Histone Modifications: Crucial Elements for Damage Response and Chromatin Restoration (original) (raw)
. Author manuscript; available in PMC: 2011 May 1.
Published in final edited form as: J Cell Physiol. 2010 May;223(2):283–288. doi: 10.1002/jcp.22060
Abstract
Eukaryotic genomes are packaged into chromatin from repeated nucleosome arrays in which DNA sequences wrap around histones. Chromatin organization has profound influence on DNA-templated processes such as transcription, DNA replication and repair. Recent studies have also revealed chromatin dynamics as an active contributor to diverse DNA damage responses. Here, we review recent progress in histone modification related to DNA damage response and post-repair chromatin restoration at the sites of DNA damage. We discuss how the timing and features of histone modifications would provide the initial as well as the final guidance for DNA damage response, and the prospect that modifications may challenge the epigenetic stability of repaired cells and serve as damage memory in chromatin.
In eukaryotic cells, genomic DNA is packaged into chromatin, a highly architectural DNA-histone complex that encodes both genetic and epigenetic information governing specific patterns of gene expression and determining cell identity (Luger, 2006). The basic building block for chromatin is the nucleosome, which consists of DNA in 147 bp length wrapped around a histone octamer, containing a tetramer of histone H3 and H4, flanked by two H2A–H2B dimers (Kornberg, 1977). Nucleosomes with linker DNAs, each about 20–60 bp, form an approximately 10 nm diameter "beads-on-a-string" structure where a linker histone H1 contacts the exit and entry of DNA strand on the nucleosome. The "beads-on-astring" structure coils into a 30 nm diameter helical structure known as the 30 nm filament. This level of chromatin structure is thought to be the form of euchromatin, which is highly dynamic and unfolds into a 10 nm fiber. Variations within nucleosomal module can be achieved by DNA methylation (Klose and Bird, 2006), histone post-translational modifications (PTMs) (Kouzarides, 2007), incorporation of distinct histone variants (Sarma and Reinberg, 2005), and association with chromatin-binding proteins (Bernstein et al, 2007). Superimposed upon DNA sequences, the histone modification and DNA methylation constitute a layer of heritable epigenetic information regulating chromatin structure and DNA accessibility.
Many different PTMs on histones include acetylation, methylation, phosphorylation, ubiquitination, et cetera (Kouzarides, 2007). These modifications occur primarily at specific positions within the amino-terminal of the histone tails. In general, lysine acetylation is related to chromatin accessibility and transcription. The effect of lysine acetylation is in part due to the neutralization of the charges of histone tails, which interact with the DNA backbone. Additionally, lysine acetylation functions through recruiting other protein factors, e.g., bromodomain proteins to a specific region of the genome, for mediating transcription and anti-silencing functions (LeRoy et al, 2008; Ladurner et al, 2003; Matangkasombut and Buratowski, 2003). Lysine methylation, on the other hand, has a different impact on transcription, depending on the positions and degree of methylation. Methylation of H3 lysine (H3K) 4 and 36 is associated with transcribed domains, whereas methylation of H3K9, H3K27 and H4K20 appears to correlate with transcriptional repression. In yeast, methylated H3K4-interacting chromodomain protein, Chd1, was found to be a component of Spt-Ada-Gcn5 acetyltransferase (SAGA) complex, and the interaction between methylated H3K4 and the Chd1 chromodomain recruits SAGA complex to chromatin (Pray-Grant et al, 2005). Human Chd1 binds to methylated H3K4 through its tandem chromodomains, linking the recognition of histone modifications to non-covalent chromatin remodeling (Sims, III et al, 2005). In contrast, methylated H3K9 and H3K27 are recognized by heterochromatin protein 1 (HP1) and Polycomb repressive complexes (PRC). The binding of HP1 and PRC to methylated H3 is influenced by the “methyl states” (Fischle et al, 2003). For example, HP1 binds to both di- and tri-methylated H3K9, while PRC2 mediates tri-methylation at H3K27 to become tri-methyl H3k27. This tri-methyl H3 mark is recognized by PRC1 that in turn ubiquitinates histone H2A at K119, whereas Ring2/Ring1b E3 ubiquitin ligase serves as the core component of PRC1 (Wang et al, 2004). In mice, PRC1 links ubiquitination of histone H2A to heritable gene silencing and X chromosome inactivation (de Napoles et al, 2004). Recent studies indicated that ubiquitinated H2A (uH2A) is enriched at high-density CpG promoters at repressed genes (Kallin et al, 2009). How H2A ubiquitination regulates gene transcription or “repressed state” of chromatin remains largely unknown.
Obviously, chromatin is intimately associated with DNA-templated cellular processes and inevitably influences transcription, DNA replication and DNA repair. While chromatin structure presents a barrier to these processes, chromatin dynamics constitutes an active component of DNA damage response (DDR). Indeed, a number of histone modifications are now known to be involved in DDR. However, the functional significance of these modifications is not fully understood. Here, we review progress in our knowledge of histone modifications in response to ionizing radiation (IR) and ultraviolet (UV) light at specifically marked damage sites in genome. We discuss the implications of these histone modifications in cell cycle regulation, epigenetic stability and damage memory in chromatin.
Histone Modifications before DNA Repair
H2A/H2AX phosphorylation
Among the known PTMs, H2A phosphorylation is very well characterized and intensively studied. The H2A phosphorylation occurs at serine 128 in yeast or at 139 of the H2AX variant in humans (Downs et al, 2000; Rogakou et al, 1998). ATM and DNA-PK function redundantly to phosphorylate H2AX after IR (Stiff et al, 2004), while ATR phosphorylates H2AX in response to replication stress and UV irradiation (Ward and Chen, 2001). In yeast, phosphorylated H2A (γH2A) can be detected as far as 50 Kb on either side of double-strand break (DSB) (Shroff et al, 2004). In human cells, γH2AX involves ~2 Mb DNA region and forms foci that are easily detectable by immunofluorescence microscopy (Rogakou et al, 1998). Phosphorylation of H2AX generates a carboxyl-terminal phospho-motif that is then recognized by the tandem BRCT (BRCA1-carboxyl-terminal) domain-containing protein, MDC1 (mediator of DNA damage checkpoint 1, MDC1), a critical scaffold for mediating downstream events (Stucki et al, 2005). The recruitment of MDC1 to γH2AX is an early event, leading to the recruitment of two downstream protein complexes: MRE11-RAD50-NBS50 (Chapman and Jackson, 2008; Melander et al, 2008; Spycher et al, 2008) and UBC13/RNF8 ubiquitin ligase complex (Wang and Elledge, 2007; Kolas et al, 2007; Huen et al, 2007; Mailand et al, 2007).
Histone ubiquitination
As a consequence of recruitment of the UBC13/RNF8 complex, γH2AX becomes ubiquitinated on chromatin surrounding the DSBs. The ubiquitination is initiated by UBC13/RNF8 ubiquitin ligase complex, and the ubiquitin conjugates are amplified by another ubiquitin ligase RNF168 (Doil et al, 2009; Stewart et al, 2009). RNF8 is recruited through interaction of its FHA domain with phosphorylated MDC1, using phosphorylated TQXF motifs in MDC1 as docking sites. Knocking down of RNF8 impairs G2/M checkpoint, and results in cellular sensitivity to low doses of IR. Thus, the MDC1- and RNF8-dependent histone ubiquitination is a critical event downstream of MDC1 binding. Ubiquitination creates K63-linked polyubiquitin chains on both γH2AX and H2A, leading to subsequent recruitment of Rap80/Abraxas/Brca1/Brcc36 complex (Wang and Elledge, 2007). Not surprisingly, Ubc13-deficient cells are compromised for DSB repair by homologous recombination (HR) (Zhao et al, 2007). Although H2AX is a target of RNF8, mutations of all lysines in H2AX neither abrogate the accumulation of ubiquitin conjugates at DSBs nor affect the recruitment of downstream factors (Doil et al, 2009). Thus, the ubiquitination of histone H2A rather than γH2AX may be the crucial event for subsequent signaling. Besides H2A and γH2AX, H2B has also been found as a substrate of RNF8 (Wu et al, 2009). However, it is not known whether H2B ubiquitination contributes to DDR.
H2A ubiquitination by UBC13/RNF8 ubiquitin ligase complex also occurs at the sites of UV-induced DNA damage (Marteijn et al, 2009). Depletion of these enzymes causes UV hypersensitivity, without affecting nucleotide excision repair (NER), suggesting that UBC13 and RNF8 are only involved in UV-induced DDR. Similar to recruitment events at DSBs, RNF8 is recruited to the sites of UV damage in a MDC1 dependent manner, but requires ATR as well as NER-generated single-stranded repair intermediates. Thus, there may be a conserved common pathway of H2A ubiquitination for both DSBs and UV lesions.
In an effort to purify and characterize histone ubiquitin ligases, Wang, H et al found an ubiquitin ligase activity capable of ubiquitinating all histones in vitro (Wang et al, 2006). The ligase was later characterized as CUL4-DDB-ROC1 complex, an enzyme that is known for DDB2 and XPC ubiquitination at UV damage sites (Sugasawa et al, 2005; El-Mahdy et al, 2006; Wang et al, 2005). A small fraction of Histone H3 and H4 (0.3% and 0.1%, respectively) is found ubiquitinated in vivo, and siRNA-mediated knockdown of CUL4A, B and DDB1 decreases the H3 and H4 ubiquitination levels. In addition, the dynamics of CUL4-DDB-ROC1-mediated H3 and H4 ubiquitination is similar to that of XPC. Indeed, further biochemical studies indicate that the H3 and H4 ubiquitination weakens the interaction between histones and DNA, and facilitates the recruitment of XPC repair factor to damaged DNA. These studies point to a role of H3 and H4 ubiquitination in chromatin disassembly at the sites of UV lesions (Wang et al, 2006).
Histone acetylation
A transient acetylation of H3 and H4 in their amino-terminal tails has been found to occur at DSBs in both mammalian and yeast cells (van and Gasser, 2005). The acetylation is mediated by TIP60 complex in mammals or its homologue NuA4, SWR1 and INO80 in yeast. The TIP60 complex comprises TIP60 HAT, SNf2-related p400, TRRAP and other components. This large multiprotein complex acetylates several lysine residues (H3 K14, K23, H4 K5, K8, K12 and K14) of core histones in vitro (Kimura and Horikoshi, 1998). Mutations of lysine residues in H4 tail confer sensitivity to DSB-generating agents (Tamburini and Tyler, 2005; Bird et al, 2002; Murr et al, 2006). TIP60 is recruited to DSBs, where it binds to and acetylates H4 with cofactor TRRAP (Ikura et al, 2007). Depletion of TRARP impairs recruitment of DNA repair protein 53BP1 and RAD51 but not MDC1, and affects DSB repair. It is not yet known how histone acetylation by TIP60 complex regulates chromatin organization. Recently, TIP60 is found to promote ubiquitination of γH2AX (Ikura et al, 2007). The acetylation appears to be a prerequisite for ubiquitination of γH2AX. Furthermore, the acetylation is mediated by a TIP60-UBC13 complex, and the acetylation leads to release of H2AX from damaged chromatin. Thus, the sequential acetylation and ubiquitination of H2AX may promote histone dynamics at DSBs.
Histone Modification after DNA Repair
In order to distinguish the distinct roles of histone modifications in the course of DDR, it is essential to separate the events occurring before and after the repair of DNA damage. It is becoming increasingly apparent that some histone modifications occur as “post repair” events, as they are linked to the restoration of chromatin occurring after DNA sequences are successfully restored. The plethora of histone modifications occurring upon DNA damage induction suggest that the chromatin in the vicinity of damaged sites undergoes substantial processing and rearrangements. Thus, it is intuitive to reason that histone modifications are critical elements in the execution of DNA damage response. The key question is whether the original chromatin organization is eventually restored faithfully or some lingering epigenetic alterations in the newly restored chromatin are set as the memory of the genomic repair?
Histone modification and DNA replication-coupled nucleosome assembly
During cell proliferation, histone modifications are tightly associated with DNA replication-coupled nucleosome assembly. As DNA replicates, the parental histones are randomly transferred onto either the leading or the lagging strand (Jackson, 1988). When the parental nucleosomes are disrupted, a nucleosome is separated into two H2A–H2B dimers and one H3–H4 tetramer. The latter is randomly transferred onto one of the nascent DNA strands. Newly synthesized H3 and H4 are deposited to nascent DNA strands to form a chromatin precursor, which then serves as the template for deposition of either new or old histone H2A and H2B (Smith and Stillman, 1991). Conceivably, the deposition of new histones is important for full restoration of nucleosome density onto two daughters DNA strands. The H3 and H4 deposition involves histone chaperones, and among them, chromatin-assembly factor 1 (CAF-1) plays a pivotal role in chromatin assembly during DNA replication and DNA repair (Hoek and Stillman, 2003; Gaillard et al, 1996; Green and Almouzni, 2003; Polo et al, 2006; Zhu et al, 2009). CAF-1 is an evolutionary conserved protein complex, consisting of p150, p60 and p48 subunits, with a unique ability to preferentially deposit newly synthesized H3 and H4 onto replicating DNA (Shibahara and Stillman, 1999). Human CAF-1 and HIRA form predeposition machineries with histone H3.1/H4 and H3.3/H4, respectively (Tagami et al, 2004). The CAF-1 predeposition machinery is necessary to mediate DNA synthesis-dependent nucleosome assembly. During DNA replication or DNA repair, CAF-1 is targeted to the sites of DNA synthesis through its direct interaction with DNA polymerase processivity factor PCNA. As the replication fork progresses, H3.1–H4 dimers are deposited onto nascent DNA strands (Benson et al, 2006). CAF-1 is aided by another H3–H4 chaperone anti-silencing function 1 (Asf1), which synergistically cooperates with CAF-1 in DNA repair- and replication-coupled chromatin assembly (Tyler et al, 1999; Mello et al, 2002).
The DNA replication-coupled nucleosome assembly is important for inheritance of epigenetic information during DNA replication and repair (Groth et al, 2007). In these processes, PCNA molecules are used as a hub to couple chromatin restoration to replication. The PCNA recruits a number of chromatin-modulating enzymes, e.g., DNA methyltransferase DNMT1 and CAF-1, to re-establish epigenetic memory based on DNA methylation and histone modifications. Both DNMT1 and CAF-1 also have the ability to recruit histone modifier enzymes for PTMs. For example, DNMT1 interacts with H3K9 methyltransferase G9a (Esteve et al, 2006) and EZH2 component of PRC2 (Vire et al, 2006), which catalyses trimethylation of H3K27. PRC2 binds to H3K27me3 and colocalizes with this modification in G1 phase, and with the sites of ongoing DNA replication (Hansen et al, 2008). The ability of PRC2 to recognize a previously established mark triggers its renewal (Margueron et al, 2009). DNMT1 also forms a complex with G9a, which is required for recruitment of G9a to replication sites and for maintenance of H3K9 methylation at epigenetically silenced rDNA repeat (Esteve et al, 2006). Unlike DNMT1, CAF-1 reproduces the nucleosome density on replicated DNA throughout the genome. Nevertheless, recent evidence indicated that CAF-1 plays a role in maintaining the repressed state of silenced chromatin (Houlard et al, 2006). In embryonic stem cells, depletion of CAF-1 p150 results in the alteration of epigenetic histone methylation marks at the level of pericentric heterochromatin. Additionally, CAF-1 is found to link replication-coupled chromatin assembly to DNA and histone H3K9 methylation (Reese et al, 2003; Sarraf and Stancheva, 2004). CAF-1 forms a replication-dependent complex with H3K9 methyltransferase SETDB1 and MBD1, a protein that binds to methylated CpG dinucleotide. This complex is required for maintenance of H3K9 methylation and stable silencing of certain genes in proliferating cells. During DNA replication, MBD1 recruits SETDB1 to CAF-1 p150 and mediates H3K9 methylation in replication-coupled chromatin assembly. A similar mechanism also operates for H3K9 methylation of newly synthesized H3 deposited by CAF-1 in pericentric heterochromatin (Loyola et al, 2009). In this case, SETDB1 associates with HP1α-CAF-1 complex and mono-methylates H3K9 on non-nucleosomal histone H3, providing H3K9me1 for further trimethylation in pericentric regions. While both HP1 and CAF-1 are reported to localize to the sites of DNA damage (Green and Almouzni, 2003; Luijsterburg et al, 2009), it is not known whether HP1α-CAF-1-SETDB1 complex works in DNA repair-dependent nucleosome assembly.
Histone H3K56 acetylation
Recent studies have uncovered a connection between H3K56 acetylation and chromatin assembly following DNA replication and DSB repair (Chen et al, 2008; Li et al, 2008). It was found in yeast that H3K56 acetylation drives Asf1-dependent re-assembly of chromatin after DSB repair (Chen et al, 2008). Mutation of K56 to glutamine, mimicking permanent acetylation, partially bypasses the requirement of Asf1 in resistance to DSB-generating agents. Nevertheless, DSB repair itself was operational in Asf1 mutants. On the other hand, lack of H3K56 acetylation in HAT Rtt109 mutants leads to persistent activation (phosphorylation) of checkpoint protein Rad53. These observations indicated that restoration of chromatin, driven by acetylated H3K56, is a signal for the completion of DSB repair. Similarly, CAF-1 is involved in acetylated H3K56-driven chromatin restoration (Li et al, 2008). H3K56 acetylation promotes the association of histone H3 with CAF-1 and Rtt109, and facilitates CAF-1-dependent nucleosome assembly. Thus, acetylated H3K56 coordinates the function of H3–H4 chaperones in nucleosome assembly.
Whether H3K56 acetylation occurs as results of DSB repair in human cells is a matter of debate (Das et al, 2009; Tjeertes et al, 2009). Das, C et al showed that DNA damaging agents, such as IR, UV, hydroxyurea and MMS, induce histone H3K56 acetylation in human cells, and histone acetyl transferase CBP/p300 is responsible for such an acetylation. Acetylated H3K56 colonizes with γH2AX immediately following IR, indicating that the acetylated H3K56 is enriched at the sites of DNA damage. Moreover, acetylated H3K56 is assembled into chromatin at the sites of DNA repair by CAF-1 and Asf1. By contrast, Tjeertes, JV et al observed that the H3K56 acetylation was rapidly and reversibly reduced in response to DNA damage (Tjeertes et al, 2009). Histone acetyl-transferase GCN5/KAT2A, rather than CBP/p300 acetylates H3K56 in vitro and in vivo. Further work is needed to clarify this inconsistency and reveal the importance of H3K56 acetylation in DDR in mammalian cells.
Histone H2A ubiquitination
An UV-induced mono-ubiquitination of histone 2A in the vicinity of DNA lesions has been described (Bergink et al, 2006). In living cells, harboring green fluorescent protein (GFP)-ubiquitin fusion, bleaching of GFP fluorophore in UV-exposed regions was followed by rapid local accumulation of GFP-ubiquitin fusion. The UV-induced ubiquitination requires functional NER, and coincides with the sequestration of PCNA, suggesting that the ubiquitination is linked to DNA repair. Since ubiquitin mutations that eliminate all lysine residues do not affect the ubiquitination, mono-ubiquitination is the most probable character of this NER-dependent process. Histone 2A is the primary target and the ubiquitination occurs at K119. Further studies suggest that the observed H2A ubiquitination may be a post-repair process related to chromatin restoration (Zhu et al, 2009). As determined by the formation of uH2A foci at local UV damage sites, dynamics of uH2A and XPC foci formation exhibits a clear temporal difference. For example, XPC foci are seen in almost all the damaged cells within 15 min and completely disappear at 4 h after local UV irradiation. On the other hand, the uH2A foci persist for 24 h post irradiation, suggesting that the formation of uH2A foci is not directly associated with damage recognition. More importantly, the H3–H4 chaperone CAF-1 is required for uH2A foci formation. Depletion of CAF-1 p60 by siRNA impairs recruitment of CAF-1 to DNA damage and eliminates uH2A foci. Moreover, the H2A ubiquitin ligase Ring2/Ring1b, a component of PRC2, is not detected at damage sites (Zhu et al, 2009). Therefore, it is very likely that H2A ubiquitination occurs at newly assembled nucleosomes, or the uH2A is deposited on DNA repair region from an existing pool.
It must be noted that the UV-induced H2A ubiquitination differs from γH2AX or H2A ubiquitination, observed following IR, in a number of ways. First, RNF8 and UBC13 are known to form K63-ubiquitin chain (Plans et al, 2006). Second, the ubiquitination by RNF8 is dependent on γH2AX and MDC1, whose BRCT domain binds γH2AX. Deficiency in γH2AX abolished RNF8 recruitment and eliminated IR-induced ubiquitination after IR (Huen et al, 2007). In contrast, γH2AX deficiency did not affect UV-induced H2A ubiquitination (Bergink et al, 2006). Therefore, H2AX ubiquitination is integral part of checkpoint signaling in response to DSBs and UV induced DNA damage, while NER-dependent H2A mono-ubiquitination is related to the chromatin restoration after repair of UV-induced lesions.
Concluding Remarks
In recent years, a remarkable progress has been made in revealing PTMs in DDR. A careful comparison of damage, modification types, and the time when PTMs occur, suggests that the PTMs help organize DDR for the prompt execution of checkpoint signaling, DNA repair, and ultimate restoration of chromatin. Ubiquitination and acetylation seem to be two pervasive modes of these modifications. Timing and features of modifications appear to be critical factors in determining the physiological effect of a specific modification. For example, H3 acetylation occurs both before and after DSB repair. The former, acetylation at H3K14 and H3K23, leads to γH2AX ubiquitination, checkpoint activation, and DNA repair (Ikura et al, 2007). While the latter, H3 acetylation at K56, leads to restoration of chromatin and termination of checkpoint activation (Chen et al, 2008). Also, the same concept seems true for histone ubiquitination. The γH2AX and H2A ubiquitination lead to checkpoint signaling, whereas the H2A ubiquitination after NER is associated with chromatin restoration. These scenarios and information to-date raise an important question whether these modifications are an extension of histone code albeit in the realm of DDR? Answer to this question lies in our future understanding of detailed molecular mechanisms by which each modification functions in specific DDR pathway.
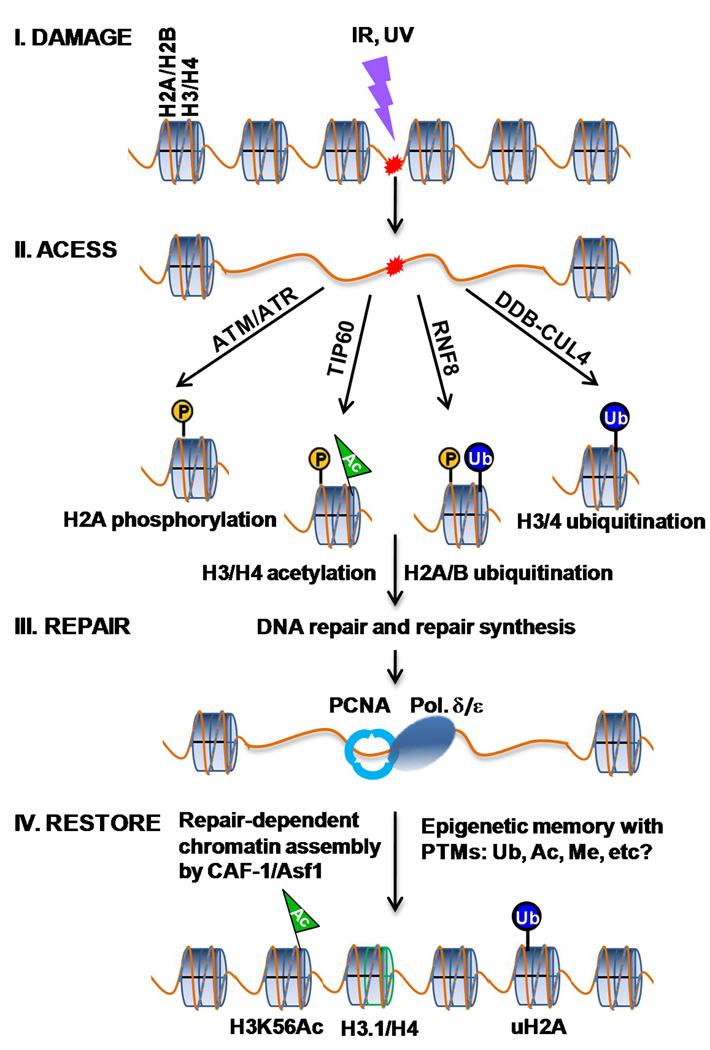
It is hypothesized that immediately upon induction of DNA damage, chromatin is locally destabilized to facilitate the access of repair machinery to DNA lesions. After DNA repair is completed, chromatin is restored to its original state in the vicinity of damage sites. This “access-repair-restore” model conceptually describes how DNA repair might occur in chromatin environment (Smerdon, 1991; Green and Almouzni, 2002). Now, histone modifications can also be integrated into this model (Fig. 1) and ask whether faithful repair of DNA sequences is always achieved with the preservation of epigenetic information? A recent study indicated that DNA damage leaves its mark on chromatin through incorporation of new H3.1 histones into the sites of UV repair (Polo et al, 2006). In the same vein, incorporations of acetylated H3K56 and uH2A into chromatin can be envisioned as analogous cellular events. Thus, these modifications too would be considered as DNA repair marks on chromatin.
Fig. 1.
Temporal interplay of histone modifications before and after DNA repair. Upon initial DNA damage, nucleosomal histones are subjected to various PTMs: H3 and H4 acetylation by TIP60 HAT, H2AX phosphorylation by ATM/ATR, γH2AX and H2A ubiquitination by RNF8, or H3 and H4 ubiquitination by DDB-CULA-ROC1. These modifications may cause nucleosome disassembly, or histone eviction, and facilitate access of the repair nanomachines to DNA lesions. When DNA repair is complete, repair-dependent chromatin assembly restores the original nucleosome density. The histones with new epigenetic marks i.e., the acetylated histone H3, the ubiquitinated H2A, and possibly the histones with other modifications are incorporated into newly assembled chromatin at the damage sites. After chromatin is restored, the checkpoint is terminated and cells resume cycling. Some PTMs would be eliminated and others retained to serve as a memory of damage, potentially challenging the epigenetic stability of the cells having undergone repair. The symbols with “P”, “Ub”, “Ac” and “Me” represent phospho, ubiquitin, acetyl and methyl groups, respectively.
Would these unique marks threaten the cell’s epigenetic stability with pathological consequences? To answer this question, it is important to know whether the specific marks constitute transient or permanent features within the cell. For instance, it has been found that USP3, a deubiquitinating enzyme, is required for deubiquitination of ubiquitin-conjugates of H2A, dephosphorylation of γH2AX, and for S phase progression after DNA damage (Nicassio et al, 2007), suggesting that ubiquitin may be removed from H2A for checkpoint recovery or adaptation. On the other hand, RNF8 ubiquitin ligase is also required for mitotic exit in cell cycle (Plans et al, 2007). Given that the H2A ubiquitination regulates gene transcription or “repressed state” of chromatin, any uH2A remaining in the repaired chromatin would be expected to have a regional effect on genomic function. Therefore, the challenge for future studies is to understand how these modifications are processed further to maintain epigenetic integrity of the cell, or if they do become epigenetic marks, how they would impact DNA-templated processes in the “once-damaged” chromatin.
Acknowledgments
We would like to thank Keisha Milum for critical reading of the manuscript, and past and present members of Wani laboratory for diligently contributing over the years in shaping our scientific thought process.
The research was sponsored by NIH grants ES2388, ES12991 and CA93413.
Abbreviations
Asf1
anti-silencing function 1
CAF-1
chromatin-assembly factor 1
DDR
DNA damage response
DSB
double-strand break
GFP
green fluorescent protein
H
histone
HP1
heterochromatin protein 1
HR
homologous recombination
IR
ionizing radiation
K
lysine
MDC1
mediator of DNA damage checkpoint 1
NER
nucleotide excision repair
PRC
polycomb repressive complex
PTM
post-translational modification
γH2AX
phosphorylated H2AX
SAGA
Spt-Ada-Gcn5 acetyltransferase
siRNA
small interference RNA
UV
ultraviolet light
uH2A
ubiquitinated H2A
Literature Cited
- Benson LJ, Gu Y, Yakovleva T, Tong K, Barrows C, Strack CL, Cook RG, Mizzen CA, Annunziato AT. Modifications of H3 and H4 during chromatin replication, nucleosome assembly, and histone exchange. J Biol Chem. 2006;281:9287–9296. doi: 10.1074/jbc.M512956200. [DOI] [PubMed] [Google Scholar]
- Bergink S, Salomons FA, Hoogstraten D, Groothuis TA, de WH, Wu J, Yuan L, Citterio E, Houtsmuller AB, Neefjes J, Hoeijmakers JH, Vermeulen W, Dantuma NP. DNA damage triggers nucleotide excision repair-dependent monoubiquitylation of histone H2A. Genes Dev. 2006;20:1343–1352. doi: 10.1101/gad.373706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128:669–681. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Bird AW, Yu DY, Pray-Grant MG, Qiu Q, Harmon KE, Megee PC, Grant PA, Smith MM, Christman MF. Acetylation of histone H4 by Esa1 is required for DNA double-strand break repair. Nature. 2002;419:411–415. doi: 10.1038/nature01035. [DOI] [PubMed] [Google Scholar]
- Chapman JR, Jackson SP. Phospho-dependent interactions between NBS1 and MDC1 mediate chromatin retention of the MRN complex at sites of DNA damage. EMBO Rep. 2008;9:795–801. doi: 10.1038/embor.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Carson JJ, Feser J, Tamburini B, Zabaronick S, Linger J, Tyler JK. Acetylated lysine 56 on histone H3 drives chromatin assembly after repair and signals for the completion of repair. Cell. 2008;134:231–243. doi: 10.1016/j.cell.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das C, Lucia MS, Hansen KC, Tyler JK. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature. 2009;459:113–117. doi: 10.1038/nature07861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Napoles M, Mermoud JE, Wakao R, Tang YA, Endoh M, Appanah R, Nesterova TB, Silva J, Otte AP, Vidal M, Koseki H, Brockdorff N. Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev Cell. 2004;7:663–676. doi: 10.1016/j.devcel.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Doil C, Mailand N, Bekker-Jensen S, Menard P, Larsen DH, Pepperkok R, Ellenberg J, Panier S, Durocher D, Bartek J, Lukas J, Lukas C. RNF168 binds and amplifies ubiquitin conjugates on damaged chromosomes to allow accumulation of repair proteins. Cell. 2009;136:435–446. doi: 10.1016/j.cell.2008.12.041. [DOI] [PubMed] [Google Scholar]
- Downs JA, Lowndes NF, Jackson SP. A role for Saccharomyces cerevisiae histone H2A in DNA repair. Nature. 2000;408:1001–1004. doi: 10.1038/35050000. [DOI] [PubMed] [Google Scholar]
- El-Mahdy MA, Zhu Q, Wang QE, Wani G, Praetorius-Ibba M, Wani AA. Cullin 4A-mediated proteolysis of DDB2 protein at DNA damage sites regulates in vivo lesion recognition by XPC. J Biol Chem. 2006;281:13404–13411. doi: 10.1074/jbc.M511834200. [DOI] [PubMed] [Google Scholar]
- Esteve PO, Chin HG, Smallwood A, Feehery GR, Gangisetty O, Karpf AR, Carey MF, Pradhan S. Direct interaction between DNMT1 and G9a coordinates DNA and histone methylation during replication. Genes Dev. 2006;20:3089–3103. doi: 10.1101/gad.1463706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischle W, Wang Y, Jacobs SA, Kim Y, Allis CD, Khorasanizadeh S. Molecular basis for the discrimination of repressive methyl-lysine marks in histone H3 by Polycomb and HP1 chromodomains. Genes Dev. 2003;17:1870–1881. doi: 10.1101/gad.1110503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard PH, Martini EMD, Kaufman PD, Stillman B, Moustacchi E, Almouzni G. Chromatin assembly coupled to DNA repair: A new role for chromatin assembly factor I. Cell. 1996;86:887–896. doi: 10.1016/s0092-8674(00)80164-6. [DOI] [PubMed] [Google Scholar]
- Green CM, Almouzni G. When repair meets chromatin. EMBO Rep. 2002;3:28–33. doi: 10.1093/embo-reports/kvf005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CM, Almouzni G. Local action of the chromatin assembly factor CAF-1 at sites of nucleotide excision repair in vivo. EMBO J. 2003;22:5163–5174. doi: 10.1093/emboj/cdg478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth A, Rocha W, Verreault A, Almouzni G. Chromatin challenges during DNA replication and repair. Cell. 2007;128:721–733. doi: 10.1016/j.cell.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Hansen KH, Bracken AP, Pasini D, Dietrich N, Gehani SS, Monrad A, Rappsilber J, Lerdrup M, Helin K. A model for transmission of the H3K27me3 epigenetic mark. Nat Cell Biol. 2008;10:1291–1300. doi: 10.1038/ncb1787. [DOI] [PubMed] [Google Scholar]
- Hoek M, Stillman B. Chromatin assembly factor 1 is essential and couples chromatin assembly to DNA replication in vivo. Proc Natl Acad Sci U S A. 2003;100:12183–12188. doi: 10.1073/pnas.1635158100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlard M, Berlivet S, Probst AV, Quivy JP, Hery P, Almouzni G, Gerard M. CAF-1 is essential for heterochromatin organization in pluripotent embryonic cells. PLoS Genet. 2006;2:e181. doi: 10.1371/journal.pgen.0020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen MS, Grant R, Manke I, Minn K, Yu X, Yaffe MB, Chen J. RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell. 2007;131:901–914. doi: 10.1016/j.cell.2007.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikura T, Tashiro S, Kakino A, Shima H, Jacob N, Amunugama R, Yoder K, Izumi S, Kuraoka I, Tanaka K, Kimura H, Ikura M, Nishikubo S, Ito T, Muto A, Miyagawa K, Takeda S, Fishel R, Igarashi K, Kamiya K. DNA damage-dependent acetylation and ubiquitination of H2AX enhances chromatin dynamics. Mol Cell Biol. 2007;27:7028–7040. doi: 10.1128/MCB.00579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson V. Deposition of newly synthesized histones: hybrid nucleosomes are not tandemly arranged on daughter DNA strands. Biochemistry. 1988;27:2109–2120. doi: 10.1021/bi00406a044. [DOI] [PubMed] [Google Scholar]
- Kallin EM, Cao R, Jothi R, Xia K, Cui K, Zhao K, Zhang Y. Genome-wide uH2A localization analysis highlights Bmi1-dependent deposition of the mark at repressed genes. PLoS Genet. 2009;5:e1000506. doi: 10.1371/journal.pgen.1000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Horikoshi M. Tip60 acetylates six lysines of a specific class in core histones in vitro. Genes Cells. 1998;3:789–800. doi: 10.1046/j.1365-2443.1998.00229.x. [DOI] [PubMed] [Google Scholar]
- Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Kolas NK, Chapman JR, Nakada S, Ylanko J, Chahwan R, Sweeney FD, Panier S, Mendez M, Wildenhain J, Thomson TM, Pelletier L, Jackson SP, Durocher D. Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science. 2007;318:1637–1640. doi: 10.1126/science.1150034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg RD. Structure of chromatin. Annu Rev Biochem. 1977;46:931–954. doi: 10.1146/annurev.bi.46.070177.004435. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Ladurner AG, Inouye C, Jain R, Tjian R. Bromodomains mediate an acetyl-histone encoded antisilencing function at heterochromatin boundaries. Mol Cell. 2003;11:365–376. doi: 10.1016/s1097-2765(03)00035-2. [DOI] [PubMed] [Google Scholar]
- LeRoy G, Rickards B, Flint SJ. The double bromodomain proteins Brd2 and Brd3 couple histone acetylation to transcription. Mol Cell. 2008;30:51–60. doi: 10.1016/j.molcel.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhou H, Wurtele H, Davies B, Horazdovsky B, Verreault A, Zhang Z. Acetylation of histone H3 lysine 56 regulates replication-coupled nucleosome assembly. Cell. 2008;134:244–255. doi: 10.1016/j.cell.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyola A, Tagami H, Bonaldi T, Roche D, Quivy JP, Imhof A, Nakatani Y, Dent SY, Almouzni G. The HP1alpha-CAF1-SetDB1-containing complex provides H3K9me1 for Suv39-mediated K9me3 in pericentric heterochromatin. EMBO Rep. 2009;10:769–775. doi: 10.1038/embor.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger K. Dynamic nucleosomes. Chromosome Res. 2006;14:5–16. doi: 10.1007/s10577-005-1026-1. [DOI] [PubMed] [Google Scholar]
- Luijsterburg MS, Dinant C, Lans H, Stap J, Wiernasz E, Lagerwerf S, Warmerdam DO, Lindh M, Brink MC, Dobrucki JW, Aten JA, Fousteri MI, Jansen G, Dantuma NP, Vermeulen W, Mullenders LH, Houtsmuller AB, Verschure PJ, van DR. Heterochromatin protein 1 is recruited to various types of DNA damage. J Cell Biol. 2009;185:577–586. doi: 10.1083/jcb.200810035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailand N, Bekker-Jensen S, Faustrup H, Melander F, Bartek J, Lukas C, Lukas J. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell. 2007;131:887–900. doi: 10.1016/j.cell.2007.09.040. [DOI] [PubMed] [Google Scholar]
- Margueron R, Justin N, Ohno K, Sharpe ML, Son J, Drury WJ, III, Voigt P, Martin SR, Taylor WR, De MV, Pirrotta V, Reinberg D, Gamblin SJ. Role of the polycomb protein EED in the propagation of repressive histone marks. Nature. 2009;461:762–767. doi: 10.1038/nature08398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteijn JA, Bekker-Jensen S, Mailand N, Lans H, Schwertman P, Gourdin AM, Dantuma NP, Lukas J, Vermeulen W. Nucleotide excision repair-induced H2A ubiquitination is dependent on MDC1 and RNF8 and reveals a universal DNA damage response. J Cell Biol. 2009;186:835–847. doi: 10.1083/jcb.200902150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matangkasombut O, Buratowski S. Different sensitivities of bromodomain factors 1 and 2 to histone H4 acetylation. Mol Cell. 2003;11:353–363. doi: 10.1016/s1097-2765(03)00033-9. [DOI] [PubMed] [Google Scholar]
- Melander F, Bekker-Jensen S, Falck J, Bartek J, Mailand N, Lukas J. Phosphorylation of SDT repeats in the MDC1 N terminus triggers retention of NBS1 at the DNA damage-modified chromatin. J Cell Biol. 2008;181:213–226. doi: 10.1083/jcb.200708210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello JA, Sillje HH, Roche DM, Kirschner DB, Nigg EA, Almouzni G. Human Asf1 and CAF-1 interact and synergize in a repair-coupled nucleosome assembly pathway. EMBO Rep. 2002;3:329–334. doi: 10.1093/embo-reports/kvf068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murr R, Loizou JI, Yang YG, Cuenin C, Li H, Wang ZQ, Herceg Z. Histone acetylation by Trrap-Tip60 modulates loading of repair proteins and repair of DNA double-strand breaks. Nat Cell Biol. 2006;8:91–99. doi: 10.1038/ncb1343. [DOI] [PubMed] [Google Scholar]
- Nicassio F, Corrado N, Vissers JH, Areces LB, Bergink S, Marteijn JA, Geverts B, Houtsmuller AB, Vermeulen W, Di Fiore PP, Citterio E. Human USP3 is a chromatin modifier required for S phase progression and genome stability. Curr Biol. 2007;17:1972–1977. doi: 10.1016/j.cub.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Plans V, Guerra-Rebollo M, Thomson TM. Regulation of mitotic exit by the RNF8 ubiquitin ligase. Oncogene. 2007 doi: 10.1038/sj.onc.1210782. [DOI] [PubMed] [Google Scholar]
- Plans V, Scheper J, Soler M, Loukili N, Okano Y, Thomson TM. The RING finger protein RNF8 recruits UBC13 for lysine 63-based self polyubiquitylation. J Cell Biochem. 2006;97:572–582. doi: 10.1002/jcb.20587. [DOI] [PubMed] [Google Scholar]
- Polo SE, Roche D, Almouzni G. New histone incorporation marks sites of UV repair in human cells. Cell. 2006;127:481–493. doi: 10.1016/j.cell.2006.08.049. [DOI] [PubMed] [Google Scholar]
- Pray-Grant MG, Daniel JA, Schieltz D, Yates JR, III, Grant PA. Chd1 chromodomain links histone H3 methylation with. Nature. 2005;433:434–438. doi: 10.1038/nature03242. [DOI] [PubMed] [Google Scholar]
- Reese BE, Bachman KE, Baylin SB, Rountree MR. The methyl-CpG binding protein MBD1 interacts with the p150 subunit of chromatin assembly factor 1. Mol Cell Biol. 2003;23:3226–3236. doi: 10.1128/MCB.23.9.3226-3236.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- Sarma K, Reinberg D. Histone variants meet their match. Nat Rev Mol Cell Biol. 2005;6:139–149. doi: 10.1038/nrm1567. [DOI] [PubMed] [Google Scholar]
- Sarraf SA, Stancheva I. Methyl-CpG binding protein MBD1 couples histone H3 methylation at lysine 9 by SETDB1 to DNA replication and chromatin assembly. Mol Cell. 2004;15:595–605. doi: 10.1016/j.molcel.2004.06.043. [DOI] [PubMed] [Google Scholar]
- Shibahara K, Stillman B. Replication-dependent marking of DNA by PCNA facilitates CAF-1-coupled inheritance of chromatin. Cell. 1999;96:575–585. doi: 10.1016/s0092-8674(00)80661-3. [DOI] [PubMed] [Google Scholar]
- Shroff R, rbel-Eden A, Pilch D, Ira G, Bonner WM, Petrini JH, Haber JE, Lichten M. Distribution and dynamics of chromatin modification induced by a defined DNA double-strand break. Curr Biol. 2004;14:1703–1711. doi: 10.1016/j.cub.2004.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims RJ, III, Chen CF, Santos-Rosa H, Kouzarides T, Patel SS, Reinberg D. Human but not yeast CHD1 binds directly and selectively to histone H3 methylated at lysine 4 via its tandem chromodomains. J Biol Chem. 2005;280:41789–41792. doi: 10.1074/jbc.C500395200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smerdon MJ. DNA repair and the role of chromatin structure. Curr Opin Cell Biol. 1991;3:422–428. doi: 10.1016/0955-0674(91)90069-b. [DOI] [PubMed] [Google Scholar]
- Smith S, Stillman B. Stepwise assembly of chromatin during DNA replication in vitro. EMBO J. 1991;10:971–980. doi: 10.1002/j.1460-2075.1991.tb08031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spycher C, Miller ES, Townsend K, Pavic L, Morrice NA, Janscak P, Stewart GS, Stucki M. Constitutive phosphorylation of MDC1 physically links the MRE11-RAD50-NBS1 complex to damaged chromatin. J Cell Biol. 2008;181:227–240. doi: 10.1083/jcb.200709008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart GS, Panier S, Townsend K, Al-Hakim AK, Kolas NK, Miller ES, Nakada S, Ylanko J, Olivarius S, Mendez M, Oldreive C, Wildenhain J, Tagliaferro A, Pelletier L, Taubenheim N, Durandy A, Byrd PJ, Stankovic T, Taylor AM, Durocher D. The RIDDLE syndrome protein mediates a ubiquitin-dependent signaling cascade at sites of DNA damage. Cell. 2009;136:420–434. doi: 10.1016/j.cell.2008.12.042. [DOI] [PubMed] [Google Scholar]
- Stiff T, O'Driscoll M, Rief N, Iwabuchi K, Lobrich M, Jeggo PA. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004;64:2390–2396. doi: 10.1158/0008-5472.can-03-3207. [DOI] [PubMed] [Google Scholar]
- Stucki M, Clapperton JA, Mohammad D, Yaffe MB, Smerdon SJ, Jackson SP. MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell. 2005;123:1213–1226. doi: 10.1016/j.cell.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Sugasawa K, Okuda Y, Saijo M, Nishi R, Matsuda N, Chu G, Mori T, Iwai S, Tanaka K, Tanaka K, Hanaoka F. UV-induced ubiquitylation of XPC protein mediated by UV-DDB-ubiquitin ligase complex. Cell. 2005;121:387–400. doi: 10.1016/j.cell.2005.02.035. [DOI] [PubMed] [Google Scholar]
- Tagami H, Ray-Gallet D, Almouzni G, Nakatani Y. Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell. 2004;116:51–61. doi: 10.1016/s0092-8674(03)01064-x. [DOI] [PubMed] [Google Scholar]
- Tamburini BA, Tyler JK. Localized histone acetylation and deacetylation triggered by the homologous recombination pathway of double-strand DNA repair. Mol Cell Biol. 2005;25:4903–4913. doi: 10.1128/MCB.25.12.4903-4913.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjeertes JV, Miller KM, Jackson SP. Screen for DNA-damage-responsive histone modifications identifies H3K9Ac and H3K56Ac in human cells. EMBO J. 2009;28:1878–1889. doi: 10.1038/emboj.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler JK, Adams CR, Chen SR, Kobayashi R, Kamakaka RT, Kadonaga JT. The RCAF complex mediates chromatin assembly during DNA replication and repair. Nature. 1999;402:555–560. doi: 10.1038/990147. [DOI] [PubMed] [Google Scholar]
- van AH, Gasser SM. The histone code at DNA breaks: a guide to repair? Nat Rev Mol Cell Biol. 2005;6:757–765. doi: 10.1038/nrm1737. [DOI] [PubMed] [Google Scholar]
- Vire E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, Morey L, Van EA, Bernard D, Vanderwinden JM, Bollen M, Esteller M, Di CL, de LY, Fuks F. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–874. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- Wang B, Elledge SJ. Ubc13/Rnf8 ubiquitin ligases control foci formation of the Rap80/Abraxas/Brca1/Brcc36 complex in response to DNA damage. Proc Natl Acad Sci U S A. 2007;104:20759–20763. doi: 10.1073/pnas.0710061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang L, Erdjument-Bromage H, Vidal M, Tempst P, Jones RS, Zhang Y. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431:873–878. doi: 10.1038/nature02985. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhai L, Xu J, Joo HY, Jackson S, Erdjument-Bromage H, Tempst P, Xiong Y, Zhang Y. Histone H3 and H4 ubiquitylation by the CUL4-DDB-ROC1 ubiquitin ligase facilitates cellular response to DNA damage. Mol Cell. 2006;22:383–394. doi: 10.1016/j.molcel.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Wang QE, Zhu Q, Wani G, El-Mahdy MA, Li J, Wani AA. DNA repair factor XPC is modified by SUMO-1 and ubiquitin following UV irradiation. Nucleic Acids Res. 2005;33:4023–4034. doi: 10.1093/nar/gki684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem. 2001;276:47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- Wu J, Huen MS, Lu LY, Ye L, Dou Y, Ljungman M, Chen J, Yu X. Histone ubiquitination associates with BRCA1-dependent DNA damage response. Mol Cell Biol. 2009;29:849–860. doi: 10.1128/MCB.01302-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao GY, Sonoda E, Barber LJ, Oka H, Murakawa Y, Yamada K, Ikura T, Wang X, Kobayashi M, Yamamoto K, Boulton SJ, Takeda S. A critical role for the ubiquitin-conjugating enzyme Ubc13 in initiating homologous recombination. Mol Cell. 2007;25:663–675. doi: 10.1016/j.molcel.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Wani G, Arab HH, El-Mahdy MA, Ray A, Wani AA. Chromatin restoration following nucleotide excision repair involves the incorporation of ubiquitinated H2A at damaged genomic sites. DNA Repair (Amst) 2009;8:262–273. doi: 10.1016/j.dnarep.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]