GABAA Receptor γ2 Subunit Mutations Linked to Human Epileptic Syndromes Differentially Affect Phasic and Tonic Inhibition (original) (raw)
Abstract
GABA acts on GABAA receptors to evoke both phasic inhibitory synaptic events and persistent, tonic currents. The γ2 subunit of the GABAA receptor is involved in both phasic and tonic signaling in the hippocampus. Several mutations of this subunit are linked to human epileptic syndromes with febrile seizures, yet it is not clear how they perturb neuronal activity. Here, we examined the expression and functional impact of recombinant γ2 in hippocampal neurons. We show that the K289M mutation has no effect on membrane trafficking and synaptic aggregation of recombinant γ2, but accelerates the decay of synaptic currents. In contrast, the R43Q mutation primarily reduces surface expression of recombinant γ2. However, it has no dominant effect on synaptic currents but instead reduces tonic GABA currents, at least in part by reducing surface expression of the α5 subunit. Our data suggests that the phenotypic specificity of mutations affecting the GABAA receptor γ2 gene may result from different actions specific to distinct modes of GABAergic signaling.
Keywords: GABA, GABAA receptor, epilepsy, hippocampus, synaptic transmission, benzodiazepine
Introduction
Two modes of inhibition are mediated by GABAA receptors. Phasic activation of postsynaptic receptors elicits a local conductance change that decays in tens of milliseconds (Jones and Westbrook, 1996). These fast synaptic events shape neuronal integration (Pouille and Scanziani, 2001) and contribute to the synchronization of neuronal ensembles (Cobb et al., 1995). A distinct, tonic activation of postsynaptic receptors is mediated by ambient GABA which may approach micromolar concentrations under normal conditions (Lerma et al., 1986) and further increase during pathological activities (Minamoto et al., 1992). Ambient GABA activates high affinity, extrasynaptic receptors that were identified in various neurons including hippocampal principal cells and interneurons (Semyanov et al., 2004; Farrant and Nusser, 2005). Tonic signals reduce neuronal input resistance and excitability (Brickley et al., 1996), control the integration of excitatory inputs (Mitchell and Silver, 2003) and modulate network excitability (Semyanov et al., 2003).
Receptors at most cortical GABAergic synapses involve combinations of α1–3, β2/3 and γ2 subunits (Brunig et al., 2002). In contrast receptors mediating tonic inhibition differ between brain structures and cell types and may involve either α4/6, β1–3 and δ subunits (Hamann et al., 2002; Nusser and Mody, 2002; Mangan et al., 2005), or α5, β3 and γ2 (Sur et al., 1998; Caraiscos et al., 2004; Prenosil et al., 2006), or both (Stell et al., 2003; Scimemi et al., 2005). Thus the γ2 subunit contributes to receptors involved in both phasic and tonic inhibition and underlies the benzodiazepine sensitivity of both modes of inhibition (Bai et al., 2001; Prenosil et al., 2006). It also affects the kinetics and conductance of GABAA receptors (Gunther et al., 1995), promotes receptor clustering and maintenance at synapses (Essrich et al., 1998), and interacts with GABA receptor associated proteins, including GABARAP (Wang et al., 1999) and GODZ (Keller et al., 2004).
Several mutations of the GABAA receptor γ2 (GABRG2) gene encoding the γ2 subunit are linked to generalized epilepsy syndromes with febrile seizures (Baulac et al., 2001; Wallace et al., 2001; Harkin et al., 2002; Kananura et al., 2002). Although deficits in GABAergic function have often been related to epilepsies, the neuronal mechanisms leading from mutation to seizure remain elusive. Functional studies in heterologous systems have provided ambiguous results. The K289M mutation affects a conserved residue in the second extracellular domain of γ2 and was first shown to reduce the amplitude of GABA currents in oocytes (Baulac et al., 2001), but studies in HEK cells demonstrate an accelerated deactivation and unchanged amplitude (Bianchi et al., 2002; Hales et al., 2006). The R43Q mutation, in the N-terminal domain, is suggested to abolish benzodiazepine sensitivity (Wallace et al., 2001), accelerate deactivation (Bowser et al., 2002) or decrease GABA currents by reducing membrane expression (Bianchi et al., 2002; Hales et al., 2006). Possibly, differences in non-neuronal expression systems are significant. Such systems lack endogenous α and β subunits, synaptic specializations and receptor-associated proteins and preclude distinguishing synaptic versus nonsynaptic effects.
We expressed GFP-tagged, recombinant γ2 in hippocampal neurons to examine the functional impact of mutations affecting the γ2 subunit. We find the K289M mutation does not affect surface expression or aggregation of GABAA receptors but accelerates the deactivation of synaptic currents without affecting tonic currents. In contrast, the R43Q mutation has no dominant effect on synaptic currents but γ2 subunit surface expression and clustering is compromised and tonic currents are reduced. Thus different mutations of GABRG2 selectively perturb tonic or phasic GABAergic signals, and defects in tonic signaling contribute to some inherited, generalized epileptic syndromes.
Materials and Methods
GFP-GABRG2 constructs
Murine γ2L tagged at the N terminus with enhanced GFP (Clontech, Mountain View, CA) was provided by Prof. S. Moss (UCL, London, UK) in the mammalian expression vector pRK5 (Kittler et al., 2000). R43Q, K289M and Q351X mutations were introduced using the QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). All constructs were verified by direct sequencing.
Hippocampal primary cultures and transfection
Preparation of hippocampal cultures followed previous protocols (Goslin and Banker, 1998). Hippocampi were dissected from E17–19 Sprague Dawley rat embryos and dissociated using 0.25% trypsin and mild trituration. Neurons were plated at a density of 2.5.104 cells/cm2 on poly-l-ornithine-coated coverslips in MEM (Invitrogen, Cergy Pontoise, France) supplemented with 10% horse serum, 2 mm glutamine and 1 mm Na-Pyruvate. After 4 h, medium was replaced by 1 ml of serum-free culture medium containing Neurobasal with B27 supplement, glutamine (2 mm) and penicillin/streptomycin. Cultures were maintained at 36°C in a humidified CO2 incubator for up to 6 weeks and fed once a week.
After 14 d, neurons were transfected with Lipofectamine 2000 (Invitrogen) according to manufacturer's instructions. Coverslips were transferred into serum-free culture medium and a mixture of 1 μg of DNA and 3 μl of lipofectamine in 60 μl of serum-free medium was added. Visualization of transfected neurons in some physiological experiments was facilitated by cotransfection of pEGFP-N1 (Clontech) at a ratio of 1:20. After 45 min incubation, coverslips were returned to the conditioned culture medium for 4–7 d before immunostaining or electrophysiology.
Immunocytochemistry
Total receptor staining.
Neurons were fixed for 15 min with 4% paraformaldehyde in PBS at room temperature (RT), rinsed in PBS and permeabilized with 0.5% Triton X-100 in PBS. After 30 min incubation in blocking buffer (2% BSA and 10% horse serum in PBS), they were incubated with primary antibody 30 min, washed in PBS and incubated in secondary antibody for 30 min. After washing in PBS, coverslips were mounted in Fluorostab (Bioscience, Emmenbrücke, Switzerland).
Surface receptor staining.
Neurons were washed and incubated for 30 min at 4°C in serum-free medium with one or several primary antibodies. Secondary antibodies were applied before fixation in 4% paraformaldehyde in PBS.
Antibodies.
Primary antibodies were guinea pig anti-α5 and -γ2 and rat anti-α1 (Mohler et al., 1995); mouse anti-GAD (GAD6; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA); rabbit anti-GFP (Invitrogen) and mouse anti-GFP (Roche Diagnostics, Meylan, France). Secondary antibodies were Cy3-conjugated anti-mouse F(ab′)2, Cy3-conjujgated anti-rabbit F(ab′)2 (Jackson ImmunoResearch, West Grove, PA) and Alexa-488-conjugated anti-guinea pig and anti-mouse (Invitrogen).
Image analysis
Fluorescence images were acquired using a Photometrics (Roper Scientific SAS, Evry, France) CoolSnap Fx cooled CCD camera mounted on an Olympus Optical (Tokyo, Japan) BX60 microscope with a 60× NA 1.25 objective using MetaVue software (Molecular Devices, Sunnyvale, CA). Receptor clusters were visualized in Photoshop. After background subtraction, somata and dendrites were manually outlined and selected areas were high pass (r = 2.0) and outline-filtered (internal level = 140). The number and unit surface of clusters in each area was then determined using particle analysis and surface measurement procedures of NIH-Image. Cluster colocalization was counted manually from level-adjusted images of live immunostaining for both GFP and either α1 or α5 subunits from 4 to 7 dendritic sections per neuron.
Anti-α5 or γ2 antibodies were applied to unfixed neurons to quantify α5 or γ2 immunostaining, respectively (see Fig. 2) (supplemental Fig. 2, available at www.jneurosci.org as supplemental material) and anti-GFP was applied after fixation to facilitate identification of transfected neurons. Fluorescent images acquired with identical parameters for all cells were digitized and processed in NIH image with no thresholding or filtering. Several dendritic sections identified by anti-GFP staining were outlined. Mean intensity and cumulative pixel intensity distributions were derived from signal intensity histograms constructed for each neuron.
Figure 2.
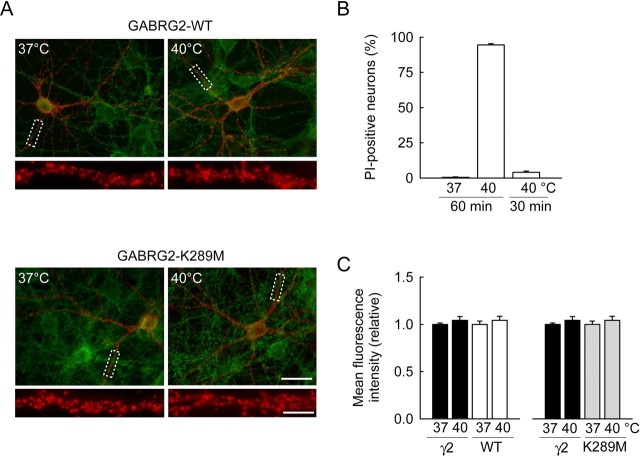
Lack of effect of elevated temperature on the membrane expression of endogenous and recombinant γ2 in hippocampal neurons. Hippocampal neurons were incubated at 40°C for 30 or 60 min. A, Fluorescent micrographs showing endogenous (green) and recombinant (red) γ2 staining performed on living neurons immediately after a 30 min incubation. No apparent difference was observed in either signal, both in neurons expressing WT or K289M GABRG2. Scale bar, 30 μm. Boxed areas from top are enlarged below to show specifically recombinant γ2 membrane staining (scale bar, 5 μm). B, Propidium iodide incubation and NeuN staining were used to quantify the proportion of neuronal cell death after exposition to elevated temperature. No apparent cell death was observed at 37°C or after a 30 min exposure to 40°C. However, massive cell death was induced by more prolonged (60 min) exposure to 40°C. Summary data from 236, 363, and 572 cells, respectively. C, Summary data of endogenous and recombinant γ2 surface immunostaining after 30 min incubation at 37 versus 40°C. Mean pixel intensities (relative) are shown for 27–53 cells in each condition, from two independent experiments. No statistical difference was observed for any staining at 40 versus 37°C (p > 0.3).
Assessment of neuronal viability was performed on cultures exposed for 30 or 60 min to either 37°C or 40°C in the presence of 5 μg/ml propidium iodide. After incubation, cultures were immediately fixed in 4% PFA, permeabilized and processed for NeuN immunostaining. Propidium iodide staining was then evaluated from 350 to 600 NeuN immunopositive cells imaged using a 20× objective.
Electrophysiology
Records were made from cultured neurons on coverslips superfused at ∼1.3 ml/min with a solution containing (in mm): 136 NaCl, 20 d-glucose, 10 HEPES, 3 MgCl2, 2 KCl, 2 CaCl2 (pH = 7.4) at 31°C. Untransfected or transfected neurons were identified by GFP fluorescence. Whole-cell recording pipettes were filled with intracellular solution containing (in mm): 120 CsMeSO4, 10 CsCl, 10 HEPES, 10 EGTA, 4 MgATP, 1.8 MgCl2, 0.4 Na3GTP (pH = 7.4). In some experiments (supplemental Fig. 3, available at www.jneurosci.org as supplemental material), CsMeSO4 was replaced by an equimolar concentration of CsCl to maximize the driving force for chloride. Currents were recorded with an Axopatch 200B amplifier (Molecular Devices), filtered at 2 kHz and digitized at 10–20 kHz. Access and input resistance as well as cell capacitance were monitored with −5 mV voltage steps.
Miniature, GABAA receptor-mediated currents (mIPSCs) were isolated by addition of NBQX (10 μm), D,L-APV (100 μm), tetrodotoxin (1 μm) and CGP52432 (10 μm). To elicit tonic GABA currents, a second patch pipette containing 1 μm GABA dissolved with all the antagonists in recording solution was positioned at ∼25 μm from the recorded cell. GABA was applied by pressure injection using a 10 s, 10 psi pulse generated by a Picospritzer III (General Valve Fairfield, NJ).
All drugs were purchased from Tocris Bioscience (Bristol, UK) or Sigma-Aldrich (Lyon, France), except tetrodotoxin (Latoxan, Valence, France). Values are mean ± SEM. Significance was tested using Mann–Whitney or Kolmogorov–Smirnov statistics in SigmaStat (Systat Software, Point Richmond, CA).
Results
Membrane expression of recombinant γ2 in hippocampal neurons
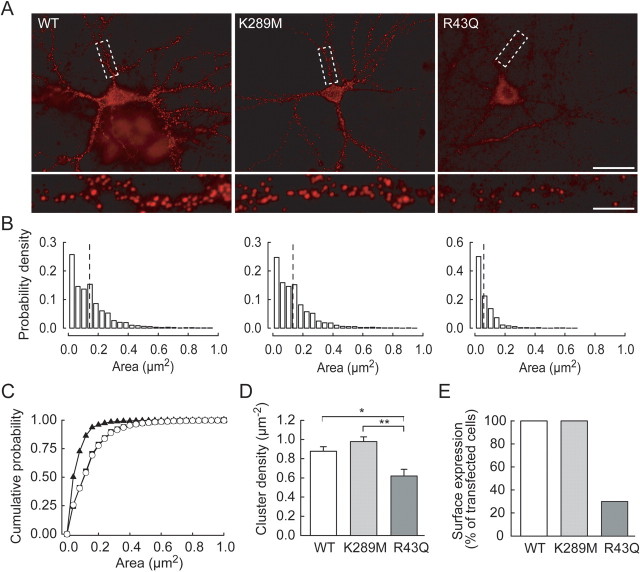
The effects of GABRG2 mutations on GABAergic signaling were examined by transfecting hippocampal neurons with wild-type (WT) or mutant GABRG2 constructs with GFP fused to their N terminus. Because the γ2 subunit is involved in postsynaptic aggregation of GABAA receptors (Essrich et al., 1998; Schweizer et al., 2003), we first examined surface expression of recombinant γ2. Membrane-inserted receptors were detected by exposing living neurons to an antibody against the extracellular GFP tag before fixation (see Materials and Methods). As expected, neurons transfected with GFP alone were not stained (supplemental Fig. 1_A_,B, available at www.jneurosci.org as supplemental material). After 4–7 d, numerous punctae of recombinant γ2 staining were detected at somatic and dendritic sites of neurons transfected with GABRG2 (Fig. 1A). Their density (0.89 ± 0.06 μm−2) and surface area (0.13 ± 0.01 μm2) were similar to those of clusters of endogenous γ2 subunits in cerebellar and hippocampal neurons (Studler et al., 2002; Sun et al., 2004) (supplemental Fig. 2, available at www.jneurosci.org as supplemental material).
Figure 1.
The R43Q but not the K289M mutation disrupts membrane expression and aggregation of the γ2 subunit in hippocampal neurons. A, Neurons transfected with wild-type (WT), K289M, or R43Q recombinant GABRG2 and labeled with anti-GFP antibody to stain surface receptors. Scale bar, 30 μm. Boxed areas from top are enlarged below. Neurons transfected with WT or K289M mutant γ2 show dense clusters of varying size, but cells transfected with the R43Q mutant exhibit only sparse, small membrane clusters. Scale bar, 5 μm. B, Distribution of areas for clusters of recombinant γ2 from 26 (WT), 25 (K289M), and 9 of 30 (R43Q) neurons. Dashed lines represent mean cluster areas for each group. Large clusters (>0.2 μm2) are essentially absent in neurons expressing the R43Q mutant. C, Cumulative distributions of cluster area show differences between WT (open circles) and R43Q (filled triangles) but not K289M mutant (filled squares; Kolmogorov–Smirnov test, p < 0.005). D, The mean density of recombinant γ2 clusters was reduced by ∼35% in neurons expressing the R43Q mutant reduction compared with WT. E, Proportion of neurons with surface expression of recombinant γ2. *p < 0.02; **p < 0.002.
Overexpression of the mutant, recombinant GABRG2 did not significantly affect the morphology or membrane properties of transfected neurons compared with neurons expressing wild-type, recombinant GABRG2 (supplemental Fig. 1_C_, available at www.jneurosci.org as supplemental material). Transfection with GABRG2 bearing the K289M mutation did not alter the density (0.95 ± 0.05 μm−2, p = 0.3), mean area (0.13 ± 0.01 μm−2, p = 0.8) or surface distribution (Kolmogorov–Smirnov test, p > 0.05) (Fig. 1C) of recombinant γ2 clusters, suggesting this mutation did not affect membrane targeting or aggregation of the γ2 subunit. In contrast, γ2 bearing the R43Q mutation was not expressed at the membrane of 21 of 30 transfected neurons. In these cells, recombinant γ2 was retained in the cytoplasm as in heterologous systems (Kang and Macdonald, 2004; Hales et al., 2005; Frugier et al., 2006). In the other 9 neurons, recombinant γ2 formed surface clusters of lower density (0.64 ± 0.08 μm−2, p < 0.03) and smaller size (0.06 ± 0.01 μm2, p < 0.001) than wild-type γ2. The reduced cluster size mostly reflected reduced numbers of larger clusters (Fig. 1B,C). Thus the K289M and R43Q mutations have different effects on membrane targeting and aggregation of the γ2 subunit in neurons. However, expression of mutant versus wild-type GABRG2 did not significantly alter the total surface expression of γ2 protein (supplemental Fig. 2, available at www.jneurosci.org as supplemental material).
A recent study conducted in heterologous cells suggested membrane expression of mutant but not wild-type γ2 may be rapidly reduced on exposure to elevated temperatures (Kang et al., 2006). We tried to replicate these experiments in hippocampal neurons, by comparing surface expression of endogenous and recombinant γ2 before and after a transient temperature increase. This analysis was restricted to the wild-type and K289M mutant γ2 because surface expression of the R43Q mutant was undetectable in a majority (70%) (Fig. 1E) of transfected neurons. We could not detect any significant change in surface expression of either endogenous or recombinant γ2 (WT or K289M) during a 30 min exposure to 40°C (Fig. 2A,C), whereas longer exposures resulted in major neuronal cell death (Fig. 2B). These results suggest temperature-dependent internalization of the K289M mutant may not contribute predominantly to the functional impact of the mutation.
Synaptic targeting of recombinant γ2
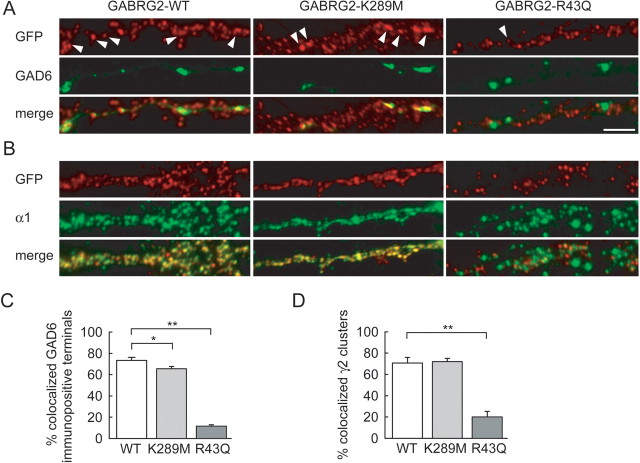
Colocalization with presynaptic markers suggests most large γ2 clusters in hippocampal neurons are expressed at postsynaptic sites (Scotti and Reuter, 2001; Christie et al., 2002; Alldred et al., 2005). The presence of mutated γ2 in synaptic receptors might affect the properties of GABAA receptor-mediated synaptic currents whereas its exclusion would reduce the number of functional postsynaptic receptors. We thus compared the synaptic localization of recombinant γ2 bearing K289M or R43Q mutations with that of wild-type γ2. The proportion of GAD-positive presynaptic varicosities apposed to recombinant γ2 clusters was slightly reduced in neurons expressing K289M GABRG2 (66 ± 2 vs 73 ± 3%, n = 21 and 14 cells, respectively) (Fig. 3A,C). However, a marked reduction was observed in neurons transfected with R43Q GABRG2 that did show a surface expression (11 ± 2%, n = 12 cells, p < 0.001). Similarly, a majority of wild-type and K289M recombinant γ2 clusters colocalized (70.7 ± 5.1 vs 71.9 ± 3.1% colocalization, p = 0.9) with the endogenous α1 subunit expressed at most GABAergic synapses onto pyramidal cells (Klausberger et al., 2002). In contrast, R43Q recombinant γ2 clusters rarely colocalized with α1 immunopositive clusters (20.0 ± 5.3% colocalization, p < 0.002) (Fig. 3B,D) or with endogenous γ2 clusters (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Thus the R43Q but not the K289M mutation seems to impede synaptic incorporation of the γ2 subunit.
Figure 3.
Synaptic and extrasynaptic aggregation of recombinant γ2. A, Dual live immunostaining shows partial colocalization of recombinant γ2 (red) and GAD6 (green). Large clusters of WT or K289M recombinant γ2 (arrowheads) are often apposed to GAD6-immunopositive terminals, but such colocalization rarely occurs for clusters of R43Q recombinant γ2. B, Similarly, ∼70% recombinant γ2 clusters (red) colocalize with α1-immunopositivity (green) in neurons expressing WT or K289M but not R43Q recombinant GABRG2. The R43Q γ2 clusters are often intercalated between α1 clusters. Scale bar, 5 μm. C, Colocalization of GAD6-immunopositive terminals with recombinant γ2 clusters from 14 (WT), 21 (K289M), and 12 (RQ) neurons. D, Colocalization of recombinant γ2-immunopositive clusters with endogenous α1 clusters from 9 (WT), 11 (K289M), and 6 (R43Q) neurons. *p < 0.05; **p < 0.002.
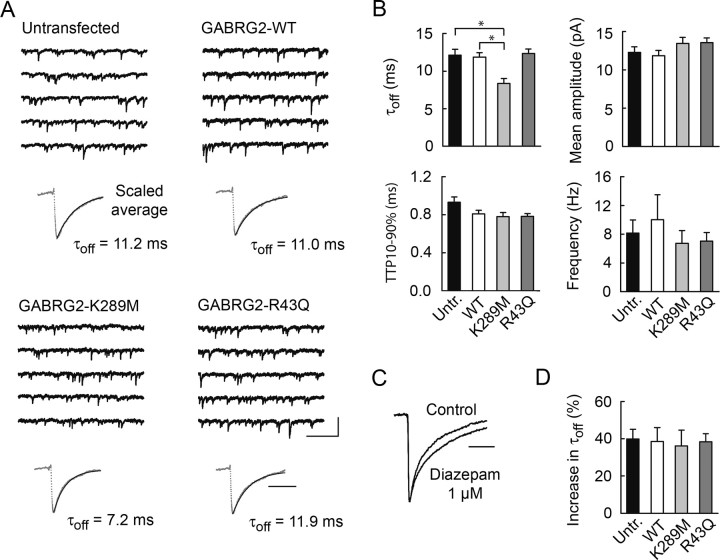
We next asked how these mutations affect GABAA receptor-mediated synaptic events. Miniature IPSCs (mIPSCs) were pharmacologically isolated by tetrodotoxin and the glutamate receptor antagonists D,L-APV and NBQX. mIPSCs of neurons expressing wild type GABRG2 were comparable in amplitude, kinetics and frequency with those of untransfected neurons (Fig. 4B) suggesting that overexpression of recombinant γ2 altered neither the properties or the number of synaptic GABAA receptors. mIPSCs of neurons expressing K289M GABRG2 had similar frequency (6.7 ± 1.8 vs 10.0 ± 3.5 Hz, p = 0.2), mean amplitude (13.5 ± 0.8 vs 13.3 ± 0.7 pA, p = 0.8) and onset kinetics (10–90% time to peak, 0.78 ± 0.04 vs 0.81 ± 0.04 ms, p = 0.6) to those recorded from neurons expressing wild-type recombinant GABRG2. However, their decay was more rapid (time constant, 8.4 ± 0.7 vs 11.9 ± 0.6 ms, p < 0.002), resulting in a reduction of the total charge transfer by ∼25%. Modeling (Poncer et al., 1996) predicts that this accelerated decay should reduce mIPSP amplitude by ∼20% near resting potential in pyramidal cells with a membrane time constant of 20–30 ms (Spruston and Johnston, 1992).
Figure 4.
Dominant effect of the K289M but not the R43Q mutation on synaptic GABA currents. A, Continuous records and scaled averages (dotted gray line) of ∼100 GABAAR-mediated, miniature IPSCs recorded from transfected and untransfected hippocampal neurons. Averaged mIPSC decay was fit by a single exponential (plain line). B, Averaged data from 9 to 14 records from transfected and untransfected cells shows no differences in mean mIPSC amplitude, 10–90% time-to-peak, or frequency. However, mIPSCs decay faster in neurons expressing the K289M mutant than in untransfected neurons or neurons expressing wild-type GABRG2. C, Scaled, averaged mIPSCs recorded in the presence of 1 μm diazepam show increased decay time constant. D, Averaged data of diazepam-induced increase in mIPSC decay time constant (τoff) in untransfected neurons, as well as neurons transfected with WT or mutant recombinant GABRG2, showing no difference between conditions. Calibration: 50 pA, 500 ms (continuous records); 20 ms (scaled averages). *p < 0.002.
The frequency, amplitude and kinetics of mIPSCs in neurons expressing GABRG2 with the R43Q mutation were similar to those of neurons expressing wild-type GABRG2 (Fig. 4B). Similarly, expression of GABRG2 with the Q351X mutation, which entirely suppressed membrane expression of recombinant γ2, did not affect mIPSC properties (n = 14 cells; data not shown). Finally, some earlier studies in heterologous cells suggested the R43Q mutation may reduce or even abolish benzodiazepine-mediated potentiation of GABAA receptor currents (Wallace et al., 2001; Bowser et al., 2002) (but see Bianchi et al., 2002). However, this seems unlikely to affect synaptic GABA currents because synaptic targeting of recombinant γ2 is primarily compromised by the R43Q mutation. Accordingly, we observed no difference in the effect of diazepam (1 μm) on synaptic currents recorded from untransfected neurons versus neurons expressing any recombinant GABRG2 (Fig. 4C,D). In all neurons, diazepam increased mIPSC decay time constant by ∼40% without affecting their peak amplitude, as previously described (Poncer et al., 1996). Mutations which impair membrane and synaptic targeting of the γ2 subunit thus have no dominant effect on synaptic GABAergic signals. Heterozygous expression of R43Q mutant γ2 thus seems not to interfere with the sorting, assembly or targeting of endogenous subunits that form hippocampal synaptic GABAA receptors.
Functional impact of recombinant γ2 on tonic, GABAA receptor-mediated inhibition
Ambient GABA also activates high-affinity, extrasynaptic receptors mediating tonic currents (Semyanov et al., 2004) that modulate network excitability (Mitchell and Silver, 2003; Glykys and Mody, 2006). Because both endogenous and recombinant γ2 were detected at extrasynaptic sites (Fig. 3), we asked whether mutations in GABRG2 also affect tonic GABA currents.
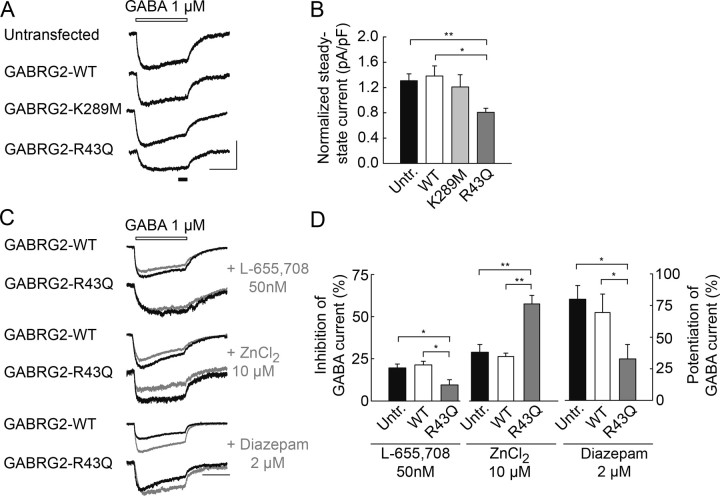
Previous studies revealed tonic GABAA-dependent currents in cultured hippocampal neurons (Bai et al., 2001). Such currents were virtually undetectable in our recordings using CsMeSO4-based internal solution. When the chloride driving force was enhanced by use of a CsCl-based internal solution, the amplitude of tonic, bicuculline sensitive currents was highly variable in amplitude, probably because of variability in local neuronal and glial cell density (supplemental Fig. 3, available at www.jneurosci.org as supplemental material). We therefore recorded responses to prolonged, focal application of 1 μm GABA, a concentration near that of ambient GABA in vivo [∼0.8 μm (Lerma et al., 1986)]. The resulting inward currents, which showed little inactivation, were normalized to the membrane capacitance of the recorded neuron to permit comparison of cells of different size. There was no difference in either the normalized charge or steady-state amplitude of GABA currents recorded from untransfected neurons or neurons transfected with wild-type GABRG2 (13.4 ± 1.0 vs 14.8 ± 1.9 pC/pF, p = 0.6 and 1.31 ± 0.11 vs 1.38 ± 0.16 pA/pF, p = 0.9, respectively) (Fig. 5A,B). This suggests that overexpression of recombinant γ2 in hippocampal neurons did not affect tonic GABA currents. The tonic current induced by GABA application was also unaffected by expression of recombinant γ2 bearing the K289M mutation (normalized steady-state current: 1.21 ± 0.19 pA/pF, p = 0.6). In contrast it was reduced in amplitude by ∼38% in neurons transfected with the R43Q mutant (normalized steady-state current = 0.81 ± 0.07 pA/pF, p < 0.05). Either the density and/or the conductance of the high affinity receptors that mediate tonic GABAA signals seems to be reduced by the R43Q but not the K289M mutation.
Figure 5.
Reduced tonic GABA currents in neurons expressing R43Q GABRG2. A, Tonic, GABAAR-mediated currents evoked by a 10 s application of 1 μm GABA (open bar). Steady-state current was measured from the last 1.5 s of the application (filled bar). B, Mean, steady-state amplitude of GABA currents normalized to cell capacitance for 29 (untransfected), 17 (WT), 14 (K289M), and 24 (R43Q) neurons. GABA current amplitude is not significantly different in untransfected neurons and cells expressing wild-type or K289M recombinant GABRG2 (p > 0.2) but is reduced by ∼40% in neurons expressing the R43Q mutant. C, Effect of L-655,708 (50 nm), ZnCl2 (10 μm), and diazepam (2 μm) on currents evoked by 1 μm GABA (open bar). L-655,708 reduces the charge of GABA currents (gray traces) by ∼20% in untransfected neurons and neurons expressing GABRG2-WT (p > 0.7) and by ∼10% in neurons expressing GABRG2-R43Q. Conversely, 10 μm ZnCl2 reduces GABA currents by 25–30% in untransfected and GABRG2-WT expressing neurons (p > 0.4) and by ∼60% in neurons expressing GABRG2-R43Q. Diazepam increased the charge of GABA currents by 70–80% in untransfected neurons and by ∼30% in neurons expressing GABRG2-R43Q. D, Summary data from 7 to 10 (L-655,708), 5 to 10 (ZnCl2) and 5 to 7 (diazepam) neurons. *p < 0.05; **p < 0.005. Calibration: 100 pA, 5 s.
Hippocampal neurons express several GABAA receptor complexes which include γ2 together with the α1, α2 or α5 subunits (Fritschy and Mohler, 1995; Pirker et al., 2000). α5-containing receptors are expressed at extrasynaptic sites (Brunig et al., 2002) and probably contribute to tonic GABA receptor mediated currents (Caraiscos et al., 2004; Scimemi et al., 2005; Prenosil et al., 2006). Reduced tonic inhibition might then reflect a decrease in membrane expression of α5-containing receptors. We assessed the contribution of these receptors to tonic GABA currents with the benzodiazepine inverse agonist L-655,708 (Quirk et al., 1996) at a concentration (50 nm) specific to α5-containing GABAA receptors (Caraiscos et al., 2004; Scimemi et al., 2005). In untransfected neurons or neurons expressing wild-type GABRG2, L-655,708 reduced the amplitude of tonic GABA-evoked currents by ∼20% (−19.6 ± 2.4 and −21.3 ± 2.2%, respectively; p = 0.7) (Fig. 5C,D). This effect was however significantly reduced in neurons expressing recombinant γ2 bearing the R43Q mutation (−9.6 ± 3.0%, p < 0.05), suggesting expression of this mutant reduces tonic, GABAA-receptor mediated currents, at least in part by reducing the contribution of α5-containing receptors. Other receptor assemblies contributing to tonic currents at synapses onto hippocampal neurons include δ-containing receptors (Stell et al., 2003; Scimemi et al., 2005) and α/β oligomeres (Mortensen and Smart, 2006). Such receptors are modulated by zinc with IC50 ∼100-fold lower than γ2-containing receptors (Saxena and Macdonald, 1996). Accordingly, we observed that application of 10 μm ZnCl2 reduced tonic GABA currents by ∼ 30% in untransfected neurons or neurons expressing wild-type GABRG2 (−28.9 ± 4.5 and −26.4 ± 2.0%, respectively, p = 0.4) (Fig. 5C,D), probably reflecting a partial contribution of γ2-lacking receptors to tonic GABA currents. In neurons expressing the R43Q mutant, this reduction of GABA currents by zinc reached 57.5 ± 5.2% (p < 0.005). Conversely, the potentiation of tonic GABA currents by diazepam (2 μm) was not significantly different in neurons expressing wild-type GABRG2 and untransfected neurons (69.4 ± 14.7% and 79.9 ± 10.9% respectively, p = 0.6) but was reduced by ∼50% in neurons expressing the R43Q mutant receptor (32.8 ± 11.8%, n = 5, p < 0.05). Our results thus suggest that expression of γ2 bearing the R43Q mutation depress tonic GABA currents in neurons by specifically reducing the contribution of α5/γ2-containing receptors.
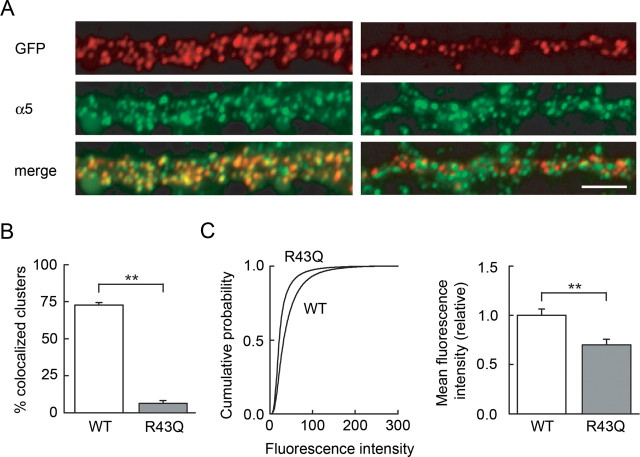
This observation might reflect either a reduced expression or altered function of α5-containing receptors. To discriminate between these possibilities, we compared the expression of the α5 subunit and recombinant γ2 in neurons expressing either wild type or R43Q mutant GABRG2. As for endogenous α1 and γ2 subunits, few recombinant R43Q γ2 clusters colocalized with endogenous α5 subunit clusters in neurons with a surface expression of the recombinant subunit (13.4 ± 4.2% vs 72.7 ± 1.8% for WT γ2, p < 0.005) (Fig. 6A,B). However, the surface expression of endogenous α5 was significantly reduced in neurons expressing R43Q mutant GABRG2 compared with WT (relative mean fluorescence intensity/pixel 1.00 ± 0.07 and. 0.70 ± 0.06 respectively, n = 14 cells each, p < 0.005) (Fig. 6C,D). These observations suggest that the reduction in tonic GABA currents induced by the R43Q mutation probably results from a reduced surface expression of other subunits contributing to tonic currents, in particular α5, rather than from functional changes in receptors incorporating mutated subunits.
Figure 6.
The R43Q mutation reduces endogenous α5 surface expression and prevents coaggregation of recombinant γ2 with α5. A, Dual live staining shows that recombinant γ2 (red) and endogenous α5 (green) subunits are partially (∼70%) colocalized in dendritic sections from neurons expressing wild-type GABRG2. In neurons expressing the R43Q mutant, few recombinant γ2 clusters colocalized with α5 clusters. Scale bar, 5 μm. B, Colocalization of recombinant γ2 clusters with α5 clusters in 11 (WT, 50 dendritic sections) and three (R43Q, 8 sections) neurons. C, Endogenous α5 surface expression in neurons transfected with WT and with R43Q recombinant GABRG2 (14 cells each). Left, Cumulative histograms show that fluorescence in dendrites outlined from postfixation, anti-GFP-stained neurons is significantly reduced in R43Q neurons compared with WT (Kolmogorov–Smirnov test, p < 0.001). Right, Mean pixel intensities averaged from the 14 cells in each group. **p < 0.005.
Discussion
We have shown that distinct mutations of the GABRG2 gene associated with generalized epilepsies with febrile seizures induce differential deficits in phasic and tonic GABAergic signaling. The K289M mutation, linked to the GEFS+ syndrome does not affect trafficking or aggregation of the GABAA receptor γ2 subunit at synaptic or extrasynaptic sites. It has a dominant effect on the kinetics of synaptic events, but does not affect tonic GABA currents. In contrast the R43Q mutation linked to febrile seizures (FS) and childhood absence epilepsy (CAE), compromises surface expression and synaptic aggregation of the γ2 subunit and reduces tonic GABA currents but does not affect GABA-mediated synaptic events.
In heterologous expression systems, the K289M mutation has been shown to reduce the amplitude (Baulac et al., 2001; Ramakrishnan and Hess, 2004) and accelerate the kinetics of GABA currents (Bianchi et al., 2002; Hales et al., 2006) and to reduce membrane expression of the γ2 subunit (Kang et al., 2006). We found no change in surface expression, synaptic or extrasynaptic clustering or coaggregation with α subunits in hippocampal neurons. The amplitude of both synaptic and tonic GABAA-receptor currents was similar in neurons transfected with either K289M mutant or WT γ2 subunits. This is consistent with a recent modeling study showing that, although the K289 residue forms part of the channel lining (O'Mara et al., 2005), the K to M mutation does not change channel conductance and so is unlikely to affect current density. However mIPSCs decayed more quickly, consistent with reduced mean open time and accelerated deactivation observed in heterologous cells (Bianchi et al., 2002; Hales et al., 2006). Because our experiments were conducted on neurons expressing endogenous, wild-type GABRG2, we conclude that acceleration of synaptic GABA currents is a dominant effect of the K289M mutation. However the degree of acceleration presumably depends on the wild-type/mutant allele ratio and may have been overestimated if GABRG2 was overexpressed in our experiments.
In heterologous systems the R43Q mutation abolished benzodiazepine sensitivity (Wallace et al., 2001; Bowser et al., 2002), increased receptor desensitization (Bowser et al., 2002), and reduced GABAA current density (Bianchi et al., 2002) as a result of impaired membrane expression (Kang and Macdonald, 2004; Hales et al., 2005). We found this mutation reduced but did not abolish surface expression of γ2 in hippocampal neurons. Even when expressed at the membrane, however, R43Q γ2 failed to form large postsynaptic clusters or aggregate with endogenous α1, α5 or γ2 subunits. This effect is consistent with a role for the highly conserved R43 residue in mediating intersubunit assembly (Hales et al., 2005). The formation of small, mostly extrasynaptic clusters of R43Q γ2 subunits in ∼30% of transfected neurons suggests the mutant subunit aggregates, perhaps as a monomer or homo-oligomer, but does not assemble with other subunits (Martinez-Torres and Miledi, 2004). Despite this deficit in γ2 trafficking and aggregation, expression of the R43Q mutant did not significantly alter mIPSCs properties. The lack of a dominant effect on synaptic currents suggests the R43Q mutant protein does not reduce the density of functional synaptic GABAA receptors. Accordingly, we found no change in the total surface expression of γ2 in neurons expressing this mutant (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). It therefore seems unlikely that the mutation, when heterozygous, might reduce synaptic currents in conditions of action-potential dependent or independent GABA release. This observation might explain the apparent absence of a synaptic phenotype in hippocampal neurons in mice bearing heterozygous, but not homozygous, R43Q mutation (Jones et al., 2006). In contrast, expression of the R43Q mutant reduced tonic GABAergic currents. This deficit in tonic signaling probably did not result from a functional alteration of receptors including the mutated γ2 because this subunit was not inserted into the membrane of ∼70% of cells and did not aggregate correctly with other GABA receptor subunits in the remaining 30%. Instead, we noted a reduced surface expression of the endogenous α5 subunit in neurons transfected with GABRG2-R43Q. This reduction might reflect a trapping of α5 with mutant R43Q γ2 in the endoplasmic reticulum (Kang and Macdonald, 2004). Beta subunits could escape such ER trapping because mutation of R43 of the γ2 subunit is thought to modify interactions with β but not α subunits (Hales et al., 2005). Alternatively, mutant R43Q γ2 may compete for α5-associated proteins, such as radixin (Loebrich et al., 2006), and so prevent aggregation of extrasynaptic receptors thus decreasing tonic inhibition (Petrini et al., 2004). Tonic GABA currents of hippocampal pyramidal cells are probably mediated by both α5β3γ2 and α4β2/3δ assemblies, in proportions that may depend on agonist concentration (Caraiscos et al., 2004; Scimemi et al., 2005; Glykys and Mody, 2006). A selective decrease in the density of α5-containing receptors might thus act to increase the relative contribution of the other receptors to tonic currents (Scimemi et al., 2005). Consistent with this hypothesis, the sensitivity of tonic GABA currents to the α5-specific benzodiazepine L-655,708 was reduced in neurons expressing R43Q γ2 whereas their sensitivity to zinc was enhanced. Our results are consistent with a selective reduction of α5-containing receptor expression at the membrane. Their contribution to tonic currents in neurons expressing R43Q γ2 may then be reduced thereby increasing the relative contribution of γ2-lacking receptors which may include α4 and δ subunits, or αβ oligomeres (Saxena and Macdonald, 1996; Scimemi et al., 2005; Mortensen and Smart, 2006).
These results show GABRG2 mutations linked to human epileptic syndromes induce deficits in either phasic or tonic GABAergic signaling in neurons. An acceleration of fast IPSCs in neurons with a slow membrane time constant will reduce both the amplitude and decay time constant of inhibitory synaptic potentials by ∼20% ((Poncer et al., 1996) and simulation data not shown). Low concentrations of penicillin or gabazine, which reduce IPSP amplitude to a similar degree but do not affect tonic inhibition (Yeung et al., 2003), induce rhythmic, synchronous activities in the hippocampus (Schneiderman, 1986). Conversely, alterations in tonic inhibition have also recently been linked with epileptogenesis. α5 subunit expression is reduced in some animal models of focal, hippocampal epilepsies (Houser and Esclapez, 2003; Scimemi et al., 2005). Although such a loss might be expected to reduce tonic inhibition, the paradoxical increase in tonic currents observed in hippocampal neurons has been attributed to a compensatory upregulation of subunits other than α5 (Scimemi et al., 2005). In contrast, tonic inhibition is reduced by ∼50% in hippocampal neurons of GABRA5−/− mice. This reduction is associated with a hippocampal hyperexcitability (Bonin et al., 2007) and the generation of epileptiform discharges in the CA3 area (Glykys and Mody, 2006). Similarly, polymorphisms of the GABRD gene which encodes the extrasynaptic GABA receptor subunit δ have recently been identified as susceptibility factors for generalized epilepsies and shown to reduce tonic GABA currents (Dibbens et al., 2004). These observations, and our results, suggest even partial reductions in tonic GABAergic inhibition may favor epileptogenesis. Reduced tonic currents should lower neuronal input conductance, thus increasing excitability and the ability of excitatory inputs to trigger firing (Brickley et al., 1996; Hamann et al., 2002; Mitchell and Silver, 2003). Such effects may be particularly critical in immature networks in which tonic GABAergic actions are more prominent than in the adult (Semyanov et al., 2003), consistent with the linkage of GABRG2 and GABRD mutations with childhood epileptic syndromes.
Both mutations studied in this work have been linked to generalized epileptic syndromes including febrile seizures (Baulac et al., 2001; Wallace et al., 2001). The K289M mutation segregated with both FS and afebrile seizures. Although the R43Q mutation segregated with both FS and CAE, it could only account alone for FS whereas interaction with other genes was required to account for CAE (Marini et al., 2003). How may these mutations, although interfering with distinct modes of GABAergic signaling, both favor the emergence of generalized seizures during febrile episodes? An earlier study conducted in heterologous cells suggested mutations of GABRG2 may confer γ2 with properties of temperature-dependent trafficking to the cell membrane that may rapidly decrease its surface expression at 40°C (Kang et al., 2006). However, surface expression of the K289M mutant γ2 was not reduced in hippocampal neurons exposed to this temperature. Instead, we suggest reduced synaptic or tonic inhibition by K289M and R43Q mutations respectively may act by lowering the threshold for ictogenesis, which may be particularly critical in conditions of elevated body temperature which leads to respiratory alkalosis and subsequent hyperexcitability (Schuchmann et al., 2006). Yet, because pH changes in the range triggered by elevated body temperature seem to have relatively minor effects on GABAA receptor channels (Krishek et al., 1996), it seems unlikely that those might exert their ictogenic effects directly through actions on GABAA receptors.
In conclusion, distinct mutations of the GABAA receptor γ2 subunit affect different aspects of GABAergic signaling. This suggests that distinct pharmaco-therapies may be appropriate for patients carrying these different mutations. However, it remains to be established how and where seizures are initiated in the presence of such deficits in phasic or tonic inhibition. Knock-in transgenic models might be needed to fully reveal causal links between reduced inhibition and epileptogenesis, and also to disclose possible compensatory mechanisms engaged during development.
Footnotes
This work was supported by Inserm, Ministère de l'Enseignement Supérieur et de Recherche, and research grants from the Fondation pour la Recherche Médicale and Fondation Electricité de France (J.C.P.). We thank Prof. Steve Moss for the mouse GFP-GABRG2 construct, Drs. Sabine Lévi and Antoine Triller for help with GABA receptor immunochemistry and comments on this manuscript, and Grégory Gauvain for assistance with hippocampal cultures.
References
- Alldred MJ, Mulder-Rosi J, Lingenfelter SE, Chen G, Luscher B. Distinct γ2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin. J Neurosci. 2005;25:594–603. doi: 10.1523/JNEUROSCI.4011-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Baulac S, Huberfeld G, Gourfinkel-An I, Mitropoulou G, Beranger A, Prud'homme JF, Baulac M, Brice A, Bruzzone R, LeGuern E. First genetic evidence of GABA(A) receptor dysfunction in epilepsy: a mutation in the gamma2-subunit gene. Nat Genet. 2001;28:46–48. doi: 10.1038/ng0501-46. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Song L, Zhang H, Macdonald RL. Two different mechanisms of disinhibition produced by GABAA receptor mutations linked to epilepsy in humans. J Neurosci. 2002;22:5321–5327. doi: 10.1523/JNEUROSCI.22-13-05321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin RP, Martin LJ, Macdonald JF, Orser BA. α5GABAA receptors regulate the intrinsic excitability of mouse hippocampal pyramidal neurons. J Neurophysiol. 2007;98:2244–2254. doi: 10.1152/jn.00482.2007. [DOI] [PubMed] [Google Scholar]
- Bowser DN, Wagner DA, Czajkowski C, Cromer BA, Parker MW, Wallace RH, Harkin LA, Mulley JC, Marini C, Berkovic SF, Williams DA, Jones MV, Petrou S. Altered kinetics and benzodiazepine sensitivity of a GABAA receptor subunit mutation [gamma 2(R43Q)] found in human epilepsy. Proc Natl Acad Sci USA. 2002;99:15170–15175. doi: 10.1073/pnas.212320199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol (Lond) 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunig I, Scotti E, Sidler C, Fritschy JM. Intact sorting, targeting, and clustering of gamma-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J Comp Neurol. 2002;443:43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, Miralles CP, De Blas AL. GABAergic innervation organizes synaptic and extrasynaptic GABAA receptor clustering in cultured hippocampal neurons. J Neurosci. 2002;22:684–697. doi: 10.1523/JNEUROSCI.22-03-00684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Dibbens LM, Feng HJ, Richards MC, Harkin LA, Hodgson BL, Scott D, Jenkins M, Petrou S, Sutherland GR, Scheffer IE, Berkovic SF, Macdonald RL, Mulley JC. GABRD encoding a protein for extra- or peri-synaptic GABAA receptors is a susceptibility locus for generalized epilepsies. Hum Mol Genet. 2004;13:1315–1319. doi: 10.1093/hmg/ddh146. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Frugier G, Coussen F, Giraud MF, Odessa MF, Emerit MB, Boue-Grabot E, Garret M. A gamma 2(R43Q) mutation, linked to epilepsy in humans, alters GABAa receptor assembly and modifies subunit composition on the cell surface. J Biol Chem. 2006;282:3819–3828. doi: 10.1074/jbc.M608910200. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABA A receptor alpha5 subunit-deficient mice. J Neurophysiol. 2006;95:2796–2807. doi: 10.1152/jn.01122.2005. [DOI] [PubMed] [Google Scholar]
- Goslin K, Banker G. Rat hippocampal neurons in low density culture. In: Banker G, Goslin K, editors. Culturing nerve cells. Cambridge, MA: MIT; 1998. pp. 251–282. [Google Scholar]
- Gunther U, Benson J, Benke D, Fritschy JM, Reyes G, Knoflach F, Crestani F, Aguzzi A, Arigoni M, Lang Y, Bluethmann H, Mohler H, Luscher B. Benzodiazepine-insensitive mice generated by targeted disruption of the gamma 2 subunit gene of gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA. 1995;92:7749–7753. doi: 10.1073/pnas.92.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales TG, Tang H, Bollan KA, Johnson SJ, King DP, McDonald NA, Cheng A, Connolly CN. The epilepsy mutation, gamma2(R43Q) disrupts a highly conserved inter-subunit contact site, perturbing the biogenesis of GABAA receptors. Mol Cell Neurosci. 2005;29:120–127. doi: 10.1016/j.mcn.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Hales TG, Deeb TZ, Tang H, Bollan KA, King DP, Johnson SJ, Connolly CN. An asymmetric contribution to gamma-aminobutyric type A receptor function of a conserved lysine within TM2–3 of alpha1, beta2, and gamma2 subunits. J Biol Chem. 2006;281:17034–17043. doi: 10.1074/jbc.M603599200. [DOI] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Harkin LA, Bowser DN, Dibbens LM, Singh R, Phillips F, Wallace RH, Richards MC, Williams DA, Mulley JC, Berkovic SF, Scheffer IE, Petrou S. Truncation of the GABA(A)-receptor gamma2 subunit in a family with generalized epilepsy with febrile seizures plus. Am J Hum Genet. 2002;70:530–536. doi: 10.1086/338710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR, Esclapez M. Downregulation of the alpha5 subunit of the GABA(A) receptor in the pilocarpine model of temporal lobe epilepsy. Hippocampus. 2003;13:633–645. doi: 10.1002/hipo.10108. [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. The impact of receptor desensitization on fast synaptic transmission. Trends Neurosci. 1996;19:96–101. doi: 10.1016/s0166-2236(96)80037-3. [DOI] [PubMed] [Google Scholar]
- Jones MV, Klahn BD, Goldschen-Ohm MP, Tan HO, Petrou S, Stenton LA. Inhibitory synaptic function and GABAA receptor kinetics in a GEFS+ epilepsy knock-in mouse (γ2R43Q) Soc Neurosci Abstr. 2006;32:328–9. [Google Scholar]
- Kananura C, Haug K, Sander T, Runge U, Gu W, Hallmann K, Rebstock J, Heils A, Steinlein OK. A splice-site mutation in GABRG2 associated with childhood absence epilepsy and febrile convulsions. Arch Neurol. 2002;59:1137–1141. doi: 10.1001/archneur.59.7.1137. [DOI] [PubMed] [Google Scholar]
- Kang JQ, Macdonald RL. The GABAA receptor γ2 subunit R43Q mutation linked to childhood absence epilepsy and febrile seizures causes retention of α1β2γ2S receptors in the endoplasmic reticulum. J Neurosci. 2004;24:8672–8677. doi: 10.1523/JNEUROSCI.2717-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JQ, Shen W, Macdonald RL. Why does fever trigger febrile seizures? GABAA receptor γ2 subunit mutations associated with idiopathic generalized epilepsies have temperature-dependent trafficking deficiencies. J Neurosci. 2006;26:2590–2597. doi: 10.1523/JNEUROSCI.4243-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CA, Yuan X, Panzanelli P, Martin ML, Alldred M, Sassoe-Pognetto M, Luscher B. The γ2 subunit of GABAA receptors is a substrate for palmitoylation by GODZ. J Neurosci. 2004;24:5881–5891. doi: 10.1523/JNEUROSCI.1037-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000;20:7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Roberts JD, Somogyi P. Cell type- and input-specific differences in the number and subtypes of synaptic GABAA receptors in the hippocampus. J Neurosci. 2002;22:2513–2521. doi: 10.1523/JNEUROSCI.22-07-02513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishek BJ, Amato A, Connolly CN, Moss SJ, Smart TG. Proton sensitivity of the GABA(A) receptor is associated with the receptor subunit composition. J Physiol (Lond) 1996;492:431–443. doi: 10.1113/jphysiol.1996.sp021319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Herranz AS, Herreras O, Abraira V, Martin del Rio R. In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res. 1986;384:145–155. doi: 10.1016/0006-8993(86)91230-8. [DOI] [PubMed] [Google Scholar]
- Loebrich S, Bahring R, Katsuno T, Tsukita S, Kneussel M. Activated radixin is essential for GABAA receptor alpha5 subunit anchoring at the actin cytoskeleton. EMBO J. 2006;25:987–999. doi: 10.1038/sj.emboj.7600995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PS, Sun C, Carpenter M, Goodkin HP, Sieghart W, Kapur J. Cultured hippocampal pyramidal neurons express two kinds of GABAA receptors. Mol Pharmacol. 2005;67:775–788. doi: 10.1124/mol.104.007385. [DOI] [PubMed] [Google Scholar]
- Marini C, Harkin LA, Wallace RH, Mulley JC, Scheffer IE, Berkovic SF. Childhood absence epilepsy and febrile seizures: a family with a GABA(A) receptor mutation. Brain. 2003;126:230–240. doi: 10.1093/brain/awg018. [DOI] [PubMed] [Google Scholar]
- Martinez-Torres A, Miledi R. Expression of functional receptors by the human gamma-aminobutyric acid A gamma 2 subunit. Proc Natl Acad Sci USA. 2004;101:3220–3223. doi: 10.1073/pnas.0308682101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamoto Y, Itano T, Tokuda M, Matsui H, Janjua NA, Hosokawa K, Okada Y, Murakami TH, Negi T, Hatase O. In vivo microdialysis of amino acid neurotransmitters in the hippocampus in amygdaloid kindled rat. Brain Res. 1992;573:345–348. doi: 10.1016/0006-8993(92)90786-9. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Mohler H, Knoflach F, Paysan J, Motejlek K, Benke D, Luscher B, Fritschy JM. Heterogeneity of GABAA-receptors: cell-specific expression, pharmacology, and regulation. Neurochem Res. 1995;20:631–636. doi: 10.1007/BF01694546. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Smart TG. Extrasynaptic alphabeta subunit GABAA receptors on rat hippocampal pyramidal neurons. J Physiol (Lond) 2006;577:841–856. doi: 10.1113/jphysiol.2006.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- O'Mara M, Cromer B, Parker M, Chung SH. Homology model of the GABAA receptor examined using Brownian dynamics. Biophys J. 2005;88:3286–3299. doi: 10.1529/biophysj.104.051664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini EM, Marchionni I, Zacchi P, Sieghart W, Cherubini E. Clustering of extrasynaptic GABA(A) receptors modulates tonic inhibition in cultured hippocampal neurons. J Biol Chem. 2004;279:45833–45843. doi: 10.1074/jbc.M407229200. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Poncer JC, Durr R, Gahwiler BH, Thompson SM. Modulation of synaptic GABAA receptor function by benzodiazepines in area CA3 of rat hippocampal slice cultures. Neuropharmacology. 1996;35:1169–1179. doi: 10.1016/s0028-3908(96)00055-x. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Prenosil GA, Schneider Gasser EM, Rudolph U, Keist R, Fritschy JM, Vogt KE. Specific subtypes of GABAA receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. J Neurophysiol. 2006;96:846–857. doi: 10.1152/jn.01199.2005. [DOI] [PubMed] [Google Scholar]
- Quirk K, Blurton P, Fletcher S, Leeson P, Tang F, Mellilo D, Ragan CI, McKernan RM. [3H]L-655,708, a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the alpha 5 subunit. Neuropharmacology. 1996;35:1331–1335. doi: 10.1016/s0028-3908(96)00061-5. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan L, Hess GP. On the mechanism of a mutated and abnormally functioning gamma-aminobutyric acid (A) receptor linked to epilepsy. Biochemistry. 2004;43:7534–7540. doi: 10.1021/bi036181+. [DOI] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Properties of putative cerebellar gamma-aminobutyric acid A receptor isoforms. Mol Pharmacol. 1996;49:567–579. [PubMed] [Google Scholar]
- Schneiderman JH. Low concentrations of penicillin reveal rhythmic, synchronous synaptic potentials in hippocampal slice. Brain Res. 1986;398:231–241. doi: 10.1016/0006-8993(86)91482-4. [DOI] [PubMed] [Google Scholar]
- Schuchmann S, Schmitz D, Rivera C, Vanhatalo S, Salmen B, Mackie K, Sipila ST, Voipio J, Kaila K. Experimental febrile seizures are precipitated by a hyperthermia-induced respiratory alkalosis. Nat Med. 2006;12:817–823. doi: 10.1038/nm1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer C, Balsiger S, Bluethmann H, Mansuy IM, Fritschy JM, Mohler H, Luscher B. The gamma 2 subunit of GABA(A) receptors is required for maintenance of receptors at mature synapses. Mol Cell Neurosci. 2003;24:442–450. doi: 10.1016/s1044-7431(03)00202-1. [DOI] [PubMed] [Google Scholar]
- Scimemi A, Semyanov A, Sperk G, Kullmann DM, Walker MC. Multiple and plastic receptors mediate tonic GABAA receptor currents in the hippocampus. J Neurosci. 2005;25:10016–10024. doi: 10.1523/JNEUROSCI.2520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti AL, Reuter H. Synaptic and extrasynaptic gamma-aminobutyric acid type A receptor clusters in rat hippocampal cultures during development. Proc Natl Acad Sci USA. 2001;98:3489–3494. doi: 10.1073/pnas.061028798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Spruston N, Johnston D. Perforated patch-clamp analysis of the passive membrane properties of three classes of hippocampal neurons. J Neurophysiol. 1992;67:508–529. doi: 10.1152/jn.1992.67.3.508. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studler B, Fritschy JM, Brunig I. GABAergic and glutamatergic terminals differentially influence the organization of GABAergic synapses in rat cerebellar granule cells in vitro. Neuroscience. 2002;114:123–133. doi: 10.1016/s0306-4522(02)00206-3. [DOI] [PubMed] [Google Scholar]
- Sun C, Sieghart W, Kapur J. Distribution of alpha1, alpha4, gamma2, and delta subunits of GABAA receptors in hippocampal granule cells. Brain Res. 2004;1029:207–216. doi: 10.1016/j.brainres.2004.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Quirk K, Dewar D, Atack J, McKernan R. Rat and human hippocampal alpha5 subunit-containing gamma-aminobutyric acidA receptors have alpha5 beta3 gamma2 pharmacological characteristics. Mol Pharmacol. 1998;54:928–933. doi: 10.1124/mol.54.5.928. [DOI] [PubMed] [Google Scholar]
- Wallace RH, Marini C, Petrou S, Harkin LA, Bowser DN, Panchal RG, Williams DA, Sutherland GR, Mulley JC, Scheffer IE, Berkovic SF. Mutant GABA(A) receptor gamma2-subunit in childhood absence epilepsy and febrile seizures. Nat Genet. 2001;28:49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]
- Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW. GABA(A)-receptor-associated protein links GABA(A) receptors and the cytoskeleton. Nature. 1999;397:69–72. doi: 10.1038/16264. [DOI] [PubMed] [Google Scholar]
- Yeung JY, Canning KJ, Zhu G, Pennefather P, MacDonald JF, Orser BA. Tonically activated GABAA receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol. 2003;63:2–8. doi: 10.1124/mol.63.1.2. [DOI] [PubMed] [Google Scholar]