Linking Molecules to Mood: New Insight Into the Biology of Depression (original) (raw)
. Author manuscript; available in PMC: 2011 May 1.
Abstract
Major depressive disorder is a heritable neuropsychiatric syndrome characterized by relatively subtle cellular and molecular alterations localized to a complex network of neural substrates. These brain regions display dynamic neuroplastic adaptations to endocrine and immunologic stimuli arising from within and outside the central nervous system. Depression’s clinical and etiological heterogeneity adds a third level of complexity, implicating different pathophysiological mechanisms in different patients with the same DSM diagnosis. Current pharmacological antidepressant treatments improve depressive symptoms through complex mechanisms that are themselves incompletely understood. This review summarizes our current knowledge of the neurobiology of depression by combining insights from human clinical studies and molecular explanations from animal models. We provide recommendations for future research, with a focus on translating today’s discoveries into improved diagnostic tests and treatments.
Introduction
There are overwhelming justifications to explore the molecular mechanisms underlying major depressive disorder: 1 in 6 individuals in the United States will develop depressive symptoms requiring treatment (1), depression significantly complicates chronic illness (2) and depression is the leading cause of disability worldwide (3). Unfortunately, the motivated laboratory neuroscientist who wishes to explore the molecular underpinnings of depression is faced with unique challenges. In contrast to the clear cut phenotypes encountered in substance dependence or obesity, the strictest guidelines for diagnosing depression include several vague elements (e.g., “insomnia or hypersomnia nearly every day”(4)), creating a challenge for the generation of animal models. Unlike Parkinson or Alzheimer disease, depression lacks any clear consensus neuropathology, rare familial genetic causes or highly penetrant vulnerability genes, providing no obvious starting points for molecular investigations. In spite of its well-known genetic risk, the search for genes involved has been frustratingly low yield. Neglecting uniquely human features of depression such as sadness, guilt and suicidality, recapitulating more accessible symptoms (e.g., anhedonia) in animal models is also not straightforward. Consequently, progress in understanding the molecular biology of depression has been extremely slow, particularly in comparison to other multifactorial syndromes such as type II diabetes mellitus and cancer. Thus, as the burden of depression continues to increase (3), depression will feature prominently during the extra years of life gained from improved outcomes in cardiovascular disease, cancer, and other domains.
And yet, it is an exciting time to be a depression researcher. Advances in molecular tools and ongoing improvements in behavioral techniques have allowed for genuinely novel insights into depression’s neurobiological correlates. Methods to capture and artificially stimulate or inhibit the electrophysiological activity of individual types of neurons in the brain in vivo have added new dimensions to available approaches, permitting us for the very first time to describe and manipulate the previously enigmatic neurophysiological correlates of concepts such as “reward” or “anxiety”. Coupled with experimental advances in treatment, we can be optimistic that developing tomorrow’s therapies will no longer rely solely on modifications of existing agents that were discovered by serendipity six decades ago. This review aims to provide a framework to interpret continuing advances in the basic science of depression.
Insights from Human Studies
While animal experiments offer a unique opportunity to test cellular and molecular hypotheses, human clinical investigation continues to provide insights about depression that are inaccessible in animals. Postmortem studies designed to capture the neuropathology of depression have largely focused on cortical and hippocampal regions, which show a number of subtle atrophic changes such as reduced neuronal size, decreased glial cell number, local reductions in dendrite densities and trophic factors (5-7). These results agree with evidence of volume loss in these regions as shown by structural magnetic resonance imaging (MRI) (8, 9). Molecular techniques such as DNA microarray profiling have been applied to regions such as the amygdala and locus ceruleus to document gene expression alterations associated with depression (10, 11). Within the coming years, we can hope for a more comprehensive list of depression’s neuropathological changes particularly with the advent of centralized brain collections, which are able to furnish larger sample sizes while simultaneously excluding traditional sources of confound, such as suicide, comorbid substance abuse or a bipolar diagnosis. When elegantly combined with animal models or neuroimaging data, these postmortem depression studies have the opportunity to demonstrate true causal relationships (12-15).
Functional MRI and positron emission tomography (PET) have shown how depressive behavior can be correlated with hypermetabolism of the subgenual cingulate cortex and amygdala (16) as well as hypometabolism of the dorsal prefrontal cortex and striatal regions (8). In an attempt to integrate these anatomic data, there have been several formulations of a “depression circuit” (Figure 1). After years of largely empirical reports, we now encroach upon the possibility of testing and refining these circuit models in humans, thanks to recent experimental interventional advances such as deep brain stimulation (DBS) and repetitive transcranial magnetic stimulation (rTMS). For cases of treatment-resistant depression, DBS has been successfully applied to the subgenual cingulate cortex (17, 18) and the ventral striatum/nucleus accumbens without permanent adverse effects (19-21). Refinements in stimulation parameters for rTMS applied to the dorsolateral prefrontal cortex have significantly improved the magnitude and endurance of observed antidepressant effects (22). While these techniques are safer than earlier rudimentary approaches to “psychosurgery”, the precise mechanisms by which DBS and rTMS act are still incompletely understood. It is not known, for example, whether the local effects of DBS work through excitation or inhibition or effects on fibers of passage (23). Recently developed optogenetic tools make it possible to activate or inhibit particular neuronal cell types, and/or their terminals within defined brain regions (24), allowing for a deeper exploration of the neurophysiological mechanisms underlying the therapeutic effects of DBS. Thus, as DBS and rTMS are scaled-down and characterized in laboratory animals, one can expect clinical improvements in patient selection, technique and localization.
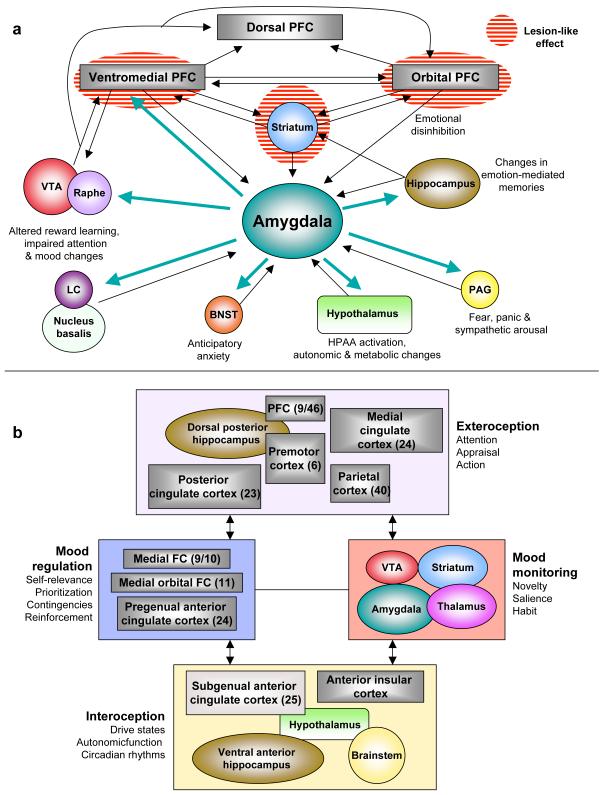
Figure 1. Two heuristic formulations of “depression circuits.”.
a) An amygdala-centric circuit (8) largely inspired by structural brain imaging and postmortem studies. According to this model, the emotional symptoms of depression can be brought about by functional impairment (“lesion-like” effects) of the striatum or prefrontal and orbital prefrontal cortex (and/or their associated white matter tracts), resulting in disinhibition of the amygdala and downstream structures. Alternatively, they can arise from functional hypersensitivity of the amygdala (turquoise arrows) which gives rise to dysregulation of prefrontal cortical structures. b) Another circuit model (138) generated with a greater emphasis on functional imaging results. The main nodes consist of four clusters of brain regions with strong anatomical connections to each other; bidirectional arrows indicate strong inter-connections. This model compartmentalizes depressive endophenotypes into exteroceptive (cognitive), interoceptive (visceral-motor), mood-regulating and mood-monitoring functions, with numbers in parentheses reflecting formal Brodmann area assignments. Both formulations should be seen as offering a simplified heuristic framework for further research into depression’s diagnosis, pathophysiology and treatment. They do not convey the cellular and molecular heterogeneity of each node within the circuit (for example, the VTA is comprised of several types of dopaminergic and GABAergic neurons defined by differences in connectivity and receptor expression). Abbreviations: VTA (ventral tegmental area), LC (locus coeruleus), BNST (bed nucleus of the stria terminalis), PFC (prefrontal cortex), PAG (periaqueductal gray).
With heritability estimates of ~40% (25), two main techniques have been utilized to explore the genetics of depression. Candidate genes, identified through an investigator’s best guess about etiological mechanisms, have been examined through linkage or genetic association studies (26). Single nucleotide polymorphisms (SNPs) in specific genes such as GNB3 (guanine nucleotide-binding protein-3) or MTHFR (methylene tetrahydrofolate reductase) have survived stringent statistical requirements of meta-analyses. While their odds ratios are too weak for diagnostics or risk stratification, these and other genes may offer new clues into disease pathophysiology (27). Genome-wide association studies (GWAS) are inherently unbiased as they currently can simultaneously examine up to one million SNPs. While such trials have identified SNPs in previously unappreciated molecules like piccolo (a presynaptic nerve terminal protein) or GRM7 (metabotropic glutamate receptor-7), these findings are themselves of relatively poor statistical significance and are not replicated across studies (26). Several explanations have been put forth, including relatively vague DSM diagnostic criteria, considerable disease heterogeneity and the relatively potent contribution of ongoing life stressors and epigenetic plasticity. Within the coming decade, we remain hopeful that newer technologies will have greater success and replicability, including whole “exome” studies (which exclude non-coding regions representing 99% of the genome), as well as whole-genome sequencing. Of course, a key unanswered question is whether these genetic data should be correlated with DSM diagnostic categories, more broadly across several DSM diagnoses, or with more carefully defined behavioral, endocrine, neurochemical or neuroimaging phenotypes. Identifying such genes will be hugely beneficial for the generation of bona fide animal models of depression.
An important insight gained from everyday clinical practice is the observation that monoamine reuptake inhibitors and other modulators of monoaminergic function improve symptoms in ~50% of depressed patients and produce a remission in 30-40% of patients (28). These data illustrate the tremendous genetic heterogeneity of treatment response and efforts are underway to identify pharmacogenetic predictors of a favorable treatment response to monoaminergic agents (29, 30). Since monoamine enhancers improve depressive symptoms, it was suggested historically that depression is caused by deficits in monoaminergic transmission (“monoamine hypothesis”), which continues to be a prominent preoccupation of the field. However, after more than a decade of PET studies (positioned aptly to quantitatively measure receptor and transporter numbers and occupancy) (31), monoamine depletion studies (which transiently and experimentally reduce brain monoamine levels) (32) as well as genetic association analyses examining polymorphisms in monoaminergic genes (27, 33, 34), there is little evidence to implicate true deficits in serotonergic, noradrenergic, or dopaminergic neurotransmission in the pathophysiology of depression. This is not surprising, as there is no a priori reason that the mechanism of action of a treatment is the opposite of disease pathophysiology (35). Thus, currently available agents likely restore mood by modulating distinct processes that are unrelated to the primary pathology of depression, just as diuretics improve the symptomatology of congestive heart failure without affecting cardiac myocytes directly. Similarly, the success of intravenous ketamine in rapidly alleviating depressive symptoms in treatment-resistant depression (36) has prompted an exploration of the cellular and neuroanatomical substrates for ketamine’s actions and the search for ketamine-like therapies that lack psychotomimetic side effects. However, formulating a “glutamatergic hypothesis of depression” is grossly simplistic and only fuels inaccurate public misconceptions of depression’s “chemical imbalance”, particularly since more than half of all neurons in the brain utilize glutamate as a neurotransmitter.
Animal Models of Depression
The design, application and relative strengths and limitations of depression models have been discussed in several reviews (1, 37). Without definitive knowledge of pathophysiological processes, these models are often evaluated for their face, construct and pharmacological validity (1), as are models of other clinically defined neuropsychiatric syndromes, such as autism and schizophrenia. Face validity judges a model’s symptomatic homology to human depression. Today’s depression models achieve this goal considerably: rodent and primate models have successfully recapitulated states of social withdrawal, hypophagia and weight loss, anhedonia, circadian changes and abnormalities of the HPA (hypothalamic-pituitary-adrenal) axis, although these phenotypes are often transient and not all present simultaneously. The more challenging construct validity requires a model to honor etiological factors implicated in depression, which are themselves not entirely understood. Most paradigms utilize some form of stress (of a physical or a psychosocial form), given the known association between independent stressful life events and depressive episodes (38). More recently, there has been a greater emphasis on recapitulating both environmental (such as stressful life events) and genetic risk factors (although these remain largely unknown) in the same model.
Pharmacological or predictive validity is met when a model’s depression-like behaviors are reversed by currently available antidepressant modalities, and several models in use today display this type of predictability with the therapeutic delay that characterizes antidepressant responses in humans. However, given that all available pharmacological agents are monoamine modulators and only a minority of patients remit following first-line therapies (28), the requirement for pharmacological reversibility is perhaps desirable but not mandatory. Since the mechanisms underlying the delayed antidepressant effects of medication and non-medication treatments (including exercise, electroconvulsive seizures, etc.) remain largely unknown, there have been several attempts to employ animal models to dissect these mechanisms (i.e., models of antidepressant action), with the caveat that these therapies are applied to laboratory animals that generally lack depression-like behavior or any particular genetic vulnerability to depression. A potential fourth criterion that has received considerably less attention is pathological validity, whereby depression-related physiological, molecular and cellular abnormalities in animals are validated by demonstrating identical changes in postmortem brain samples from depressed humans. This is a genuinely difficult requirement, but has been gaining increasing popularity with the more widespread access to postmortem samples (12-15). Ideally, this criterion might be better addressed through functional imaging studies with depressed patients, but this will require substantial improvements in molecular imaging capabilities.
From an evolutionary perspective, depression may be an analog of the involuntary defeat strategy (IDS), occurring when an animal perceives defeat in a hierarchical struggle for resources (39). Hyperarousal, psychomotor retardation, reduced motivation and sleep alterations in the setting of losing are postulated to have an adaptive advantage in that they serve to protect losers from further attack and focus cognitive resources on planning ways out of complex social problems (40, 41). Most behavioral endpoints in depression models aim to quantitatively assay some type of experimentally induced defeat or despair (Figure 2), even though this aspect of mammalian behavior is likely physiological (i.e., adaptive) rather than pathological. Additionally, while despair behavior is often extrapolated as being depression-like, it is clearly a huge inference to make from rodent models, and most stressors also produce anxiety-like changes that are exaggerated manifestations of the fight or flight response (reduced exploration, freezing, stress-induced hyperthermia, HPA axis activation, etc.). For example, repeated social subordination in mice (social defeat) leads to a long-lasting phenotype of reduced social interaction with other mice. This impairment in sociability can be interpreted as a reduced motivation to interact (an abnormality of reward) or as a heightened avoidance of novel social stimuli (a pathological anxiety response). Distinguishing between these alternative hypotheses is difficult and may even be irrelevant, particularly given the poorly defined neurobiological distinctions between anxiety and depression and their highly variable clinical presentation. In either case, the model employs a naturalistic social stress-induced behavior that is quantifiable and amenable to experimental manipulation (12-14, 42-50).
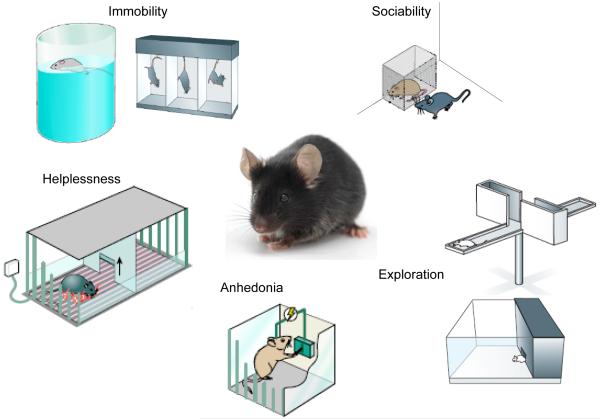
Figure 2. Common behavioral endpoints in rodent depression studies.
This figure diagrams certain widely utilized quantitative and automatable behavioral endpoints used in experiments with rats or mice as measures of depression-related behavior. They can be employed following chronic stress paradigms such as social defeat, to phenotype genetic mutant mice, to validate antidepressant treatments or as tools to localize genomic mediators of complex behaviors in QTL analyses (quantitative trait locus). The most popular endpoint is immobility, which is interpreted as a measure of behavioral despair or freezing in response to an inescapable stressor like forced swimming or tail suspension. The closely related helplessness can be inferred through the learned helplessness paradigm, where animals receive a series of inescapable electrical shocks in one compartment, and on subsequent testing days display a deficit in their motivation to avoid these shocks when a clear escape route is provided. Anhedonia in mice and rats can be measured in several ways, ranging from simple measures of sucrose preference (measuring the relative preference for a dilute solution of sucrose versus water), to preference for a high fat diet, to ICSS (intracranial self stimulation) where one directly measures motivation (lever pressing) to receive highly rewarding electrical stimulation. Reductions in exploratory behavior are often interpreted as elevations in anxiety, and can be quantified by measuring amounts of time spent in aversive portions of a field of exploration such as the open arms of the elevated plus maze (top) or the brightly illuminated portion of the light-dark box. One can also measure deficits in sociability, which may reflect impairments in natural reward or social anxiety. These assays have been employed in stress paradigms, mutant mouse models as well as models of secondary depression such as that seen, for example, with obesity, breast cancer or chronic interferon treatment. A common practice is to generate behavioral profiles by employing a broad battery of these tests following stress, genetic, or pharmacological manipulations, which can also include changes in weight asnd appetite, as well as deficits in self-grooming (deteriorations in fur coat).
The forced swim and tail suspension tests (FST, TST) are the simplest and most widely utilized models of depression and antidepressant action (Figure 2). While these approaches have been rightly criticized for involving acute stress and acute antidepressant responses, they have permitted the rapid behavioral screening of novel chemical antidepressants and the phenotyping of genetically altered mutant mice. In certain instances, they have directed the field towards fundamentally novel molecular hypotheses. For example, an antidepressant-like phenotype on the FST (decreased immobility and greater struggling/swimming) was observed in mice deficient in ASIC-1a (acid sensing ion channel 1a), a pH sensitive ion channel expressed in the brain (51). Subsequent studies have shown that ASIC-1a expressed in the amygdala participates in eliciting a fear response to a variety of aversive cues culminating in acidemia (51, 52), implicating inhibitors of ASIC-1a (a previously unappreciated target) as potential therapeutics against anxiety and depressive disorders. Analogous approaches have identified several other novel molecular targets, including p11 (a calcium binding chaperone molecule that promotes serotonin signaling through the 5HT1B receptor subtype (15)), TREK-1 (a distinct type of potassium channel that is enriched in depression-related limbic brain regions (53, 54)) and ghrelin (a stomach-derived endocrine mediator of energy homeostasis (45)), among many others.
In the following sections, we focus on neurobiological themes that exhibit therapeutic promises. The two main values of utilizing rats and mice to study depression are: 1) the ability to describe and characterize neuroplasticity with exquisite spatial and temporal precision, and 2) the opportunity to utilize molecular innovations to demonstrate the causative effects of those neuroplastic changes on assays of depression- and antidepressant-like behavior.
Neurogenic and Neurotrophic Theories
The first description of continually dividing neuronal progenitors in the adult mammalian brain offered the promise of a solution for a host of neurodegenerative disorders that until today lack definitive cures (55). Exploring the physiologic role of endogenous neurogenesis, particularly that which occurs in the hippocampal dentate gyrus, has important relevance to the study of psychiatric disease (56). The journey from a hippocampal stem cell in the subgranular zone to a mature dentate gyrus granule cell neuron with appropriate synaptic connections occurs in stages defined by specific cellular markers, with the rates of proliferation and survival modulated by numerous stimuli. Unpredictable stressors, glucocorticoids, drugs of abuse or high energy electromagnetic radiation negatively influence this process, while antidepressants, voluntary exercise or environmental enrichment accelerate adult hippocampal neurogenesis (57).
Laboratory rodents have been utilized extensively to explore the regulation and contribution of these new hippocampal neurons to depression-related phenotypes. In models of antidepressant action, cranial irradiation (which severely impairs the mitotic potential of hippocampal stem cells) and aging (another robust negative regulator of adult hippocampal neurogenesis) impair some but not all of the effects of monoamine reuptake inhibitors, (58-61), suggesting that these agents may function through neurogenesis-dependent and –independent processes (62). Clearly, only those actions of antidepressants that involve hippocampal circuitry could be mediated through enhanced neurogenesis. Indeed, one study was able to demonstrate the antidepressant effect of the direct intracerebral infusion of bone-marrow derived mesenchymal stem cells, which both themselves transform into neurons as well as generate diffusible permissive factors that accelerate endogeneous neurogenesis (63). These preliminary results support the idea that enhancing hippocampal neurogenesis (pharmacologically or by way of cellular transplantation) can serve to boost or augment the antidepressant response. At the same time, impairments in the rates of neurogenesis do not appear to be involved in the core features of depression. Mice subjected to cranial irradiation are unimpaired across several indices of depression-related behavior (58, 60). Consistent with its proposed role in hippocampal-dependent learning (55, 57), adult hippocampal neurogenesis may play a pathological role in the establishment of aversive memories of traumatic stressors and the sequelae of post-traumatic stress (64). As the field struggles to clarify the functional relevance of these new neurons, stress-induced reductions in hippocampal proliferation are best interpreted as a marker of hippocampal plasticity (which may be impaired in some types of depression).
Another widespread endpoint for assaying the effects of stress, antidepressants and genetic manipulations is measuring levels of brain-derived neurotrophic factor (BDNF) in the hippocampus. This practice, stemming from the “neurotrophic hypothesis” of depression (65), is based on three main observations: an impairment of hippocampal BDNF signaling produces certain depression-related behaviors and impairs the actions of antidepressants (66-68), experimental increases in hippocampal BDNF levels produce antidepressant-like effects (69-71) and hippocampal BDNF levels are reduced in postmortem samples from depressed humans (6). BDNF is one of numerous growth factors that have been implicated in depression, including fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), and non-acronymic VGF (1). Through modulation of their levels and downstream signaling, these growth factors appear to transduce stressors into lowered rates of adult hippocampal neurogenesis, atrophic changes and impaired synaptic plasticity of hippocampal neurons, which might (in theory) explain the cognitive impairments and hippocampal atrophy seen in depression (65).
Translating these BDNF findings may not be straightforward. Aside from the challenges associated with synthesizing a specific agonist of BDNF, enhancing BDNF function in the nucleus accumbens and amygdala can have detrimental effects on measures of anhedonia, anxiety and social interaction in rodents (1, 69). A naturally occurring single nucleotide polymorphism in BDNF (G196A, val_66_met) results in dramatic alterations in intracellular trafficking of BDNF and its activity-dependent release (1). Meta-analyses show that while the met allele marginally increases the risk for depression in men but not women, it is also associated with a better antidepressant response (72, 73). A hippocampus-specific increase in BDNF activity may improve certain cognitive symptoms of depression (58) and facilitate hippocampal neurogenesis. While we possess the technology to deliver specific genes into human brain through viral vectors (74), the beneficial effects of BDNF would have to outweigh potential negative effects on lowering seizure thresholds, altering indices of learning and memory as well as increasing the likelihood of malignant transformation (75, 76). Nevertheless, understanding the roles of these growth factors in depression’s pathophysiology remains an extremely active area of research, with an emphasis now placed on extrahippocampal trophic signaling and exploring downstream signaling pathways, which may have greater pharmaceutical application.
The Contribution of Epigenetic Modifications
Biological theories of depression’s etiology have traditionally focused on the interplay between genetic risks and environmental/social hazards, with gene x environment interactions invoked to explain how relatively weak genetic vulnerabilities combined with the right environmental triggers may lead to significant psychiatric impairment (77). However, the significant discordance of depression between monozygotic twins (who often share the same environment as well as genes), the remarkably slow progress in identifying genetic risk factors, and depression’s twofold female predominance suggest the presence of a third, non-genetic and non-environmental component to variability (78). Epigenetic modifications have been implicated as a significant contributor to this third source of variability, and are broadly divided into those that modify DNA directly (e.g., DNA methylation), those that alter histones (e.g., histone acetylation or methylation), or those that involve non-coding RNAs (such as microRNAs) that regulate gene expression (79). In changing DNA’s tertiary structure, they adjust interactions between DNA and associated proteins such as transcription factors and RNA polymerases, which ultimately alters levels of messenger RNA expressed by given genes. Pathological epigenetic events have been implicated in numerous chronic diseases (79-81), most notably in cancer, where aberrant epigenetic changes promote genetic instability (82).
Through combining animal models with an explosion of novel molecular tools, several epigenetic events have been linked to depression-related behavior and antidepressant action. In rats, offspring born to mothers that display low levels of maternal licking and grooming behavior display exaggerated corticosteroid responses to stress and increased anxiety, which are mediated in part by increased methylation (and subsequent repression) of the glucocorticoid receptor (GR) gene promoter in the hippocampus. This type of epigenetic mark is stable to adulthood, reversed by chemical augmenters of DNA methylation, and entirely dependent on the maternal behavior of the fostering rather than the biological mother (i.e., independent of germ-line transmission) (83). Early life stress applied to mice produces hypomethylation of the arginine vasopressin (AVP) gene in the hypothalamic paraventricular nucleus, resulting in hypersecretion of AVP, pathologically enhanced serum corticosterone level and increased depression-like behavior (84). Histone acetylation, a mark of active transcription, is increased at certain BDNF promoters when socially defeated mice receive a course of chronic imipramine, and this hyperacetylation event is mediated by the downregulation of histone deacetylase 5 (HDAC5) (46). While overexpression of HDAC5 in the hippocampus counteracts the effects of antidepressants, mice that are globally deficient in HDAC5 display an enhanced vulnerability to chronic stress (48).
These examples illustrate the complexity in translating these epigenetic changes into the clinic: while certain perturbations robustly alter epigenetic marks on one gene in one brain region, other brain regions may have opposing changes at distinct genes. Furthermore, most epigenetic enzymes occur in several isoforms, each with their own tissue specificity and regulatory factors (e.g., HDAC5 is part of a family of 11 HDAC isoforms that are expressed across all major organ systems (85)) further complicating the development of selective small-molecule antagonists. In spite of this complexity, epigenetic modulators show some promise as treatments for depression. In animal models, systemically or locally administered HDAC inhibitors display antidepressant properties without obvious adverse effects on health (12, 79), suggesting that HDAC inhibitors may function by modulating a global acetylation/deacetylation balance across several brain regions. Of course, histone acetylation functions in concert with several other markers of gene repression and activation, including histone methylation, phosphorylation, sumoylation and ubiquitination (85). Thus, to comprehensively describe and appreciate the intricacies of depression-related epigenetic plasticity, we can expect a continued evolution in molecular and bioinformatic techniques. Rather than examining candidate genes like BDNF or GR, the field has begun to transition toward genome-wide approaches to studying chromatin regulation (47) shifting the focus from “epigenetic marks” to “epigenomic signatures.” As these technologies characterized in mouse and rat models begin to be applied to depressed human postmortem tissue (86), the ultimate goal would be to use transcriptional and epigenetic profiling as biomarkers to distinguish clinical categories of depressive illness, to determine responsivity to various antidepressant classes, or to differentiate treatment-sensitive from treatment-resistant illness. These profiles may offer new insights into subtype-specific pathophysiology and therapies and aid in the validation of our current animal models.
The Role of Dopaminergic Reward Circuits
The dramatic reinforcing properties of direct intracranial self-stimulation (ICSS) in rodents led to the appreciation of a series of subcortical regions critical for reward and appetitive behavior (87). The two main structures implicated by ICSS are the lateral hypothalamus and medial forebrain bundle, the latter containing ascending dopaminergic projections from the ventral tegmental area (VTA) to the nucleus accumbens (87). Under baseline conditions, VTA dopaminergic neurons oscillate between tonic (low frequency regular action potentials) or phasic (bursts of action potentials) patterns of activity (88). Unexpected rewards produce a transient increase in phasic firing (encoding a “reward prediction error”), which is sufficient to reinforce antecedent behaviors (24). All major classes of abused drugs appear to “signal” a reward, at least in part, by artificially enhancing dopamine transmission in the nucleus accumbens (such as cocaine, which blocks the dopamine transporter) (87).
Given depression’s prominent features of anhedonia and appetite alterations, this circuit has become an obvious focus of attention for basic molecular and electrophysiological studies. In rodents, long-term antidepressant administration reduces the firing rates of VTA dopamine neurons (89). In contrast, psychosocial stressors activate VTA firing and increase nucleus accumbens dopamine levels (13, 49, 90), and this may represent a positive coping strategy to enhance motivation during stressful situations (87). One mechanism for this enhanced VTA excitability may be the reduced activation of the protein kinase AKT, which leads to reductions in local inhibitory neurotransmission (14). Variations in the neuroplastic adaptations expressed by these neurons may also contribute to individual differences in the responsiveness to stress. In the mouse social defeat paradigm, while stress-susceptible mice display enhanced VTA activity and subsequent BDNF release, stress-resilient mice overcome this excitability change by upregulating potassium channel subunits expressed by VTA dopamine neurons that maintain normal tonic firing rates (13, 43). Stress-induced increases in nucleus accumbens BDNF may mediate pathological reward learning, such that, following a series of aversive social encounters, the positive rewarding value of social interaction is now modified to have a negative valence (1). Enhanced mesolimbic dopaminergic signaling may explain the reported efficacy of anti-dopaminergic agents as adjunct antidepressants (91) and, by enhancing basal dopaminergic and BDNF signaling, this model may also explain the significant comorbidity of substance dependence and depressive disorders (92, 93).
Nucleus accumbens neurons, anatomically situated to integrate reward-related dopaminergic signals as well as glutamatergic input from the prefrontal cortex, hippocampus and amygdala (20), themselves display numerous stress- and antidepressant-induced changes (87). One such neuroplastic event is the modulation of CREB (cyclic AMP response element binding protein): while prolonged social isolation stress reduces CREB activity and generates a predominantly anxious phenotype (94), active stressors or drugs of abuse increase CREB activity and promote anhedonia to a host of natural and drug rewards (87). Neuroimaging studies with depressed humans show that quantitative indices of anhedonia are associated with reduced nucleus accumbens volume (95) as well as hypoactivation during simple tasks of incentive reward (96, 97). In an attempt to reverse this nucleus accumbens hypoactivation, bilateral DBS to this and nearby brain region has been successfully applied to several cases of treatment-resistant depression (Figure 3). Consistent with its centralized location, responders to DBS displayed normalized PET indices of activity in the nucleus accumbens and the larger ventral striatum, while also reducing activity in the subgenual cingulate cortex and other prefrontal cortical regions (19, 20). In rats, DBS applied to the nucleus accumbens with simultaneous electrophysiological recordings from multiple distant sites has suggested that the therapeutically relevant effects of DBS are due to the synchronization of inhibition across a network of cortical and subcortical regions (98), possibly explaining anatomically distant effects of DBS. In this way, the application and validation of DBS in depression models offers opportunities to improve our circuit models (Figure 1) and shed light on the neurobiological correlates of treatment resistance.
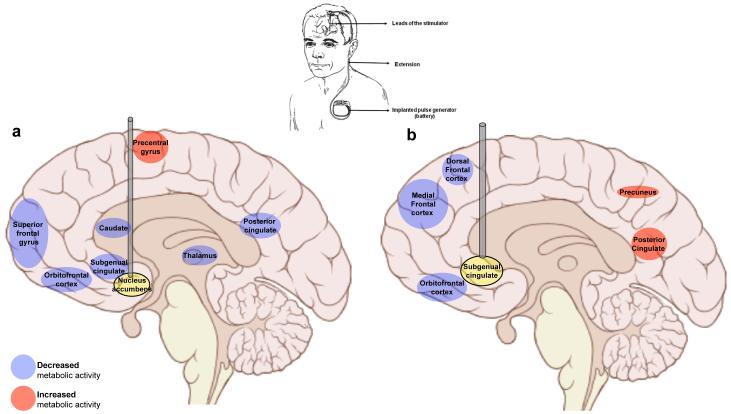
Figure 3. Effects of deep brain stimulation (DBS) on brain metabolic activity.
a) In treatment-resistant depression, DBS applied to the nucleus accumbens and nearby regions produces significant clinical improvement as well as changes in metabolic activity across an array of neural substrates (as measured by fluorodeoxyglucose positron emission tomography before and 6 months after DBS implantation) (18, 19). Among responders, reduced glucose metabolism (blue shading) was observed in the orbitofrontal cortex, superior frontal gyrus and posterior and subgenual cingulate cortical areas. b) The subgenual (also known as subcallosal) cingulate cortex is itself another DBS target for depression (17, 18), and post-DBS PET studies reveal similar patterns of decreases in frontal cortical metabolism. In addition, DBS applied here normalizes heightened blood flow to this region that is associated with depressive episodes (not shown). These data, together with results from rodent DBS studies (98), suggest that continuous DBS in these areas may alleviate severe depressive symptoms by enhancing inhibition across a circuit of cortical and subcortical structures.
Sex, Steroids and Immunity
The network of neural substrates involved in depression’s symptomatology displays a remarkable degree of plasticity to a host of peripherally derived chemical stimuli, and forwarding our understanding of the endocrinology and immunology of depression offers very exciting therapeutic avenues. Considerable research in the field has focused on a central role of a pathologically dysregulated HPA axis (1), whereby stress-induced hypercortisolemia leads to the central downregulation of GR impairing cortisol’s negative feedback, enhancing levels of CRF (corticotropin-releasing hormone) and ACTH (adrenocorticotrophic hormone) (35). This vicious cycle sustains elevated cortisol levels, possibly leading to hippocampal atrophy and reduced rates of neurogenesis, as well as predisposing depressed individuals to insulin resistance and abdominal obesity (99, 100). A large body of clinical and preclinical evidence supports this model. Depressed patients display dexamethasone non-suppression that is reversed by antidepressant treatment (101), enhanced cerebrospinal fluid levels of CRF (102) and alterations in diurnal cortisol rhythms (103). Mice that are treated chronically with glucocorticoids develop anhedonia in conjunction with other molecular correlates of depression (104). In line with these data, chronic glucocorticoid administration reduces hippocampal volume and impairs cognition in humans (105), while the GR antagonist mifepristone improves psychotic and depressive symptoms in patients with psychotic major depression (106). Antagonizing CRF signaling, particularly through the CRF1 receptor subtype, leads to strong anxiolytic effects in several rodent models (107). While the validation of CRF1 antagonists for depression and anxiety disorders remains an active area of clinical research, previously tested pharmacological prototypes have failed for a variety of reasons including off-target hepatoxicity (108, 109).
This “cortisol hypothesis” represents a vibrant part of the preclinical depression literature: with commercially available glucocorticoid immunoassays, experimental manipulations are often validated as being “prodepressant” or “antidepressant” depending on their effects on baseline or stress-induced glucocorticoid levels. However, several key points argue for a reappraisal of this practice: 1) true hypercortisolemia is rarely observed in outpatient depressed populations and may only be associated with depression severe enough to require hospitalization (110, 111); 2) depressed patients with atypical features and victims of post-traumatic stress tend to display hypocortisolemia (110, 112, 113); and 3) mice designed to display reduced central GR signaling (mimicking hypercortisolemic states) and those that centrally overexpress GR display identical behavioral and endocrinological phenotypes (114, 115). In spite of the strong immunosuppressant properties of glucocorticoids, levels of circulating pro-inflammatory cytokines (taken as a quantitative marker of systemic GR mediated signaling) are usually elevated in major depression (116), including IL-1 (interleukin-1), IL-6 and TNF-α (tumor necrosis factor α). These cytokines are themselves sufficient to impair GR signaling and, thus, rather than directly affecting HPA function, stress likely leads to glucocorticoid insufficiency through cytokine intermediates (101). Under certain circumstances, this reduced GR-mediated signaling may promote hypercortisolemia, severe insomnia and hypophagia (melancholic features), but in other conditions may lead to hypocortisolemia, hyperphagia and fatigue (atypical features). Cytokines themselves play powerful roles in depression-related neuroplasticity: chronic stress produces significant changes in immune function (117) and cytokines induce depression-like behavior when injected into rodents (118). IL-1β is one such cytokine: through the actions of the transcription factor NFκB (nuclear factor-κB), stress-induced increases in IL-1β lead to reductions in hippocampal neurogenesis and anhedonic phenotypes (119).
The greater female predisposition to depression, as well as its greater incidence in postpartum and perimenopausal periods, argue strongly for a thorough understanding of the role of gonadal hormones in affective regulation. The heightened female vulnerability to experience depressive episodes is limited to the post-pubertal and pre-menopausal period and, accordingly, much of the field’s emphasis has focused on the neurobiology of estrogen. Studies in rodent models have demonstrated that estrogen has antidepressant properties and also augments antidepressant actions of monoaminergic agents. Conversely, mice lacking aromatase (required for the generation of estrogenic steroids) or ERβ (estrogen receptor β) display aberrant stress-related behavior (120). Consistent with the broad central expression of ERβ, the antidepressant effects of estrogen signaling have been linked to several neurobiological substrates, including hippocampal neurogenesis, BDNF signaling, serotonergic neurotransmission and HPA axis function (121).
While this body of evidence may explain how significant fluctuations in hormone levels can trigger depressive episodes, it does not account for the heightened female vulnerability to depression, which is likely as much about female vulnerability factors as it may be about male resiliency factors to depressogenic stimuli. For instance, in comparison to males, female rodents display passive coping strategies and a more pronounced HPA axis activation to a variety of stressors. These features can be “masculinized” by providing testosterone during puberty, demonstrating how gender differences in behavioral physiology can be hardwired during certain critical periods (122). Ovariectomy also promotes active stress-coping, an effect that may be related to estrogen signaling within the nucleus accumbens (123). Aside from hormonal influences, it is important to recognize that gender differences also likely arise from numerous genes on sex chromosomes that are unrelated to gonadal function. Through standard genetic engineering techniques, one can create mice that are chromosomally male (i.e., XY) while having female gonads, and vice versa (Figure 4). Studies with this model have shown that while the development and maturation of male copulatory behaviors and sexually dimorphic brain structures are dependent on gonadal output, other genes on sex chromosomes independently drive other behavioral traits that are relevant to depression, including habit formation, parental and aggressive behaviors and social interaction (124, 125).
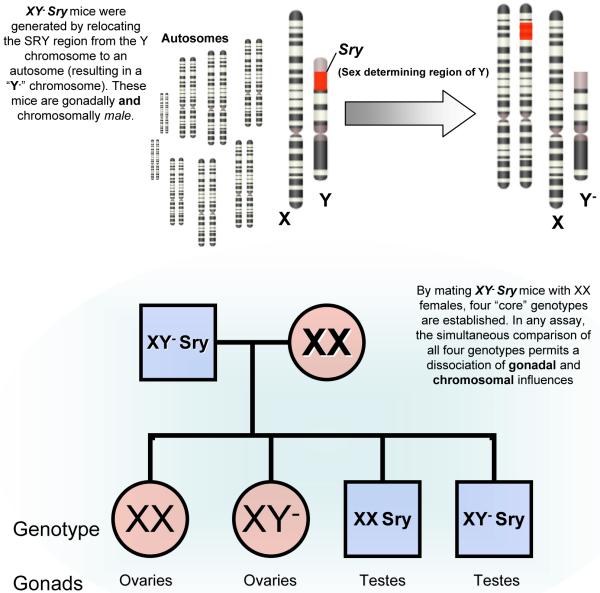
Figure 4. A genetic model to study gender differences in depression-related behavior.
The neurobiology underlying the greater female vulnerability to depression (or the relative resilience in males) remains largely unknown. The vast majority of research in the field has focused on how gonadal hormones affect stress-related behavior (i.e., estrogens, progesterones, and testosterones). However, studies using the “four core genotypes” (FCG) model shown here have illustrated that important sexually dimorphic anatomic and behavioral traits are unrelated to gonadal output and are localized to the many genes contained on sex chromosomes. The FCG mouse model was developed following a spontaneous mutation resulting in the loss of SRY (sex determining region of Y) from the Y chromosome, resulting in gonadal females. A functional SRY transgene was inserted into an autosome rescuing these mice back to gonadal males (“XY− Sry”). Mating these males with XX females results in the four core genotypes, comparisons between which have allowed for a dissociation of chromosomal and gonadal mediators of a variety of behavioral and physiological phenotypes (124). Studies utilizing the FCG mouse model generally employ gonadectomy to control for confounds related to menstrual cycling in gonadal females.
Mediators of Energy Homeostasis
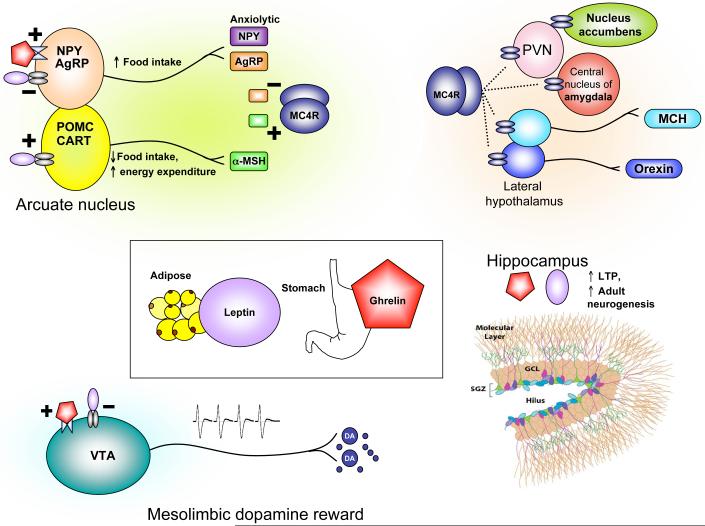
The appetite and metabolic abnormalities associated with depression and depression-related entities range from severe hypophagia and anorexia to binge-eating and obesity. A thorough understanding of such complex phenomena requires knowledge about physiological mechanisms of energy homeostasis, which refers to processes that maintain equilibrium between caloric intake and energy expenditure. In mammals, this is achieved largely through the action of circulating hormones that relay information about peripheral energy levels to the brain (126). Two such hormones that have received tremendous attention are leptin and ghrelin (Figure 5). Leptin is synthesized in white adipose tissue and is secreted in times of nutritional excess. Many obese individuals display a hyperleptinemia associated with central leptin resistance (127). In contrast, ghrelin is synthesized by gastric fundus cells and released during times of energy scarcity, and its secretion stimulates caloric intake and energy storage (126). The principal homeostatic site of action of leptin and ghrelin is the hypothalamic arcuate nucleus, where they exert anorexigenic or orexigenic effects, respectively, through a biologically elegant system of neuropeptides. Interestingly, receptors for leptin and ghrelin, as well as receptors for other feeding peptides such as MCH (melanin concentrating hormone), NPY (neuropeptide Y), AgRP (agouti-related peptide), α-MSH (α-melanocyte stimulating hormone) and orexin (hypocretin) are expressed in several depression-related limbic substrates. In rodents, chronic stress decreases serum leptin levels (128) and increases serum ghrelin (45). The systemic administration of either hormone produces antidepressant effects on the FST, enhances hippocampal neurogenesis, and improves learning and memory in behavioral and cellular (i.e., LTP or long-term potentiation) assays (45, 128-132). In contrast to their identical actions in hippocampus, dopaminergic neurons of the VTA are excited by ghrelin and inhibited by leptin (133-135), illustrating how their hypothalamic effects on appetite are complemented in the VTA through opposite modulation of reward sensitivity.
Figure 5. Mediators of energy homeostasis as targets for metabolic and affective illness.
Leptin (synthesized by white adipose tissue) and ghrelin (synthesized in the stomach) are two canonical examples of how endocrine hormones that signal information about peripheral energy state can also exert effects on depression-related behaviors. Leptin and ghrelin receptors are expressed in the hypothalamic arcuate nucleus, which contains two main types of neurons defined by their neuropeptides. These two neuronal types differentially express neuropeptides which act on the same melanocortin receptor (MC4R) with opposing effects: AgRP (an endogenous antagonist) and α-MSH (an endogenous agonist). (α-MSH is a product of the pro-opiomelanocortin or POMC gene.) AgRP neurons also express NPY, while α-MSH neurons also express CART (cocaine- and amphetamine-regulated transcript, named for its original identification). NPY and AgRP are orexigenic, while α-MSH and CART are anorexigenic. Leptin reduces food intake and increases energy expenditure by inhibiting NPY/AgRP-releasing neurons and exciting α-MSH/CART neurons. Ghrelin acts by promoting the release of NPY and AgRP. MC4Rs are expressed widely in the brain, including in the paraventricular hypothalamic nucleus (PVN, influencing the release of numerous neuropeptides including corticotropin-releasing hormone and thyrotropin-releasing hormone), the lateral hypothalamus (containing MCH- and orexin-secreting neurons which regulate food intake and arousal) as well as extrahypothalamic sites such as the nucleus accumbens and amygdala (where they are thought to influence mood regulation) (50, 139). Synthetic antagonists of MC4R are antidepressant and anxiolytic (136), as are agonists of NPY (121). In the VTA, direct infusion of leptin or ghrelin exert opposing effects on food intake through their contrasting effects on dopaminergic neuronal firing. Their VTA actions are believed to control the motivational or hedonic aspects of food intake (135) and are likely altered in other reward-related disorders such as depression and substance dependence. In contrast to the VTA, leptin and ghrelin appear to have identical effects on hippocampal plasticity: they both positively modulate LTP (long-term potentiation, an electrophysiological assay for activity-dependent synapse strengthening) as well as enhance adult hippocampal neurogenesis through receptors expressed on hippocampal progenitor cells. Abbreviations: NPY (neuropeptide Y), AgRP (agouti-related peptide), α-MSH (α-melanocyte stimulating hormone), PVN (paraventricular [hypothalamic] nucleus), MCH (melanin concentrating hormone), SGZ (subgranular zone), GCL (granule cell layer), VTA (ventral tegmental area), DA (dopamine).
In addition to persistent deficits in social interaction and anhedonia, mice subjected to chronic social defeat stress display an initial weight loss followed by a prolonged hyperphagic phase during which they rapidly regain their body weight and eventually gain more weight compared to control or stress-resilient animals. This phenomenon is at least partially mediated by both reduced serum leptin and central leptin resistance, which ultimately weakens central melanocortinergic signaling, i.e., through the MC4 receptor (Figure 5). This hypoleptinemia seems to be mediated by enhanced β3-adrenergic signaling, which promotes sympathetically-mediated lipolysis. β3-adrenergic antagonists co-administered during social defeat prevent the weight gain and reduced leptin, but worsen social deficits (50), suggesting that enhanced β3-adrenergic signaling has an adaptive function at the expense of metabolic derangements.
In this way, understanding the hedonic impact of homeostatic signals provides numerous targets for pharmaceutical development in depressive disorders, particularly in those cases associated with significant metabolic abnormalities. An obvious example would be in cases of HIV- or cancer-related cachexia, where artificially enhancing ghrelin or attenuating melanocortin signaling would have therapeutic hyperphagic and antidepressant effects. Non-peptide antagonists of the MC4 receptor have already been shown to exert antidepressant and anxiolytic effects in animal models (136). Conversely, patients with comorbid depression and obesity (137) might benefit from therapies designed to alleviate central leptin resistance (a challenging objective). Elucidating such therapies will require a deep understanding of the anatomy and physiology of leptin receptor signaling, and that of numerous other feeding peptides, which remains an exciting area of active research.
Conclusions and Future Perspectives
Today’s approaches to dissect the neurobiology of depression employ an unprecedented array of experimental techniques in humans and animals, including genome-wide DNA sequencing, chromatin immunoprecipitation to study epigenetic factors, functional brain imaging, optogenetic electrophysiological tools, viral-mediated gene transfer and an impressive assortment of genetic mutant mice. The list of molecular players involved in depression’s phenotypes has now expanded to include genes from diverse aspects of cellular physiology such as numerous neurotransmitter and neuropeptide systems, steroid hormones, neurotrophic and cytokine signaling cascades, ion channels, HDACs, circadian genes (87, 107), transcription factors (e.g., CREB NFκB, and ΔFosB (42)) and p11, among many others. Most of the new targets are derived from experiments in rodents and, while these studies are scientifically sound, the targets themselves may or may not be therapeutically relevant or feasible for human depression. Beyond the synthetic obstacles of designing safe and effective small-molecule modulators or viral vectors for use in depressed humans, a key challenge will be to prioritize these targets and develop collaborative efforts to rule them in or out at a reasonable pace.
A large and unacceptable divide continues to exist between animal studies and human clinical investigation. An important example is our appreciation of cortical contributions to depression: while human neuroimaging studies repeatedly implicate cortical subregions such as the subgenual cingulate or orbitofrontal cortex, the vast majority of rodent studies limit their analyses to the hippocampus or amygdala. While studying cortical circuits in rodents is more challenging, the human findings clearly demonstrate the high priority for this work. Many reports in the basic literature focus on drastic behavioral and neurobiological phenotypes in constitutive knockout mice, even though homozygous human “knockouts” presumably are a negligible contribution to clinical depression. As progress in delineating the genetics of depression continues, it will be crucial to complement knockout studies by examining molecular and epigenetic mechanisms underlying individual variability and understanding the cellular and physiological consequences of psychiatrically relevant human SNPs. Finally, pathological validation using postmortem brain tissue provides a crucial link between our inherently limited laboratory models and the molecular enigmas of human depression.
Human studies must also mature. Observational reports that measure serum BDNF or glucocorticoid levels can expand to include multiple measures such as serum leptin, ghrelin, and thyroid hormone, and metabolic status, to name just a few, as well as segregating patients into depressive subtypes. Brain imaging experiments continue to largely focus on volume or activity measures of particular brain regions or on monoamine receptor/transporter occupancy. It is essential to vastly expand the range of proteins that can be assessed in the living brain so that proteins that are at the heart of pathophysiological models in rodents can at last be analyzed in depressed patients. As informed clinicians and scientists in the field, we have a responsibility to expand the horizon of our investigations and constantly reassess our analytic methods and theoretical paradigms. We should look well beyond monoamines, cortisol, BDNF and the hippocampus to determine tomorrow’s novel medical and surgical therapeutic avenues for depression.
Acknowledgements
Preparation of this review was supported by grants from the National Institute of Mental Health. EJN reports consulting income from Merck Research Laboratories and PsychoGenics, and a research alliance with AstraZeneca (2007-2009). VK thanks UT Southwestern’s Medical Scientist Training Program for their support and mentorship.
References
- 1.Krishnan V, Nestler EJ. The Molecular Neurobiology of Depression. Nature. 2008;455(7215):894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O’Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P, Jr., Valvo WJ. Mood Disorders in the Medically Ill: Scientific Review and Recommendations. Biol Psychiatry. 2005;58(3):175–89. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Lopez AD, Murray CC. The Global Burden of Disease, 1990-2020. Nat Med. 1998;4(11):1241–3. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- 4.Diagnostic and Statistical Manual of Mental Disorders. 4th Edition American Psychiatric Publishing; Washington, D.C.: 2000. [Google Scholar]
- 5.Hercher C, Turecki G, Mechawar N. Through the Looking Glass: Examining Neuroanatomical Evidence for Cellular Alterations in Major Depression. J Psychiatr Res. 2009;43(11):947–61. doi: 10.1016/j.jpsychires.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Karege F, Vaudan G, Schwald M, Perroud N, Harpe R. Neurotrophin Levels in Postmortem Brains of Suicide Victims and the Effects of Antemortem Diagnosis and Psychotropic Drugs. Brain Res Mol Brain Res. 2005;136(1-2):29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 7.Soetanto A, Wilson RS, Talbot K, Un A, Schneider JA, Sobiesk M, Kelly J, Leurgans S, Bennett DA, Arnold SE. Association of Anxiety and Depression with Microtubule-Associated Protein 2- and Synaptopodin-Immunolabeled Dendrite and Spine Densities in Hippocampal Ca3 of Older Humans. Arch Gen Psychiatry. 2010;67(5):448–57. doi: 10.1001/archgenpsychiatry.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savitz J, Drevets WC. Bipolar and Major Depressive Disorder: Neuroimaging the Developmental-Degenerative Divide. Neurosci Biobehav Rev. 2009;33(5):699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Videbech P, Ravnkilde B. Hippocampal Volume and Depression: A Meta-Analysis of Mri Studies. Am J Psychiatry. 2004;161(11):1957–66. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 10.Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, Schatzberg AF, Myers RM, Akil H, Watson SJ. Altered Expression of Glutamate Signaling, Growth Factor, and Glia Genes in the Locus Coeruleus of Patients with Major Depression. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sibille E, Wang Y, Joeyen-Waldorf J, Gaiteri C, Surget A, Oh S, Belzung C, Tseng GC, Lewis DA. A Molecular Signature of Depression in the Amygdala. Am J Psychiatry. 2009;166(9):1011–24. doi: 10.1176/appi.ajp.2009.08121760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Covington HE, 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, Fass DM, Renthal W, Rush AJ, 3rd, Wu EY, Ghose S, Krishnan V, Russo SJ, Tamminga C, Haggarty SJ, Nestler EJ. Antidepressant Actions of Histone Deacetylase Inhibitors. J Neurosci. 2009;29(37):11451–60. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular Adaptations Underlying Susceptibility and Resistance to Social Defeat in Brain Reward Regions. Cell. 2007;131(2):391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan V, Han MH, Mazei-Robison M, Iniguez SD, Ables JL, Vialou V, Berton O, Ghose S, Covington HE, 3rd, Wiley MD, Henderson RP, Neve RL, Eisch AJ, Tamminga CA, Russo SJ, Bolanos CA, Nestler EJ. Akt Signaling within the Ventral Tegmental Area Regulates Cellular and Behavioral Responses to Stressful Stimuli. Biol Psychiatry. 2008;64(8):691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, Vaugeois JM, Nomikos GG, Greengard P. Alterations in 5-Ht1b Receptor Function by P11 in Depression-Like States. Science. 2006;311(5757):77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- 16.Ressler KJ, Mayberg HS. Targeting Abnormal Neural Circuits in Mood and Anxiety Disorders: From the Laboratory to the Clinic. Nat Neurosci. 2007;10(9):1116–24. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep Brain Stimulation for Treatment-Resistant Depression. Neuron. 2005;45(5):651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal Cingulate Gyrus Deep Brain Stimulation for Treatment-Resistant Depression. Biol Psychiatry. 2008;64(6):461–7. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 19.Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, Axmacher N, Lemke M, Cooper-Mahkorn D, Cohen MX, Brockmann H, Lenartz D, Sturm V, Schlaepfer TE. Nucleus Accumbens Deep Brain Stimulation Decreases Ratings of Depression and Anxiety in Treatment-Resistant Depression. Biol Psychiatry. 2010;67(2):110–6. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V. Deep Brain Stimulation to Reward Circuitry Alleviates Anhedonia in Refractory Major Depression. Neuropsychopharmacology. 2008;33(2):368–77. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 21.Malone DA, Jr., Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, Rauch SL, Rasmussen SA, Machado AG, Kubu CS, Tyrka AR, Price LH, Stypulkowski PH, Giftakis JE, Rise MT, Malloy PF, Salloway SP, Greenberg BD. Deep Brain Stimulation of the Ventral Capsule/Ventral Striatum for Treatment-Resistant Depression. Biol Psychiatry. 2009;65(4):267–75. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross M, Nakamura L, Pascual-Leone A, Fregni F. Has Repetitive Transcranial Magnetic Stimulation (Rtms) Treatment for Depression Improved? A Systematic Review and Meta-Analysis Comparing the Recent Vs. The Earlier Rtms Studies. Acta Psychiatr Scand. 2007;116(3):165–73. doi: 10.1111/j.1600-0447.2007.01049.x. [DOI] [PubMed] [Google Scholar]
- 23.Montgomery EB, Jr., Gale JT. Mechanisms of Action of Deep Brain Stimulation(Dbs) Neurosci Biobehav Rev. 2008;32(3):388–407. doi: 10.1016/j.neubiorev.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic Firing in Dopaminergic Neurons Is Sufficient for Behavioral Conditioning. Science. 2009;324(5930):1080–4. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish National Twin Study of Lifetime Major Depression. Am J Psychiatry. 2006;163(1):109–14. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- 26.Shyn SI, Hamilton SP. The Genetics of Major Depression: Moving Beyond the Monoamine Hypothesis. Psychiatr Clin North Am. 2010;33(1):125–40. doi: 10.1016/j.psc.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Leon S, Janssens AC, Gonzalez-Zuloeta Ladd AM, Del-Favero J, Claes SJ, Oostra BA, van Duijn CM. Meta-Analyses of Genetic Studies on Major Depressive Disorder. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002088. [DOI] [PubMed] [Google Scholar]
- 28.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M. Evaluation of Outcomes with Citalopram for Depression Using Measurement-Based Care in Star*D: Implications for Clinical Practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 29.Garriock HA, Kraft JB, Shyn SI, Peters EJ, Yokoyama JS, Jenkins GD, Reinalda MS, Slager SL, McGrath PJ, Hamilton SP. A Genomewide Association Study of Citalopram Response in Major Depressive Disorder. Biol Psychiatry. 2010;67(2):133–8. doi: 10.1016/j.biopsych.2009.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uher R, Huezo-Diaz P, Perroud N, Smith R, Rietschel M, Mors O, Hauser J, Maier W, Kozel D, Henigsberg N, Barreto M, Placentino A, Dernovsek MZ, Schulze TG, Kalember P, Zobel A, Czerski PM, Larsen ER, Souery D, Giovannini C, Gray JM, Lewis CM, Farmer A, Aitchison KJ, McGuffin P, Craig I. Genetic Predictors of Response to Antidepressants in the Gendep Project. Pharmacogenomics J. 2009;9(4):225–33. doi: 10.1038/tpj.2009.12. [DOI] [PubMed] [Google Scholar]
- 31.Smith DF, Jakobsen S. Molecular Tools for Assessing Human Depression by Positron Emission Tomography. Eur Neuropsychopharmacol. 2009;19(9):611–28. doi: 10.1016/j.euroneuro.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Ruhe HG, Mason NS, Schene AH. Mood Is Indirectly Related to Serotonin, Norepinephrine and Dopamine Levels in Humans: A Meta-Analysis of Monoamine Depletion Studies. Mol Psychiatry. 2007;12(4):331–59. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- 33.Muglia P, Tozzi F, Galwey NW, Francks C, Upmanyu R, Kong XQ, Antoniades A, Domenici E, Perry J, Rothen S, Vandeleur CL, Mooser V, Waeber G, Vollenweider P, Preisig M, Lucae S, Muller-Myhsok B, Holsboer F, Middleton LT, Roses AD. Genome-Wide Association Study of Recurrent Major Depressive Disorder in Two European Case-Control Cohorts. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.131. [DOI] [PubMed] [Google Scholar]
- 34.Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the Serotonin Transporter Gene (5-Httlpr), Stressful Life Events, and Risk of Depression: A Meta-Analysis. Jama. 2009;301(23):2462–71. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of Depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 36.Zarate CA, Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A Randomized Trial of an N-Methyl-D-Aspartate Antagonist in Treatment-Resistant Major Depression. Arch Gen Psychiatry. 2006;63(8):856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 37.Cryan JF, Slattery DA. Animal Models of Mood Disorders: Recent Developments. Curr Opin Psychiatry. 2007;20(1):1–7. doi: 10.1097/YCO.0b013e3280117733. [DOI] [PubMed] [Google Scholar]
- 38.Kendler KS, Karkowski LM, Prescott CA. Causal Relationship between Stressful Life Events and the Onset of Major Depression. Am J Psychiatry. 1999;156(6):837–41. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- 39.Sloman L. A New Comprehensive Evolutionary Model of Depression and Anxiety. J Affect Disord. 2008;106(3):219–28. doi: 10.1016/j.jad.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 40.Nesse RM. Is Depression an Adaptation? Arch Gen Psychiatry. 2000;57(1):14–20. doi: 10.1001/archpsyc.57.1.14. [DOI] [PubMed] [Google Scholar]
- 41.Watson PJ, Andrews PW. Toward a Revised Evolutionary Adaptationist Analysis of Depression: The Social Navigation Hypothesis. J Affect Disord. 2002;72(1):1–14. doi: 10.1016/s0165-0327(01)00459-1. [DOI] [PubMed] [Google Scholar]
- 42.Berton O, Covington HE, 3rd, Ebner K, Tsankova NM, Carle TL, Ulery P, Bhonsle A, Barrot M, Krishnan V, Singewald GM, Singewald N, Birnbaum S, Neve RL, Nestler EJ. Induction of Deltafosb in the Periaqueductal Gray by Stress Promotes Active Coping Responses. Neuron. 2007;55(2):289–300. doi: 10.1016/j.neuron.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 43.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential Role of Bdnf in the Mesolimbic Dopamine Pathway in Social Defeat Stress. Science. 2006;311(5762):864–8. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 44.Lutter M, Krishnan V, Russo SJ, Jung S, McClung CA, Nestler EJ. Orexin Signaling Mediates the Antidepressant-Like Effect of Calorie Restriction. J Neurosci. 2008;28(12):3071–5. doi: 10.1523/JNEUROSCI.5584-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM. The Orexigenic Hormone Ghrelin Defends against Depressive Symptoms of Chronic Stress. Nat Neurosci. 2008;11(7):752–3. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained Hippocampal Chromatin Regulation in a Mouse Model of Depression and Antidepressant Action. Nat Neurosci. 2006;9(4):519–25. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 47.Wilkinson MB, Xiao G, Kumar A, LaPlant Q, Renthal W, Sikder D, Kodadek TJ, Nestler EJ. Imipramine Treatment and Resiliency Exhibit Similar Chromatin Regulation in the Mouse Nucleus Accumbens in Depression Models. J Neurosci. 2009;29(24):7820–32. doi: 10.1523/JNEUROSCI.0932-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Renthal W, Maze I, Krishnan V, Covington HE, 3rd, Xiao G, Kumar A, Russo SJ, Graham A, Tsankova N, Kippin TE, Kerstetter KA, Neve RL, Haggarty SJ, McKinsey TA, Bassel-Duby R, Olson EN, Nestler EJ. Histone Deacetylase 5 Epigenetically Controls Behavioral Adaptations to Chronic Emotional Stimuli. Neuron. 2007;56(3):517–29. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 49.Anstrom KK, Miczek KA, Budygin EA. Increased Phasic Dopamine Signaling in the Mesolimbic Pathway During Social Defeat in Rats. Neuroscience. 2009;161(1):3–12. doi: 10.1016/j.neuroscience.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chuang JC, Krishnan V, Yu HG, Mason B, Cui H, Wilkinson MB, Zigman JM, Elmquist JK, Nestler EJ, Lutter M. A Beta(3)-Adrenergic-Leptin-Melanocortin Circuit Regulates Behavioral and Metabolic Changes Induced by Chronic Stress. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coryell MW, Wunsch AM, Haenfler JM, Allen JE, Schnizler M, Ziemann AE, Cook MN, Dunning JP, Price MP, Rainier JD, Liu Z, Light AR, Langbehn DR, Wemmie JA. Acid-Sensing Ion Channel-1a in the Amygdala, a Novel Therapeutic Target in Depression-Related Behavior. J Neurosci. 2009;29(17):5381–8. doi: 10.1523/JNEUROSCI.0360-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, 3rd, Welsh MJ, Wemmie JA. The Amygdala Is a Chemosensor That Detects Carbon Dioxide and Acidosis to Elicit Fear Behavior. Cell. 2009;139(5):1012–21. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heurteaux C, Lucas G, Guy N, El Yacoubi M, Thummler S, Peng XD, Noble F, Blondeau N, Widmann C, Borsotto M, Gobbi G, Vaugeois JM, Debonnel G, Lazdunski M. Deletion of the Background Potassium Channel Trek-1 Results in a Depression-Resistant Phenotype. Nat Neurosci. 2006;9(9):1134–41. doi: 10.1038/nn1749. [DOI] [PubMed] [Google Scholar]
- 54.Mazella J, Petrault O, Lucas G, Deval E, Beraud-Dufour S, Gandin C, El-Yacoubi M, Widmann C, Guyon A, Chevet E, Taouji S, Conductier G, Corinus A, Coppola T, Gobbi G, Nahon JL, Heurteaux C, Borsotto M. Spadin, a Sortilin-Derived Peptide, Targeting Rodent Trek-1 Channels: A New Concept in the Antidepressant Drug Design. PLoS Biol. 2010;8(4):e1000355. doi: 10.1371/journal.pbio.1000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kempermann G. The Neurogenic Reserve Hypothesis: What Is Adult Hippocampal Neurogenesis Good For? Trends Neurosci. 2008 doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Sahay A, Hen R. Adult Hippocampal Neurogenesis in Depression. Nat Neurosci. 2007;10(9):1110–5. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 57.Eisch AJ, Cameron HA, Encinas JM, Meltzer LA, Ming GL, Overstreet-Wadiche LS. Adult Neurogenesis, Mental Health, and Mental Illness: Hope or Hype? J Neurosci. 2008;28(46):11785–91. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-Speed Imaging Reveals Neurophysiological Links to Behavior in an Animal Model of Depression. Science. 2007;317(5839):819–23. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- 59.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of Hippocampal Neurogenesis for the Behavioral Effects of Antidepressants. Science. 2003;301(5634):805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 60.Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C. Drug-Dependent Requirement of Hippocampal Neurogenesis in a Model of Depression and of Antidepressant Reversal. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 61.Couillard-Despres S, Wuertinger C, Kandasamy M, Caioni M, Stadler K, Aigner R, Bogdahn U, Aigner L. Ageing Abolishes the Effects of Fluoxetine on Neurogenesis. Mol Psychiatry. 2009;14(9):856–64. doi: 10.1038/mp.2008.147. [DOI] [PubMed] [Google Scholar]
- 62.David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R. Neurogenesis-Dependent and -Independent Effects of Fluoxetine in an Animal Model of Anxiety/Depression. Neuron. 2009;62(4):479–93. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tfilin M, Sudai E, Merenlender A, Gispan I, Yadid G, Turgeman G. Mesenchymal Stem Cells Increase Hippocampal Neurogenesis and Counteract Depressive-Like Behavior. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.110. [DOI] [PubMed] [Google Scholar]
- 64.Lagace DC, Donovan MH, Decarolis NA, Farnbach LA, Malhotra S, Berton O, Nestler EJ, Krishnan V, Eisch AJ. Adult Hippocampal Neurogenesis Is Functionally Important for Stress-Induced Social Avoidance. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0910072107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pittenger C, Duman RS. Stress, Depression, and Neuroplasticity: A Convergence of Mechanisms. Neuropsychopharmacology. 2008;33(1):88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 66.Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. Trkb Regulates Hippocampal Neurogenesis and Governs Sensitivity to Antidepressive Treatment. Neuron. 2008;59(3):399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ. Brain-Derived Neurotrophic Factor Conditional Knockouts Show Gender Differences in Depression-Related Behaviors. Biol Psychiatry. 2007;61(2):187–97. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 68.Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of Brain-Derived Neurotrophic Factor in Specific Brain Sites Precipitates Behaviors Associated with Depression and Reduces Neurogenesis. Mol Psychiatry. 2010;15(1):80–92. doi: 10.1038/mp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, Chattarji S. Transgenic Brain-Derived Neurotrophic Factor Expression Causes Both Anxiogenic and Antidepressant Effects. Proc Natl Acad Sci U S A. 2006;103(35):13208–13. doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-Derived Neurotrophic Factor Produces Antidepressant Effects in Behavioral Models of Depression. J Neurosci. 2002;22(8):3251–61. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoshaw BA, Malberg JE, Lucki I. Central Administration of Igf-I and Bdnf Leads to Long-Lasting Antidepressant-Like Effects. Brain Res. 2005;1037(1-2):204–8. doi: 10.1016/j.brainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 72.Kato M, Serretti A. Review and Meta-Analysis of Antidepressant Pharmacogenetic Findings in Major Depressive Disorder. Mol Psychiatry. 2010;15(5):473–500. doi: 10.1038/mp.2008.116. [DOI] [PubMed] [Google Scholar]
- 73.Verhagen M, van der Meij A, van Deurzen PA, Janzing JG, Arias-Vasquez A, Buitelaar JK, Franke B. Meta-Analysis of the Bdnf Val66met Polymorphism in Major Depressive Disorder: Effects of Gender and Ethnicity. Mol Psychiatry. 2010;15(3):260–71. doi: 10.1038/mp.2008.109. [DOI] [PubMed] [Google Scholar]
- 74.Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ. Safety and Tolerability of Gene Therapy with an Adeno-Associated Virus (Aav) Borne Gad Gene for Parkinson’s Disease: An Open Label, Phase I Trial. Lancet. 2007;369(9579):2097–105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 75.Croll SD, Chesnutt CR, Greene NA, Lindsay RM, Wiegand SJ. Peptide Immunoreactivity in Aged Rat Cortex and Hippocampus as a Function of Memory and Bdnf Infusion. Pharmacol Biochem Behav. 1999;64(3):625–35. doi: 10.1016/s0091-3057(99)00122-7. [DOI] [PubMed] [Google Scholar]
- 76.Sniderhan LF, Garcia-Bates TM, Burgart M, Bernstein SH, Phipps RP, Maggirwar SB. Neurotrophin Signaling through Tropomyosin Receptor Kinases Contributes to Survival and Proliferation of Non-Hodgkin Lymphoma. Exp Hematol. 2009;37(11):1295–309. doi: 10.1016/j.exphem.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Caspi A, Moffitt TE. Gene-Environment Interactions in Psychiatry: Joining Forces with Neuroscience. Nat Rev Neurosci. 2006;7(7):583–90. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- 78.Mill J, Petronis A. Molecular Studies of Major Depressive Disorder: The Epigenetic Perspective. Mol Psychiatry. 2007;12(9):799–814. doi: 10.1038/sj.mp.4001992. [DOI] [PubMed] [Google Scholar]
- 79.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic Regulation in Psychiatric Disorders. Nat Rev Neurosci. 2007;8(5):355–67. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 80.Gluckman PD, Hanson MA, Buklijas T, Low FM, Beedle AS. Epigenetic Mechanisms That Underpin Metabolic and Cardiovascular Diseases. Nat Rev Endocrinol. 2009;5(7):401–8. doi: 10.1038/nrendo.2009.102. [DOI] [PubMed] [Google Scholar]
- 81.Graff J, Mansuy IM. Epigenetic Dysregulation in Cognitive Disorders. Eur J Neurosci. 2009;30(1):1–8. doi: 10.1111/j.1460-9568.2009.06787.x. [DOI] [PubMed] [Google Scholar]
- 82.Sharma S, Kelly TK, Jones PA. Epigenetics in Cancer. Carcinogenesis. 2010;31(1):27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McGowan PO, Meaney MJ, Szyf M. Diet and the Epigenetic (Re)Programming of Phenotypic Differences in Behavior. Brain Res. 2008;1237:12–24. doi: 10.1016/j.brainres.2008.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OF, Spengler D. Dynamic DNA Methylation Programs Persistent Adverse Effects of Early-Life Stress. Nat Neurosci. 2009;12(12):1559–66. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- 85.Renthal W, Nestler EJ. Epigenetic Mechanisms in Drug Addiction. Trends Mol Med. 2008;14(8):341–50. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Akbarian S, Huang HS. Epigenetic Regulation in Human Brain-Focus on Histone Lysine Methylation. Biol Psychiatry. 2009;65(3):198–203. doi: 10.1016/j.biopsych.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nestler EJ, Carlezon WA., Jr. The Mesolimbic Dopamine Reward Circuit in Depression. Biol Psychiatry. 2006;59(12):1151–9. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 88.Schultz W. Getting Formal with Dopamine and Reward. Neuron. 2002;36(2):241–63. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 89.Dremencov E, El Mansari M, Blier P. Effects of Sustained Serotonin Reuptake Inhibition on the Firing of Dopamine Neurons in the Rat Ventral Tegmental Area. J Psychiatry Neurosci. 2009;34(3):223–9. [PMC free article] [PubMed] [Google Scholar]
- 90.Tidey JW, Miczek KA. Social Defeat Stress Selectively Alters Mesocorticolimbic Dopamine Release: An in Vivo Microdialysis Study. Brain Res. 1996;721(1-2):140–9. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- 91.Nelson JC, Papakostas GI. Atypical Antipsychotic Augmentation in Major Depressive Disorder: A Meta-Analysis of Placebo-Controlled Randomized Trials. Am J Psychiatry. 2009;166(9):980–91. doi: 10.1176/appi.ajp.2009.09030312. [DOI] [PubMed] [Google Scholar]
- 92.Brady KT, Sinha R. Co-Occurring Mental and Substance Use Disorders: The Neurobiological Effects of Chronic Stress. Am J Psychiatry. 2005;162(8):1483–93. doi: 10.1176/appi.ajp.162.8.1483. [DOI] [PubMed] [Google Scholar]
- 93.Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic Bdnf Activity in Nucleus Accumbens with Cocaine Use Increases Self-Administration and Relapse. Nat Neurosci. 2007;10(8):1029–37. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- 94.Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iniguez SD, Cao JL, Kirk A, Chakravarty S, Kumar A, Krishnan V, Neve RL, Cooper DC, Bolanos CA, Barrot M, McClung CA, Nestler EJ. Creb Regulation of Nucleus Accumbens Excitability Mediates Social Isolation-Induced Behavioral Deficits. Nat Neurosci. 2009;12(2):200–9. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wacker J, Dillon DG, Pizzagalli DA. The Role of the Nucleus Accumbens and Rostral Anterior Cingulate Cortex in Anhedonia: Integration of Resting Eeg, Fmri, and Volumetric Techniques. Neuroimage. 2009;46(1):327–37. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, Dichter GS. Fmri of Alterations in Reward Selection, Anticipation, and Feedback in Major Depressive Disorder. J Affect Disord. 2009;118(1-3):69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced Caudate and Nucleus Accumbens Response to Rewards in Unmedicated Individuals with Major Depressive Disorder. Am J Psychiatry. 2009;166(6):702–10. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McCracken CB, Grace AA. Nucleus Accumbens Deep Brain Stimulation Produces Region-Specific Alterations in Local Field Potential Oscillations and Evoked Responses in Vivo. J Neurosci. 2009;29(16):5354–63. doi: 10.1523/JNEUROSCI.0131-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Golden SH. A Review of the Evidence for a Neuroendocrine Link between Stress, Depression and Diabetes Mellitus. Curr Diabetes Rev. 2007;3(4):252–9. doi: 10.2174/157339907782330021. [DOI] [PubMed] [Google Scholar]
- 100.McEwen BS. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 101.Pace TW, Miller AH. Cytokines and Glucocorticoid Receptor Signaling. Relevance to Major Depression. Ann N Y Acad Sci. 2009;1179:86–105. doi: 10.1111/j.1749-6632.2009.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated Concentrations of Csf Corticotropin-Releasing Factor-Like Immunoreactivity in Depressed Patients. Science. 1984;226(4680):1342–4. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 103.Keller J, Flores B, Gomez RG, Solvason HB, Kenna H, Williams GH, Schatzberg AF. Cortisol Circadian Rhythm Alterations in Psychotic Major Depression. Biol Psychiatry. 2006;60(3):275–81. doi: 10.1016/j.biopsych.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 104.Gourley SL, Wu FJ, Kiraly DD, Ploski JE, Kedves AT, Duman RS, Taylor JR. Regionally Specific Regulation of Erk Map Kinase in a Model of Antidepressant-Sensitive Chronic Depression. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brown ES. Effects of Glucocorticoids on Mood, Memory, and the Hippocampus. Treatment and Preventive Therapy. Ann N Y Acad Sci. 2009;1179:41–55. doi: 10.1111/j.1749-6632.2009.04981.x. [DOI] [PubMed] [Google Scholar]
- 106.Simpson GM, El Sheshai A, Loza N, Kingsbury SJ, Fayek M, Rady A, Fawzy W. An 8-Week Open-Label Trial of a 6-Day Course of Mifepristone for the Treatment of Psychotic Depression. J Clin Psychiatry. 2005;66(5):598–602. doi: 10.4088/jcp.v66n0509. [DOI] [PubMed] [Google Scholar]
- 107.Berton O, Nestler EJ. New Approaches to Antidepressant Drug Discovery: Beyond Monoamines. Nat Rev Neurosci. 2006;7(2):137–51. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 108.Holsboer F, Ising M. Central Crh System in Depression and Anxiety--Evidence from Clinical Studies with Crh1 Receptor Antagonists. Eur J Pharmacol. 2008;583(2-3):350–7. doi: 10.1016/j.ejphar.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 109.Rakofsky JJ, Holtzheimer PE, Nemeroff CB. Emerging Targets for Antidepressant Therapies. Curr Opin Chem Biol. 2009;13(3):291–302. doi: 10.1016/j.cbpa.2009.04.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brouwer JP, Appelhof BC, Hoogendijk WJ, Huyser J, Endert E, Zuketto C, Schene AH, Tijssen JG, Van Dyck R, Wiersinga WM, Fliers E. Thyroid and Adrenal Axis in Major Depression: A Controlled Study in Outpatients. Eur J Endocrinol. 2005;152(2):185–91. doi: 10.1530/eje.1.01828. [DOI] [PubMed] [Google Scholar]
- 111.Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, Smit JH, Zitman FG, Penninx BW. Major Depressive Disorder and Hypothalamic-Pituitary-Adrenal Axis Activity: Results from a Large Cohort Study. Arch Gen Psychiatry. 2009;66(6):617–26. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- 112.Stewart JW, Quitkin FM, McGrath PJ, Klein DF. Defining the Boundaries of Atypical Depression: Evidence from the Hpa Axis Supports Course of Illness Distinctions. J Affect Disord. 2005;86(2-3):161–7. doi: 10.1016/j.jad.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 113.Yehuda R. Status of Glucocorticoid Alterations in Post-Traumatic Stress Disorder. Ann N Y Acad Sci. 2009;1179:56–69. doi: 10.1111/j.1749-6632.2009.04979.x. [DOI] [PubMed] [Google Scholar]
- 114.Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y, Muglia LJ. Acquired Deficit of Forebrain Glucocorticoid Receptor Produces Depression-Like Changes in Adrenal Axis Regulation and Behavior. Proc Natl Acad Sci U S A. 2005;102(2):473–8. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wei Q, Lu XY, Liu L, Schafer G, Shieh KR, Burke S, Robinson TE, Watson SJ, Seasholtz AF, Akil H. Glucocorticoid Receptor Overexpression in Forebrain: A Mouse Model of Increased Emotional Lability. Proc Natl Acad Sci U S A. 2004;101(32):11851–6. doi: 10.1073/pnas.0402208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A Meta-Analysis of Cytokines in Major Depression. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 117.Miller AH, Maletic V, Raison CL. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biol Psychiatry. 2009;65(9):732–41. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as Mediators of Depression: What Can We Learn from Animal Studies? Neurosci Biobehav Rev. 2005;29(4-5):891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 119.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear Factor-{Kappa}B Is a Critical Mediator of Stress-Impaired Neurogenesis and Depressive Behavior. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Osterlund MK. Underlying Mechanisms Mediating the Antidepressant Effects of Estrogens. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbagen.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 121.Covington HE, 3rd, Vialou V, Nestler EJ. From Synapse to Nucleus: Novel Targets for Treating Depression. Neuropharmacology. 2009 doi: 10.1016/j.neuropharm.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goel N, Bale TL. Examining the Intersection of Sex and Stress in Modelling Neuropsychiatric Disorders. J Neuroendocrinol. 2009;21(4):415–20. doi: 10.1111/j.1365-2826.2009.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.LaPlant Q, Chakravarty S, Vialou V, Mukherjee S, Koo JW, Kalahasti G, Bradbury KR, Taylor SV, Maze I, Kumar A, Graham A, Birnbaum SG, Krishnan V, Truong HT, Neve RL, Nestler EJ, Russo SJ. Role of Nuclear Factor Kappab in Ovarian Hormone-Mediated Stress Hypersensitivity in Female Mice. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Arnold AP, Chen X. What Does The “Four Core Genotypes” Mouse Model Tell Us About Sex Differences in the Brain and Other Tissues? Front Neuroendocrinol. 2009;30(1):1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR. Sex Chromosome Complement Regulates Habit Formation. Nat Neurosci. 2007;10(11):1398–400. doi: 10.1038/nn1994. [DOI] [PubMed] [Google Scholar]
- 126.Lutter M, Nestler EJ. Homeostatic and Hedonic Signals Interact in the Regulation of Food Intake. J Nutr. 2009;139(3):629–32. doi: 10.3945/jn.108.097618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zigman JM, Elmquist JK. Minireview: From Anorexia to Obesity--the Yin and Yang of Body Weight Control. Endocrinology. 2003;144(9):3749–56. doi: 10.1210/en.2003-0241. [DOI] [PubMed] [Google Scholar]
- 128.Lu XY, Kim CS, Frazer A, Zhang W. Leptin: A Potential Novel Antidepressant. Proc Natl Acad Sci U S A. 2006;103(5):1593–8. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Garza JC, Guo M, Zhang W, Lu XY. Leptin Increases Adult Hippocampal Neurogenesis in Vivo and in Vitro. J Biol Chem. 2008;283(26):18238–47. doi: 10.1074/jbc.M800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Moon M, Kim S, Hwang L, Park S. Ghrelin Regulates Hippocampal Neurogenesis in Adult Mice. Endocr J. 2009;56(3):525–31. doi: 10.1507/endocrj.k09e-089. [DOI] [PubMed] [Google Scholar]
- 131.Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschop MH, Horvath TL. Ghrelin Controls Hippocampal Spine Synapse Density and Memory Performance. Nat Neurosci. 2006;9(3):381–8. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 132.Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M, Matsumiya T, Ishibashi M, Aou S, Li XL, Kohno D, Uramura K, Sougawa H, Yada T, Wayner MJ, Sasaki K. Leptin Facilitates Learning and Memory Performance and Enhances Hippocampal Ca1 Long-Term Potentiation and Camk Ii Phosphorylation in Rats. Peptides. 2006;27(11):2738–49. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 133.Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB, Horvath TL. Ghrelin Modulates the Activity and Synaptic Input Organization of Midbrain Dopamine Neurons While Promoting Appetite. J Clin Invest. 2006;116(12):3229–39. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin Regulation of the Mesoaccumbens Dopamine Pathway. Neuron. 2006;51(6):811–22. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 135.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin Receptor Signaling in Midbrain Dopamine Neurons Regulates Feeding. Neuron. 2006;51(6):801–10. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 136.Chaki S, Hirota S, Funakoshi T, Suzuki Y, Suetake S, Okubo T, Ishii T, Nakazato A, Okuyama S. Anxiolytic-Like and Antidepressant-Like Activities of Mcl0129 (1-[(S)-2-(4-Fluorophenyl)-2-(4-Isopropylpiperadin-1-Yl)Ethyl]-4-[4-(2-Met Hoxynaphthalen-1-Yl)Butyl]Piperazine), a Novel and Potent Nonpeptide Antagonist of the Melanocortin-4 Receptor. J Pharmacol Exp Ther. 2003;304(2):818–26. doi: 10.1124/jpet.102.044826. [DOI] [PubMed] [Google Scholar]
- 137.Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, Zitman FG. Overweight, Obesity, and Depression: A Systematic Review and Meta-Analysis of Longitudinal Studies. Arch Gen Psychiatry. 2010;67(3):220–9. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 138.Mayberg HS. Targeted Electrode-Based Modulation of Neural Circuits for Depression. J Clin Invest. 2009;119(4):717–25. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kishi T, Elmquist JK. Body Weight Is Regulated by the Brain: A Link between Feeding and Emotion. Mol Psychiatry. 2005;10(2):132–46. doi: 10.1038/sj.mp.4001638. [DOI] [PubMed] [Google Scholar]