Hypoxia triggers Hedgehog-mediated tumor stromal interactions in pancreatic cancer (original) (raw)
. Author manuscript; available in PMC: 2014 Jun 1.
Abstract
Pancreatic cancer is characterized by a desmoplastic reaction that creates a dense fibroinflammatory microenvironment, promoting hypoxia and limiting cancer drug delivery due to decreased blood perfusion. Here we describe a novel tumor-stroma interaction that may help explain the prevalence of desmoplasia in this cancer. Specifically, we found that activation of HIF-1α by tumor hypoxia strongly activates secretion of the sonic hedgehog ligand SHH by cancer cells which in turn causes stromal fibroblasts to increase fibrous tissue deposition. In support of this finding, elevated levels of HIF-1α and SHH in pancreatic tumors were determined to be markers of decreased patient survival. Repeated cycles of hypoxia and desmoplasia amplified each other in a feed forward loop that made tumors more aggressive and resistant to therapy. This loop could be blocked by HIF-1α inhibition, which was sufficient to block SHH production and HH signaling. Taken together, our findings suggest that increased HIF-1α produced by hypoxic tumors triggers the desmoplasic reaction in pancreatic cancer, which is then amplified by a feed forward loop involving cycles of decreased blood flow and increased hypoxia. our findings strengthen the rationale for testing HIF inhibitors may therefore represent a novel therapeutic option for pancreatic cancer.
INTRODUCTION
Pancreatic cancer is a devastating disease with the majority of patients succumbing within one year of diagnosis (1). Surgical resection offers the only curative therapy but is an option for less than 20 % of patients and yields actuarial 5-year survival rates of only about 20% (2). Treatment failure due to local recurrence and hepatic metastases can occur within 1 to 2 years after surgery (3). Other treatment options including gemcitabine and erlotinib offer only a small survival advantage (4) and the overall 5 year survival rate for patients with pancreatic cancer across all stages remains 0.4 to 4 %, making pancreatic cancer one of the top causes of death from cancer in the Western world (5).
A characteristic of pancreatic cancer is extensive desmoplasia comprising a dense stromal fibroinflammatory reaction of fibroblasts, inflammatory cells and tumor vasculature (6). Desmoplasia leads to decreased blood supply, poor drug delivery (7) and hypoxia (8). Although hypoxia presents a particularly hostile environment for cell growth, cancer cells are able to adapt and survive by increasing the expression of genes responsible for anaerobic metabolism, cell survival, metastasis, and formation of new blood vessels (9). The cellular response to hypoxia is mediated through a rapid increase in the levels of the transcription factors hypoxia inducible factor-1 (HIF-1) and HIF-2. HIFs are heterodimers of inducible α and constitutive β subunits. The importance of HIF-1α to pancreatic cancer is directly demonstrated by the resistance to chemotherapy and radiation seen in pancreatic cancer cells constitutively expressing HIF-1α, and their increased in vivo tumorigenicity (8).
Recent evidence demonstrates that expression of sonic hedgehog (SHH) ligand contributes to the formation of desmoplasia in pancreatic cancer and that paracrine hedgehog (HH) signaling plays an important role in the communication between tumor and stroma cells and promotes tumorigenesis (10–13). HH signaling is initiated by binding of SHH ligand to the Patched-1 receptor (PTCH1) that relieves repression of the transducer protein Smoothened (SMO), thus triggering activation of the GLI family of transcription factors. Genes activated by GLI include GLI-1 itself, PTCH1 and depending on the cell type, genes upregulating cell proliferation, survival, angiogenesis and through SNAIL the epithelial-mesenchyme transition of metastasis (14).
Altered HH signaling has been implicated in the development of approximately 20–25% of all cancers and has been classified into two major types (15, 16). The first type includes mutations in HH network genes resulting in HH ligand-independent tumors, they include PTCH inactivating mutations, SMO activating mutations, or loss of RENKCTD11, a GLI antagonist, in medulloblastoma (16, 17). The second type is HH ligand-dependent tumors characterized by aberrant expression of HH ligand that is observed especially in cancers of the gastrointestinal tract (including pancreas), breast, colon and prostate (16, 18). In some cases the tumors have been proposed to respond to HH ligand in an autocrine manner as HH ligand has been found to be both produced by the tumor cells and to activate them (16). In contrast, others have described paracrine models where the HH ligand is produced by the tumor cells (HH-producing cell) but acts on the fibroblasts and stellate cells in the stroma (HH-responding cells) (10–13, 19).
SHH and downstream components of the HH signaling pathway have been shown to be present in precursor lesions and primary pancreatic tumors but not in normal pancreas, suggesting that hedgehog signaling may play a role in the initiation and progression of pancreatic cancer (20–22). This concept is supported by the finding that mouse transgenic models with SHH expressed in pancreatic epithelium, together with mutant KRAS, show an enhanced incidence of pancreatic intraepithelial neoplasia and increased lethality (23). Furthermore, expression of SHH in transformed primary pancreatic ductal epithelial cells in an orthotopic model of pancreatic cancer leads to increased desmoplasia (11). Inhibition of HH signaling was shown to enhance vascular density and delivery of chemotherapy in a mouse model of pancreatic cancer (24) and to inhibit tumor initiation and metastasis in orthotopic xenografts established from human pancreatic cancer cell lines (25).
While it has become clear that aberrant activation of HH signaling either by mutations or ligand overexpression can lead to cancer, the mechanisms responsible for regulating SHH expression in human cancer are poorly understood (26–28). We show here that hypoxia, acting through HIF-1α is responsible for the increase in SHH in pancreatic cancer cells. Moreover, we show a positive correlation between HIF-1α and SHH expression in human pancreatic cancer and in a retrospective study that both predict for decreased patient survival. In addition, we found that the SHH secreted by pancreatic cancer cells in hypoxia act in a paracrine manner to increase GLI activation, and the formation of collagen 1 and fibronectin, markers of desmoplasia by fibroblasts. The studies suggest that increased HIF-1α could be an initiating factor in the desmoplasia of pancreatic cancer which is amplified by cycles of decreased blood flow, increased hypoxia and further desmoplasia.
MATERIALS AND METHODS
Reagents
Anti-HIF-1α antibody was from BD Biosciences (Sparks, MD). Anti-SHH antibody used for western blotting was from Santa Cruz Biotechnology, Inc., (Santa Cruz, California). Anti-SHH antibody used for IHC and IF staining was from Abcam Inc., (Cambridge, MA), and was specific for SHH and did not react with IHH or DHH (Figure S1, A and B). Anti-actin (I-19) antibody was from Santa Cruz Biotechnology, Inc. Anti-FLAG (anti-DDK) antibody was from Origene (Rockville, MD). Anti-collagen 1 and anti-fibronectin antibodies used for IF and IHC were from Abcam Inc. SAG was from Enzo Life Sciences (Plymouth Meeting, PA). PX-478, a HIF-1α inhibitor (29) was synthesized by the MD Anderson Translational Chemistry Core Service. GDC-0449 was from Selleck Chemicals (Houston, TX).
Cells
All cells were obtained from the American Tissue Type Culture Collection (Manassas, VA). Panc-1, SU.86.86, Capan-2 and MiaPaCa-2 are human pancreatic cancer cells. NIH-3T3/GLI-luc (SHH-Light II) (30) is a mouse NIH-3T3 cell line stably transfected with a GLI-responsive Firefly luciferase reporter and a constituitive _Renilla_-luciferase expression vector. 293 EcR SHH (31) are human embryonic kidney HEK293 cells carrying a stably integrated murine SHH under ecdysone-inducible control. Cells were grown in humidified 95% air, 5% CO2 at 37 °C in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS). All cell lines were tested to be mycoplasma free using a PCR ELISA kit (Roche Diagnostics Inc, Indianapolis, IN). Cell line lineage was confirmed by the M.D. Anderson Cancer Center Characterized Cell Line Core Service.
Human Pancreatic Cancer Immunohistochemistry
A tissue microarray (TMA) was constructed from paraffin-embedded tumor blocks using an indexed manual tissue arrayer (Advanced EDM Automation, Poway, CA). When tissue was available, cases were triple punched with 1.0 mm core needles to include 2 tumor cores and 1 adjacent normal core. Only resected pancreatic adenocarcinoma, adenosquamous and carcinoma were used in this analysis (n= 129) though not all samples were evaluated for all markers. Hematoxylin and eosin stained slides were reviewed by a pathologist (GH) and desired regions marked and transferred to the donor paraffin blocks. The master TMA blocks were sectioned at 5μm thickness and water flotation used for tissue section transfer. TMA slides were dewaxed, rehydrated and subjected to heat induced epitope retrieval with EDTA solution for 30 minutes using a BondMax™ autostainer (Leica Microsystems, Inc, Bannockburn, IL). Endogenous peroxidase was blocked and the slide was incubated for 30 minutes with HIF-1α or SHH antibody (25ug/ml) and visualized by the Bond™ Polymer Refine Detection kit (Leica Microsystems) using diaminobenzidine chromogen as substrate. Immunohistochemical staining localization was evaluated in adenocarcinoma and in peritumoral stroma separately. The vasculature was not included in the stromal component. Staining intensity was scored blinded on a scale of 0 to 3 with a score of 3 indicating strong staining. In cases where staining was heterogeneous for either compartment, the higher intensity staining was scored. Survival information was missing for 4 of the 129 patient samples in the dataset, and 11 were still alive.
Microarray
Gene expression microarray analyses were performed on RNA from MiaPaca2 wild type cells transfected with Dharmacon SMARTpool siRNA against HIF-1α (M-004018-05) or control siRNA (D-001210-05) using DharmaFECT 2 (Dharmacon, Lafayette, CO). HIF-1α knockdown efficacy was determined by Western blotting (data not shown). RNA was isolated using the Qiagen RNAeasy kit (Qiagen, Valencia, CA), and microarray analysis was carried out on an Affymetrix Human U133 Plus 2.0 chip (Affymetrix, Santa Clara, CA). All correlations are significant below the Bonferroni cutoff of P=0.00217 (0.05/23 markers =0.00217).
Pentachrome staining
Pentachrome staining was carried out using the Russell-Movat Pentachrome Stain Kit (American MasterTech, catalog #KTRMP, Lodi, CA). Slides were deparaffinized with three 30min immersion in xylene, followed by two 2 minutes rehydration rinses through an alcohol series (100%, 95%, 70%), and rinsed in distilled water. Slides were placed in Verhoeff’s Elastic Stain for 15min, followed by a 5 minutes tap water rinse and distilled water rinse. Elastic fibers were differentiated in 2% ferric chloride for 2 minutes (or until elastic fibers are defined). Slides were rinsed in distilled water and placed in 5% sodium thiosulfate for 1 minute. Slides were rinsed in tap water for 5 minutes, placed in 3% glacial acetic acid for 3 minutes, and then moved to a 1% Alcian blue solution for 15 minutes. Slides were rinsed in tap water, followed by distilled water, and placed in crocein scarlet-acid fuchsin for 2 minutes. After three rinses of distilled water and five sips in 1% glacial acetic acid slides were placed in two changes of 5% phosphotungstic acid for 3 minutes each. Slides were again dipped in 1% glacial acetic acid and dehydrated in 3 changes of absolute alcohol. Slides were placed in alcoholic saffron for 15 minutes, dehydrated in absolute alcohol, followed by xylene, and coverslipped.
qRT-PCR
Total RNA was isolated using the RNeasy RNA isolation kit and RNase-Free DNase set (Qiagen, Valencia, CA). qRT-PCR was performed using either a human hedgehog pathway array or specific human/mouse SYBR® Green primers for selected genes (SABioscience, Frederick, MD). The experiments were performed using the RT2 first strand kit and RT2 qPCR master mix Reagents (SABioscience) and analyzed in triplicates on a 7300 real time PCR System (Applied Biosystems). Normalization and analyses were carried out using β2-microglobulin (B2M) as the internal reference by the 2−ΔΔ_CT_ method (32). Significance was determined by analysis of variance or Student’s t tests where appropriate.
Transfections and stable cell lines
MiaPaca2 and Panc-2 were stably transfected with shHIF-1α using the siPORTamine and lipofectamine transfection reagents respectively. Clones were selected by growing the cells in puromycin and HIF-1α expression was analyzed by Western blotting. siRNAs to human SHH, HIF-1α, polo-like-kinase (PLK), and the non-targeting siRNAs, On Target Plus 4 (OTP4) and scramble #5 (SMARTpool, Thermo Scientific Dharmacon, Lafayette, CO) were transfected into Panc-2 cells using the lipofectamine 2000 reagent. Lipofectamine RNAiMAX (invitrogen, Carlsbad, CA) was used to transfect siRNA into Panc1 cells before plating the cells on NanoCultureR plates. Myc-DDK-HHIP (Origene, catalog number RC206868) was transfected into Panc-1 cells using lipofectamine 2000.
Western Blotting
Cells were lysed in 20 m Tris, pH 7.4, 150 m NaCl, 1% Triton X-100, 2 m EDTA, 1 m DTT, and protease inhibitors for 30 min on ice and spun at 13,000 rpm in a microcentrifuge for 15 min at 4 °C. Proteins were separated using SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked in 5% milk in PBS with 0.1% Tween 20, incubated with the appropriate antibodies according to the manufacturer’s recommendation and subjected to enhanced chemiluminescence analysis.
Assays for HH pathway activation
Activation of the HH pathway in NIH-3T3/GLI-luc fibroblasts was measured in co-cultures with pancreatic cancer cells where NIH-3T3/GLI-luc cells were plated at high density (90 × 104 cells/well in 6 well plates) with or without MiaPaca shV or MiaPaca shHIF-1α (50 × 104 cells/well) in complete media. On the next day media was replaced with DMEM containing 0.5% serum and cells were placed in normoxia or hypoxia (1% O2) for 3 additional days. Luciferase activity in cell lysates was measured using the Dual-Glo luciferase assay system (Promega). To measure activation of the HH pathway in pancreatic cancer cells, cells were transiently transfected with Cignal™ HH reporter assay kit (SABiosciences) using the lipofectamine 2000 and siPORTamine transfection reagents for Panc-1 and MiaPaca-2 cells, respectively. Luciferase was measured 48 hrs post transfection. SHH was prepared from conditioned media of 293 EcR SHH cells plated at 70% confluency and grown for 1 day in normoxia in DMEM containing 10% FBS and ponasterone. The conditioned media was then centrifuged to remove any remaining cells. For 3D assays tumor cells and fibroblasts were co-cultured on NanoCultureR plates (SCIVAX.CORP Japan). NIH-3T3/GLI-luc (10×103 cells/well), and pancreatic cancer cells (15×103 cells/well) were plated in 0.5% medium and 1% Matrigel (BD Biosciences) for 5 days. For luciferase assay, 2 μl luciferin (of 15 mg/ml stock) were added directly to media and luciferase activity was measured in Xenogen IVIS Lumina (Caliper LifeSciences). For qRT-PCR, cells were collected using a multi channel pipet and RNA was isolated.
Immunofluorescence staining
NIH-3T3/GLI-luc cells were grown to confluence on chamber glass slides with or without pancreatic tumor cells in normoxia and hypoxia. One day after plating the medium was changed to DMEM containing 0.5% FBS for additional 24–48 hours. Samples were fixed in methanol/acetone at −20°C and subsequently stained with antibodies against fibronectin (1:250) and collagen I (1:500 dilution). Alexa Fluor 488 goat anti-rabbit (1:200) (Invitrogen) was used as a secondary antibody.
In vitro translation assay
Expression of SHH, IHH and DHH from Myc-DDK-tagged ORF cDNA clones (catalog numbers RC222175, RC213565 and RC206715 respectively; Origene Technologies, Rockville, MD) was done using the TNT Quick coupled transcription/translation system (Promega, Madison WI). Western blotting showed reaction of the SHH antibody with SHH and not IHH or DHH (Fig S7).
RESULTS
Tumor HIF-1α correlates with stromal SHH in human pancreatic cancer
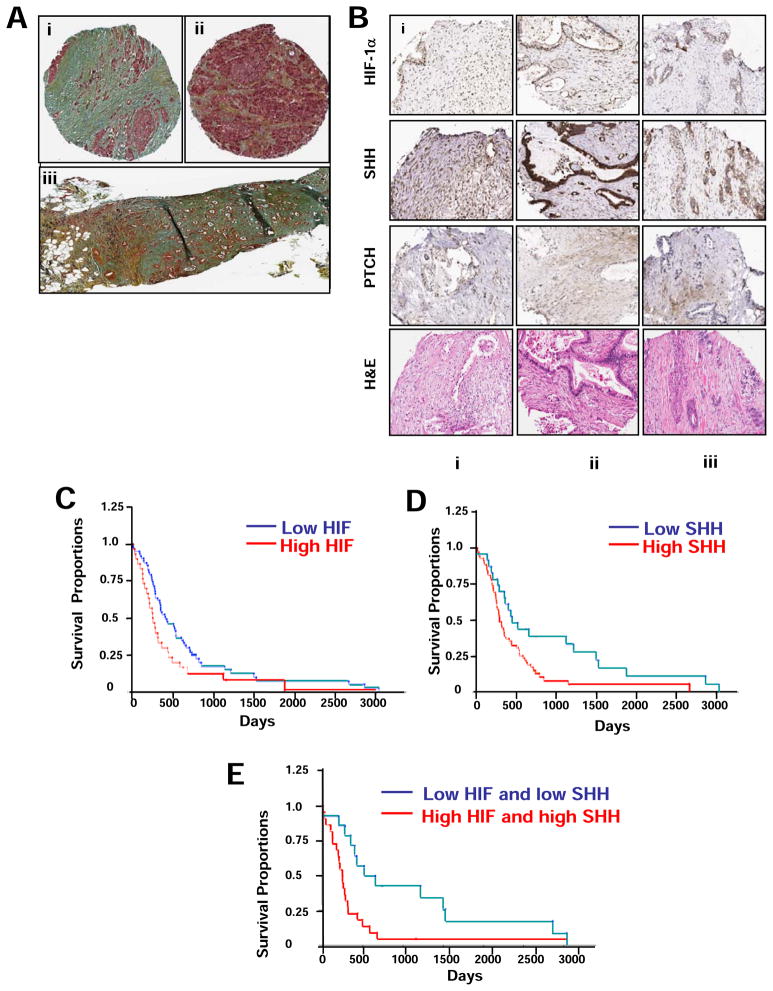
A representative immunohistochemical staining of desmoplasia in resected pancreatic ductal carcinoma is shown in Figure 1A illustrating the infiltrative tumor associated with large amounts of stroma (i) as compared to patient matched adjacent stroma with normal architecture and focal collagen bands (ii). Figure 1A iii shows metastatic pancreatic cancer with staining for elastin, collagen, mucin and infiltrative tumor. We tested the correlation between the expression of tumor nuclear HIF-1α as a marker of hypoxia, and stromal SHH as a marker for HH signaling, in pancreatic adenocarcinoma, ductal adenocarcinoma, and adenosquamous carcinoma biopsy cores scored on a tissue microarray using immunohistochemical analysis. High expression levels of SHH were detected in 63 of 98 (64%) evaluable samples, and high expression levels of HIF-1α were detected in 28 of 97 (29%) evaluable samples. Examples of staining for HIF-1α and SHH 1 in patient matched TMA cores of pancreatic adenocarcinoma cases with scoring in adenocarcinoma and stromal component are shown in Figures1B and S2. A positive correlation was found between tumor HIF-1α and stromal SHH expression (Spearman= 0.3254, P=0.0015). In addition, HIF-1α staining in the tumor positively correlated with HIF-1α staining in the stroma (Spearman= 0.5588, P<0.0001), and with stromal carbonic anhydrase IX (CAIX) staining (Spearman= 0.3187, P=0.0016) (Table 1). Other markers tested including PTCH, smooth muscle actin (SMA), and Ki-67, a marker of proliferation, Bruton’s Tyrosine Kinase (BTK), mothers against decapentaplegic (SMAD4), and RIO kinase 3 (RIOK3) did not correlate with either HIF-1α or SHH in cytoplasm or stroma (Figure 1B, and Table 1).
Figure 1. Immunohistochemical staining of desmoplasia, and of HIF-1α and SHH showing a correlation between elevated expression and decreased patient survival in pancreatic tumors.
A, Representative pentachrome staining of human pancreatic adenocarcinoma showing; (i) infiltrative tumor (pink staining) associated with large amounts of stroma (blue, green); (ii) the adjacent tumor normal pancreas interface showing islet cell hyperplasia and acini (pink) with bands of stroma (yellow, blue); (iii) metastatic pancreatic adenocarcinoma with staining scored on a scale of 1 = minimal to 5 =marked, showing elastin (black)= 1, collagen (yellow) = 3, mucin (blue) = 4 and tumor infiltrate (brown) = 4. B, IHC staining of HIF-1α, SHH and PTCH-1 in patient matched TMA cores of pancreatic adenocarcinoma cases with scoring and analyses in adenocarcinoma and stromal component. High power images are shown in Figure S1. Scores are on a scale of 0–3 with 0 and1 as low, 2 medium, and 3 high, in tumor (T) and stroma (S). HIF-1α scored (i) T=3, S=3 (ii) T=2, S=1 and (iii) T=1, S=1. SHH was scored similarly with (i) T=2, S=2; (ii) T=3, S=1 and (iii) T=1, S=1. PTCH showed predominant staining in stroma component with (i) T=3, S=1; (ii) T=1, S=2 and (iii) T=0, S=2. C, D and E, Kaplan-Meier patient survival analyses of tumor HIF-1α (nuclear) and SHH (cytoplasmic) separated into low (< 2) and high (≥2) staining scores showing C, high HIF-1α (30/93 patients) is associated by Wilcoxan signed-rank test with decreased patient survival (p = 0.012); D, high SHH (68/91) is associated with decreased survival (p = 0.043); and E, the combination of high HIF-1α and high SHH (22/36) compared to low HIF-1α, low SHH (14/36) is highly associated with decreased patient survival (p = 0.005).
Table I.
A spearman correlation analysis of IHC staining of patients tumor samples
| Marker | HIF-1α Stroma | HIF-1α Tumor | CAIX Stroma | Shh Tumor | Shh Stroma | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spearman | P-Value | Spearman | P-Value | Spearman | P-Value | Spearman | P-Value | Spearman | P-Value | |
| HIF-1α Stroma | ||||||||||
| HIF-1α Tumor | 0.5588 | 2.71E-9 | ||||||||
| CAIX Stroma | 0.4038 | 2.82E-5 | 0.3187 | 0.0016 | ||||||
| SHH Tumor | 0.3547 | 0.0004 | 0.3254 | 0.0015 | 0.1125 | 0.2802 | ||||
| SHH Stroma | 0.3972 | 0.0001 | 0.2791 | 0.0065 | 0.0934 | 0.3654 | 0.3023 | 0.0031 | ||
| PTCH Tumor | 0.1381 | 0.4512 | 0.15 | 0.4124 | −0.2597 | 0.1512 | 0.54 | 0.7692 | −0.1095 | 0.5506 |
| PTCH Stroma | −0.093 | 0.6127 | −0.1349 | 0.4617 | 0.093 | 0.6127 | 0.0221 | 0.9045 | −0.0498 | 0.7865 |
| KI-67 Nuclear | 0.2956 | 0.0052 | 0.2803 | 0.0082 | 0.0854 | 0.4287 | 0.0583 | 0.5940 | 0.1779 | 0.1013 |
| SMA Stroma | 0.2161 | 0.2124 | 0.0695 | 0.6915 | 0.1778 | 0.3068 | 0.0876 | 0.6224 | 0.2135 | 0.2253 |
| BTK Tumor | 0.453 | 0.0081 | 0.3782 | 0.03 | −0.0322 | 0.8586 | 0.1873 | 0.3046 | 0.4472 | 0.0103 |
| SMAD4 Tumor | −0.3187 | 0.0545 | −0.1458 | 0.3894 | −0.302 | 0.0693 | −0.3083 | 0.0673 | −0.3274 | 0.0513 |
High levels of tumor HIF-1α and stromal SHH are associated with decreased patient survival
To examine the effect of HIF-1α and SHH expression on survival, tumor nuclear HIF-1α and cytoplasmic SHH immunohistochemistry (IHC) scores were divided into high (≥2.0) and low (< 2.0). We found that a high HIF-1α nuclear score was associated with significantly shorter survival (median 253 compared to 405 days, P=0.03 (log rank), n=93) (Figure 1C). Similarly, a high SHH score was also associated with significantly shorter survival (median 285 compared to 445 days, P = 0.0087 (log rank), n=91) (Figure 1D). When patients with both high tumor nuclear HIF-1α and stromal SHH scores were compared with patients with both low tumor nuclear HIF-1α and stromal SHH scores, significantly shorter survival was observed (median 248 days compared to 587 days, P = 0.0032) (Figure 1E). No significant association was found between PTCH expression in either tumor or stroma, and survival (Figure S3). A Cox proportional hazards model was used to study the effect of multiple covariates simultaneously. When cytoplasmic SHH and nuclear HIF-1α expression levels are both in the model, a significant effect of cytoplasmic SHH expression on survival was found, with hazard ratio (HR) = 1.78 (p = 0.0016), whereas the dichotomized nuclear HIF-1α had no significant effect on survival (p=0.12). Because the Cox proportional hazards model effectively measures the effect of one variable at fixed levels of the other variables in the model, this might suggest that the apparent effect of HIF-1α expression alone on patient survival can be attributed to elevated SHH expression due to a positive correlation between the two factors. In univariate analysis, the conventional prognostic markers, pT Category, T Staging and the histologic grade are significant (P<0.05) while pN category and M Staging are marginally significant for overall survival (Table S1 and Figure S4).
Hypoxia induces SHH expression in pancreatic cancer cells
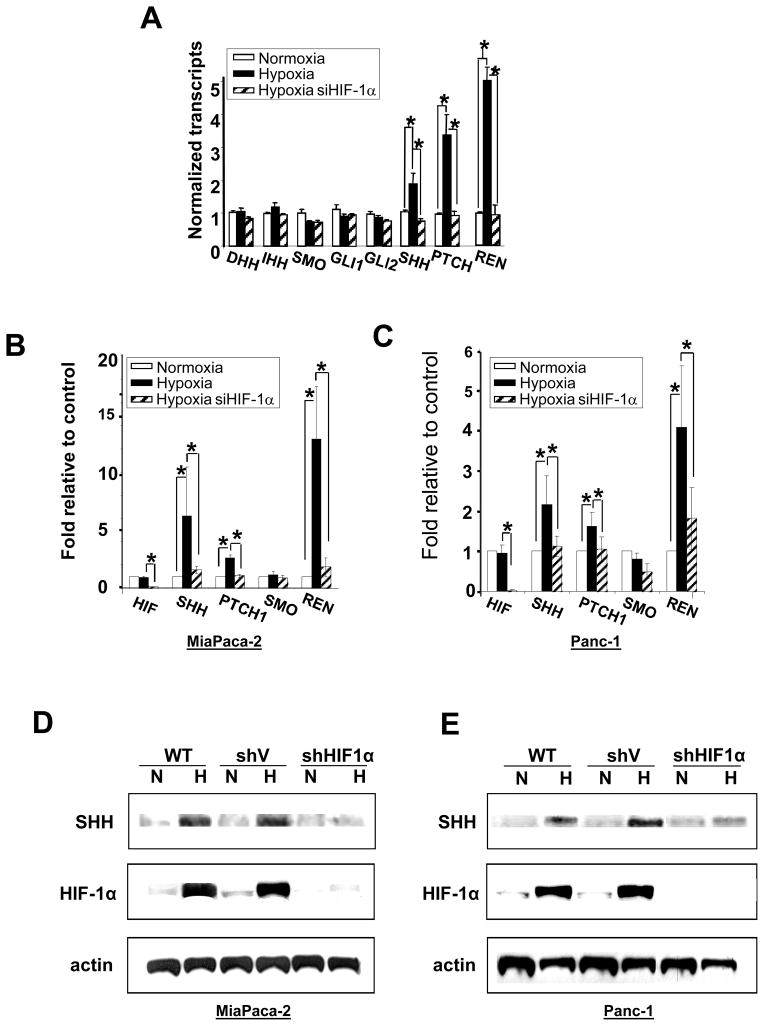
To test the hypothesis that HIF-1α/hypoxia may be responsible for mediating the increase in SHH we conducted a transcriptome microarray study using MiaPaCa-2 pancreatic cancer cells comparing genes transcribed in normoxia and 24 hours hypoxia (1% O2), and using siRNA to HIF-1α to determine which genes were HIF-1α-dependent. We found three HH pathway genes that showed significant hypoxia/HIF-1α dependent induction; SHH ligand, and two suppressors of the pathway; PTCH1 and RENKCTD11 (Figure 2A). qRT-PCR using a pathway array profiling the expression of 84 genes involved in hedgehog signaling confirmed the increased expression of SHH and RENKCTD11 in hypoxia in MiaPaCa-2 cells, while PTCH1 showed only a small although consistent increase in hypoxia (Table S2). Further qRT-PCR validation confirmed an increase in SHH and RENKCTD11 in the MiaPaca2, Panc-1, Capan-2 and Su.86.86 pancreatic cancer cell lines (Figure 2, B and C, and Figure S5, A and B).
Figure 2. Pancreatic cancer cells show increased HIF-1α-dependent expression of SHH, PTCH and RENKCTD11 in hypoxia.
A. Microarray transcriptome data from MiaPaCa-2 cells exposed to normoxia for 24 hr (white bars), hypoxia for 24 hr (black bars); or hypoxia and siHIF-1α for 24 hours (hatched bars). B. qRT-PCR validation in MiaPaCa-2 and C. Panc-1 cells. Bars are the same as in A. All values are the mean of 3 determinations, * is p < 0.05 compared to appropriate control. D, E, Western blots showing SHH and HIF-1α protein expression following 24 hours exposure of D. MiaPaca2 cells or E. Panc-1 cells to (N) normoxia or (H) hypoxia (1% O2) in wild type (WT), empty vector (shV) or shHIF-1α stably transected cells. F. Immunofluorescence staining for SHH expression (red) in MiaPaca-2 cells exposed to normoxia or hypoxia for 24 hours. Cell nuclei are stained with DAPI (blue).
Western blotting and immunofluorescence staining confirmed a hypoxia dependent increase in SHH protein (Figure 2, D, E and F) and this was shown by shRNA knockdown to be dependent on HIF-1α (Figure 2, D and E). A time course analysis of the increase in SHH showed accumulation of SHH in hypoxia over a period of 3 days in both MiaPaCa-2 and Panc-1 cells (Figure S4, C and D). Even though we observed consistent increase in RENKCTD11 RNA transcript levels in hypoxia we were not able to detect increased RENKCTD11 protein expression using Western blot or IHC analysis.
Hypoxia-induced SHH secretion by tumor cells results in paracrine SHH/GLI-1 activation in fibroblasts
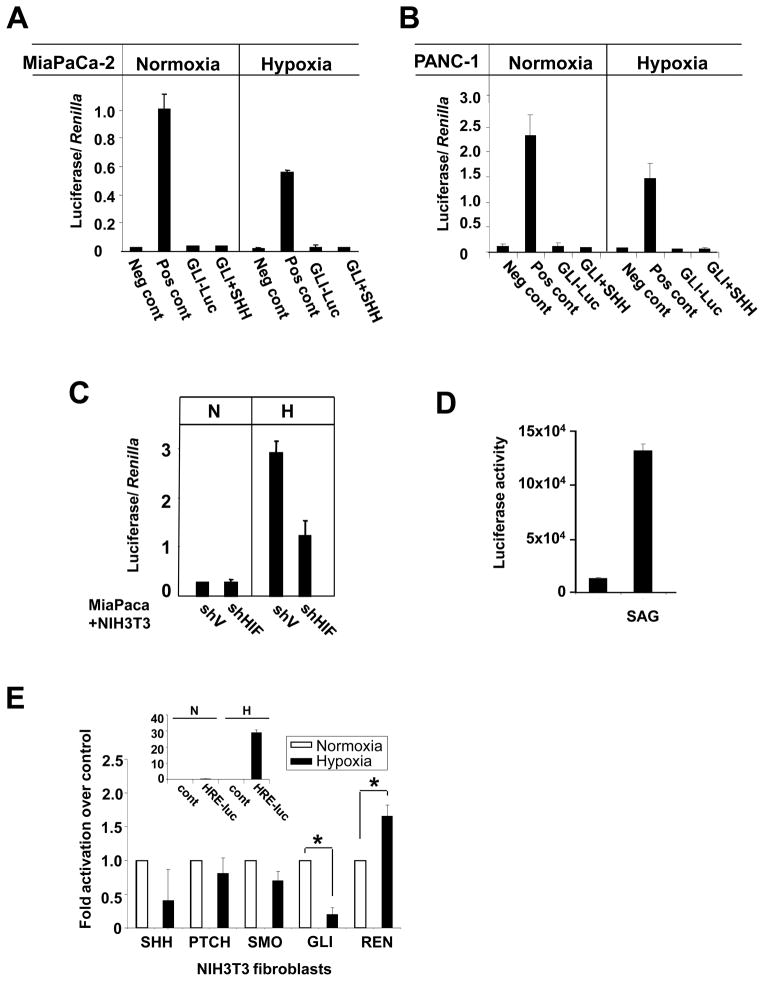
Despite the hypoxic induction of SHH, MiaPaca-2 and Panc-1 cells transfected with a GLI-luciferase reporter of HH activation showed no increase in GLI activity in either normoxia or hypoxia suggesting the absence of autocrine stimulation of the cancer cells by SHH (Figure 3A and B).
Figure 3. Hypoxia stimulates SHH formation by tumor cells and paracrine GLI-1 activation in fibroblasts.
A. A HH reporter assay in MiaPaca-2 and B. Panc-1 cells transiently transfected with a mixture of constitutively expressing Renilla luciferase and either a non-inducible Firefly luciferase reporter (Neg cont), a constitutively expressing Firefly luc reporter (Pos cont), or an inducible GLI-luc reporter (GLI-luc). Cells were treated with 293-SHH conditioned media (SHH), incubated for 16 hours in normoxia or hypoxia and assayed using a dual Luc assay. Data are presented as mean ± SD and normalized to Renilla luciferase. C. Luciferase activity in NIH-3T3/GLI-luc fibroblasts co-cultured in normoxia or hypoxia with MiaPaca-2 cells expressing either control vector (shV) or shRNA for HIF-1α (shHIF). D. Luciferase activity in NIH-3T3/GLI-luc treated with SAG in normoxia. E. RT-PCR showing no increase in transcript levels of HH components in normoxia or hypoxia in NIH-3T3 fibroblasts. Inset, hypoxia-induced activation of HIF activity in NIH-3T3 cells transfected with HRE-luc reporter.
Typically, the role of HH signaling in development is mediated by paracrine effects on mesenchymal cells. In addition, it has been shown that tumors overexpressing HH ligands activate the signaling pathway in neighboring stroma cells (10–13, 19). Hence, we next tested whether SHH secreted by pancreatic cancer cells could activate the HH pathway in fibroblasts. A 2D co-culture of MiaPaCa-2 and NIH-3T3/GLI-luc cells showed a HIF-1α dependent increase in GLI1 reporter activity in hypoxia but not normoxia (Figure 3C), while addition of SAG, a benzothiophene compound known to activate the HH pathway, stimulated the GLI reporter in NIH-3T3 under normoxic conditions (Figure 3D). RT-PCR analysis to test whether NIH-3T3 fibroblasts themselves upregulate the expression of HH pathway components in hypoxia showed no increase in SHH, PTCH, SMO, HHIP and GLI transcripts levels, (Figure 3E) despite the increase in HIF activity during hypoxia (Figure 3E inset).
HIF-1α- and SHH-dependent paracrine activation of HH pathway in a 3D co-culture system
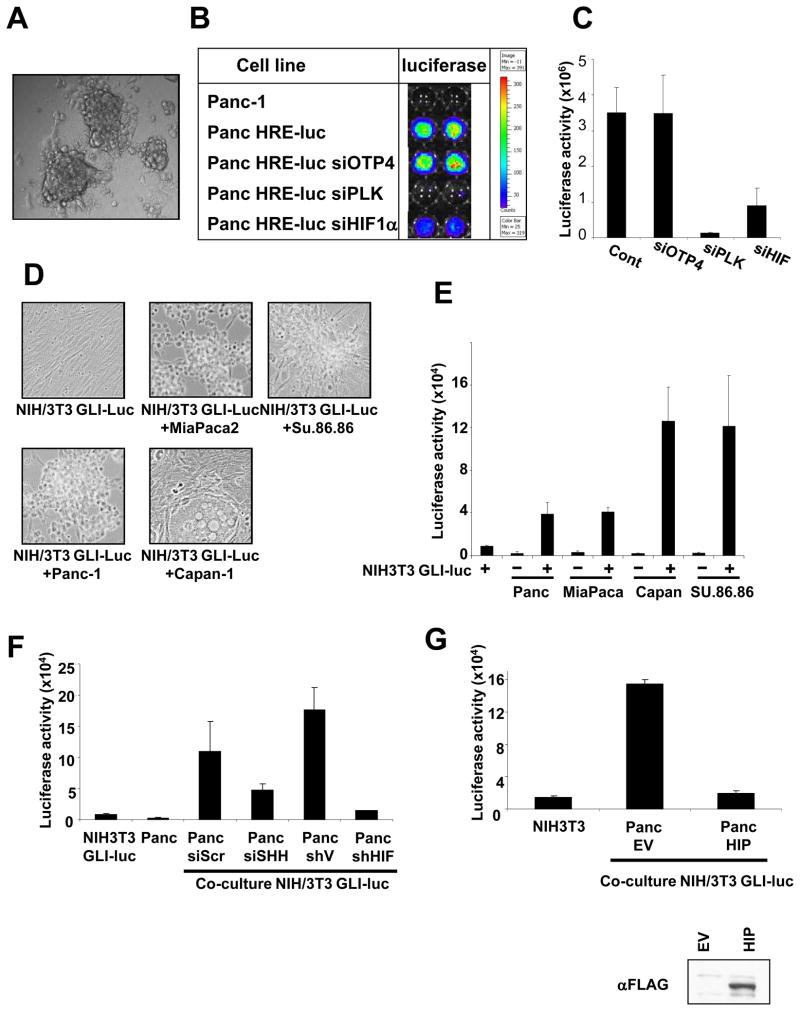
To further elucidate the mechanism of HH signaling in pancreatic cancer we used a 3D culture system which more closely simulate conditions of in vivo tumor growth. Initial characterization of the system was done with Panc-1 cells stably expressing a luciferase reporter construct under the control of a hypoxia response element (Panc HRE-luc). Panc-1 HRE-luc cells grown for 5 days in air on a 3D nano-matrix form spheres (Figure 4A) with a hypoxic core and upregulate HIF-1α activity as demonstrated by activation of the HRE-luc reporter (Figure 4B and C). Transfection of siHIF-1α inhibited the luciferase activity in the spheres (Figure 4B and C). To study HH activation in 3D co-cultures of tumor cells and fibroblasts we plated NIH-3T3/GLI-luc cells together with either Panc-1, MiaPaca-2, Capan-2 or Su.86.86 for 5 days during which they continued to proliferate and assembled into 3D structures consisting of both pancreatic cancer cells and fibroblasts (Figure 4D). GLI transactivation measured by luciferase expression was only found when the NIH-3T3/GLI-luc cells were co-cultured with Panc-1, MiaPaca-2, Capan-2 or Su.86.86 pancreatic cancer cells, but not when the NIH-3T3/GLI-luc cells were cultured alone (Figure 4E).
Figure 4. A HIF1-α- and SHH-dependent activation of hedgehog signaling in fibroblasts grown in 3D co-cultures with pancreatic cancer cells.
A. Image of hypoxic spheres formed by Panc-1 HRE-luc cells grown on 3D nano plates. B. An image of HRE-luc activity in duplicate 3D wells of Panc-1 and Panc-1 HRE-luc cells transfected with the non-targeting siRNA, siOTP4, siRNA to Polo-Like Kinase, PLK, which kills the cells, or siRNA to HIF-1α. On the right, a bar graph quantifying the luciferase counts. C. Quantitation of the luciferase activity of Panc-HRE-luc cells shown in B. D. Images of NIH-3T3/GLI-luc cells grown on 3D plates alone or in co-culture with Panc-1, MiaPaca-2, Capan-1 and Su.86.86 cells. E. Luciferase activity of NIH-3T3/GLI-luc cells grown on 3D plates with or without Panc-1, MiaPaca-2, Capan-1 and Su.86.86 cells. F. GLI-luc activity of NIH-3T3/GLI-luc fibroblasts co-cultured on 3D plates with Panc-1 cells transfected with siScr, siSHH, shV or shHIF-1α. G. GLI-luc activity of NIH-3T3/GLI-luc fibroblasts co-cultured on 3D plates with Panc-1 cells transfected with empty vector (Panc EV) or HIP (Panc HIP). Below an anti-FLAG Western blot showing the expression of HIP in the transfected Panc-1 cells.
Next, we confirmed that activation of GLI in NIH-3T3 fibroblasts co-cultured with pancreatic tumor cells is mediated by HIF-1α. We used Panc-1 cells that stably express shHIF-1α and that have undetectable levels of HIF-1α as compared to shVector cells (Figure 2D). GLI-luc expression in the NIH-3T3 fibroblasts was decreased when they were co-cultured with Panc-1 shHIF1-α cells as compared to Panc-1 shVector cells (Figure 4F). In addition, RT-PCR analysis showed that expression of HHIP and PTCH, two HH target genes, was reduced in NIH3T3 co-cultured with Panc cells transfected with siHIF-1α as compared to those transfected with siScr, suggesting that GLI activation in fibroblasts is dependent on expression of HIF-1α in the tumor cells (Figure S6).
To test whether activation of fibroblasts was mediated by SHH secreted from the tumor cells we used siRNA to SHH. Transfection of SHH targeted siRNA into Panc-1 cells reduced the level of SHH transcript by about 60% (as measured by RT-PCR) and decreased their ability to induce GLI transactivation in NIH-3T3/GLI-luc compared to transfection of a non-targeting siScr (Figure 4F). Overexpression by transfection of Panc-1 cells with the cDNA for the HH interacting protein, HIP, a regulatory component of the HH signaling pathway that localizes on the cell membrane (33) also inhibited GLI activation in NIH-3T3/GLI-luc cells (Figure 4G). In addition, expression of HHIP and PTCH was reduced in fibroblasts co-cultured with Panc-1 cells overexpressing HHIP (Figure S5). These results confirm that the SHH formed by pancreatic cancer cells is involved in mediating HH activation in fibroblasts.
HIF-1α-dependent expression of SHH by pancreatic cancer cells induces a desmoplastic reaction in fibroblasts
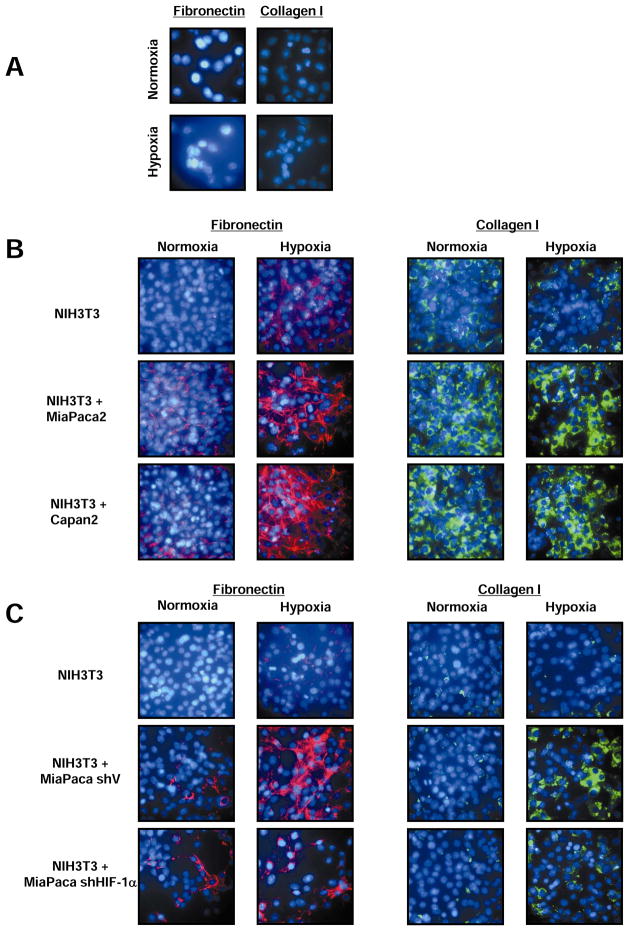
Expression of SHH has been reported to contribute to the formation of desmoplasia in pancreatic cancer (11). We therefore tested whether hypoxia and HIF-1α-induced formation of SHH by pancreatic cancer cells can induce a desmoplastic reaction in cultured fibroblasts. We performed immunofluorescence (IF) analysis of the production of two extracellular matrix proteins, fibronectin and collagen I, both of which are well characterized components of desmoplasia. There was minimal to no immunofluorescence staining of the tumor cells themselves (Figure 5A). However, in co-cultures of NIH-3T3 cells with MiaPaca2 or Capan2 cells, immunostaining for both fibronectin and collagen was stronger in hypoxia compared to normoxia (Figures 5B). The requirement for HIF-1α and SHH expression by pancreatic cancer cells for the desmoplastic reaction was demonstrated by decreased fibronectin and collagen I staining in co-cultures of NIH-3T3 cells with MiaPaca2 cells stably transfected with shHIF-1α or siSHH, compared to MiaPaca2 cells expressing shVector or siSCR, respectively (Figure 5C and S7).
Figure 5. SHH formation by pancreatic cancer cells in hypoxia induces a desmoplastic reaction in fibroblasts.
A. Absence of collagen I and fibronectin immunostaining in MiaPaca-2 cells in normoxia and hypoxia. B. Fibronectin and collagen I immunostaining in NIH-3T3 fibroblasts co-cultured with MiaPaca-2 or Capan-2 in normoxia and hypoxia. C. Fibronectin and Collagen I immunostaining in NIH-3T3 fibroblasts co-cultured with MiaPaca-2 transfected with empty vector (shV) or shRNA to HIF1-α (shHIF) in normoxia and hypoxia.
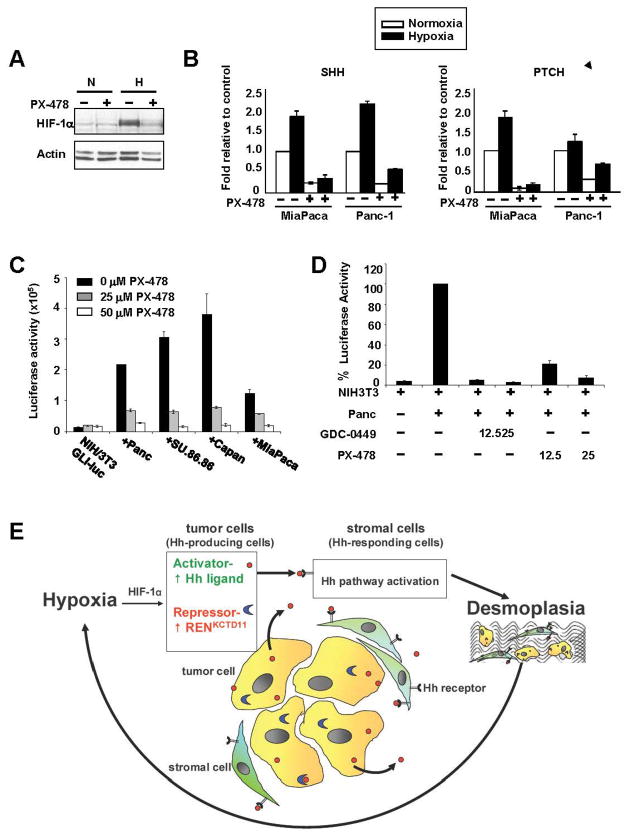
A HIF-1α inhibitor, PX-478, inhibits hedgehog signaling
Inhibiting HH signaling is an attractive approach for therapy of cancers where HH is aberrantly regulated. Therefore, we investigated whether it is possible to block the formation of HH pathway components in the tumor cells using the HIF inhibitor PX-478 (Figure 6A) (34–35). Treatment with PX-478 of Panc-1 and MiaPaca-2 cells decreased the transcript levels of SHH and PTCH in both normoxia and hypoxia (Figure 6B). In addition, PX-478 treatment of 3D co-cultures of NIH-3T3/GLI-luc cells with either Panc-1, Su.86.86, Capan-2 or MiaPaca2, resulted in complete inhibition of GLI activation (Figure 6C). Furthermore, comparing the effects of PX-478 and the SMO selective antagonist GDC-0449, in the 3D system revealed that both HIF-1α and SHH drive HH signaling in the stroma (Figure 6D). This provides a proof of concept that HIF inhibitors can be used to inhibit pancreatic cancer growth by inhibiting SHH production by hypoxic cancer cells.
Figure 6. A HIF-1α inhibitor, PX-478, inhibits hedgehog pathway activation and could break the cycles of hypoxia and desmoplasia.
A. Western blot analysis showing inhibition of HIF-1α in MiaPaca 2 cells treated with 50 μM PX-478 for 24 hours in normoxia (N) and hypoxia (H). B. qRT-PCR analysis of SHH and PTCH expression in MiaPaca-2 and Panc-1 cells treated with 50 μM PX-478 for 24 hours in normoxia (white bar) or hypoxia (black bar). C. PX-478 inhibits GLI-1 transactivation in 3D co-cultures of NIH-3T3 fibroblasts and pancreatic cancer cells. D. Inhibition of GLI-luc activity by PX-478 and GDC0449, selective SMO antagonist, in 3D co-cultures of Panc-1 and NIH-3T3/GLI-luc cells. E. The vicious cycle of hypoxia, HIF-1α and HH signaling in pancreatic cancer.
DISCUSSION
Pancreatic cancer is one of the most hypoxic solid tumors and HIF-1α levels are elevated in human primary pancreatic cancer as compared to normal pancreas (8, 35–37). Our study describes a new mechanism that ties hypoxia with the elevated expression of SHH and the desmoplasia commonly seen in pancreatic cancer. We show that hypoxia, acting through elevated HIF-1α, increases the expression of SHH in pancreatic cancer cells and that patients with high expression of HIF-1α and SHH show decreased survival. We also found that in hypoxia the SHH produced by the tumor cells, acts in a paracrine manner on fibroblasts to increase desmoplasia. We propose a new model of tumor-stroma interaction whereby multiple cycles of hypoxia, HIF-1α, HH signaling, and desmoplasia render pancreatic cancer more aggressive and resistant to therapy.
Immunohistochemical staining of human pancreatic tumors revealed a significant positive correlation between hypoxia measured by expression of HIF-1α and carbonic anhydrase IX, a downstream target of HIF-1α, in the tumor and expression of SHH in the stroma. Both high HIF-1α and high SHH scores were associated with a significantly shorter patient survival, and the outcome of combining these two markers (high HIF-1α and high SHH vs low HIF-1α and low SHH) was a highly significant prediction for decreased patient survival. No correlation was found with other markers tested including PTCH, KI-67- a proliferation marker, vimentin or SMA. The later two markers stained the stroma indiscriminately. Our results conflict with a recent report that high expression of SMA in the stroma correlates with worse prognosis (38).
We showed that in pancreatic tumor cells hypoxia increased the expression of SHH in a HIF-1α-dependent manner. Using a HH pathway specific array, we confirmed that SHH, PTCH and RENKCTD11 transcripts are modified in hypoxia. Moreover, we identified the HRE core sequence (G/A)CGTG, based on 108 core HREs found in 70 known HIF1α target genes (39) in the promoter regions (positions relative to transcriptional start) of SHH (759, 503); RENKCTD11 (491, 243, 186) and PTCH1: (272, 916). Noteworthy, hypoxia has previously been reported to increase SHH expression and induce a HH response in ischemic heart models (40–44).
Despite the fact that the NIH-3T3 fibroblasts responded to SHH by activating GLI, they themselves had low level of SHH transcript and did not induce expression of SHH in hypoxia even though they expressed active HIF-1α in hypoxia as determined by HRE-luc reporter assays. At the moment the full mechanism underlying HIF-1α induced expression of SHH and PTCH in pancreatic cells but not in fibroblasts is not fully elucidated. However, we believe that the differential activation of the Hh pathway observed is partly attributable to the experimental settings, eg 2D versus 3D. In 2D monolayer cultures cells are homogenously exposed to hypoxia, thus likely exhibit a homogenous response and a substantial transcriptional increase of Hh target genes that can be easily detected in bulk experiments. In 3D co-culture conditions, tumor cells and fibroblasts form spheroids in which cell are exposed to a gradient of hypoxia, thus Hh activation might be patchy and exhibited only by the hypoxic fraction of cells in the spheroid, therefore accounting for the modest increase of the luciferase activity and for the lack of detectable transcriptional changes assessed by the bulk RT PCR experiments.
Our finding that SHH acts in a paracrine fashion is in accord with recent evidence showing that in various cancers, including pancreatic ductal adenocarcinoma, HH ligands fail to activate signaling in tumor epithelial cells but do activate fibroblasts in the stromal microenvironment (10–13, 19). Paracrine HH signaling has been shown to support pro-tumorigenic communication between tumor cells and fibroblasts in the stroma and to promote tumor growth (16, 27). Recent work suggests that pancreatic tumor cells are not competent to transduce HH signaling because they express oncogenic KRAS. Based on these studies KRAS suppresses ciliogenesis and therefore pancreatic tumor cells are devoid of primary cilia which are required for HH signaling (10, 45, 46). In another study it was suggested that KRAS acts through dual-specificity tyrosine phosphorylation regulated kinase 1A (DYRK1B) and potentially other factors to block HH activation (46).
HH signaling is known to induce fibroblasts to produce extracellular proteins such as fibronectin and collagen I involved in desmoplasia (11). We showed that hypoxia via HIF-1α increases the expression of SHH in the pancreatic cell lines thus activating the HH pathway and desmoplasia in fibroblasts (Figure 5). These results suggested that in pancreatic cancer, hypoxia and HIF-1α increase desmoplasia by upregulating the formation of SHH by pancreatic cancer cells, which then stimulates HH signaling and collagen I and fibronectin formation by stroma fibroblast cells.
Inhibiting HH signaling is an attractive approach for therapy of cancers where HH is aberrantly regulated and was shown to enhance delivery of chemotherapy in a mouse model of pancreatic cancer (24). So far only SMO inhibitors have been tested in humans and have produced promising anti-tumor responses in early clinical studies (47–49). However, resistance to SMO inhibition has been shown to rapidly occur in medulloblastma patients due to mutations of SMO that do not affect HH signaling but disrupt the ability of the GDC-0449 inhibitor to bind SMO (50). An alternative approach, and one that would not be susceptible to this kind of resistance, is to inhibit the production of HH ligand by the tumor cell, for example by using a HIF-1α inhibitor that will prevent the hypoxia induced fibrosis caused by the response of fibroblasts to sHH. Moreover, since induction of SHH is only one of HIF’s pleiotropic effects, this further validates HIF as a promising target for cancer therapy. We have shown that the HIF-1α inhibitor PX-478, can inhibit GLI activation in fibroblasts co-cultured with pancreatic cancer cells (Figure 6), strengthening the notion that HIF-1α inhibitors may be beneficial for hypoxic and HH ligand-dependent tumors such as pancreatic and prostate cancers (32).
Based on our results, we propose the vicious cycle model of pancreatic cancer (Figure 6E) in which tumor hypoxia leads to increased SHH and desmoplasia which in turn further reduces blood supply to the tumor thus further augmenting tumor hypoxia. Repeating cycles of hypoxia and desmoplasia may be responsible for the highly hypoxic and fibrotic characteristics of pancreatic cancer. Breaking the cycle, for example with a HIF-1α inhibitor, provides an alternative approach for treatment of HH ligand-dependent cancers and may also help to enhance delivery of chemotherapy.
Supplementary Material
1
2
3
4
Acknowledgments
This work was supported by grants CA0163541, 098920, 095060, 017094, 16672-35, CA109552, 095920, and 126577 from the National Cancer Institute, and by gifts from the Perot Foundation and the Katz Family foundation. We also thank the MD Anderson siRNA Screening Service for developing the 3D culture assay system.
Footnotes
Conflict of Interest Disclosure: GP owns stock in the Oncothyreon whose agent PX-478 was used in this research.
References
- 1.Hezel AF, Bardeesy N. Prognostic markers in pancreatic ductal adenocarcinomas. Cancer Biol Ther. 2008;7:1360–61. doi: 10.4161/cbt.7.9.6955. [DOI] [PubMed] [Google Scholar]
- 2.Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 3.Griffin JF, Smalley SR, Jewell W, Paradelo JC, Reymond RD, Hassanein RE, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990;66:56–61. doi: 10.1002/1097-0142(19900701)66:1<56::aid-cncr2820660112>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Mahalingam D, Kelly KR, Swords RT, Carew J, Nawrocki ST, Giles FJ. Emerging drugs in the treatment of pancreatic cancer. Expert Opin Emerg Drugs. 2009;14:311–28. doi: 10.1517/14728210902972502. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Siege R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 6.Pandol S, Edderkaoui M, Gukovsky I, Lugea A, Gukovskaya A. Desmoplasia of pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2009;7:S44–47. doi: 10.1016/j.cgh.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6:1186–97. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 8.Koong AC, Mehta VK, Le QT, Fisher GA, Terris DJ, Brown, et al. Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys. 2000;48:919–22. doi: 10.1016/s0360-3016(00)00803-8. [DOI] [PubMed] [Google Scholar]
- 9.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–80. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 10.Baile JM, Mohr AM, Hollingsworth MA. Sonic hedgehog paracrine signaling regulates metastasis and lymphangiogenesis in pancreatic cancer. Oncogene. 2009;28:3513–25. doi: 10.1038/onc.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T, et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res. 2008;14:5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian H, Callahan CA, DuPree KJ, Darbonne WC, Ahn CP, Scales SJ, et al. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc Natl Acad Sci U S A. 2009;106:4254–59. doi: 10.1073/pnas.0813203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–10. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 14.Merchant AA, Matsui W. Targeting Hedgehog--a cancer stem cell pathway. Clin Cancer Res. 2010;16:3130–40. doi: 10.1158/1078-0432.CCR-09-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katoh Y, Katoh M. Hedgehog target genes: mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr Mol Med. 2009;9:873–86. doi: 10.2174/156652409789105570. [DOI] [PubMed] [Google Scholar]
- 16.Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sc. 2009;30:303–12. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Di Marcotullio L, Ferrett E, De Smaele E, Argenti B, Mincione C, Zazzeroni F, et al. REN(KCTD11) is a suppressor of Hedgehog signaling and is deleted in human medulloblastoma. Proc Natl Acad Sci U S A. 2004;101:10833–38. doi: 10.1073/pnas.0400690101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–51. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 19.Fan L, Pepicelli CV, Dibble CC, Catbagan W, Zarycki JL, Laciak R, et al. Hedgehog signaling promotes prostate xenograft tumor growth. Endocrinology. 2004;145:3961–70. doi: 10.1210/en.2004-0079. [DOI] [PubMed] [Google Scholar]
- 20.Feldmann G, Habbe N, Dhara S, Bish S, Alvarez H, Fendrich V, et al. Hedgehog inhibition prolongs survival in a genetically engineered mouse model of pancreatic cancer. Gut. 2008;57:1420–30. doi: 10.1136/gut.2007.148189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kayed H, Kleeff J, Osman T, Keleg S, Buchler MW, Friess H. Hedgehog signaling in the normal and diseased pancreas. Pancreas. 2006;32:119–29. doi: 10.1097/01.mpa.0000202937.55460.0c. [DOI] [PubMed] [Google Scholar]
- 22.Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasca di Magliano M, Sekine S, Ermilov A, Ferris J, Dlugosz AA, Hebrok M. Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes Dev. 2006;20:3161–73. doi: 10.1101/gad.1470806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldmann G, Fendrich V, McGovern K, Bedja D, Bisht S, Alvarez H, et al. An orally bioavailable small-molecule inhibitor of Hedgehog signaling inhibits tumor initiation and metastasis in pancreatic cancer. Mol Cancer Ther. 2008;7:2725–35. doi: 10.1158/1535-7163.MCT-08-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Xie G, Fan Q, Xie J. Activation of the hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene. 2010;29:469–81. doi: 10.1038/onc.2009.392. [DOI] [PubMed] [Google Scholar]
- 27.Morris JP, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer. 2010;10:683–95. doi: 10.1038/nrc2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasperczyk H, Baumann B, Debatin KM, Fulda S. Characterization of sonic hedgehog as a novel NF-kappaB target gene that promotes NF-kappaB-mediated apoptosis resistance and tumor growth in vivo. FASEB J. 2009;23:21–33. doi: 10.1096/fj.08-111096. [DOI] [PubMed] [Google Scholar]
- 29.Welsh S, William R, Kirkpatrick L, Paine-Murriet G, Powis G. Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1alpha. Mol Cancer Ther. 2004;3:233–44. [PubMed] [Google Scholar]
- 30.Taipale J, Chen JK, Coope MK, Wang B, Mann RK, Milenkovic L, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–09. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 31.Cooper MK, Porter JA, Young KE, Beach PA. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280:1603–07. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. 2001;25:402–08. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Chuang PT, McMahon AP. Vertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding protein. Nature. 1999;397:617–21. doi: 10.1038/17611. [DOI] [PubMed] [Google Scholar]
- 34.Koh MY, Spivak-Kroizman T, Venturin S, Welsh S, Williams RR, Kirkpatrick DL, et al. Molecular mechanisms for the activity of PX-478, an antitumor inhibitor of the hypoxia-inducible factor-1alpha. Mol Cancer Ther. 2008;7:90–100. doi: 10.1158/1535-7163.MCT-07-0463. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz DL, Bankson JA, Lemos R, Jr, Lai SY, Thittai AK, He Y, et al. Radiosensitization and stromal imaging response correlates for the HIF-1 inhibitor PX-478 given with or without chemotherapy in pancreatic cancer. Mol Cancer Ther. 2010;9:2057–67. doi: 10.1158/1535-7163.MCT-09-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buchler P, Reber HA, Buchler M, Shrinkante S, Buchler MW, Friess H, et al. Hypoxia-inducible factor 1 regulates vascular endothelial growth factor expression in human pancreatic cancer. Pancreas. 2003;26:56–64. doi: 10.1097/00006676-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–35. [PubMed] [Google Scholar]
- 38.Fujita H, Ohuchida K, Mizumoto K, Nakata K, Yu J, Kayashima, et al. Alpha-smooth muscle actin expressing stroma promotes an aggressive tumor biology in pancreatic ductal adenocarcinoma. Pancreas. 2010;39:1254–62. doi: 10.1097/MPA.0b013e3181dbf647. [DOI] [PubMed] [Google Scholar]
- 39.Wenge RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005:re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 40.Bijlsma MF, Groot AP, Oduro JP, Franken RJ, Schoenmakers SH, Peppelenbosch MP, et al. Hypoxia induces a hedgehog response mediated by HIF-1alpha. J Cell Mol Med. 2009;13:2053–60. doi: 10.1111/j.1582-4934.2008.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hwang JM, Weng YJ, Lin JA, Bau DT, Ko FY, Tsa FJ, et al. Hypoxia-induced compensatory effect as related to Shh and HIF-1alpha in ischemia embryo rat heart. Mol Cell Biochem. 2008;311:179–87. doi: 10.1007/s11010-008-9708-6. [DOI] [PubMed] [Google Scholar]
- 42.Kusano KF, Pola R, Murayama T, Curry C, Kawamoto A, Iwakura A, et al. Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signaling. Nat Med. 2005;11:1197–1204. doi: 10.1038/nm1313. [DOI] [PubMed] [Google Scholar]
- 43.Pola R, Ling LE, Aprahamian TR, Barban E, Bosch-Marce M, Curry C. Postnatal recapitulation of embryonic hedgehog pathway in response to skeletal muscle ischemia. Circulation. 2003;108:479–85. doi: 10.1161/01.CIR.0000080338.60981.FA. [DOI] [PubMed] [Google Scholar]
- 44.Wang G, Zhang Z, Xu Z, Yin H, Bai L, Ma Z, et al. Activation of the sonic hedgehog signaling controls human pulmonary arterial smooth muscle cell proliferation in response to hypoxia. Biochim Biophys Acta. 2010;1803:1359–67. doi: 10.1016/j.bbamcr.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seeley ES, Carriere C, Goetze T, Longnecker DS, Korc M. Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia. Cancer Res. 2009;69:422–30. doi: 10.1158/0008-5472.CAN-08-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lauth M, Bergstrom A, Shimokawa T, Tostar U, Jin Q, Fendrich V, et al. DYRK1B-dependent autocrine-to-paracrine shift of Hedgehog signaling by mutant RAS. Nat Struct Mol Biol. 2010;17:718–25. doi: 10.1038/nsmb.1833. [DOI] [PubMed] [Google Scholar]
- 47.Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361:1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Von Hoff DD, LaRusso PM, Rudi CM, Reddy JC, Yauch RL, Tibes R, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–72. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 49.Low JA, de Sauvage FJ. Clinical experience with Hedgehog pathway inhibitors. J Clin Oncol. 2010;28:5321–26. doi: 10.1200/JCO.2010.27.9943. [DOI] [PubMed] [Google Scholar]
- 50.Yauch RL, Dijkgraaf GJ, Alicke B, Januario T, Ahn CP, Holcomb T, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–74. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2
3
4