Control of TH17/Treg Balance by Hypoxia-inducible Factor 1 (original) (raw)
. Author manuscript; available in PMC: 2012 Sep 2.
SUMMARY
T cell differentiation into distinct functional effector and inhibitory subsets is regulated in part by the cytokine environment present at the time of antigen recognition. Here, we show that hypoxia-inducible factor 1 (HIF-1), a key metabolic sensor, regulates the balance between T regulatory (Treg) and TH17 differentiation. HIF-1α enhances TH17 development through direct transcriptional activation of RORvt, and via tertiary complex formation with RORvt and p300 recruitment to the IL17 promoter, thereby regulating TH17 signature genes. Concurrently, HIF-1α attenuates Treg development by binding Foxp3 and targeting it for proteasomal degradation. Importantly this regulation occurs under both normoxic and hypoxic conditions. Mice with HIF-1α deficient T cells are resistant to induction of TH17-dependent experimental autoimmune encephalitis associated with diminished TH17 and increased Treg cells. These findings highlight the importance of metabolic cues in T cell fate determination and suggest that metabolic modulation could ameliorate certain T cell-based immune pathologies.
INTRODUCTION
Host defense against microorganisms requires a complex network of specialized T cell populations responsible for triggering inflammation to eradicate the infection, resolving the inflammatory phase after elimination of the threat, and attenuating dysregulated or inappropriate immune responses. Despite their diverse functions, these T cell subsets largely differentiate from the same pool of precursor naïve CD4+ T cells upon stimulation by antigen in the presence of unique cytokine signals present in the microenvironment (Murphy and Reiner, 2002; Zhu et al., 2010). For instance, TH1 cells are induced by type 1 interferons and propagated by IL-12, TH2 cells require IL-4, and TH17 cells are induced by IL-6 and TGF-p and propagated by IL-23 and IL-21. The activity of all of these effector T cells is attenuated by anti-inflammatory regulatory T cells (Tregs) that inhibit T cell proliferation and autoimmune responses (Barnes and Powrie, 2009; Sakaguchi et al., 2008). Tregs can be induced from naïve T cells upon exposure to TGF-β and are propagated by IL-2 (Chen et al., 2003; Davidson et al., 2007; Rajewsky and von Boehmer, 2008).
Treg are commonly categorized into thymus-derived natural Treg (nTreg) and induced Treg (iTreg). Both iTreg and nTreg express Foxp3 as a core subset-specific transcription factor, which activates a large bank of genes that mediate the suppressive phenotype of Treg and also silences many effector T cell genes (Getnet et al., 2010; Pan et al., 2009; Sakaguchi et al., 2008). TGF-β has been shown to maintain peripheral nTreg cells that develop in the thymus, and its deficiency leads to development of early lethal autoimmunity. Moreover, TGF-β induces Foxp3 expression in peripheral naïve T cells leading to the differentiation of iTregs, which exhibit a suppressive phenotype similar to that seen in nTreg cells (Sakaguchi et al., 2008).
TH17 and Treg cells share a common requirement for TGF-p in their differentiation requirements despite expressing distinct transcriptional regulators (RORγt vs. Foxp3 respectively) and demonstrating opposing functions (inflammatory vs. anti-inflammatory)(Bettelli et al., 2006; Dong, 2008; Littman and Rudensky, 2010; Mangan et al., 2006; O'Quinn et al., 2008; Veldhoen et al., 2006). TH17 cells are important in responses mounted against extracellular bacterial infections of the intestine and the airways (Korn et al., 2009). Despite providing a benefit in these settings, TH17 can play a pathologic role in the induction of several autoimmune diseases including collagen-induced arthritis, experimental autoimmune encephalomyelitis (EAE), inflammatory bowel diseases (Fife et al., 2009; Weaver et al., 2007; Wu et al., 2009) and inflammation-induced carcinogenesis (Wu et al., 2009). In these models, Treg-mediated suppression of TH17 responses often plays a protective role against pathology associated with the disease (Ahern et al., 2010).
In addition to TGF-β, initial TH17 priming also requires the cooperative action of IL-6 signaling. Subsequently, IL-23 and IL-21 play a key role in the maintenance of TH17 differentiation by enhancing the transcription of IL-17 and other TH17 signature cytokines (Korn et al., 2009; Littman and Rudensky, 2010). Interestingly, IL-6, IL-21 and IL-23 all activate Stat3, which is critical for the effects of these cytokines on TH17 cell differentiation (Zhou et al., 2007; (Harris et al., 2007). While it is now known that Stat3 induces RORyt gene expression (Harris et al., 2007; Yang et al., 2007) - the key transcription factor required for TH17 development (Ivanov et al., 2006) - the mechanism by which this is accomplished remains unclear. Interestingly, the defect in TH17 differentiation seen in the absence of Stat3 can be only partially rescued by RORγt overexpression (Yang et al., 2008b). Furthermore, although Stat3 binds most of the genes shown to promote TH17 cell fate determination, it also binds to many genes involved in T cell survival and proliferation (Durant et al., 2010). Genes bound by RORγt, on the other hand, are fairly specific for the TH17 differentiation program. Clearly, additional regulators contribute to the control of the TH17 transcriptional program.
Interestingly, accumulating evidence suggests that TH17 and iTreg arise from a common precursor. In response to TGF-β in vitro as well as i_n vivo_, many T cells co-express RORγt and Foxp3 (Veldhoen et al., 2006; Yang et al., 2008a). Ultimately, depending on the interplay between additional environmental cues such as the relative amounts of IL-6 and TGF-β, one or the other subset emerges as the dominant phenotype. High TGF-β levels in the absence of IL-6 induce Foxp3 and repress IL-23R transcription. Foxp3 can in turn bind to the RORγt protein and antagonize its ability to bind DNA, thus pushing T cell differentiation away from the TH17 transcriptional program and towards the Treg lineage. On the other hand, pro-inflammatory cytokines such as IL-6 or IL-21 in the presence of low TGF-β activate Stat3, which overcomes Foxp3 inhibition of RORγt transcriptional activity. This leads to the up-regulation of the IL-23R, thus pushing T cell differentiation toward a TH17 fate (Zhou et al., 2008). Ultimate TH17 differentiation is associated with Foxp3 down-regulation and sustained, unopposed RORγt and Stat3 transcriptional activity, although the mechanisms underlying Foxp3 suppression during TH17 lineage commitment are not fully understood.
In this study, we explored the role of a key metabolic sensor and regulator in T cell fate determination. We report that hypoxia-inducible factor 1α (HIF-1α), the transcription factor that mediates the metabolic switch from oxidative phosphorylation to aerobic glycolysis in response to hypoxia (Semenza, 2007) in fact regulates the TH17/Treg balance. Specifically, we have discovered that HIF-1α promotes TH17 differentiation by directly inducing RORγt transcription and subsequently collaborates with RORγt to regulate downstream TH17 genes. In addition, HIF-1α inhibits Treg differentiation through an active process that targets Foxp3 protein for degradation. Given the plasticity between TH17 and Treg programs, our study sheds light on how this balance is subject to metabolic regulation and suggests new strategies to manipulate these cell lineage decisions in order to treat diseases associated with TH17/Treg imbalance.
RESULTS
HIF-1γα expression is up-regulated in a Stat3-dependent manner in T cells under Th17 skewing conditions
HIF-1 is a heterodimeric transcription factor consisting of a highly regulated oxygen-sensitive HIF-1α subunit and a constitutive present β subunit (termed HIF-1β)(Semenza, 2007). HIF-1α expression in T cells can be induced both by hypoxic and non-hypoxic stimuli, including TCR-triggered and PI3K–mediated pathways, that result in mRNA up-regulation and protein stabilization (Lukashev et al., 2006). Intrigued by a potential link between the requirement for Stat3 in the TH17 program and our previous observation that HIF-1α is a target gene for activated Stat3 in tumor cells (Harris et al., 2007; Xu et al., 2005), we tested the hypothesis that HIF-1α plays a positive role in TH17 development. We began by analyzing HIF-1α mRNA expression in helper T cell subsets using qRT-PCR and found that HIF-1α mRNA was most highly expressed in TH17 cells (Figure 1A). When naïve T cells were activated in the presence of TGF-β + IL-6 (TH17 skewing conditions), HIF-1α mRNA increased, peaked after 48 hours of culture and then began to decline (Figure 1B) relative to consistently low background signal established using HIF-1α knockout (HIF-1α−/−) T cells (generated by crossing CD4_cre_ x HIF-1α_flox/flox_ mice, termed T-HIF-1−/− mice). T-HIF-1−/− mice had normal development of CD4+ and CD8+ T cells, B cells and dendritic cells (Figure S1). Whereas wild type (WT) T cells activated under TH17-skewing conditions accumulated IL-17A and RORγt transcripts over time, the induction of these TH17 associated mRNAs by HIF-1α deficient T cells was minimal (Figure 1C and 1D). Since HIF-1α mRNA was up-regulated during in vitro TH17 differentiation and RORvt and IL-17A mRNA induction were significantly diminished in the absence of HIF-1α, we suspected that HIF-1α regulates important components of the TH17 pathway. Furthermore, we measured HIF-1α and HIF-1β protein in T cells stimulated with differing levels of IL-6 and TGF-β. While HIF-1β expression was constitutive as expected under all treatments, both TGF-β and IL-6 alone induced HIF-1α and the combination of the two generated significantly higher levels of HIF-1α protei ven under normoxic conditions (Figure 1E). As Stat3 is known to mediate TH17 lineage commitment and has previously been shown to regulate HIF-1α in tumor cell lines, we hypothesized that HIF-1α induction may be diminished in Stat3-deficient T cells activated under TH17 generating conditions. Indeed, after isolating CD4+CD62LhighCD25-(naïve) Stat3-deficient T cells from CD4-cre x Stat3flox/flox mice and culturing them under TH17 skewing conditions for 4 days, very little HIF-1α protein was detected by Western blot (Figure 1E). Furthermore, a chromatin immunoprecipitation (ChIP) experiment was carried out to test whether Stat3 directly induces HIF-1α gene expression in TH17 cells. Stat3 was directly associated with the promoter region of HIF-1α in the WT TH17-skewed T cells, but not at the promoter region of GAPDH (Figure 1F). These findings confirm that HIF-1α expression is induced in T cells during TH17 differentiation in a Stat3-dependent manner.
Figure 1. HIF-1α mRNA is up-regulated in T cells under TH17 skewing conditions in Stat3 dependent manner.
(A) Naïve (CD4+CD25-CD62Lhigh) T cells were cultured under Th-cell subset inducing conditions, and HIF-1 mRNA was detected by qRT-PCR. (B, C and D) Naïve T cells from wild type (WT, HIF-1α+/+) or CD4Cre x HIF-1flox/flox mice (ko, HIF-1α−/−) were stimulated in the presence of TGFβ and IL-6. RNA was isolated from these cells and qRT-PCR was performed at different time points during culture to measure HIF-1α (B), IL-17 (C) and RORγt (D) transcript levels. (E) WT or Stat3−/− (obtained from CD4Cre x Stat3flox/flox mice) naïve CD4+ T cells were isolated and cultured under the indicated conditions for 2 days, followed by SDS-PAGE and Western blotting using antibodies against HIF-1α (top), HIF-1β (middle) and tubulin (bottom), respectively. (F) A ChIP assay was used to examine direct Stat3 binding to the HIF-1α promoter. Panels A–D and F depict the mean + s.d. of at least three experiments while Panel E is a representative result. See also Figures S1.
HIF-1α positively regulates TH17 development at multiple levels
Further analysis of TH17 differentiation by intracellular staining was performed on WT and HIF-1α −/− T cells. We observed significant induction of IL-17A protein by WT T cells stimulated with TGF-β and IL-6 (Figure 2A). Strikingly, the pattern of IL-17A and Foxp3 protein expression in HIF-1α −/− T cells cultured under the same conditions was dramatically altered (Figure 2A, right panel). In agreement with the mRNA results from Figure 1C, these HIF-1 deficient T cells displayed a marked reduction in IL-17A+ cells. Despite this notable disparity in IL-17 induction, no significant differences in IFN-γ or IL-4 were seen between WT and HIF-1α−/− T cells under TH1 or TH2 skewing conditions (Figure S2A), and CD4+ cells from both groups showed comparable proliferative capacity (Figure S2B). These results suggest that HIF-1α deficiency specifically affects the TH17 pathway, as opposed to creating a global defect in T cell proliferation or differentiation capacity.
Figure 2. HIF-1α is required for TH17 development in vitro.
(A) Naïve T cells isolated from wild type (WT, HIF-1α+/+) or CD4Cre x HIF-1αflox/flox (HIF-1α−/−) mice were cultured under TH17 skewing conditions with anti-CD3/CD28 and TGFβ, IL-6 and anti-IFNγ, IL-12 and IL-4 antibodies for 6 days. Cells were then stained for IL-17 and Foxp3 (see Methods). Numbers represent the percentage of CD4+ cells positive for the indicated marker. (B) FACS-sorted naïve CD4+ T cells from WT or T-HIF-1α−/− mice were activated and cultured under TH17 skewing for 4 days. Total RNA was isolated and mRNA expression of IL-17, IL-17F and IL-23R genes was assessed by qRT-PCR. For each gene, expression level in HIF-1−/− T cells was set to 1. The mean + s.d. of at least 3 trials is shown. (C) Naïve CD4+ T cells were activated with anti-CD3/CD28 under either neutral (anti-IL-4 and anti-IFNγ Ig) or TH17-skewing conditions, and transduced with a bicistronic retrovirus expressing HIF-1α-GFP or GFP alone. Intracellular cytokines were stained and analyzed in GFP+ cells. Numbers represent the percentage of gated GFP+ cells. (D) Naïve T cells isolated from WT or T-HIF-1α−/− mice were activated as described for (A) under normoxia or hypoxia (see Methods). Panels C and D represent at least 2 independent experiments. See also Figure S2.
The mRNA expression of other TH17-related genes such as IL-17F and IL-23R was also much lower in HIF-1α −/− T cells than WT T cells, indicating that HIF-1α regulates multiple genes in the TH17 program (Figure 2B). Furthermore, we also noted a dramatic increase in the proportion of Foxp3+ cells in HIF-1α −/− T cells (Figure 2A). To directly assess the role of HIF-1α in TH17 programming, we used retroviral transduction to overexpress HIF-1α in naïve CD4+ cells followed by detection of IL-17A and IFN-γ by intracellular cytokine staining (ICS). The ectopic expression of HIF-1α substantially increased the percentage of IL-17A+ cells even in the absence of the TH17-driving cytokines IL-6 and TGF-β (Figure 2C). This induction of IL-17A was associated with the upregulation of both RORγt mRNA and protein (Figure S2). The IL-17A+ cell population was also significantly increased upon overexpression of HIF-1α under TH17 skewing conditions (Figure 2C, right panel). These results support the notion that HIF-1α might facilitate TH17 development through the upregulation of RORγt.
The fact that HIF-1α is a major sensor of metabolic cues, such as oxygen tension, suggests that this protein could be an important link between metabolism and T cell fate determination. To formally test this, we asked whether hypoxia, which stabilizes HIF-1α protein levels and subsequent transcriptional activity, could affect TH17 differentiation. Indeed, after TH17-skewing we observed a higher proportion of IL-17A+ cells in hypoxic compared to normoxic culture conditions. This increase was abrogated in HIF-1α −/− T cells (Figure 2D and S2E), indicating that the hypoxia-induced enhancement of TH17 differentiation is HIF-1α-dependent. These findings support the idea that metabolic cues modulate T cell differentiation and that HIF-1α is an important mediator of this effect.
HIF-1α activates RORyt transcription and cooperates with RORyt and p300 to activate the IL-17A gene during TH17 development
Next we sought to further dissect the molecular mechanism by which HIF-1α regulates TH17 development. Given that RORγt is a key transcriptional regulator of TH17 cells, and the dramatically blunted induction of RORγt observed in HIF-1α −/− T cells (Figure 1D), we determined whether HIF-1α directly regulates RORγt gene expression. We used a reporter assay with the luciferase gene under the control of the 1.1kb RORγt promoter (Figure S3A and S3B). Transfection of Jurkat cells with the RORγt promoter-driven reporter construct, together with increasing amounts of HIF-1α plasmid indeed caused corresponding increases in RORγt promoter activity, particularly upon PMA and ionomycin stimulation (Figure S3C). Analysis of the RORγt promoter sequence revealed that a hypoxia response element (HRE - a conserved HIF-1α binding site) is located in the proximal region of the RORγt promoter (Figure S3A). To further assess the importance of HIF-1α in RORγt promoter activation, we tested the reporter activity of a promoter with mutated HIF-1α binding sites (Figure S3B). The mutated constructs had much lower luciferase activity compared to that of a wild type RORγt promoter-driven luciferase reporter (Figure 3A). Likewise, the expression of a HIF-1α mutant gene lacking the DNA binding domain failed to activate the wild type RORγt promoter (Figure 3B). Since the human and mouse RORγt promoter sequences share a high degree of identity as well as a conserved HRE (Figure S3A), we also tested the importance of HIF-1α in activation of the human RORγt promoter. Luciferase assays using the human sequence demonstrated that HIF-1α activates this promoter in a similar fashion to that of the mouse (Figure S3D and S3E). These results suggest that HIF-1α can regulate RORγt gene expression.
Figure 3. HIF-1α transactivates RORγt transcription and is necessary for RORγt-driven TH17 differentiation in vitro.
(A) Jurkat T cells were co-transfected with a luciferase reporter under the control of a wild type RORγt promoter or one with a mutated HIF-1-binding site. 24hrs post-transfection, cells were either treated with PMA and ionomycin or left untreated prior to analysis of luciferase activity, which was normalized to that of Renilla luciferase. (B) Jurkat T cells were transfected with a RORγt-luciferase reporter plasmid (incorporating the RORγt promoter) along with plasmid encoding either wild type HIF-1 or a HIF-1 mutant (with a DNA binding domain deletion). 24hrs post-transfection, cells were stimulated and luciferase activity was assessed as described in (A). Data are shown for three independent experiments (mean and s.d. of triplicate transfections). (C) A ChIP assay was used to measure direct HIF-1 binding to the RORγt promoter directly. (D and E) Naïve WT or HIF-1−/− CD4+ T cells were activated with anti-CD3/anti-CD28 under neutral (anti-IL4 and anti-IFNγ) conditions, and transduced with either a bicistronic retrovirus expressing RORγt-GFP or the GFP containing empty vector. Intracellular IL-17 and Foxp3 were stained. The plots shown are gated on GFP+ cells. These experiments were repeated at least twice with consistent results. See also Figures S3.
To further dissect this mechanism, a ChIP assay was employed to test if HIF-1α directly binds to the promoter region of the RORγt gene in primary, _in vitro_-generated TH17 cells. Indeed, HIF-1α was found bind to several HREs located at the RORγt promoter, but not the promoter of the non-HIF-1α-regulated Gmpr gene (Figure 3C and Figure S4A–D). These results collectively indicate that HIF-1α directly transactivates RORγt gene expression.
Since our results suggested that RORγt acts directly on the IL-17A promoter, we asked whether RORγt could rescue TH17 development in HIF-1α−/− T cells. In HIF-1α+/+ T cells, retroviral RORγt expression induced a significantly higher proportion of IL-17A+ cells (45.7%) under non-skewing conditions, compared to control retrovirus recipients (0.72%) (Figure 3D and 3E, left panels). However, ectopic RORγt expression in HIF-1α−/− T cells induced a strikingly diminished population of IL-17A+ cells (1.33%) (Figure 3E, right panel). These results suggest that HIF-1α not only directly mediates RORγt gene expression, but also is required for the full function of RORγt in IL-17A gene transcription.
To gain further insight into how HIF-1α and RORγt co-regulate IL-17A transcription, we performed a luciferase reporter assay to determine if transient expression of HIF-1α and RORγt could activate the 1.3kb IL-17 promoter. The expression of wild type HIF-1α in the presence of RORγt resulted in significantly increased reporter gene activity over that induced by RORγt alone (Figure 4A). In striking contrast to our findings with the RORyt promoter, a HIF-1α mutant with a characterized DNA binding domain deletion (Arany et al., 1996) showed no defect in inducing reporter gene activity, which implies that DNA binding is dispensable for HIF-1α-mediated IL-17A expression. Since the DNA binding domain of HIF-1α did not appear to be required for its function in facilitating RORγt activity, we speculated that HIF-1α might physically associate with RORγt, serving as a co-activator for RORγt without direct DNA binding. To test this hypothesis, a Flag-tagged HIF-1α plasmid was co-expressed with Myc-tagged RORγt in Jurkat T cells. HIF-1α was found to co-immunoprecipitate (co-IP) with RORγt (Figure S4E). The association between HIF-1α and RORyt was further confirmed by co-IP of endogenous HIF-1α and RORyt in T cells cultured under TH17-skewing conditions (Figure 4B). To verify that the interaction between HIF-1α and RORγt was direct, we employed affinity-purified recombinant GST-fusion proteins to pull down Myc-RORγt from 293T cell lysate. GST-HIF-1α (amino acids1–80), rather than GST alone, was bound to RORγt (Figure S4F). Further testing this apparent direct association of RORγt and HIF-1α, we purified His-tagged RORγt from E. coli and a GST-pulldown assay was carried out using GST- or GST-HIF-1α fragments as indicated. Only GST fusions of the HIF-1α N-terminus (1–80 aa), but not GST alone or other HIF-1α fragments, successfully pulled down RORγt (Figure 4C). Taken together, these results suggested a direct interaction between the two proteins in vitro.
Figure 4. RORγt, HIF-1α and p300 bind to the IL-17 promoter to regulate its gene expression.
(A) A HIF-1α mutant (HIF-1-ΔDBD, with the DNA binding domain deleted) retains the capacity to activate IL-17 promoter-driven luciferase activity in the presence of RORγt. Jurkat T cells were transfected with an IL-17 promoter-driven luciferase reporter along with the indicated plasmids followed by stimulation and assessment as described for Figure 3A. Data are representative of at least 3 experiments (mean and s.d. of triplicate transfections). (B) The interaction between HIF-1 and RORγt was examined with co-immunoprecipitation. FACS-sorted CD4+ T cells were activated and cultured under TH17-skewing conditions for 5 days. The whole cell lysate were immunoprecipitated with either anti-RORγt (left panel) or anti-HIF-1α antibodies (right panel), resolved by SDS-PAGE and blotted with the indicated antibodies. (C) RORγt interacts with the N-terminus of HIF-1. His-tagged RORγt purified from E. coli was incubated with different fragments of GST-HIF-1 also purified from E. coli as indicated, followed by pull-down with GST beads, resolution by SDS-PAGE and Westen blotting with anti-RORγt (top and bottom) antibodies, or anti-GST (middle).
(D and E) FACS-sorted CD4+ T cells from WT or HIF-1−/− mice were activated under TH17-skewing conditions (as described for B) prior to harvest for ChIP assay utilizing anti-RORγt, anti-HIF-1 or anti-p300 antibodies. (F) Hypoxia enhances the activation of IL-17 promoter-driven luciferase activity by HIF-1, RORγt and p300. Jurkat T cells were transfected with a IL-17 promoter-driven luciferase reporter along with the indicated plasmids under normoxia or hypoxia, followed by stimulation and assessment as described for Figure 3B. Data are representative of at least 3 independent experiments (mean and s.d. of triplicate transfections). (G) Histone hyperacetylation at the IL-17 promoter was detected by ChIP assay in WT and HIF-1α−/− T cells under TH17 skewing conditions for 5 days. See also Figures S4–S6 and Table S1.
Because it has been shown that the transcription factor p300 is critical for HIF-1α-mediated gene activation under hypoxic conditions, we hypothesized that HIF-1α might function in a complex with RORγt by recruiting p300 to activate target genes such as IL-17A. To test this hypothesis, a ChIP assay was employed to determine whether RORγt, HIF-1α and p300 associate with the IL-17A promoter during TH17 differentiation. Indeed, we detected localized binding of RORγt, HIF-1α and p300 to the IL-17A promoter determined using primers flanking the RORγt-binding site located at the IL-17A promoter region (Figure 4D). This binding, as analyzed by ChIP, was specific since no significant signal could be detected at a non-RORγt binding region within the IL-17A promoter. Furthermore, ChIP analysis revealed that HIF-1α was recruited to several other putative RORγt binding regions, including CNS2. This recruitment of HIF-1α was dependent on RORγt since it was abrogated in T cells from RORγt knockout mice (Figure S5A–D). Additionally, we observed similar co-localized binding of RORγt, HIF-1α and p300 at the promoters of the TH17 signature genes IL-17F and IL-23R (Figure 4E). The role of the RORγt/HIF-1α/p300 complex in the activation of the IL-17A promoter was further confirmed by reporter assays in Jurkat and 293T cells. Surprisingly, co-expression of RORγt, HIF-1α and p300 led to a more than 10-fold increase in luciferase activity compared to that induced by combinations of any two of these proteins (Figure S5E and S5F). However, a HIF-1α mutant with a C-terminal deletion of the p300 binding domain (Arany et al., 1996) failed to synergize with RORγt and p300 in the activation of the IL-17A promoter, implying that p300 is required for the full function of RORγt and HIF-1α in the regulation of IL-17A gene expression (Figure S5E). In addition, IL-17A production in Jurkat T cells co-transfected with RORγt, HIF-1α and p300 was further increased under hypoxic conditions (Figure 4F). These results suggest that, while active under normoxic conditions, HIF-1α’s ability to promote IL-17A production is indeed modulated by oxygen tension. Of note, the gut, which is known to be relatively hypoxic under physiologic conditions (Koch, 2002), contains a high proportion of IL-17A–producing cells. We found that, under steady state conditions, T-HIF-1−/− mice had reduced proportions of IL-17A+ cells in the lamina propria (Figure S6A–D), indicating a potential role for HIF-1α in promoting TH17 development in the context of microbial influences.
p300 possesses histone acetyltransferase (HAT) activity (Thompson et al., 2004). In order to determine whether this HAT activity is required for the RORγt/HIF-1α /p300 complex-mediated IL-17A gene expression, we performed co-transfection studies using a p300 mutant with a HAT domain deletion (Δp300) (Youn et al., 2000). Wild type p300, but not Δp300 could synergistically enhance IL-17A promoter activity in the presence of HIF-1α and RORγt (Figure S5E). Furthermore, a HIF-1α mutant with a deletion in its p300 binding domain (Arany et al., 1996) fails to activate the IL-17A promoter. These findings indicate that RORγt and HIF-1α, through recruitment of p300 to the IL-17A promoter, acetylate histones to “open” the chromatin structure and facilitate gene expression. In support of this notion, ChIP assays revealed that histones H3 and H4 around the IL-17A promoter region were highly acetylated in WT compared to HIF-1−/−T cells under TH17-inducing conditions (Figure 4G). Similarly, hyper-acetylated histones H3 and H4 were associated with the promoter of TH17 signature genes IL-17F and IL-23R in WT, but not in HIF-1α −/−, TH17 cells (Figure S6E and S6F).
Taken together, these results demonstrate that HIF-1α plays a dual role in regulating IL-17A transcriptional activity by directly activating RORγt transcription and then associating with RORγt at the IL-17A promoter to recruit p300, thus generating a permissive chromatin structure.
HIF-1α mediates Foxp3 protein degradation during TH17 development
Although we established a role for HIF-1α in IL-17A gene regulation, it was unclear how HIF-1α deficiency caused Foxp3 protein accumulation in T cells under in vitro TH17-inducing conditions (Figure 2A). Curiously, Foxp3 mRNA levels in WT and HIF-1α −/− T cells stimulated under TH17 skewing conditions were essentially identical (Figure 5A). This implies that HIF-1α might be involved in Foxp3 protein modulation in addition to its role in the transcriptional activation of the TH17 pathway. Further evidence supporting this hypothesis comes from analysis of Foxp3 protein levels in T cells cultured under Treg skewing conditions (TGF-β plus IL-2). In concordance with our findings in T cells cultured under TH17 skewing conditions, HIF-1α −/− T cells cultured under Treg conditions also expressed substantially more Foxp3 than did WT T cells (Figure 5B and 5C). These findings suggest that under both Treg- and TH17-skewing conditions, HIF-1α down-regulates Foxp3 protein levels despite demonstrating no effect on Foxp3 mRNA levels (Figure 5A). Based on these findings, we sought to determine whether Foxp3 levels were sensitive to hypoxia. T cells were thus cultured with TGF-β and IL-2 under hypoxic conditions. Foxp3+ cells were significantly decreased under these hypoxic conditions relative to normoxia (Figure 5D). Thus, hypoxi culture conditions have opposite effects on IL-17 and Foxp3 induction. Compatible with this in vitro finding, steady state levels of Foxp3+ cells in the lamina propria of the gut, which, as indicated above (Koch, 2002), is relatively hypoxic, are significantly increased in T-HIF-1−/− mice (Figure S6A–D).
Figure 5. HIF-1α mediated Foxp3 degradation through proteasomal degradation pathways.
(A) Naïve T cells from either WT or T-HIF-1−/− mice were cultured and stimulated in the presence of TGFβ and IL-6 and qRT-PCR was performed to measure Foxp3 mRNA at different times. (B) HIF-1α−/− T cells displayed enhanced Foxp3 accumulation during in vitro Treg differentiation. Naïve T cells from HIF-1+/+ and T-HIF-1−/− were isolated by FACS and activated under Treg skewing (5ng/ml TGFβ, 100U IL-2). Cells were stained for Foxp3 after 72 hours. Shown are representative histograms for HIF-1+/+ (red line) and HIF-1−/− (blue line) cells from three experiments. An isotype control is shown in green. Numbers represent the mean percentage of Foxp3+ cells. (C) Foxp3 protein is lost upon culture of WT T cells with IL-6 but remains unchanged in HIF-1α−/− Foxp3+ T cells. Naive T cells were activated under Treg-skewing (TGFβ, 5ng/ml) with the indicated doses of IL-6 for 4 days. Western blotting was used to measure Foxp3 protein level. (D) Hypoxia reduced expression of Foxp3 by naïve T cells during in vitro differentiation. Naïve T cells isolated from Foxp3-GFP reporter mice (CD4+GFP-CD62Lhigh) were cultured under Treg skewing conditions (see B) in either a hypoxic chamber or under normoxia (blue and red lines, respectively). Numbers represent the mean percentage of CD4+ cells expressing GFP (Foxp3+) from three experiments. (E) HIF-1 interacts with Foxp3 in iTreg cells. FACS-sorted CD4+ T cells were activated and cultured under iTreg-skewing for 4 days. Cell lysates were immunoprecipitated with anti-HIF-1 (left panel) or anti-Foxp3 antibodies (right panel), followed by SDS-PAGE and western blotting. (F, G and H) HIF-1α mediates Foxp3 degradation. 293T cells were cotransfected with Foxp3 +/− ubiquitin and increasing amounts of WT HIF-1 (F) or mutant HIF-1 (p420A and p564A) (G) or a deleted ODD domain (CA5-HIF) (H)). CoIP and Western blots were probed as indicated. (I and J) Naïve T cells were cultured under Treg-skewing conditions in normoxia (N) or hypoxia (H) for 4 days. Cells were harvested and lysed, followed by immunoprecipitation with anti-Foxp3 or control IgG antibodies. The pulled down protein along with an input control were resolved by SDS-PAGE, followed by western blot. Depicted are typical findings from 3 independent experiments. See also Figure S7
We next asked if a physical interaction between Foxp3 and HIF-1α existed. Immunoprecipitation and Western blot analysis of 293 T cells co-transfected with HIF-1α and Foxp3, in fact, demonstrated a physical interaction between these two molecules (Figure S7A). This interaction between HIF-1α and Foxp3 was further confirmed by endogenous co-IP in iTreg cells (Figure 5E). The HIF-1α binding domain was further mapped to the C-terminus of Foxp3 (Figure S7B) and the Foxp3 binding domain in HIF-1α was found to be within the N-terminal 80 amino acids by GST pull-down experiments (Figure S7C). Based on these results, we sought to determine whether HIF-1α could regulate Foxp3 protein levels through a mechanism completely distinct from its classic role as a transcriptional regulator. In particular, we hypothesized that HIF-1 might target Foxp3 for degradation. Indeed, upon co-transfection of 293T cells with Foxp3 and increasing amounts of Flag-tagged HIF-1α, Foxp3 protein levels progressively decreased (Figure 5F). On the other hand, RORγt protein levels remained unchanged upon co-transfection with HIF-1α (Figure S7D). It has been well-established that ubiquitin-dependent degradation of HIF-1α itself occurs via proline hydroxylation at amino acid positions 402 and 564 by prolyl hydroxylases (PHDs). Hydroxylated HIF-1α is subsequently bound by the von Hippel-Lindau protein (VHL), which recruits the Elongin-C-Elongin-B-Cullin-2-E3-ubiquitin ligase complex, thereby targeting HIF-1α for degradation by the 26S proteasome (Semenza, 2007). We wondered whether HIF-1α’s ability to induce Foxp3 protein degradation was dependent on this very mechanism. To address this, we co-transfected 293T cells with Foxp3 and a HIF-1α mutant in which prolines 402 (p402A) and 564 (p564A) were mutated to alanines, making HIF-1α resistant to both hydroxylation by PHDs and subsequent proteasomal degradation. The expression of P402A and P564A HIF-1α failed to induce Foxp3 degradation (Figure 5G and 5H). Similar co-transfection assays using a HIF-1α plasmid containing an oxygen dependent domain (ODD) deletion (CA5-HIF-1α) further demonstrated that a non-proline hydroxylatable HIF-1α was incapable of mediating Foxp3 degradation (Figure 5G and 5H). Knockdown of PHD2 with siRNA also eliminated the ability of wild type HIF-1α to mediate the degradation of Foxp3 (Figure S8A). HIF-1α mediated Foxp3 degradation was completely inhibited in the presence of a proteasome inhibitor (Figure S8B). Furthermore, we assessed if Foxp3 itself could be ubiquitinated under hypoxic conditions in primary iTregs. Hypoxic culture significantly enhanced both Foxp3 ubiquitination and degradation in these cells (Figure 5I and 5J). Furthermore, this ubiquitin-mediated Foxp3 degradation process could be prevented by addition of the proteasomal inhibitor MG132 to iTreg cells under hypoxia. Collectively, these results are compatible with a scenario in which HIF-1α mediates Foxp3 degradation via PHD-VHL-Ubiquitin-mediated proteasomal degradation, although future studies are needed to delineate precisely how HIF-1α protein turnover dynamics in T cells modulate Foxp3 levels.
Collectively, these in vitro differentiation studies, alongside our transcriptional and biochemical analyses, suggest a model in which HIF-1α is transcriptionally activated via Stat3 signaling in differentiating T cells, resulting in the enhancement of the TH17 genetic program via a RORγt/p300-dependent mechanism and repression of the Treg transcriptional program through induced degradation of Foxp3.
Mice with HIF-1α-deficient T cells fail to mount a strong TH17 response, have increased Treg numbers and are resistant to EAE
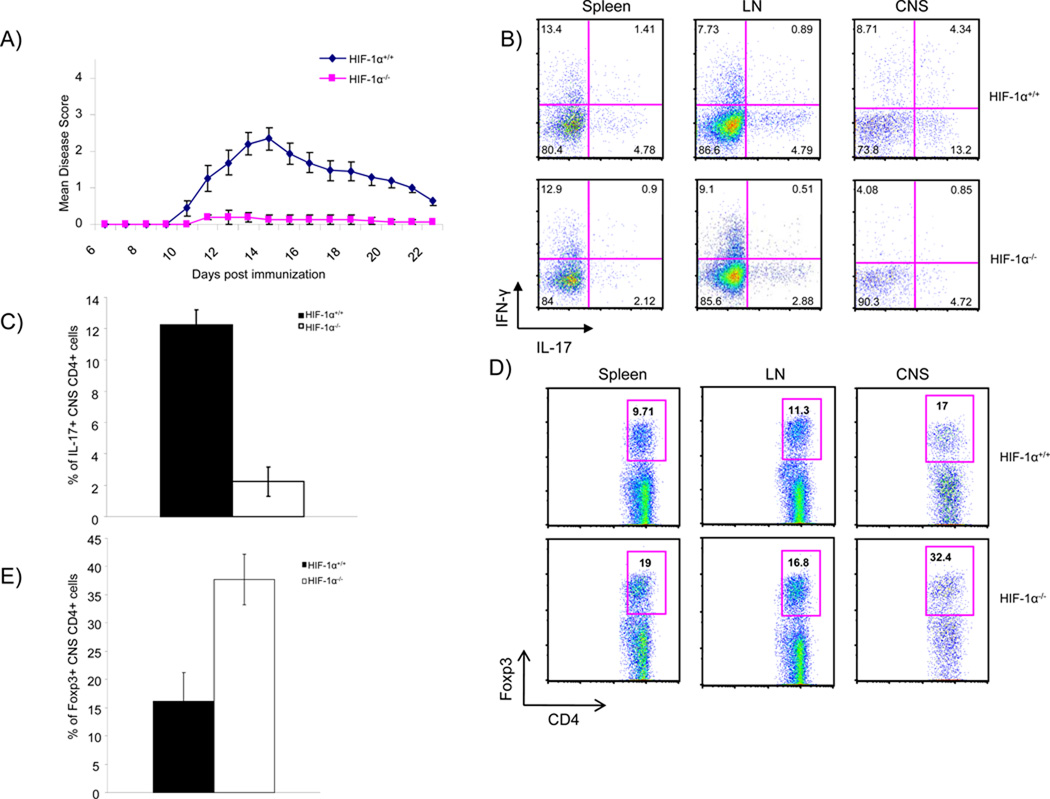
Our in vitro findings that HIF-1α regulates the TH17/Treg balance prompted us to test its role in vivo in the setting of a pathologic TH17-dependent autoimmune disease. TH17 cells are the major pathogenic population in experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis, although factors besides IL-17A and IL-17F can contribute to the disease. Immunization of B6 mice with myelin oligodendrocyte glycoprotein peptide (MOG35–55) in complete Freund’s adjuvant (CFA) induces a TH17-dependent response that induces encephalitis. After a peak of encephalitis, clinically discernible by various gradable neurologic signs (including tail paralysis, progressing to hind and front limb paralysis) the disease remits, concomitant with expansion of Tregs that can silence the TH17 encephalitic process. Strikingly, WT mice immunized with MOG35–55 developed EAE, while T-HIF-1α −/− mice were highly resistant (Figure 6A). At peak disease, lymph node-, spleen- and central nervous system (CNS)-infiltrating CD4+ T cells from WT mice contained higher proportions of IL-17+ cells compared to those of T-HIF-1α −/− mice (Figure 6B and 6C). In contrast, T-HIF-1−/− mice showed a significant increase in the percentage of CD4+ Foxp3+ cells during the recovery phase of disease (21 days post-injection), concomitant with a decrease in the proportion of IL-17+ CD4+ cells in T-HIF-1−/−animals (Figure 6D and 6E). This altered balance between Treg cells and TH17 cells exactly parallels our in vitro culture studies and suggests that HIF-1α indeed plays a role in modulating the TH17/Treg balance. Taken together, these results support the notion that HIF-1α plays a role in setting the TH17/Treg balance in a TH17-dependent disease model and that the mechanisms defined in the in vitro differentiation assays are also operative in vivo (Figure 7).
Figure 6. Mice lacking HIF-1α in CD4+ T cells are deficient in IL-17 production, have increased numbers of Foxp3 Treg and are more resistant to EAE.
(A) EAE was induced in HIF-1+/+ and T-HIF-1−/− mice by injection of MOG35–55 in CFA and Pertussis Toxin. Disease severity was monitored and scored daily. T-HIF-1−/− mice failed to develop the severe disease seen in HIF-1+/+ mice. Mean scores for HIF-1+/+ and T-HIF-1−/− mice over time are represented (+/− SEM; *p<0.05). Shown is a representative of 4 experiments (N=7–10 per group). (B, C) During peak disease (day12), draining lymph node, splenic, and CNS infiltrating T cells were recovered and stained for IL-17 and IFNγ. The mean percentage of CNS CD4+ cells during peak disease (days 12–14) positive for IL-17 was determined (C). (D and E) Similarly, during the recovery phase (day 21), tissue infiltrating cells were isolated and the percentage of CD4+ that wereFoxp3+ was found (D and E). Panels C and E present the mean (± SEM, *p<0.05) of at least 3 trials and dot plots are representative analyses.
Figure 7. A model for the multi-factorial role of HIF-1α in modulating the TH17/Treg balance.
Stat3 activation by factors such as IL-6 transcriptionally activates HIF-1. HIF-1 levels are further regulated by oxygen tension and other metabolites, representing a key molecular link between metabolic cues and T cell lineage commitment. HIF-1 directly activates RORγt gene transcription and furthermore, recruits p300 to RORγt transcription complexes to the promoters of TH17 genes (ie IL-17). These activities promote Th17 differentiation. Concomitantly, HIF-1 induces Foxp3 protein degradation via targeting for ubiquitination and proteasomal degradation.
DISCUSSION
Here we identify HIF-1α as a major player in the development of TH17 cells and further demonstrate its role in modulating the TH17:Treg balance. Specifically, HIF-1α promotes TH17 development through: a) direct transcriptional activation of RORγt, the major TH17 transcription factor, and b) direct collaboration with RORγt to activate TH17 signature genes, such as IL-17A, through mechanisms involving p300 recruitment and histone acetylation. Other transcription factors have been identified to contribute in the induction of IL-17A in TH17- polarized cells: Runx1 (Zhang et al., 2008), BATF (Schraml et al., 2009), Stat3, c-Maf (Bauquet et al., 2009), AHR (Veldhoen et al., 2008) and RORα (Yang et al., 2008b). Interestingly, like most of these factors, HIF-1α not only regulates RORγt expression at the mRNA level, it also cooperates with RORγt protein to regulate IL-17A–related genes during TH17 development. HIF-1 may promote the TH17 response in additional ways. Indeed, studies by Miossec and colleagues indicate that the hypoxia induced pathway is itself activated by IL-17A and IL-17F (Hot and Miossec, 2011), suggesting that IL-17 signaling can sustain the HIF-1 pool and thereby perpetuate an existing TH17response,
Coordinate with these transcriptional mechanisms for promotion of TH17 development, HIF-1α inhibits differentiation toward the Treg lineage through a distinct, non-transcriptional mechanism. We show that HIF-1 targets Foxp3 for ubiquitination and proteasomal degradation, using the same ubiquitin ligase system that is responsible for degradation of HIF-1α itself (Figure 7).
Our findings help to clarify the molecular events involved in lineage differentiation from the precursors of TH17 and Treg cells into the distinct T cell subsets. In addition, because HIF-1α responds to metabolic cues, our findings link metabolism to T cell differentiation and provide a basis for understanding how the metabolic environment of a T cell can modulate its fate decisions. Our studies on HIF-1α presented here and those of Delgoffe et al (Delgoffe et al., 2009; Delgoffe et al., 2011; Delgoffe and Powell, 2009) and Shi et al (Shi et al., 2011) on the mTOR pathway further link metabolic sensing and cytokine signaling in T cell fate determination.
HIF-1α is a major sensor of oxygen tension and indeed, we found that TH17 differentiation is enhanced under hypoxia in a HIF-1α-dependent manner. Interestingly, a number of studies have demonstrated that inflammatory environments are, relatively hypoxic. It is therefore likely that HIF-1α activity represents a major mechanism by which the hypoxic conditions associated with inflammation can promote TH17 differentiation. It is quite clear however that HIF-1α protein is readily detectable in TH17 cells under normoxia, and the experiments utilizing HIF-1α−/− T cells confirm that under such conditions TH17 differentiation is significantly impaired without HIF-1α. Even though HIF-1α was originally discovered as a hypoxia sensor, it is now well appreciated that HIF-1α levels can be significantly affected by many other important metabolites such as reactive oxygen species and succinate (Pouyssegur and Mechta-Grigoriou, 2006). This regulation appears to be at the level of PHD enzyme activity, which in normoxia hydroxylates specific prolines on HIF-1α, targeting it for VHL-dependent ubiquination and proteosomal degradation.
Our findings regarding the role of HIF-1α in targeting Foxp3 for degradation help explain the unresolved question of how dual TH17/Treg precursors that express both RORγt and Foxp3 eventually eliminate Foxp3 when they commit to TH17 differentiation in a STAT3-dependent fashion. Rudensky and colleagues have recently proposed that conserved noncoding sequence 2 (CNS2) in the Foxp3 gene represents a site for positive transcriptional autoregulation of Foxp3, providing a mechanism for the protein to maintain its own expression levels (Zheng et al., 2010). Thus, degradation of Foxp3 in TH17/Treg progenitors mediated by HIF-1α would be expected to eventually diminish Foxp3 gene transcriptional activity thereby antagonizing stable Foxp3 autoregulation and consequent Treg lineage commitment.
Sitkovsky and colleagues have suggested that HIF-1α activity in T cells is a general negative regulator of T cell activation (Lukashev et al., 2006). These experiments did not evaluate specific T cell differentiation decisions however. We found that neither IFN-y nor IL-4 production was significantly affected by the absence of HIF-1 in T cells cultured under Th1 or Th2 conditions, Respectively. Because TH17 cytokines can inhibit Th1/IFN-γ development in some circumstances (Luger et al., 2008), it is possible that the enhanced IFN-y production seen in HIF-1α−/− T cells under the non-skewing in vitro conditions used by Lukashev et al is in fact a reflection of diminished TH17development.
Our in vivo findings demonstrate that mice with HIF-1α−/− T cells are resistant to induction of EAE, a TH17-dependent disease whose remission is dependent upon Treg cells (Schraml et al., 2009). These experiments validate the role of HIF-1α identified by our in vitro studies and further suggest the possibility for metabolic modulation of autoimmune diseases. Indeed, there is great interest in developing HIF-1α inhibitors based on its role in cancer. It would be of worthwhile to determine if any of these inhibitors can alter the TH17:Treg balance in vivo and ameliorate TH17-dependent autoimmune diseases.
EXPERIMENTAL PROCEDURES
Mice
All animal experiments were performed in specific-pathogen-free facilities in the Johns Hopkins Animal Resource Center following national, state and institutional guidelines. Animal protocols were approved by the Johns Hopkins Animal Care and Use Committee. Foxp3-GFP mice (Fontenot et al., 2005) were kindly provided by A. Rudensky. C57BL/6 HIF-1αfl/fl mice were produced in Dr. Semenza’s lab. _Rorc_−/− mice were purchased from the Jackson Laboratory.
T Cell Differentiation
Naive T cells were purified using a FACS Aria sorter prior to stimulation with anti-CD3 /CD28 antibodies in a 24-well plate (1 and 4 µg/well, respectively; Biolegend) for 3-to-7 days. TH17 skewing conditions consisted of IMDM media supplemented with 5% FBS, 20ng/ml IL-6, 2.5ng/ml TGFβ (Peprotech), and 10ug/ml neutralizing antibodies against IFNγ, IL-4, and IL-12 (Biolegend). For hypoxia experiments, cells were cultured in a GasPak Plus anaerobic chamber (1% O2) for 20hr cycles interrupted by normoxic rest.
Quantitative RT-PCR
RNA was isolated by a miniRNA extraction kit (Qiagen). The cDNA archival kit (Applied Biosystem) was used as per the manufacturer’s instruction. Triplicate reactions were run using an ABI Prism 7500. mRNA levels were determined by comparative CT method and normalized to β-actin or 18s rRNA expression.
EAE Induction
Six-eight week old, sex matched WT and T-HIF-1α−/− littermates were injected s.c in the rear flank with 100µg MOG35–55 peptide (2HN-MEVGWYRSPFSRVVHLYRNGK-COOH) in complete Freund’s Adjuvant (Sigma) and 250 ng pertussis toxin (List Biological) was injected i.p. Mice were monitored daily and disease severity was scored.
Immunoprecipitation and western blotting
Immunoprecipitation and western blotting were performed as elsewhere (Pan et al., 2005). Immunoprecipitations were done using a Pierce Crosslink IP kit and clean-Blot IP detection system (Thermo Scientific).
Reporter Gene Assays
The luciferase reporter gene assay reagents were obtained from Promega, and the assay was performed per manufacturer’s instructions.
Chromatin Immunoprecipitation Assays
ChIP analysis was carried out according to the manufacturer’s protocol (Upstate/Millipore, Massachusetts, USA). The amount of immunoprecipitated DNA was quantified by real-time PCR with the ABI PRISM 7500 Sequence Detection System (Applied Biosystems) using SYBR Green. All primers used for ChIP assays are listed in Table S1.
GST pull-down
Cell lysate or purified protein was incubated with 3µg of affinity purified GST protein (with a GSTrap column, Amersham) in the presence of 0.2%BSA in BC100 Buffer on a rotator overnight at 4°C. Proteins were pulled down using GST beads, followed by washing, glutathione elution and resolution by SDS-PAGE.
Statistical Analysis
An unpaired student’s t test was used to determine significance (*p<0.05).
Supplementary Material
01
02
Table.
Primer Name
| HIF-1_F1 (Stat3 ChIP) |
|---|
| HIF-1_R1 (Stat3 ChIP) |
| RORγt_F2 (HIF-1 ChIP) |
| RORγt_R2 (HIF-1 ChIP) |
| RORγt_F2 (−4149 to −4208) |
| RORγt_R2 (−4149 to −4208) |
| RORγt_F3 (−6988 to −7002) |
| RORγt_R3 (−6988 to −7002) |
| RORγt_F4 (non-HIF1 binding) |
| RORγt_R4 (non-HIF1 binding) |
| IL-17A_F5 (RORγt flanking) |
| IL-17A_R5 (RORγt flanking) |
| IL-17A_F6 (non-RORγt binding ) |
| IL-17A_R6 (non-RORγt binding ) |
| IL-17A_F7 (−2913 to −2925) |
| IL-17A_R7 (−2913 to −2925) |
| IL-17A_F8 (−6128 to −6140) |
| IL-17A_R8 (−6128 to −6140) |
| IL-17A_F9 (outside CNS2) |
| IL-17A_R9 (outside CNS2) |
| IL-17F_F10 (RORγt flanking) |
| IL-17F_R10 (RORγt flanking) |
| IL-17F_F11 (non-RORγt binding) |
| IL-17F_R11 (non-RORγt binding) |
| IL-23R_F12 (RORγt flanking) |
| IL-23R_R12 (RORγt flanking) |
| IL-23R_F13 (non-RORγt binding) |
| IL-23R_R13 (non-RORγt binding) |
| IL-17A_14 (H3-Ac) |
| IL-17A_14 (H3-Ac) |
| IL-17F_F15 (H3-Ac) |
| IL-17F_R15 (H3-Ac) |
| IL-23R_F16 (H3-Ac) |
| IL-23R_R16 (H3-Ac) |
ACKNOWLEDGMENTS
We thank the members of the D. Pardoll, F. Pan, J. Powell and C. Drake laboratories for helpful discussions. We are grateful to Drs. J. Liu (Department of Pharmacology at Johns Hopkins), A. Rudensky (Memorial Sloan Kettering Cancer Center), D. Littman (NYU) for helpful suggestions and/or reagent contribution. We thank Dr. H. Wei (Dr. Semenza’s laboratory) for the HIF1fl/fl miceand genotyping primer sequences. This work was supported by grants from NIH and the Melanoma Research Alliance, the Janey Fund and Seraph Foundation, and gifts from Bill and Betty Topecer and Dorothy Needle. F. P. is a recipient of the Stewart Trust Scholar Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33:279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany Z, Huang LE, Eckner R, Bhattacharya S, Jiang C, Goldberg MA, Bunn HF, Livingston DM. An essential role for p300/CBP in the cellular response to hypoxia. Proc Natl Acad Sci U S A. 1996;93:12969–12973. doi: 10.1073/pnas.93.23.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401–411. doi: 10.1016/j.immuni.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgoffe GM, Powell JD. mTOR: taking cues from the immune microenvironment. Immunology. 2009;127:459–465. doi: 10.1111/j.1365-2567.2009.03125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- Durant L, Watford WT, Ramos HL, Laurence A, Vahedi G, Wei L, Takahashi H, Sun HW, Kanno Y, Powrie F, O'Shea JJ. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32:605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Getnet D, Grosso JF, Goldberg MV, Harris TJ, Yen HR, Bruno TC, Durham NM, Hipkiss EL, Pyle KJ, Wada S, et al. A role for the transcription factor Helios in human CD4(+)CD25(+) regulatory T cells. Mol Immunol. 2010;47:1595–1600. doi: 10.1016/j.molimm.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, Grosso JF, Yen HR, Xin H, Kortylewski M, Albesiano E, Hipkiss EL, Getnet D, Goldberg MV, Maris CH, et al. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol. 2007;179:4313–4317. doi: 10.4049/jimmunol.179.7.4313. [DOI] [PubMed] [Google Scholar]
- Hot A, Miossec P. Effects of interleukin (IL)-17A and IL-17F in human rheumatoid arthritis synoviocytes. Ann Rheum Dis. 2011;70:727–732. doi: 10.1136/ard.2010.143768. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Koch CJ. Measurement of absolute oxygen levels in cells and tissues using oxygen sensors and 2-nitroimidazole EF5. Methods Enzymol. 2002;352:3–31. doi: 10.1016/s0076-6879(02)52003-6. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashev D, Klebanov B, Kojima H, Grinberg A, Ohta A, Berenfeld L, Wenger RH, Sitkovsky M. Cutting edge: hypoxia-inducible factor 1alpha and its activation-inducible short isoform I.1 negatively regulate functions of CD4+ and CD8+ T lymphocytes. J Immunol. 2006;177:4962–4965. doi: 10.4049/jimmunol.177.8.4962. [DOI] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- O'Quinn DB, Palmer MT, Lee YK, Weaver CT. Emergence of the Th17 pathway and its role in host defense. Adv Immunol. 2008;99:115–163. doi: 10.1016/S0065-2776(08)00605-6. [DOI] [PubMed] [Google Scholar]
- Pan F, Means AR, Liu JO. Calmodulin-dependent protein kinase IV regulates nuclear export of Cabin1 during T-cell activation. Embo J. 2005;24:2104–2113. doi: 10.1038/sj.emboj.7600685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Yu H, Dang EV, Barbi J, Pan X, Grosso JF, Jinasena D, Sharma SM, McCadden EM, Getnet D, et al. Eos mediates Foxp3-dependent gene silencing in CD4+ regulatory T cells. Science. 2009;325:1142–1146. doi: 10.1126/science.1176077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouyssegur J, Mechta-Grigoriou F. Redox regulation of the hypoxia-inducible factor. Biol Chem. 2006;387:1337–1346. doi: 10.1515/BC.2006.167. [DOI] [PubMed] [Google Scholar]
- Rajewsky K, von Boehmer H. Lymphocyte development: overview. Curr Opin Immunol. 2008;20:127–130. doi: 10.1016/j.coi.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, Sahota G, Sim J, Mukasa R, Cemerski S, et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460:405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007;2007:cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1{alpha}-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PR, Wang D, Wang L, Fulco M, Pediconi N, Zhang D, An W, Ge Q, Roeder RG, Wong J, et al. Regulation of the p300 HAT domain via a novel activation loop. Nat Struct Mol Biol. 2004;11:308–315. doi: 10.1038/nsmb740. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Briggs J, Park S, Niu G, Kortylewski M, Zhang S, Gritsko T, Turkson J, Kay H, Semenza GL, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24:5552–5560. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008a;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008b;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn HD, Chatila TA, Liu JO. Integration of calcineurin and MEF2 signals by the coactivator p300 during T-cell apoptosis. Embo J. 2000;19:4323–4331. doi: 10.1093/emboj/19.16.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Qian DZ, Tan YS, Lee K, Gao P, Ren YR, Rey S, Hammers H, Chang D, Pili R, et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc Natl Acad Sci U S A. 2008;105:19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01
02