD8 T cells specific for a natural tumor antigen. Cancer Res. 61:6860–6867 [PubMed] [Google Scholar]
- Balkwill F., Mantovani A. 2001. Inflammation and cancer: back to Virchow? Lancet. 357:539–545 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- Bell D., Chomarat P., Broyles D., Netto G., Harb G.M., Lebecque S., Valladeau J., Davoust J., Palucka K.A., Banchereau J. 1999. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J. Exp. Med. 190:1417–1426 10.1084/jem.190.10.1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursalian T.E., McEarchern J.A., Law C.L., Grewal I.S. 2009. Targeting CD70 for human therapeutic use. Adv. Exp. Med. Biol. 647:108–119 10.1007/978-0-387-89520-8_7 [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Lynch K.R. 2002. FTY720: targeting G-protein-coupled receptors for sphingosine 1-phosphate in transplantation and autoimmunity. Curr. Opin. Immunol. 14:569–575 10.1016/S0952-7915(02)00374-6 [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Davis M.D., Heise C.E., Albert R., Cottens S., Hof R., Bruns C., Prieschl E., Baumruker T., Hiestand P., et al. 2002. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 277:21453–21457 10.1074/jbc.C200176200 [DOI] [PubMed] [Google Scholar]
- Colella T.A., Bullock T.N.J., Russell L.B., Mullins D.W., Overwijk W.W., Luckey C.J., Pierce R.A., Restifo N.P., Engelhard V.H. 2000. Self-tolerance to the murine homologue of a tyrosinase-derived melanoma antigen: implications for tumor immunotherapy. J. Exp. Med. 191:1221–1232 10.1084/jem.191.7.1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronella J.A., Spier C., Welch M., Trevor K.T., Stopeck A.T., Villar H., Hersh E.M. 2002. Antigen-driven oligoclonal expansion of tumor-infiltrating B cells in infiltrating ductal carcinoma of the breast. J. Immunol. 169:1829–1836 [DOI] [PubMed] [Google Scholar]
- Cose S. 2007. T-cell migration: a naive paradigm? Immunology. 120:1–7 10.1111/j.1365-2567.2006.02511.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cose S., Brammer C., Khanna K.M., Masopust D., Lefrançois L. 2006. Evidence that a significant number of naive T cells enter non-lymphoid organs as part of a normal migratory pathway. Eur. J. Immunol. 36:1423–1433 10.1002/eji.200535539 [DOI] [PubMed] [Google Scholar]
- Coussens L.M., Werb Z. 2002. Inflammation and cancer. Nature. 420:860–867 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan B.P., Gibbs K.D., Jr., Ostrand-Rosenberg S. 2006. Dendritic cells cross-dressed with peptide MHC class I complexes prime CD8+ T cells. J. Immunol. 177:6018–6024 [DOI] [PubMed] [Google Scholar]
- Drayton D.L., Ying X., Lee J., Lesslauer W., Ruddle N.H. 2003. Ectopic LT alpha beta directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J. Exp. Med. 197:1153–1163 10.1084/jem.20021761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayton D.L., Liao S., Mounzer R.H., Ruddle N.H. 2006. Lymphoid organ development: from ontogeny to neogenesis. Nat. Immunol. 7:344–353 10.1038/ni1330 [DOI] [PubMed] [Google Scholar]
- Dunn G.P., Old L.J., Schreiber R.D. 2004. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 21:137–148 10.1016/j.immuni.2004.07.017 [DOI] [PubMed] [Google Scholar]
- Friberg M., Jennings R., Alsarraj M., Dessureault S., Cantor A., Extermann M., Mellor A.L., Munn D.H., Antonia S.J. 2002. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int. J. Cancer. 101:151–155 10.1002/ijc.10645 [DOI] [PubMed] [Google Scholar]
- Fridlender Z.G., Sun J., Kim S., Kapoor V., Cheng G., Ling L., Worthen G.S., Albelda S.M. 2009. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 16:183–194 10.1016/j.ccr.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furumoto K., Soares L., Engleman E.G., Merad M. 2004. Induction of potent antitumor immunity by in situ targeting of intratumoral DCs. J. Clin. Invest. 113:774–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich D.I., Velders M.P., Sotomayor E.M., Kast W.M. 2001. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J. Immunol. 166:5398–5406 [DOI] [PubMed] [Google Scholar]
- Galkina E., Kadl A., Sanders J., Varughese D., Sarembock I.J., Ley K. 2006. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J. Exp. Med. 203:1273–1282 10.1084/jem.20052205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galon J., Costes A., Sanchez-Cabo F., Kirilovsky A., Mlecnik B., Lagorce-Pagès C., Tosolini M., Camus M., Berger A., Wind P., et al. 2006. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 313:1960–1964 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- GeurtsvanKessel C.H., Willart M.A., Bergen I.M., van Rijt L.S., Muskens F., Elewaut D., Osterhaus A.D., Hendriks R., Rimmelzwaan G.F., Lambrecht B.N. 2009. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J. Exp. Med. 206:2339–2349 10.1084/jem.20090410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F., Puig P.E., Roux S., Parcellier A., Schmitt E., Solary E., Kroemer G., Martin F., Chauffert B., Zitvogel L. 2005. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J. Exp. Med. 202:919–929 10.1084/jem.20050463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan S., Chan C.J., Cella M., Haynes N.M., Rapaport A.S., Boles K.S., Andrews D.M., Smyth M.J., Colonna M. 2008. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J. Exp. Med. 205:2965–2973 10.1084/jem.20081752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle S., Dujardin H.C., Bakocevic N., Fleige H., Danzer H., Willenzon S., Suezer Y., Hämmerling G., Garbi N., Sutter G., et al. 2009. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. J. Exp. Med. 206:2593–2601 10.1084/jem.20091472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargadon K.M., Brinkman C.C., Sheasley-O’neill S.L., Nichols L.A., Bullock T.N.J., Engelhard V.H. 2006. Incomplete differentiation of antigen-specific CD8 T cells in tumor-draining lymph nodes. J. Immunol. 177:6081–6090 [DOI] [PubMed] [Google Scholar]
- Jackaman C., Lew A.M., Zhan Y., Allan J.E., Koloska B., Graham P.T., Robinson B.W., Nelson D.J. 2008. Deliberately provoking local inflammation drives tumors to become their own protective vaccine site. Int. Immunol. 20:1467–1479 10.1093/intimm/dxn104 [DOI] [PubMed] [Google Scholar]
- Kawai K., Shahinian A., Mak T.W., Ohashi P.S. 1996. Skin allograft rejection in CD28-deficient mice. Transplantation. 61:352–355 10.1097/00007890-199602150-00003 [DOI] [PubMed] [Google Scholar]
- Kim H.J., Kammertoens T., Janke M., Schmetzer O., Qin Z., Berek C., Blankenstein T. 2004. Establishment of early lymphoid organ infrastructure in transplanted tumors mediated by local production of lymphotoxin alpha and in the combined absence of functional B and T cells. J. Immunol. 172:4037–4047 [DOI] [PubMed] [Google Scholar]
- Kirk C.J., Hartigan-O’Connor D., Mulé J.J. 2001. The dynamics of the T-cell antitumor response: chemokine-secreting dendritic cells can prime tumor-reactive T cells extranodally. Cancer Res. 61:8794–8802 [PubMed] [Google Scholar]
- Kobayashi M., Mitoma J., Nakamura N., Katsuyama T., Nakayama J., Fukuda M. 2004. Induction of peripheral lymph node addressin in human gastric mucosa infected by Helicobacter pylori. Proc. Natl. Acad. Sci. USA. 101:17807–17812 10.1073/pnas.0407503101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortylewski M., Kujawski M., Wang T., Wei S., Zhang S., Pilon-Thomas S., Niu G., Kay H., Mulé J., Kerr W.G., et al. 2005. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat. Med. 11:1314–1321 10.1038/nm1325 [DOI] [PubMed] [Google Scholar]
- Kündig T.M., Shahinian A., Kawai K., Mittrücker H.W., Sebzda E., Bachmann M.F., Mak T.W., Ohashi P.S. 1996. Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity. 5:41–52 10.1016/S1074-7613(00)80308-8 [DOI] [PubMed] [Google Scholar]
- Ladányi A., Kiss J., Somlai B., Gilde K., Fejos Z., Mohos A., Gaudi I., Tímár J. 2007. Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol. Immunother. 56:1459–1469 10.1007/s00262-007-0286-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Chin R.K., Christiansen P., Sun Y., Tumanov A.V., Wang J., Chervonsky A.V., Fu Y.X. 2006. Recruitment and activation of naive T cells in the islets by lymphotoxin beta receptor-dependent tertiary lymphoid structure. Immunity. 25:499–509 10.1016/j.immuni.2006.06.016 [DOI] [PubMed] [Google Scholar]
- Marinkovic T., Garin A., Yokota Y., Fu Y.X., Ruddle N.H., Furtado G.C., Lira S.A. 2006. Interaction of mature CD3+CD4+ T cells with dendritic cells triggers the development of tertiary lymphoid structures in the thyroid. J. Clin. Invest. 116:2622–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo A.L., Lake R.A., Lo D., Sherman L., McWilliam A., Nelson D., Robinson B.W., Scott B. 1999. Tumor antigens are constitutively presented in the draining lymph nodes. J. Immunol. 162:5838–5845 [PubMed] [Google Scholar]
- Masson F., Calzascia T., Di Berardino-Besson W., de Tribolet N., Dietrich P.Y., Walker P.R. 2007. Brain microenvironment promotes the final functional maturation of tumor-specific effector CD8+ T cells. J. Immunol. 179:845–853 [DOI] [PubMed] [Google Scholar]
- Miyagawa S., Soeda J., Takagi S., Miwa S., Ichikawa E., Noike T. 2004. Prognostic significance of mature dendritic cells and factors associated with their accumulation in metastatic liver tumors from colorectal cancer. Hum. Pathol. 35:1392–1396 10.1016/j.humpath.2004.07.018 [DOI] [PubMed] [Google Scholar]
- Morimoto K., Satoh-Yamaguchi K., Hamaguchi A., Inoue Y., Takeuchi M., Okada M., Ikeda W., Takai Y., Imai T. 2008. Interaction of cancer cells with platelets mediated by Necl-5/poliovirus receptor enhances cancer cell metastasis to the lungs. Oncogene. 27:264–273 10.1038/sj.onc.1210645 [DOI] [PubMed] [Google Scholar]
- Mueller S.N., Ahmed R. 2009. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. USA. 106:8623–8628 10.1073/pnas.0809818106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins D.W., Bullock T.N., Colella T.A., Robila V.V., Engelhard V.H. 2001. Immune responses to the HLA-A*0201-restricted epitopes of tyrosinase and glycoprotein 100 enable control of melanoma outgrowth in HLA-A*0201-transgenic mice. J. Immunol. 167:4853–4860 [DOI] [PubMed] [Google Scholar]
- Munn D.H., Sharma M.D., Hou D., Baban B., Lee J.R., Antonia S.J., Messina J.L., Chandler P., Koni P.A., Mellor A.L. 2004. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J. Clin. Invest. 114:280–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr I.W., Reel M., Oberbarnscheidt M.H., Mounzer R.H., Baddoura F.K., Ruddle N.H., Lakkis F.G. 2007. Tertiary lymphoid tissues generate effector and memory T cells that lead to allograft rejection. Am. J. Transplant. 7:1071–1079 10.1111/j.1600-6143.2007.01756.x [DOI] [PubMed] [Google Scholar]
- Nelson D.J., Mukherjee S., Bundell C., Fisher S., van Hagen D., Robinson B. 2001. Tumor progression despite efficient tumor antigen cross-presentation and effective “arming” of tumor antigen-specific CTL. J. Immunol. 166:5557–5566 [DOI] [PubMed] [Google Scholar]
- Nguyen L.T., Elford A.R., Murakami K., Garza K.M., Schoenberger S.P., Odermatt B., Speiser D.E., Ohashi P.S. 2002. Tumor growth enhances cross-presentation leading to limited T cell activation without tolerance. J. Exp. Med. 195:423–435 10.1084/jem.20010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols L.A., Chen Y., Colella T.A., Bennett C.L., Clausen B.E., Engelhard V.H. 2007. Deletional self-tolerance to a melanocyte/melanoma antigen derived from tyrosinase is mediated by a radio-resistant cell in peripheral and mesenteric lymph nodes. J. Immunol. 179:993–1003 [DOI] [PubMed] [Google Scholar]
- Niers T.M., Brüggemann L.W., Klerk C.P., Muller F.J., Buckle T., Reitsma P.H., Richel D.J., Spek C.A., Van Tellingen O., Van Noorden C.J. 2009. Differential effects of anticoagulants on tumor development of mouse cancer cell lines B16, K1735 and CT26 in lung. Clin. Exp. Metastasis. 26:171–178 10.1007/s10585-008-9227-6 [DOI] [PubMed] [Google Scholar]
- Nowak A.K., Lake R.A., Marzo A.L., Scott B., Heath W.R., Collins E.J., Frelinger J.A., Robinson B.W. 2003. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J. Immunol. 170:4905–4913 [DOI] [PubMed] [Google Scholar]
- Pagès F., Galon J., Dieu-Nosjean M.C., Tartour E., Sautès-Fridman C., Fridman W.H. 2010. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 29:1093–1102 10.1038/onc.2009.416 [DOI] [PubMed] [Google Scholar]
- Prévost-Blondel A., Zimmermann C., Stemmer C., Kulmburg P., Rosenthal F.M., Pircher H. 1998. Tumor-infiltrating lymphocytes exhibiting high ex vivo cytolytic activity fail to prevent murine melanoma tumor growth in vivo. J. Immunol. 161:2187–2194 [PubMed] [Google Scholar]
- Rabinovich G.A., Gabrilovich D., Sotomayor E.M. 2007. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 25:267–296 10.1146/annurev.immunol.25.022106.141609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennert P.D., James D., Mackay F., Browning J.L., Hochman P.S. 1998. Lymph node genesis is induced by signaling through the lymphotoxin beta receptor. Immunity. 9:71–79 10.1016/S1074-7613(00)80589-0 [DOI] [PubMed] [Google Scholar]
- Schrama D., thor Straten P., Fischer W.H., McLellan A.D., Bröcker E.B., Reisfeld R.A., Becker J.C. 2001. Targeting of lymphotoxin-alpha to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid-like tissue. Immunity. 14:111–121 10.1016/S1074-7613(01)00094-2 [DOI] [PubMed] [Google Scholar]
- Schreiber K., Rowley D.A., Riethmüller G., Schreiber H. 2006. Cancer immunotherapy and preclinical studies: why we are not wasting our time with animal experiments. Hematol. Oncol. Clin. North Am. 20:567–584 10.1016/j.hoc.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Shahinian A., Pfeffer K., Lee K.P., Kündig T.M., Kishihara K., Wakeham A., Kawai K., Ohashi P.S., Thompson C.B., Mak T.W. 1993. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 261:609–612 10.1126/science.7688139 [DOI] [PubMed] [Google Scholar]
- Sheasley-O’Neill S.L., Brinkman C.C., Ferguson A.R., Dispenza M.C., Engelhard V.H. 2007. Dendritic cell immunization route determines integrin expression and lymphoid and nonlymphoid tissue distribution of CD8 T cells. J. Immunol. 178:1512–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields J.D., Kourtis I.C., Tomei A.A., Roberts J.M., Swartz M.A. 2010. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science. 328:749–752 10.1126/science.1185837 [DOI] [PubMed] [Google Scholar]
- Shin H., Wherry E.J. 2007. CD8 T cell dysfunction during chronic viral infection. Curr. Opin. Immunol. 19:408–415 10.1016/j.coi.2007.06.004 [DOI] [PubMed] [Google Scholar]
- Shrikant P., Mescher M.F. 1999. Control of syngeneic tumor growth by activation of CD8+ T cells: efficacy is limited by migration away from the site and induction of nonresponsiveness. J. Immunol. 162:2858–2866 [PubMed] [Google Scholar]
- Sica A., Bronte V. 2007. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Invest. 117:1155–1166 10.1172/JCI31422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Garvin J.E., Koretzky G.A., Jordan M.S. 2009. T cell activation. Annu. Rev. Immunol. 27:591–619 10.1146/annurev.immunol.021908.132706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiotto M.T., Yu P., Rowley D.A., Nishimura M.I., Meredith S.C., Gajewski T.F., Fu Y.X., Schreiber H. 2002. Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity. 17:737–747 10.1016/S1074-7613(02)00480-6 [DOI] [PubMed] [Google Scholar]
- Staton T.L., Habtezion A., Winslow M.M., Sato T., Love P.E., Butcher E.C. 2006. CD8+ recent thymic emigrants home to and efficiently repopulate the small intestine epithelium. Nat. Immunol. 7:482–488 10.1038/ni1319 [DOI] [PubMed] [Google Scholar]
- von Andrian U.H., Mackay C.R. 2000. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 343:1020–1034 10.1056/NEJM200010053431407 [DOI] [PubMed] [Google Scholar]
- Wang Y.C., Zhu L., McHugh R., Sell K.W., Selvaraj P. 1995. Expression of heat-stable antigen on tumor cells provides co-stimulation for tumor-specific T cell proliferation and cytotoxicity in mice. Eur. J. Immunol. 25:1163–1167 10.1002/eji.1830250505 [DOI] [PubMed] [Google Scholar]
- Wang T., Niu G., Kortylewski M., Burdelya L., Shain K., Zhang S., Bhattacharya R., Gabrilovich D., Heller R., Coppola D., et al. 2004. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat. Med. 10:48–54 10.1038/nm976 [DOI] [PubMed] [Google Scholar]
- Watts T.H. 2005. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 23:23–68 10.1146/annurev.immunol.23.021704.115839 [DOI] [PubMed] [Google Scholar]
- Weninger W., Carlsen H.S., Goodarzi M., Moazed F., Crowley M.A., Baekkevold E.S., Cavanagh L.L., von Andrian U.H. 2003. Naive T cell recruitment to nonlymphoid tissues: a role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J. Immunol. 170:4638–4648 [DOI] [PubMed] [Google Scholar]
- Westermann J., Smith T., Peters U., Tschernig T., Pabst R., Steinhoff G., Sparshott S.M., Bell E.B. 1996. Both activated and nonactivated leukocytes from the periphery continuously enter the thymic medulla of adult rats: phenotypes, sources and magnitude of traffic. Eur. J. Immunol. 26:1866–1874 10.1002/eji.1830260830 [DOI] [PubMed] [Google Scholar]
- Wolkers M.C., Stoetter G., Vyth-Dreese F.A., Schumacher T.N. 2001. Redundancy of direct priming and cross-priming in tumor-specific CD8+ T cell responses. J. Immunol. 167:3577–3584 [DOI] [PubMed] [Google Scholar]
- Wong S.B., Bos R., Sherman L.A. 2008. Tumor-specific CD4+ T cells render the tumor environment permissive for infiltration by low-avidity CD8+ T cells. J. Immunol. 180:3122–3131 [DOI] [PubMed] [Google Scholar]
- Yu P., Lee Y., Liu W., Chin R.K., Wang J., Wang Y., Schietinger A., Philip M., Schreiber H., Fu Y.X. 2004. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat. Immunol. 5:141–149 10.1038/ni1029 [DOI] [PubMed] [Google Scholar]
- Zhang B., Bowerman N.A., Salama J.K., Schmidt H., Spiotto M.T., Schietinger A., Yu P., Fu Y.X., Weichselbaum R.R., Rowley D.A., et al. 2007. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J. Exp. Med. 204:49–55 10.1084/jem.20062056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippelius A., Bioley G., Le Gal F.A., Rufer N., Brandes M., Batard P., De Smedt M., Plum J., Speiser D.E., Cerottini J.C., et al. 2004. Human thymus exports naive CD8 T cells that can home to nonlymphoid tissues. J. Immunol. 172:2773–2777 [DOI] [PubMed] [Google Scholar]
- Zou W. 2005. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer. 5:263–274 10.1038/nrc1586 [DOI] [PubMed] [Google Scholar]