Dynamic roles of type I and type II interferons in early infection with Mycobacterium tuberculosis (original) (raw)
. Author manuscript; available in PMC: 2013 Jun 15.
Published in final edited form as: J Immunol. 2012 May 7;188(12):6205–6215. doi: 10.4049/jimmunol.1200255
Abstract
Whereas the protective role of type II interferon (IFN), or IFN-γ, against Mycobacterium tuberculosis has been established, the effects of type I IFNs are still unclear. One potential confounding factor is the overlap of function between the two signaling pathways. We used mice carrying null mutations in type I IFN receptor, type II IFN receptor or both and compared their immune responses to those of wild type mice following aerosol infection with M. tuberculosis. We discovered that, in the absence of response to IFN-γ, type I IFNs play a non-redundant protective role against tuberculosis. Mice unable to respond to both types of IFNs had more severe lung histopathology for similar bacterial loads and died significantly earlier than mice with impaired IFN-γ signaling alone. We excluded a role for type I IFN in T cell recruitment, which was IFN-γ-dependent, while both types of IFNs were required for optimal NK cell recruitment to the lungs. Type I IFN had a time-dependent influence on the composition of lung myeloid cell populations, in particular by limiting the abundance of _M. tuberculosis_-infected recruited macrophages after the onset of adaptive immunity. We confirmed that response to IFN-γ was essential to control intracellular mycobacterial growth, without any additional effect of type I IFN. Together, our results imply a model in which type I IFN limit the number of target cells that M. tuberculosis can infect in the lungs while IFN-γ enhances their ability to restrict bacterial growth.
Introduction
Both innate and adaptive immune responses are necessary to control Mycobacterium tuberculosis, but they are not sufficient to achieve sterilizing immunity. This allows the bacteria to establish chronic infection and complicates efforts to develop effective tuberculosis vaccines. Understanding the protective mechanisms underlying immune responses to M. tuberculosis and their limitations is key to developing new strategies to control the global tuberculosis epidemic.
In the armamentarium deployed by the immune system against M. tuberculosis, cytokines, interferons (IFNs) in particular, play an essential role (1). IFNs are a group of closely related cytokines, sharing transcription factors and signaling pathways, but are able to induce very diverse, even apparently opposing, immune responses. Type I IFNs, which include multiple forms of IFN-α and one IFN-β, signal through the same receptor, termed IFNAR, and have been commonly associated with innate immune responses to viruses. However, type I IFNs can also be induced by non-viral infections, during which they display diverse immunomodulatory properties (2). Type II IFN, or IFN-γ, which signals through a distinct receptor, IFNGR, is the canonical cytokine of adaptive Th1 immunity and is essential for immune responses to intracellular bacteria, including M. tuberculosis.
The absolute requirement for IFN-γ in immune control of tuberculosis is well-established in animal models (3) and humans (4). IFN-γ-dependent protection is commonly believed to act through increasing the mycobactericidal activity of macrophages, but recent work has revealed additional important regulatory roles of IFN-γ during M. tuberculosis infection (5, 6). The role of type I IFNs in tuberculosis is also incompletely understood but, in contrast to IFN-γ, there is evidence that type I IFNs actually promote rather than control M. tuberculosis infection. Production of type I IFNs by restimulated peripheral blood monocytes from patients with active, but not latent, infection (7) as well as a type I IFN-inducible transcriptional signature in blood leukocytes from patients with active tuberculosis (8) suggest a positive correlation between type I IFNs and disease. However, reports show M. tuberculosis can inhibit production of (9) and response to (10) type I IFNs, which indicate these cytokines may also benefit the host. In fact, type I IFNs have been found to have a beneficial effect against pulmonary tuberculosis in a clinical trial (11). Type I IFNs have also been suggested to be a potential therapy for infections with multidrug-resistant strains (12) or in patients with IFNGR deficiencies (13). However, exacerbation of tuberculosis has been reported in a patient receiving IFN-α treatment for viral hepatitis (14). Animal models have also shown both beneficial and deleterious effects of type I IFNs during mycobacterial infections. While type I IFNs can impair the ability of human macrophages to control the growth of Mycobacterium bovis BCG (15), IFN-β has been shown to improve BCG immunogenicity by increasing human dendritic cell (DC) maturation (16). A recent study has revealed the inhibitory role of _M. tuberculosis_-induced type I IFN upon IL-1β production, considered as essential for the control of mycobacterial infection, in human macrophages (17). These discrepancies could be attributed to the fact that type I IFNs act differently on distinct cell types (18) and therefore in vitro systems cannot reflect the sum of their effects in vivo. Likewise, studies in animal models have characterized the role of type I IFNs during mycobacterial infections and have found distinct effects, depending on the bacterial species or strain. For example, infusion of IFN-β enhanced the resistance of mice to Mycobacterium avium infection (19). In contrast, the apparently hypervirulent strain of M. tuberculosis HN878 has been reported to induce high levels of type I IFNs, and pathology in HN878-infected wild-type mice was worsened by intranasal treatment with IFN-α/β (20). Infection of Ifnar−/− mice revealed that type I IFNs were necessary for the early control of M. bovis BCG infection (21), while Ifnar−/− mice showed improved control of late infection with the HN878 strain (22). This time-dependent relationship between type I IFNs and control of tuberculosis, could also contribute to the lack of consensus on the role of these cytokines.
A crucial element to understand the role of type I IFNs in tuberculosis is their ability to influence recruitment and/or differentiation of myeloid cells (23), which are primary cellular targets of mycobacteria. Administration of the potent type I IFN inducer polyinosinic-polycytidylic acid stabilized with poly-L-lysine in vivo induced type I IFN-dependent recruitment of a CD11b+F4/80+Gr1int subset of _M. tuberculosis_-permissive macrophages (24). Finally, type I and type II IFNs have overlapping biological activities, since both can induce phosphorylation and dimerization of the transcription factor, STAT1, which can obscure their individual anti-mycobacterial contributions.
The present study was designed to clarify the role of type I IFNs during tuberculosis, while isolating the potentially confounding influence of type II IFN. We studied the immune responses of mice unable to respond to IFN-α/β, IFN-γ, or both in a model of aerosol infection. We discovered that type I IFNs play a non-redundant role in protection against M. tuberculosis. That role is masked in the presence of high levels of IFN-γ during adaptive immune responses but is essential for the optimal recruitment, differentiation and/or survival of myeloid cells in the lungs, in particular CD11cnegCD11b+Gr-1lo/neg macrophages. These observations do not exclude a model in which an excess of type I IFNs might be detrimental to control of infection, but they emphasize the need for caution if anti-tuberculosis therapies are to be based on inhibition of type I IFN signaling.
Material and methods
Mice
Wild type and IFN-γ receptor null (Ifngr−/−) mice on the C57BL/6 background were originally purchased from The Jackson Laboratory (Bar Harbor, ME). IFN-α/β receptor null (Ifnar−/−) and STAT1 null (Stat1−/−) mice, both backcrossed to C57BL/6 mice for at least 10 generations, were generously provided by Professors Jonathan Sprent (The Scripps Research Institute, La Jolla, CA) and David Levy (New York University School of Medicine) respectively. Ifnar−/− and Ifngr−/− mice were crossed to obtain double null mutant mice (Ifnar−/−Ifngr−/−). All mice were bred and maintained under specific pathogen-free conditions at the NYULMC. For infections with Mycobacterium tuberculosis, animals were housed under barrier conditions in the ABSL-3 facility at NYULMC. For tissue harvest, mice were euthanized by CO2 asphyxiation followed by cervical dislocation. All mice were between 8 and 12 weeks of age at the beginning of the experiment. All experiments were performed with the prior approval of the NYULMC Institutional Animal Care and Use Committee.
Bacterial infection
Mycobacterium tuberculosis H37Rv was grown as previously described (25). In some experiments, a strain of M. tuberculosis H37Rv expressing green fluorescent protein (Mtb-GFP) (26) was used. Mice were infected via the aerosol route, using an inhalation exposure system (Glas-Col) (26), calibrated to deliver approximately 100 colony forming units (CFU) per animal. The infectious dose was confirmed on day 1 by plating whole lung homogenates from 5 mice on Middlebrook 7H11 agar. CFU were counted after incubation at 37°C for 2–3 weeks. To determine the bacterial load throughout the infection, lungs and mediastinal lymph node were harvested, homogenized, and serial dilutions were plated on Middlebrook 7H11 agar.
Histopathology
The left lung was harvested and processed as previously described (5) for histopathological examination.
Flow cytometry and microscopy
Lungs were removed and processed into single cell suspensions for flow cytometry and enumeration of GFP-expressing M. tuberculosis per cell in specific subsets as previously described (26). Antibodies conjugated to various fluorophores and directed against the following surface markers were used: CD49b (Dx5, BD Biosciences), NK1.1 (PK136, BD Biosciences), CD4 (RM4-5, BioLegend), CD8 (53–6.7, BD Biosciences), CD62L (MEL-14, BioLegend), CD107a (1D4B, BD Biosciences), H2Kb (AF6-88.5, BioLegend), CD11c (HL3, BD Biosciences), CD11b (M1/70, BD Biosciences), Gr-1 (RB6-8C5, BD Biosciences), CD40 (HM40-3, BD Biosciences), CD80 (B7-1, BD Biosciences), CD86 (B7-2, BD Biosciences), I-A/I-E (2G9, BD Biosciences). A minimum of 200,000 events per sample, gated on single live cells using forward and side scatter parameters, was acquired using a FACScalibur and CellQuest software or an LSRII and FACSDiva software (BD Biosciences). Data were analyzed using FlowJo software (TreeStar).
ELISA measurement of cytokine production
Supernatants from whole lung homogenates harvested at various time points following M. tuberculosis infection were analyzed by ELISA using commercial kits to quantitate IFN-β (R&D Systems) and IFN-γ (BD Biosciences).
Statistics
Experiments were routinely performed two or three times or more, and one representative set of results is presented. Results are expressed as mean and standard error. Unless otherwise stated, Student’s two-tailed t-test was used to compare experimental groups, with P <0.05 considered significant.
Results
Type I IFNs play a non-redundant protective role during tuberculosis
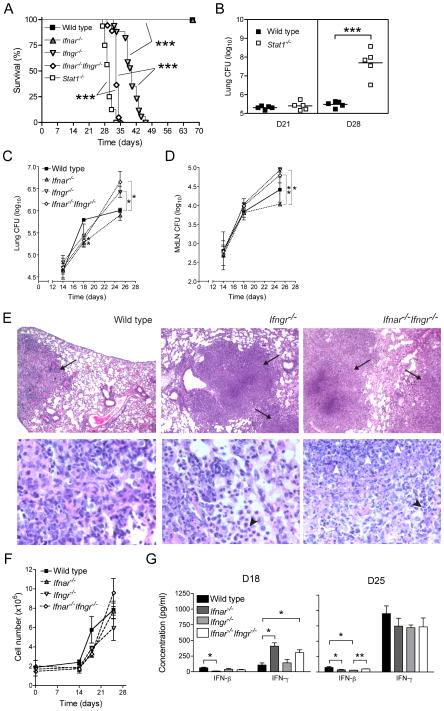
To characterize the role of type I IFNs during tuberculosis without the potential confounding presence of type II IFN, we used mice incapable of responding to both types of IFNs by lack of specific receptors (Ifnar−/−Ifngr−/−) or their common transcription factor (Stat1−/−). We first studied the consequences of a low-dose aerosol infection with M. tuberculosis on survival and lung bacterial load in Stat1−/− and Ifnar−/−Ifngr−/− mice and compared them to their single-null mutant counterparts and to wild type C57BL/6 mice. Ifnar−/−Ifngr−/− mice died significantly earlier than Ifngr−/− mice (median survival = 33 days vs. 40 days, p<0.0001; Fig. 1A) while none of the Ifnar−/− or wild type mice succumbed during the first 70 days following M. tuberculosis infection. Stat1−/− mice infected simultaneously died even earlier than Ifnar−/−Ifngr−/− mice (median survival = 30 days vs. 33 days, p<0.0001; fig. 1A). In a parallel experiment, quantitation of lung bacterial loads revealed that Stat1−/− mice harbored more than 2 log10 more bacteria in their lungs than wild type mice at day 28 post-infection, whereas the bacterial loads in the two groups were indistinguishable a week earlier (Fig. 1B). Similarly, there was no difference in lung bacterial loads in wild type, Ifnar−/−, Ifngr−/− or Ifnar−/−Ifngr−/− mice before 18 days post-infection (Fig. 1C). On day 18, all groups of receptor null mice had approximately 40% fewer bacteria in their lungs than wild type mice. By day 25, Ifnar−/− mice still had fewer M. tuberculosis in their lungs than any other groups. However, bacterial loads in the lungs of Ifngr−/− and Ifnar−/−Ifngr−/− mice increased exponentially after day 18 and by day 25, they were 3- and 6-fold higher respectively than in wild type mice. In the mediastinal lymph node, there was no difference in bacterial loads in any of the groups of mice until 25 days post-infection. At that time, the lymph node CFU mirrored that in the lungs with a significantly lower bacterial burden in Ifnar−/− mice and higher number of M. tuberculosis in the lymph nodes of Ifngr−/− and Ifnar−/−Ifngr−/− mice (Fig. 1D).
Figure 1. Type I IFNs play an early, non-redundant role in protection against M. tuberculosis.
(A) Survival of wild type (n=5), Ifngr−/− (n=20), Ifnar−/− (n=5), Ifnar−/−Ifngr−/−(n=20) and Stat1−/− (n=20) mice following aerosol infection with M. tuberculosis (Mtb); groups were compared using a log-rank test; ***: p<0.0001. (B) Bacterial load in the lungs of wild type and Stat1−/− mice (n=5) 21 and 28 days post-infection (dpi) with Mtb; ***: p<0.0001. (C) Lung and (D) mediastinal lymph node (MdLN) bacterial load in wild type, Ifngr−/−, Ifnar−/−, Ifnar−/−Ifngr−/− mice after Mtb infection; data are expressed as mean ± S.E for n=3; *: p<0.05, **: p<0.005. (E) Representative lung histopathology in wild type, Ifngr−/− and Ifnar−/−Ifngr−/− mice 28 dpi, showing granulomatous lesions (arrows), neutrophilic infiltration (black arrowheads) and tissue necrosis (white arrowheads); hematoxylin and eosin staining, original magnification: 4x (upper panels), 40x (lower panels). (F) Total lung cell number in wild type, Ifngr−/−, Ifnar−/−, Ifnar−/−Ifngr−/− mice after Mtb infection; data are expressed as mean ± S.E for n=3. (G) IFN-β and IFN-γ concentrations measured by ELISA in lung homogenate supernatants of wild type, Ifngr−/−, Ifnar−/−, Ifnar−/−Ifngr−/− mice 18 and 25 dpi; data are expressed as mean ± S.E for n=3; *: p<0.05, **: p<0.01.
Histopathological examination of the lungs revealed that, on day 25, wild type mice presented a classic granulomatous response with few limited infiltrates of inflammatory cells and with alveolar spaces retaining most of their structural integrity (Fig. 1E). In Ifngr−/− mice, the extent of the inflammatory infiltrates was noticeably greater than in wild type mice, and more neutrophilic in nature (Fig. 1E). However, those infiltrates remained regionally localized and a substantial proportion of healthy tissue remained. In contrast, in Ifnar−/−Ifngr−/− mice, infiltration of the tissue by inflammatory cells, neutrophils in particular, severely compromised the architecture of the entire lung (Fig. 1E). In addition, tissue liquefaction was observed and centers of granulomas were characterized by tissue necrosis (Fig. 1E). When we enumerated total lung cells, we observed that in the first 18 days of infection Ifnar−/−, Ifngr−/− and Ifnar−/−Ifngr−/− mice had lower cell counts, between 3.3±0.3×106 and 3.8±0.2×106 cells, than wild type mice which totaled 5.8±1.4×106 cells (Fig. 1F). At day 25, total cell yields from the lungs were more scattered between groups, Ifnar−/−Ifngr−/− mice presenting the highest cellularity, with 9.6±1.4×106 cells, consistent with the histological observations (Fig. 1E).
Finally, considering single IFN receptor null mice are able to respond to other types of IFN, we assessed the effects of IFN receptor deletion on the levels of IFNs in the lungs during TB (Fig. 1G). Early in infection, IFN-β and IFN-γ were produced in similar quantities in wild type mice, 62±10 and 107±34 pg/ml respectively on day 18. Deletion of type I IFN receptor had divergent effects on type I and type II IFN in the lungs, with a significant reduction in IFN-β (12.1±0.8 pg/ml; p=0.038) and a significant increase in IFN-γ (408±55 pg/ml; p=0.0152). Interestingly, increased levels of IFN-γ were also observed in Ifnar−/−Ifngr−/− mice (304±48 pg/ml), suggesting IFN-γ-independent mechanisms. On the other hand, deletion of type II IFN receptor had little or no effect on the respective levels of IFN-β or IFN-γ at that time of infection. By day 25, the levels of IFN-γ in the lungs of M. tuberculosis infected wild type mice rose to 950±126 pg/ml, approximately twelve-fold the level of IFN-β, which remained similar to the levels measured a week earlier (75±12 pg/ml). Deletion of IFN receptors - type I, II or both -had the same effect on decreasing the levels of IFN-β at day 25 as on day 18. Levels of IFN-γ in the lungs of _M. tuberculosis_-infected mice at day 25 were only marginally affected by the deletion of either IFN receptor.
These data show that type I IFN plays a non-redundant role, which is most readily demonstrated in the absence of IFN-γ signaling, for the control of lung bacterial burdens and histopathology, as well as survival, following M. tuberculosis infection in mice. In addition, the relationship between type I, type II IFN, bacterial control and recruitment of cells to the lungs during TB changes over time, with a transition at 3 weeks of infection during the onset of adaptive immunity, characterized by a marked increase in IFN-γ production.
Type I IFN only marginally affects T cells in lungs of M. tuberculosis-infected mice
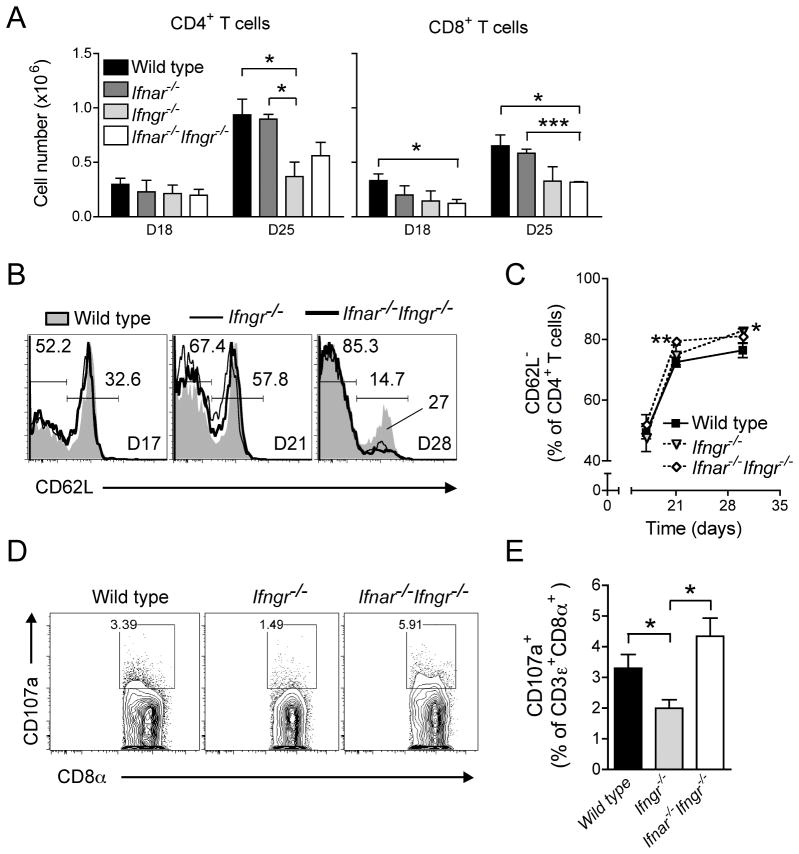
To evaluate whether the early protective effect of type I IFN responsiveness during tuberculosis is mediated through enhanced priming or trafficking of T lymphocytes, we examined populations of CD4+ and CD8+ T cells in the lungs of infected wild type and IFN receptor null mice.
At day 18 post-infection, CD4+ T cell populations were equivalent in wild type mice and those lacking type I and/or type II IFN receptors (Fig. 2A). In contrast, there were fewer CD8+ T cells in the lungs of Ifnar−/−Ifngr−/− mice compared with wild type mice, while mice lacking individual IFN receptors had intermediate numbers of CD8+ T cells in their lungs.
Figure 2. Type I IFNs have a limited impact on T cell recruitment and activation during early tuberculosis.
(A) CD4+ and CD8+ T cell numbers in the lungs of wild type, Ifngr−/−, Ifnar−/−, Ifnar−/−Ifngr−/− mice 18 and 25 days following aerosol infection with Mtb; data are expressed as mean ± S.E for n=3; *: p<0.05, ***: p<0.0001. (B) Representative flow cytometry histogram of CD62L expression on CD4+ T cells in the lungs of wild type, Ifngr−/− and Ifnar−/−Ifngr−/− mice 17, 21 and 30 dpi; data are expressed as the percentage of CD4+ T cells in each gate. (C) Frequency of CD62L− cells amongst CD4+ T cells in the lungs of wild type, Ifngr−/− and Ifnar−/−Ifngr−/− mice following Mtb infection; data are expressed as mean ± S.E for n=5; *: p<0.05, **: p<0.005. (D) Representative flow cytometry plot of CD107a expression on CD8+ T cells in the lungs of wild type, Ifngr−/− and Ifnar−/−Ifngr−/− mice 25 dpi; data are expressed as the percentage of CD107a+ amongst CD8+ T cells. (E) Frequency of CD107a+ cells amongst CD8+ T cells in the lungs of wild type, Ifngr−/− and Ifnar−/−Ifngr−/− mice 25 dpi; data are expressed as mean ± S.E for n=5; *: p<0.05.
By day 25, both CD4+ and CD8+ T cell populations had increased similarly in the lungs of wild type and Ifnar−/− mice, by 2 to 3.5-fold compared with day 18. In contrast, the number of CD4+ T cells increased minimally in Ifngr−/− mice, with 60% fewer CD4+ T cells than in the lungs of wild type mice at that time. The increase in CD4+ T cells was also limited in Ifnar−/−Ifngr−/− mice on day 25, with approximately 40% fewer CD4+ T cells than in the lungs of wild type mice. In comparison to wild type and Ifnar−/− mice, the increase of CD8+ T cell populations in the lungs of Ifngr−/− mice was also limited, independently of their capacity to respond to type I IFN. Overall, responsiveness to IFN-γ was the strongest determinant of the recruitment of CD4+ and CD8+ T cells to the lungs early after infection with M. tuberculosis; the ability of mice to respond to type I IFN had no apparent effect on T cell recruitment in the first month of infection.
Consistent with other studies using BCG (27) or M. tuberculosis (28), we found that the frequency of effector, CD4+CD62Llo/neg T cells in the lungs rose sharply during the third week of infection, from day 17 to day 21 (Fig. 2B, 2C); this was not affected by the capacity to respond to IFN-α/β or IFN-γ. However, Ifnar−/−Ifngr−/− mice displayed a significantly higher frequency of effector CD4+CD62Llo/neg T cells (79.4±0.8%; p=0.0049) in their lungs than wild type mice (72.5±1.6%) at day 21. Nine days later, the frequency of effector CD4+CD62Llo/neg T cells in the lungs had only increased slightly in all groups but was still higher in Ifnar−/−Ifngr−/− mice (81.1±2.4%) and significantly higher in Ifngr−/− mice (82.8±1.3%; p=0.0164) compared to that in wild type mice (76.4±2.4%). Wild type mice also retained a larger population of naïve CD4+CD62L+ T cells in their lungs than Ifnar−/−, Ifngr−/− or Ifnar−/−Ifngr−/− mice (Fig. 2B), possibly reflecting the higher bacterial burdens in the different groups of mice at that time.
Cytolytic CD8+ T cell activity in the lungs, as assessed by surface expression of the degranulation marker CD107a/LAMP-1 (Fig. 2D), was significantly higher in wild type (3.3±0.4%; p=0.0478) and Ifnar−/−Ifngr−/− mice (4.3±0.6%; p=0.0115) than in Ifngr−/− mice (2.0±0.3%) at day 25, indicating that responsiveness to type I IFNs is necessary for optimal development and/or degranulation of cytolytic CD8+ T cells in this context (Fig. 2E). However, since the higher frequency of CD8+ T cell degranulation did not translate to improved control of bacterial growth in the lungs, this effect of type I IFNs is clearly insufficient to provide improved control of M. tuberculosis at this stage of infection.
Overall, these results indicate that, in addition to their marginal effect on T cell recruitment to the lungs, type I IFNs play no more than a transient or secondary role in CD4+ and CD8+ T cell activation during early infection with M. tuberculosis, particularly in the context of IFN-γ responsiveness.
NK cell responses during M. tuberculosis infection require both type I and type II IFN responsiveness
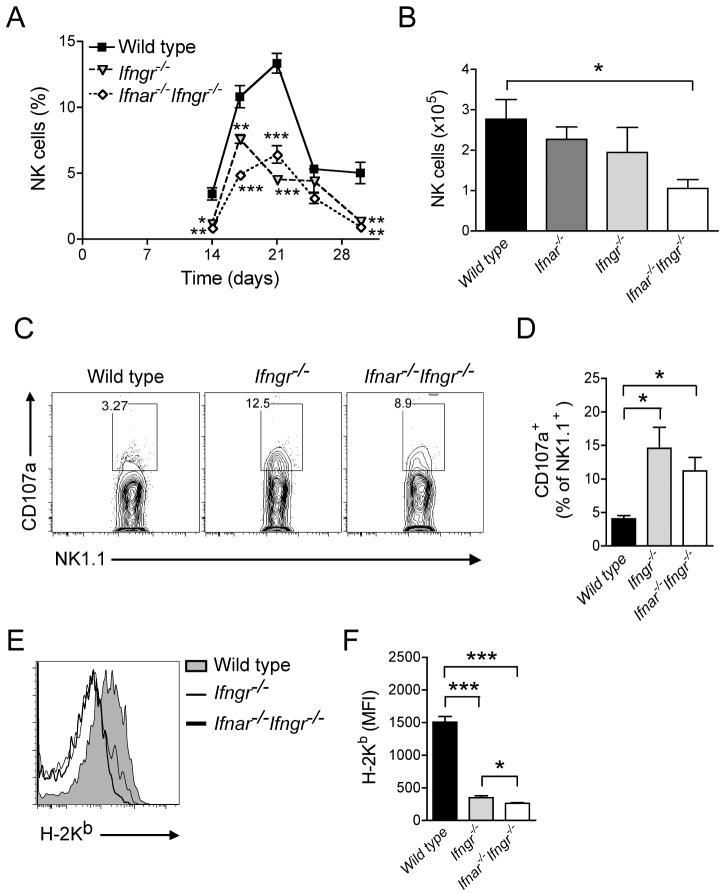
Type I IFNs have been implicated in NK cell homeostasis and activation against tumors (29) or viral infections (30), indicating that differences in NK cells might provide at least a partial explanation for the poorer control of M. tuberculosis that we observed in Ifnar−/−Ifngr−/− versus Ifngr−/− mice. To test the hypothesis that this applies during infection with M. tuberculosis, we first determined whether recruitment of NK cells to the lungs during tuberculosis was dependent on responsiveness to type I and/or type II IFNs.
In an initial experiment, we observed that CD3−Dx5+ NK cells were recruited to the lungs of mice following M. tuberculosis infection with a peak frequency at day 21 (13.3±0.7% of total lung cells), followed by a rapid decline (Fig. 3A). This recruitment peak was significantly blunted in Ifngr−/− (4.5±0.2%; p<0.0001) as well as in Ifnar−/−Ifngr−/− mice (6.4±0.7%; p=0.0001). In an additional experiment, we also determined that the number of NK cells in the lungs of Ifnar−/−Ifngr−/− mice was reduced by 60% in comparison to wild type mice 18 days post-infection (Fig. 3B). This effect was due to combined unresponsiveness to both types of interferons, as Ifnar−/− and Ifngr−/− mice only displayed a reduction of 20% and 30% of NK cell populations respectively in comparison to wild type mice.
Figure 3. Both type I and type II IFNs are required for optimal recruitment of NK cells to the lungs following M. tuberculosis infection.
(A) Frequency of NK cells in the lungs of wild type, Ifngr−/− and Ifnar−/−Ifngr−/− mice following aerosol infection with Mtb; data are expressed as mean ± S.E for n=5. (B) Number of NK cells in the lungs of wild type, Ifngr−/−, Ifnar−/−, Ifnar−/−Ifngr−/− mice 21 dpi; *: p<0.05. (C) Representative flow cytometry plot of CD107a expression on NK cells in the lungs of wild type, Ifngr−/− and Ifnar−/−Ifngr−/− mice 25 dpi; data are expressed as the percentage of CD107a+ amongst NK1.1+ cells. (D) Frequency of CD107a+ cells amongst NK cells in the lungs of wild type, Ifngr−/− and Ifnar−/−Ifngr−/− mice 25 dpi; data are expressed as mean ± S.E for n=5; *: p<0.05. (E) Representative flow cytometry histogram of MHC class I expression (H-2Kb) on myeloid dendritic cells (CD11bhiCD11c+) in the lungs of wild type, Ifngr−/− and Ifnar−/−Ifngr−/− mice 25 dpi. (F) Level of expression of MHC class I (H-2Kb) on myeloid dendritic cells in the lungs of wild type, Ifngr−/− and Ifnar−/−Ifngr−/− mice 25 dpi; data are expressed as mean fluorescence intensity (MFI) ± S.E for n=5; *: p<0.05, ***: p<0.0001.
Although NK cells were less abundant in the lungs of Ifnar−/−, Ifngr−/− or Ifnar−/−Ifngr−/− mice, their cytolytic activity as assayed by surface expression of CD107a/LAMP-1 in CD3e−NK1.1+ cells (Fig. 3C) was significantly higher in Ifngr−/− as well as in Ifnar−/−Ifngr−/− mice than in wild type mice after 25 days of M. tuberculosis infection (Fig. 3D). As NK cell degranulation can be triggered by low levels of MHC class I on target cells (31), we quantified this parameter by flow cytometry on diverse myeloid cell subsets in the lungs (Fig. 3E). Myeloid subsets were defined based on the expression of specific surface markers (Fig. 4). We observed that the level of expression of MHC class I on CD11bhiCD11c+ myeloid DC was significantly lower in Ifngr−/− (Mean Fluorescence Intensity (MFI): 351±28; p<0.0001) and Ifnar−/−Ifngr−/− mice (MFI: 262±13; p<0.0001) compared with wild type mice (MFI: 1506±87) (Fig. 3F). Similarly, low levels of MHC class I were observed on other myeloid cells from Ifnar−/−, Ifngr−/− or Ifnar−/−Ifngr−/− mice although these differences did not reach statistical significance in comparison to wild type mice (data not shown).
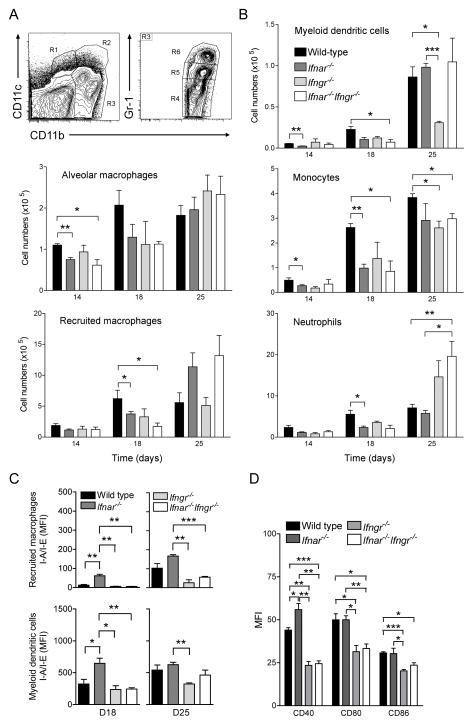
Figure 4. Type I and type II IFNs orchestrate optimal recruitment and/or differentiation and activation of lung myeloid cells during early tuberculosis.
(A) Flow cytometry gating strategy for quantification of lung myeloid cell populations following aerosol infection with M. tuberculosis. R1 (CD11bnegCD11c+): alveolar macrophages, R2 (CD11bhiCD11c+): myeloid dendritic cells, R4 (CD11cnegCD11b+Gr-1lo/neg): recruited macrophages, R5 (CD11cnegCD11b+Gr-1int): monocytes, R6 (CD11cnegCD11bhiGr-1hi): neutrophils. (B) Abundance of lung myeloid cell populations in the lungs of wild type, Ifnar−/−, Ifngr−/− and Ifnar−/−Ifngr−/− mice 14, 18 and 25 dpi; data are expressed as mean ± S.E for n=3; *: p<0.05, **: p<0.01, ***: p<0.0001. (C) Expression of MHC class II (I-A/I-E) on recruited macrophages and myeloid dendritic cells in the lungs of wild type, Ifnar−/−, Ifngr−/− and Ifnar−/−Ifngr−/− mice 18 and 25 dpi; data are expressed as mean fluorescence intensity (MFI) ± S.E for n=3; *: p<0.05, **: p<0.01, ***: p<0.0001. (D) Expression of co-stimulatory molecules, i.e. CD40, CD80 and CD86, on myeloid dendritic cells in the lungs of wild type, Ifnar−/−, Ifngr−/− and _Ifnar−/−Ifngr−/−_mice 25 days after infection with M. tuberculosis; data are expressed as mean fluorescence intensity (MFI) ± S.E for n=3; *: p<0.05, **: p<0.01, ***: p<0.0001.
These observations indicate the importance of responsiveness to type I and type II IFNs for optimal development and/or recruitment of NK cells to the lungs following M. tuberculosis infection as well as their roles in MHC class I expression and subsequent NK cell activation.
Type I and type II IFN signaling orchestrates the accumulation and activation of myeloid cells in the lung during M. tuberculosis infection
Type I and II IFNs have been reported to individually influence recruitment, differentiation and activation of myeloid cells under inflammatory conditions or during infections (23, 32). Our approach, based on single and double null mutant mice for their respective receptors, allowed us to examine how their interaction affects lung myeloid cell populations during M. tuberculosis infection.
As shown by flow cytometry (Fig. 4A), lung myeloid cell populations were dominated by CD11cnegCD11b+Gr-1lo/neg recruited macrophages and CD11cnegCD11bhiGr-1hi neutrophils in all groups of mice until day 18 (Fig. 4B). At that time, responses to either type I or type II IFN were necessary for optimal recruitment and/or differentiation of all cell types, i.e. monocytes, recruited macrophages, myeloid DC and neutrophils. On day 25 post-infection, a further increase in the myeloid DC population occurred in the lungs of wild type mice. This increase depended upon the ability of mice to respond to IFN-γ Interestingly, the deficit of myeloid DC in the lungs of Ifngr−/− mice was dependent on type I IFN signaling, as Ifnar−/−Ifngr−/− mice had a normal population of myeloid DC in their lungs, even though type I IFNs did not show any independent effect on myeloid DC recruitment and/or differentiation at this time of infection. Also on day 25 post-infection, mice unable to respond to type I IFN possessed on average twice as many recruited macrophages (Ifnar−/− mice: 11.4±3.9×105 cells, Ifnar−/−Ifngr−/− mice: 13.2±3.3×105 cells) in their lungs compared with wild type mice (5.6±1.6×105 cells), with no effect of their responsiveness to IFN-γ (Ifngr−/− mice: 5.1±0.4×105 cells). The optimal recruitment of monocytes to the lungs of _M. tuberculosis_-infected mice remained dependent on both types of IFNs at day 25, as it was earlier. Consistent with the observation by histopathology, the number of neutrophils in the lungs was significantly higher in _Ifngr−/−_and Ifnar−/−Ifngr−/− mice than in wild type or Ifnar−/− mice.
Both types of IFNs also regulated the level of activation of lung myeloid cells in vivo as measured by surface expression of MHC class II and co-stimulatory molecules. In particular, Ifnar−/− mice showed a significantly enhanced level of MHC class II on recruited macrophages (MFI: 61±8; p=0.0055) and myeloid DC (MFI: 647±81; p=0.0419) when compared to wild type mice (MFI: 12±4 and 323±73, respectively) on 18 days post-infection (Fig. 4C). That enhancement was entirely IFN-γ-dependent as recruited macrophages and myeloid DC in Ifnar−/−Ifngr−/− mice showed similar levels of MHC class II compared with wild type mice. In contrast, IFN-γ did not have any impact of its own at this early stage of M. tuberculosis infection. However, on day 25, IFN-γ responsiveness was clearly responsible for a sharp increase in MHC class II levels on recruited macrophages and for a more limited one on myeloid DC. At that time of infection, the effects of type I IFN signaling on MHC class II expression waned in both cell types. Similarly, levels of co-stimulatory molecules. i.e. CD40, CD80 and CD86, on myeloid DC were also completely dependent on the capacity of cells to respond to IFN-γ, whereas type I IFN had no noticeable effect (Fig. 4D).
Our observations reveal that the interplay between type I and type II IFNs for recruitment and/or differentiation of myeloid cells and their activation as antigen presenting cells (APC) in the lungs during early M. tuberculosis infection is biphasic. During the initial 3 weeks of infection, both IFNs act together to populate the lungs with most cell types but they have divergent effects on activation of APC. Beyond 3 weeks, type I and type II IFNs also diverge in their recruitment/differentiation potential of myeloid cells as IFN-γ becomes the predominant regulator of APC activation.
Type I IFNs determine the number of M. tuberculosis-infected macrophages in the lungs
In order to better understand the impact of type I and type II IFNs on myeloid cell populations and the increased bacterial burdens in the lungs of Ifnar−/−Ifngr−/− mice, we investigated the distribution of M. tuberculosis within specific cell subtypes as well as bacterial burdens at the single cell level.
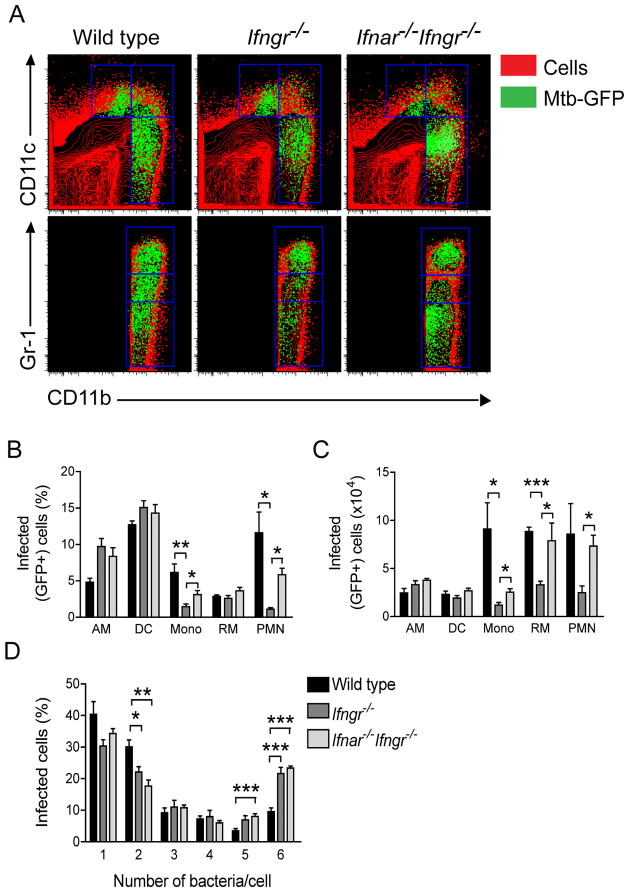
We used a GFP-expressing strain of M. tuberculosis (26), together with flow cytometry, to identify individual myeloid cells that contain bacteria (Fig. 5A). Consistent with our prior observation with wild type mice (26), on day 25, dendritic cells were the most frequently infected cells - between 12 and 15% of the population (Fig. 5B) - and there was no apparent influence of responsiveness from these cells to IFNs. Alveolar macrophages were more susceptible to M. tuberculosis infection in the absence of IFN-γ signaling, without any additional effect of type I IFNs, but these differences were not translated in absolute numbers of infected cells (Fig. 5C). In contrast, monocytes and neutrophils were infected significantly less frequently (Fig. 5B) and in smaller numbers (Fig. 5C) in Ifngr−/− mice than in wild type mice. Both in frequency and in absolute numbers, Ifnar−/−Ifngr−/− mice had a significantly more infected monocytes and neutrophils than Ifngr−/− mice. Finally, while the frequency of infected recruited macrophages was not influenced by the ability of mice to respond to IFNs, there was a clear role of type I IFNs on the absolute number of infected recruited macrophages. Significantly more infected recruited macrophages were present in the lungs of _M. tuberculosis_-infected Ifnar−/−Ifngr−/− mice (7.9±2.1×104 cells: p=0.049) than in Ifngr−/− mice (3.3±0.4×104 cells). Interestingly, wild type mice had a similar number of infected recruited macrophages (8.8±0.5×104 cells) compared with Ifnar−/−Ifngr−/− mice. Because we observed a significantly higher lung bacterial load in Ifnar−/−Ifngr−/− mice than in wild type mice (Fig. 1C), we hypothesized that, for an equal number of infected cells, the number of bacteria per cell was higher in mice unable to respond to both types of IFN. Indeed, enumeration of GFP-expressing M. tuberculosis in infected cells by fluorescence microscopy revealed that mice unable to respond to IFN-γ harbored significantly more bacteria per cell than wild type mice (Fig. 5D). It is noteworthy that this effect was not altered by the additional absence of type I IFN responsiveness.
Figure 5. Type I IFN signaling is required to limit the number of cells infected by M. tuberculosis in the lung.
(A) Representative flow cytometry plots showing the distribution of GFP-expressing M. tuberculosis overlayed onto lung myeloid cell populations as identified by expression of CD11b, CD11c and Gr-1 in wild type, _Ifngr−/−_and Ifnar−/−Ifngr−/− mice at 25 dpi. (B) Frequency of infected (GFP+) cells in the lungs of wild type, Ifngr−/− and Ifnar−/−Ifngr−/− mice (legend in figure 5D) in each myeloid cell population; AM: alveolar macrophages, DC: myeloid dendritic cells, Mono: monocytes, RM: recruited macrophages, PMN: neutrophils; data are expressed as mean ± S.E for n=5; *: p<0.05, **: p<0.01. (C) Numbers of infected (GFP+) cells in the lungs of wild type, Ifngr−/− and Ifnar−/−Ifngr−/− mice (legend in figure 5D) in each myeloid cell population; data are expressed as mean ± S.E for n=5; *: p<0.05, ***: p<0.0001. (D) Frequency of infected cells in the lungs of wild type, Ifngr−/− and Ifnar−/−Ifngr−/− mice distributed according to the number of bacteria in each cell counted by microscopy in at least 100 cells/mouse; data are expressed as mean ± S.E for n=5; *: p<0.05, **: p<0.01, ***: p<0.0001.
Together, these results explain the higher bacterial burden in the lungs, and most likely the impaired survival, of Ifnar−/−Ifngr−/− mice compared with Ifngr−/− mice. This difference appears to be due to the role of type I IFNs in the modulation of myeloid cell populations, in particular recruited macrophages. However, the decisive factor determining susceptibility to M. tuberculosis at the individual cell level in vivo is the ability to respond to IFN-γ (Fig. 6).
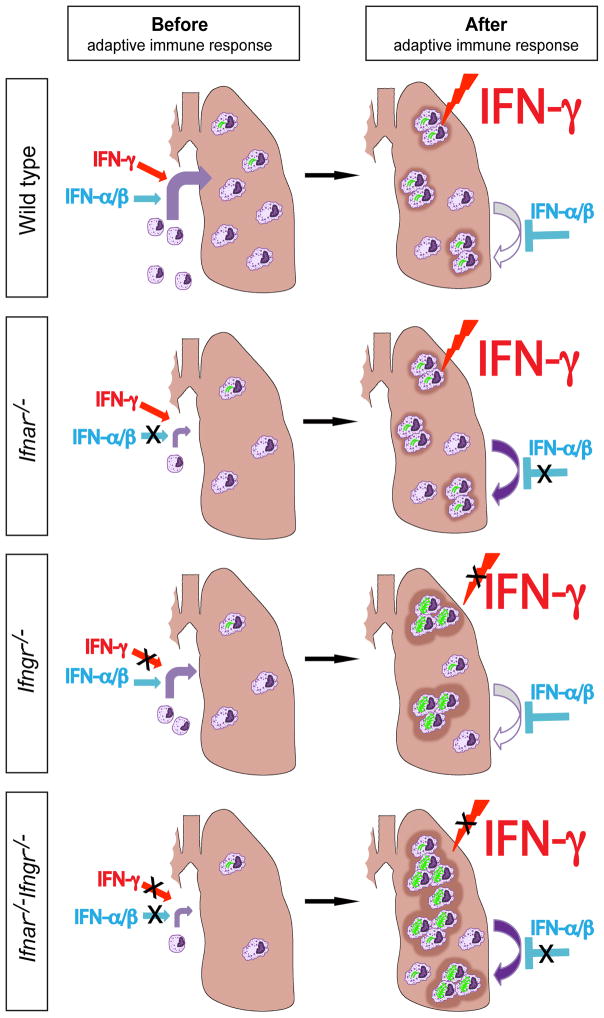
Figure 6. Interplay of IFNs controls early tuberculosis.
Schematic model of myeloid cell recruitment, evolution of granulomatous lesions and influence of type I and type II IFNs in the lungs during early tuberculosis. In wild type mice, IFN-α β and IFN-γ are produced in similar amounts during the pre-adaptive immune phase (<3 weeks) and they both contribute to the optimal recruitment, differentiation and/or survival of myeloid cells to the lungs. In _Ifnar−/−_ mice, _Ifngr−/−_ mice and, even more markedly, in _Ifnar−/−Ifngr−/−_mice, this influx of potential cellular targets of _M. tuberculosis_ is limited, leading to lower lung bacterial loads. After the onset of the adaptive immune response (>3 weeks), IFN-γ is produced in greater amounts than type I IFNs and, in wild type mice, controls bacterial growth, including via activation of individual cells; at that time, type I IFNs limit the inflammatory effects of IFN-γ. For this reason, Ifnar−/− mice have an increased population of myeloid cells in the lungs but they retain the ability to control intracellular bacteria in response to IFN-γ. In Ifngr−/− mice, cells cannot control the growth of M. tuberculosis, leading to severe granulomatous lesions and neutrophilic infiltrates. In Ifnar−/−Ifngr−/− mice, the additional defect in type I IFN signaling increases recruitment, differentiation and/or survival of potential target cells of M. tuberculosis, which translates into widespread granulomatous response that affects the whole lung and further impairs mouse survival.
Discussion
The results that we present here demonstrate that type I IFNs play a non-redundant protective role during the early stages of tuberculosis in vivo. Our data also contribute to explain the conflicting results surrounding type I IFNs in mycobacterial infections. In particular, we reveal both pro- and anti-inflammatory properties of type I IFNs in tuberculosis, a duality already observed in autoimmune diseases (33–35). The transition from pro- to anti-inflammatory effects is time-dependent and type I IFNs ultimately balance out pro-inflammatory IFN-γ by limiting the abundance of potential target cells in the lungs (Fig. 6). However, we also demonstrate these effects of type I IFNs are intricately connected to and ultimately masked by the influence of IFN-γ especially after the onset of adaptive immunity.
During the innate immune phase following M. tuberculosis infection, both types of IFN are produced in similar quantities. Type I and type II IFNs activate parallel signaling pathways, resulting in phosphorylation, nuclear translocation and binding of Signal Transcription And Transduction (STAT) molecules to IFN-γ Activated Sequence (GAS) enhancer elements located in the promoter regions of pro-inflammatory genes (2), such as MCP-1/CCL2 or iNOS. This common pro-inflammatory pathway could explain how both types of IFN act synergistically to mount an optimal immune response to tuberculosis, in particular the recruitment, differentiation and/or survival of myeloid cells in the lungs. Paradoxically, this initial increase of myeloid cell populations, e.g. dendritic cells and recruited macrophages, promotes infection by providing target cells for intracellular growth of M. tuberculosis. This phenomenon, well characterized in the zebrafish embryo model of mycobacterial infection (36), explains the lower bacterial loads in the lungs of mice unable to respond to one or both types of IFNs early in infection. These effects on myeloid cells also explain the higher susceptibility of mice treated with polyinosinic-polycytidylic acid stabilized with poly-L-lysine, which induces type I IFN-dependent recruitment of a subset of _M. tuberculosis_-permissive macrophages (24). Additionally, we observed that, when type I and II IFNs are present in similar concentrations in the lungs, type I IFN limits the expression of IFN-γ-induced MHC class II on APC. This concentration-dependant regulatory role has been described previously in vitro (37) and has been shown to be due to down-regulation of IFNGR, at least in the context of L. monocytogenes infection (38). Higher expression of MHC class II on APC in Ifnar−/− mice at 3 weeks post-infection was accompanied by a higher production of IFN-γ in the lungs but without evidence of a direct correlation, particularly since IFN-α/β has also been shown to inhibit IFN-γ production (39). In any case, it did not improve bacterial control significantly when compared to Ifnar−/−Ifngr−/− mice. Taken together, our results suggest that the availability of _M. tuberculosis_-susceptible myeloid cells in the lungs is a more decisive correlate of disease control than response to IFN-γ before the onset of adaptive immune responses.
After the onset of adaptive immune responses to tuberculosis, CD4+ and CD8+ effector T cells traffic to the lungs where they produce IFN-γ, whose concentration then exceeds by a factor of ten that of type I IFN. Under these conditions, IFN-γ becomes the predominant regulator of T cell recruitment, activation of APCs, and control of M. tuberculosis growth as demonstrated by our results in terms of bacterial load per cell. While type I IFNs do not play any detectable role in the recruitment of T cells during tuberculosis, they still contribute to the control of lung myeloid cell populations, but this time by limiting the abundance of recruited macrophages and myeloid DC.
The molecular mechanisms behind type I IFN fate decision as pro- or anti-inflammatory effectors are still largely unknown. In addition to GAS elements, type I IFN-induced transcription factors can bind to other activating elements, such as IFN-stimulated response elements (ISRE)(2). Differential usage of these activating elements can be modulated by the nature, the duration of phosphorylation and/or the abundance of STAT molecules, all mechanisms regulated by IFNs themselves (40). Under these circumstances, it is possible that, in the presence of high concentrations of IFN-γ during tuberculosis, type I IFNs switch to an anti-inflammatory regulatory role. In particular, type I IFNs can inhibit inflammasome-mediated production of IL-1β by an ISRE-dependent mechanism (41). Additionally, type I IFNs can modulate STAT1 activity in myeloid cells by inducing a STAT3-dependent pathway, thus sequestering transcription factors in STAT1:STAT3 heterodimers (42). Activated STAT3 pathway can also increase IL-10 production and amplify anti-inflammatory responses by inducing BCL3 or IL-1 receptor antagonist (43). Finally, the ubiquitous distribution of IFNAR and IFNGR implies that many cell types can respond to these cytokines but with distinct outcomes, with some cellular subsets more prone to play an anti-inflammatory role. Recently, pulmonary CD11c+ cells were shown to suppress excessive IFN-γ response and prevent excessive lung damage following _Pneumocystis_-induced chronic inflammation via type I IFN-mediated signaling (44).
At the cell population level, the anti-inflammatory role of type I IFNs during tuberculosis can be explained by a reduction in a) recruitment, b) differentiation and/or c) survival of these myeloid cell subsets. Type I IFN-induced apoptosis in macrophages infected with L. monocytogenes (45) has been suggested as a mechanism to explain the excess of spleen macrophages in Ifnar−/− mice compared to wild type mice (46). In the case of myeloid DC, we have previously shown that the abundance of myeloid DC in the lungs of _M. tuberculosis_-infected mice depends on the expression of IFNGR on non-hematopoietic cells (5), which can explain the defect observed in Ifngr−/− mice in the present study. Surprisingly, we observed a restoration of myeloid DC populations in Ifnar−/−Ifngr−/− mice. This could represent an extension of in vitro data showing that human monocyte-derived DC generated in the presence of type I IFN are more susceptible to apoptosis when exposed to activating stimuli such as IFN-γ plus bacterial products (47) and therefore, the reduced population of lung myeloid DC in the absence of IFNGR would be compensated by an increased survival in the absence of IFNAR. Alternatives to the pro-apoptotic role of type I IFN have been proposed to explain its influence on myeloid cells during infection with M. tuberculosis. In particular, in vitro studies indicate that M. tuberculosis can divert human monocyte differentiation from dendritic cells into macrophage-like host cells in an IFN-α-dependant fashion (48). However, our experimental data do not necessarily corroborate nor refute this mechanism, some of which has also been described for IFN-γ (49). Other hypotheses include anti-proliferative properties displayed by type I IFN in general (50) and on murine macrophages in particular (51). Finally, the increased lung neutrophilia, correlated with the inability to respond to IFN-γ, is a known consequence of IL-17-driven inflammation during tuberculosis (6), on which we show here that type I IFN have no additional effect.
In the present study, we also demonstrated the importance of both types of IFNs for the optimal recruitment of NK cells to the lungs during the early phase of tuberculosis. Previous work showed that IFN-α/β production in vitro by _M. tuberculosis_-infected human DC leads to chemokine-mediated recruitment of NK cells (52). Even though an earlier study questioned the role of NK cells in the control of M. tuberculosis (53), these cells have been characterized as an early, T cell-independent source of IFN-γ following infection (54) and could prove to be a valuable asset for protection in HIV/AIDS patients.
In our model, combined effects of IFN-γ on mycobactericidal activity in target cells, e.g. recruited macrophages, and of type I IFN on their abundance in the lungs contribute to the overall immune control of tuberculosis. We detected no evidence of a detrimental role of type I IFN on the ability of individual cells to control intracellular growth of M. tuberculosis in vivo. Nevertheless, our results are compatible with previous studies showing that type I IFN can negatively influence the outcome of infection when its production is imbalanced, therefore modifying the equilibrium of myeloid cell subsets in the lungs. The difference observed between the clear detrimental role of type I IFNs in other bacterial infections, such as L. monocytogenes, and the more complex situation following M. tuberculosis infection might be explained by the fact that the latter has proven to be a relatively weak inducer of type I IFNs (data not shown), as well as on the selective tropism of M. tuberculosis for professional phagocytic cells. Further studies will be required to define the conditions, i.e. type I IFN subtypes, cellular source(s), concentrations, tissue distribution, and timing, under which the effects induced by type I IFN transition from protective to deleterious. As we have shown here, these studies will also need to take into account the influence of IFN-γ. The recent discovery of a relationship between IFNs and IL-1 production during _M. tuberculosi_s infection in vivo highlights the partial overlap of function between type I and type II IFNs (18). Finally, our results provide additional information, which may help determine whether the type I IFN signature associated with active tuberculosis in patients is the cause or the consequence of a poorly controlled infection. This distinction is crucial for future treatments targeting type I IFN-signaling in tuberculosis patients as well as for patients with combined viral infections, like hepatitis C or HIV.
Acknowledgments
We thank Eleanor Allen and Tawania Fergus for their invaluable technical assistance.
This work was supported by National Institutes of Health grants R01 AI059667 and R01 AI051242.
Abbreviations used in this article
BCG
bacille Calmette-Guérin
DC
dendritic cells
IFNAR
interferon-alpha/beta receptor
IFNGR
interferon-gamma receptor
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Cooper AM, Mayer-Barber KD, Sher A. Role of innate cytokines in mycobacterial infection. Mucosal Immunol. 2011;4:252–260. doi: 10.1038/mi.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type I interferons. Nat Rev Immunol. 2012 doi: 10.1038/nri3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. The Journal of experimental medicine. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dupuis S, Doffinger R, Picard C, Fieschi C, Altare F, Jouanguy E, Abel L, Casanova JL. Human interferon-gamma-mediated immunity is a genetically controlled continuous trait that determines the outcome of mycobacterial invasion. Immunol Rev. 2000;178:129–137. doi: 10.1034/j.1600-065x.2000.17810.x. [DOI] [PubMed] [Google Scholar]
- 5.Desvignes L, Ernst JD. Interferon-gamma-responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity. 2009;31:974–985. doi: 10.1016/j.immuni.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nandi B, Behar SM. Regulation of neutrophils by interferon-{gamma} limits lung inflammation during tuberculosis infection. The Journal of experimental medicine. 2011;208:2251–2262. doi: 10.1084/jem.20110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stern JN, Keskin DB, Romero V, Zuniga J, Encinales L, Li C, Awad C, Yunis EJ. Molecular signatures distinguishing active from latent tuberculosis in peripheral blood mononuclear cells, after in vitro antigenic stimulation with purified protein derivative of tuberculin (PPD) or Candida: a preliminary report. Immunol Res. 2009;45:1–12. doi: 10.1007/s12026-008-8024-2. [DOI] [PubMed] [Google Scholar]
- 8.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, Quinn C, Blankenship D, Dhawan R, Cush JJ, Mejias A, Ramilo O, Kon OM, Pascual V, Banchereau J, Chaussabel D, O’Garra A. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simmons DP, Canaday DH, Liu Y, Li Q, Huang A, Boom WH, Harding CV. Mycobacterium tuberculosis and TLR2 agonists inhibit induction of type I IFN and class I MHC antigen cross processing by TLR9. J Immunol. 2010;185:2405–2415. doi: 10.4049/jimmunol.0904005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prabhakar S, Qiao Y, Hoshino Y, Weiden M, Canova A, Giacomini E, Coccia E, Pine R. Inhibition of response to alpha interferon by Mycobacterium tuberculosis. Infection and immunity. 2003;71:2487–2497. doi: 10.1128/IAI.71.5.2487-2497.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giosue S, Casarini M, Alemanno L, Galluccio G, Mattia P, Pedicelli G, Rebek L, Bisetti A, Ameglio F. Effects of aerosolized interferon-alpha in patients with pulmonary tuberculosis. American journal of respiratory and critical care medicine. 1998;158:1156–1162. doi: 10.1164/ajrccm.158.4.9803065. [DOI] [PubMed] [Google Scholar]
- 12.Palmero D, Eiguchi K, Rendo P, Castro Zorrilla L, Abbate E, Gonzalez Montaner LJ. Phase II trial of recombinant interferon-alpha2b in patients with advanced intractable multidrug-resistant pulmonary tuberculosis: long-term follow-up. Int J Tuberc Lung Dis. 1999;3:214–218. [PubMed] [Google Scholar]
- 13.Ward CM, Jyonouchi H, Kotenko SV, Smirnov SV, Patel R, Aguila H, McSherry G, Dashefsky B, Holland SM. Adjunctive treatment of disseminated Mycobacterium avium complex infection with interferon alpha-2b in a patient with complete interferon-gamma receptor R1 deficiency. Eur J Pediatr. 2007;166:981–985. doi: 10.1007/s00431-006-0339-1. [DOI] [PubMed] [Google Scholar]
- 14.Telesca C, Angelico M, Piccolo P, Nosotti L, Morrone A, Longhi C, Carbone M, Baiocchi L. Interferon-alpha treatment of hepatitis D induces tuberculosis exacerbation in an immigrant. J Infect. 2007;54:e223–226. doi: 10.1016/j.jinf.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Bouchonnet F, Boechat N, Bonay M, Hance AJ. Alpha/beta interferon impairs the ability of human macrophages to control growth of Mycobacterium bovis BCG. Infection and immunity. 2002;70:3020–3025. doi: 10.1128/IAI.70.6.3020-3025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giacomini E, Remoli ME, Gafa V, Pardini M, Fattorini L, Coccia EM. IFN-beta improves BCG immunogenicity by acting on DC maturation. J Leukoc Biol. 2009;85:462–468. doi: 10.1189/jlb.0908583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novikov A, Cardone M, Thompson R, Shenderov K, Kirschman KD, Mayer-Barber KD, Myers TG, Rabin RL, Trinchieri G, Sher A, Feng CG. Mycobacterium tuberculosis triggers host type I IFN signaling to regulate IL-1beta production in human macrophages. J Immunol. 2011;187:2540–2547. doi: 10.4049/jimmunol.1100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer-Barber KD, Andrade BB, Barber DL, Hieny S, Feng CG, Caspar P, Oland S, Gordon S, Sher A. Innate and Adaptive Interferons Suppress IL-1alpha and IL-1beta Production by Distinct Pulmonary Myeloid Subsets during Mycobacterium tuberculosis Infection. Immunity. 2011;35:1023–1034. doi: 10.1016/j.immuni.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denis M. Recombinant murine beta interferon enhances resistance of mice to systemic Mycobacterium avium infection. Infection and immunity. 1991;59:1857–1859. doi: 10.1128/iai.59.5.1857-1859.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manca C, Tsenova L, Bergtold A, Freeman S, Tovey M, Musser JM, Barry CE, 3rd, Freedman VH, Kaplan G. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha/beta. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5752–5757. doi: 10.1073/pnas.091096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuchtey J, Fulton SA, Reba SM, Harding CV, Boom WH. Interferon-alphabeta mediates partial control of early pulmonary Mycobacterium bovis bacillus Calmette-Guerin infection. Immunology. 2006;118:39–49. doi: 10.1111/j.1365-2567.2006.02337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ordway D, Henao-Tamayo M, Harton M, Palanisamy G, Troudt J, Shanley C, Basaraba RJ, Orme IM. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J Immunol. 2007;179:522–531. doi: 10.4049/jimmunol.179.1.522. [DOI] [PubMed] [Google Scholar]
- 23.Lee PY, Li Y, Kumagai Y, Xu Y, Weinstein JS, Kellner ES, Nacionales DC, Butfiloski EJ, van Rooijen N, Akira S, Sobel ES, Satoh M, Reeves WH. Type I interferon modulates monocyte recruitment and maturation in chronic inflammation. Am J Pathol. 2009;175:2023–2033. doi: 10.2353/ajpath.2009.090328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antonelli LR, Gigliotti Rothfuchs A, Goncalves R, Roffe E, Cheever AW, Bafica A, Salazar AM, Feng CG, Sher A. Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. The Journal of clinical investigation. 2010;120:1674–1682. doi: 10.1172/JCI40817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banaiee N, Kincaid EZ, Buchwald U, Jacobs WR, Jr, Ernst JD. Potent inhibition of macrophage responses to IFN-gamma by live virulent Mycobacterium tuberculosis is independent of mature mycobacterial lipoproteins but dependent on TLR2. J Immunol. 2006;176:3019–3027. doi: 10.4049/jimmunol.176.5.3019. [DOI] [PubMed] [Google Scholar]
- 26.Wolf AJ, Linas B, Trevejo-Nunez GJ, Kincaid E, Tamura T, Takatsu K, Ernst JD. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol. 2007;179:2509–2519. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- 27.Palendira U, Bean AG, Feng CG, Britton WJ. Lymphocyte recruitment and protective efficacy against pulmonary mycobacterial infection are independent of the route of prior Mycobacterium bovis BCG immunization. Infection and immunity. 2002;70:1410–1416. doi: 10.1128/IAI.70.3.1410-1416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kipnis A, Basaraba RJ, Orme IM, Cooper AM. Role of chemokine ligand 2 in the protective response to early murine pulmonary tuberculosis. Immunology. 2003;109:547–551. doi: 10.1046/j.1365-2567.2003.01680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swann JB, Hayakawa Y, Zerafa N, Sheehan KC, Scott B, Schreiber RD, Hertzog P, Smyth MJ. Type I IFN contributes to NK cell homeostasis, activation, and antitumor function. J Immunol. 2007;178:7540–7549. doi: 10.4049/jimmunol.178.12.7540. [DOI] [PubMed] [Google Scholar]
- 30.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annual review of immunology. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 31.Gasser S, Raulet DH. Activation and self-tolerance of natural killer cells. Immunol Rev. 2006;214:130–142. doi: 10.1111/j.1600-065X.2006.00460.x. [DOI] [PubMed] [Google Scholar]
- 32.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 33.Brod SA. Autoimmunity is a type I interferon-deficiency syndrome corrected by ingested type I IFN via the GALT system. J Interferon Cytokine Res. 1999;19:841–852. doi: 10.1089/107999099313343. [DOI] [PubMed] [Google Scholar]
- 34.Kieseier BC. The mechanism of action of interferon-beta in relapsing multiple sclerosis. CNS Drugs. 2011;25:491–502. doi: 10.2165/11591110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 35.Crow MK. Type I interferon in organ-targeted autoimmune and inflammatory diseases. Arthritis Res Ther. 2010;12(Suppl 1):S5. doi: 10.1186/ar2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volkman HE, Pozos TC, Zheng J, Davis JM, Rawls JF, Ramakrishnan L. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science (New York, NY. 2010;327:466–469. doi: 10.1126/science.1179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ling PD, Warren MK, Vogel SN. Antagonistic effect of interferon-beta on the interferon-gamma-induced expression of Ia antigen in murine macrophages. J Immunol. 1985;135:1857–1863. [PubMed] [Google Scholar]
- 38.Rayamajhi M, Humann J, Penheiter K, Andreasen K, Lenz LL. Induction of IFN-alphabeta enables Listeria monocytogenes to suppress macrophage activation by IFN-gamma. The Journal of experimental medicine. 2010;207:327–337. doi: 10.1084/jem.20091746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen KB, Cousens LP, Doughty LA, Pien GC, Durbin JE, Biron CA. Interferon alpha/beta-mediated inhibition and promotion of interferon gamma: STAT1 resolves a paradox. Nature immunology. 2000;1:70–76. doi: 10.1038/76940. [DOI] [PubMed] [Google Scholar]
- 40.Wesoly J, Szweykowska-Kulinska Z, Bluyssen HA. STAT activation and differential complex formation dictate selectivity of interferon responses. Acta Biochim Pol. 2007;54:27–38. [PubMed] [Google Scholar]
- 41.Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Forster I, Farlik M, Decker T, Du Pasquier RA, Romero P, Tschopp J. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–223. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Ho HH, Ivashkiv LB. Role of STAT3 in type I interferon responses. Negative regulation of STAT1-dependent inflammatory gene activation. The Journal of biological chemistry. 2006;281:14111–14118. doi: 10.1074/jbc.M511797200. [DOI] [PubMed] [Google Scholar]
- 43.Murray PJ. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8686–8691. doi: 10.1073/pnas.0500419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meissner N, Swain S, McInnerney K, Han S, Harmsen AG. Type-I IFN signaling suppresses an excessive IFN-gamma response and thus prevents lung damage and chronic inflammation during Pneumocystis (PC) clearance in CD4 T cell-competent mice. Am J Pathol. 2010;176:2806–2818. doi: 10.2353/ajpath.2010.091158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Connell RM, Saha SK, Vaidya SA, Bruhn KW, Miranda GA, Zarnegar B, Perry AK, Nguyen BO, Lane TF, Taniguchi T, Miller JF, Cheng G. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. The Journal of experimental medicine. 2004;200:437–445. doi: 10.1084/jem.20040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Auerbuch V, Brockstedt DG, Meyer-Morse N, O’Riordan M, Portnoy DA. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. The Journal of experimental medicine. 2004;200:527–533. doi: 10.1084/jem.20040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lehner M, Felzmann T, Clodi K, Holter W. Type I interferons in combination with bacterial stimuli induce apoptosis of monocyte-derived dendritic cells. Blood. 2001;98:736–742. doi: 10.1182/blood.v98.3.736. [DOI] [PubMed] [Google Scholar]
- 48.Mariotti S, Teloni R, Iona E, Fattorini L, Romagnoli G, Gagliardi MC, Orefici G, Nisini R. Mycobacterium tuberculosis diverts alpha interferon-induced monocyte differentiation from dendritic cells into immunoprivileged macrophage-like host cells. Infection and immunity. 2004;72:4385–4392. doi: 10.1128/IAI.72.8.4385-4392.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delneste Y, Charbonnier P, Herbault N, Magistrelli G, Caron G, Bonnefoy JY, Jeannin P. Interferon-gamma switches monocyte differentiation from dendritic cells to macrophages. Blood. 2003;101:143–150. doi: 10.1182/blood-2002-04-1164. [DOI] [PubMed] [Google Scholar]
- 50.Stark GR, I, Kerr M, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 51.Hamilton JA, Whitty GA, Kola I, Hertzog PJ. Endogenous IFN-alpha beta suppresses colony-stimulating factor (CSF)-1-stimulated macrophage DNA synthesis and mediates inhibitory effects of lipopolysaccharide and TNF-alpha. J Immunol. 1996;156:2553–2557. [PubMed] [Google Scholar]
- 52.Lande R, Giacomini E, Grassi T, Remoli ME, Iona E, Miettinen M, Julkunen I, Coccia EM. IFN-alpha beta released by Mycobacterium tuberculosis-infected human dendritic cells induces the expression of CXCL10: selective recruitment of NK and activated T cells. J Immunol. 2003;170:1174–1182. doi: 10.4049/jimmunol.170.3.1174. [DOI] [PubMed] [Google Scholar]
- 53.Junqueira-Kipnis AP, Kipnis A, Jamieson A, Juarrero MG, Diefenbach A, Raulet DH, Turner J, Orme IM. NK cells respond to pulmonary infection with Mycobacterium tuberculosis, but play a minimal role in protection. J Immunol. 2003;171:6039–6045. doi: 10.4049/jimmunol.171.11.6039. [DOI] [PubMed] [Google Scholar]
- 54.Feng CG, Kaviratne M, Rothfuchs AG, Cheever A, Hieny S, Young HA, Wynn TA, Sher A. NK cell-derived IFN-gamma differentially regulates innate resistance and neutrophil response in T cell-deficient hosts infected with Mycobacterium tuberculosis. J Immunol. 2006;177:7086–7093. doi: 10.4049/jimmunol.177.10.7086. [DOI] [PubMed] [Google Scholar]