Revisiting PI3-kinase signalling in angiogenesis (original) (raw)
Abstract
PI3Ks belong to a family of lipid kinases that comprises eight isoforms. They phosphorylate the third position of the inositol ring present in phosphatidylinositol lipids and, in turn, activate a broad range of proteins. The PI3K pathway regulates primal cellular responses, including proliferation, migration, metabolism and vesicular traffic. These processes are fundamental for endothelial cell function during sprouting angiogenesis, the most common type of blood vessel formation. Research in animal models has revealed key functions of PI3K family members and downstream effectors in angiogenesis. In addition, perturbations in PI3K signalling have been associated with aberrant vascular growth including tumour angiogenesis and vascular malformations. Together, this highlights that endothelial cells are uniquely sensitive to fluctuations in PI3K signalling. Here, we aim to update the current view on this important signalling cue in physiological and pathological blood vessel growth.
Keywords: angiogenesis, PI3K, vascular malformations
Tissue homeostasis is maintained through a functional network of blood vessels that provide nutrients and oxygen and remove the metabolic waste and carbon dioxide. Therefore, it is of little surprise that the cardiovascular system is one of the first to develop during mammalian embryogenesis. While the primitive vascular plexus arises de novo from mesoderm-derived cells (the so-called angioblasts), the majority of vessels develop by sprouting angiogenesis, a process of vessel formation from existing ones (1, 2). Upon exposure to proangiogenic stimuli, endothelial cells, which line up the inner part of the vascular tubes, undergo dynamic and complex morpho-biochemical changes that allow them to invade and expand in avascularised tissues. Endothelial cells that acquire migratory properties, referred to as tip cells, are followed by proliferative stalk cells that make up the structure of the nascent vessel. This unique plasticity of endothelium to respond, adapt and rearrange requires rigorous regulatory mechanisms which prevent from uncontrolled vascular growth, a pathological situation frequently occurring in diseases (e.g. tumour growth, vascular eye disease or overgrowth syndromes) (1, 2).
PI3K (phosphatidylinositol 3-kinase) signalling constitutes one of the key nodes that control a plethora of cellular functions, including growth, migration, actin cytoskeleton remodelling, metabolism and vesicular traffic (3, 4, 5). PI3Ks generate a pool of different phosphatidylinositol derivates, all phosphorylated at the third position of the inositol headgroup, that mediate the transduction of extracellular signals as well as the sorting of membrane vesicles (3, 4). This highly conserved family of lipid enzymes consists of eight catalytical isoforms that, based on their substrate preferences, are grouped into three main classes.
Class I PI3Ks are heterodimers, composed of one of the p110 catalytic subunits in complex with one of the regulatory subunits. Based on the type of the regulatory subunit that they bind, class I PI3Ks are further subdivided into class IA (PI3Kα, PI3Kβ, PI3Kδ) that binds to one of the five p85 regulatory isoforms and class IB (PI3Kγ) that couples with either p84 or p101 regulatory subunits. Despite differences in ways of activation, all class I PI3Ks produce phosphatidylinositol 3,4,5-trisphosphate (PtdIns(3,4,5)P3, also known as PIP3). On the other hand, the three class II isoforms, PI3K-C2α, PI3K-C2β and PI3K-C2γ, give rise to two distinct lipid products – phosphatidylinositol 3-phosphate (PtdIns3P) and phosphatidylinositol 3,4-bisphosphate (PtdIns(3,4)P2) – while the only class III isoform – Vps34 – forms only PtdIns3P (3, 4, 5).
This review focuses on the current knowledge on the role of the PI3K pathway in angiogenesis. Moreover, we will highlight the pathological consequences, when this signalling hub is deregulated in the endothelium in vivo. For more detailed aspects of the PI3K pathway and its modes of activation, we refer the reader to Ref (3, 4, 6).
The PI3K pathway in developmental angiogenesis
Genetic targeting of PI3K components and downstream effectors in mice has shed light onto the role of this signalling pathway in angiogenesis (Table 1). Overall, these series of publications have revealed important observations: (i) which genes of the PI3K pathways are essential for angiogenesis, (ii) whether they play a selective role or some redundancy exists between isoforms, (iii) how this signalling pathway orchestrates the different steps of the angiogenic cascade. Details on these phenotypes are described in the next section (Fig. 1).
Table 1.
Mouse models with a genetic inactivation of selected classes I and II PI3K signaling components with their vascular phenotypes.
| Mouse model | Lineage/tissue specificity | Vascular-related phenotype | References | |
|---|---|---|---|---|
| PI3Kα | Pik3ca−/− | Global | Embryonic lethality (E9.5–10.5) and multiple vascular defects (severe haemorrhages in head and trunk, poorly developed endocardium and cardinal vein) | (9, 10) |
| Pik3ca D933A/D933A | Global | Embryonic lethality (E10.5–12.5), growth retardation and severe vascular defects | (7) | |
| Tie2-Cre, Pik3ca Flox/Flox | Endothelium, haematopoietic cells | Embryonic lethality (E10.5–12.5) and defective angiogenic growth due to impaired cell migration | ||
| Pdgfb-CreERT2, Pik3ca D933A/Flox | Endothelium | Aberrant endothelial cell rearrangements and anastomosis during sprouting angiogenesis and reduced endothelial cell proliferation | (8) | |
| PI3Kβ | Tie2-Cre, Pik3cb Flox/Flox | Endothelium, haematopoietic cells | Viable, fertile and no overvascular defects during embryonic development and improved resistance to cardiac infarction as a result of enhanced PI3K/AKT/eNOS signalling | (7, 12) |
| PTEN | Pten−/− | Global | Embryonic lethality (E9.5) and defects in placenta development as well as cephalic and caudal regions | (87) |
| Tie2-Cre, Pten Flox/Flox | Endothelium, haematopoietic cells | Embryonic lethality (E11.5) due to cardiac muscle development failure and severe haemorrhages as a result of impaired mural cells recruitment | (15) | |
| Pdgfb-CreERT2, Pten Flox/Flox | Endothelium | Vascular hyperplasia in a retina model as a result of uncontrolled stalk cell proliferation | (16) | |
| AKT | Akt1−/− | Global | Viable, defective ischemia-induced angiogenesis and endothelial progenitor cells recruitment | (19, 21) |
| Cdh5-CreERT2, Akt1Flox/Flox | Endothelium | Hampered vessel growth and increased vessel regression in the retina | (20) | |
| FOXO | Foxo1−/− | Global | Embryonic lethality (E11), highly impaired cardiovascular and yolk sac development and abnormal vascular remodelling | (27, 28) |
| Tie2-Cre, Foxo1Flox/Flox | Endothelium, haematopoietic cells | Embryonic lethality (E11), endothelial-specific Foxo1 deletion phenocopies the global Foxo1 knockout phenotype | (29) | |
| Cdh5-Cre, Foxo1Flox/Flox | Endothelium | |||
| Tie2-Cre, Foxo1Flox/Flox | Endothelium, haematopoietic cells | Increased endothelial cell proliferation, vessel enlargement and hyperplasia in retinal vasculature | (30) | |
| Pdgfb-CreERT2, Foxo1Flox/Flox | Endothelium | |||
| Foxo3a−/− | Global | Proper embryonic vascular development and augmented neovascularisation in adults upon ischemia induction | (27) | |
| mTOR | Tie2-Cre, DeptorFlox/Flox | Endothelium, haematopoietic cells | Increased number of vessels in heart, kidney and liver as a result of increased production of proangiogenic factors (HIF-1α, VEGH-A) | (25) |
| Tie2-CreERT2, RaptorFlox/Flox | Endothelium, haematopoietic cells | Improvement of blood perfusion and hindlimb recovery in ischaemic diabetic mouse model, promotion of angiogenesis and endothelial autophagy | (26) | |
| PI3K-C2α | Pi3kc2a−/− | Global | Embryonic lethality (E10.5–11.5) due to severe vascular defects in the embryo and yolk sac | (32) |
| Tie2-Cre, Pik3c2aFlox/Flox | Endothelium, haematopoietic cells | Embryonic lethality (E16.5–18.5) and impaired VE-cadherin delivery, cell junctions assembly and endosomal traffic | ||
| Cdh5-CreERT2, Pik3c2aFlox/Flox | Endothelium | Hampered retinal vascularisation |
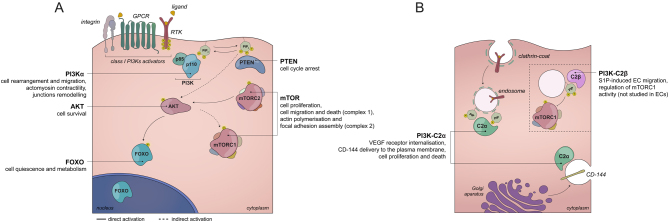
Figure 1.
PI3K signalling in endothelium. (A) Receptors activation attracts class I PI3Ks to the plasma membrane through its regulatory subunit, where the enzymatic conversion of PtdIns(4,5)P2 to PtdIns(3,4,5)P3 occurs. This lipid transduces the information by activating protein kinases, such as AKT, which in turn can activate and mediate the function of mTOR as well as FOXO transcription factors, thus triggering a multitude of cellular responses. Both, in vivo and in vitro endothelial cell-specific functions of PI3K signalling components are depicted. RTK – receptor tyrosine kinase, GPCR – G protein-coupled Receptor. (B) Activatory inputs of class II PI3Ks in the endothelium are not clear. PI3K-C2α and PI3K-C2β isoforms act as single holoenzymes at vesicular membranes, producing PtdIns(3)P and PtdIns (3,4)P2 phospholipids. While the role of PI3K-C2α in endothelial cell biology in vivo has been determined, the function of PI3K-C2β still remains obscure as most studies involved other cell types.
Class I PI3K subunits and PTEN
Among class I PI3Ks, PI3Kα predominantly governs endothelial cell behaviour during vessel growth. This is illustrated by the severe vascular defects and embryonic lethality induced by constitutive and endothelial-specific inactivation of PI3Kα (7, 8, 9, 10), a phenotype that does not occur when other class I PI3K subunits are inactivated (7). Several key functions during the expansion of the vasculature have been ascribed to PI3Kα. During vasculogenesis, it was shown that PI3Kα regulates the venous identity of endothelial cells (11). Conversely, arteriogenesis requires the suppression of PI3K signalling (12). Using mouse and zebrafish models of sprouting angiogenesis, it was identified that PI3Kα regulates endothelial cell rearrangements and junctional remodelling within the nascent tube (8, 13).
There are emerging evidences showing that other class I PI3K isoforms play a role in endothelial cells. This is the case for PI3Kβ in ischaemic hearts (14) and for PI3Kδ in inflammation (15). The inactivation of PI3Kβ in endothelial cells results in enhanced vascular endothelial growth factor (VEGF)-stimulated PI3Kα/AKT signalling and angiogenesis and, in turn, the reduction of myocardial ischaemic injury in vivo (14). This led to hypothesise that in the endothelium PI3Kβ exerts a feedback inhibition on PI3Kα. While this holds promises for PI3Kβ-targeted therapy to revascularise tissues, it still needs to be demonstrated experimentally. PI3Kδ is expressed at low levels in the endothelium under physiological status (7). Nevertheless, inflammatory cues enhance its expression, which suggest that PI3Kδ may regulate endothelial cell functions in these conditions (15). Further experiments to decipher the role of PI3Kδ in the inflamed endothelium are required.
The production of PtdIns(3,4,5)P3 is counteracted by lipid phosphatases such as PTEN (phosphatase and tensin homolog), a pivotal tumour suppressor gene (16). This is in line with the observation that the endothelial-specific loss of PTEN in mice results in deadly haemorrhages and cardiac dysfunction during early embryogenesis (17). Mechanistic studies revealed that PTEN restrains endothelial cell proliferation during critical steps of the angiogenic process. Specifically, PTEN-mediated cell cycle arrest enables both Notch-dependent stalk specification and Alk1-mediated vessel patterning (18, 19). Interestingly, PTEN regulates endothelial cell proliferation through its catalytic and nuclear scaffolding properties (18).
PI3K protein effectors
The activation of class I PI3Ks by extracellular stimuli results in the formation of PtdIns(3,4,5)P3 at the plasma membrane. This lipid transduces the chemical information by interacting with lipid-binding pleckstrin homology (PH) domains in a range of protein effectors to regulate their localisation and/or activity (3). Amongst them, the protein kinase AKT constitutes a key class I PI3K protein effector (20). This dominant role, which includes the activation of multifunctional signalling nodes such as GSK3, FOXO (Forkhead box O) and mTOR (mammalian target of rapamycin), is partially explained by the large number of AKT substrates (>100) identified until now (20). Endothelial selectivity for AKT isoforms during angiogenesis also occurs, with AKT1 playing a major role in this process (21). However, constitutive AKT1 knockout mice are viable, suggesting a certain degree of overlapping functions between AKT isoforms during vascular embryonic development (22). In sharp contrast, AKT1 is essential to sustain vessel integrity during adulthood (23).
mTOR represents another key signalling hub that converges many distinct signals, both extra- and intracellular. Amongst several inputs, mTOR can signal downstream of PI3K/AKT and act in two different multi-protein complexes referred to as mTORC1 and mTORC2, respectively (24). The generation of constitutive knockout mice of several components of mTORC1 and mTORC2 have contributed in establishing a central role of this hub in a regulation of fundamental organismal functions, including angiogenesis (24). Indeed, endothelial-specific deletion of several components of mTORC has shed light onto the autonomous role of mTOR cell in the vessel growth (25, 26). However, this is still insufficient to fully understand how and in which contexts mTOR regulates angiogenesis. Given the key role of mTOR as a metabolic sensor and the unique metabolic requirements of endothelial cells during angiogenesis, it is tempting to speculate that mTOR signalling also sustains the metabolic behaviour of the endothelium during angiogenesis.
FOXO transcription factors, another crucial component of the PI3K/AKT cascade, have also emerged as key effectors of angiogenesis. While FOXOs constitute a family of four isoforms, FOXO1 plays the main role in vascular development, with the global genetic inactivation of FOXO1 in mice leading to aberrant vascular growth and embryonic lethality (27, 28, 29). Recent data using a tamoxifen-inducible Cre strategy has showed that FOXO1 restricts endothelial cell proliferation by hampering glycolysis and oxidative phosphorylation through MYC (30).
Several studies have also showed that class I PI3K signalling regulates angiogenesis beyond AKT (8, 31). This is not surprising as it is estimated that around 10–30 proteins respond to class I PI3K lipids (4). Amongst them, a critical role of PI3Kα in regulating components of the actin machinery in the endothelium is emerging (8, 31). Noteworthy, this regulation was shown to be multifactorial, enabling a fine-tuning of the myosin light chain activity and, in turn, actin contractility. This includes both activation of the small GTPase RhoA (7, 31) and the myosin light chain phosphatase (8). Together, this highlights that the understanding of the PI3K function in the endothelium is still in its infancy and calls for the development of better tools which allow to elucidate how this signalling pathway governs angiogenesis.
Class II PI3K isoforms
The three, rather unexplored and enigmatic, isoforms of class II PI3Ks have recently received more attention (4). In particular, PI3K-C2α isoform was shown to be crucial for vasculature development in an endothelial cell-autonomous manner (32, 33). Indeed, endothelial-specific deficiency of PI3K-C2α led to embryonic lethality in mice as a result of defective angiogenesis and vessels integrity (32). The lipid kinase activity of PI3K-C2α appears to be necessary to regulate vascular development (34). On a cellular level, PI3K-C2α regulates the delivery of an essential junctional protein, VE-cadherin (CD-144), to the endothelial plasma membrane and the internalisation of activated VEGF receptors by controlling endosomal traffic (32). PI3K-C2α was also involved in primary cilium function, thus suggesting a role in shear stress sensing (33). In vitro studies have provided some insights into the role of PI3K-C2β on endothelial cells. It was shown that this isoform is required for sphingosine-1-phosphate (S1P)- and high-density lipoprotein (HDL)-induced migration (35). However, others reported that S1P-induced endothelial cell migration requires the activity of PI3K-C2α as well (36), suggesting that both isoforms might have partial overlapping functions in those cells. The expression of PI3K-C2γ is restricted to liver, pancreas and kidney, and therefore it is unlikely that it plays a role in the endothelium.
The class I PI3K pathway in pathological vessel growth
Perturbations in class I PI3K signalling have been linked to aberrant vascular growth including tumour angiogenesis and vascular malformations.
The impact of PI3K inhibitors on tumour vessels
Angiogenesis is a rate-limiting step in tumour growth; therefore, the concept of eradicating cancer by inhibiting neoangiogenesis was conceived as a unique opportunity. Nevertheless, blocking VEGF failed to improve survival as a monotherapy. Instead, a combination of anti-angiogenic drugs with a classical (untargeted) chemotherapy showed improved anti-tumour properties (37, 38, 39). These strategies were proved beneficial in renal cell carcinoma and ovarian and neuroendocrine tumours. However, other tumours such as prostate cancer, pancreatic adenocarcinoma and melanoma were resistant to anti-angiogenic therapy (37, 38). Together, this highlights the need to understand the organotypic tumour–endothelium interactions in order to improve current anti-vascular therapies.
Several studies documented that PI3K signalling sustains tumour angiogenesis either by regulating endothelial cell functions or by stimulating VEGF production (40). In line with this notion, inhibition of PI3K was shown to have an anti-angiogenic impact on a variety of preclinical models of cancer, resulting in different effects depending on the type and the dose of PI3K inhibitor used (40). A large proportion of these studies used pan-class PI3K inhibitors at high-doses, which mainly showed vascular pruning effects (41, 42, 43, 44, 45). However, PI3K inhibitors displayed a weaker anti-vascular impact than VEGF-targeted therapies (45, 46), indicating reduced applicability in these settings. Instead, low doses of PI3K inhibitors were shown to enhance vascular function and, in turn, enhance the delivery of chemotherapy to the tumour (47, 48). These studies suggest that low doses of these inhibitors may result in the so-called vessel normalisation effect (49), an approach that could be exploited to enhance drug delivery and immunotherapy influx. Yet there is no clinical data on the impact of PI3K inhibitors on tumour vessels (40). A recent clinical study combining radiotherapy and PI3K inhibitors has shown that this combination reduced tumour hypoxia compared to radiotherapy alone (50). Given that enhanced oxygenation has been proposed to be a critical biomarker for vessel normalisation (39), it is tempting to speculate that PI3K inhibitors induce a vascular normalisation effect under certain conditions (50).
Mutations of PI3K pathway in vascular malformations
Somatic genetic activation of class I PI3K is a common event in cancer, including mutational activation of PI3Kα, AKT1 and inactivation of PTEN (4, 5). Similar genetic alterations have now been found in vascular malformations, a heterogenous group of congenital diseases in which mutations are either acquired postzygotically or are present in the germline (51, 52). Activating mutations in PIK3CA and AKT mainly occur somatically as the expression of the so-called hotspots in the germline results in embryonic lethality due to vascular defects (53, 54). In contrast, germline loss of function mutations in PTEN is tolerated and leads to PTEN hamartoma tumour syndromes (PHTSs) (55). The mosaic vs germline tolerance of mutations of PI3K pathway is quite likely explained by their impact on the perturbations of the pathway, with mutations in PIK3CA and AKT1 resulting in higher aberrant activation of PI3K signalling. Thus, it is not surprising that rare and weak germline PIK3CA mutations have been detected in patients with brain overgrowth and megalencephaly-capillary malformation syndrome (MCAP) (56).
_PIK3CA_-driven vascular malformations are grouped under the umbrella of _PIK3CA_-realted overgrowth spectrum (PROS) and can manifest in three forms: (i) in single lesions such as isolated venous (54, 57, 58) and lymphatic malformations (59, 60); (ii) in combined vascular anomalies which comprise the overgrowth of more than one vascular component such as capillaries, venous an lymphatic beds (59, 61, 62); and (iii) as complex syndromes, characterised by a combination of vascular, adipose, muscle and skin overgrowth (63). This suggests that PIK3CA mutations appear at different stages during embryonic development, affecting different cell types (51, 64). Similar to epithelial cancer, the most common mutations found in vascular malformations are high activating mutations either in the helical (E542K, E545K) or in the kinase (H1047R, H1047L) domains of PIK3CA (64). Yet, there is no well-established correlation between any particular PIK3CA mutation and a phenotype. The mutational pattern is particularly interesting in megalencephaly-capillary malformation syndrome (MCAP), a condition included in the PROS classification. MCAP encompasses more widespread, but less severe, overgrowth affecting both mesoderm- and neuroectoderm-derived tissues and is usually caused by any of the wide range of less prevalent mutations that are predicted to be weakly activating. Based on the pattern of overgrowth in MCAP, causal mutations likely appear earlier in development before the divergence of mesoderm and ectoderm (65). In line with the presence of weak PIK3CA mutations, MCAP patients develop capillary malformations, a phenotype that is much less severe than venous malformations or lymphatic malformations. This suggests that the cellular consequences of activating PIK3CA mutations are dose (allele) dependent. In line with this notion, it has been recently documented that PIK3CA dose-dependent molecular reprogramming (two copies vs a single copy of PIK3CAH1047R) of humans induced pluripotent stemness (66). Mutations in other PI3K catalytic isoforms in vascular malformations have not been yet reported. For more detailed aspects of PIK3CA mutations in vascular malformations, we refer the reader to Ref (64, 67).
Similar to PIK3CA, somatic mutations in AKT were identified in vascular malformations. AKT mutations may also manifest as isolated lesions or within a complex syndrome known as Proteus syndrome (54, 68). Interestingly, activating mutations in AKT are not restricted to one isoform, as mutations in all three AKT isoforms were identified as a cause of vascular malformations, with only mutations in AKT1 being described in Proteus syndrome (54, 68).
PHTS is a severe tumour risk syndrome caused by a wide spectrum of germline-inactivating mutations in the PTEN gene with an autosomal dominant inheritance pattern. More than 30% patients with PHTS suffer from multiple vascular malformations, being predominantly of arteriovenous malformation (AVM) nature (69, 70, 71, 72, 73). These lesions consist of a direct connection between arteries and veins, which bypass the formation of the capillary network between them. In some PHTS patients, vascular malformations are noted at birth. Yet, since these malformations grow with the patient, they might not be apparent until the patient reaches an older age or a grave episode happens, such as intracranial haemorrhage. Until now, it remains elusive whether vascular malformations in PHTS patients are a result of an endothelial-intrinsic effect. It is also not known whether specific PTEN genotypes (referred to as the spectrum of PTEN mutations identified in PHTS patients) lead to the development of vascular malformations in PHTS patients. Indeed, it has been shown that PHTS patients with similar germline PTEN mutations are discordant for the appearance of vascular malformations, supporting the hypothesis of a ‘second hit’ for the development of these vascular lesions (69, 70, 73).
Activation of PI3K signalling in vascular malformations
There is some evidence that aberrant activation of PI3K is also linked to genetic alterations of extracellular receptors in vascular malformations. For instance, between 50 and 60% of sporadic venous malformations are caused by somatic activating mutations in the TEK gene, which encodes for the endothelial tyrosine-protein kinase receptor (TIE2) (74), with another 25% caused by mutations in PIK3CA (described earlier) (54, 57, 58, 64, 67). TEK mutations result in the enhanced activation of PI3K, and the inhibition of this signalling pathway prevents the growth of these lesions (75). The co-existence of TEK and PIK3CA mutations in venous malformation is rare (54, 57), which indicates that only one mutation is sufficient to cause vascular malformations. Also, it suggests that TIE2 mainly signals through PI3Kα in the endothelium. Based on this, it is tempting to speculate that orphan venous malformations (around 20%) are caused by mutations in other members of the same pathway. In a few cases in which both TEK and PIK3CA mutations have been detected in the same patient (64), it is possible that the less activating mutation arose earlier and that the endothelial cell acquired the more potent hotspot mutation later. Another possibility is that these mutations do not co-exist in the same cell. This would imply that these lesions are genetically heterogenous, as documented for cerebral cavernous malformations (76).
Another example of aberrant PI3K activation in vascular malformation was described in hereditary haemorrhagic telangiectasia (HHT), an inherited autosomal dominant vascular disorder characterised by the appearance of AVM (77). Around 90% of HHT patients were diagnosed by heterozygous inactivating mutations in members of the transforming growth factor beta (TGFβ) family. So far, inactivating mutations in the endothelial surface receptors endoglin (ENG, mutated in HHT1) and ACVRL1 (referred to as ALK1, mutated in HHT2) and the transcriptional factor SMAD4 (mutated juvenile polyposis HHT syndrome) were described (78, 79, 80). Modelling HHT in mice showed that genetic and pharmacologic inhibition of ENG, ALK1 and SMAD4 promoted aberrant activation of PI3K/AKT in endothelial cells (19, 81, 82, 83). Notably, this was also documented in human HHT1 and HHT2 lesions (83, 84). Mechanistically, the loss of expression of ALK1 in endothelial cells showed decreased PTEN activity, thus resulting in the hyperactivation of PI3K (19, 83). Intriguingly, while mutations in ALK1 and PTEN result in AVM (69), no mutations in PIK3CA or AKT1 have been reported in AVM. It is tempting to speculate that PI3K-related vascular disease manifestation is determined by the strength in the aberrant activation of the pathway.
Conclusion
Much progress has been made in the understanding of PI3K signalling pathway in the endothelium during vessel growth. Recent discoveries have demonstrated that endothelial cells are extremely sensitive to PI3K signalling and that even small perturbations in this pathway have a strong impact on the vasculature. This is further confirmed by lessons learnt from targeting this pathway in tumour angiogenesis in preclinical models where acute vs chronic inhibition of PI3K results in different outcomes. An emerging theme is that the aberrant activation of PI3K signalling as a cause of vascular malformations is more common than anticipated. Yet the origin of mutations in the PI3K pathway, when occurring somatically, remains unknown. There is a general consensus that they largely occur during embryonic development involving endothelial cells and early endothelial cell precursors. Critically, the association between aberrant PI3K activation and vascular anomalies goes beyond mutations; therefore, extending the list of patients who may benefit from PI3K-targeted pharmacological approaches. Of note, clinical data on the use of several inhibitors targeting different components of the PI3K pathway to treat vascular disorders is emerging (64, 67, 85, 86). Thus, the field calls for a debate to define the best targeted therapy for each patient. This will quite likely involve work in preclinical models to explore the efficacy, the route of administration, the dose and the duration of these treatments.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
M G lab is supported by the research grants SAF2017-89116R-P from MINECO (Spain) co-funded by European Regional Developmental Fund (ERDF), a Way to Build Europe; by the Catalan Government through the project 2017-SGR; by la Fundació Bancària ‘La Caixa’; by la Asociación Española contra el Cancer (AECC)-Grupos Traslacionales (GCTRA18006CARR); by la Fundación BBVA (Beca Leonardo a Investigadores y Creadores Culturales 2017); and by the People Programme (Marie-Curie Actions; grant agreement 317250) of the European Union’s Seventh Framework Programme FP7/2007–2013, the Marie Skłodowska-Curie (grant agreement 675392) of the European Union’s Horizon 2020 research. P K personal support was from Marie-Curie ITN Actions.
Acknowledgements
We would like to thank all members of the Vascular Biology and Signalling Group for their valuable feedback.
References
- 1.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell 2011. 873–887. ( 10.1016/j.cell.2011.08.039) [DOI] [PubMed] [Google Scholar]
- 2.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nature Reviews Molecular Cell Biology 2007. 464–478. ( 10.1038/nrm2183) [DOI] [PubMed] [Google Scholar]
- 3.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nature Reviews Molecular Cell Biology 2010. 329–341. ( 10.1038/nrm2882) [DOI] [PubMed] [Google Scholar]
- 4.Bilanges B, Posor Y, Vanhaesebroeck B. PI3K isoforms in cell signalling and vesicle trafficking. Nature Reviews Molecular Cell Biology 2019. 515–534. ( 10.1038/s41580-019-0129-z) [DOI] [PubMed] [Google Scholar]
- 5.Goncalves MD, Hopkins BD, Cantley LC. Phosphatidylinositol 3-kinase, growth disorders, and cancer. New England Journal of Medicine 2018. 2052–2062. ( 10.1056/NEJMra1704560) [DOI] [PubMed] [Google Scholar]
- 6.Graupera M, Potente M. Regulation of angiogenesis by PI3K signaling networks. Experimental Cell Research 2013. 1348–1355. ( 10.1016/j.yexcr.2013.02.021) [DOI] [PubMed] [Google Scholar]
- 7.Graupera M, Guillermet-Guibert J, Foukas LC, Phng L, Cain RJ, Salpekar A, Pearce W, Meek S, Millan J, Cutillas PR, et al. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature 2008. 662–666. ( 10.1038/nature06892) [DOI] [PubMed] [Google Scholar]
- 8.Angulo-Urarte A, Casado P, Castillo SD, Kobialka P, Kotini MP, Figueiredo AM, Castel P, Rajeeve V, Milà-Guasch M, Millan J, et al. Endothelial cell rearrangements during vascular patterning require PI3-kinase-mediated inhibition of actomyosin contractility. Nature Communications 2018. 4826 ( 10.1038/s41467-018-07172-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bi L, Okabe I, Bernard DJ, Wynshaw-Boris A, Nussbaum RL. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110alpha subunit of phosphoinositide 3-kinase. Journal of Biological Chemistry 1999. 10963–10968. ( 10.1074/jbc.274.16.10963) [DOI] [PubMed] [Google Scholar]
- 10.Lelievre E, Bourbon P, Duan L, Nussbaum RL, Fong G. Deficiency in the p110alpha subunit of PI3K results in diminished Tie2 expression and Tie2(-/-)-like vascular defects in mice. Blood 2005. 3935–3938. ( 10.1182/blood-2004-10-3955) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, Shokat KM, Stainier DYR. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science 2009. 294–298. ( 10.1126/science.1178577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren B, Deng Y, Mukhopadhyay A, Lanahan AA, Zhuang ZW, Moodie KL, Mulligan-Kehoe MJ, Byzova TV, Peterson RT, Simons M. ERK1/2-Akt1 crosstalk regulates arteriogenesis in mice and zebrafish. Journal of Clinical Investigation 2010. 1217–1228. ( 10.1172/JCI39837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicoli S, Knyphausen C, Zhu L, Lakshmanan A, Lawson N. miR-221 is required for endothelial tip cell behaviors during vascular development. Developmental Cell 2012. 418–429. ( 10.1016/j.devcel.2012.01.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Zhabyeyev P, Azad AK, Wang W, Minerath RA, DesAulniers J, Grueter CE, Murray AG, Kassiri Z, Vanhaesebroeck B, et al. Endothelial and cardiomyocyte PI3Kbeta divergently regulate cardiac remodelling in response to ischaemic injury. Cardiovascular Research 2019. 1343–1356. ( 10.1093/cvr/cvy298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitehead MA, Bombardieri M, Pitzalis C, Vanhaesebroeck B. Isoform-selective induction of human p110delta PI3K expression by TNFalpha: identification of a new and inducible PIK3CD promoter. Biochemical Journal 2012. 857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nature Reviews Molecular Cell Biology 2012. 283–296. ( 10.1038/nrm3330) [DOI] [PubMed] [Google Scholar]
- 17.Hamada K. The PTEN/PI3K pathway governs normal vascular development and tumor angiogenesis. Genes and Development 2005. 2054–2065. ( 10.1101/gad.1308805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serra H, Chivite I, Angulo-Urarte A, Soler A, Sutherland JD, Arruabarrena-Aristorena A, Ragab A, Lim R, Malumbres M, Fruttiger M, et al. PTEN mediates Notch-dependent stalk cell arrest in angiogenesis. Nature Communications 2015. 7935 ( 10.1038/ncomms8935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ola R, Dubrac A, Han J, Zhang F, Fang JS, Larrivée B, Lee M, Urarte AA, Kraehling JR, Genet G, et al. PI3 kinase inhibition improves vascular malformations in mouse models of hereditary haemorrhagic telangiectasia. Nature Communications 2016. 13650 ( 10.1038/ncomms13650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manning BD, Toker A.AKT/PKB signaling: navigating the network. Cell 2017. 381–405. ( 10.1016/j.cell.2017.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee MY, Luciano AK, Ackah E, Rodriguez-Vita J, Bancroft TA, Eichmann A, Simons M, Kyriakides TR, Morales-Ruiz M, Sessa WC. Endothelial Akt1 mediates angiogenesis by phosphorylating multiple angiogenic substrates. PNAS 2014. 12865–12870. ( 10.1073/pnas.1408472111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen WS. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes and Development 2001. 2203–2208. ( 10.1101/gad.913901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerr BA, West XZ, Kim Y, Zhao Y, Tischenko M, Cull RM, Phares TW, Peng X, Bernier-Latmani J, Petrova TV, et al. Stability and function of adult vasculature is sustained by Akt/Jagged1 signalling axis in endothelium. Nature Communications 2016. 10960 ( 10.1038/ncomms10960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saxton RA, Sabatini DM. MTOR signaling in growth, metabolism, and disease. Cell 2017. 960–976. ( 10.1016/j.cell.2017.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding Y, Shan L, Nai W, Lin X, Zhou L, Dong X, Wu H, Xiao M, Zhou X, Wang L, et al. DEPTOR deficiency-mediated mTORc1 hyperactivation in vascular endothelial cells promotes angiogenesis. Cellular Physiology and Biochemistry 2018. 520–531. ( 10.1159/000488619) [DOI] [PubMed] [Google Scholar]
- 26.Fan W, Han D, Sun Z, Ma S, Gao L, Chen J, Li X, Li X, Fan M, Li C, et al. Endothelial deletion of mTORC1 protects against hindlimb ischemia in diabetic mice via activation of autophagy, attenuation of oxidative stress and alleviation of inflammation. Free Radical Biology and Medicine 2017. 725–740. ( 10.1016/j.freeradbiomed.2017.05.001) [DOI] [PubMed] [Google Scholar]
- 27.Furuyama T, Kitayama K, Shimoda Y, Ogawa M, Sone K, Yoshida-Araki K, Hisatsune H, Nishikawa S, Nakayama K, Nakayama K, et al. Abnormal angiogenesis in FoxO1 (Fkhr)-deficient mice. Journal of Biological Chemistry 2004. 34741–34749. ( 10.1074/jbc.M314214200) [DOI] [PubMed] [Google Scholar]
- 28.Hosaka T, Biggs WH, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. PNAS 2004. 2975–2980. ( 10.1073/pnas.0400093101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dharaneeswaran H, Abid MR, Yuan L, Dupuis D, Beeler D, Spokes KC, Janes L, Sciuto T, Kang PM, Jaminet SS, et al. FOXO1-mediated activation of Akt plays a critical role in vascular homeostasis. Circulation Research 2014. 238–251. ( 10.1161/CIRCRESAHA.115.303227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilhelm K, Happel K, Eelen G, Schoors S, Oellerich MF, Lim R, Zimmermann B, Aspalter IM, Franco CA, Boettger T, et al. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature 2016. 216–220. ( 10.1038/nature16498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gambardella L, Hemberger M, Hughes B, Zudaire E, Andrews S, Vermeren S. PI3K signaling through the dual GTPase-activating protein ARAP3 is essential for developmental angiogenesis. Science Signaling 2010. ra76 ( 10.1126/scisignal.2001026) [DOI] [PubMed] [Google Scholar]
- 32.Yoshioka K, Yoshida K, Cui H, Wakayama T, Takuwa N, Okamoto Y, Du W, Qi X, Asanuma K, Sugihara K, et al. Endothelial PI3K-C2alpha, a class II PI3K, has an essential role in angiogenesis and vascular barrier function. Nature Medicine 2012. 1560–1569. [DOI] [PubMed] [Google Scholar]
- 33.Franco I, Gulluni F, Campa C, Costa C, Margaria J, Ciraolo E, Martini M, Monteyne D, De Luca E, Germena G, et al. PI3K class II alpha controls spatially restricted endosomal PtdIns3P and Rab11 activation to promote primary cilium function. Developmental Cell 2014. 647–658. ( 10.1016/j.devcel.2014.01.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alliouachene S, Bilanges B, Chaussade C, Pearce W, Foukas LC, Scudamore CL, Moniz LS, Vanhaesebroeck B. Inactivation of class II PI3K-C2alpha induces leptin resistance, age-dependent insulin resistance and obesity in male mice. Diabetologia 2016. 1503–1512. ( 10.1007/s00125-016-3963-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tibolla G, Piñeiro R, Chiozzotto D, Mavrommati I, Wheeler AP, Norata GD, Catapano AL, Maffucci T, Falasca M. Class II phosphoinositide 3-kinases contribute to endothelial cells morphogenesis. PLoS ONE 2013. e53808 ( 10.1371/journal.pone.0053808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biswas K, Yoshioka K, Asanuma K, Okamoto Y, Takuwa N, Sasaki T, Takuwa Y. Essential role of class II phosphatidylinositol-3-kinase-C2alpha in sphingosine 1-phosphate receptor-1-mediated signaling and migration in endothelial cells. Journal of Biological Chemistry 2013. 2325–2339. ( 10.1074/jbc.M112.409656) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jayson GC, Kerbel R, Ellis LM, Harris AL. Antiangiogenic therapy in oncology: current status and future directions. Lancet 2016. 518–529. ( 10.1016/S0140-6736(15)01088-0) [DOI] [PubMed] [Google Scholar]
- 38.Wong PP, Bodrug N, Hodivala-Dilke KM. Exploring novel methods for modulating tumor blood vessels in cancer treatment. Current Biology 2016. R1161–R1166. ( 10.1016/j.cub.2016.09.043) [DOI] [PubMed] [Google Scholar]
- 39.Martin JD, Seano G, Jain RK. Normalizing function of tumor vessels: progress, opportunities, and challenges. Annual Review of Physiology 2019. 505–534. ( 10.1146/annurev-physiol-020518-114700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okkenhaug K, Graupera M, Vanhaesebroeck B. Targeting PI3K in cancer: impact on tumor cells, their protective stroma, angiogenesis, and immunotherapy. Cancer Discovery 2016. 1090–1105. ( 10.1158/2159-8290.CD-16-0716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schnell CR, Stauffer F, Allegrini PR, O’Reilly T, McSheehy PMJ, Dartois C, Stumm M, Cozens R, Littlewood-Evans A, Garcia-Echeverria C, et al. Effects of the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 on the tumor vasculature: implications for clinical imaging. Cancer Research 2008. 6598–6607. ( 10.1158/0008-5472.CAN-08-1044) [DOI] [PubMed] [Google Scholar]
- 42.Soler A, Serra H, Pearce W, Angulo A, Guillermet-Guibert J, Friedman LS, Viñals F, Gerhardt H, Casanovas O, Graupera M, et al. Inhibition of the p110alpha isoform of PI 3-kinase stimulates nonfunctional tumor angiogenesis. Journal of Experimental Medicine 2013. 1937–1945. ( 10.1084/jem.20121571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murillo MM, Zelenay S, Nye E, Castellano E, Lassailly F, Stamp G, Downward J. RAS interaction with PI3K p110alpha is required for tumor-induced angiogenesis. Journal of Clinical Investigation 2014. 3601–3611. ( 10.1172/JCI74134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan TL, Choi HS, Matsui A, Benes C, Lifshits E, Luo J, Frangioni JV, Cantley LC. Class 1A PI3K regulates vessel integrity during development and tumorigenesis. PNAS 2008. 9739–9744. ( 10.1073/pnas.0804123105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soler A, Figueiredo AM, Castel P, Martin L, Monelli E, Angulo-Urarte A, Mila-Guasch M, Vinals F, Baselga J, Casanovas O, et al. Therapeutic benefit of selective inhibition of p110alpha PI3-kinase in pancreatic neuroendocrine tumors. Clinical Cancer Research 2016. 5805–5817. ( 10.1158/1078-0432.CCR-15-3051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 2009. 220–231. ( 10.1016/j.ccr.2009.01.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qayum N, Muschel RJ, Im JH, Balathasan L, Koch CJ, Patel S, McKenna WG, Bernhard EJ. Tumor vascular changes mediated by inhibition of oncogenic signaling. Cancer Research 2009. 6347–6354. ( 10.1158/0008-5472.CAN-09-0657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fokas E, Im JH, Hill S, Yameen S, Stratford M, Beech J, Hackl W, Maira S-M, Bernhard EJ, McKenna WG, et al. Dual inhibition of the PI3K/mTOR pathway increases tumor radiosensitivity by normalizing tumor vasculature. Cancer Research 2012. 239–248. ( 10.1158/0008-5472.CAN-11-2263) [DOI] [PubMed] [Google Scholar]
- 49.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nature Clinical Practice Oncology 2006. 24–40. ( 10.1038/ncponc0403) [DOI] [PubMed] [Google Scholar]
- 50.McGowan DR, Skwarski M, Bradley KM, Campo L, Fenwick JD, Gleeson FV, Green M, Horne A, Maughan TS, McCole MG, et al. Buparlisib with thoracic radiotherapy and its effect on tumour hypoxia: a phase I study in patients with advanced non-small cell lung carcinoma. European Journal of Cancer 2019. 87–95. ( 10.1016/j.ejca.2019.03.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Madsen RR, Vanhaesebroeck B, Semple RK. Cancer-associated PIK3CA mutations in overgrowth disorders. Trends in Molecular Medicine 2018. 856–870. ( 10.1016/j.molmed.2018.08.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Castillo SD, Vanhaesebroeck B, Sebire NJ. Phosphoinositide 3-kinase: a new kid on the block in vascular anomalies. Journal of Pathology 2016. 387–396. ( 10.1002/path.4802) [DOI] [PubMed] [Google Scholar]
- 53.Hare LM, Schwarz Q, Wiszniak S, Gurung R, Montgomery KG, Mitchell CA, Phillips WA. Heterozygous expression of the oncogenic Pik3ca(H1047R) mutation during murine development results in fatal embryonic and extraembryonic defects. Developmental Biology 2015. 14–26. [DOI] [PubMed] [Google Scholar]
- 54.Castel P, Carmona FJ, Grego-Bessa J, Berger MF, Viale A, Anderson KV, Bague S, Scaltriti M, Antonescu CR, Baselga E, et al. Somatic PIK3CA mutations as a driver of sporadic venous malformations. Science Translational Medicine 2016. 332ra42 ( 10.1126/scitranslmed.aaf1164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liaw D, Marsh DJ, Li J, Dahia PLM, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, Peacoke M, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nature Genetics 1997. 64–67. ( 10.1038/ng0597-64) [DOI] [PubMed] [Google Scholar]
- 56.Riviere JB, Mirzaa GM, O’Roak BJ, Beddaoui M, Alcantara D, Conway RL, St-Onge J, Schwartzentruber JA, Gripp KW, Nikkel SM, et al. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nature Genetics 2012. 934–940. ( 10.1038/ng.2331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castillo SD, Tzouanacou E, Zaw-Thin M, Berenjeno IM, Parker VER, Chivite I, Milà-Guasch M, Pearce W, Solomon I, Angulo-Urarte A, et al. Somatic activating mutations in Pik3ca cause sporadic venous malformations in mice and humans. Science Translational Medicine 2016. 332ra43 ( 10.1126/scitranslmed.aad9982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Limaye N, Kangas J, Mendola A, Godfraind C, Schlögel M, Helaers R, Eklund L, Boon L, Vikkula M. Somatic activating PIK3CA mutations cause venous malformation. American Journal of Human Genetics 2015. 914–921. ( 10.1016/j.ajhg.2015.11.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luks VL, Kamitaki N, Vivero MP, Uller W, Rab R, Bovée JV, Rialon KL, Guevara CJ, Alomari AI, Greene AK, et al. Lymphatic and other vascular malformative/overgrowth disorders are caused by somatic mutations in PIK3CA. Journal of Pediatrics 2015. 1048.e1–1054.e5. ( 10.1016/j.jpeds.2014.12.069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osborn AJ, Dickie P, Neilson DE, Glaser K, Lynch KA, Gupta A, Dickie BH. Activating PIK3CA alleles and lymphangiogenic phenotype of lymphatic endothelial cells isolated from lymphatic malformations. Human Molecular Genetics 2015. 926–938. ( 10.1093/hmg/ddu505) [DOI] [PubMed] [Google Scholar]
- 61.Kurek KC, Luks V, Ayturk U, Alomari A, Fishman S, Spencer S, Mulliken J, Bowen M, Yamamoto G, Kozakewich HW, et al. Somatic mosaic activating mutations in PIK3CA cause CLOVES syndrome. American Journal of Human Genetics 2012. 1108–1115. ( 10.1016/j.ajhg.2012.05.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keppler-Noreuil KM, Sapp JC, Lindhurst MJ, Parker VE, Blumhorst C, Darling T, Tosi LL, Huson SM, Whitehouse RW, et al. Clinical delineation and natural history of the PIK3CA-related overgrowth spectrum. American Journal of Medical Genetics: Part A 2014. 1713–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lindhurst MJ, Parker VER, Payne F, Sapp JC, Rudge S, Harris J, Witkowski AM, Zhang Q, Groeneveld MP, Scott CE, et al. Mosaic overgrowth with fibroadipose hyperplasia is caused by somatic activating mutations in PIK3CA. Nature Genetics 2012. 928–933. ( 10.1038/ng.2332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Castillo SD, Baselga E, Graupera M. PIK3CA mutations in vascular malformations. Current Opinion in Hematology 2019. 170–178. ( 10.1097/MOH.0000000000000496) [DOI] [PubMed] [Google Scholar]
- 65.Mirzaa G, Timms AE, Conti V, Boyle EA, Girisha KM, Martin B, Kircher M, Olds C, Juusola J, Collins S, et al. PIK3CA-associated developmental disorders exhibit distinct classes of mutations with variable expression and tissue distribution. JCI Insight 2016. 87623 ( 10.1172/jci.insight.87623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Madsen RR, Knox RG, Pearce W, Lopez S, Mahler-Araujo B, McGranahan N, Vanhaesebroeck B, Semple RK. Oncogenic PIK3CA promotes cellular stemness in an allele dose-dependent manner. PNAS 2019. 8380–8389. ( 10.1073/pnas.1821093116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Le Cras TD, Boscolo E. Cellular and molecular mechanism of PIK3CA-related vascular anomalies. Vascular Biology 2019. H33–H40. ( 10.1530/VB-19-0016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lindhurst MJ, Sapp JC, Teer JK, Johnston JJ, Finn EM, Peters K, Turner J, Cannons JL, Bick D, Blakemore L, et al. A mosaic activating mutation in AKT1 associated with the Proteus syndrome. New England Journal of Medicine 2011. 611–619. ( 10.1056/NEJMoa1104017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tan W-H, Baris HN, Burrows PE, Robson CD, Alomari AI, Mulliken JB, Fishman SJ, Irons MB. The spectrum of vascular anomalies in patients with PTEN mutations: implications for diagnosis and management. Journal of Medical Genetics 2007. 594–602. ( 10.1136/jmg.2007.048934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caux F, Plauchu H, Chibon F, Faivre L, Fain O, Vabres P, Bonnet F, Selma ZB, Laroche L, Gérard M, et al. Segmental overgrowth, lipomatosis, arteriovenous malformation and epidermal nevus (SOLAMEN) syndrome is related to mosaic PTEN nullizygosity. European Journal of Human Genetics 2007. 767–773. ( 10.1038/sj.ejhg.5201823) [DOI] [PubMed] [Google Scholar]
- 71.Suphapeetiporn K, Kongkam P, Tantivatana J, Sinthuwiwat T, Tongkobpetch S, Shotelersuk V. PTEN c.511C>T nonsense mutation in a BRRS family disrupts a potential exonic splicing enhancer and causes exon skipping. Japanese Journal of Clinical Oncology 2006. 814–821. ( 10.1093/jjco/hyl107) [DOI] [PubMed] [Google Scholar]
- 72.Srinivasa RN, Burrows PE. Dural arteriovenous malformation in a child with Bannayan-Riley-Ruvalcaba syndrome. American Journal of Neuroradiology 2006. 1927–1929. [PMC free article] [PubMed] [Google Scholar]
- 73.Burrows PE. Angioarchitecture of hereditary arteriovenous malformations. Seminars in Interventional Radiology 2017. 250–257. ( 10.1055/s-0037-1604298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Limaye N, Wouters V, Uebelhoer M, Tuominen M, Wirkkala R, Mulliken JB, Eklund L, Boon LM, Vikkula M. Somatic mutations in angiopoietin receptor gene TEK cause solitary and multiple sporadic venous malformations. Nature Genetics 2009. 118–124. ( 10.1038/ng.272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boscolo E, Limaye N, Huang L, Kang K, Soblet J, Uebelhoer M, Mendola A, Natynki M, Seront E, Dupont S, et al. Rapamycin improves TIE2-mutated venous malformation in murine model and human subjects. Journal of Clinical Investigation 2015. 3491–3504. ( 10.1172/JCI76004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malinverno M, Maderna C, Abu Taha A, Corada M, Orsenigo F, Valentino M, Pisati F, Fusco C, Graziano P, Giannotta M, et al. Endothelial cell clonal expansion in the development of cerebral cavernous malformations. Nature Communications 2019. 2761 ( 10.1038/s41467-019-10707-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shovlin CL. Hereditary haemorrhagic telangiectasia: pathophysiology, diagnosis and treatment. Blood Reviews 2010. 203–219. ( 10.1016/j.blre.2010.07.001) [DOI] [PubMed] [Google Scholar]
- 78.Gallione CJ, Klaus DJ, Yeh EY, Stenzel TT, Xue Y, Anthony KB, McAllister KA, Baldwin MA, Berg JN, Lux A, et al. Mutation and expression analysis of the endoglin gene in hereditary hemorrhagic telangiectasia reveals null alleles. Human Mutation 1998. 286–294. () [DOI] [PubMed] [Google Scholar]
- 79.Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon S-J, Stenzel TT, Speer M, Pericak-Vance MA, Diamond A, et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nature Genetics 1996. 189–195. ( 10.1038/ng0696-189) [DOI] [PubMed] [Google Scholar]
- 80.Gallione C, Aylsworth AS, Beis J, Berk T, Bernhardt B, Clark RD, Clericuzio C, Danesino C, Drautz J, Fahl J, et al. Overlapping spectra of SMAD4 mutations in juvenile polyposis (JP) and JP-HHT syndrome. American Journal of Medical Genetics: Part A 2010. 333–339. ( 10.1002/ajmg.a.33206) [DOI] [PubMed] [Google Scholar]
- 81.Jin Y, Muhl L, Burmakin M, Wang Y, Duchez A, Betsholtz C, Arthur H, Jakobsson L. Endoglin prevents vascular malformation by regulating flow-induced cell migration and specification through VEGFR2 signalling. Nature Cell Biology 2017. 639–652. ( 10.1038/ncb3534) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ola R, Künzel SH, Zhang F, Genet G, Chakraborty R, Pibouin-Fragner L, Martin K, Sessa W, Dubrac A, Eichmann A. SMAD4 prevents flow induced arteriovenous malformations by inhibiting casein kinase 2. Circulation 2018. 2379–2394. ( 10.1161/CIRCULATIONAHA.118.033842) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alsina-Sanchis E, García-Ibáñez Y, Figueiredo AM, Riera-Domingo C, Figueras A, Matias-Guiu X, Casanovas O, Botella LM, Pujana MA, Riera-Mestre A, et al. ALK1 loss results in vascular hyperplasia in mice and humans through PI3K activation. Arteriosclerosis, Thrombosis, and Vascular Biology 2018. 1216–1229. ( 10.1161/ATVBAHA.118.310760) [DOI] [PubMed] [Google Scholar]
- 84.Iriarte A, Figueras A, Cerdà P, Mora JM, Jucglà A, Penín R, Viñals F, Riera-Mestre A. PI3K (phosphatidylinositol 3-kinase) activation and endothelial cell proliferation in patients with hemorrhagic hereditary telangiectasia Type 1. Cells 2019. E971 ( 10.3390/cells8090971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Venot Q, Blanc T, Rabia SH, Berteloot L, Ladraa S, Duong J, Blanc E, Johnson SC, Hoguin C, Boccara O, et al. Targeted therapy in patients with PIK3CA-related overgrowth syndrome. Nature 2018. 540–546. ( 10.1038/s41586-018-0217-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parker VER, Keppler-Noreuil KM, Faivre L, Luu M, Oden NL, De Silva L, Sapp JC, Andrews K, Bardou M, Chen KY, et al. Safety and efficacy of low-dose sirolimus in the PIK3CA-related overgrowth spectrum. Genetics in Medicine 2019. 1189–1198. ( 10.1038/s41436-018-0297-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Suzuki A, de la Pompa JL, Stambolic V, Elia AJ, Sasaki T, Barrantes IdB, Ho A, Wakeham A, ltie A, Khoo W, et al. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Current Biology 1998. 1169–1178. ( 10.1016/S0960-9822(07)00488-5) [DOI] [PubMed] [Google Scholar]