Atacicept: targeting B cells in multiple sclerosis (original) (raw)
Abstract
Multiple sclerosis (MS) has traditionally been considered to be a T-cell-mediated disease. However, there is an increasing body of evidence for the involvement of B cells and autoantibodies in the pathology of this disease, providing a rationale for treatments directed against B cells. In this paper we summarize evidence for the key role of B cells in the immunopathology of MS and review data supporting the use of a novel B-cell targeted therapy, atacicept, in this condition. Atacicept is a human recombinant fusion protein that comprises the binding portion of a receptor for both BLyS (B-Lymphocyte Stimulator) and APRIL (A PRoliferation-Inducing Ligand), two cytokines that have been identified as important regulators of B-cell maturation, function and survival. Atacicept has shown selective effects on cells of the B-cell lineage, acting on mature B cells and blocking plasma cells and late stages of B-cell development while sparing B-cell progenitors and memory cells. The efficacy of atacicept in animal models of autoimmune disease and the biological activity of atacicept in patients with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) has been demonstrated. Clinical studies were initiated to investigate the safety, tolerability and efficacy of atacicept in patients with MS. An unexpected increase in inflammatory activity in one of the trials, however, led to suspension of all atacicept trials in MS.
Keywords: multiple sclerosis, B lymphocytes, B cells, treatment, immunotherapies, atacicept
Introduction
Multiple sclerosis (MS) is a progressive inflammatory disease of the central nervous system (CNS) [Lassmann et al. 2007] in which leukocytes and antibodies attack myelin sheaths and axons in the CNS, resulting in axonal demyelination, damage and destruction [Kornek et al. 2000].
The pathology of MS appears to be heterogeneous, and distinct patterns of disease have been described. Key features include the presence of T cells and macrophages, B cells, immunoglobulins (Igs) and complement activation products, myelin protein loss, oligodendrocyte destruction, and the location and extension of plaques within the CNS [Lucchinetti et al. 2000]. These patterns may reflect the underlying autoimmune pathology in the individual patient.
The exact cause of MS is still unknown, but several aetiological factors have been proposed, including environmental [Marrie, 2004], immunological [Hohlfeld and Wekerle, 2004] and genetic factors [Barcellos et al. 2003; Willer et al. 2003]. It seems probable that MS is a multifactorial disease in which environmental factors trigger an autoimmune response in genetically susceptible individuals [Handel et al. 2010; Ramagopalan et al. 2009]. Myelin basic protein and myelin oligodendrocyte glycoprotein (MOG) might represent relevant targets of autoimmune responses, but the heterogeneous nature of MS suggests that there may be many and varied targets in different individuals. Immunological mechanisms proposed to induce the autoimmune response seen in MS include [Sospedra and Martin, 2005]:
- molecular mimicry: activation of autoreactive cells by cross-reactivity between self-antigens and foreign antigens;
- bystander activation: activation of autoreactive cells by nonspecific inflammatory events that occur during infection.
Until recently, MS was considered to be primarily a T-cell-mediated disease [Sospedra and Martin, 2005]. Both CD4+ and CD8+ T cells are present in MS lesions, and have been shown to be essential features of MS pathology [Chitnis, 2007; Sospedra and Martin, 2005]. Activated CD4+ Th1 cells contribute to inflammation in the CNS and may increase the permeability of the blood–brain barrier (BBB) [Delgado and Sheremata, 2006; Hemmer et al. 2002], whereas CD8+ T cells can damage axons directly [McDole et al. 2006; Medana et al. 2001]. Existing therapies for MS aim to prevent autoimmune destruction of the CNS, and all either target T cells or produce more generalized suppression of the immune system. Likewise, therapies for many other autoimmune disorders have generally focused on suppressing or modifying T-cell responses. However, growing evidence for a central role of B cells in MS suggests that B-cell targeted therapies might represent interesting and relevant treatment strategies that may broaden our limited therapeutic armentarium.
Several potential therapies for MS that affect B cells or both T and B cells are in clinical development:
- Agents that target both T and B cells include:
- fingolimod, which affects lymphocyte trafficking [Cohen et al. 2010, Chun and Hartung, 2010; Brinkmann, 2009; Brown et al. 2007; Hemmer and Hartung, 2007; Chofflon, 2005];
- alemtuzumab, which binds surface CD52 causing profound lymphocyte depletion [Coles et al. 2008; Cree, 2006];
- teriflunomide, an antimetabolite that blocks production of T and B cells [Zeyda et al. 2005].
- Specific B-cell targeting agents include:
- rituximab, a chimeric monoclonal antibody against CD20 that is expressed on the surface of B cells, but not terminally differentiated plasma cells [Hauser et al. 2008; Simpson and Coles, 2007];
- ocrelizumab and ofatumumab, fully humanized anti-CD20 monoclonal antibody that target the same cells as rituximab, but with different binding sites [Genovese et al. 2008];
- belimumab, a humanized monoclonal antibody targeting the soluble B-cell activating factor;
- LY2127399, a fully human IgG4 monoclonal antibody targeting both membrane-bound and soluble B-cell activating factor;
- atacicept, a fusion protein that blocks plasma cell function and the late stages of B-cell development.
Rituximab, ocrelizumab, and ofatumumab produce profound, selective depletion of all circulating CD20+ B cells but do not directly affect T cells [Stüve et al. 2009; Genovese et al. 2008; Liossis and Sfikakis, 2008; Silverman, 2006].
Indirect effects on T cells have been reported, based on the observation that T-cell numbers are decreased in patients treated with rituximab [Cross et al. 2006]. In contrast, atacicept selectively inhibits antigen-driven B-cell responses and plasma cell survival, while sparing B-cell progenitors and memory cells.
In this article we summarize the evidence for B-cell involvement in MS and provide a rationale for the use of B-cell targeting therapies in this disease. The mechanism of action of atacicept will be reviewed alongside preclinical evidence of activity in animal models of MS, and early clinical results with atacicept in autoimmune diseases will be presented.
Review criteria
A search of PubMed was performed to identify English-language articles published on ‘B cell’ AND ‘multiple sclerosis’ AND ‘therapy’ in the last 10 years. Additional searches for ‘B cell’ AND ‘EAE’, and ‘atacicept’ were also performed. An informal review of these articles was then performed to identify those considered most relevant to the focus of the article (i.e. evidence for B cells in the pathogenesis of MS; targets of B cell therapy; current B-cell targeting therapies for autoimmune diseases; evidence for efficacy of B-cell targeting therapies in animal models of autoimmune disease). Additional searches were performed as needed to identify relevant articles relating to the biological functions of BLyS and APRIL, and primary papers were cited. Information on the ongoing clinical development of atacicept was sourced from published abstracts from the relevant congress, and information contained within associated posters where available.
B cells: key players in MS pathogenesis
In MS and other autoimmune diseases, B cells have traditionally been considered to play a secondary T-cell-dependent role, producing pathogenic autoreactive antibodies that promote tissue destruction by recruiting macrophages and through activation of the complement pathway [Hawker, 2008]. However, B cells may play a more central role in MS immunopathogenesis. In addition to producing antibodies, activated B cells can act as antigen-specific antigen-presenting cells for T cells; this may be crucial for rapid responses to B-cell targeted therapies. Within the T-cell–B-cell synapse, B cells produce costimulatory molecules that can influence the differentiation of T cells to Th1 or Th2 cells [Zouali, 2008]. B cells also produce cytokines (interleukin [IL]-6 and IL-10, and tumour necrosis factor [TNF]-α) that activate macrophages and, in the case of IL-6 and IL-10, stimulate further B-cell proliferation.
This key role of B cells in MS is corroborated by evidence from the experimental autoimmune encephalomyelitis (EAE) mouse model of MS. When EAE is induced in B-cell deficient mice, a characteristic feature of the disease is a lack of demyelination [Svensson et al. 2002]. There is now considerable evidence from clinical studies that also indicates that B cells play a central role in the pathogenesis of MS (Box 1).
Box 1.
Evidence supporting the key role of B cells in the pathogenesis of multiple sclerosis (MS).
| • Clonal expansion of B cells in the central nervous system (CNS) |
|---|
| • Oligoclonal bands in the cerebrospinal fluid |
| • Presence of B cells in MS lesions |
| • Autoantibody production against CNS components |
| • Presence of antibodies in MS lesions |
| • Intrathecal ectopic germinal centres |
| • Histopathological evidence of antibody-mediated pathology |
| • Efficacy of plasmapheresis and B-cell targeting therapies |
B cells and autoantibodies in the CNS in MS
Patients with MS generally have normal numbers of circulating B cells and plasma cells, but increased B-cell numbers in the CNS. B cells in the CNS of patients with MS are mainly memory cells and short-lived plasmablasts, and a consequent relative reduction in naive B cells is observed [Cepok et al. 2005; Corcione et al. 2004]. Plasmablasts persist in the cerebrospinal fluid (CSF) throughout the course of MS, and numbers of these cells correlate with intrathecal IgG synthesis and with active inflammatory parenchymal disease, as assessed by magnetic resonance imaging (MRI) [Cepok et al. 2005], suggesting that these short-lived plasmablasts could be a major effector B-cell population in MS.
The presence of oligoclonal Ig bands in the CSF is a hallmark of MS and reflects ongoing, persistent humoral immune responses. Sequence analysis of antibodies in the CSF of patients with MS has revealed a high frequency of clonally expanded B cells, providing evidence of somatic hypermutation and antigen-driven B-cell selection [Owens et al. 2006]. This clonal expansion of B cells occurs early in demyelinating disease: expanded B- and plasma-cell clones can be found in the CSF of some patients only a few months after the onset of clinically isolated syndrome (CIS) [Qin et al. 2003]. The antigen target of these antibodies has yet to be identified, but myelin components, lipids, oligodendrocyte proteins and viruses have been proposed. For example, the presence of intrathecal antimyelin IgG and IgM has been associated with rapidly progressing disease [Franciotta et al. 2008; Zouali, 2008; Lambracht-Washington et al. 2007; Kolln et al. 2006; Owens et al. 2006; Perini et al. 2006; Villar et al. 2005; Archelos et al. 2000].
Several features of MS lesions suggest that B cells and antibodies contribute to their development [Archelos et al. 2000]. B cells and plasma cells have been detected in MS lesions, indicating that B cells can cross the BBB, and their numbers are highest in active lesions and during the late stages of the disease. Autoantibodies specific for myelin components have also been found in MS lesions. In addition, the capping of surface IgG on microglia/macrophages engaged in myelin breakdown [Prineas and Graham, 1981], and the codeposition of IgG and activated complement fragments and membrane attack complexes at the borders of active MS lesions [Archelos et al. 2000], strongly implicate antibodies as effectors of demyelination. Vesicles enriched with membrane attack complex have also been demonstrated in the CSF of patients with MS [Scolding et al. 1989] indicating that complement contributes to myelin damage.
It has been suggested that different pathophysiological mechanisms in MS result in specific patterns of demyelination in individual patients [Lucchinetti et al. 2000]. Among the patterns of demyelination in active lesions described by Lucchinetti and colleagues, types I and II were histologically similar to T-cell-mediated and T-cell plus antibody-mediated EAE, respectively. Thus, antibody-mediated demyelination may be a predominant pathogenic mechanism in the formation of white-matter MS lesions in a proportion of patients [Lucchinetti et al. 2000].
B-cell activation and maturation in the CNS
The enrichment of B-cell subsets that are normally found in germinal centres of secondary lymphoid follicles has been reported within the CSF of patients with MS, indicating that B-cell affinity maturation may occur or recur in the CNS [Krumbholz et al. 2006; Cepok et al. 2005]. High levels of chemokines known to support germinal-centre formation and function are also present in the CSF of patients with MS [Scolding et al. 1989]. Indeed, ectopic lymphoid follicles have been detected in the CNS of patients with progressive MS [Serafini et al. 2004]. In a postmortem study of 29 patients with secondary progressive MS (SPMS) and seven patients with primary progressive MS (PPMS), B-cell follicles were found in the meninges entering the cerebral sulci in 41.4% of patients with SPMS, but not in patients with PPMS [Magliozzi et al. 2007].
These data suggest that an immunopathogenic mechanism exists whereby B-cell follicles developing in the brain have a major detrimental impact on the integrity of cortical structures. Emerging evidence suggests that, as the disease evolves into the progressive stage, the immune response, in particular that driven by B cells, is compartmentalized and anatomically and functionally remote from control by systemic immune factors.
Many factors that promote B-cell differentiation, function and survival have been identified within MS lesions, including TNF-α, IL-1, IL-2, IL-4, IL-6 and IL-10 [Franciotta et al. 2008]. In addition, T cells expressing CD40L (CD154), which is essential for T-cell dependent B-cell activation, have been found in MS lesions. Elevated levels of nerve growth factor, which promotes the survival of memory B cells, have also been reported in the CSF of patients with MS. B-Lymphocyte Stimulator (BLyS; also called B-cell Activating Factor of the TNF Family [BAFF]; CD257) and APRIL (A PRoliferation-Inducing Ligand; CD256) are members of the TNF superfamily that play an important role in B-cell survival and proliferation [Dillon et al. 2006; MacKay and Tangye, 2004]. BLyS is usually expressed in the brain at levels about 10% of those in lymphatic tissues. In MS lesions, however, BLyS expression is strongly upregulated to levels observed in lymphatic tissues [Krumbholz et al. 2005]. Increased APRIL expression in astrocytes has been noted in the brain of patients with MS compared with healthy controls [Thangarajh et al. 2007]. Elevated expression of both BLyS and APRIL has also been reported in the monocytes and T cells of patients with MS [Thangarajh et al. 2005, 2004]. BLyS has been found to be localized to astrocytes, close to immune cells expressing its receptor, BAFF-R [Krumbholz et al. 2005]. Thus, astrocytes appear to be a nonimmune source of BLyS, and CNS-produced BLyS may support B-cell survival in inflammatory diseases such as MS.
Further evidence for a role of B cells in the immunopathology of MS is the efficacy of B-cell- and antibody-directed therapies, such as plasmapheresis and rituximab, in treating some cases of MS [Hauser et al. 2008; Hemmer and Stüve, 2007; Weinshenker et al. 1999]. Interestingly, B cells may also promote repair in the CNS: activated B cells secrete brain-derived neurotrophic factor in the MS lesion, which is known to prevent axonal loss after neuronal injury and in neurodegenerative disorders [Kerschensteiner et al. 1999].
B-cell targeting in autoimmune disease
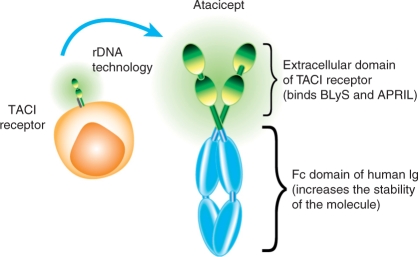
Atacicept is a novel, soluble, fully human recombinant fusion protein containing the extracellular ligand-binding portion of the TACI (Transmembrane Activator and CAML [calcium-modulator and cyclophilin-ligand]-Interactor) receptor and a modified Fc portion of human IgG [Gross et al. 2000] (Figure 1). Atacicept, therefore, contains the binding portion of a receptor that binds both BLyS and APRIL, two important cytokine regulators of B-cell maturation, function and survival. Atacicept has high affinity and specificity for these two cytokines, acting as an antagonist by preventing receptor binding [Dillon et al. 2006; Thangarajh et al. 2005; Weinshenker et al. 1999]. Atacicept was developed specifically for the treatment of B-cell-mediated diseases, and is being evaluated for the treatment of the autoimmune diseases systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA).
Figure 1.
The structure of atacicept: a recombinant fusion protein comprising the extracellular ligand-binding domain of the naturally occurring TACI (Transmembrane Activator and CAML [calcium-modulator and cyclophilin-ligand]-Interactor) receptor, linked to a recombinant Fc domain of human immunoglobulin (Ig). BLyS, B-Lymphocyte Stimulator; APRIL, A PRoliferation-Inducing Ligand.
BLyS, APRIL and their receptors
BLyS and APRIL are members of the TNF cytokine superfamily. BLyS is a potent stimulator of B-cell maturation, function and survival, and is expressed by monocytes, dendritic cells, neutrophils and T cells; BLyS exists in membrane-bound and secreted, soluble forms [Thangarajh et al. 2004; Schneider et al. 1999]. APRIL is a structural homologue of BLyS that is secreted as a soluble protein by monocytes, macrophages, dendritic cells, neutrophils and T cells [Thangarajh et al. 2004], and it shares some of the biological properties of BLyS [Dillon et al. 2006; Schneider et al. 1999].
Three receptors have been identified that have unique binding affinities for BLyS and APRIL [Marsters et al. 2000]. These are:
- TACI;
- BCMA (B-cell maturation antigen);
- BAFF-R.
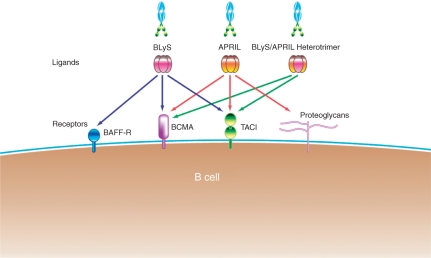
TACI and BCMA bind homotrimers of BLyS and APRIL, whereas BAFF-R binds only homotrimers of BLyS with high affinity [Roschke et al. 2002] APRIL and BLyS also form heterotrimers that bind only to TACI (Figure 2). In vitro studies with Ig-fusion proteins for all three receptors have shown that only TACI-Ig blocks the biological activity of the heterotrimeric complexes [Yan et al. 2001], indicating that these heterotrimers exert their effects via this receptor. Studies with TACI-deficient mice indicate that TACI plays a critical role in B-cell homeostasis [Seshasayee et al. 2003; Yan et al. 2001] and is a key mediator of Ig class switching [Castigli et al. 2005b]. Mutations in TACI have also been associated with common variable immunodeficiency and IgA deficiency [Castigli et al. 2005a]. TACI is, therefore, an important receptor in B-cell function and survival. The expression of the three receptors varies in different B-cell subsets: BAFF-R is expressed earliest, being present on immature B cells, but not on terminally differentiated plasma cells, whereas TACI and BCMA are expressed on mature B cells and plasma cells [Hsu et al. 2002]. BAFF-R, which is exclusively expressed on B lymphocytes, is thought to be the primary receptor through which BLyS exerts its effects on cell survival [Thompson et al. 2001].
Figure 2.
Mechanism of action of atacicept: binding of BLyS (B-Lymphocyte Stimulator) and APRIL (A Proliferation-Inducing Ligand) via their common receptor, TACI (Transmembrane Activator and CAML [calcium-modulator and cyclophilin-ligand]-Interactor). BAFF-R, B-cell Activating Factor of the TNF Family receptor; BCMA, B-Cell Maturation Antigen. Modified from Dillon et al. [2006] with permission.
BLyS and APRIL have been implicated in the pathogenesis of several autoimmune diseases including MS, SLE and RA. Both BLyS and APRIL are overexpressed, locally and systemically [Dillon et al. 2006], and circulating, biologically active, heterotrimeric forms have been detected in individuals with systemic autoimmune diseases [Roschke et al. 2002].
Mechanism of action of atacicept
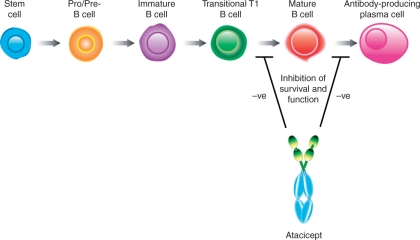
Atacicept binds to BLyS and APRIL on their cognate receptors, and has been shown to neutralize all forms of BLyS and APRIL homo/heterotrimers, inhibiting their effects on B-cell survival and key B-cell functions. Blockade of both BLyS and APRIL is needed to target antibody-secreting plasma cells, with preclinical data suggesting that BLyS and APRIL are both important for the survival of long-lived bone marrow plasma cells [Benson et al. 2008]. Atacicept indirectly targets mature B cells and short-lived antibody-producing plasma cells by inhibiting B-cell survival at stages after the T1 transitional stage, while sparing B-cell progenitors and memory B cells (Figure 3) [Dillon et al. 2006; Gross et al. 2001]. Thus, survival of short-lived plasma cells and serum Ig levels are reduced, as documented in healthy volunteers and in patients with SLE or RA [Tak et al. 2008; Dall’Era et al. 2007; Munafo et al. 2007]. Studies in mice have shown that the ability to eliminate viral infections is retained during atacicept treatment [Heffernan et al. 2008].
Figure 3.
Effect of atacicept on B cells: atacicept inhibits memory B cell survival and function, a B-cell population after the T1 transitional stage in the development of B lymphocytes. Theoretically, there might also be an inhibitory effect of atacicept on antibody-producing plasma cells. Both target cell populations are antigen experienced, whereas all stages prior to that are antigen naive.
Targeting BLyS and APRIL in disease models
In animal models, the overexpression of BLyS has been associated with autoimmune disease onset and severity [Gross et al. 2000; MacKay et al. 1999], highlighting BLyS antagonism as a potential therapeutic approach. Transgenic mice overexpressing BLyS have increased B-cell numbers and enlarged lymphoid organs, and develop an autoimmune condition similar to SLE [Do et al. 2000; MacKay et al. 1999]. These mice also develop a secondary, age-related pathology reminiscent of Sjögren’s syndrome. In contrast, mice deficient in BLyS or with a mutation in BAFF-R have reduced numbers of peripheral mature B cells but normal immature B cells and bone-marrow function [Munafo et al. 2007]. The role of APRIL in in vivo B-cell biology remains somewhat unclear. Transgenic mice deficient in APRIL retain B cells, suggesting that this growth factor does not play a critical role in B-cell development [Varfolomeev et al. 2004]. APRIL does, however, play an important role in several key B-cell functions, including antigen presentation and Ig class switching [Dillon et al. 2006].
In vivo experimental studies confirm that targeting BLyS/APRIL–TACI interactions affects B-cell responses. Overexpression of soluble TACI (TACI-Ig) in transgenic mice has been shown to result in fewer mature B cells and reduced circulating antibody levels than in wild-type mice [Gross et al. 2001]. Modulation of B-cell survival by atacicept has been demonstrated in in vivo preclinical studies. Normal mice treated with atacicept produced markedly fewer mature B cells and lower circulating antibody levels than untreated controls [Carbonatto et al. 2008; Gross et al. 2001]. Similar effects have been reported in healthy monkeys treated with atacicept [Carbonatto et al. 2008]. In mouse models of collagen-induced arthritis and of SLE, atacicept was able to inhibit the development of pathogenic B cells and antibodies, and improve disease features [Dillon et al. 2006; Gross et al. 2001]. These preclinical results indicate that atacicept may provide a novel approach to treating autoimmune diseases.
Atacicept in SLE and RA
The biological activity of atacicept has been confirmed in clinical studies in patients with SLE or RA [Nestorov et al. 2010, 2008; Munafo et al. 2007; Kalled, 2005]. The pathological role of B cells in both these autoimmune conditions has been established [Martinez-Gamboa et al. 2006; Anolik and Sanz, 2004].
The safety and tolerability of subcutaneous atacicept has been assessed in a phase Ib, multicentre, double-blind, placebo-controlled, dose-escalation study of 49 patients with SLE [Dall’Era et al. 2007]. Atacicept was well tolerated, and produced rapid and sustained reductions in circulating Igs after the first treatment dose. Effects on mature and total B-cell numbers were also seen. The effects of atacicept were most pronounced in the cohorts of patients who received repeated (4-weekly) doses. Importantly, the rate of infections did not differ between patients receiving atacicept or placebo. Recent findings in another phase Ib study of 24 patients with mild-to-moderate SLE that received three single doses, multiple doses of atacicept by intravenous route or placebo corroborated these findings [Pena-Rossi et al. 2009].
A phase Ib, exploratory, multicentre, double-blind, placebo-controlled, dose-escalating, single- and repeat-dose study has also been performed to assess the safety and pharmacokinetics of atacicept in patients with RA [Nestorov et al. 2008; Tak et al. 2008]. Subcutaneous atacicept was well tolerated, both systemically and locally, and treatment produced rapid reductions in Ig levels in the peripheral blood; the largest reduction was seen in IgM. Levels of circulating IgA, IgG and IgM rheumatoid factor were also lowered. Atacicept treatment produced biphasic effects on total (defined by CD19+), mature (CD19+, IgD+, CD27−), memory (CD19+, CD20+, CD27+, CD38−), immature (CD19+, IgD−, CD27−) and ‘naive’ (CD19+, CD20+, CD27−, CD38−) B cells. An initial dose-related increase in cell numbers followed the first dose of atacicept, which was mainly accounted for by an increase in memory B cells. A later, sustained dose-related reduction in B-cell concentrations to below predose levels was seen, with the effect on mature B-cell numbers being particularly pronounced.
In the phase Ib studies described above, atacicept was found to be well tolerated locally and systemically. The most frequently observed adverse events (AEs), both in patients with RA and those with SLE, included nausea, fatigue, headache, rhinitis, nasopharyngitis and chronic pyelonephritis. Severe AEs were not observed in patients with SLE treated with either single- or repeated-dose atacicept; the only severe AE reported in this patient population involved a patient receiving placebo treatment [Do et al. 2000]. In contrast, severe AEs were reported in 5% of patients with RA exposed to atacicept, although half were considered to be unrelated or unlikely to be related to the study drug [Carbonatto et al. 2008]. Overall, both for the patients with SLE and those with RA, the nature and frequency of AEs was similar among patients exposed to atacicept or placebo. Among patients with SLE or RA, injection-site reactions (ISRs, which includes redness and swelling) were more common in the atacicept arm of each trial than in the placebo arm [Carbonatto et al. 2008; Thompson et al. 2001]. In addition, patients with RA who received atacicept experienced more frequent skin and subcutaneous tissue disorders than those who received placebo, although the small number of patient included in the trial did not allow a causal relationship to be established [Carbonatto et al. 2008]. Good local and systemic tolerability were also observed with single-dose atacicept among healthy volunteers [Do et al. 2000]. Indeed, in the healthy-volunteer study all reported treatment-emergent AEs were transient and mild or moderate [Do et al. 2000]. Furthermore, no clinically significant changes in vital signs or laboratory parameters were observed over the course of the study [MacKay et al. 1999]. Taken together, evidence from the phase I trials in healthy volunteers and patients with systemic autoimmune diseases suggest that atacicept is generally well tolerated, but may be associated with ISRs.
Atacicept in experimental models of MS
In addition to the role of B cells in MS, several lines of evidence suggest that atacicept has therapeutic potential in this disease. BLyS and APRIL have been shown to be upregulated in peripheral blood monocytes and T cells of patients with MS [Thangarajh et al. 2005, 2004], and BLyS is strongly expressed on astrocytes within MS lesions [Krumbholz et al. 2005], suggesting that the targets of atacicept are involved in MS pathogenesis.
Administration of an antagonist of BLyS has been shown to delay the onset and reduce the severity of EAE [Pena-Rossi et al. 2009; Huntington et al. 2006]. The activity of atacicept as prophylaxis or treatment is currently being assessed in two mouse EAE models of MS: the primarily B-cell dependent model using sensitization with recombinant MOG (rMOG), and the primarily T-cell dependent MOG peptide-EAE model. In both models, prophylactic atacicept produced marked reductions in circulating mature B-cell numbers and in serum IgM and IgG levels, and significantly delayed disease onset compared with that in vehicle-treated controls. Significant reductions in disease incidence and severity were seen after the administration of atacicept in the rMOG-EAE model, which were associated with reduced B-cell infiltration into the CNS. Atacicept, therefore, can inhibit pathological B-cell-dependant processes in EAE with a consequent reduction in disease activity.
Atacicept: clinical development in MS
Two phase II studies of atacicept in MS were initiated, one in patients with MS and one in patients with optic neuritis (ON) as a first demyelinating event suggestive of MS.
The ATAMS study
The ATAMS (ATAcicept in Multiple Sclerosis) study (IMP28063, ClinicalTrials.gov identifier: NCT00642902) was designed to assess the safety and tolerability of atacicept and its effects on CNS inflammation in relapsing MS, and determine a minimally effective dose. Patients were eligible for the study if they had a diagnosis of relapsing–remitting MS (RRMS), an Expanded Disability Status Scale (EDSS) score of 0–5.5 and fulfil one of the following criteria: at least two documented relapses during the previous 2 years; at least one documented relapse in the year prior to enrolment, or at least one T1 gadolinium (Gd)-enhanced lesion on MRI at screening. The study aimed to recruit 300 patients, randomized 1:1:1:1 to receive one of three doses of subcutaneous atacicept or placebo. The primary endpoint was the mean number of T1 Gd-enhancing lesions per patient per scan between weeks 12 and 36, inclusive. Secondary endpoints included the number of new T1 hypointense lesions and the proportion of patients who remain relapse-free over the course of the study.
In the ATAMS study an increase in inflammatory disease activity was reported. This led to a suspension of all atacicept trials in MS. Patients are currently being followed up to obtain further safety information and the analysis of the resulting data will be published when available.
The ATON study
A complementary Phase II study to ATAMS was performed in patients with ON. The ATON (ATacicept in Optic Neuritis) study (IMP28156, ClinicalTrials.gov identifier: NCT00624468) was initiated to assess the safety and tolerability of atacicept in patients with ON. This study should also explore the ability of atacicept to preserve retinal nerve fibre layer (RNFL) thickness, as assessed by optical coherence tomography (OCT). Reduced RNFL thickness is associated with visual impairment and brain atrophy in MS, with increasing overall neurological impairment (as shown by changes in EDSS and Multiple Sclerosis Functional Composite scores) and with MS disease duration [Gordon-Lipkin et al. 2007; Fisher et al. 2006]. Thus, the assessment of degenerative changes of the optic nerve/RNFL by OCT can be used as a marker of CNS neurodegeneration and is a recognized model system to test neuroprotective treatment strategies [Frohmann et al. 2006]. Patients diagnosed with unilateral symptomatic ON as a CIS were randomized 1:1 to receive either subcutaneous atacicept, 150 mg every week with a 4-week loading dose, or placebo for a total of 36 weeks. The target sample size was 82 patients. The primary endpoint was the change in RNFL thickness in the affected eye between baseline and week 36. Secondary endpoints included the difference in RNFL thickness between the affected and unaffected eye, changes in macular thickness and volume over 36 weeks, visual function and the incidence of treatment-emergent AEs.
Conclusions
B cells play a key role in the pathogenesis of MS, and therefore represent a promising potential target for treatments. Currently, all approved MS treatments target T-cell-mediated processes alone or produce global effects on immune function, leaving key B-cell-mediated pathogenic processes largely unaffected. There is apparently a need for B-cell targeting therapies in MS. Such therapies may provide wider treatment options for patients with progressive disease, and may also benefit those in whom current treatments achieve suboptimal outcomes. Therapies that target novel pathways also provide the opportunity for combination therapy; it is logical to speculate that in a disease such as MS with a complicated pathology, targeting multiple pathways will be necessary to achieve optimal outcomes for patients.
The specific, selective effects of atacicept on mature B cells, and particularly plasma cells, made this drug appear as a promising candidate for targeting pathogenic B lymphocytes in MS. In contrast to other B-cell targeting therapies in development, atacicept does not cause generalized depletion of B cells, but affects only mature, antibody-secreting cells. This specific effect on mature B cells suggests that atacicept theoretically may have limited impact on the immune system leaving beneficial immune response intact. However, an unexpected increase in disease activity in the ATAMS trial led to the suspension of all atacicept trials in MS. At present, reasons for the observed increase in disease activity remain unknown but are currently under investigation. This underlines the difficulties in translating experimental insights successfully from bench to bedside [Hohlfeld and Wiendl, 2001].
Acknowledgements
The authors thank Andrea Plant PhD (Caudex Medical; supported by Merck Serono S.A. – Geneva) for editorial assistance and Patrick Küry PhD, Düsseldorf, for help with the figures.
Conflict of interest statement
Drs Hartung and Kieseier have received honoraria for lecturing, travel expenses for attending meetings, and financial support for research from BayerHealthcare, BayerSchering, Biogen Idec, Merck Serono, Novartis, Sanofi Aventis and Teva.
Dr. Hartung served as a member of the Steering Committee for the ATAMS trial.
References
- Anolik J., Sanz I. (2004) B cells in human and murine systemic lupus erythematosus. Curr Opin Rheumatol 16: 505–512 [DOI] [PubMed] [Google Scholar]
- Archelos J.J., Storch M.K., Hartung H.P. (2000) The role of B cells and autoantibodies in multiple sclerosis. Ann Neurol 47: 694–706 [PubMed] [Google Scholar]
- Barcellos L.F., Oksenberg J.R., Begovich A.B., Martin E.R., Schmidt S., Vittinghoff E., et al. (2003) HLA-DR2 dose effect on susceptibility to multiple sclerosis and influence on disease course. Am J Hum Genet 72: 710–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson M.J., Dillon S.R., Castigli E., Geha R.S., Xu S., Lam K.P., et al. (2008) Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J Immunol 180: 3655–3659 [DOI] [PubMed] [Google Scholar]
- Brinkmann, V. (2009) FTY720 (fingolimod) in multiple sclerosis: therapeutic effects in the immune and the central nervous system. Brit J Pharmacol 158: 1173–1182. [DOI] [PMC free article] [PubMed]
- Brown B.A., Kantesaria P.P., McDevitt L.M. (2007) Fingolimod: a novel immunosuppressant for multiple sclerosis. Ann Pharmacother 41: 1660–1668 [DOI] [PubMed] [Google Scholar]
- Carbonatto M., Yu P., Bertolino M., Vigna E., Steidler S., Fava L., et al. (2008) Nonclinical safety, pharmacokinetics, and pharmacodynamics of atacicept. Toxicol Sci 105: 200–210 [DOI] [PubMed] [Google Scholar]
- Castigli E., Wilson S.A., Garibyan L., Rachid R., Bonilla F., Schneider L., et al. (2005a) TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet 37: 829–834 [DOI] [PubMed] [Google Scholar]
- Castigli E., Wilson S.A., Scott S., Dedeoglu F., Xu S., Lam K.P., et al. (2005b) TACI and BAFF-R mediate isotype switching in B cells. J Exp Med 201: 35–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepok S., Rosche B., Grummel V., Vogel F., Zhou D., Sayn J., et al. (2005) Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain 128: 1667–1676 [DOI] [PubMed] [Google Scholar]
- Chitnis T. (2007) The role of CD4 T cells in the pathogenesis of multiple sclerosis. Int Rev Neurobiol 79: 43–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chofflon M. (2005) Mechanisms of action for treatments in multiple sclerosis: does a heterogeneous disease demand a multi-targeted therapeutic approach? BioDrugs 19: 299–308 [DOI] [PubMed] [Google Scholar]
- Chun, J. and Hartung, H.-P. (2010) Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol 33: 91–101. [DOI] [PMC free article] [PubMed]
- Cohen, J.A., Barkhoff, F., Comi, G., Hartung, H.P., Katri, O.B., Montalban, X. et al (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362: 402–415. [DOI] [PubMed]
- Coles A.J., Compston D.A., Selmaj K.W., Lake S.L., Moran S., Margolin D.H., et al. (2008) Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 359: 1786–1801 [DOI] [PubMed] [Google Scholar]
- Corcione A., Casazza S., Ferretti E., Giunti D., Zappia E., Pistorio A., et al. (2004) Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc Natl Acad Sci U S A 101: 11064–11069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree B. (2006) Emerging monoclonal antibody therapies for multiple sclerosis. Neurologist 12: 171–178 [DOI] [PubMed] [Google Scholar]
- Cross A.H., Stark J.L., Lauber J., Ramsbottom M.J., Lyons J.A. (2006) Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol 180: 63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall’Era M., Chakravarty E., Wallace D., Genovese M., Weisman M., Kavanaugh A., et al. (2007) Reduced B lymphocyte and immunoglobulin levels after atacicept treatment in patients with systemic lupus erythematosus: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating trial. Arthritis Rheum 56: 4142–4150 [DOI] [PubMed] [Google Scholar]
- Delgado S., Sheremata W.A. (2006) The role of CD4+ T-cells in the development of MS. Neurol Res 28: 245–249 [DOI] [PubMed] [Google Scholar]
- Dillon S.R., Gross J.A., Ansell S.M., Novak A.J. (2006) An APRIL to remember: novel TNF ligands as therapeutic targets. Nat Rev Drug Discov 5: 235–246 [DOI] [PubMed] [Google Scholar]
- Do R.K., Hatada E., Lee H., Tourigny M.R., Hilbert D., Chen-Kiang S., et al. (2000) Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J Exp Med 192: 953–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J.B., Jacobs D.A., Markowitz C.E., Galetta S.L., Volpe N.J., Nano-Schiavi M.L., et al. (2006) Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology 113: 324–332 [DOI] [PubMed] [Google Scholar]
- Franciotta D., Salvetti M., Lolli F., Serafini B., Aloisi F. (2008) B cells and multiple sclerosis. Lancet Neurol 7: 852–858 [DOI] [PubMed] [Google Scholar]
- Frohman E., Costello F., Zivadinov R., Stuve O., Conger A., Winslow H., et al. (2006) Optical coherence tomography in multiple sclerosis. Lancet Neurol 5: 853–863 [DOI] [PubMed] [Google Scholar]
- Genovese M.C., Kaine J.L., Lowenstein M.B., Del Giudice J., Baldassare A., Schechtman J., et al. (2008) Ocrelizumab, a humanized anti-CD20 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I/II randomized, blinded, placebo-controlled, dose-ranging study. Arthritis Rheum 58: 2652–2661 [DOI] [PubMed] [Google Scholar]
- Gordon-Lipkin E., Chodkowski B., Reich D.S., Smith S.A., Pulicken M., Balcer L.J., Frohman E.M., et al. (2007) Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology 69: 1603–1609 [DOI] [PubMed] [Google Scholar]
- Gross J.A., Dillon S.R., Mudri S., Johnston J., Littau A., Roque R., et al. (2001) TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. impaired B cell maturation in mice lacking BLyS. Immunity 15: 289–302 [DOI] [PubMed] [Google Scholar]
- Gross J.A., Johnston J., Mudri S., Enselman R., Dillon S.R., Madden K., et al. (2000) TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature 404: 995–999 [DOI] [PubMed] [Google Scholar]
- Handel, A.E., Giovannono G., Ebers, G.E. and Ramagopalan S.V. (2010) Environmental factors and their timing in adult-onset multiple sclerosis. Nat Rev Neurol 6: 156–166. [DOI] [PubMed]
- Hauser S.L., Waubant E., Arnold D.L., Vollmer T., Antel J., Fox R.J., et al. (2008) B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 358: 676–688 [DOI] [PubMed] [Google Scholar]
- Hawker K. (2008) B-cell targeted treatment for multiple sclerosis: mechanism of action and clinical data. Curr Opin Neurol 21(Suppl 1): S19–S25 [DOI] [PubMed] [Google Scholar]
- Heffernan J., Burleson F., Roque R., Waggie K., Carbonatto M., Ponce R., et al. (2008) The evaluation of atacicept on protective immunity in the mouse streptococcal host resistance model (abstract 1934). Toxicologist 102: 398–398 [Google Scholar]
- Hemmer B., Archelos J.J., Hartung H.P. (2002) New concepts in the immunopathogenesis of multiple sclerosis. Nat Rev Neurosci 3: 291–301 [DOI] [PubMed] [Google Scholar]
- Hemmer B., Hartung H.P. (2007) Toward the development of rational therapies in multiple sclerosis: what is on the horizon? Ann Neurol 62: 314–326 [DOI] [PubMed] [Google Scholar]
- Hemmer, B. and Stüve, O. (2007) Revised criteria for neuromyelitis optica – a new diagnostic standard? Nat Clin Pract Neurol 3: 132–133. [DOI] [PubMed]
- Hohlfeld R., Wekerle H. (2004) Autoimmune concepts of multiple sclerosis as a basis for selective immunotherapy: from pipe dreams to (therapeutic) pipelines. Proc Natl Acad Sci U S A 101(Suppl 2): 14599–14606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohlfeld R., Wiendl H. (2001) The ups and downs of multiple sclerosis therapeutics. Ann Neurol 49: 281–284 [PubMed] [Google Scholar]
- Hsu B.L., Harless S.M., Lindsley R.C., Hilbert D.M., Cancro M.P. (2002) Cutting edge: BLyS enables survival of transitional and mature B cells through distinct mediators. J Immunol 168: 5993–5996 [DOI] [PubMed] [Google Scholar]
- Huntington N.D., Tomioka R., Clavarino C., Chow A.M., Liñares D., Maña P., et al. (2006) A BAFF antagonist suppresses experimental autoimmune encephalomyelitis by targeting cell-mediated and humoral immune responses. Int Immunol 18: 1473–1485 [DOI] [PubMed] [Google Scholar]
- Kalled S.L. (2005) The role of BAFF in immune function and implications for autoimmunity. Immunol Rev 204: 43–54 [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M., Gallmeier E., Behrens L., Leal V.V., Misgeld T., Klinkert W.E., et al. (1999) Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med 189: 865–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolln J., Ren H.M., Da R.R., Zhang Y., Spillner E., Olek M., et al. (2006) Triosephosphate isomerase- and glyceraldehyde-3-phosphate dehydrogenase-reactive autoantibodies in the cerebrospinal fluid of patients with multiple sclerosis. J Immunol 177: 5652–5658 [DOI] [PubMed] [Google Scholar]
- Kornek B., Storch M.K., Weissert R., Wallstroem E., Stefferl A., Olsson T., et al. (2000) Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol 157: 267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbholz M., Theil D., Cepok S., Hemmer B., Kivisäkk P., Ransohoff R.M., et al. (2006) Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain 129: 200–211 [DOI] [PubMed] [Google Scholar]
- Krumbholz M., Theil D., Derfuss T., Rosenwald A., Schrader F., Monoranu C.M., et al. (2005) BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J Exp Med 201: 195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambracht-Washington D., O’Connor K.C., Cameron E.M., Jowdry A., Ward E.S., Frohman E., et al. (2007) Antigen specificity of clonally expanded and receptor edited cerebrospinal fluid B cells from patients with relapsing remitting MS. J Neuroimmunol 186: 164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H., Bruck W., Lucchinetti C.F. (2007) The immunopathology of multiple sclerosis: an overview. Brain Pathol 17: 210–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liossis S.N., Sfikakis P.P. (2008) Rituximab-induced B cell depletion in autoimmune diseases: potential effects on T cells. Clin Immunol 127: 280–285 [DOI] [PubMed] [Google Scholar]
- Lucchinetti C., Brück W., Parisi J., Scheithauer B., Rodriguez M., Lassmann H., et al. (2000) Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 47: 707–717 [DOI] [PubMed] [Google Scholar]
- MacKay F., Tangye S.G. (2004) The role of the BAFF/APRIL system in B cell homeostasis and lymphoid cancers. Curr Opin Pharmacol 4: 347–354 [DOI] [PubMed] [Google Scholar]
- MacKay F., Woodcock S.A., Lawton P., Ambrose C., Baetscher M., Schneider P., et al. (1999) Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med 190: 1697–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliozzi R., Howell O., Vora A., Serafini B., Nicholas R., Puopolo M., et al. (2007) Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 130: 1089–1104 [DOI] [PubMed] [Google Scholar]
- Marrie R.A. (2004) Environmental risk factors in multiple sclerosis aetiology. Lancet Neurol 3: 709–718 [DOI] [PubMed] [Google Scholar]
- Marsters S.A., Yan M., Pitti R.M., Haas P.E., Dixit V.M., Ashkenazi A., et al. (2000) Interaction of the TNF homologues BLyS and APRIL with the TNF receptor homologues BCMA and TACI. Curr Biol 10: 785–788 [DOI] [PubMed] [Google Scholar]
- Martinez-Gamboa L., Brezinschek H.P., Burmester G.R., Dorner T. (2006) Immunopathologic role of B lymphocytes in rheumatoid arthritis: rationale of B cell-directed therapy. Autoimmun Rev 5: 437–442 [DOI] [PubMed] [Google Scholar]
- McDole J., Johnson A.J., Pirko I. (2006) The role of CD8+ T-cells in lesion formation and axonal dysfunction in multiple sclerosis. Neurol Res 28: 256–261 [DOI] [PubMed] [Google Scholar]
- Medana I., Martinic M.A., Wekerle H., Neumann H. (2001) Transection of major histocompatibility complex class I-induced neurites by cytotoxic T lymphocytes. Am J Pathol 159: 809–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo A., Priestley A., Nestorov I., Visich J., Rogge M. (2007) Safety, pharmacokinetics and pharmacodynamics of atacicept in healthy volunteers. Eur J Clin Pharmacol 63: 647–656 [DOI] [PubMed] [Google Scholar]
- Nestorov I., Munafo A., Papasouliotis O., Visich J. (2008) Pharmacokinetics and biological activity of atacicept in patients with rheumatoid arthritis. J Clin Pharmacol 48: 406–417 [DOI] [PubMed] [Google Scholar]
- Nestorov I., Papasouliotis O., Pena-Rossi C., Munafo A. (2010) Pharmakokinetics and immunoglobulin response of subcutaneous and intravenous atacicept in patients with systemic lupus erythematosus. J Pharmac Sci 99: 524–538 [DOI] [PubMed] [Google Scholar]
- Owens G.P., Bennett J.L., Gilden D.H., Burgoon M.P. (2006) The B cell response in multiple sclerosis. Neurol Res 28: 236–244 [DOI] [PubMed] [Google Scholar]
- Pena-Rossi C., Nasonov E., Stanislav M., Yakusevich V., Ershova O., Lomareva N., et al. (2009) An exploratory dose-escalating study investigating the safety, tolerability, pharmacokinetics and pharmacodynamics of intravenous atacicept on patients with systemic lupus erythematosus. Lupus 18: 547–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perini P., Ranzato F., Calabrese M., Battistin L., Gallo P. (2006) Intrathecal IgM production at clinical onset correlates with a more severe disease course in multiple sclerosis. J Neurol Neurosurg Psychiatry 77: 953–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser C.M. (2006) The multiple sclerosis trait and the development of multiple sclerosis: genetic vulnerability and environmental effect. Clin Neurol Neurosurg 108: 227–233 [DOI] [PubMed] [Google Scholar]
- Prineas J.W., Graham J.S. (1981) Multiple sclerosis: capping of surface immunoglobulin G on macrophages engaged in myelin breakdown. Ann Neurol 10: 149–158 [DOI] [PubMed] [Google Scholar]
- Qin Y., Duquette P., Zhang Y., Olek M., Da R.R., Richardson J., et al. (2003) Intrathecal B-cell clonal expansion, an early sign of humoral immunity, in the cerebrospinal fluid of patients with clinically isolated syndrome suggestive of multiple sclerosis. Lab Invest 83: 1081–1088 [DOI] [PubMed] [Google Scholar]
- Ramagopalan, S.V., Dyment, D.A. and Ebers, G.C. (2009) Environmental factors and their timing in adult-onset multiple sclerosis. Trends Neurosci 649: 1–8. [DOI] [PubMed]
- Roschke V., Sosnovtseva S., Ward C.D., Hong J.S., Smith R., Albert V., et al. (2002) BLyS and APRIL form biologically active heterotrimers that are expressed in patients with systemic immune-based rheumatic diseases. J Immunol 169: 4314–4321 [DOI] [PubMed] [Google Scholar]
- Schneider P., MacKay F., Steiner V., Hofmann K., Bodmer J.L., Holler N., et al. (1999) BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med 189: 1747–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolding N.J., Morgan B.P., Houston W.A., Linington C., Campbell A.K., Compston D.A., et al. (1989) Vesicular removal by oligodendrocytes of membrane attack complexes formed by activated complement. Nature 339: 620–622 [DOI] [PubMed] [Google Scholar]
- Serafini B., Rosicarelli B., Magliozzi R., Stigliano E., Aloisi F. (2004) Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol 14: 164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshasayee D., Valdez P., Yan M., Dixit V.M., Tumas D., Grewal I.S., et al. (2003) Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity 18: 279–288 [DOI] [PubMed] [Google Scholar]
- Silverman G.J. (2006) Therapeutic B cell depletion and regeneration in rheumatoid arthritis: emerging patterns and paradigms. Arthritis Rheum 54: 2356–2367 [DOI] [PubMed] [Google Scholar]
- Simpson B.S., Coles A.J. (2007) Rationale for cytotoxic monoclonal antibodies in MS. Int MS J 14: 48–56 [PubMed] [Google Scholar]
- Sospedra M., Martin R. (2005) Immunology of multiple sclerosis. Annu Rev Immunol 23: 683–747 [DOI] [PubMed] [Google Scholar]
- Stüve, O., Leussink, V.I., Fröhlich R., Hemmer B., Hartung H.P., Menge T. and Kieseier, B.C. (2009) Long-term B lymphocyte depletion with rituximab in patients with relapsing-remitting multiple sclerosis. Arch Neurol 66: 259–261. [DOI] [PubMed]
- Svensson L., Abdul-Majid K.-B., Bauer J., Lassmann H., Harris R.A., Holmdahl R., et al. (2002) A comparative analysis of B cell-mediated myelin oligodendrocyte glycoprotein-experimental autoimmune encephalomyelitis pathogenesis in B cell-deficient mice reveals an effect on demyelination. Eur J Immunol 32: 1939–1946 [DOI] [PubMed] [Google Scholar]
- Tak P.P., Thurlings R.M., Rossier C., Nestorov I., Dimic A., Mircetic V., et al. (2008) Atacicept in patients with rheumatoid arthritis: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating, single- and repeated-dose study. Arthritis Rheum 58: 61–72 [DOI] [PubMed] [Google Scholar]
- Thangarajh M., Gomes A., Masterman T., Hillert J., Hjelmstrom P. (2004) Expression of B-cell-activating factor of the TNF family (BAFF) and its receptors in multiple sclerosis. J Neuroimmunol 152: 183–190 [DOI] [PubMed] [Google Scholar]
- Thangarajh M., Masterman T., Hillert J., Moerk S., Jonsson R. (2007) A proliferation-inducing ligand (APRIL) is expressed by astrocytes and is increased in multiple sclerosis. Scand J Immunol 65: 92–98 [DOI] [PubMed] [Google Scholar]
- Thangarajh M., Masterman T., Rot U., Duvefelt K., Brynedal B., Karrenbauer V.D., et al. (2005) Increased levels of APRIL (a proliferation-inducing ligand) mRNA in multiple sclerosis. J Neuroimmunol 167: 210–214 [DOI] [PubMed] [Google Scholar]
- Thompson J.S., Bixler S.A., Qian F., Vora K., Scott M.L., Cachero T.G., et al. (2001) BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science 293: 2108–2111 [DOI] [PubMed] [Google Scholar]
- Varfolomeev E., Kischkel F., Martin F., Seshasayee D., Wang H., Lawrence D., et al. (2004) APRIL-deficient mice have normal immune system development. Mol Cell Biol 24: 997–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar L.M., Sádaba M.C., Roldán E., Masjuan J., González-Porqué P., Villarrubia N., et al. (2005) Intrathecal synthesis of oligoclonal IgM against myelin lipids predicts an aggressive disease course in MS. J Clin Invest 115: 187–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker B.G., O’Brien P.C., Petterson T.M., Noseworthy J.H., Lucchinetti C.F., Dodick D.W., et al. (1999) A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol 46: 878–886 [DOI] [PubMed] [Google Scholar]
- Willer C.J., Dyment D.A., Risch N.J., Sadovnick A.D., Ebers G.C. (2003) Twin concordance and sibling recurrence rates in multiple sclerosis. Proc Natl Acad Sci U S A 100: 12877–12882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M., Wang H., Chan B., Roose-Girma M., Erickson S., Baker T., et al. (2001) Activation and accumulation of B cells in TACI-deficient mice. Nat Immunol 2: 638–643 [DOI] [PubMed] [Google Scholar]
- Zeyda M., Poglitsch M., Geyeregger R., Smolen J.S., Zlabinger G.J., Hörl W.H., et al. (2005) Disruption of the interaction of T cells with antigen-presenting cells by the active leflunomide metabolite teriflunomide: involvement of impaired integrin activation and immunologic synapse formation. Arthritis Rheum 52: 2730–2739 [DOI] [PubMed] [Google Scholar]
- Zouali M. (2008) B lymphocytes—chief players and therapeutic targets in autoimmune diseases. Front Biosci 13: 4852–4861 [DOI] [PubMed] [Google Scholar]