Role of regulatory b cells in neuroimmunologic disorders (original) (raw)
Abstract
B lymphocytes augment the immune response by producing antibodies and activating T cells by antigen presentation. Recent studies have highlighted a specific and functionally significant B‐cell subset that could downregulate excessive immune and inflammatory responses through a vast array of inhibitory cytokines, such as interleukin (IL)‐10 and transforming growth factor‐β (TGF‐β). This subset of B cells is generally referred to as regulatory B cells (Bregs). In addition, recent studies have shown that IL‐35‐producing Bregs also play a role in downregulation of immunity. Diverse phenotypes of Bregs have been proposed to underlie human disorders and their animal models. Most studies have focused on the role of different subsets of Bregs and Bregs‐associated molecules such as IL‐10, TGF‐β, and IL‐35 in the pathogenesis of neuroimmunologic disorders. Furthermore, Bregs exert regulatory function mainly through suppressing the differentiation of Th1/Th17 cells and promoting regulatory T‐cell expansion. Reduced presence of Bregs is reportedly associated with progression of several neuroimmunologic disorders. This Review summarizes the current knowledge on the role of Bregs in neuroimmunologic disorders, including multiple sclerosis, neuromyelitis optica, and myasthenia gravis. © 2016 The Authors. Journal of Neuroscience Research Published by Wiley Periodicals, Inc.
Keywords: regulatory B cells, IL‐10, TGF‐β, IL‐35, neuroimmunologic disorders
B cells, as precursors of plasmablasts and plasma cells, are thought to upregulate humoral immune response by producing antibodies and activating T cells by antigen presentation. Recent work has revealed a specific protective role of B cells in modulating the immune response under pathogenic conditions (Tedder, 2015). These specific B‐cell subsets can downmodulate the immune response through production of anti‐inflammatory cytokines such as interleukin (IL)‐10 and transforming growth factor‐β (TGF‐β; Mizoguchi and Bhan, 2006; DiLillo et al., 2010; Kalampokis et al., 2013; Yang et al., 2013; Candando et al., 2014; Goode et al., 2014). These subsets of B cells are generally referred to as regulatory B cells (Bregs). Recent studies also suggest that Bregs are related to the pathogenesis in several immune‐related disorders (Blair et al., 2010; Noh et al., 2010; Olkhanud et al., 2011; Furuzawa‐Carballeda et al., 2013, 2014; Wilde et al., 2013; Daien et al., 2014; He et al., 2014; Hua et al., 2014; L. Wang et al., 2014; Aybar et al., 2015; de Masson et al., 2015; Zhu et al., 2015). Bregs are known mainly for suppressing the pathogenic Th1/Th17 cells and promoting regulatory T‐cell (Treg) expansion, thereby allowing Bregs to exert their regulatory function. The lack or loss of Bregs has been shown to be associated with progression of several neuroimmunologic diseases, such as multiple sclerosis (MS; Knippenberg et al., 2011; de Andres et al., 2014), neuromyelitis optica (NMO; Quan et al., 2013), and myasthenia gravis (MG; Sun et al., 2014). More importantly, recent articles have described a new IL‐35‐producing B‐cell (i35‐Breg) subset that appears to downregulate the immune response through production of IL‐35 (Shen et al., 2014; R.X. Wang et al., 2014; Egwuagu and Yu, 2015). Bregs comprise several immunophenotypically distinct B‐cell lineages identifiable by the production of the immunomodulatory cytokines IL‐10, TGF‐β, and IL‐35. However, the precise phenotypic characterization and signaling molecules of Bregs remain unclear. Additional insights into the role and characteristics of Bregs may well provide new therapeutic targets in patients with neuroimmunologic disorders. This Review provides a summary of the current state of knowledge on the role of Bregs in neuroimmunologic disorders.
PHENOTYPIC CHARACTERIZATION OF Bregs
Phenotypic Characterization of Mice Bregs
Because no precise phenotypic characteristics or signaling molecules of Bregs exist, the best strategy for identifying Bregs would be by intracellular staining for IL‐10. However, this process involves fixing and permeabilizing cells, which may affect the functional characterization of Bregs. So exact cell surface phenotypes and markers are key to the identification of Bregs. Some of the cell surface phenotypes that are reportedly specific to Bregs in mice, related to their capacity to produce IL‐10, are summarized in Table 1.
Table 1.
Phenotypic Characterization of Regulatory B Cells in Mice and Humans
| Species | Phenotypes/markers | Relevance to disorders/modelsa | References |
|---|---|---|---|
| Mice | CD19+CD5+CD1dhi | CHS, EAE | Matsushita et al., 2008; Yanaba et al., 2008 |
| CD1dhiCD21hiCD23−CD24hiIgMhiIgDlo | IBD | Wei et al., 2005 | |
| TIM‐1+B | EAE | Ding et al., 2011 | |
| CD1dhiCD21hiCD23+CD24hilgMhiIgD+ | Experimental arthritis | Evans et al., 2007 | |
| CD19+CD25+B220+ | Breast cancer | Olkhanud et al., 2011 | |
| Human | CD19+CD5+CD1dhi | NMO, CHB, CHC | Wang et al., 2014a; Yang et al., 2015 |
| CD19+CD5+Foxp3+ | Normal subjects | Noh et al., 2010 | |
| CD19+CD24hiCD27+ | cGVHD, RA, SS, SLE | Iwata et al., 2011; Daien et al., 2014; de Masson et al., 2015 | |
| CD19+CD25+ | MS | de Andres et al., 2014 | |
| CD19+CD38+CD1d+IgM+CD147+ | Solid tumors | Lindner et al., 2013 | |
| CD5+CD24hiCD38hi | AAV | Aybar et al., 2015 | |
| CD19+CD24hiCD38hi | MS, NMO, SLE, RA, SS, pemphigus, ITP, AAV | Blair et al., 2010; Furuzawa‐Carballeda et al., 2013; Wilde et al., 2013; Daien et al., 2014; Hua et al., 2014; Zhu et al., 2015 |
IL‐10‐producing spleen B cells were restricted to a unique CD1dhiCD5+ Breg subset that was absent in Cd19–/– mice. This relatively rare CD19+CD5+CD1dhi Breg subset has been named B10 cells because it is attributable to IL‐10 production (Yanaba et al., 2008). In earlier studies, splenic marginal zone (MZ) B cells (CD1dhiCD21hiCD23−CD24hiIgMhiIgDlo) were shown to produce IL‐10 and inhibit the development of inflammatory bowel disease in animal models (Wei et al., 2005; Mauri and Bosma, 2012). Moreover, transitional 2‐MZ precursor (T2‐MZP) B cells (CD1dhiCD21hiCD23+CD24hilgMhiIgD+) are known to inhibit the progression of arthritis. The negative regulation of T2‐MZP cells apparently depends on IL‐10 secretion, given that T2‐MZP cells from IL‐10–/– mice failed to protect against the development of arthritis (Evans et al., 2007; Mauri and Bosma, 2012). T‐cell immunoglobulin domain and mucin domain‐1 (TIM‐1) have been shown to identify more than 70% of spleen IL‐10‐producing B cells. TIM‐1 was expressed by a large number of IL‐10‐producing regulatory B cells in all major B‐cell subsets (Ding et al., 2011). TIM‐1‐deficient B cells have been shown to enhance Th1/Th17 responses and to aggravate experimental autoimmune encephalomyelitis (EAE; Xiao et al., 2015). Bregs are thought to exert their suppressive effect through the production of inhibitory cytokines. Much progress has been made in the identification of Bregs through studies in mice.
Phenotypic Characterization of Human Bregs
The current state of knowledge on the phenotypes and markers of human Bregs (Blair et al., 2010; Iwata et al., 2011; Furuzawa‐Carballeda et al., 2013; Daien et al., 2014; L. Wang et al., 2014) is presented in Table 1. Human Bregs have been shown to share some of the phenotypic characteristics with previously defined markers in mice (Correale et al., 2008; Yanaba et al., 2008). Human IL‐10‐expressing Bregs were shown to be normally present in low but readily identifiable numbers in the peripheral blood and spleen, a finding similar to that observed in mice (Iwata et al., 2011; Kalampokis et al., 2013). After ex vivo stimulation with phorbol myristate acetate and ionomycin plus brefeldin‐A, human Bregs were shown to block protein production (Candando et al., 2014). This method provides an indirect assessment of the active Bregs associated with human autoimmune disorders because Bregs could not be observed directly ex vivo (Candando et al., 2014).
Bregs from peripheral blood were shown to produce more IL‐10 after stimulation by CD40 compared with that after stimulation by both B‐cell receptor (BCR) and CD40. Furthermore, Bregs were shown to secrete markedly less IL‐10 in MS patients compared with healthy controls under both conditions (Duddy et al., 2007).
Iwata et al. (2011) described a subset of human Bregs with CD19+CD24hiCD27+ that played an immune regulatory role and appeared to impair functionally patients with systemic lupus erythematosus (SLE). Furthermore, this subset of Bregs showed a reduced capacity for secreting IL‐10 after CD40 engagement in SLE patients compared with healthy controls (Iwata et al., 2011). B cells that express granzyme B revealed a CD19+CD38+CD1d+IgM+CD147+ phenotype in the microenvironment of various solid tumors. The establishment of this regulatory phenotype in solid tumor infiltrates was shown to suppress antitumor immune responses (Lindner et al., 2013). Human Bregs are known to have a high expression level of CD48 and CD148 markers. CD48 is a marker for B‐cell activation, and CD148 is considered a marker for human memory B cells (Kalampokis et al., 2013). Phenotypes that are unique to Bregs have not yet been identified; nonetheless, Bregs are thought to modulate immunity negatively through complex mechanisms in human autoimmune disorders.
FUNCTIONS OF Bregs
Bregs Exercise Function Through IL‐10
There are several mechanisms by which Bregs exert their regulatory function during immune response (Fig. 1). IL‐10 is an immunoregulatory cytokine that plays a pivotal role in controlling excessive inflammation and downregulating the immune response (Lemoine et al., 2009; Yao et al., 2013). IL‐10‐producing Bregs are known to regulate macrophage and dendritic cell activation negatively (Kalampokis et al., 2013). According to Flores‐Borja et al. (2013), CD19+CD24hiCD38hi Bregs were shown to downregulate Th1/Th17 response. Carter et al. (2011) showed that mice specifically lacking IL‐10‐producing B cells developed a progressive arthritis and exhibited an increased frequency in inflammatory Th1/Th17 cells compared with wild‐type (WT) mice. Furthermore, adoptive transfer of Bregs to IL‐10–/– mice was shown to reduce the frequencies of Th1/Th17 pathogenic cells and suppress autoreactive inflammation. Overall, Bregs appear to suppress the differentiation of Th1/Th17 cells and enhance Th2 polarization, resulting in decreased T‐cell activation (Lampropoulou et al., 2008; Kalampokis et al., 2013).
Figure 1.
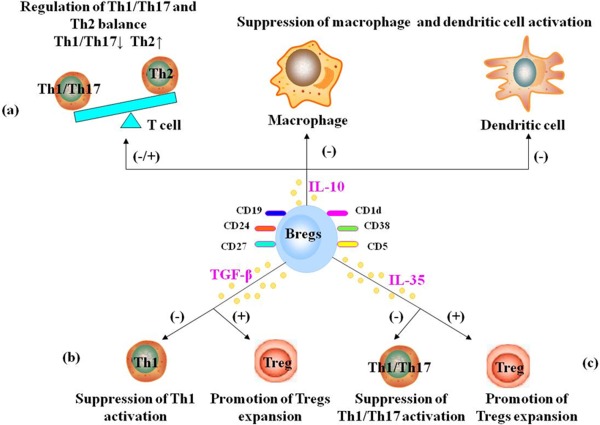
Bregs play their roles in immune response through IL‐10, TGF‐β, and/or IL‐35. a: IL‐10‐producing Bregs appear to suppress the differentiation of Th1/Th17 cells and enhance Th2 polarization. IL‐10‐producing Bregs also suppress macrophage and dendritic cells activation. b: TGF‐β‐producing Bregs appear to suppress Th1 activation and promote Tregs expansion. c: i35‐Bregs appear to suppress Th1/ Th17 activation and promote Tregs expansion. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In an experimental study, mice with IL‐10‐deficient B cells did not fully recover from EAE, which prompted the notion that IL‐10‐producing Bregs could suppress inflammation in autoimmune disorders (Wolf et al., 1996). The transfer of WT IL‐10‐producing Bregs ameliorated the inflammation and severity of EAE, whereas the transfer of Bregs from IL‐10–/– mice had no such effect. Consequently, disease recovery is attributed predominantly to autoantigen‐reactive IL‐10‐producing Bregs (Wolf et al., 1996). These specific IL‐10‐producing Bregs are also referred to as B10 cells (Kalampokis et al., 2013). Several studies have shown a significant decrease in B10 cells in human neuroimmunologic disorders (Knippenberg et al., 2011; Quan et al., 2013; Sun et al., 2014).
BCR‐induced signals appear to be essential for B10 cells to function effectively. Early studies of CD19 transgenic mice showed that Cd19–/– mice had impaired BCR signaling and decreased B10 cells, whereas overexpression of CD19 in mice resulted in an increased number of B10 cells (Yanaba et al., 2008). B‐cell‐mediated immune inhibition was shown to involve antigen‐specific B‐cell activation through both BCR and CD40 signaling (Hilgenberg et al., 2014). Nonetheless, these signals were inadequate to stimulate the production of IL‐10, given that the naïve B cells did not produce inhibitory cytokine IL‐10, suggesting that they were not responsible for the suppression of disease after stimulation by BCR and CD40 (Lampropoulou et al., 2008, 2010).
Several studies showed marked increases in secretion of IL‐10 by naïve B cells after stimulation with agonists of Toll‐like receptor (TLR) signaling, which is also known to trigger the inhibitory response (Lampropoulou et al., 2008, 2010). Mice carrying B‐cell‐restricted deficiency in the MyD88 signaling pathway, an essential signaling adaptor protein for most TLR‐developed chronic relapses of EAE, were clinically indistinguishable from mice lacking IL‐10 production by B cells. TLR signaling in B cells appears to be a requirement for B‐cell‐mediated suppressive function in the pathogenesis of diseases (Lampropoulou et al., 2008). The suppressive function of B cells is thought to be initiated by a two‐step process, TLR signaling and subsequent amplification through BCR and CD40 signaling (Yao et al., 2013). In another crucial recent discovery, Yoshizaki et al. (2012) showed that CD40 signals could induce Breg expansion into functional IL‐10‐producing effector‐cell generation through IL‐21‐dependent cognate interactions.
Bregs Exercise Function Through IL‐35
IL‐35, a recently identified cytokine that belongs to the IL‐12 family, is a potent anti‐inflammatory cytokine produced by Tregs and Bregs (Collison et al., 2010; Mauri and Nistala, 2014; Xiang and Xie, 2015). A novel i35‐Breg subpopulation was shown to downregulate the immune response by inhibiting Th1/Th17 cells (Choi et al., 2015). This new key player appears promising and could open new horizons for treating human autoimmune disorders in the future (Shen et al., 2014; R.X. Wang et al., 2014; Egwuagu and Yu, 2015).
B‐cell‐derived IL‐35 is thought to be an important regulator of T‐cell‐mediated autoimmunity. In one study, mice lacking expression of IL‐35 in B cells lost their ability to recover completely from EAE in contrast to the WT mice (Shen et al., 2014). This study also demonstrated secretion of IL‐35 by B cells after TLR4 and CD40 stimulation. Although TLR4 triggered the formation of IL‐10‐producing Bregs, such an effect was not observable after costimulation by TLR4 and CD40 (Shen et al., 2014).
Treatment of mice with IL‐35 has been shown to restrain harmful immune responses in an animal model of uveitis. Furthermore, mice that were lacking IL‐35 or had dysfunctional IL‐35 signaling (comprising IL‐12Rβ2/IL‐27Rα subunits) showed a poor expansion of Bregs and manifested progression of uveitis. After adoptive transfer of recombinant IL‐35, however, Bregs induced by IL‐35 suppressed uveitis, inhibiting the pathogenic Th1/Th17 cells and promoting Tregs expansion. Furthermore, IL‐35 induced the conversion of human B cells into Bregs, suggesting that IL‐35 could be one possible molecule for treating autoimmune disorders by virtue of its effect in enhancing Bregs function (R.X. Wang et al., 2014).
Bregs Exercise Function Through TGF‐β
Bregs have been shown to regulate negatively the immune response through the production of the anti‐inflammatory cytokine TGF‐β (Holan et al., 2014; Vadasz and Toubi, 2014). Bregs were shown to downregulate pathogenic Th1 immunity effectively through anti‐inflammatory TGF‐β (Tian et al., 2001). Furthermore, Bregs were also shown to promote graft survival by enhancing Tregs expansion, possibly through TGF‐β production (Lee et al., 2014). TGF‐β can inhibit T‐cell immunity by directly suppressing cytokine production by effector T cells or indirectly downregulating the activity of antigen‐presenting cells (Tian et al., 2001; Holan et al., 2014; Lee et al., 2014). It is interesting to note that the adoptive transfer of TGF‐β‐producing Bregs, generated in vitro by lipopolysaccharide stimulation, appeared to inhibit the development of diabetes in mice by inducing apoptosis of the effector T cells (Tian et al., 2001).
Bregs IN NEUROIMMUNOLOGIC DISORDERS
EAE and MS
The established MS animal model EAE is a Th1‐mediated demyelinating autoimmune disease of the central nervous system (CNS). B cells are known to be involved in the pathogenesis of EAE. EAE can typically be induced in mice either by immunization with myelin antigens such as myelin oligodendrocyte glycoprotein (MOG) or by adoptive transfer of autoantigen‐specific CD4+ T cells (Ray et al., 2011; Robinson et al., 2014). In this regard, EAE has been widely used to study the pathogenesis and potential treatments for MS (Ray et al., 2011; Duffy et al., 2014; Pierson et al., 2014; Robinson et al., 2014). The regulatory effects of B cells on EAE were demonstrated almost 20 years ago. Mice with B‐cell deficiency failed to recover fully from EAE (Wolf et al., 1996). When B cells from WT mice had been depleted by monoclonal antibody (mAb) CD20 7 days before EAE induction, it was found that the total number of CD4+ T cells in the CNS was significantly expanded, and disease symptoms were exacerbated. However, the adoptive transfer of IL‐10‐producing regulatory CD5+CD1dhi potent B cells, but not of other B cells, suppressed disease symptoms (Fillatreau et al., 2002; Matsushita et al., 2008). Additional experimental data supported similar effects in EAE that had been treated with a B‐targeted mAb that blocks CD22 (Matsushita et al., 2010). Additionally, B‐cell depletion during the progression of EAE dramatically ameliorated symptoms and the inflammatory response (Matsushita et al., 2008). Another study identified plasmablasts in the draining lymph nodes as the IL‐10‐producing Bregs that negatively regulate EAE inflammation. EAE progression was enhanced by their deletion in mice lacking plasmablasts (Matsumoto et al., 2014). In addition, infiltrating T cells were significantly reduced in B‐cell‐deficient mice that had been treated with E2 and Bregs. Thus, coadministration of Bregs and E2 might provide a promising future immunotherapy for treatment of EAE (Zhang et al., 2015a, 2015b).
Phenotypic characterization of Bregs in human neuroimmunologic disorders is presented in Table 2. MS is primarily a chronic progressive autoimmune demyelinating disease, caused by migration of T cells in the CNS (Compston and Coles, 2008; Roosendaal and Barkhof, 2015). Cumulative evidence emphasizes the significance of B‐cell subsets in the pathogenesis (Lucchinetti et al., 2000) and therapy (Keegan et al., 2005) of MS. Specifically, Bregs play an important role in the pathophysiological basis of MS (Krumbholz and Meinl, 2014). Helminth infection in MS patients induced more Bregs capable of dampening the immune response through production of high levels of IL‐10 (Correale et al., 2008; Correale and Equiza, 2014). In a recent study, fewer IL‐10 producing B cells were found in the peripheral blood of MS patients during both relapse and remission compared with healthy controls (Knippenberg et al., 2011).
Table 2.
Phenotypic Characterization of Bregs in Human Neuroimmunologic Disorders
| Neuroimmunologic disorders | Changes in various phenotype Bregs | References |
|---|---|---|
| MS | ↑CD19+CD25+ Bregs in relapsing MS vs. remitting MS | Knippenberg et al., 2011; de Andres et al., 2014; Michel et al., 2014 |
| ↑CD19+CD25+ Bregs in remitting MS vs. HC | ||
| –CD19+CD24hiCD38hi Bregs in MS vs. HC | ||
| ↓CD19+IL10+ Bregs in MS vs. HC | ||
| NMO | ↓CD19+CD24hiCD38hi Bregs in NMO vs. MS | Quan et al., 2013; Yang et al., 2015 |
| ↓CD19+CD24hiCD38hi Bregs in NMO vs. HC | ||
| ↓CD19+CD5+CD1dhi Bregs in NMO vs. MS | ||
| ↓CD19+CD5+CD1dhi Bregs in NMO vs. MS | ||
| MG | ↓CD19+CD24hiCD38hi Bregs in MG vs. HC | Sun et al., 2014 |
| ↓CD19+CD5+CD1dhi Bregs in MG vs. HC |
However, contradictory findings have been reported by researchers describing other studies in which normal frequency and phenotype of Bregs was observed in the peripheral blood of patients with MS (Michel et al., 2014). No differences were observed between MS patients and healthy controls in the frequency and function of CD19+CD24hiCD38hi Bregs, suggesting that the lack of peripheral Bregs may not contribute to the pathophysiology of MS (Michel et al., 2014). However, these discrepancies could be due to differences in the protocols in terms of time of cell culture and nature of stimulation, both of which have been shown to have an impact on cytokine secretion by B cells.
Researchers identified a new type of Bregs (CD19+CD25+), in low but readily appreciable numbers, in human peripheral circulation of MS patients and healthy controls. In one study, MS patients had a higher frequency of circulating CD19+CD25+ B cells during relapse compared with those in remission. The frequency of circulating CD19+CD25+ Bregs was also higher in MS patients in remission compared with healthy controls (de Andres et al., 2014). Understanding the complex role of Bregs will help to illuminate the immunopathogenesis of MS, which may offer new opportunities for targeting MS.
NMOSD
NMO is a severe inflammatory demyelinating disorder of the CNS that is characterized by typical attacks of optic neuritis and longitudinally extended transverse myelitis (Wingerchuk et al., 2007, 2015). Several studies have indicated a crucial role of B cells in the pathogenesis of NMO‐spectrum disorder (NMOSD; Krumbholz and Meinl, 2014; Bennett et al., 2015). The central role of autoantibodies against water channel protein aquaporin 4 (AQP4; Lennon et al., 2004) and MOG (Probstel et al., 2015) in the pathogenesis of NMOSD has provided a new hope for treatment of this rapidly disabling disorder.
A few researchers have reported on the role of Bregs in the pathogenesis of NMOSD. Recently, two articles from Chinese researchers indicated significantly lower frequencies of different subsets of Bregs in patients with NMOSD compared with both MS patients and healthy controls (Quan et al., 2013; Yang et al., 2015). One of these studies demonstrated significantly lower frequencies of CD19+CD24hiCD38hi Bregs and IL‐10 levels in NMOSD. Additionally, the frequency of CD19+CD24hiCD38hi Bregs was even lower in the NMOSD patients who tested positive for AQP4 antibody compared with those testing negative for AQP4 antibody (Quan et al., 2013).
Another study appeared to mirror these findings in that the proportion of CD19+CD5+CD1dhi Bregs was significantly less in NMOSD patients compared with those in MS patients and healthy controls. Furthermore, the proportion of CD19+CD5+CD1dhi Bregs in CD19+B cells was lower in AQP4 antibody‐positive NMOSD patients than that in antibody‐negative patients (Yang et al., 2015). These findings appear to implicate the reduced expression of Bregs in the pathogenesis of neuroautoimmune disorders.
MG
MG is a B‐cell‐mediated neuroautoimmune disorder in which the main target autoantigens are either the muscle‐specific tyrosine kinase (MuSK) or the acetylcholine receptor (AchR) at the postsynaptic membrane of the neuromuscular junction, leading to muscle weakness and fatigue (Vincent et al., 2001; Guptill et al., 2015). CD72 has been demonstrated to act as a B‐cell‐inhibitory receptor in several autoimmune disorders (Smith et al., 2004; Nakano et al., 2007). Preliminary studies have shown that CD72 appears to be significantly decreased in MG patients and negatively correlates with anti‐AchR‐IgG levels. This suggests an inhibitory effect of CD72 on B‐cell activation in MG and implicates CD72 in the causation of MG (Lu et al., 2013).
It has recently been demonstrated that the frequency and function of Bregs (CD19+CD24hiCD38hi phenotype as well as CD19+CD5+CD1dhi phenotype) were both reduced in MG patients compared with healthy controls. In addition, the frequency and function of Bregs were associated with disease activity (Sun et al., 2014). Another study appeared to mirror these findings in that a lower percentage of B10 cells was detected in patients with MuSK MG (Guptill et al., 2015). Thus, Bregs may serve as a marker for disease activity in MG patients and is a potential future therapeutic target.
CONCLUSIONS
Available evidence indicates that Bregs can downregulate excessive inflammatory responses in neuroimmunologic disorders. Because of the obvious limitations of obtaining human peripheral blood samples, there are only a few studies on regulatory B cells have been published, except for the most common neuroimmunologic diseases, such as MS, NMOSD, and MG. Undoubtedly, there will be further significant advances in other important neuroimmunologic diseases in the coming years. We equally anticipate the precise identification of additional Bregs subpopulations that produce different cytokines or mediate distinct functions. However, whether i35‐Bregs and IL‐10‐producing B cells are overlapping subpopulations or simply represent different stages of B cells is yet to be understood, and this must be addressed in the future. We must understand how to expand Bregs efficiently in vitro and how to achieve maximal regulatory function of B cells. This crucial knowledge will open the gates for a promising, novel approach to treatment of human immune‐related disorders.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest.
ROLE OF AUTHORS
Primary authorship: JH, LS. Significant contributing authorship and editing of intellectual content: XF, ZW, YC. Critical review and editing of intellectual content: JZ, TJ.
SIGNIFICANCE B cells are thought to upregulate humoral immune response by producing antibodies and activating T cells by antigen presentation. This Review provides a succinct synopsis of the current literature and highlights a specific and functionally significant subset of B cells that can downregulate immune response through inhibitory cytokines such as interleukin (IL)‐10, IL‐35, and transforming growth factor‐β. This subset of B cells is generally referred to as regulatory B cells (Bregs). Additional research is required for understanding how to expand Bregs efficiently in vitro to achieve maximal regulatory function of B cells. This crucial knowledge will open the gates for a promising, novel approach to treatment of human neuroimmunologic disorders.
REFERENCES
- Aybar LT, McGregor JG, Hogan SL, Hu Y, Mendoza CE, Brant EJ, Poulton CJ, Henderson CD, Falk RJ, Bunch DO. 2015. Reduced CD5+CD24hiCD38hi and interleukin 10+ regulatory B cells in active antineutrophil cytoplasmic autoantibody‐associated vasculitis permit increased circulating autoantibodies. Clin Exp Immunol 180:178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JL, O'Connor KC, Bar‐Or A, Zamvil SS, Hemmer B, Tedder TF, von Budingen HC, Stuve O, Yeaman MR, Smith TJ, Stadelmann C. 2015. B lymphocytes in neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm 2:e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair PA, Norena LY, Flores‐Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. 2010. CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 32:129–140. [DOI] [PubMed] [Google Scholar]
- Candando KM, Lykken JM, Tedder TF. 2014. B10 cell regulation of health and disease. Immunol Rev 259:259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter NA, Vasconcellos R, Rosser EC, Tulone C, Munoz‐Suano A, Kamanaka M, Ehrenstein MR, Flavell RA, Mauri C. 2011. Mice lacking endogenous IL‐10‐producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol 186:5569–5579. [DOI] [PubMed] [Google Scholar]
- Choi J, Leung PS, Bowlus C, Gershwin ME. 2015. IL‐35 and autoimmunity: a comprehensive perspective. Clin Rev Allergy Immunol 49:327–332. [DOI] [PubMed] [Google Scholar]
- Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, Rehg JE, Jones ML, Ni HT, Artis D, Turk MJ, Vignali DA. 2010. IL‐35‐mediated induction of a potent regulatory T cell population. Nat Immunol 11:1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston A, Coles A. 2008. Multiple sclerosis. Lancet 372:1502–1517. [DOI] [PubMed] [Google Scholar]
- Correale J, Equiza TR. 2014. Regulatory B cells, helminths, and multiple sclerosis. Methods Mol Biol 1190:257–269. [DOI] [PubMed] [Google Scholar]
- Correale J, Farez M, Razzitte G. 2008. Helminth infections associated with multiple sclerosis induce regulatory B cells. Ann Neurol 64:187–199. [DOI] [PubMed] [Google Scholar]
- Daien CI, Gailhac S, Mura T, Audo R, Combe B, Hahne M, Morel J. 2014. Regulatory B10 cells are decreased in patients with rheumatoid arthritis and are inversely correlated with disease activity. Arthritis Rheum 66:2037–2046. [DOI] [PubMed] [Google Scholar]
- de Andres C, Tejera‐Alhambra M, Alonso B, Valor L, Teijeiro R, Ramos‐Medina R, Mateos D, Faure F, Sanchez‐Ramon S. 2014. New regulatory CD19+CD25+ B‐cell subset in clinically isolated syndrome and multiple sclerosis relapse. Changes after glucocorticoids. J Neuroimmunol 270:37–44. [DOI] [PubMed] [Google Scholar]
- de Masson A, Bouaziz JD, Le Buanec H, Robin M, O'Meara A, Parquet N, Rybojad M, Hau E, Monfort JB, Branchtein M, Michonneau D, Dessirier V, Sicre de Fontbrune F, Bergeron A, Itzykson R, Dhedin N, Bengoufa D, Peffault de Latour R, Xhaard A, Bagot M, Bensussan A, Socie G. 2015. CD24hiCD27 and plasmablast‐like regulatory B cells in human chronic graft‐versus‐host disease. Blood 125:1830–1839. [DOI] [PubMed] [Google Scholar]
- DiLillo DJ, Matsushita T, Tedder TF. 2010. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci 1183:38–57. [DOI] [PubMed] [Google Scholar]
- Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, Chalasani G, Sayegh MH, Najafian N, Rothstein DM. 2011. Regulatory B cells are identified by expression of TIM‐1 and can be induced through TIM‐1 ligation to promote tolerance in mice. J Clin Invest 121:3645–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, Kim HJ, Bar‐Or A. 2007. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol 178:6092–6099. [DOI] [PubMed] [Google Scholar]
- Duffy SS, Lees JG, Moalem‐Taylor G. 2014. The contribution of immune and glial cell types in experimental autoimmune encephalomyelitis and multiple sclerosis. Mult Scler Int 2014:285245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egwuagu CE, Yu CR. 2015. Interleukin 35‐producing B cells (i35‐Breg): a new mediator of regulatory B‐cell functions in CNS autoimmune diseases. Crit Rev Immunol 35:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JG, Chavez‐Rueda KA, Eddaoudi A, Meyer‐Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C. 2007. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol 178:7868–7878. [DOI] [PubMed] [Google Scholar]
- Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. 2002. B cells regulate autoimmunity by provision of IL‐10. Nat Immunol 3:944–950. [DOI] [PubMed] [Google Scholar]
- Flores‐Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA, Mauri C. 2013. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med 5:173ra23. [DOI] [PubMed] [Google Scholar]
- Furuzawa‐Carballeda J, Hernandez‐Molina G, Lima G, Rivera‐Vicencio Y, Ferez‐Blando K, Llorente L. 2013. Peripheral regulatory cells immunophenotyping in primary Sjogren's syndrome: a cross‐sectional study. Arthritis Res Ther 15:R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuzawa‐Carballeda J, Sanchez‐Guerrero J, Betanzos JL, Enriquez AB, Avila‐Casado C, Llorente L, Hernandez‐Molina G. 2014. Differential cytokine expression and regulatory cells in patients with primary and secondary Sjogren's syndrome. Scand J Immunol 80:432–440. [DOI] [PubMed] [Google Scholar]
- Goode I, Xu H, Ildstad ST. 2014. Regulatory B cells: the new “it” cell. Transplant Proc 46:3–8. [DOI] [PubMed] [Google Scholar]
- Guptill JT, Yi JS, Sanders DB, Guidon AC, Juel VC, Massey JM, Howard JF Jr, Scuderi F, Bartoccioni E, Evoli A, Weinhold KJ. 2015. Characterization of B cells in muscle‐specific kinase antibody myasthenia gravis. Neurol Neuroimmunol Neuroinflamm 2:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Qian H, Liu Y, Duan L, Li Y, Shi G. 2014. The roles of regulatory B cells in cancer. J Immunol Res 2014:215471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgenberg E, Shen P, Dang VD, Ries S, Sakwa I, Fillatreau S. 2014. Interleukin‐10‐producing B cells and the regulation of immunity. Curr Top Microbiol Immunol 380:69–92. [DOI] [PubMed] [Google Scholar]
- Holan V, Zajicova A, Javorkova E, Trosan P, Chudickova M, Pavlikova M, Krulova M. 2014. Distinct cytokines balance the development of regulatory T cells and interleukin‐10‐producing regulatory B cells. Immunology 141:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua F, Ji L, Zhan Y, Li F, Zou S, Chen L, Gao S, Li Y, Chen H, Cheng Y. 2014. Aberrant frequency of IL‐10‐producing B cells and its association with Treg/Th17 in adult primary immune thrombocytopenia patients. Biomed Res Int 2014:571302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, Hall RP, St Clair EW, Tedder TF. 2011. Characterization of a rare IL‐10‐competent B‐cell subset in humans that parallels mouse regulatory B10 cells. Blood 117:530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalampokis I, Yoshizaki A, Tedder TF. 2013. IL‐10‐producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res Ther 15(Suppl 1):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan M, Konig F, McClelland R, Bruck W, Morales Y, Bitsch A, Panitch H, Lassmann H, Weinshenker B, Rodriguez M, Parisi J, Lucchinetti CF. 2005. Relation between humoral pathological changes in multiple sclerosis and response to therapeutic plasma exchange. Lancet 366:579–582. [DOI] [PubMed] [Google Scholar]
- Knippenberg S, Peelen E, Smolders J, Thewissen M, Menheere P, Cohen Tervaert JW, Hupperts R, Damoiseaux J. 2011. Reduction in IL‐10 producing B cells (Breg) in multiple sclerosis is accompanied by a reduced naive/memory Breg ratio during a relapse but not in remission. J Neuroimmunol 239:80–86. [DOI] [PubMed] [Google Scholar]
- Krumbholz M, Meinl E. 2014. B cells in MS and NMO: pathogenesis and therapy. Semin Immunopathol 36:339–350. [DOI] [PubMed] [Google Scholar]
- Lampropoulou V, Hoehlig K, Roch T, Neves P, Calderon Gomez E, Sweenie CH, Hao Y, Freitas AA, Steinhoff U, Anderton SM, Fillatreau S. 2008. TLR‐activated B cells suppress T cell‐mediated autoimmunity. J Immunol 180:4763–4773. [DOI] [PubMed] [Google Scholar]
- Lampropoulou V, Calderon‐Gomez E, Roch T, Neves P, Shen P, Stervbo U, Boudinot P, Anderton SM, Fillatreau S. 2010. Suppressive functions of activated B cells in autoimmune diseases reveal the dual roles of Toll‐like receptors in immunity. Immunol Rev 233:146–161. [DOI] [PubMed] [Google Scholar]
- Lee KM, Stott RT, Zhao G, SooHoo J, Xiong W, Lian MM, Fitzgerald L, Shi S, Akrawi E, Lei J, Deng S, Yeh H, Markmann JF, Kim JI. 2014. TGF‐beta‐producing regulatory B cells induce regulatory T cells and promote transplantation tolerance. Eur J Immunol 44:1728–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine S, Morva A, Youinou P, Jamin C. 2009. Regulatory B cells in autoimmune diseases: how do they work? Ann N Y Acad Sci 1173:260–267. [DOI] [PubMed] [Google Scholar]
- Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG. 2004. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364:2106–2112. [DOI] [PubMed] [Google Scholar]
- Lindner S, Dahlke K, Sontheimer K, Hagn M, Kaltenmeier C, Barth TF, Beyer T, Reister F, Fabricius D, Lotfi R, Lunov O, Nienhaus GU, Simmet T, Kreienberg R, Moller P, Schrezenmeier H, Jahrsdorfer B. 2013. Interleukin‐21‐induced granzyme B‐expressing B cells infiltrate tumors and regulate T cells. Cancer Res 73:2468–2479. [DOI] [PubMed] [Google Scholar]
- Lu J, Li J, Zhu TQ, Zhang L, Wang Y, Tian FF, Yang H. 2013. Modulation of B‐cell regulatory molecules CD22 and CD72 in myasthenia gravis and multiple sclerosis. Inflammation 36:521–528. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. 2000. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 47:707–717. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Baba A, Yokota T, Nishikawa H, Ohkawa Y, Kayama H, Kallies A, Nutt SL, Sakaguchi S, Takeda K, Kurosaki T, Baba Y. 2014. Interleukin‐10‐producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity 41:1040–1051. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. 2008. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest 118:3420–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita T, Horikawa M, Iwata Y, Tedder TF. 2010. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late‐phase immunopathogenesis. J Immunol 185:2240–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri C, Bosma A. 2012. Immune regulatory function of B cells. Ann Rev Immunol 30:221–241. [DOI] [PubMed] [Google Scholar]
- Mauri C, Nistala K. 2014. Interleukin‐35 takes the “B” line. Nat Med 20:580–581. [DOI] [PubMed] [Google Scholar]
- Michel L, Chesneau M, Manceau P, Genty A, Garcia A, Salou M, Elong Ngono A, Pallier A, Jacq‐Foucher M, Lefrere F, Wiertlewski S, Soulillou JP, Degauque N, Laplaud DA, Brouard S. 2014. Unaltered regulatory B‐cell frequency and function in patients with multiple sclerosis. Clin Immunol 155:198–208. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A, Bhan AK. 2006. A case for regulatory B cells. J Immunol 176:705–710. [DOI] [PubMed] [Google Scholar]
- Nakano S, Morimoto S, Suzuki J, Mitsuo A, Nakiri Y, Katagiri A, Nozawa K, Amano H, Tokano Y, Hashimoto H, Takasaki Y. 2007. Downregulation of CD72 and increased surface IgG on B cells in patients with lupus nephritis. Autoimmunity 40:9–15. [DOI] [PubMed] [Google Scholar]
- Noh J, Choi WS, Noh G, Lee JH. 2010. Presence of Foxp3‐expressing CD19+CD5+ B cells in human peripheral blood mononuclear cells: human CD19+CD5+Foxp3+ regulatory B cell (Breg). Immune Netw 10:247–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, Biragyn A. 2011. Tumor‐evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T‐regulatory cells. Cancer Res 71:3505–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson ER, Stromnes IM, Goverman JM. 2014. B cells promote induction of experimental autoimmune encephalomyelitis by facilitating reactivation of T cells in the central nervous system. J Immunol 192:929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probstel AK, Rudolf G, Dornmair K, Collongues N, Chanson JB, Sanderson NS, Lindberg RL, Kappos L, de Seze J, Derfuss T. 2015. Anti‐MOG antibodies are present in a subgroup of patients with a neuromyelitis optica phenotype. J Neuroinflamm 12:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan C, Yu H, Qiao J, Xiao B, Zhao G, Wu Z, Li Z, Lu C. 2013. Impaired regulatory function and enhanced intrathecal activation of B cells in neuromyelitis optica: distinct from multiple sclerosis. Mult Scler 19:289–298. [DOI] [PubMed] [Google Scholar]
- Ray A, Mann MK, Basu S, Dittel BN. 2011. A case for regulatory B cells in controlling the severity of autoimmune‐mediated inflammation in experimental autoimmune encephalomyelitis and multiple sclerosis. J Neuroimmunol 230:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson AP, Harp CT, Noronha A, Miller SD. 2014. The experimental autoimmune encephalomyelitis (EAE) model of MS: utility for understanding disease pathophysiology and treatment. Handbook Clin Neurol 122:173–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosendaal SD, Barkhof F. 2015. Imaging phenotypes in multiple sclerosis. Neuroimage Clin N Am 25:83–96. [DOI] [PubMed] [Google Scholar]
- Shen P, Roch T, Lampropoulou V, O'Connor RA, Stervbo U, Hilgenberg E, Ries S, Dang VD, Jaimes Y, Daridon C, Li R, Jouneau L, Boudinot P, Wilantri S, Sakwa I, Miyazaki Y, Leech MD, McPherson RC, Wirtz S, Neurath M, Hoehlig K, Meinl E, Grutzkau A, Grun JR, Horn K, Kuhl AA, Dorner T, Bar‐Or A, Kaufmann SH, Anderton SM, Fillatreau S. 2014. IL‐35‐producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 507:366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Gordon TP, Macardle PJ. 2004. Increased expression of the B‐cell‐regulatory molecule CD72 in primary Sjogren's syndrome. Tissue Antigens 63:255–259. [DOI] [PubMed] [Google Scholar]
- Sun F, Ladha SS, Yang L, Liu Q, Shi SX, Su N, Bomprezzi R, Shi FD. 2014. Interleukin‐10 producing‐B cells and their association with responsiveness to rituximab in myasthenia gravis. Muscle Nerve 49:487–494. [DOI] [PubMed] [Google Scholar]
- Tedder TF. 2015. B10 cells: a functionally defined regulatory B cell subset. J Immunol 194:1395–1401. [DOI] [PubMed] [Google Scholar]
- Tian J, Zekzer D, Hanssen L, Lu Y, Olcott A, Kaufman DL. 2001. Lipopolysaccharide‐activated B cells downregulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J Immunol 167:1081–1089. [DOI] [PubMed] [Google Scholar]
- Vadasz Z, Toubi E. 2014. The many faces of B regulatory cells. Isr Med Assoc J 16:631–633. [PubMed] [Google Scholar]
- Vincent A, Palace J, Hilton‐Jones D. 2001. Myasthenia gravis. Lancet 357:2122–2128. [DOI] [PubMed] [Google Scholar]
- Wang L, Qiu J, Yu L, Hu X, Zhao P, Jiang Y. 2014. Increased numbers of CD5+CD19+CD1dhighIL‐10+ Bregs, CD4+Foxp3+ Tregs, CD4+CXCR5+Foxp3+ follicular regulatory T (TFR) cells in CHB or CHC patients. J Transl Med 12:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, Wingfield PT, Kim SH, Egwuagu CE. 2014. Interleukin‐35 induces regulatory B cells that suppress autoimmune disease. Nat Med 20:633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B, Velazquez P, Turovskaya O, Spricher K, Aranda R, Kronenberg M, Birnbaumer L, Braun J. 2005. Mesenteric B cells centrally inhibit CD4+ T cell colitis through interaction with regulatory T‐cell subsets. Proc Natl Acad Sci U S A 102:2010–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde B, Thewissen M, Damoiseaux J, Knippenberg S, Hilhorst M, van Paassen P, Witzke O, Cohen Tervaert JW. 2013. Regulatory B cells in ANCA‐associated vasculitis. Ann Rheum Dis 72:1416–1419. [DOI] [PubMed] [Google Scholar]
- Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. 2007. The spectrum of neuromyelitis optica. Lancet Neurol 6:805–815. [DOI] [PubMed] [Google Scholar]
- Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, de Seze J, Fujihara K, Greenberg B, Jacob A, Jarius S, Lana‐Peixoto M, Levy M, Simon JH, Tenembaum S, Traboulsee AL, Waters P, Wellik KE, Weinshenker BG. 2015. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 85:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SD, Dittel BN, Hardardottir F, Janeway CA Jr. 1996. Experimental autoimmune encephalomyelitis induction in genetically B‐cell‐deficient mice. J Exp Med 184:2271–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang XG, Xie Q. 2015. IL‐35: a potential therapeutic target for controlling hepatitis B virus infection. J Dig Dis 16:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Brooks CR, Sobel RA, Kuchroo VK. 2015. Tim‐1 is essential for induction and maintenance of IL‐10 in regulatory B cells and their regulation of tissue inflammation. J Immunol 194:1602–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. 2008. A regulatory B‐cell subset with a unique CD1dhiCD5+ phenotype controls T‐cell‐dependent inflammatory responses. Immunity 28:639–650. [DOI] [PubMed] [Google Scholar]
- Yang F, Huang D, Cheng C, Wu W. 2015. [Proportion and significance of CD1dhiCD5+CD19+ regulatory B cell in peripheral blood of patients with neuromyelitis optica]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 31:375–378. [PubMed] [Google Scholar]
- Yang M, Rui K, Wang S, Lu L. 2013. Regulatory B cells in autoimmune diseases. Cell Mol Immunol 10:122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Simard AR, Shi FD, Hao J. 2013. IL‐10‐producing lymphocytes in inflammatory disease. Int Rev Immunol 32:324–336. [DOI] [PubMed] [Google Scholar]
- Yoshizaki A, Miyagaki T, DiLillo DJ, Matsushita T, Horikawa M, Kountikov EI, Spolski R, Poe JC, Leonard WJ, Tedder TF. 2012. Regulatory B cells control T‐cell autoimmunity through IL‐21‐dependent cognate interactions. Nature 491:264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Benedek G, Bodhankar S, Lapato A, Vandenbark AA, Offner H. 2015a. IL‐10 producing B cells partially restore E2‐mediated protection against EAE in PD‐L1 deficient mice. J Neuroimmunol 285:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lapato A, Bodhankar S, Vandenbark AA, Offner H. 2015b. Treatment with IL‐10 producing B cells in combination with E2 ameliorates EAE severity and decreases CNS inflammation in B‐cell‐deficient mice. Metab Brain Dis 30:1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu HQ, Xu RC, Chen YY, Yuan HJ, Cao H, Zhao XQ, Zheng J, Wang Y, Pan M. 2015. Impaired function of CD19+CD24hiCD38hi regulatory B cells in patients with pemphigus. Br J Dermatol 172:101–110. [DOI] [PubMed] [Google Scholar]