Role of Histone N-Terminal Tails and Their Acetylation in Nucleosome Dynamics (original) (raw)
Abstract
Histone N-terminal tails are central to the processes that modulate nucleosome structure and function. We have studied the contribution of core histone tails to the structure of a single nucleosome and to a histone (H3-H4)2 tetrameric particle assembled on a topologically constrained DNA minicircle. The effect of histone tail cleavage and histone tail acetylation on the structure of the nucleoprotein particle was investigated by analyzing the DNA topoisomer equilibrium after relaxation of DNA torsional stress by topoisomerase I. Removal of the H3 and H4 N-terminal tails, as well as their acetylation, provoked a dramatic change in the linking-number difference of the (H3-H4)2 tetrameric particle, with a release of up to 70% of the negative supercoiling previously constrained by this structure. The (H3-H4)2 tetramers containing tailless or hyperacetylated histones showed a striking preference for relaxed DNA over negatively supercoiled DNA. This argues in favor of a change in tetramer structure that constrains less DNA and adopts a relaxed flat conformation instead of its left-handed conformation within the nucleosome. In contrast neither removal or hyperacetylation of H3 and H4 tails nor removal or hyperacetylation of H2A and H2B N-terminal tails affected the nucleosome structure. This indicates that the globular domain of H2A and H2B is sufficient to stabilize the tailless or the hyperacetylated (H3-H4)2 tetramer in a left-handed superhelix conformation. These results suggest that the effect of histone tail acetylation that facilitates transcription may be mediated via transient formation of an (H3-H4)2 tetrameric particle that could adopt an open structure only when H3 and/or H4 tails are hyperacetylated.
In eukaryotes, chromatin structure plays a major role in all aspects of DNA metabolism including transcription, replication, and repair. Changes in accessibility of DNA to nucleases in response to different stimuli reveal that its structure is dynamic. This involves alteration in composition and structure of chromatin fibers and nucleosomes. A nucleosome, the fundamental repeating structural unit of chromatin, consists of an octamer of core histones and 147 bp of DNA wrapped around the octamer in a left-handed superhelix. The histone octamer has a tripartite structure (1), which is organized as a histone (H3-H4)2 tetramer flanked by two H2A-H2B dimers (47). Core histones display N-terminal tails whose sequences are highly conserved from yeast to human. Genetic studies with yeast cells have demonstrated that these tails are essential, since the simultaneous deletion of H3 and H4 tails or the simultaneous deletion of H2A and H2B tails is lethal (25). Involvement of histone tails in the control of gene expression may result from two nonmutually exclusive mechanisms: (i) changes in structure or composition of the nucleosome and/or the chromatin fiber and (ii) modulation of the interaction of histone tails with regulatory factors (19).
The N-terminal tails of the four core histones are targets for posttranslational modifications such as acetylation, methylation, and phosphorylation (for a review, see reference 10) that correlate with changes in gene activity. It is usually proposed that histone tail acetylation, which takes place on lysines, results in a change in chromatin structure (52). Contribution of histone tails to nucleosome structure and nucleosome arrays has been demonstrated (2, 14, 15, 45), but the mechanisms involved in acetylation-mediated transcriptional regulation remain to be elucidated.
High-resolution crystal structure analysis of nucleosome core particles did not show organized domains within the protruding N-terminal tails. Five amino acids of the H3 N-terminal tail and eight amino acids of the H2B N-terminal tail, which form a random coil segment, pass between the gyres of the DNA superhelix; in addition, four amino acids of the H2A N-terminal tail are bound to the minor groove on the outside of the superhelix (27). In yeast cells, it has been shown that these regions of the tails (immediately adjacent to helix α1 of H2A, H2B, and H4) that contact DNA are involved in the repression of basal transcription (24), suggesting that these parts of the histone tails contribute to nucleosome structure. However, these tail regions are not post-translationally modified, making unlikely their involvement in gene activity modulation via histone acetylation. Contrasting with the X-ray crystallography results, circular-dichroism experiments revealed that the N-terminal tails of H3 and H4 adopt a highly structured conformation in the nucleosome (4). The apparently contradictory results from structural studies on the organization of the distal part of the tails do not allow any conclusion with respect to their possible contribution to the structure of the nucleosome or the chromatin fiber.
Transient changes in nucleosome composition may provide another mechanism of local modulations of chromatin structure. The lability of the interaction between H2A-H2B dimers and (H3-H4)2 tetramers may have a significance for the nucleosome-conformational changes observed in cells. Indeed, actively transcribing chromatin is depleted in H2A-H2B dimers (3) that are rapidly exchanged (21, 26). Factors such as nucleoplasmin or NAP1 interact with H2A-H2B dimers and stimulate the binding of regulatory factors to their cognate DNA targets assembled in chromatin via, most probably, removal of the H2A-H2B dimers (7, 50). Furthermore, FACT (for facilitates chromatin transcription), a protein complex that facilitates chromatin-specific transcription elongation (35), also interacts preferentially with H2A-H2B dimers within the nucleosome, suggesting that nucleosome structures depleted in H2A-H2B dimers might be formed in cells by chromatin-remodeling complexes. Supporting this last hypothesis, deletion of one of the H2A or H2B genes suppresses in vivo the defects due to mutations of the SWI/SNF chromatin-remodeling complex (20).
We investigated the contribution of histone tails to the structure of a single nucleosome or (H3-H4)2 tetrameric particle that may represent an intermediate in chromatin remodeling. Nucleoprotein complexes were assembled on topologically constrained DNA minicircles. DNA topology analysis provided a sensitive method to detect structural changes in DNA associated with the core particle (51). Our results show that neither removal of the four histone N-terminal tails nor their acetylation had any significant effect on the nucleosome structure. In contrast, removal of the tails of histones H3 and H4 as well as their acetylation had a dramatic effect on the structure of the (H3-H4)2 tetrameric particle. These findings suggest that the effect of histone acetylation on chromatin structure may involve a transient formation of (H3-H4)2 tetrameric particles.
MATERIALS AND METHODS
Tissue culture and histone acetylation.
Jurkat cells were grown in RPMI medium supplemented with 5% fetal calf serum and antibiotics up to a density of 450,000 cells/ml. Trichostatin A (TSA, 500 ng/ml) was added, and the cells were collected after 10 h (final cell density, 800,000 cells/ml). L12-10 cells were grown in RPMI medium supplemented with 10% fetal calf serum, glutamine, and antibiotics up to a density of 106 cells/ml and treated with 10 mM butyrate for 18 h, and the cells were harvested (final cell density, 2.5 × 106 cells/ml).
Preparation of topologically constrained minicircles.
To generate minicircles, we used a 359-bp fragment that originates from a _Bam_HI digest of plasmid pUC(359.3). This fragment contains 256 bp of 5S ribosomal DNA and was derived from the 357-bp fragment described in reference 11. The 357-bp fragment was end filled at the unique _Taq_I site to generate the 359-bp fragment. The 359-bp fragment was cloned as a tandem repeat in pUC18 at the _Bam_HI site generating the construct pUC(359.3). After 32P end labeling, the fragment was purified, and the different DNA topoisomers were prepared as described in reference 54.
Chromatin preparation.
Nuclei were isolated from duck erythrocytes, according to the method of Bates et al. (5). Purified nuclei were suspended in 15 mM Tris HCl (pH 7.5)–15 mM NaCl–60 mM KCl–0.15 mM spermine–0.5 mM spermidine–10 mM β-mercaptoethanol–0.25 mM phenylmethylsulfonyl fluoride (PMSF)–0.1 mM EDTA–0.34 M sucrose. The nucleus suspension (at an optical density at 600 nm [OD600] of 10 to 15) was adjusted to 1 mM CaCl2, and the nuclei were digested by micrococcal nuclease to produce mainly mono- and dinucleosomes (typically 100 U of micrococcal nuclease per ml for 10 min at 37°C). The digestion was stopped by addition of EDTA (5 mM final concentration), and nuclei were collected and lysed in 1 mM EDTA. The supernatant containing the soluble chromatin was collected by centrifugation and adjusted to 650 mM NaCl by dropwise addition of a 5 M solution to dissociate H1. Soluble chromatin (500 μl) was layered on a 30-ml 5 to 28% sucrose gradient in 10 mM Tris HCl (pH 7.5)–1 mM EDTA–650 mM NaCl–5 mM β-mercaptoethanol–0.2 mM PMSF and centrifuged in an SW28 rotor (Beckman) for 20 h at 27,500 rpm at 6°C. After centrifugation the fractions containing the mono- and dinucleosomes were pooled, dialyzed against 20 mM KPO4 (pH 7.4)–20 mM NaCl–5 mM β-mercaptoethanol and concentrated with polyethylene glycol (PEG) 6-8000 to reach an OD260 between 15 and 20. Samples were dialyzed against the same buffer.
Digestion of the core particles with clostripain.
Clostripain (Sigma) was solubilized in 1 mM calcium acetate–2.5 mM dithiothreitol and left overnight at 4°C, and histone N-terminal tail cleavage was performed as described in reference 13. Treatment of the core particles for 30 min at 37°C with 1 U of clostripain per mg of histone resulted in the removal of H3-H4 N-terminal tails, along with three amino acids of the H2A N-terminal tail (mild clostripain digestion). Treatment of the core particles for 1 h at 37°C with 20 U of clostripain per mg of histone removed H2A and H2B N-terminal tails but also generated internal cuts in H3-H4 (extensive clostripain digestion). Digestion was stopped by addition of 1 mM TLCK (_N_α-_p_-tosyl-l-lysine chloromethyl ketone [Sigma]).
Histone purification.
Intact, acetylated or cleaved histones were purified by hydroxylapatite chromatography as described in reference 17. H2A-H2B dimers and (H3-H4)2 tetramers were concentrated by centrifugation in Centricon 10 microconcentrators (Amicon). Samples were dialyzed against 10 mM Tris HCl (pH 7.5)–2 M NaCl–5 mM MgCl2–0.2 mM EDTA–0.2 mM PMSF–0.5 mM β-mercaptoethanol. Histone preparations were aliquoted and stored at −80°C.
Chromatin reconstitution.
To generate nucleosomes and (H3-H4)2 tetrameric nucleoprotein particles containing either intact, acetylated or tailless histones, histone octamers or tetramers were assembled on topologically constrained DNA circles according to the salt jump method (43) as described in reference 18, using a histone-DNA weight ratio (_r_W) of 0.2. To investigate the influence of either deletion or acetylation of all the N-terminal tails on the nucleosome particle prior to particle assembly, purified intact or tailless (H3-H4)2 tetramers were recombined with purified, intact or tailless H2A-H2B dimers. Nucleosomes and tetrameric particles were assembled on topoisomer −3 or −2. The choice of a particular topoisomer was based on the fact that it is not present in the final topoisomer equilibrium after relaxation of the particle with topoisomerase I. This avoids errors on the calculation of DNA linking number difference (ΔLkP) due to contaminating incompletely relaxed particles. For relaxation studies, nucleoprotein particle preparations were adjusted to 50 mM Tris HCl (pH 7.5)–0.1 mM EDTA–50 mM KCl–5 mM MgCl2–100 μg of bovine serum albumin per ml, and the DNA was relaxed by incubation with 800 to 1,000 U of calf thymus topoisomerase I (Gibco BRL-Life Technologies)/ml at 37°C for 1 h (53).
PAGE.
Purified histones were characterized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE). The level of acetylation of H3 and H4 was determined with acid-urea acrylamide gels (36). Proteins were visualized either with a fluoroimager (Molecular Dynamics) using Sypro orange (Interchim) staining or by Coomassie blue staining.
Octameric and tetrameric nucleoprotein particles were subjected to electrophoresis at room temperature in 4% polyacrylamide (acrylamide-bisacrylamide, 29:1 [wt/wt]) slab gels (0.15 by 17 by 18 cm) in Tris-EDTA buffer. Gels were prerun for 1 h at 200 to 250 V before samples were loaded, and electrophoresis was then performed at the same voltage for 3 to 4 h with extensive buffer recirculation. Gels were dried and autoradiographed at −80°C. In preparative relaxation experiments, the amounts of samples loaded were different for unrelaxed (controls) and relaxed particles. To be able to purify the DNA topoisomers from the relaxed particles, fourfold more material than for the controls was loaded. These “chromatin” gels were dried without heating to allow reswelling of excised gel slices and elution of DNA. Unless otherwise stated, naked DNA was electrophoresed at room temperature in 4% polyacrylamide (acrylamide-bisacrylamide, 19:1 [wt/wt]) slab minigels (0.15 by 10 by 8 cm) for 2 h at 100 V in 20 mM sodium acetate–2 mM EDTA–40 mM Tris-acetate (pH 7.8). When required for the separation of DNA topoisomers, chloroquine (125 μM) was included. The radioactivity in the bands was quantitated in the dried gels using a phosphorimager (Fuji PC-Bas).
Calculation of ΔLkP.
ΔLkP was calculated from the amounts of the different topoisomers after relaxation of the particle, as previously described (17), using an Lk0 of 34 for the 359-bp fragment. The number n of independent reconstitution experiments is indicated in Table 1.
TABLE 1.
Comparison of ΔLkP values for the different nucleoprotein particles
| Particle | Mean ΔLkP ± SD (n) |
|---|---|
| Nucleosome (duck erythrocytes) | −1.05 ± 0.020 (7) |
| Nucleosome (mouse L12-10 cells) | −1.03 ± 0.010 (2) |
| Nucleosome, tailless H3-H4 | −1.03 ± 0.016 (5) |
| Nucleosome, tailless H2A-H2B | −1.03 ± 0.003 (2) |
| Nucleosome, tailless H3-H4, H2A-H2B | −1.02 ± 0.013 (2) |
| Nucleosome, butyrate treated | −1.05 ± 0.016 (2) |
| Nucleosome, TSA treated | −1.03 ± 0.004 (2) |
| Tetrameric particle | −0.62 ± 0.060 (8) |
| Tetrameric particle, tailless H3-H4 | −0.16 ± 0.060 (3) |
| Tetrameric particle, TSA treated | −0.37 ± 0.030 (3) |
| Tetrameric particle, butyrate treated | −0.27 ± 0.001 (2) |
RESULTS
Cleavage of histone N-terminal tails by clostripain.
To remove specifically N-terminal tails of all four core histones, we used clostripain, a protease that cuts preferentially the four histone N-terminal tails and leaves intact the tail regions preceding the first α-helix that contacts the DNA and the C-terminal tails (13).
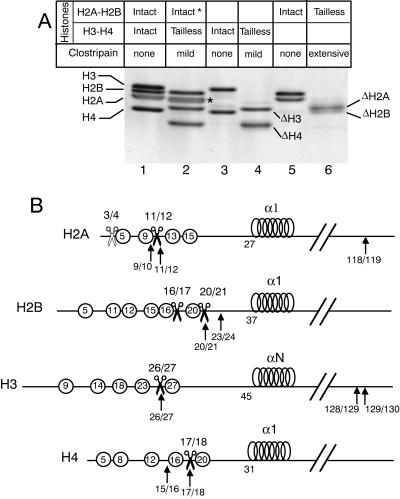
A purified chromatin fraction containing mono- and dinucleosomes was treated with clostripain and analyzed by sodium dodecyl sulfate-PAGE (Fig. 1A). As expected (13), mild treatment with clostripain resulted in quantitative conversion of H3 and H4 (Fig. 1A, lane 1) to faster migrating species (Fig. 1A, lane 2) that reflect the specific removal of histone H3 and H4 tails. Note that under the experimental conditions used, the band corresponding to H2A migrated as a doublet (Fig. 1A, lane 2, band marked with a star), while migration of the band corresponding to H2B remained unchanged. This shows the partial removal of 3 amino acids from the H2A tail (Fig. 1A and B) while H2B remained intact (13). These results confirm that a mild clostripain treatment of the core particles can be used to obtain histone preparations containing tailless H3 and H4 without any significant digestion of H2A and H2B (13).
FIG. 1.
Histone tail cleavage after clostripain treatment of core particles. (A) PAGE analysis of intact and clostripain-treated histones. Lane 1, control histone octamers; lane 2, mild clostripain digestion of core particles, resulting in the removal of H3 and H4 N-terminal tails and of the partial removal of three amino acids from H2A tail (*); lane 3, control (H3-H4)2 tetramer; lane 4, purified tailless (H3-H4)2 tetramer; lane 5, control H2A-H2B dimers; lane 6, tailless H2A-H2B dimers purified from core particles submitted to extensive clostripain digestion. (B) Schematic representation of the four histones. The circles with numbers indicate the position of lysines. The scissors mark the clostripain cutting sites. For H2A, white scissors, mild clostripain treatment; black scissors, extensive clostripain treatment. The black arrows mark the trypsin cutting sites. The positions of the cutting sites are from reference 12.
All our attempts failed to remove simultaneously and completely the tails of the four histones by digesting core particles with clostripain. Indeed, upon removal of H3-H4 tails, there was almost no digestion of H2A-H2B tails, and on the other hand, extensive clostripain digestion, allowing the removal of H2A-H2B tails, generated internal cuts in H3. To overcome this problem, we performed separate mild and extensive clostripain digestions of the core particles (Fig. 1A). The mild digestion allowed us to purify tailless H3-H4 (Fig. 1A, lane 4), whereas the extensive digestion permitted the production of tailless H2A-H2B (Fig. 1A, lane 6). For H2A and H2B, the exact positions of the cuts after extensive clostripain treatment were determined by sequencing the N-terminal regions of the proteins after separation by acrylamide gel electrophoresis. H2A was cut at a single position between amino acids 11 and 12, while H2B underwent two cuts between amino acids 16 and 17 and 20 and 21, generating a mixture of two cleaved H2B subpopulations. To obtain octamers containing the four histones with all N-terminal tails cleaved, it was necessary to produce separately tailless H3-H4 (mild clostripain treatment) and tailless H2A-H2B (extensive clostripain treatment) and recombine them after purification.
H3 and H4 tails stabilize (H3-H4)2 tetramer structure in a left-handed conformation.
Changes in DNA topology, such as ΔLkP of DNA minicircles wrapped around a histone surface, can be used to study DNA structural changes due to protein binding (51). Furthermore, this approach can be used to monitor changes of protein conformation that result in consequent changes in DNA topology (37). Nucleoprotein particles were assembled on a constrained DNA minicircle, and then the remaining torsional stress was relaxed by topoisomerase I. After deproteinization and restoration of the torsional stress absorbed by the particle, DNA topoisomers obtained were analyzed by PAGE and quantified.
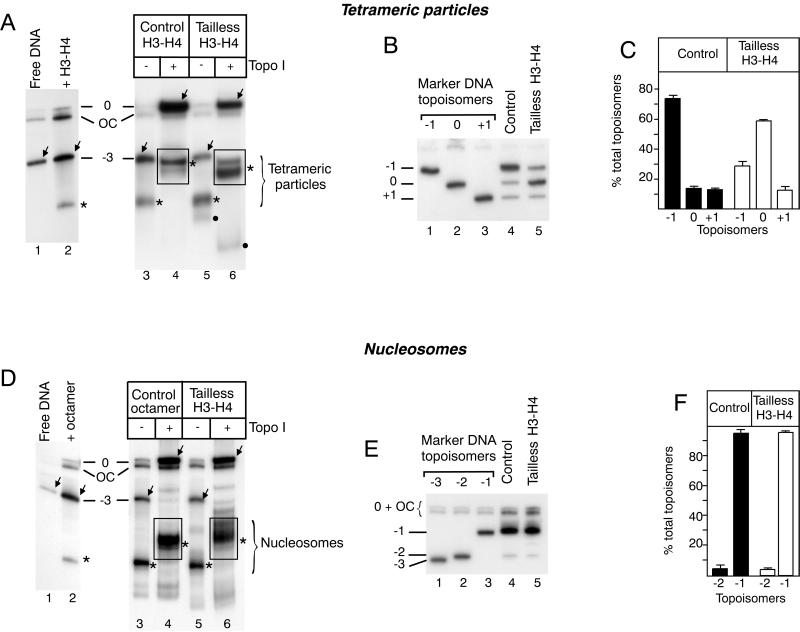
Since the (H3-H4)2 tetramer plays a central role in the establishment of nucleosomal structure (39) and since a structural transition of the tetrameric particle has been demonstrated (16, 17), we investigated the influence of histone tails on the structure of the tetrameric particle (Fig. 2A through C). Intact (H3-H4)2 tetramers (Fig. 2A, lanes 2, 3, and 4) or (H3-H4)2 tetramers containing tailless H3-H4 (Fig. 2A, lanes 5 and 6) were assembled on DNA minicircles. Following DNA relaxation by topoisomerase I, a quantitative mobility shift of both the nucleoprotein particle and free DNA was observed (Fig. 2A, compare lanes 3 and 4 to lanes 5 and 6). The bands corresponding to the relaxed nucleoprotein complexes were eluted and deproteinized, and topoisomer equilibrium (reflecting thermal fluctuations of the structure) was analyzed by electrophoresis. Topoisomerase I treatment of particles containing intact tetramers resulted in the appearance of a characteristic set of DNA topoisomers (Fig. 2B [lane 4], Fig. 2C, and Table 1) with a majority (74% ± 5% of the total 32P label) of the topoisomer containing one negative superhelical turn (topoisomer −1), along with approximately the same amount of topoisomers containing one positive superhelical turn (topoisomer +1; 11% ± 2%) or no superhelical turn (topoisomer 0; 15% ± 4%). Since the tetramer can adopt several structural conformations (left handed, flat, and right handed) and accommodates both negative and positive DNA supercoiling (16), this result indicates that the majority of the intact tetrameric particle adopts a left-handed conformation. When the clostripain-treated tetramers were used, topoisomerase I relaxation revealed a dramatic change in the topoisomer equilibrium (Fig. 2B [lane 5] and 2C) with a majority of topoisomer 0 (58% ± 1% of the total 32P label). The ΔLkP values were calculated for each independent relaxation experiment, from the amounts of topoisomers, as previously described (17). These values are summarized in Table 1. The dramatic change in the ΔLkP values resulting from H3-H4 N-terminal tail cleavage suggests a structural transition of the tetramer from a left-handed to a more relaxed flat conformation in the absence of these tails.
FIG. 2.
Effect of N-terminal histone tails on the structure of tetrameric (A through C) and nucleosomal (D through F) particles. (A) Intact (H3-H4)2 tetramers (lanes 2 through 4) and tailless (H3-H4)2 (lanes 5 and 6) were purified, and tetrameric particles were reconstituted on DNA topoisomers −3. After reconstitution, samples were treated with topoisomerase I (lanes 4 and 6). The bands corresponding to the nucleoprotein particles are marked with stars, and those corresponding to free DNA are marked with arrows. The bands within the rectangles were excised, and DNA was purified to analyze the DNA topoisomer equilibrium obtained after relaxation. The faint bands labeled with black dots in lanes 5 and 6 correspond to tetramer aggregates. (B) DNA topoisomer analysis. The electrophoretic migration was performed in the presence of 125 μM chloroquine to resolve DNA topoisomer 0 from open circles. The migration positions of the different naked DNA topoisomers are indicated on the left. Lanes 1 through 3, marker DNA topoisomers; lane 4, control sample; lane 5, tailless tetrameric particle. (C) Quantitation of topoisomers. The same data were used for the calculations of ΔLkP values summarized in Table 1. The number of independent experiments is indicated in the table. (D) Control histone octamers (lanes 2 through 4) and core histones containing tailless H3 and H4 (lanes 5 and 6) were assembled on DNA topoisomer −3. Samples were untreated (lanes 3 and 5) or treated with topoisomerase I (lanes 4 and 6) to relax the extranucleosomal DNA loop. The bands within the rectangles (lanes 4 and 6) were treated as described for panel A. Labeling of the bands is the same as in panel A. (E) DNA topoisomer analysis. Lanes 1 through 3, marker DNA topoisomers; lane 4, control sample; lane 5, sample from clostripain-treated histones. (F) Quantitation of the topoisomers, as in panel C.
The H2A-H2B dimers stabilize the nucleosome in a left-handed conformation, regardless of the presence of histone H3 and H4 tails.
We then investigated the effect of H2A-H2B on H3-H4 N-terminal tail-mediated stabilization of the left-handed conformation (Fig. 2D through F). Intact histone octamers (Fig. 2D, lanes 2, 3, and 4) and octamers containing tailless H3 and H4 (Fig. 2D, lanes 5 and 6) were analyzed using the same experimental design (Fig. 2E). In the presence as well as in the absence of H3-H4 tails, DNA topoisomer −1 represented, respectively, 95% ± 2.9 and 96% ± 1% of total DNA. Although in the same experiment tail cleavage always led to a slight variation in topoisomer distribution resulting in a decrease in ΔLkP values, the statistical analysis of the results did not show a significant change in topoisomer equilibrium (Fig. 2F) and ΔLkP values (Table 1). This result indicates that in the presence of H2A-H2B dimers, the H3 and H4 N-terminal tails do not contribute significantly to mononucleosome structure.
H2A and H2B tails do not contribute to the stabilization of the nucleosome in a left-handed conformation.
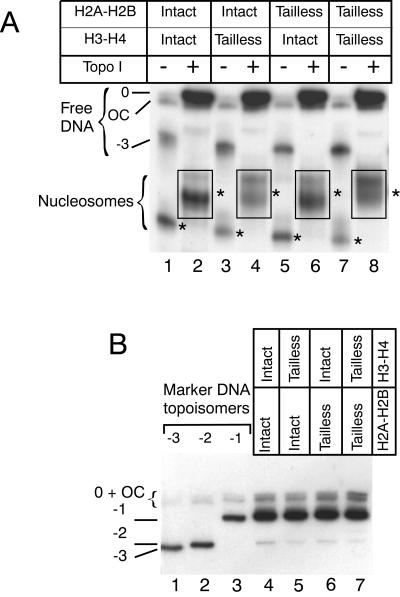
The left-handed conformation of a nucleosome containing a tailless (H3-H4)2 tetramer may result from a contribution of H2A-H2B dimer tails to the structure of the particle. To investigate the contribution of H2A and H2B N-terminal tails to the nucleosome structure, we purified intact and tailless (H3-H4)2 tetramers and H2A-H2B dimers (Fig. 1). We used them to reconstitute, on topologically constrained DNA minicircles, particles containing either tailless or intact (H3-H4)2 tetramers, tailless or intact H2A-H2B dimers, or both (Fig. 3). After relaxation with topoisomerase I (Fig. 3A, lanes 2, 4, 6, and 8), the topoisomer equilibrium was analyzed (Fig. 3B). Neither the removal of H3-H4 tails (Fig. 3B, lane 5) nor the removal of H2A-H2B tails (Fig. 3B, lane 6) nor the simultaneous removal of all histone tails (Fig. 3B, lane 7) resulted in a change of DNA topoisomer equilibrium. The removal of all histone tails had no significant effect on the structure of a single nucleosome, demonstrating that the globular parts of H2A and/or H2B are sufficient to overcome the effect of H3 and/or H4 tail cleavage.
FIG. 3.
Role of N-terminal histone tails in the structure of a nucleosome. (A) Nucleosomes were assembled on DNA topoisomer −3, using intact histones (lanes 1 and 2), tailless (H3-H4)2 tetramers and intact H2A-H2B dimers (lanes 3 and 4), intact (H3-H4)2 tetramers and tailless H2A-H2B dimers (lanes 5 and 6), and tailless (H3-H4)2 tetramers and tailless H2A-H2B dimers (lanes 7 and 8). Samples in lanes 2, 4, 6, and 8 were treated with topoisomerase I. The bands corresponding to the nucleoprotein particles are labeled with stars. The bands within the rectangles were excised, and the DNA topoisomer equilibrium was analyzed as described in Fig. 2. (B) DNA topoisomer analysis. The analysis was performed as described in the legend to Fig. 2. Lanes 1 through 3, marker DNA topoisomers. The migration position of the different naked DNA topoisomers is indicated on the left, and labeling is the same as in Fig. 2.
Histone tail acetylation affects the structure of the tetrameric particle but not the structure of the nucleosome.
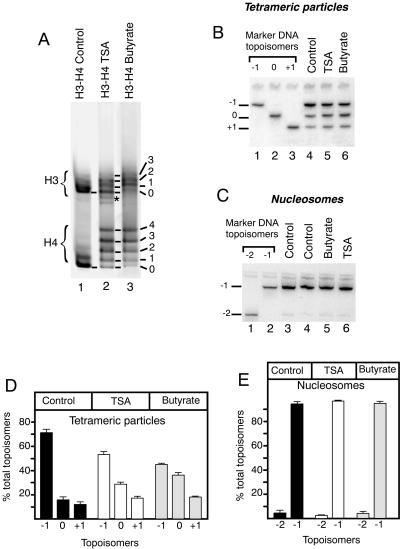
To investigate the effects of histone tail acetylation on the structure of a single nucleosome, we used the same methodology as for the investigation of the contribution of histone tails to the nucleoprotein particle structure. Hyperacetylated histones were isolated from cells treated with a histone deacetylase inhibitor, either TSA for 10 h or butyrate for 18 h. (H3-H4)2 tetramers and H2A-H2B dimers were purified separately. The acetylation levels of H3 and H4 were monitored by acid-urea acrylamide gel electrophoresis (Fig. 4A, lanes 2 and 3). Both treatments led to hyperacetylation of H3 and H4, containing up to four acetyl residues per H4 molecule. As a result of the difference in treatment time with the histone deacetylase inhibitors, H3 and H4 isolated from cells treated with butyrate were acetylated to a higher extent than those isolated from TSA-treated cells (Fig. 4A, compare lanes 2 and 3). These histones were used to reconstitute tetrameric particles on DNA topoisomer −2, and the topoisomer equilibrium was analyzed after relaxation (Fig. 4B). Comparison of Fig. 4B lanes 5 and 6 with lane 4 revealed a significant change in topoisomer distribution (summarized in Fig. 4D). Compared to the control, the acetylation of H3-H4 tails led to a decrease in the amount of DNA topoisomer −1 (70% ± 3% of the total DNA for the control, 54% ± 3% for histones from TSA-treated cells, and 45% ± 1% for histones from butyrate-treated cells). This was accompanied by a concomitant increase in the proportions of topoisomers 0 (19% ± 1% of the total DNA for the control, 29% ± 1% for histones from TSA-treated cells, and 36% ± 2% for histones from butyrate-treated cells) and +1 (11% ± 2% of the total DNA for the control, 17% ± 4% for histones from TSA-treated cells, and 18% ± 1% for histones from butyrate-treated cells). The change in topoisomer distribution was more pronounced with the histones isolated from the highly acetylated butyrate-treated cells than with histones isolated from TSA-treated cells that were acetylated at a lesser extent. This resulted in significant changes in ΔLkP values (Table 1).
FIG. 4.
Effect of histone N-terminal tail acetylation on the structure of tetrameric particles and nucleosomes. (A) Characterization of acetylated histones by acetate-urea PAGE. Lane 1, control hypoacetylated duck (H3-H4)2 tetramers; lane 2, (H3-H4)2 tetramers purified from Jurkat cells treated with TSA; lane 3, (H3-H4)2 tetramers purified from mouse L1210 cells treated with butyrate. The star marks a slight contamination with H2A-H2B dimers in the sample isolated from the TSA-treated cells. The numbers refer to the number of acetyl groups per histone molecule. (B) Analysis of DNA topoisomer equilibrium after relaxation of tetrameric particles assembled on DNA topoisomer −2; lane 4, control hypoacetylated tetramers (duck histones); lane 5, tetramers from TSA-treated cells; lane 6, tetramers from butyrate-treated cells; lanes 1 through 3, marker DNA topoisomers. (C) Analysis of DNA topoisomer equilibrium after relaxation of nucleosomes assembled on DNA topoisomer −3. Lane 3, control hypoacetylated nucleosomes (duck histones); lane 4, control hypoacetylated nucleosomes (histones from L1210 cells); lane 5, hyperacetylated nucleosomes (histone octamers from L1210 cells treated by butyrate); lane 6, hyperacetylated nucleosomes (tetramers and dimers from TSA-treated Jurkat cells); lanes 1 and 2, marker DNA topoisomers. (D through E) Quantitation of topoisomers after relaxation of tetrameric particles and nucleosomes, respectively. For details see the legend to Fig. 2C.
To determine if H2A-H2B dimers were able to counteract the effect of histone tail acetylation, as they did for tail cleavage, we reconstituted nucleosomes on DNA topoisomer −3, using histone octamers purified from duck erythrocytes (Fig. 4C, lane 3), from untreated L1210 cells (Fig. 4C, lane 4), from butyrate-treated L1210 cells (lane 5), or (H3-H4)2 tetramers and H2A-H2B dimers purified separately from TSA-treated Jurkat cells (Fig. 4C, lane 6). After assembly and relaxation with topoisomerase I, the DNA topoisomers were quantitated (Fig. 4E) and found to be very similar. The main component was topoisomer −1, along with DNA topoisomer −2. Topoisomer −1 represented 95% ± 3% of the total DNA for particles containing hypoacetylated histones, 97% ± 0.3% for particles containing histones from TSA-treated cells, and 95% ± 2% for particles containing histones from butyrate-treated cells. This analysis reveals that, as shown for tail cleavage, histone tail acetylation has no significant effect on the structure of the nucleosome.
DISCUSSION
In this study, we have investigated the influence of histone N-terminal tail removal or acetylation on the structure of a single nucleosome or tetrameric (H3-H4)2 particle assembled on a topologically constrained DNA minicircle. Upon relaxation with topoisomerase I of the DNA constraints within the particle, changes in protein conformation can be monitored by changes in DNA topoisomer equilibrium. This method was successfully used to monitor changes in conformation of DNA-binding proteins (28) or protein complexes, such as the nucleosome (37).
Our results indicate that removal of the four histone N-terminal tails had little effect on the structure of a single nucleosome since the ΔLkP value (Table 1) is not significantly affected (ΔLkP = −1.03 ± 0.016 instead of −1.05 ± 0.02 for the control). In contrast, removal of H3 and H4 tails (Table 1) is accompanied by a dramatic change in the linking-number difference of the (H3-H4)2 tetrameric particles (ΔLkP = −0.16 ± 0.06 instead of −0.62 ± 0.06 for the control). The DNA topoisomer equilibrium resulting from the relaxation of control tetrameric particles with topoisomerase I includes three adjacent DNA topoisomers (16): −1, 0, and +1 for the 359-bp DNA circle used here (Fig. 2A). In this case DNA topoisomer −1 is the major one, and DNA topoisomers 0 and +1 are only minor. This equilibrium reflects the prevalence of the left-handed conformation over the flat conformation (represented by DNA topoisomer 0) or the right-handed conformation (represented by DNA topoisomer +1). The (H3-H4)2 tetramer is known to be highly flexible and to oscillate between these three conformations (16, 17). This equilibrium is altered by removal of H3 and H4 N-terminal tails. The tailless tetramer shows a strong preference for DNA topoisomer 0 over −1 and +1, with a ΔLkP value close to zero (Fig. 2A). The change in DNA topoisomer equilibrium following histone tail removal results probably from alteration of the histone tetramer conformation, inducing a modification in DNA writhe, rather than from a change in DNA pitch, due to the absence of histone tail intercalation that might occur with intact histones. This is supported by two sets of data: first, the affinity of the tailless (H3-H4)2 tetramer for DNA topoisomer 0 is higher than that of the intact tetramer (data not shown); and second, the presence of H2A-H2B dimers abolishes the differences in topoisomer equilibrium induced by the cleavage of H3 and H4 tails (Fig. 2 and 3).
Our results confirm a very recent report (42) in which the authors have investigated the role of histone tails in chiral transition of the tetrameric particle. Consistent with our data, they found that removal of the N-terminal tails by trypsin digestion provoked an opening of the tetrameric particle. These authors have also investigated the effect of histone tail acetylation on the structure of the tetrameric particle. Although they conclude from their calculations that the free energy of the particle was decreased, topological analysis did not show differences between particles containing nonacetylated or acetylated histones.
Contrasting with this report, we found that hyperacetylation of H3 and H4 (Table 1 and Fig. 4) resulted in a change of the structure of the tetrameric particle revealed by the increase of ΔLkP up to −0.27 ± 0.001 for tetrameric particles containing H3-H4 isolated from butyrate-treated cells. The increase in acetylation levels of H3 and H4 enhanced structural changes of the tetrameric particle. A moderate acetylation (1 or 2 acetyl groups per molecule) had no effect on the ΔLkP value (data not shown), while the increase in acetylation (compare histones purified from TSA- or butyrate-treated cells [Table 1]) resulted in a more dramatic change of the tetrameric particle structure that adopts a conformation more relaxed than the control. Moreover, the acetylation heterogeneity probably leads to an underestimation of its real effect on the structure of the tetrameric particle. The lack of effect of a moderate histone acetylation on the structure of the tetrameric particle might explain the discrepancy between our results and those from Sivolob et al. (42) that used histones bearing mainly two acetyl groups.
Our study favors a role for H3-H4 N-terminal tails in stabilizing the structure of the tetrameric particle in a left-handed superhelical conformation, a structure that has an increased affinity for H2A-H2B dimers (16). It also demonstrates that histone H3-H4 tail acetylation or removal has a similar effect on the structure of the tetrameric particle.
In the absence of histone N-terminal tails or upon their acetylation, the globular domains of H2A and H2B stabilize the (H3-H4)2 tetramer in a left-handed superhelix, in a process that does not involve H2A-H2B N-terminal tails (Fig. 3). This indicates that within the nucleosome, the contribution of H3 and H4 tails to DNA wrapping is not essential, while it becomes critical in the H3-H4 tetrameric particle. Our results show that H2A-H2B N-terminal tails do not contribute significantly to the structure of a single nucleosome. Several lines of evidence support a different role for H2A-H2B and H3-H4 tails. Genetic studies have demonstrated that the simultaneous deletion of H2A-H2B histone tails is lethal and cannot be complemented by H3-H4 tails and vice versa (25, 41).
It has been proposed, based on X-ray crystallography data, that histone tails are mainly involved in nucleosome-nucleosome interactions that could play a role in compacting the chromatin fiber. Structural data show an interaction between the H4 N-terminal tail and an acidic patch within the H2A-H2B dimer of the neighboring nucleosome (27). In the crystals, however, nucleosomes adopted an orientation different from their orientation in the chromatin fiber. This interaction between the H4 tail and H2A-H2B may have favored the formation of such crystals, and its relevance in chromatin fiber structure remains to be established. It is usually proposed that histone tail acetylation results in a change in chromatin structure. Nevertheless, the exact mechanisms linking histone acetylation and transcriptional activation remain to be elucidated. Norton et al. have shown in vitro, using subsaturated circular DNA templates, that acetylated nucleosomes constrain 20% fewer superhelical turns than unmodified nucleosomes (31, 32). In contrast, in vivo there was no detectable effect of histone acetylation on the DNA topology of a simian virus 40 minichromosome (29). In vitro, with templates harboring a nucleosome density close to that found in the cell, it was shown that hyperacetylated chromatin resembles unmodified chromatin although it displayed a high degree of conformational flexibility, revealing profound alterations of histone-DNA interactions (22). Acetylation of histone N-terminal tails has been shown to increase the binding of transcription factors to nucleosomal DNA (48) and facilitate transcription initiation (30). These observations suggest that histone acetylation affects not only chromatin fiber compaction but also the nucleosome structure. Supporting this, studies on oligonucleosomal templates have shown that a significant part of the observed effects of histone acetylation on transcription takes place at the nucleosome level, with interactions of the histone terminal domains with DNA probably hampering the action of the transcription machinery (8).
One possible nucleosome structural change linked with transcriptional activation could be the formation of tetrameric particles. A number of observations support the formation of such particles in vivo. In Archaea in which transcription initiation conforms to the eukaryal paradigm, the existence of tetrameric particles has been demonstrated. The archaeal histones are similar to H3 and H4 without N- and C-terminal tails, but homologues of H2A and H2B have not been found. It has been proposed that the basic structural unit of archaeal chromatin is a tetrameric particle (38).
In eukaryotes, various observations support the existence of such (H3-H4)2 tetrameric particles in cells. In vivo, transcription by RNA polymerase II is accompanied by the formation of split particles whose exact composition remains to be determined (23), and it has been shown that RNA polymerase II associates preferentially with nucleosomes depleted in H2A-H2B dimers (3). Interestingly, it was recently demonstrated in yeast that transcription by RNA polymerase II but not by the highly processive T7 polymerase generates such structures on the same gene (40). The targeting by RNA polymerase II but not by T7 polymerase of chromatin-remodeling complexes facilitating transcription to the transcribed gene may explain this difference. Moreover, hyperacetylated active chromatin isolated from cells by chromatography on mercury-agarose was enriched in extended nucleosomal structures (49). In vitro such extended structures were formed as a result of a low-salt-induced structural transition (6). This structural transition was inhibited by histone cross-linking. It was proposed that within these extended structures, conformationally altered H3-H4s retain DNA contacts, while contacts with H2A-H2B are sacrificed or replaced (46). Proteins such as nucleoplasmin (7) or NAP1 (50), which increase the binding of regulatory factors to their target within chromatin, and FACT, which facilitates RNA elongation, interact preferentially with H2A-H2B dimers. Furthermore, covalent cross-linking of the core histones blocked FACT activity (35). One hypothesis could be that ATP-dependent chromatin-remodeling complexes remaining to be identified promote directly or indirectly the transient formation of tetrameric particles.
On the other hand, against a role of tetrameric particles, in vitro experiments have shown that the highly processive SP6 and T7 phage polymerases can transcribe through nucleosomes (9, 34), although nucleosome cross-linking decreased the processivity (33, 34). In addition, transcription of a short DNA fragment containing one nucleosome can be transcribed in vitro by yeast RNA polymerase III, and this is accompanied by a displacement of the entire nucleosome without its disruption (44).
Here we show that histone hyperacetylation provokes a conformational change of the tetrameric (H3-H4)2 particle but not of the nucleosome. The effect of histone hyperacetylation on transcription could be mediated by the formation, at least transiently, of tetrameric particles. Acetylation of H3 and H4 N-terminal tails could cause an opening of this particle, increasing the accessibility of DNA for regulatory factors and/or polymerase complexes and could provide a mechanism for the establishment of an “open” active chromatin structure. This does not exclude an additional role for histone tails in chromatin fiber compaction.
ACKNOWLEDGMENTS
We are grateful to A. Hamiche for his valuable contribution to the design of the experimental approach, to J. L. Baneres and J. Parello for their advice and help with histone tail cleavage by clostripain, to M. Grigoriev and D. Trouche for stimulating discussions, to K. D. Carr, M. Grigoriev, D. Trouche, and L. Vandel for critically reading the manuscript, and to C. Monod for linguistic corrections.
H.R.-F. has been awarded a grant from the Ligue Nationale Contre le Cancer as a member of an Equipe Labellisée La Ligue. This work was supported in part by the Association de la Recherche contre le Cancer, the GIP Fonds de Recherche HMR, and le Conseil de Région Midi-Pyrénées. V.M. is the recipient of a fellowship from the Ligue Nationale Contre le Cancer.
REFERENCES
- 1.Arents G, Burlingame R W, Wang B C, Love W E, Moudrianakis E N. The nucleosomal core histone octamer at 3.1 A resolution: a tripartite protein assembly and a left-handed superhelix. Proc Natl Acad Sci USA. 1991;88:10148–10152. doi: 10.1073/pnas.88.22.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausio J, Dong F, van Holde K E. Use of selectively trypsinized nucleosome core particles to analyze the role of the histone “tails” in the stabilization of the nucleosome. J Mol Biol. 1989;206:451–463. doi: 10.1016/0022-2836(89)90493-2. [DOI] [PubMed] [Google Scholar]
- 3.Baer B W, Rhodes D. Eukaryotic RNA polymerase II binds to nucleosome cores from transcribed genes. Nature. 1983;301:482–488. doi: 10.1038/301482a0. [DOI] [PubMed] [Google Scholar]
- 4.Baneres J L, Martin A, Parello J. The N tails of histones H3 and H4 adopt a highly structured conformation in the nucleosome. J Mol Biol. 1997;273:503–508. doi: 10.1006/jmbi.1997.1297. [DOI] [PubMed] [Google Scholar]
- 5.Bates D L, Butler P J, Pearson E C, Thomas J O. Stability of the higher-order structure of chicken-erythrocyte chromatin in solution. Eur J Biochem. 1981;119:469–476. doi: 10.1111/j.1432-1033.1981.tb05631.x. [DOI] [PubMed] [Google Scholar]
- 6.Burch J B, Martinson H G. The roles of H1, the histone core and DNA length in the unfolding of nucleosomes at low ionic strength. Nucleic Acids Res. 1980;8:4969–4987. doi: 10.1093/nar/8.21.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Li B, Workman J L. A histone-binding protein, nucleoplasmin, stimulates transcription factor binding to nucleosomes and factor-induced nucleosome disassembly. EMBO J. 1994;13:380–390. doi: 10.1002/j.1460-2075.1994.tb06272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chirinos M, Hernandez F, Palacian E. Repressive effect on oligonucleosome transcription of the core histone tail domains. Biochemistry. 1998;37:7251–7259. doi: 10.1021/bi9729817. [DOI] [PubMed] [Google Scholar]
- 9.Clark D J, Felsenfeld G. A nucleosome core is transferred out of the path of a transcribing polymerase. Cell. 1992;71:11–22. doi: 10.1016/0092-8674(92)90262-b. [DOI] [PubMed] [Google Scholar]
- 10.Davie J R. Covalent modifications of histones: expression from chromatin templates. Curr Opin Genet Dev. 1998;8:173–178. doi: 10.1016/s0959-437x(98)80138-x. [DOI] [PubMed] [Google Scholar]
- 11.Duband-Goulet I, Carot V, Ulyanov A V, Douc-Rasy S, Prunell A. Chromatin reconstitution on small DNA rings. IV. DNA supercoiling and nucleosome sequence preference. J Mol Biol. 1992;224:981–1001. doi: 10.1016/0022-2836(92)90464-u. [DOI] [PubMed] [Google Scholar]
- 12.Dumuis-Kervabon A, Encontre I, Etienne G, Jauregui-Adell J, Mery J, Mesnier D, Parello J. A chromatin core particle obtained by selective cleavage of histones by clostripain. EMBO J. 1986;5:1735–1742. doi: 10.1002/j.1460-2075.1986.tb04418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Encontre I, Parello J. Chromatin core particle obtained by selective cleavage of histones H3 and H4 by clostripain. J Mol Biol. 1988;202:673–676. doi: 10.1016/0022-2836(88)90296-3. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher T M, Hansen J C. Core histone tail domains mediate oligonucleosome folding and nucleosomal DNA organization through distinct molecular mechanisms. J Biol Chem. 1995;270:25359–25362. doi: 10.1074/jbc.270.43.25359. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Ramirez M, Rocchini C, Ausio J. Modulation of chromatin folding by histone acetylation. J Biol Chem. 1995;270:17923–17928. doi: 10.1074/jbc.270.30.17923. [DOI] [PubMed] [Google Scholar]
- 16.Hamiche A, Carot V, Alilat M, De Lucia F, O'Donohue M F, Revet B, Prunell A. Interaction of the histone (H3-H4)2 tetramer of the nucleosome with positively supercoiled DNA minicircles: potential flipping of the protein from a left- to a right-handed superhelical form. Proc Natl Acad Sci USA. 1996;93:7588–7593. doi: 10.1073/pnas.93.15.7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamiche A, Richard-Foy H. The switch in the helical handedness of the histone (H3-H4)2 tetramer within a nucleoprotein particle requires a reorientation of the H3-H3 interface. J Biol Chem. 1998;273:9261–9269. doi: 10.1074/jbc.273.15.9261. [DOI] [PubMed] [Google Scholar]
- 18.Hamiche A, Schultz P, Ramakrishnan V, Oudet P, Prunell A. Linker histone-dependent DNA structure in linear mononucleosomes. J Mol Biol. 1996;257:30–42. doi: 10.1006/jmbi.1996.0144. [DOI] [PubMed] [Google Scholar]
- 19.Hartzog G A, Winston F. Nucleosomes and transcription: recent lessons from genetics. Curr Opin Genet Dev. 1997;7:192–198. doi: 10.1016/s0959-437x(97)80128-1. [DOI] [PubMed] [Google Scholar]
- 20.Hirschhorn J N, Brown S A, Clark C D, Winston F. Evidence that SNF2/SWI2 and SNF5 activate transcription in yeast by altering chromatin structure. Genes Dev. 1992;6:2288–2298. doi: 10.1101/gad.6.12a.2288. [DOI] [PubMed] [Google Scholar]
- 21.Jackson V. In vivo studies on the dynamics of histone-DNA interaction: evidence for nucleosome dissolution during replication and transcription and a low level of dissolution independent of both. Biochemistry. 1990;29:719–731. doi: 10.1021/bi00455a019. [DOI] [PubMed] [Google Scholar]
- 22.Krajewski W A, Becker P B. Reconstitution of hyperacetylated, DNase I-sensitive chromatin characterized by high conformational flexibility of nucleosomal DNA. Proc Natl Acad Sci USA. 1998;95:1540–1545. doi: 10.1073/pnas.95.4.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee M S, Garrard W T. Transcription-induced nucleosome ‘splitting’: an underlying structure for DNase I sensitive chromatin. EMBO J. 1991;10:607–615. doi: 10.1002/j.1460-2075.1991.tb07988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenfant F, Mann R K, Thomsen B, Ling X, Grunstein M. All four core histone N-termini contain sequences required for the repression of basal transcription in yeast. EMBO J. 1996;15:3974–3985. [PMC free article] [PubMed] [Google Scholar]
- 25.Ling X, Harkness T A, Schultz M C, Fisher-Adams G, Grunstein M. Yeast histone H3 and H4 amino termini are important for nucleosome assembly in vivo and in vitro: redundant and position-independent functions in assembly but not in gene regulation. Genes Dev. 1996;10:686–699. doi: 10.1101/gad.10.6.686. [DOI] [PubMed] [Google Scholar]
- 26.Louters L, Chalkley R. Exchange of histones H1, H2A, and H2B in vivo. Biochemistry. 1985;24:3080–3085. doi: 10.1021/bi00334a002. [DOI] [PubMed] [Google Scholar]
- 27.Luger K, Mader A W, Richmond R K, Sargent D F, Richmond T J. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 28.Lutter L C, Halvorson H R, Calladine C R. Topological measurement of protein-induced DNA bend angles. J Mol Biol. 1996;261:620–633. doi: 10.1006/jmbi.1996.0488. [DOI] [PubMed] [Google Scholar]
- 29.Lutter L C, Judis L, Paretti R F. Effects of histone acetylation on chromatin topology in vivo. Mol Cell Biol. 1992;12:5004–5014. doi: 10.1128/mcb.12.11.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nightingale K P, Wellinger R E, Sogo J M, Becker P B. Histone acetylation facilitates RNA polymerase II transcription of the Drosophila hsp26 gene in chromatin. EMBO J. 1998;17:2865–2876. doi: 10.1093/emboj/17.10.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norton V G, Imai B S, Yau P, Bradbury E M. Histone acetylation reduces nucleosome core particle linking number change. Cell. 1989;57:449–457. doi: 10.1016/0092-8674(89)90920-3. [DOI] [PubMed] [Google Scholar]
- 32.Norton V G, Marvin K W, Yau P, Bradbury E M. Nucleosome linking number change controlled by acetylation of histones H3 and H4. J Biol Chem. 1990;265:19848–19852. [PubMed] [Google Scholar]
- 33.O'Neill T E, Roberge M, Bradbury E M. Nucleosome arrays inhibit both initiation and elongation of transcripts by bacteriophage T7 RNA polymerase. J Mol Biol. 1992;223:67–78. doi: 10.1016/0022-2836(92)90716-w. [DOI] [PubMed] [Google Scholar]
- 34.O'Neill T E, Smith J G, Bradbury E M. Histone octamer dissociation is not required for transcript elongation through arrays of nucleosome cores by phage T7 RNA polymerase in vitro. Proc Natl Acad Sci USA. 1993;90:6203–6207. doi: 10.1073/pnas.90.13.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orphanides G, Wu W H, Lane W S, Hampsey M, Reinberg D. The chromatin-specific transcription elongation factor FACT comprises human SPT16 and SSRP1 proteins. Nature. 1999;400:284–288. doi: 10.1038/22350. [DOI] [PubMed] [Google Scholar]
- 36.Panyim S, Chalkley R. A new histone found only in mammalian tissues with little cell division. Biochem Biophys Res Commun. 1969;37:1042–1049. doi: 10.1016/0006-291x(69)90237-x. [DOI] [PubMed] [Google Scholar]
- 37.Prunell A. A topological approach to nucleosome structure and dynamics: the linking number paradox and other issues. Biophys J. 1998;74:2531–2544. doi: 10.1016/S0006-3495(98)77961-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reeve J N, Sandman K, Daniels C J. Archaeal histones, nucleosomes, and transcription initiation. Cell. 1997;89:999–1002. doi: 10.1016/s0092-8674(00)80286-x. [DOI] [PubMed] [Google Scholar]
- 39.Ruiz-Carrillo A, Jorcano J L. An octamer of core histones in solution: central role of the H3-H4 tetramer in the self-assembly. Biochemistry. 1979;18:760–768. doi: 10.1021/bi00572a004. [DOI] [PubMed] [Google Scholar]
- 40.Sathyanarayana U G, Freeman L A, Lee M S, Garrard W T. RNA polymerase-specific nucleosome disruption by transcription in vivo. J Biol Chem. 1999;274:16431–16436. doi: 10.1074/jbc.274.23.16431. [DOI] [PubMed] [Google Scholar]
- 41.Schuster T, Han M, Grunstein M. Yeast histone H2A and H2B amino termini have interchangeable functions. Cell. 1986;45:445–451. doi: 10.1016/0092-8674(86)90330-2. [DOI] [PubMed] [Google Scholar]
- 42.Sivolob A, De Lucia F, Alilat M, Prunell A. Nucleosome dynamics. VI. Histone tail regulation of tetrasome chiral transition. A relaxation study of tetrasomes on DNA minicircles. J Mol Biol. 2000;295:55–69. doi: 10.1006/jmbi.1999.3302. [DOI] [PubMed] [Google Scholar]
- 43.Stein A, Whitlock J P, Jr, Bina M. Acidic polypeptides can assemble both histones and chromatin in vitro at physiological ionic strength. Proc Natl Acad Sci USA. 1979;76:5000–5004. doi: 10.1073/pnas.76.10.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Studitsky V M, Clark D J, Felsenfeld G. A histone octamer can step around a transcribing polymerase without leaving the template. Cell. 1994;76:371–382. doi: 10.1016/0092-8674(94)90343-3. [DOI] [PubMed] [Google Scholar]
- 45.Tse C, Sera T, Wolffe A P, Hansen J C. Disruption of higher-order folding by core histone acetylation dramatically enhances transcription of nucleosomal arrays by RNA polymerase III. Mol Cell Biol. 1998;18:4629–4638. doi: 10.1128/mcb.18.8.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uberbacher E C, Ramakrishnan V, Olins D E, Bunick G J. Neutron scattering studies of nucleosome structure at low ionic strength. Biochemistry. 1983;22:4916–4923. doi: 10.1021/bi00290a007. [DOI] [PubMed] [Google Scholar]
- 47.Van Holde K E. Chromatin. New York, N.Y: Springer-Verlag; 1989. [Google Scholar]
- 48.Vettese-Dadey M, Grant P A, Hebbes T R, Crane-Robinson C, Allis C D, Workman J L. Acetylation of histone H4 plays a primary role in enhancing transcription factor binding to nucleosomal DNA in vitro. EMBO J. 1996;15:2508–2518. [PMC free article] [PubMed] [Google Scholar]
- 49.Walker J, Chen T A, Sterner R, Berger M, Winston F, Allfrey V G. Affinity chromatography of mammalian and yeast nucleosomes. Two modes of binding of transcriptionally active mammalian nucleosomes to organomercurial-agarose columns, and contrasting behavior of the active nucleosomes of yeast. J Biol Chem. 1990;265:5736–5746. [PubMed] [Google Scholar]
- 50.Walter P P, Owen-Hughes T A, Cote J, Workman J L. Stimulation of transcription factor binding and histone displacement by nucleosome assembly protein 1 and nucleoplasmin requires disruption of the histone octamer. Mol Cell Biol. 1995;15:6178–6187. doi: 10.1128/mcb.15.11.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White J H, Gallo R M, Bauer W R. Closed circular DNA as a probe for protein-induced structural changes. Trends Biochem Sci. 1992;17:7–12. doi: 10.1016/0968-0004(92)90417-8. [DOI] [PubMed] [Google Scholar]
- 52.Wolffe A P, Kurumizaka H. The nucleosome: a powerful regulator of transcription. Prog Nucleic Acid Res Mol Biol. 1998;61:379–422. doi: 10.1016/s0079-6603(08)60832-6. [DOI] [PubMed] [Google Scholar]
- 53.Zivanovic Y, Duband-Goulet I, Schultz P, Stofer E, Oudet P, Prunell A. Chromatin reconstitution on small DNA rings. III. Histone H5 dependence of DNA supercoiling in the nucleosome. J Mol Biol. 1990;214:479–495. doi: 10.1016/0022-2836(90)90195-R. [DOI] [PubMed] [Google Scholar]
- 54.Zivanovic Y, Goulet I, Prunell A. Properties of supercoiled DNA in gel electrophoresis. The V-like dependence of mobility on topological constraint. DNA-matrix interactions. J Mol Biol. 1986;192:645–660. doi: 10.1016/0022-2836(86)90282-2. [DOI] [PubMed] [Google Scholar]