Intra-axonal mechanisms driving axon regeneration (original) (raw)
. Author manuscript; available in PMC: 2021 Aug 1.
Abstract
Traumatic injury to the peripheral and central nervous systems very often causes axotomy, where an axon loses connections with its target resulting in loss of function. The axon segments distal to the injury site lose connection with the cell body and degenerate. Axotomized neurons in the periphery can spontaneously mount a regenerative response and reconnect to their denervated target tissues, though this is rarely complete in humans. In contrast, spontaneous regeneration rarely occurs after axotomy in the spinal cord and brain. Here, we concentrate on the mechanisms underlying this spontaneous regeneration in the peripheral nervous system, focusing on events initiated from the axon that support regenerative growth. We contrast this with what is known for axonal injury responses in the central nervous system. Considering the neuropathy focus of this special issue, we further draw parallels and distinctions between the injury-response mechanisms that initiate regenerative gene expression programs and those that are known to trigger axon degeneration.
INTRODUCTION
It is well established that axons in the peripheral nervous system (PNS) regenerate more effectively than those of the central nervous system (CNS). The poor regeneration in the CNS is due to both extrinsic and intrinsic factors, and much effort has been devoted to understanding how PNS neurons regenerate with the goal of targeting those mechanisms to increase regeneration after brain and spinal cord injuries. Injury to PNS axons initiates a robust intrinsic growth program (Huebner and Strittmatter, 2009). The differences in intrinsic growth capacity between PNS and CNS axons become quite clear when examining dorsal root ganglion (DRG) neurons, which have both peripherally and centrally projecting axons. Injuring the peripherally projecting axon of these neurons activates a regeneration program but injuring the centrally projecting DRG axons does not activate this regeneration program (McQuarrie and Grafstein, 1973; Neumann and Woolf, 1999). However, if the peripheral axon is injured first (termed a ‘conditioning lesion’), the central axon is able to regenerate after a subsequent injury (Chong et al., 1999; Neumann and Woolf, 1999; Richardson and Issa, 1984). This conditioning effect is also seen in the PNS when a nerve is injured proximal to the initial conditioning lesion, indicating that the growth potential can be broadly changed by peripheral nerve injury (Forman et al., 1980). Using a similar in vivo conditioning PNS axotomy followed by sensory neuron culture, Smith and Skene showed that a transcriptional switch underlies this conditioning-effect by activating a growth-promoting gene expression program (Smith and Skene, 1997). Consistent with this, injection of non-hydrolysable, cell-permeable cyclic AMP into the DRG, allows injured DRG neurons to regenerate axons after severing their central axons (Neumann et al., 2002; Qiu et al., 2002). These early observations compiled with many other lines of evidence argue that initiation of a neuronal growth program involves a sequence of events that transforms the neuron from a normal maintenance/function mode to a re-growth mode. Axonal injury is the first event in this sequence, and PNS axotomy precipitates axon-to-soma signals that support the transition from maintenance/function to growth mode. Localized events within the axon contribute to transformation to this growth mode, and soma-to-axon signaling further support axon regeneration after injury. We will address these axon-initiated mechanisms in the paragraphs below, focusing on how injury is signaled to the soma (axon-to-soma signaling) and how the soma alters axon growth (soma-to-axon signaling) with a particular focus on PNS neurons.
I. Axoplasmic increase in Calcium is an early response to initiate regenerative growth programs.
Calcium ion (Ca2+) influx into axons is one of the earliest changes following axotomy. This increase in axoplasmic Ca2+ is needed to reseal the injured axonal membrane, locally activate the Ca2+-dependent protease calpain, and initiate retrograde signaling mechanisms to communicate with the cell body (Figure 1A) (Bradke et al., 2012; Detrait et al., 2000; Rishal and Fainzilber, 2010). In cultured adult DRG neurons, removing Ca2+ from the media reduces neurite growth showing that Ca2+ influx has an important role in regulating axonal growth (Chierzi et al., 2005). In vivo, Ca2+ chelation significantly reduces axon regeneration in C. elegans (Ghosh-Roy et al., 2010). Despite the importance of Ca2+ entry, the regenerative effects of Ca2+ on PNS axons is not seen in the centrally projecting DRG axons (Hervera et al., 2019), so there may be discrete differences between PNS and CNS axons in this regard. Ca2+ increase occurs through openings in the membrane caused by the physical injury to the axon and by reversal of the sodium/calcium exchanger and voltage gated ion channels (Kamber et al., 2009; Kulbatski et al., 2004; Ziv and Spira, 1997). Ca2+ entry through voltage-gated calcium channels after injury is caused by membrane depolarization due to activation of gamma aminobutyric acid A (GABAA) and N-methyl-D-aspartate (NMDA) receptors (Toyoda et al., 2003), and the voltage-gated ion channel function is needed for restoring axon membrane integrity shortly after injury (Nehrt et al., 2007). In the cockroach giant axon, it was found that Ca2+, through the activation of phospholipase A2, was required for membrane re-sealing after transection (Yawo and Kuno, 1985). Ca2+-mediated activation of cysteine proteases, like calpain and calmodulin, also plays an important role in resealing the axon membrane (Xie and Barrett, 1991).
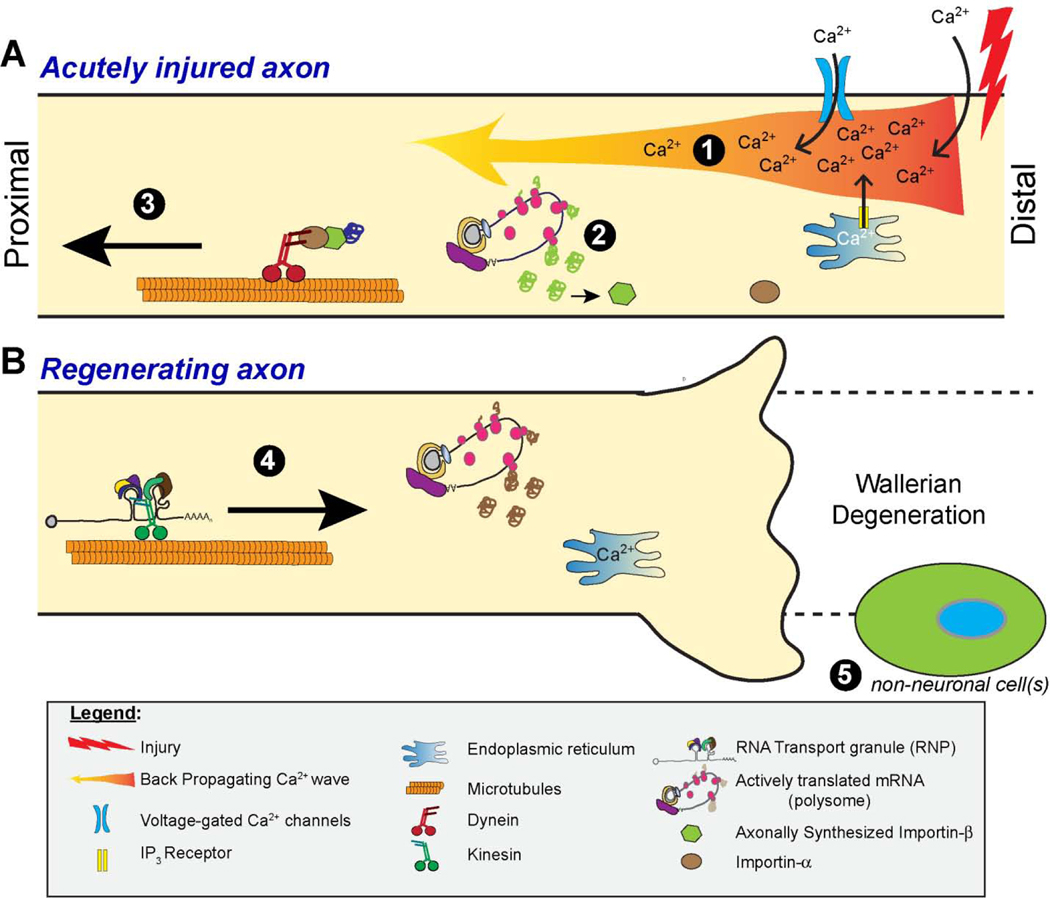
Figure 1 – Schematic of axon intrinsic signaling mechanisms that contribute to axon regeneration.
A, Depiction of back-propagating Ca2+ wave and localized effects of injury-induced Ca2+ influx into the axon are shown on top. Ca2+ entry triggers a retrograde Ca2+-mediated/dependent axon-to-soma signal (1) and activates localized protein synthesis (2) that provides a second means for axon-to-soma signaling through protein transport (3).
B, Ensuing regeneration-promoting mechanisms are depicted on the bottom, with soma-to-axon signaling through anterograde transport of mRNAs and proteins (4) with subsequent translational regulation of these axonal mRNAs. In addition to Ca2+ influx and release from intra-cellular stores, the extracellular environment, including non-neuronal cells resident and infiltrating into the nerve after injury (5), have the potential modulate localized translation events and mRNA transport.
Increase in axoplasmic Ca2+ is also a critical mediator of Wallerian degeneration in the severed distal axon stump (Conforti et al., 2014). The first phase of Wallerian degeneration begins with a short term Ca2+ wave which leads to a cascade of events including the depletion of nicotinamide adenine dinucleotide (NAD+) and adenosine triphosphate (ATP) as well as disruption of mitochondrial function and release of reactive oxygen species (ROS) (For review see Rosell and Neukomm, 2019). A second feed-forward release of Ca2+ stores from mitochondria and endoplasmic reticulum (ER) has been posited to instigate axon fragmentation during Wallerian degeneration (Barrientos et al., 2011; Villegas et al., 2014). This implies that the proximal axon must be able to buffer the Ca2+ increase relative to the distal axon stump. Work in cultured Aplysia neurons has shown that the proximal tip of severed axons have a Ca2+ concentration gradient with higher levels typical of extracellular spaces near the injury and lower levels more typical of the intracellular environment moving a few microns back towards the soma. This leads to a gradient of protease activity that is required for growth cone formation, and a new growth cone forms approximately mid-way along this Ca2+ gradient (Khoutorsky and Spira, 2005; Spira et al., 2003; Ziv and Spira, 1997). Interestingly, depletion of ER Ca2+ is well known to activate the unfolded protein response (UPR), since ER chaperone proteins needed for folding nascently synthesized proteins are Ca2+-dependent (Harding et al., 2002). Several lines of evidence indicate that localized activation of UPR in axons is needed for initiating regenerative responses (Onate et al., 2016; Ying et al., 2015). Attenuating Ca2+ release by blocking ER inositol triphosphate receptors (IP3R) and ryanodine receptors (RyR) reduces axonal outgrowth after peripheral nerve injury suggesting that regeneration of PNS axons is, at least in part, dependent on the release of ER Ca2+ stores (Ohtake et al., 2018). A decrease in mRNA translation is a critical component of the UPR, as UPR induces an inhibitory phosphorylation of the translation factor eIF2α that has been shown to occur locally in axons (Vuppalanchi et al., 2012). Similar to non-neuronal cells, translation of some mRNAs is paradoxically upregulated despite (or because of) eIF2α phosphorylation. Intra-axonal translation of the mRNA encoding the transcription factor Luman/CREB3 under UPR conditions provides an axon-to-soma signal for altering neuronal gene expression (Ying et al., 2014; Ying et al., 2015). Intra-axonal translation of Calreticulin (Calr) mRNA through UPR-associated eIF2α phosphorylation locally supports growth cone extension (Pacheco et al., 2020). As we outline below, increase in axoplasmic Ca2+ following injury is also needed for translation of axonal mRNAs whose protein products form a retrograde signaling complex to link signaling components to retrograde transport motors (Figure 1A).
Increased intracellular Ca2+ contributes to the generation of cyclic adenosine monophosphate (cAMP) and activation of protein kinase A (PKA), protein kinase C (PKC), and dual leucine zipper kinase 1 (DLK-1) that all positively contribute to axon regeneration (Mar et al., 2014). Ca2+ release from internal stores is also needed for a back-propagating Ca2+ wave that provides very rapid axon-to-soma signaling to activate PKCμ in the soma resulting in phosphorylation and nuclear exit of histone deacetylase (HDAC) 5 (Figure 1A) (Cho et al., 2013). HDAC5 subsequently localizes to axons where it can deacetylate α-tubulin, suggesting that axon-to-soma Ca2+ signaling also supports the dynamic cytoskeleton locally needed for axon regeneration (Cho and Cavalli, 2012; Cho et al., 2013). Ca2+ signaling from axons can also modulate phosphatase activity needed for dephosphorylating HDAC3 (Hervera et al., 2019). These changes in HDAC3 and HDAC5 phosphorylation status (and localization for HDAC5) provide a Ca2+-dependent axon-to-soma regulation of gene expression through modifying the acetylation status of histones (Cho et al., 2013; Hervera et al., 2019).
Despite the clearly positive effects of Ca2+ entry and intracellular store release on the early stages of axon regeneration, it should be noted that increases in axoplasmic Ca2+ can be detrimental for axon regeneration. Excessive levels of intra-axonal Ca2 can trigger axon degeneration in intact axons reminiscent of Wallerian degeneration (Stirling and Stys, 2010). Continued expression of the α2δ2 subunit of voltage-gated Ca2+ channel (Cacna2d2) in axotomized PNS neurons negatively affects their regeneration, and blocking the channel function with pregabalin facilitates axon growth and regeneration in both PNS and CNS axons (Tedeschi et al., 2016). ER Ca2+ release subsequent to IP3R activation triggers degeneration of the distal severed axon segments (Barrientos et al., 2011; Villegas et al., 2014), and work from the Segal lab points to IP3R-mediated ER Ca2+ release as a mechanism of axon degeneration in chemotherapy-induced peripheral neuropathies (Figure 2) (Pease-Raissi et al., 2017). It has also been shown that loss of the sterile alpha and TIR motif-containing 1 (SARM1) protein, which plays an important role in axon degeneration associated with Wallerian degeneration, prevents a second wave of Ca2+ elevation thereby attenuating axon degeneration after injury (Tian et al., 2020). Taken together, these observations suggest that elevations of axonal Ca2+ must be critically balanced and timed to initiate regenerative responses after axonal injury, as too much or too long of a Ca2+ elevation could precipitate axon degeneration.
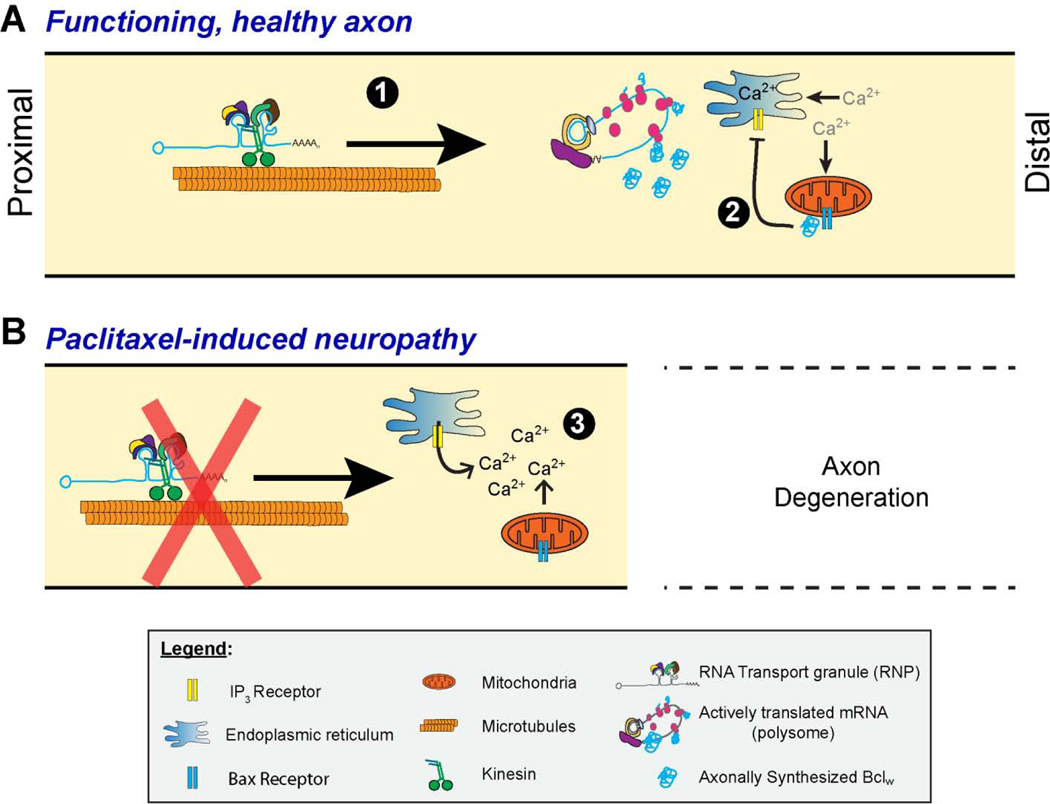
Figure 2 – Schematic of axon intrinsic signaling mechanisms that contribute to axon degeneration.
Schematic representing potential overlapping mechanisms for preventing Ca2+ store release from ER in mature functioning axons (top) and the loss of this mechanism with paclitaxel-induced peripheral neuropathy (Pease-Raissi et al., 2017). Axonally synthesized Bclw prevents or attenuates activation of IP3R along the ER (A, 1–2). Activation of IP3R (B, 3) was shown to be involved in degeneration Wallerian in severed axons (Villegas et al., 2014) and depletion of ER Ca2+ stores can trigger the UPR (Malhotra and Kaufman, 2007). As outlined in the text, UPR activation in axons is linked to some of the injury-associated translation events seen after the traumatic axonal injury depicted in Figure 1.
II. Axotomy alters axon-to-soma signals to initiate a neuronal regeneration program.
Retrograde signaling through transport of proteins provides another axon-to-soma signaling mechanism to regulate gene expression (Figure 1A). Importin-β1 mRNA (Kpnb1) is locally translated in axons after injury. The newly synthesized Importin-β1 forms an obligate heterodimer with Importin-α family proteins, with the heterodimer linking signaling cargoes containing nuclear localization signals (NLS) to the retrograde transport motor protein dynein (Hanz et al., 2003). This was a surprising finding as these importin proteins (also known as Karyopherins) were thought to only transport cargo proteins across the nuclear pore. Nonetheless, the mRNA encoding the Ran binding protein 1 (RanBP1) is also locally translated after axotomy, and RanBP1 protein facilitates Importin-β/α heterodimer formation by releasing Importin-α from other interacting proteins, a mechanism that was also thought to be restricted to the nuclear pore region (Yudin et al., 2008). Intra-axonal translation of Kpnb1 and RanBP1 mRNAs requires the injury-induced axoplasmic Ca2+ increase outlined above, thereby providing a sequence of elevated Ca2+ → axonal mRNA translation → axon-to-soma signaling (Hanz et al., 2003; Yudin et al., 2008).
Transcription factors are one of the likely cargos for retrograde transport motor proteins and complexes after axonal injury. Though it is not known if Importin-β/α system is involved in retrograde transport for all, some of these retrogradely transported transcription factors are clearly synthesized in axons after injury. Coupled with nuclear transport, these provide an on-demand axon-to-nucleus signaling mechanism for changing transcriptional programs after PNS axotomy. Signal transducer and activator of transcription 3 (STAT3) is one of those locally synthesized transcription factors. Nascently synthesized, retrogradely transported phospho-STAT3 supports survival of DRG neurons following PNS axotomy (Ben-Yaakov et al., 2012). Obviously, survival of the injured neuron is needed for functional regeneration so axonally synthesized STAT3 supports regeneration. Peroxisome proliferator-activated receptor gamma (PPARγ), which has well characterized roles in adipogenesis and glucose homeostasis, is similarly synthesized in PNS axons after axotomy and the retrogradely transported protein is needed for axon regeneration (Lezana et al., 2016). UPR induction after axotomy triggers translation of axonal Luman and Xbp1 mRNAs (Onate et al., 2016; Ying et al., 2014). Luman protein is retrogradely transported to the soma, where its nuclear functions support new gene transcription for axon growth (Ying et al., 2014). Interestingly, Xbp1 mRNA must be spliced before it can encode a functional transcription factor, and Ohate et al. (2016) show that this splicing event occurs in axons after injury, with overexpression of the spliced Xbp1 increasing axon regeneration. However, it should be emphasized that UPR-induced translational activation in axons is not limited to transcription factor mRNAs, as the mRNAs encoding the ER chaperone proteins Calr and Grp78 (also called BiP) are also translated with UPR activation in axons (Vuppalanchi et al., 2012) and recent work has shown that Protein kinase RNA-like endoplasmic reticulum kinase (PERK) is needed for Calr mRNA translation in axons after injury (Pacheco et al., 2020). Intra-axonal synthesis of transcription factor proteins for axonto-nuclear signaling is also not limited to regenerating axons. For example, nerve growth factor (NGF) activates translation of cAMP-responsive element-binding protein (Creb) mRNA in developing sensory axons, and the retrogradely transported CREB protein is needed for NGF-dependent neuronal survival (Cox et al., 2008). However, Creb mRNA was not detected in sympathetic axons (Andreassi et al., 2010), and it does not appear to be a prominent player in adult DRG axons in our hands. Thus, which axon-to-nuclear signals generated is likely unique to different physiological settings and different neuronal types.
Retrogradely transported kinases, which are resident in axons before injury, can also modulate activity of nuclear transcription factors. Axotomy induced activation of extracellular-regulated kinase (ERK) provides one such signal. Here, phospho-ERK binds to importin β1 through a scaffold provided by a proteolytic fragment of Vimentin (Vim) protein, which also prevents ERK dephosphorylation during its transport to the soma where it activates the ETS Transcription Factor ELK1 (Perlson et al., 2005). Vim mRNA is known to be locally translated in axons (Willis et al., 2005), and the proteolytic Vim fragment is generated through a Ca2+-dependent activation of calpain in axons after nerve injury (Perlson et al., 2005). DLK provides an axon-to-soma link to the nuclear transcription factor c-Jun and helps to modulate transport of other injury-induced signals. Sustained phosphorylation of c-Jun after PNS nerve injury is associated with increased regeneration (Kenney and Kocsis, 1998), and DLK knockdown decreases the axotomy-induced c-Jun phosphorylation in DRG after sciatic nerve injury (Holland et al., 2016). Further DLK activation after injury contributes to the retrograde transport of phosphorylated STAT3 (Shin et al., 2012). Notably, Ca2+-dependent axon-to-soma signaling through local translation of Vim mRNA has also been detected in CNS neurons after exposure to Aβ1–42 (Walker et al., 2018), so at least some of these axon intrinsic signaling mechanisms may extend to the CNS.
III. Mechanisms for gene regulation by axotomy-induced axon-to-nucleus signaling.
The molecular consequences for injury-induced axon-to-soma signaling have been demonstrated for both back-propagating Ca2+ wave and Importin-β/α transport, with large cohorts of genes upregulated or down regulated after axotomy altered by disruption of HDAC5 phosphorylation/nuclear export or Importin-β/α transport (Cho et al., 2013; Perry et al., 2012). This indicates that axon-to-soma signaling not only activates gene programs for axon growth but also represses axon gene expression programs associated with neuronal maintenance/function. Presumably, the axon-derived cargoes of the Importin-β/α complex provide these functions by directly or secondarily regulating transcription factor activity. Histone modification and changes in DNA methylation after injury provide a more indirect modulation of neuronal gene expression. Modifications of histones and DNA have been shown to play important roles in axon regeneration by affecting chromatin structure, thereby modifying chromatin access for transcriptional machinery following injury in both the PNS and CNS (Palmisano and Di Giovanni, 2018). Further, the Ca2+-dependent loss of phospho-HDAC5 from the nucleus was correlated with an increase in the expression of genes associated with transcriptional regulation, so this axon-to-nuclear signaling affects transcription of transcriptional regulators (Cho et al., 2013).
The histone acetyltransferases have also been shown to acetylate transcription factors. Acetylation of lysine residues in the C-terminus of the transcription factor p53 causes an upregulation of Gap43 mRNA during facial motor neuron axon regeneration (Tedeschi et al., 2009). This p53 effect required acetyl transferase activity of the CREB binding protein (CBP). It has also been shown that inducing hyperacetylation of histones by inhibiting HDACs in cultured CNS neurons increases neurite outgrowth in a transcription-dependent fashion (Gaub et al., 2010). Also, axonal HDAC targets can extend beyond histones, as indicated above for HDAC5 targeting acetyl-α-tubulin. HDAC6 is also known to deacetylate α-tubulin, but in contrast to HDAC5 its activity attenuates rather than supports axon growth (Rivieccio et al., 2009). We recently showed that HDAC6 also deacetylates Miro1 in axons, and decreases axon growth by attenuating mitochondrial transport and membrane potential (Kalinski et al., 2019).
Considering that nuclear depletion of HDAC5 induces growth-promoting gene expression, it follows that an upregulation of histone acetyltransferases could similarly increase axon regeneration. Consistent with this notion, overexpression of the histone acetyltransferase p300 in RGCs increases regeneration after optic nerve injury (Gaub et al., 2011). Work from the di Giovanni lab recently showed activation of the related acetyl-transferase CBP increases axon regeneration in both PNS and CNS neurons through modulation of gene expression (Hutson et al., 2019). Interestingly, environmental enrichment triggered an increase in CBP activity that also supported axon regeneration after spinal cord injury (Hutson et al., 2019). It is not clear if this is a retrograde signal derived from the environmental enrichment or soma-restricted mechanism, but these observations emphasize that modulation of histone acetylation after injury brings a potential therapeutic strategy for improving axon regeneration. It should be noted that histone methylation can also impact transcriptional regulation by either inducing or repressing transcription depending on the residues methylated (Wahane et al., 2019). Axon-to-soma modulation of histone methylation status has not been addressed in neurons, but repression of the polycomb complex that catalyzes methylation of H3 at lysine 27 histone attenuates expression of regeneration promoting genes in Schwann cells (Ma et al., 2018).
DNA methylation also contributes to axon regeneration potential and this epigenetic mechanism is specifically regulated by PNS axon injury. In general, DNA hypermethylation is associated with gene repression while hypomethylation is associated with gene activation; however, this does not hold true for all differentially methylated genes after axotomy (Lindner et al., 2014; Puttagunta et al., 2014). Axotomy of the peripheral axons of DRG neurons, not the central axons, leads to an increase in expression of Ten-eleven translocation 3 protein (Tet3), which demethylates DNA by catalyzing the conversion of 5-methylcytosine to 5-hydroxymethylcytosine (5hmC) (Loh et al., 2017; Weng et al., 2017). This injury-induced upregulation of Tet3 requires retrograde Ca2+ signaling, and it leads to an increase in the expression of regeneration-associated genes (RAGs) and axon regeneration (Weng et al., 2017). As mentioned above, DNA methylation can lead to gene silencing and this mechanism has been recently linked to the development of neuropathic pain after nerve injury. After a neuropathic pain inducing peripheral nerve injury, DNA methyltransferase 3α (DNMT3α) mRNA and protein are significantly increased in DRG neurons during the early phase after injury, which in turn contributes to the development of neuropathic pain by down regulating the expression of the Kv1.2 potassium channel (Zhao et al., 2017). In a bone cancer pain model, DNMTs are also increased in the spinal dorsal horn ipsilateral to the bone cancer afflicted limb and inhibiting DNMTs in the spinal cord attenuates the development of pain hypersensitivity associated with a bone cancer pain model by increasing Kv1.2 expression further providing a link between gene silencing of potassium channels due to DNA methylation and neuropathic pain (Miao et al., 2017). DNMT3α is also responsible for silencing the expression of mu and kappa opioid receptors in DRG neurons after neuropathic pain inducing peripheral nerve injury, thereby reducing the response of these animals to opioid analgesics (Sun et al., 2017).
Levels of the epigenetic regulator ubiquitin-like containing PHD ring finger 1 (UHRF1), which plays an important role in DNA methylation and subsequently axon regeneration, increase after peripheral nerve injury. Knocking down UHRF1 in spot cultures of DRG neurons leads to an impairment in axon regeneration, suggesting that some gene silencing is required for axon regeneration (Oh et al., 2018). UHRF1 works to promote axon regeneration after peripheral axotomy by effectively silencing the transcription of phosphatase and tensin homolog (PTEN) and RE1 silencing transcription factor (REST) through methylation of their promoter regions (Oh et al., 2018). Taken together, these and other observations indicate that injury-induced axon-to-soma signaling alters somal gene expression through both direct transcriptional and epigenetic mechanisms, with the combination undoubtedly responsible for the broad change in gene expression programs needed to shift from a maintenance/function to regeneration phenotype.
IV. Post-transcriptional regulation of gene expression during nerve regeneration.
Role of localized mRNAs in axon growth and regeneration –
Regulation of gene expression extends to synthesis of proteins, and many lines of evidence indicate that neuronal mRNAs are subjected to several different levels of post-transcriptional control. Indeed, localizing mRNAs to axons, modifying an mRNA’s translational efficiency, or altering survival of an mRNA can dictate when a protein is introduced, where the proteins is introduced, and how much of that protein is generated. Intra-axonal translation of mRNAs was mentioned above for the Kpnb1, Ranbp1, Vim, and some transcription factors, but this mechanism also extends to mRNAs whose proteins products directly regulate axon growth (Figure 1A). For example, axonally synthesized proteins are needed for growth cone formation after in vitro axotomy in DRG neurons (Chierzi et al., 2005). Though it is not clear which nascently synthesized proteins are needed for the growth cone formation, Pacheco et al. (2020) recently showed that translation of axonal Calr mRNA supports initiation of axon growth in axotomized DRG neurons (Pacheco et al., 2020). Axonal Calr translation is increased by phosphorylation of eIF2α (Vuppalanchi et al., 2012), and, as an avid Ca2+ binding protein (Gelebart et al., 2005), this raises the intriguing possibility that axonal Calr protein allows growth cone formation by chelating axoplasmic Ca2+. The injury induced axon-to-soma signaling can also change the repertoire of mRNAs in axons, with mRNAs encoding RAGs showing increased transport into axons following axotomy (Figure 1A) (Kar et al., 2018). Subcellular localization of mRNAs is dictated by sequence motifs within the mRNAs (_cis_-elements) that are recognized by RNA Binding Proteins (RBPs; _trans_-factors) to form ribonucleotide protein (RNP) complex. These RNPs associate with kinesin for anterograde transport into axons, with the moving RNPs referred to ‘transport granules’. So far, no unique themes for _cis_-element mRNA sequences have been identified that allow predicting whether an mRNA will localize, and studies in cultured neurons, and recently in vivo, have identified hundreds to thousands of axonally localized mRNAs (Briese et al., 2016; Gumy et al., 2011; Hafner et al., 2019; Kar et al., 2018; Minis et al., 2014; Rotem et al., 2017; Shigeoka et al., 2016; Zivraj et al., 2010). However, relatively few RBPs have been defined for axonal mRNAs. We recently used experimentally defined _cis_-elements from several axonal mRNAs to expand knowledge of axonal RBPs. These proteomics results showed that multiple axonal RBPs can interact with the same _cis_-element, but also emphasize that many RBPs that were thought to be restricted to the nucleus localize into PNS axons (Lee et al., 2018). Interestingly, axonal localization hnRNPs K, H1, and F increases after axotomy and knockdown of these and related RBPs points to roles in axon growth (Lee et al., 2018), further emphasizing the role of axonal mRNA-RBP interactions in axon regeneration. Table 1 summarizes several of the identified axonal mRNA localizing _cis-_elements and corresponding RBPs. A few of these warrant further description for their potential roles in axon growth and regeneration and to emphasize the complexities of RBP-mRNA and RBP-RBP interactions in axons.
Table 1:
Known axonal RNA motifs determining axonal mRNA localization. Beyond the localizing _cis_-elements outlined below, Martinez et al. (2019) recently reported that RNA binding motifs for Pumilo proteins (Pum1 and Pum2) serve as signals to retain mRNAs in cell bodies of PNS and CNS neurons.
| _Cis-_element | _Trans-_factors(s) | Neuron Type | Reference |
|---|---|---|---|
| Actb - 54 nt Zip code element | Zip Code Binding Protein 1 (ZBP1)* | (Fibroblasts) Cortical Sensory | (Kislauskis et al., 1994; Willis et al., 2007; Zhang et al., 1999) |
| Gap43 - 3’ UTR ARE | ZBP1 and HuD* | Sensory Neurons | (Donnelly et al., 2011; Yoo et al., 2013) |
| Hmgb1 - 3’UTR 60 nt motif | hnRNP K* | Sensory Neurons | (Lee et al., 2018) |
| Kpnb1 - 34 nt MAIL motif | Nucleolin (Ncl) | Sensory | (Perry et al., 2016) |
| Kappa-Opioid receptor - 3’ UTR | Cop1b | Sensory | (Bi et al., 2007) |
| mTor - 3’UTR | Nucleolin (Ncl) SMN-miR interaction** | Sensory Hippocampal & cortical** | (Kye et al., 2014; Terenzio et al., 2018) |
| Nrn1 - 3’ UTR ARE | SMN and HuD | Hippocampal | (Akten et al., 2011; Merianda et al., 2013) |
| Nrn1 - 5’ UTR 104 nt motif | hnRNP H1 and hnRNP F* | Sensory Neurons | (Merianda et al., 2013) |
| Tau - 3’ UTR 240 nt motif | ZBP1, HuD, and G3BP | Neural differentiated PC12 cells and P19 cells | (Aronov et al., 1999; Aronov et al., 2002; Atlas et al., 2004) |
Zip Code Binding Protein 1 (ZBP1, also called IGF1 binding protein 1 [IMP1]) was shown to bind a 54 nucleotide element in the 3’UTR of β-Actin (Actb) mRNA for localization to the periphery of migrating fibroblasts and growing axons (Kislauskis et al., 1994; Zhang et al., 1999). Introducing a transgene with the Actb 3’UTR into mouse PNS neurons decreased axonal levels of Actb and Gap43 mRNAs and decreased axon regeneration after axotomy (Donnelly et al., 2011). Thus, ZBP1 is needed for axonal transport of both of these mRNAs, and limited availability of ZBP1 in adult PNS neurons restricts axon regeneration capacity (Donnelly et al., 2011). This also emphasizes that ZBP1 binds to multiple mRNAs, and RNA co-IP studies in non-neuronal cells support this idea but also emphasize that ZBP1 interacts with many other RBPs (Jonson et al., 2007). Such RNA-RBP interactomes in other cellular systems have been defined as “RNA regulons” (Keene, 2007). Splicing Factor Proline And Glutamine Rich (SFPQ), which co-assembles with Bcl w and LaminB2 mRNAs into RNPs for axonal localization (Cosker et al., 2016), and Nucleolin, which is needed for transport of Kpnb1 and mTor mRNAs (Perry et al., 2016; Terenzio et al., 2018), provide two more examples. Bclw and LaminB2 proteins support survival of axons (Cosker et al., 2016; Yoon et al., 2012), while Importin-β1 and mTOR are injury response proteins that help to initiate axon regeneration responses (Perry et al., 2016; Terenzio et al., 2018). Though some headway has been made in identifying axonal RNA regulons, much more systematic analyses of the axonal mRNAs bound to axonal RBPs are needed to fully understand how RBP-mRNA interactions contribute to axon regeneration.
RNA-seq analyses of axonal hnRNP K, F, and H1 immunoprecipitates define RNA regulons supporting axon growth by bioinformatics (Lee et al., 2018), but these need to be distinguished for direct vs. indirect interactions as the transport RNPs can have many different RBPs. For example, axonal Actb mRNA has been shown to interact with ZBP1, hnRNP R, spinal motor neuron (SMN) and HuD (also called Elavl4) proteins (Glinka et al., 2010; Kim et al., 2015b; Rossoll et al., 2003; Tiruchinapalli et al., 2003). While hnRNP R and ZBP1 contribute to Actb mRNA axonal localization in motor neurons, ZBP1 and HuD are necessary for axonal localization in sensory neurons (Glinka et al., 2010; Kim et al., 2015b; Rossoll et al., 2002). ZBP1’s interaction with Gap43 mRNA requires HuD binding to the AU rich element (ARE) in Gap43 mRNA’s 3’UTR (Yoo et al., 2013). The complement of proteins binding to the individual mRNAs likely determine its fate for subcellular localization, but mRNAs may behave differently in different neuron types as suggested by hnRNP R and HuD roles in Actb localization in motor vs. sensory neurons. Further differences in the axonal transcriptome of neuron types and physiological conditions will undoubtedly be uncovered by systematic analyses of transcriptomes and axonal RNA regulons. For example, the mRNA encoding γ-Actin (Actg) does not localize to axons of cortical or sensory neurons (Bassell et al., 1998; Willis et al., 2005), but it does localize to axons of embryonic motor neurons (Moradi et al., 2017). Similar to Actb and other axonal mRNAs, the 3’UTR of Actg drives its localization in motor neurons (Moradi et al., 2017). The molecular basis of this differential Actg localization is not known beyond the functions of its 3’UTR, but this will likely be explained by different populations of RBPs interacting with this Actg 3’UTR in motor vs. sensory and cortical neurons. Also of interest is that the Hengst lab very recently uncovered a mechanism restricting mRNAs from axonal localization; specifically, they found that the Pumilio 1 and 2 (Pum1 and Pum2) binding element (PBE)-containing mRNAs are restricted to the soma of developing neurons (Martinez et al., 2019). It will be telling to see if this PBE-based mechanism is differentially regulated in different neuron types and physiological conditions.
Translational control for axon growth and regeneration –
Several lines of evidence indicate that mRNAs are stored until needed in PNS axons, which provides an on-demand system for generating new proteins locally in response to different stimuli. Evidence for this is seen with near immediate axonal translation of the injury-response gene mRNAs after axotomy that are outlined above (Figure 1A) (e.g., Importin-β1, RanBP1, STAT3 (Ben-Yaakov et al., 2012; Hanz et al., 2003; Yudin et al., 2008)). Extracellular stimuli that axons encounter have also been shown to activate translation of different axonal mRNAs. For example, translation of axonal RhoA and Cofilin mRNAs is up regulated by growth inhibitory signals (Piper et al., 2006; Wu et al., 2005) and Actb, the actin nucleating Arp2/3 complex activator WAVE1, and the actin complex stabilizer Cortactin mRNAs are upregulated by neurotrophins (Spillane et al., 2011; Willis et al., 2007). Exposure and withdrawal of NGF was shown to trigger the translation of axonal Pafah1b1 (Lis1) mRNA, with the end result determined by whether or not the mRNA is bound to Adenomatous Polyposis Coli (APC) protein (Villarin et al., 2016). APC has been shown to localize mRNAs to microtubule plus-ends, and spatially orchestrate protein synthesis in axons and growth cones (Preitner et al., 2014).
Two general mechanisms have been uncovered beyond the direct RBP-mRNA interactions to account for how stimuli, including axonal injury, can recruit mRNAs into translation. The Flanagan lab published a provocative mechanism where mRNAs and translational machinery interact with the cytoplasmic tail of the transmembrane receptor deleted in colon cancer (DCC). Binding of Netrin ligand to DCC receptor led to dissociation of these mRNAs and formation of actively elongating polysomes (Tcherkezian et al., 2010). Elegant work from the Holt lab very recently extended this observation to other axon guidance receptors (Koppers et al., 2019). We recently showed that the axonal mRNA interaction with the stress granule (SG) associated protein, Ras-GAP SH3 domain Binding Protein1 (G3BP1), provides a mechanism for activating translation of quiescent axonal mRNAs after axotomy (Sahoo et al., 2018a). Given the link to injury and regeneration, we will expand on this mechanism below; however, the possibility that receptor-mediated axonal mRNA storage contributes to regeneration has not been tested, so we do not intend to imply that mechanism is not relevant for regeneration (or axon degeneration).
SGs are used by other cell types to store untranslated mRNAs after exposure to oxidative or metabolic stress (Anderson and Kedersha, 2008). Several SG proteins have been documented in PNS axons (Murashov et al., 2007; Price et al., 2006). We found that the classic SG proteins G3BP1, Fragile X mental retardation syndrome-related protein 1 (FXR1), Fragile X mental retardation protein (FMRP), T-cell restricted intra-cellular antigen 1 (TIA1), and Human antigen-R (HuR) form SG-like structures in PNS axons under basal conditions and mRNAs are associated with these axonal SG-like structures (Sahoo et al., 2018a). Much effort for the study of SG proteins and their aggregation has been focused on neurodegenerative diseases, particularly amyotrophic lateral sclerosis and frontotemporal dementia (Mackenzie et al., 2017). G3BP1-containing granules were also recently shown to hitchhike on moving lysosomes to reach distal axons (Liao et al., 2019). In both mammalian and C. elegans axons, the SG protein granules rapidly increase in size after axotomy. For C. elegans blocking this aggregation through depletion of TIAR2, the orthologue of mammalian TIA1, increased axonal regeneration (Andrusiak et al., 2019). For mammalian PNS neurons, blocking G3BP1 aggregation by expression of G3BP1 acidic domain or a cell-permeable peptide from the acidic domain increased axonal protein synthesis and accelerated in vivo axon regeneration (Sahoo et al., 2018a). These data emphasize that SG proteins act to attenuate axon growth rates. Interestingly, aggregation of SG proteins has been demonstrated in several neurodegenerative conditions, including aggregation in axons (Khalfallah et al., 2018; Maziuk et al., 2017; McDonald et al., 2011), and mutations in TIA1 protein in amyotrophic lateral sclerosis suggest SG proteins aggregation can cause neuron loss (Mackenzie et al., 2017). With regards to the topic of this special issue, the possibility that SG protein aggregation occurs in peripheral neuropathies has not been addressed.
As implied by the text above and Table 1, regulatory elements or motifs within mRNAs determine their post-translational regulation, including recognition by the non-coding RNAs (ncRNAs) outlined in the next section. Stimuli from the environment of the axon have been shown to impact axonal mRNA transport and translation. This has been documented for axon guidance cues in developing axons and for trophic factor stimulation of mature axons (Sahoo et al., 2018b). For example, NGF-stimulated axon growth requires translation of axonal Par3 mRNA in developing sensory neurons (Hengst et al., 2009) and growth attenuation by CSPGs requires translation of axonal RhoA mRNA (Walker et al., 2012). Willis et al. (2007) showed altered transport for entire cohorts of mRNAs in adult sensory neurons in response to growth promoting vs. growth-inhibiting stimuli, with specific mRNAs accumulating or being depleted from focal stimulation points (Willis et al., 2007). The signals triggered by these extracellular stimuli likely impact functions of the RBPs or combinations of RBPs bound to these mRNAs. It will be important to understand how RBP-driven mechanisms intersect with the translational control pathways discussed herein and considering how we might utilize these mechanisms for neural repair strategies.
Contributions of non-coding RNAs to axon growth and regeneration –
Numerous studies have shown the role of non-coding RNA (ncRNA)-mediated mechanisms, particularly through micro-RNAs (miRs), in neuronal development, including critical roles in neural induction/patterning, neural precursor cell proliferation, migration, differentiation, axon growth and synaptogenesis (Cho et al., 2019; Motti et al., 2012). miRs work by inhibiting translation of target mRNAs or triggering degradation of target mRNAs, but it should be noted that in silico predicted mRNA targets for a given miR must be validated to ensure that the mRNA is actually a target (e.g., secondary/tertiary RNA structure or interacting proteins could hide an miR binding site in an mRNA). Nonetheless, an extensive body of evidence also supports roles for miRs in neuronal injury and disease (Cho et al., 2019). The Murashov lab showed that genetic ablation of dicer, a central regulator of miR biogenesis, impeded sciatic nerve regeneration, attenuating anatomical, physiological and functional recovery (Wu et al., 2012). A number of studies using both microarray and deep sequencing approaches have identified miRs whose levels are affected in both spinal cord and sciatic nerve injury (Table 2) (Motti et al., 2017; Strickland et al., 2011a; Strickland et al., 2011b; Wu and Murashov, 2013; Yu et al., 2011; Zhang et al., 2011; Zhou et al., 2011). By integrating the results from the miR profiles with the transcriptomics data from injured DRGs, candidate molecular pathways affected by the regulated miRs were identified. These pathways represent functions in neuronal differentiation and activation of stem cell niches intrinsic growth capacity, Schwann cellneuron communication, cellular morphogenesis, cell polarity and cytoskeletal reorganization (Motti et al., 2017; Strickland et al., 2011a; Yu et al., 2011). We outline a few examples of miRs with validated neuronal mRNA targets involved in axon growth and regeneration below, but refer the reader to the references herein and in Table 2 for a more formative description of the injury-regulated miRs and putative targets.
Table 2:
Micro-RNAs affecting axonal growth
| Neuron/tissue source | Approach | Pathway (Target mRNAs) | Reference |
|---|---|---|---|
| Spinal cord (36 miRs) – T12-T13 spinal cord contusion (1–14 days after-injury) | Microarray | De-differentiation of surviving neurons, emergent activation of stem cell niches. | (Strickland et al., 2011a) |
| DRGs (20 miRs) –sciatic nerve transection (7 days after-injury) | Microarray | Ras/Raf/ERK [Sprouty 2 (miR-21)] | (Strickland et al., 2011b) |
| DRGs (21 miRs)- sciatic nerve transection – (7 days after-injury) | Microarray | Slit-Robo-srGAP [Robo2 (miR-145), srGAP (miR-214)] | (Zhang et al., 2011) |
| DRGs (201 miRs) and Proximal stump (225 miRNAs) – sciatic nerve transection (0–14 days after-injury) | RNA-seq | Intrinsic growth capacity, Schwann cellneuron communication | (Yu et al., 2011) |
| DRGs (26 miRs) - sciatic nerve transection (0–14 days after-injury) | Microarray | cAMP/CREB-based signaling [Pten (miR-222)] | (Zhou et al., 2011) |
| DRGs (19 miRs) – sciatic nerve crush (4 days after-injury) | Microarray | Wnt/β-catenin signaling pathway [Kremen 1 (miR-431)] | (Wu and Murashov, 2013) |
| DRGs (49 miRs) – sciatic nerve crush (7 days after-injury) | RNA-seq | Morphogenesis, polarity, GTPase regulatory activity, actin cytoskeleton reorganization and neural tube formation | (Motti et al., 2017) |
| Sciatic nerve axoplasm (141 miRs; 10 pre-miRNA) – sciatic nerve crush (miRs: 1–14 days after-injury; pre-miRNA: 7 days after-injury) | RNA-seq | ER stress response, cytoskeleton dynamics, vesicle formation | (Kim et al., 2015a; Phay et al., 2015) |
Several validated miR-mRNA target pairs have been identified for neural injury and regeneration responses. miR-21 that is upregulated in DRGs after injury has been shown to modulate Sprouty2 mRNA levels (Strickland et al., 2011b). Sprouty2 is a Ras/Raf/ERK pathway inhibitor that blocks brain-derived neurotrophic factor (BDNF)-induced neuronal differentiation and survival (Gross et al., 2007), so decreasing Sprouty2 would increase pro-growth signaling from BDNF. miR-21 was also shown to be upregulated in injured spinal cord after exercise, which is known to increase functional recovery after spinal cord injury (Liu et al., 2012). Peripheral nerve injury led to down regulation of miR-145 and −214 that regulate the levels of Robo2 and srGAP2 proteins to increase neurite growth and regeneration (Zhang et al., 2011). PNS nerve injury increases miR-222 levels that promotes neurite growth by regulating levels of PTEN (Zhou et al., 2012). Previous studies from the He lab have shown that PTEN knockout supports axon regeneration after optic nerve injury and spinal cord injury (Park et al., 2010). miR-21 mentioned above is also predicated to target PTEN (Liu et al., 2012), though this has not been validated with miR overexpression and knockdown studies. miR-431 is upregulated in DRGs after sciatic nerve crush and targets the Wnt signaling inhibitor Kermen 1 to promote axon regeneration in DRGs (Wu and Murashov, 2013). Wnt signaling has been linked to spinal cord injury and regeneration, so it will be of interest to determine if miR-431 contributes to CNS axon regeneration (Fernandez-Martos et al., 2011; Gonzalez-Fernandez et al., 2014). Together, these and other studies emphasize the role(s) for ncRNAs in PNS and CNS injury responses and regeneration.
The above-mentioned studies focused on miRNAs present in soma of injured neurons or did not distinguish between somal and axonal roles. Work in cultured neurons has clearly shown that miRs localize into axons (Gumy et al., 2011; Natera-Naranjo et al., 2010). Phay et al. (2015) employed deep sequencing (RNA-seq) to identify miRs present in axoplasmic extracts from injured and regenerating rodent sciatic nerves; they found 141 axonal miRNAs with altered levels in injured relative to uninjured axons. Previous studies have shown that axons contain enzymes needed for miR biogenesis and precursor-miRNAs (pre-miR) can be locally processed and function to modulate axonal activity and function (Aschrafi et al., 2008; Aschrafi et al., 2012; Hengst and Jaffrey, 2007; Kar et al., 2013). This raises the appealing hypothesis that axonal injury may locally control miR function by regulating processing of pre-miR into mature miRs within axons. Consistent with this, Kim et al. (2015) showed the presence of pre-miRs in sciatic nerve axons, and they found that injury can indeed trigger processing to generate mature miRs from these precursors (2015). Recently it was shown that some pre-miRs are actively trafficked to distal axons of RGC neurons by hitchhiking on late endosomes/lysosomes, and these pre-miRs are then processed into mature miRs upon exposure to axon guidance cues (Corradi et al., 2020). As we point out below, this endosome/lysosome mediated transport of RNAs, including pre-miRs, could be affected in peripheral neuropathies. Taken together, there is substantial evidence that, changes in both the axonal and somal miR populations play an important role in modulating regenerative capacity after injury and may serve as potential targets for increasing growth of injured axons. Further systematic studies will be needed to fully understand the dynamics of miR function in neurons and how these miRs impact injury-induced gene expression at both global and local levels. The post-transcriptional mechanisms outlined in this section and above emphasize that mRNAs lead a rich life after transcription and restricting study to only levels of RAG mRNAs gives an incomplete picture of the protein changes that are driving regeneration.
V. Growth cone formation and cytoskeletal dynamics for axon growth.
A critical feature of successful axonal regeneration is the ability to initiate and support axon extension. Although there are reports of axon regeneration in the apparent absence of a growth cone (Jin et al., 2016), a general assumption is that the injured axon forms a growth cone that is the site of axon extension. Indeed, one aspect that differentiates injured PNS from CNS axons is the ability to form growth cones in the PNS rather than retraction bulbs as seen in the CNS (Tom et al., 2004). As outlined above, growth cone formation requires Ca2+ influx and in vitro work suggests that intra-axonal protein synthesis is also needed (Blanquie and Bradke, 2018; Chierzi et al., 2005; Erturk et al., 2007; Kamber et al., 2009). The growth cone is comprised of the following components: the peripheral domain (P domain), which includes dynamic F-actin filled filopodia and lamellipodia, a more stable central domain (C domain) containing microtubules, organelles and vesicles, and a transitional domain between the P and C domains containing the motor protein myosin II (Dent et al., 2011). Axonal outgrowth through the growth cone involves three stages: protrusion, engorgement, and consolidation. For protrusion, F-actin filled filopodia and lamellipodia extend from the leading edge of the growth cone (Lowery and Van Vactor, 2009). During engorgement, dynamic microtubules in the C domain enter the newly formed protrusions bringing along vesicles and organelles (Lowery and Van Vactor, 2009). Consolidation occurs with depolymerization of F-actin in the neck of the growth cone with the axon shaft established by compaction of microtubules (Lowery and Van Vactor, 2009). Regulation of the dynamics of these cytoskeletal components by extracellular stimuli is a prominent feature of developmental axon guidance and contributes to axon regeneration; further, work linking the tubulin binding protein APC to mRNAs brings a potential mechanism to alter the proteome of the growth cone (Preitner et al., 2014).
Cytoskeletal reorganization and dynamic cytoskeletal elements in the axon are essential for growth cone formation after nerve injury; local availability of monomer proteins for polymerization of microfilaments and microtubules, levels and activity of binding proteins, and post-translational modifications drive polymerization and stabilization/destabilization of these cytoskeletal elements in the distal axon/growth cone (Dent et al., 2011). Actin dynamics in the filopodia and lamellipodia are needed for growth cone navigation (Geraldo and Gordon-Weeks, 2009) and microfilament turnover in the P domain is necessary for growth cone motility (Difato et al., 2011). Actin depolymerizing factor (ADF)/cofilin is one of the proteins responsible for actin depolymerization in axons and is necessary for regeneration of the centrally projecting DRG axons after a conditioning lesion (Tedeschi et al., 2019), and notably ADF/Cofilin is locally translated in axons (Bellon et al., 2017; Piper et al., 2006; Willis et al., 2007). Axotomy of RGCs causes F-actin destabilization and the Doublecortin and Doublecortin-like kinases help stabilize F-actin in injured axons (Nawabi et al., 2015).
Microtubule dynamics in the growth cone are essential for axon extension (Hur et al., 2012). Acetylated α-tubulin tends to be more prevalent in regions of stable microtubules, while tyrosinated α-tubulin is more prevalent in regions with dynamic microtubules. In PNS neurons, levels of acetylated α-tubulin decrease in axons near the injury site with a concomitant increase in tyrosinated α-tubulin (Cho and Cavalli, 2012). Attenuating tyrosination of α-tubulin significantly reduces PNS sensory axon regeneration by decreasing retrograde injury signaling and delaying the activation of a pro-regenerative program (Song et al., 2015). HDAC inhibitors can block tubulin deacetylation and reduce PNS axon growth; as noted above, HDAC5 localization into distal axons after injury is thought to trigger deacetylation of axonal α-tubulin in the PNS. Tubulin deacetylation is responsible for supporting dynamic microtubules thereby contributing to growth cone dynamics needed to support axon outgrowth, at least in PNS neurons (Cho and Cavalli, 2012). This injury-induced tubulin deacetylation is specific to PNS neurons and does not occur to the same extent in the CNS (Cho and Cavalli, 2012). Intriguingly, microtubule stabilization has been shown to increase regeneration in CNS axons following spinal cord injury (Ruschel et al., 2015) and inhibition of HDAC6, which increases axonal levels of acetylated α-tubulin, promotes axon growth on non-permissive substrates that are part of the growth inhibitory environment of the injured CNS (Rivieccio et al., 2009). The recent report that HDAC6 deacetylates Miro1 in axons (Kalinski et al., 2019) emphasizes that HDAC6, and likely HDAC5, has other axonal substrates that contribute to its effects on axon growth. Also, the divergent effects of microtubule stability and post-translational modifications in PNS and CNS neurons may reflect differences between neuron types or distinct requisites of regenerating in permissive (PNS) vs. non-permissive environments (CNS). In addition, changes in the status of acetylation and tyrosination of α-tubulin also affects the interaction of motor proteins – e.g., Kinesin-1 and-3 have been reported to differentially prefer acetylated or tyrosinated microtubules, respectively (Cai et al., 2009; Guardia et al., 2016; Lipka et al., 2016). These findings raise the possibility that post-translational modifications of α-tubulin affect the subcellular transport and localization of organelles and RNP granules, which in turn affects the local proteome and influence axon integrity and regenerative ability. Notably, inhibition of HDAC6 is axon protective in both inherited and acquired peripheral neuropathies (Benoy et al., 2018; d’Ydewalle et al., 2011; Krukowski et al., 2017; Van Helleputte et al., 2018), and HDAC6 has been shown to interact with as well as target protein synthesis machinery and RBPs outlined above (Cao et al., 2019; Kwon et al., 2007; Mo et al., 2018). Thus, HDAC6 provides a clear mechanistic intersection for pathways involved in axon regeneration and axon degeneration.
VI. Contributions of non-neuronal cells in nerve injury and axon regeneration.
The sections above regard the axon as an entity dependent solely on the soma for responses to injury and ability to regenerate. However, the axon clearly is impacted by and senses its local environment, and non-neuronal cells as well as factors secreted by those clearly impact injury responses and axon growth (Figure 1A). For example, Schwann cells have a major impact on regeneration. Indeed, preventing Schwann cells from migrating in regenerating nerves impairs axon regeneration (Chen et al., 2005). Work from Toth et al. (2009) showed that secretion of calcitonin gene-related polypeptide (CGRP) from axons supports Schwann cell migration (Toth et al., 2009). Growth factors secreted by denervated Schwann cells serve as tropic stimuli for axon regrowth (Gordon, 2009). For example, Schwann cell secretion of BDNF in injured nerves activates axonal Tropomyosin Receptor Kinase B (TrkB) to support regenerative growth of motor axons through axon-to-soma signaling (English et al., 2014). Interestingly, evidence points to unique gene expression signatures of Schwann cells from motor vs. sensory nerves (Brushart et al., 2013; Hoke et al., 2006), so the Schwann cell may be tuned to support growth of its surrounding axons. Schwann cells in the PNS as well as oligodendrocytes in the CNS serve as a nutrient source for the distal axon by providing lactate and pyruvate to feed into axonal ATP production (Jha and Morrison, 2018). Finally, there is some evidence that Schwann cells can transfer macromolecules to axons, as Schwann cell-derived ribosomes have been detected in injured and regenerating axons (Court et al., 2008; Court et al., 2011), and a recent study suggested that glia are a major source of axonal ribosomes after axotomy (Muller et al., 2018). Schwann cell-derived exosomes have further been shown to increase axon growth in PNS neurons (Lopez-Verrilli et al., 2013). It is appealing to hypothesize that these exosomes transfer mRNAs or ncRNAs to axons, though what exactly is the growth promoting content of these Schwann cell packets can only be inferred at this point (Lopez-Leal and Court, 2016). Together, the above and other observations point to Schwann cell-to-axon signals that can support axon growth, function, and survival, and raise the potential for reciprocal axon-to-Schwann cell signaling.
Inflammatory cells infiltrate the nerve and directly or indirectly impact nerve regeneration. An inflammatory response can occur within a few minutes to hours after injury and is important for axon regeneration (Benowitz and Popovich, 2011). Granulocyte and monocyte infiltration into injured PNS nerves was suggested to contribute to regeneration by producing growth-supporting neurotrophic factors (Barrette et al., 2008). In line with this, exogenous interleukin (IL)-6 and interferon γ (IFNγ) increase neurotrophin-mediated axon outgrowth in DRG explants (Golz et al., 2006). Macrophage-derived IL-1 promotes Schwann cell synthesis of NGF in the distal nerve after injury (Lindholm et al., 1987). Macrophage infiltration into the DRG after peripheral nerve injury neurons also impacts sensory axon regeneration, and release of the C-C motif chemokine ligand 2 (CCL2) recruits macrophages to denervated DRGs (Lindborg et al., 2018; Niemi et al., 2013). Inflammatory cell-derived factors can also directly affect regeneration by acting on neurons. Leukemia inhibitory factor (LIF), a member of the gp130 cytokine family, plays an important role in signaling the immediate inflammatory cell infiltration into the injury site (Sugiura et al., 2000). The increased axon regeneration seen after a conditioning-injury is blunted in LIF knockout mice (Cafferty et al., 2001). LIF was shown to be retrogradely transported in sensory neurons after injection into the sciatic nerve indicating LIF could directly drive axon-to-soma injury signaling effects (Lindholm et al., 1987). Optic nerve injury causes a significant increase in IL-6 expression, and it can increase RGC survival and neurite outgrowth (Leibinger et al., 2013) as well as contribute to injury-conditioning responses in the PNS (Cafferty et al., 2004). IL-6 administration led to increased levels of regeneration-associated genes RAGs such as Sprr1a, Galanin, and Gap43 mRNAs in RGCs raising the possibility for IL-6 activating axon-to-nuclear signaling (Leibinger et al., 2013). Inflammatory cell-derived IL-2, IL-10, tumor necrosis factor-β, and transforming growth factor-β have also been linked to peripheral nerve injury (Fregnan et al., 2012), and some of these factors may affect CNS regeneration (Tsarouchas et al., 2018). Of note, work from the Price lab has shown that IL-6 can affect protein synthesis in intact sensory axons and trigger axon-to-nucleus signaling through localized CREB synthesis after application of neuropathic pain-invoking stimuli (Melemedjian et al., 2010; Melemedjian et al., 2014 ). Thus, regeneration-supportive inflammatory vs. pathologic inflammatory responses in the PNS could be determined by synergy between cytokine and neurotrophin signaling pathways as suggested by Vidal et al. (2013).
VII. Axon intrinsic signaling mechanisms and peripheral neuropathies
With reference to this issue of Brain Research, it should be noted that the injury response mechanisms outlined above overlap with the pathophysiology of some neuropathic conditions. We have attempted to raise these in the sections above, but appealing links between axonal mRNA transport/translation and axon function warrant further attention. In particular, alterations in axonal mRNA transport have been demonstrated in a chemotherapy-induced peripheral neuropathy. The Segal lab found that axonal transport of RNA granules containing the RBP SFPQ is decreased with paclitaxel exposure, thereby decreasing axonal synthesis of the anti-apoptotic protein Bclw whose RNA these granules transport (Figure 2) (Pease-Raissi et al., 2017). Axonal Bclw contributes to axon survival by binding to IP3R on ER preventing Ca2+ release (Figure 2) (Pease-Raissi et al., 2017). Axonal levels of the mRNAs for Mitofusin 2 (Mfn2), Opa1 Mitochondrial Dynamin Like GTPase, and Dynamin-1-like protein (Drp1), proteins that function in mitochondrial fusion and fission, are similarly decreased after exposure to paclitaxel (Bobylev et al., 2015). These observations indicate axonal protein synthesis can be disrupted in acquired neuropathies, and considering the proteins involved, this can directly affect axon survival and function. As we outline below, cellular mechanisms impacted in inherited neuropathies, including the varying forms of Charcot Marie Tooth (CMT) neuropathies, could have direct impact on the growth-associated axon-intrinsic functions outlined above.
Work in several labs now points to co-transport of axonal mRNAs and translational machinery on endocytic and lysosomal vesicles (Cioni et al., 2019; Liao et al., 2019; Rangaraju et al., 2019). This may help to explain earlier works pointing to association of RBPs (e.g., SMN and HTT) with membrane-bound organelles and organelle associated proteins (e.g., CopB1) binding to localized mRNAs (Bi et al., 2007; Custer et al., 2013; Custer et al., 2019; Savas et al., 2010; White et al., 2015). With regards to neuropathies, mutations of a number of proteins that cause demyelinating CMT are engaged in varying steps of the endocytic pathway that generates vesicles, including endocytosis, endosomal recycling, and endosome-to-lysosome trafficking (For review see Lee et al., 2017). Disrupted endocytic trafficking due to CMT-associated mutations leads to dysregulation of a number of critical intracellular signaling pathways (e.g., ERK, JNK1, AKT, and PI3K/AKT) that in turn can impact axonal maintenance/function through Schwann cell-to-axon signaling mechanisms (Fledrich et al., 2014; Kohl et al., 2010; Massa et al., 2006). More directly, the Holt lab recently showed that Rab7a mutants associated with CMT2B in RGC axons directly impairs axonal protein synthesis with loss of axon integrity (Cioni et al., 2019). The causative gene for CMT4J, Fat-induced Gene 4 (Fig4 also called Sac3) that encodes a phosphoinostide 5-phosphatase that regulates intracellular levels of PI(3,5)P2 and PI(5)P, plays a critical role in endosome-to-lysosome maturation and trafficking. Fig4-deficient animals show abnormal endosomes and lysosomes, suggesting the involvement of impaired endolysosomal trafficking in CMT4J pathogenesis (Chow et al., 2007; Lenk et al., 2011; Winters et al., 2011). It will be intriguing to see if RNA trafficking and axonal translation are disrupted in CMT4J and other inherited neuropathies.
Mitochondrial function is critical for maintaining axon integrity and mutations in proteins that cause altered mitochondrial transport and function can result in axonal forms of CMT (Kalmar et al., 2017; Rocha et al., 2018). These include MFN2 and HSPB1 whose mRNAs are also localized into PNS (Bobylev et al., 2018; Willis et al., 2005; Willis et al., 2007). mRNAs for several nuclear-encoded mitochondrial proteins also localize into the axons and their localized translation helps to maintain mitochondrial function in distal axons (Aschrafi et al., 2016; Fazal et al., 2019; Kaplan et al., 2009; Yoon et al., 2012). Similarly axonal synthesis of LaminB supports axonal integrity at least in part by supporting mitochondrial function (Yoon et al., 2012). Decreased mitochondrial respiration with resulting fall in nicotinamide adenine dinucleotide (NAD) has long been linked to Wallerian degeneration and likely contributes to other forms of axon degeneration (Conforti et al., 2014). Observations that mitochondrial ATP synthesis supports local mRNA translation during plasticity and axon branching (Rangaraju et al., 2019; Spillane et al., 2012) and axonally synthesized proteins supporting mitochondrial function (Aschrafi et al., 2012; Cioni et al., 2019; Natera-Naranjo et al., 2012) bring an interesting bidirectional network that can support axon integrity, function, and branching. The possibility that this axon-intrinsic network plays a role in the pathophysiology of peripheral neuropathy may bring new therapeutic targets to prevent axon loss and/or to encourage branching of residual axons that can reinnervate target tissues and help to restore some function.
CONCLUSION
Much progress has been made to understand how the intrinsic growth program of axons is initiated after injury and the critical mechanisms that allow the PNS to regenerate. Injury of the distal axon provides multiple paths for axon-to-soma and axon-to-nucleus signaling that shift the neuron’s phenotype to one supporting regeneration. This subsequently provides soma-to-axon signaling that is needed for effective regeneration and eventual functional recovery. Clearly more effort is needed to determine the intricacies of how these mechanisms support regeneration and how they are regulated. This is particularly true if this knowledge will be leveraged to develop effective therapies to improve neural repair in the PNS and CNS. As we note above, loss of some of these mechanisms can also contribute to peripheral neuropathic conditions, with clear links between axonal mRNA transport and translation to chemotherapy-induced peripheral neuropathy and very intriguing potential links to inherited peripheral neuropathies like CMT. Therapies targeting some of these mechanisms may indeed provide dual functions for neuropathic conditions by supporting axon survival/integrity as well as encouraging axon branching for reinnervation of denervated target tissues. However, we need a much better understanding for how broadly axonal RNA transport/translation or other axon-intrinsic mechanisms outlined above extend to inherited and acquired neuropathic conditions.
HIGHLIGHTS FOR REVIEW.
- Axonal injury in the PNS invokes axon-to-soma and axon-to-nucleus signals through back-propogating wave of Ca2+ and retrograte transport of signaling proteins that change gene expression to support regeneration through transcriptional and epigenetic mechansims.
- Part of the retrograde signaling is generated through localized synthesis Importin-β protein and transcription factors, whose intra-axonal translation is activated by increased axoplasmic Ca2+
- Subsequent soma-to-axon signaling helps to support axon growth by providing both proteins and mRNAs, with the latter used to locally generate proteins to support growth.
- Injured CNS axons share some of the same signaling mechanisms but do not appear to invoke these with traumatic injury, which may help to explain the decreased regeneration capacity of CNS as compared to PNS neurons.
- Axon degeneration can also be triggered by increase in axoplasmic Ca2+, particularly by release from intracellular stores including the ER. Interestingly, depletion of ER Ca2+ can trigger an unfolded protein response locally in axons which helps to initiate axon regeneration mechanisms with traumatic injury but also leads to axon death in chemotherapy induced neuropathy.
Our manuscript discusses these mechanisms and draws parallels between injury-response that leads to successful axon regeneration vs. known mechanisms of axon degeneration.
Acknowledgements –
The authors have been supported by the following grants from the following agencies in research directly related to the topic of this review: NIH/NINDS (K01-NS105879 to TS; R01-NS089633 and R01-NS041596 to JLT), NSF (MCB-1020970 to JLT), Department of Defense, US Army Medical Research and Development (W81XWH-13-1-0308 to JLT), the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (to JLT), South Carolina Spinal Cord Injury Research Fund (2018 PD-01 to PKS), and the UofSC Research Office ASPIRE program (to ANK). JLT is the incumbent South Carolina SmartState Endowed Chair in Childhood Neurotherapeutics at the Univ. of South Carolina.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akten B., et al. , 2011. Interaction of survival of motor neuron (SMN) and HuD proteins with mRNA cpg15 rescues motor neuron axonal deficits. Proc Natl Acad Sci U S A. 108, 10337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Kedersha N., 2008. Stress granules: the Tao of RNA triage. Trends Biochem Sci. 33, 141–50. [DOI] [PubMed] [Google Scholar]
- Andreassi C., et al. , 2010. An NGF-responsive element targets myo-inositol monophosphatase-1 mRNA to sympathetic neuron axons. Nat Neurosci. 13, 291–301. [DOI] [PubMed] [Google Scholar]
- Andrusiak MG, et al. , 2019. Inhibition of axon regeneration by liquid-like TIAR-2 granules. Neuron. 104, 290–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronov S., Marx R., Ginzburg I., 1999. Identification of 3’UTR region implicated in tau mRNA stabilization in neuronal cells. J Mol Neurosci. 12, 131–45. [DOI] [PubMed] [Google Scholar]
- Aronov S., et al. , 2002. Visualization of translated tau protein in the axons of neuronal P19 cells and characterization of tau RNP granules. J Cell Sci. 115, 3817–27. [DOI] [PubMed] [Google Scholar]
- Aschrafi A., et al. , 2008. MicroRNA-338 regulates local cytochrome c oxidase IV mRNA levels and oxidative phosphorylation in the axons of sympathetic neurons. J Neurosci. 28, 12581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschrafi A., et al. , 2012. MicroRNA-338 regulates the axonal expression of multiple nuclear-encoded mitochondrial mRNAs encoding subunits of the oxidative phosphorylation machinery. Cell Mol Life Sci. 69, 4017–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschrafi A., et al. , 2016. A heterogeneous population of nuclear-encoded mitochondrial mRNAs is present in the axons of primary sympathetic neurons. Mitochondrion. 30, 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas R., et al. , 2004. The insulin-like growth factor mRNA binding-protein IMP-1 and the Ras-regulatory protein G3BP associate with tau mRNA and HuD protein in differentiated P19 neuronal cells. J Neurochem. 89, 613–26. [DOI] [PubMed] [Google Scholar]
- Barrette B., et al. , 2008. Requirement of myeloid cells for axon regeneration. J Neurosci. 28, 9363–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos SA, et al. , 2011. Axonal degeneration is mediated by the mitochondrial permeability transition pore. J Neurosci. 31, 966–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassell GJ, et al. , 1998. Sorting of beta-actin mRNA and protein to neurites and growth cones in culture. J Neurosci. 18, 251–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellon A., et al. , 2017. miR-182 regulates Slit2-mediated axon guidance by modulating the local translation of a specific mRNA. Cell Rep. 18, 1171–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yaakov K., et al. , 2012. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J. 31, 1350–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz LI, Popovich PG, 2011. Inflammation and axon regeneration. Curr Opin Neurol. 24, 577–83. [DOI] [PubMed] [Google Scholar]
- Benoy V., et al. , 2018. HDAC6 is a therapeutic target in mutant GARS-induced Charcot-Marie-Tooth disease. Brain. 141, 673–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi J., et al. , 2007. Copb1-facilitated axonal transport and translation of kappa opioid-receptor mRNA. Proc Natl Acad Sci USA. 104, 13810–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanquie O., Bradke F., 2018. Cytoskeleton dynamics in axon regeneration. Curr Opin Neurobiol. 51, 60–69. [DOI] [PubMed] [Google Scholar]
- Bobylev I., et al. , 2015. Paclitaxel inhibits mRNA transport in axons. Neurobiol Dis. 82, 321–331. [DOI] [PubMed] [Google Scholar]
- Bobylev I., et al. , 2018. Depletion of Mitofusin-2 causes mitochondrial damage in cisplatin-induced neuropathy. Mol Neurobiol. 55, 1227–1235. [DOI] [PubMed] [Google Scholar]
- Bradke F., Fawcett JW, Spira ME, 2012. Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nat Rev Neurosci. 13, 183–93. [DOI] [PubMed] [Google Scholar]
- Briese M., et al. , 2016. Whole transcriptome profiling reveals the RNA content of motor axons. Nucleic Acids Res. 44, e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM, et al. , 2013. Schwann cell phenotype is regulated by axon modality and central-peripheral location, and persists in vitro. Exp Neurol. 247, 272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, et al. , 2001. Leukemia inhibitory factor determines the growth status of injured adult sensory neurons. J Neurosci. 21, 7161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, et al. , 2004. Conditioning injury-induced spinal axon regeneration fails in interleukin-6 knock-out mice. J Neurosci. 24, 4432–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D., et al. , 2009. Single molecule imaging reveals differences in microtubule track selection between Kinesin motors. PLoS Biol. 7, e1000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., et al. , 2019. Pseudophosphatase MK-STYX alters histone deacetylase 6 cytoplasmic localization, decreases its phosphorylation, and increases detyrosination of tubulin. Int J Mol Sci. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YY, et al. , 2005. Axon and Schwann cell partnership during nerve regrowth. J Neuropathol Exp Neurol. 64, 613–22. [DOI] [PubMed] [Google Scholar]
- Chierzi S., et al. , 2005. The ability of axons to regenerate their growth cones depends on axonal type and age, and is regulated by calcium, cAMP and ERK. Eur J Neurosci. 21, 2051–62. [DOI] [PubMed] [Google Scholar]
- Cho KHT, et al. , 2019. Emerging roles of miRNAs in brain development and perinatal brain injury. Front Physiol. 10, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y., Cavalli V., 2012. HDAC5 is a novel injury-regulated tubulin deacetylase controlling axon regeneration. EMBO J. 31, 3063–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y., et al. , 2013. Injury-induced HDAC5 nuclear export is essential for axon regeneration. Cell. 155, 894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong MS, et al. , 1999. Axonal regeneration from injured dorsal roots into the spinal cord of adult rats. J Comp Neurol. 410, 42–54. [PubMed] [Google Scholar]
- Chow CY, et al. , 2007. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature. 448, 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioni JM, et al. , 2019. Late endosomes act as mRNA translation platforms andsustain mitochondria in axons. Cell. 176, 56–72 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L., Gilley J., Coleman MP, 2014. Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nat Rev Neurosci. 15, 394–409. [DOI] [PubMed] [Google Scholar]
- Corradi E., et al. , 2020. Axonal precursor miRNAs hitchhike on endosomes and locally regulate the development of neural circuits. EMBO J. 39, e102513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosker KE, et al. , 2016. The RNA-binding protein SFPQ orchestrates an RNA regulon to promote axon viability. Nat Neurosci. 19, 690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court FA, et al. , 2008. Schwann cell to axon transfer of ribosomes: toward a novel understanding of the role of glia in the nervous system. J Neurosci. 28, 11024–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court FA, et al. , 2011. Morphological evidence for a transport of ribosomes from Schwann cells to regenerating axons. Glia. 59, 1529–39. [DOI] [PubMed] [Google Scholar]
- Cox LJ, et al. , 2008. Intra-axonal translation and retrograde trafficking of CREB promotes neuronal survival. Nat Cell Biol. 10, 149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custer SK, et al. , 2013. Dilysine motifs in exon 2b of SMN protein mediate binding to the COPI vesicle protein alpha-COP and neurite outgrowth in a cell culture model of spinal muscular atrophy. Hum Mol Genet. 22, 4043–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custer SK, et al. , 2019. Abnormal Golgi morphology and decreased COPI function in cells with low levels of SMN. Brain Res. 1706, 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Ydewalle C., et al. , 2011. HDAC6 inhibitors reverse axonal loss in a mouse model of mutant HSPB1-induced Charcot-Marie-Tooth disease. Nat Med. 17, 968–74. [DOI] [PubMed] [Google Scholar]
- Dent EW, Gupton SL, Gertler FB, 2011. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detrait E., et al. , 2000. Plasmalemmal repair of severed neurites of PC12 cells requires Ca2+ and synaptotagmin. J Neurosci Res. 62, 566–573. [DOI] [PubMed] [Google Scholar]
- Difato F., et al. , 2011. The formation of actin waves during regeneration after axonal lesion is enhanced by BDNF. Sci Rep. 1, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly CJ, et al. , 2011. Limited availability of ZBP1 restricts axonal mRNA localization and nerve regeneration capacity. EMBO J. 30, 4665–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Wilhelm JC, Ward PJ, 2014. Exercise, neurotrophins, and axon regeneration in the PNS. Physiology (Bethesda). 29, 437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erturk A., et al. , 2007. Disorganized microtubules underlie the formation of retraction bulbs and the failure of axonal regeneration. J Neurosci. 27, 9169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazal FM, et al. , 2019. Atlas of subcellular RNA localization revealed by APEX-Seq. Cell. 178, 473–490 e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Martos CM, et al. , 2011. Differential expression of Wnts after spinal cord contusion injury in adult rats. PLoS One. 6, e27000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fledrich R., et al. , 2014. Soluble neuregulin-1 modulates disease pathogenesis in rodent models of Charcot-Marie-Tooth disease 1A. Nat Med. 20, 1055–61. [DOI] [PubMed] [Google Scholar]
- Forman DS, et al. , 1980. Time course of the condition lesion effect on axonal regeneration. Brain Res. 182, 180–185. [DOI] [PubMed] [Google Scholar]
- Fregnan F., et al. , 2012. Role of inflammatory cytokines in peripheral nerve injury. Neural Regen Res. 7, 2259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaub P., et al. , 2010. HDAC inhibition promotes neuronal outgrowth and counteracts growth cone collapse through CBP/p300 and P/CAF-dependent p53 acetylation. Cell Death Differ. 17, 1392–408. [DOI] [PubMed] [Google Scholar]
- Gaub P., et al. , 2011. The histone acetyltransferase p300 promotes intrinsic axonal regeneration. Brain. 134, 2134–48. [DOI] [PubMed] [Google Scholar]
- Gelebart P., Opas M., Michalak M., 2005. Calreticulin, a Ca2+-binding chaperone of the endoplasmic reticulum. Int J Biochem Cell Biol. 37, 260–6. [DOI] [PubMed] [Google Scholar]
- Geraldo S., Gordon-Weeks PR, 2009. Cytoskeletal dynamics in growth-cone steering. J Cell Sci. 122, 3595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh-Roy A., et al. , 2010. Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J Neurosci. 30, 3175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka M., et al. , 2010. The heterogeneous nuclear ribonucleoprotein-R is necessary for axonal beta-actin mRNA translocation in spinal motor neurons. Hum Mol Genet. 19, 1951–66. [DOI] [PubMed] [Google Scholar]
- Golz G., et al. , 2006. The cytokine/neurotrophin axis in peripheral axon outgrowth. Eur J Neurosci. 24, 2721–30. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Fernandez C., et al. , 2014. Wnts are expressed in the spinal cord of adult mice and are differentially induced after injury. J Neurotrauma. 31, 565–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T., 2009. The role of neurotrophic factors in nerve regeneration. Neurosurg Focus. 26, E3. [DOI] [PubMed] [Google Scholar]
- Gross I., et al. , 2007. Sprouty2 inhibits BDNF-induced signaling and modulates neuronal differentiation and survival. Cell Death Differ. 14, 1802–12. [DOI] [PubMed] [Google Scholar]
- Guardia CM, et al. , 2016. BORC functions upstream of kinesins 1 and 3 to coordinate regional movement of lysosomes along different microtubule tracks. Cell Rep. 17, 1950–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumy LF, et al. , 2011. Transcriptome analysis of embryonic and adult sensory axons reveals changes in mRNA repertoire localization. RNA. 17, 85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner AS, et al. , 2019. Local protein synthesis is a ubiquitous feature of neuronal pre- and postsynaptic compartments. Science. 364, eaau3644. [DOI] [PubMed] [Google Scholar]
- Hanz S., et al. , 2003. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 40, 1095–104. [DOI] [PubMed] [Google Scholar]
- Harding HP, et al. , 2002. Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol. 18, 575–99. [DOI] [PubMed] [Google Scholar]
- Hengst U., Jaffrey SR, 2007. Function and translational regulation of mRNA in developing axons. Semin Cell Dev Biol. 18, 209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengst U., et al. , 2009. Axonal elongation triggered by stimulus-induced local translation of a polarity complex protein. Nature cell biology. 11, 1024–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervera A., et al. , 2019. PP4-dependent HDAC3 dephosphorylation discriminates between axonal regeneration and regenerative failure. EMBO J. 38, e101032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke A., et al. , 2006. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci. 26, 9646–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland SM, et al. , 2016. Palmitoylation controls DLK localization, interactions and activity to ensure effective axonal injury signaling. Proc Natl Acad Sci U S A. 113, 763–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner EA, Strittmatter SM, 2009. Axon regeneration in the peripheral and central nervous systems. Results Probl Cell Differ. 48, 339–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur EM, Saijilafu, Zhou FQ., 2012. Growing the growth cone: remodeling the cytoskeleton to promote axon regeneration. Trends Neurosci. 35, 164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson TH, et al. , 2019. CBP-dependent histone acetylation mediates axon regeneration induced by environmental enrichment in rodent spinal cord injury models. Sci Transl Med. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Morrison BM, 2018. Glia-neuron energy metabolism in health and diseases: New insights into the role of nervous system metabolic transporters. Exp Neurol. 309, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin LQ, et al. , 2016. Protein synthetic machinery and mRNA in regenerating tips of spinal cord axons in lamprey. J Comp Neurol. 524, 3614–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonson L., et al. , 2007. Molecular composition of IMP1 ribonucleoprotein granules. Mol Cell Proteomics. 6, 798–811. [DOI] [PubMed] [Google Scholar]
- Kalinski AL, et al. , 2019. Deacetylation of Miro1 by HDAC6 blocks mitochondrial transport and mediates axon growth inhibition. J Cell Biol. 218, 1871–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmar B., et al. , 2017. Mitochondrial deficits and abnormal mitochondrial retrograde axonal transport play a role in the pathogenesis of mutant HSP27-induced Charcot Marie Tooth disease. Hum Mol Genet. 26, 3313–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamber D., Erez H., Spira ME, 2009. Local calcium-dependent mechanisms determine whether a cut axonal end assembles a retarded endbulb or competent growth cone. Exp Neurol. 219, 112–25. [DOI] [PubMed] [Google Scholar]
- Kaplan BB, et al. , 2009. Axonal protein synthesis and the regulation of local mitochondrial function. Results Probl Cell Differ. 48, 225–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar A., Lee S., Twiss J., 2018. Expanding Axonal transcriptome brings new functions for axonally synthesized proteins in health and Ddisease. The Neuroscientist. 24, 111–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar AN, et al. , 2013. Intra-axonal synthesis of eukaryotic translationinitiation factors regulates local protein synthesis and axon growth in rat sympathetic neurons. J Neurosci. 33, 7165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene JD, 2007. RNA regulons: coordination of post-transcriptional events. Nat Rev Genet. 8, 533–43. [DOI] [PubMed] [Google Scholar]
- Kenney AM, Kocsis JD, 1998. Peripheral axotomy induces long-term c-Jun amino-terminal kinase-1 activation and activator protein-1 binding activity by c-Jun and JunD in adult rat dorsal root ganglia In vivo. J Neurosci. 18, 1318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalfallah Y., et al. , 2018. TDP-43 regulation of stress granule dynamics in neurodegenerative disease-relevant cell types. Sci Rep. 8, 7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoutorsky A., Spira ME, 2005. Calcium-activated proteases are critical for refilling depleted vesicle stores in cultured sensory-motor synapses of Aplysia. Learn Mem. 12, 414–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HH, et al. , 2015a. Identification of precursor microRNAs within distal axons of sensory neuron. J Neurochem. 134, 193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HH, et al. , 2015b. Different motif requirements for the localization zipcode element of beta-actin mRNA binding by HuD and ZBP1. Nucleic Acids Res. 43, 7432–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kislauskis EH, Zhu X., Singer RH, 1994. Sequences responsible for intracellular localization of beta-actin messenger RNA also affect cell phenotype. J Cell Biol. 127, 441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl B., et al. , 2010. MCP-1/CCL2 modifies axon properties in a PMP22-overexpressing mouse model for Charcot-Marie-tooth 1A neuropathy. Am J Pathol. 176, 1390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppers M., et al. , 2019. Receptor-specific interactome as a hub for rapid cue-induced selective translation in axons. Elife. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krukowski K., et al. , 2017. HDAC6 inhibition effectively reverses chemotherapy-induced peripheral neuropathy. Pain. 158, 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulbatski I., Cook DJ, Tator CH, 2004. Calcium entry through L-type calcium channels is essential for neurite regeneration in cultured sympathetic neurons. J Neurotrauma. 21, 357–74. [DOI] [PubMed] [Google Scholar]
- Kwon S., Zhang Y., Matthias P., 2007. The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response. Genes Dev. 21, 3381–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kye MJ, et al. , 2014. SMN regulates axonal local translation via miR-183/mTOR pathway. Hum Mol Genet. 23, 6318–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., et al. , 2018. hnRNPs binding to the axonal localization motifs of Nrn1 and HMGB1 mRNAs define growth-associated RNA regulons. Mol Cell Proteomics. 17, 2091–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Chin LS, Li L., 2017. Dysregulation of ErbB receptor trafficking and signaling in demyelinating Charcot-Marie-Tooth disease. Mol Neurobiol. 54, 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibinger M., et al. , 2013. Interleukin-6 contributes to CNS axon regeneration upon inflammatory stimulation. Cell Death Dis. 4, e609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenk GM, et al. , 2011. Pathogenic mechanism of the FIG4 mutation responsible for Charcot-Marie-Tooth disease CMT4J. PLoS Genet. 7, e1002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezana JP, et al. , 2016. Axonal PPARgamma promotes neuronal regeneration after injury. Dev Neurobiol. 76, 688–701. [DOI] [PubMed] [Google Scholar]
- Liao YC, et al. , 2019. RNA granules hitchhike on lysosomes for long-distance transport, using Annexin A11 as a molecular tether. Cell. 179, 147–164 e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindborg JA, et al. , 2018. Molecular and cellular identification of the immune response in peripheral ganglia following nerve injury. J Neuroinflammation. 15, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D., et al. , 1987. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature. 330, 658–9. [DOI] [PubMed] [Google Scholar]
- Lindner R., et al. , 2014. DNA methylation temporal profiling following peripheral versus central nervous system axotomy. Sci Data. 1, 140038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka J., et al. , 2016. Microtubule-binding protein doublecortin-like kinase 1 (DCLK1) guides kinesin-3-mediated cargo transport to dendrites. EMBO J. 35, 302–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., et al. , 2012. Exercise modulates microRNAs that affect the PTEN/mTOR pathway in rats after spinal cord injury. Exp Neurol. 233, 447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh YE, et al. , 2017. Comprehensive mapping of 5-hydroxymethylcytosine epigenetic dynamics in axon regeneration. Epigenetics. 12, 77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Leal R., Court FA, 2016. Schwann cell exosomes mediate neuron-glia communication and enhance axonal regeneration. Cell Mol Neurobiol. 36, 429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Verrilli MA, Picou F., Court FA, 2013. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 61, 1795–806. [DOI] [PubMed] [Google Scholar]
- Lowery LA, Van Vactor D., 2009. The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol. 10, 332–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma KH, et al. , 2018. Polycomb repression regulates Schwann cell proliferation and axon regeneration after nerve injury. Glia. 66, 2487–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, et al. , 2017. TIA1 mutations in amyotrophic lateral sclerosis and frontotemporal dementia promote phase separation and alter stress granule dynamics. Neuron. 95, 808–816 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ, 2007. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 18, 716–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar FM, et al. , 2014. CNS axons globally increase axonal transport after peripheral conditioning. J Neurosci. 34, 5965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JC, et al. , 2019. Pum2 shapes the transcriptome in developing axons through retention of target mRNAs in the cell body. Neuron. 104, 931–946 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa R., et al. , 2006. Overexpression of ErbB2 and ErbB3 receptors in Schwann cells of patients with Charcot-Marie-tooth disease type 1A. Muscle Nerve. 33, 342–9. [DOI] [PubMed] [Google Scholar]
- Maziuk B., Ballance HI, Wolozin B., 2017. Dysregulation of RNA binding protein aggregation in neurodegenerative disorders. Front Mol Neurosci. 10, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald KK, et al. , 2011. TAR DNA-binding protein 43 (TDP-43) regulates stress granule dynamics via differential regulation of G3BP and TIA-1. Hum Mol Genet. 20, 1400–10. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG, Grafstein B., 1973. Axon outgrowth enhanced by a previous nerve injury. Arch Neurol. 29, 53–5. [DOI] [PubMed] [Google Scholar]
- Melemedjian OK, et al. , 2010. IL-6- and NGF-induced rapid control of protein synthesis and nociceptive plasticity via convergent cignaling to the eIF4F complex. J Neurosci. 30, 15113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melemedjian OK, et al. , 2014. Local translation and retrograde axonal transport of CREB regulates IL-6-induced nociceptive plasticity. Mol Pain. 10, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merianda TT, et al. , 2013. Axonal localization of neuritin/CPG15 mRNA in neuronal populations through distinct 5’ and 3’ UTR elements. J Neurosci. 33, 13735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao XR, et al. , 2017. DNMT3a contributes to the development and maintenance of bone cancer pain by silencing Kv1.2 expression in spinal cord dorsal horn. Mol Pain. 13, 1744806917740681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minis A., et al. , 2014. Subcellular transcriptomics-dissection of the mRNA composition in the axonal compartment of sensory neurons. Dev Neurobiol. 74, 365–81. [DOI] [PubMed] [Google Scholar]
- Mo Z., et al. , 2018. Aberrant GlyRS-HDAC6 interaction linked to axonal transport deficits in Charcot-Marie-Tooth neuropathy. Nat Commun. 9, 1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi M., et al. , 2017. Differential roles of alpha-, beta-, and gamma-actin in axon growth and collateral branch formation in motoneurons. J Cell Biol. 216, 793–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motti D., Bixby JL, Lemmon VP, 2012. MicroRNAs and neuronal development. Semin Fetal Neonatal Med. 17, 347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motti D., et al. , 2017. Identification of miRNAs involved in DRG neurite outgrowth and their putative targets. FEBS Lett. 591, 2091–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller K., et al. , 2018. A predominantly glial origin of axonal ribosomes after nerve injury. Glia. 66, 1591–1610. [DOI] [PubMed] [Google Scholar]
- Murashov AK, et al. , 2007. RNAi pathway is functional in peripheral nerve axons. FASEB J. 21, 656–70. [DOI] [PubMed] [Google Scholar]
- Natera-Naranjo O., et al. , 2010. Identification and quantitative analyses of microRNAs located in the distal axons of sympathetic neurons. RNA. 16, 1516–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natera-Naranjo O., et al. , 2012. Local translation of ATP synthase subunit 9 mRNA alters ATP levels and the production of ROS in the axon. Mol Cell Neurosci. 49, 263–70. [DOI] [PubMed] [Google Scholar]
- Nawabi H., et al. , 2015. Doublecortin-Like Kinases promote neuronal survival and induce growth cone reformation via distinct mechanisms. Neuron. 88, 704–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehrt A., et al. , 2007. The critical role of voltage-dependent calcium channel in axonal repair following mechanical trauma. Neuroscience. 146, 1504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S., Woolf CJ, 1999. Regeneration of dorsal column fibers into and beyond lesion site following adult spinal cord injury. Neuron. 23, 83–91. [DOI] [PubMed] [Google Scholar]
- Neumann S., et al. , 2002. Regeneration of sensory axons within injured spinal cord induced by intraganglionic cAMP elevations. Neuron. 34, 885–93. [DOI] [PubMed] [Google Scholar]
- Niemi JP, et al. , 2013. A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve regeneration. J Neurosci. 33, 16236–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh YM, et al. , 2018. Epigenetic regulator UHRF1 inactivates REST and growth suppressor gene expression via DNA methylation to promote axon regeneration. Proc Natl Acad Sci U S A. 115, E12417–E12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake Y., et al. , 2018. Axonal activation of the unfolded protein response promotes axonal regeneration following peripheral nerve injury. Neuroscience. 375, 34–48. [DOI] [PubMed] [Google Scholar]
- Onate M., et al. , 2016. Activation of the unfolded protein response promotes axonal regeneration after peripheral nerve injury. Sci Rep. 6, 21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco A., et al. , 2020. Mechanism and role of the intra-axonal Calreticulin translation in response to axonal injury. Exp Neurol. 323, 113072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmisano I., Di Giovanni S., 2018. Advances and Limitations of Current Epigenetic Studies Investigating Mammalian Axonal Regeneration. Neurotherapeutics. 15, 529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KK, et al. , 2010. PTEN/mTOR and axon regeneration. Exp Neurol 223, 45–50. [DOI] [PubMed] [Google Scholar]
- Pease-Raissi SE, et al. , 2017. Paclitaxel Reduces Axonal Bclw to Initiate IP3R1-Dependent Axon Degeneration. Neuron. 96, 373–386 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlson E., et al. , 2005. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron. 45, 715–26. [DOI] [PubMed] [Google Scholar]
- Perry RB, et al. , 2012. Subcellular knockout of importin beta1 perturbs axonal retrograde signaling. Neuron. 75, 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RB, et al. , 2016. Nucleolin-mediated RNA localization regulates neuron growth and cycling cell size. Cell Rep. 16, 1664–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phay M., Kim HH, Yoo S., 2015. Dynamic Change and Target Prediction of Axon-Specific microRNAs in Regenerating Sciatic Nerve. PLoS One. 10, e0137461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phay M., Kim HH, Yoo S., 2018. Analysis of piRNA-Like Small Non-coding RNAs present in axons of adult sensory neurons. Mol Neurobiol. 55, 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M., et al. , 2006. Signaling mechanisms underlying Slit2-induced collapse of Xenopus retinal growth cones. Neuron. 49, 215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preitner N., et al. , 2014. APC is an RNA-binding protein, and its interactome provides a link to neural development and microtubule assembly. Cell. 158, 368–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, et al. , 2006. The RNA binding and transport proteins staufen and fragile X mental retardation protein are expressed by rat primary afferent neurons and localize to peripheral and central axons. Neuroscience. 141, 2107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttagunta R., et al. , 2014. PCAF-dependent epigenetic changes promote axonal regeneration in the central nervous system. Nat Commun. 5, 3527. [DOI] [PubMed] [Google Scholar]
- Qiu J., et al. , 2002. Spinal axon regeneration induced by elevation of cAMP. Neuron. 34, 895–903. [DOI] [PubMed] [Google Scholar]
- Rangaraju V., Lauterbach M., Schuman EM, 2019. Spatially stable mitochondrial compartments fuel local translation during plasticity. Cell. 176, 73–84 e15. [DOI] [PubMed] [Google Scholar]
- Richardson PM, Issa VM, 1984. Peripheral injury enhances central regeneration of primary sensory neurones. Nature. 309, 791–3. [DOI] [PubMed] [Google Scholar]
- Rishal I., Fainzilber M., 2010. Retrograde signaling in axonal regeneration. Exp Neurol. 223, 5–10. [DOI] [PubMed] [Google Scholar]
- Rivieccio MA, et al. , 2009. HDAC6 is a target for protection and regeneration following injury in the nervous system. Proc Natl Acad Sci U S A. 106, 19599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha AG, et al. , 2018. MFN2 agonists reverse mitochondrial defects in preclinical models of Charcot-Marie-Tooth disease type 2A. Science. 360, 336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell AL, Neukomm LJ, 2019. Axon death signalling in Wallerian degeneration among species and in disease. Open Biology. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossoll W., et al. , 2002. Specific interaction of Smn, the spinal muscular atrophy determining gene product, with hnRNP-R and gry-rbp/hnRNP-Q: a role for Smn in RNA processing in motor axons? Hum Mol Genet. 11, 93–105. [DOI] [PubMed] [Google Scholar]
- Rossoll W., et al. , 2003. Smn, the spinal muscular atrophy-determining gene product, modulates axon growth and localization of beta-actin mRNA in growth cones of motoneurons. J Cell Biol. 163, 801–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotem N., et al. , 2017. ALS along the axons - expression of coding and noncoding RNA differs in axons of ALS models. Sci Rep. 7, 44500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruschel J., et al. , 2015. Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science. 348, 347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo PK, et al. , 2018a. Axonal G3BP1 stress granule protein limits axonal mRNA translation and nerve regeneration. Nature Communications. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo PK, et al. , 2018b. Axonal mRNA transport and translation at a glance. J Cell Sci. 131 pii, jcs196808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savas JN, et al. , 2010. A role for huntington disease protein in dendritic RNA granules. J Biol Chem. 285, 13142–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeoka T., et al. , 2016. Dynamic axonal translation in developing and mature visual circuits. Cell. 166, 181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JE, et al. , 2012. Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron. 74, 1015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DS, Skene JH, 1997. A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J Neurosci. 17, 646–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W., et al. , 2015. Tubulin-tyrosine Ligase (TTL)-mediated Increase in Tyrosinated alpha-Tubulin in Injured Axons Is Required for Retrograde Injury Signaling and Axon Regeneration. J Biol Chem. 290, 14765–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane M., et al. , 2011. The actin nucleating Arp2/3 complex contributes to the formation of axonal filopodia and branches through the regulation of actin patch precursors to filopodia. Devel Neurobiol. 71, 747–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane M., et al. , 2012. Nerve growth factor-induced formation of axonal filopodia and collateral branches involves the intra-axonal synthesis of regulators of the actin-nucleating Arp2/3 complex. J Neurosci. 32, 17671–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira ME, et al. , 2003. Critical calpain-dependent ultrastructural alterations underlie the transformation of an axonal segment into a growth cone after axotomy of cultured Aplysia neurons. J Comp Neurol. 457, 293–312. [DOI] [PubMed] [Google Scholar]
- Stirling DP, Stys PK, 2010. Mechanisms of axonal injury: internodal nanocomplexes and calcium deregulation. Trends Mol Med. 16, 160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland ER, et al. , 2011a. MicroRNA dysregulation following spinal cord contusion: implications for neural plasticity and repair. Neurosci. 186, 146–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland IT, et al. , 2011b. Axotomy-induced miR-21 promotes axon growth in adult dorsal root ganglion neurons. PLoS One. 6, e23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura S., et al. , 2000. Leukaemia inhibitory factor is required for normal inflammatory responses to injury in the peripheral and central nervous systems in vivo and is chemotactic for macrophages in vitro. Eur J Neurosci. 12, 457–66. [DOI] [PubMed] [Google Scholar]
- Sun L., et al. , 2017. Nerve injury-induced epigenetic silencing of opioid receptors controlled by DNMT3a in primary afferent neurons. Pain. 158, 1153–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkezian J., et al. , 2010. Transmembrane receptor DCC associates with protein synthesis machinery and regulates translation. Cell. 141, 632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi A., et al. , 2009. A p53-CBP/p300 transcription module is required for GAP-43 expression, axon outgrowth, and regeneration. Cell Death Differ. 16, 543–54. [DOI] [PubMed] [Google Scholar]
- Tedeschi A., et al. , 2016. The Calcium Channel Subunit Alpha2delta2 Suppresses Axon Regeneration in the Adult CNS. Neuron. 92, 419–434. [DOI] [PubMed] [Google Scholar]
- Tedeschi A., et al. , 2019. ADF/Cofilin-Mediated Actin Turnover Promotes Axon Regeneration in the Adult CNS. Neuron. 103, 1073–1085 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenzio M., et al. , 2018. Locally translated mTOR controls axonal local translation in nerve injury. Science. 359, 1416–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W., Czopka T., Lopez-Schier H., 2020. Systemic loss of Sarm1 protects Schwann cells from chemotoxicity by delaying axon degeneration. Commun Biol. 3, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiruchinapalli DM, et al. , 2003. Activity-dependent trafficking and dynamic localization of zipcode binding protein 1 and beta-actin mRNA in dendrites and spines of hippocampal neurons. J Neurosci. 23, 3251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom VJ, et al. , 2004. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J Neurosci. 24, 6531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth CC, et al. , 2009. Locally synthesized calcitonin gene-related Peptide has a critical role in peripheral nerve regeneration. J Neuropathol Exp Neurol. 68, 326–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda H., et al. , 2003. Induction of NMDA and GABAA receptor-mediated Ca2+ oscillations with KCC2 mRNA downregulation in injured facial motoneurons. J Neurophysiol. 89, 1353–62. [DOI] [PubMed] [Google Scholar]
- Tsarouchas TM, et al. , 2018. Dynamic control of proinflammatory cytokines Il-1beta and Tnf-alpha by macrophages in zebrafish spinal cord regeneration. Nat Commun. 9, 4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Helleputte L., et al. , 2018. Inhibition of histone deacetylase 6 (HDAC6) protects against vincristine-induced peripheral neuropathies and inhibits tumor growth. Neurobiol Dis. 111, 59–69. [DOI] [PubMed] [Google Scholar]
- Vidal PM, et al. , 2013. The role of “anti-inflammatory” cytokines in axon regeneration. Cytokine Growth Factor Rev. 24, 1–12. [DOI] [PubMed] [Google Scholar]
- Villarin JM, et al. , 2016. Local synthesis of dynein cofactors matches retrograde transport to acutely changing demands. Nat Commun. 7, 13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas R., et al. , 2014. Calcium release from intra-axonal endoplasmic reticulum leads to axon degeneration through mitochondrial dysfunction. J Neurosci. 34, 7179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuppalanchi D., et al. , 2012. Lysophosphatidic acid differentially regulates axonal mRNA translation through 5’UTR elements. Mol Cell Neurosci. 50, 136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahane S., et al. , 2019. Epigenetic Regulation Of Axon Regeneration and Glial Activation in Injury Responses. Front Genet. 10, 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BA, Ji SJ, Jaffrey SR, 2012. Intra-axonal translation of RhoA promotes axon growth inhibition by CSPG. J Neurosci. 32, 14442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CA, et al. , 2018. Abeta1–42 triggers the generation of a retrograde signaling complex from sentinel mRNAs in axons. EMBO Rep. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng YL, et al. , 2017. An Intrinsic Epigenetic Barrier for Functional Axon Regeneration. Neuron. 94, 337–346 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JA 2nd, et al. , 2015. Huntingtin differentially regulates the axonal transport of a sub-set of Rab-containing vesicles in vivo. Hum Mol Genet. 24, 7182–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D., et al. , 2005. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J Neurosci. 25, 778–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis DE, et al. , 2007. Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. J Cell Biol. 178, 965–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters JJ, et al. , 2011. Congenital CNS hypomyelination in the Fig4 null mouse is rescued by neuronal expression of the PI(3,5)P(2) phosphatase Fig4. J Neurosci. 31, 17736–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., et al. , 2012. Dicer-microRNA pathway is critical for peripheral nerve regeneration and functional recovery in vivo and regenerative axonogenesis in vitro. Exp Neurol. 233, 555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Murashov AK, 2013. MicroRNA-431 regulates axon regeneration in mature sensory neurons by targeting the Wnt antagonist Kremen1. Front Mol Neurosci. 6, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KY, et al. , 2005. Local translation of RhoA regulates growth cone collapse. Nature. 436, 1020–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie XY, Barrett JN, 1991. Membrane resealing in cultured rat septal neurons after neurite transection: evidence for enhancement by Ca(2+)-triggered protease activity and cytoskeletal disassembly. J Neurosci. 11, 3257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawo H., Kuno M., 1985. Calcium dependence of membrane sealing at the cut end of the cockroach giant axon. J Neurosci. 5, 1626–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z., Misra V., Verge VM, 2014. Sensing nerve injury at the axonal ER: activated Luman/CREB3 serves as a novel axonally synthesized retrograde regeneration signal. Proc Natl Acad Sci U S A. 111, 16142–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z., et al. , 2015. The unfolded protein response and cholesterol biosynthesis link Luman/CREB3 to regenerative axon growth in sensory neurons. J Neurosci. 35, 14557–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S., et al. , 2013. A HuD-ZBP1 ribonucleoprotein complex localizes GAP-43 mRNA into axons through its 3’ untranslated region AU-rich regulatory element. J Neurochem. 126, 792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BC, et al. , 2012. Local translation of extranuclear lamin B promotes axon maintenance. Cell. 148, 752–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B., et al. , 2011. Profile of microRNAs following rat sciatic nerve injury by deep sequencing: implication for mechanisms of nerve regeneration. PLoS One. 6, e24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin D., et al. , 2008. Localized regulation of axonal RanGTPase controls retrograde injury signaling in peripheral nerve. Neuron. 59, 241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HL, Singer RH, Bassell GJ, 1999. Neurotrophin regulation of beta-actin mRNA and protein localization within growth cones. J Cell Biol. 147, 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, et al. , 2011. MicroRNAs 144, 145, and 214 are down-regulated in primary neurons responding to sciatic nerve transection. Brain Res. 1383, 62–70. [DOI] [PubMed] [Google Scholar]
- Zhao JY, et al. , 2017. DNA methyltransferase DNMT3a contributes to neuropathic pain by repressing Kcna2 in primary afferent neurons. Nat Commun. 8, 14712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., et al. , 2011. Early changes of microRNAs expression in the dorsal root ganglia following rat sciatic nerve transection. Neurosci Lett. 494, 89–93. [DOI] [PubMed] [Google Scholar]
- Zhou S., et al. , 2012. microRNA-222 targeting PTEN promotes neurite outgrowth from adult dorsal root ganglion neurons following sciatic nerve transection. PLoS One. 7, e44768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv N., Spira M., 1997. Localized and transient elevations of intracellular Ca2+ induce dedifferentiation of axonal segments into growth cones. J Neurosci. 17, 3568–3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivraj KH, et al. , 2010. Subcellular Profiling Reveals Distinct and Developmentally Regulated Repertoire of Growth Cone mRNAs. J Neurosci. 30, 15464–15478. [DOI] [PMC free article] [PubMed] [Google Scholar]