Once a killer, always a killer: from cytotoxic T cell to memory cell (original) (raw)
. Author manuscript; available in PMC: 2010 Nov 20.
Summary
The control of the differentiation pathways followed by responding CD8+ T cells to produce protective memory cells has been intensely studied. Recent developments have identified heterogeneity at the effector cytotoxic T-lymphocyte level within which a bona fide memory cell precursor has emerged. The challenge now is to identify the cellular and molecular factors that control this developmental pathway. This review considers aspects of the regulation of the induction of effectors, the transition of effectors to memory cells, and the dynamics of the memory population.
Keywords: T cells, infectious diseases, cytokines, cytokine receptors, cell differentiation
Introduction
Induction of cytotoxic activity by CD8+ T cells has long been a topic of immunologic interest. Ever since cytotoxic activity was initially identified using alloantigen-activated thymus-derived cells (1, 2), the process of cell-mediated cytotoxicity has been studied in much detail. Many of the earliest studies examined cytotoxic activity from the point of view of transplantation, because cytotoxic activity poses a significant obstacle to successful transplantation. Of course, the potential for cytotoxic activity to mediate tumor rejection is a major focus of cytotoxic T-lymphocyte (CTL) research. Once the exquisite specificity of CTL was clarified (3, 4), the possibility of CTL induction by vaccination to prevent infectious disease and limit tumor progression has been further explored. Furthermore, the description of cytotoxic granule exocytosis and identification of cytotoxic effector molecules, such as perforin and granzymes (5), began the definition of the molecular basis of CTL and natural killer cell-mediated cytotoxicity. Nonetheless, much still remains to be learned regarding the cytotoxic process. This includes transcriptional, translational, and post-translational control of components of the cytotoxic machinery. In addition, the cellular mechanisms and even the precise array of proteins utilized by CTLs to kill antigen-bearing target cells are far from completely defined. More recently, additional effector molecules have been identified. These include granulysin, which exhibits lytic activity toward tumors and bacteria (6). Thus, the armamentarium that CTLs bring to bear on their opponents is complex, and the governance of the components of the system continues to be analyzed.
The linkage between CD8+ memory T cells and the induction of cytotoxic activity has been known for many years. In the early days of CTL research, ‘memory’ CD8+ T cells were generated in vitro by resting allogeneic mixed lymphocyte reaction (MLR) cultures (7–10). In fact, these cultures mimicked to a reasonable extent what we now know from more sophisticated approaches. Responding MLR-induced T cells underwent initial blastogenesis followed by a contraction/death phase and a return to what appeared to be small, resting lymphocytes with low cytotoxic activity. Upon restimulation with the correct major histocompatibility complex (MHC), rapid reactivation of the MLR-induced ‘memory’ cells occurred with the induction of cytotoxic activity. Thus, the concept of ‘resting’ memory CTLs emerged. Since that time, our ability to detect and analyze antigen-specific effector and memory T cells at the cellular and molecular levels has improved by leaps and bounds because of many technological innovations including multicolor flow cytometric analysis and cell sorting, T-cell receptor (TCR) transgenic mice (11, 12), adoptive transfer of TCR transgenic cells (13), gene-targeted knockout mice (14–18), conditional knockout mice using cre-recombinase (19), and MHC class I and class II tetramer technology (20, 21). These technological advances have enabled us to garner a much more in depth knowledge of CD8+ T-cell responses and the relationship of naive, effector, and memory CD8+ T cells. Therefore, this review focuses on the developmental linkage between the induction of effector function and the generation of memory CD8+ T cells.
Induction of effector function is rapid and linked to memory T-cell development
Whether a naive CD8+ T cell transits through a requisite effector CTL stage prior to developing into a memory cell has been a long-standing question (22). The importance of the question is certainly germane to developmental immunology, but knowing the pathway of memory T-cell development could also allow clinically relevant manipulation of the system. An early study using TCR transgenic cells in an adoptive transfer system concludes that memory CD8+ T cells could be derived from effector cells (23). Subsequently, results from in vitro studies show that limited cell division is linked to the induction of CTL activity and formation of memory CD8+ T cells (24). Our work and that of others has also shown a tight linkage between induction of effector function and the generation of memory in both CD4+ and CD8+ T cells responding to infection. Limiting the level of inflammation or the strength of TCR signaling during the initiation of the T-cell response still allows effector induction and the magnitude of the effector response correlates with the level of memory development (25–27). The underlying problem with these studies is that they did not make definitive lineage designations and therefore were unable to accurately determine the fate of a given single cell. Thus, the bulk of the CTL population may exhibit cytotoxic abilities, but a small subset of cells lacking effector function may be obscured by the larger CTL population, and this small population of cells lacking cytotoxic abilities may preferentially survive to become memory cells. In addition, if sufficient ‘signal strength’ is related to both efficient effector induction and memory development, then again, the evidence remains only correlative.
Defining the naive to effector to memory transition
Attempts at addressing the issue of the precursor–product relationship between effector and memory CD8+ T cells have been made in a variety of ways over the past several years. For example, a recent report suggests that asymmetric division very early during the response results in development of separate effector and memory lineages (28). Thus, following initial activation, the first cell division produces one daughter cell with long-lived memory characteristics and another with short-lived effector abilities. The data definitively show that asymmetric division occurs as a result of engagement of the CD8+ T cell with an antigen-bearing antigen-presenting cell (APC). Less obvious is how the lineage relationships delineated after the initial divisions would be maintained throughout the remainder of the primary response leading into early memory development. It is not clear how downstream or serial interactions of responding CD8+ T cells with antigen-bearing APC, which most certainly occur (26, 29–31), would affect such a developmental scheme. This system also employed adoptive transfer of large numbers of clonal, high avidity CD8+ T cells, a necessary evil for this type of study but one that is known to affect response outcomes and memory development (32, 33). This scenario of effector/memory dichotomy at the first cell division is also hard to reconcile with what is now known about effector subset heterogeneity (34), but nonetheless such a process cannot be categorically ruled out for a subset of responders. The fact that a subset of human and mouse memory CD8+ T cells, termed effector memory cells (TEM) exhibit direct ex vivo lytic activity and constitutively express granzymes and perforin (35–37), can be used to argue that such cells are likely derived from effector cells. But what about the remainder of the memory population, the ‘central’ memory subset (TCM) that does not exert constitutive effector function? Did these cells also transit through an effector phase? Several lines of evidence suggest that the answer is yes.
More recent support for a CTL memory differentiation model whereby a naive cell must pass through an obligatory cytotoxic effector stage before becoming a memory cell comes from a recent study using a genetic tagging scheme to study the effector cell to memory cell pathway (38). In this system, effector cells are indelibly marked by virtue of cre-recombinase-mediated activity controlled by the granzyme B promoter. Thus, when CD8+ T cells are activated in response to infection with influenza virus, cre-recombinase is expressed which then cleaves a sequence encoding a stop codon present in the targeted and ubiquitously expressed Rosa locus thereby allowing expression of yellow fluorescent protein (YFP). These events result in the long-term expression of YFP in any cell that had previously expressed granzyme B and therefore cre-recombinase. The results indicate that memory CD8+ T cells are derived from cells that had been transcriptionally active at the granzyme B locus. Thus, upon challenge a proportion of the secondary responders express YFP, identifying them as memory T cells derived from the primary response that had transited through an effector phase. However, this study does not prove that all memory cells are derived from effector cells as the system is inefficient and not all granzyme expressing primary effector cells express cre-recombinase. Yet, the findings delineate the stochastic nature of the CD8+ T-cell response with regard to initial events specifying downstream consequences.
It may also be worthwhile to consider what defines an effector CD8+ T cell. Historically, mediating lytic activity identified a CD8+ T cell as an effector. Later, the ability to produce cytokines became an additional hallmark of effector function. For the most part, lytic activity and cytokine production are coincident, although there are exceptions (39, 40). Staining for intracellular proteins such as perforin or granzyme B that are known to be involved in lytic activity is oftentimes used as a surrogate marker for effector function. In addition, assays measuring degranulation are also employed to identify putative effectors (41). However, granzyme B, degranulation, or other surrogates may not always faithfully correlate with effector function. For example, granzyme A and B may not always participate in lytic activity (42). Similarly, while effector and memory cells rapidly degranulate when TCR is triggered, the contents of the cytotoxic granules are deterministic of the level of cytolytic function (43). Cytotoxic granule contents can also mediate protection against infection in unconventional ways such as the recently described action of granzyme B on inactivation of a herpes virus protein that is essential to pathogenesis (44). Granulysin, which has direct lytic activity on microbes and cells, is another granule component and is made by human, but not mouse CD8+ T cells and NK cells (6, 45). Therefore, whenever possible, direct measurement of cytolytic activity is preferable to the use of detection of surrogate molecules or processes.
Arguments have also been made with respect to the relationship between effector CD4+ T cells and memory development (46). Although this issue will not be addressed in detail in this review, the authors of a recent study that employed a clever genetic approach also concluded that memory CD4+ T cells can be derived from interferon-γ-producing primary effectors (47), but whether this is also true for T-helper 2 (Th2), Th17, and T-follicular helper cells remains unresolved. Thus, both effector CD4+ and CD8+ T cells retain the ability to differentiate into memory cells.
Early events during effector priming initiate memory development
The quest for the identity of a true memory precursor continues although significant progress in the characterization of early events specifying memory development has been made in recent years. The possibility that a subset of naive CD8+ T cells are predestined to become memory cells or that heterogeneity at the naive cell level might affect effector versus memory cell development has been discussed over the years. However, a recent study shows that predestiny or heterogeneity at the naive CD8+ T-cell level is not deterministic, because a single naive CD8+ T cell upon challenge with Listeria monocytogenes (LM) gives rise to heterogeneous effector and memory populations (48). Thus, stochastic events downstream of the initial activation of a single naive CD8+ T cell must determine which pathway a particular daughter cell chooses to follow into effector and memory subset development. It should be noted that the transferred naive CD8+ T cell is of course of a single avidity and a normal population of naive responders would be heterogeneous at least in terms of avidity. Additionally, the time at which a naive T cell enters the response may also affect the differentiation characteristics of that cell (49, 50). Moreover, under normal circumstances, the precursor frequency of naive CD8+ T cells controls competition between clones and affects response outcomes (51). Thus, multiple factors at the cellular level are important for controlling the complex orchestration of the naive to effector cell transition.
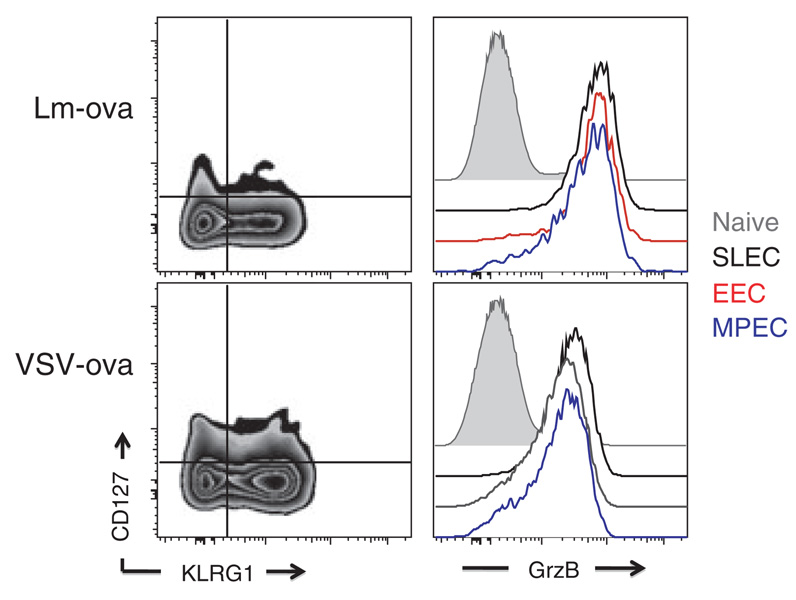
Identification of memory CD8+ T-cell precursors has been a longstanding goal in the field. Our earlier work shows that expression of IL-7Rα by CD8+ T cells responding to virus infection is critical for memory development (52). IL-7Rα expression correlates with enhanced or sustained expression of intracellular anti-apoptotic survival molecules including bcl-2 (52, 53). However, a recent report showed that IL-7Rα expression alone is not deterministic of memory development, because overexpressing IL-7Rα in responding CD8+ T cells did not lead to enhanced memory generation (54). IL-7 may also not be essential for memory CD8+ T-cell development (55), suggesting that thymic stromal lymphopoietin, whose receptor consists of IL-7Rα and a type-specific β-chain (56), may play a role in memory development. More recently, the combination of IL-7Rα expression along with the expression of killer cell lectin-like receptor G1 (KLRG1) has been used in seminal studies defining heterogeneity at the level of effector CD8+ T cells (57, 58). IL-7Rαhigh KLRG1low cells represent memory precursor effector cells (MPECs), while cells lacking IL-7Rα and expressing KLRG1 represent short-lived effector cells (SLECs). Our work (J.J. Obar and L. Lefranc¸ois, unpublished data) and that of others (34, 59) identified an additional subset, termed early effector cells (EECs) that lack both KLRG1 and IL7Rα expression. Transfer of purified EECs results in the development of MPEC and SLEC, identifying EECs as the earliest identifiable memory and effector precursors. Furthermore, we hypothesize that the EEC population likely comprises a heterogeneous population, possibly at the genetic level (i.e., epigenetic modifications), that perhaps contains cells already committed to the MPEC and SLEC lineages. Additional support for the linear differentiation model comes from the demonstration that nearly all cells of the EEC subset, as well as both the SLEC and MPEC subsets derived from the EEC population, express granzyme B (Fig. 1). Furthermore, SLECs and MPECs are also able to produce cytokines and mediate cytotoxic activity (57, 58).
Fig. 1. Granzyme B is expressed by all effector CD8+ T-cell subsets.
C57BL/6 mice were infected i.v. with either 103 colony-forming unit Lm-ova or 105 PFU vesicular stomatitis virus-ova. Five days later, Ova/Kb-specific CD8+ T cells in the spleen were phenotyped using the cell surface molecules KLRG1 and CD127 to identify the early effector cells (EECs), short-lived effector cells (SLECs), and memory precursor effector cells (MPEC) populations. Intracellular granzyme B levels were then examined within each population and compared with naive CD8+ T cells (CD11alow CD44low). Granzyme B levels for naive CD8+ T cells are displayed in the filled gray histogram, while the granzyme B levels in the Ova/Kb-specific effector CD8+ T-cell populations are in the open histograms: EECs (red line), SLECs (black line), and MPECs (blue line).
While KLRG1 serves as a useful marker to identify effector subsets, the functional relevance of KLRG1 expression in vivo is yet to be determined. KLRG1 was originally identified as a molecule expressed by rat mast cells and was termed mast cell function-associated antigen (MAFA) (60, 61). In these studies, KLRG1 was shown to inhibit immunoglobulin E (IgE)-mediated mast cell activation (61, 62). Subsequently, the gene encoding the human ortholog of MAFA was identified in the NK cell gene complex and was shown to be expressed in human basophils, mast cells, and NK cells (63). KLRG1 is a C-type lectin and a type-II transmembrane protein with an immunoreceptor tyrosine-based inhibitory motif (ITIM) present in the cytoplasmic tail (63). Further work demonstrates that KLRG1 is expressed by replicatively senescent mouse and human effector/memory CD8+ T cells (64, 65). As the SLEC population responds poorly to secondary infections (57, 58), does KLRG1-E-cadherin interaction limit the proliferative capacity of cells expressing this molecule? Or does KLRG1 expression participate in the process of the overall genetic programing of SLEC that serves to limit their replicative abilities? Thus far, a KLRG1-deficient mouse has not been described. However, a recent analysis of human CTLs shows that KLRG1-mediated events maintain active negative signaling through interaction with E-cadherin (66). Blocking the binding of KLRG1 to E-cadherin resulted in enhanced Akt phosphorylation, proliferation, and induction of cyclins (66). Furthermore, KLRG1 has been shown to inhibit IL-2 production from T-cell hybridomas when engaged by E-cadherin (67). These experiments were performed with highly differentiated CD28−CD27− CD8+ T cells (66) or T-cell hybridomas (67), thus whether KLRG1 plays a similar negative regulatory role in primary effector CD8+ T cells in vivo remains to be tested. Additionally, KLRG1 may mediate ‘reverse’ signaling through E-cadherin in APCs, which results in limited production of inflammatory cytokines (IL-6 and tumor necrosis factor-α) and enhanced IL-10 production (67). Nonetheless, the available results imply that negative regulation via KLRG1 and E-cadherin is an ongoing event that serves to control the magnitude and/or the longevity of the effector phase of the CD8+ T-cell immune response, particularly under inflammatory conditions.
Regulation of the dynamics of effector subset composition
Multiple signals are required to mount a productive CD8+ T-cell response to infection or vaccination. The ‘three-signal’ model accounts for signaling via (i) the TCR; (ii) CD28 or perhaps other costimulatory molecules; and (iii) receptors for inflammatory cytokines, particularly IL-12 and type I IFNα (68). It is now clear that IL-12 and IFNα are potent growth factors for effector CD8+ T cells (69–72). Moreover, the importance of IFNα versus IL-12 in a given response is dictated by the pathogen. Thus, the CD8+ T-cell response to lymphocytic choriomeningitis virus (LCMV) infection is highly IFNα dependent, and this infection induces large amounts of IFNα. Whereas CD8+ T-cell responses to infection with vesicular stomatitis virus (VSV), LM, or vaccinia virus (VV) are less IFNα dependent, and these infections induce lower levels of IFNα. Nevertheless, IFNα still provides a growth advantage to CD8+ T cells responding to these pathogens (70). Furthermore, both IFNα and IL-12 are required for optimal expansion of CD8+ T cells responding to LM or VSV infection (71, 73, J.J. Obar and L. Lefrancois, unpublished results).
How both IFNα and IL-12 control CD8+ T-cell effector subset differentiation has been recently examined. While the overall expansion of CD8+ T cells is decreased when the CD8+ T cells lack IFNα or IL-12 receptors (71), both cytokines appear to preferentially enhance SLEC development (57, 74; J.J. Obar and L. Lefrancois, unpublished results) (Table 1). The loss of SLEC results in concomitant increases in MPEC, but interestingly an increase in EEC also occurs. The latter finding may indicate that IL-12 and IFNα could play a role in differentiation of EEC toward the SLEC pathway (Fig. 2). The fact that IL-12 action is needed very early in the response (74) supports this possibility. Both IL-12 and IFNα likely also promote survival, as well as expansion of the effector CD8+ T cells (69–73, 75, 76). Additionally, IFNγ produced during infection regulates the expansion, survival, and differentiation of CD8+ T cells. IFNγ can provide signals directly to the CD8+ T cell enhancing their proliferation and survival (77). Furthermore, IFNγ production appears to promote IL-12 production from APCs, which thereby enhances the CD8+ T-cell response and subsequent differentiation into SLECs (74). Thus, inflammatory cytokines have a dramatic impact on the overall expansion, survival, and differentiation of CD8+ T cells.
Table 1.
Factors regulating effector cell heterogeneity
| | Outcome | Reference(s) | | | ----------------------- | ------------------------------------------- | ------------------------------------------------------------------------------ | | Cytokines | | | | IL-2 | SLEC differentiationEffector cell expansion | (86) | | IL-12 | SLEC differentiation | (57) | | Effector cell expansion | (75, 76) | | | IL-15 | SLEC survival | (57, 89) | | Memory cell turnover | (102, 135) | | | TGFβ | SLEC apoptosis | (59) | | IFNα/β | SLEC differentiationEffector cell survival | Joshua J. Obar and Leo Lefrancois, unpublished results(69, 70) | | IFNγ | SLEC differentiation | (74) | | Transcription factors | | | | T-bet | SLEC differentiation | (57) | | Blimp1 | SLEC differentiation | (84, 85) | | Xbp-1 | SLEC formation | (136) | | Gfi-1 | Represses IL-7rα | (137) | | GABPα | Enhances IL-7rα | (137) | | Other | | | | OX40 | Promotes MPEC formation | (138) | | CD4 ‘help’ | SLEC differentiation | (86) | | Adjuvants or drugs | | | | IL-2/αIL-2 complex | Enhances SLEC accumulation | (89) | | IL-7/αIL-7 complex | Enhances MPEC accumulation | (89) | | IL-15/IL-15rα complex | Enhances SLEC accumulation | (89) | | Rapamycin | Enhances MPEC formation | (82) | | Metaformin | Enhances memory formation | (83) | | CpG ODN | Enhances SLEC formation | (57, 74, 80) |
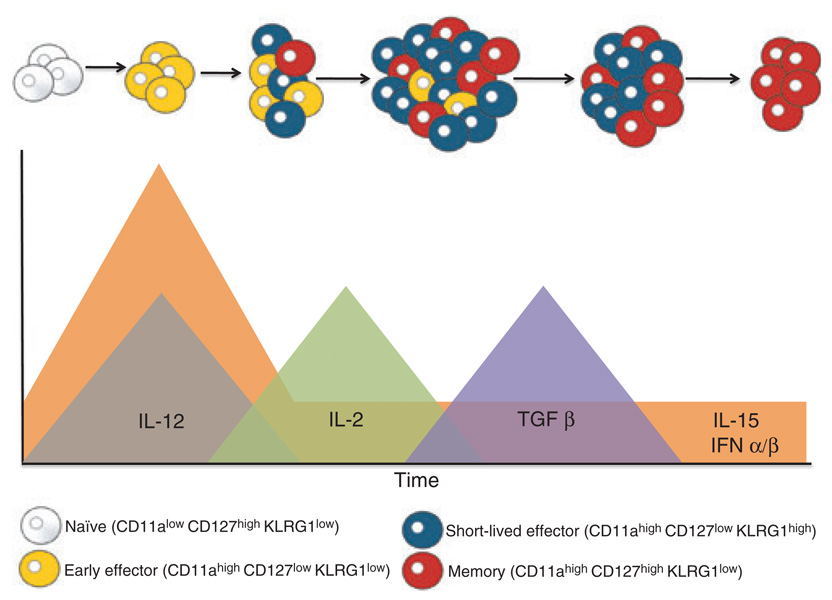
Fig. 2. Linear differentiation model of effector and memory CD8+ T cells.
Early activation of the naive CD8+ T-cell population results in the generation of the early effector cell (EEC) population, which begins differentiation into short-lived effector cells (SLEC) and memory precursor effector cells (MPECs). These early differentiation events (i.e., the first 3–4 days) correspond to times when high levels of interleukin (IL)-12, IL-15, and interferon-α/-β are found. The next cytokine that appears to work on the responding CD8+ T-cell population is IL-2, as the high-affinity IL-2 receptor is only expressed transiently during this time period (i.e., days 3–5). IL-2 not only works to amplify the whole CD8+ T-cell population but also enhances the differentiation of the EEC population to SLECs. Around the peak of the CD8+ T-cell response, high levels of transforming growth factor (TGF)-β can be found. TGFβ will oppose the actions of homeostatic levels of IL-15 and promote the preferential apoptosis of the EEC and SLEC populations leaving only the MPEC population. The levels of IL-15 in the tissues will also regulate this process as SLECs are exquisitely dependent on IL-15 for their survival, whereas the MPEC population is more dependent on IL-15 for long-term homeostatic proliferation and is dependent on IL-7 for survival.
One potential means of enhancing the efficacy of vaccines is the use of an adjuvant, such as Toll-like receptor (TLR) ligands (78). TLR ligands are known to enhance immune responses by enhancing APC maturation enabling better antigen presentation and the secretion of numerous inflammatory signals (79). As such, the inflammatory signals resulting from TLR ligand administration can modulate the EEC/MPEC/SLEC ratios (57, 74, 80). For example, TLR9 ligation induces the production of IL-12 and/or IFNα, thereby enhancing SLEC development (57, 74, 80), but whether this holds true for other TLR ligands remains unknown. Thus, depending on the scenario, the use of adjuvants to enhance vaccination could be counterproductive. While the primary response induced by an adjuvant may be robust, the preferential generation of SLECs could limit protective memory CD8+ T-cell development. Therefore, a balance must be reached such that an appropriate level of inflammation is induced to drive a sufficiently robust productive response, but also such that the inflammation is limited to the extent that memory development is not impaired. This principle is demonstrated by vaccination with peptide coated activated dendritic cells (DCs), which results in the preferential and rapid development of IL-7Rαhigh memory-like cells. The resulting cells are able to quickly respond to challenge, showing that they functionally resemble bona fide memory cells (81). A similar process occurs when LM infection is limited by antibiotic treatment. That is, although primary CD8+ T-cell expansion is blunted in this situation, IL-7Rαhigh memory–phenotype cells rapidly appear, and these cells are able to respond to recall antigen (27). Limiting LM infection also limits SLEC development (57). More recently, a low dose of rapamycin, which is typically used as an immunosuppressive drug, was shown to enhance MPEC formation through limiting inflammatory signals received by the CTL (82). Furthermore, regulation of AMP-activated kinase (AMPK) signaling by administration of metaformin demonstrates a similar increase in memory formation (83). Both of these novel ‘adjuvants’ (rapamycin and metaformin) appear to work by limiting the ability of the responding CD8+ T cells to sense the inflammatory milieu, supporting the notion that a highly inflammatory environment supports SLEC development, while low inflammatory environments promote MPEC formation. Importantly, the use of rapamycin and metaformin did not impact the overall expansion of CD8+ T cells, making them extremely attractive as adjuvants.
In addition to IL-12, IFNα/β, and IFNγ, multiple other factors control effector subset development (Table 1). For the most part the factors that have been defined appear to be those that control responses to inflammation and therefore effect SLEC development or survival. Furthermore, the transcription factors through which inflammatory cytokines may be operating have also begun to be identified. For example, the expression level of the transcription factor T-bet helps determine the SLEC/MPEC ratio (57). High levels of IL-12 drive heightened T-bet expression resulting in enhanced SLEC development. Conversely, the reduced T-bet levels present when inflammation is limited favors MPEC generation (57). Additionally, Blimp1 has recently been identified as another transcription factor that promotes SLEC differentiation (84, 85). We have recently demonstrated that IL-2 enhances SLEC differentiation (86), which could be mediated via upregulation of Blimp1 (87). Thus, we are beginning to garner a more complete understanding of how inflammatory mediators work to influence the development of an immunologic response.
IL-7 and IL-15 γc cytokine involvement in effector and memory CD8+ T-cell development and survival
Cytokines that signal via receptors containing the common γ chain (γc) are critically important for induction and maintenance of effector and memory CD8+ T cells. In particular, the role of the cytokines IL-2, IL-7, and IL-15 in CD8+ T-cell biology has been studied in substantial detail (88). As discussed above, the expression of IL-7Rα, though not necessarily IL-7, is important primarily after initial CD8+ T-cell expansion and is essential for memory cell development and survival. Nevertheless, although the overall magnitude of the response is normal in the absence of IL-7, the response of adoptively transferred OT-I cells responding to LM-ova infection is heavily skewed toward SLEC and away from MPEC generation/survival (89). Recent reports have demonstrated that γc cytokine activity can be enhanced by precomplexing the cytokine with cytokine-specific antibodies (IL-2 and IL-7) or soluble high-affinity receptor (IL-15) (90–92). Treatment of mice with IL-7/αIL-7 complexes during the contraction phase of a CD8+ T-cell response results in preferential survival of MPEC (89). This result indicates that antibody-complexed IL-7 selectively acts on those cells expressing IL-7Rα. In case of IL-15, the action of the cytokine in vivo is mediated via the unusual process of transpresentation (93). In the process of transpresentation, CD8+ T cells responding to IL-15 need not express the high-affinity IL-15Rα, but must express the IL-2/15Rβ and γc (94–97). Cells producing IL-15 and concomitantly expressing IL-15Rα, such as DCs, transpresent IL-15 to apposing cells (95, 96, 98–101). Precisely how IL-15 and IL-15Rα are controlled during a dynamic infection-driven CD8+ T-cell response is not entirely clear. In any case, the contraction phase of the response following infection of IL-15−/− mice with either virus or bacteria is dramatically enhanced, beginning earlier and resulting with only a small number of cells surviving the crash (89, 102). Interestingly, despite the requirement for IL-15 in the proliferative maintenance of CD8+ memory T cells, SLECs appear to be especially sensitive to the action of IL-15 and are more rapidly lost than MPECs in the absence of IL-15 (57, 89). Treatment with superagonistic IL-15/IL-15Rα or IL-2/αIL-2 complexes also enhances SLEC formation (89). The dichotomy in IL-15 action on SLECs versus MPECs may be related to the effect of the cytokine on survival versus proliferation of one subset versus another. Additionally, this dichotomy could be a result of the sensitivity of SLECs to transforming growth factor (TGF)-β-induced apoptosis, which can be rescued by IL-15 (59). IL-15 is responsible for homeostatic proliferation of memory CD8+ T cells, while IL-15 during the primary response may be more involved in survival of SLECs and opposing the actions of TGFβ (Fig. 2). Indeed, SLECs proliferate poorly in response to IL-2/αIL-2 or IL-15/IL-15Rα complexes, suggesting that these agents are more important for assisting in survival of this subset (89).
The link between CD4+ T-cell help, costimulation, and IL-2 activity in regulating CD8+ T-cell responses
Priming of CD8+ T cells can occur in multiple ways depending on the infection or vaccination scheme. In some cases, the requirement for particular cytokines or costimulators may be bypassed via activation and involvement of alternate pathways (103–105). While some CD8+ T-cell responses require CD4+ T-cell help, others apparently do not. This is also true for certain costimulators. In particular, CD40–CD40L interactions are essential for some CD8+ T-cell responses but dispensable for others (103–105). The level of inflammation (i.e., the type of pathogen or vaccine), the amount of antigen, the precursor frequency of responding CD8+ T cells, and the route of infection all participate in determining the factors required to mount a successful CD8+ T-cell response. In general, the cellular, soluble factors, and costimulatory requirements for a CD8+ T-cell response are defined by their ability to cause robust expansion of responding CD8+ T cells. However, some factors may not participate via provision of a growth stimulus, but rather by augmenting, or dampening, cellular functions such as effector function or homing. Because of the concerted often simultaneous efforts of multiple cellular and soluble factors, it is oftentimes difficult to assign precise functions to one participant versus another. Additionally, as is often the case, one factor may affect the action of another by upregulation of necessary receptors or activation of a distinct cellular subset.
The original definition of CD4+ T-cell help for CD8+ T-cell responses centered on the production of IL-2 by CD4+ T cells (106). Later studies suggested that the way in which CD4+ T cells helped CD8+ T cells was through activation of APC and upregulation of CD40 (107–111). The initial activation of APCs would then allow naive CD8+ T cells to subsequently encounter activated APCs, resulting in priming and expansion. Thus, in this scheme of a temporal bridge between CD4+ and CD8+ T-cell activation, CD4-derived IL-2 did not appear to play a role in CD8+ T-cell activation. The reality of the situation no doubt contains elements of both models, and the interpretation of the original results may now be seen in the light of recent developments. For example, new findings indicate that rather than CD4+ cells expressing CD40L that interacts with CD40 on APCs, APCs can be induced by viral infection to express CD40L that then interacts with CD40 expressed by CD8+ T cells, even in the absence of CD4+ T cells (112). Thus, there are at least two pathways for driving CD8+ T-cell effector development: (i) CD4 dependent, in which CD4+ T cells may activate the DC but also will provide IL-2 to activated CD8+ T cells and (ii) CD4 independent, in which APCs activated via TLR or infection are able to directly drive CD8+ T-cell activation. The latter scenario may also require IL-2, perhaps through autocrine production. Indeed, our results from the analysis of the largely CD4+ T-cell independent CD8+ T-cell response to VSV infection demonstrate a partial reliance on IL-2 for optimal expansion and SLEC generation (86). By comparison, the CD4-dependent CD8+ T-cell response to intraperitoneal VV infection is IL-2 dependent but does not require CD40 (86). The latter result implies that in some situations, CD4+ T-cell help operates through provision of IL-2 rather than via APC activation. The route of infection may also affect the CD4+ T-cell dependence of the CD8+ T-cell response. Thus, the overall expansion of CD8+ T cells responding to intranasal VV infection is normal in the absence of CD4+ T cells (113). However, the resulting memory cells are functionally defective because of heightened programed death-1 (PD-1) expression (113). Overall, the available results indicate that the mechanism of CD4+ T-cell help cannot yet be codified, and the precise factors involved in ‘help’ need be examined for each immunization condition.
The kinetics of the requirement for IL-2 during the CD8+ T-cell response is also instructive. IL-2 is not required for initiation of cell cycle in responding CD8+ T cells (114, 115). Both autocrine and paracrine IL-2 effects have been observed. When IL-2-deficient CD8+ T cells are transferred into IL-2 competent hosts, the CD8+ T-cell response is actually greater than that mounted by IL-2-competent CD8+ T cells, particularly in non-lymphoid tissues. Moreover, IL-2 competent CD8+ T cells can ‘rescue’ IL-2-deficient T cells, demonstrating paracrine transfer of cytokine among ‘brothers’. These results suggest that IL-2 may play a negative role in dampening the response, but in these studies an analysis of the EEC/MPEC/SLEC subsets was not performed. Rather than being involved during early immune response initiation, IL-2 acts during the late primary response, but still prior to the peak of the expansion phase (Figs 2 and 3). At those later time points, IL-2 will serve to maximize CD8+ T-cell growth and promote survival (86, 115). Furthermore, maximal IL-2-mediated growth activity correlates with maximum expression of IL-2Rα (CD25), which is required for formation of the high-affinity IL-2 receptor. Of particular interest is the finding that CD4+ T cells augment CD25 expression (86). Whether this effect is through IL-2-mediated upregulation of CD25 is not yet known, although in vitro data support this possibility (116) and administration of IL-2 to helpless CD8+ T cells rescued CD25 expression (86).
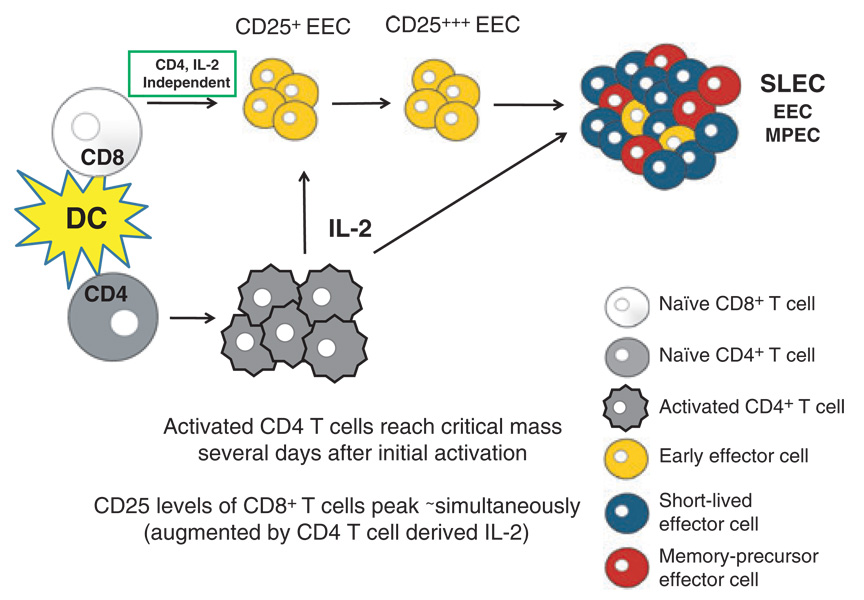
Fig. 3. Model for the role of interleukin (IL)-2 production from ‘helper’ CD4+ T cells in effector CD8+ T-cell differentiation and expansion.
Activation of both CD4+ and CD8+ T cells by dendritic cells will occur simultaneously in lymphoid organs. For both CD4+ and CD8+ T cells, activation and subsequent proliferation is mediated by T-cell receptor:pMHC (peptide: major histocompatibility complex) interactions and costimulatory molecules, such as CD28:CD80/86. When the ‘helper’ CD4+ T-cell population reaches a critical mass enough paracrine IL-2 will be produced to ‘help’ maintain the expansion and differentiation of CD8+ T-cell responders. IL-2 will act on the CD25+ early effector cells (EECs) population and further upregulate the expression of CD25, at which time it will help direct the differentiation of the EEC. IL-2 will promote the preferential differentiation toward short-lived effector cells, but is likely important in the expansion of all effector cells.
In addition to enhancing CD8+ T-cell expansion and survival, both CD4+ T-cell help and IL-2 have also been implicated in imprinting CD8+ T cells during the primary response. In this scenario, CD4+ T cells and/or IL-2 affect the responding CD8+ T cells not through providing growth signals, but via programing of the CD8+ T cells to allow the resultant memory cells to respond to secondary antigenic challenge (113, 117–120). However, later work suggests that CD4+ T cells are important not for programing but for maintenance of CD8+ T-cell memory after the expansion phase by an as yet undefined mechanism (121). Other studies have variously shown depending on the immunogen or pathogen employed that CD4+ T cells are neither essential for primary nor recall responses, only needed in the primary response, or only needed in the secondary response (122–126). Similarly, IL-2 apparently ‘programs’ primary LCMV or LM-specific CD8+ T cells to generate competent memory cells (120) or is required as a growth factor in both the primary and secondary response to acute LCMV infection (127). The reason for the discrepancies between the different studies is not clear, as at least in some cases the response to the same pathogen was being analyzed. In our studies, we examined the role of CD4+ T-cell help and IL-2 in the development of the three main CD8+ T-cell effector subsets: EECs, MPECs, and SLECs. The primary CD8+ T-cell response to infection with VV intraperitoneally or LM is CD4+ T-cell dependent, while the VSV CD8+ T-cell response is CD4+ T-cell independent. In the absence of CD4+ T cells, early expansion of LM or VV-specific CD8+ T cells is normal, but continued expansion does not occur leading to development of a reduced memory pool. In CD25−/− CD8+ T cells, the major defect observed during the effector phase is the development and/or survival of SLECs. Concomitant increases in the proportion of EECs and MPECs are also evident in CD25−/− effector CD8+ T cells. Interestingly, although based on overall expansion the CD8+ T-cell response to VSV infection is CD4+ T-cell independent, SLEC generation is partially defective when CD4+ T cells or IL-2Rα signaling is absent during the VSV infection. The effect on the overall response is likely not as dramatic as observed with LM infection, because of the fact that many fewer SLEC are generated in response to VSV versus LM infection (J.J. Obar and L. Lefrançois, unpublished observation). In addition, during all three infections (VSV, LM, and VV), CD4+ T cells are required for maximal induction of CD25 expression by responding CD8+ T cells (86). Thus, the degree of dependence of the total CD8+ T-cell response on CD4+ T cells and IL-2 is linked, at least in part, to the relative proportions of EECs, MPECs, and SLECs that are produced in response to different infections or immunogens. Considering that ‘helpless’ or CD25-deficient memory CD8+ T cells are reportedly defective, we had predicted that MPEC would be preferentially disturbed by the absence of CD4+ T cells or IL-2Rα signaling. Yet, SLECs are selectively lost in these situations. Moreover, in CD4+ T-cell-depleted mice or in intact mixed bone marrow chimeras produced with CD25−/− and wildtype BM, both helpless and CD25−/− memory CD8+ T cells undergo a robust expansion upon challenge that is equal to or greater than the response of wildtype memory CD8+ T cells (86, 123). However, as in the primary response, SLEC generation is blunted in the recall response of CD25−/− CD8+ T cells.
Our hypothesis then is that CD4+ and CD8+ T-cell activation occur essentially simultaneously. In certain infections, initial APC activation is mediated via TLR signaling or other pathogen-associated molecular pattern-sensing mechanisms. As CD4+ T-cell expansion is believed to be considerably less robust than CD8+ T-cell expansion (128, 129), CD4+ T-cell numbers will lag behind CD8+ T-cell numbers during the early phases of the response. Once a critical mass of CD4+ T cells is available, then sufficient IL-2 is produced to provide help to the responding CD8+ T cells via CD25 upregulation, leading to enhancement of proliferation (Fig. 3). In other cases, such as herpes simplex virus I infection, the CD8+ T-cell response requires ‘licensing’ of DCs by CD4+ T cells in the first couple days after infection (125), although CD4+ T-cell-derived IL-2 may also play a role in CD8+ T-cell expansion.
Memory CD8+ T cells and cytotoxicity
The passage of naive CD8+ T cells through an effector stage results in permanent genetic, phenotypic, and functional changes that can be detected in the memory CD8+ T-cell population (36, 37, 130–132). One of the functions that is retained by a subset of memory cells is the ability to mediate direct ex vivo cytotoxic activity (36, 37). Those cells that retain direct ex vivo cytotoxic activity are termed TEM cells and are preferentially localized to non-lymphoid tissues, such as the lung and intestinal mucosa (37, 133). Additionally, in the mouse spleen a population of TEM cells and a non-lytic TCM cell population are found, both of which can convert to cytotoxic memory cells upon entry into non-lymphoid tissues (134). With secondary and tertiary boosts, the TEM cell population progressively increases in lymphoid and non-lymphoid tissues (133). Nearly all tertiary memory CD8+ T cells, except those in the LN, express granzyme B (133). These findings reinforce the linear differentiation model as retention of lytic activity by memory cells is unlikely to have occurred without passage through an effector stage in the primary response. We have also examined the effector subsets and their protective abilities in secondary and tertiary CD8+ T-cell responses to infection with VSV and LM. Early after a secondary challenge, the majority of the effector cells are made up of KLRG1high cells, but a substantial portion of these also express IL-7R α double-positive effector cell (DPEC) (Joshua J. Obar and Leo Lefrançois, unpublished data). After several months the memory population was comprised of roughly equal proportions of SLEC, DPEC, and MPEC phenotype subsets. Given these findings, the moniker of ‘short-lived’ may not apply in secondary responses. Nonetheless, challenge of each of the memory subsets revealed a hierarchy of responsiveness in terms of the ability to mount a proliferative response: MPEC > DPEC > SLEC. The ability to respond via proliferation also correlated to the protective capacity of each subset. Thus, KLRG1 expression correlated with reduced proliferative and protective abilities, further supporting the concept that KLRG1 may be mediating a negative regulatory effect on the response.
Onward through the fog
As discussed here, the fog is beginning to lift on the mysteries surrounding memory T-cell development. Several of the factors regulating effector CD8+ T-cell heterogeneity have been identified, and this knowledge has set the stage to delve deeper into the control of the effector phase and the ensuing development of memory CD8+ T-cell subsets. Molecular and genetic analyses of the earliest effector cell populations are likely to reveal considerable heterogeneity. Studying the causal effects of this heterogeneity will eventually allow us to identify the very earliest events required for effector subset specification. This analysis will in turn provide the means to determine the precise pathway from effector ‘stem cells’ to memory CD8+ T-cell generation.
Acknowledgements
The authors thank the past and present members of the Lefrançois lab who contributed to the work discussed in this review and thank Dr. Lynn Puddington for many insightful discussions. Work in the Lefrançois lab has been supported by NIH grants (AI051583, AI076457, AI041576, AI078289, P01 AI0561 72, and F32 AI074277 to J.J.O.).
References
- 1.Brunner KT, Mauel J, Cerottini JC, Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 1968;14:181–196. [PMC free article] [PubMed] [Google Scholar]
- 2.Cerottini JC, Nordin AA, Brunner KT. In vitro cytotoxic activity of thymus cells sensitized to alloantigens. Nature. 1970;227:72–73. doi: 10.1038/227072a0. [DOI] [PubMed] [Google Scholar]
- 3.Zinkernagel RM, Doherty PC. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature. 1974;251:547–548. doi: 10.1038/251547a0. [DOI] [PubMed] [Google Scholar]
- 4.Bevan MJ. In radiation chimeras, host H-2 antigens determine immune responsiveness of donor cytolytic cells. Nature. 1977;269:417–418. doi: 10.4049/jimmunol.176.1.677. [DOI] [PubMed] [Google Scholar]
- 5.Henkart PA, Catalfamo M. CD8+ effector cells. Adv Immunol. 2004;83:233–252. doi: 10.1016/S0065-2776(04)83007-4. [DOI] [PubMed] [Google Scholar]
- 6.Krensky AM, Clayberger C. Biology and clinical relevance of granulysin. Tissue Antigens. 2009;73:193–198. doi: 10.1111/j.1399-0039.2008.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersson LC, Hayry P. Specific priming of mouse thymus-dependent lymphocytes to allogeneic cells in vitro. Eur J Immunol. 1973;3:595–599. doi: 10.1002/eji.1830030913. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald HR, Engers HD, Cerottini JC, Brunner KT. Generation of cytotoxic T lymphocytes in vitro. II. Effect of repeated exposure to alloantigens on the cytotoxic activity of long-term mixed leukocyte cultures. J Exp Med. 1974;140:718–730. doi: 10.1084/jem.140.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDonald HR, Sordat B, Cerottini JC, Brunner KT. Generation of cytotoxic T lymphocytes in vitro. IV. Functional activation of memory cells in the absence of DNA synthesis. J Exp Med. 1975;142:622–636. doi: 10.1084/jem.142.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lefrancois L, Klein JR, Paetkau V, Bevan MJ. Antigen-independent activation of memory cytotoxic T cells by interleukin 2. J Immunol. 1984;132:1845–1850. [PubMed] [Google Scholar]
- 11.Kisielow P, Teh HS, Bluthmann H, von Boehmer H. Positive selection of antigen-specific T cells in the thymus by restricting MHC molecules. Nature. 1988;335:730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- 12.Berg LJ, Pullen AM, Fazekas de St GB, Mathis D, Benoist C, Davis MM. Antigen/MHC-specific T cells are preferentially exported from the thymus in the presence of their MHC ligand. Cell. 1989;58:1035–1046. doi: 10.1016/0092-8674(89)90502-3. [DOI] [PubMed] [Google Scholar]
- 13.Kearney ER, Walunas TL, Karr RW, et al. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J Immunol. 1995;155:1032–1036. [PubMed] [Google Scholar]
- 14.Koller BH, et al. Germ-line transmission of a planned alteration made in a hypoxanthine phosphoribosyltransferase gene by homologous recombination in embryonic stem cells. Proc Natl Acad Sci USA. 1989;86:8927–8931. doi: 10.1073/pnas.86.22.8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koller BH, Smithies O. Inactivating the beta 2-microglobulin locus in mouse embryonic stem cells by homologous recombination. Proc Natl Acad Sci USA. 1989;86:8932–8935. doi: 10.1073/pnas.86.22.8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans MJ. Potential for genetic manipulation of mammals. Mol Biol Med. 1989;6:557–565. [PubMed] [Google Scholar]
- 17.Mansour SL, Thomas KR, Capecchi MR. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988;336:348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- 18.Thomas KR, Capecchi MR. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in mid-brain and cerebellar development. Nature. 1990;346:847–850. doi: 10.1038/346847a0. [DOI] [PubMed] [Google Scholar]
- 19.Gu H, Zou YR, Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993;73:1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- 20.Altman JD, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 21.Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 23.Bruno L, Kirberg J, von Boehmer H. On the cellular basis of immunological T cell memory. Immunity. 1995;2:37–43. doi: 10.1016/1074-7613(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 24.Opferman JT, Ober BT, Ashton-Rickardt PG. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science. 1999;283:1745–1748. doi: 10.1126/science.283.5408.1745. [DOI] [PubMed] [Google Scholar]
- 25.Lefrançois L, Marzo A, Williams K. Sustained response initiation is required for T cell clonal expansion but not for effector or memory development in vivo. J Immunol. 2003;171:2832–2839. doi: 10.4049/jimmunol.171.6.2832. [DOI] [PubMed] [Google Scholar]
- 26.Blair DA, Lefrancois L. Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells. Proc Natl Acad Sci USA. 2007;104:15045–15050. doi: 10.1073/pnas.0703767104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badovinac VP, Harty JT. Manipulating the rate of memory CD8+ T cell generation after acute infection. J Immunol. 2007;179:53–63. doi: 10.4049/jimmunol.179.1.53. [DOI] [PubMed] [Google Scholar]
- 28.Chang JT, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 29.Mempel TR, Henrickson SE, von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 30.Celli S, Lemaitre F, Bousso P. Real-time manipulation of T cell-dendritic cell interactions in vivo reveals the importance of prolonged contacts for CD4+ T cell activation. Immunity. 2007;27:625–634. doi: 10.1016/j.immuni.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 31.Khanna KM, McNamara JT, Lefrancois L. In situ imaging of the endogenous CD8 T cell response to infection. Science. 2007;318:116–120. doi: 10.1126/science.1146291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamann D, Baars PA, Rep MH, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 37.Masopust D, Vezys V, Marzo AL, Lefranc¸ois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 38.Bannard O, Kraman M, Fearon DT. Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science. 2009;323:505–509. doi: 10.1126/science.1166831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otten GR, Germain RN. Split anergy in a CD8+ T cell: receptor-dependent cytolysis in the absence of interleukin-2 production. Science. 1991;251:1228–1231. doi: 10.1126/science.1900952. [DOI] [PubMed] [Google Scholar]
- 40.Vezys V, Olson S, Lefrançois L. Expression of intestine-specific antigen reveals novel pathways of CD8 T cell tolerance induction. Immunity. 2000;12:505–514. doi: 10.1016/s1074-7613(00)80202-2. [DOI] [PubMed] [Google Scholar]
- 41.Betts MR, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 42.Regner M, Pavlinovic L, Koskinen A, Young N, Trapani JA, Mullbacher A. Cutting edge: rapid and efficient in vivo cytotoxicity by cytotoxic T cells is independent of granzymes A and B. J Immunol. 2009;183:37–40. doi: 10.4049/jimmunol.0900466. [DOI] [PubMed] [Google Scholar]
- 43.Wolint P, Betts MR, Koup RA, Oxenius A. Immediate cytotoxicity but not degranulation distinguishes effector and memory subsets of CD8+ T cells. J Exp Med. 2004;199:925–936. doi: 10.1084/jem.20031799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clayberger C. Cytolytic molecules in rejection. Curr Opin Organ Transplant. 2009;14:30–33. doi: 10.1097/MOT.0b013e32831c8462. [DOI] [PubMed] [Google Scholar]
- 46.Seder RA, Ahmed R. Similarities and differences in CD4(+) and CD8(+) effector and memory T cell generation. Nat Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 47.Harrington LE, Janowski KM, Oliver JR, Zajac AJ, Weaver CT. Memory CD4 T cells emerge from effector T-cell progenitors. Nature. 2008;452:356–360. doi: 10.1038/nature06672. [DOI] [PubMed] [Google Scholar]
- 48.Stemberger C, Huster KM, Koffler M, et al. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27:985–997. doi: 10.1016/j.immuni.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 49.D’Souza WN, Hedrick SM. Cutting edge: Latecomer CD8 T cells are imprinted with a unique differentiation program. J Immunol. 2006;177:777–781. doi: 10.4049/jimmunol.177.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Catron DM, Rusch LK, Hataye J, Itano AA, Jenkins MK. CD4+ T cells that enter the draining lymph nodes after antigen injection participate in the primary response and become central-memory cells. J Exp Med. 2006;203:1045–1054. doi: 10.1084/jem.20051954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schluns KS, Kieper WC, Jameson SC, Lefranc¸ois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 53.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 54.Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci USA. 2007;104:11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klonowski KD, Williams KJ, Marzo AL, Lefrancois L. Cutting edge: IL-7-independent regulation of IL-7 receptor alpha expression and memory CD8 T cell development. J Immunol. 2006;177:4247–4251. doi: 10.4049/jimmunol.177.7.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pandey A, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nature Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 57.Joshi NS, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanjabi S, Mosaheb MM, Flavell RA. Opposing effects of TGF-beta and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity. 2009;31:131–144. doi: 10.1016/j.immuni.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guthmann MD, Tal M, Pecht I. A new member of the C-type lectin family is a modulator of the mast cell secretory response. Int Arch Allergy Immunol. 1995;107:82–86. doi: 10.1159/000236938. [DOI] [PubMed] [Google Scholar]
- 61.Guthmann MD, Tal M, Pecht I. A secretion inhibitory signal transduction molecule on mast cells is another C-type lectin. Proc Natl Acad Sci USA. 1995;92:9397–9401. doi: 10.1073/pnas.92.20.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rong X, Pecht I. Clustering the mast cell function-associated antigen (MAFA) induces tyrosyl phosphorylation of the Fc epsilonRI-beta subunit. Immunol Lett. 1996;54:105–108. doi: 10.1016/s0165-2478(96)02657-0. [DOI] [PubMed] [Google Scholar]
- 63.Butcher S, Arney KL, Cook GP. MAFA-L, an ITIM-containing receptor encoded by the human NK cell gene complex and expressed by basophils and NK cells. Eur J Immunol. 1998;28:3755–3762. doi: 10.1002/(SICI)1521-4141(199811)28:11<3755::AID-IMMU3755>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 64.Voehringer D, Blaser C, Brawand P, Raulet DH, Hanke T, Pircher H. Viral infections induce abundant numbers of senescent CD8 T cells. J Immunol. 2001;167:4838–4843. doi: 10.4049/jimmunol.167.9.4838. [DOI] [PubMed] [Google Scholar]
- 65.Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1) Blood. 2002;100:3698–3702. doi: 10.1182/blood-2002-02-0657. [DOI] [PubMed] [Google Scholar]
- 66.Henson SM, et al. KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood. 2009;113:6619–6628. doi: 10.1182/blood-2009-01-199588. [DOI] [PubMed] [Google Scholar]
- 67.Banh C, Fugere C, Brossay L. Immunoregulatory functions of KLRG1 cadherin interactions are dependent on forward and reverse signaling. Blood. 2009;114:5299–5306. doi: 10.1182/blood-2009-06-228353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Curtsinger JM, et al. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- 69.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thompson LJ, Kolumam GA, Thomas S, Murali-Krishna K. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J Immunol. 2006;177:1746–1754. doi: 10.4049/jimmunol.177.3.1746. [DOI] [PubMed] [Google Scholar]
- 71.Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J Immunol. 2009;182:2786–2794. doi: 10.4049/jimmunol.0803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aichele P, Unsoeld H, Koschella M, Schweier O, Kalinke U, Vucikuja S. CD8 T cells specific for lymphocytic choriomeningitis virus require type I IFN receptor for clonal expansion. J Immunol. 2006;176:4525–4529. doi: 10.4049/jimmunol.176.8.4525. [DOI] [PubMed] [Google Scholar]
- 73.Keppler SJ, Theil K, Vucikuja S, Aichele P. Effector T-cell differentiation during viral and bacterial infections: role of direct IL-12 signals for cell fate decision of CD8(+) T cells. Eur J Immunol. 2009;39:1774–1783. doi: 10.1002/eji.200839093. [DOI] [PubMed] [Google Scholar]
- 74.Cui W, Joshi NS, Jiang A, Kaech SM. Effects of Signal 3 during CD8 T cell priming: Bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine. 2009;27:2177–2187. doi: 10.1016/j.vaccine.2009.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 76.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 77.Whitmire JK, Tan JT, Whitton JL. Interferon-gamma acts directly on CD8+ T cells to increase their abundance during virus infection. J Exp Med. 2005;201:1053–1059. doi: 10.1084/jem.20041463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Medzhitov R, Janeway CA., Jr An ancient system of host defense. Curr Opin Immunol. 1998;10:12–15. doi: 10.1016/s0952-7915(98)80024-1. [DOI] [PubMed] [Google Scholar]
- 79.van Duin D, Medzhitov R, Shaw AC. Triggering TLR signaling in vaccination. Trends Immunol. 2006;27:49–55. doi: 10.1016/j.it.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 80.Pham NL, Badovinac VP, Harty JT. A default pathway of memory CD8 T cell differentiation after dendritic cell immunization is deflected by encounter with inflammatory cytokines during antigen-driven proliferation. J Immunol. 2009;183:2337–2348. doi: 10.4049/jimmunol.0901203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 82.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pearce EL, Walsh MC, Cejas PJ, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 85.Rutishauser RL, et al. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Obar JJ, et al. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc Natl Acad Sci USA. 2010;107:193–198. doi: 10.1073/pnas.0909945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gong D, Malek TR. Cytokine-dependent Blimp-1 expression in activated T cells inhibits IL-2 production. J Immunol. 2007;178:242–252. doi: 10.4049/jimmunol.178.1.242. [DOI] [PubMed] [Google Scholar]
- 88.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 89.Rubinstein MP, et al. IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood. 2008;112:3704–3712. doi: 10.1182/blood-2008-06-160945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15Ra immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177:6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rubinstein MP, et al. Converting IL-15 to a superagonist by binding to soluble IL-15Ra. Proc Natl Acad Sci. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 93.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 94.Burkett PR, et al. IL-15Ralpha expression on CD8+ T cells is dispensable for T cell memory. Proc Natl Acad Sci USA. 2003;100:4724–4729. doi: 10.1073/pnas.0737048100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schluns KS, Klonowski KD, Lefranc¸ois L. Trans-regulation of memory CD8 T cell prolferation by IL-15Ra+ bone marrow-derived cells. Blood. 2004;103:988–994. doi: 10.1182/blood-2003-08-2814. [DOI] [PubMed] [Google Scholar]
- 96.Sandau MM, Schluns KS, Lefrancois L, Jameson SC. Cutting edge: transpresentation of IL-15 by bone marrow-derived cells necessitates expression of IL-15 and IL-15R alpha by the same cells. J Immunol. 2004;173:6537–6541. doi: 10.4049/jimmunol.173.11.6537. [DOI] [PubMed] [Google Scholar]
- 97.Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koka R, et al. Interleukin (IL)-15Ra-deficient natural killer cells survive in normal but not IL-15Ra-deficient mice. J Exp Med. 2003;197:977–984. doi: 10.1084/jem.20021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schluns KS, Nowak EC, Cabrera-Hernandez A, Puddington L, Lefrancois L, Aguila HL. Distinct cell types control lymphoid subset development by means of IL-15 and IL-15 receptor alpha expression. Proc Natl Acad Sci USA. 2004;101:5616–5621. doi: 10.1073/pnas.0307442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stonier SW, Ma LJ, Castillo EF, Schluns KS. Dendritic cells drive memory CD8 T-cell homeostasis via IL-15 transpresentation. Blood. 2008;112:4546–4554. doi: 10.1182/blood-2008-05-156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mortier E, et al. Macrophage- and dendritic-cell-derived interleukin-15 receptor alpha supports homeostasis of distinct CD8(+) T cell subsets. Immunity. 2009;31:811–822. doi: 10.1016/j.immuni.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 102.Schluns KS, Williams K, Ma A, Zheng XX, Lefrançois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 103.Mescher MF, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 104.Khanolkar A, Badovinac VP, Harty JT. CD8 T cell memory development: CD4 T cell help is appreciated. Immunol Res. 2007;39:94–104. doi: 10.1007/s12026-007-0081-4. [DOI] [PubMed] [Google Scholar]
- 105.Zhang S, Zhang H, Zhao J. The role of CD4 T cell help for CD8 CTL activation. Biochem Biophys Res Commun. 2009;384:405–408. doi: 10.1016/j.bbrc.2009.04.134. [DOI] [PubMed] [Google Scholar]
- 106.Keene JA, Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J Exp Med. 1982;155:768–782. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Borrow P, et al. CD40L-deficient mice show deficits in antiviral immunity and have an impaired memory CD8(+) CTL response. J Exp Med. 1996;183:2129–2142. doi: 10.1084/jem.183.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40- CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 109.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 110.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 111.Buhlmann JE, Gonzalez M, Ginther B, et al. Sustained expansion of CD8+ T cells requires CD154 expression by Th cells in acute graft versus host disease. J Immunol. 1999;162:4373–4376. [PubMed] [Google Scholar]
- 112.Johnson S, et al. Selected Toll-like receptor ligands and viruses promote helper-independent cytotoxic T cell priming by upregulating CD40L on dendritic cells. Immunity. 2009;30:218–227. doi: 10.1016/j.immuni.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fuse S, et al. Recall responses by helpless memory CD8+ T cells are restricted by the up-regulation of PD-1. J Immunol. 2009;182:4244–4254. doi: 10.4049/jimmunol.0802041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.D’Souza WN, Schluns KS, Masopust D, Lefrançois L. Essential role for IL-2 in the regulation of antiviral extralymphoid CD8 T cell responses. J Immunol. 2002;168:5566–5572. doi: 10.4049/jimmunol.168.11.5566. [DOI] [PubMed] [Google Scholar]
- 115.D’Souza WN, Lefrancois L. IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J Immunol. 2003;171:5727–5735. doi: 10.4049/jimmunol.171.11.5727. [DOI] [PubMed] [Google Scholar]
- 116.Smith KA, Cantrell DA. Interleukin 2 regulates its own receptors. Proc Natl Acad Sci USA. 1985;82:864–868. doi: 10.1073/pnas.82.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 118.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 119.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shedlock DJ, Whitmire JK, Tan J, MacDonald AS, Ahmed R, Shen H. Role of CD4 T cell help and costimulation in CD8 T cell responses during Listeria monocytogenes infection. J Immunol. 2003;170:2053–2063. doi: 10.4049/jimmunol.170.4.2053. [DOI] [PubMed] [Google Scholar]
- 123.Marzo AL, et al. Fully functional memory CD8 T cells in the absence of CD4 T cells. J Immunol. 2004;173:969–975. doi: 10.4049/jimmunol.173.2.969. [DOI] [PubMed] [Google Scholar]
- 124.Bachmann MF, et al. Cutting edge: distinct roles for T help and CD40/CD40 ligand in regulating differentiation of proliferation-competent memory CD8+ T cells. J Immunol. 2004;173:2217–2221. doi: 10.4049/jimmunol.173.4.2217. [DOI] [PubMed] [Google Scholar]
- 125.Smith CM, et al. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol. 2004;5:1143–1148. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- 126.Agnellini P, Wiesel M, Schwarz K, Wolint P, Bachmann MF, Oxenius A. Kinetic and mechanistic requirements for helping CD8 T cells. J Immunol. 2008;180:1517–1525. doi: 10.4049/jimmunol.180.3.1517. [DOI] [PubMed] [Google Scholar]
- 127.Bachmann MF, Wolint P, Walton S, Schwarz K, Oxenius A. Differential role of IL-2R signaling for CD8(+) T cell responses in acute and chronic viral infections. Eur J Immunol. 2007;37:1502–1512. doi: 10.1002/eji.200637023. [DOI] [PubMed] [Google Scholar]
- 128.Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, Shen H. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J Immunol. 2002;168:1528–1532. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- 129.Moon JJ, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 131.Intlekofer AM, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 132.Northrop JK, Thomas RM, Wells AD, Shen H. Epigenetic remodeling of the IL-2 and IFN-gamma loci in memory CD8 T cells is influenced by CD4 T cells. J Immunol. 2006;177:1062–1069. doi: 10.4049/jimmunol.177.2.1062. [DOI] [PubMed] [Google Scholar]
- 133.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 134.Marzo AL, Yagita H, Lefrancois L. Cutting edge: migration to nonlymphoid tissues results in functional conversion of central to effector memory CD8 T cells. J Immunol. 2007;179:36–40. doi: 10.4049/jimmunol.179.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Becker TC, Wherry EJ, Boone D, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kamimura D, Bevan MJ. Endoplasmic reticulum stress regulator XBP-1 contributes to effector CD8+ T cell differentiation during acute infection. J Immunol. 2008;181:5433–5441. doi: 10.4049/jimmunol.181.8.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chandele A, Joshi NS, Zhu J, Paul WE, Leonard WJ, Kaech SM. Formation of IL-7 Ralphahigh and IL-7Ralphalow CD8 T cells during infection is regulated by the opposing functions of GABPalpha and Gfi-1. J Immunol. 2008;180:5309–5319. doi: 10.4049/jimmunol.180.8.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mousavi SF, et al. OX40 costimulatory signals potentiate the memory commitment of effector CD8+ T cells. J Immunol. 2008;181:5990–6001. doi: 10.4049/jimmunol.181.9.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]