Nonrenewal of Neurons in the Cerebral Neocortex of Adult Macaque Monkeys (original) (raw)
Abstract
The concept that, after developmental periods, neocortical neurons become numerically stable and are normally nonrenewable has been challenged by a report of continuous neurogenesis in the association areas of the cerebral cortex in the adult Macaque monkey. Therefore, we have reexamined this issue in two different Macaque species using the thymidine analog bromodeoxyuridine (BrdU) as an indicator of DNA replication during cell division. We found several BrdU+/NeuN+ (neuronal nuclei) double-labeled cells, but cortical neurons, distinguished readily by their size and cytological and immunohistochemical properties, were not BrdU positive. We examined in detail the frontal cortex, where it is claimed that the largest daily addition of neurons has been made, but did not see migratory streams or any sign of addition of new neurons. Thus, we concluded that, in the normal condition, cortical neurons of adult primates, similar to other mammalian species, are neither supplemented nor renewable.
Keywords: adult neurogenesis, cerebral cortex, primates, DNA synthesis, glial markers, 3D immunohistochemistry
Most neurons of the mammalian brain are generated from neuroepithelial cells near cerebral ventricles during well defined developmental stages and then act as substrates of neural circuitry throughout life (Rakic, 1985). However, recent findings of adult neurogenesis in selected structures of the mammalian brain present the possibility that this concept does not apply for all brain regions, in particular, the neural circuits of the olfactory bulb and dentate gyrus of the hippocampus, which may be changing their basic circuitry with the arrival of newly born neurons (Rochefort et al., 2002; van Praag et al., 2002). In addition, the application of new staining methods in the adult Macaque monkeys uncovered some new neurons in the olfactory bulb (Kornack and Rakic, 2001b) and dentate gyrus of the hippocampus (Gould et al., 1999a; Kornack and Rakic, 1999).
In terms of the cerebral neocortex, studies in various mammalian species that range from mice and rats to ferrets and cats have indicated that neurogenesis in this structure stops after well defined developmental periods (Hicks and D'Amato, 1968; Caviness and Sidman, 1973; Luskin and Shatz, 1985; Jackson et al., 1989; Takahashi et al., 1996). Furthermore, no sign of neuronal renewal has been found in the cortex of adult rodents (Magavi et al., 2000). An extensive analysis of Macaque monkeys exposed to [3H]thymidine indicated that the neocortical neurons in primates are generated prenatally (Rakic, 1974) and not normally renewed during adulthood (Rakic, 1985, 2002a). It was suggested that a stable population of neurons in the cortex might be an important mechanism for continuity of learning and preserving memory over a lifetime (Rakic, 1985). Contrary to this theory, Gould and colleagues assert, on the basis of labeling with the thymidine analog bromodeoxyuridine (BrdU), that streams of new neurons are generated continuously and added to the association neocortex of adult Macaque monkeys (Gould et al., 1999b), where many form connections and survive for weeks before dying (Gould et al., 2001). The daily addition of thousands of new neurons to the principal sulcus alone (Gould et al., 1999b) in the absence of net growth indicated high turnover. To verify this claim, Kornack and Rakic (2001a) have performed experiments using the BrdU method in adult Macaque monkeys. They found BrdU-labeled non-neuronal cells, but they did not detect newly born neurons in the neocortex, nor did they find the stream of migrating neurons from the proliferative subventricular zone to the principal sulcus of the prefrontal cortex (Kornack and Rakic, 2001a). This discrepancy in basic findings required a reexamination in other laboratories (Nowakowski and Hayes, 2000).
Therefore, we addressed the question of whether new neurons are generated and continuously added in the neocortex of juvenile and young adult Macaque monkeys using BrdU methods for labeling DNA synthesis in the dividing brain cells (Nowakowski et al., 1989) that were used by previous investigators. We examined in detail the frontal cortex, where it has been previously claimed that the largest daily addition of neurons is made (Gould et al., 1999b). We selected juvenile and young adult monkeys because they presumably have more neuronal plasticity and to avoid the possibility of BrdU labeling during unscheduled DNA synthesis in the process of cell death (apoptosis) in aged animals (Yang et al., 2001). We also avoided high doses of BrdU to avoid its possible mutagenic and stimulation effect on DNA synthesis (Nowakowski and Hayes, 2001; Rakic, 2002b). To identify the phenotypes of BrdU-labeled cells in the neocortex, an immunohistochemical analysis was performed with neuronal markers NeuN, a transcriptional factor that is expressed in the nucleus and cytoplasm of neurons (Mullen et al., 1992), and doublecortin (DCX) (Gleeson et al., 1999; Nacher et al., 2001) and also with markers of other non-neuronal cell types. GFAP and S-100β (astroglial cell), O4 (oligodendrocyte), and Iba-1 (microglial cell) (Ito et al., 1998) were used.
Materials and Methods
Animals. Two young adult female Macaca fascicularis (Cynomolgus monkey; 5 years of age) and two juvenile female M. fuscata (Japanese monkey; 2 years of age) were used. Monkeys were housed at the Primate Research Institute of Kyoto University and were maintained in individual cages with up to 16 cages facing each other in a single room. Thus, the monkeys could see and interact with other monkeys. In addition, Japanese monkeys were provided with a piece of wood that was hung in the cage as a playing tool and a food pickup toy that was placed in front of the cage every day for several hours. The size of the toy was 60 × 60 cm with 480 holes, half of which were covered with a piece of plate so that the monkey needed to use its fingers to carefully pick up the food (Saguinus, Monmouth, IL). The food used was raisins, and the monkeys usually picked up most of the raisins within several hours. The monkeys were cared for according to the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health and the_Guide for Care and Use of Laboratory Primates_ published by the Primate Research Institute, Kyoto University.
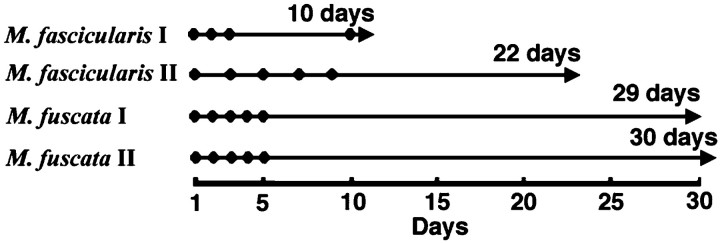
Injections of BrdU. Monkeys were injected with BrdU (Wako, Osaka, Japan) dissolved in 0.9% NaCl with 0.007m NaOH. Two M. fascicularis were injected intravenously, whereas the M. fuscata received intraperitoneal injections. The first M. fascicularis(M. fascicularis I) received once-daily BrdU injections for 3 consecutive days and one more injection after another 7 d with a dose of 100 mg/kg body weight per injection; it was perfused 10 d after the initial BrdU injection, i.e., 1 day after the final BrdU injection (Fig. 1). The second M. fascicularis (M. fascicularis II) received five once-daily BrdU injections every other day with a dose of 75 mg/kg per injection; then, it was perfused 22 d after the initial BrdU injection, i.e., 14 d after the final BrdU injection. The first_M. fuscata_ (M. fuscata I) received once-daily BrdU injections for 5 consecutive days with a dose of 75 mg/kg per injection; it was perfused 29 d after the initial BrdU injection, i.e., 25 d after the final BrdU injection. The second M. fuscata (M. fuscata II) received the same series of BrdU injections as M. fuscata I, but it was perfused 30 d after the initial BrdU injection, i.e., 26 d after the final BrdU injection. All animals were killed by intracardiac perfusion with 4% paraformaldehyde. All removed brains were postfixed with fresh 4% paraformaldehyde for 2 d at 4°C. This combination of injections and perfusion schedule allowed the exposure of newly generated cells as well as the ability to follow their migration and differentiation to the cortex as done on the hippocampus (Markakis and Gage, 1999).
Fig. 1.
BrdU injection schedule. The day of BrdU injection is represented by the filled circle, and the day of perfusion is represented by the arrowhead. M. fascicularis I received once-daily BrdU injections for 3 consecutive days and one more injection after 7 d and then was perfused 1 d after the final injection. M. fascicularis II received five once-daily injections every other day and then was perfused 14 d after the final injection.M. fuscata I and M. fuscata II received once-daily injections for 5 consecutive days. Then, M. fuscata I and M. fuscata II were perfused 25 or 26 d after the final injection, respectively. The_number_ of days represents the period between the first injection and perfusion.
Preparation of tissue sections. Postfixed brains were dissected into right and left hemispheres. Each hemisphere was cut into blocks of 5 mm. The brain blocks were put into O.C.T. Compound (Sakura, Tokyo, Japan), and frozen at −80°C. The frozen blocks were sliced into 40 μm coronal sections with a cryostat (MICROM, Walldorf, Germany). The sliced sections were preserved in a cryoprotectant solution (30% ethylene glycol, 30% glycerol in 0.05m phosphate buffer) at −20°C until they were processed.
Immunohistochemistry. Sections were washed twice with Tris-buffered saline (TBS) for 10 min. Sections were then treated in 10m citric acid buffer at 90°C for 5 min. The heated sections were left at room temperature for 30 min and washed with TBS for 10 min. The sections were incubated in 1m HCl at 37°C for 30 min and neutralized by rinsing with 0.1 m borate buffer and washed twice with TBS. The sections were then blocked with a blocking solution (5% normal donkey serum and 0.3% Triton X-100 in TBS) for 30 min at room temperature with gentle shaking. The blocked sections were reacted with primary antibodies at 4°C. The concentration of Triton X-100 in the blocking solution dissolving primary antibodies with 1 or 3 d reaction time was 0.3 or 0.1%, respectively. Monoclonal rat anti-BrdU antibody (Harlan, Leicestershine, UK) with a 1:200 dilution and monoclonal mouse anti-BrdU (Becton Dickinson, Franklin Lake, NJ) at 1:33 were used. Some antibodies to identify cell types were also used. As antibodies to the neuronal marker, monoclonal mouse anti-NeuN antibody (1:1000; Chemicon, Temecula, CA) and polyclonal goat anti-DCX antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA) were used. The reaction time of NeuN was 1 d and that of DCX was 3 d. As antibodies to the astroglial marker, polyclonal rabbit anti-GFAP antibody (1:10,000; Dako, Carpinteria, CA) and polyclonal rabbit anti-S-100β antibody (1:5000; Swant, Bellinzona, Switzerland) were used. As the antibody to oligodendrocyte marker, monoclonal mouse IgM anti-O4 antibody (1:10; Chemicon) was used. Then, as the antibody to microglial marker, polyclonal rabbit IgG anti-Iba-1 antibody (1:250; kind gift from S. Kohsaka, National Institute of Neuroscience, Tokyo, Japan) was used. The reaction time of GFAP, O4, and Iba-1 was 1 d and that of S-100β and O4 was 3 d. After immunoreaction with these primary antibodies, sections were washed with TBS. The sections were then reacted with fluorochromo-conjugated secondary antibodies. As secondary antibodies, Alexa 488 (1:1000; Molecular Probes, Eugene, OR) and rhodamine Red-X (1:200; Jackson ImmunoResearch, West Grove, PA) were used. Similarly as with the primary antibody, the sections were put into secondary antibodies dissolved in the blocking solution and incubated for 2 hr at room temperature with gentle shaking. The sections were then washed with TBS. The stained sections were mounted on glass slides and incubated for 15 min in DAPI (Sigma, St. Louis, MO) dissolved in 0.1% Triton X-100 in TBS and then coverslipped by using Immu-Mount (Shandon, Pittsburgh, PA) with 2% 1,4-diazabiccydo-2,2,2-octane (Sigma).
Confocal imaging and data analysis. Stained sections were observed using confocal laser scanning microscopy (TCS SP2; Leica, Wetzlar, Germany). Obtained image data were processed using image processing software (LCS; Leica) and reconstructed to three-dimensional (3D) images. With this software, a cross section of the scanning area can be confirmed, and the acquired image data can be analyzed in greater detail.
Quantification of BrdU-labeled cells. Three coronal sections of the anterior, middle, and posterior parts of the principal sulcus and central sulcus were prepared. Then, BrdU-labeled cells were counted in both banks of the principal sulcus and central sulcus by using a fluorescent microscopy (Olympus BX50, Tokyo, Japan). Furthermore, in the ventral part of area 14 (rectus gyrus) on the section of the principal sulcus, BrdU-labeled cells were also counted.
Results
As reported by previous investigators, we observed a number of BrdU-labeled cells in the cerebral cortex of each monkey (Table1). The region of rectus gyrus (area 14) in juvenile M. fuscata II had the highest frequency of BrdU+ cells (88.7 cells per cubic millimeter) among all the cortical regions. The principal sulcus of the frontal association cortex had a higher frequency of BrdU-labeled cells than the motor cortex and the somatosensory cortex. BrdU-labeled cells were detected in the white matter as well as the cortex. Among the four monkeys, M. fascicularis I had the highest frequency of BrdU-labeled cells in the white matter. However, we did not detect any stream of BrdU-labeled cells within the white matter, which might indicate cell migration from the subventricular zone (SVZ) to the cerebral cortex. In the SVZ, all four monkeys had BrdU-labeled cells, but the largest number of BrdU-labeled cells appeared to be in the SVZ of M. fascicularis I, which had a single injection of BrdU 24 hr before it was killed (data not shown). In the cerebral cortex, some of the BrdU-labeled cells were clearly glial and endothelial cells. To determine the phenotypes of these BrdU-labeled cells, we used glial cell markers. As a result, few BrdU+ cells (<1%) were colabeled with S-100β, the astroglial marker, and then few BrdU+ cells (< 1%) expressed Iba-1, the microglial marker (Fig.2A–J).
Table 1.
Number of BrdU-labeled cells in the frontal cortex of Macaque monkeys
| Animal | Age (years) | BrdU injections | Number of BrdU-labeled cells per cubic millimeter | |||||
|---|---|---|---|---|---|---|---|---|
| Number of injections | Survival time1-a | Principal sulcus1-b | Rectus gyrus1-c | Central sulcusd | ||||
| Cortex | WM | Cortex | Cortex | WM | ||||
| M. fascicularis I | 5 | 4 | 10 days | 28.6 | 43.4 | 27.7 | 19.3 | 24.7 |
| M. fascicularis II | 5 | 5 | 21 days | 27.2 | 30.5 | 39.2 | 23.3 | 19.6 |
| _M. fuscata_I | 2 | 5 | 29 days | 25.1 | 36.4 | 54.7 | 18.1 | 16.9 |
| M. fuscata II | 2 | 5 | 30 days | 48.5 | 35.2 | 88.7 | 33.9 | 17.5 |
Fig. 2.
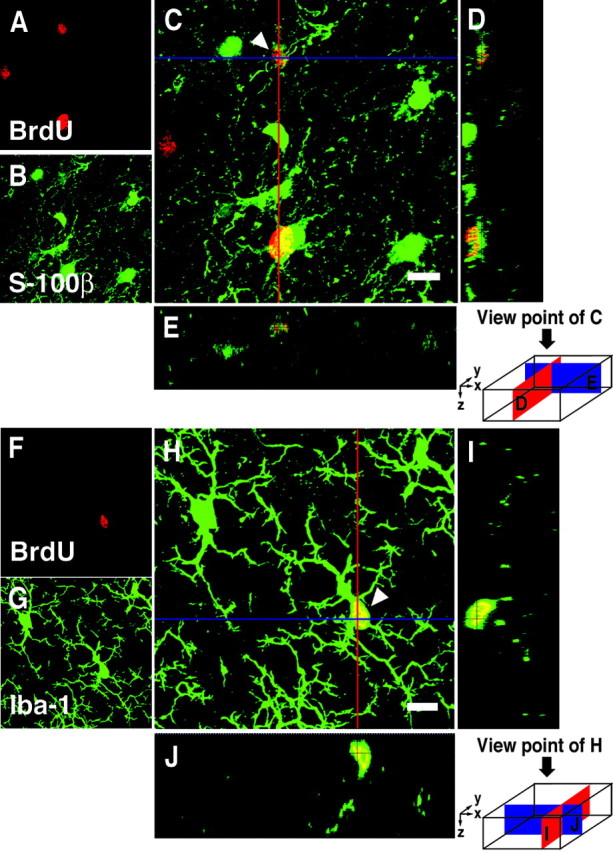
3D images of BrdU+ cells double-labeled with glial markers in the prefrontal cortex of Macaque monkeys.A–E, A cell double-labeled with BrdU and S-100β (astroglial marker indicated by arrowhead).A–C, Images projected from a stack of 42 optical slices at a 0.5 μm interval. A, BrdU (red).B, S-100β (green).C, Overlay. D, The_y_–z cross section of the red line of C. E, The_x_–z cross section of the blue line of C. F–J, A cell double-labeled with BrdU and Iba-1 (microglial marker indicated by arrowhead).F–H, Images projected from a stack of 53 optical slices at a 0.5 μm interval. F, BrdU (red).G, Iba-1 (green).H, Overlay. I, The_y_–z cross section of the red line of H. J, The_x_–z cross section of the blue line of H. Scale bars, 8 μm.
To investigate the possibility that BrdU+ cells are colabeled with the neuronal marker, sections were double-stained with BrdU and NeuN. Some BrdU-labeled nuclei appeared to be colabeled with NeuN and, on initial inspection of our material, could be indicative of newly generated neurons (Fig. 3A). In such cases, we performed a detailed confocal z-series analysis, which revealed that most of these BrdU-labeled nuclei actually belonged to cells that were closely apposed to neurons but were themselves immunonegative for NeuN (Fig. 3B–I). This part of our findings is very similar to the results reported by Kornack and Rakic (2001a). Those flat BrdU+ cells, which are usually sticking to the cell body of neurons, are anatomically known to be satellite glial cells. In the prefrontal cortex of a young adult M. fascicularis II or juvenile M. fuscata II, 37.4 or 35.7%, respectively, of all BrdU+ cells were satellite glial cells, which were not stained with glial markers such as GFAP, S-100β, O4, and Iba-1.
Fig. 3.
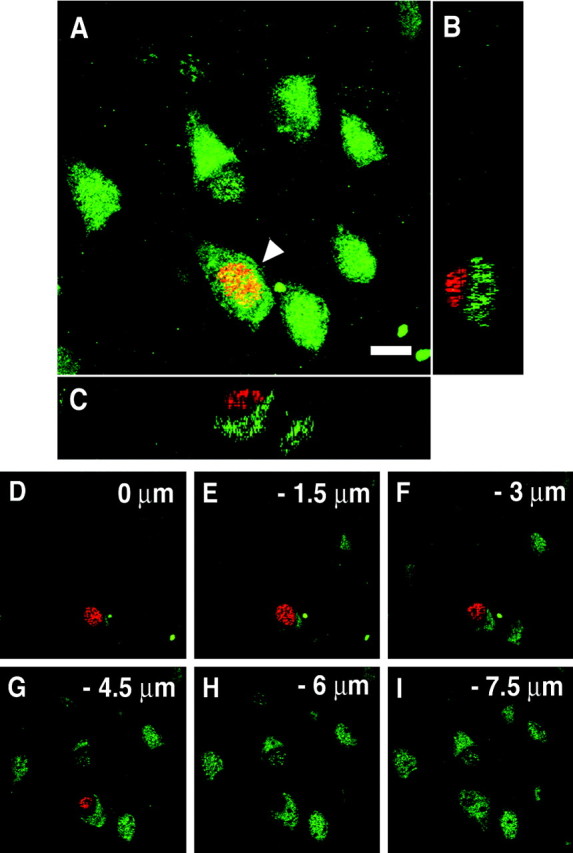
3D images of a BrdU-labeled satellite glial cell.A, An image projected from a stack of 36 optical slices at a 0.5 μm interval. A cell double-labeled with BrdU (red) and NeuN (green) appears to be found (arrowhead). Scale bar, 8 μm.B, C, In fact, in the cross sections, the cell is not double-labeled. A BrdU-labeled cell is closely apposed to the soma of a NeuN-labeled neuron. B, The_y_–z cross section of A.C, The x_–_z cross section of A. D–I, Six different optical slices at a 1.5 μm interval of A also indicate that these are two separate cells.
To characterize and quantify the remaining population of BrdU+ cells, we performed an extensive double-staining immunohistochemical analysis of the prefrontal cortex as well as other areas of the cerebral neocortex. We found only 18 BrdU+/NeuN+ cells in the >500 cortical slices from four monkeys. Because each slice possesses between 300 and 1400 BrdU+ cells, this incidence of BrdU+/NeuN+ cells is between 18 in 150,000 and 18 in 700,000 (0.0026–0.012%). However, because each animal received four or five BrdU injections, the number labeled per day has to be considerably smaller. Nine of 18 BrdU+/NeuN+ cells were localized in the principal sulcus, and five were observed in the ventral part of area 14. However, the localization of NeuN protein was limited to the cell nucleus (Figs. 4A–E,5A–J), which was not the case with the surrounding neurons. Furthermore, the oval, triangular, or spindle-shaped nuclei of these cells were significantly smaller than of any cortical neurons in the same tissue. Because NeuN may not be a definitive neuronal marker (Wolf et al., 1997; Teo et al., 1999), we also performed anti-DCX staining, which labels perinuclear cytoplasm of migrating young neurons (Gleeson et al., 1999; Nacher et al., 2001). With this method, we observed two BrdU+ cells that were weakly costained with this marker in >100 slices examined (Fig.4F–J). This is an incidence of <2 in 30,000 which is a lower rate of double-positive cells than we detected with NeuN and BrdU. In addition, those BrdU+/DCX+ cells were substantially less intensely stained with DCX antibody than the migrating cells observed in the olfactory bulb of the very same animals (see below). At any rate, DCX antibody was reported to stain nondividing cells in the brain regions where there are no new neurons, such as the corpus callosum (Nacher et al., 2001), and thus those faintly stained cells cannot be taken as evidence for migrating neurons in the cortex.
Fig. 4.

3D images of BrdU+ cells in the prefrontal cortex of a juvenile Macaque monkey. A–E, A cell double-labeled with BrdU and NeuN (arrowhead). The localization of NeuN protein in the double-labeled cell is limited to the cell nucleus. A, BrdU (red).B, NeuN (green). C, Overlay. D, The y_–_z_section view of C. E, The_x_–_z section view of C.F–J, A cell double-labeled with BrdU and DCX (arrowhead). The DCX staining of the double-labeled cell is weak. F, BrdU (red). G, DCX (green). H, Overlay.I, The y_–_z section view of H. J, The_x_–z section view of H. Scale bars, 8 μm.
Fig. 5.
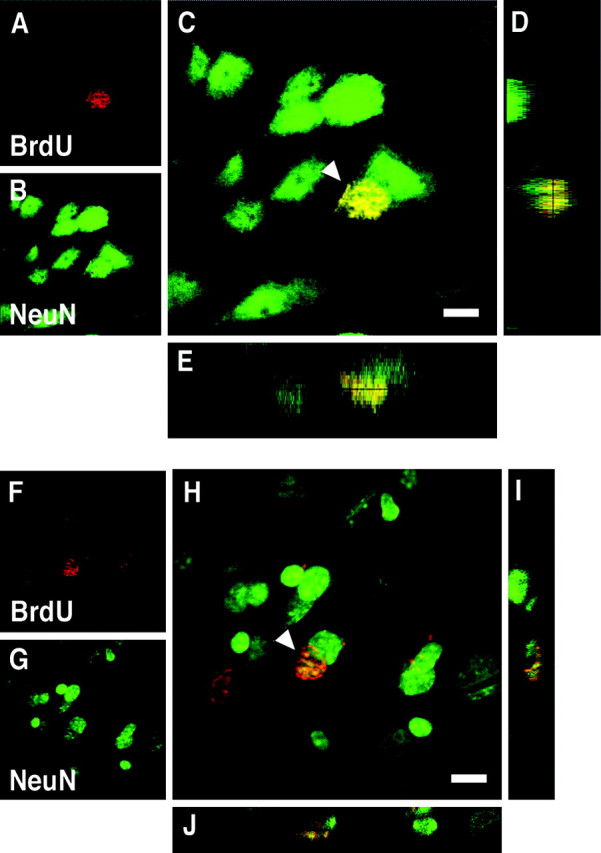
3D images of cells double-labeled with BrdU and NeuN in the prefrontal cortex of a young adult Macaque monkey.A–E, A cell double-labeled with BrdU and NeuN (arrowhead) in the prefrontal cortex of M. fascicularis I. The localization of NeuN protein in the double-labeled cell is limited to the nucleus. A, BrdU (red). B, NeuN (green). C, Overlay.D, The y_–_z section view of C. E, The_x_–z section view of C.F–J, A cell double-labeled with BrdU and NeuN (arrowhead) in the prefrontal cortex of M. fascicularis II. As in M. fascicularis I, the distribution of NeuN protein is localized in the nucleus.F, BrdU (red). G, NeuN (green). H, Overlay.I, The y_–_z section view of H. J, The_x_–z section view of H. Scale bars, 8 μm.
To check the validity and reliability of our methods, we examined the presence and nature of BrdU-labeled cells in the olfactory bulb where new neurons have been reported to exist in monkeys (Kornack and Rakic, 2001b) by using juvenile M. fuscata. Indeed, in the olfactory bulb we observed BrdU+/NeuN+ cells situated around the glomerular layer of the olfactory bulb (Fig.6A–E). Judging from their size and shape, these cells seemed to be interneurons. We usually observed several BrdU+/NeuN+ cells per sagittal section of the olfactory bulb, clearly demonstrating the presence of a small but consistent number of newborn neurons in the olfactory bulb in this species. This incidence is therefore at least 100 times greater than the incidence of BrdU+/NeuN+ neurons in the prefrontal cortex. This finding stands in contrast to the lack of such cells in the cerebral neocortex of the very same specimens stained with the same method. Importantly, we observed a number of BrdU+/DCX+ double-stained cells located just away from the subventricular zone of the olfactory bulb (Fig. 6F–J). The DCX staining of these cells was strong and located over the cytoplasm, equivalent to the staining of the neighboring neurons. Together, our comparative analysis reveals a small but steady cell turnover of interneurons in the olfactory bulb and the absence of such turnover in the cortical areas examined in the same specimens. This indicates that the methods that we have used are suitably sensitive to detect neuron production when it is present.
Fig. 6.
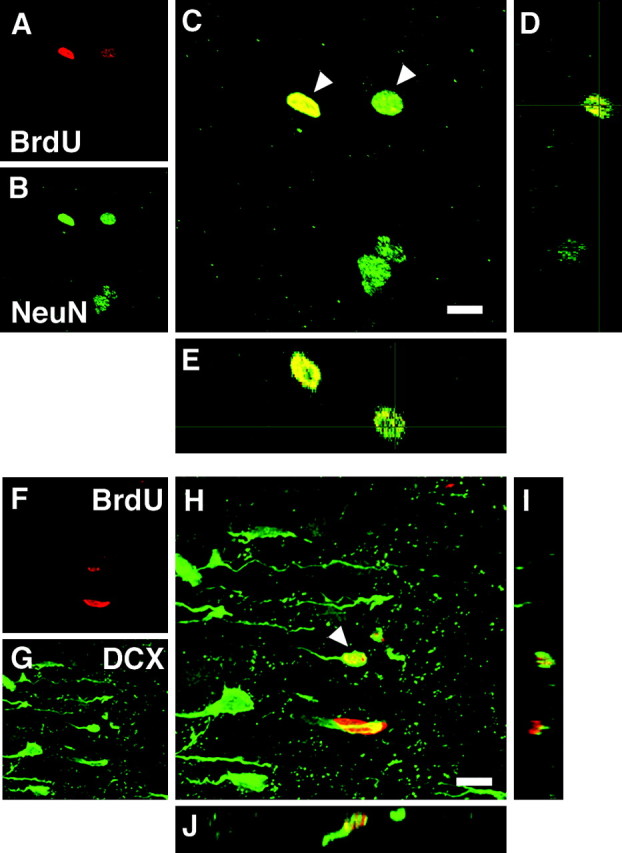
3D images of BrdU+ cells in the olfactory bulb of a juvenile Macaque monkey. A–E, Two cells double-labeled with BrdU and NeuN (arrowheads) are located around the glomerular layer. A, BrdU (red). B, NeuN (green). C, Overlay.D, The y_–_z section view of C. E, The_x_–z section view of C.F–J, A cell double-labeled with BrdU and DCX (arrowhead) is located on the rostral migratory stream as it enters the olfactory bulb. F, BrdU (red). G, DCX (green). H, Overlay.I, The y_–_z section view of H. J, The_x_–z section view of H. Scale bars, 8 μm.
Discussion
The present study was conducted in two different species of Macaque monkeys that have been maintained in an environment enriched with toys and social interactions. We applied the most advanced methods of labeling new cells and their phenotypes. Our comprehensive and detailed analysis shows that although there is an easily detectable amount of cell proliferation that leads to BrdU-labeled cells in the cerebral cortex, we could not unequivocally identify a single, newly produced neuron. Thus, our results support the concept that neuronal populations of the cerebral neocortex in healthy, sexually mature primates become stable and are normally nonrenewable (Rakic, 1985;Kornack and Rakic, 2001a). Most of the BrdU-labeled cells in the neocortex were non-neuronal, and few (<2%) of them were stained with glial markers such as S-100β and Iba-1. Satellite glial cells (35–38%), which generally are closely adhered to the surface of neocortical neurons, turned out not to be stained with any of the glial markers that we used (GFAP, S-100β, O4, and Iba-1). These satellite glial cells have been observed previously in the substantia nigra of adult rat (Lie et al., 2002). They also did not determine the phenotype of these satellite glial cells. In the end, we could not determine the phenotype of the rest (∼60%) of the BrdU-labeled cells.
Of a total of several hundred thousand BrdU-labeled cells observed, only 18 NeuN and 2 DCX double-labeled cells could be found. Importantly, the size, shape, and staining properties of these cells differed from the neurons in the adjacent neocortex, and the double labeling of these cells was qualitatively different from that which we observed in the olfactory bulb. Furthermore, DCX protein can be expressed in differentiating neurons and does not necessarily indicate that the immunostained neuron is new or migrating (Nacher et al., 2001). This casts doubt on the identity of any of these cells as newly produced neurons. Thus, our extensive examination of the tissue from juvenile and young adult Macaque monkeys did not reveal evidence of the turnover of neurons in the prefrontal or any other area of the cerebral neocortex.
What cell type are the small BrdU+/NeuN+ cells that are occasionally encountered within the primate cerebral cortex? One possibility is that some of them are degenerating neurons that increase their DNA synthesis in response to damage (Sanes and Okun, 1972; Klein et al., 2002) or as a component of naturally occurring programmed cell death (Yang et al., 2001). Second, the BrdU label might come from the blood-derived stem cells fused with neurons (Steindler and Pincus, 2002; Terada et al., 2002; Wurmser and Gage, 2002). Third, some cells could be intrinsic multipotent progenitors that express this protein but are normally prevented from differentiating into mature cortical neurons in the cortical environment (Kukekov et al., 1999; Gage, 2000; Laywell et al., 2000). Finally, there is the possibility that some of them are glial progenitors that potentially give rise to NeuN+ tumors (Wolf et al., 1997; Teo et al., 1999). Importantly, even if they are neurons, the incidence would be very small (perhaps, one to two neurons per day for the large portion of the prefrontal cortex that we sampled) and is consistent with the early report that the production of new neurons in the primate brain and in particular the neocortex is extremely “limited” compared with the high rate of neuronal renewal observed in brains of fish, amphibians, and some birds (Rakic, 1985).
It should be emphasized that neocortical neurons in other mammalian species, including rodents, are also generated during a restricted developmental period (for review, see Rakic, 2002a). Except for the report on granule cells in the hippocampus (Eriksson et al., 1998), there are no signs of natural neuronal turnover in the human forebrain (Seress et al., 2001). However, it was reported recently that in the neocortex of adult rodents neurogenesis could be induced under a specific neurodegenerative condition, in particular after a photolytic deletion of projection neurons with the use of the retrograde transport of a dye and laser illumination (Magavi et al., 2000). A small number of potential neuronal precursors may exist in the primate cerebral cortex, but they do not normally differentiate into cortical neurons attributable to local conditions (Kirschenbaum et al., 1994;Pincus et al., 1998; Gage, 2000; Laywell et al., 2000; Steindler and Pincus, 2002). Such cells theoretically could be induced to differentiate into neurons by way of support of the conducive neurogenetic microenvironment (Leavitt et al., 1999; Laywell et al., 2000; Song et al., 2002). Therefore, if a technique and growth factor(s) capable of inducing appropriate neuronal differentiation are developed, neuronal replacement therapies for neurodegenerative disease may become possible through manipulation of endogenous neural precursors even in areas such as the primate cerebral neocortex, where neuronal replacement does not normally occur.
Footnotes
This study is supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan. We thank Dr. Jose Antonio Campos-Ortega and Dr. Yukio Hirata for informative discussion and technical advice. We also thank Dr. Shinichi Kohsaka for kindly providing an anti-Iba-1 antibody.
Correspondence should be addressed to Tatsuhiro Hisatsune, Department of Integrated Biosciences, Graduate School of Frontier Sciences, The University of Tokyo, Bioscience Building 402, 5-1-5 Kashiwanoha, Kashiwa, Chiba 277-8562, Japan. E-mail:hisatsune@k.u-tokyo.ac.jp.
References
- 1.Caviness VS, Jr, Sidman RL. Time of origin or corresponding cell classes in the cerebral cortex of normal and reeler mutant mice: an autoradiographic analysis. J Comp Neurol. 1973;148:141–151. doi: 10.1002/cne.901480202. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 3.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 4.Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- 5.Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci USA. 1999a;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould E, Reeves AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999b;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 7.Gould E, Vail N, Wagers M, Gross CG. Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc Natl Acad Sci USA. 2001;98:10910–10917. doi: 10.1073/pnas.181354698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hicks SP, D'Amato CJ. Cell migrations to the isocortex in the rat. Anat Rec. 1968;160:619–634. doi: 10.1002/ar.1091600311. [DOI] [PubMed] [Google Scholar]
- 9.Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localization of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 10.Jackson CA, Peduzzi JD, Hickey TL. Visual cortex development in the ferret. I. Genesis and migration of visual cortical neurons. J Neurosci. 1989;9:1242–1253. doi: 10.1523/JNEUROSCI.09-04-01242.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirschenbaum B, Nedergaard M, Preuss A, Barami K, Fraser RA, Goldman SA. In vitro neuronal production and differentiation by precursor cells derived from the adult human forebrain. Cereb Cortex. 1994;4:576–589. doi: 10.1093/cercor/4.6.576. [DOI] [PubMed] [Google Scholar]
- 12.Klein JA, Longo-Guess CM, Rossmann MP, Seburn KL, Hurd RE, Frankel WN, Bronson RT, Ackerman SL. The harlequin mouse mutation downregulates apoptosis-inducing factor. Nature. 2002;419:367–374. doi: 10.1038/nature01034. [DOI] [PubMed] [Google Scholar]
- 13.Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci USA. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kornack DR, Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science. 2001a;294:2127–2130. doi: 10.1126/science.1065467. [DOI] [PubMed] [Google Scholar]
- 15.Kornack DR, Rakic P. The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc Natl Acad Sci USA. 2001b;98:4752–4757. doi: 10.1073/pnas.081074998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kukekov VG, Laywell ED, Suslov O, Davies K, Scheffler B, Thomas LB, O'Brien TF, Kusakabe M, Steindler DA. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol. 1999;156:333–344. doi: 10.1006/exnr.1999.7028. [DOI] [PubMed] [Google Scholar]
- 17.Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci USA. 2000;97:13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leavitt BR, Hernit-Grant CS, Macklis JD. Mature astrocytes transform into transitional radial glia within adult mouse neocortex that supports directed migration of transplanted immature neurons. Exp Neurol. 1999;157:43–57. doi: 10.1006/exnr.1999.6982. [DOI] [PubMed] [Google Scholar]
- 19.Lie DC, Dziewczapolski G, Willhoite AR, Kaspar BK, Shults CW, Gage FH. The adult substantia nigra contains progenitor cells with neurogenic potential. J Neurosci. 2002;22:6639–6649. doi: 10.1523/JNEUROSCI.22-15-06639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luskin MB, Shatz CJ. Neurogenesis of the cat's primary visual cortex. J Comp Neurol. 1985;242:611–631. doi: 10.1002/cne.902420409. [DOI] [PubMed] [Google Scholar]
- 21.Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 22.Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406:449–460. [PubMed] [Google Scholar]
- 23.Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 24.Nacher J, Crespo C, McEwen BS. Doublecortin expression in the adult rat telencephalon. Eur J Neurosci. 2001;14:629–644. doi: 10.1046/j.0953-816x.2001.01683.x. [DOI] [PubMed] [Google Scholar]
- 25.Nowakowski RS, Hayes NL. New neurons: extraordinary evidence or extraordinary conclusion? Science. 2000;288:771. doi: 10.1126/science.288.5467.771a. [DOI] [PubMed] [Google Scholar]
- 26.Nowakowski RS, Hayes NL. Stem cells: the promises and pitfalls. Neuropsychopharmacology. 2001;25:799–804. doi: 10.1016/S0893-133X(01)00379-7. [DOI] [PubMed] [Google Scholar]
- 27.Nowakowski RS, Lewin SB, Miller MW. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- 28.Pincus DW, Goodman RR, Fraser RA, Nedergaard M, Goldman SA. Neural stem and progenitor cells: a strategy for gene therapy and brain repair. Neurosurgery. 1998;42:858–867. doi: 10.1097/00006123-199804000-00103. [DOI] [PubMed] [Google Scholar]
- 29.Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- 30.Rakic P. Limits of neurogenesis in primates. Science. 1985;227:1054–1056. doi: 10.1126/science.3975601. [DOI] [PubMed] [Google Scholar]
- 31.Rakic P. Adult corticogenesis: an evaluation of the evidence. Nat Rev Neurosci. 2002a;3:65–71. doi: 10.1038/nrn700. [DOI] [PubMed] [Google Scholar]
- 32.Rakic P. Adult neurogenesis in mammals: an identity crisis. J Neurosci. 2002b;22:614–618. doi: 10.1523/JNEUROSCI.22-03-00614.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanes JR, Okun LM. Induction of DNA synthesis in cultured neurons by ultraviolet light or methyl methane sulfonate. J Cell Biol. 1972;53:587–590. doi: 10.1083/jcb.53.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seress L, Abraham H, Tornoczky T, Kosztolanyi G. Cell formation in the human hippocampal formation from mid-gestation to the late postnatal period. Neuroscience. 2001;105:831–843. doi: 10.1016/s0306-4522(01)00156-7. [DOI] [PubMed] [Google Scholar]
- 36.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 37.Steindler DA, Pincus DW. Stem cells and neuropoiesis in the adult human brain. Lancet. 2002;359:1047–1054. doi: 10.1016/S0140-6736(02)08096-0. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi T, Nowakowski RS, Caviness VS. The leaving or Q fraction of the murine cerebral proliferative epithelium: a general model of neocortical neuronogenesis. J Neurosci. 1996;16:6183–6196. doi: 10.1523/JNEUROSCI.16-19-06183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teo JG, Gultekin SH, Bilsky M, Gutin P, Rosenblum MK. A distinctive glioneuronal tumor of the adult cerebrum with neuropil-like (including “rosetted”) islands: report of 4 cases. Am J Surg Pathol. 1999;23:502–510. doi: 10.1097/00000478-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 41.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolf HK, Buslei R, Blumcke I, Wiestler OD, Pietsch T. Neural antigens in oligodendrogliomas and dysembryoplastic neuroepithelial tumors. Acta Neuropathol (Berl) 1997;94:436–443. doi: 10.1007/s004010050730. [DOI] [PubMed] [Google Scholar]
- 43.Wurmser AE, Gage FH. Stem cells: cell fusion causes confusion. Nature. 2002;416:485–487. doi: 10.1038/416485a. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y, Geldmacher DS, Herrup K. DNA replication precedes neuronal cell death in Alzheimer's disease. J Neurosci. 2001;21:2661–2668. doi: 10.1523/JNEUROSCI.21-08-02661.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]