Senescence in tumours: evidence from mice and humans (original) (raw)
. Author manuscript; available in PMC: 2013 Jun 5.
Published in final edited form as: Nat Rev Cancer. 2010 Jan;10(1):51–57. doi: 10.1038/nrc2772
Preface
Cellular senescence is a stress response that stably blocks proliferation and whose importance in cancer is increasingly recognized. Senescence is prevalent in premalignant tumours and progression to malignancy requires evading senescence. Malignant tumours, however, may still undergo senescence by interventions that restore tumour suppressors or inactivate oncogenes. Senescent tumour cells can be cleared by immune cells and this may result in efficient tumour regression. Standard chemotherapy also has the potential to induce senescence, which may underlie part of its therapeutic activity. While these concepts are well supported in mouse models, it remains ahead to translate them to clinical oncology.
Introduction
The initial description of cellular senescence by Hayflick and collaborators was based on the meticulous analysis of normal human cells grown in vitro 1. They found that, in contrast to cancer cells, normal cells have a finite proliferative capacity that ends in a stable and long-term cell cycle arrest, characterized by lack of response to growth factors, sustained metabolic activity, and changes in cell morphology2. The molecular basis for this response has been intensively studied and it is now considered a combination of at least three mechanisms, namely, telomere shortening, upregulation of the CDKN2A locus (which encodes INK4A and ARF), and accumulation of DNA damage, whose relative contribution to senescence depends on the cell type and the cell culture conditions2.
More than a decade ago now, a phenotype similar to senescence was unexpectedly observed in normal cells grown in vitro upon overexpression of an oncogenic version of HRAS (HRASG12V)3. Normal cells forced to express high levels of the oncogene, rather than increasing their proliferation, stopped dividing and suffered morphological and molecular changes indistinguishable from senescence3. Two crucial tumour suppressors, INK4A and p53, were shown to be upregulated in oncogenically-stressed cells and to be responsible for the cell cycle arrest imposed on these cells. In this manner, the concept of oncogene-induced senescence (OIS) emerged as a putative tumour suppressor mechanism, similar to the better-known phenomenon of oncogene-induced apoptosis4. The occurrence of cellular senescence within murine and human tumours was originally reported in a series of studies describing the presence of markers of senescence (see Box1) in premalignant tumours and their absence in malignant ones5-9. Since then, numerous additional investigations have further refined our understanding of the role of senescence during tumorigenesis. In this review, we discuss the current in vivo evidence linking senescence with tumour suppression.
BOX 1: Markers of senescence in vivo.
While senescent cells in vitro usually adopt a large and flat morphology, senescent tumour cells in vivo present the morphology associated with the corresponding tumour stage. Therefore, molecular markers are necessary to qualify a lesion as senescent. Despite the efforts deployed by many laboratories and the increasing interest in defining cellular senescence, there is still a paucity of robust markers of cellular senescence54. The single most accepted and widely used marker is the staining for beta-galactosidase assessed at a suboptimal pH of 6.0 (senescence-associated beta-galactosidase, SABG)55. Despite the existence of exceptions17, 18, this is among the most robust markers for senescent cells56, 57. In vivo, it has been used to demonstrate senescence induction in a wide variety of cancer settings (Tables 1 and 2).
Other classical markers of oncogene-induced senescence (OIS) are the very same proteins involved in the mechanism of cell growth arrest. The products of the CDKN2A locus (INK4A and ARF) have proved useful markers of senescence in vivo, sometimes even in the absence of a positive SABG17. For some particular models of in vivo senescence, other cell cycle regulators are more informative, such as p21 and p2722, 24. Along the same lines, molecules involved in the DNA damage response (such as γH2AX) or in the formation of senescence-associated heterochromatin foci (such as HP1γ), have been used as surrogate markers of the process (Tables 1 and 2).
DNA microarray analysis of senescence has also provided new markers of senescence, such as DEC1 and DCR2 7, that have been used successfully in the identification of in vivo senescence (Tables 1 and 2).
All of these markers however, do not offer compelling evidence of senescence induction if not combined with the concomitant identification of lower levels of proliferation, as typically determined with Ki67 or BrdU labelling.
Triggers of tumour cell senescence
The oncogene used in the original description of OIS in vitro was HRASG12V 3, and soon after, the Raf/Mek pathway downstream of Ras was revealed as the most relevant one leading to senescence10, 11. These seminal observations in vitro were among the first to be validated in vivo using mouse models with inducible endogenous oncogenes (Figure 1 and Table 1). In particular, endogenous KrasG12V was shown to trigger senescence during the early stages of lung and pancreatic tumorigenesis driven by this oncogene7. Subsequent studies by three different laboratories using similar mouse models based on endogenous KrasG12D have confirmed these observations in premalignant lesions of the lung (S. Ryeom, personal communication) and pancreas12 (C. Carriere and M. Korc, personal communication). However, other investigators have not found evidence for senescence in _KrasG12D_-driven lung lesions13 or _KrasG12D_-driven pancreatic lesions (M. Caldwell and D. Tuveson, personal communication). Understanding the basis for these discrepancies will hopefully shed additional light onto the early stages of tumorigenesis. Importantly, senescence has been also observed in lung tumours and melanocytic nevi [SEE GLOSSARY] when using mice carrying endogenous BrafV600E, an oncogene from the Raf family14, 15. The other two Ras family members, NRAS and HRAS, also induce senescence in vivo. In particular, transgenic expression of NrasG12D in lymphoid tissue results in lymphocytes highly susceptible to senescence following chemotherapy5. In the case of HRAS, transgenic inducible expression of HrasG12V in the mammary gland leads to hyperproliferation when the oncogene is expressed at low levels, but to tumour cell senescence when the oncogene is highly expressed16. The latter observations might have important implications for our understanding of the mechanism of induction of cellular senescence (discussed below). Other mouse models of oncogenic Hras expression, either from its endogenous promoter or targeted to the bladder epithelium, have certified the existence of tumour cell senescence17, 18. In the case of chemically-induced skin papillomas, which are associated with HRAS oncogenic activation, senescence has also been documented7, 19 and is mediated by the downstream activation of p38 MAP kinase20.
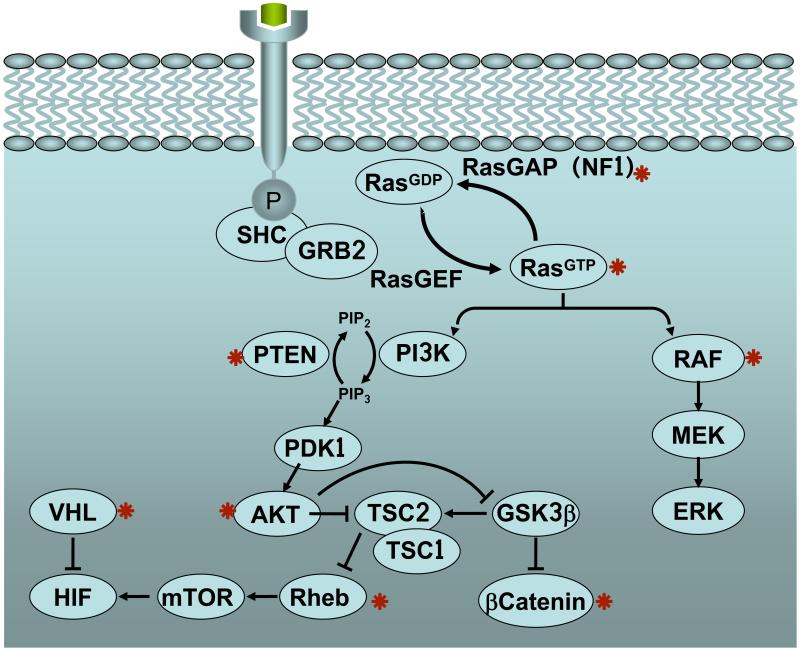
Figure 1.
Signalling pathways and oncogenes (marked by a red asterisk) whose activation leads to senescence induction in vivo.
Table 1. Mouse models of tumour cell senescence.
| Senescence inducing event | Tissue/Tumour | Evidence of senescence | Refs. | |
|---|---|---|---|---|
| Oncogeneactivation | HrasG12V | Mammary tumours,bladder tumours,DMBA/TPA skinpapillomas | SABG,γH2AX, p53,INK4A, p21,ARF, low Ki67 | 16-20 |
| KrasG12V | Lung adenomas,Pancreatic intraductalneoplasias | SABG, INK4A,DEC1, DCR2,INK4B, HP1γ,low Ki67 | 7 | |
| NrasG12D | Lymphoproliferativedisorders | SABG | 5 | |
| BrafV600E | Nevi, Lung adenomas, | SABG, DEC1,ARF, low Ki67 | 14, 15 | |
| Rheb | Prostate PIN | SABG, lowKi67 | 23 | |
| E2f3 | Pituitary hyperplasia | SABG, INK4A,ARF, SAHFs,low BrdU | 8 | |
| Akt1 | Prostate PIN | SABG, p27,HP1α, HP1γ,low BrdU | 22 | |
| β-catenin | Thymus | SABG, INK4B,INK4A, DEC1,CTSF, CDH16,low BrdU | 25 | |
| Oncogeneinactivation | Myc | Lymphoma,osteosarcoma, livercarcinoma, lungcarcinoma | SABG, INK4B,p21, H3K9me3 | 44, 45 |
| Tumoursuppressorinactivation | Pten | Prostate PIN | ARF, p53, p21,SABG, lowKi67 | 6 |
| Rb | Thyroid C celladenomas | SABG,H3K9me3,HP1γ, INK4A | 58 | |
| Vhl | Kidney | SABG, p27,DCR2 | 24 | |
| Tumoursuppressoractivation | p53 | Sarcomas, livercarcinomas | SABG, INK4B,INK4A DCR2,low P-histoneH3, low Ki67 | 39, 40 |
In addition to the Ras oncogenes and their proximal downstream kinases, distal effectors of the Ras pathway, such as the E2f family of transcription factors21, can also induce senescence. For example, expression of an inducible E2f3 transgene in the intermediate lobe of the pituitary of mice causes an initial burst of proliferation, but then cells stop dividing, acquire markers of senescence and do not form tumours8.
The PI3K/Akt pathway also plays a crucial role in the generation of proliferative signals. Specific genetic ablation of the gene encoding PTEN, a phosphatase that opposes PI3K activity, in mouse prostate leads to prostate intraepithelial neoplasia (PIN) with features of cellular senescence6. Similarly, targeted expression of AKT1 in prostate leads to formation of PIN lesions, which show markers of cellular senescence22. Akt signalling branches out into multiple pathways but, interestingly, mice overexpressing RHEB, which links Akt to mTOR, also produces PIN lesions positive for senescence in the prostate23. Another example of cellular senescence triggered by the loss of a tumour suppressor is provided by a mouse model of conditional deletion of Vhl, a tumour suppressor frequently mutated in human renal cell carcinomas. Loss of VHL leads to senescence in the kidney associated with increased levels of the cell cycle inhibitor p27 24. Finally, transgenic expression of a stabilized form of β-catenin in lymphocytes, rather than triggering lymphomagenesis, results in DNA damage, followed by senescence and apoptosis25.
In summary, during the last years senescence has moved from the realm of in vitro cultured cells to the complexity of experimental mouse tumours driven by a variety of oncogenic pathways (see Figure 1 and Table 1). Importantly, the analysis of human tumours is starting to provide interesting examples of senescence.
Senescence in human tumours
The observation of tumour cell senescence has not been restricted to mouse models, but has also been reported in humans (Table 2). In fact, melanocyte senescence associated to the presence of oncogenic BRAFV600E was part of the initial reports on cellular senescence in vivo9. Similarly, human PIN lesions express markers of senescence providing a nice correlate to the mouse models of neoplastic prostate lesions6. The tumour suppressor NF1 constitutes another interesting example of cellular senescence in humans26. Loss-of-function mutation of NF1 underlies the familial cancer syndrome known as neurofibromatosis type I. NF1 encodes a Ras GTPase-activating protein that is a negative regulator of Ras activity. Thus, absence of NF1 results in hyperactivated Ras signalling and formation of neoplastic lesions known as neurofibromas, which were shown to express markers of senescence26. Finally, premalignant human colon adenomas also show features of senescence associated with the presence of DNA damage markers27 and with the expression of p53β (a p53 isoform) and INK4A28. The above data are highly suggestive of tumour cell senescence playing an important role not only in mouse models of tumorigenesis but also in human cancer. Nonetheless, these studies need to be extended to more types of human cancer.
Table 2. Human tumours showing cell senescence.
| Associated oncogenic event | Tissue/Tumour | Evidence of senescence | Refs. |
|---|---|---|---|
| NF1 inactivation | Dermal neurofibromas | SABG, INK4A | 26 |
| BRAFV600E | Nevi | SABG, INK4A, low Ki67 | 29 |
| not determined | Prostate PIN | SABG, CXCR2 | 6, 52 |
| not determined | Colon adenomas | SABG, INK4A, IL8 | 27, 28,53 |
Current concepts on tumour senescence
Senescence is characteristic of premalignant tumour stages
One of the first lessons derived from the analysis of senescence in tumours is its close association with the premalignant stages of tumorigenesis, but its absence from malignant tumours. Indeed, the original identification of senescent tumour cells was obtained on lung adenomas, pancreatic intraductal neoplasias, prostate intraepithelial neoplasias and melanocytic nevi, which are all pre-malignant tumours6, 7, 9. In contrast, senescence was absent in their corresponding malignant stages, namely, lung adenocarcinomas, pancreatic ductal adenocarcinomas, prostate adenocarcinomas and melanomas, respectively6, 7, 29. All these evidences strongly suggest a role of senescence as a barrier to tumour progression.
Senescence is a tumour suppressive mechanism
Evidence for the tumour suppressor role of senescence has been obtained with mouse models of cancer triggered by ablation of the tumour suppressor Pten in the prostate6 or oncogenic Nras expression in the haematopoietic system5. In both cases, the oncogenic initiating event led to the development of senescent premalignant lesions with little evidence of apoptosis. Interestingly, full-blown malignancy and loss of senescence markers occurred when the oncogenic event was combined with simultaneous deletion of mediators of the senescence response, such as the tumour suppressor p53 6 or the histone methyltransferase of lysine 9 in histone 3 known as SUV39H1 5. This histone methyltransferase is involved in the formation of heterochromatin and could be relevant for the formation of senescence-associated heterochromatin foci (or SAHFs, [SEE GLOSSARY]), which are domains of silenced chromatin considered important for the senescent phenotype 30. The association between senescence and tumour suppression has been subsequently supported in other mouse cancer models, such as BRAFV600E-induced lung tumours14, BRAFV600E-induced melanomas31, and HRASG12V-induced mammary tumours16. In these cases, genetic deletion of Cdkn2a (encoding INK4A and ARF) or Trp53 (which encodes p53), abrogated senescence and allowed progression to malignant stages, providing a compelling case for a causal link between the induction of senescence by INK4A, ARF and p53 and tumour suppression14, 16, 31.
Not all the senescent premalignant stages are, however, strictly dependent on INK4A, ARF or p53. In this regard, senescent pre-lymphomagenic thymocytes in mice with β-catenin expression showed a stable senescent response even in the absence of p53, although loss of this tumour suppressor allowed progression to lymphoma25. Similarly, the absence of INK4A favoured BRAFV600E-induced melanomas, but nevi with detectable senescence were still produced15. This is in agreement with data in humans indicating that not all the cells in senescent nevi are positive for INK4A, and also with the fact that individuals from a Dutch family with hereditary melanoma carrying inactivating mutations in both copies of the CDKN2A locus still develop nevi9. Finally, deletion of Vhl in the mouse kidney results in senescence with associated increase in p27, but not of INK4A, ARF or p53, suggesting that other cell cycle regulators can also be engaged by aberrant oncogenic activation to implement senescence24. Similarly, transgenic AKT1-driven PIN lesions also show increased expression of p27 associated to senescence22. Moreover, when these transgene was expressed in combination with genetic deletion of Cdkn1b (the gene encoding p27), senescence was absent and mice developed invasive prostate cancer22. Together, these observations point to redundant mechanisms of senescence whose relevance depends on the tissue type and the driving oncogenes.
Levels of oncogene activity determine the outcome of senescence
It is now well established that induction of senescence by oncogenic Ras in vitro only occurs when the oncogene is overexpressed, but not when the oncogene is expressed at its normal levels13, 32. As a matter of fact, normal levels of expression of oncogenic KRAS are for the most part inconsequential, with little or no signs of activation of its canonical downstream effectors Erk and Akt, and with modest effects on proliferation13, 32. This is also the case at the organismal level and mice expressing oncogenic Kras from its endogenous promoter are largely normal and present a normal tissue architecture (aside from the eventual development of a few lung tumours after prolonged latency)13, 32. The little or minimal phenotype of oncogenic KRAS in cells and tissues likely reflects the operation of negative feedback loops that counteract the effect of oncogenic Ras on its downstream effectors. According to this view, the signalling produced by oncogenic Ras only becomes tumorigenic when the negative feedback loops are cancelled or when they are surpassed by upregulation of the oncogene above its normal expression levels. In support of this, when HRASG12V expression was carefully titrated on a mouse model16, moderate overexpression of HRASG12V produced focal hyperplasias that did not lead to tumours, whereas high overexpression led to low-grade tumours with senescent markers. Interestingly, tumours with high levels of oncogene expression only progressed to full-blown carcinomas when senescence was cancelled by the absence of INK4A, ARF or p53 16.
Conceptually similar observations have been made using other mouse models. In particular, expression of oncogenic HrasG12V from its endogenous locus recapitulates the complex phenotypes of the Costello syndrome [SEE GLOSSARY], a human developmental disorder produced by germline mutations in HRAS17, 33. Costello syndrome increases the susceptibility to develop papillomas, rhabdomyosarcomas and bladder carcinomas34. Interestingly, mice with germline HrasG12V were prone to developing skin papillomas and angiosarcomas, which were invariably associated with DNA amplification of the oncogene and senescence17. Moreover, a zebrafish model expressing oncogenic hrasG12V 35 also recapitulates phenotypes of human Costello syndrome and high susceptibility to tumour development, and, interestingly, senescent cells can be detected in heart and brain35.
Overall, a picture emerges by which normal or low levels of oncogenic expression are inconsequential or result in hyperplasias that do not lead to tumours; while, high levels of oncogenic signalling are necessary to produce premalignant tumours and the engagement of senescence. The signal amplifying events during tumorigenesis can consist in increased gene copy number, increased transcriptional activity of the oncogene promoter, or loss of negative regulators of the oncogenic signalling.
Finally, it should be warned that the above ideas are derived from the study of Ras oncogenes and may not apply to all oncogenes and cell types. For example, physiological levels of BRAFV600E appear to be sufficient to trigger senescence in in vitro grown melanocytes9.
Tumour suppressors inducing and preventing senescence
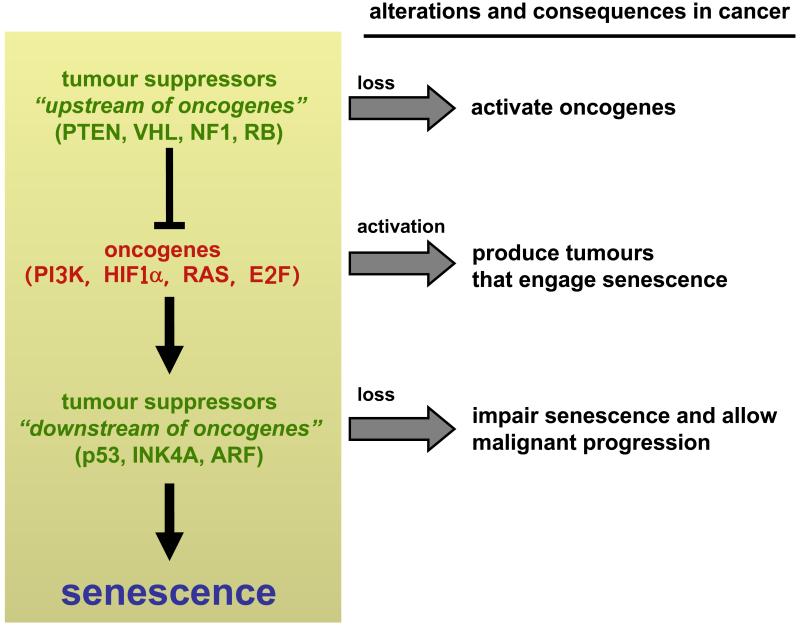
From work on mouse models, an interesting distinction emerges among tumour suppressors regarding their role in senescence: some of them function “upstream of oncogenes” and prevent senescence; while others act “downstream of oncogenes” and induce senescence (Figure 2). In particular, tumour suppressors PTEN, VHL, NF1 and RB constitutively oppose pro-oncogenic signals emanating, respectively, from PI3K, HIF1α, Ras, or E2f. Consequently, their deletion results in excessive proliferative signalling that eventually leads to senescence. On the other hand, the tumour suppressors p53, INK4A and ARF monitor the presence of oncogenic signalling, which activates them and triggers senescence. In agreement with the sensor function of these tumour suppressors, their expression or activity under normal, non-stressful, conditions is generally very low, but they become strongly expressed and/or activated after oncogenic signalling2. Therefore, there are tumour suppressors that basally control oncogenes and whose activity is constitutively necessary to prevent oncogene-induced senescence (PTEN, VHL, NF1 and RB) (“upstream of oncogenes”), while others (p53, INK4A and ARF) act occasionally and inducibly when oncogenic signalling is unleashed (“downstream of oncogenes”) (Figure 2). In this context, telomere shortening can also be considered among the last group of tumour suppressors. Specifically, critically short telomeres occur upon excessive proliferation and trigger senescence to prevent tumour formation36, 37 (reviewed in 38).
Figure 2.
Tumour suppressors can be grouped into two categories depending on their effect on senescence. Some tumour suppressors (at the top of the cascade, i.e. “upstream of oncogenes”) prevent excessive oncogenic signalling and their deletion in normal cells triggers senescence. Other tumour suppressors (“downstream of oncogenes”) sense excessive oncogenic signalling and induce senescence, and their absence in tumour cells allows progression to malignancy.
Senescence as anti-cancer therapy
As mentioned above, progression to malignancy involves bypassing or inhibiting critical mediators of senescence, however, this does not mean that malignant cells have completely lost their capacity to undergo senescence. Given the tumour suppressive potential of senescence, it is of interest to test the efficacy of senescence-inducing interventions for the treatment of cancer. Mouse models have provided support to the predicted therapeutic capacity of senescence-inducing treatments, and a few human studies are also suggestive of chemotherapeutic responses mediated by senescence.
Senescence after restoration of tumour suppression
Two recent reports addressed the anti-tumour efficacy of restoring the tumour suppressive function of p53 using mouse models in which p53 can be switched off and back on again. Both spontaneous and radiation-induced tumour development were assessed using different genetic approaches to ablate and then restore p53 function. The majority of sarcomas, lymphomas and liver carcinomas that developed in the absence of p53 regressed after p53 restoration39, 40. Interestingly, while massive apoptosis accounted for lymphoma regression, sarcomas and liver carcinomas regressed in association with a potent senescent response. Tumour regression by senescence was accompanied by the presence of tumour infiltrating neutrophils, macrophages and natural killer cells40. This suggests that senescent tumour cells, in contrast to non-senescent tumour cells, are efficiently cleared out by immune cells. Another interesting point from these studies is that artificial expression of p53 only triggers senescence or apoptosis in tumour cells, but is inconsequential in normal tissues39. The reason for this discrimination is that the aberrant context of tumour cells constitutively generates p53-activating signals; whereas in non-tumoural tissues, mere expression of p53 is not followed by its activation. Together, these experimental data offer support for the idea that development of drugs targeted at restoring p53 function in tumours might provide effective means of restricting tumour growth by senescence and even promoting tumour cell clearance, while sparing normal tissues. Such p53 restoring drugs are been developed (reviewed in 41) and some of their preliminary characterizations lend support for a senescence-inducing effect on tumour cells42, 43.
Senescence in response to oncogene inactivation
Restoring lost or inactive tumour suppressors is not the only possible therapeutic intervention to induce senescence. Studies aimed at determining the necessity of oncogenic signalling for the maintenance of the malignant phenotype have also shown the potency of cellular senescence in controlling tumour growth. Mouse models of Myc transgenic expression under inducible tissue-specific promoters showed that the maintenance of MYC-initiated hepatocellular carcinomas, lymphomas or osteosarcomas depends on the continuous expression of the oncogene44. Interestingly, switching-off Myc transgenic expression in these variety of tumors caused rapid regression accompanied by cellular senescence44. Even more, inactivation of crucial senescence mediators, such as INK4A, RB or p53, abolished senescence and tumour regression44. Therefore, targeting critical oncogenes can reactivate senescence and induce tumour regression even in full-blown malignancies.
Oncogene inactivation may also induce cellular senescence when the targeted oncogene is not the tumour-initiating event. This is the case of KRASG12D-initiated lung carcinomas, where inactivation of the three Myc paralogs, MYC, NMYC and LMYC, by means of an artificial dimerization partner known as Omomyc, results in tumour regression in association with apoptosis and senescence45. Interestingly, Myc inactivation during 1 month only had mild adverse effects on normal tissues that were fully reversed upon interruption of Omomyc expression45. These results are remarkable because they show that senescence can be engaged in established tumours even by targeting molecules other than the actual initiating oncogene, leading to tumour regression while sparing normal cells. Based on this, it is reasonable to expect that therapeutic interventions aimed at targeting molecules required to support tumour growth would also lead to cellular senescence induction.
Senescence-inducing chemotherapy
Current anti-tumour strategies are designed to kill cancer cells, although they are often limited by pro-survival alterations present in cancer cells. Senescence-inducing drugs, by attacking tumour cells from a different angle, might prove effective alone or in combination with classical therapeutic approaches, and might offer an opportunity to reduce the toxicity of chemotherapy. Mouse models of chemotherapy have shown that MYC-initiated lymphomas respond to cyclophosphamide by inducing tumour cell senescence mediated by INK4A and p53 and this correlated with a better prognosis following chemotherapy46.
In the case of human cancer cells grown in vitro, classical chemotherapy often induces senescence at moderate doses and apoptosis at higher ones47. Chemotherapy-induced regression of human cancers is not always explained by an apoptotic response (see for example 48), and it is conceivable that senescence could play an important role in chemotherapy, as it has been shown in mouse cancer models. In this regard, two reports analyzing senescence markers in biopsies from lung and breast cancer patients after neoadjuvant chemotherapy have observed chemotherapy-induced senescence and its association to treatment success49, 50. More recently, the analysis of prostate cancer biopsies has shown that previous treatment of patients with chemotherapy induces markers of senescence51.
A note of caution for senescence-inducing therapies
As a note of caution, we must bear in mind the potential problems that might arise from senescence-inducing therapies. It is conceivable that cancer cells in a senescence-like status might remain as “dormant tumour cells” and represent a dangerous potential for tumour relapse. In this regard it is important to gain deeper knowledge of the mechanisms responsible for senescent tumour cell clearance.
In addition, senescent cells show a robust secretory phenotype known as SASP (for senescence-associated secretory phenotype), in which the cells release a number of pro-inflammatory cytokines, chemokines and tissue remodelling enzymes. Some of these factors, such as IL-6 and IL-8, serve a cell autonomous function that reinforces senescence in a paracrine manner52, 53. It can be speculated that another role of SASP could be to stimulate the clearance of senescent cells by the immune system. Finally, as a word of caution, SASP components may also dangerously stimulate malignant phenotypes of nearby tumour cells51.
Future prospects
Cancer mouse models have demonstrated the occurrence of senescence associated with pre-malignant stages of neoplastic transformation and its critical function in preventing tumour progression. Interestingly, senescent tumour cells are not only growth arrested but can be also cleared by phagocytic cells39. Following on this, senescence-inducing drugs could represent an ideal opportunity to increase the arsenal of anti-cancer weapons. Finally, studies on human cancer samples should establish whether senescence is or is not relevant for cancer progression and therapeutic responses.
At a glance.
- Senescence is a stress response prevalent in the aberrant environment of tumours.
- Senescent cells are incapable of further proliferation and therefore tumour cell senescence is a brake to tumour progression.
- A large body of evidence in mouse models indicates that in pre-malignant tumours most cells are senescent, thus explaining the slow growth and low malignancy of these tumours. There are also examples of senescence in human pre-malignant tumours.
- A class of tumour suppressors monitors stress signals and their activation triggers senescence, most notably, p53, INK4a and ARF. Their loss or inactivation is associated with impaired senescence, unleashing malignant progression.
- Malignant tumours, despite their impaired ability to undergo senescence, can still be forced into senescence if critical oncogenic pathways are disabled or tumour suppressors are restored.
- Senescent tumour cells are rapidly cleared by immune cells resulting in efficient tumour regression.
- Senescence constitutes a new end-point that can be of relevance for the development of new drugs, for prognosis, or for the evaluation of therapeutical treatments.
Glossary
Nevi
Benign skin lesions of melanocytes that are thought to be senescent
SAHFs
Senescence-associated heterochromatin foci are highly condensed chromatin regions established during senescence and thought to serve as silencing domains
Costello syndrome
Complex developmental syndrome with distinctive craniofacial features and predisposition to neoplasia development caused by activating germline mutations in HRAS
Author Biographies
Manuel Collado
Manuel Collado is a staff investigator within the Tumour Suppression Group at the Spanish National Cancer Research Centre (CNIO) in Madrid, Spain. He received his Ph.D. in 1997 from the Universidad Autónoma de Madrid, Spain. He completed postdoctoral training at the Ludwig Institute for Cancer Research, in London, U.K. and at Memorial Sloan Kettering Cancer Center in New York, U.S.A., working on cellular senescence and the PI3K/AKT pathway and the cyclin-dependent kinase inhibitor p27. He joined Manuel Serranós laboratory in 2001 where he has been working on oncogene-induced senescence and tumour suppression.
Manuel Serrano
Manuel Serrano is principal investigator at the Spanish National Cancer Research Centre (CNIO) in Madrid, Spain, where he leads the Tumour Suppression Group. He received his Ph.D. in 1991 from the Universidad Autónoma de Madrid, Spain. He did his postdoctoral training at Cold Spring Harbor Laboratory in New York, U.S.A., where he identified the cyclin-dependent kinase inhibitor, INK4A, and described for the first time the process of oncogene-induced senescence. He returned to Spain in 1997 to lead his own research group, first at the Spanish National Center of Biotechnology (CNB) in Madrid, and since 2003 at the CNIO.
References
- 1.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–33. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. Original description of oncogene-induced senescence in in vitro cultured primary human and mouse cells after overexpression of oncogenic HRAS. Prompted the concept of cellular senescence as a tumour suppressor mechanism.
- 4.Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–15. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- 5.Braig M, et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–5. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–30. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collado M, et al. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 8.Lazzerini Denchi E, Attwooll C, Pasini D, Helin K. Deregulated E2F activity induces hyperplasia and senescence-like features in the mouse pituitary gland. Mol Cell Biol. 2005;25:2660–72. doi: 10.1128/MCB.25.7.2660-2672.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michaloglou C, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–4. doi: 10.1038/nature03890. References 5-9 report for the first time the existence of senescence associated with the pre-malignant stage of tumorigenesis both in mouse tumour models and in human neoplastic lesions.
- 10.Lin AW, et al. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 1998;12:3008–19. doi: 10.1101/gad.12.19.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu J, Woods D, McMahon M, Bishop JM. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 1998;12:2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morton JP, et al. Mutant but not knockout p53 drives metastatic pancreatic cancer. Proc Natl Acad Sci U S A. 2009 in press. [Google Scholar]
- 13.Tuveson DA, et al. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell. 2004;5:375–87. doi: 10.1016/s1535-6108(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 14.Dankort D, et al. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes Dev. 2007;21:379–84. doi: 10.1101/gad.1516407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhomen N, et al. Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell. 2009;15:294–303. doi: 10.1016/j.ccr.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Sarkisian CJ, et al. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat Cell Biol. 2007;9:493–505. doi: 10.1038/ncb1567. This paper elegantly demonstrates that tumour formation by oncogenic HRAS requires high levels of expression of the oncogene, whereas low levels do not lead to tumours. The tumours produced by high HRAS only progress to a pre-malignant stage due to the engagement of senescence. Cancellation of p53 or INK4A/ARF eliminates senescesce and allows progression to full malignancy.
- 17.Chen X, et al. Endogenous expression of Hras(G12V) induces developmental defects and neoplasms with copy number imbalances of the oncogene. Proc Natl Acad Sci U S A. 2009;106:7979–84. doi: 10.1073/pnas.0900343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mo L, et al. Hyperactivation of Ha-ras oncogene, but not Ink4a/Arf deficiency, triggers bladder tumorigenesis. J Clin Invest. 2007;117:314–25. doi: 10.1172/JCI30062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamakoshi K, et al. Real-time in vivo imaging of p16Ink4a reveals cross talk with p53. J Cell Biol. 2009;186:393–407. doi: 10.1083/jcb.200904105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun P, et al. PRAK is essential for ras-induced senescence and tumor suppression. Cell. 2007;128:295–308. doi: 10.1016/j.cell.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 21.Sears RC, Nevins JR. Signaling networks that link cell proliferation and cell fate. J Biol Chem. 2002;277:11617–20. doi: 10.1074/jbc.R100063200. [DOI] [PubMed] [Google Scholar]
- 22.Majumder PK, et al. A prostatic intraepithelial neoplasia-dependent p27 Kip1 checkpoint induces senescence and inhibits cell proliferation and cancer progression. Cancer Cell. 2008;14:146–55. doi: 10.1016/j.ccr.2008.06.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nardella C, et al. Aberrant Rheb-mediated mTORC1 activation and Pten haploinsufficiency are cooperative oncogenic events. Genes Dev. 2008;22:2172–7. doi: 10.1101/gad.1699608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young AP, et al. VHL loss actuates a HIF-independent senescence programme mediated by Rb and p400. Nat Cell Biol. 2008;10:361–9. doi: 10.1038/ncb1699. [DOI] [PubMed] [Google Scholar]
- 25.Xu M, et al. Beta-catenin expression results in p53-independent DNA damage and oncogene-induced senescence in prelymphomagenic thymocytes in vivo. Mol Cell Biol. 2008;28:1713–23. doi: 10.1128/MCB.01360-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Courtois-Cox S, et al. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell. 2006;10:459–72. doi: 10.1016/j.ccr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartkova J, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–7. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 28.Fujita K, et al. p53 isoforms Delta133p53 and p53beta are endogenous regulators of replicative cellular senescence. Nat Cell Biol. 2009;11:1135–42. doi: 10.1038/ncb1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray-Schopfer VC, et al. Cellular senescence in naevi and immortalisation in melanoma: a role for p16? Br J Cancer. 2006;95:496–505. doi: 10.1038/sj.bjc.6603283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narita M, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–16. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 31.Goel VK, et al. Melanocytic nevus-like hyperplasia and melanoma in transgenic BRAFV600E mice. Oncogene. 2009;28:2289–98. doi: 10.1038/onc.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guerra C, et al. Tumor induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell. 2003;4:111–20. doi: 10.1016/s1535-6108(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 33.Schuhmacher AJ, et al. A mouse model for Costello syndrome reveals an Ang II-mediated hypertensive condition. J Clin Invest. 2008;118:2169–79. doi: 10.1172/JCI34385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7:295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- 35.Santoriello C, et al. Expression of H-RASV12 in a zebrafish model of Costello syndrome causes cellular senescence in adult proliferating cells. Dis Model Mech. 2009;2:56–67. doi: 10.1242/dmm.001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cosme-Blanco W, et al. Telomere dysfunction suppresses spontaneous tumorigenesis in vivo by initiating p53-dependent cellular senescence. EMBO Rep. 2007;8:497–503. doi: 10.1038/sj.embor.7400937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldser DM, Greider CW. Short telomeres limit tumor progression in vivo by inducing senescence. Cancer Cell. 2007;11:461–9. doi: 10.1016/j.ccr.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng Y, Chan SS, Chang S. Telomere dysfunction and tumour suppression: the senescence connection. Nat Rev Cancer. 2008;8:450–8. doi: 10.1038/nrc2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ventura A, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–5. doi: 10.1038/nature05541. This and the following reference (40) made used of sophisticated genetically manipulated mice engineered to switch off and on again tumour suppressor p53. Tumours developed in the absence of p53 were efficiently controlled and regressed after re-expression of p53 and, for some of the tumour types, the mechanism restraining tumour progression was demonstrated to be cellular senescence.
- 40.Xue W, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–60. doi: 10.1038/nature05529. This paper added to the previous one the concept of senescent tumour cell clearance. The authors identified cells of the innate immune system as responsible for clearing out tumour cells that were held in check by cellular senescence after p53 restoration and this mechanism accounts for the disappearance of the tumour masses.
- 41.Brown CJ, Lain S, Verma CS, Fersht AR, Lane DP. Awakening guardian angels: drugging the p53 pathway. Nature Reviews Cancer. 2009;9:862–73. doi: 10.1038/nrc2763. [DOI] [PubMed] [Google Scholar]
- 42.Efeyan A, et al. Induction of p53-dependent senescence by the MDM2 antagonist nutlin-3a in mouse cells of fibroblast origin. Cancer Res. 2007;67:7350–7. doi: 10.1158/0008-5472.CAN-07-0200. [DOI] [PubMed] [Google Scholar]
- 43.Kumamoto K, et al. Nutlin-3a activates p53 to both down-regulate inhibitor of growth 2 and up-regulate mir-34a, mir-34b, and mir-34c expression, and induce senescence. Cancer Res. 2008;68:3193–203. doi: 10.1158/0008-5472.CAN-07-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu CH, et al. Cellular senescence is an important mechanism of tumor regression upon c-Myc inactivation. Proc Natl Acad Sci U S A. 2007;104:13028–33. doi: 10.1073/pnas.0701953104. An elegant demonstration of the concept that elimination of an oncogene necessary for tumour maintenance, in this case MYC, may result in tumour regression associated to senescence.
- 45.Soucek L, et al. Modelling Myc inhibition as a cancer therapy. Nature. 2008;455:679–83. doi: 10.1038/nature07260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmitt CA, et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell. 2002;109:335–46. doi: 10.1016/s0092-8674(02)00734-1. [DOI] [PubMed] [Google Scholar]
- 47.Roninson IB. Tumor cell senescence in cancer treatment. Cancer Res. 2003;63:2705–15. [PubMed] [Google Scholar]
- 48.Cleator S, Parton M, Dowsett M. The biology of neoadjuvant chemotherapy for breast cancer. Endocr Relat Cancer. 2002;9:183–95. doi: 10.1677/erc.0.0090183. [DOI] [PubMed] [Google Scholar]
- 49.Roberson RS, Kussick SJ, Vallieres E, Chen SY, Wu DY. Escape from therapy-induced accelerated cellular senescence in p53-null lung cancer cells and in human lung cancers. Cancer Res. 2005:2795–803. doi: 10.1158/0008-5472.CAN-04-1270. [DOI] [PubMed] [Google Scholar]
- 50.te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 2002;62:1876–83. [PubMed] [Google Scholar]
- 51.Coppe JP, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–68. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Acosta JC, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–18. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 53.Kuilman T, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–31. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 54.Collado M, Serrano M. The power and the promise of oncogene-induced senescence markers. Nat Rev Cancer. 2006;6:472–6. doi: 10.1038/nrc1884. [DOI] [PubMed] [Google Scholar]
- 55.Dimri GP, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kurz DJ, Decary S, Hong Y, Erusalimsky JD. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci. 2000;113(Pt 20):3613–22. doi: 10.1242/jcs.113.20.3613. [DOI] [PubMed] [Google Scholar]
- 57.Yang NC, Hu ML. The limitations and validities of senescence associated-beta-galactosidase activity as an aging marker for human foreskin fibroblast Hs68 cells. Exp Gerontol. 2005;40:813–9. doi: 10.1016/j.exger.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 58.Shamma A, et al. Rb Regulates DNA damage response and cellular senescence through E2F-dependent suppression of N-ras isoprenylation. Cancer Cell. 2009;15:255–69. doi: 10.1016/j.ccr.2009.03.001. [DOI] [PubMed] [Google Scholar]