Toll-like receptor (TLR) and Inflammasome Actions in the Central Nervous System: New and Emerging Concepts (original) (raw)
. Author manuscript; available in PMC: 2013 Jul 1.
Published in final edited form as: Trends Immunol. 2012 Apr 21;33(7):333–342. doi: 10.1016/j.it.2012.03.001
Abstract
During the past ten years, much attention has been focused towards elucidating the impact of Toll-like receptors (TLRs) in CNS innate immunity. TLR signaling triggers the transcriptional activation of pro-IL-1β and pro-IL-18 that are processed into their active forms by the inflammasome. Recent studies have demonstrated inflammasome involvement during CNS infection, autoimmune disease, and injury. This review will address inflammasome actions within the CNS and how cooperation between TLR and inflammasome signaling may influence disease outcome. In addition, the concept of alternative inflammasome functions independent of IL-1 and IL-18 processing are considered in the context of CNS disease.
Keywords: Inflammasome, Toll-like Receptor, Microglia, IL-1β, IL-18, neurodegeneration, bacterial meningitis, brain abscess
Neuroinflammation and TLR/inflammasome crosstalk in the CNS
The healthy central nervous system (CNS), while once considered immune privileged, is now recognized as a site where immune surveillance occurs [1]. CNS infection or injury elicits immune responses resulting from activated resident parenchymal populations, as well as peripheral immune cell infiltrates after the blood brain barrier (BBB) is compromised. CNS inflammation can be either neuroprotective or destructive depending on 1) the type of insult; 2) the intensity and duration of the inflammatory response; and 3) the composition of inflammatory infiltrates.
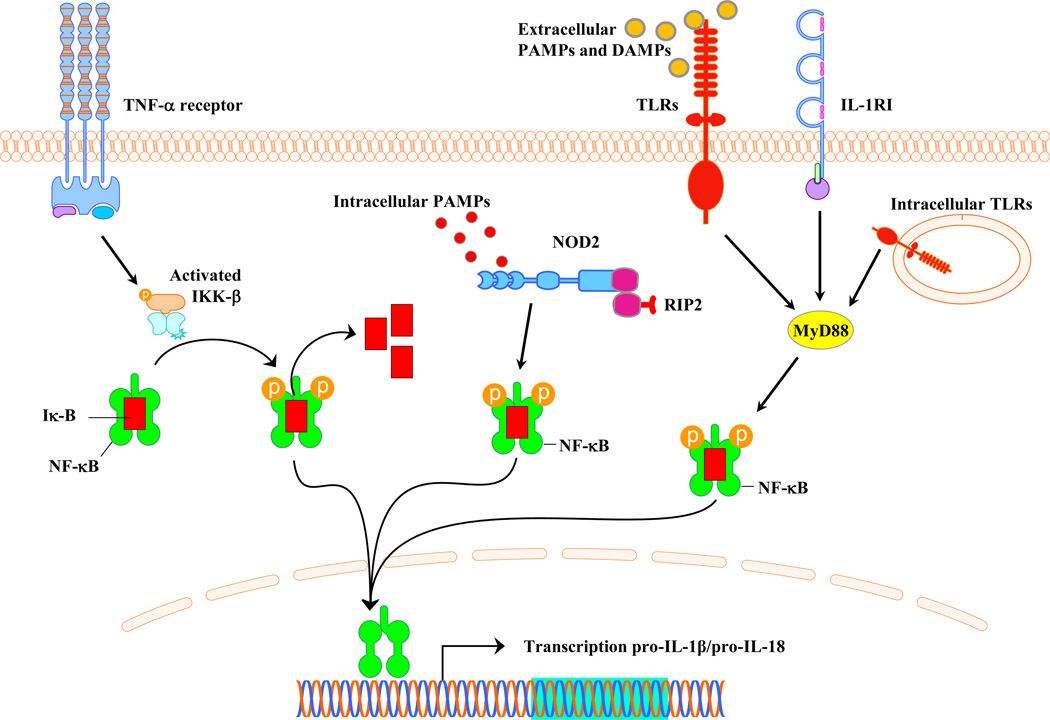
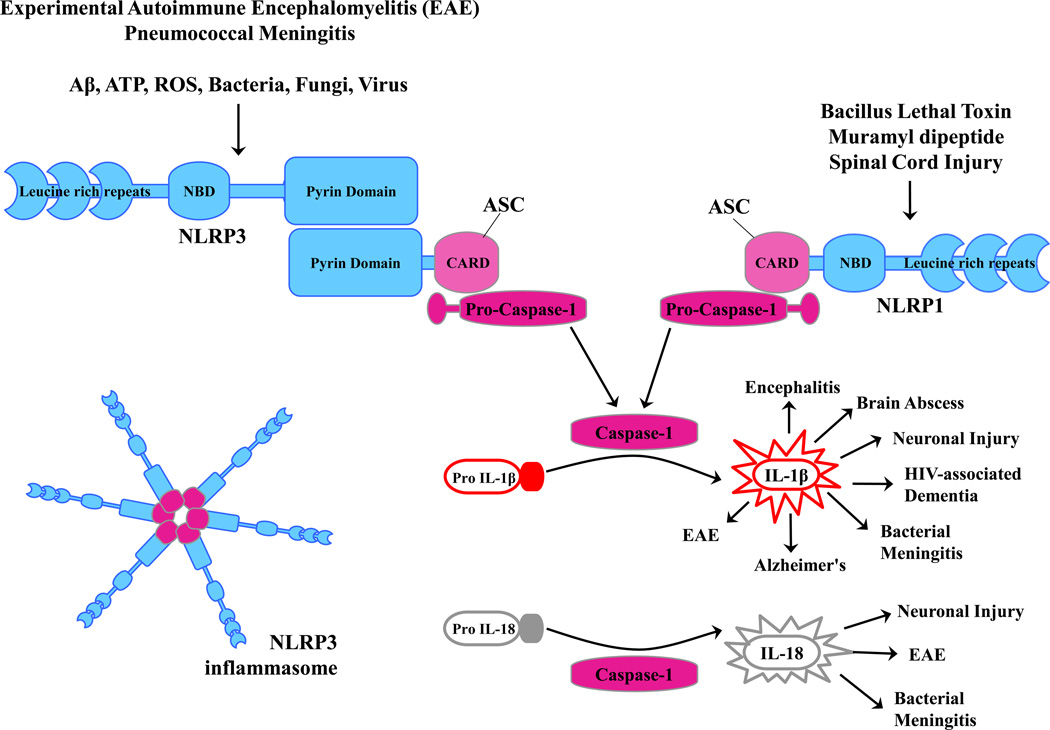
Innate immune activation in the CNS can be triggered by numerous pathways after recognition of invading pathogens and/or tissue damage by pattern recognition receptor (PRRs). Much attention has focused in recent years on a two-signal model mediated by Toll-like receptors (TLRs) and Nod-like receptors (NLRs), the latter of which forms the inflammasome that is responsible for pro-IL-1β and pro-IL-18 processing. A total of 13 and 10 TLRs have been identified in humans and mice, respectively, that detect pathogen-associated molecular patterns (PAMPs) expressed on broad microbial subclasses to maximize receptor usage [2]. In addition, TLRs recognize endogenous molecules, referred to as danger-associated molecular patterns (DAMPs), which are typically sequestered from the immune system but released during tissue pathology [2]. With the exception of TLR3, TLR engagement leads to MyD88 recruitment and subsequent NF-κB- and MAPK-mediated transcriptional activation of inflammatory mediators (Fig. 1). Whereas TLRs are membrane spanning receptors, NLRs are cytoplasmic sensors that oligomerize to form a platform for the inflammasome, a multi-subunit complex that processes pro-IL-1β and pro-IL-18 into their mature forms via caspase-1 action (Fig. 2). Many NLRs possess an N-terminal pyrin domain that interacts with the pyrin domain of ASC to bridge the complex to pro-caspase-1; however, other NLRs contain a CARD domain that allows for direct binding to pro-caspase-1 (Fig. 2).
Figure 1. Pathways involved in pro-IL-1β and pro-IL-18 expression.
Induction of pro-IL-1β and pro-IL-18 occurs via TLR, NOD, or TNFR signaling in response to extracellular PAMPs, cytoplasmic PAMPs, or TNF-α, respectively.
Figure 2. Formation of NLRP3 or NLRP1 inflammasomes.
Structurally, NLRs contain leucine rich repeats and a nucleotide binding domain (NBD). Only certain NLRs possess an N-terminal pyrin domain (i.e. NLRP3), whereas others do not (i.e. NLRP1). The N-terminal pyrin domain of NLRP3 interacts with the pyrin domain of ASC that serves to bridge the complex to pro-caspase-1. NLRP3 inflammasome oligomerization and subsequent caspase-1 activation is triggered by a diverse array of stimuli, including Aβ, ATP, ROS (reactive oxygen species), as well as various bacterial, fungal, and viral products.
A well-characterized outcome of TLR and inflammasome cooperation is IL-1β and IL-18 secretion. Two signals are required for this process; namely the transcriptional induction of pro-IL-1β and pro-IL-18 by TLR or nucleotide-binding oligomerization domain-containing protein (NOD) signaling, followed by proteolytic processing via inflammasome activation [3]. Signal 1 can be elicited by TLR ligands or TNF-α (Fig. 1), whereas numerous molecules can provide signal 2, including various pore-forming toxins, amyloid-beta (Aβ), ATP, K+ efflux, as well as silica and uric acid crystals [4–6]. Of the 22 NLR genes identified in humans, NLRP3 is the best characterized inflammasome (Fig. 2), and the fact that numerous structurally distinct stimuli are capable of initiating NLRP3 inflammasome action has led to the theory that NLRP3 senses a generic “danger” signal postulated to arise from lysosomal damage [7, 8]. This contrasts with other NLRs that respond to a more restricted stimulus repertoire [9].
Parenchymal microglia are the main cell type that contribute to CNS innate immune signaling and express the complete repertoire of identified TLRs as well as several inflammasome-related molecules, which together endows the cell with potent inflammatory capacity [10, 11]. In addition to maintaining CNS homeostasis and providing neuronal support, astrocytes are also capable of contributing to innate immune responses through a limited TLR repertoire [10, 12]. However, inflammasome activity in astrocytes remains unclear.
Interleukin-1β and IL-18 are implicated in the pathophysiology of numerous neurodegenerative diseases, including multiple sclerosis (MS) and Alzheimer’s disease (AD), as well as a number of CNS infections ranging from bacterial meningitis, brain abscess, and HIV-associated dementia [13–15]. IL-1 and IL-18 also regulate the induction of adaptive immunity, and both cytokines have been shown to influence disease development in experimental autoimmune encephalomyelitis (EAE), an animal model for MS [16, 17]. Although the roles of inflammasomes are becoming increasingly well-defined, insights into inflammasome biology in the CNS is only just emerging. Here we discuss how interactions between TLRs and the inflammasome mediate responses to both pathogen-associated and endogenous triggers during CNS pathology (Box 1). We also examine recent findings describing inflammasome actions that extend beyond its traditional role in IL-1β/IL-18 processing.
Box 1: Molecular triggers and regulation of TLR and inflammasome activation.
The identity of TLR ligands is relatively well-defined in the context of CNS infectious diseases. In contrast, TLR agonists during neurodegeneration/autoimmunity remain elusive. Some possible examples of the latter include heat shock proteins and extracellular matrix fragments liberated during inflammation or tissue remodeling [2]. Likewise, the molecular triggers leading to inflammasome activation are unknown. Extrapolating from existing literature, it is likely that generalized intracellular signals induced after cell damage/stress are sensed by cytoplasmic NLRs to initiate inflammasome activation.
During the course of many CNS diseases, the immune response not only facilitates pathogen clearance/tissue remodeling via TLR signaling, but may also cause bystander damage to surrounding parenchyma. This may trigger the release of self-antigens or DAMPs, many of which would not typically be exposed to the extracellular milieu. For example, during CNS infection, TLRs might play a dual role in ligand recognition, first by facilitating responses to the inciting pathogen and upon tissue destruction, recognizing newly liberated self-antigens, a scenario termed the “pathogen-necrosis-autoantigen triad” [100]. Newly liberated self-antigens may serve as direct triggers for TLRs to perpetuate the inflammatory response. This mechanism could increase the possibility of autoimmunity, although this remains speculative.
Negative regulatory signals controlling TLR/inflammasome activity are important for maintaining cytokine production at sufficient levels during conditions when triggering stimuli are abundant. IL-1β is a potent cytokine with pleiotropic actions, many of which can be deleterious if the cytokine is produced in excess. With regard to the attenuation of TLR signaling, several negative regulators have been identified, including splice variants for adaptors, ubiquitination, transcriptional regulators, and microRNA [2, 101]. At the level of the inflammasome, pyrin-only domain proteins (POPs), CARD-only domain proteins (COPs), and ASC splice variants have been reported to attenuate inflammasome activity [101, 102]. In addition, ASC has also been shown to inhibit NF-κB signaling, a major transcription factor for inducing pro-IL-1β and pro-IL-18 [103]. However, most of these studies have been conducted in non-CNS cell types/tissues and little information is available to date regarding the negative regulation of TLR or inflammasome action in the context of CNS inflammation. Additional studies are warranted to address this point, since it may prove beneficial from a therapeutic perspective to augment negative regulatory pathways to fine-tune receptor activity during an in vivo inflammatory setting.
TLR and inflammasome activation during CNS infectious diseases
Significant information is available regarding TLR involvement during CNS infectious diseases, likely because microbial ligands were the first triggers identified for these receptors. In contrast, fewer studies have examined inflammasome involvement with NLR-deficient mice, although this can be inferred with the use of models deficient in IL-1 and IL-18 action. Upon CNS invasion, pathogens are sensed by resident microglia and astrocytes by PRRs, such as TLRs, which trigger localized inflammatory cascades leading to the recruitment of peripheral leukocytes to participate in pathogen clearance. In pneumococcal meningitis, many studies have focused on TLR2, where receptor loss diminished inflammatory mediator expression and cellular influx [18–21]. Despite a reduction in molecules implicated in the pathophysiology of bacterial meningitis, TLR2 KO mice exhibited increased bacterial burdens and worsening of disease. CNS bacterial burdens were also elevated in MyD88 KO mice during pneumococcal meningitis; however, pathological inflammation was less severe, which was evident by decreases in the expression of several proinflammatory mediators, cellular influx into the CNS, BBB permeability, and edema [22]. Given the key role of IL-1β in the pathophysiology of bacterial meningitis [23], the phenotype of MyD88 KO mice probably originates, in part, from lack of IL-1R signaling in addition to the failure to trigger inflammatory mediator expression. This highlights the need for a core TLR-driven pathway to control bacterial replication within the meninges; however, if not tightly regulated this same response can exert adverse effects. Therefore, timing the delivery of a negative regulator of TLR signaling may prove beneficial to thwart untoward effects of inflammation subsequent to bacterial clearance. In contrast to bacterial meningitis, IL-1β action is critical for survival and pathogen containment during bacterial abscess formation in the CNS parenchyma [24], which was corroborated by the finding that MyD88 KO animals were exquisitely sensitive to infection, exhibiting decreased immune infiltrates and inflammatory mediator release [25]. Studies with bone marrow chimeras revealed a key role for MyD88 in CNS intrinsic cells for eliciting WT inflammatory responses during early brain abscess development [26]. TLR2 regulates bacterial burdens, immune infiltrates, and inflammatory mediator production during brain abscess development [27–29]. However, the less severe phenotypes in TLR2 compared to MyD88 KO mice suggests the coordinate actions of multiple TLR/IL-1R/IL-18R pathways.
Compared to TLRs, less information in available regarding inflammasome actions during CNS infection. The importance of caspase-1 in S. pneumoniae meningitis has been investigated using both caspase-1 KO mice and pharmacologic inhibition of caspase-1 activity, where both led to a reduction in IL-1β levels concomitant with decreased proinflammatory mediator expression and leukocyte influx into the cerebral spinal fluid (CSF) [30]. Of note, a recent study revealed that caspase-1-deficient mice on the C57LB/6 background harbor a second mutation in caspase-11 [31]. Therefore, caution should be used when interpreting results with these animals since caspase-11 can also process pro-IL-1β. The NLRP3 inflammasome is implicated in mediating tissue damage during pneumococcal meningitis, probably via IL-1β release [19]. In particular, disease severity, intracranial pressure, and CSF leukocytosis were inhibited in both NLRP3 and ASC KO mice, although IL-1β expression was not reported in this study [19]. Interestingly, despite improvements in meningitis severity, bacterial burdens remained equivalent between NLRP3 KO, ASC KO, and WT mice [19]. In this context, it is not clear what mechanisms are employed for bacterial eradication; however, from a clinical perspective this would be achieved with aggressive antibiotic therapy. Therefore, preventing overactive, damaging inflammation within the meningeal space by targeting IL-1 activity may prove to be a beneficial adjunctive therapy. It is intriguing that IL-1β exerts pathological actions during meningitis, yet the cytokine is essential for protection during CNS parenchymal infection [24]. The reason(s) responsible for these differences remain to be determined but may be explained, in part, by IL-1β effects on edema formation, which are better tolerated in the parenchyma compared to the meninges.
Numerous studies have demonstrated the requirement for various TLRs in triggering microglial activation after exposure to intact pathogens or PAMPs [10]. In comparison, less information is available regarding TLR crosstalk. A recent report demonstrated that TLR2–TLR9 interactions limited IL-12 family member production in microglia upon exposure to intact Gram-positive bacteria but not TLR2 PAMPs [32]. Likewise, the synthetic TLR7 agonist, imiqimod, could inhibit TLR9-induced cytokine/chemokine secretion in microglia and astrocytes and TLR7-deficient microglia displayed enhanced cytokine responses to CpG ODN stimulation [33]. Additional studies examining TLR crosstalk are needed since pathogens harbor numerous PAMPs that can engage multiple TLRs in the same cell. Defining TLR combinations and dominant receptors may enable their therapeutic targeting.
Recent studies show that IL-1β production by microglia is regulated by the NLRP3 inflammasome after exposure to live S. aureus [34]. Inflammasome activation was partially mediated by bacterial hemolysins (α- and γ-toxins) and extracellular ATP. Interestingly, IL-18 concentrations were unaffected by the loss of NLRP3, ASC, or caspase-1 activity, which indicates that IL-18 processing is regulated by a novel inflammasome-independent mechanism in response to live bacteria [34]. It is not clear if pro-IL-18 processing in microglia is broadly inflammasome-independent, or if this occurs in a stimulus-dependent manner.
An unresolved question is whether astrocytes possess inflammasome activity since there is conflicting data on whether astrocytes produce IL-1β. There is also discrepancy about whether astrocytes express TLR4 [35, 36]; its detection in vitro may originate from low numbers of microglia that persist in primary astrocyte cultures [37]. A recent study utilizing highly purified astrocytes obtained by a stringent differential shaking protocol or gancyclovir to kill HSVtk-expressing microglia has established that mouse astrocytes do not express detectable TLR4 [38]. Likewise, astrocytes do not respond to LPS unless in the presence of microglia, and this was attributed to both cell-cell contact and soluble factors, since the addition of microglial-conditioned medium was capable of sensitizing astrocytes to subsequent LPS exposure. This confirms an earlier report, where exposure of FACS-enriched astrocytes to the Gram-negative pathogen C. koseri was not capable of eliciting IL-1β production, although robust chemokine synthesis was detected [39]. These results highlight the importance of glial crosstalk in dictating astrocyte cytokine production and the fact that studies examining purified microglia and astrocytes should be complemented with co-culture studies to appreciate the influence of intercellular interactions.
When considering how TLR/inflammasome pathways contribute to sensing various pathogens, one must take into account the route of infection, growth phase of the pathogen, and pathogen niche that can conceivably influence the importance of TLR/inflammasome recognition. Further understanding of the role of inflammasome components during various CNS infections may identify molecular pathways that can be exploited therapeutically.
Effects of TLR signaling and inflammasome activation during CNS neurodegeneration/injury
Numerous autoimmune and neurodegenerative disorders, such as MS and AD, as well as CNS injury responses, including spinal cord injury (SCI) and stroke, are typified by an inflammatory component. Central to these diseases is IL-1 secretion, although other inflammatory mediators also play a role. Less is known regarding the biological consequences of IL-18 during CNS pathology, although the cytokine is expressed in several neuroinflammatory conditions including bacterial and viral infections, EAE, stroke, and SCI [40].
TLRs: Sensing the Toll on CNS damage
Studies examining TLR involvement during neurodegenerative diseases have increased in recent years as more evidence supporting DAMP triggers has emerged. Not surprisingly, the outcomes of TLR action vary widely, with either neuroprotective or destructive effects reported depending on the disease model. Neurological function and Aβ levels were moderately reduced after TLR2 or MyD88 KO bone marrow transfer into irradiated Aβ transgenic mice [41, 42], revealing a role for these molecules in infiltrating myeloid cells. It remains unclear whether these results are mediated by macrophages or microglia because of issues related to irradiation effects on the BBB [43, 44] but the modest impact of peripherally-derived myeloid cells suggests both populations are likely involved. This possibility is supported by studies where AD transgenic mice were crossed with MyD88-deficient animals revealing more robust effects on Aβ loads [45]. However, our understanding of TLR action in AD remains incomplete because another report demonstrated that amyloid precursor protein (APP)/TLR2-deficient mice had more severe neurological impairment despite delayed Aβ deposition [46]. These inconsistencies may result, in part, from different AD mouse models crossed with TLR-deficient animals. Microglia are a main source of IL-1β in AD, and signal 1 in response to Aβ may be provided by TLR engagement because multiple TLRs have been implicated in Aβ recognition [46, 47]. Recently, statins have been shown to augment TLR/MyD88-dependent proinflammatory responses in microglia through cholesterol modulation [48], which may have untoward consequences in the context of neurodegeneration.
Several studies have suggested that TLRs can impact disease severity during EAE [49, 50]; however, the identity of putative TLR ligands is elusive. As infectious etiologies have been suggested to trigger/exacerbate MS, PAMP triggering of TLRs may occur [51]; alternatively, DAMPs released in response to cell stress or injury may be involved (Box 1). The TLR2 ligand peptidoglycan (PGN) has been detected in the brains of non-human primates with demyelinating disease as well as in MS patients [52, 53]. However, other reports show that TLR4- and TLR9-deficient mice exhibit more severe EAE symptoms, suggesting protective roles for these TLRs during disease [54]. Although the role of TLRs in MS and EAE is not straightforward, there is consensus that MyD88 KO mice are resistant to EAE induction [50, 54]. Therefore, it is likely that other MyD88-dependent receptors, such as IL-1R and IL-18R, also influence disease progression, which is supported by several studies [13, 16, 17, 55].
Following SCI, MyD88 deficiency led to reduced neutrophil and type I inflammatory monocyte infiltration [56]; however, both TLR2 and TLR4 play a protective role in SCI by regulating inflammation, gliosis, and demyelination, demonstrating the importance of unidentified endogenous DAMPs that trigger beneficial effects following CNS injury [57]. In a model of axonal transaction in the entorhinal cortex, proinflammatory mediator expression and leukocyte recruitment were reduced in MyD88 KO animals [58]. This is likely attributed to TLR recognition of DAMPs because similar observations were made in TLR2 but not IL-1R, IL-18R, or TLR4 KO mice [58, 59]. However, the long-term consequences of MyD88 or TLR2 loss were not examined.
TLR signaling also influences tissue injury following CNS ischemia. TLR stimulation prior to an ischemic event leads to a phenomenon known as preconditioning, where inflammatory damage is significantly attenuated in response to ischemic challenge [60]. Furthermore, TLR231 deficient mice are protected against ischemic brain damage, which may be mediated, in part, by a reduction in pro-IL-1β/IL-18 production [61]. The collective evidence to date has revealed a role for TLR action during sterile inflammation and injury. Although the DAMPs that trigger TLR activation in these disease models remain obscure, the finding that functional outcomes result from TLR loss suggests these pathways may be amenable to therapeutic manipulation to favor repair processes.
Inflammasome actions during neurodegeneration/autoimmune disease
Compared to TLRs, our knowledge of inflammasome involvement during neurodegeneration is limited. A recent report has demonstrated that Aβ leads to NLRP3 inflammasome activation and pro-IL-1β processing by microglia in both a caspase-1- and cathepsin B-dependent manner [62]. It is not clear if other NLRs can serve as molecular platforms for IL-1β processing in response to Aβ and the contribution of key inflammasome components has not been tested in AD animal models. Based on the central role of IL-1β in AD pathology [63], a prediction is that plaque burden and disease severity would be attenuated in inflammasome-deficient AD mouse models. NLRP1 inflammasome expression was increased in aged rats, which correlated with elevated IL-1β and IL-18 levels and age-related cognitive decline [64]; however, a direct cause-and-effect relationship remains to be defined. Interestingly, a recent report has revealed evidence of an association between single nucleotide variations in the NLRP1 gene and AD [65].
The requirement for NLRP3 during EAE is controversial. NLRP3 has been shown to exacerbate disease severity and immune infiltrates during both active EAE and in a cuprizone model of demyelination through IL-18 action [13, 17]. However, a separate study found no role for NLRP3 in active EAE, but showed reduced disease severity in ASC KO mice [66], the latter an adaptor for bridging NLRP3 to caspase-1. ASC KO animals were more protected from EAE than caspase-1 KO mice, suggesting that ASC has activity independent of the inflammasome. The basis for these conflicting data is unknown. Although there is agreement for caspase-1 involvement in EAE, most studies were performed in caspase-1-deficient mice that possess a second mutation in caspase-11 [31]. Therefore, it is unclear whether one or both of these caspases are responsible for disease progression. Future studies are needed to identify upstream mediators (i.e. alternative NLRs) and signaling pathways in addition to other downstream effectors that act in concert to regulate neuroinflammation during EAE.
The role of IL-1β in EAE is also unclear. A recent study reported no protection against EAE in IL-1β KO mice; however, this may be due to residual actions of IL-1α at the IL-1R [13, 67], which is supported by earlier reports demonstrating that EAE incidence was significantly lower in IL-1R1 KO mice [16, 68, 69]. The majority of evidence suggests that IL-1β promotes an encephalogenic Th17 response during EAE, providing indirect evidence for inflammasome activation that agrees with recent studies in caspase-1 KO mice [55, 70]. However, IL-1 has also been implicated in remyelination via tropic effects on oligodendrocyte progenitors [71]. Therefore, the timing, duration, and intensity of inflammasome action probably dictate whether outcomes are beneficial or destructive.
After SCI pro-IL-1β and pro-IL-18 are rapidly processed by the NLRP1 inflammasome, which includes the physical association of ASC, caspase-1, and caspase-11 [72]. Inhibition of ASC resulted in tissue sparing and some functional improvement after SCI, implying a detrimental role for inflammasome activity. This is an intriguing relationship, because NLRP1 directly interacts with caspase-1 via homotypic CARD interactions. Therefore, the involvement of ASC in this inflammasome complex appears unusual and remains to be clarified. However, a study has shown that ASC is not required for NLRP1-dependent caspase-1 activation but can enhance this process [73]. Since NLRP1 action was not inhibited in SCI [72], the effects of ASC may occur through interactions with NLRP1 or alternative ASC-dependent NLRs, such as NLRP3, which remains an open question.
Less is known about the role of the inflammasome during ischemia, although similar to SCI the available evidence supports detrimental effects. Neutralization of NLRP1 inflammasome activity diminished IL-1β secretion and resulted in less tissue pathology following thromboembolic stroke [74]. During traumatic brain injury, rapid IL-1β and IL-18 processing occurred via the NLRP1 inflammasome in a caspase-1-dependent manner; however, ASC inhibition also resulted in a significant reduction in pathology [75]. These results bear a striking resemblance to those obtained in SCI, suggesting a conserved NLRP1 pathway is elicited after injury that exerts adverse effects on tissue homeostasis.
Recent evidence has revealed an association between inflammasome activation and autophagy, the latter of which delivers damaged organelles and proteins from the cytoplasm to lysosomes for clearance [76]. Induction of NLRP3 or AIM2 inflammasomes in macrophages triggered autophagy, which served as a negative feedback loop to limit inflammasome action [77]. Interestingly, several reports have demonstrated that mitochondrial damage and mtDNA release induce cytoplasmic NLRP3 inflammasome activation and autophagy [78–80]. Currently, no studies have examined the potential relationship between autophagy and inflammasome activation in the context of CNS disease. This relationship may prove critical for our understanding of neurodegenerative diseases typified by inclusion formation, such as AD, Huntington disease, and lysosomal storage diseases, where defects in autophagy have been suggested [81, 82].
It is clear that TLR and inflammasome actions impact the course of neurodegenerative and autoimmune diseases in the CNS based on the phenotypes obtained with receptor-deficient mouse models. What remains elusive is the identity of the DAMP signals, which are less well-characterized compared to the infectious triggers for TLR/inflammasome action.
Concluding remarks
TLRs play key roles in CNS infectious diseases, primarily by eliciting proinflammatory cytokine production leading to pathogen clearance. These receptors also impact neurodegenerative diseases, although the TLR ligands remain elusive. In this case, TLR actions can be either beneficial (SCI), detrimental (EAE) or mixed (AD). NLRP3 inflammasome activity during EAE appears to exacerbate disease severity, although some discrepancies exist, whereas it plays an important role in bacterial clearance in pneumococcal meningitis. Numerous issues remain to be addressed in this field, a few of which are mentioned in the “Outstanding Questions” box.
Existing literature suggests that the strength of the inflammatory insult may dictate the magnitude of TLR/inflammasome involvement. For example, in CNS bacterial infection models, MyD88-deficient mice exhibit dramatic alterations in inflammatory mediator secretion, immune cell recruitment, and activation [22, 25]. In contrast, more modest phenotypes are observed with MyD88- or TLR2-deficient animals during neurodegenerative diseases (i.e. AD) [41, 83], which generally agrees with the milder inflammatory milieu elicited compared to infections. Similar trends are emerging with regard to inflammasome involvement but additional studies are warranted before any conclusions can be drawn.
In many instances, the cellular source of inflammasome activity during CNS infection or injury is not known. Inflammasome expression/activity has been reported in several cell types including microglia, astrocytes, and even neurons [34, 62, 72, 84]. For the latter two, the functional significance remains to be determined but could conceivably extend beyond pro-IL-1β processing, which appears plausible because neither cell type is associated with robust IL-1β production. CNS inflammasome activity and subsequent IL-1β release is a mechanism to augment inflammatory cascades during injury/infection. Typically, IL-1β is considered detrimental to CNS homeostasis during bacterial meningitis, Alzheimer’s Disease, and EAE; however, the cytokine is important for host defense during bacterial brain abscess development. IL-18 is also processed by the inflammasome and is implicated in EAE pathogenesis [13, 17] but additional studies are needed to assess its role during other CNS diseases. Therefore, the context, timing, and concentrations of IL-1β and IL-18 likely play key roles in regulating beneficial versus detrimental effects during CNS inflammatory responses.
Although inflammasome action within the CNS is an emerging field, available evidence allows a few comparisons to be drawn with inflammasomes in peripheral tissues. For example, NLRP3 regulates inflammation during dextran sulfate sodium (DSS)-induced colitis and EAE via IL-18 action [17, 85, 86]. The NLRP1 inflammasome has been implicated in SCI and stroke, implying responses to DAMPs. This is intriguing since other studies have identified Bacillus anthracis and muramyl dipeptide as triggers of NLRP1 inflammasome activation [73, 87], and implies the action of a distinct molecular trigger.
Although inflammasome activity represents the predominant pathway for pro-IL-1β processing, it is clear that other mechanisms also contribute, as demonstrated by unchanged IL-1β or IL-18 concentrations during CNS inflammation despite inflammasome inhibition [13, 17, 88]. Recent studies have shown that cathepsin B participates in pro-IL-1β cleavage in microglia in response to diverse stimuli [34, 62]; however, the precise mechanism that enables a lysosomal enzyme to access the cytoplasmic compartment to impact cytokine processing remains unclear. One possibility is that these stimuli cause lysosomal damage and subsequent cathepsin B release, similar to the mechanism proposed during the intracellular accumulation of crystals [7, 89, 90]. In this case, cathepsin B could cleave a substrate in the cytoplasm that generates a NLRP3 ligand, degrade an endogenous NLRP3 inhibitor, or directly cleave pro-IL-1β in the cytosol. S. pneumoniae also triggers pro-IL-1β cleavage via cathepsin B and pharmacological inhibition of cathepsin B activity afforded slight protection during pneumococcal meningitis, although IL-1β levels in vivo were not investigated [19]. Caspase-8 has been linked to pro-IL-1β processing in response to TLR3 and TLR4 ligands [91] and a recent study demonstrated that fungal recognition by dectin-1 triggered pro-IL-1β processing by a noncanonical caspase-8 inflammasome [92]. Interestingly, caspase-8 inflammasome activation did not require fungal internalization unlike the canonical NLRP3-dependent pathway, yet both were ASC-dependent. These findings are intriguing from two perspectives; first, they highlight the diversity of mechanisms employed for IL-1β production. Second, since caspase-8 inflammasome activation can be triggered by extracellular fungi, this provides an immediate source of IL-1β until the canonical NLRP3-dependent pathway can be elicited following pathogen internalization. Since numerous fungal pathogens target the CNS, it will be interesting to determine whether similar noncanonical pathways are triggered to amplify IL-1β production during infection.
Research in non-CNS models of infection/injury has revealed novel inflammasome-independent actions for ASC that can be informative when considering the possibility of similar events in the context of CNS disease. First, ASC regulates the expression of cytokines/chemokines that are not processed by the inflammasome, as demonstrated by reductions in TNF-α, CCL3, CCL4, CCL13, and CCL20 expression in THP-1 cells following siRNA inhibition of ASC that occur independently of NLRP3, NLRC4, and caspase-1 [93]. It was recently demonstrated in a model of chronic Mycobacterium tuberculosis infection that granuloma formation and host defense required ASC but not NLRP3 or caspase-1 activity [94]. Whether ASC requires caspase-1 activity for the genesis of protective CNS antibacterial immunity remains to be determined. ASC also influences adaptive immunity by post-transcriptional regulation of the actin cytoskeleton, affecting phagocytosis and T cell chemotaxis [13, 17, 95, 96]. The adjuvant MF-59 induces humoral immunity to influenza vaccination in an ASC-dependent, inflammasome-independent manner [97] and two separate studies of antigen-induced arthritis revealed immune defects in ASC but not NLRP3 or caspase-1 KO mice that were attributed to ASC-dependent effects on cell-mediated immunity [98, 99].
Although recent studies have begun to investigate mechanisms of inflammasome activation within the CNS, much remains to be learned. One intriguing matter relates to functions for the inflammasome that extend beyond its traditional role in pro-IL-1β and pro-IL-18 processing. Indeed, it seems reasonable to conclude that the inflammasome did not evolve for the sole purpose of cytokine cleavage based on its complexity in subunit composition and widespread repertoire of inflammatory triggers. The fact that the NLRP3 inflammasome responds to such a wide array of ligands that lack any structural similarities, indicates that it senses a universal signal that might result from cellular stress, which is emphasized by its role in CNS infectious and non-infectious diseases. These possibilities wait evaluation in future studies.
Box 2: Outstanding questions.
- Understand TLR cooperativity during CNS disease.
- Characterize conditions whereby TLR/inflammasome actions are beneficial or detrimental for functional outcomes following CNS injury/infection.
- Identify means whereby inflammatory responses are tailored for PAMPs/DAMPs when the majority of TLRs identified to date utilize similar signaling pathways. A related question can be raised with regard to inflammasomes, in particular NLRP3, which has been shown to be activated by a multitude of stimuli with no known structural similarities.
- Elucidate mechanisms of TLR-inflammasome crosstalk in the context of CNS disease.
- Assess whether TLR and/or inflammasome activity is more prominent in infiltrating bone marrow-derived versus CNS-intrinsic populations to resolve discrepancies that exist in different disease models.
- Discover novel pathways whereby the inflammasome regulates CNS disease independent of cytokine processing.
- Explore the potential involvement of alternative inflammasomes during CNS injury/infection (i.e. NLRP1, NLRC4, NLRC5, AIM-2).
- Identify novel therapeutic agents to manipulate TLR/inflammasome activity in vivo.
- Characterize inflammasome-independent mechanisms for IL-1β and IL-18 processing.
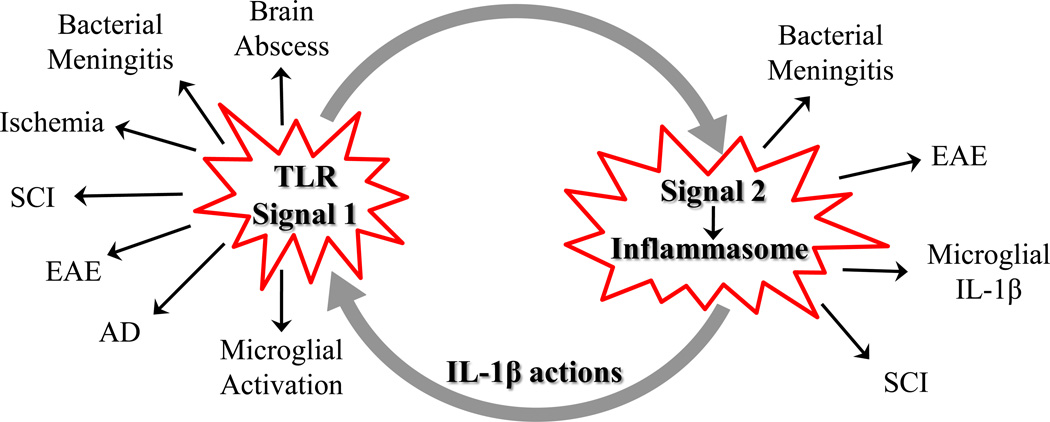
Figure 3. TLR-inflammasome crosstalk.
TLR and inflammasome action have been implicated in numerous infectious and neurodegenerative diseases, which are linked by the requirement for signal 1 (TLR) and signal 2 (inflammasome activation) to elicit IL-1β production. In turn, IL-1β can feed back to augment TLR-mediated effects.
Table 1.
TLR and NLR activation during CN S infection, injury and autoimmunity
| Pathology | TLR Activation | NLR Activation | References | |
|---|---|---|---|---|
| Alzheimer’s Disease | Excessive production & processing of Aβ and phosphorylation of tau protein resulting in plaques and tangles | Aβ aggregates bind CD14, Aβ can activate microglial TLR2 and TLR4 | Oligomeric/fibrillar Aβ leads to NLRP3 inflammasome activation in microglia and processing of pro-IL-1β in a caspase-1-dependent manner | [46, 62, 104] |
| Multiple Sclerosis | Reactive glia and infiltrating T cells trigger inflammatory processes and subsequent oligodendrocyte and axonal destruction | MyD88 signaling via various TLRs influences EAE severity. Role of individual TLRs controversial | ASC, caspase-1 and IL-1β signaling are important for EAE progression, role of NLRP3 is unclear | [13, 16, 17, 50, 54, 66, 70, 105] |
| Spinal Cord Injury | Robust microglial activation with infiltrating monocyte & macrophages influencing pathology | Endogenous “danger signals” trigger beneficial effects through TLR2 & 4 signaling following SCI | SCI-induced processing of IL-1β requires NALP1, ASC, caspase-1 and caspase-11. ASC loss protective in SCI | [56, 57, 72] |
| Neuronal Injury (Stroke & TBI) | Neuronal excitability, neurotoxin production, leukocyte infiltration, microglia activation and astrogliosis | TLR preconditioning attenuates inflammatory damage. However, TLR2 or 4 KO protects against brain damage in stroke model | Increased expression of IL-1β in cerebrospinal fluid. NLRP1, ASC neutralization leads to reduced pathology. | [60, 61, 74, 75] |
| Bacterial Meningitis | Inflammation of the leptomeninges resulting in tissue and vascular injury and increased intracranial pressure | MyD88 and TLR2 play protective role in meningitis | Disease severity significantly reduced in NLRP3- and ASC-deficient animals | [18–20, 22] |
| Bacterial Abscess | Necrosis, peripheral immune infiltrates, glial activation, and fibrotic capsule formation | Complex roles for TLRs in disease pathogenesis, while MyD88 is essential for protective immunity | NLRP3 inflammasome activation involved in IL-1β production by microglia | [25, 28, 29, 106] |
ACKNOWLEDGEMENTS
We acknowledge researchers who have contributed to this field, whose work was not cited because of space restrictions. This work was supported by supported by the NIH National Institute of Neurological Disorders and Stroke (NINDS) R01 NS040730 and NS053487 to T.K. R.H. is supported by a UNMC Graduate Student Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Greenwood J, et al. Review: leucocyte-endothelial cell crosstalk at the blood-brain barrier: a prerequisite for successful immune cell entry to the brain. Neuropathol Appl Neurobiol. 2011;37:24–39. doi: 10.1111/j.1365-2990.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 3.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 4.Petrilli V, et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 5.Dostert C, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craven RR, et al. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One. 2009;4:e7446. doi: 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroder K, et al. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327:296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- 9.Martinon F, et al. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 10.Kielian T. Toll-like receptors in central nervous system glial inflammation and homeostasis. J Neurosci Res. 2006;83:711–730. doi: 10.1002/jnr.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58:253–263. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- 12.Farina C, et al. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Jha S, et al. The inflammasome sensor, NLRP3, regulates CNS inflammation and demyelination via caspase-1 and interleukin-18. J Neurosci. 2010;30:15811–15820. doi: 10.1523/JNEUROSCI.4088-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin WS, Mrak RE. Interleukin-1 in the genesis and progression of and risk for development of neuronal degeneration in Alzheimer's disease. J Leukoc Biol. 2002;72:233–238. [PMC free article] [PubMed] [Google Scholar]
- 15.Zwijnenburg PJ, et al. IL-1 receptor type 1 gene-deficient mice demonstrate an impaired host defense against pneumococcal meningitis. J Immunol. 2003;170:4724–4730. doi: 10.4049/jimmunol.170.9.4724. [DOI] [PubMed] [Google Scholar]
- 16.Sutton C, et al. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gris D, et al. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J Immunol. 2010;185:974–981. doi: 10.4049/jimmunol.0904145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Echchannaoui H, et al. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J Infect Dis. 2002;186:798–806. doi: 10.1086/342845. [DOI] [PubMed] [Google Scholar]
- 19.Hoegen T, et al. The NLRP3 Inflammasome Contributes to Brain Injury in Pneumococcal Meningitis and Is Activated through ATP-Dependent Lysosomal Cathepsin B Release. J Immunol. 2011;187:5440–5451. doi: 10.4049/jimmunol.1100790. [DOI] [PubMed] [Google Scholar]
- 20.Koedel U, et al. Toll-like receptor 2 participates in mediation of immune response in experimental pneumococcal meningitis. J Immunol. 2003;170:438–444. doi: 10.4049/jimmunol.170.1.438. [DOI] [PubMed] [Google Scholar]
- 21.Klein M, et al. Innate immunity to pneumococcal infection of the central nervous system depends on toll-like receptor (TLR) 2 and TLR4. J Infect Dis. 2008;198:1028–1036. doi: 10.1086/591626. [DOI] [PubMed] [Google Scholar]
- 22.Koedel U, et al. MyD88 is required for mounting a robust host immune response to Streptococcus pneumoniae in the CNS. Brain. 2004;127:1437–1445. doi: 10.1093/brain/awh171. [DOI] [PubMed] [Google Scholar]
- 23.Koedel U, et al. New understandings on the pathophysiology of bacterial meningitis. Curr Opin Infect Dis. 2010;23:217–223. doi: 10.1097/QCO.0b013e328337f49e. [DOI] [PubMed] [Google Scholar]
- 24.Kielian T, et al. IL-1 and TNF-alpha play a pivotal role in the host immune response in a mouse model of Staphylococcus aureus-induced experimental brain abscess. J Neuropathol Exp Neurol. 2004;63:381–396. doi: 10.1093/jnen/63.4.381. [DOI] [PubMed] [Google Scholar]
- 25.Kielian T, et al. MyD88-dependent signals are essential for the host immune response in experimental brain abscess. J Immunol. 2007;178:4528–4537. doi: 10.4049/jimmunol.178.7.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garg S, et al. MyD88 expression by CNS-resident cells is pivotal for eliciting protective immunity in brain abscesses. ASN Neuro. 2009;1 doi: 10.1042/AN20090004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vidlak D, et al. Roles of Toll-like receptor 2 (TLR2) and superantigens on adaptive immune responses during CNS staphylococcal infection. Brain Behav Immun. 2011;25:905–914. doi: 10.1016/j.bbi.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kielian T, et al. Toll-like receptor 2 modulates the proinflammatory milieu in Staphylococcus aureus-induced brain abscess. Infect Immun. 2005;73:7428–7435. doi: 10.1128/IAI.73.11.7428-7435.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stenzel W, et al. Both TLR2 and TLR4 are required for the effective immune response in Staphylococcus aureus-induced experimental murine brain abscess. Am J Pathol. 2008;172:132–145. doi: 10.2353/ajpath.2008.070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koedel U, et al. Role of Caspase-1 in experimental pneumococcal meningitis: Evidence from pharmacologic Caspase inhibition and Caspase-1-deficient mice. Ann Neurol. 2002;51:319–329. doi: 10.1002/ana.10103. [DOI] [PubMed] [Google Scholar]
- 31.Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 32.Holley MM, et al. Toll-like receptor 2 (TLR2)-TLR9 crosstalk dictates IL-12 family cytokine production in microglia. Glia. 2012;60:29–42. doi: 10.1002/glia.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butchi NB, et al. Interactions between TLR7 and TLR9 agonists and receptors regulate innate immune responses by astrocytes and microglia. Glia. 2010;58:650–664. doi: 10.1002/glia.20952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanamsagar R, et al. Inflammasome activation and IL-1β/IL-18 processing are influenced by distinct pathways in microglia. J Neurochem. 2011 doi: 10.1111/j.1471-4159.2011.07481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorina R, et al. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFkappaB signaling, MAPK, and Jak1/Stat1 pathways. Glia. 2011;59:242–255. doi: 10.1002/glia.21094. [DOI] [PubMed] [Google Scholar]
- 36.Bsibsi M, et al. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- 37.Saura J. Microglial cells in astroglial cultures: a cautionary note. J Neuroinflammation. 2007;4:26. doi: 10.1186/1742-2094-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holm TH, et al. Microglia are required for astroglial toll-like receptor 4 response and for optimal TLR2 and TLR3 response. Glia. 2012;60:630–638. doi: 10.1002/glia.22296. [DOI] [PubMed] [Google Scholar]
- 39.Liu S, Kielian T. MyD88 is pivotal for immune recognition of Citrobacter koseri and astrocyte activation during CNS infection. J Neuroinflammation. 2011;8:35. doi: 10.1186/1742-2094-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alboni S, et al. Interleukin 18 in the CNS. J Neuroinflammation. 2010;7 doi: 10.1186/1742-2094-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu S, et al. TLR2 Is a Primary Receptor for Alzheimer's Amyloid beta Peptide To Trigger Neuroinflammatory Activation. J Immunol. 2012;188:1098–1107. doi: 10.4049/jimmunol.1101121. [DOI] [PubMed] [Google Scholar]
- 42.Hao W, et al. Myeloid differentiation factor 88-deficient bone marrow cells improve Alzheimer's disease-related symptoms and pathology. Brain. 2011;134:278–292. doi: 10.1093/brain/awq325. [DOI] [PubMed] [Google Scholar]
- 43.Ajami B, et al. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 44.Ajami B, et al. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 45.Lim JE, et al. MyD88 deficiency ameliorates beta-amyloidosis in an animal model of Alzheimer's disease. Am J Pathol. 2011;179:1095–1103. doi: 10.1016/j.ajpath.2011.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richard KL, et al. Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1–42 and delay the cognitive decline in a mouse model of Alzheimer's disease. J Neurosci. 2008;28:5784–5793. doi: 10.1523/JNEUROSCI.1146-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reed-Geaghan EG, et al. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci. 2009;29:11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Der Putten C, et al. Statins amplify TLR-induced responses in microglia via inhibition of cholesterol biosynthesis. Glia. 2012;60:43–52. doi: 10.1002/glia.21245. [DOI] [PubMed] [Google Scholar]
- 49.Miranda-Hernandez S, et al. Role for MyD88, TLR2 and TLR9 but Not TLR1, TLR4 or TLR6 in Experimental Autoimmune Encephalomyelitis. J Immunol. 2011;187:791–804. doi: 10.4049/jimmunol.1001992. [DOI] [PubMed] [Google Scholar]
- 50.Prinz M, et al. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J Clin Invest. 2006;116:456–464. doi: 10.1172/JCI26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salvetti M, et al. Epstein-Barr virus and multiple sclerosis. Curr Opin Neurol. 2009;22:201–206. doi: 10.1097/WCO.0b013e32832b4c8d. [DOI] [PubMed] [Google Scholar]
- 52.Visser L, et al. Phagocytes containing a disease-promoting Toll-like receptor/Nod ligand are present in the brain during demyelinating disease in primates. Am J Pathol. 2006;169:1671–1685. doi: 10.2353/ajpath.2006.060143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schrijver IA, et al. Bacterial peptidoglycan and immune reactivity in the central nervous system in multiple sclerosis. Brain. 2001;124:1544–1554. doi: 10.1093/brain/124.8.1544. [DOI] [PubMed] [Google Scholar]
- 54.Marta M, et al. Unexpected regulatory roles of TLR4 and TLR9 in experimental autoimmune encephalomyelitis. Eur J Immunol. 2008;38:565–575. doi: 10.1002/eji.200737187. [DOI] [PubMed] [Google Scholar]
- 55.Li Q, et al. Endothelial IL-1R1 is a critical mediator of EAE pathogenesis. Brain Behav Immun. 2011;25:160–167. doi: 10.1016/j.bbi.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pineau I, et al. Astrocytes initiate inflammation in the injured mouse spinal cord by promoting the entry of neutrophils and inflammatory monocytes in an IL-1 receptor/MyD88-dependent fashion. Brain Behav Immun. 2010;24:540–553. doi: 10.1016/j.bbi.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 57.Kigerl KA, et al. Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J Neurochem. 2007;102:37–50. doi: 10.1111/j.1471-4159.2007.04524.x. [DOI] [PubMed] [Google Scholar]
- 58.Babcock AA, et al. Signaling through MyD88 regulates leukocyte recruitment after brain injury. J Immunol. 2008;181:6481–6490. doi: 10.4049/jimmunol.181.9.6481. [DOI] [PubMed] [Google Scholar]
- 59.Babcock AA, et al. Toll-like receptor 2 signaling in response to brain injury: an innate bridge to neuroinflammation. J Neurosci. 2006;26:12826–12837. doi: 10.1523/JNEUROSCI.4937-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marsh BJ, et al. Toll-like receptor signaling in endogenous neuroprotection and stroke. Neuroscience. 2009;158:1007–1020. doi: 10.1016/j.neuroscience.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang SC, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Halle A, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shaftel SS, et al. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation. 2008;5:7. doi: 10.1186/1742-2094-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mawhinney LJ, et al. Heightened inflammasome activation is linked to age-related cognitive impairment in Fischer 344 rats. BMC Neurosci. 2011;12:123. doi: 10.1186/1471-2202-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pontillo A, et al. NALP1/NLRP1 Genetic Variants are Associated With Alzheimer Disease. Alzheimer Dis Assoc Disord. 2011 doi: 10.1097/WAD.0b013e318231a8ac. [DOI] [PubMed] [Google Scholar]
- 66.Shaw PJ, et al. Critical role for PYCARD/ASC in the development of experimental autoimmune encephalomyelitis. J Immunol. 2010;184:4610–4614. doi: 10.4049/jimmunol.1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Jong BA, et al. Production of IL-1beta and IL-1Ra as risk factors for susceptibility and progression of relapse-onset multiple sclerosis. J Neuroimmunol. 2002;126:172–179. doi: 10.1016/s0165-5728(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 68.Chung Y, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsuki T, et al. Abnormal T cell activation caused by the imbalance of the IL-1/IL-1R antagonist system is responsible for the development of experimental autoimmune encephalomyelitis. Int Immunol. 2006;18:399–407. doi: 10.1093/intimm/dxh379. [DOI] [PubMed] [Google Scholar]
- 70.Lalor SJ, et al. Caspase-1-processed cytokines IL-1beta and IL-18 promote IL-17 production by gammadelta and CD4 T cells that mediate autoimmunity. J Immunol. 2011;186:5738–5748. doi: 10.4049/jimmunol.1003597. [DOI] [PubMed] [Google Scholar]
- 71.Mason JL, et al. Interleukin-1beta promotes repair of the CNS. J Neurosci. 2001;21:7046–7052. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Rivero Vaccari JP, et al. A molecular platform in neurons regulates inflammation after spinal cord injury. J Neurosci. 2008;28:3404–3414. doi: 10.1523/JNEUROSCI.0157-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Faustin B, et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 74.Abulafia DP, et al. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J Cereb Blood Flow Metab. 2009;29:534–544. doi: 10.1038/jcbfm.2008.143. [DOI] [PubMed] [Google Scholar]
- 75.de Rivero Vaccari JP, et al. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J Cereb Blood Flow Metab. 2009;29:1251–1261. doi: 10.1038/jcbfm.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levine B, et al. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi CS, et al. Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol. 2012;13:255–263. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shimada K, et al. Oxidized Mitochondrial DNA Activates the NLRP3 Inflammasome during Apoptosis. Immunity. 2012 doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakahira K, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou R, et al. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 81.Schultz ML, et al. Clarifying lysosomal storage diseases. Trends Neurosci. 2011;34:401–410. doi: 10.1016/j.tins.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kragh CL, et al. Autophagy in dementias. Brain Pathol. 2012;22:99–109. doi: 10.1111/j.1750-3639.2011.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lim JE, et al. MyD88 Deficiency Ameliorates beta-Amyloidosis in an Animal Model of Alzheimer's Disease. Am J Pathol. 2011 doi: 10.1016/j.ajpath.2011.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Silverman WR, et al. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 2009;284:18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bauer C, et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192–1199. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- 86.Zaki MH, et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nour AM, et al. Anthrax lethal toxin triggers the formation of a membrane-associated inflammasome complex in murine macrophages. Infect Immun. 2009;77:1262–1271. doi: 10.1128/IAI.01032-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barker BR, et al. Cross-regulation between the IL-1beta/IL-18 processing inflammasome and other inflammatory cytokines. Curr Opin Immunol. 2011 doi: 10.1016/j.coi.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martinon F, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 91.Maelfait J, et al. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J Exp Med. 2008;205:1967–1973. doi: 10.1084/jem.20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gringhuis SI, et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13:246–254. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- 93.Taxman DJ, et al. The NLR adaptor ASC/PYCARD regulates DUSP10, mitogen-activated protein kinase (MAPK), and chemokine induction independent of the inflammasome. J Biol Chem. 2011;286:19605–19616. doi: 10.1074/jbc.M111.221077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McElvania Tekippe E, et al. Granuloma formation and host defense in chronic Mycobacterium tuberculosis infection requires PYCARD/ASC but not NLRP3 or caspase-1. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012320. e12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ippagunta SK, et al. The inflammasome adaptor ASC regulates the function of adaptive immune cells by controlling Dock2-mediated Rac activation and actin polymerization. Nat Immunol. 2011;12:1010–1016. doi: 10.1038/ni.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taxman DJ, et al. Cutting edge: ASC mediates the induction of multiple cytokines by Porphyromonas gingivalis via caspase-1-dependent and -independent pathways. J Immunol. 2006;177:4252–4256. doi: 10.4049/jimmunol.177.7.4252. [DOI] [PubMed] [Google Scholar]
- 97.Ellebedy AH, et al. Inflammasome-independent role of the apoptosis-associated speck-like protein containing CARD (ASC) in the adjuvant effect of MF59. Proc Natl Acad Sci U S A. 2011;108:2927–2932. doi: 10.1073/pnas.1012455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kolly L, et al. Inflammatory role of ASC in antigen-induced arthritis is independent of caspase-1, NALP-3, and IPAF. J Immunol. 2009;183:4003–4012. doi: 10.4049/jimmunol.0802173. [DOI] [PubMed] [Google Scholar]
- 99.Ippagunta SK, et al. Inflammasome-independent role of apoptosis-associated speck like protein containing a CARD (ASC) in T cell priming is critical for collagen-induced arthritis. J Biol Chem. 2010;285:12454–12462. doi: 10.1074/jbc.M109.093252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Esen N, Kielian T. Toll-like receptors in brain abscess. Curr Top Microbiol Immunol. 2009;336:41–61. doi: 10.1007/978-3-642-00549-7_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Coll RC, O'Neill LA. New insights into the regulation of signalling by toll-like receptors and nod-like receptors. J Innate Immun. 2010;2:406–421. doi: 10.1159/000315469. [DOI] [PubMed] [Google Scholar]
- 102.Matsushita K, et al. A splice variant of ASC regulates IL-1beta release and aggregates differently from intact ASC. Mediators Inflamm. 2009;2009 doi: 10.1155/2009/287387. 287387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stehlik C, et al. The PAAD/PYRIN-family protein ASC is a dual regulator of a conserved step in nuclear factor kappaB activation pathways. J Exp Med. 2002;196:1605–1615. doi: 10.1084/jem.20021552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Holley MMaKT. Th1 and Th17 Cells Regulate Innate Immune Responses and Bacterial Clearance during Central Nervous System Infection. J Immunol. doi: 10.4049/jimmunol.1101660. (in review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Covacu R, et al. TLR activation induce s TNF-alpha production from adult neural stem/progenitor cells. J Immunol. 2009;182:6889–6895. doi: 10.4049/jimmunol.0802907. [DOI] [PubMed] [Google Scholar]
- 106.Lathia JD, et al. Toll-like receptor 3 is a negative regulator of embryonic neural progenitor cell proliferation. J Neurosci. 2008;28:13978–13984. doi: 10.1523/JNEUROSCI.2140-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]