The Neural Rejuvenation Hypothesis of Cocaine Addiction (original) (raw)
. Author manuscript; available in PMC: 2015 Aug 1.
Published in final edited form as: Trends Pharmacol Sci. 2014 Jun 20;35(8):374–383. doi: 10.1016/j.tips.2014.05.005
Abstract
A leading hypothesis guiding current molecular and cellular research of drug addiction conceptualizes key aspects of addiction as a form of memory, in which common neuroplasticity mechanisms that mediate normal learning and memory processes are “hijacked” by exposure to drugs of abuse to produce pathologic addiction-related memories. Such addiction-related memories are particularly robust and long-lasting and once formed, less amenable to updating. Here, we propose the Neural Rejuvenation Hypothesis of Cocaine Addiction: that repeated exposure to drugs of abuse induces some plasticity mechanisms that are normally associated with brain development within the brain’s reward circuitry, which mediate the highly efficient and unusually stable memory abnormalities that characterize addiction.
Keywords: cocaine, addiction, silent synapse, NMDA receptor, synaptic plasticity, CREB, dendritic spine
Addiction and memory: the neural rejuvenation hypothesis
Despite its complex nature, drug addiction is at its core an acquired behavioral state formed in vulnerable individuals after they repeatedly experience cascades of emotional and motivational extremes during bouts of drug exposure and withdrawal. A major focus of the field has, therefore, been on identifying and characterizing drug-induced neuroadaptations in the relevant brain regions important for addiction1,2. An interesting observation over the years is the implication of many widely reported mechanisms of neuroplasticity, known to mediate diverse aspects of normal learning and memory, in the development of drug addiction3. Another interesting consensus that is equally applicable to the drug addiction and learning and memory fields, is that while it has been possible to identify numerous molecular and cellular adaptations involved in either or both phenomena, it has not yet been possible to understand the biological basis underlying the uniquely strong memories and habits such as those that characterize a drug-addicted state or other forms of powerful emotional memory.
In 1931, Edwin Holt suggested in his well-known essay, entitled “Animal drive and the learning process,” that some embryonic or developmental mechanisms might be used during learning4. It is generally true that younger brains are better in forming memories and, by analogy, are more vulnerable to the plastic changes that underlie addiction5,6. These considerations raise the possibility that addiction, and other forms of extremely durable emotional memory, are mediated in part by mechanisms normally involved in development. In other words, drugs of abuse awaken and then utilize in key brain regions highly efficient plasticity mechanisms, which normally occur during development, to produce abnormally robust and stable forms of memories related to addiction.
The goal of this article is to review an increasing body of experimental evidence to support this “rejuvenation” hypothesis of drug addiction: namely, that exposure to drugs of abuse reopens juvenile forms of plasticity at the molecular, cellular, and circuitry levels within the brain’s reward pathways (Box 1), and that through drug-induced neural rejuvenation and subsequent re-maturation, strong and durable maladaptive plastic changes are formed to drug-associated memories7-9. We focus on cocaine-induced plasticity in the nucleus accumbens (NAc) to illustrate the general theme of the rejuvenation hypothesis. Related mechanisms induced by other drugs of abuse and in other brain regions will be discussed more briefly to demonstrate the widespread role of juvenile-like plasticity in addiction.
Box 1. Brain reward pathways, the nucleus accumbens (NAc), and addiction.
In 1954, James Olds and Peter Milner found that electrical stimulation of certain brain regions appeared to give pleasure to experimental animals122. Later it was found that the same brain regions functioned similarly in humans. These brain regions form several relatively separate neural circuits that are thought to mediate reward-associated behavioral responses, and thus called the brain reward pathways. Among these pathways, the mesolimbic dopamine pathway, comprised of the reciprocal projection between the forebrain region nucleus accumbens (NAc) and the midbrain region ventral tegmental area (VTA), has been most extensively examined because of its clear role in responding to, predicting, and pursuing rewards. In the 1970s, Gordon Mogenson and colleagues proposed that the NAc is an interface between emotion and behavior; it functions to prioritize emotional and motivational arousals for behavioral output (reviewed in 123). This notion has since been supported by extensive experimental evidence. Drug addiction can be viewed as an emotional and motivational disorder, which develops after repeated exposure to drugs of abuse, and is expressed as compulsive and persistent drug seeking and taking. The NAc has been implicated in both the development and expression of addictive behaviors. An overly simplified scenario is that exposure to drugs of abuse induces persistent changes in the NAc, with some of these changes causing the NAc to preferentially prioritize drug-related emotional and motivational arousals into behaviors.
Insights from molecular results
At the transcriptional level
The NAc (see Box 1) is a critical component of the brain’s reward pathways that mediate addiction-related behavioral abnormalities10,11. Two prominent and extensively studied transcriptional responses in the NAc after exposure to cocaine or other drugs of abuse are activation of CREB and accumulation of ΔFosB, two transcriptional factors that regulate synaptogenesis and circuitry development12,13.
CREB is rapidly but transiently activated in the NAc upon initial exposure to cocaine or other drugs of abuse, and can be activated repeatedly upon chronic drug exposure13,14. During brain development, CREB and its downstream targets are among the central signaling pathways that regulate the survival, maturation, and integration of newly generated neurons into established neural networks15. Particularly during synaptogenesis, activation of CREB is essential for the growth of new axons, formation of new postsynaptic dendritic spines, prevention of non-specific synaptogenesis, and refinement of synaptic connections16-19. Within the NAc, activation of CREB upregulates several functional clusters of genes, in particular, genes associated with synaptic development, neuronal growth, and cell adhesion20. These genes, once repetitively transcribed and translated following repeated exposure to cocaine or other drugs of abuse, may provide essential construction blocks for the re-shaping of NAc circuits.
Unlike CREB, whose activation is transient, ΔFosB, because of its unusual stability, accumulates gradually in response to repeated exposure to cocaine or other abused drugs12,21. ΔFosB has been proposed to contribute importantly to certain long-lasting cellular and behavioral alterations following drug exposure. Upon upregulation of ΔFosB, NAc neurons express a large number of genes related to synaptic and circuitry development, in particular, glutamate NMDA receptors (NMDARs) and several other components of excitatory synapses20. NMDARs play a critical role in synaptogenesis22. As such, ΔFosB-induced NMDAR regulation may participate in the formation of new excitatory synapses and new circuits following exposure to cocaine or other drugs of abuse (see below).
Several other transcription factors, including c-Fos23 and NFκB24, as well as numerous forms of epigenetic regulation21, induced by exposure to cocaine are also potentially important, but are not discussed in this article. Rather, activation of CREB- and ΔFosB-signaling is used here as a prototype of how diverse types of transcriptional mechanisms contribute to the reemergence of developmental forms of plasticity in the NAc after exposure to cocaine or other drugs of abuse.
At the neurotrophic level
Chip-based gene screening shows that expression levels of neurotrophins as well as their receptors are increased in the NAc of human cocaine abusers25. In the neonatal brain, neurotrophins are expressed at high levels in the brain sites where new synapses are formed and new circuits are defined26. In the developed brain, expression of neurotrophins can be re-induced during reparative processes after brain injury26. Following exposure to cocaine or other drugs of abuse, one of the neurotrophins, brain-derived neurotrophic factor (BDNF), is upregulated in the NAc shell, ventral tegmental area (VTA), and related reward regions27-29 and directly implicated in cocaine self-administration, conditioned place preference, and locomotor sensitization30.
During brain development, BDNF promotes synapse formation both pre- and postsynaptically. Presynaptically, BDNF promotes axonal branching and organization31. On postsynaptic side, BDNF promotes dendritic arborization32, increases the number, size, and motility of dendritic spines33-35, and enhances synthesis of synaptic proteins36. In animal models in which BDNF is overexpressed an increased number of synapses and increased number of docked neurotransmitter vesicles are observed37.
The pro-synaptogenesis effects of BDNF are likely mediated by activation of the BDNF receptor, TrkB38,39, a type of tyrosine receptor kinase. After binding to BDNF, TrkB activates several intracellular and nuclear signaling pathways, among which is the ERK signaling cascade that directly phosphorylates CREB Ser 133 to induce CREB activation. Given the demonstrated role of CREB in dendritic spine formation, synaptic connectivity, expression of synaptic proteins, and synaptic plasticity, the pro-synaptogenesis effects of BDNF are probably mediated in part by BDNF’s activation of CREB40-42. Likewise, one of the promoter regions of the Bdnf gene is activated by CREB43, creating a feed-forward loop that controls the synaptic effects of BDNF and CREB. Other signaling pathways downstream of BDNF, such as AKT-mTOR as just one example, are also likely involved in the regulation of synaptic structure and function and warrant further study.
In animal studies, increased levels of Bdnf transcripts are observed in the NAc and related brain regions immediately after acute or repeated i.p. injections of cocaine28,44. However, increased protein levels of BDNF in the NAc build gradually during withdrawal from cocaine self-administration, achieving maximum levels only after 30 days of withdrawal27. This “incubation” pattern (see below) suggests that upregulation of BDNF might be a delayed downstream consequence of CREB activation after exposure to cocaine or, alternatively, partly independent of CREB activation and mediated by different mechanisms either locally within NAc or in several afferent brain regions.
Taken together, exposure to cocaine or other drugs of abuse induces alterations in transcription factors and neurotrophins, which have been intrinsically implicated in synaptogenesis and circuitry development in the developing brain. Because of these and other processes, dormant developmental mechanisms in the adult brain may be reawakened in response to drugs of abuse to restructure existing synapses or even form new synapses in the NAc and other addiction-associated brain regions. As discussed below, excitatory synapses in the NAc and other reward regions exhibit several characteristic developmental features after cocaine exposure.
Rejuvenation of excitatory synapses
Re-enrichment of GluN2B-containing NMDARs
Excitatory synapses in the NAc, formed from projections from several limbic and paralimbic brain regions (Fig. 1), are targets of drugs of abuse to induce addition-related behaviors.45 At a fully functional excitatory synapse, there are two major types of ionotropic glutamate receptors, NMDARs and AMPA receptors (AMPARs). Whereas AMPARs serve as the main mediator of excitatory synaptic transmission, NMDARs mainly function by regulating synaptic transmission through their coupled intracellular signaling mechanisms. In many brain regions, a signature feature of newborn excitatory synapses is the enrichment in GluN2B-containing NMDARs46. During synaptogenesis, GluN2B NMDARs may function to drive the formation of postsynaptic structures; overexpression of these receptors substantially increases the number of dendritic spines47. During synapse maturation, these receptors are thought to maintain the immature status of excitatory synapses, allowing a highly fine-tuned synaptic maturation to occur48,49. Synaptic GluN2B NMDARs are gradually replaced by GluN2A NMDARs along the course of brain development46, and this switch reflects the maturation of excitatory synapses. A major functional difference between GluN2A and GluN2B NMDARs is that the latter possess a substantially higher ability to activate Ca2+/calmodulin-dependent protein kinase II (CaMKII) signaling, which is strongly implicated in mediating activity-dependent synaptic strengthening50.
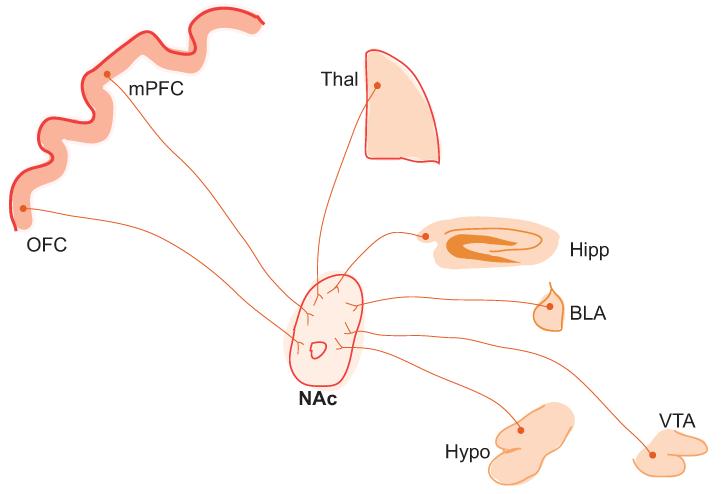
Figure 1. Diagram showing major limbic and paralimbic glutamatergic projections to the NAc.
mPFC, the medial prefrontal cortex which comprises the infralimbic and prelimbic PFC; OFC, the orbitofrontal cortex; Hipp, the hippocampus; BLA, the basolateral amygdala; and Thal, the thalamus. Other major inputs to the NAc include the VTA (ventral tegmental area) and Hypo (hypothalamus), which send dopaminergic and peptidergic inputs to the NAc, although some of these projections may also be glutamatergic.
Most NMDARs in the developed brain are believed to be tetramers, containing two GluN1 and two GluN2 subunits. Following non-contingent exposure to cocaine, the cell surface levels of GluN1 and GluN2B subunits in NAc neurons is increased, accompanied by an increase in the ratio of GluN2B to GluN2A NMDAR-mediated synaptic currents51. These results suggest that excitatory synapses in the NAc become re-enriched in GluN2B-containing NMDARs following repeated exposure to cocaine. Additional evidence suggests that cocaine-induced re-enrichment of synaptic GluN2B is mediated by activation of CREB52. The genes encoding GluN1 and GluN2B, but not GluN2A, contain a CREB-binding site, which can be activated upon CREB activation53. In NAc slice cultures, activation of CREB increases protein levels of GluN1 and GluN2B subunits, but not GluN2A subunits52,54. In cocaine-experienced rats, preventing cocaine-induced activation of CREB in NAc neurons prevents cocaine-induced increases in the GluN2B/GluN2A ratio at excitatory synapses52.
In addition to CREB, increased levels of ΔFosB selectively prolong the decay kinetics of NMDAR excitatory postsynaptic currents (EPSCs) in a subpopulation of NAc neurons (those expressing D1 dopamine receptors), a biophysical change of NMDARs consistent with an increase in GluN2B NMDARs55. It is not clear whether CREB and ΔFosB share the same signaling cascades or operate independently in regulating synaptic expression of GluNR2B NMDARs.
Interestingly, the re-enrichment of GluN2B at NAc excitatory synapses may exhibit delayed and sometimes dynamic patterns, depending on the cocaine regimen56. Furthermore, synaptic re-enrichment of GluN2B is also observed after exposure to other drugs of abuse, such as heroin and nicotine57,58.
In general, younger synapses exhibit a higher ability to undergo experience-dependent plastic changes likely because different and presumably more efficient plasticity mechanisms are employed, some involving NMDARs59-61. In addition, internal or external experience may influence the stoichiometric ratio of GluN2B/2A of NMDARs to dictate the direction of synaptic plasticity (i.e., potentiation or depression). This has been extensively demonstrated in the visual cortex, in which excitatory synapses become more prone to undergo LTP in animals with little visual stimulation (animals reared in dark)62,63. This form of metaplasticity is proposed to be mediated by an increase in the GluN2B/2A ratio induced by dark-rearing, and preventing the shift of GluN2B/2A by knocking out GluN2A subunits erases the shift of the long-term potentiation (LTP) threshold in dark-reared animals63-66. Thus, a logical extension of these results is that an increase in the relative weight of GluN2B subunits in synaptic NMDARs facilitates the induction of LTP at excitatory synapses. If so, cocaine-induced upregulation of synaptic GluN2B NMDARs may contribute to the observed LTP-like effects at NAc synapses after cocaine withdrawal67 or during cue- or cocaine priming-induced reinstatement of cocaine-seeking behavior after withdrawal68,69.
Re-emergence of GluN3A NMDARs
In addition to extensively expressed GluN1 and GluN2 subunits, non-canonical GluN3A subunits are also found in some NMDARs, which are highly expressed during early development, but sharply down-regulated and remains low in adulthood70. Following a single i.p. injection of cocaine in young adult mice, many GluN3A-GluN2B NMDARs are detected in VTA dopamine neurons71. Both GluN3A72 and GluN2B49 are known to limit synaptic insertion of AMPARs during brain development. Although it is intriguing to speculate that cocaine-induced upregulation of GluN3A/GluN2B NMDARs may function to retain VTA excitatory synapses in the relatively “unsaturated”, immature status during brain development, empirical results suggest otherwise. It is shown that cocaine-induced GluN1/GluN3A/GluN2B triheteromers promote synaptic accumulation of atypical, GluR2-lacking AMPARs in VTA dopamine neurons71.
Silent synapses
Along with the overall cocaine-induced re-enrichment of GluN2B NMDARs in the NAc, further evidence suggests that at least a portion of these newly synthesized GluN2B NMDARs exist in AMPAR-silent excitatory synapses that are likely newly generated upon cocaine exposure9,51,52.
AMPAR-silent excitatory synapses (Box 2) have a prominent developmental signature; they are highly enriched in the neonatal brain and decrease to a very low level after development73. In these synapses, only NMDAR-mediated responses are stably detected; AMPARs, if any, are highly labile and not reliably detected through evoked EPSCs74. As such, silent synapses can be functionally regarded as AMPAR-silent, NMDAR-only synapses. Because synaptic insertion of AMPARs serves as a critical mechanism through which excitatory synapses are potentiated, silent synapses, which are “primed” to undergo both electrophysiological strengthening and anatomical growth75, have been proposed as highly efficient plasticity substrates that may serve as one of the key cellular mechanisms for the high learning ability of young brains76-80.
Box 2. Silent synapses.
The concept of silent synapse was first raised by the finding of “ineffective synapses”, at which activation of presynaptic fibers fails to trigger postsynaptic responses124. Some of these ineffective synapses were later found to possess typical morphology of synapses, but with either a non-functional postsynaptic membrane or non-functional presynaptic release125,126, thus rendering them “silent”. Focusing on hippocampal CA1 neurons, two studies were published in 1995, demonstrating a form of AMPAR-silent excitatory synapses77,93. This form of silent synapse possesses fully functional presynaptic release, which can trigger reliable NMDAR-mediated responses postsynaptically, but AMPAR-mediated responses are minimal. Because of their magnesium-mediated blockade, NMDARs conduct very little current at resting membrane potentials. As such, these synapses are often silent when the postsynaptic neurons dwell in their resting state. Silent synapses have received a great deal of attention for their role in long-term potentiation (LTP), with experience-dependent unsilencing of silent synapses via the recruitment of AMPARs resulting in postsynaptic strengthening of synaptic transmission76. Silent synapses were subsequently found to be extremely abundant in the developing brain but diminish in adulthood73. Because of this and other properties, silent synapses are thought to represent newly generated synaptic contacts, but convincing experimental results are still lacking76. Abnormal generation of silent synapses in the adult brain has been implicated in a variety of brain diseases127.
With respect to cocaine-generated silent synapses, Thomas, Malenka and colleagues initially showed that in mice with repeated injections of cocaine and a withdrawal period, re-exposure to cocaine decreases the AMPAR/NMDAR ratio at NAc excitatory synapses128. They proposed that this cocaine-induced long-term depression (LTD)-like effect may be accompanied by generation of AMPAR-silent synapses128. Subsequent studies from the Wolf laboratory showed that the cell surface levels of AMPARs in NAc neurons are not altered after short-term cocaine withdrawal, suggesting that the potentially altered AMPAR/NMDAR ratio during early withdrawal periods is likely due to changes in NMDARs129. These studies, together with related work in hippocampus77,80,93, paved the road toward the demonstration of cocaine-induced synaptic insertion of GluN2B NMDARs and generation of silent synapses in the NAc51,52. In contrast to early withdrawal periods, the cell surface and synaptic levels of AMPARs, particularly calcium-permeable AMPARs (CP-AMPARs), are increased in the NAc after prolonged withdrawal from cocaine self-administration130,131. Recent results suggest that some of these newly inserted CP-AMPARs are recruited by cocaine-generated silent synapses during cocaine withdrawal, resulting in maturation of these synapses and remodeling of affected NAc circuits102.
Following repeated i.p. injections of cocaine, a large portion of silent synapses are detected in the NAc51. These cocaine-generated silent synapses appear to be present at most NAc excitatory inputs52, and are enriched in GluN2B NMDARs51. These findings suggest that exposure to cocaine not only rejuvenates excitatory synapses, but also creates highly efficient plasticity substrates to form new and potentially more durable adaptive changes. Taken together with the observations that NAc excitatory synapses exhibit an enhanced ability to undergo LTP immediately after cocaine exposure81, and that the excitatory synaptic strength is greatly increased after long-term withdrawal from cocaine exposure45, a recent review proposes that generation of silent synapses is a form of metaplasticity induced by cocaine to prime NAc excitatory synapses for strengthening during withdrawal9.
Whereas silent synapses can be generated either by a complete internalization of AMPARs or by insertion of NMDARs in new synaptic contacts, further analysis supports the latter possibility for cocaine-generated silent synapses (Fig. 2). First, cocaine-induced generation of synapses in NAc is accompanied by an increased number of dendritic spines, suggesting that new, synapse-like structures are formed82,83. Most of the new spines appear to be immature thin spines, as opposed to more mature mushroom-shaped spines (but see below)84. Second, cocaine-induced generation of silent synapses is abolished upon blockade of cocaine-induced activation of CREB52, and likely ∆FosB too85,86; both transcriptional factors have been critically implicated in synaptogenesis. Third, cocaine-induced generation of silent synapses is accompanied by the insertion of new NMDARs, and inhibiting newly inserted NMDARs disables cocaine-generated silent synapses51, suggesting that these silent synapses are formed in an NMDAR-driven manner rather than via the internalization of AMPARs at pre-existing synapses. Finally, cocaine-generated silent synapses are enriched in GluN2B NMDARs9,51,52, the type of NMDARs that are highly enriched in nascent synapses74,87,88. Thus, cocaine-induced generation of silent synapses appears to involve the formation of new synapses (Figs. 2, 3) and strengthening of affected neural circuits. Whereas certain forms of experience-dependent synaptic plasticity in the adult brain may be achieved by local events involving small numbers of affected synapses, silent synapse-based plasticity induced in NAc by chronic cocaine exposure seems to occur more generally and involves activation of transcription factors, upregulation of GluN2B NMDARs, synaptic recruitment of high-conductance AMPARs (see below), and generation of new spines and new synapses for circuitry remodeling.
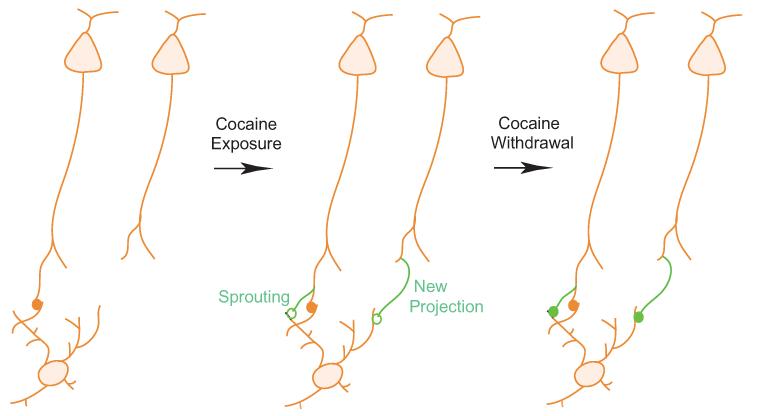
Figure 2. Hypothesized scheme showing generation of new synapses in the nucleus accumbens following repeated exposure to cocaine.
Left: NAc neurons receive excitatory synaptic input from several projection sites (see Figure 1). Middle: Repeated exposure to cocaine generates nascent, immature (open green circles) excitatory synaptic contacts on NAc neurons. These nascent synaptic contacts may be generated through the growth of new spines on NAc neurons (see Figure 3) in conjunction with: i) axonal sprouting from the same presynaptic terminals that have existing synaptic innervations on the same dendritic area; or ii) newly grown axons from a different population of projection neurons that do not normally have innervations on the same dendritic area of these NAc neurons. Right: During long-term withdrawal from cocaine exposure, a subset of newly generated excitatory synapses develop from their immature form to a more mature form (filled green circles), thus potentially creating new circuits.
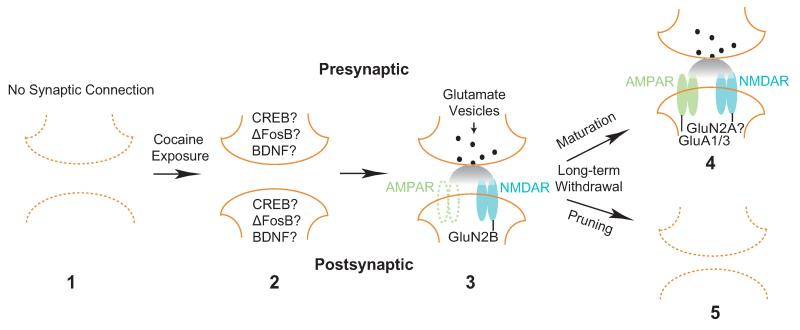
Figure 3. Scheme of a silent synapse-based hypothesis of cocaine-induced synaptic formation following repeated cocaine exposure.
According to this hypothesis, exposure to cocaine generates nascent immature excitatory synapses de novo in the NAc. 1) The potential cocaine-induced nascent synapses are either dormant (inactive) or do not exist before exposure to cocaine. 2) Exposure to cocaine activates signaling cascades, likely involving CREB, ∆FosB, BDNF, and numerous synaptogenesis molecules, both in the NAc to mediate postsynaptic adaptations and in innervating glutamatergic neurons to mediate presynaptic adaptations. 3) During cocaine exposure, initial synaptic function is acquired within these newly generated synaptic contacts by insertion of GluN2B-enriched NMDARs. AMPARs are either not inserted or inserted together with NMDARs but are highly labile and not functionally stable. Thus, these newly generated synapses are AMPAR-silent at the functional level. 4 and 5) During long-term withdrawal from cocaine exposure, some of these cocaine-induced silent synapses develop into fully functional excitatory synapses by recruiting calcium-permeable AMPRARs (CP-AMPARs) (4), whereas others are pruned away (5). Through maturation of a portion of silent synapses, cocaine experience can induce new neural circuits in the NAc.
Potential synaptogenesis
It has long been thought that experience triggers synapse formation89 (Box 3), a notion supported for example by observations in sensory cortices in which the structural dynamics associated with synapse formation and elimination occurs in response to sensory experience90-92. Whereas modification of existing synapses is an important means to change synaptic and circuitry properties, generation of new synapses might create new circuits that mediate distinct informational flow for newly acquired associations. This is particularly relevant to the development of drug addiction, during which new multi-dimensional associations are established in subjects who have never before experienced drugs of abuse.
Box 3. Synaptogenesis.
Synaptogenesis is a critical process for the development of neural circuitry. An important initial step is for presynaptic terminals (e.g., axonal growth cone) and postsynaptic spines being oriented toward one another to achieve a precise and effective connection, a process likely involving numerous target-derived factors, both diffusible and membrane-associated132. Such pre- and postsynaptic membranes are bound together through cell-adhesion molecules133 and other activity-induced factors134. This is followed by structural refining of newly formed synapses, including clustering of postsynaptic receptors and stabilization of the presynaptic release machinery22. The pre- and postsynaptic properties of newly generated synapses are subject to continuous evolution in response to internal and external factors.
It is important to take one step back and realize that, whether drug-induced or developmentally regulated, it remains debatable whether silent synapses represent an initial stage in synaptogenesis. On the one hand, a large portion of excitatory synapses are silent synapses at early developmental stages, and most mature excitatory synapses are dominated by AMPARs73,76. Silent synapses recruit AMPARs in an activity-dependent manner73,77,93,94, and such unsilencing of silent synapses and the corresponding increase of AMPAR/NMDAR ratio signal the maturation of excitatory synapses76. On the other hand, several studies demonstrate that during developmental synaptogenesis AMPARs and NMDARs arrive at excitatory synapses at the same time95,96. In a recent study using two-photon glutamate uncaging at single spines, newly generated dendritic spines exhibit AMPAR-mediated activity that is comparable to their neighboring mature spines97. Furthermore, AMPAR-silent spines are detectable and are not correlated with the age of the spines97. Therefore, if all excitatory synapses are generated with both AMPARs and NMDARs present, why is there such a high level of silent synapses detected in the developing brain?
There are at least two scenarios that may reconcile these seemingly discrepant results. The first is that both AMPARs and NMDARs are delivered together to newly formed synapses95,96, but AMPARs are highly labile74, are prone to be internalized upon basal synaptic activity98, and thus become silent shortly after being generated. Some silent synapses re-express/stabilize AMPARs when experiencing activity-dependent regulation and eventually evolve into fully matured synapses. In this scenario, the initially internalized AMPARs may serve as a readily available pool for AMPARs, with synaptic GluN2B NMDARs functioning to retain the silent nature of the synapses until activity-dependent regulation49. The second scenario involves two types of new synapses/spines that are generated in different ways. Due to technical difficulties, most two-photon work has focused to date on relatively large, mushroom-like spines, whereas thin, filopodia-like spines have not yet been sufficiently examined. It is clear that the level of AMPARs is strongly correlated with spine volume, whereas such correlation is lacking or very weak for NMDARs99-101. It is possible that new excitatory synapses are generated in two subtypes, one as relatively large spines containing both AMPARs and NMDARs, and the other as thin spines with NMDARs only. It is notable that, following exposure to cocaine, the numbers of both thin and large spines in the NAc are increased, with increases in thin spines possibly predominating at early withdrawal time points and increases in mushroom spines predominating at later times of withdrawal 52,84. However, this remains controversial and varies with different cocaine treatment regimens, with a careful, comprehensive analysis of spine induction after cocaine self-administration not yet available. As such, if the second scenario stands, it would appear that both types of synaptogenesis are initiated in cocaine-experienced animals. Nonetheless, nascent or not, silent synapses are a signature property of the developing brain, and generation of these structures represents the neural rejuvenation in response to cocaine exposure.
Silent synapse-based re-development of NAc circuitry
Once generated, silent synapses face two cellular fates: to be pruned away by metabolic turnover, or to undergo a maturation process and evolve into fully functional synapses. There is now evidence supporting the latter possibility. Indeed, it has been shown that generation and subsequent maturation of silent synapses are crucial cellular cascades triggered by cocaine exposure to re-develop key neural circuits in order to establish addiction-associated behaviors.
Such silent synapse-based re-development of an NAc circuit is exemplified in our recent study focusing on the projection of basolateral amygdala (BLA) glutamatergic neurons to the NAc. Within this projection, a large number of silent synapses are first generated soon after cocaine self-administration. Over a month of cocaine withdrawal, functional AMPARs are gradually recruited to these silent synapses, which convert them to becoming fully functional102. Further results show that AMPARs that un-silence silent synapses are atypical, Ca2+-permeable (CP) receptors102. Insertion of CP-AMPARs not only strengthens synaptic strength, but also potentially creates new synaptic signaling pathways, evokes new plasticity rules, and redefines the transmission properties of the circuits67,103. Thus, if cocaine-generated silent synapses are indeed nascent synaptic contacts, unsilencing of these silent synapses by recruiting CP-AMPARs may create new BLA-to-NAc informational flow much different from that carried by pre-existing BLA- to-NAc projections (Fig. 3).
Collectively, the BLA-to-NAc projection undergoes a two-phase reorganization following cocaine exposure: generation of silent synapses upon exposure, followed by the gradual unsilencing of these silent synapses during prolonged cocaine withdrawal. What behavioral consequences are mediated by this silent synapse-based reorganization of BLA-to-NAc projections? This circuit has long been implicated in many forms of cue-induced motivated behaviors104-108 including cue-induced drug seeking109. In rodent models of addiction and relapse, the progressive increase in cue-induced cocaine seeking during withdrawal—termed incubation of cocaine craving (Box 4)— exhibits two-phases with a time course that approximates the generation and maturation of silent synapses. Particularly, soon after cocaine self-administration when silent synapses are generated, animals establish the initial association between cocaine intake and cocaine-associated cues such that, by the end of the training, the cues alone (without cocaine) are sufficient to induce cocaine seeking. Over the course of withdrawal during which silent synapses are unsilenced, cue-induced cocaine seeking progressively increases110,111. To examine the role of silent synapse-based reorganization of BLA-to-NAc projections in cocaine incubation, we induced internalization of CP-AMPARs selectively from BLA-to-NAc synapses after cocaine withdrawal102. This in vivo manipulation does not eliminate cocaine-induced silent synapses, but re-silences those already unsilenced synapses, pushing the synaptic state back to the first phase. Reversing the maturation of silent synapses within the BLA-to-NAc projection reverses the intensification (incubation) of cue-induced cocaine seeking after drug withdrawal102.
Box 4. Incubation of cue-induced cocaine craving.
Drug craving is an emotional and motivational urge to consume drugs. In animal models, craving has been inferred from behavioral responses, such as pressing a lever that was previously associated with cocaine delivery. It was first hypothesized135 and subsequently observed in animal models110,136 that cue-induced cocaine craving progressively increases during a period of drug abstinence. This intensification process, which is often referred to as ‘incubation of craving’, has also been observed in animals exposed to methamphetamine137, alcohol138, nicotine139, or sucrose140. These incubation models identify the withdrawal period as a crucial substrate for addiction-related behavioral abnormalities as well as a possible therapeutic window during which to intervene to reduce drug craving and relapse.
These findings raise several interesting questions: 1) Previous results suggest that silent synapses are also generated in response to cocaine exposure within other glutamatergic projections to the NAc, which arise from several limbic and paralimbic brain regions and play differential roles in the development and maintenance of addiction51,52. Does silent synapse-based rejuvenation also occur in other projections, and if so, what are the corresponding behavioral consequences? 2) It has been known that different drug exposure patterns produce very different behavioral consequences. Is silent synapse-based synaptic and circuitry re-development triggered similarly by different drug treatment regimens? 3) Exposures to different classes of drugs of abuse exert very different pharmacological and physiological effects initially but they all lead to addition eventually. Is silent synapse-based synaptic and circuitry rejuvenation triggered by exposure to other drugs of abuse? 4) During brain development, organized synaptic pruning is an essential step after synaptogenesis to build efficient neural networks. In addition to maturation, are some cocaine-generated silent synapses pruned away as part of circuitry remodeling during cocaine withdrawal? 5) Can other behavioral experiences influence the maturation or potential pruning of cocaine-generated silent synapses to achieve therapeutic effects? Addressing these questions will substantially deepen our understanding about how synapses and neural circuitry evolve to mediate drug addiction.
Other rejuvenated neuronal substrates
Inhibitory synapses
Emerging evidence reveals that inhibitory synaptic transmission also undergoes certain degrees of rejuvenation following exposure to cocaine. Fast GABAergic inhibitory synaptic transmission is primarily mediated by GABAA receptors, the composition of which is under developmental control. For example, ɑ2/3 subunit-enriched GABAA receptors are predominant during early developmental stages and are gradually replaced by ɑ1 subunit-enriched GABA receptors in the adult112,113 A. This developmental switch of GABAA receptor subunit composition substantially accelerates the activation and inactivation rate of inhibitory postsynaptic currents (IPSCs), presumably improving the precision of temporal inhibition over the neural circuit.
Following exposure to cocaine, whole brain levels of ɑ1 subunits are reduced114, a change that can be regarded as reversing the development of GABAA receptors. By contrast, we showed recently that chronic cocaine induces in the NAc expression levels of ɑ1 subunits, with no change in ɑ2 subunits, and that this is accompanied by an increased frequency of spontaneous IPSCs of NAc neurons115. Also, blockade of GABAA receptors in NAc prevents the development of sensitized locomotor responses to repeated cocaine. While these findings suggest that a cocaine-induced reemergence of a developmental process controlling GABAA function may also occur in the NAc, further work is needed to identify the brain areas that do show the cocaine-induced suppression of ɑ1 expression and recapitulation of a developmental mode of plasticity as well as its cellular and behavioral consequences.
Neurogenesis
While promoting synaptogenesis, exposure to drugs of abuse has been shown to dampen adult neurogenesis in the hippocampus. Neurogenesis and neurogenesis-related migration of progenitor cells in the adult brain are reliably observed predominantly in the subgranular zone (SGZ) on the border of the granule cell layer in the hippocampus, and the subventricular zone along the walls of the lateral ventricles116-119. It has been consistently demonstrated that exposure to opiates, nicotine, alcohol, or cocaine decreases the proliferation or differentiation of neural progenitor cells within the hippocampal SGZ120,121. Experimentally reducing adult hippocampal neurogenesis confers vulnerability in animal models of cocaine addiction, whereas behavioral stimulations such as voluntary wheel running that enhance neurogenesis reduce behavioral responses elicited by cocaine or other drugs of abuse120. Despite these important results, more work is needed to establish the precise role of adult neurogenesis in drug addiction. It is likely neurogenesis per se does not carry intrinsic pro- or anti-addiction information, however, impairment of the neural networks formed from newly plugged-in neurons may contribute to drug-associated memories. It will be interesting in future studies to examine how drugs of abuse—through the regulation of neurogenesis—influence specific hippocampal circuits and their connections with other limbic brain regions.
Concluding remarks
Experimental evidence suggests that exposure to cocaine rejuvenates specific neural substrates, awakening certain dormant developmental plasticity mechanisms to form unusually strong and long-lasting maladaptive changes that underlie key aspects of drug-related memories. Cocaine-induced neuronal rejuvenation is followed by subsequent re-maturation processes during drug withdrawal, which contributes to the strengthening and perpetuation of the resulting synaptic, circuitry, and behavioral abnormalities. Such a role for developmental mechanisms is likely to be highly region-specific. We focused here on the effects of cocaine on the NAc, which has been the focus of most studies to date. Similar studies of other drugs of abuse and many other reward-related brain regions are now a high priority for the field. The notion that drugs of abuse first trigger and then utilize developmental plasticity mechanisms offers a new way to understand and explore the cellular and circuitry processes underlying the development of drug addiction and to use this evolving information to generate improved treatments for addictive disorders.
Highlights.
- Addiction may be partly mediated by mechanisms normally involved in development
- Juvenile substrates of molecular/neural plasticity reemerge after cocaine exposure
- Rejuvenated neural circuits are highly plastic and mature during cocaine withdrawal
- This creates strong and durable maladaptive changes underlying addiction
Acknowledgement
Related work from the authors was supported by NIH DA023206, DA030379, and DA034856 (YD); and DA008227 and DA014133 (EJN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors have no conflict of interest to declare.
References
- 1.Shaham Y, Hope BT. The role of neuroadaptations in relapse to drug seeking. Nat Neurosci. 2005;8:1437–1439. doi: 10.1038/nn1105-1437. [DOI] [PubMed] [Google Scholar]
- 2.Kalivas PW. How do we determine which drug-induced neuroplastic changes are important? Nat Neurosci. 2005;8:1440–1441. doi: 10.1038/nn1105-1440. [DOI] [PubMed] [Google Scholar]
- 3.Nestler EJ. Common molecular and cellular substrates of addiction and memory. Neurobiology of learning and memory. 2002;78:637–647. doi: 10.1006/nlme.2002.4084. [DOI] [PubMed] [Google Scholar]
- 4.Holt EB. Animal Drive and the Learning Process. Edwin Holt. 1931 [Google Scholar]
- 5.Ehrlich ME, et al. Periadolescent mice show enhanced DeltaFosB upregulation in response to cocaine and amphetamine. J Neurosci. 2002;22:9155–9159. doi: 10.1523/JNEUROSCI.22-21-09155.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong WC, et al. Adolescents are more vulnerable to cocaine addiction: behavioral and electrophysiological evidence. J Neurosci. 2013;33:4913–4922. doi: 10.1523/JNEUROSCI.1371-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Creed MC, Luscher C. Drug-evoked synaptic plasticity: beyond metaplasticity. Current opinion in neurobiology. 2013;23:553–558. doi: 10.1016/j.conb.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Huang YH, et al. An unusual suspect in cocaine addiction. Neuron. 2013;80:835–836. doi: 10.1016/j.neuron.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee BR, Dong Y. Cocaine-induced metaplasticity in the nucleus accumbens: Silent synapse and beyond. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wise RA. The role of reward pathways in the development of drug dependence. Pharmacol Ther. 1987;35:227–263. doi: 10.1016/0163-7258(87)90108-2. [DOI] [PubMed] [Google Scholar]
- 11.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 13.Chao J, Nestler EJ. Molecular neurobiology of drug addiction. Annu Rev Med. 2004;55:113–132. doi: 10.1146/annurev.med.55.091902.103730. [DOI] [PubMed] [Google Scholar]
- 14.Carlezon WA, Jr., et al. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- 15.Merz K, et al. CREB in adult neurogenesis--master and partner in the development of adult-born neurons? The European journal of neuroscience. 2011;33:1078–1086. doi: 10.1111/j.1460-9568.2011.07606.x. [DOI] [PubMed] [Google Scholar]
- 16.Aguado F, et al. The CREB/CREM transcription factors negatively regulate early synaptogenesis and spontaneous network activity. J Neurosci. 2009;29:328–333. doi: 10.1523/JNEUROSCI.5252-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 18.Lonze BE, et al. Apoptosis, axonal growth defects, and degeneration of peripheral neurons in mice lacking CREB. Neuron. 2002;34:371–385. doi: 10.1016/s0896-6273(02)00686-4. [DOI] [PubMed] [Google Scholar]
- 19.Murphy DD, Segal M. Morphological plasticity of dendritic spines in central neurons is mediated by activation of cAMP response element binding protein. Proc Natl Acad Sci U S A. 1997;94:1482–1487. doi: 10.1073/pnas.94.4.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- 21.Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waites CL, et al. Mechanisms of vertebrate synaptogenesis. Annu Rev Neurosci. 2005;28:251–274. doi: 10.1146/annurev.neuro.27.070203.144336. [DOI] [PubMed] [Google Scholar]
- 23.Guez-Barber D, et al. FACS identifies unique cocaine-induced gene regulation in selectively activated adult striatal neurons. J Neurosci. 2011;31:4251–4259. doi: 10.1523/JNEUROSCI.6195-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russo SJ, et al. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J Neurosci. 2009;29:3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albertson DN, et al. Distinctive profiles of gene expression in the human nucleus accumbens associated with cocaine and heroin abuse. Neuropsychopharmacology. 2006;31:2304–2312. doi: 10.1038/sj.npp.1301089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grimm JW, et al. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filip M, et al. Alterations in BDNF and trkB mRNAs following acute or sensitizing cocaine treatments and withdrawal. Brain research. 2006;1071:218–225. doi: 10.1016/j.brainres.2005.11.099. [DOI] [PubMed] [Google Scholar]
- 29.Graham DL, et al. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- 30.Lobo MK, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen-Cory S, Fraser SE. Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature. 1995;378:192–196. doi: 10.1038/378192a0. [DOI] [PubMed] [Google Scholar]
- 32.McAllister AK, et al. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 33.Tyler WJ, Pozzo-Miller L. Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones. The Journal of physiology. 2003;553:497–509. doi: 10.1113/jphysiol.2003.052639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horch HW, et al. Destabilization of cortical dendrites and spines by BDNF. Neuron. 1999;23:353–364. doi: 10.1016/s0896-6273(00)80785-0. [DOI] [PubMed] [Google Scholar]
- 35.Ji Y, et al. Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nat Neurosci. 2010;13:302–309. doi: 10.1038/nn.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tartaglia N, et al. Protein synthesis-dependent and -independent regulation of hippocampal synapses by brain-derived neurotrophic factor. The Journal of biological chemistry. 2001;276:37585–37593. doi: 10.1074/jbc.M101683200. [DOI] [PubMed] [Google Scholar]
- 37.Aguado F, et al. BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/Cl- co-transporter KCC2. Development. 2003;130:1267–1280. doi: 10.1242/dev.00351. [DOI] [PubMed] [Google Scholar]
- 38.Minichiello L, et al. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24:401–414. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]
- 39.Chen X, et al. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Impey S, et al. Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell. 2004;119:1041–1054. doi: 10.1016/j.cell.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 41.Arthur JS, et al. Mitogen- and stress-activated protein kinase 1 mediates cAMP response element-binding protein phosphorylation and activation by neurotrophins. J Neurosci. 2004;24:4324–4332. doi: 10.1523/JNEUROSCI.5227-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu B, et al. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:401–416. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- 43.Tao X, et al. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 44.Fumagalli F, et al. Repeated exposure to cocaine differently modulates BDNF mRNA and protein levels in rat striatum and prefrontal cortex. The European journal of neuroscience. 2007;26:2756–2763. doi: 10.1111/j.1460-9568.2007.05918.x. [DOI] [PubMed] [Google Scholar]
- 45.Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Zundert B, et al. Receptor compartmentalization and trafficking at glutamate synapses: a developmental proposal. Trends Neurosci. 2004;27:428–437. doi: 10.1016/j.tins.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- 48.Gambrill AC, Barria A. NMDA receptor subunit composition controls synaptogenesis and synapse stabilization. Proc Natl Acad Sci U S A. 2011;108:5855–5860. doi: 10.1073/pnas.1012676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gray JA, et al. Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: single-cell NMDA receptor subunit deletion in vivo. Neuron. 2011;71:1085–1101. doi: 10.1016/j.neuron.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Halt AR, et al. CaMKII binding to GluN2B is critical during memory consolidation. The EMBO journal. 2012;31:1203–1216. doi: 10.1038/emboj.2011.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang YH, et al. In vivo cocaine experience generates silent synapses. Neuron. 2009;63:40–47. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown TE, et al. A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J Neurosci. 2011;31:8163–8174. doi: 10.1523/JNEUROSCI.0016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klein M, et al. Cloning and characterization of promoter and 5′-UTR of the NMDA receptor subunit epsilon 2: evidence for alternative splicing of 5′-non-coding exon. Gene. 1998;208:259–269. doi: 10.1016/s0378-1119(98)00005-5. [DOI] [PubMed] [Google Scholar]
- 54.Huang YH, et al. CREB modulates the functional output of nucleus accumbens neurons: a critical role of N-methyl-D-aspartate glutamate receptor (NMDAR) receptors. The Journal of biological chemistry. 2008;283:2751–2760. doi: 10.1074/jbc.M706578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grueter BA, et al. FosB differentially modulates nucleus accumbens direct and indirect pathway function. Proc Natl Acad Sci U S A. 2013;110:1923–1928. doi: 10.1073/pnas.1221742110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schumann J, Yaka R. Prolonged withdrawal from repeated noncontingent cocaine exposure increases NMDA receptor expression and ERK activity in the nucleus accumbens. J Neurosci. 2009;29:6955–6963. doi: 10.1523/JNEUROSCI.1329-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gipson CD, et al. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci U S A. 2013;110:9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shen H, et al. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc Natl Acad Sci U S A. 2011;108:19407–19412. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee H-K, et al. NMDA receptor-independent long-term depression correlates with successful aging in rats. Nature Neuroscience. 2005;8:1657–1659. doi: 10.1038/nn1586. [DOI] [PubMed] [Google Scholar]
- 60.Yasuda H, et al. A developmental switch in the signaling cascades for LTP induction. Nat Neurosci. 2003;6:15–16. doi: 10.1038/nn985. [DOI] [PubMed] [Google Scholar]
- 61.Maffei A, Turrigiano G. The age of plasticity: developmental regulation of synaptic plasticity in neocortical microcircuits. Progress in brain research. 2008;169:211–223. doi: 10.1016/S0079-6123(07)00012-X. [DOI] [PubMed] [Google Scholar]
- 62.Kirkwood A, et al. Experience-dependent modification of synaptic plasticity in visual cortex. Nature. 1996;381:526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- 63.Philpot BD, et al. Evidence for altered NMDA receptor function as a basis for metaplasticity in visual cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:5583–5588. doi: 10.1523/JNEUROSCI.23-13-05583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quinlan EM, et al. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nature neuroscience. 1999;2:352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- 65.Quinlan EM, et al. Bidirectional, experience-dependent regulation of N-methyl-D- aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci U S A. 1999;96:12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Philpot BD, et al. Obligatory role of NR2A for metaplasticity in visual cortex. Neuron. 2007;53:495–502. doi: 10.1016/j.neuron.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Frontiers in molecular neuroscience. 2012;5:72. doi: 10.3389/fnmol.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gipson CD, et al. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77:867–872. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gipson CD, et al. Rapid, transient synaptic plasticity in addiction. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wong HK, et al. Temporal and regional expression of NMDA receptor subunit NR3A in the mammalian brain. The Journal of comparative neurology. 2002;450:303–317. doi: 10.1002/cne.10314. [DOI] [PubMed] [Google Scholar]
- 71.Yuan T, et al. Expression of Cocaine-Evoked Synaptic Plasticity by GluN3A-Containing NMDA Receptors. Neuron. 2013;80:1025–1038. doi: 10.1016/j.neuron.2013.07.050. [DOI] [PubMed] [Google Scholar]
- 72.Roberts AC, et al. Downregulation of NR3A-containing NMDARs is required for synapse maturation and memory consolidation. Neuron. 2009;63:342–356. doi: 10.1016/j.neuron.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Durand GM, et al. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- 74.Groc L, et al. AMPA signalling in nascent glutamatergic synapses: there and not there! Trends Neurosci. 2006;29:132–139. doi: 10.1016/j.tins.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 75.Philpot BD, Zukin RS. Synapse-specific metaplasticity: to be silenced is not to silence 2B. Neuron. 2010;66:814–816. doi: 10.1016/j.neuron.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Isaac JT, et al. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 78.Isaac JT, et al. Silent glutamatergic synapses in the mammalian brain. Can J Physiol Pharmacol. 1999;77:735–737. [PubMed] [Google Scholar]
- 79.Malenka RC, Nicoll RA. Silent synapses speak up. Neuron. 1997;19:473–476. doi: 10.1016/s0896-6273(00)80362-1. [DOI] [PubMed] [Google Scholar]
- 80.Marie H, et al. Generation of silent synapses by acute in vivo expression of CaMKIV and CREB. Neuron. 2005;45:741–752. doi: 10.1016/j.neuron.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 81.Yao WD, et al. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron. 2004;41:625–638. doi: 10.1016/s0896-6273(04)00048-0. [DOI] [PubMed] [Google Scholar]
- 82.Kolb B, et al. Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proc Natl Acad Sci U S A. 2003;100:10523–10528. doi: 10.1073/pnas.1834271100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 84.Russo SJ, et al. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maze I, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robison AJ, et al. Behavioral and structural responses to chronic cocaine require a feedforward loop involving DeltaFosB and calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell. J Neurosci. 2013;33:4295–4307. doi: 10.1523/JNEUROSCI.5192-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dingledine R, et al. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 88.Washbourne P, et al. Rapid recruitment of NMDA receptor transport packets to nascent synapses. Nat Neurosci. 2002;5:751–759. doi: 10.1038/nn883. [DOI] [PubMed] [Google Scholar]
- 89.Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annual review of physiology. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- 90.Kleim JA, et al. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci. 1996;16:4529–4535. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Knott GW, et al. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron. 2002;34:265–273. doi: 10.1016/s0896-6273(02)00663-3. [DOI] [PubMed] [Google Scholar]
- 92.Darian-Smith C, Gilbert CD. Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature. 1994;368:737–740. doi: 10.1038/368737a0. [DOI] [PubMed] [Google Scholar]
- 93.Liao D, et al. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 94.Petralia RS, et al. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat Neurosci. 1999;2:31–36. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- 95.Friedman HV, et al. Assembly of new individual excitatory synapses: time course and temporal order of synaptic molecule recruitment. Neuron. 2000;27:57–69. doi: 10.1016/s0896-6273(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 96.Hall BJ, Ghosh A. Regulation of AMPA receptor recruitment at developing synapses. Trends Neurosci. 2008;31:82–89. doi: 10.1016/j.tins.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 97.Zito K, et al. Rapid functional maturation of nascent dendritic spines. Neuron. 2009;61:247–258. doi: 10.1016/j.neuron.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xiao MY, et al. Creation of AMPA-silent synapses in the neonatal hippocampus. Nat Neurosci. 2004;7:236–243. doi: 10.1038/nn1196. [DOI] [PubMed] [Google Scholar]
- 99.Matsuzaki M, et al. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Noguchi J, et al. Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron. 2005;46:609–622. doi: 10.1016/j.neuron.2005.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sobczyk A, et al. NMDA receptor subunit-dependent [Ca2+] signaling in individual hippocampal dendritic spines. J Neurosci. 2005;25:6037–6046. doi: 10.1523/JNEUROSCI.1221-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee BR, et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16:1644–1651. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shepherd JD. Memory, plasticity and sleep - A role for calcium permeable AMPA receptors? Frontiers in molecular neuroscience. 2012;5:49. doi: 10.3389/fnmol.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ambroggi F, et al. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cador M, et al. Involvement of the amygdala in stimulus-reward associations: interaction with the ventral striatum. Neuroscience. 1989;30:77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- 106.Setlow B, et al. Disconnection of the basolateral amygdala complex and nucleus accumbens impairs appetitive pavlovian second-order conditioned responses. Behavioral neuroscience. 2002;116:267–275. doi: 10.1037//0735-7044.116.2.267. [DOI] [PubMed] [Google Scholar]
- 107.Shiflett MW, Balleine BW. At the limbic-motor interface: disconnection of basolateral amygdala from nucleus accumbens core and shell reveals dissociable components of incentive motivation. The European journal of neuroscience. 2010;32:1735–1743. doi: 10.1111/j.1460-9568.2010.07439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stuber GD, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 110.Neisewander JL, et al. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pickens CL, et al. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wisden W, et al. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fritschy JM, et al. Switch in the expression of rat GABAA-receptor subtypes during postnatal development: an immunohistochemical study. J Neurosci. 1994;14:5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu NY, et al. Mediating effect of dopamine D3 receptors on Jak2 and GABAAalpha1 expression in mouse brains induced by cocaine. Chinese medical journal. 2007;120:910–914. [PubMed] [Google Scholar]
- 115.Kennedy PJ, et al. Class I HDAC inhibition blocks cocaine-induced plasticity by targeted changes in histone methylation. Nat Neurosci. 2013;16:434–440. doi: 10.1038/nn.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. The Journal of comparative neurology. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- 117.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nature medicine. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 118.Manganas LN, et al. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318:980–985. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Messier B, Leblond CP. Cell proliferation and migration as revealed by radioautography after injection of thymidine-H3 into male rats and mice. The American journal of anatomy. 1960;106:247–285. doi: 10.1002/aja.1001060305. [DOI] [PubMed] [Google Scholar]
- 120.Mandyam CD, Koob GF. The addicted brain craves new neurons: putative role for adult-born progenitors in promoting recovery. Trends Neurosci. 2012;35:250–260. doi: 10.1016/j.tins.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eisch AJ, et al. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci U S A. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. Journal of comparative and physiological psychology. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 123.Mogenson GJ, et al. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 124.Merrill EG, Wall PD. Factors forming the edge of a receptive field: the presence of relatively ineffective afferent terminals. The Journal of physiology. 1972;226:825–846. doi: 10.1113/jphysiol.1972.sp010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Charpier S, et al. “Latent” inhibitory connections become functional during activity- dependent plasticity. Proc Natl Acad Sci U S A. 1995;92:117–120. doi: 10.1073/pnas.92.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lin JW, Faber DS. Synaptic transmission mediated by single club endings on the goldfish Mauthner cell. II. Plasticity of excitatory postsynaptic potentials. J Neurosci. 1988;8:1313–1325. doi: 10.1523/JNEUROSCI.08-04-01313.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hanse E, et al. AMPA-silent synapses in brain development and pathology. Nat Rev Neurosci. 2013;14:839–850. doi: 10.1038/nrn3642. [DOI] [PubMed] [Google Scholar]
- 128.Thomas MJ, et al. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- 129.Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Conrad KL, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McCutcheon JE, et al. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31:5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Koob GF, et al. Tail-pinch stimulation: sufficient motivation for learning. Science. 1976;194:637–639. doi: 10.1126/science.982032. [DOI] [PubMed] [Google Scholar]
- 133.See RE, et al. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology. 2001;154:301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- 134.O’Dell LE, et al. Behavioral effects of psychomotor stimulant infusions into amygdaloid nuclei. Neuropsychopharmacology. 1999;20:591–602. doi: 10.1016/S0893-133X(98)00083-9. [DOI] [PubMed] [Google Scholar]
- 135.Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Archives of general psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- 136.Grimm JW, et al. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shepard JD, et al. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biological psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 138.Bienkowski P, et al. Time-dependent changes in alcohol-seeking behaviour during abstinence. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2004;14:355–360. doi: 10.1016/j.euroneuro.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 139.Abdolahi A, et al. Incubation of nicotine seeking is associated with enhanced protein kinase A-regulated signaling of dopamine- and cAMP-regulated phosphoprotein of 32 kDa in the insular cortex. The European journal of neuroscience. 2010;31:733–741. doi: 10.1111/j.1460-9568.2010.07114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Grimm JW, et al. Incubation of sucrose craving: effects of reduced training and sucrose pre-loading. Physiology & behavior. 2005;84:73–79. doi: 10.1016/j.physbeh.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]