Intracellular Proton-mediated Activation of TRPV3 Channels Accounts for the Exfoliation Effect of α-Hydroxyl Acids on Keratinocytes (original) (raw)
Background: Little is known about how α-hydroxyl acids (AHAs) widely cause exfoliation to expose fresh skin cells.
Results: Transient receptor potential vanilloid 3 (TRPV3) channel in keratinocytes is potently activated by intracellular acidification induced by glycolic acid.
Conclusion: TRPV3-mediated proton-sensing and cell death in keratinocytes may serve as a molecular basis for the cosmetic use of AHAs.
Significance: We describe a novel mechanism by which TRPV3 is activated by intracellular protons.
Keywords: Acidosis, Cell Death, Gating, Ion Channels, Keratinocytes, 2-APB, HaCaT, α-Hydroxyl Acids, pH, Skin
Abstract
α-Hydroxyl acids (AHAs) from natural sources act as proton donors and topical compounds that penetrate skin and are well known in the cosmetic industry for their use in chemical peels and improvement of the skin. However, little is known about how AHAs cause exfoliation to expose fresh skin cells. Here we report that the transient receptor potential vanilloid 3 (TRPV3) channel in keratinocytes is potently activated by intracellular acidification induced by glycolic acid. Patch clamp recordings and cell death assay of both human keratinocyte HaCaT cells and TRPV3-expressing HEK-293 cells confirmed that intracellular acidification led to direct activation of TRPV3 and promoted cell death. Site-directed mutagenesis revealed that an N-terminal histidine residue, His-426, known to be involved in 2-aminoethyl diphenylborinate-mediated TRPV3 activation, is critical for sensing intracellular proton levels. Taken together, our findings suggest that intracellular protons can strongly activate TRPV3, and TRPV3-mediated proton sensing and cell death in keratinocytes may serve as a molecular basis for the cosmetic use of AHAs and their therapeutic potential in acidic pH-related skin disorders.
Introduction
α-Hydroxyl acids (AHAs)3 are a group of organic carboxylic compounds that act as proton donors and have profound effects on keratinization of the skin. AHAs are well known in the cosmetic industry for their use in chemical peels (1, 2), and for the reduction of wrinkles and the signs of aging, and improving the overall look and feel of the skin (3–5). As topical compounds, AHAs are thought to penetrate the skin and cause exfoliation and the exposure of fresh skin cells. Glycolic acid, the smallest AHA molecule with the greatest bioavailability, penetrates the skin most easily, accounting for its popularity in cosmetic applications (6–8). The bioavailability of glycolic acid usually depends on its concentration and hence the pH level it produces (9–12). Despite the importance and prevalence of glycolic acid and other AHAs, the mechanism by which they are sensed by the skin is poorly understood.
TRPV3 is a Ca2+-permeable cation channel that is primarily expressed in keratinocytes of the skin (13, 14), as well as in sensory neurons (15, 16) where its activation causes a feeling of warmth. Indeed, transient receptor potential vanilloid 3 (TRPV3) is activated by moderate heating (>33 °C) that is perceived by human as warm (14–17). Organic chemicals such as 2-aminoethyl diphenylborinate (2-APB) (18, 19) and its structurally related compounds (20), plant-derived compounds such as camphor, thymol, and carvacrol (21, 22), and unsaturated fatty acids can activate or potentiate TRPV3 (23). The expression of TRPV3 has been demonstrated in primary rodent keratinocytes and immortalized skin cells such as human HaCaT cells and mouse 308 keratonocytes (14–16, 19, 24–28), where it plays an important role in skin physiology such as thermosensation and nociception (22, 29). Targeted deletion of TRPV3 in mice abolishes TRPV3 currents in keratinocytes. Noticeably, TRPV3 knock-out causes a wavy hair/curly whisker phenotype and erythroderma (also known as the red man syndrome), indicating that the TRPV3 channel is necessary and important for normal hair morphogenesis and epidermal barrier formation (30). Spontaneous autosomal dominant mutations in TRPV3 that result in constitutive channel activity can also cause hairlessness (31), dermatitis, and inflammatory skin lesions in rodents (32–34). We have recently identified three genetic gain-of-function mutations located in the S4–S5 linker and C terminus of TRPV3 that cause Olmsted syndrome, characterized by bilateral multilating palmoplantar keratoderma and periorificial keratotic plaques with severe itching at lesions (35). All these investigations indicate that overactive TRPV3 mediates an important role in skin physiology and pathophysiology. Furthermore, the TRPV3-activator carvacrol has been shown to boost collagen expression in the skin (36). Interestingly, glycolic acid also promotes collagen synthesis in skin (7, 9, 37), indicating a role for TRPV3 in glycolic acid-mediated processes (7, 9, 37). Taken together, these observations raise the question of whether AHAs can affect the skin physiology by directly modulating the function of TRPV3 channel. To understand the molecular mechanism by which topical AHAs affect the epidermis and keratinization of the skin, we investigated the proton effect on TRPV3 function.
Here we report that glycolic acid can strongly activate the TRPV3 channel. This activation is partially mediated by intracellular protons that act on His-426, located at the distal N terminus. Our findings demonstrate a novel gating mechanism by which TRPV3 is directly activated by intracellular acidification, likely accounting for the cosmetic effect of AHAs on keratinization of the skin.
MATERIALS AND METHODS
cDNA Constructs and Cell Culture
Human keratinocyte HaCaT cells and HEK-293 cells were cultured in a DMEM supplemented with 10% FBS at 37 °C with 5% CO2. They were passaged every 6–24 h, and plated onto glass coverslips coated with 0.1 mg/ml of poly-l-lysine for improving cell adhesion and subsequent patch recordings. Transient transfections were made using Lipofectamine 2000 (Invitrogen). TRP channel cDNAs, including mouse TRPV1, mouse TRPV2, mouse TRPV3, TRPV3-E631K/D641K, and TRPV3-H426N mutants, human TRPV3, mouse TRPV4, and the TRPV4-N456H/W737R mutant were fused at the C terminus with the cDNA encoding an eYFP, as previously described (38). Attachment of eYFP, used as a marker for identification of transfected cells did not affect channel function. For each transfection, 1 μg of individual cDNA were used and for the coexpression of TRPV3 and TRPV3-E631K/D641K, equal amounts of each cDNA (1 μg) were used. All mutants were generated by overlap PCR and confirmed by DNA sequencing. Electrophysiological experiments were performed between 24 and 48 h after transfection.
Electrophysiology
Whole cell and single-channel currents were recorded using a HEKA EPC10 amplifier with PatchMaster software (HEKA). Patch pipettes were pulled from borosilicate glass and fire polished to a resistance of ∼2 MΩ. Membrane potential was held at 0 mV unless stated otherwise. Currents were elicited by a protocol consisting of a 300-ms step to +80 mV, followed by a 300-ms step to −80 mV at 1-s intervals. Current amplitude was analyzed at +80 mV. During whole cell recording the capacity current was minimized by the amplifier circuitry, and the series resistance was compensated by 80%. All experiments were conducted at ∼22 °C. The dose-response relationship and the closing rate were determined using a solution exchanger RSC-200 with seven separate tubes to deliver different concentrations of protons. The tube number sent by the solution exchanger was fed into an analog input port of the EPC10 patch-clamp amplifier and recorded simultaneously with current. The stable current amplitude at different concentrations was recorded.
Solutions and Chemicals
Both pipette solution and bath solution contained 130 mm NaCl and 0.2 mm EDTA. Glycolic acid solution contained 100 mm glycolic acid, 30 mm NaCl, and 0.2 mm EDTA. For solutions with pH > 6.0, 3 mm HEPES was added; for solutions with pH ≤ 6.0, HEPES was replaced with 3 mm MES. All the chemicals, including 2-APB for electrophysiological experiments were from Sigma.
Cell Death Assay
HEK-293 cells and HaCaT cells grown in 6-well culture plates were randomly divided into different groups and then treated with 20 mm glycolic acid with or without modifying agents at pH 7.4 or 5.5. 12 h later, cells were washed three times with PBS and incubated in PBS containing Hoechst or propidium iodide (PI) for 10 min. Cell images of four random fields of view demonstrating total cells (Hoechst-stained nuclei) and dead cells (PI-stained nuclei) were taken and counted on a microscope (Olympus IX71) using a digital camera. Cell death was determined by averaging the percentage of dead cells (PI/Hoechst staining) in each plate.
Data Analysis
All data are shown as mean ± S.E. G-V curves were fitted to a single Boltzmann function.
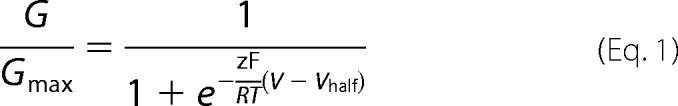
Where G/Gmax is the normalized conductance, z is the equivalent gating charge, _V_half is the half-activation voltage, F is the Faraday's constant, R is the gas constant, and T is the temperature in Kelvin. The relative conductance (G/_G_max) was obtained by normalizing the tail currents (after 2-ms hyperpolarization to −100 mV from a given voltage) to the maximum tail current. The dose-response relationship was fitted to the Hill equation.
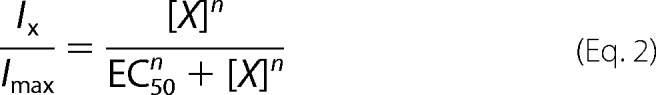
Where Ix is the steady-state TRPV current in the presence of concentration [_x_], _I_max is the maximal current amplitude. EC50 is the concentration for the half-maximal effect and is replaced by IC50 if the effect is inhibitory. Statistical significance, determined by Student's t test, is indicated as: *, p < 0.05; **, p < 0.01; ***, p < 0.001; and n.s., no significant difference.
RESULTS
Glycolic Acid Activates TRPV3 Expressed in HEK-293 Cells
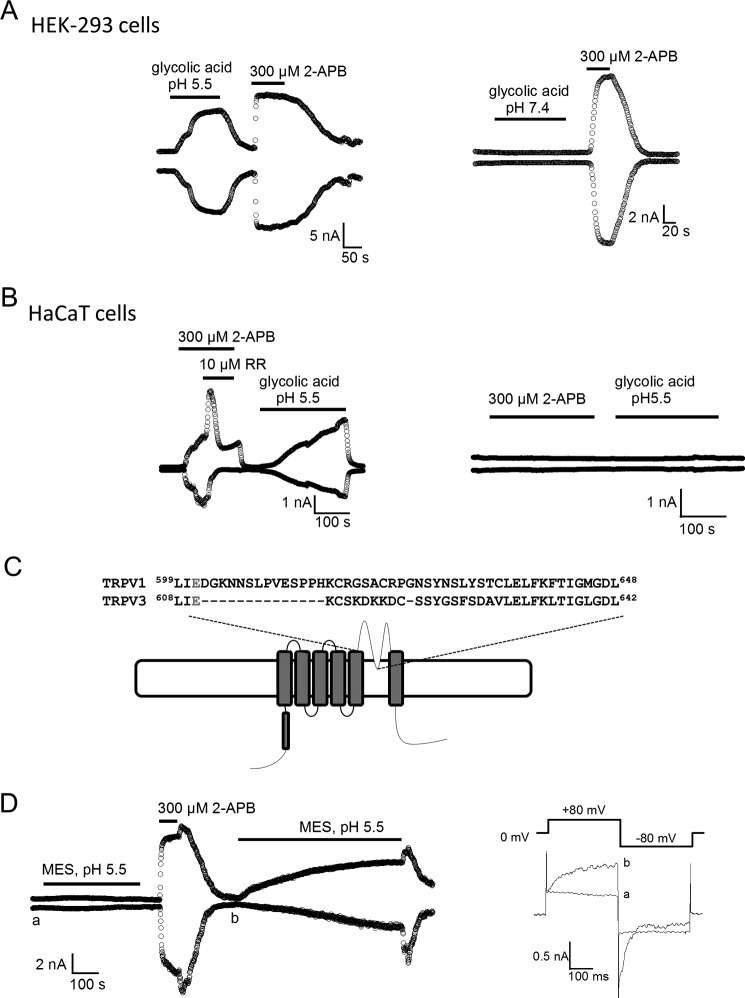
To examine whether TRPV3 can be directly activated by proton released from glycolic acid, we conducted patch clamp recordings of HEK-293 cells transfected with TRPV3 cDNA. Whole cell recordings of channel-expressing cells gave rise to a robust response to extracellular glycolic acid (100 mm, pH 5.5) (Fig. 1A, left panel). The glycolic acid effect developed slowly, and could be washed out slowly after switching to a normal bath solution. Subsequent application of TRPV3 channel opener 2-APB (300 μm) elicited an instantaneous TRPV3 current from the same cells, confirming the presence of TRPV3 channels. The glycolic acid-induced current was only present at acidic pH levels. Application of the same concentration of glycolic acid buffered at pH 7.4 failed to activate TRPV3, whereas a robust TRPV3 current was seen upon subsequent application of 2-APB (Fig. 1A, right panel). These observations indicate that extracellular glycolic acid at low pH (5.5 but not 7.4) caused the activation of TRPV3.
FIGURE 1.
Glycolic acid-induced activation of TRPV3 in HaCaT and HEK-293 cells. A, representative whole cell recordings of HEK-293 cells expressing TRPV3 in the presence of 100 mm glycolic acid at pH 5.5 (left panel) or pH 7.4 and 300 μm 2-APB (right panel). B, left panel, representative whole cell TRPV3-like currents were evoked by 300 μm 2-APB and 100 mm glycolic acid at pH 5.5 in HaCaT cells; as a control, cells that had no response to 2-APB also had no response to glycolic acid (right panel). C, sequence alignment between the pore regions of TRPV1 and TRPV3. The Glu-601 pH site in TRPV1 and its corresponding Glu-610 in TRPV3 are highlighted in red. D, time course of whole cell TRPV3 currents evoked by a MES-buffered solution at pH 5.5 and subsequent application of 300 μm 2-APB. After washout of 2-APB, a slow developing current was evoked by the pH 5.5 solution (left panel). Comparison of current traces labeled a and b obtained at the time indicated showed the remaining channel activity of TRPV3 by 2-APB (right panel).
Glycolic Acid Induces TRPV3-like Currents in Human Keratinocytes
To confirm that endogenous TRPV3 channels could be activated by glycolic acid, we performed whole cell patch clamp recordings of HaCaT cells, a human keratinocyte cell line. Consistent with reports that TRPV3 is highly expressed and functional in a small subset of keratinocytes (14, 25, 39), application of 300 μm 2-APB evoked a TRPV3-like current in 8 of 53 HaCaT cells (Fig. 1B, left panel), whereas most of the recorded cells did not respond to 2-APB (or glycolic acid at pH 5.5) (Fig. 1B, right panel). To further confirm the identity of this current, we used a nonspecific voltage-dependent antagonist of TRP channels, ruthenium red. 10 μm Ruthenium red blocked the 2-APB-induced inward current, but potentiated the outward current, consistent with characteristic ruthenium red-mediated blockage of TRPV3 (19, 30). Both 2-APB activation and ruthenium red blockage of TRPV3 could be reversed by washing out the reagents (Fig. 1B, left panel). Interestingly, only for cells showing a 2-APB-induced current, further application of a solution containing 100 mm glycolic acid (pH 5.5) resulted in a slow-developing, nondesensitizing current (Fig. 1B, left panel). In contrast, 2-APB-insensitive cells showed no response to the glycolic acid-containing solution (Fig. 1B, right panel). These results indicated that the TRPV3-like current recorded in HaCaT cells resembled the heterologous expression of TRPV3 function (18, 40), providing evidence that TRPV3 is activated by glycolic acid in human keratinocyte HaCaT cells.
Because a glutamate residue (at the extracellular end of S5) involved in extracellular proton activation of TRPV1 is also conserved in TRPV3, we wondered whether extracellular application of glycolic acid could activate TRPV3 through a similar mechanism (41) (Fig. 1C). To test the effect of extracellular protons on TRPV3, we utilized an acidic bath solution (pH 5.5) buffered by MES, a membrane impermeable acid. Application of this solution for at least 5 min failed to induce a current response in cells expressing TRPV3 (Fig. 1D). Subsequent application of 2-APB (300 μm) induced a robust, rapid TRPV3 current activation from the same cells, confirming the presence of the channel (Fig. 1D). This observation indicates that extracellular proton cannot activate the TRPV3 channel, consistent with a previous report (16). Surprisingly, when a MES-buffered acidic bath solution (pH 5.5) was used after 2-APB application, a slowly developing current very similar to that elicited by glycolic acid was observed (Fig. 1D). We noticed that after 2-APB treatment, the TRPV3 channels maintained a low activity level (Fig. 1D, right). As TRPV3 is a nonselective and calcium-permeable cation channel with substantial proton permeability, protons can pass through the open channel to induce cytosolic acidosis (42). The substantial permeation effect raised the possibility that protons could be activating TRPV3 from the intracellular side. Intracellular activation of TRPV3 by proton would be consistent with results from the glycolic acid experiments, because that glycolic acid is a weak AHA acid, and in solution it exists as an equilibrium of the charged and neutral forms: HG ↔ H+ + G−. At low pH levels (high [H+]), the equilibrium shifts toward the left and glycolic acid exists in the neutral, membrane permeable form; whereas at high pH levels (low [H+]), the equilibrium shifts toward the right and glycolic acid exists in the charged, membrane impermeable form. Once the protonated neutral glycolic acid diffuses across the cell membrane into the cell, it re-equilibrates to release a free proton, causing intracellular acidification (12, 43). Can intracellular acidification activate TRPV3? To address this question, we performed inside-out patch clamp recordings of HEK-293 cells expressing TRPV3.
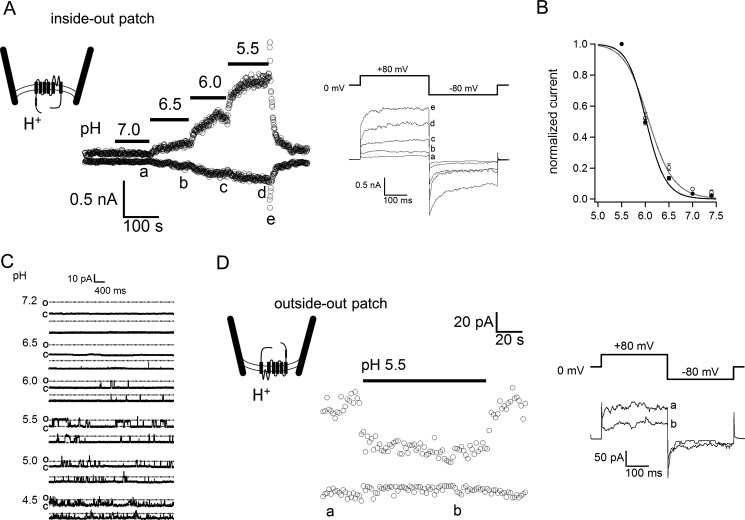
Intracellular Acidification Activates TRPV3 Expressed in HEK-293 Cells
A low, basal TRPV3 activity was observed when inside-out patches were excised and exposed to a pH 7.4 bath solution (Fig. 2A). Decreasing the bath pH from 7.4 to 5.5 resulted in a gradual increase of TRPV3 current in a pH-dependent manner (Fig. 2, A and B), indicating that intracellular acidification could indeed directly activate the channel. Activation of TRPV3 current could be seen at pH 6.5 and reached a maximum at approximately pH 5.5 (Fig. 2, A and B). Single channel recordings of TRPV3 current further confirmed that intracellular protons increased the open channel probability (Fig. 2C). Further acidification (such as pH 4.5) led to current inhibition, which was partially due to reduction of the single-channel conductance (Fig. 2C and see below). In contrast, when we performed outside-out patch clamp recordings, we observed that extracellular proton did not activate any TRPV3 current. Instead, there was a slight inhibition of the basal TRPV3 activity, which was apparently due to a reduction of the single-channel current amplitude (Fig. 2C). The observation further confirms that TRPV3 activation by proton originates from the intracellular side. As additional controls, we tested other temperature-sensitive TRPV channels (TRPV1, TRPV2, and TRPV4) and confirmed that they were not activated by intracellular protons.
FIGURE 2.
Intracellular proton activation of TRPV3 in a pH-dependent manner. A, representative current traces were recorded from an inside-out patch facing solutions of various pH levels (left panel). The current traces labeled as a to e were obtained at the time indicated (right panel). B, dose-response curve for proton activation of TRPV3. Baseline-subtracted currents were normalized to steady-state currents at pH 5.5. The smooth curve represents a fit of the Hill equation with pH½ = 6.1 ± 0.01, and Hill slope at 1.7 ± 0.5 (n = 5). C, inside-out patch recordings of single TRPV3 channel expressed in HEK293 cells in response to different pH values. D, a representative current trace recorded from an outside-out patch in response to a bath solution of pH 5.5 (left). The current traces labeled as a and b were obtained at the time indicated (right panel).
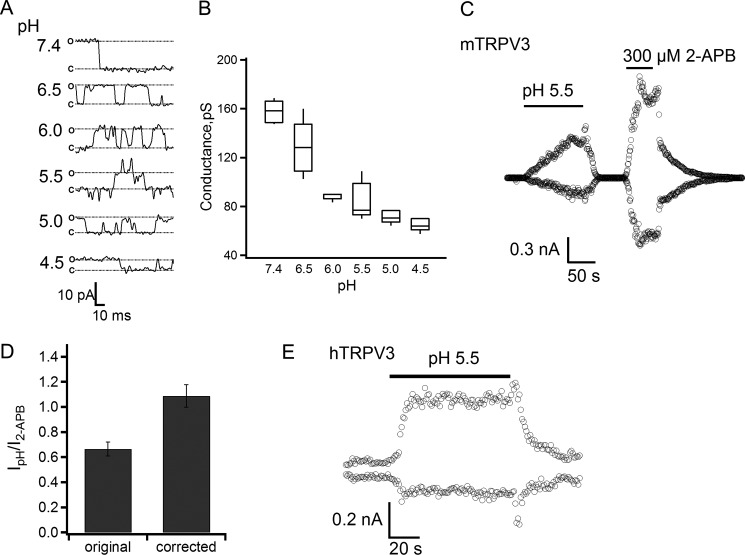
Intracellular Protons Are a Potent Activator for TRPV3
To test the efficiency of proton in activating TRPV3, we conducted single-channel recordings in the inside-out configuration at a wide pH range to evaluate the permeation effect of proton that partially masked its gating effect. These recordings showed that protons dose dependently inhibited the single-channel current amplitude (Fig. 3A). At physiological pH, the single-channel conductance was estimated to be 157.7 ± 4.0 pS (n = 5), similar to that previously reported (19, 38). At pH 5.5, intracellular protons reduced the single-channel conductance by almost 50%, to 84.1 ± 6.9 pS (Fig. 3B), indicating that the gating effect was indeed partially masked. For this reason, we used the pH-dependent single-channel conductance to adjust the macroscopic currents as shown in Fig. 3C. The corrected current amplitudes were much higher after removing the permeation effect, reflecting a significant gating effect of intracellular protons. For comparison, at a saturating concentration (300 μm) of 2-APB, TRPV3 activation reached an open probability of about 73%, as estimated by noise analysis (44). Based on the relative current amplitudes of the proton-elicited current and the 2-APB-elicited current, at pH 5.5 proton potentiated TRPV3 to an open probability of about 90% (Fig. 3D). We also observed that intracellular protons activated large currents in HEK-293 cells transfected with the human TRPV3 channel, indicating that activation by protons is a conserved property of TRPV3 channels (Fig. 3E). Thus, our data demonstrate that intracellular protons are a strong activator for TRPV3.
FIGURE 3.
Protons are an efficient opener of the TRPV3 channel. A, representative single-channel currents recorded at the indicated intracellular pH levels from inside-out patches at +80 mV. B, box-and-whisker plot of the single-channel conductance versus the corresponding intracellular pH values, n = 5–6. The whisker top, box top, line inside the box, box bottom, and whisker bottom represent the maximum, 75th percentile, median, 25th percentile, and minimum value of each pool of conductance measurements, respectively. C, a representative current trace recorded from an inside-out patch evoked by pH 5.5 solution and 300 μm 2-APB in mTRPV3-expressing HEK-293 cells. D, average current (left bar) evoked by the pH 5.5 solution was normalized to the response evoked by 300 μm 2-APB. The right bar represents the same data after correction for proton-induced reduction in single-channel conductance, so that the height of the bar directly reflects the relative open probability, n = 4. E, a representative current trace recorded from an inside-out patch evoked by a pH 5.5 solution from a hTRPV3-expressing cell.
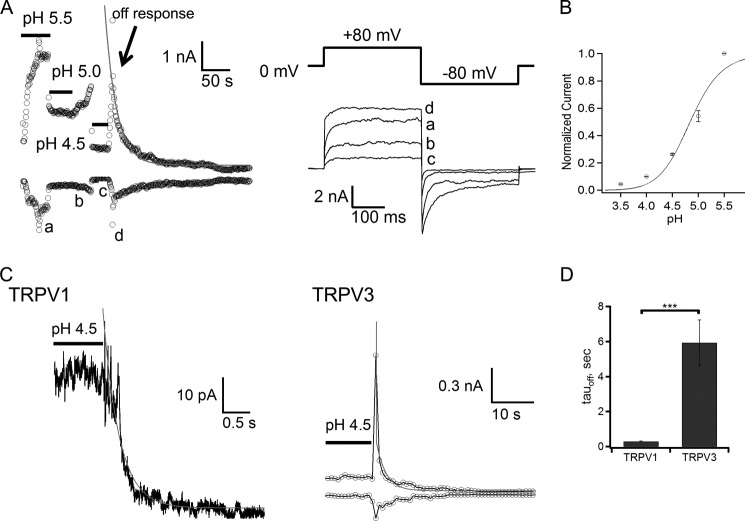
Off-response Property of Proton-induced Activation of TRPV3
Our results showed that pH-induced TRPV3 activation appeared to saturate at pH 5.5 (Fig. 2B). Further decreasing bath pH to 5 or 4.5 resulted in suppression of the steady-state TRPV3 current in a dose-dependent manner (Fig. 4, A and B), indicating a dominant inhibitory effect on conductance. More interestingly, at these low pH levels (high [H+]) robust acidic-induced transient off-responses were clearly observed upon removal of the acid, and the amplitude of the off-response almost reached the same level as the current at pH 5.5 (Fig. 4A). This implies that the open probability of TRPV3 at pH 4.5 could reach the maximum level, but inhibition in single-channel conductance and other potential proton-sensitive site(s) contributed to the decline of overall current amplitude. Removal of conductance inhibition transiently revealed the full scale of the activation effect of protons. The off-response thus reflected the dual effects of intracellular protons on TRPV3, indicating the off gating transition is much slower than the speed of the acid-base neutralization reaction.
FIGURE 4.
The off-response property of TRPV3. A, a representative current trace recorded from an inside-out patch facing solutions of various pH values (left panel). The current traces labeled as a–d were obtained at the time indicated (right panel). The off-response was obtained at pH 4.5. B, proton dose-response curve for proton inhibition of TRPV3. Baseline-subtracted currents were normalized to steady-state currents at pH 5.5. The Hill equation was used for curve fitting with pH½ = 4.84 ± 0.09, and Hill slope at 1.4 ± 0.3 (n = 8). C, single exponential fits for the off-response of TRPV1 (left panel) and TRPV3 (right panel). TRPV1 current was recorded from an outside-out patch evoked by pH 4.5 solution, and TRPV3 current was recorded from an inside-out patch evoked by pH 4.5 solution. D, comparison of off-response time constants of TRPV1 and TRPV3, p < 0.001, n = 6–8.
To better clarify the off-response of TRPV3, we conducted comparative measurements for the off-response of TRPV1 induced by removal of the extracellular acidic solution from outside-out patches (45, 46). A small and rapid off-response was observed in TRPV1 after acid exposure (pH 4.5). The time constant of the closing process was less than 310 ms (Fig. 4, C and D), consistent with the observation of the transient gating process of TRPV1 (47). We then measured the closing rate of the proton-induced gating of TRPV3 in the presence of a pH 4.5 bath solution. The closing rate was significantly slower, with a time constant of 7.2 ± 2.2 s (n = 6) (Fig. 4C). Activation of TRPV3 displays a unique property known as sensitization due to hysteresis of opening the gate (14–16, 48, 49). It is likely that the strong hysteresis of TRPV3 gating leads to the noticeably slower off-response.
Leftward Shift of the Voltage-dependent Activation Curve of TRPV3 by Protons
TRPV3 activation exhibits weak voltage dependence in a nonphysiological, highly depolarized voltage range. However, other stimuli such as temperature change or binding of ligands can shift the voltage-dependent activation range toward physiologically relevant potentials to gate the channel (50–52). We therefore tested the effect of intracellular proton on voltage-dependent activation of TRPV3. In inside-out patches, TRPV3 currents were elicited using a voltage step protocol ranging from −100 to +300 mV at different levels of intracellular pH (Fig. 5A). At pH 7.4, there was very little channel activity even at a membrane potential of +300 mV, and the midpoint of voltage activation (_V_½) was estimated as 182.7 ± 2.0 mV (Fig. 5B). Increasing the proton concentration to pH 5.5 induced a large increase of TRPV3 current (Fig. 5A, right panel) and a significant leftward shift of the activation curve, with the _V_½ value shifted to 94.4 ± 5.2 mV (Fig. 5B). The shift resulted in enhanced channel activity at physiological voltages. These data suggest that, similar to heat and 2-APB, intracellular proton can increase TRPV3 activity by both direct activation and a shift of the voltage dependence of the channel.
FIGURE 5.
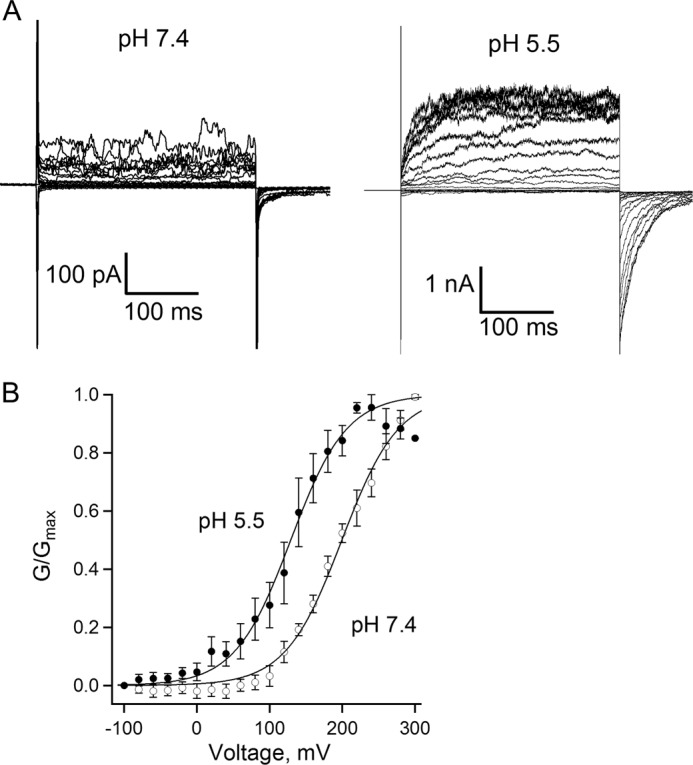
A leftward shift of voltage-dependent activation of TRPV3 by intracellular protons. A, representative currents recorded from inside-out patches at pH 7.4 or 5.5. Patches were held at 0 mV, steps from −100 to 300 mV in an increment of 20 mV for 300 ms, and then back to −100 for 100 ms. B, normalized steady-state conductance curves at pH 7.4 and 5.5, constructed from the tail currents. The _V_½ value, determined from fitting the data to a Boltzmann function, shifted about 70 mV, from +199.4 ± 1.8 mV at pH 7.4 (open circles) to +129.1 ± 2.1 mV at pH 5.5 (filled circles), n = 5 each.
Reduction of Intracellular Proton-activated Currents in a TRPV3-H426N Mutant
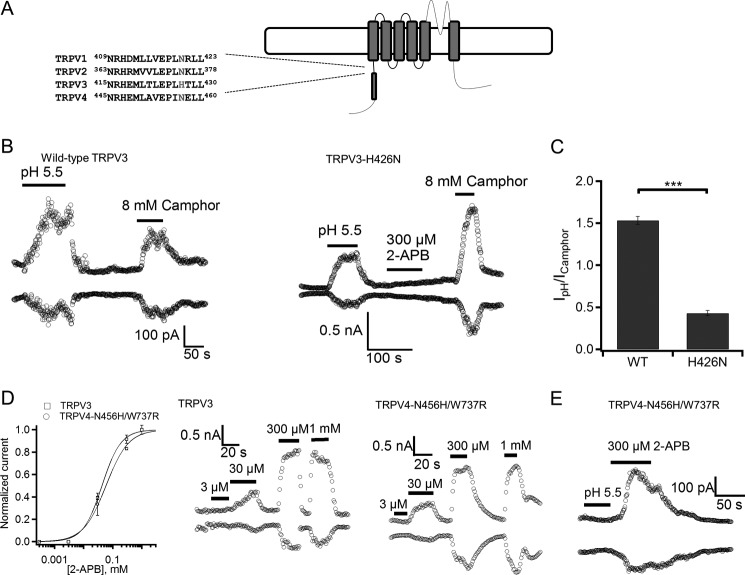
To identify residues responsible for intracellular proton activation of TRPV3, we focused on amino acids having side chain p_Ka_ values in a physiologically relevant range (i.e. His, Glu, and Asp residues that have a p_Ka_ value of 6.0, 4.1, and 4.0, respectively). The His residues are good candidates because His has a p_Ka_ value closest to the half-maximal pH (pH½) for TRPV3 activation. Based on the sequence alignment (Fig. 6A), His-426 in TRPV3 is unique, as the corresponding residue in the intracellular insensitive TRPV1, TRPV2, or TRPV4 is Asn. We noticed that His-426 in TRPV3 is important for 2-APB activation (53), and decided to determine whether His-426 is also critical for the response of the channel to intracellular protons. For the TRPV3-H426N mutant, the 2-APB response was deficient, but the mutant retained normal camphor sensitivity (53). To evaluate the response of mutant TRPV3 to intracellular acidification, we compared the current induced by proton at pH 5.5 to that by 8 mm camphor (Fig. 6B). The current amplitude ratio between them was calculated to be 1.51 for wild-type TRPV3, versus 0.46 for the H426N mutant, indicating a partial loss of proton activation for the H426N mutant and the role of His-426 in pH sensing (Fig. 6C).
FIGURE 6.
TRPV3-H426N mutation affects proton activation. A, sequence alignment of the N-terminal MPR of mTRPV1, mTRPV2, mTRPV3, and mTRPV4. The amino acid histidine involved in 2-APB sensing is indicated in red. B, representative currents obtained from inside-out patches with HEK-293 cells expressing wild-type (WT) TRPV3 (left panel) or mutant TRPV3 H426N (right panel) evoked by pH 5.5 solution, 8 mm camphor, or 300 μm 2-APB. C, a comparison of the ratio between the current evoked by pH 5.5 solution and current evoked by 8 mm camphor from WT TRPV3 and the TRPV3-H426N mutant, p < 0.001, n = 3–4. D, concentration-response curve of the 2-APB activated response in WT TRPV3 and TRPV4-N456H/W737R mutant (left panel), n = 5. Representative whole cell currents obtained from HEK-293 cells expressing WT TRPV3 (middle panel) and the TRPV4-N456H/W737R mutant (right panel) evoked by various concentrations of 2-APB. E, a representative current trace recorded from an inside-out patch evoked by pH 5.5 solution and currents evoked by 300 μm 2-APB in HEK-293 cells expressing the TRPV4-N456H/W737R mutant.
To further investigate whether protons and 2-APB used the same pathway to influence TRPV activation, we generated a 2-APB-sensitive TRPV4 mutant by introducing a histidine mutation at the equivalent position of His-426 and an addition of Trp to Arg mutation at position 737 (TRPV4-N456H/W737R) (53), and tested the proton sensitivity of this TRPV4 mutant. The TRPV4-N456H/W737R mutant and wild-type TRPV3 showed a similar dose-response relationship to 2-APB (EC50 of 42.7 ± 0.3 and 58.7 ± 0.8 μm, respectively) (Fig. 6D). However, the TRPV4-N456H/W737R mutant channel showed no activation response to intracellular acidification (Fig. 6E). The fact that we introduced 2-APB sensitivity but not proton sensitivity into TRPV4 implies that the mechanisms for pH sensing and 2-APB sensing are distinct. Hence our data suggest that His-426 in TRPV3 is involved in sensing both 2-APB and proton, whereas additional residues may participate in either proton binding or subsequent conformational changes.
Glycolic Acid Induces Cytotoxicity in Both HEK-293 Cells Expressing TRPV3 and HaCaT Cells
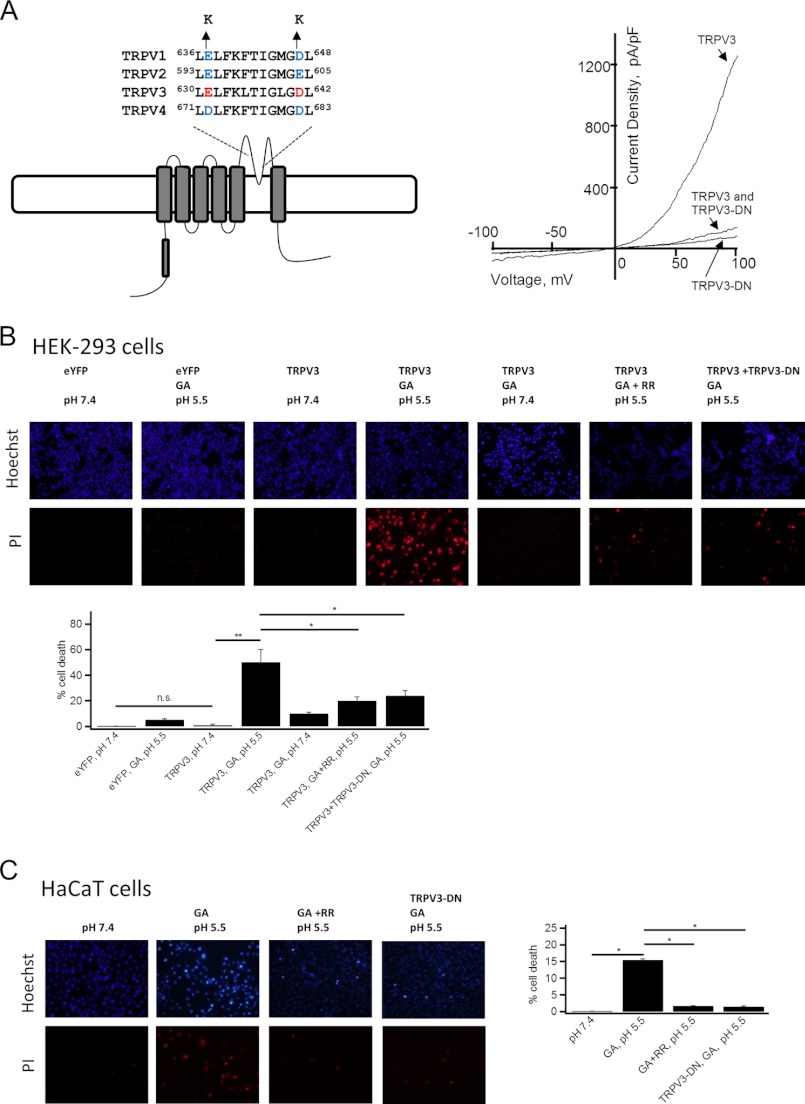
Based on the observation that glycolic acid potentially activates TRPV3 channel via intracellular acidosis, we asked whether TRPV3 could mediate glycolic acid-induced cell toxicity. We applied glycolic acid to HEK-293 cells expressing TRPV3 and measured cell viabilities using a PI staining assay. Cell death was determined by quantification of Hoechst-stained cells (total) versus PI-labeled cells (dead) after treatment with 20 mm glycolic acid for 12 h. Exposure to glycolic acid at pH 5.5 showed a significant increase in cell death with HEK-293 cells expressing TRPV3, as compared with cells expressing eYFP (negative control) (Fig. 7B). As expected, cells expressing TRPV3 and treated with glycolic acid at pH 7.4 did not show a significant increase in cell death. Although there is a lack of highly selective TRPV3 inhibitor, the toxicity was greatly attenuated by application of 10 μm ruthenium red, a nonspecific TRP channel blocker, or by co-transfection of the TRPV3-E631K/D641K double mutant (54, 55) that exerted a strong dominant-negative effect on rescuing the overactive TRPV3 channel (Fig. 7, A and B). These results are consistent with the idea that glycolic acid induces cell damage by activating TRPV3. In addition, we tested the effect of TRPV3 activation on HaCaT cells. As shown in Fig. 7C, HaCaT cells treated with glycolic acid at pH 5.5 showed increased cell death, as compared with HaCaT cells at pH 7.4, consistent with the data obtained with HEK-293 cells expressing TRPV3. Thus, our results confirmed the involvement of TRPV3 in mediating the cellular actions of glycolic acid. Our findings are also consistent with the anti-proliferative effects of glycolic acid in HaCaT cells (56) as well as the report that TRPV3 activation induces cell death in human outer root sheath keratinocytes (27).
FIGURE 7.
Contribution of TRPV3 activation to glycolic acid-mediated cell death in HEK293 and HaCaT cells. A, sequence alignment of pore helix and selectivity filter for mTRPV1, mTRPV2, mTRPV3, and mTRPV4. The conserved negatively charged residues causing dominant-negative effects when substituted with lysine are indicated in red or blue (left panel). Typical normalized currents to cell membrane capacitance evoked by 300 μm 2-APB and measured by a voltage ramp from −100 to +100 mV in 100 ms from cells expressing wild-type TRPV3 and TRPV3-E631K/D641K (TRPV3-DN) mutant alone or co-expressing wild-type TRPV3 and TRPV3-DN mutant at 1:1 ratio (right panel). B, top panels show representative images of Hoechst staining (upper images) and PI labeling (lower images) of HEK-293 cells expressing eYFP, TRPV3 alone, or co-expressing TRPV3 and TRPV3-DN mutant at 1:1 ratio following he indicated interventions. The bottom panel shows a bar graph of statistical analysis, n = 3. C, representative images of Hoechst staining and PI labeling of HaCaT cells following the indicated interventions (left panels), and statistical analysis of cell death quantified by PI labeling over Hoechst staining cells in each corresponding group (left images), n = 4. Statistical significance is indicated as: *, p < 0.05; **, p < 0.01; n.s., no significant difference.
DISCUSSION
Weak acids from normal cellular metabolites, whereas not toxic under physiological conditions, can lead to intracellular acidification in a concentration and extracellular pH-dependent fashion (12, 43, 57–59). In this study, we demonstrated that glycolic acid-induced intracellular acidification could lead to TRPV3 activation. We suggest that glycolic acid may cause intracellular acidification in two ways (Fig. 8). In its neutral form, glycolic acid may cross the cell membrane to reach the cytosol where it re-equilibrates and releases the proton. Alternatively, protons can pass through the open TRPV3 channel or other proton-permeant pathways. These two processes may work cooperatively in TRPV3-expressing cells, as an increase in intracellular proton concentration through the first pathway would activate TRPV3 to promote the second pathway. As TRPV3 has a high Ca2+ permeability, elevated TRPV3 activity may cause intracellular Ca2+ overload that affects many aspects of cellular physiology and eventually causes cell death. Our findings reveal a novel mechanism by which TRPV3 is regulated by intracellular acidification through a coupling between cytosolic proton and Ca2+. This mechanism may help explain the cosmetic effect of weak acids on proliferation and an early phase of apoptosis in keratinocytes for skin renewal (Fig. 8). Therefore, pH sensing by TRPV3 may serve as an important mechanism in medical applications of topical AHAs such as glycolic acid on the skin.
FIGURE 8.
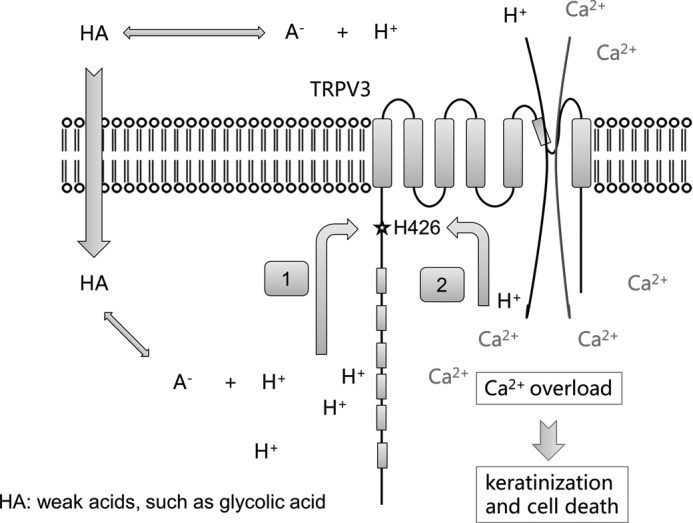
A model for functional activation of TRPV3 by AHAs. Weak acids such as glycolic acid can diffuse across the cell membrane in the protonated form, and then re-equilibrate to generate a free proton, causing intracellular acidification and activation of TRPV3 (pathway 1). Protons can also pass through activated TRPV3 or other proton-permeable channels to induce intracellular acidification and TRPV3 activation (pathway 2). TRPV3 activation mediates Ca2+ influx, leading to cytosolic Ca2+ overload, keratinization, and cell death.
The detailed molecular mechanism underlying intracellular proton activation of TRPV3 remains to be elucidated. Our results suggest that His-426 plays an important role in proton-mediated activation of TRPV3. His-426 resides in the N-terminal membrane-proximal region (MPR), located between the last ankyrin repeat domain and the first transmembrane domain. MPR is an important region involved in different modes of TRPV1–4 activation. Especially, His-426 in the MPR of TRPV3 plays a critical role in 2-APB-induced channel activation (53). The corresponding amino acid, His-378, in the MPR of TRPV1 has been shown to affect the intracellular alkalization-induced activation (60). Yao et al. (61) recently reported that MPR might be the heat sensor of TRPVs, as swapping MPRs between TRPV channels led to a switch of the temperature sensitivity. These results suggest MPR is a shared functional element important for the activation of TRPV channels. Protonation of His-426 in the MPR is thus expected to affect TRPV3 activity by directly interacting with the activation process. However, we cannot rule out other potential site(s) besides His-426 that may be involved in the proton sensing.
Besides providing a viable explanation of the cosmetic effect of topical AHAs, what is the physiological and pathophysiological significance of TRPV3 proton sensing? Variations in the pH level occurs in normal physiological processes such as respiratory and metabolic acidosis and alkalosis; it can be much more severe under pathological conditions such as tissue damage, inflammation, and ischemia (62). Cell acidification can serve as a concomitant of cell death (63) and a means to detect painful conditions, especially in deep tissues where the temperature is expected to remain constant (64). A number of TRP channels have been shown to be quite sensitive to changes in extracellular pH (65, 66). Although intracellular acidification-mediated inhibition has been observed in TRPV5 and TRPM2 channels (67, 68), our findings represent the first demonstration of intracellular acidification-mediated activation by a member of the TRPV channel family.
Besides being expressed in skin keratinocytes, TRPV3 is also expressed in various areas of the nervous system such as dorsal root ganglion, trigeminal ganglion, spinal cord, and brain (14, 16). Acidosis is a noxious condition associated with inflammation, ischemia, or defective acid containment. Among several acid-sensitive ion channels, acid-sensing ion channels and TRPV1 have been proposed to sense acid-mediated nociception (69–71). Acid-sensing ion channels detect moderate decreases in extracellular pH (72), whereas TRPV1 is activated only by severe acidosis resulting in pH values below 6 (41). Both acid-sensing ion channels and TRPV1 exhibit very rapid desensitization and only produce a transient current response (73–75). How neurons can detect long lasting acid environments remains largely unknown. Although TRPV3 cannot sense the extracellular protons directly, we show in the present study that it can sensitively report intracellular acidification. Because TRPV3 mediates responses to warm temperatures above 33 °C, TRPV3 confers a basal activity at body temperatures that might allow protons to enter the cell and further activate the channel at elevated extracellular proton levels, thus acting as a primary pH sensor. Interestingly, Miyamoto et al. (42) recently reported that TRPV3 regulates nitric-oxide syntheses in the skin through a pH-dependent fashion. The activation of TRPV1 or other proton-permeable channels that are coexpressed with TRPV3 in many cell types may further facilitate cytosolic acidification (76), supporting a role for TRPV3 in acid sensing, acid-induced pain, and acid-evoked feedback regulation of homeostatic reactions. For these reasons, TRPV3 may be an attractive target for the development of analgesic drugs to relieve acidic pain (77).
An interesting observation of the present study was that increasing the concentration of intracellular protons beyond pH 5.5 evoked little TRPV3 current, but a robust transient response was observed when the acidic solution was replaced. This off-response has been suggested to have a potential association with sour taste sensation in mammals (21, 78–81). We showed here that this off-response is predominantly due to the removal of inhibition effects of intracellular protons on single-channel conductance. It reveals that the gating process underlying recovery from low pH-induced activation is quite slow. Our data also suggest that the strong inhibition of the TRPV3 current at highly acidic pH levels may not be accounted for by simple conductance inhibition, indicating that an inhibitory effect of proton on channel gating may also exist. A second pH sensing domain or general charge effects on the TRP domain may contribute to this inhibition (82).
In summary, we have demonstrated that intracellular protons can strongly activate TRPV3, revealing a coupling between cytosolic protons and calcium. When the channel is sensitized, extracellular proton can efficiently pass through the channel pore to further open the channel, so that TRPV3 can detect both intracellular and extracellular acidosis under physiological or pathophysiological conditions. The sensitivity of TRPV3 to acidosis may explain the cosmetic effect of topical AHAs and provide a new target for pain medication and human skin diseases caused by TRPV3 gain-of-function mutations (35).
Acknowledgments
We thank our laboratory members Yiquan Tang, Jun Su, and Xiling Bian for discussion. We also thank J. M. Wang for consistent support during this research.
*
This work was supported, in whole or in part, by National Institutes of Health Grant R01NS072377 (to J. Z.) and Research Grant 30970919 from the National Science Foundation of China, the Ministry of Education of China 111 Project China (B07001) (to K. W. W.), and the China Scholarship Council (to X. C.).
3
The abbreviations used are:
AHA
α-hydroxyl acid
TRPV3
transient receptor potential vanilloid 3
2-APB
2-aminoethyl diphenylborinate
PI
propidium iodide
MPR
membrane-proximal region.
REFERENCES
- 1.Vidt D. G., Bergfeld W. F. (1997) Cosmetic use of α-hydroxy acids. Cleveland Clin. J. Med. 64, 327–329 [DOI] [PubMed] [Google Scholar]
- 2.Tung R. C., Bergfeld W. F., Vidimos A. T., Remzi B. K. (2000) α-Hydroxy acid-based cosmetic procedures. Guidelines for patient management. Am. J. Clin. Dermatol. 1, 81–88 [DOI] [PubMed] [Google Scholar]
- 3.Clark C. P., 3rd (1996) α-Hydroxy acids in skin care. Clin. Plast. Surg. 23, 49–56 [PubMed] [Google Scholar]
- 4.Kurtzweil P. (1998) α-Hydroxy acids for skin care. FDA Consum. 32, 30–35 [PubMed] [Google Scholar]
- 5.Roenigk H. H., Jr. (1995) Treatment of the aging face. Dermatol. Clin. 13, 245–261 [PubMed] [Google Scholar]
- 6.Moy L. S., Murad H., Moy R. L. (1993) Glycolic acid peels for the treatment of wrinkles and photoaging. J. Dermatol. Surg. Oncol. 19, 243–246 [DOI] [PubMed] [Google Scholar]
- 7.Moy L. S., Howe K., Moy R. L. (1996) Glycolic acid modulation of collagen production in human skin fibroblast cultures in vitro. Dermatol. Surg. 22, 439–441 [DOI] [PubMed] [Google Scholar]
- 8.Bergfeld W., Tung R., Vidimos A., Vellanki L., Remzi B., Stanton-Hicks U. (1997) Improving the cosmetic appearance of photoaged skin with glycolic acid. J. Am. Acad. Dermatol. 36, 1011–1013 [DOI] [PubMed] [Google Scholar]
- 9.Kim S. J., Won Y. H. (1998) The effect of glycolic acid on cultured human skin fibroblasts. Cell proliferative effect and increased collagen synthesis. J. dermatol. 25, 85–89 [PubMed] [Google Scholar]
- 10.Thueson D. O., Chan E. K., Oechsli L. M., Hahn G. S. (1998) The roles of pH and concentration in lactic acid-induced stimulation of epidermal turnover. Dermatol. Surg. 24, 641–645 [DOI] [PubMed] [Google Scholar]
- 11.DiNardo J. C., Grove G. L., Moy L. S. (1996) Clinical and histological effects of glycolic acid at different concentrations and pH levels. Dermatol. Surg. 22, 421–424 [DOI] [PubMed] [Google Scholar]
- 12.Gutknecht J., Tosteson D. C. (1973) Diffusion of weak acids across lipid bilayer membranes. Effects of chemical reactions in the unstirred layers. Science 182, 1258–1261 [DOI] [PubMed] [Google Scholar]
- 13.Hamamoto T., Takumida M., Hirakawa K., Takeno S., Tatsukawa T. (2008) Localization of transient receptor potential channel vanilloid subfamilies in the mouse larynx. Acta Otolaryngolog. 128, 685–693 [DOI] [PubMed] [Google Scholar]
- 14.Peier A. M., Reeve A. J., Andersson D. A., Moqrich A., Earley T. J., Hergarden A. C., Story G. M., Colley S., Hogenesch J. B., McIntyre P., Bevan S., Patapoutian A. (2002) A heat-sensitive TRP channel expressed in keratinocytes. Science 296, 2046–2049 [DOI] [PubMed] [Google Scholar]
- 15.Xu H., Ramsey I. S., Kotecha S. A., Moran M. M., Chong J. A., Lawson D., Ge P., Lilly J., Silos-Santiago I., Xie Y., DiStefano P. S., Curtis R., Clapham D. E. (2002) TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418, 181–186 [DOI] [PubMed] [Google Scholar]
- 16.Smith G. D., Gunthorpe M. J., Kelsell R. E., Hayes P. D., Reilly P., Facer P., Wright J. E., Jerman J. C., Walhin J. P., Ooi L., Egerton J., Charles K. J., Smart D., Randall A. D., Anand P., Davis J. B. (2002) TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418, 186–190 [DOI] [PubMed] [Google Scholar]
- 17.Cheng W., Yang F., Liu S., Colton C. K., Wang C., Cui Y., Cao X., Zhu M. X., Sun C., Wang K., Zheng J. (2012) Heteromeric heat-sensitive transient receptor potential channels exhibit distinct temperature and chemical response. J. Biol. Chem. 287, 7279–7288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu H. Z., Gu Q., Wang C., Colton C. K., Tang J., Kinoshita-Kawada M., Lee L. Y., Wood J. D., Zhu M. X. (2004) 2-Aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J. Biol. Chem. 279, 35741–35748 [DOI] [PubMed] [Google Scholar]
- 19.Chung M. K., Lee H., Mizuno A., Suzuki M., Caterina M. J. (2004) 2-Aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J. Neurosci. 24, 5177–5182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colton C. K., Zhu M. X. (2007) 2-Aminoethoxydiphenyl borate as a common activator of TRPV1, TRPV2, and TRPV3 channels. Handb. Exp. Pharmacol., 173–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu H., Delling M., Jun J. C., Clapham D. E. (2006) Oregano, thyme, and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 9, 628–635 [DOI] [PubMed] [Google Scholar]
- 22.Moqrich A., Hwang S. W., Earley T. J., Petrus M. J., Murray A. N., Spencer K. S., Andahazy M., Story G. M., Patapoutian A. (2005) Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 307, 1468–1472 [DOI] [PubMed] [Google Scholar]
- 23.Hu H. Z., Xiao R., Wang C., Gao N., Colton C. K., Wood J. D., Zhu M. X. (2006) Potentiation of TRPV3 channel function by unsaturated fatty acids. J. Cell. Physiol. 208, 201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bang S., Yoo S., Yang T. J., Cho H., Kim Y. G., Hwang S. W. (2010) Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple anti-nociception. Br. J. Pharmacol. 161, 707–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung M. K., Lee H., Mizuno A., Suzuki M., Caterina M. J. (2004) TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J. Biol. Chem. 279, 21569–21575 [DOI] [PubMed] [Google Scholar]
- 26.Chung M. K., Lee H., Caterina M. J. (2003) Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J. Biol. Chem. 278, 32037–32046 [DOI] [PubMed] [Google Scholar]
- 27.Borbíró I., Lisztes E., Tóth B. I., Czifra G., Oláh A., Szöllosi A. G., Szentandrássy N., Nánási P. P., Péter Z., Paus R., Kovács L., Bíró T. (2011) Activation of transient receptor potential vanilloid-3 inhibits human hair growth. J. Investig. Dermatol. 131, 1605–1614 [DOI] [PubMed] [Google Scholar]
- 28.Bang S., Yoo S., Yang T. J., Cho H., Hwang S. W. (2010) Farnesyl pyrophosphate is a novel pain-producing molecule via specific activation of TRPV3. J. Biol. Chem. 285, 19362–19371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang S. M., Lee H., Chung M. K., Park U., Yu Y. Y., Bradshaw H. B., Coulombe P. A., Walker J. M., Caterina M. J. (2008) Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J. Neurosci. 28, 13727–13737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng X., Jin J., Hu L., Shen D., Dong X. P., Samie M. A., Knoff J., Eisinger B., Liu M. L., Huang S. M., Caterina M. J., Dempsey P., Michael L. E., Dlugosz A. A., Andrews N. C., Clapham D. E., Xu H. (2010) TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 141, 331–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asakawa M., Yoshioka T., Matsutani T., Hikita I., Suzuki M., Oshima I., Tsukahara K., Arimura A., Horikawa T., Hirasawa T., Sakata T. (2006) Association of a mutation in TRPV3 with defective hair growth in rodents. J. Investig. Dermatol. 126, 2664–2672 [DOI] [PubMed] [Google Scholar]
- 32.Yoshioka T., Imura K., Asakawa M., Suzuki M., Oshima I., Hirasawa T., Sakata T., Horikawa T., Arimura A. (2009) Impact of the G573S substitution in TRPV3 on the development of allergic and pruritic dermatitis in mice. J. Investig. Dermatol. 129, 714–722 [DOI] [PubMed] [Google Scholar]
- 33.Steinhoff M., Bíró T. (2009) A TR(I)P to pruritus research. Role of TRPV3 in inflammation and itch. J. Investig. Dermatol. 129, 531–535 [DOI] [PubMed] [Google Scholar]
- 34.Imura K., Yoshioka T., Hirasawa T., Sakata T. (2009) Role of TRV3 in immune response to development of dermatitis. J. Inflamm. 6, 17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin Z., Chen Q., Lee M., Cao X., Zhang J., Ma D., Chen L., Hu X., Wang H., Wang X., Zhang P., Liu X., Guan L., Tang Y., Yang H., Tu P., Bu D., Zhu X., Wang K., Li R., Yang Y. (2012) Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am. J. Hum. Genet. 90, 558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J., Jung E., Yu H., Kim Y., Ha J., Kim Y. S., Park D. (2008) Mechanisms of carvacrol-induced expression of type I collagen gene. J. Dermatol. Sci. 52, 160–169 [DOI] [PubMed] [Google Scholar]
- 37.Montell C. (2010) Preventing a Perm with TRPV3. Cell 141, 218–220 [DOI] [PubMed] [Google Scholar]
- 38.Cheng W., Yang F., Takanishi C. L., Zheng J. (2007) Thermosensitive TRPV channel subunits coassemble into heteromeric channels with intermediate conductance and gating properties. J. Gen. Physiol. 129, 191–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bang S., Yoo S., Yang T., Cho H., Hwang S. (2012) Br. J. Pharmacol. 165, 683–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caterina M. J., Schumacher M. A., Tominaga M., Rosen T. A., Levine J. D., Julius D. (1997) The capsaicin receptor. A heat-activated ion channel in the pain pathway. Nature 389, 816–824 [DOI] [PubMed] [Google Scholar]
- 41.Jordt S. E., Tominaga M., Julius D. (2000) Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc. Natl. Acad. Sci. U.S.A. 97, 8134–8139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyamoto T., Petrus M. J., Dubin A. E., Patapoutian A. (2011) TRPV3 regulates nitric-oxide synthase-independent nitric oxide synthesis in the skin. Nat. Commun. 2, 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutknecht J. (1992) Aspirin, acetaminophen, and proton transport through phospholipid bilayers and mitochondrial membranes. Mol. Cell. Biochem. 114, 3–8 [DOI] [PubMed] [Google Scholar]
- 44.Sigworth F. J. (1980) The variance of sodium current fluctuations at the node of Ranvier. J. Physiol. 307, 97–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baumann T. K., Martenson M. E. (2000) Extracellular protons both increase the activity and reduce the conductance of capsaicin-gated channels. J. Neurosci. 20, RC80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welch J. M., Simon S. A., Reinhart P. H. (2000) The activation mechanism of rat vanilloid receptor 1 by capsaicin involves the pore domain and differs from the activation by either acid or heat. Proc. Natl. Acad. Sci. U.S.A. 97, 13889–13894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao J., Liu B., Qin F. (2010) Kinetic and energetic analysis of thermally activated TRPV1 channels. Biophys. J. 99, 1743–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu B., Yao J., Zhu M. X., Qin F. (2011) Hysteresis of gating underlines sensitization of TRPV3 channels. J. Gen. Physiol. 138, 509–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao R., Tang J., Wang C., Colton C. K., Tian J., Zhu M. X. (2008) Calcium plays a central role in the sensitization of TRPV3 channel to repetitive stimulations. J. Biol. Chem. 283, 6162–6174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nilius B., Talavera K., Owsianik G., Prenen J., Droogmans G., Voets T. (2005) Gating of TRP channels. A voltage connection? J. Physiol. 567, 35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Voets T., Droogmans G., Wissenbach U., Janssens A., Flockerzi V., Nilius B. (2004) The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 430, 748–754 [DOI] [PubMed] [Google Scholar]
- 52.Matta J. A., Ahern G. P. (2007) Voltage is a partial activator of rat thermosensitive TRP channels. J. Physiol. 585, 469–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu H., Grandl J., Bandell M., Petrus M., Patapoutian A. (2009) Two amino acid residues determine 2-APB sensitivity of the ion channels TRPV3 and TRPV4. Proc. Natl. Acad. Sci. U.S.A. 106, 1626–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iwata Y., Katanosaka Y., Arai Y., Shigekawa M., Wakabayashi S. (2009) Dominant-negative inhibition of Ca2+ influx via TRPV2 ameliorates muscular dystrophy in animal models. Hum. Mol. Genet. 18, 824–834 [DOI] [PubMed] [Google Scholar]
- 55.Liedtke W., Tobin D. M., Bargmann C. I., Friedman J. M. (2003) Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 100, 14531–14536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davidge K. S., Motterlini R., Mann B. E., Wilson J. L., Poole R. K. (2009) Carbon monoxide in biology and microbiology. Surprising roles for the ”Detroit perfume.“ Adv. Microb. Physiol. 56, 85–167 [DOI] [PubMed] [Google Scholar]
- 57.Maidorn R. P., Cragoe E. J., Jr., Tannock I. F. (1993) Therapeutic potential of analogues of amiloride. Inhibition of the regulation of intracellular pH as a possible mechanism of tumor selective therapy. Br. J. Cancer 67, 297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karuri A. R., Dobrowsky E., Tannock I. F. (1993) Selective cellular acidification and toxicity of weak organic acids in an acidic microenvironment. Br. J. Cancer 68, 1080–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y. Y., Chang R. B., Allgood S. D., Silver W. L., Liman E. R. (2011) A TRPA1-dependent mechanism for the pungent sensation of weak acids. J. Gen. Physiol. 137, 493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dhaka A., Uzzell V., Dubin A. E., Mathur J., Petrus M., Bandell M., Patapoutian A. (2009) TRPV1 is activated by both acidic and basic pH. J. Neurosci. 29, 153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao J., Liu B., Qin F. (2011) Modular thermal sensors in temperature-gated transient receptor potential (TRP) channels. Proc. Natl. Acad. Sci. U.S.A. 108, 11109–11114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boron W. F. (2005) in Medical Physiology (Boron W. F., Boulpaep E. L., eds) pp. 633–653, Elsevier Saunders, Philadelphia, PA [Google Scholar]
- 63.Gottlieb R. A., Nordberg J., Skowronski E., Babior B. M. (1996) Apoptosis induced in Jurkat cells by several agents is preceded by intracellular acidification. Proc. Natl. Acad. Sci. U.S.A. 93, 654–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tominaga M., Caterina M. J., Malmberg A. B., Rosen T. A., Gilbert H., Skinner K., Raumann B. E., Basbaum A. I., Julius D. (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21, 531–543 [DOI] [PubMed] [Google Scholar]
- 65.Ryu S., Liu B., Yao J., Fu Q., Qin F. (2007) Uncoupling proton activation of valloinoid receptor TRPV1. J. Neurosci. 27, 12797–12807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aneiros E., Cao L., Papakosta M., Stevens E., Phillips S., Grimm C. (2011) EMBO J. 30, 994–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Du J., Xie J., Yue L. (2009) Modulation of TRPM2 by acidic pH and the underlying mechanisms for pH sensitivity. J. Gen. Physiol. 134, 471–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeh B. I., Kim Y. K., Jabbar W., Huang C. L. (2005) Conformational changes of pore helix coupled to gating of TRPV5 by protons. EMBO J. 24, 3224–3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leffler A., Mönter B., Koltzenburg M. (2006) The role of the capsaicin receptor TRPV1 and acid-sensing ion channels (ASICS) in proton sensitivity of subpopulations of primary nociceptive neurons in rats and mice. Neuroscience 139, 699–709 [DOI] [PubMed] [Google Scholar]
- 70.Smith E. S., Lewin G. R. (2009) Nociceptors, a phylogenetic view. J. Comp. Physiol. A Neuroethol. Sen. Neural Behav. Physiol. 195, 1089–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jones N. G., Slater R., Cadiou H., McNaughton P., McMahon S. B. (2004) Acid-induced pain and its modulation in humans. J. Neurosci. 24, 10974–10979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shimada S., Yamamura H., Ueda T., Yamamoto T., Ugawa S. (2004) Functional analysis of acid sensing ion channels. Nihon Shinkei Seishin Yakurigaku Zasshi 24, 243–246 [PubMed] [Google Scholar]
- 73.Moran M. M., McAlexander M. A., Bíró T., Szallasi A. (2011) Transient receptor potential channels as therapeutic targets. Nat. Rev. Drug Discov. 10, 601–620 [DOI] [PubMed] [Google Scholar]
- 74.Nassini R., Materazzi S., De Siena G., De Cesaris F., Geppetti P. (2010) Transient receptor potential channels as novel drug targets in respiratory diseases. Curr. Opin. Investig. Drugs 11, 535–542 [PubMed] [Google Scholar]
- 75.Gunthorpe M. J., Szallasi A. (2008) Peripheral TRPV1 receptors as targets for drug development. New molecules and mechanisms. Curr. Pharmaceut. Design 14, 32–41 [DOI] [PubMed] [Google Scholar]
- 76.Hellwig N., Plant T. D., Janson W., Schäfer M., Schultz G., Schaefer M. (2004) TRPV1 acts as proton channel to induce acidification in nociceptive neurons. J. Biol. Chem. 279, 34553–34561 [DOI] [PubMed] [Google Scholar]
- 77.Reilly R. M., Kym P. R. (2011) Analgesic potential of TRPV3 antagonists. Curr. Top. Med. Chem. 11, 2210–2215 [DOI] [PubMed] [Google Scholar]
- 78.Danilova V., Danilov Y., Roberts T., Tinti J. M., Nofre C., Hellekant G. (2002) Sense of taste in a new world monkey, the common marmoset. Recordings from the chorda tympani and glossopharyngeal nerves. J. Neurophysiol. 88, 579–594 [DOI] [PubMed] [Google Scholar]
- 79.DeSimone J. A., Callaham E. M., Heck G. L. (1995) Chorda tympani taste response of rat to hydrochloric acid subject to voltage-clamped lingual receptive field. Am. J. Physiol. 268, C1295–1300 [DOI] [PubMed] [Google Scholar]
- 80.Lin W., Ogura T., Kinnamon S. C. (2002) Acid-activated cation currents in rat vallate taste receptor cells. J. Neurophysiol. 88, 133–141 [DOI] [PubMed] [Google Scholar]
- 81.Inada H., Kawabata F., Ishimaru Y., Fushiki T., Matsunami H., Tominaga M. (2008) Off-response property of an acid-activated cation channel complex PKD1L3-PKD2L1. EMBO Rep. 9, 690–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Valente P., Fernández-Carvajal A., Camprubí-Robles M., Gomis A., Quirce S., Viana F., Fernández-Ballester G., González-Ros J. M., Belmonte C., Planells-Cases R., Ferrer-Montiel A. (2011) Membrane-tethered peptides patterned after the TRP domain (TRPducins) selectively inhibit TRPV1 channel activity. FASEB J. 25, 1628–1640 [DOI] [PubMed] [Google Scholar]