Spatial control of exocytosis (original) (raw)
. Author manuscript; available in PMC: 2012 Jun 6.
Abstract
During many key biological processes, exocytosis is confined to distinct regions of the plasma membrane. Spatial control of exocytosis correlates with altered membrane skeleton dynamics and assembly of local membrane microdomains. These domains act as local stages for the assembly and the regulation of molecular complexes (targeting patches) that mediate vesicle–membrane fusion. Furthermore, local activation of signaling pathways reinforces formation of these patches and might effect global repositioning of the secretory pathway toward sites of localized exocytosis.
Introduction
Exocytosis, the fusion of intracellular membrane vesicles with the plasma membrane, occurs constitutively in all eukaryotic cells and is upregulated in some cell types in response to extrinsic stimuli (e.g. neurotransmitters and hormones). Membrane fusion is mediated by soluble _N_-ethylmaleimide-sensitive factor (NSF) attachment protein receptors (SNAREs) found on transport vesicles (v-SNAREs) and their target membrane (t-SNAREs) [1]. In addition to the soluble proteins α-SNAP and NSF, which are responsible for the dissociation of SNARE complexes, several signaling molecules, cytoskeletal components and plasma-membrane-associated proteins and lipids have been implicated in the control of membrane fusion [2].
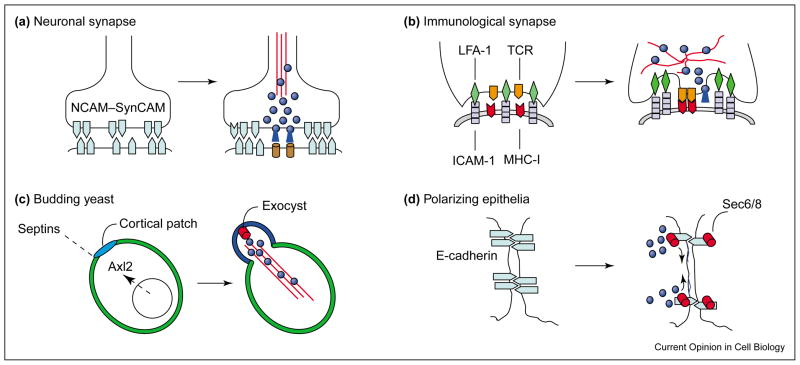
Spatial control of exocytosis underlies many key biological processes (Figure 1). In neurons, exocytosis of secretory granules and vesicles is confined to the synaptic cleft and takes place within a millisecond time frame [2]. During immune surveillance, contact between an antigen-presenting cell (APC) and a cytotoxic T cell leads to formation of the immunological synapse, and vesicle transport of soluble agents and membrane proteins is confined within this zone of cell–cell contact [3]. During cell migration and wound healing, exocytosis is tightly coupled to local changes in plasma membrane tension and membrane-cytoskeleton dynamics [4]. During cell division, localized exocytosis is thought to mediate ingression of the cleavage furrow [5,6]. Development of cell polarity is accompanied by directed exocytosis at specialized sites of membrane growth [7].
Figure 1.
Molecular cues and membrane domains of localized exocytosis. (a) During development of the neuronal synapse, neural adhesion molecules (e.g. NCAM, SynCAM [light blue]) stabilize initial neurite–neurite contacts and interact with Golgi-derived organelles and cytoskeleton-binding proteins for the formation of the synaptic cleft. Exocytosis of secretory granules and vesicles (dark blue) is confined to this region. (b) During immune surveillance, T-cell receptors (TCRs, orange) and LFA-1 (green) cell adhesion molecules interact with MHC I (red) and ICAM-1 (lilac) molecules for the formation of the immunological synapse. In the mature immunological synapse (right), exocytosis (dark blue) is confined to an outer domain of adhesion molecules separate from the central cluster of proteins (orange, red) involved in TCR signaling. (c) In budding yeast, both extrinsic (disassembly of the septin ring) and intrinsic (cell-cycle-dependent expression of Axl2p) cues lead to the formation of a cortical patch (mid blue) of proteins (see text for full details) and to recruitment of the exocyst (red). (d) In polarizing epithelia, plasma membrane recruitment of the exocyst (red) requires Ca2+-dependent cell–cell adhesion, and initially, is spatially confined within detergent-insoluble puncta of E-cadherin (light blue). However, the exocyst is restricted later to the tight junction as cells become more polarized. Overall, specialized molecular complexes (e.g. the exocyst [Sec6/8] complex, SNARE proteins) are assembled within distinct membrane domains to achieve localized delivery and fusion of intracellular vesicles. These domains appear to be the result of local changes in intramembrane and membrane-skeleton dynamics.
Here, we review recent evidence and dissect the spatial control of exocytosis as a process that relies on a number of factors: molecular cues that define membrane domains for localized exocytosis; local assembly and regulation of molecular complexes (targeting patches) that mediate membrane fusion; and cytoskeleton tracks and membrane carriers that connect the _trans_-Golgi network (TGN) with targeting patches of the plasma membrane.
Molecular cues and membrane domains regulating spatial control of exocytosis
Spatial control of exocytosis implies that vesicle fusion does not occur randomly on the plasma membrane. With the advent of evanescent wave microscopy, also termed ‘total internal reflection fluorescence microscopy’ (TIR-FM), single vesicle fusion events have been imaged and analyzed for constitutive and regulated exocytosis, which are considered to either require or not require a specific signal for vesicle fusion, respectively. Although constitutive exocytosis in COS-1 cells appears to occur all over the ventral plasma membrane with no preferred sites of fusion [8], constitutive exocytosis in PtK2 cells is non-random and fusion events are spatially constrained within micron-sized ‘hot spots’ [9•]. TIR-FM imaging of regulated secretion in neurons and neuroendocrine cells has clearly revealed sites, termed ‘active zones’, at which repeated vesicle docking and fusion occur [10,11]. Evidence for spatially restricted sites of constitutive exocytosis is supported by clustering of t-SNARE (syntaxin 4) proteins in the plasma membrane of BHK cells [12••]. Similar to their syntaxin counterparts in neurosecretory PC12 cells, maintenance of these clusters of syntaxin 4 depends on cholesterol and might reflect sites of preferential vesicle docking and fusion.
The minimal protein machinery required for exocytosis (i.e. the SNARE proteins) interacts with phospholipids [13] and is associated with cholesterol-dependent (‘lipid-raft’) membrane microdomains [12••,14]. However, spatial control of exocytosis might not be specified only by clustering of SNARE proteins in lipid rafts. External positional cues that alter intramembrane and membrane-skeleton dynamics within particular regions of the plasma membrane appear to be involved in several examples of localized exocytosis (Figure 1).
During synaptogenesis in murine hippocampal neurons, clusters of neural adhesion molecule NCAM180 are trapped at the site of two contacting neurites [15•] (Figure 1a). NCAM-mediated cell–cell adhesion not only stabilizes initial contacts between neurites but also restricts Golgi-derived organelles to those cell–cell contacts via the membrane–skeleton protein spectrin [15•]. Synaptic cell adhesion molecule (SynCAM), a brain-specific synaptic adhesion molecule with a cytoplasmic tail that interacts with PDZ-domain proteins, has also been shown to induce synapse formation in hippocampal neurons and in non-neuronal cells [16]. Synaptic size and strength can be further modulated by neural activity, which induces redistribution of β-catenin into spines, where β-catenin mediates the association of another cell adhesion molecule, E-cadherin, with the actin cytoskeleton [17].
Similarly, during formation of the immunological synapse (Figure 1b), small-scale clusters of T-cell receptors (TCRs) interact with the corresponding peptide–MHC complexes on the APC, initiating signaling pathways that lead to the conversion of the cell adhesion molecule β2-integrin (LFA-1) into its high-avidity form, and the recruitment of talin, which mediates β2-integrin association with the actin cytoskeleton [18]. In the mature immunological synapse, exocytosis is confined to an outer domain of adhesion molecules separate from the central cluster of proteins involved in TCR signaling [19]. In addition to talin, the actin-binding protein ezrin and the actin polymerization factor coronin-1 also appear to be involved in formation of a distinct domain of cytoskeleton-anchored transmembrane proteins at the periphery of the contact zone [20].
The role of membrane-skeleton domains in restricting sites of exocytosis is also exemplified in polarized growth of Saccharomyces cerevisiae. In haploid cells, bud site selection, which leads to formation of the daughter cell, correlates with assembly of a cortical patch of proteins with specialized functions (see below) adjacent to the preceding bud site. Assembly of this patch is temporally controlled by cell-cycle-dependent expression of genes such as AXL2 [21], and is thought to be spatially defined by septins, novel components of the membrane skeleton that form a ring structure before cytokinesis [22•] (Figure 1c). The cortical patch comprises Bud3p, Bud4p, Axl1p and the transmembrane protein Axl2p (reviewed in [22•]). The cytoplasmic domain of Axl2p binds Bud5p [23], a GDP/GTP exchange factor for the Ras-like GTPase Bud1p, which, in turn, interacts with upstream regulators (Cdc24p and Cdc42p) of the actin cytoskeleton [22•]. Moreover, the GTP-bound form of Cdc42p associates with Sec3p [24•], a spatial landmark for the assembly of the exocyst (or Sec6/8 complex), a multicomponent complex (Sec3p, Sec5p, Sec6p, Sec8p, Sec10p, Sec15p, Exo70p and Exo84p) localized to sites of bud growth [25,26]. Sec3p was recently found to interact with Iqg1p, which also appears to be involved in bud-site selection through its interaction with Bud4p [27•]. Localization of Sec3p is independent of the actin cytoskeleton and other components of the exocyst, but subsequent polarization of the actin cytoskeleton may contribute to the assembly of the exocyst [28], which has also been implicated in the cell separation of Schizosaccharomyces pombe during cytokinesis [29].
In polarizing epithelia, plasma membrane recruitment of the exocyst requires Ca2+-dependent cell–cell adhesion, and is spatially confined within detergent-insoluble puncta of E-cadherin [30] (Figure 1d). Similarly, in non-motile fibroblasts, the Sec6/8 complex is restricted to sites of cell–cell contact, where it co-localizes with adhesion molecules and actin-associated proteins [31]. In fully polarized epithelia, the Sec6/8 complex localizes to the lateral plasma membrane [30,32,33••], and antibodies against Sec8 inhibit vesicle fusion with the basal-lateral, but not apical membrane [30]. Vesicle fusion has yet to be examined at sites of cell–cell contact, but the overall molecular picture is reminiscent to cadherin-mediated synaptogenesis in hippocampal neurons (see above; [15•]) where the Sec6/8 complex is localized to the presynaptic membranes of newly formed synapses [34].
Although assembly of the exocyst appears to correlate with extrinsic and intrinsic positional cues (e.g. cadherin-mediated cell adhesion and cell-cycle-dependent gene expression) and local rearrangements of the membrane skeleton, how it interacts with the SNARE machinery is unknown. Recent studies indicate that the exocyst belongs to a wider family of ‘quarterfoil’ molecular complexes including the conserved oligomeric Golgi complex (Sec34/35 complex) and the Golgi-associated retrograde protein complex (Vps52/53/54, also known as Vps53), which act as molecular tethers for vesicle docking before SNARE-mediated fusion with target membranes [35••]. Hence, in concert with the SNARE machinery or independently, the exocyst might specify sites of vesicle docking and fusion at membrane-skeleton patches of the plasma membrane.
Assembly of targeting patches: local machineries and global regulators
According to the SNARE hypothesis, specificity of membrane fusion is determined by physicochemical properties of SNARE proteins (v-/t-SNARE pairing), which can be further modulated by their distribution within distinct subcellular compartments and by their interaction with regulatory proteins [36]. In addition, exocytosis can be spatially controlled by differential distribution of SNARE proteins within distinct domains of the plasma membrane. Evidence for this type of mechanism comes from studies of exocytosis in polarized epithelia. Plasma membrane t-SNARE proteins syntaxin 3 and 4 are confined to the apical and basal-lateral surfaces of polarized epithelial cells, respectively [37,38]. Three-dimensional imaging of post-Golgi vesicle traffic reveals that exocytosis of basal-lateral marker proteins occurs exclusively within the upper regions of the lateral membrane [33••]. By contrast, apical marker proteins do not fuse anywhere within the basal-lateral membrane. However, upon microtubule disruption apical marker proteins appear to fuse with the basal membrane, correlating with unconfined relocalization of syntaxin 3 throughout the apical and basal-lateral domains of microtubule-disrupted cells [33••].
How is exocytosis of basal-lateral marker proteins confined to the upper regions of the lateral membrane while syntaxin 4 occupies the entire plane of the membrane? Strict localization of the Sec6/8 complex to the apex of the lateral membrane [30,32] hints at its importance as a spatial regulator of exocytosis, and is consistent with its role in exocytosis at the tip of the daughter bud of S. cerevisiae in which t-SNARE proteins (Sso1p, Sso2p and Sec9p) are uniformly distributed over the mother–daughter cell surface [39,40]. It should be noted that Kreitzer et al. [33••] observed the Sec6/8 complex throughout the lateral membrane, but this difference with previous studies has yet to be reconciled. Aside from the Sec6/8 complex, fusion of membrane vesicles with the lateral membrane could be regulated by SNARE-interacting molecules such as the mammalian homologue of the Drosophila tumor suppressor protein Mlgl (lethal [2] giant larvae). The phosphorylated form of Mlgl is localized to the lateral membrane of polarized MDCK cells, where it physically interacts with syntaxin 4 [41].
SNARE complex assembly is regulated by the Sec1/Munc18 (SM)-related proteins and Rab GTPases [2]. SM proteins bind to the amino-terminal domain of syntaxins (syntaxin 1–4), and thus regulate interaction of that domain with the central SNARE motif, which is responsible for the formation of a SNARE core complex [42,43]. Masking of the central SNARE motif by its most amino-terminal domain results in an inactive or closed SNARE conformation. Accumulation of Sec1p at sites of active exocytosis in budding yeast (i.e. at the tips of small buds, or at mother–daughter necks [44]) and restricted localization of Munc18-2 to the apical membrane of polarized epithelia where it interacts with syntaxin 3 [45] suggest that these proteins might participate in the spatial control of exocytosis. SM proteins are further subjected to phosphorylation (by protein kinase C and cyclin-dependent kinase 5) and their interaction with syntaxins is modulated by the Rab3 effector RIM, and possibly through other SM-interacting molecules such as Mints (also known as X11-like proteins), adaptor proteins of conserved carboxy-terminal PTB and PDZ domains, and the Doc2 (double C2) family of proteins that contain Ca2+- and phospholipids-binding domains (reviewed in [2]).
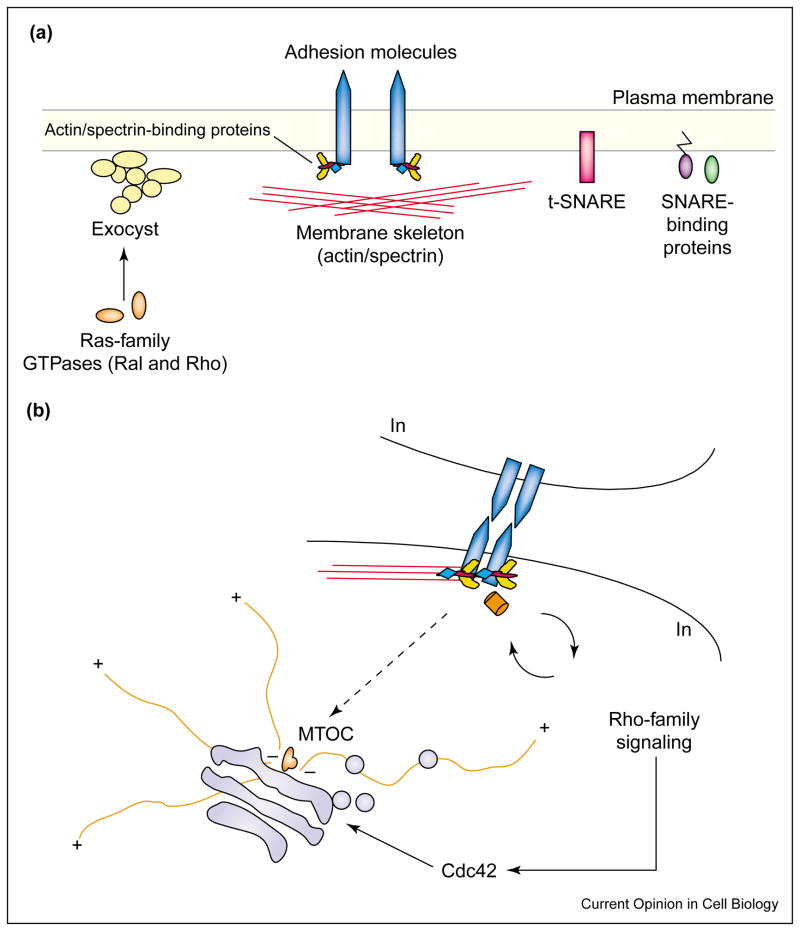
In addition to their interaction with SM proteins, Rab GTPases and other small GTP-binding proteins of the Ras superfamily (e.g. Ral and Rho) interact with the exocyst and may impose spatial control on SNARE complex assembly and/or function (Figure 2a). In budding yeast, the exocyst component Sec15p interacts with the GTP-bound form of Sec4p, a Rab protein associated with secretory vesicles [46]. In mammalian cells, the GTP-bound form of Ral was recently shown to associate with Sec5p [47,48•]. Disruption of Ral expression and function results in disruption of exocyst assembly [48•], random distribution of basal-lateral marker proteins over the entire cell surface of polarized epithelia [48•], and suppression of regulated exocytosis in synaptosomes [48•] and PC12 cells [49]. In fibroblasts, inhibition of RalA binding to Sec5p prevents formation of filopodia [50]. Finally, Rho1 in its GTP-bound form also interacts with the exocyst component Sec3p, and rho1 mutant alleles affect localization of the exocyst in the budding yeast [51•].
Figure 2.
Assembly of targeting patches and inside–out orientation of the secretory pathway. (a) Molecular hardware for assembly of a targeting patch. Targeting patches (SNAREs [pink], SNARE-binding proteins [purple, green], and the exocyst [yellow]) are assembled within distinct regions of the plasma membrane, as defined through the interaction of adhesion molecules (blue) with the membrane skeleton (red). Targeting patch assembly is further reinforced by cell-adhesion-activated signaling. SNARE proteins and components of the exocyst interact with Rab GTPases and other small GTP-binding proteins of the Ras superfamily (e.g. Ral and Rho [orange]). (b) Outside–in cues and inside–out orientation of the secretory pathway. Budding of vesicles from the Golgi complex (pale blue) and their transport along the cytoskeleton (orange) can be influenced by the same molecular events that trigger and reinforce the assembly of a targeting patch. Rho family GTPases (dark orange; RhoA, Rac1, Cdc42) are activated during cadherin-mediated cell–cell adhesion, and Cdc42 has been shown to influence protein export from the Golgi complex. In addition, cadherin-mediated cell adhesion induces stabilization of the microtubule minus ends; and during the formation of the immunological synapse, the MTOC (orange) is drawn vectorially to sites of cell–cell contact (dashed arrow).
Receptor-activated signaling is also likely to affect localized exocytosis. During receptor-stimulated activation, lipid products such as phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphatidylinositol 3,4,5-trisphosphate (PIP3) are asymmetrically distributed within the cytoplasmic leaflet of the plasma membrane. PIP2 and PIP3 interact with proteins that effect cytoskeleton assembly and with molecules involved in membrane fusion during regulated exocytosis (see the review by Bankaitis and Morris, this issue). Interestingly, the Sec6/8 complex has been shown to co-immunoprecipitate with signaling and Ca2+-transport proteins [52], but how lipid asymmetry and signaling cascades are linked to localized exocytosis is unclear.
Cytoskeleton tracks and membrane carriers make the connection
While a targeting patch marks the site of exocytic membrane fusion, transport vesicles that depart the Golgi complex must be directionally oriented toward the plasma membrane and sites of restricted exocytosis. Although vesicles could slowly reach their final destination by random diffusion, imaging of post-Golgi traffic indicates that vesicles travel towards the plasma membrane along curvilinear paths that overlap with microtubules [53]. In addition, proteins that are destined to different domains of the plasma membrane (apical versus basal-lateral) travel within separate membrane carriers [9•]. Transport of secretory vesicles is also coupled to the orientation of the actin cytoskeleton in budding yeast [40], where bundles of actin filaments (actin cables) transverse mother–daughter cell (bud) cytoplasm and converge on sites of active exocytosis [54].
Selectivity in transport vesicle delivery could be achieved by the following mechanisms: first, distinct groups of cargo proteins are individually packaged into single vesicles (protein sorting); and second, through their interaction with molecular motors, vesicles travel along cytoskeleton tracks to connect with targeting patches at the plasma membrane. Protein sorting during export from the Golgi complex is well documented and several signals have been identified to interact with clathrin adaptor protein complexes, presumably toward the formation of distinct vesicles [55]. How these signals result in sorting of specific proteins into vesicles and in polarized exocytosis is unclear, but post-Golgi vesicle transport depends on the microtubule motor protein kinesin [56] and a novel kinesin protein (KIF13A) has been shown to interact with the AP-1 adaptor complex [54]. In budding yeast, the actin motor protein Myo2p drives movement of secretory vesicles along actin cables [40,54], and myosin II is also implicated in the release of vesicles from the TGN of mammalian cells [57].
Budding of vesicles from the Golgi complex and their transport along the cytoskeleton could be influenced by the same molecular events that trigger and reinforce the assembly of a targeting patch (Figure 2b). Hence, activation mechanisms (e.g. cadherin-activated signaling) for localized exocytosis (e.g. neuronal, immunological synapse) would bear upon mechanisms of protein sorting and vesicle budding at the TGN [58]. Rho-family GTPases (RhoA, Rac1 and Cdc42) are activated during cadherin-mediated cell–cell adhesion [59], and Cdc42 has been shown to influence exit of basal-lateral marker proteins from the Golgi complex [60,61•]. Protein kinase D is also suggested to regulate fission from the TGN [62]. Furthermore, during formation of the immunological synapse, the microtubule-organizing center (MTOC) is drawn vectorially to the site of cell–cell contact [63••], and cadherin-mediated cell adhesion induces stabilization of the microtubule minus ends [64]. Organelles of the late secretory pathway (e.g. the Golgi complex and the endocytic recycling compartment) are positioned along the MTOC, and the microtubule motor protein KIFC3 appears to act synergistically with dynein for proper positioning of the Golgi complex [65]. Therefore, the entire secretory apparatus might reorient toward sites of localized exocytosis in a fashion similar to the orientation of the mitotic spindle during cell division.
In addition to classical pathways of exocytosis, novel routes of vesicle transport are now being discovered. In the Drosophila imaginal disk epithelium, novel exocytic vesicles, termed ‘argosomes’, are derived from the basal-lateral membrane and travel long distances through the disk epithelium [66•]. In dendritic cells, vesicular-tubular structures that emanate from lysosomes appear to dock and fuse with the plasma membrane [67•]. The rediscovery of the lysosome as a secretory organelle and its role in calcium-dependent exocytosis of nonsecretory cells [68•] blur the distinction between constitutive and regulated exocytosis. In addition, a new type of vesicles, termed ‘enlargosomes’, was recently implicated in the calcium-induced exocytosis of secretory and non-secretory cells [69]. Hence, in future studies, it will be interesting to see how these non-classical routes of membrane traffic connect with plasma membrane domains of localized exocytosis.
Conclusions
Spatial control of exocytosis correlates with altered membrane-skeleton dynamics and assembly of local membrane microdomains of distinct structural and functional organization. These domains act as local stages for the activation of signaling pathways, which contribute to the formation of a targeting patch and could effect global repositioning of the secretory apparatus. Most of this model derives from studies in tissue culture cells and from paradigms of regulated exocytosis. Thus, it remains unclear if in a living organism constitutive exocytosis obeys similar rules and mechanisms. Does constitutive exocytosis represent a specialized case of regulated exocytosis? If yes, why is it important to confine membrane fusion within distinct regions of the plasma membrane? Real-time imaging of vesicle fusion on cell-free bona fide plasma membranes should allow the molecular dissection and reconstitution of localized exocytosis. Furthermore, much has to be gained from the study of new proteins that until recently were the unknown components of vesicle fusion and transport. Overall, spatial control of exocytosis requires the understanding of molecular mechanisms that identify localized sites of membrane fusion, and how they are linked to intracellular mechanisms of vesicle targeting and fusion. Intergrading outside–in cues and inside–out paths of exocytosis will greatly aid our current understanding.
Update
The role of cytoskeleton tracks in constitutive exocytosis was demonstrated recently by a study showing that disruption of microtubules not only alters the shape and directionality of membrane vesicles but also reduces the efficiency of the fusion process (an increase in the number of incomplete fusion events was observed as more fusion events were required for the discharge of cargo proteins into the plasma membrane) [70]. According to the same study, disruption of the actin cytoskeleton decreased the average time that a vesicle was docked to the plasma membrane, but had no gross effect on vesicle transport and fusion [70]. On the same topic, transport of two distinct populations of vesicles, both of which are targeted to the apical membrane of epithelial cells, was shown recently to occur in two distinct modes (along actin tracks and in an actin-independent fashion), depending on their cargo proteins and their association with actin-binding motor proteins [71].
A flurry of recent papers has revisited the role of the Sec6/8 complex in regulated exocytosis, and new studies have attempted to pinpoint the role of localized exocytosis in cell division. In Drosophila, mutations in the ecocyst subunit Sec5 inhibited neuronal growth, but had no effect on synaptic transmission [72]. This observation further suggests that vesicle fusion at the neuronal synapse is mechanistically distinct from vesicle fusion during neurite growth. However, in a different type of regulated exocytosis, the Sec6/8 complex assembles at the plasma membrane of fat cells in response to insulin, and its presence appears to be required for the translocation of the insulin-responsive glucose transporter Glu4 from intracellular membranes to the cell surface [73]. Finally, a recent study indicated that a SNARE-mediated membrane fusion event is required for the final step of cytokinesis (abscission), when the midbody, a cytoplasmic bridge that connects two daughter cells, is torn apart [74].
Acknowledgments
We apologize to our colleagues for having to omit many references that detail the studies reported in this review, owing to space constraints. Work from the Nelson laboratory is supported by National Institutes of Health grant GM35527 to WJ Nelson. ET Spiliotis is a fellow of the Jane Coffin Childs Memorial Fund for Medical Research.
Abbreviations
MTOC
microtubule organizing center
NCAM
neural adhesion molecule
NSF
_N_-ethylmaleimide-sensitive factor
SM
Sec1/Munc18
SNARE
soluble NSF attachment protein receptor
TGN
_trans_-Golgi network
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol. 2001;2:98–106. doi: 10.1038/35052017. [DOI] [PubMed] [Google Scholar]
- 2.Rizo J, Sudhof TC. Snares and Munc18 in synaptic vesicle fusion. Nat Rev Neurosci. 2002;3:641–653. doi: 10.1038/nrn898. [DOI] [PubMed] [Google Scholar]
- 3.Bossi G, Trambas C, Booth S, Clark R, Stinchcombe J, Griffiths GM. The secretory synapse: the secrets of a serial killer. Immunol Rev. 2002;189:152–160. doi: 10.1034/j.1600-065x.2002.18913.x. [DOI] [PubMed] [Google Scholar]
- 4.Sheetz MP. Cell control by membrane–cytoskeleton adhesion. Nat Rev Mol Cell Biol. 2001;2:392–396. doi: 10.1038/35073095. [DOI] [PubMed] [Google Scholar]
- 5.Finger FP, White JG. Fusion and fission: membrane trafficking in animal cytokinesis. Cell. 2002;108:727–730. doi: 10.1016/s0092-8674(02)00668-2. [DOI] [PubMed] [Google Scholar]
- 6.Bednarek SY, Falbel TG. Membrane trafficking during plant cytokinesis. Traffic. 2002;3:621–629. doi: 10.1034/j.1600-0854.2002.30904.x. [DOI] [PubMed] [Google Scholar]
- 7.Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- 8.Schmoranzer J, Goulian M, Axelrod D, Simon SM. Imaging constitutive exocytosis with total internal reflection fluorescence microscopy. J Cell Biol. 2000;149:23–32. doi: 10.1083/jcb.149.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9•.Keller P, Toomre D, Diaz E, White J, Simons K. Multicolour imaging of post-Golgi sorting and trafficking in live cells. Nat Cell Biol. 2001;3:140–149. doi: 10.1038/35055042. Multicolor imaging is combined with total internal reflection fluorescence microscopy to image segregation and trafficking of apical and basal-lateral cargo in living cells. Apical and basal-lateral marker proteins are transported within separate membrane carriers and sites of preferred exocytosis (hot spots) are revealed in the plasma membrane of PtK2 cells. [DOI] [PubMed] [Google Scholar]
- 10.Oheim M, Loerke D, Stuhmer W, Chow RH. The last few milliseconds in the life of a secretory granule. Docking, dynamics and fusion visualized by total internal reflection fluorescence microscopy (TIRFM) Eur Biophys J. 1998;27:83–98. doi: 10.1007/s002490050114. [DOI] [PubMed] [Google Scholar]
- 11.Zenisek D, Steyer JA, Almers W. Transport, capture and exocytosis of single synaptic vesicles at active zones. Nature. 2000;406:849–854. doi: 10.1038/35022500. [DOI] [PubMed] [Google Scholar]
- 12••.Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, Jahn R. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J. 2001;20:2202–2213. doi: 10.1093/emboj/20.9.2202. This study demonstrates non-uniform distribution of SNARE proteins on the plasma membrane of neurosecretory cells and of cells that do not possess a regulated secretory pathway. Syntaxins are shown to be clustered in large (200 nm), cholesterol-dependent domains at which secretory vesicles preferentially dock and fuse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner ML, Tamm LK. Reconstituted syntaxin1a/SNAP25 interacts with negatively charged lipids as measured by lateral diffusion in planar supported bilayers. Biophys J. 2001;81:266–275. doi: 10.1016/S0006-3495(01)75697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chamberlain LH, Burgoyne RD, Gould GW. SNARE proteins are highly enriched in lipid rafts in PC12 cells: implications for the spatial control of exocytosis. Proc Natl Acad Sci USA. 2001;98:5619–5624. doi: 10.1073/pnas.091502398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Sytnyk V, Leshchyns’ka I, Delling M, Dityateva G, Dityatev A, Schachner M. Neural cell adhesion molecule promotes accumulation of TGN organelles at sites of neuron-to-neuron contacts. J Cell Biol. 2002;159:649–661. doi: 10.1083/jcb.200205098. This study suggests a molecular mechanism for cell-adhesion-mediated synaptogenesis. Golgi-derived organelles are linked via spectrin to clusters of neural adhesion molecule, which are trapped at sites of initial neurite-to-neurite contact. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- 17.Murase S, Mosser E, Schuman EM. Depolarization drives beta-catenin into neuronal spines promoting changes in synaptic structure and function. Neuron. 2002;35:91–105. doi: 10.1016/s0896-6273(02)00764-x. [DOI] [PubMed] [Google Scholar]
- 18.Freiberg BA, Kupfer H, Maslanik W, Delli J, Kappler J, Zaller DM, Kupfer A. Staging and resetting T cell activation in SMACs. Nat Immunol. 2002;3:911–917. doi: 10.1038/ni836. [DOI] [PubMed] [Google Scholar]
- 19.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 20.Roumier A, Olivo-Marin JC, Arpin M, Michel F, Martin M, Mangeat P, Acuto O, Dautry-Varsat A, Alcover A. The membrane–microfilament linker ezrin is involved in the formation of the immunological synapse and in T cell activation. Immunity. 2001;15:715–728. doi: 10.1016/s1074-7613(01)00225-4. [DOI] [PubMed] [Google Scholar]
- 21.Lord M, Yang MC, Mischke M, Chant J. Cell cycle programs of gene expression control morphogenetic protein localization. J Cell Biol. 2000;151:1501–1512. doi: 10.1083/jcb.151.7.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Casamayor A, Snyder M. Bud-site selection and cell polarity in budding yeast. Curr Opin Microbiol. 2002;5:179–186. doi: 10.1016/s1369-5274(02)00300-4. This is a review of recent findings on the roles of cortical tags, GTPases and the cytoskeleton in the generation and maintenance of cell polarity in yeast. [DOI] [PubMed] [Google Scholar]
- 23.Marston AL, Chen T, Yang MC, Belhumeur P, Chant J. A localized GTPase exchange factor, Bud5, determines the orientation of division axes in yeast. Curr Biol. 2001;11:803–807. doi: 10.1016/s0960-9822(01)00230-5. [DOI] [PubMed] [Google Scholar]
- 24•.Zhang X, Bi E, Novick P, Du L, Kozminski KG, Lipschutz JH, Guo W. Cdc42 interacts with the exocyst and regulates polarized secretion. J Biol Chem. 2001;276:46745–46750. doi: 10.1074/jbc.M107464200. This report shows that polarized localization of the exocyst is controlled by Cdc42. In vitro studies indicate that Sec3p interacts directly with Cdc42 in its GTP-bound form. The authors propose that Cdc42 coordinates the machinery of vesicle docking and the actin cytoskeleton for polarized secretion. [DOI] [PubMed] [Google Scholar]
- 25.TerBush DR, Maurice T, Roth D, Novick P. The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- 26.Guo W, Grant A, Novick P. Exo84p is an exocyst protein essential for secretion. J Biol Chem. 1999;274:23558–23564. doi: 10.1074/jbc.274.33.23558. [DOI] [PubMed] [Google Scholar]
- 27•.Osman MA, Konopka JB, Cerione RA. Iqg1p links spatial and secretion landmarks to polarity and cytokinesis. J Cell Biol. 2002;159:601–611. doi: 10.1083/jcb.200205084. Iqg1p, which is a target/effector for Cdc42p, is shown to interact with spatial landmark proteins for exocytosis (Sec3p) and marker proteins for axial bud-site selection in Saccharomyces cerevisiae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finger FP, Hughes TE, Novick P. Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell. 1998;92:559–571. doi: 10.1016/s0092-8674(00)80948-4. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Tang X, Liu J, Trautmann S, Balasundaram D, McCollum D, Balasubramanian MK. The multiprotein exocyst complex is essential for cell separation in Schizosaccharomyces pombe. Mol Biol Cell. 2002;13:515–529. doi: 10.1091/mbc.01-11-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Scheller RH, Nelson WJ. Sec6/8 complex is recruited to cell–cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell. 1998;93:731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- 31.Yeaman C, Grindstaff KK, Wright JR, Nelson WJ. Sec6/8 complexes on trans-Golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells. J Cell Biol. 2001;155:593–604. doi: 10.1083/jcb.200107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipschutz JH, Guo W, O’Brien LE, Nguyen YH, Novick P, Mostov KE. Exocyst is involved in cystogenesis and tubulogenesis and acts by modulating synthesis and delivery of basolateral plasma membrane and secretory proteins. Mol Biol Cell. 2000;11:4259–4275. doi: 10.1091/mbc.11.12.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33••.Kreitzer G, Schmoranzer J, Low SH, Li X, Gan Y, Weimbs T, Simon SM, Rodriguez-Boulan E. Three-dimensional analysis of post-Golgi carrier exocytosis in epithelial cells. Nat Cell Biol. 2003;5:126–136. doi: 10.1038/ncb917. Exocytosis of basal-lateral marker proteins is shown to occur exclusively within the upper half of the lateral membrane. Apical marker proteins do not fuse anywhere within the basal-lateral membrane, and, only upon microtubule disruption, appear to fuse with the basal membrane, correlating with unconfined localization of syntaxin 3 throughout the apical and basal-lateral domains of microtubule-disrupted cells. [DOI] [PubMed] [Google Scholar]
- 34.Hazuka CD, Foletti DL, Hsu SC, Kee Y, Hopf FW, Scheller RH. The Sec6/8 complex is located at neurite outgrowth and axonal synapse-assembly domains. J Neurosci. 1999;19:1324–1334. doi: 10.1523/JNEUROSCI.19-04-01324.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35••.Whyte JR, Munro S. Vesicle tethering complexes in membrane traffic. J Cell Sci. 2002;115:2627–2637. doi: 10.1242/jcs.115.13.2627. In this review, the authors outline recent findings indicating a new family of ‘quaterfoil’ tethering complexes, which may interact with a set of fourfold-symmetric components, such as the core domain of a trans SNARE complex. [DOI] [PubMed] [Google Scholar]
- 36.McNew JA, Parlati F, Fukuda R, Johnston RJ, Paz K, Paumet F, Sollner TH, Rothman JE. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature. 2000;407:153–159. doi: 10.1038/35025000. [DOI] [PubMed] [Google Scholar]
- 37.Low SH, Chapin SJ, Weimbs T, Komuves LG, Bennett MK, Mostov KE. Differential localization of syntaxin isoforms in polarized Madin–Darby canine kidney cells. Mol Biol Cell. 1996;7:2007–2018. doi: 10.1091/mbc.7.12.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, Low SH, Miura M, Weimbs T. SNARE expression and localization in renal epithelial cells suggest mechanism for variability of trafficking phenotypes. Am J Physiol Renal Physiol. 2002;283:F1111–1122. doi: 10.1152/ajprenal.00185.2002. [DOI] [PubMed] [Google Scholar]
- 39.Brennwald P, Kearns B, Champion K, Keranen S, Bankaitis V, Novick P. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 1994;79:245–258. doi: 10.1016/0092-8674(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 40.Finger FP, Novick P. Spatial regulation of exocytosis: lessons from yeast. J Cell Biol. 1998;142:609–612. doi: 10.1083/jcb.142.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musch A, Cohen D, Yeaman C, Nelson WJ, Rodriguez-Boulan E, Brennwald PJ. Mammalian homolog of Drosophila tumor suppressor lethal (2) giant larvae interacts with basolateral exocytic machinery in Madin–Darby canine kidney cells. Mol Biol Cell. 2002;13:158–168. doi: 10.1091/mbc.01-10-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dulubova I, Sugita S, Hill S, Hosaka M, Fernandez I, Sudhof TC, Rizo J. A conformational switch in syntaxin during exocytosis: role of Munc18. EMBO J. 1999;18:4372–4382. doi: 10.1093/emboj/18.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Misura KM, Scheller RH, Weis WI. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature. 2000;404:355–362. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- 44.Carr CM, Grote E, Munson M, Hughson FM, Novick PJ. Sec1p binds to SNARE complexes and concentrates at sites of secretion. J Cell Biol. 1999;146:333–344. doi: 10.1083/jcb.146.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riento K, Galli T, Jansson S, Ehnholm C, Lehtonen E, Olkkonen VM. Interaction of Munc-18-2 with syntaxin 3 controls the association of apical SNAREs in epithelial cells. J Cell Sci. 1998;111:2681–2688. doi: 10.1242/jcs.111.17.2681. [DOI] [PubMed] [Google Scholar]
- 46.Guo W, Roth D, Walch-Solimena C, Novick P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 1999;18:1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brymora A, Valova VA, Larsen MR, Roufogalis BD, Robinson PJ. The brain exocyst complex interacts with RalA in a GTP-dependent manner: identification of a novel mammalian Sec3 gene and a second Sec15 gene. J Biol Chem. 2001;276:29792–29797. doi: 10.1074/jbc.C100320200. [DOI] [PubMed] [Google Scholar]
- 48•.Moskalenko S, Henry DO, Rosse C, Mirey G, Camonis JH, White MA. The exocyst is a Ral effector complex. Nat Cell Biol. 2002;4:66–72. doi: 10.1038/ncb728. Activated Ral, a member of the Ras-family of GTPases, is shown to interact directly with exocyst component Sec5. Disruption of Ral expression and function results in disruption of exocyst assembly, mislocalization of basal-lateral marker proteins, and suppression of regulated exocytosis in synaptosomes and PC12 cells. [DOI] [PubMed] [Google Scholar]
- 49.Polzin A, Shipitsin M, Goi T, Feig LA, Turner TJ. Ral-GTPase influences the regulation of the readily releasable pool of synaptic vesicles. Mol Cell Biol. 2002;22:1714–1722. doi: 10.1128/MCB.22.6.1714-1722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugihara K, Asano S, Tanaka K, Iwamatsu A, Okawa K, Ohta Y. The exocyst complex binds the small GTPase RalA to mediate filopodia formation. Nat Cell Biol. 2002;4:73–78. doi: 10.1038/ncb720. [DOI] [PubMed] [Google Scholar]
- 51•.Guo W, Tamanoi F, Novick P. Spatial regulation of the exocyst complex by Rho1 GTPase. Nat Cell Biol. 2001;3:353–360. doi: 10.1038/35070029. Similar to the role of Cdc42 (Zhang et al. [2001] [24•]), the GTP-bound form of Rho1 is shown to interact with Sec3. Mutant alleles of rho1 affect localization of Sec3 and other components of the exocyst. [DOI] [PubMed] [Google Scholar]
- 52.Shin DM, Zhao XS, Zeng W, Mozhayeva M, Muallem S. The mammalian Sec6/8 complex interacts with Ca2+ signaling complexes and regulates their activity. J Cell Biol. 2000;150:1101–1112. doi: 10.1083/jcb.150.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toomre D, Keller P, White J, Olivo JC, Simons K. Dual-color visualization of trans-Golgi network to plasma membrane traffic along microtubules in living cells. J Cell Sci. 1999;112:21–33. doi: 10.1242/jcs.112.1.21. [DOI] [PubMed] [Google Scholar]
- 54.Pruyne D, Bretscher A. Polarization of cell growth in yeast. J Cell Sci. 2000;113:571–585. doi: 10.1242/jcs.113.4.571. [DOI] [PubMed] [Google Scholar]
- 55.Ait Slimane T, Hoekstra D. Sphingolipid trafficking and protein sorting in epithelial cells. FEBS Lett. 2002;529:54–59. doi: 10.1016/s0014-5793(02)03183-6. [DOI] [PubMed] [Google Scholar]
- 56.Kreitzer G, Marmorstein A, Okamoto P, Vallee R, Rodriguez-Boulan E. Kinesin and dynamin are required for post-Golgi transport of a plasma-membrane protein. Nat Cell Biol. 2000;2:125–127. doi: 10.1038/35000081. [DOI] [PubMed] [Google Scholar]
- 57.Musch A, Cohen D, Rodriguez-Boulan E. Myosin II is involved in the production of constitutive transport vesicles from the TGN. J Cell Biol. 1997;138:291–306. doi: 10.1083/jcb.138.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Donaldson JG, Lippincott-Schwartz J. Sorting and signaling at the Golgi complex. Cell. 2000;101:693–696. doi: 10.1016/s0092-8674(00)80881-8. [DOI] [PubMed] [Google Scholar]
- 59.Yap AS, Kovacs EM. Direct cadherin-activated cell signaling: a view from the plasma membrane. J Cell Biol. 2003;160:11–16. doi: 10.1083/jcb.200208156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kroschewski R, Hall A, Mellman I. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat Cell Biol. 1999;1:8–13. doi: 10.1038/8977. [DOI] [PubMed] [Google Scholar]
- 61•.Musch A, Cohen D, Kreitzer G, Rodriguez-Boulan E. Cdc42 regulates the exit of apical and basolateral proteins from the trans-Golgi network. EMBO J. 2001;20:2171–2179. doi: 10.1093/emboj/20.9.2171. In this report, mutants of Cdc42 are shown to inhibit export of basal-lateral marker proteins from the Golgi complex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liljedahl M, Maeda Y, Colanzi A, Ayala I, Van Lint J, Malhotra V. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell. 2001;104:409–420. doi: 10.1016/s0092-8674(01)00228-8. [DOI] [PubMed] [Google Scholar]
- 63••.Kuhn JR, Poenie M. Dynamic polarization of the microtubule cytoskeleton during CTL-mediated killing. Immunity. 2002;16:111–121. doi: 10.1016/s1074-7613(02)00262-5. Modulated polarization microscopy and three-dimensional image reconstruction were used to image microtubule-organizing center (MTOC) movement to the immunological synapse. The MTOC is drawn vectorially by a microtubule sliding mechanism to the contact site between the cytotoxic T cell and its target cell. [DOI] [PubMed] [Google Scholar]
- 64.Chausovsky A, Bershadsky AD, Borisy GG. Cadherin-mediated regulation of microtubule dynamics. Nat Cell Biol. 2000;2:797–804. doi: 10.1038/35041037. [DOI] [PubMed] [Google Scholar]
- 65.Xu Y, Takeda S, Nakata T, Noda Y, Tanaka Y, Hirokawa N. Role of KIFC3 motor protein in Golgi positioning and integration. J Cell Biol. 2002;158:293–303. doi: 10.1083/jcb.200202058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66•.Greco V, Hannus M, Eaton S. Argosomes: a potential vehicle for the spread of morphogens through epithelia. Cell. 2001;106:633–645. doi: 10.1016/s0092-8674(01)00484-6. This study reveals a novel type of membrane exovesicles, argosomes, that disperse the morphogen protein Wingless over large distances through the Drosophila imaginal disk epithelium. [DOI] [PubMed] [Google Scholar]
- 67•.Chow A, Toomre D, Garrett W, Mellman I. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature. 2002;418:988–994. doi: 10.1038/nature01006. This study reveals surprising transport of MHC II molecules from lysosomes to the plasma membrane. In live dendritic cells, GFP-tagged MHC II molecules were observed to exit lysosomes in tubular carriers that go on to fuse with the plasma membrane. [DOI] [PubMed] [Google Scholar]
- 68•.Andrews NW. Lysosomes and the plasma membrane: trypanosomes reveal a secret relationship. J Cell Biol. 2002;158:389–394. doi: 10.1083/jcb.200205110. In this review, recent evidence on the role of lysosomes as regulated secretory compartments that interact directly with the plasma membrane is discussed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Borgonovo B, Cocucci E, Racchetti G, Podini P, Bachi A, Meldolesi J. Regulated exocytosis: a novel, widely expressed system. Nat Cell Biol. 2002;4:955–962. doi: 10.1038/ncb888. [DOI] [PubMed] [Google Scholar]
- 70.Schmoranzer J, Simon SM. Role of microtubules in fusion of post-Golgi vesicles to the plasma membrane. Mol Biol Cell. 2003;14:1558–1569. doi: 10.1091/mbc.E02-08-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacob R, Heine M, Alfalah M, Naim HY. Distinct cytoskeletal tracks direct individual vesicle populations to the apical membrane of epithelial cells. Curr Biol. 2003;13:607–612. doi: 10.1016/s0960-9822(03)00188-x. [DOI] [PubMed] [Google Scholar]
- 72.Murthy M, Garza D, Scheller RH, Schwarz TL. Mutations in the exocyst component Sec5 disrupt neuronal membrane traffic, but neurotransmitter release persists. Neuron. 2003;37:433–447. doi: 10.1016/s0896-6273(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 73.Inoue M, Chang L, Hwang J, Chiang SH, Saltiel AR. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature. 2003;422:629–633. doi: 10.1038/nature01533. [DOI] [PubMed] [Google Scholar]
- 74.Low SH, Li X, Miura M, Kudo N, Quinones B, Weimbs T. Syntaxin 2 and endobrevin are required for the terminal step of cytokinesis in mammalian cells. Dev Cell. 2003;4:753–759. doi: 10.1016/s1534-5807(03)00122-9. [DOI] [PubMed] [Google Scholar]