Fear and the Defense Cascade: Clinical Implications and Management (original) (raw)
Supplemental digital content is available in the text.
Keywords: collapsed immobility, defense cascade, defense responses, fear behaviors, fight, flaccid immobility, flight, freeze, freezing, fright, quiescent immobility, threat-induced fainting, tonic immobility
Abstract
Evolution has endowed all humans with a continuum of innate, hard-wired, automatically activated defense behaviors, termed the defense cascade. Arousal is the first step in activating the defense cascade; flight or fight is an active defense response for dealing with threat; freezing is a flight-or-fight response put on hold; tonic immobility and collapsed immobility are responses of last resort to inescapable threat, when active defense responses have failed; and quiescent immobility is a state of quiescence that promotes rest and healing. Each of these defense reactions has a distinctive neural pattern mediated by a common neural pathway: activation and inhibition of particular functional components in the amygdala, hypothalamus, periaqueductal gray, and sympathetic and vagal nuclei. Unlike animals, which generally are able to restore their standard mode of functioning once the danger is past, humans often are not, and they may find themselves locked into the same, recurring pattern of response tied in with the original danger or trauma. Understanding the signature patterns of these innate responses—the particular components that combine to yield the given pattern of defense—is important for developing treatment interventions. Effective interventions aim to activate or deactivate one or more components of the signature neural pattern, thereby producing a shift in the neural pattern and, with it, in mind-body state. The process of shifting the neural pattern is the necessary first step in unlocking the patient’s trauma response, in breaking the cycle of suffering, and in helping the patient to adapt to, and overcome, past trauma.
In The Expression of the Emotions in Man and Animals (1872), Darwin1 argued that human expressions of emotion resembled those of lower animals and that emotions are adaptive because they prompt action responses that are beneficial to the organism. Positive emotions promote social-engagement behaviors, whereas negative emotions, many of which are activated by threat, invoke defense responses.2,3 Writing in 1908, McDougall4 described the various instinctual behaviors that accompanied the emotions of fear, anger, and disgust. Building on McDougall’s ideas, Cannon (1915)5 wrote his landmark book, Bodily Changes in Pain, Hunger, Fear and Rage, describing the bodily changes that occurred in the context of emotional excitement. That work is best remembered for elaborating the concept of fight or flight.* In 1920, Rivers7 (a physician working with officers suffering from shell shock during the First World War) described five danger instincts: flight, aggression, manipulative activity, immobility, and collapse.† Subsequent research with animals determined that, depending on the degree of threat and the distance between the predator and prey, distinct responses—freezing, flight or fight, tonic immobility, and quiescent immobility—proceed sequentially along a continuum, termed the defense cascade.‡,2,8,9,17 Researchers likewise began to use the defense cascade to define the progressive defense/fear responses in humans.2,15,18,19
In evolutionary terms the responses that make up the defense cascade are primitive emotional states—coordinated patterns of motor-autonomic-sensory response—that are available to be automatically activated in the context of danger. Emotions are played out “in the theatre of the body.”20(p 28) For humans, the activation of defense responses—the sudden change in motor and physiological state—may be experienced as overwhelming, and beyond conscious control. In clinical practice these phenomena are common, and they occur across a broad range of disorders and clinical presentations: posttraumatic stress disorder (PTSD), peritraumatic reactions (as in physical or sexual assaults, or following accidents or natural disasters), complex trauma, borderline personality disorder, and states of intense distress potentially leading to self-harm.21 As every clinician knows, these different states are difficult to understand (what is the underlying dynamic?), difficult to identify and differentiate (what exactly is this state, and how does it differ from other states?), and difficult to manage and treat.
The first goal of this article is to examine the defense responses through the lens of neuroscience and to elaborate a model that explains their brain and body mechanisms. For this purpose we conducted wide-ranging searches for relevant literature on PubMed; identified research from, and sometimes communicated with, laboratories and clinical groups worldwide conducting relevant research; and retrieved and tracked references to seminal articles in the history of the defense cascade. The second goal is to use that model to understand different clinical presentations and phenomena, and to determine appropriate treatment and management of patients.
Central to the analytical framework for this article is the defense cascade. All defense responses in the animal model of the defense cascade—arousal, freezing, flight or fight, tonic immobility, collapsed immobility, and quiescent immobility—are responses to threat mediated by neural circuits involving the extended amygdala, hypothalamus, periaqueductal gray (PAG), ventral pontine tegmentum, ventral and dorsal medulla, and spinal cord.22–25 Each defense response has a signature neural pattern that corresponds to a combination of activated connections within a descending neural network (see Figures 1 and 2). This descending network terminates at the level of the effector organs, where it controls a somatomotor component (which involves skeletal muscle), an autonomic/visceromotor component (which involves the viscera), and a pain-processing component. Changes in the patterns of activity of that network mediate the defense cascade and define the different types of defense responses that, taken together, form the defense repertoire of mammalian species. In any particular situation the defense response will be a function of the species-specific defense repertoire,8 genetic variations among strains,26 characteristics of the threat, and context in which it occurs, all influenced by individual differences.§,27
Figure 1.
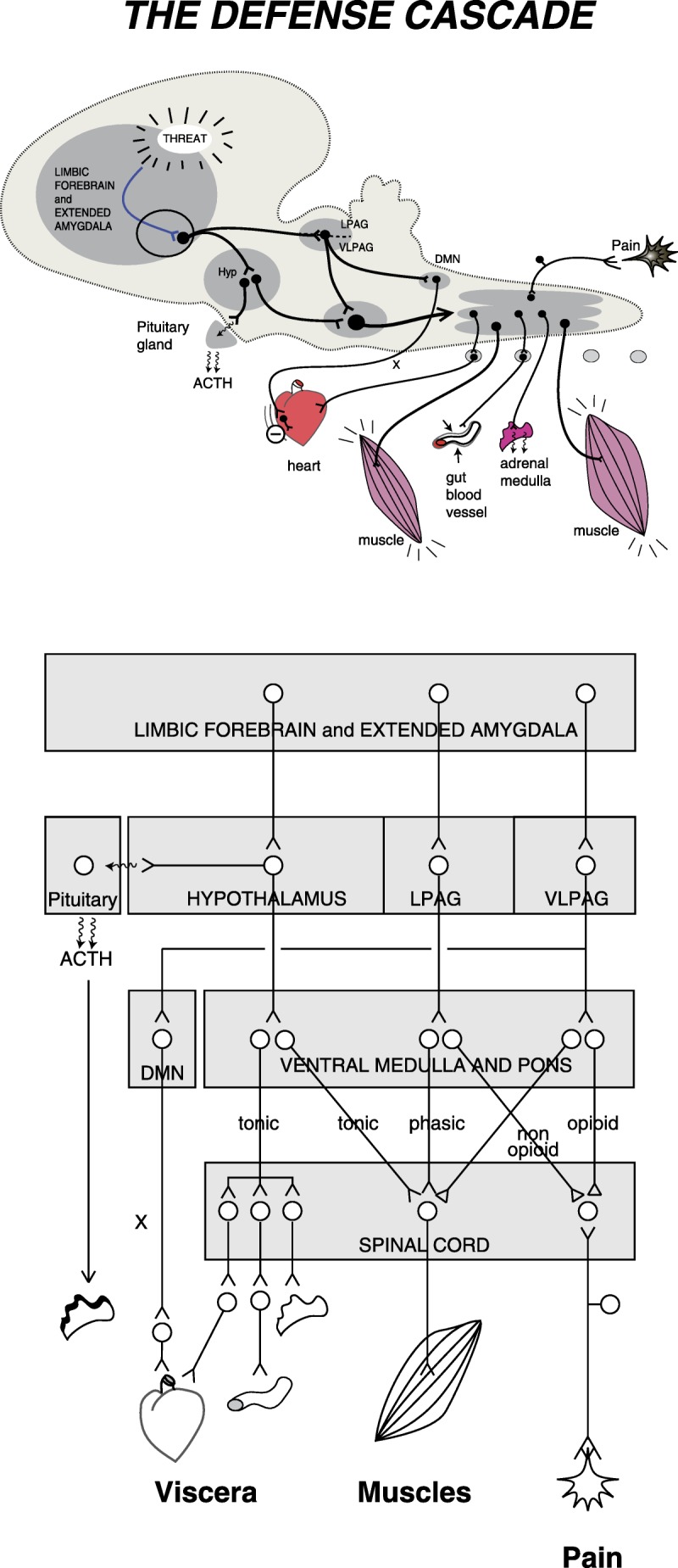
The defense cascade. Schematic views of the descending pathways connecting brain and spinal cord structures to some of the peripheral organs involved in the expression of defense behaviors. The upper panel shows the structures and pathways on a side view of a stylized mammalian brain. The bottom panel is a block diagram of the same information with more details. ACTH, adrenocorticotropic hormone; DMN, dorsal motor nucleus of the vagus; Hyp, hypothalamus; LPAG, lateral periaqueductal gray; VLPAG, ventrolateral periaqueductal gray; X, vagus nerve.
Figure 2.
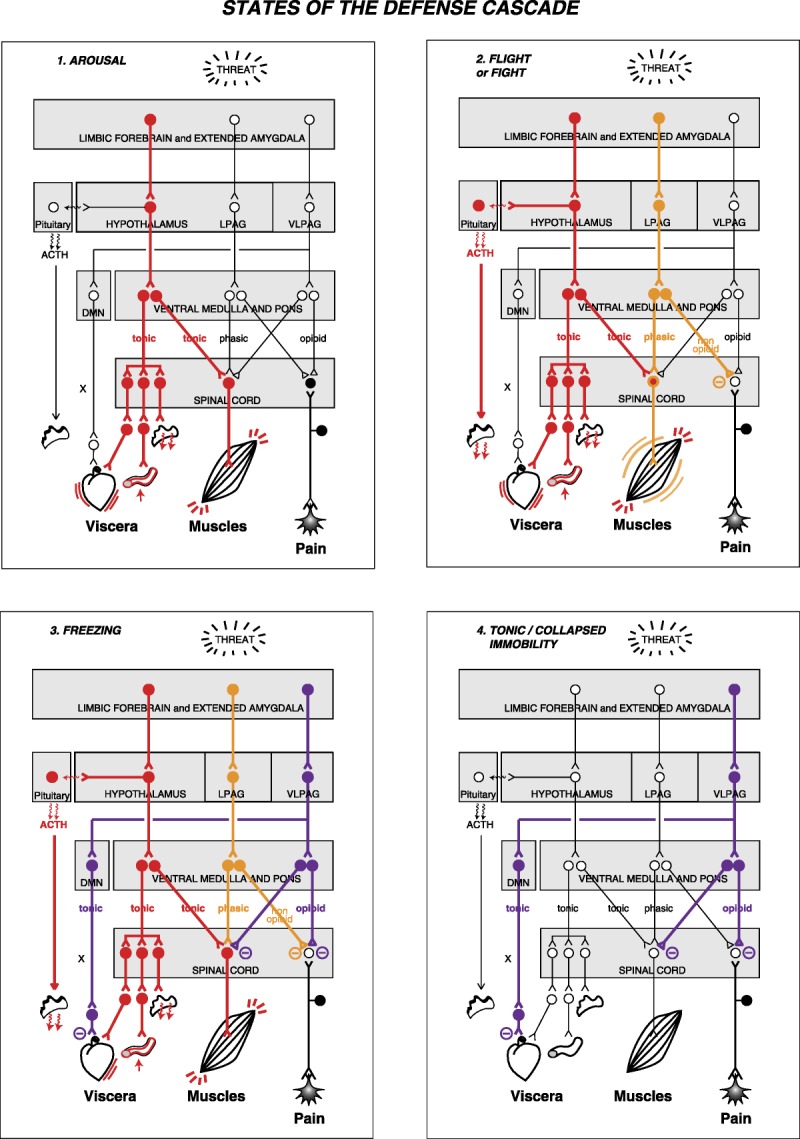
States of the defense cascade. The diagram depicts the states of arousal, flight or fight, freezing, and tonic/collapsed immobility in terms of patterns of neural activity in the different structures and pathways of the defense cascade network. I. Arousal, the first step to the activation of the defense cascade, can be viewed as the activation of the hypothalamus pathway. II. Fight or flight involves the activation of the hypothalamus and lateral periaqueductal gray. III. Freezing—flight or fight put on hold—involves activation of the following: hypothalamus pathway; unmyelinated vagal pathway from the dorsal motor nucleus (which opposes the sympathetic activation); lateral periaqueductal gray; and ventrolateral periaqueductal gray (which opposes activation of the lateral periaqueductal gray). IV. Tonic/collapsed immobility involves activation of the unmyelinated vagal pathway from the dorsal motor nucleus and of the ventrolateral periaqueductal gray pathway. In tonic/collapsed immobility the hypothalamus pathway is not activated. The filled circles depict activated neurons, whereas the open circles depict non-activated neurons. ACTH, adrenocorticotropic hormone; DMN, dorsal motor nucleus of the vagus; Hyp, hypothalamus; LPAG, lateral periaqueductal gray; VLPAG, ventrolateral periaqueductal gray; X, vagus nerve.
As noted previously, each defense response is accompanied by changes in pain processing and sensory processing. Adaptations in pain processing—in particular, the different roles that analgesia plays in each separate defense response—ensures that the animal is able to remain fully focused on the threat and to respond self-protectively, and that the animal’s attention is not distracted by aversive body states such as injuries. Non-opioid analgesia accompanies the “active” defense responses (flight or fight), and opioid analgesia accompanies the “passive” defense responses (freezing, tonic immobility, collapsed immobility, and quiescent immobility).28–30 Because opiates induce a state of well-being, it is probable that, during the passive defense responses, opioid analgesia functions on a subjective level to mitigate the intensity of subjective fear. Whereas pain processing has been extensively studied, comparatively little is known about the detailed dynamics of sensory processing during defensive mind-body states; of necessity, our scientific discussion of sensory processing as such (in the first nonclinical section of the article) is therefore limited.** Further information about pain processing during states of defense can be found in Lanius and colleagues (2014).33
Finally, the animal and human defense cascades differ in several respects. For humans, the model includes collapsed immobility,††,7,16,35 which is characterized by bradycardia combined with hypotonicity of skeletal muscles. Various writers have used other terms to refer to collapsed immobility: collapse,7,21 flaccid immobility,16,35 faint,16,35 fear-induced fainting,16 vasovagal syncope,36 neurocardiogenic syncope,37 and fainting in the context of a blood phobia.16,35,38,39 To increase precision, we (the authors) use the term collapsed immobility to identify threat-induced fainting mediated by neural circuits involving the extended amygdala, hypothalamus, and periaqueductal gray, as in tonic immobility (see below), with the addition of cerebral ischemia mediating a loss of muscle tone and changes in consciousness. Another difference is that we identify freezing to be a flight-or-fight response put on hold. With these modifications, the defense cascade in humans involves the following action patterns or mind-body states: (1) arousal, the first step in activating the defense cascade; (2) flight or fight, an active defense response for dealing with threat; (3) freezing, which is a flight-or-fight response put on hold; (4) tonic immobility, a response to inescapable threat, or a strategy of last resort, when active defense responses have failed; (5) collapsed immobility, a variant of tonic immobility, in which muscle tone is lost and consciousness is compromised secondary to bradycardia-induced cerebral hypoxia;‡‡ and (6) quiescent immobility, a state of quiescence that promotes rest and healing. This order differs from conceptualizations based on the distance of the predatory threat—in which freezing is discussed before flight or fight.2,8,9,17 As indicated above, the reason for reversing the order of the first two patterns or states is that freezing is best understood as an inhibited flight-or-fight response, which therefore needs to be discussed first.
The human model is also more complex because humans make subjective representations of body states and endow their experiences with meaning, and because humans use their minds to create internally generated representations of threat—images of feeling states and events from the past or images of the imagined future—which, like real external threats, have the capacity to activate the body’s defense systems in the absence of external threat. Fear states can therefore be induced by combinations of internal and external triggers, some of which will be accessible to conscious processing, and some not.42 In this context it is important to note that, although we focus primarily on the role of phylogenetically old circuits underpinning innate animal and human defense responses, in humans these circuits are embedded within, and interact with, a broad array of more recently evolved neural circuits and networks involved in emotion regulation. Whenever necessary, these newer elements will be integrated into our discussion.
AROUSAL
Arousal is the first, necessary step in activating the defense cascade in both animals and humans (see Figures 1 and 2 and Supplemental Text Box 1, http://links.lww.com/HRP/A8, for more detail). It sometimes leads straight into the flight-or-fight response or, more commonly, into the freeze response. In some circumstances, arousal may also be followed directly by tonic immobility or collapsed immobility, specifically in circumstances where the latter responses have been primed by past experience. The hypothalamus plays a major role in arousal by increasing tone both in the sympathetic branch of the autonomic (visceromotor) nervous system and in the somatomotor nervous system (i.e., the striated muscles) (see Figures 1 and 2 and Supplemental Text Box 1, http://links.lww.com/HRP/A8). In states of high arousal, sympathetic activation causes vasoconstriction of blood vessels that supply the salivary glands, resulting in a dry mouth, increased tone in the proximal laryngeal muscles (alongside the back and postural muscles), and, in turn, a high-pitched voice (see Vignette 2). In brief, all muscles, both smooth and striated, increase in tone;41,43 heart rate and respiration become more rapid; and posture is stabilized. The body is prepared for action.
Vignette 1:§§ Arousal
Svetlana, a 35-year-old doctor in the midst of litigation, presented with a request for help in managing physiological symptoms of arousal. Her symptoms included sweating, a rapid heart rate, hyperventilation, and a sense of panic. Because of muscle tension in her back, neck, and calves, she found it difficult to settle down at night, and she experienced more myoclonic jerks as she was trying to get to sleep. Her sleep pattern was characterized by multiple arousals, during which she ruminated about the litigation. In the preceding months she had changed her eating pattern to multiple small meals because the food felt like a rock inside her stomach, as if she was unable to digest it. On this new dietary regime, she was losing roughly a kilogram a month.
Vignette 2: Arousal
An officer from the Second World War described his experience of increased arousal whenever he heard artillery and mortar fire: “One’s mouth goes dry and black, and a strange squeaking or quacking comes out, joined sometimes with a stammer. Very hard for a field-grade officer to keep his dignity when that happens.”44(p 278)
FLIGHT OR FIGHT: THE ANIMAL MODEL
Flight-or-fight responses are active defense responses—coordinated patterns of emotional-behavioral-physiological response—that are activated when animals are confronted with imminent danger, such as being actively pursued or attacked by a predator. Studies suggest that flight or fight is the sum total of distinct components activated concurrently: a somatomotor (skeletal muscle) component, a visceromotor (autonomic) component, and a pain-modulation component (see Figures 1 and 2).24,45,46
Skeletal Muscle (Somatomotor) Activation
Flight or fight is mediated through the lateral periaqueductal gray (LPAG), which activates the basic, stereotypical motor patterns of flight or fight—for example, attack, running, treading, burying (see Figures 1 and 2).24,28,47,48 Direct projections from the amygdala and limbic forebrain activate specific areas within the LPAG to produce these basic patterns, which control not only limb muscles but also laryngeal muscles resulting in snarling, growling, and howling.24,48,49 The LPAG, in turn, activates premotor centers in the pons and medulla, which then activate motor networks in the spinal cord or brain stem.28,47 Concomitant activation of cortical loops with the basal ganglia and cerebellum may then modulate those basic patterns, depending on the context and the overall strategy of defense.50
Pattern of Autonomic (Visceromotor) Activation
Sympathetic
Activation of autonomic centers in the dorsal hypothalamus causes a generalized sympathetic response that includes activation of the heart (increased heart rate and cardiac output) and increased vascular resistance in the viscera, which increases the perfusion pressure of tissues, especially the muscles, heart, and brain.24,28 Sympathetic activation of the adrenal medulla, which causes the release of circulating catecholamines, acts to amplify the sympathetic response. Sympathetic efferents to the gut inhibit routine digestive functions. The same hypothalamic regions also increase respiration to facilitate gas exchange through the lungs, in parallel to the increased perfusion of active tissues.
Parasympathetic
At the same time that cardiac sympathetic tone is increased, vagal cardiac parasympathetic tone is reduced. According to current neurophysiological models,3,51 this process is mediated primarily by the efferent vagal fibers that originate from the nucleus ambiguous and that fire in synchrony with the respiratory cycle (respiratory-related cardiac vagal efferents) (see Supplemental Text Box 2, http://links.lww.com/HRP/A9).
Pain Processing
Flight or fight involves non-opioid analgesia,28,29 which can be evoked by activation of the LPAG.28 Projections to the spinal cord block ascending pain signals.30
FLIGHT OR FIGHT IN HUMANS
Vignette 3. Flight or fight
Kitti was a 10-year-old girl living with her adopted parents on a country property. Kitti had suffered physical and sexual abuse when in the care of her biological mother. Sometimes when she was out shopping with her foster mother, Kitti would mistake a passing stranger to be her biological mother. At night, her memories now activated, Kitti would “see” her biological mother looking at her through her bedroom window. Faced with this threat, Kitti would run out of the house and into the paddocks. If her parents tried to stop her, Kitti would hit, kick, and bite them in her frantic efforts to get away.
Vignette 4. Flight or fight
Jeremy, a veteran of the 2003 war in Iraq, presented for his first therapy appointment in 2009. His apprehension was evident from the moment he entered the waiting room: he scanned the empty room repeatedly and jumped at the slightest sound. During the assessment Jeremy became unnerved by the process, and in response to a clumsily asked question, his fear gave way to rage. He stood up suddenly, pushed his chair violently to the back of the room, and stood glaring at the therapist. Jeremy’s face and body communicated his anger and readiness for action: his eyebrows were lowered and pulled together, his brow was furrowed, his nostrils were flared, his mouth was ajar to reveal his teeth, his lips were thin and tense, his breathing was heavy, and his large frame shook with pent-up energy. He screamed at the therapist to “back off.”
When the misunderstanding regarding the therapist’s clumsy question was resolved, Jeremy started to settle. He stated that this kind of response had been occurring with increasing frequency since returning from active service in Iraq. Jeremy reported that when out in public, he was constantly on the lookout for “trouble” and that, unfortunately, he often found it. He described a number of physical altercations—he had attacked other men on the basis of perceived provocation—that had occurred over the last 12 months. He told the therapist that although he could recall his initial angry response, he would then lose track of time. When he became self-aware again, he would find himself towering over the vanquished man lying on the ground.
Flight or fight*** is common. Traumatized or emotionally disturbed children often respond to commonplace stressors or traumatic triggers by running away or by exploding into a violent rage (see Vignette 3). In the chapter “Anger and Hatred” in The Expression of the Emotions in Man and Animals, Darwin described this mind-body state in the following words: “when in a violent rage,” human children “roll on the ground on their backs or bellies, screaming, kicking, scratching or biting everything within reach.”1(p 236) In adults, flight-or-fight responses are commonly seen in traumatized individuals—for example, those with PTSD—whose hyperaroused state may shift into episodes of overwhelming rage (fight) or into escape from situations or contexts that trigger cognitive or somatic reminders of past trauma (flight).52
In response to trauma scripts, patients with the reexperiencing/hyperarousal response—as in PTSD—show decreased activation in anterior brain regions implicated in regulating arousal and emotion (ventromedial prefrontal cortex and rostral anterior cingulate cortex), decreased activation in the thalamus and occipital cortices, and increased activity in the amygdala and insula.53–58 The increased amygdalar activation increases the probability that defense programs mediated by the amygdala-hypothalamus-PAG circuits (flight or fight; freezing) will be activated. And because the insula is involved in neural representations of body state—including acute sympathetic arousal—increased activity there reflects the individual’s hyperaroused body state.54,57,59
THE FREEZE RESPONSE: THE ANIMAL MODEL
The freeze response is also referred to as attentive immobility, hyper-reactive immobility, and reactive immobility. It has been most extensively studied in rodents45,60 and monkeys (see Figure 3).61–63 Freezing occurs in the context of predatory threats—detection of a predator—or in laboratory situations where the animal is reexposed to a context or discrete cues that have previously been associated with an aversive event.9,46,60,61 In predator-prey interactions, this attentive immobility functions to decrease the likelihood of detection since the visual cortex of mammalian carnivores is programmed to detect moving objects.16 Attentive immobility enables the animal to continue scanning the environment and readies the animal for an active response such as flight or fight.16,45,61 In the laboratory situation, the fourth author has observed rats to freeze for periods as long as 20 minutes. In wild rodents, freezing up to a period of 60 minutes—at which point the researcher had to interrupt his observations—has been described.†††,34 There can be marked differences in freezing within species across different genetic strains and research paradigms.61,64,65
Figure 3.

Freezing in a rat. The rat is stopped in midmovement. Despite being immobilized, the rat remains alert; it continues to scan the environment; and its body is tense and poised for action. Its ears are flattened. If the predator attacks, freezing will give way to flight, and the rat will attempt to dart away to safety.
The components of the freeze response are described below and are visually depicted in Figures 1 and 2.
Skeletal Muscle (Somatomotor) Activation
Freezing is a flight-or-fight response put on hold (see Figures 1 and 2). Activation of the ventrolateral periaqueductal gray (VLPAG)—the VLPAG brake—by the central nucleus of the amygdala imposes immobility, canceling any movement and forcing the animal to stay put.46 In effect, the VLPAG puts a brake on LPAG output, thereby preventing expression of the flight-or-fight motor patterns (except vocalizations) triggered by the LPAG, but leaves intact the pathways coming from the hypothalamus that set muscle tone (see Figures 1 and 2).46 Despite being immobilized, muscle tone is high: this combination results in the characteristic freeze response. In the rat, respiration during freezing is very rapid until the rat begins to vocalize ultrasonically, at which point the respiratory rate drops precipitously because ultrasonic vocalizations require long periods of expiration.
The pathways mediating immobility downstream to the VLPAG are not well understood. Based on current knowledge, the likely pathways for VLPAG outputs are as follows: the outputs may relay in the rostral ventral medulla to modulate premotor neurons that project to the spinal cord, or they may relay in the rostral ventral midbrain onto dopaminergic neurons of the substantia nigra; in either case, those neurons would modulate, in turn, motor loops in the striatum (basal ganglia), producing immobility.66
Pattern of Autonomic (Visceromotor) Activation
Freezing involves a coactivation of sympathetic and parasympathetic components.
Sympathetic
Sympathetic activation of the heart, lungs, gut, and other visceral tissues occurs in the same way as described for the flight-or-fight response and as shown in Figures 1 and 2.
Parasympathetic
According to Porges’s polyvagal theory,3 vagal tone—mediated by respiration-related vagal efferents from the nucleus ambiguous (NA) to the heart—is withdrawn. Instead, activation of non-respiration-related vagal efferents from the dorsal motor nucleus (DMN) takes place alongside sympathetic activation, opposes the sympathetic activation, and can cause a sudden drop in heart rate (see Supplemental Text Box 2, http://links.lww.com/HRP/A9). This drop is usually referred to as fear bradycardia.‡‡‡,34 In most cases, however, the coactivation of the parasympathetic component will generate a bradycardic effect, manifesting as an attenuated tachycardia or with no change in heart rate.68–70 This DMN-vagal inhibition of the heart is the autonomic equivalent of the immobility imposed on the somatomotor system. The cardiac brake is released when the animal switches back to flight or fight.46 A number of parallel pathways that include projections from the amygdala and VLPAG to the DMN28 are likely to mediate this inhibitory vagal response.
Pain Processing
The integrated freeze response includes an opioid-mediated analgesia,28,29 which is itself mediated by the PAG and the rostral ventromedial medulla pain circuit.71
The sum total of the above-described processes is a frightened animal that is stopped in midmovement, highly aroused, and primed to respond, but that is not yet active.34,45,60 If the predator attacks or, for example, the researcher attempts to pick up the rat, freezing gives way to flight or fight. The move to release the VLPAG and vagal brakes—that is, to switch from freezing to flight or fight—is probably initiated by the amygdala and brought about by an inhibition of the central nucleus of the amygdala, the main controller of the VLPAG.
THE FREEZE RESPONSE IN HUMANS
Vignette 5. Freezing
Mary, a 10-year-old girl, was at home when the kerosene that the family used to heat the house caught fire. Mary’s mother picked up the burning can and shouted at Mary to open the door. Mary froze: her eyes wide in fear, her gaze fixated on the burning can, her body tense and tight—a statue caught in mid-stance. Her mother attempted to leave the room without the assistance of Mary, who remained immobilized. As Mary’s mother opened the door, the burning can fell from her hands, setting fire to Mary’s puppies, which were positioned just outside the doorway. Roused into action, Mary ran to her mother to help put out the fire and possibly save her puppies.
Vignette 6. Freezing
Jian, a retired policeman, had worked in an area plagued by gangs. During his service, several of his friends had been badly injured there, and many had experienced high levels of fear when deployed on projects associated with the gangs. Although these events had taken place a long time ago, Jian remained vigilant and described his fear response in the following way. “I’ll be walking along the street with my wife and I will see one of them—the body shape, the hairdo, the skin color, or the tattoo—in the distance. I stop. Conversation is suspended. I completely switch off from the person next to me. My body tenses. A cold shiver runs through me. My heart pounds, and I sweat. I am rooted to the spot. My focus is on the person, and I watch their every move. Nothing else exists. Are they steering away from me or toward me? Who are they? What are they doing? Are they looking at me? Why are they here? I stand there a long while, my body tense, rooted to the spot, analyzing. I stand there until I am satisfied that they are not following me. Then I come back to myself and move away. But my body takes hours to settle down. And that only happens when I am sure that they are not following me. If they come toward me, I am out of there.”
Freezing in humans is a transient state that occurs at the very beginning of the threat experience and that involves heightened attention, enhanced vigilance to threat cues, and an activated, tense body poised for action. Typically a short-lived phenomenon (often lasting only a few seconds), it is accessible for conscious processing and subjective representation. Stilling of the body, measured by reductions in body sway72 and coupled with a drop in heart rate,73 has been interpreted as the human equivalent of freezing. Decreased body sway and heart rate (bradycardic effect) have been demonstrated in response to threatening stimuli (pictures of angry faces or of mutilation), social-threat stimuli, and states of anticipatory anxiety.73–81 Functional magnetic resonance imaging studies—performed while negatively arousing (aversive) stimuli, including pictures of mutilation and injury in humans, are being processed—provide clear evidence of PAG activation82,83 and suggest increased functional connectivity between the amygdala and PAG, coupled with a bradycardic effect.84 Individuals with a history of aversive life events show enhanced body stilling to aversive stimuli, suggesting that the freeze response can by primed by experience.76
TONIC IMMOBILITY: THE ANIMAL MODEL
Tonic immobility is a phylogenetically old defense response that occurs in a large number of species: insects, crustaceans, fish, amphibians, reptiles, birds, and mammals, including primates and humans.8,85 The long-held uncertainty about the nature of this defense response is reflected by the many different names used to describe it (see Supplemental Text Box 3, http://links.lww.com/HRP/A10). Tonic immobility is usually a terminal defense used when flight or fight has failed, and the animal has been caught by a predator. Its function is to deactivate the predator’s killing reflex or to discourage consumption, as many predators are reluctant to eat dead meat (see Figure 4).16,45,52,85 In some species or strains within species, however, tonic immobility may be the front-line defense response to extreme threat, even when the animal is not restrained.8,26,86 In laboratory settings, tonic immobility is elicited under conditions in which restraint and fear co-occur—for example, turning the animal upside down and restraining it until it stops struggling. Although the psychophysiological correlates of tonic immobility vary somewhat from one species to the next, the key clinical features are summarized in Supplemental Text Box 3, http://links.lww.com/HRP/A10.
Figure 4.
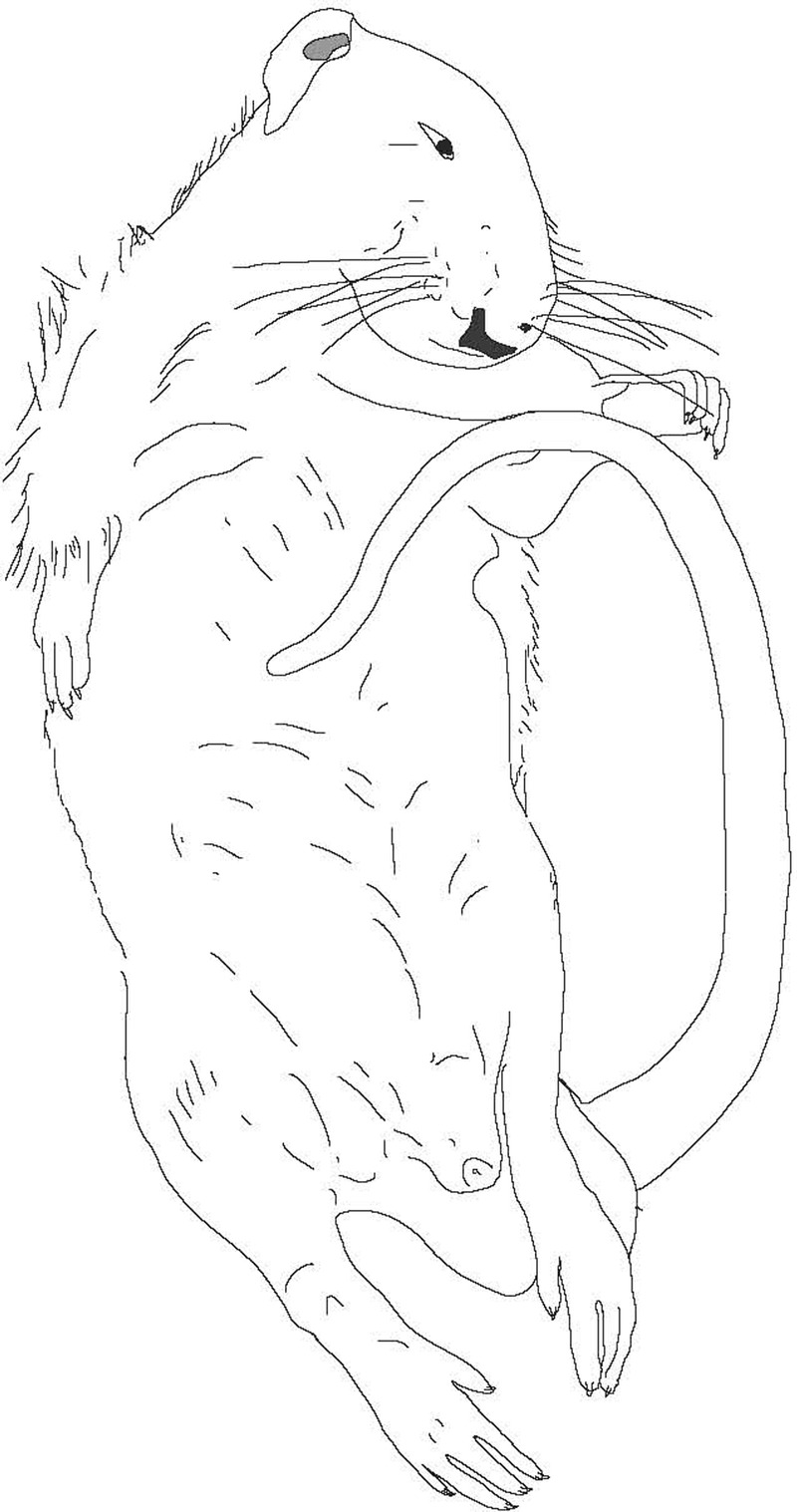
Tonic immobility in a rat. The trunk and limbs are rigid and may be held in unusual or awkward postures. The body can often be manipulated (waxy flexibility). The eyes may be closed or open. If the latter, the rat will have a glassy, unfocused gaze. Because the animal has the appearance of being dead, tonic immobility is also known, following Darwin’s terminology, as feigning death.87
Tonic immobility is a shutdown response mediated by phylogenetically old areas of the brain that appear to activate only when newer structures such as the amygdala are deactivated88 and when freezing and flight or fight are switched off.89–92 Tonic immobility can be induced in animals without a cerebrum.93 The components that make up tonic immobility are described below and are depicted visually in Figures 1 and 2.
The Sensory Component
Tactile sensory, proprioceptive, and visceral afferents, coupled with fear, trigger the tonic immobility response. The act of struggling with a predator elicits strong tactile sensory and proprioceptive stimuli.8,89 The VLPAG receives signals from deep somatic (muscle, joint) or visceral tissues via the dorsal horn of the spinal cord, from the parabrachial nucleus,25,94,95 and from the vagal sensory nucleus (the solitary track nucleus).28,96 Tonic immobility appears to be triggered when these sensory inputs reach a critical threshold.
The Skeletal Muscle (Somatomotor) Component
The “waxy” immobility that characterizes tonic immobility is also mediated by activation of the VLPAG.28,47,97 Because tonic immobility occurs when freezing and flight or fight are switched off, it can be conceptualized as the unhindered expression of VLPAG output (see Figures 1 and 2).47 Further downstream, descending projections either via the ventral medulla98 or possibly directly to the motor neurons of the ventral horn of the spinal cord99 mediate the immobility and loss of the righting reflex§§§ that characterize tonic immobility. A more complex and recently discovered inhibitory pathway ascending from the VLPAG to the basal ganglia via the rostromedial tegmental nucleus and the dopaminergic neurons of the substantia nigra may also contribute to this immobility.****,66
Pattern of Autonomic (Visceromotor) Activation
Sympathetic
Onset of tonic immobility in mammals is associated with a withdrawal of sympathetic activity, as is well documented in dogs and rats.100,101
Parasympathetic
Many of the clinical and physiological features of tonic immobility—bradycardia, life-threatening arrhythmias, decrease in temperature, decrease in respiration, and defecation—appear to reflect parasympathetic activity from the dorsal motor nucleus to the heart, lungs, and defense programs run by the enteric nervous system (see Supplemental Text Box 2, http://links.lww.com/HRP/A9). According to polyvagal theory,3 the bradycardia seen during tonic immobility is mediated by the subpopulation of cardiac vagal neurons in the DMN—non-respiration-related neurons that are intermittently active102—that are also activated in orienting and freezing. The defense strategy of tonic immobility is potentially lethal,101 as has been reported in many studies (see Supplemental Text Box 3, http://links.lww.com/HRP/A10). Dissection and ECG studies in dogs and rats, respectively, suggest that these deaths result from a parasympathetic surge combined with sympathetic withdrawal.100,101 In the dog, activation of a very small number of preganglionic efferent cardiac vagal fibers can initiate significant bradycardia and even cardiac arrest (Armour JA, personal communication, 2001)†††† (see Supplemental Text Box 2, http://links.lww.com/HRP/A9). These autonomic changes are synchronized by the VLPAG, whose activation produces immobility, a fall in blood pressure, and bradycardia (see Supplemental Text Box 2, http://links.lww.com/HRP/A9).47
Pain Processing
The antinociceptive response elicited during tonic immobility is opioid mediated103,104 and involves activation of the PAG and the rostral ventromedial medulla pain circuit.71
TONIC IMMOBILITY IN HUMANS
Vignette 7. Tonic Immobility
Sylvia, a nine-year-old girl born to a drug-addicted mother, was placed in her father’s care after she was found on the streets, at four years of age, eating food from garbage cans. In addition to severe neglect, Sylvia had been sexually abused by her mother’s boyfriends and had been exposed to domestic violence. Sylvia experienced a pervasive fear that she would somehow be separated from her father. At times, just the mention of a longer separation would elicit a shutdown response. Sylvia would stop talking, she would go pale, and her facial expression would become blank. Her body would go still, and be cool and clammy to the touch. Her father described it in the following words, “It is as if she is not at home, and when I look into her eyes, it’s like looking into nothingness into the back of her eyes.” On some of these occasions, Sylvia would start saying odd things, like “Daddy, you look so far away, it’s like you’re on the other side of the wall.” Sylvia’s father used gentle touch to help Sylvia shift out of such episodes.
Vignette 8. Tonic Immobility
Agata was a 32-year-old woman who, during childhood, had experienced neglect and physical and sexual abuse both within and outside of her family. In therapy sessions, when memories of sexual abuse were triggered, Agata would become pale and quiet. Her gaze would become unfocused and disconnected, and she would find it difficult to speak: her words emerged broken and incoherent. The therapist experienced the transference as a thick trance-like state within which it was difficult to think. The therapist would verbally identify Agata’s state and talk to her in gentle, soothing tones, breathing slowly and calmly. Over time, Agata learned to focus on the therapist’s voice and use it as a means of shifting herself back to the present, thereby shortening the episodes to a period of a few minutes. Agata described her experience in the following way. “I saw the face of my abuser looking at me. My body went cold, and I was paralyzed, locked in eye contact with his angry face, and disconnected from myself. I felt both trapped and distanced from myself.”
Vignette 9. Tonic Immobility
Paulo, a 21-year-old army recruit stationed in Iraq, recalled his first experience of a firefight. While under insurgent fire in an armored troop carrier, his commander ordered the soldiers in the vehicle to dismount and engage the insurgents with small-arms fire. After finding a position of cover, Paulo came under direct fire from small arms and rocket-propelled grenades. He recalled lying behind a fallen power pole, immobilized by fear and feeling strangely detached from the situation. He was unable to lift his head, move his limbs, or aim his rifle. He recalled a sensation of being drawn to the ground, a heavy sensation that he could not resist. Despite hearing his commander on a radio giving him instructions, he was unable to respond. After an indeterminate period of time, he recalled gaining a sense of control over his body when a fellow soldier joined him, repeatedly hit him on his helmet, and told him to return fire, which he was then able to do.‡‡‡‡ After a two-hour gun battle in which he took part, his troop returned to their base unharmed. It was only then that he realized he had been incontinent of both urine and feces.
In humans, tonic immobility may be elicited in a number of different scenarios: when the individual is cornered and perceives that neither escape nor fighting is possible; as a response of last resort when there is physical contact with a perpetrator and flight or fight is not possible or has failed; or as the individual’s first-line response to trauma (or to recurrent memories of trauma), due either to priming in the context of previous experience or to other individual differences. Tonic immobility can occur from standing, seated, or supine positions. The dearth of studies of tonic immobility in humans make it difficult to clarify whether these variants of tonic immobility (acute responses and primed or habitual responses) are all mediated primarily by activation of neural circuits involving the extended amygdala, hypothalamus, and periaqueductal gray, or whether tonic immobility responses that are habitual may potentially also be activated via an alternate route—namely, the basal ganglia circuits involved in habitual behavior, also termed the dorsal striatal habit memory system.105,106
Tonic immobility has been most often described in the sexual assault literature, where it is referred to as rape-induced paralysis,107 and also in accounts given by shell-shocked soldiers, plane/car crash victims, and survivors of physical assault or attacks by wild animals (see Supplemental Text Box 3, http://links.lww.com/HRP/A10). According to individual accounts, tonic immobility in humans appears to present as a loss of the ability to move or call out and is thought to occur when a person is in imminent or actual (and great) danger, when a threshold of sympathetic arousal has been reached, but when escape or winning a fight is not possible or is perceived as not possible. Victims describe subjective experiences of fear, immobility, coldness, numbness and analgesia, uncontrollable shaking, eye closure, and dissociation (derealization and depersonalization), as well as a sense of entrapment, inescapability, futility, or hopelessness. This subjective experience parallels that of animals in tonic immobility. What is (presumably) different is that human victims also typically retain a vivid memory of the event.
Tonic immobility is also sometimes—but not always—included within the construct of peritraumatic reactions (which include symptoms of panic, dissociation, and sometimes tonic immobility).107–109 Because tonic immobility includes a subjective experience of derealization and depersonalization, it has sometimes been conceptualized as a subtype of dissociation (see Supplemental Text Box 4, http://links.lww.com/HRP/A11). Lanius (2014)110 hypothesizes that symptoms of derealization and depersonalization during tonic immobility and other dissociative states may be mediated by kappa opioids—also known as dynorphins—because their activation has been documented in experiments of tonic immobility in animals111–113 and because they are known to cause disturbances in the perception of space and time, abnormal visual experiences, disturbances in body-image perception, and depersonalization and derealization in humans.114,115 It is also possible that changes in cerebral blood flow, secondary to bradycardia, contribute to perceptual disturbances.116,117 Some clinicians have proposed that catatonia may be a fear response that is phylogenetically related to tonic immobility118 (see Supplemental Text Box 5, http://links.lww.com/HRP/A12). A key difficulty in assessing the potential overlaps with these clinical constructs is the dearth of studies that utilize standardized questions about the individual’s subjective experience of immobility or that directly assess motor and autonomic state.
A review of available data suggests that tonic immobility, peritraumatic dissociation, and dissociative PTSD may be mediated by a shared neural network involving amygdala deactivation, absence of sympathetically mediated arousal symptoms (decreased skin conductance and an absence of heart rate increases), parasympathetic activation (a decrease in heart rate in some studies, presumably reflecting a DMN-mediated bradycardia), and analgesia.27,56,119–126 Imaging studies using traumatic scripts have demonstrated that patients with dissociative PTSD have the opposite response pattern from patients with the reexperiencing/hyperarousal PTSD (as described above in “Flight or Fight in Humans”). The dissociative pattern of response involves hyperactivity of anterior brain regions associated with arousal and emotion regulation§§§§ (medial prefrontal cortex; rostral and dorsal anterior cingulate cortex),55,120 lack of amygdala activation to trauma narrative (the amygdala is inhibited by the anterior brain regions previously mentioned),120 changes in connectivity and activation of the right anterior insula,55,127,128 and increased activity in areas in the temporal cortices. Interestingly, Lanius and colleagues55 reported that a third of patients showed both types of responses in a single experimental session: reexperiencing symptoms in response to one script and dissociative symptoms in response to another. These results highlight that traumatized individuals can seamlessly shift from one mind-body state to another, and may show a complex pattern of symptom presentation.
COLLAPSED IMMOBILITY: THE HUMAN MODEL
Collapsed immobility was first identified by Rivers7 nearly a century ago and was only recently (2004) added to the human defense cascade by Bracha.16 Many different names are used as synonyms for collapsed immobility, including collapse,7,21 flaccid immobility,16,35 faint,16,35 fear-induced fainting,16 vasovagal syncope,36 neurocardiogenic syncope,37 and fainting in the context of a blood phobia.16,35,38,39 Although certain animal species—for example, rabbits, opossums, tegus lizards, and hummingbirds***** (see Figure 5 and Supplemental Text Box 3, http://links.lww.com/HRP/A10)—sometimes respond to forced restraint or capture with collapsed immobility rather than tonic immobility, the upright posture of humans makes them especially prone to the bradycardia-induced hypoxia that leads to collapsed immobility.34
Figure 5.

Collapsed immobility in an opossum. The trunk and limbs are limp and immobile. The animal has the appearance of being dead. The term death feint has been used to describe collapsed immobility in animals.85
The same neural network mediates both tonic immobility (characterized by a waxy hypertonicity) and collapsed immobility (characterized by a loss of muscle tone). Like tonic immobility, collapsed immobility can occur from standing, seated, or supine positions, although it is most likely in the standing or seated positions. In collapsed immobility, as in tonic immobility, a DMN parasympathetic surge results in sudden bradycardia or asystole. Unlike what happens in tonic immobility, however, the associated decrease in cerebral blood flow is greater in collapsed immobility and leads to hypoxia. This hypoxic brain state disrupts the signals from the brain stem that ordinarily maintain muscle tone, rendering the individual both immobile and collapsed. In response to brain hypoxia, the individual also experiences a change in his or her level of consciousness, ranging from compromised consciousness to a complete loss of consciousness (syncope).116,117 In some individuals cerebral hypoxia can compromise inhibitory control and manifest as increased anxiety, panic, weeping, or moaning.116,117 Individual variations in physiology and in sensitivity to hypoxia116 are likely to determine whether the immobility response will be tonic or collapsed immobility.
Vignette 10. Collapsed Immobility
Adoni, a 10-year-old boy living with his mother and stepfather, presented with episodes of fainting. The relationship between Adoni’s parents had deteriorated, and Adoni described the verbal and physical violence between them in graphic detail. The visual and auditory images of the violence disrupted Adoni’s sleep at night and intruded into his mind during the day. Subsequent to each outbreak of violence—some of which needed police intervention—Adoni’s anxiety would escalate, and he would experience a fainting episode or a series of fainting episodes. Adoni had no memory of these episodes: he would wake up and find himself lying on the ground.
Vignette 11. Collapsed Immobility
Bettina, a 23-year-old university student, had been emotionally neglected as a child and adolescent. Throughout childhood, Bettina’s key psychological strategy for managing distressing emotions was to avoid conflict with others and to push distressing emotions out of mind. Following a fight with her boyfriend, Betinna began to suffer from fainting spells: at times, following the faint, Bettina would display arrhythmic jerking of her arms of legs. When Bettina visited her doctor, her faints were reproduced on blood taking: Bettina became suddenly pale, clammy, and cold, and her doctor noticed a sudden collapse of her veins before the faint.
Vignette 12. Collapsed Immobility
Jean-Luc, a 20-year-old science student, was asked to prepare pithed frogs for a physiology practical class. Pithing consists in sliding a 10-cm rod (pith) from the first vertebra down into the vertebral column and then up into the cranial cavity to destroy both the spinal cord and the brain before removing organs (e.g., the heart). The procedure had to be done quickly and with great precision. This was Jean-Luc’s first attempt, and he was obviously afraid of missing and of hurting the animal. As he grabbed the slimy frog in one hand and the pith in the other, he was already sweating and shaking. He bent the head of the frog, inserted the pith, pushed it down toward the vertebral column, but missed. The pith went down the thorax and abdomen, piercing the lungs and diaphragm. Terrified, he tried again but missed a second time, and a third. Suddenly, Jean-Luc’s body began to jerk (myoclonic jerks), and he fainted. A few minutes later he recovered consciousness, pale and shaken.
Vignette 13: Collapsed Immobility
When a shell had exploded in his vicinity while on the front lines during the First World War, Roland fainted and soiled his pants. He was hospitalized and eventually sent home with a diagnosis of shell shock. Many years later Roland still avoided festivities that involved firework displays. The sound of fireworks made him panicky, and he would sometimes lose consciousness and soil himself. His friends noted that in such circumstances Ronald typically became panicked and sweaty, turned pale, fell to his knees, and then slid into unconsciousness. Sometimes his body trembled as it lay on the ground.
Vignette 14: Collapsed Immobility
Danae was 14-year-old adolescent with left cerebral atrophy of unknown origin (unchanging over time) and a history both of absence seizures (well controlled on medication) and non-epileptic seizures (twitching and tonic/clonic-like movements). Danae’s non-epileptic seizures had occurred in the context of episodes of high arousal involving anxiety and hyperventilation. During these episodes her pCO2 had dropped to 20 mm Hg (from a baseline of 34 mm Hg), leading to changes in consciousness and abnormal movements. Danae had learned to prevent these episodes by controlling her breathing. Subsequently, however—in the context of school examinations, when she became overwhelmed by fear and anxiety—she presented with episodes of sudden fainting accompanied by incontinence. EEG telemetry remained the same, and the episodes went away following the examination period. It was explained to the family that when sympathetic arousal reached a threshold, the parasympathetic nerve to the heart and the bladder could be activated. This caused Danae to faint and her bladder to release.
Vignette 15: Collapsed Immobility
“As army surgeon, I had once to be present at the execution of some brigands. It was a summary judgment. A major of the bersaglieri [a distinguished unit of the Italian army] put a few questions to one or two, then turning to the captain said simply: ‘Shoot them.’ Some were dumbfounded and stood open-mouthed, petrified; others seemed indifferent. I remember one lad, of scarcely twenty years of age, who mumbled replies to a few questions, then remained silent, in the position of a man warding off a fatal blow, with lifted arms, extended palms, the neck drawn between the shoulders, the head held sideways, the body bent and drawn backwards. When he heard the dreadful word, he emitted a shrill, heart-rending cry of despair, looked around him, as though eagerly seeking something, then turned to flee and rushed with outspread arms against a wall of the court, writhing, and scratching it as though trying to force an entrance between the stones, like a [polyp] clinging to a rock. After a few screams and contortions, he suddenly sank to the ground, powerless and helpless, like a log. He was pale and trembled as I have never seen anyone tremble since; it seemed as though the muscles had been turned to a jelly which was shaken in all directions.”130(p 145)
QUIESCENT IMMOBILITY: THE ANIMAL MODEL
Quiescent immobility is a reaction to “deep or inescapable” pain, chronic injury, injury by a predator, or defeat by a conspecific, and to states of exhaustion (where recuperation is needed) after a period of acute stress, once the animal has returned to a safe environment.46,47,131,132 It differs from pain responses to noxious stimulation of surface areas of the body, which trigger an active defense response—namely, moving the body part away from the source of pain. In rodents, quiescent immobility involves the cessation of all ongoing spontaneous activity, hyporeactivity (absence of orientation, startle response, and vocalization), hypotension, and bradycardia.47,131 Given the model presented in this article, quiescent immobility should be understood as yet another variant of VLPAG-mediated immobility, whose neural components are detailed in the earlier section on tonic immobility.
QUIESCENT IMMOBILITY IN HUMANS
In humans, like animals, quiescent immobility is an adaptive response that occurs in response to severe visceral, skeletal, muscle, or joint pain (see Vignette 16) or to a stressful event (see Vignette 17). When quiescent immobility is prolonged beyond the period needed for physical healing, it becomes maladaptive. It is possible that some chronic pain and fatigue syndromes—for example, complex regional pain syndrome type 1—may represent quiescent immobility in maladaptive forms. Because of the dearth of literature specifically addressing quiescent immobility in humans, it is not possible to explore this subject further at this time.
Vignette 16. Quiescent Immobility Following Visceral Pain
Hendrik, a 60-year-old academic, had avoided gall bladder surgery despite recurrent episodes of abdominal pain, belching, not eating, and stools of no color—which occurred, over a period of decades, whenever he passed a gall stone. The episode that precipitated surgery—involving the passage of a larger stone that became impacted in the bile duct and that eventually exited into the duodenum through a fistula—occurred during a holiday when Hendrik was unable to access medical help. “The pain was severe. As it moved it cut through me like a blade. For four days I didn’t eat, I scarcely drank, and I stayed absolutely still.”
Vignette 17. Quiescent Immobility Following Emotional Pain
Phillip, a 40-year-old accountant, had spent many years recovering from a childhood and adolescence characterized by neglect and abuse. He was an only child, and his mother struggled to raise him despite her emotional dysregulation and her drug and alcohol problems. Phillip described his mother’s behavior as unpredictable and often violent. He coped by distancing himself from his mother and seeking solace in work and in his intimate relationship. In recent months, Phillip’s mother unexpectedly called him, seeking connection and forgiveness. He reported little in the way of emotional reaction, but his partner noted that he subsequently experienced hypersomnia, anergia, amotivation, social withdrawal, reduced appetite, and weight loss. He found the experience puzzling and denied symptoms of depression. He found that the feelings passed after several days. Only in hindsight did he attribute it to the contact with his mother.
THE DEFENSE CASCADE AND IMPLICATIONS FOR CLINICAL PRACTICE
In this final clinical section of the article we touch upon the types of interventions—and possible mechanisms underlying these interventions—that can be used by clinicians and patients to manage the mind-body states that are the human expression of the defense cascade. It must be noted upfront that many treatments for trauma-related symptoms, including those related to the defense cascade, are in their infancy and that there is a pressing need for research to assess their efficacy and elucidate their underlying mechanisms. That said, we and other clinicians have found the interventions presented here to be useful and important in helping patients manage their defense responses.
In this section we utilize the terms defense responses, defense states, mind-body states, and action patterns interchangeably to refer to coordinated patterns of motor-autonomic-sensory response that are accompanied by a subjective component—the individual’s subjective experience of his or her body state. In addition, we use the term somatic in its original meaning from the Greek, to refer to the body as a whole. In this context, the term somatic interventions refer to bottom-up interventions that involve the body and that utilize all types of sensory inputs: those originating within the body (interoceptive133 and proprioceptive134) and those from outside the body (sight, sound, light touch). Interoceptive inputs are mediated by autonomic afferents (see Supplemental Text Box 6, http://links.lww.com/HRP/A13), proprioceptive inputs are mediated by proprioceptive afferents, and sensory inputs from outside the body are mediated by the classic and special sensory pathways. We use the term _sensorimotor_—as in sensorimotor interventions†††††—in a broad way to refer to body interventions that involve both sensory (interoceptive, proprioceptive, and classic sensory) and motor components. Our neural systems perspective—which conceptualizes the extended amygdala-hypothalamic-PAG circuits as embedded in a larger neural network involved in somatic and emotion regulation (see Supplemental Text Box 6, http://links.lww.com/HRP/A13)—suggests that there may be multiple ways, both nonpharmacological and pharmacological, in which activity and connectivity in the neural network may be modulated either to decrease the probability that the defense responses will be activated or to facilitate shifts of mind-body state.
Before proceeding further, we highlight the importance of the therapeutic relationship itself: in working with traumatized patients, therapists typically use that relationship as a means of helping patients to regulate their physiological arousal. Because the social-engagement system is interconnected with the autonomic regulation of the heart and lungs, the process of engaging with the patient (even in small ways through the use of gaze, tone of voice, or rhythm) can promote a concurrent shift in sympathovagal balance—upregulation of vagal activity and downregulation of sympathetic activity—to a mind-body state of interpersonal connectedness and physiological calm.3,139,140 This use of the therapeutic relationship—the dyadic regulation of affect—builds on developmental processes in which the attachment figure acts as a psychobiological regulator, and regulation is a dyadic interpersonal achievement.141,142 Different models of therapy have different ways of describing this safe, physiologically calm, therapeutic dimension, whether defined spatially or interpersonally: secure base, intersubjective relational context, relational or psychobiological attunement, or the dyadic regulation of affect. Whatever its description, this dimension represents the interpersonal space within which all other therapeutic work with traumatized patients is done. It is in that setting that the patient learns to regulate arousal and tolerate intense emotions that would otherwise trigger high states of arousal and potentially activate the defense cascade. When defense responses are activated in the context of therapy, therapists use a range of interventions, all building upon the therapeutic relationship, to help patients shift out of the given defense state and to return, eventually, to a state of calm.
CLINICAL INTERVENTIONS THAT DECREASE AROUSAL
Because innate defense responses are activated in contexts of high arousal, interventions that decrease arousal are used by therapists to help patients maintain arousal within an optimal range—not too high and not too low—also called the window of tolerance.143(p 26) From a neural-systems perspective, arousal-decreasing interventions can be understood as modifying mind-body states via a number of mechanisms: (1) change in sympathovagal balance, (2) downregulation of the hypothalamic-pituitary-adrenal (HPA) axis, and (3) downregulation of limbic activity or reactivity. As described below, interventions may utilize a somatic approach (which targets the body proper), a brain approach that targets limbic areas (working, in effect, from the bottom up), or a brain approach that targets cortical areas involved in emotion/somatic regulation (working, in effect, from the top down).
Somatic Approaches to Decreasing Arousal
Arousal can be modulated by somatic interventions that increase vagal tone. Breathing interventions—including controlled breathing, slow-breathing techniques (from yoga and other Eastern disciplines), coherence breathing, brahmari breathing (“bee breath,” also from yoga), and breathing at ones resonant frequency—utilize a decrease in respiratory rate, prolonged expiration (pranayama), breathing against airway resistance, or vibration of the airway to reduce physiological arousal144–146 (see Supplemental Text Box 7, http://links.lww.com/HRP/A14). The mechanism by which voluntary breathing, as in these exercises, helps to restore sympathovagal balance is not well understood.
In general, the gating of vagal activity by respiration is mediated centrally; cardiac vagal neurons from the nucleus ambiguous sit in close proximity to neurons involved in respiration and modulate heart function in synchrony with the respiratory cycle, with the consequence that if the breathing rate slows, vagal activity is automatically upregulated.102,145 It appears likely that in patients suffering from emotional disorders, this central respiratory-gating mechanism may be dysregulated and that training in slow breathing may reset the mechanism by strengthening vagal tone. The other dimensions of breathing interventions—for example, the use of resistance or vibrations—are likely to engage a different mechanism, the peripheral activation of vagal lung afferents.145,147 Although it is not yet known how this peripheral activation of vagal afferents works to influence the central respiratory-gating mechanism, such activation will, in theory, activate central parasympathetic representations and deactivate sympathetic-related regions and neurotransmitter systems at higher levels (see Supplemental Text Box 7, http://links.lww.com/HRP/A14).59,139,148–150 Importantly, many of the interventions that mothers use to soothe infants—patting, rocking, singing—are likely to rely on the activation of interoceptive, proprioceptive, and classic sensory afferents.
Acupuncture has long been used in Asian medicine to treat stress-related disorders, and it is increasingly being used in Western medicine.151 Studies suggest that acupuncture may function by downregulating brain systems involved in activating the HPA axis and the sympathetic arm of the autonomic system.152–154
Voluntary regular exercise is another means of lowering arousal, apparently by modulating amygdala reactivity155,156 (see Supplemental Text Box 1, http://links.lww.com/HRP/A8). The muscle contraction that occurs during exercise also causes intracellular perturbations that disturb metabolic homeostasis, thereby promoting a broad range of adaptive responses and increasing overall resilience in response to stress.157,158 Even gentle movement is likely to have a significant impact; yoga, tai chi, qigong, and progressive muscle relaxation, practiced along with breathing and mindfulness interventions, are integral components of many healing traditions.144 Since, in the short term, exercise increases arousal (though the ultimate goal and result is to decrease arousal), some patients will need to start with forms of exercise, such as gentle yoga posture or other exercises with simple movements, that raise arousal in small increments. Sudden large increases in arousal may have the unwanted effect of mimicking sensations experienced during trauma and may consequently trigger flight or fight, tonic immobility, or collapsed immobility.
Bottom-Up Brain Approaches to Decreasing Arousal
Pharmacological agents used to treat anxiety disorders—benzodiazepines, clonidine, propranolol, selective serotonin reuptake inhibitors, and dual serotonin and norephinephrine reuptake inhibitors—are thought to inhibit the amygdala and other limbic structures by acting on alpha, beta, GABA, or serotonin receptors in the amygdala, hypothalamus, or PAG.159–162 The disadvantage of medications is that, unlike nonpharmacological interventions, they involve no skill-building element that would help individuals to actively induce shifts in brain-body state or that would, in effect, train their brains to process information in different ways. Subsequent discontinuation of medication—and with it, the removal of limbic inhibition—will potentially leave the individual prone to experiencing strong limbic reactivity to new stressors, without having brought about any long-term changes in information processing.160 But if medication is complemented by other interventions that are capable of effecting long-term change, the use of medication for a determinate period may help the patient to maintain arousal at a manageable level while the other treatment proceeds.
Numerous other interventions—which utilize implicit training techniques designed to modify attention and attentional biases to threat stimuli—are currently being developed.163,164
Top-Down Brain Approaches to Decreasing Arousal
Mindfulness meditation appears both to enhance prefrontal cortex (PFC) activity and downregulate amygdala activity.165,166 Mindfulness has been defined as a particular form of attention: an open-focus attention that involves the ability to direct and maintain a nonjudgmental, moment-by-moment, accepting awareness of the present—canvassing thoughts, feelings, and physical sensations—without attending to any one sensory object in particular.167 Because mindfulness engages different networks from those that are harnessed during narrative generation and ruminative thoughts about the self,166 the practice of mindfulness enables individuals to enter a mind-body state that is different from mind-body states connected with trauma and from states in which individuals ruminate about the traumatic past. Many trauma therapists use a form of mindfulness—_somatic micro-tracking_—to help patients attend to minute shifts in somatic state and to allow themselves to experience defensive action patterns, or to finish enacting them, in an accepting and nonjudgmental way.136,138,143,168,169
Various other techniques—for example, “open focus,” a form of EEG biofeedback—use shifts in attention to induce high-amplitude alpha waves in the EEG, which are associated with reduced brain-body arousal.170 Whereas the beta range (13–50 Hz) is associated with narrow attention and is the zone in which we carry out task-focused activities, the alpha range (8–12 Hz) is produced during alert relaxed states, including states of mindfulness and other states characterized by open-focus attention, and the theta range (4–8 Hz) is produced when deeply relaxed, day dreaming, or falling asleep.170,171 EEG studies reveal a significant increase in alpha and theta activity during mindfulness meditation.171
Finally, interventions that attempt to verbally facilitate reappraisal of the individual’s threat expectancies (as in cognitive therapy without the exposure component‡‡‡‡‡),172,173 that target imaginary rescripting of the trauma narrative,174 or that induce and maintain a mind state of optimism, compassion, hope, and expectancy (as in spiritual beliefs in a loving and protective Other)169,175–177 may function to reduce arousal by downregulating the HPA axis,178 downregulating sympathetic activation,172 and modulating immune function to respond in more adaptive ways.175,179,180 Importantly, because the HPA axis, sympathetic system, and immune system interact reciprocally as part of a larger stress-regulation system, downregulation of one system will have a correlative impact on the functioning of the other systems.41,181
CLINICAL INTERVENTIONS THAT TARGET THE PROCESSING OF TRAUMATIC MEMORIES
Since memories of past trauma can activate the body’s arousal systems—resulting in arousal, the precondition of activating the defense cascade—interventions that target memory processing and that aim to desensitize the individual to the traumatic memories are an integral component of many approaches to therapy for past trauma, including cognitive-behavioral therapy (CBT), eye movement desensitization and reprocessing (EMDR), and tapping. When traumatic memories are successfully processed, the physiological markers of arousal likewise decrease.160,182–186 Dynamic psychotherapists also see themselves as working toward the same goals; that is, in successful therapies, patients come to understand, tolerate, and reinterpret traumatic memories.187
CBT interventions for past trauma are thought to work by engaging the PFC-hippocampus-amygdala system (see Supplemental Text Box 6, http://links.lww.com/HRP/A13). These interventions—which potentially include repeated exposure to traumatic memories and cognitive-restructuring techniques that aim to teach more realistic appraisals of the trauma—are thought to be a form of extinction learning in which conditioned fear responses are inhibited by new learning of safe associations, a process that involves top-down inhibition of the amygdala by the PFC and hippocampus.188 PFC function has been found to be enhanced during CBT interventions.160,182 A subgroup of patients—those who demonstrate excessive recruitment of the amygdala and intense somatic arousal—do not respond therapeutically to CBT interventions.189–191 Instead, their arousal response is paradoxically amplified during the intervention—habituation does not occur—thereby reinforcing the aversive nature of the memory and increasing the probability that flight or fight, tonic immobility, or collapsed immobility may be activated. In an effort to address this problem, some treatment programs implement phase-based approaches that assess affect-regulation skills or tolerance of intense interoceptive sensations prior to CBT or other forms of exposure therapy.187,192–194
Eye movement desensitization and reprocessing also uses imaginary exposure—bringing the unresolved memory and its affective and somatic components into mind—to trigger sympathetic arousal, and asks the individual to hold the memory while tracking the therapist’s fingers or moving his or her eyes from side to side in response to bilateral tones.195–197 Although the mechanisms underpinning EMDR have yet to be identified, neuroimaging studies document changes in processing and brain connectivity following EMDR treatment, with a discernible shift in firing from prefrontal and limbic regions to the fusiform and visual cortex during exposure to the traumatic script.198,199 Arousal in response to the traumatic script also decreases.183–186 For a discussion of possible mechanisms underlying EMDR, see Lanius and Bergmann (2014).200
Like CBT and EMDR, the tapping technique—also known as energy psychology or the emotional freedom technique—uses imaginal exposure along with three additional elements: awareness of body state, the act of tapping a series of acupuncture points on the body, and repeating certain phrases related to the negative event in an accepting way.201 The putative mechanisms underlying this intervention are unknown, and further outcome studies are needed.
What is intriguing about EMDR and tapping is the use of movement and somatic tracking—the patient is asked to become aware of somatic sensations—as key elements of these interventions. Because action tendencies and somatic states are so closely tied to traumatic states, it is possible that engagement of motor and sensory systems facilitates changes in the processing of trauma-related material.
Clinical Interventions Specific to Managing Defensive Mind-Body States
In this final section we briefly touch upon interventions that can be used within the therapy context to help patients manage the other specific mind-body states—freeze, flight or fight, tonic immobility, or collapsed immobility—that make up the human defense cascade. Akin to traumatic memories (fixed and repeating visual memories), defensive mind-body states can be conceptualized as a set of fixed and repeating action patterns, visceromotor memories, dispositional representations,§§§§§ or procedural representations that arose in response to the original trauma, that are automatically reactivated again and again by environmental triggers reminiscent of the trauma, and that remain uncompleted, unprocessed, and stuck in time.202
When defensive mind-body states are conceptualized in this way, it follows that interventions that help patients process these fixed action patterns will need to be delivered on a somatic (body) level, enabling the processing of the interoceptive sensations and movements that make up the action pattern, and freeing the patient from reenacting the given action pattern time and time again. Sensations—the subjective representations of specific patterns of autonomic and sensory activation—need to be mindfully tracked until they dissipate and are replaced by new sensations.135–138,169,203 The fixed action pattern needs to be mindfully and slowly brought to completion, thereby disrupting its “stuck” quality and allowing it to be replaced by new action patterns (see Vignette 17).135–138,203
From a clinical perspective, the defense cascade can be understood as a hierarchical behavioral framework in which patients typically shift from one action pattern to another action pattern in an established order. Likewise, recovery would be expected to follow the reverse order. That is, in our clinical work, we have found that patients in tonic immobility, for example, are most likely to shift into a state of flight or fight or extreme arousal because in humans, either of these states can precede tonic immobility (see Vignettes 15 and 18). Patients’ individual patterns of shift can often be identified by asking the patient to carefully describe their somatic experiences. More generally, understanding the progression of different states in the defense cascade will enable the therapist to make predictions (both in general and, in time, for each individual patient) and to help patients understand, and be less frightened and surprised by, the sudden shifts from one body state to another. In both children and adults, animal stories can be used to explain these mind-body states.135
Vignette 18: Shifts Between Defensive Mind-Body States
Awa was a 35-year-old woman who, as a child, had been sexually abused by her grandfather, neglected by her drug-addicted parents, and subjected to a range of other dangers while roaming the streets as a child. Anxious about the upcoming anniversary of her grandfather’s death—she was amnestic with regard to the previous year’s anniversary—Awa requested and obtained a planned admission to ensure that she would not be alone. On the day marking the anniversary, Awa woke up complaining of agitation and hypersensitivity to sounds: she paced around the garden and startled when her nurse spoke to her (a state of increased arousal). When Awa’s therapist arrived for a scheduled session, Awa was sitting very still on her bed and staring out the window with a glazed look in her eye. Her pulse was 46 beats a minute. The therapist spoke softly to inform Awa of her presence, and asked Awa to focus on her feet and to press her feet into the ground so that she could feel its firmness. She then asked Awa to focus on her breathing, and sat with her while they breathed in synchrony together. Awa’s breathing became suddenly more abrupt, and her gaze more focused. Her hands became clenched into fists (a state of flight or fight). The therapist asked Awa to focus on the movement that wanted to happen and to follow it in a mindful way.
Shifting out of Tonic Immobility
Patients who enter a state of tonic immobility are disconnected from the therapist and also disconnected from the self. Most interventions that aim to help patients shift out of the state of tonic immobility involve the use of bottom-up somatic strategies—coupled with mindfulness—to heighten somatosensory signals. For patients who have lost awareness of body state, the goal is to reactivate representations of body state—by focusing on interoceptive, proprioceptive, or touch sensations—thereby allowing the patient to become aware of his or her body (see Supplemental Text Box 6, http://links.lww.com/HRP/A13).59,204 For patients who are acutely aware of sensations but who are unable to move, the goal is to initiate a progressive approach that will, in stages, lead to the termination of tonic immobility. The patient would begin by attending to interoceptive signals (e.g., the sensation of breathing), then to the movements associated with those interoceptive signals (e.g., the movement of the chest during breathing), and then to the proprioceptive signals indicating, for example, patterns of muscle tension or body postures—ultimately leading the patient to enact the movements associated with these patterns or postures. In this way the therapist uses a step-wise approach that helps the patient focus on interoceptive signals as a precursor to the proprioceptive signals associated with small movements or attempts to move.
Commonly used sensorimotor interventions help patients focus attention on interoceptive, proprioceptive, and classic sensory sensations as a means of orientating them back to the here and now—often referred to as grounding interventions.136,138,143,203,205,206 As a means of improving patients’ awareness of interoceptive sensations, they can be asked to attend to and track internal body sensations. In this context, patients may be asked to put one hand on the abdomen and the other hand over the heart (the intensity of sensations can be accentuated by pressure from the fingers or by the addition of humming/chanting****** to induce vibrations within the chest and abdomen). Attention to exteroceptive sensations may be facilitated by having patients tap or rub parts of the body, by having them feel water running or air moving over the body, and so on. As noted previously, therapists of all orientations utilize eye gaze and voice to connect with patients and to cut through the state of disconnection that typifies tonic immobility. Commonly used grounding interventions also include feeling one’s feet on the ground (which can be accentuated by having patients stomp their feet, massage their legs, or shift the body’s weight to the toes, heels, and sides of the feet), feeling the chair pressing on one’s back, and feeling the firmness of the wall with one’s hands.
Theoretically, movement is incompatible with tonic immobility and will, of necessity, induce a change of mind-body state.207 Motor interventions commonly used to help patients shift out of states of tonic immobility—or to prevent that state from being activated when distressing memories are being processed during the session—include the following: standing up (to increase proprioceptive signals), walking, raising the arms or stretching the body in some way, conducting therapy while the patient is sitting on a ball (which requires constant attention to proprioceptive signals to keep one’s balance), and as further discussed in the section below on freezing, the actual enactment of sensed motor-dispositional representations (of running away, punching, kicking, and other protective actions) that cannot be completed while in tonic immobility.136,143 Importantly, some patients will find it easier to feel their bodies if they are moving.208
The practice of mindfulness—attending fully to the somatic sensation or body movement—is a key component of all these interventions and is likely to amplify their effect. But what is the mechanism? As has long been known, focusing one’s attention onto a body part increases blood flow in that part of the body,1,209,210 and increased blood flow, especially coupled with mindful attention to the body, would activate additional autonomic afferent inputs back to the brain, increasing neural activity at multiple levels of representation, including the insulae, where subjective representations of body state are thought to be activated (see Supplemental Text Box 6, http://links.lww.com/HRP/A13).59,211 Thus, in theory, mindful acceptance and exploration of body sensations further amplifies body signals—thereby bringing the insula back on line, while simultaneously engaging the PFC, thereby allowing for top-down modulation of the amygdala. The practice of fostering a state of engaged, but nonreactive, curiosity (mindfulness) helps the individual decouple the physical sensations from the associated feelings of fear.136,138,169
Importantly, as was suggested earlier, breaking the state of tonic immobility may sometimes plunge the individual into flight or fight136 or, alternatively, into a state of very high arousal (see Vignette 18). Also as suggested earlier, the therapist and patient may need to prepare for that possibility.
Shifting out of Freezing
Freezing—flight or fight put on hold—is a mind-body state characterized by a narrow focus of attention: the individual is focused on the threat (see Vignettes 5 and 6) and is primed to respond, though not yet active. Shifting out of the freeze response is likely to land the patient in flight or fight or, alternatively, to return the patient to a state of high arousal. In the therapy room, therapists can utilize various interventions to help patients manage freeze states. The choice of intervention will depend on the patient’s capacity to tolerate the intense body sensations that are integral to the state of freezing.
One strategy is to direct and assist the patient to mindfully observe his or her body sensations and to track them or “breathe into” them, until they have subsided and transformed.136,137,169 Levine136 emphasizes that it is not uncommon for patients to experience tingling, trembling, shaking, or limb jerking as they track their body sensations. Importantly, in this situation patients need to attend solely to body sensations, and to put aside any emotions, memories, and cognitions, thereby confining processing to a sensorimotor level. Attention to concurrent emotions, memories, and cognitions is likely to overwhelm the patient, escalating arousal. The therapist may also ask the patient to become aware of any action patterns—patterns of muscle tension or an impulse to enact a particular movement—which the patient may then be asked to track and act out to completion in a slow, mindful way. The repeated practice of these sensorimotor interventions enables the individual to learn that the freeze state is temporary and will pass.136,169
Another strategy—especially when a patient is unable to tolerate the intensity of body sensations—is to use interventions that function to expand the focus of attention. Because freezing involves a narrowed focus of attention, opening the focus of attention can function to dissipate the freeze state or to limit it to shorter time frames. Exercises that can help patients hone the skill of contracting and expanding their attention include the following: attending to objects in the room and noticing their color and texture or the space between objects; attending to an imaged scene that heralds safety; and simply attending to the therapist’s calm voice. Also helpful are meditation exercises that practice narrowing and widening the focus of attention, and open-focus biofeedback exercises, in which the individuals use EEG biofeedback to improve their capacity to induce the high-amplitude alpha waves that typify open-focus attention. Once patients have gained some skill at shifting attention, they can be taught to use oscillation techniques†††††† to move back and forth between the trauma-related somatic state and some alternative state (of safety), thereby enabling them to process the somatic state incrementally.
Because freeze states are generally so transitory, it is unlikely that the therapist will have much of an opportunity to work with a patient while in that state (even if one can be triggered in the safety of the therapy room).What can be done in therapy, however, is to prepare the patient to deal with freezing. That is, the patient will need to acquire and practice the requisite skills, in preparation for using them in real time, away from therapy.
Managing Flight or Fight
Flight-or-fight states involve a disposition to act (run, hit, bite, kill) and are coupled with strong negative emotions (fear, anger) and an upregulated body state (pounding heart, tense muscles). In and of themselves, flight-or-fight states can be frightening and difficult to tolerate. Concern that others may be harmed can add to the individual’s fear of entering into this mind-body state. Like freezing, the flight-or-fight mind-body state is characterized by a narrow range of attention in which the individual is focused on responding to the threat (or, in therapy, the body state associated with it). Everything else recedes into the background (see Vignette 4).
Flight-or-fight states are easier to manage in therapy if the patient and therapist are able to identify early somatic precursors (movements or sensations that herald the emergence of this action pattern)—for example, the furrowing of the eyebrows, the tensing of the jaw, or the clenching of a fist, any or all of which may occur prior to the emergence of the full pattern. When those precursors are identified, the therapist can interrupt the patient’s narrative and direct the patient to take a mindful stance and track the somatic sensations—putting aside emotions, memories, and cognitions—until the defense state resolves. In some cases the patient may need to implement breathing or grounding interventions that help downregulate arousal. If the patient is unable to tolerate his or her body sensations or is unable to take a mindful stance, implementing interventions that widen the focus of attention can be used to exit flight or fight. In that situation, as earlier described, oscillation techniques can be used to help the patient process flight-or-fight states incrementally.136,143
Managing Collapsed Immobility
In clinical practice, collapsed immobility presents as episodes of fainting (syncope) or alternately as a loss of muscle tone accompanied by a compromised level of consciousness (pre-syncope). Fainting is the result of compromised cerebral function secondary to decreased oxygen or glucose availability, which can be caused by various mechanisms, of which collapsed immobility is just one. Thus, the first step in managing fainting is to exclude possible medical causes (e.g., cardiac pathology, hypoglycemia, orthostatic problems). Once the clinician is satisfied that the fainting episodes are not medical in origin and are therefore likely to be associated with stress or threat, it is useful to clarify whether the fainting results primarily from stress-induced hyperventilation or should be understood, instead, as an instance of collapsed immobility. A clinical assessment of hyperventilation coupled with a formal hyperventilation challenge and measurement of transdermal carbon dioxide can be helpful in determining what mechanism is primarily responsible.41
In cases where the fainting is likely to reflect collapsed immobility—that is, the likely mechanism is activation of neural circuits involving the extended amygdala, hypothalamus, and PAG, with cardiac vagal neurons in the DMN being activated downstream—the next step is to implement interventions that increase the patient’s safety. Although collapsed immobility may occur with little warning, some patients are able to pick up subtle warning signs—visual blurring, sweating, nausea, warmth, light-headedness, and fatigue—and are able to prevent the syncope by positioning themselves on the floor and lifting their feet. Other patients are able to identify specific trauma-related triggers—current or past—that precipitate collapsed immobility, and may be able to take appropriate precautions. Another option is to implement general arousal-decreasing interventions (as discussed above in “Clinical Interventions That Decrease Arousal”) to reduce the probability that the DMN cardiac vagal pathway will be activated (since the DMN pathway is activated in high-arousal contexts). In some cases clinicians may trial a selective serotonin reuptake inhibitor, serotonin/norephinephrine reuptake inhibitor, or even beta blocker to decrease arousal. Finally, as with other trauma-related conditions or disorders, subsequent treatment will need to address the traumatic-related issues—past or present—that are triggering the patient’s episodes of collapsed immobility.
One additional twist is that stress-related hyperventilation, even when not in itself sufficient to cause fainting, may actually be increasing the patient’s susceptibility to episodes of collapsed immobility. Drawing on animal research, Porges3 postulates that hypoxic conditions—of which hyperventilation-induced cerebral hypoxia is an example—increase the probability that the sporadically active102 cardiac vagal neurons in the DMN will be activated, resulting in an episode of collapsed immobility. Controlling hyperventilation episodes via body scans212 and the use of breath techniques41 may indirectly decrease the frequency of collapsed immobility episodes.
CONCLUSION
This article, a collaboration among clinicians (a child and family therapist, cognitive-behavioral therapist, and psychotherapist) and a neuroscientist (the fourth author), integrates current neurophysiological findings into a model for understanding the defense responses that make up the defense cascade. We hope that this model, coupled with the clinical vignettes and complementary discussions of the implications for clinical practice, will help clinicians to recognize and differentiate these defense states in their daily work, and that our analysis will provide them with ideas and options for treatment, so as to unlock the patient’s pattern of trauma response and break the cycle of suffering. We also hope that the availability of an integrated model and its vocabulary will allow clinicians to engage in more productive conversations not only with scientists and other clinicians, but with their patients.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgments
We would like to thank our patients for sharing their stories with us and for teaching us about trauma responses. We especially thank Hendrik (pseudonym), Jean-Luc (pseudonym), Mary (pseudonym) and her mother, and Danae (pseudonym) and her parents for allowing us to use their stories. The other vignettes in the article are amalgams of similar cases. We thank Mathew Coleman for contributing vignette amalgams to this article from his work with war veterans, Sue Foley for her contribution to the vignettes, Veronica Chandler for her line drawings of the freezing rat, the rat in tonic immobility, and the opossum in collapsed immobility, and Mathew Coleman and Megan Chambers for their feedback on earlier drafts of this article. We thank Drew (J. A.) Armour, Christopher Cain, and Erno Hermans for putting aside time to respond to the first author’s questions via email.
* The concept of “fight or flight” was firmly established in the first, 1915 edition of Bodily Changes in Pain, Hunger, Fear and Rage, where Cannon wrote that “the emotion of fear is associated with the instinct for flight, and the emotion of anger or rage with the instinct for fighting or attack.”5(p 187) In both the first and second (1929)6 editions, Cannon referred to these two instincts in a variety of ways: “struggle or flight,”5(p 202) “flight or conflict,”5(p 205) “fighting or flight,”5(p 211) and “fighting or escape that accompany or follow distress or fear or rage.”5(p 202) The authors are unsure when and by whom the catchy term fight or flight was introduced when referring to Cannon’s earlier work. We (the authors) use the term flight or fight because it better captures the tendency of most mammals to flee, rather than to fight.
† Rivers’s term aggression is equivalent to fight; manipulative activity referred to complex behaviors that functioned to overcome or avoid danger; his term immobility is equivalent to freezing; and his term collapse encompassed both tonic and collapsed immobility (see his examples of a seal in tonic immobility and a human in collapsed immobility).7
‡ William Halse Rivers,7 in 1920, was the first to propose a continuum of the instincts of self-preservation, at the end of which were instincts pertaining to the protection of the animal or person from danger—flight, aggression, manipulative activity, immobility, or collapse (including both tonic immobility and collapsed immobility). Subsequently, in 1967, Stanley Ratner8 proposed a continuum of innate fear reactions that included startle, watchfulness (freezing), and, at the extreme end of the continuum, prolonged immobility (tonic immobility). He did not include flight or fight—described as such by Cannon in 19156—in his continuum. Subsequently, a number of researchers noted that defense behaviors changed in a patterned manner as a predator approached (sometimes referred to as predatory imminence).9–13 These efforts to describe responses to predatory imminence included freezing and flight or fight but did not include tonic immobility. Subsequent animal research took place in silos, with some researchers focusing on the responses identified in relation to predatory imminence and others focusing on tonic immobility. In 1997 and 2000, Peter Lang and colleagues2,14 introduced the phrase defense cascade and used the notion of predatory imminence to investigate human behavior. And in 2004, Stefan Bracha15,16 developed a broader human model that included not only the freeze, flight, and flight responses associated with predatory imminence but also tonic and collapsed immobility.
§ The study by Lanius and colleagues27 investigated the responses of a husband and wife to traumatic script-driven imagery following a car accident in which they had been trapped, had watched a child burn to death, and had feared that they, too, would die. Whereas the husband experienced intense anxiety, arousal, and escape-focused cognitions (flight and fight), the wife experienced both numbness and a subjective sense of immobility (tonic immobility).
** For clinical articles regarding the primacy of data-driven processing—the processing of sensory impressions and perceptual characteristics—during trauma, see Halligan and colleagues (2003)31 and Ehring and colleagues (2008).32
†† We utilize the term collapsed immobility to refer specifically to immobility accompanied by a loss of muscle tone, or flaccidity. By contrast, Rivers (1920)7 used the term collapse to refer to both tonic and collapsed immobility responses, as described in vignettes within his chapter about the danger instincts. More recently, Baldwin (2013)21 adopted Rivers’s term collapse in his article about trauma responses. Baldwin appears to use the term inconsistently to refer to bradycardia, to freeze responses (as in Hofer [1970]34), and to Baldwin’s own construct of freeze-fright. It is not clear how Baldwin’s construct of freeze-fright relates the well-established constructs of freezing and tonic immobility (also referred to as fright by Bracha16) in the broader literature.
‡‡ We do not use Bracha’s catchy mnemonic—freeze, flight, fight, fright, _faint_—to describe the defense cascade,16 because the terms fright and effroi are commonly used by researchers to refer to individuals’ experience of subjective fear rather than as synonyms for tonic immobility.40 We also do not use the term faint, because fainting can be caused by many mechanisms unrelated to the neural circuits mediating the defense responses. Likewise, the term fear-induced fainting is nonspecific because such fainting can be caused by cerebral ischemia secondary to vagal mechanisms or by fear-induced hyperventilation.41
§§ Of the vignettes in the article, the majority are amalgams from the authors’ clinical practices (Vignettes 1, 3, 4, 6–11, 13, 17, and 18); four (Vignettes 5, 12, 14, and 16) are from patients who kindly gave consent to be included in the article; and two (Vignettes 2 and 15) are quoted from the published literature.
*** Some authors equate avoidance of threat with flight or fight. Here we use flight or fight to refer only to situations where flight or fight is actively engaged.
††† In his wonderful article about prolonged immobility in wild rodents, Hofer34 utilizes the term tonic immobility for immobility responses that are now referred to as freezing and the term feigned death for what is now termed tonic immobility. Hofer’s descriptions of these states and the differences between them are very clear.
‡‡‡ Fear bradycardia was first termed fright bradycardia by Abbot Gaunt and Carl Gans in 1969.67
§§§ The righting reflex refers to postural adjustments needed to correct the body’s orientation when it is displaced from its upright position.
**** In Parkinson’s disease, loss of these dopaminergic neurons in the substantia nigra, rather than their inhibition, is responsible for immobility.
†††† Andrew (Drew) Armour, now retired, was a professor in the Department of Anatomy and Neurobiology at Dalhousie University. He studied the autonomic system in dogs.
‡‡‡‡ As noted by Rodger McLean (1922–2009), an Australian Flying Officer in the Second World War, officers who trained pilots were able to break tonic immobility states in trainees—while at the controls, learning to fly—by using a firm voice devoid of fear to issue simple orders that the men had already learned and that were automatic: “flaps,” “raise the stick,” “rudder.” McLean mentioned that he was told this tip by an older pilot, and it saved his life on several occasions. Trainers who had not learned the tip sometimes went down with the plane when trainees could not “unstick.”
§§§§ Animal EEG data suggest that tonic immobility is associated with a biphasic cortical response: increased cortical arousal during induction and early stages of tonic immobility and decreased cortical arousal in later stages (see Supplemental Text Box 3). How these findings translate to humans is not known (see Supplemental Text Box 3 for a discussion).
††††† Therapies that focus primarily on movement and sensation-focused mindedness are referred to by a number of names, including somatic experiencing, SIBAM (sensation, image, behavior, affect, meaning) model of bottom-up processing, and sensorimotor approach to psychotherapy.135–138
‡‡‡‡‡ Cognitive-behavioral therapy is an umbrella term that includes both cognitive (e.g., identification and challenging of thoughts and beliefs) and behavioral (e.g., exposure and behavioral activation) components.
§§§§§ Damasio20 uses the term dispositional representations to describe patterns of neurological activity that dispose the individual to behave in particular ways. Other terms used to refer to the same concept include action patterns, innate motor responses, patterned states of muscular tension in readiness for action, and preparatory protective actions.136,137
****** In Eastern traditions the voo or om sounds are commonly used.136,144
†††††† In these techniques, the focus of attention is shifted back and forth between the body and open focus (or, for example, between a sensation in one part of the body and a different sensation in another part of the body). Levine136 refers to the process of moving between aversive mind-body states and states of calm/well-being as the process of pendulation.
Original manuscript received 23 February 2014; revised manuscript received 7 July 2014, accepted for publication subject to revision 15 September 2014; revised manuscript received 20 September 2014.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.harvardreviewofpsychiatry.org).
REFERENCES
- 1.Darwin C. The expression of the emotions in man and animals. 4th ed London: John Murray, 1872. [Google Scholar]
- 2.Lang PJ, Simons RF, Balaban MT. Attention and orienting: sensory and motivational processes. Mahwah, NJ: Erlbaum, 1997. [Google Scholar]
- 3.Porges SW. The polyvagal theory: neurophysiological foundations of emotions, attachment, communication, and self-regulation. New York: Norton, 2011. [Google Scholar]
- 4.McDougall W. Introduction to social psychology. London: Methuen, 1908. [Google Scholar]
- 5.Cannon WB. Bodily changes in pain, hunger, fear and rage: an account of recent researches into the function of emotional excitement. New York: D. Appleton, 1915. [Google Scholar]
- 6.Cannon WB. Bodily changes in pain, hunger, fear and rage: an account of recent researches into the function of emotional excitement. 2nd ed New York: D. Appleton, 1929. [Google Scholar]
- 7.Rivers WHR. The danger-instincts. In: Instinct and the unconscious: a contribution to a biological theory of the psycho-neuroses. London: Cambridge University Press, 1920: 52– 60. [Google Scholar]
- 8.Ratner SC. Comparative aspects of hypnosis. In: Gordon JE, ed. Handbook of clinical experimental hypnosis. New York: Macmillan, 1967. [Google Scholar]
- 9.Fanselow MS, Lester LS. A functional behavioristic approach to aversely motivated behavior: predatory imminence as a determinate of the topology of defensive behavior. In: Bolles RC, Beecher MD, eds. Evolution and learning. Hillsdale, NJ: Erlbaum, 1988: 185– 211. [Google Scholar]
- 10.Gray JA. The psychology of fear and stress. 2nd ed Cambridge: Cambridge University Press, 1988. [Google Scholar]
- 11.Blanchard RJ, Blanchard DC. Attack and defense in rodents as ethoexperimental models for the study of emotion. Prog Neuropsychopharmacol Biol Psychiatry 1989; 13 suppl: S3– 14. [DOI] [PubMed] [Google Scholar]
- 12.Fanselow MS. The adaptive function of conditioned defensive behaviour: an ecological approach to Pavlovian stimulus substitution theory. In: Blanchard RJ, Brain PF, Blanchard DC, Parmigiani S, eds. Ethoexperimental approaches to the study of behavior. Boston: Kluwer Academic, 1989: 151– 66. [Google Scholar]
- 13.Myer JS. Some effects of noncontingent aversive stimulation. In: Brush FR, ed. Aversive conditioning and learning. New York: Academic, 1971: 469– 536. [Google Scholar]
- 14.Lang RJ, David M, Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord 2000; 61: 137– 59. [DOI] [PubMed] [Google Scholar]
- 15.Bracha HS, Ralston TC, Matsukawa JM, Williams AE, Bracha AS. Does “fight or flight” need updating? Psychosomatics 2004; 45: 448– 9. [DOI] [PubMed] [Google Scholar]
- 16.Bracha HS. Freeze, flight, fight, fright, faint: adaptationist perspectives on the acute stress response spectrum. CNS Spectr 2004; 9: 679– 85. [DOI] [PubMed] [Google Scholar]
- 17.Blanchard DC, Blanchard RJ. Ethoexperimental approaches to the biology of emotion. Ann Rev Psychol 1988; 39: 43– 68. [DOI] [PubMed] [Google Scholar]
- 18.Mobbs D, Petrovic P, Marchant JL, et al. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science 2007; 317: 1079– 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mobbs D, Marchant JL, Hassabis D, et al. From threat to fear: the neural organization of defensive fear systems in humans. J Neurosci 2009; 29: 12236– 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damasio AR. Looking for Spinoza: joy, sorrow, and the feeling brain. Orlando, FL: Harcourt, 2003. [Google Scholar]
- 21.Baldwin DV. Primitive mechanisms of trauma response: an evolutionary perspective on trauma-related disorders. Neurosci Biobehav Rev 2013; 37: 1549– 66. [DOI] [PubMed] [Google Scholar]
- 22.Choi JS, Cain CK, LeDoux JE. The role of amygdala nuclei in the expression of auditory signaled two-way active avoidance in rats. Learn Mem 2010; 17: 139– 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gozzi A, Jain A, Giovanelli A, et al. A neural switch for active and passive fear. Neuron 2010; 67: 656– 66. [DOI] [PubMed] [Google Scholar]
- 24.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 1988; 8: 2517– 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci 1994; 17: 379– 89. [DOI] [PubMed] [Google Scholar]
- 26.Popova NK, Barykina NN, Plyusnina TA, Alekhina TA, Kolpakov VG. Expression of the startle reaction in rats genetically predisposed towards different types of defensive behavior. Neurosci Behav Physiol 2000; 30: 321– 5. [DOI] [PubMed] [Google Scholar]
- 27.Lanius RA, Hopper JW, Menon RS. Individual differences in a husband and wife who developed PTSD after a motor vehicle accident: a functional MRI case study. Am J Psychiatry 2003; 160: 667– 9. [DOI] [PubMed] [Google Scholar]
- 28.Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev 2001; 25: 669– 78. [DOI] [PubMed] [Google Scholar]
- 29.da Silva LF, Coimbra NC, Menescal-de-Oliveira L. Rostral ventromedial medulla modulates nociception and tonic immobility behavior through connections with the A7 catecholaminergic region. Behav Brain Res 2012; 233: 422– 7. [DOI] [PubMed] [Google Scholar]
- 30.Nuseir K, Heidenreich BA, Proudfit HK. The antinociception produced by microinjection of a cholinergic agonist in the ventromedial medulla is mediated by noradrenergic neurons in the A7 catecholamine cell group. Brain Res 1999; 822: 1– 7. [DOI] [PubMed] [Google Scholar]
- 31.Halligan SL, Michael T, Clark DM, Ehlers A. Posttraumatic stress disorder following assault: the role of cognitive processing, trauma memory, and appraisals. J Consul Clin Psychol 2003; 71: 419– 31. [DOI] [PubMed] [Google Scholar]
- 32.Ehring T, Ehlers A, Cleare AJ, Glucksman E. Do acute psychological and psychobiological responses to trauma predict subsequent symptom severities of PTSD and depression? Psychiatry Res 2008; 161: 67– 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lanius UF, Paulsen SL, Corrigan FM, eds. Neurobiology and treatment of traumatic dissociation: toward an embodied self. New York: Springer, 2014. [Google Scholar]
- 34.Hofer MA. Cardiac and respiratory function during sudden prolonged immobility in wild rodents. Psychosom Med 1970; 32: 633– 47. [DOI] [PubMed] [Google Scholar]
- 35.Bracha HS, Bracha AS, Williams AE, Ralston TC, Matsukawa JM. The human fear-circuitry and fear-induced fainting in health individuals—the paleolithic-threat hypothesis. Clin Auton Res 2005; 15: 238– 41. [DOI] [PubMed] [Google Scholar]
- 36.Gowers WR. A lecture on vaso-vagal attacks. Lancet 1907; 1: 1551– 4. [Google Scholar]
- 37.Grubb BP. Neurocardiogenic syncope. In: Grubb BP, Olshansky B, eds. Syncopy: mechanisms and management. Armonk, NY: Futura, 1998: 73– 106. [Google Scholar]
- 38.Bienvenu OJ, Eaton WW. The epidemiology of blood-injection-injury phobia. Psychol Med 1998; 28: 1129– 36. [DOI] [PubMed] [Google Scholar]
- 39.Ost LG, Sterner U, Lindahl IL. Physiological responses in blood phobics. Behav Res Ther 1984; 22: 109– 17. [DOI] [PubMed] [Google Scholar]
- 40.Vaiva G, Brunet A, Lebigot F, et al. Fright (effroi) and other peritraumatic responses after a serious motor vehicle accident: prospective influence on acute PTSD development. Can J Psychiatry 2003; 48: 395– 401. [DOI] [PubMed] [Google Scholar]
- 41.Kozlowska K. Stress, distress, and bodytalk: co-constructing formulations with patients who present with somatic symptoms. Harv Rev Psychiatry 2013; 21: 314– 33. [DOI] [PubMed] [Google Scholar]
- 42.Felmingham K, Kemp AH, Williams L, et al. Dissociative responses to conscious and non-conscious fear impact underlying brain function in post-traumatic stress disorder. Psychol Med 2008; 38: 1771– 80. [DOI] [PubMed] [Google Scholar]
- 43.Chaitow L, Bradley D, Gilbert C. Multidisciplinary approaches to breathing pattern disorders. London: Churchill Livingstone, 2002. [Google Scholar]
- 44.Fussell P. Wartime: understanding and behavior in the Second World War. Oxford: Oxford University Press, 1989. [Google Scholar]
- 45.Fanselow MS. Neural organisation of defensive behavioural system responsible for fear. Psychon Bull Rev 1994; 1: 429– 38. [DOI] [PubMed] [Google Scholar]
- 46.Walker P, Carrive P. Role of ventrolateral periaqueductal gray neurons in the behavioral and cardiovascular responses to contextual conditioned fear and poststress recovery. Neuroscience 2003; 116: 897– 912. [DOI] [PubMed] [Google Scholar]
- 47.Carrive P. The periaqueductal gray and defensive behavior: functional representation and neuronal organization. Behav Brain Res 1993; 58: 27– 47. [DOI] [PubMed] [Google Scholar]
- 48.Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. J Comp Neurol 1991; 303: 121– 31. [DOI] [PubMed] [Google Scholar]
- 49.Zhang SP, Davis PJ, Bandler R, Carrive P. Brain stem integration of vocalization: role of the midbrain periaqueductal gray. J Neurophysiol 1994; 72: 1337– 56. [DOI] [PubMed] [Google Scholar]
- 50.Kandel ER, Schwartz JH, Jessell TM. Principles of neural science. 4th ed New York: McGraw-Hill, 2000. [Google Scholar]
- 51.Taylor EW, Al-Ghamdi MS, Ihmied IH, Wang T, Abe AS. The neuranatomical basis of central control of cardiorespiratory interactions in vertebrates. Exp Physiol 2001; 86: 771– 6. [DOI] [PubMed] [Google Scholar]
- 52.Cantor C. Evolution and posttraumatic stress: disorders of vigilance and defence. London; New York: Routledge, 2005. [Google Scholar]
- 53.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164: 1476– 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayes JP, Vanelzakker MB, Shin LM. Emotion and cognition interactions in PTSD: a review of neurocognitive and neuroimaging studies. Front Integ Neurosci 2012; 6: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lanius RA, Hopper JW. Reexperiencing/hyperaroused and dissociative states in posttraumatic stress disorder. Psychiatr Times 2008;31 Oct. http://www.psychiatrictimes.com/articles/reexperiencinghyperaroused-and-dissociative-states-posttraumatic-stress-disorder. [Google Scholar]
- 56.Lanius RA, Vermetten E, Loewenstein RJ, et al. Emotion modulation in PTSD: clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry 2010; 167: 640– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pitman RK, Rasmusson AM, Koenen KC, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 2012; 13: 769– 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shin LM, McNally RJ, Kosslyn SM, et al. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. Am J Psychiatry 1999; 156: 575– 84. [DOI] [PubMed] [Google Scholar]
- 59.Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci 2011; 1225: 72– 82. [DOI] [PubMed] [Google Scholar]
- 60.Carrive P. Dual activation of cardiac sympathetic and parasympathetic components during conditioned fear to context in the rat. Clin Exp Pharmacol Physiol 2006; 33: 1251– 4. [DOI] [PubMed] [Google Scholar]
- 61.Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science 1989; 243: 1718– 21. [DOI] [PubMed] [Google Scholar]
- 62.Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neuroscience 2004; 24: 5506– 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shackman AJ, Fox AS, Oler JA, Shelton SE, Davidson RJ, Kalin NH. Neural mechanisms underlying heterogeneity in the presentation of anxious temperament. Proc Natl Acad Sci U S A 2013; 110: 6145– 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Graham LK, Yoon T, Lee HJ, Kim JJ. Strain and sex differences in fear conditioning: 22 kHz ultrasonic vocalizations and freezing in rats. Psychol Neurosci 2009; 2: 219– 25. [Google Scholar]
- 65.Smith DR, Gallagher M, Stanton ME. Genetic background differences and nonassociative effects in mouse trace fear conditioning. Learn Mem 2007; 14: 597– 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron 2009; 61: 786– 800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gaunt AS, Gans C. Diving bradycardia and withdrawal bradycardia in Caiman crocodilus. Nature 1969; 223: 207– 8. [DOI] [PubMed] [Google Scholar]
- 68.Berntson GG, Cacioppo JT, Quigley KS. Autonomic determinism: the modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychol Rev 1991; 98: 459– 87. [DOI] [PubMed] [Google Scholar]
- 69.Iwata J, LeDoux JE. Dissociation of associative and nonassociative concomitants of classical fear conditioning in the freely behaving rat. Behav Neurosci 1988; 102: 66– 76. [DOI] [PubMed] [Google Scholar]
- 70.Nijsen MJ, Croiset G, Diamant M, et al. Conditioned fear-induced tachycardia in the rat: vagal involvement. Eur J Pharmacol 1998; 350: 211– 22. [DOI] [PubMed] [Google Scholar]
- 71.Tortorici V, Aponte Y, Acevedo H, Nogueira L, Vanegas H. Tolerance to non-opioid analgesics in PAG involves unresponsiveness of medullary pain-modulating neurons in male rats. Eur J Neurosci 2009; 29: 1188– 96. [DOI] [PubMed] [Google Scholar]
- 72.Rocchi L, Chiari L, Cappello A. Feature selection of stabilometric parameters based on principal component analysis. Med Biol Eng Comput 2004; 42: 71– 9. [DOI] [PubMed] [Google Scholar]
- 73.Azevedo TM, Volchan E, Imbiriba LA, et al. A freezing-like posture to pictures of mutilation. Psychophysiology 2005; 42: 255– 60. [DOI] [PubMed] [Google Scholar]
- 74.Hillman CH, Rosengren KS, Smith DP. Emotion and motivated behavior: postural adjustments to affective picture viewing. Biol Psychol 2004; 66: 51– 62. [DOI] [PubMed] [Google Scholar]
- 75.Facchinetti LD, Imbiriba LA, Azevedo TM, Vargas CD, Volchan E. Postural modulation induced by pictures depicting prosocial or dangerous contexts. Neurosci Lett 2006; 410: 52– 6. [DOI] [PubMed] [Google Scholar]
- 76.Hagenaars MA, Stins JF, Roelofs K. Aversive life events enhance human freezing responses. J Exp Psychol Gen 2012; 141: 98– 105. [DOI] [PubMed] [Google Scholar]
- 77.Hagenaars MA, Roelofs K, Stins JF. Human freezing in response to affective films. Anxiety Stress Coping 2014; 27: 27– 37. [DOI] [PubMed] [Google Scholar]
- 78.Lopes FL, Azevedo TM, Imbiriba LA, et al. Freezing reaction in panic disorder patients associated with anticipatory anxiety. Depress Anxiety 2009; 26: 917– 21. [DOI] [PubMed] [Google Scholar]
- 79.Sanchez-Navarro JP, Martinez-Selva JM, Roman F. Uncovering the relationship between defence and orienting in emotion: cardiac reactivity to unpleasant pictures. Int J Psychophysiol 2006; 61: 34– 46. [DOI] [PubMed] [Google Scholar]
- 80.Stins JF, Beek PJ. Effects of affective picture viewing on postural control. BMC Neurosci 2007; 8: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roelofs K, Hagenaars MA, Stins J. Facing freeze: social threat induces bodily freeze in humans. Psychol Sci 2010; 21: 1575– 81. [DOI] [PubMed] [Google Scholar]
- 82.Buhle JT, Kober H, Ochsner KN, et al. Common representation of pain and negative emotion in the midbrain periaqueductal gray. Soc Cogn Affect Neurosci 2013; 8: 609– 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Satpute AB, Wager TD, Cohen-Adad J, et al. Identification of discrete functional subregions of the human periaqueductal gray. Proc Natl Acad Sci U S A 2013; 110: 17101– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hermans EJ, Henckens MJ, Roelofs K, Fernandez G. Fear bradycardia and activation of the human periaqueductal grey. NeuroImage 2012; 66C: 278– 87. [DOI] [PubMed] [Google Scholar]
- 85.Marks IM. Fears, phobias, and rituals: panic, anxiety, and their disorders. New York: Oxford University Press, 1987. [Google Scholar]
- 86.Marx BP, Forsyth JP, Gallup GG, Fusé T, Lexington JM. Tonic immobility as an evolved predator defense: implications for sexual assault survivors. Clin Psychol 2008; 15: 74– 90. [Google Scholar]
- 87.Darwin C. Journal of researches into the geology and natural history of the various countries visited by H.M.S. Beagle [The voyage of the Beagle]. London: Henry Colburn, 1839. [Google Scholar]
- 88.Jackson JH. Evolution and dissolution of the nervous system. In: Taylor J, ed. Selected writings of John Hughlings Jackson. London: Staples, 1958: 45– 118. [Google Scholar]
- 89.Klemm WR. Neurophysiologic studies of the immobility reflex (“animal hypnosis”). Neurosci Res (NY) 1971; 4: 165– 212. [PubMed] [Google Scholar]
- 90.Leite-Panissi CRA, Monassi CR, Menescal-de-Oliveira L. Role of amygdaloid nuclei in the modulation of tonic immobility in guinea pigs. Physiol Behav 1999; 67: 717– 24. [DOI] [PubMed] [Google Scholar]
- 91.Leite-Panissi CR, Coimbra NC, Menescal-de-Oliveira L. The cholinergic stimulation of the central amygdala modifying the tonic immobility response and antinociception in guinea pigs depends on the ventrolateral periaqueductal gray. Brain Res Bull 2003; 60: 167– 78. [DOI] [PubMed] [Google Scholar]
- 92.Ramos C, Leite-Panissi R, Monassi R, Menescal-De-Oliveira L. Role of the amygdaloid nuclei in the modulation of tonic immobility in guinea pigs. Physiol Behav 1999; 67: 717– 24. [DOI] [PubMed] [Google Scholar]
- 93.Schwarz BE, Bickford RG. Electroencephalographic changes in animals under the influence of hypnosis. J Nerv Ment Dis 1956; 124: 433– 40. [DOI] [PubMed] [Google Scholar]
- 94.Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol 2002; 87: 251– 8. [DOI] [PubMed] [Google Scholar]
- 95.Menescal-de-Oliveira L, Hoffmann A. The parabrachial region as a possible region modulating simultaneously pain and tonic immobility. Behav Brain Res 1993; 56: 127– 32. [DOI] [PubMed] [Google Scholar]
- 96.Clement CI, Keay KA, Podzebenko K, Gordon BD, Bandler R. Spinal sources of noxious visceral and noxious deep somatic afferent drive onto the ventrolateral periaqueductal gray of the rat. J Comp Neurol 2000; 425: 323– 44. [DOI] [PubMed] [Google Scholar]
- 97.Vieira EB, Menescal-de-Oliveira L, Leite-Panissi CR. Functional mapping of the periaqueductal gray matter involved in organizing tonic immobility behavior in guinea pigs. Behav Brain Res 2011; 216: 94– 9. [DOI] [PubMed] [Google Scholar]
- 98.Kerman IA, Akil H, Watson SJ. Rostral elements of sympatho-motor circuitry: a virally mediated transsynaptic tracing study. J Neurosci 2006; 26: 3423– 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mouton LJ, Holstege G. The periaqueductal gray in the cat projects to lamina VIII and the medial part of lamina VII throughout the length of the spinal cord. Exp Brain Res 1994; 101: 253– 64. [DOI] [PubMed] [Google Scholar]
- 100.Reese WG, Newton JE, Angel C. Induced immobility in nervous and normal pointer dogs. J Nerv Ment Dis 1982; 170: 605– 13. [DOI] [PubMed] [Google Scholar]
- 101.Richter CP. On the phenomenon of sudden death in animals and man. Psychosom Med 1957; 19: 191– 8. [DOI] [PubMed] [Google Scholar]
- 102.Taylor EW, Jordan D, Coote JH. Central control of the cardiovascular and respiratory systems and their interactions in vertebrates. Physiol Rev 1999; 79: 855– 916. [DOI] [PubMed] [Google Scholar]
- 103.da Silva LF, Menescal-de-Oliveira L. Role of opioidergic and GABAergic neurotransmission of the nucleus raphe magnus in the modulation of tonic immobility in guinea pigs. Brain Res Bull 2007; 72: 25– 31. [DOI] [PubMed] [Google Scholar]
- 104.Leite-Panissi CR, Rodrigues CL, Brentegani MR, Menescal-De-Oliveira L. Endogenous opiate analgesia induced by tonic immobility in guinea pigs. Braz J Med Biol Res 2001; 34: 245– 50. [DOI] [PubMed] [Google Scholar]
- 105.Elliott AE, Packard MG. Intra-amygdala anxiogenic drug infusion prior to retrieval biases rats towards the use of habit memory. Neurobiol Learn Mem 2008; 90: 616– 23. [DOI] [PubMed] [Google Scholar]
- 106.Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science 1999; 286: 1745– 9. [DOI] [PubMed] [Google Scholar]
- 107.Foa EB, Rothbaum BO. Treating the trauma of rape. New York: Guilford, 1998. [Google Scholar]
- 108.Rocha-Rego V, Fiszman A, Portugal LC, et al. Is tonic immobility the core sign among conventional peritraumatic signs and symptoms listed for PTSD? J Affect Disord 2009; 115: 269– 73. [DOI] [PubMed] [Google Scholar]
- 109.Maia DB, Nobrega A, Marques-Portella C, et al. Peritraumatic tonic immobility is associated with PTSD symptom severity in Brazilian police officers: a prospective study. Rev Bras Psiquiatr 2015; 37: 49– 54. [DOI] [PubMed] [Google Scholar]
- 110.Lanius UF. Dissociation and endogenous opioids: a foundational role. In: Lanius UF, Paulsen SL, Corrigan FM, eds. Neurobiology and treatment of traumatic dissociation: toward an embodied self. New York: Springer, 2014: 81– 104. [Google Scholar]
- 111.McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology 2006; 31: 1241– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci 2003; 23: 5674– 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci 2001; 21: 7397– 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dortch-Carnes J, Potter DE. Bremazocine: a kappa-opioid agonist with potent analgesic and other pharmacologic properties. CNS Drug Revs 2005; 11: 195– 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Walsh SL, Strain EC, Abreu ME, Bigelow GE. Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphone in humans. Psychopharmacology 2001; 157: 151– 62. [DOI] [PubMed] [Google Scholar]
- 116.Engel GL, Ferris EB, Logan M. Hyperventilation: analysis of clinical symptomatology. Ann Intern Med 1947; 27: 683– 704. [DOI] [PubMed] [Google Scholar]
- 117.Lempert T, Bauer M, Schmidt D. Syncope: a videometric analysis of 56 episodes of transient cerebral hypoxia. Ann Neurol 1994; 36: 233– 7. [DOI] [PubMed] [Google Scholar]
- 118.Moskowitz AK. “Scared stiff”: catatonia as an evolutionary-based fear response. Psychol Rev 2004; 111: 984– 1002. [DOI] [PubMed] [Google Scholar]
- 119.Hopper JW, Frewen PA, van der Kolk BA, Lanius RA. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J Trauma Stress 2007; 20: 713– 25. [DOI] [PubMed] [Google Scholar]
- 120.Lanius RA, Williamson PC, Boksman K, et al. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol Psychiatry 2002; 52: 305– 11. [DOI] [PubMed] [Google Scholar]
- 121.Lanius RA, Williamson PC, Hopper J, et al. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol Psychiatry 2003; 53: 204– 10. [DOI] [PubMed] [Google Scholar]
- 122.Lanius RA, Bluhm R, Lanius U, Pain C. A review of neuroimaging studies in PTSD: heterogeneity of response to symptom provocation. J Psychiatr Res 2006; 40: 709– 29. [DOI] [PubMed] [Google Scholar]
- 123.Lader MH. The psychophysiology of mental illness. London: Routledge & Kegan Paul, 1975. [Google Scholar]
- 124.Griffin MG, Resick PA, Mechanic MB. Objective assessment of peritraumatic dissociation: psychophysiological indicators. Am J Psychiatry 1997; 154: 1081– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ludascher P, Valerius G, Stiglmayr C, et al. Pain sensitivity and neural processing during dissociative states in patients with borderline personality disorder with and without comorbid posttraumatic stress disorder: a pilot study. J Psychiatr Neurosci 2010; 35: 177– 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sierra M, Berrios GE. Depersonalization: neurobiological perspectives. Biol Psychiatry 1998; 44: 898– 908. [DOI] [PubMed] [Google Scholar]
- 127.Hopper JW, Frewen P, Sack M, Lanius RA, van der Kolk BA. The responses to script-driven imagery scale (RSDI): assessment of state posttraumatic symptoms for psychobiological and treatment research. J Psychopathol Behav Assess 2007; 29: 249– 68. [Google Scholar]
- 128.Lanius RA, Williamson PC, Bluhm RL, et al. Functional connectivity of dissociative responses in posttraumatic stress disorder: a functional magnetic resonance imaging investigation. Biol Psychiatry 2005; 57: 873– 84. [DOI] [PubMed] [Google Scholar]
- 129.Nuwer R. A hungry little squatter: a lizard interloper presents challenge in Florida. N Y Times 2014;August 5:D1. http://www.nytimes.com/2014/08/05/science/a-lizard-interloper-presents-challenge-in-florida.html?emc=eta1&_r=0.
- 130.Mosso A. Fear. London: Longmans, Green, 1896. [Google Scholar]
- 131.Depaulis A, Keay KA, Bandler R. Quiescence and hyporeactivity evoked by activation of cell bodies in the ventrolateral midbrain periaqueductal gray of the rat. Exp Brain Res 1994; 99: 75– 83. [DOI] [PubMed] [Google Scholar]
- 132.Bandler R, Depaulis A. Midbrain periaqueductal gray control of defensive behavior in the cat and rat. In: Depaulis A, Bandler R, eds. The midbrain periaqueductal gray matter: functional, anatomical, and neurochemical organization. New York: Plenum, 1991: 175– 98. [Google Scholar]
- 133.Cameron OG. Interoception: the inside story—a model for psychosomatic processes. Psychosom Med 2001; 63: 697– 710. [DOI] [PubMed] [Google Scholar]
- 134.Laskowski ER, Newcomer-Aney K, Smith J. Proprioception. Phys Med Rehabil Clin N Am 2000; 11: 323– 40, vi. [PubMed] [Google Scholar]
- 135.Levine PA, Kline M. Trauma through a child’s eyes. Berkeley, CA; Lyons, CO: North Atlantic & ERGOS Institute, 2007. [Google Scholar]
- 136.Levine PA. In an unspoken voice: how the body releases trauma and restores goodness. Berkeley, CA: North Atlantic, 2010. [Google Scholar]
- 137.Ogden P, Minton K. Sensorimotor approach to processing traumatic memory. In: Figley CR, ed. Brief treatments for the traumatized. Westport, CT: Greenwood, 2002: 125– 47. [Google Scholar]
- 138.Ogden P. Embedded relational mindfulness: a sensorimotor psychotherapy perspective on the treatment of trauma. In: Follette VM, Briere J, Rozelle D, Hopper JW, Rome DI, eds. Mindfulness-oriented interventions for trauma: integrating contemplative practices. New York: Guilford, 2015. [Google Scholar]
- 139.Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci 2005; 9: 566– 71. [DOI] [PubMed] [Google Scholar]
- 140.Uvanas-Moberg K, Arn I, Magnusson D. The psychobiology of emotion: the role of the oxytocinergic system. Int J Behav Med 2005; 12: 59– 65. [DOI] [PubMed] [Google Scholar]
- 141.Schore AN. Affect dysregulation and disorders of the self. New York: Norton, 2003. [Google Scholar]
- 142.Hofer MA. Early relationships as regulators of infant physiology and behavior. Acta Paediatr Suppl 1994; 397: 9– 18. [DOI] [PubMed] [Google Scholar]
- 143.Ogden P, Minton K, Pain C. Trauma and the body: a sensorimotor approach to psychotherapy. New York: Norton, 2006. [Google Scholar]
- 144.Brown RP, Gerbarg PL. Sudarshan Kriya yogic breathing in the treatment of stress, anxiety, and depression: part I—neurophysiologic model. J Altern Complement Med 2005; 11: 189– 201. [DOI] [PubMed] [Google Scholar]
- 145.Eckberg DL. The human respiratory gate. J Physiol 2003; 548: 339– 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gevirtz R. Resonant frequency training to restore homeostasis for treatment of psychophysiological disorders. Biofeedback 2000; 27: 7– 9. [Google Scholar]
- 147.Taha BH, Simon PM, Dempsey JA, Skatrud JB, Iber C. Respiratory sinus arrhythmia in humans: an obligatory role for vagal feedback from the lungs. J Appl Physiol (1985) 1995; 78: 638– 45. [DOI] [PubMed] [Google Scholar]
- 148.Devous MD, Husain M, Harris TS, Rush AJ. Effects of VNS on regional cerebral blood flow in depressed subjects. Eur Psychiatry 2002; 17: 113– 4. [Google Scholar]
- 149.Streeter CC, Gerbarg PL, Saper RB, Ciraulo DA, Brown RP. Effects of yoga on the autonomic nervous system, gamma-aminobutyric-acid, and allostasis in epilepsy, depression, and post-traumatic stress disorder. Med Hypotheses 2012; 78: 571– 9. [DOI] [PubMed] [Google Scholar]
- 150.Woodbury DM, Woodbury JW. Effects of vagal stimulation on experimentally induced seizures in rats. Epilepsia 1990; 31 suppl 2: S7– 19. [DOI] [PubMed] [Google Scholar]
- 151.Hollifield M, Sinclair-Lian N, Warner TD, Hammerschlag R. Acupuncture for posttraumatic stress disorder: a randomized controlled pilot trial. J Nerv Ment Dis 2007; 195: 504– 13. [DOI] [PubMed] [Google Scholar]
- 152.Eshkevari L, Permaul E, Mulroney SE. Acupuncture blocks cold stress-induced increases in the hypothalamus-pituitary-adrenal axis in the rat. J Endocrinol 2013; 217: 95– 104. [DOI] [PubMed] [Google Scholar]
- 153.Li QQ, Shi GX, Xu Q, Wang J, Liu CZ, Wang LP. Acupuncture effect and central autonomic regulation. Evid Based Complement Alternat Med 2013; 2013: 267959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.McDonald JL, Cripps AW, Smith PK, Smith CA, Xue CC, Golianu B. The anti-inflammatory effects of acupuncture and their relevance to allergic rhinitis: a narrative review and proposed model. Evid Based Complement Alternat Med 2013; 2013: 591796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fox JH, Hammack SE, Falls WA. Exercise is associated with reduction in the anxiogenic effect of mCPP on acoustic startle. Behav Neurosci 2008; 122: 943– 8. [DOI] [PubMed] [Google Scholar]
- 156.Greenwood BN, Fleshner M. Exercise, stress resistance, and central serotonergic systems. Exerc Sport Sci Rev 2011; 39: 140– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Barres R, Yan J, Egan B, et al. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab 2012; 15: 405– 11. [DOI] [PubMed] [Google Scholar]
- 158.Fleshner M. Physical activity and stress resistance: sympathetic nervous system adaptations prevent stress-induced immunosuppression. Exerc Sport Sci Rev 2005; 33: 120– 6. [DOI] [PubMed] [Google Scholar]
- 159.Arce E, Simmons AN, Lovero KL, Stein MB, Paulus MP. Escitalopram effects on insula and amygdala BOLD activation during emotional processing. Psychopharmacology 2008; 196: 661– 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat Rev Neurosci 2008; 9: 788– 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lonergan MH, Olivera-Figueroa LA, Pitman RK, Brunet A. Propranolol’s effects on the consolidation and reconsolidation of long-term emotional memory in healthy participants: a meta-analysis. J Psychiatr Neurosci 2013; 38: 222– 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Stahl SM, Muntner N. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. 3rd ed Cambridge; New York: Cambridge University Press, 2008. [Google Scholar]
- 163.Bar-Haim Y. Research review: attention bias modification (ABM): a novel treatment for anxiety disorders. J Child Psychol Psychiatry 2010; 51: 859– 70. [DOI] [PubMed] [Google Scholar]
- 164.Sharpe L, Ianiello M, Dear BF, Nicholson Perry K, Refshauge K, Nicholas MK. Is there a potential role for attention bias modification in pain patients? Results of 2 randomised, controlled trials. Pain 2012; 153: 722– 31. [DOI] [PubMed] [Google Scholar]
- 165.Desbordes G, Negi LT, Pace TW, Wallace BA, Raison CL, Schwartz EL. Effects of mindful-attention and compassion meditation training on amygdala response to emotional stimuli in an ordinary, non-meditative state. Front Hum Neurosci 2012; 6: 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Farb NA, Segal ZV, Mayberg H, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc Cogn Affect Neurosci 2007; 2: 313– 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kabat-Zinn J. Mindfulness-based interventions in context: past, present, and future. Clin Psychol 2003; 10: 144– 56. [Google Scholar]
- 168.Fosha D, Paivio SC, Gleiser K, Ford JD. Experiential and emotion-focused therapy. In: Courtois CA, Ford JD, eds. Treating complex traumatic stress disorders: an evidence-based guide. New York: Guilford, 2009: 286– 311. [Google Scholar]
- 169.Hopper JW. Harnessing the seeking, satisfaction, and embodiment circuitries in contemplative approaches to trauma. In: Follette VM, Briere J, Rozelle D, Hopper JW, Rome DI, eds. Mindfulness-oriented interventions for trauma: integrating contemplative practices. New York: Guilford, 2015. [Google Scholar]
- 170.Fehmi L, Robbins J. The open-focus brain: harnessing the power of attention to heal mind and body. Boston: Trumpeter, 2007. [Google Scholar]
- 171.Chiesa A, Serretti A. Mindfulness based cognitive therapy for psychiatric disorders: a systematic review and meta-analysis. Psychiatry Res 2011; 187: 441– 53. [DOI] [PubMed] [Google Scholar]
- 172.Gross JJ. Emotion regulation: taking stock and moving forward. Emotion 2013; 13: 359– 65. [DOI] [PubMed] [Google Scholar]
- 173.Lovibond PF. Learning and anxiety: a cognitive perspective. In: Schachtman TR, Reilly S, eds. Associative learning and conditioning theory: human and non-human applications. New York: Oxford University Press, 2011: 104– 20. [Google Scholar]
- 174.Holmes EA, Arntz A, Smucker MR. Imagery rescripting in cognitive behaviour therapy: images, treatment techniques and outcomes. J Behav Ther Exp Psychiatry 2007; 38: 297– 305. [DOI] [PubMed] [Google Scholar]
- 175.Davidson RJ, Kabat-Zinn J, Schumacher J, et al. Alterations in brain and immune function produced by mindfulness meditation. Psychosom Med 2003; 65: 564– 70. [DOI] [PubMed] [Google Scholar]
- 176.Rosmarin DH, Bigda-Peyton JS, Kertz SJ, Smith N, Rauch SL, Bjorgvinsson T. A test of faith in God and treatment: the relationship of belief in God to psychiatric treatment outcomes. J Affect Disord 2013; 146: 441– 6. [DOI] [PubMed] [Google Scholar]
- 177.Weng HY, Fox AS, Shackman AJ, et al. Compassion training alters altruism and neural responses to suffering. Psychol Sci 2013; 24: 1171– 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jobin J, Wrosch C, Scheier MF. Associations between dispositional optimism and diurnal cortisol in a community sample: when stress is perceived as higher than normal. Health Psychol 2014; 33: 382– 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Brydon L, Walker C, Wawrzyniak AJ, Chart H, Steptoe A. Dispositional optimism and stress-induced changes in immunity and negative mood. Brain Behav Immun 2009; 23: 810– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Segerstrom SC, Taylor SE, Kemeny ME, Fahey JL. Optimism is associated with mood, coping, and immune change in response to stress. J Pers Soc Psychol 1998; 74: 1646– 55. [DOI] [PubMed] [Google Scholar]
- 181.Chrousos GP. Stress and disorders of the stress system. Nat Revs Endocrinol 2009; 5: 374– 81. [DOI] [PubMed] [Google Scholar]
- 182.Nishith P, Duntley SP, Domitrovich PP, Uhles ML, Cook BJ, Stein PK. Effect of cognitive behavioral therapy on heart rate variability during REM sleep in female rape victims with PTSD. J Trauma Stress 2003; 16: 247– 50. [DOI] [PubMed] [Google Scholar]
- 183.Barrowcliff AL, Gray NS, MacCulloch S, Freeman TC, MacCulloch MJ. Horizontal rhythmical eye movements consistently diminish the arousal provoked by auditory stimuli. Br J Clin Psychol 2003; 42: 289– 302. [DOI] [PubMed] [Google Scholar]
- 184.Barrowcliff AL, Gray NS, Freeman TC, MacCulloch MJ. Eye-movements reduce the vividness, emotional valence and electrodermal arousal associated with negative autobiographical memories. J Forens Psychiatr Psychol 2004; 15: 325– 45. [Google Scholar]
- 185.Pagani M, Di Lorenzo G, Monaco L, et al. Pretreatment, intratreatment, and posttreatment EEG imaging of EMDR: methodology and preliminary results from a single case study. J EMDR Prac Res 2011; 5: 42– 56. [Google Scholar]
- 186.Church D, Yount G, Brooks AJ. The effect of emotional freedom techniques on stress biochemistry: a randomized controlled trial. J Nerv Ment Dis 2012; 200: 891– 6. [DOI] [PubMed] [Google Scholar]
- 187.Van der Hart O, Nijenhuis ERS, Steele K. The haunted self: structural dissociation and the treatment of chronic traumatization. New York: Norton, 2006. [Google Scholar]
- 188.Harvey AG, Bryant RA, Tarrier N. Cognitive behaviour therapy for posttraumatic stress disorder. Clin Psychol Rev 2003; 23: 501– 22. [DOI] [PubMed] [Google Scholar]
- 189.Bryant RA, Felmingham K, Kemp A, et al. Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post-traumatic stress disorder. Psychol Med 2008; 38: 555– 61. [DOI] [PubMed] [Google Scholar]
- 190.Bradley R, Greene J, Russ E, Dutra L, Westen D. A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry 2005; 162: 214– 27. [DOI] [PubMed] [Google Scholar]
- 191.Carter J, Gerbarg PL, Brown RP, et al. Multi-component yoga breath program for Vietnam veteran post traumatic stress disorder: randomized controlled trial. J Trauma Stress Disord Treat 2013; 2: 1– 10. [Google Scholar]
- 192.Cloitre M, Courtois CA, Charuvastra A, Carapezza R, Stolbach BC, Green BL. Treatment of complex PTSD: results of the ISTSS expert clinician survey on best practices. J Trauma Stress 2011; 24: 615– 27. [DOI] [PubMed] [Google Scholar]
- 193.Cloitre M, Stovall-McClough KC, Nooner K, et al. Treatment for PTSD related to childhood abuse: a randomized controlled trial. Am J Psychiatry 2010; 167: 915– 24. [DOI] [PubMed] [Google Scholar]
- 194.Wald J, Taylor S. Efficacy of interoceptive exposure therapy combined with trauma-related exposure therapy for posttraumatic stress disorder: a pilot study. J Anxiety Disord 2007; 21: 1050– 60. [DOI] [PubMed] [Google Scholar]
- 195.Ehlers A, Bisson J, Clark DM, et al. Do all psychological treatments really work the same in posttraumatic stress disorder? Clin Psychol Rev 2010; 30: 269– 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.American Psychiatric Association. Guidelines for the psychiatric treatment of acute stress disorder and posttraumatic stress disorder. Washington, DC: APA, 2004. [Google Scholar]
- 197.Australian Centre for Posttraumatic Mental Health. Australian guidelines for the treatment of adults with acute stress disorder and posttraumatic stress disorder. ACPMH: Melbourne, 2007. [Google Scholar]
- 198.Pagani M, Di Lorenzo G, Verardo AR, et al. Neurobiological correlates of EMDR monitoring—an EEG study. Plos One 2012; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pagani M, Hogberg G, Fernandez I, Siracusano A. Correlates of EMDR therapy in functional and structural neuroimaging: a critical summary of recent findings. J EMDR Pract Res 2013; 7: 29– 38. [Google Scholar]
- 200.Lanius UF, Bergmann U. Dissociation, EMDR, and adaptive information processing: The role of sensory stimulation and sensory awareness. In: Lanius UF, Paulsen SL, Corrigan FM, eds. Neurobiology and treatment of traumatic dissociation: toward an embodied self. New York: Springer, 2014: 213– 42. [Google Scholar]
- 201.Church D, Hawk C, Brooks AJ, et al. Psychological trauma symptom improvement in veterans using emotional freedom techniques: a randomized controlled trial. J Nerv Ment Dis 2013; 201: 153– 60. [DOI] [PubMed] [Google Scholar]
- 202.van der Kolk BA, van der Hart O. Pierre Janet and the breakdown of adaptation in psychological trauma. Am J Psychiatry 1989; 146: 1530– 40. [DOI] [PubMed] [Google Scholar]
- 203.Ogden P, Fisher J. Integrating body and mind: sensorimotor psychotherapy and treatment of dissociation, defense, and dysregulation. In: Lanius UF, Paulsen SL, Corrigan FM, eds. Neurobiology and treatment of traumatic dissociation: toward an embodied self. New York: Springer, 2014: 399– 422. [Google Scholar]
- 204.Damasio AR. The feeling of what happens: body and emotion in the making of consciousness. New York: Harcourt Brace, 1999. [Google Scholar]
- 205.Benham E. Coping strategies: a psychoeducational approach to post-traumatic symptomatology. J Psychosoc Nurs Ment Health Serv 1995; 33: 30– 5. [DOI] [PubMed] [Google Scholar]
- 206.Chu JA. Controlling post-traumatic and dissociative symptoms. In: Rebuilding shattered lives: the responsible treatment of complex post-traumatic and dissociative disorders. New York: Wiley, 1998. [Google Scholar]
- 207.LeDoux JE, Gorman JM. A call to action: overcoming anxiety through active coping. Am J Psychiatry 2001; 158: 1953– 5. [DOI] [PubMed] [Google Scholar]
- 208.Segal ZV, Williams JGG, Teasdale JD. Mindfulness-based cognitive therapy for depression: a new approach to preventing relapse. New York: Guilford, 2002. [Google Scholar]
- 209.Posner MI. Attention in cognitive neuroscience: an overview. In: Gazzaniga MS, Bizzi E, eds. The cognitive neurosciences. Cambridge, MA: MIT Press, 1995: 615– 24. [Google Scholar]
- 210.Cohen BH. The motor theory of voluntary thinking. In: Davidson RJ, Schwartz GE, Shapiro D, eds. Consciousness and self-regulation, advances in research and theory. New York: Plenum, 1986. [Google Scholar]
- 211.Craig AD. Interoception and emotion: a neuroanatomical perspective. In: Lewis M, Haviland-Jones JM, Barrett LF, eds. Handbook of emotions. 3rd ed New York: Guilford, 2010: 272– 88. [Google Scholar]
- 212.Dreeben SJ, Mamberg MH, Salmon P. The MBSR body scan in clinical practice. Mindfulness 2013; 4: 394– 401. [Google Scholar]