Topography of the ISW2–nucleosome complex: insights into nucleosome spacing and chromatin remodeling (original) (raw)
Abstract
Linker DNA was found to be critical for the specific docking of ISW2 with nucleosomes as shown by mapping the physical contacts of ISW2 with nucleosomes at base-pair resolution. Hydroxyl radical footprinting revealed that ISW2 not only extensively interacts with the linker DNA, but also approaches the nucleosome from the side perpendicular to the axis of the DNA superhelix and contacts two disparate sites on the nucleosomal DNA from opposite sides of the superhelix. The topography of the ISW2–nucleosome was further delineated by finding which of the ISW2 subunits are proximal to specific sites within the linker and nucleosomal DNA regions by site-directed DNA photoaffinity labeling. Although ISW2 was shown to contact ∼63 bp of linker DNA, a minimum of 20 bp of linker DNA was required for stable binding of ISW2 to nucleosomes. The remaining ∼43 bp of flanking linker DNA promoted more efficient binding under competitive binding conditions and was functionally important for enhanced sliding of nucleosomes when ISW2 was significantly limiting.
Keywords: chromatin, chromatin remodeling, ISW2, ISWI, nucleosome
Introduction
The ISWI family is part of a larger family of ATP-dependent chromatin remodeling complexes that include the SWI/SNF, CHD or Mi-2, and INO80 families (Eisen et al, 1995; Woodage et al, 1997; Shen et al, 2000; Becker and Horz, 2002). In yeast (ISW1 and ISW2) and humans (hSNF2H and hSNF2L), there are two distinct ISWI genes encoding similar but not identical catalytic subunits (Okabe et al, 1992; Elfring et al, 1994; Aihara et al, 1998; Tsukiyama et al, 1999; Kent et al, 2001). In Drosophila, there is only one ISWI gene that is assembled into three distinct complexes referred to as NURF, CHRAC, and ACF (Langst and Becker, 2001b). The Isw2 polypeptide is associated with Itc1 to form the ISW2 complex, whereas Isw1 is associated with different subunits to form two distinct complexes referred to as ISW1a containing Ioc3 and ISW1b containing Ioc2 and Ioc4 (Vary et al, 2003). The hSnf2H protein is a part of four complexes that differ in function and size (Hakimi et al, 2002; Santoro et al, 2002; Zhou et al, 2002).
ISWI is involved in the global regulation of chromatin structure as mutant ISWI alters the overall structure of the X chromosome in Drosophila (Deuring et al, 2000). In yeast, both ISW1 and ISW2 have been shown by indirect labeling to be involved in the positioning of short nucleosomal arrays at a variety of gene regulatory regions (Kent et al, 2001). ISW2 is also involved in the repression of early meiotic gene expression and is recruited to specific regions by the Ume6 repressor (Goldmark et al, 2000; Fazzio et al, 2001). ISW2 forms a repressive chromatin structure independent of histone deacetylation by Rpd3. The alteration of chromatin structure by ISWI complexes appears to occur without disrupting the nucleosome structure and may function through mobilizing nucleosomes and potentially creating repressive higher ordered arrays (Langst et al, 1999; Kassabov et al, 2002). ISW2 can actively displace Gal4 from its binding site (Kassabov et al, 2002) and ISW1 is involved in the eviction of TBP from the PHO80 promoter (Moreau et al, 2003). ISW2 and ISW1 along with CHD1 have also been shown to be important for proper termination of transcription by RNA polymerase II (Alen et al, 2002; Morillon et al, 2003).
Many but not all of the ISWI complexes have been shown to slide nucleosomes and create uniformly spaced nucleosomal arrays (Hamiche et al, 1999; Langst et al, 1999; Langst and Becker, 2001b; Kassabov et al, 2002). ISW2 has been shown to space nucleosomes every ∼200 bp, whereas ISW1a spaces nucleosomes every ∼175 bp and ISW1b has little spacing activity (Tsukiyama et al, 1999; Vary et al, 2003). The underlying basis of the different spacing activities of these complexes is not clear. The Drosophila NURF complex has no spacing activity, whereas CHRAC and ACF complexes can efficiently create regularly spaced nucleosomal arrays.
Several of these complexes have been shown using mononucleosomes as substrates to have a sliding directional preference. The ISWI protein was shown to slide preferentially mononucleosomes positioned in the center of DNA fragments toward the ends or slightly off the ends of DNA, whereas the same protein as part of the CHRAC or ACF complex preferentially slides nucleosomes from the ends to the center of DNA (Langst et al, 1999; Langst and Becker, 2001a). The various subunits of CHRAC appear to modulate the activity of the catalytic ISWI subunit as evident by a change in sliding direction and efficiency. The ISW2 complex has a similar directional preference for sliding nucleosomes from the end to the center as does the CHRAC and ACF complex (Kassabov et al, 2002). NURF has been shown to slide nucleosomes toward the thermodynamically preferred position on DNA, but it is uncertain if this property is universally shared with the other ISWI complexes (Kang et al, 2002). None of the ISWI complexes have been shown to move nucleosomes in trans from one DNA to another (i.e. octamer transfer) as has been for human SWI/SNF and the yeast RSC complexes (Langst et al, 1999; Lorch et al, 1999; Phelan et al, 2000).
ISWI requires the N-terminal tail of histone H4 for stimulating ATPase activity and inducing nucleosome mobility (Clapier et al, 2001) and yet in another report ISWI was shown not to bind nucleosome core particles (Brehm et al, 2000). ISWI was shown to bind to linker DNA asymmetrically by DNase I protection such that only one and not the other linker DNA was protected (Langst and Becker, 2001a).
It is important to know more about how ISW2 interacts with nucleosomes to find what determines the direction of nucleosome sliding and why it evenly spaces nucleosomes in arrays. ISW2 was found to bind and slide more readily nucleosomes with linker DNAs ⩾67 bp under limiting conditions. The topography of the ISW2–nucleosome complex was examined by determining where ISW2 contacts linker DNA and the nucleosome core particle DNA at base-pair resolution by hydroxyl radical footprinting. Next, the ISW2 subunits associated at the different nucleosomal and linker DNA regions were revealed by site-specific DNA photoaffinity labeling. These data indicate the importance of the Itc1 subunit in determining the direction and extent to which nucleosomes are slid and how the linker DNA likely promotes the proper positioning of ISW2 onto the nucleosome.
Results
ISW2 binds the nucleosome at three distinct regions
DNase I and hydroxyl radical footprinting of ISW2 bound to nucleosome revealed that ISW2 makes extensive contact with linker DNA and only limited contact with nucleosomal DNA. Nucleosomes were assembled for DNase I footprinting with 250 bp of end-labeled DNA with the nucleosome positioned at one end of the DNA (103 bp of linker DNA, 0-N-103) using a DNA sequence designed to position nucleosomes uniquely into one translational position (see Supplementary data). Saturating amounts of ISW2 were added to nucleosomes (1.7- to 2.5-fold excess) as observed by gel shift assay with 80% or greater of the nucleosomes bound by ISW2 (Figure 1A). ISW2 protected ∼63 bp in the linker DNA region immediately adjacent to the nucleosome and not at the region farthest away from the nucleosome (Figure 1B, compare lanes 1 and 2 with lanes 3 and 4, and Figure 1C). The region between 123–136 bp from the dyad axis is less protected than the remainder of the ISW2 bound region possibly due to reduced affinity of ISW2 for these sites. Next, hydroxyl radical footprinting was performed where the linker DNA was on the other side of the nucleosome positioning sequence and with a slightly shorter linker DNA (75-N-0) to further probe the interactions of ISW2 with the core nucleosome. The ISW2–nucleosome and nucleosome alone complexes were stable under the hydroxyl radical footprinting conditions as shown by gel shift assay (Figure 1A, lanes 4–7). The linker DNA region was protected along with a region spanning 20 bp just inside the nucleosome at the entry site and another 10–20 bp region centered two helical turns away from the dyad axis (Figure 1B, compare lanes 9 and 12 to 8 and 11, and Figure 1C). The discrete protection sites shown in these footprinting experiments clearly indicated the specificity with which ISW2 binds the nucleosome and demonstrated that there are three regions in the nucleosome that are contacted by ISW2, namely, the linker DNA, entry site, and near the dyad axis. This multiplicity of contact sites is consistent with ISW2 being able to bind to the core nucleosome (i.e. the internal contact regions), but the overall binding of ISW2 to the nucleosome is enhanced in the presence of linker DNA of sufficient length. The hydroxyl radical footprinting shows that ISW2 binds the nucleosome from one side perpendicular to the superhelical axis and that the two contacts on the nucleosomal DNA are on opposite sides of the superhelical axis (Figure 2A). The contacts with linker DNA and within the nucleosome were shown not to be DNA sequence specific, as placing linker DNA of an entirely different sequence on the other side of the nucleosome positioning sequence shifted the ISW2 footprint (0-N-67, results not shown). The protection nearest the dyad axis moved to the other side of the dyad as well as the protection near the entry site, consistent with the change in orientation of the linker DNA.
Figure 1.
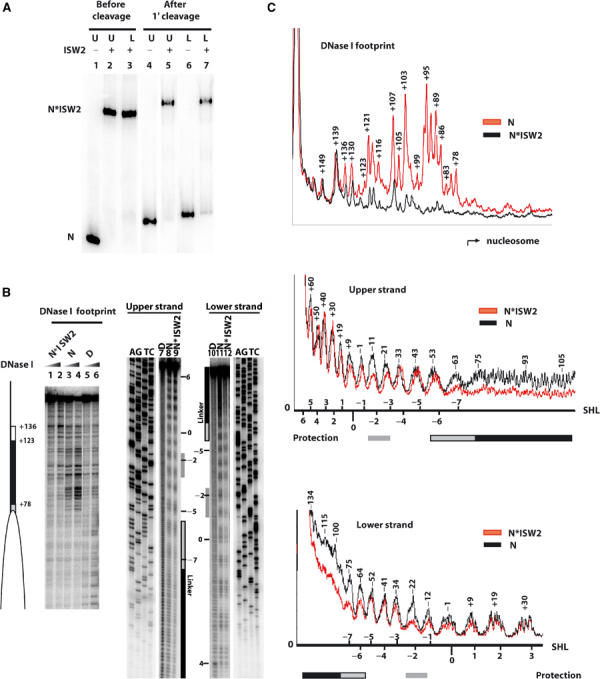
ISW2 binds to three distinct sites on nucleosomes. (A) Sucrose gradient-purified nucleosomes (40 nM) assembled (75-N-0) onto end-labeled 222 bp DNA (upper and lower refer to the opposite strands of DNA) were incubated with 100 nM ISW2. After spin column purification, samples were loaded onto a 4% native polyacrylamide gel before (lanes 1–3) and after (lanes 4–7) cleavage with hydroxyl radicals. Bands correspond to nucleosome alone (N) and nucleosome bound by ISW2 (N*ISW2). (B) End-positioned mononucleosomes reconstituted with either a 250 bp (0-N-103, 90 nM, lanes 1–6) or 222 bp end-labeled DNA (75-N-0, 40 nM, lanes 7–12) were incubated with 150 and 100 nM of ISW2, respectively. Samples were analyzed on a 6.5% polyacrylamide gel containing 8 M urea along with the sequencing ladder of the same DNA, and lanes 1, 2, 9, and 12 all contained ISW2 and nucleosomes. The white ellipse indicates the nucleosome position, and the white and black rectangles indicate the region of ISW2 protection on the linker DNA for DNase I footprinting. For the hydroxyl radical footprinting, the nucleosomal superhelical positions are indicated on the side, and the black (linker DNA) and gray (nucleosomal) boxes indicate regions of ISW2 protection. (C) Quantitation of the DNase I and hydroxyl radical protection patterns of nucleosomes with (N*ISW2) and without (N) ISW2 added are shown. The numbers above the peaks indicate the number of base pairs from the dyad axis. The superhelical location (SHL) of the nucleosome is depicted for the hydroxyl radical footprinting for both upper and lower strands.
Figure 2.
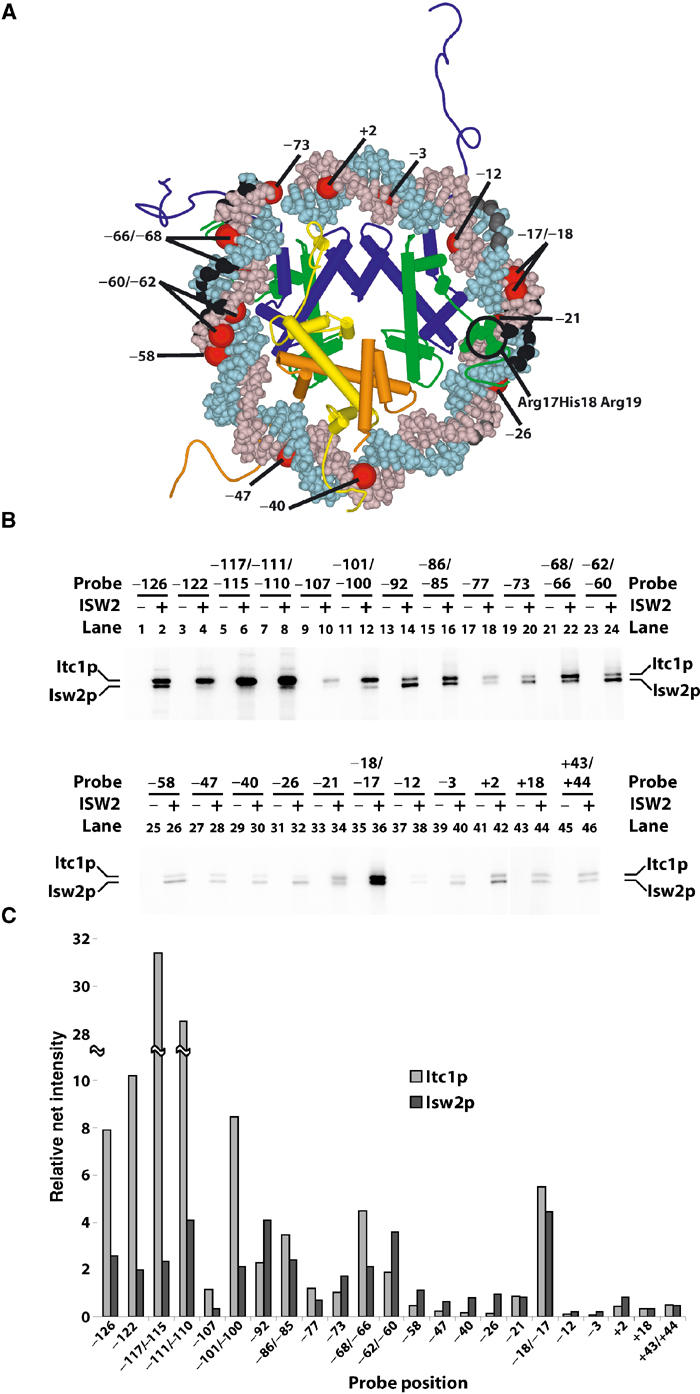
Identification of the ISW2 subunits that bind to different sites in the nucleosome. (A) The positions of the ribose residues protected from hydroxyl radical cleavage by ISW2 in the nucleosome are black (strong protection) or gray (weaker protection). The location of photoreactive nucleotides used to probe for ISW2 interactions within the nucleosome core particle is shown in red. Only one superhelical turn of DNA around the nucleosome is depicted. The epitope (R17H18R19) in the H4 tail known to stimulate ATPase activity of ISWI is space filled in green. (B) Site-specific DNA photoaffinity labeling of ISW2 subunits. Nucleosomes (75-N-0) assembled with a series of photoreactive probes (222 bp) were incubated with or without ISW2 (72 nM, >90% of nucleosomes bound), UV-irradiated, and digested with DNase I and S1 nuclease as described. The probe position numbers indicate the site of incorporated photoreactive nucleotides with reference to the dyad axis. (C) Quantitative analysis of crosslinked ISW2 subunits Itc1 and Isw2 at each probe position is shown with the probe positions indicated below.
ISW2 interactions near the dyad axis are with the minor groove of DNA, as revealed by hydroxyl radical footprinting of both DNA strands (Figures 1C and 2A). Hydroxyl radical targets the C-5 and C-4 positions in the ribose moiety as depicted in Figure 2A with space-filled regions in black (Balasubramanian et al, 1998). The observed protections are near the dyad axis spanning the minor groove region at superhelix location −2 (SHL −2) (Luger et al, 1997). Less protection was observed on the adjacent sites suggesting some overlap, but clearly the primary contact was with the minor groove. The nucleosome crystal structure and crosslinking data indicate that the histone H4 tail interacts with DNA in this region (Luger et al, 1997; Ebralidse et al, 1988; Davey et al, 2002). Those residues of H4 known to be involved in the remodeling activity of Drosophila ISWI are immediately adjacent to this strongly protected region, suggesting that the part of ISW2 bound to this region may be recruited by the N-terminal tail of H4 (Clapier et al, 2001, 2002). The N-terminal tail of histone H3 may also be involved in ISW2 binding near the entry site due to its proximity.
Which subunits of ISW2 interact with nucleosomal and linker DNA?
The subunits of ISW2 that interact with these three regions of the nucleosome were determined by site-specific DNA photoaffinity labeling. A series of DNA probes containing photoreactive nucleotides at different sites within the linker DNA or throughout the nucleosome from the entry site to the dyad axis were synthesized using an immobilized DNA template, site-specific primers, and enzymatic incorporation of photoreactive nucleotides (Sengupta et al, 1999). Each DNA probe had incorporated only 1–2 photoreactive nucleotides per probe and was localized at one particular site. The locations of these modified sites from the entry site to the dyad axis are depicted in Figure 2A and are correlated to the crystal structure of the nucleosome core particle based on the high-resolution mapping of the nucleosome translational position and the regions of DNA shown by hydroxyl radical footprinting to be on the exposed surface of the nucleosome.
In the far linker DNA region 126–100 bp from the dyad axis, the Itc1 subunit of ISW2 was exclusively crosslinked (Figure 2B, even lanes 2–12). Isw2 and Itc1 were both shown to be close to the linker DNA about 20 bp from the entry site of the nucleosome (bp −92 to −73; even lanes 14–20). Likewise, at the entry site of the nucleosome, the two subunits of ISW2 were crosslinked efficiently, corresponding to the location of one of the ISW2 internal contacts on the nucleosome at bp −68/−66 and −62/−60 (lanes 22 and 24). The 10–20 bp region located two helical turns from the dyad axis contacted both Isw2 and Itc1 as shown by being equally crosslinked at this region (lane 36). At an equal distance from the dyad axis in the other direction at bp +18, neither ISW2 subunit was efficiently crosslinked. The overall efficiencies of crosslinking Isw2 and Itc1 at the various positions corresponded well with our hydroxyl radical and DNase I fooprinting shown in Figure 1B in that the most efficient crosslinking of ISW2 subunits was evident in the linker region, entry site, and near the dyad axis (Figure 2C). Although ISW2 crosslinking is reduced in the region not protected by ISW2 between bp −54 and −39, it is nevertheless significant that the Isw2 subunit is almost exclusively the only subunit that was photoaffinity labeled in this region (even lanes 28–32). Crosslinking of primarily Isw2 in this region indicates that Isw2 is likely close, but not close enough, to be efficiently crosslinked or to be footprinted. The overall position of the Isw2 and Itc1 subunits is illustrated in Figure 7A relative to the core nucleosome.
Figure 7.
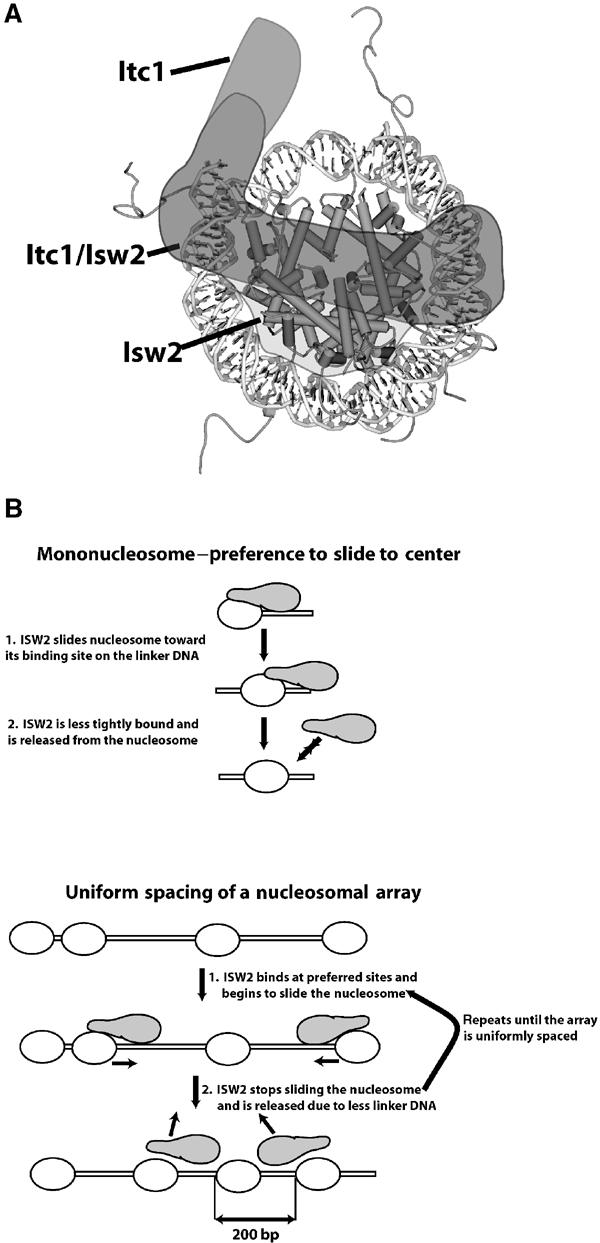
Sliding and spacing model of nucleosome by ISW2. (A) The spatial orientation of ISW2 on the nucleosome based on the crosslinking and footprinting data is depicted with the positions of Itc1 and Isw2 shown in lighter shades of gray and the merge of the two subunits shown in darker gray. (B) A model is presented to explain why ISW2 preferentially slides mononucleosomes to the center of DNA and uniformly spaces nucleosomes at 200 bp intervals in nucleosomal arrays.
The proximity of Itc1 to linker DNA and the ISW2 footprint suggests that Itc1 determines the direction of nucleosome sliding by orienting ISW2 complex on the nucleosome. Owing to the degree of symmetry of the nucleosome, the other two regions contacted by ISW2 within the nucleosome core particle would not be sufficient for the observed unique and orientation-specific binding of ISW2 to an end-positioned nucleosome.
Effect of linker DNA length on ISW2 binding to nucleosomes
The binding affinities of ISW2 for nucleosomes with varied lengths of linker DNA available at only one end of the nucleosome were measured by gel shift assay (Figure 3). Nucleosomes were reconstituted with homogenous DNA containing the positioning sequence and a specified length of linker DNA before being purified through a sucrose gradient. The length of the linker DNA was shown to be important, as ISW2 bound nucleosomes with 20 bp of linker DNA (167 bp DNA) three times and with 67 bp linker DNA (214 bp DNA) five times better than nucleosome core particles (Figure 3, lanes 2–5, 7–10, and 12–15). The largest increase in affinity of ISW2 for nucleosomes occurred with the addition of 20 bp of linker DNA to the nucleosome core particle. More linker DNA (+47 bp) increased the overall affinity of ISW2 for nucleosomes less than two-fold.
Figure 3.
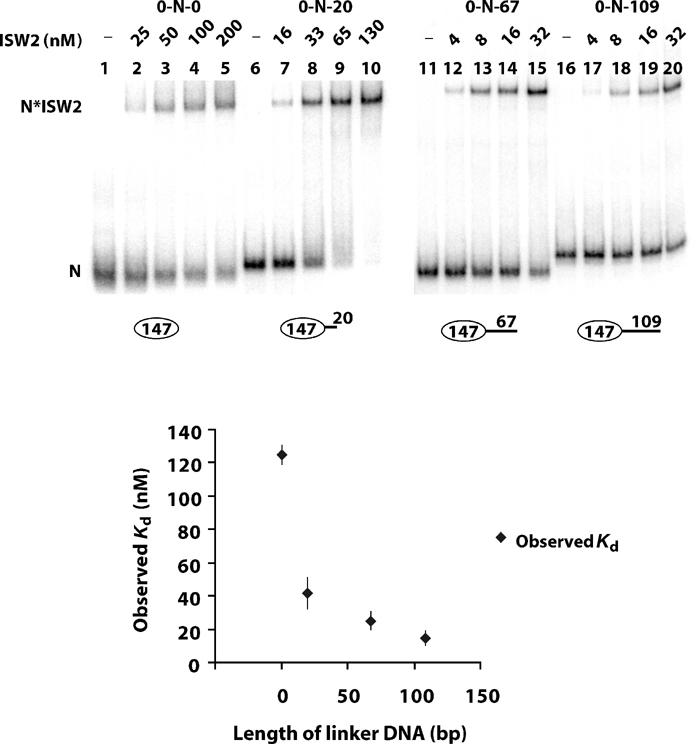
Affinity of ISW2 for end-positioned nucleosomes with different linker DNA lengths. Increasing amounts of ISW2 complex (shown above the lanes) were incubated with homogeneous sucrose gradient-purified mononucleosomes (6 nM, lanes 1–5; 2 nM, lanes 6–15; 2.6 nM, lanes 16–20; upper panel). Lanes 1–10 and 11–20 were run on different gels and hence show differences in migration of nucleosome and nucleosome–ISW2 complexes between the sets. These results were repeated more than once with similar results as shown by the standard deviations in the graph. The assemblies used are depicted below the lanes and are as follows: lanes 1–5 (0-N-0); lanes 6–10 (0-N-20); lanes 11–15 (0-N-67); and lanes 16–20 (0-N-109).
Although the extra 47 bp only modestly increased the overall equilibrium binding of ISW2 to nucleosomes, the extra linker DNA may increase the rate at which ISW2 binds nucleosome so as to facilitate the preferential loading of ISW2 onto nucleosomes with longer linker DNAs. Competition experiments were conducted with free DNA or oligonucleosomes to better discriminate differences of ISW2 binding to nucleosomes with varied linker DNA lengths (Figure 4). Nucleosomes with 67 and 109 bp linker DNAs were five times more effective at binding ISW2 than nucleosomes with 20 bp linker DNA in the presence of competitor DNA (Figure 4A). Five times more competitor DNA (12.5 versus 2.5 ng) had to be added to nucleosomes with 67 or 109 bp linker DNA than those with 20 bp of linker DNA to have comparable levels of competition for ISW2 binding (Figure 4A, compare lanes 3–5 with lanes 8–10 and 13–15). No significant difference in affinity was observed between nucleosomes with 67 or 109 bp linker DNAs. Similar results were also obtained when competing with HeLa oligonucleosomes such that five times more oligonucleosomes had to be added to nucleosomes with 67 bp of linker DNA than to nucleosomes with 20 bp of linker DNA to achieve comparable levels of competition (Figure 4B, compare lanes 3–6 and lanes 9–12).
Figure 4.
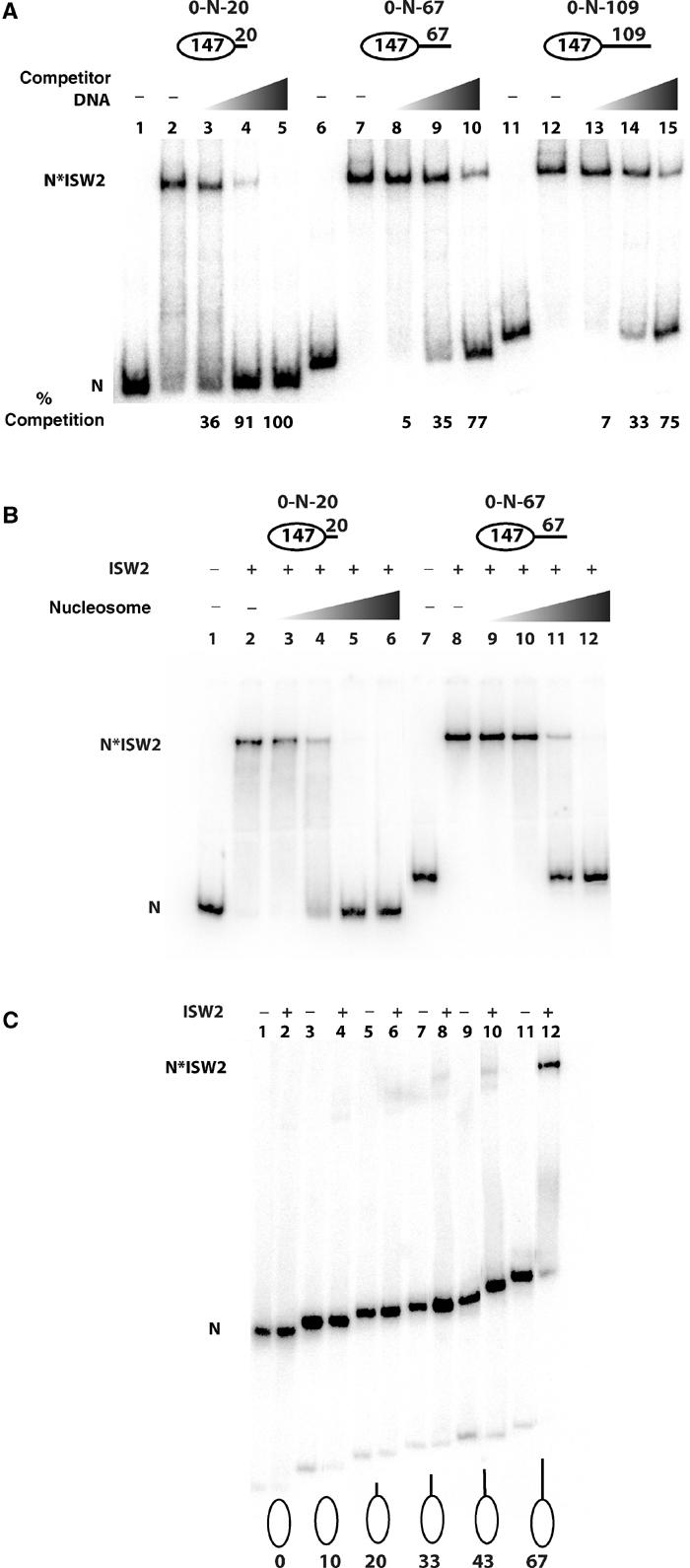
Effect of linker DNA on ISW2 binding to nucleosomes under competitive conditions. (A) Sucrose gradient-purified end-positioned nucleosomes—0-N-20 (2 nM), lanes 1–5; 0-N-67 (2 nM), lanes 6–10; 0-N-109 (2.6 nM), lanes 11–15)—were incubated with ISW2 (66 nM, lanes 2–5, 7–10, and 12–15) and increasing amounts of competitor DNA (2.5 ng, lanes 3, 8, and 13; 12.5 ng, lanes 4, 9, and 14; 62.5 ng, lanes 5, 10, and 15). Lanes 1, 6, and 11 are with nucleosome alone and the amount of ISW2 competed from nucleosomes is shown below. (B) Sucrose gradient-purified end-positioned nucleosomes (0.5 pmol) assembled on radiolabeled 167 bp DNA (0-N-20, lanes 2–6) and 214 bp DNA (0-N-67, lanes 8–12) were incubated with 1.5 pmol of ISW2 in the presence of increasing amounts of HeLa oligonucleosomes (0.023 μg, lanes 3 and 9; 0.115 μg, lanes 4 and 10; 0.58 μg, lanes 5 and 11; 2.88 μg, lanes 6 and 12). (C) End-positioned nucleosomes (70 fmol) with different lengths of linker DNA were incubated with 75 nM ISW2 in a 25 μl reaction containing some competitor oligonucleosome and free DNA. Samples were analyzed on a 4% native polyacrylamide gel. The different nucleosome constructs are shown with the number indicating the length of the linker DNA present (i.e. 0-N-0, lanes 1 and 2; 0-N-10, lanes 3 and 4; 0-N-20, lanes 5 and 6; 0-N-33, lanes 7 and 8; 0-N-43, lanes 9 and 10; 0-N-67, lanes 11 and 12).
More binding reactions were carried out in which carrier sonicated salmon sperm DNA was reconstituted along with the desired labeled DNA into nucleosomes creating conditions similar to those with competing free DNA and/or oligonucleosomes (Figure 4C). The labeled nucleosomes ranged from having no linker DNA to 10, 20, 30, 43, and 67 bp of linker DNA to determine more precisely the required optimal length of linker DNA. A fixed amount of ISW2 was added to these reactions and at a concentration sufficient to bind nucleosomes with 67 bp of linker DNA. Under these conditions, linker DNAs of 43 bp or less were not sufficient to promote significant binding of ISW2 (Figure 4C, compare lane 12 with lanes 2, 4, 6, 8, and 10). The optimal binding of ISW2 under these competitive conditions is likely to be more similar to that found in vivo and indicates that 63 bp of linker DNA is important for efficient binding of ISW2 under limiting or competitive conditions. All of the following experiments were carried out under conditions similar to those described in Figure 4C. In other data, the extra length of linker DNA was found to be required for the ISW2 footprint near the dyad axis and that 20 bp of linker DNA was insufficient (results not shown). These results illustrate that not only does the longer linker DNA enhance binding but it also promotes the specific binding of ISW2 onto the surface of the nucleosome.
Linker DNA determines the direction of nucleosome sliding by ISW2
The enhanced binding of ISW2 to nucleosomes with linker DNA of sufficient length was shown to be functionally important by comparing the extent of sliding on nucleosomes with different linker DNA lengths. ATP and various amounts of ISW2 were added to nucleosomes with 33, 67, or 142 bp of linker DNA and the amount of nucleosome sliding was determined by gel shift assay (Figure 5A). At the lower concentration of ISW2, nucleosomes with 33 bp of linker DNA were slid three-fold less efficiently than nucleosomes with 67 or 142 bp of linker DNA (Figure 5A, compare lane 2 to lanes 5 and 8). As the ISW2 concentration increased two-fold, the difference in sliding was less pronounced, although still noticeable (compare lane 3 to lanes 6 and 9). ISW2 was able to slide nucleosomes with all three different linker DNA lengths, but the overall efficiency of sliding was enhanced with nucleosomes having 67 bp or longer of linker DNA. These results also showed that ISW2 can readily slide nucleosomes away from one of the highest affinity nucleosome binding sites known, demonstrating that ISW2 does not slide nucleosomes to their thermodynamically preferred position (see also Figure 6).
Figure 5.
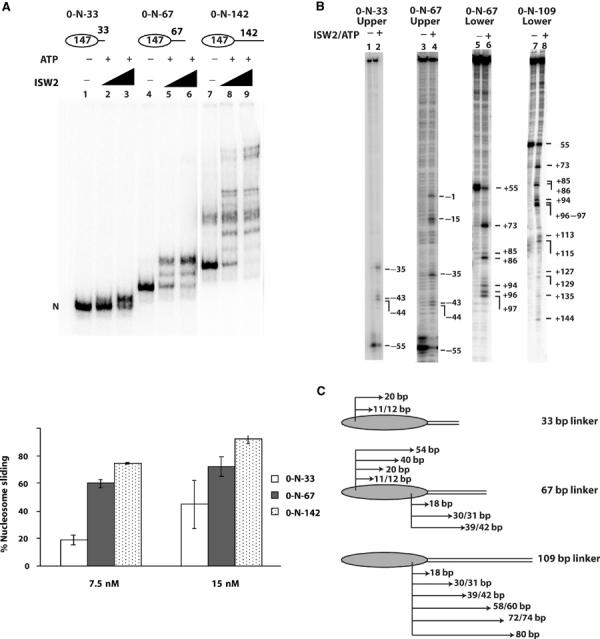
Nucleosome sliding was enhanced by increasing linker DNA length. (A) Nucleosomes (50 nM) with 33 bp (0-N-33, lanes 1–3), 67 bp (0-N-67, lanes 4–6), and 142 bp (0-N-142, lanes 7–9) of linker DNA were separated on a 5% native polyacrylamide gel (60:1, acrylamide:bis) after addition of ISW2 (7.5 and 15 nM) and ATP (400 μM). The percentage of slid nucleosomes was determined by measuring the amount of nucleosomes remaining at the original position. (B) Nucleosomes slid along DNA to similar sites upon remodeling with ISW2 irrespective of the linker DNA length. End-positioned nucleosomes, 3.1 pmol (0-N-33, lanes 1 and 2; 0-N-67, lanes 3–6; 0-N-109, lanes 7–8), with the DNA fragments labeled at the 5′ end of either the upper (lanes 1–4) or lower strand (lanes 5–8) were incubated with 0.3 pmol of ISW2 with or without 300 μM ATP at 30°C for 30 min. High-resolution mapping of positions was performed as described and the numbering on the side indicates the position of the cut site relative to the original dyad axis. (C) The translational movement of 0-N-33, 0-N-67, and 0–109 nucleosomes after ISW2 remodeling is shown as indicated by changes in the position of histone H2B residue relative to DNA. The arrows and numbers show how many base pairs the nucleosomes have slid (i.e. new cut sites) after remodeling.
Figure 6.
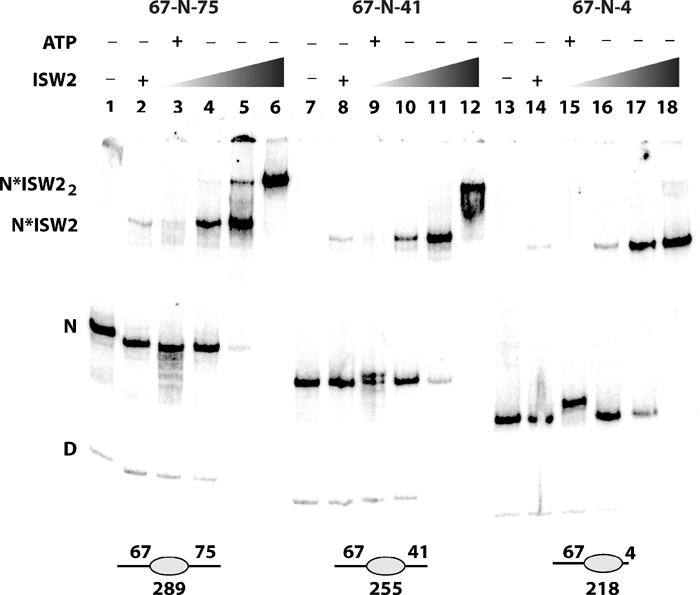
Effect of nucleosomes having two linker DNAs on ISW2 binding and sliding. The 289 bp (67-N-75, lanes 1–6), 255 bp (67-N-41, lanes 7–12), and 218 bp (67-N-4, lanes 13–18) nucleosomes (50 nM) were incubated with increasing amounts of ISW2 (8 nM, lanes 2, 3, 8, 9, 14, and 15; 25 nM, lanes 4, 10, and 16; 75 nM, lanes 5, 11, and 17; 150 nM, lanes 6, 12, and 18) with or without ATP (400 nM) and then separated by native polyacrylamide gel electrophoresis. D and N indicate the position of free DNA and nucleosome in the gel shift, and N*ISW2 and N*ISW22 indicate where one and two molecules of ISW2 are bound to the nucleosome.
The steps in sliding nucleosomes with various lengths of linker DNA were determined by high-resolution mapping of the nucleosome translational position (Figure 5B and C). There was a striking similarity of the steps with nucleosomes containing shorter and longer linker DNA. In this procedure, the modified H2B residue 53 cuts the DNA in a strand-specific manner, such that the two H2B sites cut opposite strands (Kassabov et al, 2002, 2003). Nucleosomes were slid 11, 12, and 20 bp toward the linker DNA when 33 bp of linker DNA was present (Figure 5B, lane 2, and Figure 5C, upper strand of 33 bp linker). These same steps were also evident with 67 bp of linker DNA when monitoring the same strand along with longer steps of 40 and 54 bp (Figure 5B, lane 4, and Figure 5C, upper strand of 67 bp linker). When the other H2B site nearest the linker DNA was mapped, steps of 18, 30, 31, and 39–42 were found (Figure 5B, lane 7, and Figure 5C, lower strand of 67 bp linker). Likewise, when nucleosomes with even longer linker DNA of 109 bp were used, steps found with 33 and 67 bp linker DNA (18, 30/31, and 39–42) were present as well as still longer steps of 58–60, 72–74, and 80 bp (Figure 5B, lane 8, and Figure 5C, lower strand of 109 bp linker). These results demonstrate that nucleosomes slide toward where ISW2 is bound to the linker DNA.
Potentially, the absence of mobilized nucleosomes with shorter linker DNA lengths could be due to the mobilized nucleosomes being less stable than nucleosomes slid with longer linker DNAs. If this were true, then mobilizing nucleosomes with longer linker DNAs would be expected to not have these intermediate positions that are less stable and are therefore more difficult to detect. The high-resolution mapping however showed that the intermediate positions are as easily observed with the longer linker DNAs as the further slid positions and that they correspond to the same positions as those observed with shorter linker DNAs. These data therefore suggest that the reason as to why not as much sliding is observed with the shorter linker DNA is not because the slid products are not thermodynamically stable enough to be observed, but because ISW2 has more difficulty with binding in a productive manner than those with longer linker DNAs. Furthermore, if an important thermodynamic factor caused the shorter linker DNA nucleosomes to slide stably, then such a factor should not be influenced by the concentration of ISW2. The differences in the efficiency of sliding were reduced as the concentration of ISW2 was increased and this along with the binding data suggest that the differences in binding are likely the reason for the differences in sliding.
Free ISWI had been shown earlier not to bind core nucleosomes (Brehm et al, 2000), whereas other reports indicated that ISWI does bind core nucleosomes, although no discrete complexes were detected by gel shift assay (Whitehouse et al, 2003). Unlike these earlier studies, ISW2 (native whole complex) bound core nucleosomes in a similar manner as those with linker DNA, as evident by the complexes having the same discrete electrophoretic mobility, although it bound core nucleosomes with a lower affinity requiring more ISW2 for comparable binding. DNA length dependence for ISWI binding to free DNA and for stimulation of its ATPase activity has been recently observed by T Owen-Hughes and colleagues, and correlates well with the effect shown here of a minimal amount of linker DNA length needed for stable binding of ISW2 to nucleosomes (Whitehouse et al, 2003).
Next, the effect of having two instead of one linker DNA per nucleosome was examined by assembling nucleosomes with a fixed length of linker DNA (67 bp) on one side with 4, 41, or 75 bp of linker DNA on the other side (Figure 6). Nucleosomes with 4 and 67 bp of linker DNA (67-N-4) bound one molecule of ISW2 as shown with the complex having the same electrophoretic mobility at different extents of ISW2 binding (compare lanes 14 and 16–18). The relative affinity of ISW2 for nucleosomes was slightly increased as the other linker DNA was increased from 4 to 41 or 75 bp; however, the mobility of the complex was not changed except at even higher concentrations of ISW2 (compare lanes 2, 4, and 5 or lanes 8, 10, and 11 to lanes 14, 16, and 17). These indicate that primarily a single molecule of ISW2 tends to bind even when two linker DNAs are present, although a second ISW2 did bind with an excess of ISW2 (lanes 6 and 12).
Nucleosome sliding assays on these three nucleosomal substrates not only showed that ISW2 preferentially slides nucleosomes from the ends of DNA toward the center, but that it can to a lesser extent slide nucleosomes away from a central position depending on having sufficient linker DNA available. Nucleosomes positioned toward one end of a 218 bp DNA (67-N-4) were slid completely from the end to a more central position by ISW2 (Figure 6, lanes 13 and 15). High-resolution mapping results, like those shown previously, demonstrated that these nucleosomes were slid 30–31 and 39–42 bp from their original to an almost central position (i.e. 37-N-34 or 27-N-44, results not shown). Only one slid species is visible in Figure 6, as the ISW2 concentration is sufficient for sliding to go to completion and hence only the final product is detected (27-N-44). The final length of linker DNA would be 34–46 bp in the slid nucleosome and would be suboptimal for ISW2 binding and sliding as observed earlier. These data would suggest that the nucleosome becomes trapped in the central position, because after sliding it no longer has sufficient length of linker DNA for optimal ISW2 binding and sliding of the nucleosome under these competitive conditions. When the length of the linker DNA is increased to form a more centrally positioned nucleosome (67-N-75), more movement away from the central position was observed (Figure 6, compare lane 3 with lane 15). High-resolution mapping of the slid positions found that the 289 bp nucleosomes were slid in either direction consistent with DNase I footprinting showing that both linker DNAs were equally likely to bind ISW2 (results not shown). The enhanced sliding observed away from the central position is consistent with the longer linker DNA helping to enhance the binding of ISW2 and thus sliding. Earlier results with recombinant ISWI indicated that ISWI slid nucleosomes toward its binding site; however, one difference was that ISWI was apparently able to recognize asymmetry in the nucleosome and bind preferentially to one linker and not the other in a centrally positioned nucleosome and therefore slid in only one direction (Langst and Becker, 2001a). Our data are not consistent with ISW2 recognizing any asymmetry in the nucleosome, but rather it is able to bind either linker DNA and thereby slide nucleosomes in either direction from the center.
A large amount of nucleosomes still remain in the central position with the 289 bp DNA in spite of significant movement away from the central nucleosome. This can be explained in a model where nucleosomes equilibrate toward the center because as the nucleosome moves forward on one linker DNA, the length of linker DNA progressively becomes shorter thereby promoting the dissociation of ISW2 (Figure 7B, upper panel). ISW2 can subsequently rebind the other linker DNA as it is of a more optimal length and slide the nucleosome back toward the center. Equilibrium would therefore favor sliding toward the center with a lesser amount slid toward the ends. When nucleosomes were placed at an intermediate location between the center and the end with 67 and 41 bp of linker DNA (67-N-41), nucleosomes were preferentially slid toward the 67 bp linker to a more central position and less toward the 41 bp linker at an end position (Figure 6, lanes 7 and 9) consistent with our model. High-resolution mapping of nucleosomes with linkers of different lengths showed similar results of preferential sliding toward the longer linker DNA (data not shown). Ultimately, the extent to which nucleosomes are slid in an array may be determined by the steric hindrance imposed by ISW2 binding ∼63 bp of linker DNA and would be consistent with the observed regular 200 bp spacing of nucleosome arrays by ISW2 under similar conditions (see Figure 7B, lower panel; Tsukiyama et al, 1999).
Discussion
We have examined the features of the nucleosome important for ISW2 binding so as to understand how ISW2 remodels nucleosomes and why it creates regularly spaced nucleosomal arrays and slides mononucleosomes in a preferred direction. Linker DNA was shown to promote ISW2 binding to the nucleosome and orient the ISW2–nucleosome complex such that the nucleosome is slid in the direction of the bound linker DNA. The length of the linker DNA affects the affinity of ISW2 for the nucleosome, which in turn regulates the extent to which nucleosomes slide, thereby causing mononucleosomes to slide preferentially to the center of DNA and to space nucleosomes evenly in an array (see Figure 7B). The noncatalytic subunit Itc1 of ISW2 is implicated to be involved in these processes due to its binding the more distal segment of the linker DNA as shown by DNA photoaffinity labeling. The extensive binding of Itc1 and to a lesser degree Isw2 to 63 bp of linker DNA likely serves as a steric block to prevent physically the sliding of nucleosomes too close together. Itc1 is expected to serve a regulatory role as free ISWI is itself sufficient to remodel nucleosomes. The different direction of free ISWI for sliding nucleosomes to DNA ends is likely because of the absence of its Itc1 homolog such that it no longer requires linker DNA to slide and can therefore slide nucleosomes to the extreme end of DNA or even slightly off the end (Langst and Becker, 2001a). ISWI would rapidly shuttle the nucleosome back and forth to either end with no pausing at the center such that a steady state of end-positioned nucleosomes would be created. We also show that ISW2 can efficiently slide nucleosomes off one of the highest affinity nucleosome positioning sequences known, thus eliminating the possibility that nucleosome sliding for ISW2 is directed toward thermodynamically preferred positions.
The high-resolution footprint of ISW2 bound to the nucleosome reveals two major contacts of ISW2 with the surface of the nucleosome core particle that are likely to be functionally relevant for chromatin remodeling by ISW2. ISW2 was shown to make two disparate contacts on opposite sides of the nucleosomal DNA superhelix, one near the entry site and the other 20 bp away from the dyad axis. The H4 tail is likely to be involved in the recruitment of ISW2 at the site 20 bp from the dyad axis due to its close proximity (Ebralidse et al, 1988; Luger et al, 1997; Davey et al, 2002). The functional relevance of the contacts near the dyad axis have been implicated previously in studies where the histone H4 N-terminal tail was shown to be required for the ATPase and remodeling activities of Drosophila CHRAC, ISWI, and NURF (Hamiche et al, 1999; Clapier et al, 2001, 2002). These studies showed that not only was the H4 tail critical, but that its location in the nucleosome is also important such that the H4 tail was not able to activate CHRAC or ISWI when swapped on to the N-terminus of other histones in the nucleosome. Not surprisingly, others have not been able to show directly that ISWI can contact the H4 tail alone as it is just one of several contacts that contribute to the stable binding of ISWI to the nucleosome (Clapier et al, 2002). Likewise, the interaction of ISW2 near the entry site of the nucleosome may be mediated through interactions with the H3 histone tail. Because of the physical separation of the binding sites on the nucleosome and the absence of additional footprints within the nucleosomal DNA region, it appears that ISW2 binds across the surface of the nucleosome not bound by DNA in order to form a bridge between these two binding sites as depicted in Figure 7A.
Both structural and functional data with the C-terminus of ISWI from Drosophila suggested that it may interact with the histone tails of H3 and H4 similar to that suggested from our data. The C-terminus of ISWI contains two domains, a SANT and a SANT-like domain (referred to as the SLIDE domain), that are crucial for nucleosome binding (Grune et al, 2003). The SLIDE domain is suggested to bind to the major groove of DNA with potential interactions from the minor groove, based on superimposing the SLIDE domain with the known structure of the c-Myb repeat R3 bound to DNA. Grune et al favor a model of the SLIDE domain interacting with the H4 tail and binding nucleosomal DNA 1.5 helical turns from the dyad axis, whereas the SANT domain would interact with the histone H3 tail. Due to the location of the H3 and H4 histone tails, the C-terminus of ISWI could potentially bind the histone H3 and H4 tails on the same side of the nucleosome or bridge the surface of the nucleosome perpendicular to the DNA superhelix to interact with H4 and H3 tails on opposite sides of the nucleosome as observed for ISW2. However, the structure of the C-terminus (100 Å × 20 Å) is such that the SANT and SLIDE domains could only bind to the histone H3 and H4 tails located on the same side of the nucleosome due to distance constraints (Grune et al, 2003). It seems likely from our data that either the SANT domain may not bind the H3 tail near the entry site or that the SLIDE domain does not bind near the dyad axis through interactions with the H4 tail. Further work will be needed to determine if the SANT or SLIDE domains of ISW2 are bound at either of these protected sites on the nucleosome. Also the contacts at these two locations in the nucleosome with the native ISW2 complex are not exclusively with the Isw2 or ISWI-like subunit, as the Itc1 subunit was equally crosslinked at these sites. Our data show that contacts with DNA near the histone H4 tail are also primarily through the minor groove and not the major groove.
Materials and methods
Affinity purification of ISW2 complex
The ISW2 complex was purified from Saccharomyces cerevisiae strain YTT480, bearing a FLAG™ epitope tag at the 3′ end of the ISW2 gene as described (Tsukiyama et al, 1999). Protein purity and quantitation were determined by analyzing samples on a 4–20% gradient SDS–PAGE, staining with colloidal blue (OWL Pro-Blue™) and comparing to known bovine serum albumin (BSA) standards.
Plasmids and DNA probes
The nucleosome positioning sequence 601 was obtained from plasmid pGEM-3Z/601 (Lowary and Widom, 1998). DNAs with 601 and varying extents of flanking regions were synthesized by PCR. DNAs were radiolabeled using T4 polynucleotide kinase and [γ-32P]ATP (6000 Ci/mmol) and purified with the QIAquick PCR purification kit (Qiagen). Biotinylated single-stranded DNA template bound to streptavidin-coated Dynabeads (Dynal)™ was used to generate end-labeled DNA probes as described previously (Kassabov et al, 2002).
Nucleosome reconstitution
Mononucleosomes were assembled at 37°C with 8.5–10 μg of recombinant octamers, 100–200 fmol of labeled DNA, 10 μg of sonicated salmon sperm DNA, and 1.8 M NaCl in a starting volume of 10 μl, and serial dilution (Lorch et al, 1987). Nucleosome assemblies were analyzed on a 5% native polyacrylamide gel (acrylamide:bisacrylamide; 60:1) in 0.2 × TBE with buffer recirculation at 4°C. Homogeneous reconstitutions were in a similar manner with the omission of sonicated salmon sperm DNA and the addition of ∼10 μg of the appropriate unlabeled PCR DNA. Homogeneous assemblies were purified by 5–25% sucrose gradient centrifugation. The concentrations of the homogeneous assemblies were determined based on the DNA content in the purified sample.
Mapping of H2B53 contacts on DNA
Serine 53 in histone H2B was replaced with cysteine by site-directed mutagenesis and introduced into the recombinant octamer by refolding with wild-type H2A, H3, and H4 as described (Kassabov et al, 2002). The refolded octamer was reconstituted by the serial dilution method with end-labeled DNA. The unique cysteine residue of H2B was coupled with _p_-azidophenacyl bromide (APB, Fluka), sliding assays were performed as described above and crosslinked using a UV transilluminator at 312 nM for 1 min (Kassabov et al, 2002).
Nucleosome binding and sliding assays
In a 25 μl reaction, ISW2 was prebound to nucleosomes by incubation at 30°C for 30 min in 25 mM HEPES–KOH (pH 7.6), 5 mM MgCl2, 0.1 mM EDTA, 10% glycerol, 50 mM KCl, and 0.1 μg/μl BSA. The dissociation constants (_K_D) of ISW2 for the different nucleosomes were calculated from the linear range of the plot of the percentage of nucleosome bound to ISW2 as a function of the molar concentration of ISW2. Sliding assays had ATP added to a final concentration of 500 μM and incubated for an additional 10 min. DNase I footprinting of ISW2 was carried out with concentrations of DNase I in the range of 2–16 × 10−4 U in a 25 μl binding reaction. The samples were incubated for 2 min at 25°C after addition of DNase I and the reaction was terminated by addition of EDTA to a final concentration of 20 mM (Sambrook and Russel, 2001). Samples were extracted with phenol:chloroform (24:1), ethanol precipitated and analyzed on a 6.5% polyacrylamide gel containing 8 M urea.
Hydroxyl radical footprinting
Sucrose gradient-purified nucleosomes assembled on a high-affinity nucleosome positioning sequence were used for this purpose. ISW2 binding reaction was carried out in a 25 μl reaction as described (Kassabov et al, 2002) without the addition of ATP. Glycerol and sucrose were removed from nucleosome alone and ISW2–nucleosome complexes by rapid gel filtration using a Sephacryl S-200 spin column. The cleavage reaction was performed as described (Tullius et al, 1987) except that the final concentrations of Fe(II), H2O2, ascorbate, and EDTA were 280 μM, 0.17%, 5.7 mM, and 220 μM, respectively, and the reaction was terminated by addition of 100 μl of 5 M ammonium acetate, 5 mM thiourea, and 10 mM EDTA.
Site-specific DNA photoaffinity crosslinking
A series of 23 site-specific photoreactive DNA probes were synthesized as described previously (Sengupta et al, 1999). All probes were 222 bp long such that when reconstituted into nucleosomes there was 75 bp of linker DNA on an end-positioned nucleosome. Single or double photoreactive nucleotide analogs (AB-dATP, AB-dCTP, or AB-dUTP) were incorporated at positions ranging from −126, which is in the linker region 53 bp from the edge of nucleosome, to the far inside position +43/+44. All probes were radioactively labeled with one or two α-32P-labeled nucleotides incorporated adjacent to the photoreactive nucleotide analogs. Purified photoreactive probes (66 fmol) were used to reconstitute nucleosome core particles with the salt dilution method as described (Kassabov et al, 2002), except that reaction volumes were scaled down four-fold. Nucleosomes (0.82 pmol) were incubated with or without 1.8 pmol of purified ISW2 complex at 30°C for 30 min in a 25 μl reaction containing 25 mM K-Hepes (pH 7.6), 40 mM KCl, 12 mM NaCl, 5 mM MgCl2, 5% glycerol, and 0.1 mg/ml BSA. A 4 μl volume was analyzed on a 4% native polyacrylamide gel (acrylamide:bisacrylamide, 38.9:1.1, in 0.5 × TBE) to assess ISW2 binding. In all experiments, greater than 90% of nucleosomes were bound to ISW2. Reactions were irradiated for 2 min (310 nm, 2.65 mW/cm2), and then digested with 4.6 U of DNase I and 20 U of S1 nuclease sequentially as described (Sengupta et al, 1999). Samples were analyzed by 4–20% SDS–PAGE and crosslinked ISW2 subunits visualized by phosphorimaging.
Supplementary Material
Supplementary Data
Acknowledgments
We thank Toshio Tsukiyama for providing us the yeast strain yTT480. We also thank Jon Widom for 601 DNA, Karolin Luger and Tim Richmond for providing us the histone expression plasmids, and Jim Persinger for DNA probe preparation. We thank all the members of the Bartholomew lab for their suggestions and comments. This work was supported by Public Health Service grant GM 48413 from the National Institute of Health and the American Cancer Society grant RPG-99-199-01-GMC.
References
- Aihara T, Miyoshi Y, Koyama K, Suzuki M, Takahashi E, Monden M, Nakamura Y (1998) Cloning and mapping of SMARCA5 encoding hSNF2H, a novel human homologue of Drosophila ISWI. Cytogenet Cell Genet 81: 191–193 [DOI] [PubMed] [Google Scholar]
- Alen C, Kent NA, Jones HS, O'Sullivan J, Aranda A, Proudfoot NJ (2002) A role for chromatin remodeling in transcriptional termination by RNA polymerase II. Mol Cell 10: 1441–1452 [DOI] [PubMed] [Google Scholar]
- Balasubramanian B, Pogozelski WK, Tullius TD (1998) DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone. Proc Natl Acad Sci USA 95: 9738–9743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker PB, Horz W (2002) ATP-dependent nucleosome remodeling. Annu Rev Biochem 71: 247–273 [DOI] [PubMed] [Google Scholar]
- Brehm A, Langst G, Kehle J, Clapier CR, Imhof A, Eberharter A, Muller J, Becker PB (2000) dMi-2 and ISWI chromatin remodelling factors have distinct nucleosome binding and mobilization properties. EMBO J 19: 4332–4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapier CR, Langst G, Corona DF, Becker PB, Nightingale KP (2001) Critical role for the histone H4 N terminus in nucleosome remodeling by ISWI. Mol Cell Biol 21: 875–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapier CR, Nightingale KP, Becker PB (2002) A critical epitope for substrate recognition by the nucleosome remodeling ATPase ISWI. Nucleic Acids Res 30: 649–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CA, Sargent DF, Luger K, Maeder AW, Richmond TJ (2002) Solvent mediated interactions in the structure of the nucleosome core particle at 1.9 Å resolution. J Mol Biol 319: 1097–1113 [DOI] [PubMed] [Google Scholar]
- Deuring R, Fanti L, Armstrong JA, Sarte M, Papoulas O, Prestel M, Daubresse G, Verardo M, Moseley SL, Berloco M, Tsukiyama T, Wu C, Pimpinelli S, Tamkun JW (2000) The ISWI chromatin-remodeling protein is required for gene expression and the maintenance of higher order chromatin structure in vivo. Mol Cell 5: 355–365 [DOI] [PubMed] [Google Scholar]
- Ebralidse KK, Grachev SA, Mirzabekov AD (1988) A highly basic histone H4 domain bound to the sharply bent region of nucleosomal DNA. Nature 331: 365–367 [DOI] [PubMed] [Google Scholar]
- Eisen JA, Sweder KS, Hanawalt PC (1995) Evolution of the SNF2 family of proteins: subfamilies with distinct sequences and functions. Nucleic Acids Res 23: 2715–2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfring LK, Deuring R, McCallum CM, Peterson CL, Tamkun JW (1994) Identification and characterization of Drosophila relatives of the yeast transcriptional activator SNF2/SWI2. Mol Cell Biol 14: 2225–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzio TG, Kooperberg C, Goldmark JP, Neal C, Basom R, Delrow J, Tsukiyama T (2001) Widespread collaboration of Isw2 and Sin3-Rpd3 chromatin remodeling complexes in transcriptional repression. Mol Cell Biol 21: 6450–6460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmark JP, Fazzio TG, Estep PW, Church GM, Tsukiyama T (2000) The Isw2 chromatin remodeling complex represses early meiotic genes upon recruitment by Ume6p. Cell 103: 423–433 [DOI] [PubMed] [Google Scholar]
- Grune T, Brzeski J, Eberharter A, Clapier CR, Corona DF, Becker PB, Muller CW (2003) Crystal structure and functional analysis of a nucleosome recognition module of the remodeling factor ISWI. Mol Cell 12: 449–460 [DOI] [PubMed] [Google Scholar]
- Hakimi MA, Bochar DA, Schmiesing JA, Dong Y, Barak OG, Speicher DW, Yokomori K, Shiekhattar R (2002) A chromatin remodelling complex that loads cohesin onto human chromosomes. Nature 418: 994–998 [DOI] [PubMed] [Google Scholar]
- Hamiche A, Sandaltzopoulos R, Gdula DA, Wu C (1999) ATP-dependent histone octamer sliding mediated by the chromatin remodeling complex NURF. Cell 97: 833–842 [DOI] [PubMed] [Google Scholar]
- Kang JG, Hamiche A, Wu C (2002) GAL4 directs nucleosome sliding induced by NURF. EMBO J 21: 1406–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassabov SR, Henry NM, Zofall M, Tsukiyama T, Bartholomew B (2002) High Resolution mapping of changes in histone–DNA contacts of nucleosomes remodeled by ISW2. Mol Cell Biol 22: 7524–7534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassabov SR, Zhang B, Persinger J, Bartholomew B (2003) SWI/SNF unwraps, slides, and rewraps the nucleosome. Mol Cell 11: 391–403 [DOI] [PubMed] [Google Scholar]
- Kent NA, Karabetsou N, Politis PK, Mellor J (2001) In vivo chromatin remodeling by yeast ISWI homologs Isw1p and Isw2p. Genes Dev 15: 619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langst G, Becker PB (2001a) ISWI induces nucleosome sliding on nicked DNA. Mol Cell 8: 1085–1092 [DOI] [PubMed] [Google Scholar]
- Langst G, Becker PB (2001b) Nucleosome mobilization and positioning by ISWI-containing chromatin-remodeling factors. J Cell Sci 114: 2561–2568 [DOI] [PubMed] [Google Scholar]
- Langst G, Bonte EJ, Corona DF, Becker PB (1999) Nucleosome movement by CHRAC and ISWI without disruption or _trans_-displacement of the histone octamer. Cell 97: 843–852 [DOI] [PubMed] [Google Scholar]
- Lorch Y, LaPointe JW, Kornberg RD (1987) Nucleosomes inhibit the initiation of transcription but allow chain elongation with the displacement of histones. Cell 49: 203–210 [DOI] [PubMed] [Google Scholar]
- Lorch Y, Zhang M, Kornberg RD (1999) Histone octamer transfer by a chromatin-remodeling complex. Cell 96: 389–392 [DOI] [PubMed] [Google Scholar]
- Lowary PT, Widom J (1998) New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning. J Mol Biol 276: 19–42 [DOI] [PubMed] [Google Scholar]
- Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ (1997) Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 389: 251–260 [DOI] [PubMed] [Google Scholar]
- Moreau JL, Lee M, Mahachi N, Vary J, Mellor J, Tsukiyama T, Goding CR (2003) Regulated displacement of TBP from the PHO8 promoter in vivo requires Cbf1 and the Isw1 chromatin remodeling complex. Mol Cell 11: 1609–1620 [DOI] [PubMed] [Google Scholar]
- Morillon A, Karabetsou N, O'Sullivan J, Kent N, Proudfoot N, Mellor J (2003) Isw1 chromatin remodeling ATPase coordinates transcription elongation and termination by RNA polymerase II. Cell 115: 425–435 [DOI] [PubMed] [Google Scholar]
- Okabe I, Bailey LC, Attree O, Srinivasan S, Perkel JM, Laurent BC, Carlson M, Nelson DL, Nussbaum RL (1992) Cloning of human and bovine homologs of SNF2/SWI2: a global activator of transcription in yeast S. cerevisiae. Nucleic Acids Res 20: 4649–4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan ML, Schnitzler GR, Kingston RE (2000) Octamer transfer and creation of stably remodeled nucleosomes by human SWI-SNF and its isolated ATPases. Mol Cell Biol 20: 6380–6389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russel DW (2001) Molecular Cloning. A Laboratory Manual, 3rd edn, pp 17.14 Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Santoro R, Li J, Grummt I (2002) The nucleolar remodeling complex NoRC mediates heterochromatin formation and silencing of ribosomal gene transcription. Nat Genet 32: 393–396 [DOI] [PubMed] [Google Scholar]
- Sengupta SM, Persinger J, Bartholomew B, Peterson CL (1999) Use of DNA photoaffinity labeling to study nucleosome remodeling by SWI/SNF. Methods 19: 434–446 [DOI] [PubMed] [Google Scholar]
- Shen X, Mizuguchi G, Hamiche A, Wu C (2000) A chromatin remodelling complex involved in transcription and DNA processing. Nature 406: 541–544 [DOI] [PubMed] [Google Scholar]
- Tsukiyama T, Palmer J, Landel CC, Shiloach J, Wu C (1999) Characterization of the imitation switch subfamily of ATP-dependent chromatin-remodeling factors in Saccharomyces cerevisiae. Genes Dev 13: 686–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullius TD, Dombroski BA, Churchill ME, Kam L (1987) Hydroxyl radical footprinting: a high-resolution method for mapping protein–DNA contacts. Methods Enzymol 155: 537–558 [DOI] [PubMed] [Google Scholar]
- Vary JC Jr, Gangaraju VK, Qin J, Landel CC, Kooperberg C, Bartholomew B, Tsukiyama T (2003) Yeast Isw1p forms two separable complexes in vivo. Mol Cell Biol 23: 80–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse I, Stockdale C, Flaus A, Szczelkun MD, Owen-Hughes T (2003) Evidence for DNA translocation by the ISWI chromatin-remodeling enzyme. Mol Cell Biol 23: 1935–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodage T, Basrai MA, Baxevanis AD, Hieter P, Collins FS (1997) Characterization of the CHD family of proteins. Proc Natl Acad Sci USA 94: 11472–11477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Santoro R, Grummt I (2002) The chromatin remodeling complex NoRC targets HDAC1 to the ribosomal gene promoter and represses RNA polymerase I transcription. EMBO J 21: 4632–4640 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data