Unique and redundant functions of NKp46+ ILC3s in models of intestinal inflammation (original) (raw)
Song et al. generate mice selectively lacking NKp46+ ILC3s to demonstrate that in the absence of T cells, NKp46+ILC3s are sufficient to promote inflammatory monocyte accumulation in CD40-induced colitis via production of GM-CSF. In T cell–sufficient mice, the lack of NKp46+ILC3s did not impact C. rodentium infection.
Abstract
Group 3 ILCs (ILC3s) are innate sources of IL-22 and IL-17 and include lymphoid tissue-inducer (LTi)-like and NKp46+ subsets. Both depend on RORγt and aryl hydrocarbon receptor, but NKp46+ILC3s also require Notch and T-bet for their development and are transcriptionally distinct. The extent to which these subsets have unique functions, especially in the context of T cell– and B cell–sufficient mice, remains largely unclear. To investigate the specific function of NKp46+ILC3s among other ILC3 subsets and T cells, we generated mice selectively lacking NKp46+ILC3s or all ILC3s and crossed them to T cell–deficient mice, thus maintaining B cells in all mice. In mice lacking T cells, NKp46+ILC3s were sufficient to promote inflammatory monocyte accumulation in the anti-CD40 innate colitis model through marked production of GM-CSF. In T cell–competent mice, lack of NKp46+ILCs had no impact on control of intestinal C. rodentium infection, whereas lack of all ILC3s partially impaired bacterial control. Thus, NKp46+ILC3s have a unique capacity to promote inflammation through GM-CSF–induced accumulation of inflammatory monocytes, but are superseded by LTi-like ILC3s and T cells in controlling intestinal bacterial infection.
Innate lymphoid cells (ILCs) are a heterogeneous population of lymphocytes that lack antigen-specific receptors and respond to changes in the tissue cytokine microenvironment by secreting effector cytokines (Rankin et al., 2013a; Diefenbach et al., 2014; Artis and Spits, 2015; Eberl et al., 2015). Based on the cytokines secreted and transcription factors required for their development, ILCs are divided into three major groups: ILC1s produce IFN-γ in response to IL-12, IL-15, and IL-18 and depend on T-bet for development; ILC2s secrete IL-5 and IL-13 in response to IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) and require GATA3, RORα, and Bcl11b; ILC3s produce IL-22, IL-17, and granulocyte macrophage-colony stimulating factor (GM-CSF) when stimulated with IL-23 and/or IL-1β and require RORγt and aryl hydrocarbon receptor (AHR). These major ILC groups are heterogeneous and include distinct subsets that more subtly differ in phenotype, transcriptional profile, tissue distribution, and developmental requirements (Robinette et al., 2015).
ILC3s have been associated with many immune responses in the gastrointestinal tract. They provide defense against intestinal infections by various pathogenic bacteria, such as Citrobacter rodentium (C. rodentium). This protective effect is chiefly mediated by secretion of IL-22, which prompts epithelial cells to produce antimicrobial peptides that kill bacteria (Zheng et al., 2008; Tumanov et al., 2011; Guo et al., 2014), as well as fucosylated proteins that are shed in the intestinal lumen and modify bacterial metabolism, thereby attenuating bacterial virulence (Pickard et al., 2014). ILC3s also promote intestinal homeostasis in the steady state through multiple mechanisms. These include containing intestinal microbiota through IL-22 secretion (Sonnenberg et al., 2012), inducing T cell tolerance to bacterial antigens through MHC class II presentation (Hepworth et al., 2013; Hepworth et al., 2015), and sustaining the tolerogenic function of intestinal DCs through GM-CSF secretion (Mortha et al., 2014). Furthermore, ILC3s control T cell–dependent and –independent IgA production through lymphotoxins (Kruglov et al., 2013), as well as marginal zone B cell Ig production (Magri et al., 2014). ILC3s can also protect intestinal stem cells from immune-mediated tissue damage in graft versus host disease (Dudakov et al., 2015). In some cases, ILC3s have deleterious roles. For example, ILC3s have been implicated in _Helicobacter hepaticus_–driven innate colitis, which is associated with an increased production of IL-17 and IFN-γ in the colon (Buonocore et al., 2010). Inappropriate activation of ILC3s has also been shown to induce intestinal damage through excessive IL-22–induced production of neutrophil chemoattractants by epithelial cells, resulting in accumulation of neutrophils and tissue destruction (Eken et al., 2014).
In mice, ILC3s include two major subsets (Diefenbach et al., 2014; Eberl et al., 2015). Lymphoid tissue-inducer (LTi)-like ILC3s have phenotypic features overlapping with those of fetal LTi cells that promote lymphoid organogenesis, including lack of lineage markers along with expression of CD127 and lymphotoxins. Some cells within this subset express the chemokine receptor CCR6 and are thought to have a distinct progenitor from CCR6– LTi-like ILC3s (Vonarbourg et al., 2010; Constantinides et al., 2014; Klose et al., 2014). However, both CCR6+ and CCR6– ILC3s require RORγt and AHR for development. NKp46+ILC3s express the NK cell marker NKp46 in addition to CD127 and require AHR (Kiss et al., 2011; Lee et al., 2012; Qiu et al., 2012), Notch (Lee et al., 2012) and T-bet for development (Sciumé et al., 2012; Klose et al., 2013; Rankin et al., 2013b). Under certain inflammatory conditions, NKp46+ILC3s down-regulate expression of RORγt, increase T-bet expression and become a source of IFN-γ (Vonarbourg et al., 2010). These cells, known as ex-RORγt ILC3s, may contribute to host-defense against certain bacterial infections, such as Salmonella thyphymurium (Klose et al., 2013). LTi-like and NKp46+ILC3s also differ in their distribution within the intestine; LTi-like ILC3s are found throughout the intestine in association with lymphoid follicles; NKp46+ILC3s are diffuse in the lamina propria and their distribution is dependent on the expression of the chemokine receptor CXCR6 (Satoh-Takayama et al., 2014). Notably, LTi-like ILC3s and NKp46+ ILC3s share expression of some core genes compared with other ILCs, such as IL-22, but are also transcriptionally distinct (Reynders et al., 2011; Robinette et al., 2015).
To date, it is unclear whether NKp46+ and LTi-like ILC3 subsets are redundant or have specialized functions. Moreover, the functions of NKp46+ and LTi-like ILC3s have been frequently examined in immunodeficient mice that lack adaptive responses. To address these gaps in our understanding of ILC biology, we examined novel mouse lines that lack ILC3s. RORγt- and AHR-deficient mice lack ILC3s and have been used as ILC3-deficient models. These transcription factors, however, function in a variety of cells types, including other lymphocytes and nonhematopoietic populations, making it difficult to unambiguously assess the specific functions of ILC3s in vivo. To overcome this problem, we generated conditional knock-out mice in which AHR or RORγt deficiency is restricted to RORγt- and NKp46-expressing cells, respectively, obtaining mice that lack all ILC3 subsets or only NKp46+ILC3s. Moreover, these strains were crossed to a strain that lacks αβ and γδ T cells to evaluate ILC3 functions in B cell–sufficient mice in the presence or absence of T cells.
We examined these mouse lines in two models of intestinal inflammation, including a commonly used model of colitis and an infection model. The anti-CD40 model of colitis induces pathology caused by hyperactivation of innate immunity, but can only be used in immunocompromised mice (Uhlig et al., 2006). In this model, we found that ILC3s elicited robust inflammation and accumulation of pathogenic Ly6C+ inflammatory monocytes that were dependent on excessive production of GM-CSF and IL-22. Moreover, NKp46+ILC3s were sufficient to induce inflammation in this model, demonstrating a nonredundant inflammatory function that was partially caused by their enhanced capacity to secrete GM-CSF in vivo. We also examined ILC3-deficient mouse lines in host defense against C. rodentium. ILC3s were essential in T cell–deficient mice but partially redundant in T cell–competent mice. However, the NKp46+ILC3 subset was always redundant, both in T cell–competent and T cell–deficient mice. In conclusion, NKp46+ILC3s have a unique role in GM-CSF–mediated recruitment and activation of inflammatory monocytes during innate intestinal inflammation, but are redundant for clearance of C. rodentium, whether T cells are present or absent.
RESULTS
Generation of mice that lack all ILC3s and NKp46+ILC3s
We identified ILC3s in mouse intestine as CD45+Lin–Thy1+RORγt+ cells. As previously shown (Vonarbourg et al., 2010), ILC3s were generally more abundant in the small intestine than in cecum and colon (Fig. S1 A). Based on NKp46 expression, ILC3s were distinguished into NKp46+ILC3s and LTi-like ILC3s (NKp46–) subsets. LTi-like ILC3s subsets were further divided in CCR6+ and CCR6– subsets (Fig. S1 A and not depicted; Diefenbach et al., 2014; Eberl et al., 2015). To engineer mice with specific deletions of ILC3s, we crossed Ahrf/f mice to Rorc(γt)-cre mice (AR mice) and Rorc(γt)f/f mice to NKp46-cre mice (RN mice). AR mice had a marked reduction of all ILC3s (Fig. S1 B). Moreover, because RORγt is transiently expressed during thymic lymphocyte development, AR mice also lacked AHR in T cells (unpublished data), which may affect some functions of TH17 and TH22 cells, including IL-22 secretion, in vitro but not in vivo (Martin et al., 2009; Duarte et al., 2013; Qiu et al., 2013; unpublished data). RN mice selectively lack NKp46+ILC3s (which also include ex-RORγt+ ILC3s; Fig. S1 B) and, therefore, are suitable to study the functions of NKp46+ILC3s in otherwise immunocompetent mice. AR and RN mice were then crossed with Tcrb−/−Tcrd−/− (Tcrbd−/−) mice to obtain mice that lack ILC3s and T cells (ART mice; Fig. S1, C and D), or NKp46+ILC3s and T cells (RNT mice; Fig. S1 C and S1 D). Thus, AR, RN, ART and RNT mice can be used to parse unique and redundant functions of ILC3s and T cells, while preserving B cells in all mice.
Lack of ILC3s curbs intestinal inflammation in anti–CD40-induced colitis
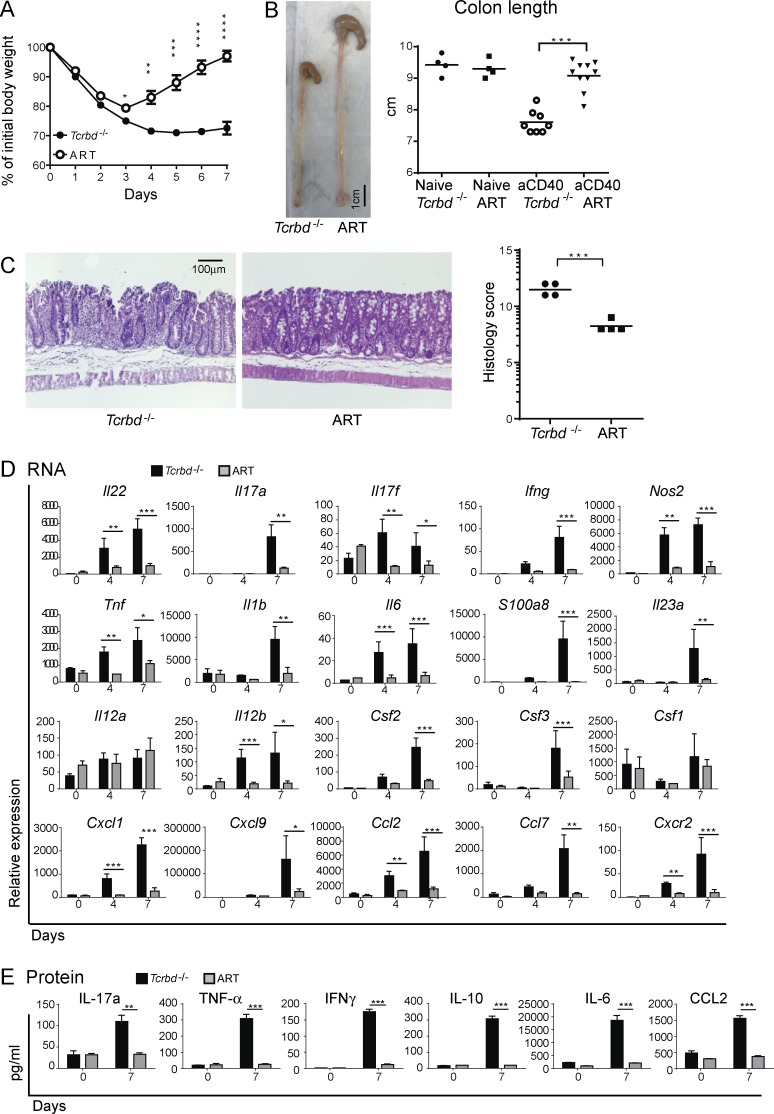
To explore the role of ILC3s in intestinal inflammation, we used a model of colitis induced by injection of anti-CD40 into mice that lack adaptive immunity, such as _Rag1_-deficient mice (Uhlig et al., 2006). In this model, anti-CD40 causes inappropriate and unrestricted activation of myeloid cells, leading to massive release of inflammatory cytokines. Colitis and mucosal immunopathology depend on IL-23, whereas IL-12 promotes wasting disease and elevation of serum inflammatory mediators. Although the anti-CD40 model is usually performed in _Rag_-deficient mice that lack both T and B cells, we chose to examine the role of ILC3 in Tcrbd−/− mice, which lack T cells but are B cell competent. After anti-CD40 injection, Tcrbd−/− mice quickly lost body weight and remained severely underweight for the duration of the experiment (7 d; Fig. 1 A). In contrast, ART mice lost less weight and quickly recovered, beginning at day 4. At day 7 after treatment, less pronounced colonic shortening and milder histopathology was evident in ART mice than in Tcrbd−/− mice (Fig. 1, B and C), whereas there was no difference between Tcrbd−/− and ART naive animals (unpublished data).
Figure 1.
ILC3 deficiency protects mice from anti-CD40–induced colitis. ART and Tcrbd−/− mice were injected with anti-CD40. (A) Mice were weighed daily, from day 0 to 7 p.i. (B) Colons were harvested and overall lengths measured 7 d p.i. Colon lengths were also assessed in Ahrf/f x Tcrbd−/− (flox control mice) and Rorγt-cre x Tcrbd−/− (Cre control mice; not depicted). A and B represent five independent experiments with 4–10 mice/group per experiment. (C) Colons were stained with hematoxylin and eosin and scored for severity of colitis on day 7, where a higher score corresponds to increased pathology. Data represent two independent experiments with four to five mice/group. (D) mRNA expression of inflammatory mediators. Colons were collected from ART and Tcrbd−/− mice at day 4 and 7 after anti-CD40 injection and the indicated transcripts were measured via quantitative RT-PCR. All gene expression values were normalized to Gapdh. (E) Protein expression of inflammatory mediators. Colonic lamina propria was processed at day 7 after anti-CD40 treatment; 106 cells were isolated and cultured for 4 h. The indicated cytokines were measured by cytometric bead array of the culture supernatants. (D and E) Data represent two independent experiments with five mice/group. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, Student’s t test. Bars represent mean ± SEM.
A marked increase in IL-22, the inflammatory mediator S100A8, the enzyme NOS2 that produces nitric oxide, as well as many inflammatory cytokines, such as IL-17a/f, IFN-γ, TNF, IL-1β, IL-23p19, IL-12p40, and IL-6, was obvious in the intestines of Tcrbd−/− mice injected with anti-CD40 (Fig. 1, D and E). Notably, IL-12p35 expression was not elevated, indicating a more predominant pathogenic role for IL-23 than for IL-12 in intestinal inflammation. Chemoattractants and growth factors for neutrophils and inflammatory monocytes, such as CXCL1, CCL2, G-CSF, and GM-CSF, were also increased. The induction in inflammatory mediators was paralleled by an increase of IL-10, which may reflect the activation of feedback mechanisms for reducing inflammation. Lower amounts of all these cytokines and chemokines were detected in the intestines of ART mice in comparison with the Tcrbd−/− mice (Fig. 1, D and E). We conclude that ART mice exhibit significantly less clinical, pathological, and biochemical features of intestinal inflammation than do Tcrbd−/− mice after administration of anti-CD40.
Lack of ILC3s curtails intestinal accumulation of Ly6C+ monocytes and neutrophils
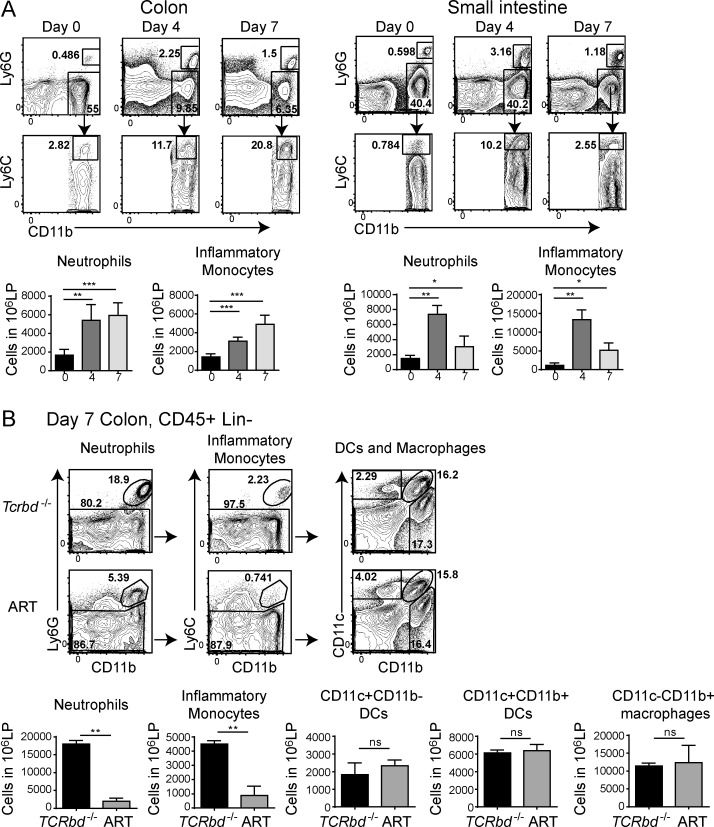
Upon induction of anti-CD40–mediated colitis, CD11b+Ly6G+ neutrophils and CD11b+Ly6G–Ly6C+ monocytes accumulated in the intestines of Tcrbd−/− mice, peaked at day 4 in the small intestine and sustained up to day 7 in the colon (Fig. 2 A). ART mice had fewer intestinal neutrophils and inflammatory monocytes than did Tcrbd−/− mice (Fig. 2 B and not depicted), whereas there was no difference between neutrophils and inflammatory monocytes in untreated mice (unpublished data). Intestinal CD11c+ DCs (including CD11b+ and CD11b– subsets) and CD11c–CD11b+ macrophages were found at similar frequencies in the colons of Tcrbd−/− and ART mice, both before and after anti-CD40 (Fig. 2 B and not depicted).
Figure 2.
Evaluation of intestinal myeloid populations in ART and Tcrbd−/− mice treated with anti-CD40. (A) Tcrbd−/− mice were treated with anti-CD40 and sacrificed at days 0, 4, and 7. Cells were purified from colonic and small intestinal lamina propria. Ly6G+ neutrophils and Ly6G–Ly6Chi inflammatory monocytes were distinguished within the population of live CD45+CD19–CD11b+ cells. Representative plots (top) and neutrophil and monocyte numbers among 106 total leukocytes (bottom) are indicated. Data represent three independent experiments with two mice/time point. (B) Colonic lamina propria cells from Tcrbd−/− and ART mice were collected at day 7 after anti-CD40 treatment. Live, CD45+CD19– cells were analyzed for expression of Ly6G, Ly6C, CD11c, and CD11b. Data represent five independent experiments with two mice/group. Representative plots (top) and total numbers of neutrophils, monocytes, and DCs among 106 total leukocytes (bottom) are indicated. *, P < 0.05; **, P < 0.01; ***, P < 0.001, student’s t test. Bars represent mean ± SEM.
We also assessed the capacity of intestinal myeloid cells to produce inflammatory cytokines. In Tcrbd−/− mice injected with anti-CD40, both neutrophils and inflammatory monocytes produced IL-1β and TNF at day 7 p.i.; inflammatory monocytes also produced IL-6 (unpublished data). DC and resident macrophages produced small amounts of these cytokines. A marked reduction in cytokine production by inflammatory monocytes, but not neutrophils, was noted in ART mice (unpublished data). These data indicate that lack of ILC3s results in accumulation of fewer neutrophils and inflammatory monocytes, which is consistent with the reduced intestinal content of CXCL1, CCL2, GM-CSF, and G-CSF observed in ART mice (Fig. 1, D and E). Moreover, lack of ILC3s selectively diminishes cytokine secretion by inflammatory monocytes, most likely due to reduced availability of GM-CSF (Fig. 1 D), which has been shown to promote monocytic functions (Griseri et al., 2012). We conclude that ILC3s have an important function in recruiting and activating Ly6C+ inflammatory monocytes in the absence of T cells.
Adoptive transfer of intestinal ILC3s into lymphopenic mice is sufficient to enable innate immune colitis
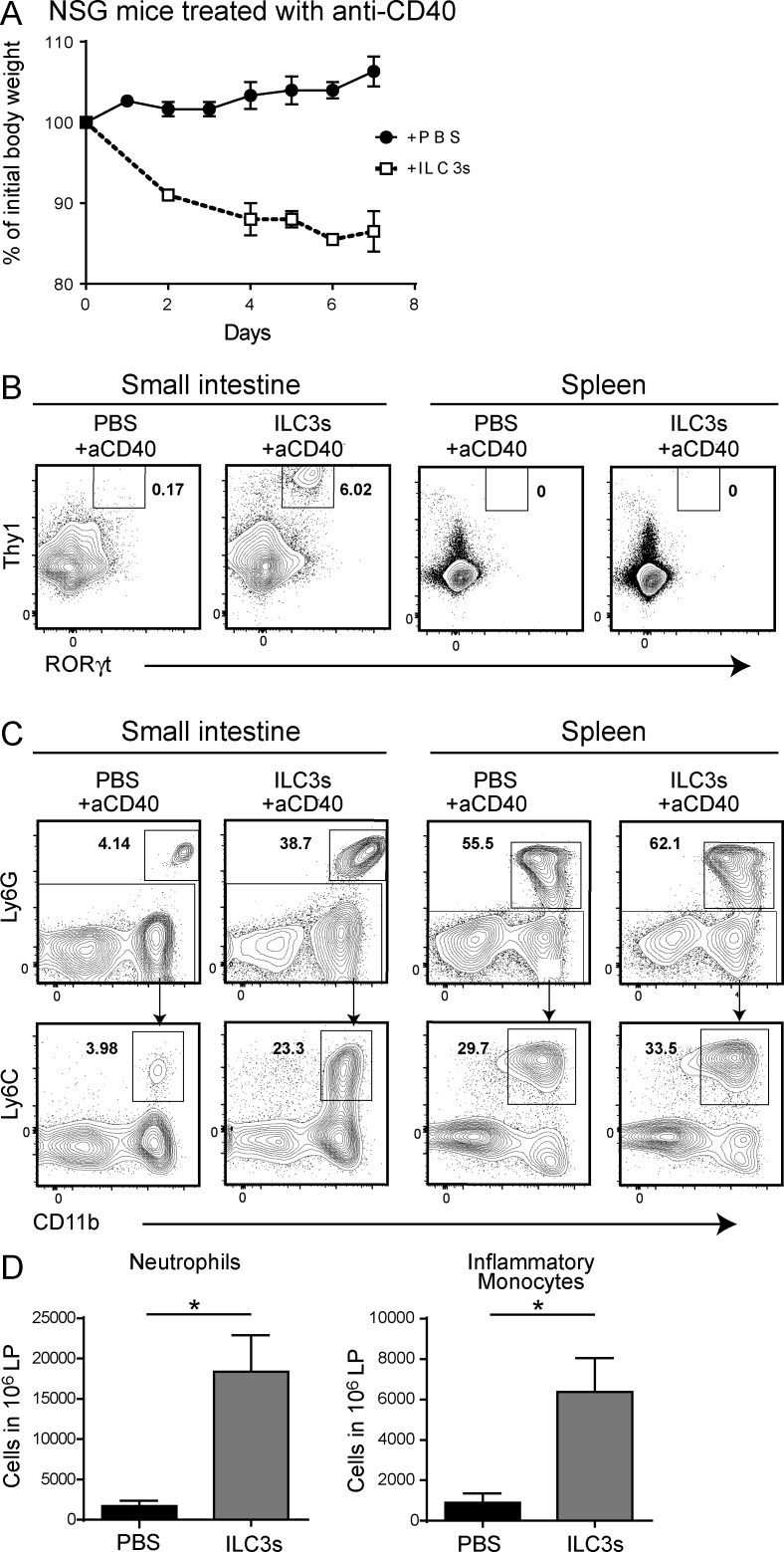
To corroborate the proinflammatory function of ILC3s in anti-CD40 colitis we performed adoptive transfer experiments. We isolated ILC3s from the small intestine of _Rag1_−/− mice (unpublished data), transferred them into NOD/SCID x γc−/− (NSG) mice, and waited 2 or 8 wk to allow cells to repopulate the intestine. Next, we induced colitis by injection of anti-CD40 and examined weight loss and intestinal pathology after 1 wk. NSG mice reconstituted with ILC3s for 8 wk quickly lost weight and remained underweight for the duration of the experiment (Fig. 3 A). In these mice, ILC3s reconstituted the intestine, whereas no ILC3s were detected in the spleen (Fig. 3 B). Weight loss was paralleled by a significant recruitment of neutrophils and inflammatory monocytes (Fig. 3, C and D). In contrast, NSG mice that had not been reconstituted or were reconstituted only for 2 wk before colitis induction showed no weight loss, absence of ILC3s in the intestine or spleen, and no intestinal recruitment of neutrophils or monocytes (unpublished data). These results conclusively demonstrate a proinflammatory function for ILC3s in anti-CD40–induced colitis.
Figure 3.
Adoptively transferred ILC3s cause severe colitis in NSG mice. 2 × 104 small intestinal ILC3s (CD45+Lin–Thy1+CD127+ cells) sort-purified from Rag1−/− mice were intravenously injected into NSG mice. (A) After 8 wk, mice were treated with anti-CD40, and body weights were measured daily, from 0 to 7 d p.i. Data represent two independent experiments with four mice/group. (B) Cells were purified from the small intestinal lamina propria. ILC3s were identified as CD45+Lin−Thy1+RORγt+ cells. Data represent three independent experiments with two mice/group. (C) Ly6G+ neutrophils and Ly6G–Ly6Chi inflammatory monocytes were distinguished within the population of live CD45+CD19–CD11b+ cells and (D) absolute numbers quantified. Data are representative of three experiments with two mice/group (C) or two independent experiments with five mice/group (D). *, P < 0.05, Student’s t test. Bars represent mean ± SEM.
Depletion of inflammatory monocytes protects mice from anti-CD40–induced colitis
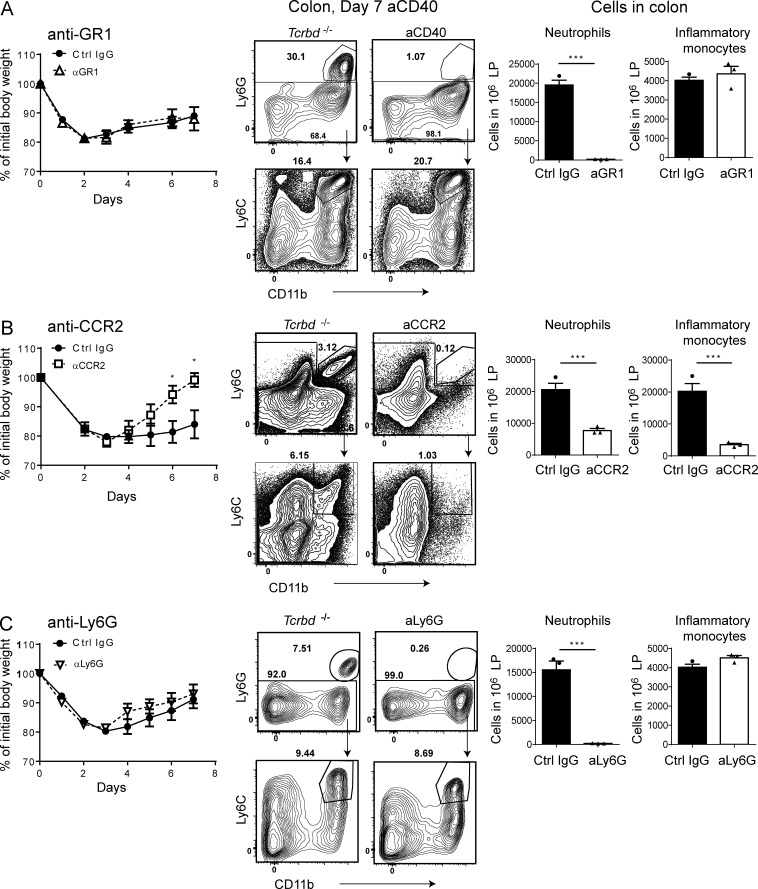
Our data suggested that ILC3s promote intestinal inflammation by inducing recruitment and activation of both neutrophils and inflammatory monocytes, which may work in concert to drive inflammation and tissue damage. To test this hypothesis, we attempted to deplete both cell types by using an anti-Ly6G/Ly6C (GR1) antibody (Daley et al., 2008). We found that administration of the antibody had no protective effect, as disease severity was unchanged compared with control treatment (Fig. 4 A). However, a close examination of the depleting efficacy of anti-GR1 administration revealed that this antibody successfully depleted both neutrophils and inflammatory monocytes in blood and spleen (not depicted), whereas only neutrophils were effectively depleted in the intestine (Fig. 4 A). Inflammatory monocytes persisted in the intestine even after 5 d of daily injections of the antibody. These results suggested that neutrophils are not critical in inducing intestinal inflammation during anti-CD40–induced colitis, but instead, that inflammatory monocytes may be important.
Figure 4.
Impact of inflammatory monocytes and neutrophils depletion on anti-CD40–induced colitis. Tcrbd−/− mice were treated with (A) anti-GR1 (RB6-8C5) or control IgG at days −1, 1, 3, and 5; (B) anti-CCR2 (MC-21) or control IgG at days −4, −1, 2, 3, and 4; (C) anti-Ly6G (1A8), or control IgG at days −1, 1, 3, and 5. Anti-CD40 was injected at day 0. (A–C) Numbers of intestinal neutrophils and inflammatory monocytes among 106 total leukocytes present in the colon are indicated for each antibody treatment to indicate the efficacy of depletion. *, P < 0.05; ***, P < 0.001, Student’s t test. Data are representative of three experiments (A and C) or two experiments (B) with four to five mice/group. Bars represent mean ± SEM.
To test this hypothesis, we tried to selectively deplete inflammatory monocytes using an antibody for CCR2 (MC-21), which is a selective marker of these cells (Shi and Pamer, 2011). Anti-CCR2–treated mice had less severe disease, lost less body weight, and recovered faster than did mice treated with a control antibody (Fig. 4 B). As expected, anti-CCR2 treatment resulted in significantly lower numbers of intestinal inflammatory monocytes (Fig. 4 B). However, anti-CCR2–treated mice also had fewer intestinal neutrophils. Given that CCR2 is not expressed by neutrophils, it is likely that this reduction is secondary to diminished accumulation of inflammatory monocytes, which are known to release cytokines and chemokines that recruit neutrophils, such as IL-1β (Rogers et al., 1994). To definitively assess the contribution of neutrophils to anti-CD40–induced colitis, we selectively depleted neutrophils using an anti-Ly6G antibody. We found that neutrophil depletion did not attenuate disease severity (Fig. 4 C). Our results indicate that inflammatory monocytes have a predominant pathogenic role over neutrophils in anti-CD40–induced colitis and, therefore, ILC3s may promote colitis primarily by inducing accumulation and activation of inflammatory monocytes in the intestine.
Neutralization of GM-CSF and IL-22 ameliorates anti-CD40–induced colitis
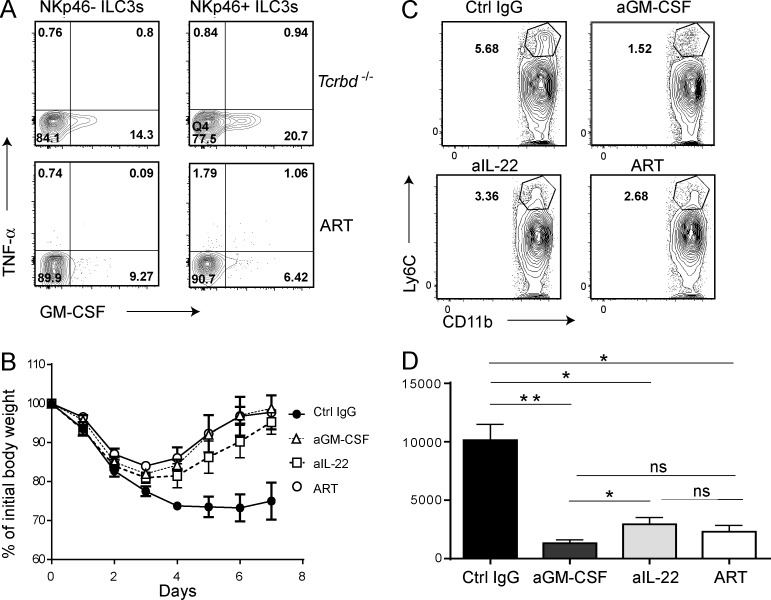
We asked how ILC3s drive accumulation of Ly6C+ monocytes in the intestine and promote subsequent inflammation. We and others have previously shown that ILC3s produce GM-CSF (Cella et al., 2009; Mortha et al., 2014). Moreover, it has been shown that GM-CSF drives extramedullary expansion of inflammatory monocytes from granulocyte-monocyte progenitors (GMPs) during inflammation (Griseri et al., 2012). Based on these data and the finding that GM-CSF was substantially reduced in ART mice compared with Tcrbd−/− controls (Fig. 1 D) after anti-CD40 injection, we hypothesized that ILC3s drive colitis by producing GM-CSF required for the expansion, survival, and function of inflammatory monocytes. Consistent with this hypothesis, we observed that anti-CD40 induced robust production of GM-CSF by ILC3s, particularly by NKp46+ILC3s (Fig. 5 A). We next assessed disease severity and accumulation of Ly6C+ monocytes during anti-CD40–induced colitis in Tcrbd−/− mice treated with an anti–GM-CSF–neutralizing antibody. Tcrbd−/− mice receiving anti–GM-CSF recovered as quickly as ART mice, whereas Tcrbd−/− treated with a control antibody showed persistent weight loss (Fig. 5 B). Recovery was paralleled by a significant reduction of intestinal Ly6C+ monocytes (Fig. 5, C and D).
Figure 5.
Impact of GM-CSF and IL-22 neutralization on anti-CD40–induced colitis. (A) Representative plots show intracellular content of GM-CSF in LTi-like ILC3s (NKp46–) and NKp46+ILC3s isolated from the small intestines of Tcrbd−/− and ART mice, 6 d after treatment with anti-CD40. Cells were surface stained to identify NKp46+ and NKp46− ILC3s; GM-CSF and TNF content were determined after 4-h culture in the presence of monensin. Data represent three independent experiments with two mice/group. (B and C) Tcrbd−/− mice were treated with anti–GM-CSF (MP1-22E9), anti–IL-22 (8E11), or control IgG at days 0, 2, 4, and 6. ART mice received no neutralizing antibodies. Anti-CD40 was injected into all mice at day 0. (B) Mice were weighed daily, from 0 to 7 d p.i. Data represent three independent experiments with five mice/group. (C) Representative plots of intestinal inflammatory monocytes present in the colon of antibody-treated mice. (D) Quantification of inflammatory monocytes as represented in (C). C and D represent three independent experiments with two mice/group. *, P < 0.05; **, P < 0.01, Student’s t test. Bars represent mean ± SEM.
Because all ILC3s produce IL-22, which can promote inflammation by stimulating epithelial cell secretion of chemoattractants for myeloid cells (Ouyang et al., 2008), we asked whether IL-22 has a pathogenic role in this model. Neutralization of IL-22 with a specific antibody reduced the severity of anti-CD40–induced colitis (Fig. 5 B) and the accumulation of Ly6C+ monocytes (Fig. 5 C) to a degree similar though significantly less than anti-GM-CSF (Fig. 5 D). We conclude that ILC3s promote colitis through secretion of GM-CSF and IL-22. GM-CSF directly promotes the accumulation of inflammatory myeloid cells; IL-22 likely acts indirectly, by stimulating epithelial cells to secrete myeloid cell chemoattractants (Ouyang et al., 2008).
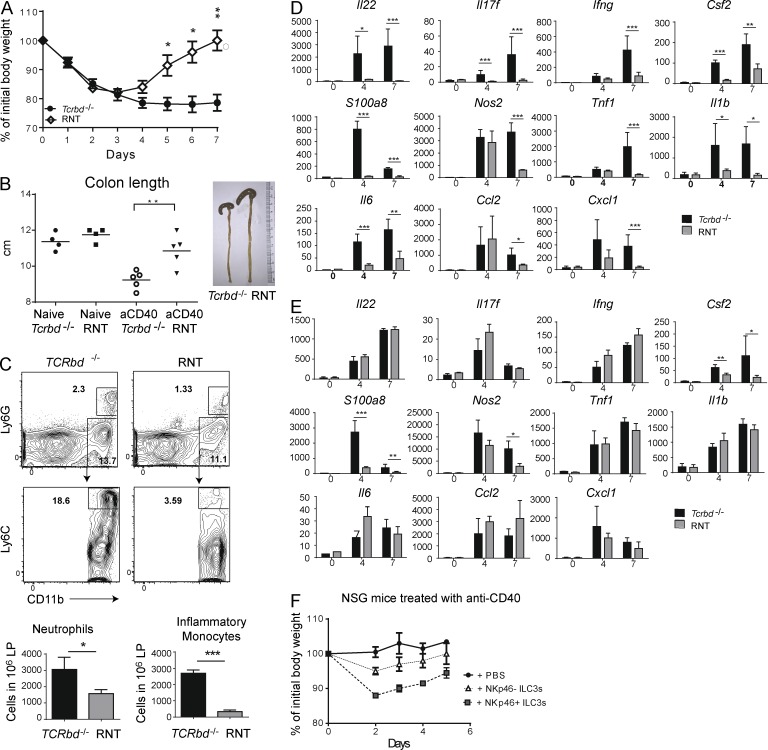
Lack of NKp46+ILC3s is sufficient to protect mice from anti-CD40–induced colitis
To determine whether NKp46+ and LTi-like ILC3s have unique or redundant roles in the pathogenesis of intestinal inflammation induced by anti-CD40, we examined the anti-CD40 model in RNT mice. After anti-CD40 injection, remarkably, RNT mice phenocopied the ART mice: they lost less weight and recovered earlier than Tcrbd−/− mice (Fig. 6 A). Moreover, RNT mice exhibited less colonic shortening than Tcrbd−/− mice (Fig. 6 B). Recruitment of neutrophils and inflammatory monocytes was also reduced (Fig. 6 C). The content of IL-22, S100A8, NOS2, IL-17F, IFN-γ, TNF, IL-1β, IL-6, and GM-CSF in the small intestine of RNT mice was markedly lower than in Tcrbd−/− mice (Fig. 6 D). Consistent with the presence of fewer NKp46+ILC3s in the colon than small intestine and less efficient depletion in this organ (Fig. S1 D), reduction of NKp46+ILC3s in RNT mice had less impact on many inflammatory mediators, which remained elevated (Fig. 6 E). However, GM-CSF was significantly reduced in colon, as were indicators of epithelia cell inflammation, such as S100A8 and NOS2. To test the differential ability of NKp46+ILC3s and LTi-like ILC3s to specifically induce colitis, we isolated both subsets and independently transferred them into NSG mice, as we had previously done with bulk ILC3s. NSG mice reconstituted with NKp46+ILC3s lost more weight than mice reconstituted with NKp46– ILC3s (Fig. 6 F). Thus, lack of NKp46+ILC3s is necessary and sufficient to protect mice from anti-CD40–induced colitis, suggesting a crucial role of NKp46+ILC3s in intestinal inflammation due to inappropriate secretion of GM-CSF and, in part, IL-22 and subsequent activation and recruitment of inflammatory monocytes.
Figure 6.
Lack of NKp46+ ILC3s curbs inflammation during anti-CD40–induced colitis. RNT and Tcrbd−/− mice were injected with anti-CD40. (A) Mice were weighed daily after antibody administration. Data represent five independent experiments with five mice/group. (B) Colons from Tcrbd−/− and RNT mice were isolated 7 d after anti-CD40 injection and total lengths measured. Data represent three independent experiments with four to five mice/group. (C) Cells were purified from the small intestine LP. Ly6G+ neutrophils and Ly6G–Ly6Chi inflammatory monocytes were distinguished within the population of live CD45+CD19–CD11b+ cells. Representative plots (left) and total neutrophil and monocyte numbers among 106 total leukocytes (right) are indicated. Data represent three independent experiments with two mice/group. Small intestines (D) and colons (E) were collected at day 7 after anti-CD40 injection, RNA was extracted and cytokine content was measured by quantitative RT-PCR. Y axis demonstrates relative transcript abundance and x axis shows day p.i. Data in D and E represent three independent experiments with five mice/group (F) NKp46+ ILC3 (CD45+Thy1+CD127+NK1.1–KLRG1–NKp46+) and LTi-like ILC3 (CD45+Thy1+CD127+ NK1.1–KLRG1–NKp46–) subsets from small intestine lamina propria were sort purified from Rag1−/− mice and intravenously injected into NSG mice. After 8 wk, mice were treated with anti-CD40, and body weights were measured daily, from 0 to 7 d p.i. Data represent two independent experiments with five mice/group. *, P < 0.05; **, P < 0.01; ***, P < 0.001, Student’s t test. Bars represent mean ± SEM.
Given the robust and unregulated inflammation in the anti-CD40 model, it possible that the physiological role of ILC3s as a mucosal sentinels via their production of IL-22 and GM-CSF become hijacked and improperly proinflammatory. Indeed, we noticed that ART and NRT mice injected with anti-CD40 have larger and heavier spleens than Tcrbd−/− mice (unpublished data). We also detected bacterial growth in the spleens of ART and RNT mice but not Tcrbd−/− mice (unpublished data), which corresponded to higher serum levels of TNF and IFN-γ (unpublished data). These results suggest that although ILC3s have deleterious local effects during inappropriate intestinal inflammation, they may be important for maintaining intestinal barrier function and preventing bacterial translocation, at least in T cell–deficient mice.
Partial redundancy of ILC3s and T cells in C. rodentium infection
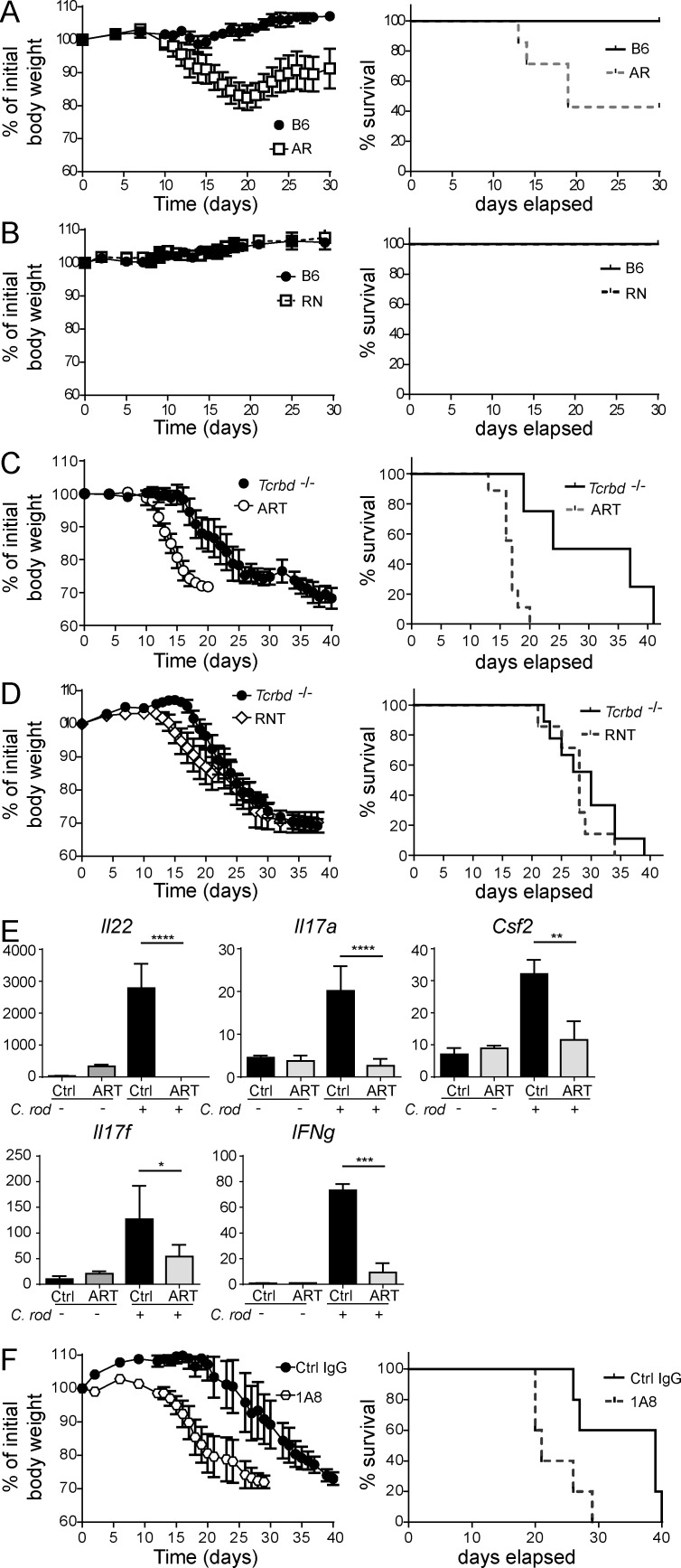
Many studies in mice have demonstrated that ILC3s mediate protection from C. rodentium infection, mainly through the production of IL-22. However, most of these studies have examined the impact of ILC3s in immunodeficient mice, like _Rag_-deficient mice, which lack important adaptive sources of IL-22, such as TH17, TH22, and γδ T cells (Sutton et al., 2009). Moreover, the role of ILC3s has been frequently assessed by anti-Thy1–mediated depletion, a method that depletes all ILCs and NK cells. To assess the specific contributions of ILC3s, particularly NKp46+ILC3s, and T cells during C. rodentium infection, we compared WT, AR, NR, ART, RNT, and Tcrbd−/− mice. As expected, AR infected with C. rodentium lost weight and ∼50% died by day 35 p.i., whereas WT mice were resistant to infection (Fig. 7 A). Because AR mice lack ILC3s but may also have defects in TH17 and TH22 cells, we attributed this susceptibility to concurrent deficits in ILC3s and T cells. However, RN mice were resistant to infection, just like WT control mice, suggesting that NKp46+ILC3s are unnecessary to control C. rodentium in the presence of T cells (Fig. 7 B). To tease apart the contributions of ILC3s and T cells, we further analyzed Tcrbd−/−, ART, and RNT mice. Tcrbd−/− mice were highly susceptible to infection, with marked weight loss and 100% mortality at ∼ day 40 p.i.; in comparison, ART mice had accelerated body weight loss and 100% mortality by day 20 (Fig. 7 C). Thus, both T cells and ILC3s contribute to antibacterial defense. Conversely, RNT mice and Tcrbd−/− controls were equally susceptible to C. rodentium infection (Fig. 7 D); body weight loss and mortality post-infection overlapped in RNT and Tcrbd−/− mice. Thus, NKp46+ILC3s alone are not required to resist C. rodentium challenge even in the absence of T cells, presumably because LTi-like ILC3s provide sufficient protection.
Figure 7.
Contribution of ILC3s to defense against C. rodentium infection. (A–D) C. rodentium (2 × 109) was administrated by oral gavage to 8–12-wk-old B6 (A and B), AR (A), RN (B), Tcrbd−/− (C and D), ART (C), and RNT (D) mice. Body weight and survival were monitored from 0 to 40 d p.i. (A–D) Data represent at least three independent experiments with 5–10 mice/group. (E) RNA was isolated from colons of Tcrbd−/− and ART mice at day 7 after injection; Il22, Il17a, Il17f, Ifng, and Csf2 were measured by RT-PCR. All gene expression values were normalized to Gapdh. Data represent three independent experiments with five mice/group. (F) Neutrophil depletion in Tcrbd−/− mice accelerates lethality to C. rodentium infection. Mice were infected and neutrophil depletion performed every 3 d by administration of 100 µg of anti-Ly6G (1A8) antibody. (E and F) Data are representative of three independent experiments with five mice/group. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, Student’s t test.
We more broadly investigated the mechanisms by which ILC3s contribute to C. rodentium susceptibility in ART mice. ART mice had markedly less IL-22 in the colon at day 7 p.i. than did Tcrbd−/− mice. Levels of IL-17A/F, IFN-γ, and GM-CSF were also reduced in the colons of ART mice (Fig. 7 E). Furthermore, significantly fewer CD11b+Ly6G+ neutrophils accumulated in the colons of ART mice than in the colons of Tcrbd−/− mice after C. rodentium infection (unpublished data). Because neutrophils are known to be important mediators of C. rodentium clearance (Wang et al., 2010; Kamada et al., 2015), we focused on the potential impact of limited neutrophil accumulation in ART mice on the outcome of infection. Depletion of neutrophils in Tcrbd−/− mice using anti-Ly6G (1A8) during C. rodentium infection caused increased disease severity, recapitulating the phenotype seen in ART mice (Fig. 7 F). Thus, ILC3s contribute to protection against C. rodentium infection in Tcrbd−/− by promoting neutrophil accumulation. We conclude that functionally competent ILC3s are essential to resist C. rodentium when T cell responses are also compromised. When T cells are present, ILC3s have limited impact and NKp46+ILC3s are dispensable.
DISCUSSION
NKp46+ and LTi-like ILC3s are two distinct subsets of ILC3s that have been shown to diverge in their development. Although both subsets depend on RORγt and AHR, NKp46+ILC3s also require Notch and T-bet, whereas LTi-like ILC3s do not. Because NKp46+ILC3s express both RORγt and T-bet, they have functional plasticity such that, under certain conditions, they down-regulate RORγt, up-regulate T-bet, and produce IFN-γ (Klose et al., 2013; Rankin et al., 2013b). Despite the knowledge that ILC3 subsets developmentally and transcriptionally diverge, it remains unclear the extent to which they are functionally distinct. Our study advances the functional characterization of NKp46+ILC3s by demonstrating their capacity to secrete GM-CSF and promote the accumulation and activation of inflammatory monocytes. This functional property of NKp46+ILC3s is evident in the anti-CD40 model of colitis, as demonstrated by the marked intracellular content of GM-CSF in NKp46+ILC3s, as well as the reduction of disease severity after deletion of NKp46+ILC3s in RNT mice or neutralization of GM-CSF in Tcrbd−/− mice. Thus, NKp46+ILC3s are necessary and sufficient to induce intestinal pathology when inappropriately activated. A previous study showed that IL-1β stimulates ILC3s to produce GM-CSF (Mortha et al., 2014). However, incubation of NKp46+ILC3s with IL-1β was not sufficient to elicit GM-CSF in vitro. Similarly, human CD56+NKp44+ILC3s did not secrete GM-CSF in response to IL-1β (unpublished data). Given these data, we postulate that induction of GM-CSF secretion requires multiple cytokines, including those induced by anti-CD40 stimulation of myeloid cells, rather than a single cytokine. Engagement of one or more cell surface receptors on NKp46+ILC3s, including NKp46, may also contribute to GM-CSF secretion.
GM-CSF and inflammatory monocytes have been identified as the culprits mediating tissue damage in a model of experimental autoimmune encephalomyelitis (Becher and Segal, 2011). Moreover, GM-CSF was shown to promote disease in a T cell transfer model of colitis by inducing excessive accumulation of granulocyte-monocyte progenitors (GMP) and consequent extramedullary production of neutrophils and inflammatory monocytes (Griseri et al., 2012). Inflammatory monocytes, in turn, promote tissue damage and dysbiosis (Zigmond et al., 2012; Seo et al., 2015). Our study shows, for the first time, that the pathogenesis of anti-CD40–induced innate immune colitis is also GM-CSF dependent. GM-CSF acts primarily by recruiting and activating inflammatory monocytes. We used CCR2 depletion and GM-CSF neutralization experiments to this end. A previous study proposed that ILC3s cause intestinal damage in the anti-CD40 model of colitis by recruiting neutrophils (Eken et al., 2014). We also observed that ILC3s induce neutrophil accumulation by stimulating the release of neutrophil chemoattractants by monocytes and epithelial cells. However, neutrophil depletion had no impact on colitis, which suggests that inflammatory monocytes dominate the pathogenic process. Because inflammatory monocytes provide an important source of IL-23 and IL-1β that stimulate ILC3s (Cella et al., 2009; Longman et al., 2014), recruitment of inflammatory monocytes likely generates a positive feedback loop that amplifies inflammation. Notably, this inflammation may reflect the physiological function of ILC3 to maintain epithelial homeostasis and support wound healing, which is hijacked in immunocompromised mice by a strong activating inflammatory stimulus such as CD40 cross-linking.
Our study genetically defines NKp46+ILC3s as a colitogenic cell subset in the anti-CD40–induced model. A previous study by Buonocore et al. (2010) showed that colitis is induced by a colonic NKp46− Thy1+ Sca1high subset that is probably unrelated to the ILC subsets that we examined, as in our experiments colitogenic cells are primarily NKp46+ RORγt+ ILC3s. In another study, Vonarbourg et al. (2010) indicated that NKp46+ RORγt− cells can induce colitis after adoptive transfer. These cells include RORγt+ cells that have converted into RORγt− cells (ex-RORγt cells), but may also include ILC1s and perhaps NK cells. By deleting NKp46+ Rorγt+ cells in RNT mice, we also eliminate ex-RORγt NKp46+ cells (which represent ∼30–50% of NKp46+NK1.1+ cells in our colony; unpublished data) but not NK cells or ILC1s. Thus, our study highlights that a spectrum of ILC subsets contribute to anti-CD40 colitis, perhaps through conversion, as well as independent functions. Moreover, both of the aforementioned studies indicate that colitis is induced by IFN-γ–producing cells (Buonocore et al., 2010; Vonarbourg et al., 2010), which may be an additional mechanism beside the pathogenic role of ILC3-derived GM-CSF that we identified here.
Remarkably, neutralization of IL-22 also eased disease severity in this colitis model. Thus, although IL-22 is generally thought to have a protective function on the intestinal mucosa, our results indicate that in some circumstances IL-22 promotes inflammation. IL-22 was recently shown to induce transcription of IL-18 in epithelial cells (Muñoz et al., 2015). Our own data suggest that IL-22 may also induce transcription of IL-33 (unpublished data). Moreover, IL-22 stimulates epithelial cells to produce chemokines that attract myeloid cells (Rutz et al., 2014). Finally, IL-22 activates STAT3, a transcription factor induced by many inflammatory cytokines that has been implicated in chronic inflammation and cancer (Ghoreschi et al., 2011). Thus, IL-22 may cause inflammation, perhaps when it is secreted in excess and/or acts in concert with other stimuli that activate IL-18, IL-33, and myeloid cells. The pathogenic function of IL-22 in the anti-CD40 model is also consistent with results from a different model of colitis induced by adoptive transfer of T reg cell-depleted CD45RBlo memory T cells into Rag-deficient mice (Kamanaka et al., 2011). Elucidating how this dual functionality of IL-22 is achieved may lead to a better understanding of inflammatory bowel diseases.
In contrast to their pathogenic role in anti-CD40 colitis, neither NKp46+ILC3s nor ILC3s in general had an impact on the course of DSS-induced colitis (unpublished data), whether it was induced in immunocompetent or T cell–depleted mice. DSS treatment damages the intestinal epithelium, thereby exposing immune cells of the lamina propria to the intestinal microbiota and its products. It is possible that DSS-induced colitis is relatively independent of GM-CSF–mediated activation of inflammatory monocytes and that other pathways, such as activation of local macrophages or other cells of the innate immune system by intestinal microbiota, are predominant in this model.
Given that many studies have focused on the role of ILCs in immunocompromised mice, particularly _Rag_-deficient mice, it remains largely unclear the extent to which many ILCs have unique functions apart from T cells and B cells. Our study parses the role of ILC3s and T cells, both of which provide important sources of IL-22 for antibacterial defense, in host defense against C. rodentium infection in the presence of B cells. We found that ILC3s have a marked impact on host defense when T cells are absent, consistent with previous studies (Sonnenberg et al., 2011; Basu et al., 2012). However, whereas ART mice were more susceptible to infection than Tcrbd−/− mice, RNT mice and Tcrbd−/− mice were equally susceptible, suggesting that LTi-like ILC3s are sufficient to control C. rodentium infection, whereas the NKp46+ILC3s are redundant. Perhaps LTi-like ILC3s are sufficient because they are strategically located close to sites at which C. rodentium bacteria adhere and cause attaching and effacing lesions, particularly epithelial cells located deep within intestinal crypts. Consistent with a previous study (Basu et al., 2012), the impact of ILC3s in host defense was limited in the presence of T cells, as T cells provide an important source of IL-22. Lack of all ILC3s partially increased susceptibility to infection, whereas lack of NKp46+ILC3s did not. Thus, NKp46+ILC3s are redundant in the control of C. rodentium infection. Collectively, our results suggest that it will be important in the future to assess the role of ILCs in mice with intact T and B cell responses.
In conclusion, we have identified nonredundant and redundant functions of intestinal NKp46+ILC3s. In colitis induced by excessive activation of innate immunity, NKp46+ILC3s have a prominent role in secreting GM-CSF and IL-22, both of which directly and indirectly induce the recruitment of inflammatory monocytes, thus igniting intestinal inflammation. However, LTi-like ILC3s supersede during infection with C. rodentium, rendering NKp46+ILC3s completely redundant, in the presence or absence of T cells. Recent studies have shown that LTi-like ILC3s expressing MHC class II in the mesenteric lymph nodes promote T cell tolerance (Hepworth et al., 2013, 2015). Thus, it is important to define the circumstances under which ILC3s promote inflammation through GM-CSF and IL-22 secretion rather than T cell tolerance and determine whether these conditions occur in human inflammatory bowel diseases.
MATERIALS AND METHODS
Mice.
Ahrf/f (Walisser et al., 2005), Rorc(γt)-cre (Eberl and Littman, 2004), Rorc(γt)f/f (Eberl and Littman, 2004), NKp46-cre (Narni-Mancinelli et al., 2011), and Rosa26-stop-YFP reporter (Rosa26stop-YFP/+; Srinivas et al., 2001) mice were kept in specific pathogen–free conditions at the animal facility of Washington University. All animal studies were approved by the Washington University Animal Studies Committee.
Anti-CD40–induced colitis.
6–10-week-old age- and gender-matched mice were injected i.p. with 100 µg of anti-CD40 antibody. Body weight was measured daily, and mice were dissected at day 4 or day 7 after injection. Colons were removed, cleaned, and processed, as previously described (Lee et al., 2012).
C. rodentium infection.
8–12-week-old age and gender-matched mice were orally administrated 2 × 109 C. rodentium, strain DBS100 (ATCC), as previously described (Cella et al., 2009). Body weight and mortality were measured until day 40. In some cases, mice were sacrificed at day 7 and colons were removed for RNA analysis and flow cytometry.
Flow cytometry.
Cells were isolated from the colon and small intestine lamina propria by Percoll-gradient method and surface stained with the indicated antibodies. Conjugated antibodies for staining were purchased from eBioscience, BD, and BioLegend. For intracellular cytokine staining, cells were cultured with complete RPMI medium containing monensin for 4–6 h. Cells were then fixed and stained according to manufacturer’s instructions, using an intracellular cytokine staining kit (eBioscience).
Cytokine measurements.
Purified colon lamina propria cells were counted, and 106 cells were cultured in complete RPMI for 4–6 h or overnight. Cytokines were measured by bead array with the Mouse Inflammation or Mouse Th1/Th2/Th17 CBA kit (BD).
Histology.
Colons were washed with PBS, and fixed in 10% phosphate-buffered formalin overnight and then transferred to 70% ethanol. Fixed tissues were then paraffin-embedded, sectioned, and stained with hematoxylin and eosin (H&E). Severity of inflammation was scored blinded, as previously described. Two intestinal pieces from the same mouse were scored and the cumulative score is plotted (Satpathy et al., 2013).
Depletions.
Tcrbd−/− mice were injected i.p. with either 20 µg of anti-CCR2 (MC-21) daily, 200 µg of anti-Ly6G (1A8) every other day, 100 µg of anti-GR1 (RB6-8C5) every other day, or control IgG. Anti-CD40 was injected at day 0. Anti–GM-CSF (MPI-22E9) and anti–IL-22 (8E11) used for neutralization experiments were obtained from Bioxcell and Genentech, respectively.
Supplementary Material
Supplemental Material
Acknowledgments
The authors thank Jennifer Bando and Victor Cortez for valuable discussion and editing of the manuscript and Eric Vivier for the generous gift of NKp46-Cre mice.
This work was supported by grants from the National Institutes of Health (1U01AI095542, R01DE021255, and R21CA16719) to M. Colonna and 1RO1CA176695 to M. Cella.
J.S. Lee is employed by Merck. The authors declare no additional competing financial interests.
Author contributions: C. Song and J.S. Lee performed experiments. R.D. Newberry and T.S. Stappenbeck performed histological and imaging analyses; S.G. generated and maintained mouse colonies. M.L. Robinette analyzed data. M. Mack provided critical reagents. M. Colonna and M. Cella conceived and supervised the project and wrote the manuscript with assistance from C. Song and M.L. Robinette. All authors provided critical comments.
Footnotes
Abbreviations used:
AHR
aryl hydrocarbon receptor
GM-CSF
granulocyte macrophage-colony stimulating factor
GMP
granulocyte-monocyte progenitor
ILC
innate lymphoid cell
LTi
lymphoid tissue-inducer
TSLP
thymic stromal lymphopoietin
References
- Artis D., and Spits H.. 2015. The biology of innate lymphoid cells. Nature. 517:293–301. 10.1038/nature14189 [DOI] [PubMed] [Google Scholar]
- Basu R., O’Quinn D.B., Silberger D.J., Schoeb T.R., Fouser L., Ouyang W., Hatton R.D., and Weaver C.T.. 2012. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity. 37:1061–1075. 10.1016/j.immuni.2012.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B., and Segal B.M.. 2011. T(H)17 cytokines in autoimmune neuro-inflammation. Curr. Opin. Immunol. 23:707–712. 10.1016/j.coi.2011.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonocore S., Ahern P.P., Uhlig H.H., Ivanov I.I., Littman D.R., Maloy K.J., and Powrie F.. 2010. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 464:1371–1375. 10.1038/nature08949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Fuchs A., Vermi W., Facchetti F., Otero K., Lennerz J.K., Doherty J.M., Mills J.C., and Colonna M.. 2009. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 457:722–725. 10.1038/nature07537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides M.G., McDonald B.D., Verhoef P.A., and Bendelac A.. 2014. A committed precursor to innate lymphoid cells. Nature. 508:397–401. 10.1038/nature13047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley J.M., Thomay A.A., Connolly M.D., Reichner J.S., and Albina J.E.. 2008. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 83:64–70. 10.1189/jlb.0407247 [DOI] [PubMed] [Google Scholar]
- Diefenbach A., Colonna M., and Koyasu S.. 2014. Development, differentiation, and diversity of innate lymphoid cells. Immunity. 41:354–365. 10.1016/j.immuni.2014.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte J.H., Di Meglio P., Hirota K., Ahlfors H., and Stockinger B.. 2013. Differential influences of the aryl hydrocarbon receptor on Th17 mediated responses in vitro and in vivo. PLoS ONE. 8:e79819 10.1371/journal.pone.0079819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudakov J.A., Hanash A.M., and van den Brink M.R.. 2015. Interleukin-22: immunobiology and pathology. Annu. Rev. Immunol. 33:747–785. 10.1146/annurev-immunol-032414-112123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl G., and Littman D.R.. 2004. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 305:248–251. 10.1126/science.1096472 [DOI] [PubMed] [Google Scholar]
- Eberl G., Colonna M., Di Santo J.P., and McKenzie A.N.. 2015. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 348:aaa6566 10.1126/science.aaa6566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eken A., Singh A.K., Treuting P.M., and Oukka M.. 2014. IL-23R+ innate lymphoid cells induce colitis via interleukin-22-dependent mechanism. Mucosal Immunol. 7:143–154. 10.1038/mi.2013.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreschi K., Laurence A., Yang X.P., Hirahara K., and O’Shea J.J.. 2011. T helper 17 cell heterogeneity and pathogenicity in autoimmune disease. Trends Immunol. 32:395–401. 10.1016/j.it.2011.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griseri T., McKenzie B.S., Schiering C., and Powrie F.. 2012. Dysregulated hematopoietic stem and progenitor cell activity promotes interleukin-23-driven chronic intestinal inflammation. Immunity. 37:1116–1129. 10.1016/j.immuni.2012.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Qiu J., Tu T., Yang X., Deng L., Anders R.A., Zhou L., and Fu Y.X.. 2014. Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity. 40:25–39. 10.1016/j.immuni.2013.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth M.R., Monticelli L.A., Fung T.C., Ziegler C.G., Grunberg S., Sinha R., Mantegazza A.R., Ma H.L., Crawford A., Angelosanto J.M., et al. 2013. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 498:113–117. 10.1038/nature12240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth M.R., Fung T.C., Masur S.H., Kelsen J.R., McConnell F.M., Dubrot J., Withers D.R., Hugues S., Farrar M.A., Reith W., et al. 2015. Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4+ T cells. Science. 348:1031–1035. 10.1126/science.aaa4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada N., Sakamoto K., Seo S.U., Zeng M.Y., Kim Y.G., Cascalho M., Vallance B.A., Puente J.L., and Núñez G.. 2015. Humoral immunity in the gut selectively targets phenotypically virulent attaching-and-effacing bacteria for intraluminal elimination. Cell Host Microbe. 17:617–627. 10.1016/j.chom.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamanaka M., Huber S., Zenewicz L.A., Gagliani N., Rathinam C., O’Connor W. Jr., Wan Y.Y., Nakae S., Iwakura Y., Hao L., and Flavell R.A.. 2011. Memory/effector (CD45RB(lo)) CD4 T cells are controlled directly by IL-10 and cause IL-22-dependent intestinal pathology. J. Exp. Med. 208:1027–1040. 10.1084/jem.20102149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss E.A., Vonarbourg C., Kopfmann S., Hobeika E., Finke D., Esser C., and Diefenbach A.. 2011. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 334:1561–1565. 10.1126/science.1214914 [DOI] [PubMed] [Google Scholar]
- Klose C.S., Kiss E.A., Schwierzeck V., Ebert K., Hoyler T., d’Hargues Y., Göppert N., Croxford A.L., Waisman A., Tanriver Y., and Diefenbach A.. 2013. A T-bet gradient controls the fate and function of CCR6-RORγt+ innate lymphoid cells. Nature. 494:261–265. 10.1038/nature11813 [DOI] [PubMed] [Google Scholar]
- Klose C.S., Flach M., Möhle L., Rogell L., Hoyler T., Ebert K., Fabiunke C., Pfeifer D., Sexl V., Fonseca-Pereira D., et al. 2014. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 157:340–356. 10.1016/j.cell.2014.03.030 [DOI] [PubMed] [Google Scholar]
- Kruglov A.A., Grivennikov S.I., Kuprash D.V., Winsauer C., Prepens S., Seleznik G.M., Eberl G., Littman D.R., Heikenwalder M., Tumanov A.V., and Nedospasov S.A.. 2013. Nonredundant function of soluble LTα3 produced by innate lymphoid cells in intestinal homeostasis. Science. 342:1243–1246. 10.1126/science.1243364 [DOI] [PubMed] [Google Scholar]
- Lee J.S., Cella M., McDonald K.G., Garlanda C., Kennedy G.D., Nukaya M., Mantovani A., Kopan R., Bradfield C.A., Newberry R.D., and Colonna M.. 2012. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat. Immunol. 13:144–151. 10.1038/ni.2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longman R.S., Diehl G.E., Victorio D.A., Huh J.R., Galan C., Miraldi E.R., Swaminath A., Bonneau R., Scherl E.J., and Littman D.R.. 2014. CX3CR1+ mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J. Exp. Med. 211:1571–1583. 10.1084/jem.20140678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri G., Miyajima M., Bascones S., Mortha A., Puga I., Cassis L., Barra C.M., Comerma L., Chudnovskiy A., Gentile M., et al. 2014. Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells. Nat. Immunol. 15:354–364. 10.1038/ni.2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B., Hirota K., Cua D.J., Stockinger B., and Veldhoen M.. 2009. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 31:321–330. 10.1016/j.immuni.2009.06.020 [DOI] [PubMed] [Google Scholar]
- Mortha A., Chudnovskiy A., Hashimoto D., Bogunovic M., Spencer S.P., Belkaid Y., and Merad M.. 2014. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 343:1249288 10.1126/science.1249288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz M., Eidenschenk C., Ota N., Wong K., Lohmann U., Kühl A.A., Wang X., Manzanillo P., Li Y., Rutz S., et al. 2015. Interleukin-22 induces interleukin-18 expression from epithelial cells during intestinal infection. Immunity. 42:321–331. 10.1016/j.immuni.2015.01.011 [DOI] [PubMed] [Google Scholar]
- Narni-Mancinelli E., Chaix J., Fenis A., Kerdiles Y.M., Yessaad N., Reynders A., Gregoire C., Luche H., Ugolini S., Tomasello E., et al. 2011. Fate mapping analysis of lymphoid cells expressing the NKp46 cell surface receptor. Proc. Natl. Acad. Sci. USA. 108:18324–18329. 10.1073/pnas.1112064108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W., Kolls J.K., and Zheng Y.. 2008. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 28:454–467. 10.1016/j.immuni.2008.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard J.M., Maurice C.F., Kinnebrew M.A., Abt M.C., Schenten D., Golovkina T.V., Bogatyrev S.R., Ismagilov R.F., Pamer E.G., Turnbaugh P.J., and Chervonsky A.V.. 2014. Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature. 514:638–641. 10.1038/nature13823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J., Heller J.J., Guo X., Chen Z.M., Fish K., Fu Y.X., and Zhou L.. 2012. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 36:92–104. 10.1016/j.immuni.2011.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J., Guo X., Chen Z.M., He L., Sonnenberg G.F., Artis D., Fu Y.X., and Zhou L.. 2013. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity. 39:386–399. 10.1016/j.immuni.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin L., Groom J., Mielke L.A., Seillet C., and Belz G.T.. 2013a. Diversity, function, and transcriptional regulation of gut innate lymphocytes. Front. Immunol. 4:22 10.3389/fimmu.2013.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin L.C., Groom J.R., Chopin M., Herold M.J., Walker J.A., Mielke L.A., McKenzie A.N., Carotta S., Nutt S.L., and Belz G.T.. 2013b. The transcription factor T-bet is essential for the development of NKp46+ innate lymphocytes via the Notch pathway. Nat. Immunol. 14:389–395. 10.1038/ni.2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynders A., Yessaad N., Vu Manh T.P., Dalod M., Fenis A., Aubry C., Nikitas G., Escalière B., Renauld J.C., Dussurget O., et al. 2011. Identity, regulation and in vivo function of gut NKp46+RORγt+ and NKp46+RORγt- lymphoid cells. EMBO J. 30:2934–2947. 10.1038/emboj.2011.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinette M.L., Fuchs A., Cortez V.S., Lee J.S., Wang Y., Durum S.K., Gilfillan S., and Colonna M.. Immunological Genome Consortium. 2015. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat. Immunol. 16:306–317. 10.1038/ni.3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H.W., Tripp C.S., Schreiber R.D., and Unanue E.R.. 1994. Endogenous IL-1 is required for neutrophil recruitment and macrophage activation during murine listeriosis. J. Immunol. 153:2093–2101. [PubMed] [Google Scholar]
- Rutz S., Wang X., and Ouyang W.. 2014. The IL-20 subfamily of cytokines—from host defence to tissue homeostasis. Nat. Rev. Immunol. 14:783–795. 10.1038/nri3766 [DOI] [PubMed] [Google Scholar]
- Satoh-Takayama N., Serafini N., Verrier T., Rekiki A., Renauld J.C., Frankel G., and Di Santo J.P.. 2014. The chemokine receptor CXCR6 controls the functional topography of interleukin-22 producing intestinal innate lymphoid cells. Immunity. 41:776–788. 10.1016/j.immuni.2014.10.007 [DOI] [PubMed] [Google Scholar]
- Satpathy A.T., Briseño C.G., Lee J.S., Ng D., Manieri N.A., Kc W., Wu X., Thomas S.R., Lee W.L., Turkoz M., et al. 2013. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nat. Immunol. 14:937–948. 10.1038/ni.2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciumé G., Hirahara K., Takahashi H., Laurence A., Villarino A.V., Singleton K.L., Spencer S.P., Wilhelm C., Poholek A.C., Vahedi G., et al. 2012. Distinct requirements for T-bet in gut innate lymphoid cells. J. Exp. Med. 209:2331–2338. 10.1084/jem.20122097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S.U., Kamada N., Muñoz-Planillo R., Kim Y.G., Kim D., Koizumi Y., Hasegawa M., Himpsl S.D., Browne H.P., Lawley T.D., et al. 2015. Distinct commensals induce interleukin-1β via NLRP3 inflammasome in inflammatory monocytes to promote intestinal inflammation in response to injury. Immunity. 42:744–755. 10.1016/j.immuni.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., and Pamer E.G.. 2011. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 11:762–774. 10.1038/nri3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg G.F., Monticelli L.A., Elloso M.M., Fouser L.A., and Artis D.. 2011. CD4+ lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 34:122–134. 10.1016/j.immuni.2010.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg G.F., Monticelli L.A., Alenghat T., Fung T.C., Hutnick N.A., Kunisawa J., Shibata N., Grunberg S., Sinha R., Zahm A.M., et al. 2012. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 336:1321–1325. 10.1126/science.1222551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C.S., William C.M., Tanabe Y., Jessell T.M., and Costantini F.. 2001. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1:4 10.1186/1471-213X-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton C.E., Lalor S.J., Sweeney C.M., Brereton C.F., Lavelle E.C., and Mills K.H.. 2009. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 31:331–341. 10.1016/j.immuni.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Tumanov A.V., Koroleva E.P., Guo X., Wang Y., Kruglov A., Nedospasov S., and Fu Y.X.. 2011. Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge. Cell Host Microbe. 10:44–53. 10.1016/j.chom.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlig H.H., McKenzie B.S., Hue S., Thompson C., Joyce-Shaikh B., Stepankova R., Robinson N., Buonocore S., Tlaskalova-Hogenova H., Cua D.J., and Powrie F.. 2006. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 25:309–318. 10.1016/j.immuni.2006.05.017 [DOI] [PubMed] [Google Scholar]
- Vonarbourg C., Mortha A., Bui V.L., Hernandez P.P., Kiss E.A., Hoyler T., Flach M., Bengsch B., Thimme R., Hölscher C., et al. 2010. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt(+) innate lymphocytes. Immunity. 33:736–751. 10.1016/j.immuni.2010.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walisser J.A., Glover E., Pande K., Liss A.L., and Bradfield C.A.. 2005. Aryl hydrocarbon receptor-dependent liver development and hepatotoxicity are mediated by different cell types. Proc. Natl. Acad. Sci. USA. 102:17858–17863. 10.1073/pnas.0504757102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Koroleva E.P., Kruglov A.A., Kuprash D.V., Nedospasov S.A., Fu Y.X., and Tumanov A.V.. 2010. Lymphotoxin beta receptor signaling in intestinal epithelial cells orchestrates innate immune responses against mucosal bacterial infection. Immunity. 32:403–413. 10.1016/j.immuni.2010.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Valdez P.A., Danilenko D.M., Hu Y., Sa S.M., Gong Q., Abbas A.R., Modrusan Z., Ghilardi N., de Sauvage F.J., and Ouyang W.. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14:282–289. 10.1038/nm1720 [DOI] [PubMed] [Google Scholar]
- Zigmond E., Varol C., Farache J., Elmaliah E., Satpathy A.T., Friedlander G., Mack M., Shpigel N., Boneca I.G., Murphy K.M., et al. 2012. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 37:1076–1090. 10.1016/j.immuni.2012.08.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material