MOF and H4 K16 Acetylation Play Important Roles in DNA Damage Repair by Modulating Recruitment of DNA Damage Repair Protein Mdc1 (original) (raw)
Abstract
MOF (MYST1) is the major enzyme to catalyze acetylation of histone H4 lysine 16 (K16) and is highly conserved through evolution. Using a conditional knockout mouse model and the derived mouse embryonic fibroblast cell lines, we showed that loss of Mof led to a global reduction of H4 K16 acetylation, severe G2/M cell cycle arrest, massive chromosome aberration, and defects in ionizing radiation-induced DNA damage repair. We further showed that although early DNA damage sensing and signaling by ATM were normal in _Mof_-null cells, the recruitment of repair mediator protein Mdc1 and its downstream signaling proteins 53bp1 and Brca1 to DNA damage foci was completely abolished. Mechanistic studies suggested that _Mof_-mediated H4 K16 acetylation and an intact acidic pocket on H2A.X were essential for the recruitment of Mdc1. Removal of Mof and its associated proteins phenocopied a charge-neutralizing mutant of H2A.X. Given the well-characterized H4-H2A trans interactions in regulating higher-order chromatin structure, our study revealed a novel chromatin-based mechanism that regulates the DNA damage repair process.
In eukaryotes, DNA is packaged with core histones and other nonhistone chromosomal proteins into several orders of chromatin structure with increasing compaction. Many cellular processes, including transcription, DNA replication, and DNA damage repair (DDR), are regulated in the context of chromatin. Recent studies have shown that histone modification (e.g., RNF8 and RNF168) and chromatin-remodeling activities (e.g., INO80 and SWR1) facilitate the accumulation and function of DNA repair proteins at the damage foci (52). Most of the regulations are achieved at the level of nucleosomes. Specifically, chromatin regulatory activities can either alter nucleosome structure and location or modulate histone-DNA contacts to promote association of _trans_-acting factors with DNA and recruit important components of the signaling cascade to DNA damage repair centers (16). Among them, the function of Saccharomyces Cerevisiae histone acetyltransferase (HAT) NuA4 and its mammalian homolog, Tip60, have been well characterized in this process (45, 52). Mutations in NuA4 or its lysine substrates on histone H4 tail in yeast led to increased sensitivity to DNA-damaging reagents and impaired double-strand repair by nonhomologous end joining (NHEJ) (3, 11). In higher eukaryotes, it has been shown that Tip60 regulates DNA repair through acetylation of both H2A and H2A.X, which facilitates polyubiquitination and dynamic exchange of H2A.X at the damage foci, and of histone H4 at lysine 5 (H4 K5), H4 K8, and H4 K12, which facilitates nucleosome remodeling and establishes less condensed nucleosome arrays (42, 44). Although regulation of DNA damage repair by higher-order chromatin structures has been proposed in these studies, mechanistic details remain unclear.
Unlike most histone modifications, H4 K16 acetylation (H4 K16ac) is unique for regulating higher-order chromatin structures beyond the level of nucleosomes. It was first reported in the crystal structure of nucleosome particles by Luger et al. that a basic region in the H4 tail (amino acids 14 to 19) is involved in the internucleosome interaction with an acidic pocket on the H2A-H2B surface in the crystal lattice (28). Subsequent studies showed that this H4-H2A interaction is essential for folding of nucleosome arrays into secondary and tertiary chromatin structures (5). The conclave acidic surface on H2A (and most H2A variants) is formed by several highly conserved negatively charged residues. Neutralizing these negative charges by mutations or by binding of a herpesvirus-derived latency-associated nuclear antigen (LANA) peptide promotes salt-induced chromatin condensation in vitro and apparent heterochromatination in vivo (6, 55). Acetylation of K16, which neutralizes the positive charge on the basic patch of H4, weakens the interaction of the H4 tail with the acidic pocket on H2A and reduces the propensity of nucleosome arrays to self-associate into the 30-nm chromatin fiber (21, 37, 40). In addition to this well-characterized function, H4 K16 acetylation was also shown to block long-range internucleosome interactions imposed by linker histones (21). Despite extensive biochemical and biophysical studies of H4 K16 acetylation in higher-order chromatin structures in vitro, its physiological implications in the control of important nuclear processes in vivo remain unclear.
The major enzyme that acetylates histone H4 K16 in mammals is MOF (also called MYST1 or KAT8) (25, 33). It is a highly conserved MYST family HAT, sharing the same domain structure and sequence homology with Tip60 (51, 53). Unlike most HATs that targets multiple sites on histones, MOF activity on nucleosomes is restricted to K16 on the histone H4 tail and is tightly regulated in two distinct complexes: the MOF-MSL complex and the MOF-MSL1v1 complex (reviewed in reference 25). In addition to H4 K16, the MOF-MSL1v1 complex, but not the MOF-MSL complex, also acetylates nonhistone substrates such as p53 and plays important roles in transcription activation (26). Despite extensive biochemical characterization (10, 26, 41, 48), the in vivo function of Mof in higher eukaryotes was not well understood. In vivo studies using mouse models showed that Mof is essential for vertebrate development and that constitutive ablation of Mof leads to peri-implantation lethality in mouse embryos (14, 49). In one study, _Mof_-null mice showed a marked delay in developmental progression with proliferation arrest and death at about E7.5 (14). In the second study, all _Mof_−/− embryos failed to develop beyond the expanded blastocyst stage and died at about the implantation stage (E4.5) (49). In both studies, _Mof_-null embryos showed massive abnormal chromatin aggregations (14, 49), suggesting a crucial role for Mof in the maintenance of chromatin structures. However, the early lethality of _Mof_-knockout mice prevented detailed loss-of-function studies for Mof in a defined genetic background in higher eukaryotes.
For in-depth study of the functions of Mof and histone H4 K16ac in the DNA damage response, we have established a conditional _Mof_-knockout mouse model. In this model, the floxed Mof allele can be deleted by 4-hydroxytamoxifen (4-OHT)-induced expression of Cre recombinase. Using the derived Mof+/+ _Cre_-positive (Cre+) and _Mof_−/− Cre+ mouse embryonic fibroblast (MEF) cell lines, we found that Mof is essential for global H4 K16 acetylation, cell proliferation, and maintenance of genome stability. Importantly, Mof was required for efficient repair of DNA damage induced by ionizing radiation (IR). Unlike Tip60, Mof did not affect ATM activation or γH2A.X accumulation at the damage foci. Instead, it is crucial for the recruitment of repair mediator proteins such as Mdc1 (mediator of DNA damage checkpoint 1), 53bp1 (p53 binding protein 1), and Brca1 (breast cancer type 1 susceptibility protein). Further mechanistic studies revealed that Mof and H4 K16 acetylation probably regulate the DNA repair process by modulating interactions of the H4 tail with the acidic pocket of H2A.X. Loss of H4 K16 acetylation or neutralization of the charge of acidic pocket on H2A.X abolished Mdc1 binding at the DNA damage foci. Together, our results demonstrated a novel mechanism for the chromatin-mediated DNA damage repair process.
MATERIALS AND METHODS
Generation of conditional _Mof_-knockout mice.
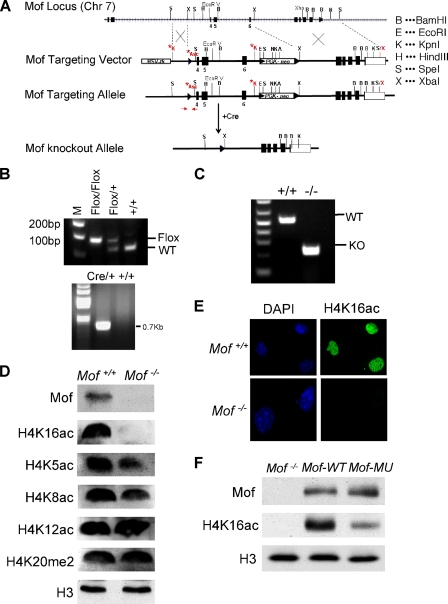
To generate conditional _Mof_-knockout mice, a targeting vector was constructed (see Fig. 1). This vector was introduced into R1 embryonic stem cells via electroporation. Three homologous recombinants were obtained. The embryonic stem cells that carry _Mof_flox/+ alleles were injected into C57BL/6 blastocysts. The chimeric mice were backcrossed with C57BL/6 to get F1 mice (_Mof_flox/+). _Mof_flox/+ mice were backcrossed six times onto the C57BL/6 background. Homozygotes were generated by crossing heterozygote _Mof_flox/+ mice with CAGG Cre-ER mice. The double heterozygotes were crossed to generate _Mof_flox/flox CAGG Cre-ER mice. All animal experiments were carried out according to the guidelines for the care and use of laboratory animals of the University of Michigan. The mice used in this study were genotyped by PCR analysis. The primers and their sequences were as follows: primer Mof-F, AACGTGGCATTGATTATTG; primer Mof-R, GCTTCTTTCTCATCCTGGTAG; primer Cre-s, GCCAGCTAAACATGCTTCATC; and primer Cre-a, ATTGCCCCTGTTTCACTATCC. The multiplex PCR produces a 150-bp band for the wild-type (WT) allele, a 200-bp band for the floxed allele, and both bands for heterozygous mice; and the Cre primer produces a 727-bp band.
FIG. 1.
Generation of conditional _Mof_-knockout alleles. (A) Scheme for generating conditional Mof alleles. The targeting vector was constructed to have a loxP site inserted between exons 3 and 4 and a neomycin gene selection cassette flanked by two loxP sites introduced between exons 6 and 7. The primers (forward and reverse) used for genotyping are highlighted in red. (B) Genotyping of germ line _Mof_flox/flox Cre-ER mice. (Top) PCR genotyping of genomic DNA from mice was performed with the primers depicted in panel A. The floxed allele gives a 200-bp product, while the wild-type allele gives a 150-bp product. (Bottom) PCR genotyping of CAGG Cre-ER allele, which gives a 727-bp product. (C) RT-PCR analyses of Mof transcripts after introduction of Cre recombinase by 4-OHT treatment. Truncated Mof mRNA (219 bp) was detected in _Mof_−/− cells, whereas the wild-type transcript is 530 bp. (D) Immunoblots for proteins in Mof+/+ and _Mof_−/− primary MEFs. The antibodies used are indicated on the right. Histone H3 was used as the loading control. (E) Immunofluorescence for H4 K16 acetylation in primary MEFs of Mof+/+ and _Mof_−/−. (F) Immunoblot for H4 K16ac for _Mof_−/− expressing wild-type Mof or the Mof mutant (MU) E350Q rescue construct. Histone H3 is blotted as the loading control.
Reverse transcription-PCRs (RT-PCRs) were performed to detect truncated Mof transcripts after 4-OHT treatment. The primers are (i) AAGAACCGACTCGCACTGAC (sense) and (ii) AAGACAAAGGGCTCCACATC (antisense). The wild-type Mof transcript produces a 522-bp band, and the _Mof_-knockout transcript produces a 219-bp band.
Antibodies.
The antibodies used in this study include anti-H3 Ser10phos (Cell Signaling), anti-acetyl-histone H4 K16 (07-329; Millipore), anti-ubiquityl-histone H2A clone E6C5 (05-678; Millipore), anti-p19Arf (M156; Millipore), anti-histone H3 (Millipore), anti-γH2AX (active motif, 39117), anti-H2A (active motif, 39209), anti-histone H4 dimethyl Lys20 (active motif, 39539), anti-acetyl-histone H4 K12 (active motif, 39165), anti-acetyl-histone H4 K5 (active motif, 39583), anti-acetyl-histone H4 K8 (active motif, 39171), anti-ATM Ser1981p clone 10H11.E.12 (active motif, 39529), anti-p16Ink4a (abA300-992A; abcam), anti-MOF (MYST1) (BL2118; Bethyl Labs), anti-TIP60 (N-17; Santa Cruz), anti-p21(C-19; Santa Cruz), anti-β-actin (Cell Signaling), anti-p53S15p (Cell Signaling), anti-Smc1S957p (Cell Signaling), anti-Chk2 clone 7 (Cell Signaling), and anti-Chk1S345p (Cell Signaling). Anti-Brca1, anti-53bp1, anti-Mdc1, and anti-γH2AX were described previously (18a).
Growth and analysis of MEFs.
MEFs were isolated from E13.5 embryos and grown under standard culture conditions. The _Mof_flox/flox CAGG Cre-ER MEF cells were cultured in the presence of 200 nM 4-OHT for 4 days to delete Mof before each experiment. The population doubling time was measured using the 3T9 protocol described previously (50). Cells from passage 7 (p7) of wild-type and _Mof_-knockout MEFs were used to obtain growth curves. For genome instability studies, metaphase chromosomes were prepared as described previously. The glass slides were then stained with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen). Images were acquired on an Olympus BX-61 microscope and viewed with SKYview software (ASI).
SA-β-Gal assay.
Passage 8 MEFs were evaluated for cellular senescence using a senescence-associated-β-galactosidase (SA-β-Gal) staining kit (senescence cell staining kit; Sigma). SA-β-galactosidase staining was performed in the 24-well plate format, according to the manufacturer's instructions. Senescent cells are stained blue.
Cell cycle analysis.
Primary MEFs (passages 5 and 6) were harvested and stained with propidium iodide (PI; 10 μg/ml in 0.5% bovine serum albumin [BSA] in 1× phosphate-buffered saline [PBS]) for 30 min at 37°C. The samples were run on a BD LSRII flow cytometer and analyzed using the BD FACS Diva program (version 6.0) and ModFit32 program. To identify cells in M phase, MEFs were fixed in 70% ethanol (following 1 h of recovery after exposure to 5 Gy IR or to no IR as a control) and stained using anti-phosphorylated H3 Ser10 (Cell Signaling). After incubation with secondary anti-rabbit IgG-AlexaFluor488 (1:200 in 0.5% BSA in PBS, 1 h at room temperature), cells were stained with 50 μg/ml PI. Flow cytometry was performed using a BD LSRII flow cytometer and were analyzed using the BD FACS Diva program (version 6.0) and the WinList3 (version 2) program.
Immunofluorescence.
MEFs were grown on coverslips (2 × 104 cells per well in a 24-well dish) and fixed in 4% paraformaldehyde for 10 min. Fixed cells were washed with PBS and permeabilized in 0.5% Triton X-100 for 5 min. After 1 h of blocking with 5% fetal bovine serum (FBS) in PBS, cells were stained with primary antibodies diluted in PBS (0.05% FBS) for 2 h at room temperature and then washed and incubated in secondary antibody conjugated with fluorophores. Cells were stained with DAPI, mounted on glass slides, and analyzed using a fluorescent microscope (Zeiss).
Neutral comet assay.
Strand break repair was analyzed by single-cell agarose gel electrophoresis under neutral conditions, as described previously (54). Cells were irradiated (80 Gy) and harvested immediately or at 1 h or 6 h postexposure. The images were analyzed for comet tail moment using CometScore software. At least 40 images were processed for each time point for each cell line.
Recombination assays.
The dividing line between green fluorescent protein (GFP; homologous recombination)-positive and -negative cells was set to the background level for the GFP-positive cells in the internal control (not transfected with I-SceI). This gate was then applied to the I-SceI-positive counterparts to determine homologous recombination efficiency.
Transient transfection and knockdown.
Transfection was performed in 60-mm dishes, using Lipofectamine 2000 (Invitrogen) transfection for _Mof_flox/flox _CAGG Cre-ER_-positive MEF cells, according to the manufacturer's instruction. The short hairpin RNA (shRNA) for Msl1v1 and small interfering RNA (siRNA) for Msl1 were used to knock down the expression of Msl1v1 and Msl1, respectively (sequences are available upon request). _Mof_−/− MEF cells transfected with the pLentilox RSV-Puro (UM vector core) vector, which expresses either FLAG-tagged WT or E350Q (mutant) Mof, followed by a 3-day selection with puromycin (200 ng/ml).
Acetyltransferase assays.
Acetyltransferase assays were performed as described previously (10). Recombinant proteins (∼2 μg) were used. For all reactions, equivalent amounts of MOF-MSL1v1 protein (less than 100 ng) were used. Reactions were carried out at 30°C for 1 h in the presence of [3H]acetyl coenzyme A ([3H]acetyl-CoA).
RESULTS
Generation of conditional knockout alleles for Mof.
In order to study the functions of Mof in vivo, we generated the conditional Mof alleles that can be inactivated through Cre/_loxP_-mediated deletion. The targeting vector was constructed to have a loxP site inserted between exons 3 and 4. A neomycin resistance gene selection cassette flanked by two loxP sites was introduced between exons 6 and 7 (Fig. 1 A). Thus, exons 4 to 6 will be deleted upon the expression of Cre recombinase (Fig. 1A). This region encodes part of the catalytic MYST domain of Mof (amino acids 173 to 257) and is essential for the Mof acetyltransferase activity (data not shown). The _Mof_flox/+ mice obtained were then crossed with CAGG Cre-ER transgenic mice, which ubiquitously express Cre recombinase in the presence of 4-OHT (17). The heterozygotes for both Cre-ER and floxed Mof alleles were intercrossed to get _Cre-ER_-positive _Mof_flox/flox, _Mof_flox/+, and Mof+/+ mice (Fig. 1B). Primary MEF cell lines were derived from E13.5 Mof+/+ Cre + and _Mof_flox/flox Cre + embryos to carry out the studies described herein.
Mof knockout leads to global loss of H4 K16 acetylation.
Upon 4-OHT treatments, about 95% of the _Mof_flox/flox Cre + cells have complete deletion of floxed Mof alleles. RNAs and proteins were extracted from these cells 4 days after 4-OHT treatment for further analyses. As shown in Fig. 1C, a shorter transcript of Mof was detected by RT-PCR in _Mof_−/− Cre + cells, consistent with the removal of exons 4 to 6. Immunoblotting showed that the full-length Mof protein was absent in _Mof_−/− Cre + cells (Fig. 1D). Notably, we did not detect any truncated Mof protein using an antibody recognizing the first 50 amino acids of Mof (data not shown). It is likely that deletion of amino acids 173 to 257 of Mof destabilized the Mof protein in vivo. Consistent with previous reports that Mof is the major H4 K16 acetyltransferase in mammals (10, 41, 48), Mof deletion led to global reduction of H4 K16 acetylation in MEF cells, as demonstrated by both immunoblotting (Fig. 1D) and immunofluorescence assay (Fig. 1E). Expressing wild-type Mof in the _Mof_-knockout cells greatly enhanced H4 K16 acetylation (Fig. 1F). In contrast, cells expressing a Mof E350Q mutant that is mostly defective in substrate binding (29) had significantly lower levels of H4 K16ac. The effect of Mof knockout on H4 K16ac is highly specific because acetylation of other lysine residues on the H4 tail was not affected (Fig. 1D). Loss of Mof and H4 K16 acetylation did not affect the dimethylation of H4 K20, a nearby lysine residue in the same basic patch region of the H4 tail (Fig. 1D).
_Mof_-knockout cells have general defects in cell proliferation.
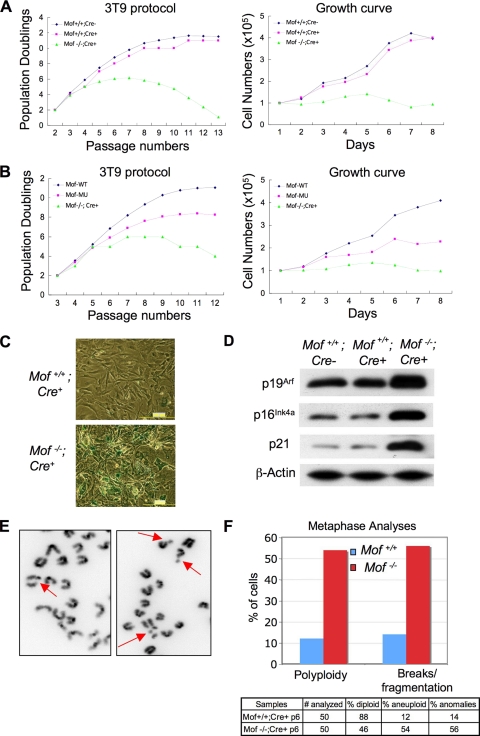
Characterization of the _Mof_−/− Cre + cells revealed that Mof played important roles in cell proliferation. Using a 3T9 protocol for the cell proliferation assay (50), _Mof_−/− Cre + cells exhibited decreased rates of population doublings after p5 compared to the rate for wild-type (Mof+/+ _Cre_-negative [_Cre−_]) primary cells (Fig. 2 A, left). Cell growth almost completely stopped by p7 for _Mof_−/− Cre + cells (Fig. 2A, right). The slow growth of _Mof_−/− Cre + cells was not a result of 4-OHT treatment, since Mof+/+ Cre + cells show population doubling and growth similar to those of Mof+/+ Cre − cells (Fig. 2A). The proliferation defects of _Mof_−/− Cre + cells can be completely rescued by wild-type Mof and only partially by Mof E350Q (Fig. 2B). The partial rescue by Mof E350Q was probably due to the residual acetyltransferase activity of this mutant (Fig. 1F). Furthermore, more than 50% of p7 _Mof_−/− Cre + cells stained positive for SA-β-Gal, suggesting premature senescence (Fig. 2C, bottom). In contrast, very few SA-β-Gal stain-positive cells were found at this stage in control Mof+/+ Cre + cells (Fig. 2C, top). Consistent with the SA-β-Gal staining results, we found dramatic upregulation of several senescence markers, such as p16Ink4a, p19Arf, and p21 (7), in _Mof_−/− Cre + cells (Fig. 2D). An increase in cell death for _Mof_−/− Cre + cells compared to that for wild-type cells was also observed in late passages.
FIG. 2.
Phenotypic analyses of _Mof_-knockout cells. (A) (Left) Cell proliferation assay for Mof+/+ Cre− (blue), Mof+/+ Cre+ (pink), and _Mof_−/− Cre + (green) primary MEF cells using a 3T9 protocol. Cells were passaged, and 9 × 105 cells were reseeded every 3 days on a 60-mm plate. (Right) Growth curves for Mof+/+ Cre−, Mof+/+ Cre+, and _Mof_−/− Cre + primary MEF cells (p7) were obtained by counting the number of cells every day. Cell numbers were plotted as a function of the passage number. (B) Cell proliferation assay (left) and growth curve analysis (right) were performed as described for panel A with _Mof_−/− Cre + and _Mof_−/− Cre + cells rescued with WT or mutant (MU) Mof E350Q. p3 instead of p2 cells were used as the starting point for the rescue experiments. (C) Cellular senescence assay with SA-β-galactosidase staining for Mof+/+ Cre+ and _Mof_−/− Cre + primary MEFs. Most of the _Mof_−/− Cre + MEF cells are positively stained. (D) Immunoblot analyses for the senescence markers p19Arf, p16Ink4a, and p21 in Mof+/+ Cre+ and _Mof_−/− Cre + p5 MEF cells. The antibodies used are indicated on the left. The immunoblot for β-actin was used as the loading control. (E) Representative pictures of chromosome breaks and fragments in _Mof_−/− Cre + cells. Chromatin breaks and fragments are indicated by arrows. (F) Summary of spontaneous genome instability in Mof+/+ Cre+ and _Mof_−/− Cre + primary MEFs. Metaphase spreads were stained with DAPI and analyzed for aneuploidy and chromosome anomalies.
Mof is important for genome integrity.
We next examined the genome stability of primary _Mof_−/− Cre + cells by karyotype analysis. The p6 Mof+/+ Cre+ and _Mof_flox/flox Cre+ MEF cells were treated with 4-OHT to delete Mof. Metaphase chromosomes from two independent _Cre_-positive Mof+/+ or _Mof_−/− cell lines were prepared and analyzed for chromosome anomalies. Although mitotic chromosome condensation was normal, we found an increase in aneuploidy and polyploidy (4N) as well as higher frequencies of chromosome breaks or fragmentations in _Mof_−/− cells (Fig. 2E, indicated by arrows). As summarized in Fig. 2F, 54% of _Mof_−/− cells but only 12% of wild-type cells demonstrated aneuploidy or polyploidy by p6. Furthermore, 56% of _Mof_−/− cells but only 14% of wild-type cells contained chromosome breaks (Fig. 2F). In most cases, multiple chromosome breaks or gaps were found in each metaphase. The chromosome anomalies increased dramatically with increasing numbers of cell divisions. Almost 20% more abnormal metaphases were observed in p6 _Mof_−/− cells than in p5 cells (data not shown). The accumulation of spontaneous genome instability in _Mof_−/− cells suggests that Mof plays important roles in maintaining genome integrity in higher eukaryotes.
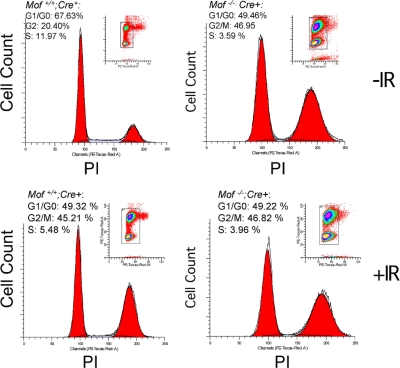
_Mof_-null cells show severe G2/M arrest in cell cycle.
The increase in chromosome anomalies in _Mof_−/− cells may result from defects in either cell cycle checkpoint or DNA repair. We first analyzed the cell cycle index of unsynchronized Mof+/+ and _Mof_−/− cell cultures. Fluorescence-activated cell sorter (FACS) analysis showed that a much higher percentage of _Mof_−/− cells was in G2/M phase (∼47% versus 20% for Mof+/+ cells) (Fig. 3, top). In relation to this, there were fewer _Mof_−/− cells in G1 and S phases of the cell cycle (Fig. 3, top). When exposed to DNA-damaging agents such as 5 Gy IR, _Mof_−/− cells showed no IR-dependent changes in the cell cycle index (Fig. 3, bottom). Under similar conditions, wild-type MEF cells demonstrated IR-induced changes in both G2/M and S phases of the cell cycle: an almost 25% increase in the G2/M-phase population and a 6.5% decrease in the S-phase population (Fig. 3, bottom). Given the persistent G2/M-phase arrest both under normal culture conditions and under conditions of IR induction, it is likely that the genome instability observed in _Mof_-null cells is due to failure to repair damaged DNA.
FIG. 3.
Mof knockout led to G2/M arrest in cell cycle. Cell cycle analyses for Mof+/+ (left) and _Mof_−/− (right) primary MEFs with or without 5 Gy IR treatment, as indicated on the right. DNA was stained by PI and measured by flow cytometry. The cell cycle index is indicated on the upper left corner of each panel.
_Mof_-null cells are deficient in DNA damage repair after ionizing radiation.
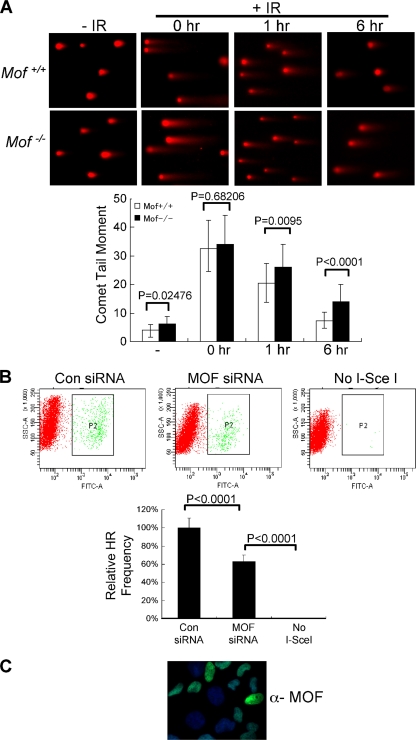
To directly examine whether _Mof_−/− cells are defective in DNA repair, we first tested if repair by NHEJ is affected in _Mof_−/− cells after DNA damage. Using the neutral comet assay (54), both wild-type and _Mof_−/− cells were either mock treated (−IR) or exposed to 80 Gy IR (+IR) and harvested at various time points postexposure (Fig. 4 A). Although induction of DNA breaks was similar for wild-type and _Mof_-knockout cells, _Mof_−/− cells repaired the damaged DNA at a lower rate (Fig. 4A). Compared to the results for wild-type cells, _Mof_−/− cells showed a modest decrease in repair efficiency (P < 0.0001), having a higher percentage of unrepaired DNA in the comet tail by 6 h (Fig. 4A, bottom). Similar moderate defects in DNA repair were also observed for _Mof_−/− cells using a pulsed-field gel electrophoresis-based double-strand break (DSB) assay after IR (data not shown). In addition to NHEJ, we also examined the function of MOF in homology-directed repair (HR). To this end, we performed a gene conversion assay using a previously established U2OS cell line with integrated direct repeat-GFPs (DR-GFPs) (18). In this assay, we transfected cells with a replication-defective adenovirus expressing I-SceI together with either control siRNAs or MOF siRNAs. Expression of the endonuclease I-SceI in the cells leads to double-strand breaks in the DR-GFP reporter which are repaired by HR and which produce GFP-positive cells (36). Thus, the efficiency of HR repair is measured by flow cytometry for GFP-positive cells. As a negative control, cells transfected with adenovirus containing no I-SceI were analyzed in parallel. As shown in Fig. 4B, cells treated with MOF siRNA consistently produced 40% fewer GFP-positive cells than cells treated with control siRNAs (P < 0.0001). The knockdown efficiency for MOF siRNA in U2OS cells was ∼50% (Fig. 4C). Taken together, Mof is likely to play important roles in IR-induced DNA damage repair. During the revision of the manuscript, similar results were reported by Sharma et al. for 293 cells treated with MOF siRNAs (39).
FIG. 4.
DSB repair defects in _Mof_−/− cells. (A) (Top) Representative images of neutral comet assays for Mof+/+ and _Mof_−/− cells. Cells were treated with 80 Gy IR and harvested at 0 h, 1 h, or 6 h after IR treatment, as indicated on top. Cells without IR treatment were used as controls. (Bottom) Unrepaired DNA was quantified as comet tail moment using CometScore software. At least 40 cells per sample were analyzed, and average values are presented. Error bars represent standard deviations. The Student t test was performed for Mof+/+ and _Mof_−/− cells at each time point, and P values are indicated. (B) Loss of Mof impairs homologous recombination repair. (Top) U2OS cells with integrated DR-GFP reporter genes were treated with either control (Con) or MOF siRNAs and then transfected with adenovirus expressing I-SceI, as indicated. Cells without I-SceI transfection were used as the negative control. A population of GFP-positive cells was separated from the rest of the cells by a P2 box in each plot. SSC, side scatter; FITC, fluorescein isothiocyanate. (Bottom) Quantitative summary of HR repair. The percentage of GFP-positive cells in each sample was normalized from the number of GFP-positive cells in the control siRNA-treated population. The Student t test was performed for control and MOF siRNA-treated cells, and P values are indicated on top. (C) Immunofluorescence using anti-Mof antibody for U2OS cells treated with MOF siRNA. About 50% of the cells have a reduced level of Mof. These cells were used in the HR assays whose results are presented in panel B. DAPI was used as the counterstain.
IR-induced DNA damage leads to a global increase in H4 K16 acetylation.
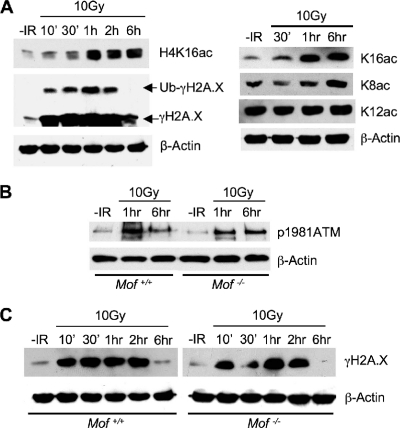
To further study the potential involvement of Mof in DNA damage repair and the underlying mechanism, we decided to examine whether IR treatment leads to changes in _Mof_-mediated H4 K16 acetylation in wild-type MEF cells. Interestingly, we found that global H4 K16 acetylation was highly induced ∼1 h after 10-Gy IR exposure (Fig. 5 A). Importantly, this induction was mostly specific for H4 K16 acetylation on the H4 tail, since no significant increase in the level of acetylation of other H4 lysine sites was detected after IR treatment (Fig. 5A, right). Of note, the increase in the level of H4 K16ac occurred after H2A.X phosphorylation, which was upregulated 10 min after IR exposure (Fig. 5A). We could not detect any damage focus-specific increase in H4 K16ac by immunofluorescence, probably due to the strong nuclear staining for H4 K16ac in cells prior to IR treatment (data not shown).
FIG. 5.
Mof knockout does not affect ATM activation or H2A.X phosphorylation after IR. (A) Immunoblots for H4 K16 acetylation and other histone modifications were tested for wild-type MEF cells at various time points after a 10-Gy IR treatment. Antibodies are indicated on the right. The immunoblot for β-actin was used as the loading control. (B and C) Immunoblot for ATM Ser1981 phosphorylation (B) or H2A.X phosphorylation (C), as indicated on the right. Total proteins were prepared from Mof+/+ and _Mof_−/− cells at the indicated times (on top) after exposure to 10 Gy IR. Proteins from cells without IR treatment were used as negative controls. The immunoblot for β-actin was used as the loading control.
Mof and H4 K16 acetylation do not affect ATM signaling or H2A.X phosphorylation.
The increase in the level of H4 K16 acetylation at 1 h after IR suggests that Mof may function at a later stage relative to the times of ATM activation and H2A.X phosphorylation. To test whether Mof affects ATM signaling, we compared the level and kinetics of IR-induced ATM Ser1981 phosphorylation as well as γH2A.X in Mof+/+ and _Mof_−/− cells by immunoblotting. As shown in Fig. 5B, Mof knockout did not affect either the level or the kinetics of ATM autophosphorylation (Fig. 5B). Furthermore, phosphorylation of important targets of the ATM signal transduction cascade, such as H2A.X, Chk1, Smc1, and p53 (23), was not affected in _Mof_−/− cells (Fig. 5C and data not shown). Of note, downregulation of γH2A.X was not affected by Mof deletion at later stages of the IR response. As shown in Fig. 5C, at 6 h after exposure to IR the level of γH2A.X in _Mof_−/− cells decreased to a level similar to that in wild-type cells. This observation is distinct from those for cells with null mutations of the closely related Tip60 (24) or the Tip60 complex component Trrap (32). Loss of function for either protein leads to delayed downregulation of γH2A.X at the DNA damage foci, an observation attributed to slower chromatin dynamics (12, 20, 24, 32). The fact that Mof and Tip60 have different effects on γH2A.X argues that they probably function at different steps in the DNA damage response.
Mof deletion abolished IR-induced recruitment of Mdc1, 53bp1, and Brca1 to DNA damage foci.
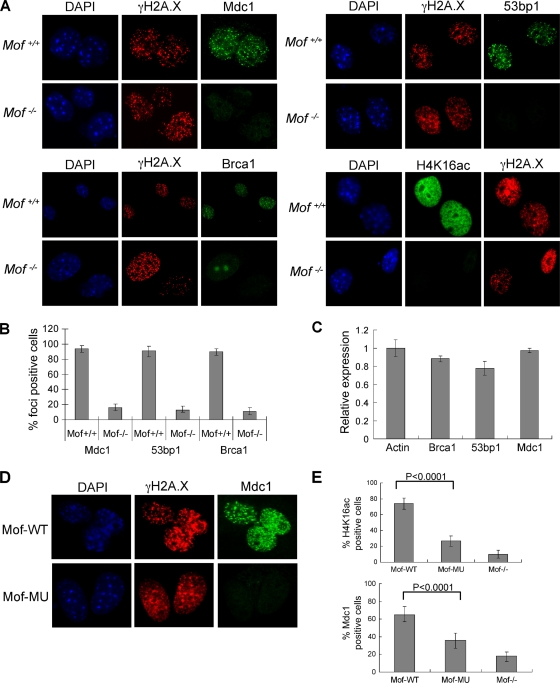
We next investigated whether Mof regulates downstream events, such as the recruitment of repair mediator proteins such as Mdc1, 53bp1, and Brca1 to the DNA damage foci. Loading of these repair proteins to sites of DNA double-strand breaks serves to amplify DNA damage signaling and activate checkpoint controls (52). This process can be monitored by immunofluorescence for rapid formation of distinct IR-induced foci (IRIFs). To this end, we treated Mof+/+ and _Mof_−/− cells with 10 Gy IR and performed immunostaining with antibodies against γH2A.X, Mdc1, 53bp1, and Brca1 2 h after treatment. Consistent with the immunoblotting results, IR-induced γH2A.X focus formation was not affected in _Mof_−/− cells (Fig. 6 A). In contrast, IRIF formation for Mdc1, 53bp1, and Brca1 was mostly abolished in _Mof_−/− cells (Fig. 6A), with more than 90% of cells staining negative for IRIFs (Fig. 6B). Of note, Brca1 was redistributed in the nuclei of _Mof_−/− cells after IR, which is distinct from the distributions of Mdc1 and 53bp1 (Fig. 6A). The mitigated recruitments for Mdc1, 53bp1, and Brca1 were not due to reduced expression of these genes (Fig. 6C) or changed recruitment kinetics (data not shown). It is important to point out that the effect of Mof deletion on Mdc1 recruitment is distinct from the effects of other histone-modifying activities (e.g., those of Tip60, Rnf168, and Rnf8), which mostly affect steps downstream of Mdc1 binding (52). To confirm that Mdc1 binding to γH2A.X foci depends on Mof acetyltransferase activity, we transfected _Mof_flox/flox Cre + cells with either wild-type Mof or Mof E350Q. The cells were then treated with 4-OHT to delete endogenous Mof. As shown in Fig. 6D and E, wild-type Mof significantly increased the numbers of H4 K16ac and Mdc1-positive cells in the population compared to those in the Mof mutant population (P < 0.0001), which had only a partial increase in the numbers of Mdc1 foci due to residual HAT activity (Fig. 6E). The positively stained cells (∼5%) in _Mof_−/− culture were probably due to incomplete deletion of endogenous Mof in these cells.
FIG. 6.
Mof and its acetyltransferase activity are essential for recruitment of repair proteins after IR. (A) Immunofluorescence for different repair mediator proteins (i.e., Mdc1, 53bp1, and Brca1) in Mof+/+ and _Mof_−/− MEFs. Cells were fixed 2 h after a 10-Gy IR treatment and immunostained with antibodies, as indicated on top. As controls, H4 K16ac was stained for Mof+/+ and _Mof_−/− MEFs (bottom right). DAPI and H2A.X were used as the counterstains in all experiments. (B) The percentage of focus-positive cells was scored for each antibody, and the averages of three independent experiments are presented. Error bars represent standard deviations. (C) Expression of Mdc1, 53bp1, and Brca1 was largely unaffected in _Mof_−/− MEFs. Ratios of mRNA levels in _Mof_−/− MEFs versus Mof+/+ MEFs were presented after normalization to β-actin levels. Error bars represent standard deviations from three independent RT-PCRs. (D) Immunofluorescence for γH2A.X and Mdc1 in _Mof_−/− cells rescued with WT Mof or mutant (MU) Mof. (E) (Top) Percentage of H4 K16ac-positive cells as an indicator of the rescue efficiency of WT Mof and mutant Mof. (Bottom) Percentage of Mdc1 focus-positive _Mof_−/− and _Mof_−/− cells rescued with WT Mof or mutant (MU) Mof. An average of three independent experiments was performed for each antibody. Error bars represent standard deviations.
Mdc1 recruitment is affected by both Mof complexes during DNA damage response.
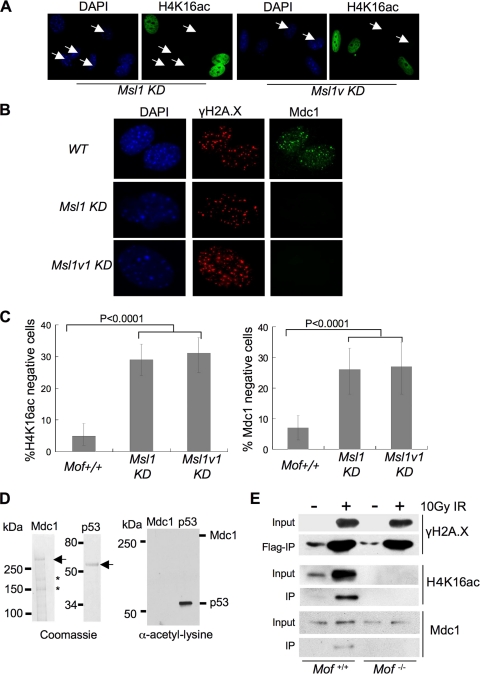
We previously demonstrated that Mof in mammalian cells resides in two distinct complexes: Mof-Msl and Mof-Msl1v1 (10, 26). While both Mof complexes are able to acetylate nucleosomal H4 K16, Mof-Msl1v1 has an expanded substrate spectrum and is capable of acetylating nonhistone substrates, such as p53 (4, 26). To test which Mof complex is important for Mdc1 recruitment and to distinguish the contributions of histone versus nonhistone acetylation, we examined Mdc1 IRIF formation in MEF cells treated with either Msl1 siRNAs or Msl1v1 shRNAs. As shown in Fig. 7, about 30% of the MEF cells showed reduced H4 K16ac after siRNA and shRNA treatment for Msl1 and Msl1v1, respectively. The reduction of H4 K16ac was closely correlated with disappearance of the Mdc1 IRIF after DNA damage in both knockdown cells (Fig. 7B and C). In fact, there was no significant difference between Msl1 and Msl1v1 siRNA/shRNA treatments (Fig. 7C). This result suggests that both Mof complexes are able to regulate Mdc1 recruitment, probably through the shared H4 K16 acetylation activity. Consistently, we found that Mdc1 was not acetylated by the Mof-Msl1v1 complex in vitro (Fig. 7D). To further examine the importance of H4 K16 acetylation on Mdc1 recruitment and its binding to γH2A.X (43), we introduced FLAG-H2A.X into _Mof_flox/flox Cre + cells. _Mof_−/− cells stably expressing FLAG-H2A.X were then derived by 4-OHT treatments to remove floxed Mof alleles. Both cell lines (_Mof_flox/flox and _Mof_−/−) were treated with 10 Gy ionizing radiation, and mononucleosomes from these cells were purified and subject to FLAG immunoprecipitation (IP). Since the majority of FLAG-H2A.X was phosphorylated after IR, FLAG IP would enrich γH2A.X-containing mononucleosomes and its binding proteins. As shown in Fig. 7E, H4 K16 acetylation was also enriched in γH2A.X-containing mononucleosomes after exposure to IR. Interestingly, while Mdc1 binding to dual modified mononucleosomes was clearly detected (Fig. 7E, second lane), it was abolished when nucleosomes were isolated from _Mof_-knockout cells (Fig. 7E, fourth versus second lane). These results strongly argue that H4 K16 acetylation by Mof directly affects Mdc1 binding to γH2A.X.
FIG. 7.
Both Msl1 and Msl1v1 knockdown (KD) affect the recruitment of Mdc1 after IR. (A) Immunofluorescence of H4 K16ac for MEF cells treated with Msl1 siRNA or Msl1v1 shRNA. In each case, about 30% of cells show reduced H4 K16ac in the population (arrowheads). (B) Immunofluorescence of γH2A.X and Mdc1 in wild-type and Msl1- or Msl1v1-knockdown MEFs. Representative pictures were selected for presentation. (C) Scores for H4 K16ac-negative (left) and Mdc1 focus-negative (right) cells in Msl1- and Msl1v1-knockdown MEF cells. Mof+/+ MEF cells were used as the control. An average of three independent experiments was performed for each antibody. Error bars represent standard deviations. The Student t test was performed, and P values (<0.0001) are indicated on top. (D) (Left) Coomassie stain of the purified recombinant Mdc1 or p53 proteins (as indicated on top). Both proteins were purified through the FLAG epitope. *, breakdown products of Mdc1. (Right) In vitro HAT assays using Mdc1 or p53 as the substrate and the MOF-Msl1v1 complex as the enzyme. [3H]acetyl-CoA was used as the cofactor. (E) Immunoblots for γH2A.X, Mdc1, and H4 K16ac, as indicated on the right. Mononucleosomes from Mof+/+ and _Mof_−/− MEFs were purified after micrococcal nuclease digestion, and H2A.X-containing nucleosomes were further purified by FLAG IP.
Interactions between the H4 tail and the acidic patch on H2A.X are important for Mdc1 recruitment.
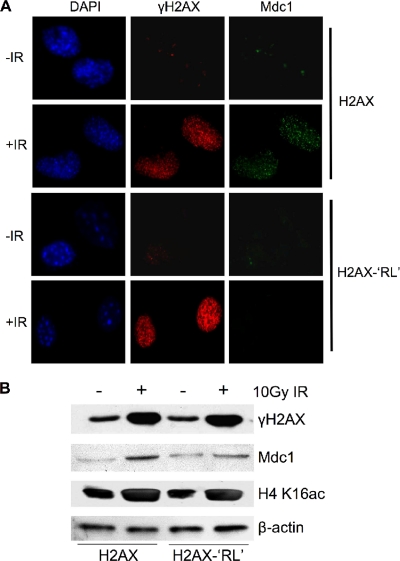
Mdc1 has previously been shown to bind directly to γH2A.X through its C-terminal BRCT domain (27, 43). The requirement of H4 K16ac for this interaction raised an interesting question: how is this effect achieved? It is well characterized that K16 resides in a basic region of H4 tail, which is critical for the inter- and intranucleosome interactions with an acidic pocket on the surface of H2A. The H4-H2A interaction is key for the fiber-fiber associations that contribute to formation of high-order chromatin structures in vitro (5). This interaction is regulated by H4 K16 acetylation (37, 40). Since the acidic pocket is highly conserved in H2A.X, we reasoned that if defects in Mdc1 recruitment in _Mof_-knockout cells are due to perturbation of interactions of H4-H2A.X, we will see a similar effect if we neutralize the charges of the acidic pocket on H2A.X. To test this hypothesis, we mutated key residues (E91 and E92) in the acidic pocket region of H2A.X to R91L92 (RL sequence). Since the RL sequence is naturally found on an H2A variant, H2A.Bbd, it does not affect the folding of mutant H2A.X. More importantly, structure studies have shown that E91 and E92 of H2A.X are not involved in the binding of the BRCT domain of Mdc1 to H2A.X (43). We stably introduced both wild-type H2A.X and the H2A.X-RL mutant into H2A.X −/− cells. Upon 10 Gy IR exposure, cells expressing exogenous H2A.X or H2A.X-RL showed indistinguishable (i.e., according to intensity and focus numbers) immunostaining for γH2A.X, suggesting that the mutant did not affect the integration of H2A.X into chromatin and its phosphorylation by ATM/ATR family kinases (Fig. 8 A). Interestingly, IRIF for Mdc1 was completely abolished in cells expressing the H2A.X-RL mutant, while it was normal in the presence of wild-type H2A.X (Fig. 8A). Consistently, the level of chromatin-associated Mdc1 was also decreased in H2A.X-RL cells (Fig. 8B). As controls, similar levels of H4 K16ac and γH2A.X were detected in both cell lines (Fig. 8B). These results suggest that Mdc1 recruitment to DNA damage foci is likely to be controlled by trans interactions between the H4 tail and the acidic pocket of H2A.X, which are subject to regulation by Mof-mediated H4 K16 acetylation.
FIG. 8.
Inter- or intranucleosomal interactions between H4 and H2A.X are important for Mdc1 recruitment to DNA damage foci. (A) Immunofluorescence for γH2A.X and Mdc1 in H2A.X −/− cells stably expressing either wild-type or H2A.X-RL 2 h after a 10-Gy IR treatment. Error bars represent standard deviations from three independent RT-PCRs. (B) Immunoblots for chromatin-associated proteins, as indicated on the right. Proteins were extracted 2 h after a 10-Gy IR treatment.
DISCUSSION
In this study, we have established a conditional mouse knockout model for Mof and derived 4-OHT-inducible Cre-ER MEF cell lines for Mof+/+ and _Mof_−/−. Using these cell lines, we demonstrate that Mof and H4 K16 acetylation are important for maintaining genome integrity. Loss of Mof and H4 K16 acetylation led to spontaneous genome instability, severe cell cycle arrest at G2/M phase (Fig. 2 and 3), and defects in both NHEJ- and HR-mediated DNA repair (Fig. 4). Importantly, although Mof deletion does not affect ATM autophosphorylation or phosphorylation of H2A.X and other targets in the ATM signaling cascade, the recruitment of repair mediator proteins Mdc1, 53bp1, and Brca1 to DNA damage foci are abolished in _Mof_−/− cells (Fig. 5). Mechanistic studies show that H4 K16 acetylation may influence γH2A.X signaling through the intra- or internucleosomal interactions with the acidic patch on H2A.X. Together, we have demonstrated a novel mechanism for the chromatin-mediated DNA damage response.
MOF is implicated in the DDR process by several earlier studies (15, 39, 48). For example, it has been reported that MOF interacts with proteins such as the MORF-related gene on chromosome 15 (MRG15) (12, 35), Ruvb1/2 (10, 20), and DNA-dependent protein kinase catalytic subunit (DNA-PKcs) (39) that are important for DNA damage repair. Cellular assays using cells treated with MOF siRNAs or with overexpression of a dominant-negative MOF mutant also show that MOF is involved in multiple steps of the DNA damage repair process (15, 39, 47). However, RNA interference-based loss of function studies for MOF in the DNA damage response sometimes give rise to conflicting results that need to be clarified. Most notably, MOF was reported to be important for IR-induced ATM and H2A.X phosphorylation at an early stage of DSB in some studies (15, 48), while it was shown to have opposite effects on these processes in others (47). These discrepancies are probably due to differences in RNA interference knockdown efficiency, the different immortalized cell lines used, and/or differences in IR conditions (i.e., dosage, time course, etc). Similar discrepancies in the Mof function in cell cycle control are also found in the literature (15, 48). Using cells carrying conditional alleles for Mof allows us, for the first time, to study the function of Mof in DNA damage repair in primary cell cultures with a well-defined genetic background. We find that Mof does not affect the earlier steps of the damage response, such as ATM signaling and γH2A.X focus formation, in response to 10-Gy IR exposure (although we cannot rule out its effects in cells treated with a low dosage of IR). Instead, it specifically affects the binding of repair mediator protein Mdc1 to γH2A.X as well as the recruitment of other downstream repair mediator proteins, such as 53bp1 and Brca1 (Fig. 6). Consistent with the role of Mof at a later stage of DDR, we find that an IR-induced global increase in the level of H4 K16 acetylation occurs approximately 1 h after irradiation in primary MEF cells (Fig. 5A). This increase in H4 K16 acetylation is likely a result of IR-induced Mof association with chromatin but not changes in Mof expression (39). Of note, a smaller percentage of _Mof_-knockout cells have foci for Mdc1, 53bp1, and Brca1, which are related to spontaneous DNA damage during the normal cell cycle (data not shown). However, it remains inconclusive whether they underlie the significant spontaneous genome instability observed for these cells or are simply a result of a reduced percentage of S-phase cells in _Mof_−/− culture (Fig. 3).
Previous studies have shown that Mof plays important roles in transcription regulation through acetylating both H4 K16 and nonhistone substrates, such as transcription factor p53 (26). In addition, Mof also interacts with other chromatin-modifying activities that are important for establishing a euchromatic environment (10). Thus, it is important to establish whether the effects of Mof knockout on DNA damage repair are direct and whether they are due to loss of H4 K16 acetylation. Our study provides several lines of evidence to support direct roles of Mof and H4 K16 acetylation in the recruitment of key repair mediator protein Mdc1 to repair foci: (i) Mof knockout does not affect expression of the Mdc1, 53bp1, or Brca1 gene (Fig. 6C). This result is consistent with a recent report that Mof knockdown in 293 cells does not affect most genes in the DNA damage repair pathway (39). (ii) The defects in Mdc1 recruitment can be rescued by expressing exogenous wild-type Mof but are rescued significantly less so by expressing the Mof mutant E350Q (Fig. 6D and E). The E350Q mutation of Mof has reduced HAT activity without affecting Mof complex formation. (iii) Upregulation of H4 K16 acetylation (Fig. 5A) and its enrichment in γH2A.X-containing mononucleosomes (Fig. 7E) are observed at 1 h post-IR. Importantly, dual modifications of H4 K16ac and H2A.X phosphorylation are required for Mdc1 association with isolated nucleosomes (Fig. 7E). (iv) Mdc1 cannot be acetylated by the Mof-Msl1v1 complex, ruling out its regulation by _Mof_-mediated posttranslational modifications (Fig. 7D). (v) Last, but not least, Mdc1 recruitment is regulated by both the Mof-Msl and Mof-Msl1v1 complexes (Fig. 7). Despite their distinct functions in transcription regulation and nonhistone substrate acetylation, both Mof complexes are important for acetylating H4 K16 in vivo (26). Taken together, our results presented here strongly argue that Mof and its H4 K16 acetyltransferase activity are directly involved in the DNA damage repair process at the step of repair protein recruitment.
The coordination between the DNA repair machinery and chromatin remodeling activities has been extensively studied (52). DNA damage elicits immediate formation of repair centers containing gigadalton-sized protein assemblies of many repair proteins (38, 46). The formation and spreading of such assemblies require a permissible chromatin environment, facilitated by ATP-dependent chromatin-remodeling activities, such as those of the INO80 complex and histone-modifying enzymes (8, 22). In particular, histone acetyltransferase Tip60 has been reported to be intensely involved in DDR (42, 45). Tip60 is a highly conserved MYST family HAT and a close homologue of MOF. It targets histone H4 (except H4 K16) and H2A tails. It was found that the Tip60 complex functions at multiple steps of DDR. It directly (i) affects ATM autophosphorylation and activation (44); (ii) facilitates γH2A.X eviction or exchange at damage sites (19, 24); (iii) recruits ribonucleotide reductase and influences DNA repair through a chromatin-independent mechanism (34); and (iv) importantly, modulates loading of 53bp1, but not Mdc1, to the damage foci after IR (32). These functions are in contrast to those of MOF, despite their sequence homology. In this study, we find that _Mof_-knockout cells have normal ATM autophosphorylation and γH2A.X kinetics after IR-induced DNA damage (Fig. 5). However, the recruitment of repair proteins Mdc1 and its downstream effectors is completely abolished (Fig. 6). This effect depends on the activity of Mof on H4 K16 acetylation (see above). Inactivation of the acetyltransferase activity of Mof by either mutating its HAT domain or knocking down the key regulatory components of the Mof complexes dramatically reduces focus formation by Mdc1 (Fig. 6 and 7). Given the different substrate specificities for Mof and Tip60 (13, 19, 51), the distinct phenotypes that we have observed in _Mof_-knockout cells probably reflect the unique role of H4 K16 acetylation in DNA damage repair compared to the roles of other histone acetylation events.
In contrast to other chromatin-remodeling activities involved in DNA damage repair (31), Mof is likely to influence the repair process through regulating higher-order chromatin structures. This attributes to H4 K16 acetylation the crucial role of regulating internucleosome interactions between the basic patch (amino acids 14 to 19) on the histone H4 tail and the acidic pocket on the H2A-H2B surface in chromatin fibers (5, 9, 28, 30). Biochemical and biophysical studies have shown that unacetylated H4 K16 is required to achieve the maximum propensity of nucleosome arrays to fold into secondary or tertiary chromatin structures in vitro (40). On the contrary, 30% H4 K16 acetylation alleviates compaction of 30 nm of chromatin fiber to a greater degree than deletion of the H4 N-terminal tail (37). Thus, it is highly likely that loss of Mof reduces accessibility of nucleosomes to repair proteins such as Mdc1 by increasing the compaction of chromatin structures at the damage foci. In support of this, we find that a charge-neutralizing mutation, H2A.X-RL, which abolishes the H4 tail interaction and which is shown to facilitate heterochromatination throughout the nuclei (55), recapitulates the effect of Mof knockout in Mdc1 recruitment (Fig. 8A). This result suggests that _Mof_-mediated H4 K16 acetylation probably regulates DNA damage repair through affecting the inter- and intranucleosome interactions of H4-H2A tails, which are essential for establishing the proper higher-order chromatin structure. Our study described here also points to an interesting and unexpected role for the acidic pocket of H2A.X in the signaling cascade of DNA damage responses. It has previously been shown that the acidic pocket of H2A is used by proteins or protein complexes to associate with nucleosome particles (1, 2). Since blocking the acidic pocket by interacting proteins (e.g., LANA) has the same effect as the charge-neutralizing mutations (i.e., H2A.X-RL) (6, 55), it will be interesting to test whether proteins that bind to this region of H2A.X block the recruitment of DNA repair proteins and regulate the DNA damage response in the same way as Mof. Future studies on the acidic pocket of H2A.X and its interacting proteins are necessary to access the generality of this novel mechanism for regulating the DNA damage repair process.
Acknowledgments
We thank Thomas Saunders at the UM ES Core for helping with the generation of _Mof_-knockout mice and Zhenkun Lou at Mayo Clinic for providing Mdc1 antibody. We thank Melanie Adams-Cioaba and Yahong Guo in the J.M. laboratory for purifying 53bp1, Yiping Wu and Jeffrey Buis in the D.F. laboratory, and Jiaxue Wu in the X.Y. laboratory for advice on DNA repair assays.
This work was supported by NIH funds to D.F., X.Y., and Y.D. and an American Cancer Society Research Scholar grant to Y.D.
Footnotes
▿
Published ahead of print on 13 September 2010.
REFERENCES
- 1.Barbera, A. J., J. V. Chodaparambil, B. Kelley-Clarke, V. Joukov, J. C. Walter, K. Luger, and K. M. Kaye. 2006. The nucleosomal surface as a docking station for Kaposi's sarcoma herpesvirus LANA. Science 311**:**856-861. [DOI] [PubMed] [Google Scholar]
- 2.Barbera, A. J., J. V. Chodaparambil, B. Kelley-Clarke, K. Luger, and K. M. Kaye. 2006. Kaposi's sarcoma-associated herpesvirus LANA hitches a ride on the chromosome. Cell Cycle 5**:**1048-1052. [DOI] [PubMed] [Google Scholar]
- 3.Bird, A. W., D. Y. Yu, M. G. Pray-Grant, Q. Qiu, K. E. Harmon, P. C. Megee, P. A. Grant, M. M. Smith, and M. F. Christman. 2002. Acetylation of histone H4 by Esa1 is required for DNA double-strand break repair. Nature 419**:**411-415. [DOI] [PubMed] [Google Scholar]
- 4.Cai, Y., J. Jin, S. K. Swanson, M. D. Cole, S. H. Choi, L. Florens, M. P. Washburn, J. W. Conaway, and R. C. Conaway. 2010. Subunit composition and substrate specificity of a MOF-containing histone acetyltransferase distinct from the male-specific lethal (MSL) complex. J. Biol. Chem. 285**:**4268-4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caterino, T. L., and J. J. Hayes. 2007. Chromatin structure depends on what's in the nucleosome's pocket. Nat. Struct. Mol. Biol. 14**:**1056-1058. [DOI] [PubMed] [Google Scholar]
- 6.Chodaparambil, J. V., A. J. Barbera, X. Lu, K. M. Kaye, J. C. Hansen, and K. Luger. 2007. A charged and contoured surface on the nucleosome regulates chromatin compaction. Nat. Struct. Mol. Biol. 14**:**1105-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collado, M., M. A. Blasco, and M. Serrano. 2007. Cellular senescence in cancer and aging. Cell 130**:**223-233. [DOI] [PubMed] [Google Scholar]
- 8.Cowell, I. G., N. J. Sunter, P. B. Singh, C. A. Austin, B. W. Durkacz, and M. J. Tilby. 2007. gammaH2AX foci form preferentially in euchromatin after ionising-radiation. PLoS One 2**:**e1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorigo, B., T. Schalch, A. Kulangara, S. Duda, R. R. Schroeder, and T. J. Richmond. 2004. Nucleosome arrays reveal the two-start organization of the chromatin fiber. Science 306**:**1571-1573. [DOI] [PubMed] [Google Scholar]
- 10.Dou, Y., T. A. Milne, A. J. Tackett, E. R. Smith, A. Fukuda, J. Wysocka, C. D. Allis, B. T. Chait, J. L. Hess, and R. G. Roeder. 2005. Physical association and coordinate function of the H3 K4 methyltransferase MLL1 and the H4 K16 acetyltransferase MOF. Cell 121**:**873-885. [DOI] [PubMed] [Google Scholar]
- 11.Downs, J. A., S. Allard, O. Jobin-Robitaille, A. Javaheri, A. Auger, N. Bouchard, S. J. Kron, S. P. Jackson, and J. Cote. 2004. Binding of chromatin-modifying activities to phosphorylated histone H2A at DNA damage sites. Mol. Cell 16**:**979-990. [DOI] [PubMed] [Google Scholar]
- 12.Garcia, S. N., B. M. Kirtane, A. J. Podlutsky, O. M. Pereira-Smith, and K. Tominaga. 2007. Mrg15 null and heterozygous mouse embryonic fibroblasts exhibit DNA-repair defects post exposure to gamma ionizing radiation. FEBS Lett. 581**:**5275-5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grienenberger, A., B. Miotto, T. Sagnier, G. Cavalli, V. Schramke, V. Geli, M. C. Mariol, H. Berenger, Y. Graba, and J. Pradel. 2002. The MYST domain acetyltransferase Chameau functions in epigenetic mechanisms of transcriptional repression. Curr. Biol. 12**:**762-766. [DOI] [PubMed] [Google Scholar]
- 14.Gupta, A., T. G. Guerin-Peyrou, G. G. Sharma, C. Park, M. Agarwal, R. K. Ganju, S. Pandita, K. Choi, S. Sukumar, R. K. Pandita, T. Ludwig, and T. K. Pandita. 2008. The mammalian ortholog of Drosophila MOF that acetylates histone H4 lysine 16 is essential for embryogenesis and oncogenesis. Mol. Cell. Biol. 28**:**397-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta, A., G. G. Sharma, C. S. Young, M. Agarwal, E. R. Smith, T. T. Paull, J. C. Lucchesi, K. K. Khanna, T. Ludwig, and T. K. Pandita. 2005. Involvement of human MOF in ATM function. Mol. Cell. Biol. 25**:**5292-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harper, J. W., and S. J. Elledge. 2007. The DNA damage response: ten years after. Mol. Cell 28**:**739-745. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi, S., and A. P. McMahon. 2002. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol. 244**:**305-318. [DOI] [PubMed] [Google Scholar]
- 18.Huang, J., M. S. Huen, H. Kim, C. C. Leung, J. N. Glover, X. Yu, and J. Chen. 2009. RAD18 transmits DNA damage signalling to elicit homologous recombination repair. Nat. Cell Biol. 11**:**592-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Huen, M. S., R. Grant, I. Manke, K. Minn, X. Yu, M. B. Yaffe, and J. Chen. 2007. RUF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell 131**:**901-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikura, T., S. Tashiro, A. Kakino, H. Shima, N. Jacob, R. Amunugama, K. Yoder, S. Izumi, I. Kuraoka, K. Tanaka, H. Kimura, M. Ikura, S. Nishikubo, T. Ito, A. Muto, K. Miyagawa, S. Takeda, R. Fishel, K. Igarashi, and K. Kamiya. 2007. DNA damage-dependent acetylation and ubiquitination of H2AX enhances chromatin dynamics. Mol. Cell. Biol. 27**:**7028-7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jha, S., E. Shibata, and A. Dutta. 2008. Human Rvb1/Tip49 is required for the histone acetyltransferase activity of Tip60/NuA4 and for the downregulation of phosphorylation on H2AX after DNA damage. Mol. Cell. Biol. 28**:**2690-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kan, P. Y., T. L. Caterino, and J. J. Hayes. 2009. The H4 tail domain participates in intra- and internucleosome interactions with protein and DNA during folding and oligomerization of nucleosome arrays. Mol. Cell. Biol. 29**:**538-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, J. A., M. Kruhlak, F. Dotiwala, A. Nussenzweig, and J. E. Haber. 2007. Heterochromatin is refractory to gamma-H2AX modification in yeast and mammals. J. Cell Biol. 178**:**209-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitagawa, R., and M. B. Kastan. 2005. The ATM-dependent DNA damage signaling pathway. Cold Spring Harbor Symp. Quant. Biol. 70**:**99-109. [DOI] [PubMed] [Google Scholar]
- 24.Kusch, T., L. Florens, W. H. Macdonald, S. K. Swanson, R. L. Glaser, J. R. Yates III, S. M. Abmayr, M. P. Washburn, and J. L. Workman. 2004. Acetylation by Tip60 is required for selective histone variant exchange at DNA lesions. Science 306**:**2084-2087. [DOI] [PubMed] [Google Scholar]
- 25.Li, X., and Y. Dou. 2010. New perspectives for the regulation of acetyltransferase MOF. Epigenetics 5**:**185-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, X., L. Wu, C. A. Corsa, S. Kunkel, and Y. Dou. 2009. Two mammalian MOF complexes regulate transcription activation by distinct mechanisms. Mol. Cell 36**:**290-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lou, Z., K. Minter-Dykhouse, X. Wu, and J. Chen. 2003. MDC1 is coupled to activated CHK2 in mammalian DNA damage response pathways. Nature 421**:**957-961. [DOI] [PubMed] [Google Scholar]
- 28.Luger, K., A. W. Mader, R. K. Richmond, D. F. Sargent, and T. J. Richmond. 1997. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 389**:**251-260. [DOI] [PubMed] [Google Scholar]
- 29.Marmorstein, R., and S. Y. Roth. 2001. Histone acetyltransferases: function, structure, and catalysis. Curr. Opin. Genet. Dev. 11**:**155-161. [DOI] [PubMed] [Google Scholar]
- 30.Megee, P. C., B. A. Morgan, and M. M. Smith. 1995. Histone H4 and the maintenance of genome integrity. Genes Dev. 9**:**1716-1727. [DOI] [PubMed] [Google Scholar]
- 31.Morrison, A. J., and X. Shen. 2006. Chromatin modifications in DNA repair. Results Probl. Cell Differ. 41**:**109-125. [DOI] [PubMed] [Google Scholar]
- 32.Murr, R., J. I. Loizou, Y. G. Yang, C. Cuenin, H. Li, Z. Q. Wang, and Z. Herceg. 2006. Histone acetylation by Trrap-Tip60 modulates loading of repair proteins and repair of DNA double-strand breaks. Nat. Cell Biol. 8**:**91-99. [DOI] [PubMed] [Google Scholar]
- 33.Neal, K. C., A. Pannuti, E. R. Smith, and J. C. Lucchesi. 2000. A new human member of the MYST family of histone acetyl transferases with high sequence similarity to Drosophila MOF. Biochim. Biophys. Acta 1490**:**170-174. [DOI] [PubMed] [Google Scholar]
- 34.Niida, H., Y. Katsuno, M. Sengoku, M. Shimada, M. Yukawa, M. Ikura, T. Ikura, K. Kohno, H. Shima, H. Suzuki, S. Tashiro, and M. Nakanishi. 2010. Essential role of Tip60-dependent recruitment of ribonucleotide reductase at DNA damage sites in DNA repair during G1 phase. Genes Dev. 24**:**333-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pardo, P. S., J. K. Leung, J. C. Lucchesi, and O. M. Pereira-Smith. 2002. MRG15, a novel chromodomain protein, is present in two distinct multiprotein complexes involved in transcriptional activation. J. Biol. Chem. 277**:**50860-50866. [DOI] [PubMed] [Google Scholar]
- 36.Pierce, A. J., R. D. Johnson, L. H. Thompson, and M. Jasin. 1999. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 13**:**2633-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson, P. J., W. An, A. Routh, F. Martino, L. Chapman, R. G. Roeder, and D. Rhodes. 2008. 30 nm chromatin fibre decompaction requires both H4-K16 acetylation and linker histone eviction. J. Mol. Biol. 381**:**816-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.San Filippo, J., P. Sung, and H. Klein. 2008. Mechanism of eukaryotic homologous recombination. Annu. Rev. Biochem. 77**:**229-257. [DOI] [PubMed] [Google Scholar]
- 39.Sharma, G. G., S. So, A. Gupta, R. Kumar, C. Cayrou, N. Avvakumov, U. Bhadra, R. K. Pandita, M. H. Porteus, D. J. Chen, J. Cote, and T. K. Pandita. 2010. MOF and histone H4 acetylation at lysine 16 are critical for DNA damage response and double-strand break repair. Mol. Cell. Biol. 30**:**3582-3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shogren-Knaak, M., H. Ishii, J. M. Sun, M. J. Pazin, J. R. Davie, and C. L. Peterson. 2006. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science 311**:**844-847. [DOI] [PubMed] [Google Scholar]
- 41.Smith, E. R., C. Cayrou, R. Huang, W. S. Lane, J. Cote, and J. C. Lucchesi. 2005. A human protein complex homologous to the Drosophila MSL complex is responsible for the majority of histone H4 acetylation at lysine 16. Mol. Cell. Biol. 25**:**9175-9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Squatrito, M., C. Gorrini, and B. Amati. 2006. Tip60 in DNA damage response and growth control: many tricks in one HAT. Trends Cell Biol. 16**:**433-442. [DOI] [PubMed] [Google Scholar]
- 43.Stucki, M., J. A. Clapperton, D. Mohammad, M. B. Yaffe, S. J. Smerdon, and S. P. Jackson. 2005. MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell 123**:**1213-1226. [DOI] [PubMed] [Google Scholar]
- 44.Sun, Y., X. Jiang, S. Chen, N. Fernandes, and B. D. Price. 2005. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc. Natl. Acad. Sci. U. S. A. 102**:**13182-13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun, Y., X. Jiang, and B. D. Price. Tip60: connecting chromatin to DNA damage signaling. Cell Cycle 9**:**930-936. [DOI] [PMC free article] [PubMed]
- 46.Sung, P., and H. Klein. 2006. Mechanism of homologous recombination: mediators and helicases take on regulatory functions. Nat. Rev. Mol. Cell Biol. 7**:**739-750. [DOI] [PubMed] [Google Scholar]
- 47.Taipale, M., and A. Akhtar. 2005. Chromatin mechanisms in Drosophila dosage compensation. Prog. Mol. Subcell. Biol. 38**:**123-149. [DOI] [PubMed] [Google Scholar]
- 48.Taipale, M., S. Rea, K. Richter, A. Vilar, P. Lichter, A. Imhof, and A. Akhtar. 2005. hMOF histone acetyltransferase is required for histone H4 lysine 16 acetylation in mammalian cells. Mol. Cell. Biol. 25**:**6798-6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas, T., M. P. Dixon, A. J. Kueh, and A. K. Voss. 2008. Mof (MYST1 or KAT8) is essential for progression of embryonic development past the blastocyst stage and required for normal chromatin architecture. Mol. Cell. Biol. 28**:**5093-5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Todaro, G. J., and H. Green. 1963. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 17**:**299-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Utley, R. T., and J. Cote. 2003. The MYST family of histone acetyltransferases. Curr. Top. Microbiol. Immunol. 274**:**203-236. [DOI] [PubMed] [Google Scholar]
- 52.van Attikum, H., and S. M. Gasser. 2009. Crosstalk between histone modifications during the DNA damage response. Trends Cell Biol. 19**:**207-217. [DOI] [PubMed] [Google Scholar]
- 53.Voss, A. K., and T. Thomas. 2009. MYST family histone acetyltransferases take center stage in stem cells and development. Bioessays 31**:**1050-1061. [DOI] [PubMed] [Google Scholar]
- 54.Wu, J., M. J. Prindle, G. R. Dressler, and X. Yu. 2009. PTIP regulates 53BP1 and SMC1 at the DNA damage sites. J. Biol. Chem. 284**:**18078-18084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou, J., J. Y. Fan, D. Rangasamy, and D. J. Tremethick. 2007. The nucleosome surface regulates chromatin compaction and couples it with transcriptional repression. Nat. Struct. Mol. Biol. 14**:**1070-1076. [DOI] [PubMed] [Google Scholar]