Adaptable P body physical states differentially regulate bicoid mRNA storage during early Drosophila development (original) (raw)
Summary
Ribonucleoprotein condensates can exhibit diverse physical states in vitro and in vivo. Despite considerable progress, the relevance of condensate physical states for in vivo biological function remains limited. Here, we investigated the physical properties of processing bodies (P bodies) and their impact on mRNA storage in mature Drosophila oocytes. We show that the conserved DEAD-box RNA helicase Me31B forms viscous P body condensates, which adopt an arrested physical state. We demonstrate that structurally distinct proteins and protein-protein interactions, together with RNA, regulate the physical properties of P bodies. Using live imaging and in situ hybridization, we show that the arrested state and integrity of P bodies support the storage of bicoid (bcd) mRNA and that egg activation modulates P body properties, leading to the release of bcd for translation in the early embryo. Together, this work provides an example of how physical states of condensates regulate cellular function in development.
Keywords: biomolecular condensates, phase separation, processing bodies, mRNA regulation, axis patterning, Drosophila, intrinsically disordered regions, in vivo imaging, ribonucleoprotein
Graphical abstract
Highlights
- •
P bodies adopt an arrested physical state in the mature oocyte - •
Multivalent interactions and structurally distinct proteins regulate P body properties - •
The arrested state and integrity of P bodies support the storage of bicoid mRNA - •
Egg activation modulates P body properties and leads to release of bicoid mRNA
Sankaranarayanan et al. show that P bodies, conserved ribonucleoprotein condensates found in Drosophila oocytes, are regulated by specific structural features and weak multivalent interactions. In vivo, P bodies adopt an arrested physical state that is critical for the storage of the bicoid patterning mRNA until egg activation.
Introduction
Many biochemical reactions in the cytoplasm of eukaryotic cells require regulation in space and time. The organization of specific reactions in the dense cytoplasmic environment is achieved through membrane-bound and membrane-less organelles. Classic membrane-bound organelles, such as the nucleus and endoplasmic reticulum, are stable micro-environments that are enclosed by membranes. However, membrane-less organelles, such as stress granules, P bodies, and nuclear bodies, which are typically composed of nucleic acids and proteins, have been shown to provide an additional level of cellular organization (Buchan and Parker, 2009; Banani et al., 2017; Shin and Brangwynne, 2017; Boeynaems et al., 2018). More generally, the designation biomolecular condensates is used to describe cellular assemblies characterized by the non-stoichiometric concentration of biomacromolecules, of which membrane-less organelles are one such example (Brangwynne et al., 2009; Li et al., 2012; Hubstenberger et al., 2013; Wang et al., 2014; Nott et al., 2015; Zhang et al., 2015; Feric et al., 2016; Hyman et al., 2014; Brangwynne et al., 2015; Banani et al., 2017; Lyon et al., 2021).
Ribonucleoprotein (RNP) complexes are an abundant and conserved class of biomolecular condensates. Found both in the cytoplasm and the nucleus, RNP condensates exhibit a wide range of physical states, ranging from dynamic liquids to stable solids (Kroschwald et al., 2015, 2018; Weber, 2017; Weber and Brangwynne, 2012; Woodruff et al., 2017). Some examples of diverse physical states observed in vivo include liquid-like P granules in Caenorhabditis elegans (C. elegans) embryos (Brangwynne et al., 2009; Wang et al., 2014), viscous nucleoli in Xenopus laevis oocytes (Brangwynne et al., 2011; Feric et al., 2016; Mitrea et al., 2016), and solid-like Balbiani bodies vertebrate oocytes (Boke et al., 2016). Despite considerable progress, the relationship between the physical states of biomolecular condensates and their in vivo function remains poorly understood.
RNP condensates are often linked with localized RNA translational regulation, which enables cells to spatiotemporally regulate protein synthesis (Medioni et al., 2012). Specialized cells, including neurons and oocytes, frequently depend on this mode of post-transcriptional regulation to control gene expression (Jung et al., 2014; Kloc and Etkin, 2005). More specifically, transcriptionally inactive oocytes, such as those in Drosophila melanogaster, rely on prolonged storage and translational control of maternally deposited transcripts for body axes patterning during development (Lasko, 2012; Tadros and Lipshitz, 2009).
One mechanism for regulating RNA metabolism involves P bodies, an evolutionarily conserved class of cytoplasmic biomolecular condensates (Sheth and Parker, 2003; Andrei et al., 2005; Kedersha et al., 2005; Eulalio et al., 2007; Parker and Sheth, 2007; Buchan, 2014; Hubstenberger et al., 2017; Luo et al., 2018). Previous studies on P bodies have highlighted their roles in RNA storage and translational repression, since P bodies are devoid of ribosomes (Hubstenberger et al., 2017; Weil et al., 2012). A conserved component of P bodies is the ATP-dependent DEAD-box RNA helicase: DDX6 in humans, Dhh1 in yeast, CGH-1 in C. elegans, and Maternal expression at 31B (Me31B) in Drosophila. Me31B is required for early Drosophila development and is estimated to be present at concentrations of ∼7.5 μM in the egg (Götze et al., 2017). During oogenesis, Me31B is known to associate with and differentially regulate several axis-patterning maternal mRNAs (Nakamura et al., 2001; Weil et al., 2012; Kaneuchi et al., 2015; Tadros and Lipshitz, 2009; York-Andersen et al., 2015). Failure to regulate these and many other mRNAs can lead to severe developmental defects (Lasko, 2012). However, the mechanisms that underlie how transcripts are maintained and translationally controlled by P bodies are not well understood.
To examine the in vivo basis of mRNA regulation, we employ a multidisciplinary approach to investigate the physical properties and in vivo functions of P bodies in mature Drosophila oocytes. Real-time live imaging reveals that P body condensates adopt a highly viscous and arrested physical state in mature oocytes, and their integrity depends on electrostatic and hydrophobic interactions, along with RNA and the actin cytoskeleton. Using in silico, in vitro, and in vivo assays, we demonstrate that intrinsically disordered regions (IDRs) in Me31B and the disordered P body protein Trailer hitch (Tral) independently regulate the assembly and physical properties of Me31B condensates. Using live imaging and single-molecule fluorescent in situ hybridization (smFISH), we demonstrate that the arrested state and integrity of P bodies is critical for the storage of bcd mRNA, which is later released for translation at egg activation. Finally, we show that P bodies in the early embryo are smaller and highly dynamic than in the oocyte, and do not co-localize with translationally active bcd mRNAs. Together, our results highlight an in vivo role for adaptable P body physical states in mRNA regulation during development.
Results
Me31B forms viscous P body condensates, which adopt an arrested physical state in mature oocytes
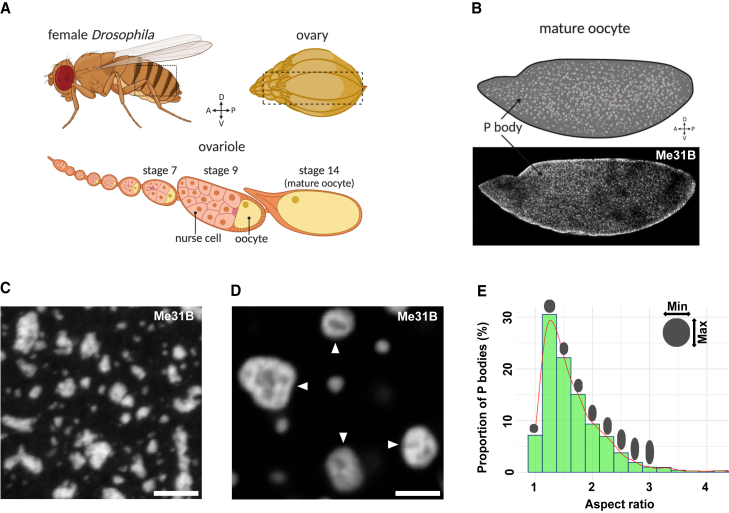
Several maternal mRNAs are thought to be stored and regulated by P bodies throughout Drosophila oogenesis (Lin et al., 2008; Nakamura et al., 2001; Weil et al., 2012). However, a mechanistic understanding of how P bodies accomplish this function remains unclear. To examine the physical state of P bodies, we isolated living-stage 14-egg chambers (hereafter referred to as mature oocytes) from female Drosophila (Figure 1A). Live imaging of Me31B::GFP revealed that P bodies are typically micron-sized condensates with varying morphologies (Figures 1B and 1C). We also found that most P bodies have internal subdomains suggestive of a heterogeneous organization (Figure 1D). Quantification of P body aspect ratios over time showed that P bodies have predominantly irregular morphologies (Figure 1E) compared with liquid-like condensates, such as P granules and stress granules (Brangwynne et al., 2009; Patel et al., 2015). Time-lapse imaging also demonstrated that P bodies undergo continuous rearrangements, mostly progressing from amorphous to spherical morphologies, as exemplified by their aspect ratio analysis over the observed timescales (Figure 2A). Additionally, P bodies undergo fusion and fission events, which are hallmarks of a dynamic state (Figures 2B, 2B′, and S1). However, the longer timescale of these events suggests that P bodies in mature Drosophila oocytes are less dynamic compared with liquid-like condensates.
Figure 1.
Me31B forms heterogeneous P body condensates in the mature oocyte
(A) Schematic of a Drosophila female ovary and ovariole. Each female contains two ovaries, each comprising 16–18 ovarioles. Each ovariole can be thought of as an assembly line for the production of mature oocytes. The oocyte is supported by a collection of nurse cells until the late stages of oogenesis. Created with BioRender.com.
(B–E) Mature oocyte (∼ 0.5 mm in length) expressing Me31B::GFP.
(B) Cartoon depicting P body distribution in the mature oocyte and confocal image of a whole mature oocyte showing P bodies throughout the cytoplasm. The concentration of P bodies at the cortex is, in part, due to this being a cross section image.
(C) Increased magnification of P bodies in the mid-lateral area of the oocyte reveals they exhibit diverse morphologies and sizes. Maximum projection 10 μm.
(D) Representative image of P bodies exhibiting multiple subdomains (white arrowheads) indicative of heterogeneous internal organization.
(E) Aspect ratio analysis of individual P bodies (>1 μm) showing an uneven range of P body morphology (n = 20). Scale bar, 5 μm (C), 2 μm (D).
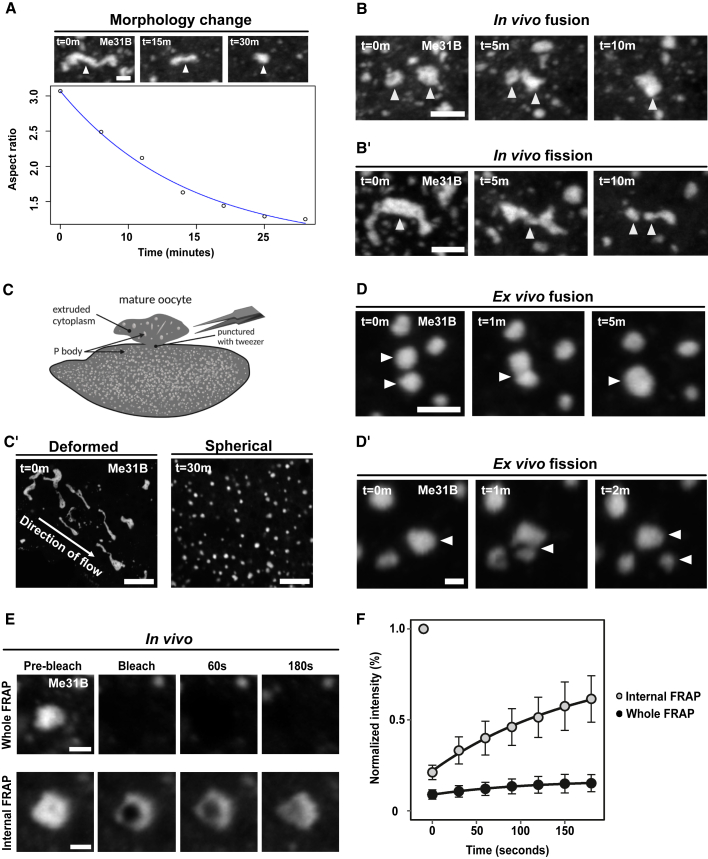
Figure 2.
P bodies adopt a less dynamic and arrested physical state
(A–E) Mature oocyte expressing Me31B::GFP.
(A) Time series of a P body displaying elastic behavior, starting in an extended state (t = 0 min) and subsequently relaxing toward a spherical morphology (t = 30 min). Plot of individual P body (n = 10) A.R over time showing relaxation from extended (A.R∼3) to spherical morphology (A.R∼1).
(B) Time series of two in vivo P bodies undergoing coalescence (white arrowheads) (n = 20).
(B′) Time series of a single in vivo P body undergoing fission to form two distinct condensates (white arrowheads) (n = 20).
(C) Cartoon depicting cytoplasmic extrusion of P bodies into halocarbon oil (ex vivo) induced by puncturing the outer membrane of the mature oocyte. Created with BioRender.com.
(C′) Ex vivo P bodies displaying stretched elastic morphologies shortly after extrusion (t = 0 min). Over time, extruded P bodies relax into homogeneous spherical condensates (t = 30 min, n = 25).
(D) Time series of ex vivo P bodies undergoing coalescence (white arrowheads) (n = 20).
(D′) Time series of ex vivo extruded P bodies undergoing fission (white arrowheads) (n = 5).
(E) Time series of whole FRAP of P body shows minimal recovery, whereas internal FRAP of P body shows increased recovery of Me31B fluorescence.
(F) P body recovery profiles after whole FRAP (n = 20) and internal FRAP (n = 24) (mean, standard deviation).
Scale bar, 2.5 μm (A–D′), 10 μm (C′), 1.5 μm (E).
See also Figures S1 and S2.
The cytoplasm of mature oocytes is packed with yolk granules and complex cytoskeletal structures. To determine if the slow P body dynamics are an intrinsic property or dependent on the oocyte cytoplasmic environment, we developed an ex vivo assay, whereby we extruded the cytoplasm into halocarbon oil (Figure 2C). Importantly, this approach does not promote P body dissolution, rather, extruded P bodies initially exhibit irregular morphologies but become spherical over the observed timescale (Figure 2C′). We also show that the extruded P bodies undergo fusion and fission events (Figures 2D and 2D′), but these are faster than in vivo, likely due to the absence of cytoplasmic crowding and cytoskeletal structures. Taken together, our data suggest that P bodies are slowly rearranging condensates, and that this physical property is inherent to P bodies in mature Drosophila oocytes.
Next, we performed fluorescence recovery after photobleaching (FRAP) on whole P bodies (whole FRAP) to examine the mobility of Me31B between the cytoplasm and the P body. This analysis revealed that Me31B localized to P bodies exhibited limited or no recovery (Figures 2E and 2F). To assess if this is a general property of P bodies, we performed whole FRAP of P bodies in earlier stages of oogenesis. The recovery patterns and the proportion of immobile Me31B were similar to those observed in mature oocytes (Figures S2A and S2B). Due to the limited exchange of Me31B between P bodies and the cytoplasm, we refer to this as the arrested state of P bodies.
To further explore Me31B dynamics, we tested if Me31B can rearrange within P bodies by assessing the mobility of Me31B after photobleaching within a region inside the P body (internal FRAP) (Figures 2E and 2F). Measurements revealed considerable recovery of fluorescence compared with whole FRAP (Figure S2C). Despite a high mobile fraction, the rate of recovery indicates that the dynamics of Me31B within P bodies is slow (Figure S2D). We further derived an apparent viscosity in the range of ∼700 Pa.s for P bodies from their internal recovery kinetics. Although this estimate should be treated qualitatively, the value is at least two orders of magnitude larger than those reported for liquid-like condensates (Alshareedah et al., 2021; Brangwynne et al., 2009). Overall, these data show that P bodies in mature oocytes adopt a viscous and arrested physical state.
Multivalent interactions, RNA, and the actin cytoskeleton regulate P body physical properties
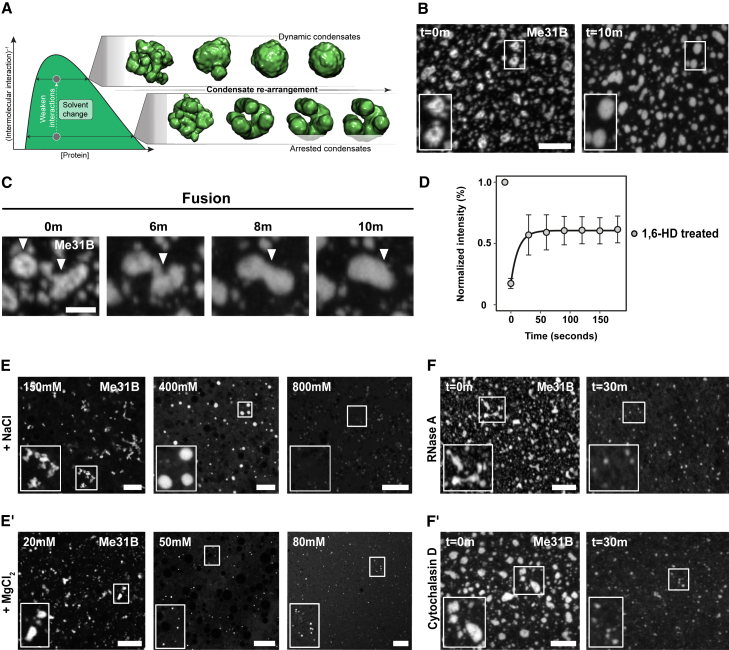
Previous work has shown that activation of the mature oocyte results in an influx of monovalent and divalent ions, release of stored mRNAs, and reorganization of the actin cytoskeleton (York-Andersen et al., 2015, 2020; Kaneuchi et al., 2015). Therefore, we wondered if these factors could regulate the physical properties of P bodies in the mature oocyte prior to egg activation. Various interactions have been shown to contribute to RNP condensation, including hydrophobic and electrostatic interactions (Brangwynne et al., 2015; Dzuricky et al., 2020; Kato et al., 2012; Murthy et al., 2019; Nott et al., 2015; Pak et al., 2016; Riback et al., 2017). The interactions that are thought to drive P body assembly can be interpreted through the lens of a pseudo two-component phase diagram (Figure 3A). In particular, by changing the solution conditions to weaken the interactions that contribute to P body assembly, theory and simulations predict an increase in internal mobility and more spherical shaped condensates, as shown previously for protein-RNA condensates (Boeynaems et al., 2019).
Figure 3.
P body physical properties are regulated by hydrophobic and electrostatic interactions along with RNA and actin
(A) Schematized phase diagram in which protein concentration extends along the x axis, whereas molecular interaction strength extends across the y axis. Inset shows snapshots from coarse-grained simulations performed at distinct positions along the y axis.
(B, C, and E–F′) Mature oocyte expressing Me31B::GFP.
(B) The addition of 5% 1,6-HD causes P bodies to transform from amorphous to spherical morphology within 10 min and results in the loss of internal heterogeneity. Maximum projection 5 μm.
(C) Time series shows two P bodies undergoing coalescence following the addition of 1,6-HD (n = 30). Maximum projection 5 μm.
(D) Whole FRAP recovery profile of 1,6-HD-treated P bodies showing rapid fluorescence recovery (n = 12) (mean, standard deviation).
(E) The addition of varying concentrations of NaCl results in diverse physical states of extruded P bodies ranging from sticky (150 mM) to liquid-like (400 mM) and diffuse state (800 mM). Single plane image.
(E′) Treatment with MgCl2 results in the dissociation of extruded P bodies at concentrations significantly lower than NaCl. Single plane image.
(F) Treatment with 500 ng/μl RNase A) or (F′) 10 μg/μl cytochalasin- D (depolymerizes actin) causes P body dissociation, resulting in smaller condensates. Maximum projection 10 μm.
n = 5 experimental repeats for (E–F').
Scale bar, 5 μm (B, C, and E–F′).
See also Figure S3.
To test if hydrophobic interactions are required for in vivo P body integrity, we treated mature oocytes with the aliphatic alcohol 1,6-hexanediol (1,6-HD), a compound identified originally in the context of attenuating hydrophobic interactions (Ribbeck and Görlich, 2002; Patel et al., 2007). The addition of 1,6-HD resulted in the transformation of P bodies toward a more spherical shape, an increase in fusion events, and, ultimately, the dissolution of condensates over time (Figures 3B, 3C, S3A, and S3B). These results support a model in which 1,6-HD weakens the multivalent interactions that contribute to P body physical state and integrity. To further test if 1,6-HD leads to a transition from an arrested to a more dynamic state, we performed whole FRAP on 1,6-HD-treated P bodies. Consistent with our model, P bodies exhibited rapid and sustained recovery (Figures 3D, S3C, and S3D). Although 1,6-HD-treated P bodies only exhibited appreciable recovery up to ∼60%, this is likely due to their dissolution occurring simultaneously. Although 1,6-HD has been shown to affect mechanical properties in cultured cells, we did not observe any noticeable phenotypes in the mature oocytes over the observed timescales (Wheeler et al., 2016). Taken together, our results suggest that hydrophobic interactions contribute to regulating the arrested state of P bodies, which, in turn, maintains their integrity.
Next, we examined if electrostatic interactions contribute to P body physical properties by testing the impact of monovalent (NaCl) or divalent salts (MgCl2). At low concentrations of NaCl, P bodies assemble into clusters, whereas at high concentrations they dissociate (Figure 3E). However, at intermediate concentration ranges (300–600 mM), P bodies adopt spherical morphologies, consistent with a more dynamic state. These results are supportive of a model in which electrostatic interactions, like hydrophobic interactions, play a role in dictating physical properties and can be tuned up or down by decreasing or increasing the monovalent salt concentrations, respectively.
Interestingly, the addition of 20 mM MgCl2 had no apparent effect on P body integrity; yet, a small increase in concentrations as low as 50 mM MgCl2 resulted in their complete dissociation (Figure 3E′). This relative sensitivity to divalent cations implies an effect beyond simply ionic strength. Collectively, these data suggest that changes in salt concentration can alter P body integrity, consistent with the morphology and state of P bodies that we observe following ex vivo egg activation or in the early embryo (York-Andersen et al., 2015).
Given the importance of electrostatic interactions, we asked if P body integrity was regulated exclusively by protein-protein interactions, or if protein-RNA interactions also contributed. Previous biochemical studies have shown that P body proteins differentially interact with Me31B in an RNA-dependent or -independent manner (Nakamura et al., 2004). To test the effect of RNA on P body integrity, we treated mature oocytes with RNase A, which leads to P body dissociation into smaller-sized condensates (Figure 3F). The incomplete dissolution implies that in vivo P body integrity is largely dependent on protein-protein interactions, but this does not exclude a contribution from protein-RNA interactions.
Finally, to examine the role of actin in regulating P body integrity, mature oocytes were treated with cytochalasin D, a commonly used actin depolymerizing agent. This treatment resulted in the dissociation of P bodies in 30 min, consistent with our data from ex vivo egg activation (Figure 3F′). Since the actin cytoskeleton is commonly involved in RNP anchoring, we tested if the dissociated P body particles exhibited altered spatial dynamics (Medioni et al., 2012; Weil et al., 2008). Particle displacement analysis showed that cytochalasin-D-treated P bodies were significantly more mobile than untreated ones (Figure S3E). Taken together, these results indicate that multiple factors regulate P body integrity, properties, and dynamics in the mature oocyte.
IDRs regulate the physical state of Me31B condensates in vitro
Having identified multiple external factors in the regulation of P body integrity, we next asked if sequence features within the Me31B protein may be regulating P body physical state. Me31B contains an ATP-binding and folded helicase domain, flanked by short N- and C-terminal IDRs (Figure 4A). Although the function of the helicase domain is well studied, much less is known about the function of the disordered regions. Since Me31B is an essential in vivo protein, we adopted an in vitro approach to examine the role of these disordered regions.
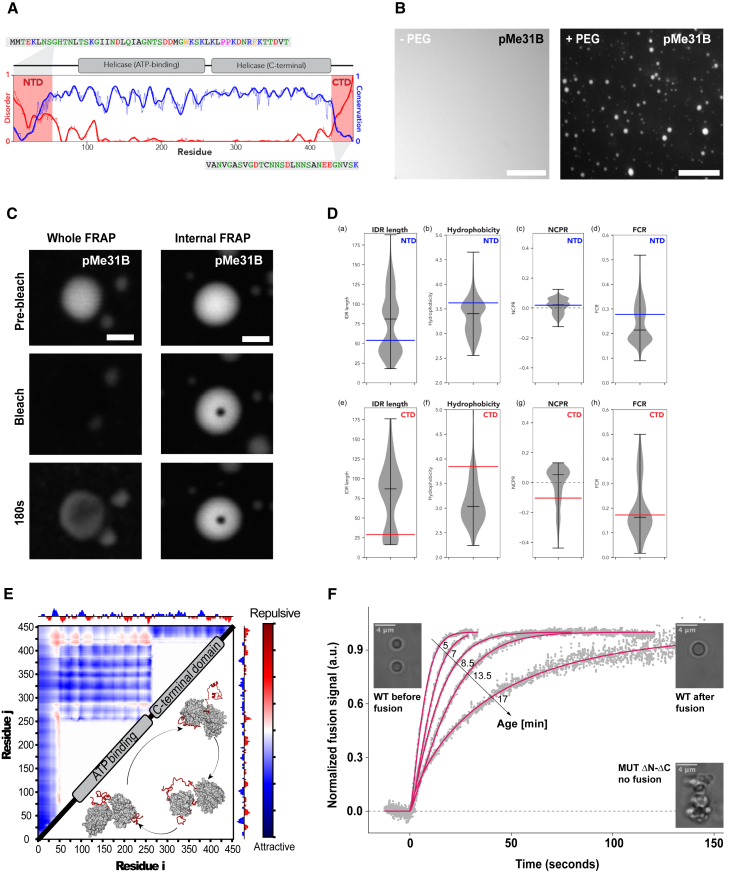
Figure 4.
Deletion of IDRs results in aggregate-like Me31B condensates in vitro
(A) Overview of disordered, conservation, and domain architecture for Me31B. Conservation calculated across 566 orthologous sequences. N-terminal domain (NTD) and C-terminal domain (CTD), sequences are highlighted, with an atomistic model of the full-length protein shown in panel E.
(B) Purified GFP-Me31B (pMe31B) at 7.5 μM is diffuse on its own but forms phase separated spherical condensates in the presence of 1% PEG (n = 10). Maximum projection 5 μm.
(C) Time series of pMe31B condensates subjected to FRAP experiments. Whole P body photobleaching shows moderate fluorescence recovery, whereas internal FRAP shows no recovery (n = 10).
(D) Violin plots quantify density of IDR length (A/E), hydrophobicity (B/F), net charge per residue, (C/G) and fraction of charged residues (D/H) for the N-terminal IDRs (A–D) or C-terminal IDRs (E-H). Blue or red bars define the associated value for the Me31B IDR in the N- or C-terminal IDR, respectively.
(E) Summary of all-atom simulations. Normalized inter-residue distance is shown with cooler colors reflecting attractive interactions and warmer colors reflecting repulsive interactions.
(F) Fusion of pMe31B condensates (magenta) at different time points post condensation, quantified by dual-TRAP optical tweezers. pMe31B ΔN-ΔC condensates (dashed line) do not fuse and rapidly aggregate with each other (n = 20).
Scale bar, 5 μm (B), 1 μm (C).
See also Figure S4.
We first tested if the purified recombinant Me31B (GFP-Me31B) can undergo condensation in vitro. Although Me31B is diffuse at physiological protein concentrations (7.5 μM), upon addition of a crowding agent, which mimics the oocyte cytoplasmic environment (1% PEG), Me31B formed spherical condensates (Figure 4B). We repeated this experiment using an alternative crowder (1% Ficoll) and confirmed that Me31B condensation does not depend on the specific chemical properties of the crowding agent (Figure S4A). Time-lapse imaging revealed that Me31B forms micron-sized spherical condensates, suggestive of a liquid-like state (Figure S4B). To examine Me31B mobility, we performed both whole FRAP and internal FRAP on freshly formed condensates. To our surprise, these condensates showed little or no recovery after photobleaching, indicating that Me31B condensates are present in an arrested physical state similar to in vivo P bodies (Figure 4C).
Next, we wondered what role IDRs might have in Me31B condensation. Previous work has shown that IDRs in DEAD-box helicases can contribute to RNP condensate formation and physical state, a property determined by sequence composition and length (Elbaum-Garfinkle et al., 2015; Hondele et al., 2019; Nott et al., 2015). We assessed conservation across a set of DDX6 orthologs (including Me31B), revealing that folded domains are highly conserved, whereas IDR length and sequence varied substantially (Figure 4D). Taken broadly, our results imply that Me31B and its orthologs may show differences in condensate formation tuned by their IDRs.
To better understand how the IDRs might contribute to function, we performed all-atom simulations of full-length Me31B, which revealed that both IDRs adopt a heterogeneous ensemble of states (Figure 4E). Interestingly, both N- and C-terminal IDRs interacted transiently and relatively non-specifically with the surface of the folded domains. These contacts were mediated through electrostatic and hydrophobic interactions (Figures S4C and S4D). Rather than acting as drivers of self-assembly, our simulations suggest the possibility that IDRs play a modulatory role.
To test for the modulatory influence of IDRs, we purified recombinant Me31B with the complete N- and C-terminal IDRs deleted (Me31BΔN-ΔC). We then used dual-trap optical tweezers to quantitatively measure the rate of condensate fusion events, thus providing a readout of their physical properties (Jahnel et al., 2011). We show that full-length Me31B condensates initially exhibit rapid fusion events; however, these decrease over time (Figure S4E). In contrast, Me31BΔN-ΔC condensates rapidly self-assembled into aggregate-like structures (Figures 4F and S4F). These results demonstrate that the IDRs tune the physical properties of Me31B condensates by attenuating the strong interactions established among the interacting folded domains.
Tral is key to regulating organization of P bodies in the mature oocyte
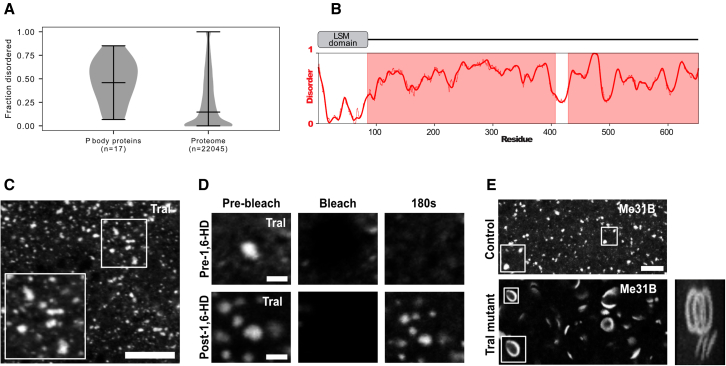
In addition to Me31B, several other proteins localize to or are found to be enriched within P bodies (Lin et al., 2008). Given the importance of disordered regions within Me31B, we hypothesized that the many IDRs found in P body proteins could potentially act as lubricants to regulate P body assembly and organization through interactions with structured proteins. To test this, we first performed disorder prediction across the set of known P body proteins to estimate the proportion of structured versus disordered regions (Figure 5A). Approximately 50% of all residues found within P body proteins are predicted to be disordered, highlighting the structural heterogeneity of components within P bodies. Among the proteins enriched with intrinsic disorder is Tral, a member of the LSM protein family (RAP55 in vertebrates, CAR-1 in C. elegans), which is known to interact directly with Me31B, function in Drosophila axis patterning, and is predicted to be largely disordered with the exception of an N-terminal LSM domain (Figure 5B) (Bouveret et al., 2000; Götze et al., 2017; Hara et al., 2018; McCambridge et al., 2020; Monzo et al., 2006; Tritschler et al., 2007, 2008, 2009; Wang et al., 2017).
Figure 5.
Absence of Tral alters P body morphology in the mature oocyte
(A) Comparison of fraction disorder in known Drosophila P body proteins (left) compared with whole Drosophila proteome (right). The average fraction disorder of the 17 proteins (Table S1) associated with the P body is over 99.9% more disordered than any possible random sized-matched set of proteins taken from the D. melanogaster proteome.
(B) Schematic of Tral domain architecture containing a structured LSM domain followed by a long stretches of highly disordered regions.
(C and D) Mature oocyte expressing GFP::Tral.
(C) Tral localizes to P body condensates with diverse morphologies and sizes, distributed throughout the oocyte cytoplasm (n = 20). Maximum projection 7 μm.
(D) Time series of FRAP experiments on GFP::Tral condensates before and after treatment with 1,6-HD (n = 13).
(E) Mature oocytes expressing Me31B::GFP (wild-type control) displaying close to spherical P body condensates. In the absence of Tral (Tral mutant), Me31B forms aberrant rod and donut-shaped P body condensates. Panel on right is a 3-D projection merge of a single donut (∼2.5 μm) and rod (∼1 μm) showing that they are distinct shapes (n = 20 mature oocytes). Maximum projection 5 μm.
Scale bar, 5 μm (C), 1.5 μm (D), 3 μm (E).
Therefore, we tested the role of Tral in P body regulation in vivo. Mature oocytes expressing GFP::Tral showed that Tral associates with P body condensates, albeit smaller in size than Me31B condensates (Figure 5C). Next, we asked whether TraI shows similar properties to that of Me31B, which would suggest that these two proteins are associated with the same physical state. Indeed, despite being structurally distinct from Me31B, whole FRAP and 1,6-HD experiments on Tral were consistent with our results for Me31B (Figure 5D). This supports a model in which Me31B and TraI are strongly coupled within P bodies, likely through direct interaction.
Since Me31B is essential for Drosophila oogenesis, we tested if Tral is required to regulate Me31B-labeled P bodies in the mature oocyte. Remarkably, in Tral mutants, P bodies have dramatically different morphologies and form rod and planar donut-shaped assemblies (Figure 5E), implying a gain of anisotropy in the underlying molecular arrangement of the condensate. The formation of apparently ordered (or partially ordered) assemblies is reminiscent of liquid-crystalline formation, as observed in the synaptonemal complex or in specific mutants of the plant protein FLOE1 (Dorone et al., 2021; Rog et al., 2017). These results suggest that, despite being structurally distinct, Tral and Me31B contribute to the organization of P bodies through synergistic interactions.
The arrested state of P bodies regulates bcd mRNA storage
Our data show that P bodies in the mature Drosophila oocyte are present in a viscous and arrested physical state. Since P bodies in the mature oocyte contain maternal mRNAs that are stored and translationally regulated over long periods, we hypothesized that the arrested physical state of P bodies could facilitate this function.
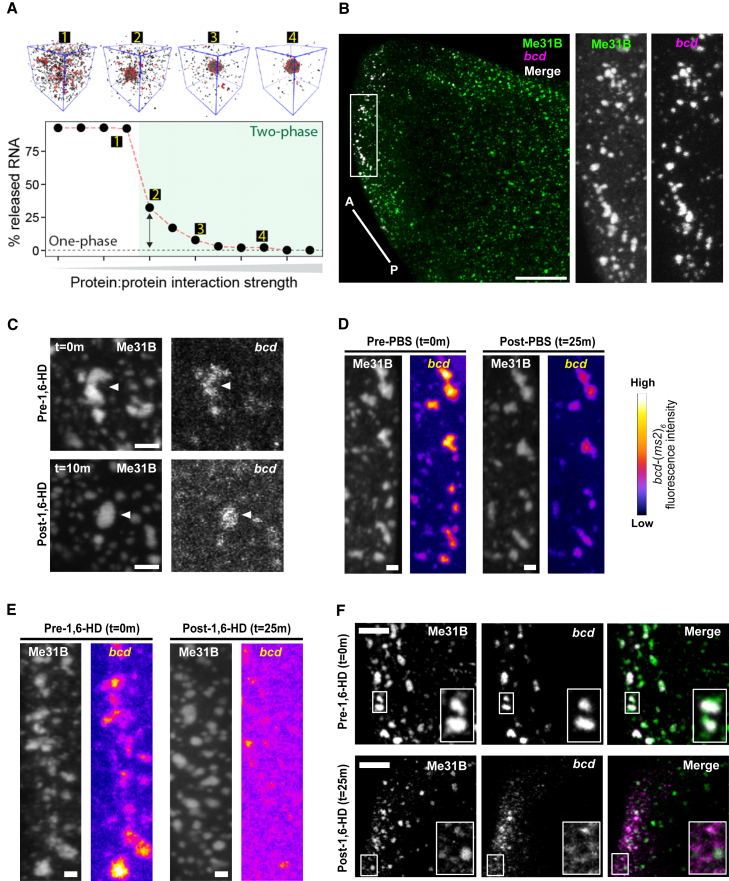
To test the hypothesis, we first developed a simple coarse-grained model in which protein and RNA will co-assemble to form condensates in silico (Figures 6A and S4G). In our model, protein and RNA molecules possess attractive protein-protein and protein-RNA interactions that form multicomponent condensates. Condensate stability depends on both the strength of protein-protein and protein-RNA interactions, such that over the concentration range examined, both species are necessary for condensation. In simulations where the protein-protein interaction strength is systematically weakened, we observe a loss of condensate integrity and a concomitant release of RNA into the dilute phase. These simulations predict that condensate integrity can be viewed as a proxy for RNA storage.
Figure 6.
Altering P body physical state leads to premature loss of bcd mRNA
(A) Coarse-grained simulations performed with 50 RNA molecules 800 protein molecules in which condensate assembly is driven by both protein-protein and protein-RNA interactions. See also Figure S4G.
(B) Mature oocyte expressing Me31B::GFP, labeled with GFP-Booster and smFISH for bcd mRNA. P bodies and bcd mRNA co-localize at the anterior region. Inset shows a zoomed in version of bcd mRNA and P bodies (n = 10 mature oocytes). Maximum projection 5 μm.
(C–E) Mature oocytes expressing Me31B::GFP, hsp83-MCP-RFP, and _bcd_-(ms2)6.
(C) The addition of 1,6-HD causes P bodies and bcd mRNA to initially adopt a spherical shape (t = 10 min), suggestive of a more dynamic physical state (n = 30). Maximum projection, 5 μm.
(D) The addition of PBS does not affect the co-localization of bcd mRNA with P bodies. Although fluorescence of bcd at t = 25 min decreases, this is likely due to photobleaching, the cytoplasmic distribution of bcd mRNA remains similar to t = 0 min (blue) (n = 35). Maximum projection 5 μm.
(E) Extended exposure to 1,6-HD results in the dispersion of bcd mRNAs, whereas P bodies remain condensed (t = 25 min) (n = 55). Maximum projection 5 μm.
(F) Mature oocyte expressing Me31B::GFP, labeled with GFP-Booster and smFISH for bcd mRNA, before and after treatment with 1,6-HD. At t = 0 min, 98% of bcd mRNA particles are co-localized with P bodies. Following treatment with 1,6-HD (t = 25 min), bcd mRNA particles disperse and are not always co-localized with P bodies (52%) (n = 10 mature oocytes). Maximum projection 5 μm.
Scale bar, 10 μm (A), 5 μm (B and F), 1.5 μm (C–E).
See also Figure S5.
We first tested this prediction in live oocytes by asking if the addition of 1,6-HD could trigger the release of bcd mRNA, a well-established example of long-term storage, which is known to localize to P bodies in the mature oocyte (Figure 6B). Upon 1,6-HD treatment of mature oocytes, P bodies became more spherical, consistent with a loss of P body integrity and a transition into a more dynamic state (Figure 6C). Furthermore, in line with our predictions, whereas P bodies remained relatively condensed, MS2-labeled bcd mRNA association with P bodies reduced dramatically post-1,6-HD treatment in comparison with phosphate buffered saline (PBS)-treated oocytes (Figures 6D, 6E, S5A, and S5B). Although we did observe a small reduction in bcd fluorescence in PBS-only-treated oocytes, this effect is likely due to the composition of monovalent salts present in PBS, in addition to the photobleaching effect (Figure S5A).
Since the live imaging of bcd mRNA with the MS2 system yields a lower signal to noise ratio than fixed samples labeled with dyes, we sought to confirm these results using smFISH coupled with immunofluorescence. In the untreated mature oocytes, bcd was concentrated in P bodies and we did not detect any free bcd mRNA that was not associated with Me31B. Alternatively, in the 1,6-HD-treated mature oocytes, more than 50% of the bcd mRNA particles were not associated with Me31B (Figure 6F). Together, these results suggest that the transition induced by 1,6-HD leads to the release of bcd mRNA, eventually resulting in P body dissolution.
Egg activation modulates P body properties and results in the release of bcd mRNA in the early embryo
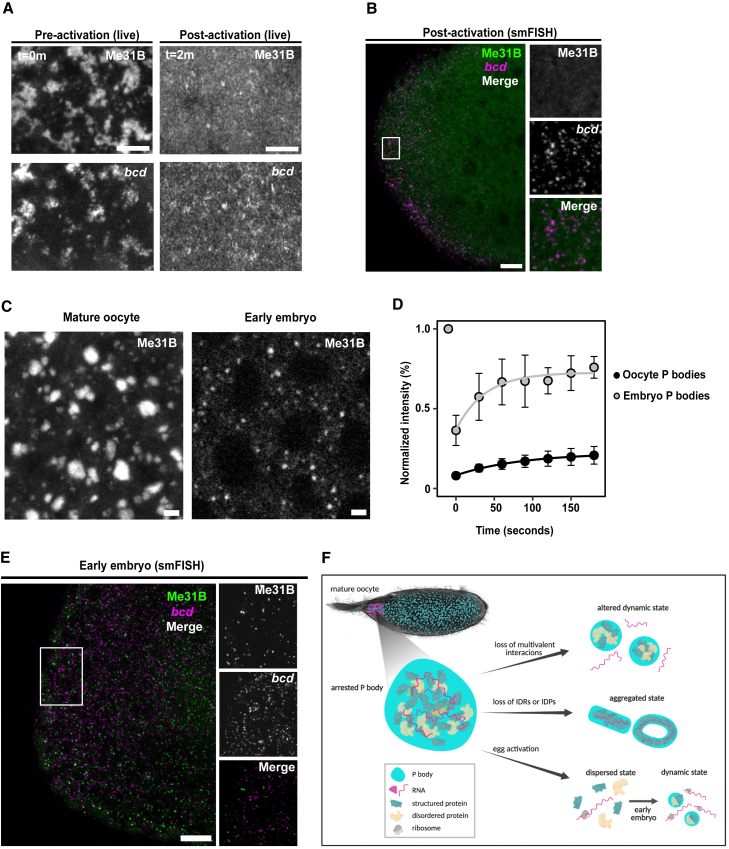
To explore the relationship between RNA release and P body integrity in a physiological context, we examined P bodies and bcd mRNA at egg activation and in the early embryo. The process of egg activation is a conserved step in animal development, and previous work in Drosophila has shown that egg activation alone results in the widespread translation of maternal mRNAs, including bcd (Eichhorn et al., 2016).
To test if egg activation affects P body integrity and bcd mRNA association, we utilized a well-established buffer (activation buffer [AB]) to activate mature oocytes ex vivo. Importantly, the addition of AB mimics downstream cellular and molecular changes observed in vivo (Krauchunas and Wolfner, 2013; York-Andersen et al., 2015, 2020). Upon treatment with AB, both P bodies and bcd mRNAs underwent a rapid dispersion, consistent with a loss of P body integrity and the simultaneous release of bcd mRNA (Figure 7A). We also confirmed the loss of association between P bodies and bcd using smFISH analysis of ex vivo activated oocytes, which showed a dispersed distribution of bcd mRNA particles at the anterior, whereas Me31B was diffused (Figure 7B). This finding is consistent with data arguing that the translation of bcd mRNA only occurs when the mRNA is no longer inside P bodies (Eichhorn et al., 2016; Weil et al., 2012). Together, these results suggest that P bodies facilitate the storage of mRNAs, such as bcd, which are later released for translation, following P body dispersion at egg activation.
Figure 7.
Egg activation modulates P body properties in the early embryo
(A–C and E) Mature oocytes expressing Me31B::GFP.
(A) The addition of activation buffer results in simultaneous dispersion of P bodies and bcd mRNA from condensed (t = 0 min) to diffused state (t = 2 min) (n = 0 mature oocytes). Maximum projection 5 μm.
(B) smFISH of activated oocytes stained for Me31B using a GFP-Booster and bcd shows diffuse P bodies and dispersed distribution of bcd mRNAs. Inset shows a zoomed in version of bcd mRNA and P body distribution (n = 10 activated oocytes). Maximum projection 5 μm.
(C) P bodies in the mature oocyte are larger than those in the early embryo (n = 50 early embryos). Max projection 3 μm.
(D) P body recovery profiles after whole FRAP of P bodies in the mature oocyte and early embryo. Mobile fraction for P bodies in the early embryo is 57% compared with 15% in the mature oocyte (n = 20 mature oocytes, n = 8 early embryos) (mean, standard deviation).
(E) smFISH of early embryos stained for Me31B using a GFP-Booster and bcd shows no co-localization of P bodies and bcd mRNAs. Inset shows a zoomed in version of bcd mRNA and P body distribution (n = 10 early embryos). Maximum projection 5 μm.
(F) P bodies (cyan) distributed throughout the mature Drosophila oocyte adopt an arrested physical state. The assembly, organization, and physical properties of P bodies are regulated by multivalent interactions between structured proteins (green) and intrinsically disordered proteins (IDP, yellow), as well as RNAs (magenta). The loss of these interactions alters the physical state of P bodies. At egg activation, P bodies disperse and release stored RNA for translation. In early embryogenesis, P bodies re-condense but are more dynamic and do not co-localize with RNAs. Created with BioRender.com.
Scale bar, 2 μm (A and C), 5 μm (B, D and E).
See also Figure S4.
Following egg activation and fertilization, P body-associated proteins have been observed in early embryos; however, details of their physical properties are not known (Lin et al., 2008; Patel et al., 2016). Live imaging in the early embryo reveals P bodies that are smaller, with increased spatial mobility, and more spherical in shape than those in mature oocytes (Figures 7C and S5C–S5E). To test if embryonic P bodies exhibit an arrested state, we performed whole FRAP. In contrast to P bodies in the mature oocyte, P bodies in the early embryo exhibited rapid recovery of fluorescence and a high proportion of mobile Me31B (Figure 7D). These results collectively show that the P bodies from the early embryo are fundamentally different from those in the mature oocyte, despite being referred with the same name.
Finally, we tested if the modified P bodies in the early embryo reassociate with bcd mRNA, which is continuously translated in early embryogenesis. Using smFISH, we found that bcd mRNA particles are not associated with the re-formed P bodies, consistent with expectations for translationally active mRNAs (Figure 7E). In addition, we visualized hunchback (hb) mRNA, a zygotic gene required for embryo pattern formation and a downstream target of Bcd protein (Crauk and Dostatni, 2005). Interestingly, hb mRNA is also not associated with P bodies in the early embryo (Figure S5F). Taken together, the modified properties of P bodies in the early embryo suggest a change in P body function during the oocyte-to-embryo transition.
Discussion
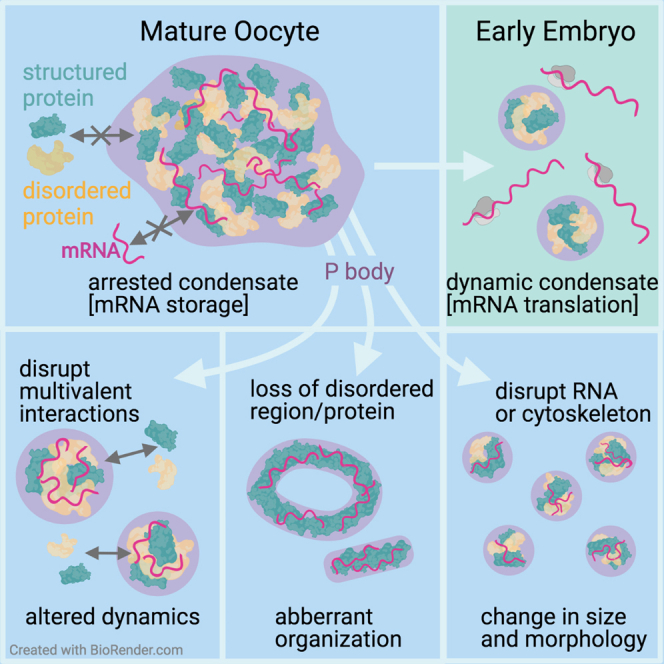
Over the last decade, biomolecular condensates have emerged as a key principle in cellular organization. Although changes in condensate physical properties have been examined extensively in vitro, the in vivo relevance of physical states has been explored to a lesser extent. Here, we demonstrate that a combination of intrinsic (multivalent interactions, presence of IDRs) and extrinsic (RNA, actin, and disordered proteins) factors can regulate the integrity and the arrested physical state of P bodies, both of which contribute to the storage of bcd mRNA in mature oocytes (Figure 7F). We also show that P bodies exhibit modified properties in the early embryo. We support a model whereby multivalent interactions, modular protein regions, and cellular factors trigger changes in the physical states of RNP condensates to facilitate differential mRNA outcomes during development.
Although dynamic, liquid-like states have been observed for many biomolecular condensates, there is a growing repertoire of functionally important and dynamically arrested condensates (Boke et al., 2016; Brangwynne et al., 2011; Hubstenberger et al., 2013; Woodruff et al., 2017). Balbiani bodies, for instance, adopt a solid-like physical state, which is thought to facilitate prolonged storage of macromolecules in dormant vertebrate oocytes (Boke et al., 2016). P bodies in Drosophila oocytes exhibit a physical state that allows internal mobility but prevents the exchange of proteins between the P body and the cytoplasm. Analogous states have been observed in the germline P bodies of arrested C. elegans oocytes (Hubstenberger et al., 2013), suggesting that the viscous properties of RNP condensates could be an evolutionarily conserved mechanism to temporally regulate mRNAs essential for normal development. Importantly, such physical states of RNP condensates may be preserved across other specialized cell types, such as neurons. For example, mRNAs stored and translationally repressed in neuronal RNP condensates are temporarily translated in an activity-induced manner at specific synapses, thereby influencing short-term or long-term memory (Puthanveettil, 2013; Rajasethupathy et al., 2009). Although it is not clear how this translation is regulated, models suggest that RNP granules switch between liquid-like and solid-like states to facilitate differential translation control (Bakthavachalu et al., 2018; Majumdar et al., 2012; Sudhakaran and Ramaswami, 2017).
Prior to this work, it was unclear how mRNAs could be subjected to efficient storage and differential release at distinct stages of oogenesis without obviously disrupting the integrity of P bodies (Sankaranarayanan and Weil, 2020). Previous work using cryo-immunoelectron microscopy on ultrathin frozen sections showed that maternal mRNAs are dynamically partitioned according to their translational status in mid-stage oocyte P bodies. Specifically, mRNAs that are being translated are enriched on the edge of P bodies with the cytoplasmic polyadenylation element-binding (CPEB) protein, oo18 RNA-binding protein (Orb), and ribosomes (Davidson et al., 2016; Weil et al., 2012). Alternatively, stored mRNAs that reside inside of mid-stage oocyte P bodies are repressed until later stages of development. Our data demonstrate that P body properties can be modified by attenuating multivalent hydrophobic or electrostatic interactions. Therefore, stored mRNAs could be subjected to controlled release through modulating the integrity of P bodies in response to developmental and molecular cues.
One striking observation is the influence of disordered regions in regulating the physical state of P body condensates. Conventional wisdom posits that IDRs contribute weak multivalent interactions that are essential for condensation. However, our results offer an alternative model—rather than driving assembly, IDRs may also function to modulate the physical state of condensates by counteracting the interactions driven by adhesive contact sites on folded domains. This model echoes prior work on the yeast prion protein Sup35, where the loss of N-terminal disordered regions leads to robust aggregation of the folded C-terminal domain, whereas the full-length protein rapidly assembles into dynamic condensates (Franzmann et al., 2018). The role of IDRs in the formation or regulation of RNP condensates is context dependent; the DEAD-box helicase eIF4A lacks any appreciable IDRs but serves to regulate stress granule formation in cells (Tauber et al., 2020). However, in structured proteins, which possess unusually short, disordered regions, as in Me31B, we speculate that the disordered regions may have emerged to modulate the physical states of RNP condensates.
Another key determinant that regulates biomolecular condensate formation and physical properties is multivalency. Condensates such as P bodies contain hundreds of diverse RNP components, which likely serve as a major source of multivalent interactions. Although structured and disordered RNA-binding proteins have been investigated previously, how they influence the overall property of condensates in vivo is unclear. Using Me31B and Tral, our results indicate that structurally distinct proteins synergistically interact to regulate P bodies during Drosophila oogenesis. These data also agree with observations reported for Tral and Me31B orthologs in arrested C. elegans oocytes (Hubstenberger et al., 2013), suggesting that the underlying physical interactions between RNP components may be evolutionarily conserved. Overall, our in vivo and in vitro data indicate that IDRs act as lubricants to regulate the overall physical state and organization of P bodies through interactions with structured proteins.
The oocyte-to-embryo transition is accompanied by large-scale changes in the cytoplasm, including the translation of stored mRNAs and widespread post-translational modifications (PTMs) (Eichhorn et al., 2016; Hara et al., 2018). In fact, Me31B is known to be phosphorylated and ubiquitinated in the early embryo (Hara et al., 2018; Zavortink et al., 2020). Such modifications have been shown to alter the physical properties of RNP condensates in vitro (Hofweber and Dormann, 2019; Owen and Shewmaker, 2019; Schisa and Elaswad, 2021). Our data from the early embryo suggest that PTMs may be influencing the modification of P body physical state and function post-egg activation.
One open question relates to the molecular differences between P bodies in the oocyte and early embryo. In vitro studies have shown that RNA can modulate the properties of condensates, including their size and dynamics (Garcia-Jove Navarro et al., 2019; Roden and Gladfelter, 2021). Our in vivo data showing smaller-sized oocyte P bodies after RNase A treatment and the absence of actively translating mRNAs in embryonic P bodies led us to speculate that a decrease in the abundance, or absence, of RNAs is likely contributing to changes in P body size and dynamics.
Finally, biochemical and molecular evidence suggest that P body proteins, including Me31B and Tral, change primary function from translational repression to degradation in the early embryo (Wang et al., 2017). We suspect the changes that accompany the dissolution of P bodies at egg activation, and their re-condensation in the embryo, reflect developmentally required transitions in mRNA regulation (Hubstenberger et al., 2013; Kato and Nakamura, 2012; Wang et al., 2017). In line with this model, we propose a general framework whereby developmental cues coordinate molecular interactions and large-scale cytoplasmic modifications to regulate mRNAs via adaptable RNP physical states.
Limitations of the study
Our work shows that the arrested physical state of P bodies facilitates the storage of bcd mRNA in the mature oocyte. Whether such a state also facilitates mRNA storage during earlier stages of oogenesis, or in other cells, remains to be determined. Additionally, more mRNAs need to be tested to expand the relevance of the arrested state. Another limitation is the use of 1,6-HD to study P body physical state and bcd regulation. Although 1,6-HD has been commonly used to attenuate hydrophobic interactions, it is not a naturally occurring cellular factor. Therefore, identifying cellular components that interfere with P body properties and studying the consequences of premature release of stored mRNAs is worthy of further investigation. Finally, we note that although 1,6-HD treatment results in the release of bcd from P bodies, we did not detect bcd translation (data not shown). This is likely due either to the requirement of a translational activator (or loss of a translational repressor) to initiate translation at egg activation or to the ability of 1,6-HD to impair kinase and phosphatase activities, which are thought to regulate translation of mRNAs (Düster et al., 2021). Whether or not the release of mRNA from P bodies alone is sufficient for translation continues to be an important area of investigation in the future.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| GFP-Booster Alexa Fluor 488 | Chromotek | Cat# gb2AF488-10 |
| Bacterial and virus strains | ||
| Sf9 cells | Expression systems | Cat#94-001F |
| Subcloning Efficiency DH5α Competent Cells | Invitrogen | Cat#18265017 |
| Chemicals, peptides, and recombinant proteins | ||
| Cytochalasin-D | Sigma-Aldrich | Cat# C8273 |
| 1,6 Hexanediol (1,6-HD) | Sigma-Aldrich | Cat# 240117 |
| RNase A | Roche | Cat# RNASEA-RO |
| cOmplete Protease Inhibitor Cocktail, EDTA-free | Roche | Cat#5056489001 |
| 1X Dulbecco’s Phosphate Buffered Saline (PBS) solution without MgCl2 | Sigma-Aldrich | Cat# D8537 |
| Potassium chloride | Merck | Cat#104935 |
| DTT | Fermentas LifeSciences | Cat#R0862 |
| Benzonase | Produced in-house | N/A |
| Tris | Carl Roth | Cat# 5429 |
| Amylose resin | NEB | Cat# E8021S |
| EDTA | Roche | Cat# 105063 |
| Pipes | Applichem | Cat# A1079 |
| monoGFP | Produced in-house | N/A |
| Activation Buffer (AB) | York-Andersen et al., 2015 | N/A |
| Schinder’s Drosophila medium | Gibco | Cat# 21720024 |
| ‘Wash Buffer A’ for Stellaris RNA FISH | LG Biosearch Technologies | Cat# SMF-WA1-60 |
| ‘Hybridisation Buffer’ for Stellaris RNA FISH | LG Biosearch Technologies | Cat# SMF-HB1-10 |
| ‘Probe Hybridisation Buffer’ for HCR V3.0 | Molecular Instruments | Bundled with custom probe set |
| ‘Probe Wash Buffer’ for HCR V3.0 | Molecular Instruments | Bundled with custom probe set |
| ‘Amplification Buffer’ for HCR V3.0 | Molecular Instruments | Bundled with custom probe set |
| SlowFade Diamond Antifade Mountant with DAPI | Thermofisher Scientific | S36934 |
| Protease inhibitor cocktail | Roche | Cat# CO-RO |
| Amylose Resin | New England Biolabs | Cat# E8021S |
| PreScission Protease GST 3C | GE Life Sciences | Cat# GE27-0843-01 |
| Polyethene Glycol – 2000 | Merck | Cat#817018 |
| Recombinant GFP-Me31B protein | This paper | N/A |
| Recombinant GFP-Me31BΔN-ΔC protein | This paper | N/A |
| Critical commercial assays | ||
| Size exclusion chromatography using a HiLoad 16/600 Superdex 200 pg | GE Life Sciences | Cat# GE28-9893-35 |
| Amicon Ultra-0.5 Centrifugal Filter Unit | Millipore | Cat# UFC5030 |
| Deposited data | ||
| Raw set of disordered regions from the Drosophila proteome | This paper | https://github.com/holehouse-lab/supportingdata/tree/master/2021/sankaranarayanan_me31b_2021 |
| Experimental models: Organisms/strains | ||
| D. melanogaster: y[1] w[∗]; P{w[+mC]=PTT-GB}me31B[CB05282](Me31B::GFP) | Bloomington Drosophila Stock Centre (Buszczak et al., 2007) | BDSC: 51530FlyBase: FBst0051530 |
| D. melanogaster: y,w, bcd-(ms2)6 (18), bcd-(ms2)6 (4); hsp83-MCP-RFP(4a) | Weil et al., 2006 | N/A |
| D. melanogaster: GFP::Tral | Drosophila Genomics Resource Centre (Morin et al., 2001) | DGRC: 110584Flytrap:G00089;DGRC:110584;RRID:DGGR_110584 |
| D. melanogaster: y[1]; P{y[+mDint2] w[BR.E.BR]=SUPor-P} tral[KG08052] ry[506] / TM3, Sb[1] Ser[1](tral1) | Bloomington Drosophila Stock Centre (Wilhelm et al., 2005) | BDSC: 14933FlyBase: FBgn0041775 |
| D. melanogaster: w[1118]; Df(3L)ED4483, P{w[+mW.Scer\FRT.hs3]=3’.RS5+3.3’}ED4483/ TM6C, cu[1] Sb[1] | Bloomington Drosophila Stock Centre (Wilhelm et al., 2005) | BDSC: 8070FlyBase: FBab0035731 |
| Oligonucleotides | ||
| Custom Stellaris FISH Probes for the 3’UTR of bcd RNA | Stellaris | See Table S2 (supplemental information). |
| Molecular Instruments Custom HCR Probe for hb RNA | Molecular Instruments | DNA Custom kitDrosophila melanogasterlot number: 2690/B795GenBank: NM_169234.2, Alexa647, v3.0 kits |
| Recombinant DNA | ||
| Recombinant GFP-Me31B plasmid | This paper | N/A |
| Recombinant GFP-Me31BΔN-ΔC plasmid | This paper | N/A |
| Software and algorithms | ||
| ImageJ | Schneider et al., 2012 | https://imagej.nih.gov/ij/ |
| ImageJ Plugin Trackmate | Tinevez et al., 2017 | https://imagej.net/plugins/trackmate/ |
| Rstudio/ R software | RStudio Team 2021 | http://www.rstudio.com/. |
| HullRad | Fleming and Fleming, 2018 | N/A |
| ABSINTH implicit solvent model | Vitalis and Pappu, 2009 | N/A |
| CAMPARI Monte Carlo simulation (v3.0) | http://campari.sourceforge.net/V3/index.html | N/A |
| SWISS-MODEL | Waterhouse et al., 2018 | N/A |
| SOURSOP | https://soursop.readthedocs.io/ | N/A |
| MDTraj | McGibbon et al., 2015 | N/A |
| DSSP Algorithm | Kabsch and Sander, 1983 | N/A |
| Protfasta | https://protfasta.readthedocs.io/ | N/A |
| Metapredict | Emenecker et al., 2021 | N/A |
| LocalCIDER | Holehouse et al., 2017 | N/A |
| PIMMS simulation engine | Martin et al., 2020 | N/A |
| Other | ||
| Iberian recipe fly food | Produced in-house | N/A |
| Oil 10 S (95 Series Halocarbon Oil) | VWR Chemicals | N/A |
| Olympus FV3000 Confocal Laser Scanning Microscope | Olympus | FV3000 |
| DeltaVision Core widefield microscope | Applied Precision, LLC | DeltaVision Core |
| Custom built dual-trap optical tweezer instrument | Jahnel et al., 2011 | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact Timothy T. Weil (tw419@cam.ac.uk)
Materials availability
Reagents generated in this study are available upon request.
Experimental model and subject details
Drosophila stocks
The following transgenic lines were used in this paper:
Me31B::GFP (BDSC 51530, (Buszczak et al., 2007)), _h_sp83-MCP-RFP and _bcd_-(ms2)6 (Weil et al., 2006), GFP::Tral (DGRC 110584, (Morin et al., 2001)), tral 1 (BDSC 14933) and Df(3L)ED4483 (BDSC 8070) (Wilhelm et al., 2005).
Fly stocks were maintained at 25°C on Iberian recipe fly food as per standard procedure. Randomly selected, healthy, adult female flies typically 2-3 days after eclosing, with the required genotype for each experiment, that had not been subjected to previous experimental procedures were fed on yeast for two days at 25°C prior to dissection of ovaries or collection of embryos. For fattening, approximately 20 females and 10 males were put in a vial together. For embryo collection, 50-100 females and 20-40 males were placed in a cage together.
Method details
Oocyte sample preparation
Mature oocytes from fattened female flies were dissected (Weil et al., 2012b; Derrick et al., 2016) into 10S oil (95 halocarbon) on a 22 mm by 40 mm cover slip for live imaging. For extrusion assays, membranes of dissected mature oocytes were poked and ruptured using sharp forceps to extrude the oocyte contents into the oil. Extruded material was then subjected to live imaging.
Live imaging
Live imaging of in vivo and ex vivo P bodies, including all Fluorescent Recovery After Photobleaching experiments, were performed on the Olympus FV3000 microscope using the 1.35 NA, 60X silicone objective at a room temperature of 20°C (Note: subtle changes in temperature can affect P body recovery kinetics). For all in vivo experiments, P bodies in the anterior to mid-lateral region of the mature oocyte were imaged. Live imaging of recombinant Me31B condensates, induced on 35mm glass bottom MatTek dishes, was performed on the DeltaVision Core widefield microscope using a 1.4 NA, 60X oil immersion objective.
Pharmacological treatments
Mature oocytes mounted in oil on a 22 mm by 40 mm coverslip and set up under the microscope were treated with one or two drops of 10 μg/ml cytochalasin-D (Sigma-Aldrich) or 5% 1,6-HD (Sigma-Aldrich) or 500 ng/ml RNase A (Sigma-Aldrich) mixed in 1X Dulbecco’s Phosphate-buffered saline (PBS) solution without MgCl2 (Sigma-Aldrich), or home-made activation buffer (3.3 mM NaH2PO4, 16.6 mM KH2PO4, 10 mM NaCl, 50 mM KCl, 5% PEG 8000, 2 mM CaCl2, pH 6.4; York-Andersen et al. 2015) using a glass pipette. Me31B or Tral labeled P bodies before and after treatment were then imaged. For salt experiments, mature oocytes were extruded into various concentrations of MgCl2 or NaCl mixed in 1X PBS for 15 minutes before being subjected to live imaging. In the case of excessive movement of the oocytes or extruded material during addition of solutions, the focal plane of interest was adjusted accordingly, and imaging was performed.
Protein purification
The plasmid backbones for the production viruses and the SF9 insect cells for the purification of the recombinant proteins were provided by the protein purification facility at the MPI-CBG in Dresden. The recombinant Me31B, (wild-type (WT) and Me31BΔN-ΔC (mutant)), were cloned using established cloning techniques, tagged with a monomeric GFP (produced in-house), expressed in and purified from SF9 insect cells using the FlexiBAC baculovirus vector system (Lemaitre et al., 2019). Cell lysis was performed using a LM20 microfluidizer in lysis buffer containing 50 mM Tris/HCl pH 7.6, 2 mM EDTA, 1x EDTA-containing protease inhibitor cocktail (Roche), 1 M KCl, 5% glycerol, 3 μg/L benzonase (degrades nucleic acids), 1 mM DTT. The soluble lysate fraction was collected after centrifugation for 1 hour at 16000 rpm (Beckman Coulter JA-25.50) at 4°C. MBP-tagged protein was captured by gravity flow affinity chromatography using amylose resin (New England Biolabs). Captured protein was washed with wash buffer (50 mM Tris/HCl pH 7.6, 2 mM EDTA,1 M KCl, 5% glycerol, 1 mM DTT, 3 ug/L benzonase) and eluted using wash buffer containing 20 mM maltose. The eluted protein was incubated with GST 3C – PreScission protease (1:50) at room temperature for 2 hours to cleave off affinity tags. Samples were applied to size exclusion chromatography using a HiLoad 16/600 Superdex 200 pg (GE Life Sciences) on an Akta pure chromatography system in 50 mM Tris/HCl pH 7.6, 2 mM EDTA,1 M KCl, 5% glycerol, 1 mM DTT. Proteins were finally concentrated using an Amicon Ultra centrifugal-500-30K filter at 4000 xg. Aliquots were flash frozen and stored at -80°C.
N terminal IDR sequence: MTEKLNSGHTNLTSKGIINDLQIAGNTSDDMGWKSKLKLPPKDNRFKTT
C-terminal IDR sequence: SVGDTCNNSDLNNSANEEGNVSK
In vitro condensation assay
Stored protein samples were thawed and spun at 5,000 rpm for a minute to remove any residual precipitates. To induce Me31B condensates (WT and mutant), 7.5 μM recombinant GFP-Me31B protein was added to an Eppendorf tube containing the condensation buffer (50 mM KCl, 20 mM PIPES, pH 7, 1% PEG-2K). Note: Gentle tapping of the tube induced spherical condensates. Mixing the content with a pipette tip was avoided as it prevented droplet formation.
Optical tweezer experiments
Condensate fusions for wildtype or mutant condensates were quantified using a custom built dual-trap optical tweezer instrument (Jahnel et al., 2011). Condensates were induced in the condensation buffer containing 5% PEG-2K at 20 μM Me31B protein concentration for both WT and mutant condensates. Post condensation, two condensates were trapped using separate optical traps and brought into close contact to induce fusion.
Fluorescence recovery after photobleaching
For whole FRAP, Me31B/Tral labeled P bodies or in vitro Me31B condensates were entirely photobleached for 5 seconds using 40% laser intensity from the 405 nm laser channel. For internal FRAP, a small region within Me31B labeled in vivo P bodies or in vitro Me31B condensates was photobleached for 5 seconds using 40% laser intensity from the 405 nm laser channel. Time lapse series of Me31B fluorescence recovery was recorded every 30 seconds (in vivo P bodies) or 10 seconds (in vitro Me31B condensates) using the pre-bleach imaging parameters (minimal laser intensity using the 488 nm laser channel, 2 Airy unit pinhole, 20482048 pixels).
Single molecule fluorescence in situ hybridization
Fly preparation: Ovaries from fattened female flies were dissected into 5% 1,6-HD(Sigma-Aldrich) dissolved in 1x PBS and teased apart to allow for permeation of the oocytes by 5% 1,6-HD. Oocytes were then incubated for 30 minutes before being transferred into and incubated in Schneider’s Drosophila medium (Gibco) for 1 hour, oocytes were then fixed as below.
Fixation: Ovaries from fattened female flies were dissected into Schneider’s Drosophila medium and teased apart before being fixed in 1 ml of 4% paraformaldehyde for 15 minutes at room temperature. Oocytes were washed thoroughly with 0.2% PBST before hybridization. Embryos collected for 1.5 hours were dechorionated with 50% household bleach. The embryos were then washed thoroughly and were fixed in a solution containing 500 μl of 4% paraformaldehyde and 500 μl heptane at room temperature for 15 minutes, the paraformaldehyde was replaced with 100% methanol, and this was shaken vigorously to pop the vitelline membrane. All liquid was removed, and the embryos were rinsed in methanol, before being washed in 0.2% PBST.
Stellaris RNA fluorescence in situ hybridization: Custom Stellaris FISH Probes were designed against the 3’ UTR of bcd mRNA (GenBank: NM_057477, GenBank: NM_169157, GenBank: NM_169159, GenBank: NM_176411, GenBank: NM_176410) by utilizing the Stellaris RNA FISH Probe Designer (Biosearch Technologies, Inc., Petaluma, CA) available online at www.biosearchtech.com/stellarisdesigner. Drosophila oocytes and embryos were hybridized with the bcd mRNA Stellaris RNA FISH Probe set labeled with Quasar 570 (Biosearch Technologies, Inc.), following the manufacturer’s instructions for ‘Drosophila embryos’ available online at www.biosearchtech.com/stellarisprotocols.
Protocol adapted from Trovisco et al. (2016), briefly, fixed oocytes and embryos were washed using ‘Wash Buffer A’ before being hybridized with 500 nM Quasar 570 -conjugated antisense Stellaris probes for bcd RNA in hybridization buffer at 37 degrees overnight. The oocytes and embryos were then re-washed in ‘Wash Buffer A’ before being washed with 0.2% PBST. The sample was then incubated in GFP-Booster Alexa Fluor 488 (1:500 (Chromotek)) at room temperature for 1 hour in 1% PBST. Embryos were washed well with 0.2% PBST before being mounted in SlowFade Diamond Antifade Mountant with DAPI (Thermofisher Scientific).
Custom Stellaris FISH Probes for the 3’UTR of bcd RNA, adapted from Trovisco et al. (2016), see Table S2.
In situ hybridization chain reaction V3.0
Protocol adapted from molecular instruments (Choi et al., 2018). Drosophila embryos were hybridized with 2 μl of odd and even HCR probes for hb mRNA in 100 μl 30% hybridization buffer at 37 degrees overnight. The sample was then washed with 30% probe wash buffer at 37 degrees and SSCT at room temperature. 2 μl of B4 Hairpin 1 and Hairpin 2 conjugated to an Alexa 647 were snap-cooled by heating to 90 degrees and cooled in the dark before being added to 100 μl of amplification buffer, this was added to the Drosophila embryos and incubated in the dark for 2 hours. The sample was then washed with SSCT at room temperature followed by 0.2% PBST before the addition of GFP-booster 488 (1:500 (Chromotek)) at room temperature for 1 hour in 1% PBST. Embryos were washed well with 0.2% PBST before being mounted in Slowfade Diamond with DAPI (Thermofisher Scientific).
Quantification and statistical analysis
Optical tweezer experiments
For quantifying the scaled fusion time for WT condensates, firstly, a relaxation time constant was derived from the fusion process over time. The scaled fusion time was then calculated by dividing the relaxation time constant by condensate radii to express the fusion time as a function independent of condensate size. For mutant condensates, due to their rapid aggregation post condensation, fusion was not quantifiable.
Fluorescence recovery after photobleaching
Mean fluorescence intensities were estimated using the Fiji ImageJ software. For whole FRAP analysis, background correction was performed by dividing Me31B fluorescent intensities of bleached condensates by fluorescent intensities of unbleached, cytoplasm. For internal FRAP, background correction was performed by dividing Me31B fluorescent intensities of bleached region within condensates by fluorescent intensities of whole condensates.
For all FRAP series, statistical analysis, curve fitting and plotting was performed using Rstudio/R software. Data for each condition was averaged and standard deviation was calculated where applicable. Recovery fitting of the normalized mean intensity as function of time was fitted by the least square analysis to determine fit to the single exponential equation: Normalized intensity = P×(1−e(−t/τ)) +y0 where y0 is the recovery plateau, t is time, τ is the time constant and P is the amplitude of the fluorescence change. To infer the spatiotemporal pattern of internal Me31B fluorescence recovery, kymographs were generated using the ImageJ plugin ‘reslice’ by measuring fluorescence across of a region of interest over time.
All statistical analysis was completed in R/R Studio, the distribution of all data sets were analyzed in R studio before statistical analysis to assure the data met the assumptions for the appropriate statistical test. Statistical analysis of the difference in recovery kinetics P bodies between the three stages of oogenesis (stage 7, stage 12 and stage 14) used a Students t-test (Figure S2).
Fluorescence intensity measurements
Analysis of Me31B::GFP and _bcd_-RFP fluorescence before and after PBS and 1,6-HD treatment was performed using ImageJ processing software. Identical imaging parameters were utilized during imaging and measurement of fluorescence using ‘analyze particles’ and ‘measure’ feature on ImageJ. Individual Me31B and bcd particles were manually counted and analyzed before and after treatment with 1,6-HD. Random particles (bcd and Me31B together) were analyzed at different time points to avoid any bias.
Aspect ratio, circularity and particle area
P body aspect ratio, circularity, and area were measured using the ‘analyze particles’ and ‘measure’ features in ImageJ. Aspect ratio values are measured as the ratio of the major axis of a particle to the minor axis of a particle which gives an estimate of particle morphology. Circularity refers to the “roundedness” of a particle (and is calculated using the formula - circularity = 4pi (area/perimeter2) which gives an estimate of particle shape.
All statistical analysis was completed in R/R Studio, the distribution of all data sets were analyzed in R studio before statistical analysis to assure the data met the assumptions for the appropriate statistical test. Statistical analysis of the difference in area and circularity of P bodies between the oocyte and embryo used a Two-Sampled Wilcoxon (Mann-Whitney) test. Statistical analysis of the difference in circularity of P bodies in the oocyte before and after treatment with 1,6-HD used a Wilcoxon signed-rank test.
Apparent viscosity estimation
Protocol was adapted from: (Hubstenberger et al., 2013). P body viscosity was estimated from internal FRAP recovery kinetics. Apparent diffusion of ∼ 0.00071 μm2/s was estimated from the calculated half-maximum (62.79s) using the equation: D≈0.224ω2/ t1/2, where ω is the radius of the bleach region, t is the time. Using the equation η=(KbT)6πRhD, where Rh is the hydrodynamic radius (An approximate hydrodynamic radius of Me31B was estimated based on our all-atom simulations, with the hydrodynamic radius calculated using HullRad (Fleming and Fleming, 2018), and T is the temperature at which experiments were conducted (21°C), the apparent viscosity was estimated.
Particle displacement analysis
P bodies were tracked and their displacement calculated before and after the addition of Activation Buffer (AB) or Cytochalasin D. Prior to tracking the images were pre-processed with a rolling ball background subtraction. Individual P bodies were then tracked using the FIJI plugin Trackmate (Tinevez et al., 2017). A simple linear tracker was used to determine particle tracks, and statistical analysis of the track displacement was completed in R/R Studio, using a Wilcoxon Signed Rank Test. The distribution of the data was analyzed in R studio before statistical analysis to assure the data met the assumptions for the statistical test.
All-atom simulations
All-atom simulations were run with the ABSINTH implicit solvent model and the CAMPARI Monte Carlo simulation (V3.0)
(http://campari.sourceforge.net/V3/index.html) and with the ion parameters from previously published work (Mao and Pappu, 2012; Vitalis and Pappu, 2009). Preferential sampling is used such that the backbone dihedral angles of folded domains are held fixed, while all sidechain dihedral angles, and the backbone dihedrals of folded proteins are fully sampled. In this way, we a priori ensure that the folded domains remain folded. While the combination of ABSINTH and CAMPARI is well-established route to obtain reliable ensembles of disordered regions, more positional restraints on folded domains have been used previously applied to obtain good agreement with experiment (Cubuk et al., 2021; Martin and Mittag, 2018; Martin et al., 2020; Newcombe et al., 2018).
Starting structures were generated first by constructing homology models of Me31B based on the DDX6 structure (PDB: 4CT5) using SWISS-MODEL (Waterhouse et al., 2018). N- and C-terminal IDRs were constructed using CAMPARI. For all simulations, disordered regions were started from randomly generated non-overlapping random-coil conformations, with each replica using a unique starting structure. Monte Carlo simulations evolve the system via a series of moves that perturb backbone and sidechain dihedral angles along with the rigid-body coordinates of both polypeptides and explicit ions. Simulation analysis was performed using SOURSOP (https://protfasta.readthedocs.io/) and MDTraj (McGibbon et al., 2015). The protein secondary structure was assessed using the DSSP algorithm (Kabsch and Sander, 1983).
Contact score analysis was performed by assessing the fraction of simulations in which two residues were in direct contact, a distance calibrated as 5.0 Å or shorter between heavy atoms. This fraction was divided by the analogous fraction computed from simulations in which all attractive molecular interactions (solvation effects, electrostatics, attractive component of the Lennard-Jones potential) were set to 0.0, in the so-called excluded volume (EV) limit (Holehouse et al., 2015).
All simulations were run at 10 mM NaCl, and PEG 310 K. Fifty independent simulations were run for a total of 80 million Monte Carlo steps with 5 million steps for equilibration. The system state saved every 100,000 steps. Each simulation generated 750 frames, generating a final ensemble of 37,500 frames. Where included, error bars are standard error of the mean over the fifty independent simulations.
Bioinformatics
Disordered regions were calculated using both Mobidb-lite (Necci et al., 2017) and with metapredict (Emenecker et al., 2021; Piovesan et al., 2021). Disordered regions were identified using consensus scores from Mobidib-lite with a minimum IDR length of 25 residues and 3 or more predictors predicting a region to be disordered. The raw set of disordered regions from the Drosophila proteome, along with analogous data for P body proteins is provided in the supplementary repository: https://github.com/holehouse-lab/supportingdata/tree/master/2021/sankaranarayanan_me31b_2021.
Sequence analysis was performed using localCIDER (Holehouse et al., 2017).
Coarse-grained simulations
Coarse-grained simulations were performed with the PIMMS simulation engine (Martin et al., 2020)https://paperpile.com/c/pRQFLB/sMrm+DzJc. Lattice-based Monte Carlo simulations afford a computationally tractable approach to sample systems with coexisting liquid phases, as has been applied in several different contexts (Boeynaems et al., 2019; Fei et al., 2017; Feric et al., 2016; Martin et al., 2020). Monte Carlo moves include chain translate, rotate, and local/global pivot moves.
Simulations were run using a simple representation scheme in which Me31B was represented as a five-bead model made up of two N-terminal beads, a single central bead, and two C-terminal beads (Figure S4G-A). In this way, the protein consists of intrinsically disordered region (IDR) beads and ordered domain (OD) beads. RNA is represented as a 20-bead homopolymer (Figure S4G-A). We emphasize that these models are designed to describe a class of phenomenon, as opposed to capturing features specific to Me31B over RNA binding proteins. Our simplification of RNA and protein not-withstanding, these simple models allow us to interrogate general behavior.
The strength of interactions between the three bead types is shown in Figures S4G-A and S4G-B. Units are in per kT (where k=1). The core key files and parameter files used to run these simulations are provided at https://github.com/holehouse-lab/supportingdata/tree/master/2021/sankaranarayanan_me31b_2021.
Protein:RNA and RNA:RNA interaction strengths are held fixed across all simulations, while the protein:protein interaction strength is systematically altered across the simulations shown in Figures S4G-A and S4G-B. The specific interaction strengths were chosen to qualitatively reflect insights from experimental work - i.e. OD:OD interaction is stronger than OD:IDR interaction, with IDR:IDR interaction being the weakest. We also assume both OD and IDR beads can interact with RNA, and that RNA:RNA interaction is repulsive. As a final note, we anticipate that RNA:RNA interactions plays an additional role in P body stability, assembly, and disassembly. However, for our initial model, absent of other specific information, we avoided adding more tunable parameters to develop a simple yet physically reasonable.
All simulations were run with 800 Me31B protein molecules. Simulations with RNA were also run with 50 RNA molecules. These numbers were chosen to ensure that reasonable statistics on droplet recruitment could be obtained with a sufficiently large system where bona fide condensation occurs. Simulations were run on a 60 x 60 x 60 lattice with periodic boundary conditions, and simulation analysis was performed on the terminal 20% of the frames. Simulations were run for around 2.5 billion Monte Carlo moves, and three independent replicas were performed, such that error bars are the standard error of the mean on these replicas.
Additional resources
No additional resources provided
Acknowledgments
We thank the Bloomington Drosophila Stock Center, Ilan, Davis, Liz Gavis, James Wilhelm, and William Chia for fly stocks. We are grateful to Paul Conduit for experimental advice; Titus Franzmann for advice on FRAP analysis; the Zoology Imaging Facility for assistance and support with microscopy; and funding from the University of Cambridge ISSF (097814) and the Wellcome Trust (200734/Z/16/Z) (to T.T.W.), INLAKS, the Cambridge Trust scholarship (to M.S.), the BBSRC DTP scholarship (to E.L.W.), William H. Danforth Plant Science Fellowship (to R.J.E), and the Technische Universität Dresden and the European Research Council (PhaseAge, No. 725836) (to S.A).
Author contributions
Conceptualization, M.S. and T.T.W.; methodology, M.S., A.S.H., and T.T.W.; investigation, majority, M.S.; investigation, simulations and in silico modeling, R.J.E. and A.S.H.; investigation, protein purification, I.R.E.A.T.; investigation, optical tweezer, M.J.; investigation, smFISH and tracking, E.L.W.; formal analysis, M.W.; writing – original draft, M.S. and T.T.W.; writing – review & editing, M.S., A.S.H., S.A., and T.T.W.; funding acquisition, M.W., A.S.H., S.A., and T.T.W.; supervision, A.S.H., S.A., and T.T.W.
Declarations of interests
S.A. is an advisor on the scientific advisory board of Dew point Therapeutics. A.S.H. is a scientific consultant with Dewpoint Therapeutics.
Published: October 15, 2021
Footnotes
Contributor Information
M. Sankaranarayanan, Email: msnsankar18@gmail.com.
Timothy T. Weil, Email: tw419@cam.ac.uk.
Supplemental information
Document S1. Figures S1–S5 and Tables S1
Document S2. Article plus supplemental information
Data and code availability
- •
All other data reported in this paper will be shared by the lead contact upon request. - •
All original code has been deposited at [https://github.com/holehouse-lab/supportingdata/tree/master/2021/sankaranarayanan_me31b_2021] and is publicly available as of the date of publication. - •
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Alshareedah I., Thurston G.M., Banerjee P.R. Quantifying viscosity and surface tension of multicomponent protein-nucleic acid condensates. Biophys. J. 2021;120:1161–1169. doi: 10.1016/j.bpj.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrei M.A., Ingelfinger D., Heintzmann R., Achsel T., Rivera-Pomar R., Lührmann R. A role for eIF4E and eIF4E-transporter in targeting mRNPs to mammalian processing bodies. RNA. 2005;11:717–727. doi: 10.1261/rna.2340405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakthavachalu B., Huelsmeier J., Sudhakaran I.P., Hillebrand J., Singh A., Petrauskas A., Thiagarajan D., Sankaranarayanan M., Mizoue L., Anderson E.N. RNP-granule assembly via ataxin-2 disordered domains is required for long-term memory and neurodegeneration. Neuron. 2018;98:754–766.e4. doi: 10.1016/j.neuron.2018.04.032. [DOI] [PubMed] [Google Scholar]
- Banani S.F., Lee H.O., Hyman A.A., Rosen M.K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017;18:285–298. doi: 10.1038/nrm.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeynaems S., Alberti S., Fawzi N.L., Mittag T., Polymenidou M., Rousseau F., Schymkowitz J., Shorter J., Wolozin B., Van Den Bosch L. Protein phase separation: a new phase in cell biology. Trends Cell Biol. 2018;28:420–435. doi: 10.1016/j.tcb.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeynaems S., Holehouse A.S., Weinhardt V., Kovacs D., Van Lindt J., Larabell C., Van Den Bosch L., Das R., Tompa P.S., Pappu R.V., Gitler A.D. Spontaneous driving forces give rise to protein−RNA condensates with coexisting phases and complex material properties. Proc. Natl. Acad. Sci. USA. 2019;116:7889–7898. doi: 10.1073/pnas.1821038116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boke E., Ruer M., Wühr M., Coughlin M., Lemaitre R., Gygi S.P., Alberti S., Drechsel D., Hyman A.A., Mitchison T.J. Amyloid-like self-assembly of a cellular compartment. Cell. 2016;166:637–650. doi: 10.1016/j.cell.2016.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouveret E., Rigaut G., Shevchenko A., Wilm M., Séraphin B. A Sm-like protein complex that participates in mRNA degradation. EMBO J. 2000;19:1661–1671. doi: 10.1093/emboj/19.7.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne C.P., Eckmann C.R., Courson D.S., Rybarska A., Hoege C., Gharakhani J., Jülicher F., Hyman A.A. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324:1729–1732. doi: 10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- Brangwynne C.P., Mitchison T.J., Hyman A.A. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc. Natl. Acad. Sci. USA. 2011;108:4334–4339. doi: 10.1073/pnas.1017150108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne C.P., Tompa P., Pappu R.V. Polymer physics of intracellular phase transitions. Nature Phys. 2015;11:899–904. [Google Scholar]
- Buchan J.R., Parker R. Eukaryotic stress granules: the ins and outs of translation. Mol. Cell. 2009;36:932–941. doi: 10.1016/j.molcel.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan J.R. mRNP granules. Assembly, function, and connections with disease. RNA Biol. 2014;11:1019–1030. doi: 10.4161/15476286.2014.972208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buszczak M., Paterno S., Lighthouse D., Bachman J., Planck J., Owen S., Skora A.D., Nystul T.G., Ohlstein B., Allen A. The carnegie protein trap library: a versatile tool for Drosophila developmental studies. Genetics. 2007;175:1505–1531. doi: 10.1534/genetics.106.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.M.T., Schwarzkopf M., Fornace M.E., Acharya A., Artavanis G., Stegmaier J., Cunha A., Pierce N.A. Third-generation in situ hybridization chain reaction: multiplexed, quantitative, sensitive, versatile, robust. Development. 2018;145:dev165753. doi: 10.1242/dev.165753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crauk O., Dostatni N. Bicoid determines sharp and precise target gene expression in the Drosophila embryo. Curr. Biol. 2005;15:1888–1898. doi: 10.1016/j.cub.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Cubuk J., Alston J.J., Incicco J.J., Singh S., Stuchell-Brereton M.D., Ward M.D., Zimmerman M.I., Vithani N., Griffith D., Wagoner J.A. The SARS-CoV-2 nucleocapsid protein is dynamic, disordered, and phase separates with RNA. Nat. Commun. 2021;12:1936. doi: 10.1038/s41467-021-21953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A., Parton R.M., Rabouille C., Weil T.T., Davis I. Localized translation of gurken/TGF-α mRNA during axis specification is controlled by access to orb/CPEB on processing bodies. Cell Rep. 2016;14:2451–2462. doi: 10.1016/j.celrep.2016.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrick C.J., York-Andersen A.H., Weil T.T. Imaging calcium in Drosophila at egg activation. J. Vis. Exp. 2016;114:e54311. doi: 10.3791/54311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorone Y., Boeynaems S., Flores E., Jin B., Hateley S., Bossi F., Lazarus E., Pennington J.G., Michiels E., De Decker M. A prion-like protein regulator of seed germination undergoes hydration-dependent phase separation. Cell. 2021;184:4284–4298.e27. doi: 10.1016/j.cell.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Düster R., Kaltheuner I.H., Schmitz M., Geyer M. 1,6-hexanediol, commonly used to dissolve liquid-liquid phase separated condensates, directly impairs kinase and phosphatase activities. J. Biol. Chem. 2021;296:100260. doi: 10.1016/j.jbc.2021.100260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzuricky M., Rogers B.A., Shahid A., Cremer P.S., Chilkoti A. De novo engineering of intracellular condensates using artificial disordered proteins. Nat. Chem. 2020;12:814–825. doi: 10.1038/s41557-020-0511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichhorn S.W., Subtelny A.O., Kronja I., Kwasnieski J.C., Orr-Weaver T.L., Bartel D.P. mRNA poly(A)-tail changes specified by deadenylation broadly reshape translation in Drosophila oocytes and early embryos. eLife. 2016;5:1–24. doi: 10.7554/eLife.16955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaum-Garfinkle S., Kim Y., Szczepaniak K., Chen C.C.-H., Eckmann C.R., Myong S., Brangwynne C.P. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc. Natl. Acad. Sci. U S A. 2015;112:7189–7194. doi: 10.1073/pnas.1504822112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emenecker R.J., Griffith D., Holehouse A.S. metapredict: a fast, accurate, and easy-to-use predictor of consensus disorder and structure. bioRxiv. 2021 doi: 10.1101/2021.05.30.446349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A., Behm-Ansmant I., Schweizer D., Izaurralde E. P-body formation is a consequence, not the cause, of RNA-mediated gene silencing. Mol. Cell. Biol. 2007;27:3970–3981. doi: 10.1128/MCB.00128-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei J., Jadaliha M., Harmon T.S., Li I.T.S., Hua B., Hao Q., Holehouse A.S., Reyer M., Sun Q., Freier S.M. Quantitative analysis of multilayer organization of proteins and RNA in nuclear speckles at super resolution. J. Cell Sci. 2017;130:4180–4192. doi: 10.1242/jcs.206854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feric M., Vaidya N., Harmon T.S., Mitrea D.M., Zhu L., Richardson T.M., Kriwacki R.W., Pappu R.V., Brangwynne C.P. Coexisting liquid phases underlie nucleolar subcompartments. Cell. 2016;165:1686–1697. doi: 10.1016/j.cell.2016.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming P.J., Fleming K.G. HullRad: fast calculations of folded and disordered protein and nucleic acid hydrodynamic properties. Biophys. J. 2018;114:856–869. doi: 10.1016/j.bpj.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzmann T.M., Jahnel M., Pozniakovsky A., Mahamid J., Holehouse A.S., Nüske E., Richter D., Baumeister W., Grill S.W., Pappu R.V. Phase separation of a yeast prion protein promotes cellular fitness. Science. 2018;359:eaao5654. doi: 10.1126/science.aao5654. [DOI] [PubMed] [Google Scholar]
- Garcia-Jove Navarro M., Kashida S., Chouaib R., Souquere S., Pierron G., Weil D., Gueroui Z. RNA is a critical element for the sizing and the composition of phase-separated RNA–protein condensates. Nat. Commun. 2019;10:3230. doi: 10.1038/s41467-019-11241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götze M., Dufourt J., Ihling C., Rammelt C., Pierson S., Sambrani N., Temme C., Sinz A., Simonelig M., Wahle E. Translational repression of the Drosophila nanos mRNA involves the RNA helicase Belle and RNA coating by Me31B and Trailer hitch. RNA. 2017;23:1552–1568. doi: 10.1261/rna.062208.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M., Lourido S., Petrova B., Lou H.J., Von Stetina J.R., Kashevsky H., Turk B.E., Orr-Weaver T.L. Identification of PNG kinase substrates uncovers interactions with the translational repressor TRAL in the oocyte-to-embryo transition. eLife. 2018;7:1–19. doi: 10.7554/eLife.33150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofweber M., Dormann D. Friend or foe-Post-translational modifications as regulators of phase separation and RNP granule dynamics. J. Biol. Chem. 2019;294:7137–7150. doi: 10.1074/jbc.TM118.001189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holehouse A.S., Das R.K., Ahad J.N., Richardson M.O.G., Pappu R.V. CIDER: resources to analyze sequence-ensemble relationships of intrinsically disordered proteins. Biophys. J. 2017;112:16–21. doi: 10.1016/j.bpj.2016.11.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holehouse A.S., Garai K., Lyle N., Vitalis A., Pappu R.V. Quantitative assessments of the distinct contributions of polypeptide backbone amides versus side chain groups to chain expansion via chemical denaturation. J. Am. Chem. Soc. 2015;137:2984–2995. doi: 10.1021/ja512062h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondele M., Sachdev R., Heinrich S., Wang J., Vallotton P., Fontoura B.M.A., Weis K. DEAD-box ATPases are global regulators of phase-separated organelles. Nature. 2019;573:144–148. doi: 10.1038/s41586-019-1502-y. [DOI] [PubMed] [Google Scholar]
- Hubstenberger A., Courel M., Bénard M., Souquere S., Ernoult-Lange M., Chouaib R., Yi Z., Morlot J.B., Munier A., Fradet M. P-body purification reveals the condensation of repressed mRNA regulons. Mol. Cell. 2017;68:144–157.e5. doi: 10.1016/j.molcel.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Hubstenberger A., Noble S.L., Cameron C., Evans T.C. Translation repressors, an RNA helicase, and developmental cues control RNP phase transitions during early development. Dev. Cell. 2013;27:161–173. doi: 10.1016/j.devcel.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman A.A., Weber C.A., Julicher F. Liquid-liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 2014;30:39–58. doi: 10.1146/annurev-cellbio-100913-013325. [DOI] [PubMed] [Google Scholar]
- Jahnel M., Behrndt M., Jannasch A., Schäffer E., Grill S.W. Measuring the complete force field of an optical trap. Opt. Lett. 2011;36:1260–1262. doi: 10.1364/OL.36.001260. [DOI] [PubMed] [Google Scholar]
- Jung H., Gkogkas C.G., Sonenberg N., Holt C.E. Remote control of gene function by local translation. Cell. 2014;157:26–40. doi: 10.1016/j.cell.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Kaneuchi T., Sartain C.V., Takeo S., Horner V.L., Buehner N.A., Aigaki T., Wolfner M.F. Calcium waves occur as Drosophila oocytes activate. Proc. Natl. Acad. Sci. USA. 2015;112:791–796. doi: 10.1073/pnas.1420589112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M., Han T.W., Xie S., Shi K., Du X., Wu L.C., Mirzaei H., Goldsmith E.J., Longgood J., Pei J. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell. 2012;149:753–767. doi: 10.1016/j.cell.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Nakamura A. Roles of cytoplasmic RNP granules in intracellular RNA localization and translational control in the Drosophila oocyte. Dev. Growth Differ. 2012;54:19–31. doi: 10.1111/j.1440-169X.2011.01314.x. [DOI] [PubMed] [Google Scholar]
- Kedersha N., Stoecklin G., Ayodele M., Yacono P., Lykke-Andersen J., Fritzler M.J., Scheuner D., Kaufman R.J., Golan D.E., Anderson P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 2005;169:871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloc M., Etkin L.D. RNA localization mechanisms in oocytes. J. Cell Sci. 2005;118:269–282. doi: 10.1242/jcs.01637. [DOI] [PubMed] [Google Scholar]
- Krauchunas A.R., Wolfner M.F. Molecular changes during egg activation. Curr. Top. Dev. Biol. 2013;102:267–292. doi: 10.1016/B978-0-12-416024-8.00010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroschwald S., Maharana S., Mateju D., Malinovska L., Nüske E., Poser I., Richter D., Alberti S. Promiscuous interactions and protein disaggregases determine the material state of stress-inducible RNP granules. eLife. 2015;4:e06807. doi: 10.7554/eLife.06807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroschwald S., Munder M.C., Maharana S., Franzmann T.M., Richter D., Ruer M., Hyman A.A., Alberti S. Different material states of Pub1 condensates define distinct modes of stress adaptation and recovery. Cell Rep. 2018;23:3327–3339. doi: 10.1016/j.celrep.2018.05.041. [DOI] [PubMed] [Google Scholar]
- Lasko P. mRNA localization and translational control in Drosophila oogenesis. Cold Spring Harb. Perspect. Biol. 2012;4:1–15. doi: 10.1101/cshperspect.a012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre R.P., Bogdanova A., Borgonovo B., Woodruff J.B., Drechsel D.N. FlexiBAC: a versatile, open-source baculovirus vector system for protein expression, secretion, and proteolytic processing. BMC Biotechnol. 2019;19:20. doi: 10.1186/s12896-019-0512-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Banjade S., Cheng H.C., Kim S., Chen B., Guo L., Llaguno M., Hollingsworth J.V., King D.S., Banani S.F. Phase transitions in the assembly of multivalent signalling proteins. Nature. 2012;483:336–340. doi: 10.1038/nature10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M.D., Jiao X., Grima D., Newbury S.F., Kiledjian M., Chou T.B. Drosophila processing bodies in oogenesis. Dev. Biol. 2008;322:276–288. doi: 10.1016/j.ydbio.2008.07.033. [DOI] [PubMed] [Google Scholar]
- Luo Y., Na Z., Slavoff S.A. P-bodies: composition, properties, and functions. Biochemistry. 2018;57:2424–2431. doi: 10.1021/acs.biochem.7b01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon A.S., Peeples W.B., Rosen M.K. A framework for understanding the functions of biomolecular condensates across scales. Nat. Rev. Mol. Cell Biol. 2021;22:215–235. doi: 10.1038/s41580-020-00303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A., Cesario W.C., White-Grindley E., Jiang H., Ren F., Khan M.R., Li L., Choi E.-M., Kannan K., Guo F. Critical role of amyloid-like oligomers of Drosophila Orb2 in the persistence of memory. Cell. 2012;148:515–529. doi: 10.1016/j.cell.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Mao A.H., Pappu R.V. Crystal lattice properties fully determine short-range interaction parameters for alkali and halide ions. J. Chem. Phys. 2012;137:064104. doi: 10.1063/1.4742068. [DOI] [PubMed] [Google Scholar]
- Martin E.W., Holehouse A.S., Peran I., Farag M., Incicco J.J., Bremer A., Grace C.R., Soranno A., Pappu R.V., Mittag T. Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. Science. 2020;367:694–699. doi: 10.1126/science.aaw8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E.W., Mittag T. Relationship of sequence and phase separation in protein low-complexity regions. Biochemistry. 2018;57:2478–2487. doi: 10.1021/acs.biochem.8b00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCambridge A., Solanki D., Olchawa N., Govani N., Trinidad J.C., Gao M. Comparative proteomics reveal Me31B’s interactome dynamics, expression regulation, and assembly mechanism into germ granules during Drosophila germline development. Sci. Rep. 2020;10:564. doi: 10.1038/s41598-020-57492-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGibbon R.T., Beauchamp K.A., Harrigan M.P., Klein C., Swails J.M., Hernández C.X., Schwantes C.R., Wang L.P., Lane T.J., Pande V.S. MDTraj: a modern open library for the analysis of molecular dynamics trajectories. Biophys. J. 2015;109:1528–1532. doi: 10.1016/j.bpj.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medioni C., Mowry K., Besse F. Principles and roles of mRNA localization in animal development. Development. 2012;139:3263–3276. doi: 10.1242/dev.078626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrea D.M., Cika J.A., Guy C.S., Ban D., Banerjee P.R., Stanley C.B., Nourse A., Deniz A.A., Kriwacki R.W. Nucleophosmin integrates within the nucleolus via multi-modal interactions with proteins displaying R-rich linear motifs and rRNA. eLife. 2016;5:e13571. doi: 10.7554/eLife.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzo K., Papoulas O., Cantin G.T., Wang Y., Yates J.R., Sisson J.C. Fragile X mental retardation protein controls trailer hitch expression and cleavage furrow formation in Drosophila embryos. Proc. Natl. Acad. Sci. USA. 2006;103:18160–18165. doi: 10.1073/pnas.0606508103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin X., Daneman R., Zavortink M., Chia W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc. Natl. Acad. Sci. USA. 2001;98:15050–15055. doi: 10.1073/pnas.261408198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy A.C., Dignon G.L., Kan Y., Zerze G.H., Parekh S.H., Mittal J., Fawzi N.L. Molecular interactions underlying liquid−liquid phase separation of the FUS low-complexity domain. Nat. Struct. Mol. Biol. 2019;26:637–648. doi: 10.1038/s41594-019-0250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A., Amikura R., Hanyu K., Kobayashi S. Me31B silences translation of oocyte-localizing RNAs through the formation of cytoplasmic RNP complex during Drosophila oogenesis. Development. 2001;128:3233–3242. doi: 10.1242/dev.128.17.3233. [DOI] [PubMed] [Google Scholar]
- Nakamura A., Sato K., Hanyu-Nakamura K. Drosophila cup is an eIF4E binding protein that associates with Bruno and regulates oskar mRNA translation in oogenesis. Dev. Cell. 2004;6:69–78. doi: 10.1016/s1534-5807(03)00400-3. [DOI] [PubMed] [Google Scholar]
- Necci M., Piovesan D., Dosztányi Z., Tosatto S.C.E. MobiDB-lite: fast and highly specific consensus prediction of intrinsic disorder in proteins. Bioinformatics. 2017;33:1402–1404. doi: 10.1093/bioinformatics/btx015. [DOI] [PubMed] [Google Scholar]
- Newcombe E.A., Ruff K.M., Sethi A., Ormsby A.R., Ramdzan Y.M., Fox A., Purcell A.W., Gooley P.R., Pappu R.V., Hatters D.M. Tadpole-like conformations of huntingtin exon 1 are characterized by conformational heterogeneity that persists regardless of polyglutamine length. J. Mol. Biol. 2018;430:1442–1458. doi: 10.1016/j.jmb.2018.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nott T.J., Petsalaki E., Farber P., Jervis D., Fussner E., Plochowietz A., Craggs T.D., Bazett-Jones D.P., Pawson T., Forman-Kay J.D. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol. Cell. 2015;57:936–947. doi: 10.1016/j.molcel.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen I., Shewmaker F. The role of post-translational modifications in the phase transitions of intrinsically disordered proteins. Int. J. Mol. Sci. 2019;20:5501. doi: 10.3390/ijms20215501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak C.W., Kosno M., Holehouse A.S., Padrick S.B., Mittal A., Ali R., Yunus A.A., Liu D.R., Pappu R.V., Rosen M.K. Sequence determinants of intracellular phase separation by complex coacervation of a disordered protein. Mol. Cell. 2016;63:72–85. doi: 10.1016/j.molcel.2016.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R., Sheth U. P bodies and the control of mRNA translation and degradation. Mol. Cell. 2007;25:635–646. doi: 10.1016/j.molcel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Patel A., Lee H.O., Jawerth L., Maharana S., Jahnel M., Hein M.Y., Stoynov S., Mahamid J., Saha S., Franzmann T.M. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell. 2015;162:1066–1077. doi: 10.1016/j.cell.2015.07.047. [DOI] [PubMed] [Google Scholar]
- Patel P.H., Barbee S.A., Blankenship J.T. GW-bodies and P-bodies constitute two separate pools of sequestered non-translating RNAs. PLoS One. 2016;11:e0150291. doi: 10.1371/journal.pone.0150291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S.S., Belmont B.J., Sante J.M., Rexach M.F. Natively unfolded nucleoporins gate protein diffusion across the nuclear pore complex. Cell. 2007;129:83–96. doi: 10.1016/j.cell.2007.01.044. [DOI] [PubMed] [Google Scholar]
- Piovesan D., Necci M., Escobedo N., Monzon A.M., Hatos A., Mičetić I., Quaglia F., Paladin L., Ramasamy P., Dosztányi Z. MobiDB: intrinsically disordered proteins in 2021. Nucleic Acids Res. 2021;49:D361–D367. doi: 10.1093/nar/gkaa1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthanveettil S.V. RNA transport and long-term memory storage. RNA Biol. 2013;10:1765–1770. doi: 10.4161/rna.27391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasethupathy P., Fiumara F., Sheridan R., Betel D., Puthanveettil S.V., Russo J.J., Sander C., Tuschl T., Kandel E. Characterization of small RNAs in Aplysia reveals a role for miR-124 in constraining synaptic plasticity through CREB. Neuron. 2009;63:803–817. doi: 10.1016/j.neuron.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riback J.A., Katanski C.D., Kear-Scott J.L., Pilipenko E.V., Rojek A.E., Sosnick T.R., Drummond D.A. Stress-triggered phase separation is an adaptive, evolutionarily tuned response. Cell. 2017;168:1028–1040.e19. doi: 10.1016/j.cell.2017.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbeck K., Görlich D. The permeability barrier of nuclear pore complexes appears to operate via hydrophobic exclusion. EMBO J. 2002;21:2664–2671. doi: 10.1093/emboj/21.11.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden C., Gladfelter A.S. RNA contributions to the form and function of biomolecular condensates. Nat. Rev. Mol. Cell Biol. 2021;22:183–195. doi: 10.1038/s41580-020-0264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rog O., Köhler S., Dernburg A.F. The synaptonemal complex has liquid crystalline properties and spatially regulates meiotic recombination factors. eLife. 2017;6:e21455. doi: 10.7554/eLife.21455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan M., Weil T.T. Granule regulation by phase separation during Drosophila oogenesis. Emerg. Top. Life Sci. 2020;4:343–352. doi: 10.1042/ETLS20190155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisa J.A., Elaswad M.T. An emerging role for post-translational modifications in regulating RNP condensates in the germ line. Front. Mol. Biosci. 2021;8:658020. doi: 10.3389/fmolb.2021.658020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth U., Parker R. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science. 2003;300:805–808. doi: 10.1126/science.1082320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y., Brangwynne C.P. Liquid phase condensation in cell physiology and disease. Science. 2017;357:eaaf4382. doi: 10.1126/science.aaf4382. [DOI] [PubMed] [Google Scholar]
- Sudhakaran I.P., Ramaswami M. Long-term memory consolidation : the role of RNA- binding proteins with prion-like domains. RNA Biol. 2017;14:568–586. doi: 10.1080/15476286.2016.1244588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros W., Lipshitz H.D. The maternal-to-zygotic transition : a play in two acts. Development. 2009;136:3033–3042. doi: 10.1242/dev.033183. [DOI] [PubMed] [Google Scholar]
- Tauber D., Tauber G., Khong A., Van Treeck B., Pelletier J., Parker R. Modulation of RNA condensation by the DEAD-box protein eIF4A. Cell. 2020;180:411–426.e16. doi: 10.1016/j.cell.2019.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinevez J.Y., Perry N., Schindelin J., Hoopes G.M., Reynolds G.D., Laplantine E., Bednarek S.Y., Shorte S.L., Eliceiri K.W. TrackMate: an open and extensible platform for single-particle tracking. Methods. 2017;115:80–90. doi: 10.1016/j.ymeth.2016.09.016. [DOI] [PubMed] [Google Scholar]
- Tritschler F., Braun J.E., Eulalio A., Truffault V., Izaurralde E., Weichenrieder O. Structural basis for the mutually exclusive anchoring of P Body components EDC3 and Tral to the DEAD box protein DDX6/Me31B. Mol. Cell. 2009;33:661–668. doi: 10.1016/j.molcel.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Tritschler F., Eulalio A., Helms S., Schmidt S., Coles M., Weichenrieder O., Izaurralde E., Truffault V. Similar modes of interaction enable trailer hitch and EDC3 to associate with DCP1 and Me31B in distinct protein complexes. Mol. Cell. Biol. 2008;28:6695–6708. doi: 10.1128/MCB.00759-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritschler F., Eulalio A., Truffault V., Hartmann M.D., Helms S., Schmidt S., Coles M., Izaurralde E., Weichenrieder O. A divergent Sm Fold in EDC3 proteins mediates DCP1 binding and P-body targeting. Mol. Cell. Biol. 2007;27:8600–8611. doi: 10.1128/MCB.01506-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trovisco V., Belaya K., Nashchekin D., Irion U., Sirinakis G., Butler R., Lee J.J., Gavis E.R., St Johnston D. bicoid mRNA localises to the Drosophila oocyte anterior by random Dynein-mediated transport and anchoring. eLife. 2016;5:e17537. doi: 10.7554/eLife.17537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitalis A., Pappu R.V. ABSINTH: a new continuum solvation model for simulations of polypeptides in aqueous solutions. J. Comput. Chem. 2009;30:673–699. doi: 10.1002/jcc.21005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.T., Smith J., Chen B.C., Schmidt H., Rasoloson D., Paix A., Lambrus B.G., Calidas D., Betzig E., Seydoux G. Regulation of RNA granule dynamics by phosphorylation of serine-rich, intrinsically disordered proteins in C. elegans. eLife. 2014;3:e04591. doi: 10.7554/eLife.04591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Ly M., Lugowski A., Laver J.D., Lipshitz H.D., Smibert C.A., Rissland O.S. ME31B globally represses maternal mRNAs by two distinct mechanisms during the Drosophila maternal-to-zygotic transition. eLife. 2017;6:1–22. doi: 10.7554/eLife.27891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse A., Bertoni M., Bienert S., Studer G., Tauriello G., Gumienny R., Heer F.T., de Beer T.A.P., Rempfer C., Bordoli L. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–W303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber S.C. Sequence-encoded material properties dictate the structure and function of nuclear bodies. Curr. Opin. Cell Biol. 2017;46:62–71. doi: 10.1016/j.ceb.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Weber S.C., Brangwynne C.P. Getting RNA and protein in phase. Cell. 2012;149:1188–1191. doi: 10.1016/j.cell.2012.05.022. [DOI] [PubMed] [Google Scholar]
- Weil T.T., Forrest K.M., Gavis E.R. Localization of bicoid mRNA in late oocytes is maintained by continual active transport. Dev. Cell. 2006;11:251–262. doi: 10.1016/j.devcel.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Weil T.T., Parton R., Davis I., Gavis E.R. Changes in bicoid mRNA anchoring highlight conserved mechanisms during the oocyte-to-embryo transition. Curr. Biol. 2008;18:1055–1061. doi: 10.1016/j.cub.2008.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil T.T., Parton R.M., Davis I. Preparing individual Drosophila egg chambers for live imaging. J. Vis. Exp. 2012;60:e3679. doi: 10.3791/3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil T.T., Parton R.M., Herpers B., Soetaert J., Veenendaal T., Xanthakis D., Dobbie I.M., Halstead J.M., Hayashi R., Rabouille C., Davis I. Drosophila patterning is established by differential association of mRNAs with P bodies. Nat. Cell Biol. 2012;14:1305–1313. doi: 10.1038/ncb2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler J.R., Matheny T., Jain S., Abrisch R., Parker R. Distinct stages in stress granule assembly and disassembly. eLife. 2016;5:e18413. doi: 10.7554/eLife.18413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm J.E., Buszczak M., Sayles S. Efficient protein trafficking requires trailer hitch, a component of a ribonucleoprotein complex localized to the ER in Drosophila. Dev. Cell. 2005;9:675–685. doi: 10.1016/j.devcel.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Woodruff J.B., Ferreira Gomes B., Widlund P.O., Mahamid J., Honigmann A., Hyman A.A. The centrosome is a selective condensate that nucleates microtubules by concentrating tubulin. Cell. 2017;169:1066–1077.e10. doi: 10.1016/j.cell.2017.05.028. [DOI] [PubMed] [Google Scholar]
- York-Andersen A.H., Hu Q., Wood B.W., Wolfner M.F., Weil T.T. A calcium-mediated actin redistribution at egg activation in Drosophila. Mol. Reprod. Dev. 2020;87:293–304. doi: 10.1002/mrd.23311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York-Andersen A.H., Parton R.M., Bi C.J., Bromley C.L., Davis I., Weil T.T. A single and rapid calcium wave at egg activation in Drosophila. Biol. Open. 2015;4:553–560. doi: 10.1242/bio.201411296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavortink M., Rutt L.N., Dzitoyeva S., Henriksen J.C., Barrington C., Bilodeau D.Y., Wang M., Chen X.X.L., Rissland O.S. The E2 Marie Kondo and the CTLH E3 ligase clear deposited RNA binding proteins during the maternal-to-zygotic transition. eLife. 2020;9:e53889. doi: 10.7554/eLife.53889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Elbaum-Garfinkle S., Langdon E.M., Taylor N., Occhipinti P., Bridges A.A., Brangwynne C.P., Gladfelter A.S. RNA controls PolyQ protein phase transitions. Mol Cell. 2015;60:220–230. doi: 10.1016/j.molcel.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Document S1. Figures S1–S5 and Tables S1
Document S2. Article plus supplemental information
Data Availability Statement
- •
All other data reported in this paper will be shared by the lead contact upon request. - •
All original code has been deposited at [https://github.com/holehouse-lab/supportingdata/tree/master/2021/sankaranarayanan_me31b_2021] and is publicly available as of the date of publication. - •
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.