The role of vitamin D in regulating the iron-hepcidin-ferroportin axis in monocytes (original) (raw)
Abstract
Chronic kidney disease affects 40% of adults aged 65 and older. Anemia of CKD is present in 30% of patients with CKD and is associated with increased cardiovascular risk, decreased quality of life, and increased mortality. Hepcidin-25 (hepcidin), the key iron regulating hormone, prevents iron egress from macrophages and thus prevents normal recycling of the iron needed to support erythropoiesis. Hepcidin levels are increased in adults and children with CKD. Vitamin D insufficiency is highly prevalent in CKD and is associated with erythropoietin hyporesponsiveness. Recently, hepcidin levels were found to be inversely correlated with vitamin D status in CKD. The aim of this study was to investigate the role of vitamin D in the regulation of hepcidin expression in vitro and in vivo. This study reports that 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), the hormonally active form of vitamin D, is associated with decreased hepcidin and increased ferroportin expression in lipopolysaccharide (LPS) stimulated THP-1 cells. 1,25(OH)2D3 also resulted in a dose-dependent decrease in pro-hepcidin cytokines, IL-6 and IL-1β, release in vitro. Further, we show that high-dose vitamin D therapy impacts systemic hepcidin levels in subjects with early stage CKD. These data suggest that improvement in vitamin D status is associated with lower systemic concentrations of hepcidin in subjects with CKD. In conclusion, vitamin D regulates the hepcidin-ferroportin axis in macrophages which may facilitate iron egress. Improvement in vitamin D status in patients with CKD may reduce systemic hepcidin levels and may ameliorate anemia of CKD.
Keywords: Vitamin D, Hepcidin, Ferroportin, NRAMP1, IL-6, IL-1β, Macrophage, Chronic kidney disease, Anemia of inflammation
Introduction
Vitamin D insufficiency is common in patients with chronic kidney disease (CKD) [1], with a prevalence rate of up to 80% of all patients with CKD stage 3 or worse [2]. Optimal vitamin D status is important in patients with CKD to regulate parathyroid hormone (PTH) concentrations [3], [4], [5] for optimal bone health and prevention of osteomalacia and for potential cardioprotective effects [3], [6], [7]. Recent reports have established an association between vitamin D insufficiency and anemia in patients with CKD [8], [9], [10]; however, the role for vitamin D in the regulation of anemia has not been fully explained.
Iron is one of the key nutrients involved in the pathophysiology of anemia of CKD. The absorption and recycling of iron is under control of the hepcidin-ferroportin axis in humans [11], [12]. Elevated hepcidin level inhibits iron uptake from the gut and sequesters iron in the reticuloendothelial system [13]. Macrophages engulf senescent red blood cells and, therefore, play a central role in iron recycling. Hepcidin retains iron in macrophages by binding to its receptor ferroportin, the only iron exporter, causing its internalization and degradation, consequently preventing iron egress from macrophages to circulation [14]. In inflammatory states such as CKD, hepcidin antimicrobial peptide (referred to as hepcidin) is elevated [15], [16]. Two cytokines (IL-1β and IL-6) are commonly elevated in CKD and stimulate hepcidin production from the liver and macrophages [17], [18], [19], [20]. Hepcidin prevents iron egress from macrophages and thus prevents normal recycling of the iron needed to support erythropoiesis [21], [22], [23]. Additionally, reduced kidney function likely prevents efficient hepcidin clearance from the plasma [8], [24]. Recent investigations show that vitamin D concentrations [assessed by serum 25-hydroxyvitamin D (25(OH)D)] are inversely associated with hepcidin concentrations and positively associated with hemoglobin and iron concentrations [8], [9], [10], [24], [25].
Given the high prevalence of vitamin D insufficiency in patients with CKD and the potential link between vitamin D and anemia, iron and hepcidin concentrations, we hypothesized that vitamin D therapy could improve expression of iron regulating proteins in macrophages in vitro which would translate into improved circulating hepcidin concentrations in humans. We examined the three key iron regulating proteins, hepcidin, NRAMP1 (the endosomal iron transporter that transfers recycled iron from the late endosome to the cytosol) [26], [27], and ferroportin, the only known cellular iron exporter [28], [29], in addition to other pro-hepcidin cytokines in monocytic cell cultures in vitro. In order to translate these findings to humans, we conducted a pilot study to examine the impact of high-dose vitamin D on circulating hepcidin concentrations.
Materials and methods
Macrophage cell culture and stimulation
THP-1 macrophage-like monocytic cells obtained from ATCC (Manassas, VA) were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 50 μg/ml penicillin and 50 IU/ml of streptomycin. Freshly grown cells were harvested and adjusted to 1 million cells/ml and transferred into 12-well tissue culture plates at 2 ml/well. THP-1 cells were cultured with 1,25(OH)2D3 (Sigma Aldrich, St. Louis, MO) doses ranging from 5 nM to 40 nM and incubated overnight. THP-1 monocytic cells differentiate into macrophage phenotype upon vitamin D exposure [30]. To induce inflammation, cells were exposed to lipopolysaccharide (LPS) (20 ng/ml) and further incubated for 6 h at 37 °C. LPS from Neisseria meningitidis serogroup B was purified and quantified as previous described [31]. Cell suspensions were centrifuged and supernatants were removed and saved at −20 °C for cytokine measurements. Harvested THP-1 cells were washed with phosphate buffered saline (PBS) then placed in RLT buffer (Qiagen; Hilden, Germany) containing 1% β-mercaptoethanol, passed over QiaShredder columns, and the resulting lysates were saved at −80 °C for mRNA extraction.
RNA isolation, quantitative real-time PCR and gene expression analysis
RNA was isolated using RNeasy Mini kits (Qiagen) following the manufacturer's instructions, as previously described [32]. Briefly, cell lysates saved in RLT buffer were mixed in 70% ethanol then passed over RNeasy columns. Columns were washed and treated with 10 μl of RNase-free DNase (Qiagen) for 15 min at room temperature prior to RNA extraction, followed by additional washing and centrifugation. RNA was eluted in 35 μl of RNase-free water, then was reverse transcribed to cDNA using QuantiTect® Reverse Transcription kit (Qiagen) following the manufacturer's instructions. Relative gene expression was determined by quantitative RT-PCR performed on resulting cDNA using SYBR Green (Promega; Madison, WI) following the manufacturer's instructions. The mRNA level was calculated in reference to β-actin, and fold change gene expression was calculated in reference to vehicle treated controls using the ΔΔCT method. Results were normalized to vehicle-treated cells which were used as controls for basal gene expression level. The following primers were used for qRT-PCR reactions: human hepcidin 5′-GACCAGTGGCTCTGTTTTCC-3′ and 5′-CACATCCCACACTTTGATCG-3′; human NRAMP1 5′-GCGAGGTCTGCCATCTCTAC-3′ and 5′-GTGTCCACGATGGTGATGAG-3′; human LL-37 5′-CACAGCAGTCACCAGAGGATTG-3′ and 5′-GGCCTGGTTGAGGGTCACT-3′; human β-actin 5′-TCTTCCAGCCTTCCTTCCT-3′ and 5′-AGCACTGTGTTGGCGTACAG-3′. Ferroportin QuantiTect primers (Hs_SLC40A1_1_SG) were purchased from Qiagen.
Cytokine release quantification
Cytokines IL-6 and IL-1β released from THP-1 cells were quantified by DuoSet ELISA (R&D Systems, Minneapolis, MN) as previously described [31], [33].
Hepcidin-25 measurements
Antibody labeling: Anti-hepcidin monoclonal antibodies were adjusted to an approximate concentration of 2 mg/ml and were Biotin- and MSD-SulfoTag (Meso Scale Discovery (MSD), Gaithersburg, MD, USA) labeled according to manufacturer's protocols. Capture antibody was biotin-labeled with Thermo no-weigh EZ Link Sulfo-NHS-LC Biotin with a 20-fold molar excess of biotin. Conjugate antibody was labeled with MSD Sulfotag NHS Ester with a 12-fold molar excess of ruthenium. Following the labeling reactions, antibodies were extensively dialyzed to remove unbound label.
Hepcidin electrochemiluminescence [34] immunoassay: The hepcidin sandwich assay [29] was performed on MSD Streptavidin 96-well plates that were washed three times with TBST (Tris buffered saline containing 10 mmol/l Tris pH 7.40, 150 mmol/l NaCl with 1 ml Tween 20/l) and blocked with 1% Bovine serum albumin (Sigma, St. Louis, MO, USA) in TBS for 1 h at room temperature. Following washing of the plate, 25 μl of biotin-labeled capture antibody (4 μg/ml) was added and allowed to bind to the plate for one hour with gentle shaking. Afterward, the wells were washed three times with TBST, and 100 μl of hepcidin standards consisting of varying concentrations of hepcidin protein in assay buffer consisting of 50 mmol/l HEPES, pH 7.40, 150 mmol/l NaCl, 1 ml/l Triton X-100, 5 mmol/l EDTA, and 5 mmol/l EGTA and 0.1% BSA, which was supplemented with 100 μg/ml Heterophilic Blocking Reagent (Scantibodies, Santee, CA, USA) were added to the wells to generate a calibration curve. Plasma samples were diluted 1:50 in the same assay buffer, added to their respective wells, and incubated for 1 h at room temperature with gentle rocking. Following aspiration, wells were washed 3 times with TBST, and 25 μl of 0.1 μg/ml ruthenium-labeled conjugate hepcidin-specific detection antibody were added to the wells, which were incubated for 1 h at room temperature. The plate was again washed three times with TBST, and 150 μl of 2X-MSD Read Buffer T was added to the wells. The plate was then read on an MSD SECTOR Imager 6000 reader, which recorded ruthenium electrochemiluminescence. Concentrations of hepcidin in samples were interpolated against a standard curve made up of reference standard hepcidin (Eli Lilly and Company, Indianapolis, IN, USA) using a 4 PL fit (Meso Scale Discovery Workbench).
Pilot clinical study design
We obtained serum from subjects with early stage CKD (stages 2/3) who completed an IRB-approved, double-blind, randomized, placebo-controlled trial of oral vitamin D3 (cholecalciferol, 50,000 IU weekly for 12 weeks, followed by 50,000 IU every other week for 40 weeks) or matching placebo for one year. CKD staging was defined by an estimated glomerular filtration rate (eGFR) of 60–89 ml/min/1.73 m2 and 30–59 ml/min/1.73 m2 for stages 2 and 3, respectively, calculated using the Modification of Diet in Renal Disease Study equation [35]. The primary endpoint of this study was serum 25(OH)D and PTH, the results of which have been published [3], [36]. All subjects provided informed consent for evaluation of their blood samples for future sub-studies to explore the impact of high-dose vitamin D on a variety of health outcomes. The clinicaltrials.gov registration number was NCT00781417. This sub-study includes only subjects with available paired serum specimens for hepcidin measurements at baseline and a three month follow-up visit (N = 38). Serum 25(OH)D was measured with a chemiluminescent assay (Immunodiagnostic Systems iSYS automated machine; Fountain Hills, AZ).
Statistical analysis
The mean values ± SD and P values (Student t test) of at least three independent determinations were calculated with Microsoft Excel software for the in vitro data. For the clinical data, descriptive statistics were performed. Differences between groups (vitamin D vs placebo) were determined with two-group _t_-tests, Mann–Whitney U tests (for variables that were not normally distributed), or chi-square tests. Spearman's rank correlation was used to determine the relationship between the percent change in serum 25(OH)D3 and the percent change in serum hepcidin. Clinical data were analyzed with JMP® Pro 10.0.0 (SAS Institute Inc., Cary, NC, USA) using two-sided tests and assuming a 5% significance level.
Results
Vitamin D (1,25(OH)2D3) is associated with decreased hepcidin and increased ferroportin mRNA expression in THP-1 cells exposed to LPS
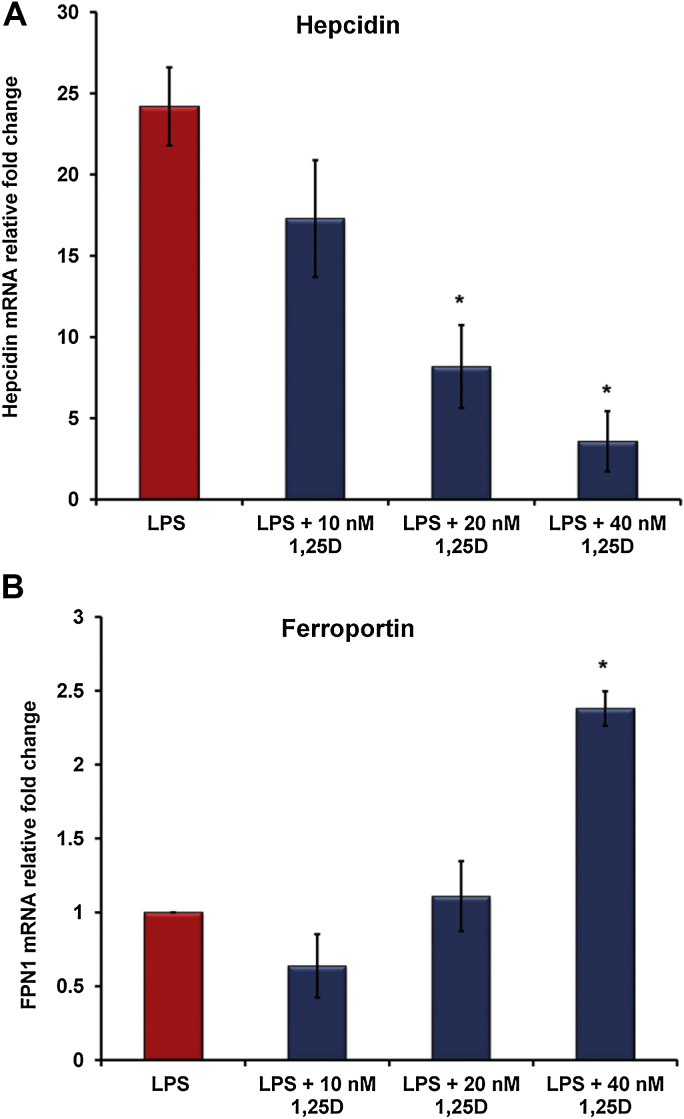
Lipopolysaccharide is a known inducer of hepcidin expression in macrophages and hepatocytes [18], [19]. The effect of the hormonally active vitamin D (1,25(OH)2D3) on regulating hepcidin and ferroportin gene expression in LPS-stimulated THP-1 cells was investigated. We found that 1,25(OH)2D3 suppressed hepcidin mRNA expression in LPS-stimulated THP-1 cells in a dose-dependent manner (Fig. 1A). In contrast, 1,25(OH)2D3 significantly increased ferroportin mRNA expression at 40 nM dose but not at lower doses (Fig. 1B). These data suggest that vitamin D regulates the hepcidin-ferroportin axis in macrophages exposed to LPS, thereby facilitating iron transport during states of inflammation.
Figure 1.
Vitamin D regulates hepcidin-ferroportin axis in LPS-stimulated macrophages. THP-1 cells were treated with increasing doses of 1,25(OH)2D3 overnight prior to LPS (20 ng/ml) exposure for 6 h. In vitro 1,25(OH)2D3 down-regulates hepcidin (A) and up-regulates ferroportin (B) expression in LPS-stimulated human THP-1 macrophages. Gene expression was assessed by quantitative RT-PCR. *P < 0.05. 1,25D: 1,25(OH)2D3.
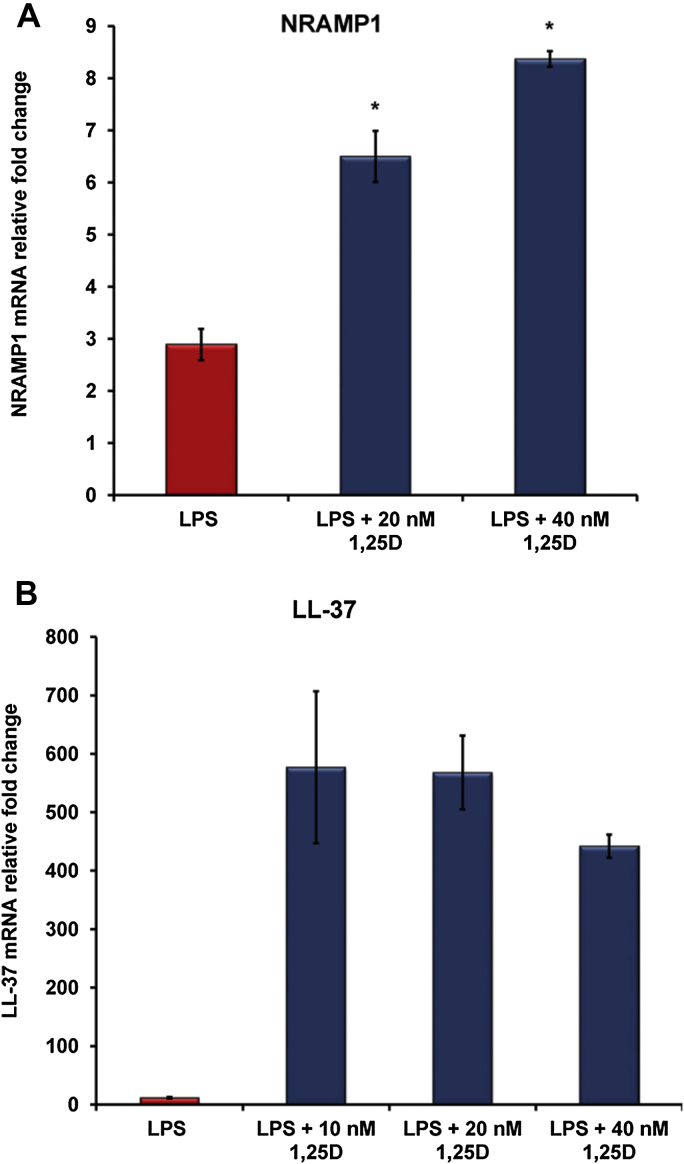
Vitamin D (1,25(OH)2D3) is associated with increased expression of NRAMP1, the endosomal iron transporter
NRAMP1, the endosomal iron transporter that plays a significant role in cellular iron homeostasis, is a direct target gene for vitamin D. As a positive control to our results that vitamin D regulates the hepcidin-ferroportin axis in THP-1 cells exposed to LPS, we examined the expression of NRAMP1 and LL-37, genes known to be induced by vitamin D [26], [30]. As expected, our data confirm that 1,25(OH)2D3 significantly induced NRAMP1 mRNA expression (Fig. 2A) and potently induced LL-37 mRNA expression in LPS-stimulated THP-1 cells (Fig. 2B).
Figure 2.
Vitamin D induces NRAMP1, the endosomal iron transporter, and LL-37 host defense peptide in macrophages. THP-1 cells were treated with increasing doses of 1,25(OH)2D3 overnight prior to LPS (20 ng/ml) exposure for 6 h. In vitro 1,25(OH)2D3 up-regulates NRAMP1 (A) and potently induces LL-37 (B) gene expression in LPS-stimulated human THP-1 macrophages. Gene expression was assessed by quantitative RT-PCR. *P < 0.05. 1,25D: 1,25(OH)2D3.
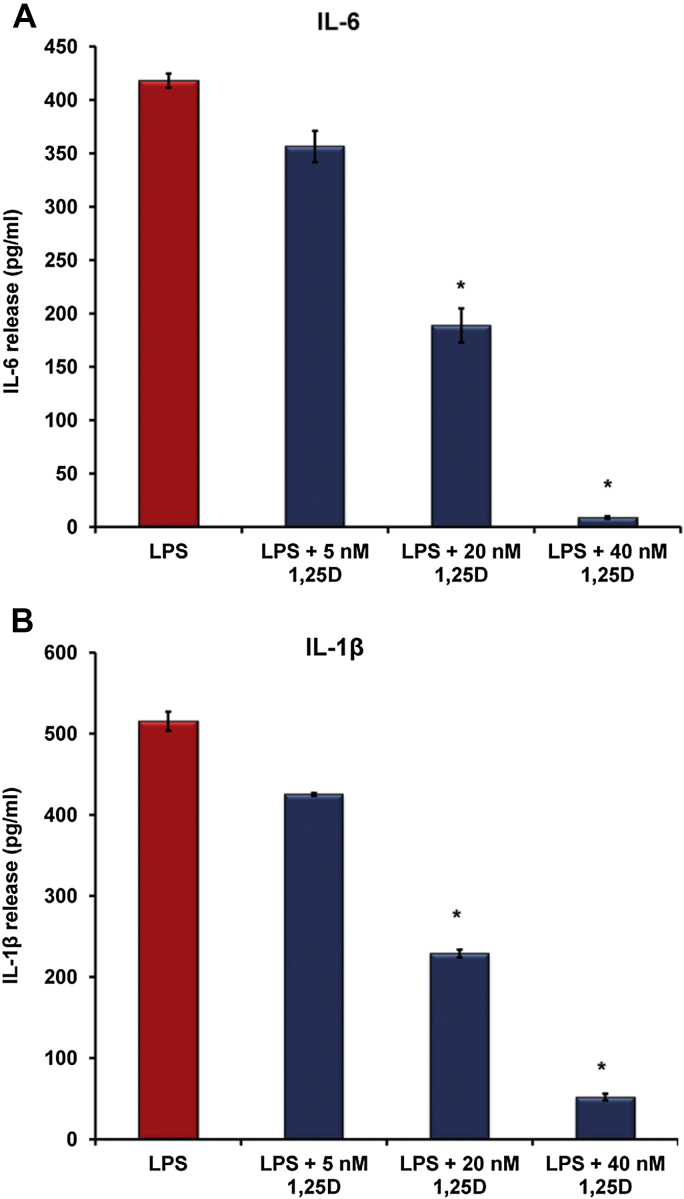
Vitamin D lowers inflammatory cytokines release from LPS-stimulated macrophages in vitro
LPS is a major inducer of inflammatory cytokine release from macrophages including pro-hepcidin cytokines, IL-6 and IL-1β [33]. We examined whether vitamin D may also decrease the release of these inflammatory cytokines from macrophages. We measured cytokine concentrations in the cultured media from THP-1 cells in the presence of increasing concentrations of 1,25(OH)2D3 prior to LPS exposure and found a dose-dependent decrease in IL-6 (Fig. 3A) and IL-1β release in vitro (Fig. 3B). Therefore, vitamin D may be associated with suppression of hepcidin expression by directly reducing pro-hepcidin cytokines release.
Figure 3.
Vitamin D reduces pro-hepcidin cytokine release from LPS-stimulated macrophages. THP-1 cells were treated with increasing doses of 1,25(OH)2D3 overnight prior to LPS (20 ng/ml) exposure for 6 h. In vitro 1,25(OH)2D3 (1,25D) reduces IL-6 (A) and IL-1β (B) release from THP-1 cells exposed to 20 ng/ml of LPS. Cytokines release was measured by ELISA. *P < 0.05. 1,25D: 1,25(OH)2D3.
Vitamin D effects on hepcidin concentrations in patients with stages 2 and 3 CKD
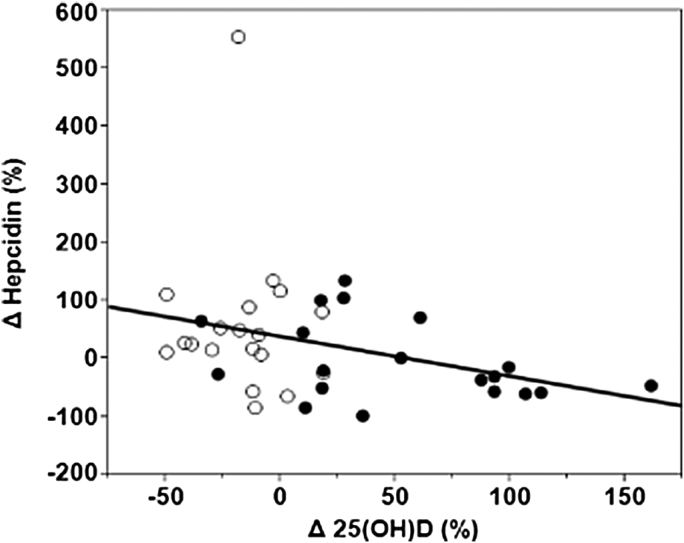
Baseline demographic and biochemical characteristics for the subjects evaluated in the pilot study are listed in Table 1. Coincidently, baseline serum 25(OH)D concentrations were lower in the vitamin D supplemented group. Baseline systemic hepcidin levels were similar for the vitamin D supplemented and placebo groups. The percent change from baseline to 3 months in serum 25(OH)D concentrations was inversely associated with the percent change in serum hepcidin concentrations (Spearman rho = −0.38, P = 0.02) (Fig. 4, open circles represent patients receiving placebo. Filled circles represent patients receiving vitamin D).
Table 1.
Baseline demographic characteristics of subjects with early stage CKD
| Variable | Vitamin D (n = 19) | Placebo (n = 19) | P |
|---|---|---|---|
| Age (y) | 62.5 ± 11.0 | 62.0 ± 8.7 | 0.89 |
| BMI (kg/m2) | 33.2 ± 5.4 | 32.1 ± 8.0 | 0.63 |
| Male [n (%)] | 19 (100.0) | 17 (89.5) | 0.15 |
| African American (%) | 9 (47.4) | 9 (47.4) | 1.00 |
| eGFR (ml/min/1.73 m2) | 59.4 ± 14.4 | 62.3 ± 16.0 | 0.57 |
| CKD Stage 2/3 (N) | 9/10 | 8/11 | 0.74 |
| Hypertension (%) | 17 (89.5) | 17 (89.5) | 1.00 |
| Diabetes (%) | 17 (89.5) | 13 (68.4) | 0.11 |
| 25(OH)D (ng/ml) | 27.5 ± 6.3 | 32.9 ± 8.5 | 0.03 |
| Hepcidin (ng/ml)a | 8.5 (5.5, 17.7) | 11.9 (6.8, 13.8) | 0.90 |
Figure 4.
Relationship between percent change in serum hepcidin and percent change in 25(OH)D from baseline to 3 months. Open circles represent patients receiving placebo. Filled circles represent patients receiving vitamin D. Spearman's rho = −0.38, P = 0.02.
Discussion
Macrophages play a central role in iron metabolism and in host defense [14], [37]. Macrophages are also major producers of inflammatory cytokines. Here we report that vitamin D treatment is associated with reduced hepcidin expression in vitro and in vivo. Our in vitro studies show that vitamin D is associated with decreased hepcidin while increasing ferroportin and NRAMP1 mRNA expression in a dose-dependent manner in human monocytic THP-1 cells in the presence of an inflammatory stimulus (i.e. exposure to LPS). This study also shows that vitamin D is associated with reduced concentration of hepcidin stimulatory cytokines, IL-6 and IL-1β, from cultured THP-1 cells exposed to LPS. Taken together, these data suggest that vitamin D may have an important role in regulating cellular iron homeostasis via the hepcidin-ferroportin-NRAMP1 axis in macrophages to facilitate iron egress during inflammation. Our in vivo pilot study shows that an increase in serum 25(OH)D concentration is associated with a decrease in serum hepcidin in subjects with early stage CKD. Our data suggest that vitamin D therapy may improve altered iron homeostasis associated with anemia of CKD in vitamin D deficient patients.
Our study is in agreement with recent studies showing that vitamin D suppresses hepcidin expression in macrophages [38], [39]. Bacchetta and co-workers show that vitamin D significantly suppressed hepcidin expression in monocytes by 0.5-fold and provides evidence that vitamin D directly downregulates hepcidin expression as they identified a VDRE binding site on human hepcidin promoter [39]. Adding to the findings of this previous study, we examined the effect of vitamin D on hepcidin expression in monocytes during inflammation (i.e. in LPS-stimulated THP-1 cells). LPS is a well-known inducer of hepcidin and can mimic in vivo inflammatory conditions since microbial translocation is commonly associated with chronic diseases including CKD [40], [41], [42], [43]. Our data clearly demonstrate that vitamin D significantly reduced IL-6 and IL-1β release (major inflammatory cytokines that are elevated in CKD) [44], [45], [46] from LPS-induced THP-1 cells. Elevated IL-6 levels are associated with poor clinical outcomes and with anemia of chronic disease since IL-6 is a direct inducer of hepcidin [47]. Therefore, we postulate that reducing circulating IL-6 levels would lead to a reduction in hepcidin expression in liver hepatocytes, the major source of hepcidin, as well as in macrophages [48]. Elevation in circulating IL-6 levels have been reported in patients with late stage CKD where anemia of CKD is highly prevalent [47]. Further, LPS is a major component of microbial translocation seen during chronic inflammation [40], [41], [42], [43]. LPS induces both hepcidin and IL-6 expression whereas LL-37 binds and neutralizes LPS activity [49]. Therefore, we postulate that high-dose vitamin D therapy suppresses hepcidin expression directly, as shown by Bacchetta et al. [39], and indirectly by reducing pro-hepcidin inflammatory cytokines IL-6 and IL-1β.
Further, we recently reported that vitamin D status assessed as serum 25(OH)D is inversely correlated with the inflammatory chemokine, MCP-1, in vivo and in vitro [3], [36]. MCP-1 is found to be associated with serum hepcidin and macrophage iron in patients with metabolic syndrome alterations [50]. Other studies reported that vitamin D suppressed the release of inflammatory cytokines from monocytes and macrophages [51]. The mechanism by which vitamin D exerts anti-inflammatory effects is recently proposed to be mediated by microRNA called miR-155 [52]. MicroRNAs are noncoding RNAs that control genes expression by repressing mRNA translation. Specifically, miR-155 encoded by bic gene is a critical regulator of TLR signaling in macrophages. miR-155 targets SOCS1 (cytokine signaling protein 1), the negative feedback regulator of cytokine release, therefore high miR-155 expression is associated with a heightened cytokine release from macrophages. Increased expression of miR-155 is reported in primary monocytes and macrophages from SLE and in atherosclerotic plaque [53], [54], [55], [56], [57]. Vitamin D is reported to promote the negative feedback regulation of LPS-mediated signaling by targeting miR-155-suppressor of cytokine signaling protein 1 (SOCS1) in macrophages since miRNA-155 is highly upregulated by toll-like receptors (TLR) ligands, i.e. LPS and microbial translocation and downregulated by vitamin D [52], [58].
In addition to miRNA-155, vitamin D may regulate the immune system by inducing autophagy and regulating endoplasmic stress. Autophagy is a conserved process by which cells recycle macromolecules, thus playing an essential role in cellular homeostasis and in host defense [59], [60], [61], [62]. Campbell et al. demonstrated that vitamin D enhanced autophagy, which inhibited HIV and Mycobacterium tuberculosis infection in macrophages [61]. Autophagy induction reduces cytokines and other inflammatory mediators release from LPS-stimulated macrophages [63]. Further, endoplasmic reticulum (ER) stress is shown to dysregulate cellular iron homeostasis by inducing hepcidin expression [64]. Recent report shows that vitamin D relieves ER stress, which would be another possible mechanism by which vitamin D reduces hepcidin expression [65], [66]. Elucidation of these mechanisms is worthy of further investigation.
In this pilot study we show that an increase in serum 25(OH)D concentrations is associated with a decrease in hepcidin levels in subjects with early stage CKD enrolled in a high-dose vitamin D trial. Our clinical finding supports our in vitro data that suggest vitamin D lowers hepcidin expression directly and/or indirectly by decreasing pro-hepcidin cytokines. Bacchetta and co-workers also demonstrated that high-dose vitamin D therapy reduced blood hepcidin-25 levels in healthy donors [39]. The relationship between vitamin D and hepcidin may explain the link between low vitamin D status and anemia in children [67]. These findings are clinically significant as a large majority of patients with CKD have vitamin D insufficiency [2], and approximately 40% of patients with CKD stage 4 have anemia [68]. Correction of vitamin D as a potential adjunctive therapy in treatment of anemia of CKD is attractive given the relatively inexpensive cost, favorable safety profile, and the potential to reduce the dependence on other more expensive therapies such as erythropoietin stimulating agents. Very few studies have been conducted to evaluate vitamin D as a therapy for anemia of CKD. Goicoechea et al. demonstrated in 28 patients on hemodialysis and severe hyperparathyroidism that intravenous calcitriol, the hormonally active form of vitamin D, reduced the need for erythropoietin therapy [69]. Similarly, Albitar et al. demonstrated in a prospective study of 12 patients, treatment with alfacalcidol, an analog compound of 1,25(OH)2D, improved anemia in patients on hemodialysis [70].
The strength and novelty of this study was the evaluation of the effect of vitamin D on suppressing hepcidin expression in LPS-stimulated monocytes indicating the efficacy of vitamin D on regulating hepcidin during inflammation, a common hallmark of CKD. In addition to hepcidin and ferroportin, this study investigated the impact of vitamin D on NRAMP1, the endosomal iron transporter. A limitation was the use of immortalized human monocytic THP-1 cells rather than primary peripheral monocytes from subjects with CKD. A limitation of our clinical pilot study was that this was a secondary analysis in CKD patients with available serum for measurement of hepcidin; therefore, we may have been limited in statistical power. Regardless, our clinical data support our in vitro findings of a hepcidin-lowering effect of vitamin D.
In summary, this study reports that high-dose vitamin D impacts hepcidin expression in vitro and in vivo. Vitamin D is associated with alterations of the hepcidin-ferroportin axis in monocytes exposed to LPS and leads to a reduction of pro-hepcidin cytokine, IL-6 and IL-1β, therefore facilitating iron egress during inflammation. This in vitro observation appears to be supported by our early translational pilot study in subjects with early CKD where changes in vitamin D status induced by high-dose oral vitamin D therapy impacted changes in systemic hepcidin levels.
Acknowledgment
This work is supported in part by grants from Emory-Egleston Children's Research Center and Center for Pediatric Nanomedicine of Emory + Children's Pediatrics Research Center to S.M.Z, and by T32 DK007298-32S1 to J.A.A. S.M.Z. especially acknowledges the support of N. McCarty (Center for Cystic Fibrosis Research, Emory University School of Medicine) for providing the research facility and laboratory space where part of this work was conducted. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
References
- 1.Mallbris L., O'Brien K.P., Hulthen A., Sandstedt B., Cowland J.B., Borregaard N. Neutrophil gelatinase-associated lipocalin is a marker for dysregulated keratinocyte differentiation in human skin. Exp Dermatol. 2002;11(6):584–591. doi: 10.1034/j.1600-0625.2002.110611.x. [DOI] [PubMed] [Google Scholar]
- 2.LaClair R.E., Hellman R.N., Karp S.L., Kraus M., Ofner S., Li Q. Prevalence of calcidiol deficiency in CKD: a cross-sectional study across latitudes in the United States. Am J Kidney Dis. 2005;45(6):1026–1033. doi: 10.1053/j.ajkd.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez J.A., Law J., Coakley K.E., Zughaier S.M., Hao L., Shahid Salles K. High-dose cholecalciferol reduces parathyroid hormone in patients with early chronic kidney disease: a pilot, randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2012;96(3):672–679. doi: 10.3945/ajcn.112.040642. 3417221 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wasse H., Huang R., Long Q., Zhao Y., Singapuri S., McKinnon W. Very high-dose cholecalciferol and arteriovenous fistula maturation in ESRD: a randomized, double-blind, placebo-controlled pilot study. J Vasc Access. 2013;0(0):0. doi: 10.5301/jva.5000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandra P., Binongo J.N., Ziegler T.R., Schlanger L.E., Wang W., Someren J.T. Cholecalciferol (vitamin D3) therapy and vitamin D insufficiency in patients with chronic kidney disease: a randomized controlled pilot study. Endocr Pract. 2008;14(1):10–17. doi: 10.4158/EP.14.1.10. 2654595 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Judd S.E., Tangpricha V. Vitamin D therapy and cardiovascular health. Curr Hypertens Rep. 2011;13(3):187–191. doi: 10.1007/s11906-011-0190-2. [DOI] [PubMed] [Google Scholar]
- 7.Ullah M.I., Uwaifo G.I., Nicholas W.C., Koch C.A. Does Vitamin D deficiency cause hypertension? Current evidence from clinical studies and potential mechanisms. Int J Endocrinol. 2010;2010:1–11. doi: 10.1155/2010/579640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carvalho C., Isakova T., Collerone G., Olbina G., Wolf M., Westerman M. Hepcidin and disordered mineral metabolism in chronic kidney disease. Clin Nephrol. 2011;76(2):90–98. doi: 10.5414/cn107018. [DOI] [PubMed] [Google Scholar]
- 9.Perlstein T.S., Pande R., Berliner N., Vanasse G.J. Prevalence of 25-hydroxyvitamin D deficiency in subgroups of elderly persons with anemia: association with anemia of inflammation. Blood. 2011;117(10):2800–2806. doi: 10.1182/blood-2010-09-309708. [DOI] [PubMed] [Google Scholar]
- 10.Icardi A., Paoletti E., De Nicola L., Mazzaferro S., Russo R., Cozzolino M. Renal anaemia and EPO hyporesponsiveness associated with vitamin D deficiency: the potential role of inflammation. Nephrol Dial Transplant. 2013;28(7):1672–1679. doi: 10.1093/ndt/gft021. [DOI] [PubMed] [Google Scholar]
- 11.Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117(17):4425–4433. doi: 10.1182/blood-2011-01-258467. 3099567 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun C.C., Vaja V., Babitt J.L., Lin H.Y. Targeting the hepcidin-ferroportin axis to develop new treatment strategies for anemia of chronic disease and anemia of inflammation. Am J Hematol. 2012;87(4):392–400. doi: 10.1002/ajh.23110. 3653431 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganz T., Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med. 2011;62:347–360. doi: 10.1146/annurev-med-050109-142444. [DOI] [PubMed] [Google Scholar]
- 14.Ganz T. Macrophages and systemic iron homeostasis. J Innate Immun. 2012;4(5–6):446–453. doi: 10.1159/000336423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaritsky J., Young B., Gales B., Wang H.J., Rastogi A., Westerman M. Reduction of serum hepcidin by hemodialysis in pediatric and adult patients. Clin J Am Soc Nephrol. 2010;5(6):1010–1014. doi: 10.2215/CJN.08161109. 2879302 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babitt J.L., Lin H.Y. Molecular mechanisms of hepcidin regulation: implications for the anemia of CKD. Am J Kidney Dis. 2010;55(4):726–741. doi: 10.1053/j.ajkd.2009.12.030. 2905036 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganz T., Olbina G., Girelli D., Nemeth E., Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112(10):4292–4297. doi: 10.1182/blood-2008-02-139915. [DOI] [PubMed] [Google Scholar]
- 18.Nemeth E., Rivera S., Gabayan V., Keller C., Taudorf S., Pedersen B.K. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271–1276. doi: 10.1172/JCI20945. 398432 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee P., Peng H., Gelbart T., Wang L., Beutler E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci U S A. 2005;102(6):1906–1910. doi: 10.1073/pnas.0409808102. 548537 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wrighting D.M., Andrews N.C. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108(9):3204–3209. doi: 10.1182/blood-2006-06-027631. 1895528 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pigeon C., Ilyin G., Courselaud B., Leroyer P., Turlin B., Brissot P. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276(11):7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 22.Park C.H., Valore E.V., Waring A.J., Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276(11):7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 23.Krause A., Neitz S., Magert H.J., Schulz A., Forssmann W.G., Schulz-Knappe P. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480(2–3):147–150. doi: 10.1016/s0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- 24.Zaritsky J., Young B., Wang H.J., Westerman M., Olbina G., Nemeth E. Hepcidin–a potential novel biomarker for iron status in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1051–1056. doi: 10.2215/CJN.05931108. 2689881 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cabrera A.R., Cuneo K.C., Desjardins A., Sampson J.H., McSherry F., Herndon J.E., 2nd Concurrent stereotactic radiosurgery and bevacizumab in recurrent malignant gliomas: a prospective trial. Int J Radiat Oncol Biol Phys. 2013;86(5):873–879. doi: 10.1016/j.ijrobp.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 26.Roig E.A., Richer E., Canonne-Hergaux F., Gros P., Cellier M.F. Regulation of NRAMP1 gene expression by 1alpha,25-dihydroxy-vitamin D(3) in HL-60 phagocytes. J Leukoc Biol. 2002;71(5):890–904. [PubMed] [Google Scholar]
- 27.Rodrigues P.N., Gomes S.S., Neves J.V., Gomes-Pereira S., Correia-Neves M., Nunes-Alves C. Mycobacteria-induced anaemia revisited: a molecular approach reveals the involvement of NRAMP1 and lipocalin-2, but not of hepcidin. Immunobiology. 2011;216(10):1127–1134. doi: 10.1016/j.imbio.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Nemeth E., Tuttle M.S., Powelson J., Vaughn M.B., Donovan A., Ward D.M. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 29.De Domenico I., Ward D.M., Langelier C., Vaughn M.B., Nemeth E., Sundquist W.I. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell. 2007;18(7):2569–2578. doi: 10.1091/mbc.E07-01-0060. 1924807 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cellier M., Shustik C., Dalton W., Rich E., Hu J., Malo D. Expression of the human NRAMP1 gene in professional primary phagocytes: studies in blood cells and in HL-60 promyelocytic leukemia. J Leukoc Biol. 1997;61(1):96–105. doi: 10.1002/jlb.61.1.96. [DOI] [PubMed] [Google Scholar]
- 31.Zughaier S.M., Tzeng Y.L., Zimmer S.M., Datta A., Carlson R.W., Stephens D.S. Neisseria meningitidis lipooligosaccharide structure-dependent activation of the macrophage CD14/Toll-like receptor 4 pathway. Infect Immun. 2004;72(1):371–380. doi: 10.1128/IAI.72.1.371-380.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zughaier S.M. Neisseria meningitidis capsular polysaccharides induce inflammatory responses via TLR2 and TLR4-MD-2. J Leukoc Biol. 2011;89(3):469–480. doi: 10.1189/jlb.0610369. 3040465 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zughaier S.M., Zimmer S.M., Datta A., Carlson R.W., Stephens D.S. Differential induction of the toll-like receptor 4-MyD88-dependent and -independent signaling pathways by endotoxins. Infect Immun. 2005;73(5):2940–2950. doi: 10.1128/IAI.73.5.2940-2950.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leclercq A., Houard X., Philippe M., Ollivier V., Sebbag U., Meilhac O. Involvement of intraplaque hemorrhage in atherothrombosis evolution via neutrophil protease enrichment. J Leukoc Biol. 2007;82(6):1420–1429. doi: 10.1189/jlb.1106671. [DOI] [PubMed] [Google Scholar]
- 35.Levey A.S., Coresh J., Greene T., Stevens L.A., Zhang Y.L., Hendriksen S. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 36.Alvarez J.A., Zughaier S.M., Law J., Hao L., Wasse H., Ziegler T.R. Effects of high-dose cholecalciferol on serum markers of inflammation and immunity in patients with early chronic kidney disease. Eur J Clin Nutr. 2013;67(3):264–269. doi: 10.1038/ejcn.2012.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J., Pantopoulos K. Regulation of cellular iron metabolism. Biochem J. 2011;434(3):365–381. doi: 10.1042/BJ20101825. 3048577 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bell S.J. The effect of VitaminD3 on hepcidin and IL-8 expression in monocytes. J Hematol. 2013;2(1):1–7. [Google Scholar]
- 39.Bacchetta J., Zaritsky J.J., Sea J.L., Chun R.F., Lisse T.S., Zavala K. Suppression of iron-regulatory hepcidin by vitamin D. J Am Soc Nephrol. 2014;25:564–572. doi: 10.1681/ASN.2013040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Layoun A., Santos M.M. Bacterial cell wall constituents induce hepcidin expression in macrophages through MyD88 signaling. Inflammation. 2012;35(4):1500–1506. doi: 10.1007/s10753-012-9463-4. [DOI] [PubMed] [Google Scholar]
- 41.Wang L., Harrington L., Trebicka E., Shi H.N., Kagan J.C., Hong C.C. Selective modulation of TLR4-activated inflammatory responses by altered iron homeostasis in mice. J Clin Invest. 2009;119(11):3322–3328. doi: 10.1172/JCI39939. 2769199 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Theurl I., Theurl M., Seifert M., Mair S., Nairz M., Rumpold H. Autocrine formation of hepcidin induces iron retention in human monocytes. Blood. 2008;111(4):2392–2399. doi: 10.1182/blood-2007-05-090019. [DOI] [PubMed] [Google Scholar]
- 43.Ramezani A., Raj D.S. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. 2013 doi: 10.1681/ASN.2013080905. 24231662 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCarley P. The KDOQI clinical practice guidelines and clinical practice recommendations for treating anemia in patients with chronic kidney disease: implications for nurses. Nephrol Nurs J. 2006;33(4):423–426. 45; quiz 7–8. [PubMed] [Google Scholar]
- 45.Malyszko J., Malyszko J.S., Bachorzewska-Gajewska H., Poniatowski B., Dobrzycki S., Mysliwiec M. Neutrophil gelatinase-associated lipocalin is a new and sensitive marker of kidney function in chronic kidney disease patients and renal allograft recipients. Transplant Proc. 2009;41(1):158–161. doi: 10.1016/j.transproceed.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 46.Malyszko J., Bachorzewska-Gajewska H., Sitniewska E., Malyszko J.S., Poniatowski B., Dobrzycki S. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in non-diabetic patients with stage 2-4 chronic kidney disease. Ren Fail. 2008;30(6):625–628. doi: 10.1080/08860220802134607. [DOI] [PubMed] [Google Scholar]
- 47.Pecoits-Filho R., Barany P., Lindholm B., Heimburger O., Stenvinkel P. Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant. 2002;17(9):1684–1688. doi: 10.1093/ndt/17.9.1684. [DOI] [PubMed] [Google Scholar]
- 48.Wang F.D., Zhou D.B., Li S.L., Wang X., Zhang J.P., Duan M.H. Effect of recombinant human erythropoietin on hepcidin mRNA expression in patients with multiple myeloma. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2011;19(2):390–394. [PubMed] [Google Scholar]
- 49.Zughaier S.M., Shafer W.M., Stephens D.S. Antimicrobial peptides and endotoxin inhibit cytokine and nitric oxide release but amplify respiratory burst response in human and murine macrophages. Cell Microbiol. 2005;7(9):1251–1262. doi: 10.1111/j.1462-5822.2005.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valenti L., Dongiovanni P., Motta B.M., Swinkels D.W., Bonara P., Rametta R. Serum hepcidin and macrophage iron correlate with MCP-1 release and vascular damage in patients with metabolic syndrome alterations. Arterioscler Thromb Vasc Biol. 2011;31(3):683–690. doi: 10.1161/ATVBAHA.110.214858. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y., Leung D.Y., Richers B.N., Liu Y., Remigio L.K., Riches D.W. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188(5):2127–2135. doi: 10.4049/jimmunol.1102412. 3368346 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y., Liu W., Sun T., Huang Y., Wang Y., Deb D.K. 1,25-dihydroxyvitamin D promotes negative feedback regulation of TLR signaling via targeting microRNA-155-SOCS1 in macrophages. J Immunol. 2013;190(7):3687–3695. doi: 10.4049/jimmunol.1203273. 3608760 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katsushima F., Takahashi A., Sakamoto N., Kanno Y., Abe K., Ohira H. Expression of micro-RNAs in peripheral blood mononuclear cells from primary biliary cirrhosis patients. Hepatol Res. 2013 doi: 10.1111/hepr.12198. [DOI] [PubMed] [Google Scholar]
- 54.Wang H., Peng W., Ouyang X., Li W., Dai Y. Circulating microRNAs as candidate biomarkers in patients with systemic lupus erythematosus. Transl Res. 2012;160(3):198–206. doi: 10.1016/j.trsl.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 55.Leng R.X., Pan H.F., Qin W.Z., Chen G.M., Ye D.Q. Role of microRNA-155 in autoimmunity. Cytokine Growth Factor Rev. 2011;22(3):141–147. doi: 10.1016/j.cytogfr.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Wang G., Tam L.S., Li E.K., Kwan B.C., Chow K.M., Luk C.C. Serum and urinary cell-free MiR-146a and MiR-155 in patients with systemic lupus erythematosus. J Rheumatol. 2010;37(12):2516–2522. doi: 10.3899/jrheum.100308. [DOI] [PubMed] [Google Scholar]
- 57.Li Y.C., Chen Y., Liu W., Thadhani R. MicroRNA-mediated mechanism of vitamin D regulation of innate immune response. J Steroid Biochem Mol Biol. 2013 doi: 10.1016/j.jsbmb.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen N.K., Chong T.W., Loh H.L., Lim K.H., Gan V.H., Wang M. Negative regulatory responses to metabolically triggered inflammation impair renal epithelial immunity in diabetes mellitus. J Mol Med (Berl) 2013;91(5):587–598. doi: 10.1007/s00109-012-0969-x. 3644409 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jo E.K. Innate immunity to mycobacteria: vitamin D and autophagy. Cell Microbiol. 2010;12(8):1026–1035. doi: 10.1111/j.1462-5822.2010.01491.x. [DOI] [PubMed] [Google Scholar]
- 60.Yuk J.M., Shin D.M., Lee H.M., Yang C.S., Jin H.S., Kim K.K. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6(3):231–243. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 61.Campbell G.R., Spector S.A. Vitamin D inhibits human immunodeficiency virus type 1 and Mycobacterium tuberculosis infection in macrophages through the induction of autophagy. PLoS Pathog. 2012;8(5):e1002689. doi: 10.1371/journal.ppat.1002689. 3349755 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campbell G.R., Spector S.A. Toll-like receptor 8 ligands activate a vitamin D mediated autophagic response that inhibits human immunodeficiency virus type 1. PLoS Pathog. 2012;8(11):e1003017. doi: 10.1371/journal.ppat.1003017. 3499571 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zughaier S., Karna P., Stephens D., Aneja R. Potent anti-inflammatory activity of novel microtubule-modulating brominated noscapine analogs. PLoS One. 2010;5(2):e9165. doi: 10.1371/journal.pone.0009165. 2820095 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vecchi C., Montosi G., Zhang K., Lamberti I., Duncan S.A., Kaufman R.J. ER stress controls iron metabolism through induction of hepcidin. Science. 2009;325(5942):877–880. doi: 10.1126/science.1176639. 2923557 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Riek A.E., Oh J., Bernal-Mizrachi C. 1,25(OH)(2) vitamin D suppresses macrophage migration and reverses atherogenic cholesterol metabolism in type 2 diabetic patients. J Steroid Biochem Mol Biol. 2013;136:309–312. doi: 10.1016/j.jsbmb.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Riek A.E., Oh J., Sprague J.E., Timpson A., de las Fuentes L., Bernal-Mizrachi L. Vitamin D suppression of endoplasmic reticulum stress promotes an antiatherogenic monocyte/macrophage phenotype in type 2 diabetic patients. J Biol Chem. 2012;287(46):38482–38494. doi: 10.1074/jbc.M112.386912. 3493893 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Atkinson M.A., Melamed M.L., Kumar J., Roy C.N., Miller E.R., 3rd, Furth S.L. Vitamin D, race, and risk for anemia in children. J Pediatr. 2014;164:153–158. doi: 10.1016/j.jpeds.2013.08.060. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Astor B.C., Muntner P., Levin A., Eustace J.A., Coresh J. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988-1994) Arch Intern Med. 2002;162(12):1401–1408. doi: 10.1001/archinte.162.12.1401. [DOI] [PubMed] [Google Scholar]
- 69.Goicoechea M., Vazquez M.I., Ruiz M.A., Gomez-Campdera F., Perez-Garcia R., Valderrabano F. Intravenous calcitriol improves anaemia and reduces the need for erythropoietin in haemodialysis patients. Nephron. 1998;78(1):23–27. doi: 10.1159/000044877. [DOI] [PubMed] [Google Scholar]
- 70.Albitar S., Genin R., Fen-Chong M., Serveaux M.O., Schohn D., Chuet C. High-dose alfacalcidol improves anaemia in patients on haemodialysis. Nephrol Dial Transplant. 1997;12(3):514–518. doi: 10.1093/ndt/12.3.514. [DOI] [PubMed] [Google Scholar]