Location, location, location: Tissue resident memory T cells in mice and humans (original) (raw)
. Author manuscript; available in PMC: 2019 Oct 5.
Published in final edited form as: Sci Immunol. 2019 Apr 5;4(34):eaas9673. doi: 10.1126/sciimmunol.aas9673
Abstract
The discovery of T cells resident in diverse tissues has altered our understanding of adaptive immunity to encompass site-specific responses mediated by tissue-adapted memory T cells throughout the body. Here, we discuss the key phenotypic, transcriptional, and functional features of these tissue-resident memory T cells (TRM) as established in mouse models of infection and translated to humans by novel tissue sampling approaches. Integration of findings from mouse and human studies may hold the key to unlocking the potential of TRM for promoting tissue immunity and preventing infection.
Introduction
The ability of the immune system to maintain memory of previous antigen encounters is the basis for vaccines, long-term immunity, and health. Immunological memory was originally found to be maintained within the blood, through long-lived antibody responses in serum and within a specialized population of circulating memory T cells. The identification of heterogeneous subsets of memory T cells based on expression of the lymph node (LN) homing receptor CCR7 delineating CCR7hi central-memory (TCM) and CCR7lo effector-memory (TEM) cells (1) suggested that T cell migration beyond blood could be a major determinant for memory maintenance and/or protective immunity. Investigations in mouse models showed diverse distribution of memory T cells in multiple anatomical sites—including secondary lymphoid organs, mucosal, and barrier tissues (2, 3)—suggesting continuous surveillance. Subsequent to these earlier findings, a series of studies in mice demonstrated that subpopulations of CD4+ or CD8+TEM remain resident in tissues such as lung, skin, and gut long after infection resolution (4–7). These key studies laid the foundation for the designation of tissue-resident memory T cells (TRM) as a new subset of memory T cells that provides localized protective immunity and immunosurveillance in tissues.
Most of our current understanding regarding the differentiation, maintenance, and function of TRM stems from in vivo studies in mouse models of infection. Initial studies characterizing TRM in the mouse lungs, skin, and female reproductive tract (FRT) established tissue residence by using in vivo assays for tissue retention and further confirmed TRM as mediators of in situ protective responses (7–12). Molecular characterization of the phenotypic, functional, and transcriptional features of these tissue-retained mouse memory T cells have together defined the TRM subset. Broadly speaking, CD4+ and CD8+TRM can be distinguished from circulating counterparts based on expression of the T cell activation and retention marker CD69 and the αE integrin CD103 for subsets of CD8+TRM in mucosal and barrier sites. TRM were further found to exhibit a transcriptional profile distinct from circulating memory T cells, with differential expression of key transcription factors (TFs) (13–15). Together, these seminal studies in mice defined a new paradigm for T cell–mediated immunity and a novel memory T cell subset that mediates localized, tissue-intrinsic surveillance and protective immunity, extending previously held views of memory T cells as a circulating and broadly surveilling population.
Given the emerging importance of TRM in mouse models, it is essential to assess human T cell immunity through the lens of tissue localization and long-term tissue residence. However, blood is the major sample site for human immune cell studies; obtaining tissue samples from living individuals is limited to biopsies or surgical resections along the healthy margins of diseased organs. We have extensively characterized tissues obtained from previously healthy organ donors for the study of immune cells (16–24), demonstrating that this type of tissue resource effectively reveals snapshots of tissue immunity throughout all stages of life. In both organ donor tissue and surgical resections, TRM-phenotype cells expressing CD69 +/−CD103 have been identified in virtually every tissue examined, including lungs, liver, pancreas, lymphoid tissues, genital mucosa, the gastrointestinal tract (stomach, jejunum, ileum, and colon), bone marrow (BM), and in brain obtained from autopsies (25–31). Transcriptional profiling of CD69+memory T cells from human lungs, spleen, liver, and other sites has demonstrated a conserved transcriptional profile distinct from blood memory T cells that exhibits key features with mouse TRM (18, 32, 33). The study of TRM in human tissues has also revealed an association with protective immunity and specific disease states, such as inflammatory disorders and autoimmunity (34, 35).
There has been considerable debate in the field on the translational potential of genetically inbred mouse models for studying immune responses and immunological memory within tissue sites. Although mouse models cannot recapitulate the length and diversity of exposures to pathogens that takes place over many decades in humans, the extent to which this difference affects the generalizability of findings on tissue immunity in mice is not known. A recently proposed solution to this issue has been the use of outbred mice obtained from pet stores, also referred to as “dirty” mice (36). Several immune parameters in dirty mice align more closely with adult humans, including having abundant TRM populations in lymphoid and nonlymphoid tissues (36–38). However, it is not yet clear how effectively dirty mice recapitulate human immune responses, in general, and whether use of dirty mice needs to supplant studies that use inbred strains.
In this Review, we will discuss how tissue residency is defined for mouse and human T cells and the identification of TRM in both species. We will highlight studies that characterize TRM phenotype and tissue-specific adaptations of TRM across different sites, many of which are conserved in mice and humans (Table 1). Furthermore, we will discuss key similarities and differences between the transcriptional regulation and formation of TRM in mice and humans. Last, we will review studies that investigate TRM function in disease contexts involving novel sampling and both current and future strategies for targeting TRM in vaccines.
Table 1. A comparison between mouse and human tissue-resident memory T cells.
Ab, antibody; BM, bone marrow; LN, lymph nodes; SG, salivary gland; TRM, tissue-resident memory T cells.
| Mice | Humans | |
|---|---|---|
| Techniques to define tissue residency | Parabiosis (7, 12, 39–41); in vivo labeling (7, 8, 11, 46); Ab depletion (10, 47, 48); FTY720 (11, 49–52); transplantation (4–6, 53); transcriptional profiling (13–15, 32, 83). | Transcriptional profiling (18, 22, 23, 32, 33, 71, 93); Ab depletion (54); Transplantation (55–57). |
| Canonical phenotype | CD69 +/−CD103 | CD69 +/− CD103 |
| Accessory markers | CD101, CD49a, PD-1, CXCR6, CLA, LFA1, CD11a, CXCR3, CCR10 (7, 14, 53, 69, 78, 89). | CD101, CD49a, PD-1, CXCR6, CLA, CCR8 (18, 27, 32, 33, 74, 93, 94). |
| TFs | Hobit and Blimp1 (13); Runx3, CD8+TRM (83); Notch, CD103+ CD8+TRM maintenance (32). | Hobit, Blimp1, Runx3 (18, 33); Notch/RBPj enriched on lung TRM (32, 33, 57) |
| Tissue maintenance | Maintained over months: skin (12, 91), gut (6), brain (64), lung (CD4+TRM) (11, 48), liver (89), SG (108). Wane over months: lung CD8+TRM (91). | Maintained over years: skin (56), intestine (55), lung (57). TRM frequency maintained over life (17, 24). |
| Features in nonlymphoid tissues | Skin CD103+/−CD4+TRM most frequent in naïve mice (45); epidermal CD103+CD8+TRM and dermal CD103+/−CD4+TRM accrue in infected mice (65). Liver CD8+TRM lack CD103 (89). | Epidermal CD103+CD4+ TRM and dermal CD103–CD4+ TRM most frequent in healthy skin (66). Liver CD8+TRM express CD103 (27). |
| Features in lymphoid tissues | LN CD8+TRM infrequent (40), more abundant in dirty mice (38). BM CD8+T cells recirculate (39). | Large TRM pool (18, 22, 71), LN-specific transcriptional profile (22). BM CD69+CD4+T cells quiescent, broad specificities (26). |
| Role in immunity | Protective in viral (5, 7, 43, 90), bacterial (49, 67, 73, 104, 107), parasitic (89) and fungal (106) infections. | Correlation of antigen-specific TRM abundance to viral control (27, 71, 113). |
| Potential target for vaccination | Demonstrable (50, 52, 89, 98, 119–124) | Promising |
Defining tissue residency for T cells
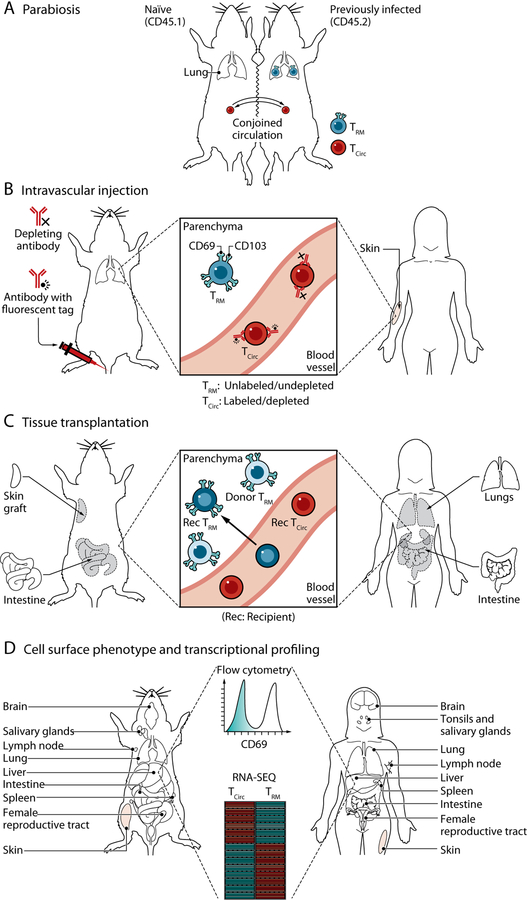
In contrast with circulating memory T cell populations that survey peripheral tissues through the blood and lymph, the term “tissue resident” refers to the retention of memory T cells localized in tissues that are maintained independent of circulating counterparts. There are various experimental methods used to determine the recirculation potential of T cells (Fig. 1 and Table 1), including parabiotic surgery, in vivo antibody labeling, T cell depletional strategies, tissue transplantation, and phenotypic or transcriptional profiling. Parabiotic surgery, in which the blood circulation of two individual mice are conjoined, allows for the assessment of T cell migration from one parabiont to the other (Fig. 1A). Whereas blood T cells typically reach homeostasis between partner mice within a week, resident T cells do not equilibrate and remain in specific tissues of one mouse (7, 12, 39–41). Parabiosis experiments have been instrumental in defining TRM as an independent population of memory T cells in tissues, although there are caveats to this approach. Not all circulating blood cells equilibrate between parabionts equally, such as short-lived neutrophils, dendritic cell (DC) precursors, and inflammatory monocytes (42). Furthermore, inflammation from the procedure may effectively recruit and retain circulating memory T cells in tissues, blurring distinctions between circulatory and resident subsets in certain sites (43–45).
Fig. 1. Approaches for the study of TRM in mice and humans.
(A) Parabiosis. Parabiotic surgery, shown here as the conjoining of the skin between two congenic mice, results in anastomosis of the vasculature assess in vivo circulatory potential. TRM (generated by previous infection in one of the mice) fail to equilibrate between parabionts, providing evidence for tissue residency. (B) In vivo antibody labeling. Intravascular injection of fluorescently labeled or depleting antibodies targets cells in vascular compartments, leaving those outside of the circulation (within the tissue parenchyma) protected from antibody labeling. (C) Tissue transplantation. Analysis of donor and recipient T cells within transplanted organs and tissues allow assessment of retention of donor T cells within (and outside) the graft and influx of recipient cells from circulation. (D) Cell phenotype and transcriptional profile. Expression of CD69, CD103, and other TRM accessory markers (Table 1) by means of flow cytometry is frequently used to define TRM in human tissues or mouse models of infection. Transcriptional profiling (such as microarray or RNA-sequencing) has been used to define gene signatures and TFs associated with tissue residency. Credit: A Kitterman/Science Immunology
A less laborious approach to identifying TRM in mice is through in vivo labeling of circulating cells with fluorochrome-conjugated antibodies by means of intravascular injection, which rapidly marks all cells in or accessible to the vasculature (Fig. 1B). T cells that are tissue resident at the time of injection are “protected” from circulating antibody and have been shown to localize in different tissue niches (7, 8, 11, 46). This method can therefore serve as a rapid assessment of localization and residence of T cells, particularly in mucosal sites, although is less useful for TRM in BM and liver sinusoids (8). Another related technique to distinguish circulating T cells from TRM based on accessibility to antibody infusion is administering T cell depletional antibodies or agents that promote lymphopenia and prevent T cell egress. Treatment of mice with anti-Thy-1 depletional antibodies effectively eliminates circulating T cells while sparing TRM, as assessed in the FRT and lung (10, 47, 48). In addition, treatment of mice with FTY720, an S1PR1 agonist, causes peripheral lymphopenia and prevents tissue egress, resulting in depletion of circulating T cells from tissues while maintaining CD4+ and CD8+TRM at similar numbers to control-treated mice, as in the lung and FRT (11, 49–52). Last, transplantation of peripheral tissues, such as sections of the skin or intestine into congenic or naive mice (Fig. 1C), also permits the assessment of residency potential and persistence of putative donor TRM in the graft (4–6, 53). The findings from these and other studies in mice have demonstrated that TRM are a distinct, noncirculating population of long-lived memory T cells.
Similar criteria by using parabiosis and in vivo labeling to establish tissue residency cannot be applied to human T cells, and cell surface phenotyping combined with transcriptional profiling is often relied on to designate TRM populations (Fig. 1D). However, certain clinical situations involving T cell depletional therapies and sampling from organ and composite tissue transplantation has provided evidence for the persistence of tissue memory T cells maintained distinct from circulating counterparts (Table 1). Treatment of cutaneous T cell lymphoma patients with anti-CD52 depleting antibodies (alemtuzumab) eliminated circulating T cells from the blood but spared a persisting resident population of CD4+ and CD8+ memory T cells in the skin (54). Transplantation of human lymphocyte antigen (HLA)–disparate organs that contain endogenous TRM has created a natural experiment for assessing potential persistence of donor-derived TRM and development of tissue T cell populations from circulating recipient T cells (Fig. 1C). In intestinal transplants, donor-derived T cells were detected both in circulation and within the intestinal graft up to a year after transplant with TRM phenotype cells in the intestinal graft (55). Similarly, epidermal CD8+T cells of donor origin in face transplant recipients were observed up to 2 years after transplantation (56). A recent study in lung transplantation identified the exclusive persistence of donor-derived TRM phenotype cells >1 year after transplantation in the transplanted lung but not in the recipient blood (57), providing definitive evidence for tissue-specific retention of human TRM. Together, these results indicate that human TRM persist in the tissue niche long term, similar to mouse TRM.
Distinguishing features of TRM
Defining cell surface markers that distinguish TRM from circulating memory T cell subsets in both mouse and human tissues remains an ongoing endeavor. CD69, originally defined as an early T cell activation marker, is expressed by a large proportion of tissue memory CD4+ and CD8+ T cells in mice and humans and is generally considered a canonical TRM marker on the basis of both functional and transcriptional evidence. Functionally, CD69 serves as a signal for tissue retention by binding to and sequestering the sphingosine-1-phosphate receptor (S1PR1), which is required for tissue egress (58–60); down-regulation of the TF KLF2, which controls S1PR1 expression, is essential for TRM formation in mice (61). Whereas transient expression of CD69 on recently activated effector T cells similarly serves to retain them in the LNs (58), CD69 expression by TRM is constitutive and not associated with expression of activation markers like CD25, CD38, and HLA-DR (18, 22). In addition, the majority of mouse CD8+TRM retained in tissues during parabiosis studies express CD69 (40). Importantly, sorting for CD69 expression on human tissue memory T cells defines a TRM transcriptional profile on the basis of homology to mouse TRM that is distinct from that of CD69– memory T cells in tissues and blood (18). Interestingly, genetic deletion of CD69 in mice results in a reduction (but not ablation) of CD8+TRM in the skin and lung (14, 41) and does not affect CD4+TRM formation (62). Taken together, these findings establish that although CD69 expression can identify TRM in tissues, CD69 expression per se is not necessarily sufficient for TRM formation.
CD103, the αE- subunit of the αEβ7-integrin that binds E-cadherin expressed on epithelial cells (63), is expressed by a subset of mouse and human CD8+memory T cells in mucosal and barrier tissue sites (5, 16, 64, 65). CD103 expression by TRM is more limited in lineage and tissue location compared with CD69. In humans and mice, CD8+ TRM within mucosal tissues—including the skin, lungs, salivary glands, and small intestines—are enriched for CD103 expression (14, 17, 32, 41, 65–68) likely because E-cadherin:αEβ7-integrin interactions anchor CD103+TRM in specific locations within the mucosal epithelium. Accordingly, the formation of CD8+TRM in the skin of CD103-deficient mice are reduced in number and exhibit increased motility compared with wild-type controls (14, 69). However, CD8+ TRM in lymphoid organs such as BM, spleen, and LN do not express CD103 at steady-state (18, 70, 71), possibly because of limited interactions with epithelial cells in these sites. The role of CD103 on CD4+TRM is less clear; many CD4+TRM in mice and humans do not express CD103 (18, 43, 66, 72, 73), although substantial populations of CD103+CD4+TRM can be detected in lungs, intestines, and skin (18, 45, 65, 66). Transcriptional up-regulation of ITGAE (CD103) was identified as a core signature marker for both CD8+ and CD4+ TRM in humans, based on up-regulated expression in CD69+ compared with CD69– memory T cells (18). Therefore, CD69 expression along with CD103 in certain sites reliably distinguishes mouse and human TRM from their circulating counterparts.
A growing catalog of molecules that control homing, migration, and function have been found to be differentially expressed on TRM compared with circulating TEM cells based on additional profiling on the cellular and transcriptional level (Table 1). In terms of homing and chemokine receptors, mouse and human CD4+ and CD8+TRM express the integrin CD49a, most often in the lung and skin (5, 14, 18, 74, 75). CD49a (integrin α1) binds to CD29 (integrin β1) to from VLA-1, an integrin specific to collagen (76), suggesting that CD49a may play an important role in adhesion of TRM near collagen-rich basement membrane of the epithelium. Accordingly, antibody blockade or genetic deletion of VLA-1 in mice results in impaired retention of CD8+ TRM in peripheral tissues (77). CD49a expression can also delineate subsets of human skin TRM with distinct cytokine profiles (74). CXCR6, a chemokine receptor which binds CXCL16, is another core signature marker of TRM in both human tissues (18) and in the mouse skin and liver, where it promotes CD8+TRM establishment (69, 78). TRM also exhibit down-regulated expression of CX3CR1 compared with circulating memory T cells in mice and humans (18, 79), indicating that precise regulation of homing and migration are required for TRM persistence.
Functionally, TRM differ in several respects from circulating memory T cells. Mouse and human CD4+ and CD8+TRM exhibit elevated levels of transcripts encoding inflammatory cytokines and cytotoxicity-associated genes compared with circulating memory T cells (13, 14, 18, 23, 32, 74), suggesting a poised state for rapid effector function upon activation. Interestingly, CD4+ and CD8+ TRM also express multiple surface molecules associated with inhibition of T cell function, including PD-1 (18, 27, 32), a key marker of T cell exhaustion associated with functional hyporesponsiveness in chronic infection and tumors (80). Human TRM also express CD101 (18), shown to inhibit proliferation and interleukin-2 (IL-2) production (81). Similarly, mouse skin TRM generated from herpes simplex virus (HSV) infection express inhibitory receptors PD-1, Tim-3, LAG3, CTLA4, and CD101 (82). It is tempting to speculate that the constitutive expression inhibitory receptors on TRM may be a critical adaptation to prevent excessive activation or inflammation in the tissue niche. Coexpression of proinflammatory markers in the context of increased expression inhibitory molecules by TRM may enable TRM to be self-regulating in the tissue site.
Mechanisms for TRM generation and maintenance
The designation of TRM as a distinct subset with its own transcriptional profile raises an important question concerning the identity of the TFs that drive TRM formation (Table 1). Several TFs—including Hobit, Blimp, Runx3, and Notch—were found in mouse infection models to promote CD8+ TRM formation and were identified on the basis of up-regulated expression of the TF or its transcriptional targets in TRM relative to circulating memory T cells (13, 32, 83). The TFs that drive human TRM formation remain as yet undefined. NOTCH1/RBPJ is up-regulated in human TRM as part of the core gene signature (18, 32, 33), whereas ZNF683(Hobit) transcripts are expressed at low levels in human TRM, albeit with up-regulated expression in lung CD8+TRM compared with circulating memory T cells (18, 32, 33). However, high-resolution single-cell transcriptomic profiling of human donor and recipient-derived T cells in airway samples of lung transplant recipients revealed up-regulated expression of ZNF683 and RUNX3 in long-lived donor TRM relative to recipient T cells that enter the lung from circulation (57). Importantly, RBPJ was up-regulated in recipient-derived TRM rather than those from the lung donor, suggesting that Notch signaling may be involved in newly formed rather than “mature” TRM. This finding also raises the important issue that sampling human TRM persisting in tissues may identify up-regulated factors required for TRM maintenance rather than development. Investigating gene expression of newly established human TRM, in the context of organ transplantation or within skin lesions for example, may be an effective strategy to capture TF activity that drives TRM formation in humans.
The specific signals required for the long-term maintenance of TRM with diverse tissues remains an active area of investigation in mouse models. TRM up-regulate receptors for the homeostatic cytokines IL-7 and IL-15 (17, 84), similar to circulating memory T cells, suggesting that these cytokines may also contribute to TRM maintenance. IL-15 is essential for long-term maintenance and/or survival for some CD8+ TRM populations in tissues such as in the skin, lungs, and salivary glands but not for other sites, including the intestines and FRT (72, 84–86). In the skin, both IL-7 and IL-15 together maintain CD8+TRM, whereas IL-7 alone is required for CD4+ TRM persistence (87). Furthermore, CD103+CD8+ TRM in the intestines and skin require tumor growth factor–β (TGF-β) for long-term retention, likely for promoting CD103 expression that anchors the cells to the epithelium (14, 88). Although the specific requirements for TRM maintenance throughout diverse tissues are incompletely understood, these studies suggest that TRM in different tissues may require distinct signals for their maintenance and survival.
After their formation, TRM can stably persist in many tissue sites, including the skin, gut, brain, liver, and lung (6, 11, 12, 64, 89). Their longevity, however, may depend on the tissue of residence and host factors. Strikingly, in the lungs of mice infected with influenza A virus (IAV), CD8+ TRM were found to diminish over time owing to apoptosis and a lack of replenishment from circulating TEM cells (90, 91). Lung CD4+ TRM also generated by IAV persisted for several months after infection and did not require replenishment from circulation over the short term (11, 48). Moreover, lung CD4+ TRM generated from allergen exposure maintained constant numbers and the ability to mediate immunopathology over months in vivo, whereas numbers of CD8+TRM underwent rapid attrition (47, 48). These findings suggest different requirements for maintenance of mouse CD8+ and CD4+ TRM.
In human tissues, there is evidence that TRM in certain sites are long-lived. The overall proportion of TRM is set early in life and is maintained at a constant frequency for a particular site (lung, intestines, and secondary lymphoid organs) for decades of life into old age (17, 19, 24). Although this analysis does not assess antigen-specific populations, these results reveal homeostatic maintenance of stable TRM populations. Lung TRM of donor origin in transplanted lungs were found to persist for up to 15 months after transplantation in a number of recipients (57), providing direct evidence that human lung TRM can persist in vivo. TRM in the lung and other human tissues exhibit lower frequencies of Ki67+ cells indicative of proliferating cells as compared with circulating T cells in blood and other sites (17), suggesting lower rates of turnover of tissue as compared with circulating T cells. In human skin, there is evidence for long-term maintenance of antigen-specific TRM, based on studies of skin lesions in psoriasis, an inflammatory skin condition. Lesions and flare-ups are known to occur at the same location over years, and this is associated with the presence of clonal populations of skin TRM that produce IL-17 (92). It is possible that the continuous exposure of human tissues to multiple microbial and nonmicrobial antigens over time may create environments that promote prolonged TRM maintenance that may not be recapitulated in conventional inbred mouse models.
Tissue localization and adaptations of TRM
TRM are broadly distributed throughout the body and have been identified in almost as many human tissues as in mice, including the skin, lungs, intestines, salivary glands, brain, liver, and lymphoid organs (16–18, 25, 27–29, 31, 32, 54, 71, 75). Importantly, the extent of CD69 and CD103 expression by CD4+ and CD8+ TRM is a feature of the specific tissue that is highly conserved between individuals (17, 18, 22). This conservation of tissue-specific TRM phenotypes across individuals suggests distinct influences of the microenvironment on TRM, although tissue influences on TRM function, longevity, and homeostasis are only beginning to be defined for mice and humans. In particular, T cells in skin, lung, and lymphoid organs exhibit distinct properties as described below and diagrammed in Fig. 2.
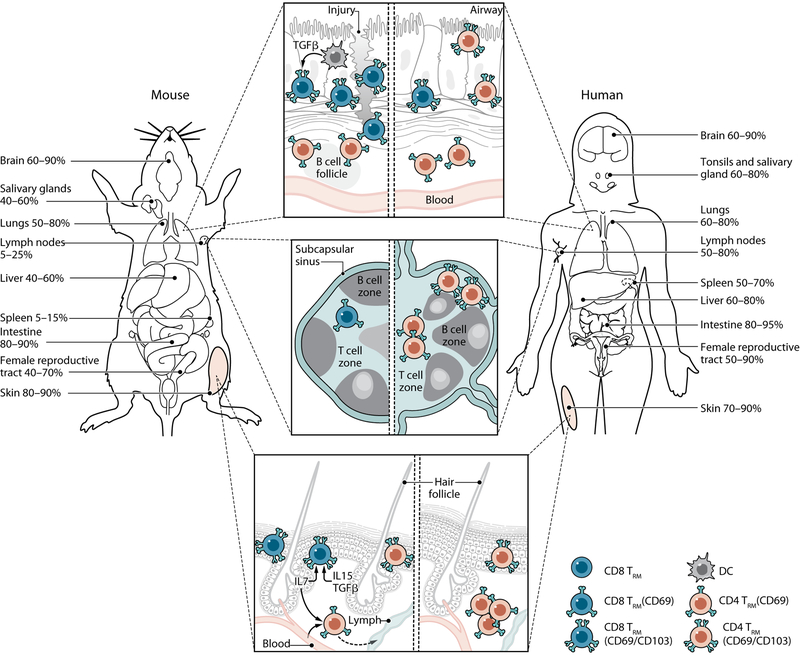
Fig. 2. TRM compartmentalization and tissue niches in mice and humans.
Noncirculating TRM take up residence in distinct mucosal, lymphoid, and barrier sites throughout mice and human tissues. Percentages in each tissue denote the frequency of T cells that exhibit surface markers indicating residency (CD69 +/−CD103) in both humans and mouse models of infection with references indicated. (Top) In mice, CD103+CD8+ TRM accumulate in within the lung epithelium or areas of tissue injury and are maintained by DCs producing TGF-β. CD4+TRM localize around B cell follicles. In humans, CD103+CD8+ TRM accumulate in the epithelium, and CD103–CD4+TRM are common in the lamina propria. (Middle) LN TRM are infrequent in conventional specific pathogen–free mice, lack CD103 and reside primarily near the subcapsular sinus. LN CD4+ and CD8+ TRM in humans are more abundant as compared with mice. (Bottom) CD103+CD8+ TRM in previously infected mice are the most prevalent skin TRM population, reside in the epidermis, and are maintained by IL-7, IL-5, and TGF-β signals. A proportion of CD4+ T cells expressing CD69 in the dermis retain their ability to recirculate. In humans, dermal CD103–CD4+ TRM are the most prevalent population, whereas CD103+CD8+ TRM predominate in the epidermis. Credit: A Kitterman/Science Immunology
In healthy human skin, the majority of TRM are dermal CD4+CD69+CD103– cells, with the epidermis containing mixed populations of CD4+ and CD8+ TRM expressing CD103 (66). These TRM express high levels of the cutaneous lymphocyte antigen (CLA) and specific chemokine receptors such as CCR4, and a proportion up-regulates the chemokine receptor CCR8 upon entry into the human skin niche (93, 94). In naïve mice, skin CD103+/−CD4+ TRM are also most prevalent (45), although large numbers of CD103+CD8+ TRM accumulate in the epidermis after infection (65). Notably, a large proportion of dermal CD4+memory T cells retain their ability to circulate (45). The chemokine receptors CXCR3, CXCR6, and CCR10 mediate formation and/or maintenance of skin TRM in mice (14, 69). A recent study in mice also found that skin TRM may alter their metabolism within specific sites; CD8+ TRM acquire a program of exogenous lipid uptake and increased oxidative metabolism to persist in the skin niche and mediate immunity (95). Whether this metabolic reprogramming of TRM is a general mechanism for tissue adaptation or specific to lipid-rich epithelial tissues remains to be determined.
The lung tissue niche contains abundant populations of CD4+ and CD8+ TRM in humans and mice (7, 11, 16, 33, 40, 75, 96). Similar to the skin, optimal formation of CD8+ TRM in the mouse lungs needs cross-priming DCs within the local LN (97). Once in the lung niche, CD103+ DCs facilitate CD103 up-regulation and maintenance of CD8+ TRM through the production of TGF-β (98). Notably, CD4+ and CD8+ TRM display distinct clustering patterns within the tissue. CD8+ TRM localize within specific niches of tissue regeneration after lung injury (termed repair-associated memory depots) that aid their formation and maintenance, whereas CD4+ TRM localize to the airways or around B cell follicles (11, 41). Together, these studies in mice suggest that the lung tissue niche is a distinct and highly dynamic site for TRM generation, although whether this aspect of the lung environment in humans is conserved remains to be determined.
Studies in secondary lymphoid tissues of mice and humans demonstrate that CD4+ and CD8+ TRM are present in LNs and spleen (17, 70, 71). In conventional inbred mouse models, the majority of virus-specific T cells within lymphoid tissue are circulating with <10% resident by parabiosis (40). In humans, a much higher proportion (30 to 50%) of CD4+ and CD8+ T cells express phenotypes and transcriptional profiles of TRM cells in the spleen and LNs (17, 18, 22). Interestingly, dirty mice possess a similarly increased number of TRM in their LNs relative to conventional mice (36), suggesting that TRM accumulation in lymphoid tissue may be a function of antigen exposure. Human LN CD8+ TRM exhibit phenotypic, functional, and epigenetic signatures associated with tissue residency (22, 71). However, human LN TRM also exhibit an organ-specific signature compared with other sites with increased expression of TFs TCF-1 and LEF-1, T-follicular helper cell markers CXCR5 and CXCR4, and reduced expression of effector molecules (22). This human LN-specific profile for memory CD8+ T cells is transcriptionally similar to a population of memory CD8+ T cells identified in a chronic infection model in mice, defined by expression of CXCR5 and TCF-1 and high proliferative capacity (99). Accordingly, human LN memory CD8+ T cells also exhibit higher proliferative capacity than their counterparts in other tissues (22). These results suggest that LNs may represent a niche for long-term TRM maintenance and quiescence.
The BM is another lymphoid niche that supports the long-term maintenance of memory T cells. Human and mouse memory T cells expressing CD69 in the absence of other markers of activation can be found in large numbers in the BM; 10 to 30% of memory T cells in mice and up to 60% of TEM cells in human BM express CD69 but not CD103 (26, 100). Whether memory CD8+ T cells take up residence in the mouse BM is unclear because parabiosis experiments demonstrate that these cells equilibrate between host and partner (39), although memory CD4+T cells appear to be retained in the BM niche over the long term (101). Interestingly, genetic knockout studies in mice have shown that CD69-deficiency reduces the accumulation of CD4+ T cells in the BM, suggesting that CD69 plays a role in T cell retention in the BM (100). In humans, BM memory T cells exhibit biased specificities for pathogens usually encountered during childhood (26), and in some individuals, BM is enriched for cytomegalovirus (CMV)–specific T cells (102), suggesting compartmentalized maintenance. Further studies are needed to determine the localization of human BM TRM and their maintenance in niches.
Together, these studies in specific sites highlight that both local microenvironmental signals and availability of tissue-specific niches play critical roles in the generation and maintenance of TRM in tissues.
Role in immunity to infections
TRM act as immune sentinels and rapidly respond to infection by orchestrating local protective immune responses to eliminate pathogens. Because of their localization directly in tissues, they are able to immediately recognize infected cells or their cognate antigens presented by antigen-presenting cells, triggering a cascade of pro-inflammatory effector functions directly in situ (10). Mouse models have been highly informative in dissecting the contributions of CD4+ and CD8+ TRM in protective immunity to a growing number of infectious pathogens—including viruses, bacteria, fungi, and parasites (Table 1)—in diverse sites such as mucosal and barrier sites (lung, FRT, skin, and intestines), liver, salivary glands, and brain. In the lung, CD4+ and/or CD8+ TRM mediate optimal protection against respiratory viruses such as influenza and respiratory syncytial virus (RSV), whereas circulating memory T cells appear to be dispensable (7, 90, 103). Lung CD4+TRM also provide essential protection to bacterial pathogens such as Mycobacterium tuberculosis and Bordetella pertussis (49, 104). In the FRT, CD4+ TRM in the genital mucosa were critical in protection against infection by the clinically relevant pathogen HSV-1 (43) and also mediate local viral clearance in the mouse model of systemic lymphocytic choriomeningitis virus (LCMV) infection (9, 10). CD8+TRM in the skin confer enhanced protection against local infection by HSV-1 or vaccinia virus compared with circulating memory T cells (5, 12, 105) and also protection to fungal infection such as Candida albicans, a common skin pathogen (106). Both intestinal CD4+ and CD8+ TRM contribute to protection against oral infection with the intestinal pathogen Listeria monocytogenes (67, 73), whereas CD4+ TRM elicit optimal protection against Salmonella infection (107). In other sites, liver TRM protect against liver infection with malaria parasites (89), salivary gland CD8+ TRM control CMV infection (108), and brain CD8+ TRM can clear LCMV during secondary challenge, independent of circulating memory T cells (109). Together, these findings demonstrate the importance of both CD4+ and CD8+ TRM in tissue-localized immunity to pathogens, with CD8+TRM more prevalent in viral and parasitic infections whereas CD4+ TRM mediate protection to bacterial and fungal invaders.
Determining mechanisms by which TRM mediate protective immunity is an active area of investigation and may differ depending on the tissue site and the cellular components interacting with TRM; these specific factors have not yet been identified. TRM in the skin and FRT were shown to proliferate in situ after local viral or antigenic challenge (52, 82, 110), but whether proliferation was required for protection is not clear. Production of interferon-γ (IFN-γ) by TRM has been demonstrated in a number of viral infection models to be required for protection in the FRT (9, 43), skin (111), and lung (112), whereas IL-17 is required for antifungal responses in the skin (106). Direct cytotoxicity can also be important for protection because brain CD8+ TRM require both perforin and IFN-γ for optimal clearance of LCMV infection (109). Further studies are needed to further identify the targets of TRM-mediated effector function.
There is substantial evidence that TRM play key roles in orchestrating protective immune responses in humans as well. A variety of virus-specific CD4+ or CD8+ TRM have been identified in human tissues, including those specific to CMV (102), RSV (113), Epstein-Barr virus (29, 114), HSV (115), hepatitis B virus (HBV) (27), human immunodeficiency virus (HIV) (71, 116), and IAV (96, 117), demonstrating that both acute and chronic viral infections generate and shape the pool of CD8+ TRM in tissues. Tissue biopsies of human skin during HSV reactivation also show that cytotoxic CD8+ TRM are in direct contact with virally infected cells, suggesting their involvement in antiviral control (118). Importantly, several studies have correlated enhanced control of viral infection with increased populations of virus-specific TRM in human tissues. During experimental RSV infection in human volunteers, RSV-specific CD8+ TRM in airway samples correlated with reduced viral load (113). Similarly, in liver biopsies from HBV-infected patients, the frequency of hepatic CD8+ TRM that produce IL-2 was highest in patients with well-controlled infections (27). Last, HIV-positive individuals who spontaneously controlled viral replication showed higher frequencies of HIV-specific CD8+ TRM in their LNs compared with HIV-positive individuals with progressive infection (71).
TRM as a target of vaccination
The ultimate goal in translating the fundamental knowledge of TRM revealed in mouse models to humans is to develop novel vaccines for promoting protective immunity. Accordingly, preclinical mouse models of vaccination and infection have shown promising outcomes when targeting TRM responses (Table 1). Intranasal administration of a live-attenuated IAV (LAIV) vaccine generated long-term virus-specific CD4+ and CD8+ TRM in the lungs of mice, which mediated heterosubtypic protection independent of circulating memory T cells or neutralizing antibodies (50). Importantly, parenteral administration of inactivated virus or LAIV failed to generate TRM responses and provide cross-strain protection (50), demonstrating that both route and vaccine formulation (live-attenuated virus) are key determinants for TRM formation. Vaccination with Bacille Calmette-Guérin (BCG) in mice similarly demonstrated that mucosal but not subcutaneous administration of BCG generated protective TRM in the airway (119). Delivery of vaccine vectors to specific tissues has also proved successful in inducing protective TRM immune responses, including those that use IAV vectors expressing HIV antigens (120) and HPV pseudovirus that encode antigens from HSV (121) and RSV (52). These results emphasize that the current immunization approaches that administer vaccines parenterally may be less effective in generating TRM-mediated protection compared with tissue-targeted approaches.
Another immunization approach to generate TRM, designated “prime and pull,” combines vaccination (prime) with local administration of chemokines or adjuvants to recruit TRM precursors to target tissues (pull). Subcutaneous immunization with an attenuated strain of HSV-2 coupled with topically applied chemokines to the vaginal mucosa generated virus-specific CD8+ TRM cells that protected mice from lethal HSV-2 challenge in the FRT (122, 123). Variations of this approach combine prime and pull into a single inoculum, such as by using antigen complexed to antibodies targeting tissue-specific DC populations (98). A particularly successful strategy in mice used a hepatocyte-specific adenovirus expressing malaria antigens to target TRM formation in the liver and prevent liver-stage malaria and has now progressed to phase I clinical trials in humans (124).
Together, these studies provide promising proof-of-principle results that protective TRM can be generated through vaccination. Whether TRM-based vaccines can be applied to humans to prevent acute and/or chronic infections will likely be determined in the coming years. Therapeutically generated TRM must also conform to the homeostatic balance of tolerance and effector responses within tissues to prevent immunopathology. The capacity of individual human tissues to generate and maintain TRM and the minimum threshold of TRM to provide protection are essential questions that will inform optimization of protective immunity in the next generation of vaccines.
Concluding remarks
The discovery of T cells as residents in tissues has initiated a paradigm shift in the way we study and understand T cell–mediated immunity: from a circulating and surveilling population transiting through tissues to sentinels maintained in diverse anatomic compartments. Focusing the study of T cells to tissues not only changed the way we analyze mouse models of infection and immunity but posed particular challenges in studying TRM in humans, necessitating new types of sampling and tissue acquisition. Studying TRM in single-infection models in mice has served to define the key phenotypic, functional, and transcriptional features of TRM that translate to TRM in human tissue sites. Continuing to integrate human and mouse studies will be paramount in the development of strategies that harness the full potential of TRM to promote tissue immunity.
References and Notes
- 1.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A, Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Masopust D, Vezys V, Marzo AL, Lefrancois L, Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417. (2001). [DOI] [PubMed] [Google Scholar]
- 3.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK, Visualizing the generation of memory CD4 T cells in the whole body. Nature 410, 101–105. (2001). [DOI] [PubMed] [Google Scholar]
- 4.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR, Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science 319, 198–202 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Gebhardt T et al. , Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 10, 524–530 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Masopust D et al. , Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med 207, 553–564 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teijaro JR et al. , Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol 187, 5510–5514 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson KG et al. , Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc 9, 209–222 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schenkel JM et al. , T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 346, 98–101 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schenkel JM, Fraser KA, Vezys V, Masopust D, Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol 14, 509–513 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner DL et al. , Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol 7, 501–510 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang X et al. , Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature 483, 227–231 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackay LK et al. , Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 352, 459–463 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Mackay LK et al. , The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol 14, 1294–1301 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Wakim LM et al. , The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol 189, 3462–3471 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sathaliyawala T et al. , Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 38, 187–197 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thome JJ et al. , Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell 159, 814–828 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar BV et al. , Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep 20, 2921–2934 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thome JJ et al. , Early-life compartmentalization of human T cell differentiation and regulatory function in mucosal and lymphoid tissues. Nat Med 22, 72–77 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granot T et al. , Dendritic Cells Display Subset and Tissue-Specific Maturation Dynamics over Human Life. Immunity 46, 504–515 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carpenter DJ et al. , Human immunology studies using organ donors: Impact of clinical variations on immune parameters in tissues and circulation. Am J Transplant 18, 74–88 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miron M et al. , Human Lymph Nodes Maintain TCF-1(hi) Memory T Cells with High Functional Potential and Clonal Diversity throughout Life. J Immunol 201, 2132–2140 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar BV et al. , Functional heterogeneity of human tissue-resident memory T cells based on dye efflux capacities. JCI Insight 3, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Senda T et al. , Microanatomical dissection of human intestinal T-cell immunity reveals site-specific changes in gut-associated lymphoid tissues over life. Mucosal Immunol 12, 378–389 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Booth JS et al. , Characterization and functional properties of gastric tissue-resident memory T cells from children, adults, and the elderly. Front Immunol 5, 294 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okhrimenko A et al. , Human memory T cells from the bone marrow are resting and maintain long-lasting systemic memory. Proc Natl Acad Sci U S A 111, 9229–9234 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pallett LJ et al. , IL-2(high) tissue-resident T cells in the human liver: Sentinels for hepatotropic infection. J Exp Med 214, 1567–1580 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong MT et al. , A High-Dimensional Atlas of Human T Cell Diversity Reveals Tissue-Specific Trafficking and Cytokine Signatures. Immunity 45, 442–456 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Woon HG et al. , Compartmentalization of Total and Virus-Specific Tissue-Resident Memory CD8+ T Cells in Human Lymphoid Organs. PLoS Pathog 12, e1005799 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swaims-Kohlmeier A et al. , Progesterone Levels Associate with a Novel Population of CCR5+CD38+ CD4 T Cells Resident in the Genital Mucosa with Lymphoid Trafficking Potential. J Immunol 197, 368–376 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smolders J et al. , Tissue-resident memory T cells populate the human brain. Nat Commun 9, 4593 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hombrink P et al. , Programs for the persistence, vigilance and control of human CD8(+) lung-resident memory T cells. Nat Immunol 17, 1467–1478 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Oja AE et al. , Trigger-happy resident memory CD4(+) T cells inhabit the human lungs. Mucosal Immunol 11, 654–667 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Clark RA, Resident memory T cells in human health and disease. Sci Transl Med 7, 269rv261 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muruganandah V, Sathkumara HD, Navarro S, Kupz A, A Systematic Review: The Role of Resident Memory T Cells in Infectious Diseases and Their Relevance for Vaccine Development. Front Immunol 9, 1574 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beura LK et al. , Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532, 512–516 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reese TA et al. , Sequential Infection with Common Pathogens Promotes Human-like Immune Gene Expression and Altered Vaccine Response. Cell Host Microbe 19, 713–719 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beura LK et al. , T Cells in Nonlymphoid Tissues Give Rise to Lymph-Node-Resident Memory T Cells. Immunity 48, 327–338 e325 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klonowski KD et al. , Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity 20, 551–562 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Steinert EM et al. , Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell 161, 737–749 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takamura S et al. , Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J Exp Med 213, 3057–3073 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu K et al. , Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol 8, 578–583 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Iijima N, Iwasaki A, T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 346, 93–98 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nolz JC, Harty JT, IL-15 regulates memory CD8+ T cell O-glycan synthesis and affects trafficking. J Clin Invest 124, 1013–1026 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collins N et al. , Skin CD4(+) memory T cells exhibit combined cluster-mediated retention and equilibration with the circulation. Nat Commun 7, 11514 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson KG et al. , Cutting edge: intravascular staining redefines lung CD8 T cell responses. J Immunol 189, 2702–2706 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hondowicz BD et al. , Interleukin-2-Dependent Allergen-Specific Tissue-Resident Memory Cells Drive Asthma. Immunity 44, 155–166 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turner DL et al. , Biased Generation and In Situ Activation of Lung Tissue-Resident Memory CD4 T Cells in the Pathogenesis of Allergic Asthma. J Immunol 200, 1561–1569 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilk MM et al. , Lung CD4 Tissue-Resident Memory T Cells Mediate Adaptive Immunity Induced by Previous Infection of Mice with Bordetella pertussis. J Immunol 199, 233–243 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Zens KD, Chen JK, Farber DL, Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight 1, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Florido M et al. , Pulmonary immunization with a recombinant influenza A virus vaccine induces lung-resident CD4(+) memory T cells that are associated with protection against tuberculosis. Mucosal Immunol, (2018). [DOI] [PubMed] [Google Scholar]
- 52.Cuburu N et al. , Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. J Clin Invest 122, 4606–4620 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malik BT et al. , Resident memory T cells in the skin mediate durable immunity to melanoma. Sci Immunol 2, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clark RA et al. , Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci Transl Med 4, 117ra117 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zuber J et al. , Bidirectional intragraft alloreactivity drives the repopulation of human intestinal allografts and correlates with clinical outcome. Sci Immunol 1, eaah3732 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lian CG et al. , Biomarker evaluation of face transplant rejection: association of donor T cells with target cell injury. Mod Pathol 27, 788–799 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Snyder ME, Finlayson MO, Connors T, Dogra P, Farber DL, Generation and persistence of human tissue-resident memory T cells in lung transplantation. Science Immunol. 4, eaav5581 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shiow LR et al. , CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440, 540–544 (2006). [DOI] [PubMed] [Google Scholar]
- 59.Pham TH, Okada T, Matloubian M, Lo CG, Cyster JG, S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity 28, 122–133 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mackay LK et al. , Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J Immunol 194, 2059–2063 (2015). [DOI] [PubMed] [Google Scholar]
- 61.Skon CN et al. , Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol 14, 1285–1293 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ugur M, Schulz O, Menon MB, Krueger A, Pabst O, Resident CD4+ T cells accumulate in lymphoid organs after prolonged antigen exposure. Nat Commun 5, 4821 (2014). [DOI] [PubMed] [Google Scholar]
- 63.Cepek KL et al. , Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature 372, 190–193 (1994). [DOI] [PubMed] [Google Scholar]
- 64.Wakim LM, Woodward-Davis A, Bevan MJ, Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci U S A 107, 17872–17879 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gebhardt T et al. , Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 477, 216–219 (2011). [DOI] [PubMed] [Google Scholar]
- 66.Watanabe R et al. , Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med 7, 279ra239 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sheridan BS et al. , Oral infection drives a distinct population of intestinal resident memory CD8(+) T cells with enhanced protective function. Immunity 40, 747–757 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hofmann M, Pircher H, E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc Natl Acad Sci U S A 108, 16741–16746 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zaid A et al. , Chemokine Receptor-Dependent Control of Skin Tissue-Resident Memory T Cell Formation. J Immunol 199, 2451–2459 (2017). [DOI] [PubMed] [Google Scholar]
- 70.Schenkel JM, Fraser KA, Masopust D, Cutting edge: resident memory CD8 T cells occupy frontline niches in secondary lymphoid organs. J Immunol 192, 2961–2964 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buggert M et al. , Identification and characterization of HIV-specific resident memory CD8(+) T cells in human lymphoid tissue. Sci Immunol 3, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Strutt TM et al. , IL-15 supports the generation of protective lung-resident memory CD4 T cells. Mucosal Immunol 11, 668–680 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Romagnoli PA et al. , Differentiation of distinct long-lived memory CD4 T cells in intestinal tissues after oral Listeria monocytogenes infection. Mucosal Immunol 10, 520–530 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheuk S et al. , CD49a Expression Defines Tissue-Resident CD8(+) T Cells Poised for Cytotoxic Function in Human Skin. Immunity 46, 287–300 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Purwar R et al. , Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLoS One 6, e16245 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roberts AI, Brolin RE, Ebert EC, Integrin alpha1beta1 (VLA-1) mediates adhesion of activated intraepithelial lymphocytes to collagen. Immunology 97, 679–685 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ray SJ et al. , The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity 20, 167–179 (2004). [DOI] [PubMed] [Google Scholar]
- 78.Tse SW, Radtke AJ, Espinosa DA, Cockburn IA, Zavala F, The chemokine receptor CXCR6 is required for the maintenance of liver memory CD8(+) T cells specific for infectious pathogens. J Infect Dis 210, 1508–1516 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gerlach C et al. , The Chemokine Receptor CX3CR1 Defines Three Antigen-Experienced CD8 T Cell Subsets with Distinct Roles in Immune Surveillance and Homeostasis. Immunity 45, 1270–1284 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wherry EJ, T cell exhaustion. Nat Immunol 12, 492–499 (2011). [DOI] [PubMed] [Google Scholar]
- 81.Soares LR, Tsavaler L, Rivas A, Engleman EG, V7 (CD101) ligation inhibits TCR/CD3-induced IL-2 production by blocking Ca2+ flux and nuclear factor of activated T cell nuclear translocation. J Immunol 161, 209–217 (1998). [PubMed] [Google Scholar]
- 82.Park SL et al. , Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat Immunol 19, 183–191 (2018). [DOI] [PubMed] [Google Scholar]
- 83.Milner JJ et al. , Runx3 programs CD8(+) T cell residency in non-lymphoid tissues and tumours. Nature 552, 253–257 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mackay LK et al. , T-box Transcription Factors Combine with the Cytokines TGF-beta and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity 43, 1101–1111 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Schenkel JM et al. , IL-15-Independent Maintenance of Tissue-Resident and Boosted Effector Memory CD8 T Cells. J Immunol 196, 3920–3926 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Verbist KC, Field MB, Klonowski KD, Cutting edge: IL-15-independent maintenance of mucosally generated memory CD8 T cells. J Immunol 186, 6667–6671 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adachi T et al. , Hair follicle-derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat Med 21, 1272–1279 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang N, Bevan MJ, Transforming growth factor-beta signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity 39, 687–696 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fernandez-Ruiz D et al. , Liver-Resident Memory CD8(+) T Cells Form a Front-Line Defense against Malaria Liver-Stage Infection. Immunity 45, 889–902 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Wu T et al. , Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol 95, 215–224 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Slutter B et al. , Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci Immunol 2, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matos TR et al. , Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing alphabeta T cell clones. J Clin Invest 127, 4031–4041 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCully ML et al. , CCR8 Expression Defines Tissue-Resident Memory T Cells in Human Skin. J Immunol 200, 1639–1650 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clark RA et al. , The vast majority of CLA+ T cells are resident in normal skin. J Immunol 176, 4431–4439 (2006). [DOI] [PubMed] [Google Scholar]
- 95.Pan Y et al. , Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 543, 252–256 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pizzolla A et al. , Influenza-specific lung-resident memory T cells are proliferative and polyfunctional and maintain diverse TCR profiles. J Clin Invest 128, 721–733 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iborra S et al. , Optimal Generation of Tissue-Resident but Not Circulating Memory T Cells during Viral Infection Requires Crosspriming by DNGR-1(+) Dendritic Cells. Immunity 45, 847–860 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wakim LM, Smith J, Caminschi I, Lahoud MH, Villadangos JA, Antibody-targeted vaccination to lung dendritic cells generates tissue-resident memory CD8 T cells that are highly protective against influenza virus infection. Mucosal Immunol 8, 1060–1071 (2015). [DOI] [PubMed] [Google Scholar]
- 99.Im SJ et al. , Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shinoda K et al. , Type II membrane protein CD69 regulates the formation of resting T-helper memory. Proc Natl Acad Sci U S A 109, 7409–7414 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tokoyoda K et al. , Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity 30, 721–730 (2009). [DOI] [PubMed] [Google Scholar]
- 102.Gordon CL et al. , Tissue reservoirs of antiviral T cell immunity in persistent human CMV infection. J Exp Med 214, 651–667 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Morabito KM et al. , Intranasal administration of RSV antigen-expressing MCMV elicits robust tissue-resident effector and effector memory CD8+ T cells in the lung. Mucosal Immunol 10, 545–554 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sakai S et al. , Cutting edge: control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T cells. J Immunol 192, 2965–2969 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mackay LK et al. , Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci U S A 109, 7037–7042 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Park CO et al. , Staged development of long-lived T-cell receptor alphabeta TH17 resident memory T-cell population to Candida albicans after skin infection. J Allergy Clin Immunol 142, 647–662 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Benoun JM et al. , Optimal protection against Salmonella infection requires noncirculating memory. Proc Natl Acad Sci U S A 115, 10416–10421 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thom JT, Weber TC, Walton SM, Torti N, Oxenius A, The Salivary Gland Acts as a Sink for Tissue-Resident Memory CD8(+) T Cells, Facilitating Protection from Local Cytomegalovirus Infection. Cell Rep 13, 1125–1136 (2015). [DOI] [PubMed] [Google Scholar]
- 109.Steinbach K et al. , Brain-resident memory T cells represent an autonomous cytotoxic barrier to viral infection. J Exp Med 213, 1571–1587 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Beura LK et al. , Intravital mucosal imaging of CD8(+) resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nat Immunol 19, 173–182 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ariotti S et al. , T cell memory. Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science 346, 101–105 (2014). [DOI] [PubMed] [Google Scholar]
- 112.McMaster SR, Wilson JJ, Wang H, Kohlmeier JE, Airway-Resident Memory CD8 T Cells Provide Antigen-Specific Protection against Respiratory Virus Challenge through Rapid IFN-gamma Production. J Immunol 195, 203–209 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jozwik A et al. , RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat Commun 6, 10224 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hislop AD et al. , Tonsillar homing of Epstein-Barr virus-specific CD8+ T cells and the virus-host balance. J Clin Invest 115, 2546–2555 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Posavad CM et al. , Enrichment of herpes simplex virus type 2 (HSV-2) reactive mucosal T cells in the human female genital tract. Mucosal Immunol 10, 1259–1269 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kiniry BE et al. , Detection of HIV-1-specific gastrointestinal tissue resident CD8(+) T-cells in chronic infection. Mucosal Immunol 11, 909–920 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Piet B et al. , CD8(+) T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung. J Clin Invest 121, 2254–2263 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu J et al. , Immune surveillance by CD8alphaalpha+ skin-resident T cells in human herpes virus infection. Nature 497, 494–497 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Perdomo C et al. , Mucosal BCG Vaccination Induces Protective Lung-Resident Memory T Cell Populations against Tuberculosis. MBiol. 7, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tan HX et al. , Induction of vaginal-resident HIV-specific CD8 T cells with mucosal prime-boost immunization. Mucosal Immunol 11, 994–1007 (2018). [DOI] [PubMed] [Google Scholar]
- 121.Cuburu N et al. , Topical herpes simplex virus 2 (HSV-2) vaccination with human papillomavirus vectors expressing gB/gD ectodomains induces genital-tissue-resident memory CD8+ T cells and reduces genital disease and viral shedding after HSV-2 challenge. J Virol 89, 83–96 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shin H, Iwasaki A, A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491, 463–467 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shin H, Kumamoto Y, Gopinath S, Iwasaki A, CD301b+ dendritic cells stimulate tissue-resident memory CD8+ T cells to protect against genital HSV-2. Nat Commun 7, 13346 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gola A et al. , Prime and target immunization protects against liver-stage malaria in mice. Sci Transl Med 10, (2018). [DOI] [PubMed] [Google Scholar]