Genomewide Scans of Complex Human Diseases: True Linkage Is Hard to Find (original) (raw)
Abstract
Many “complex” human diseases, which involve multiple genetic and environmental determinants, have increased in incidence during the past 2 decades. During the same time period, considerable effort and expense have been expended in whole-genome screens aimed at detection of genetic loci contributing to the susceptibility to complex human diseases. However, the success of positional cloning attempts based on whole-genome screens has been limited, and many of the fundamental questions relating to the genetic epidemiology of complex human disease remain unanswered. Both to review the success of the positional cloning paradigm as applied to complex human disease and to investigate the characteristics of the whole-genome scans undertaken to date, we created a database of 101 studies of complex human disease, which were found by a systematic Medline search (current as of December 2000). We compared these studies, concerning 31 different human complex diseases, with regard to design, methods, and results. The “significance” categorizations proposed by Lander and Kruglyak were used as criteria for the “success” of a study. Most (66.3% [_n_=67]) of the studies did not show “significant” linkage when the criteria of Lander and Kruglyak (1995) were used, and the results of studies of the same disease were often inconsistent. Our analyses suggest that no single study design consistently produces more-significant results. Multivariate analysis suggests that the only factors independently associated with increased study success are (a) an increase in the number of individuals studied and (b) study of a sample drawn from only one ethnic group. Positional cloning based on whole-genome screens in complex human disease has proved more difficult than originally had been envisioned; detection of linkage and positional cloning of specific disease-susceptibility loci remains elusive.
Introduction
Complex human diseases involve multiple, interacting genetic and environmental determinants (Weeks and Lathrop 1995). Many such diseases have increased in incidence during the past 2 decades in the developed nations and are of major clinical and economic significance. During the same period, the genetic etiology of many complex human diseases has been increasingly emphasized as a means of better understanding their pathogenesis, with the ultimate goal of improving preventive strategies, diagnostic tools, and therapies (Risch 2000). During the past decade, considerable effort and expense have been expended in whole-genome screens aimed at detection of genetic loci contributing to the susceptibility to complex human diseases.
Positional cloning begins with the identification of a chromosomal region that is transmitted within families, along with the disease phenotype of interest (genetic linkage). Positional cloning has been extremely useful in the identification of genes responsible for diseases with simple Mendelian inheritance, such as cystic fibrosis (Zielenski and Tsui 1995). The ultimate goal of positional cloning is to identify sequence variants within the coding or controlling regions of a gene associated with the phenotype of interest. However, the success of such positional cloning attempts has been limited, and most of the fundamental questions relating to the genetic epidemiology of complex human disease remain unanswered. In contrast to what has been found for monogenic traits, the results have often been disappointing or even inconsistent (Rao 2001; Terwilliger and Goring 2000). The large amount of linkage data generated from whole-genome screens is difficult to synthesize and interpret.
Both to review the success of the positional cloning paradigm as applied to complex human disease and to investigate the characteristics of the whole-genome scans undertaken to date, we created a database of 101 whole-genome scans of complex human disease, which were found by a systematic Medline search. All studies considered complex diseases in humans, described a complete whole-genome scan (often excluding X and Y chromosomes) and were published in peer-reviewed scientific journals. This database, current as of December 2000, includes the majority of genome scans undertaken in humans to date. We compared these studies, concerning 31 different human complex diseases, with regard to design, methods, and relative “success.”
Given the large number of whole-genome scans performed to date—and the concomitant enormous expenditure of research resources—a systematic review of the success and characteristics of such studies seems timely. We hypothesized that such a review might assist researchers to find the appropriate design for their future research work and might also facilitate the evaluation of publications in this field. The principal aims of this study were to review the success of whole-genome scanning as a strategy for gene discovery in complex human disease and to compare and analyze methodical differences related to success in gene localization.
Material and Methods
Database
A database was created containing the following information: publication details (author, title, source, year of study, and main trait); phenotypic traits investigated; study design (environmental factors, number of associated traits, sibling recurrence risk ratio [λS], prevalence, ascertainment background, phenotyping procedure, and family structure); details of study population (ethnic background and use of inbred/outbred populations); sample-size details (number of probands, individuals, families, and sib pairs); genotyping methods (type of markers, number of markers, mean polymorphism information content, mean heterozygosity, mean spacing of markers, and missing values); statistical methods; and results obtained (number of positive markers concerning the individual threshold, with localization and marker term; maximum LOD score or Z score, and minimum P value). When a “second-stage” (e.g., an additional sample of families or additional markers) was reported within the same publication, the database was supplemented with the relevant additional information.
The following initial keywords were used for the search: “genome-wide scan,” “genome-wide screen,” and “genome-wide search.” This entailed the reading and discarding of several thousand titles and several hundreds of abstracts of studies of monogenic diseases or animal models. The search was expanded by use of other terms found to be commonly used in published whole-genome scans (e.g., “susceptibility loci,” “genomic scan,” and “genome screen”). The database was checked by examination of both the discussion section and the reference list of the publications found in our initial search, allowing the completeness of our database of genome scans in each specific field to be validated. The database was current through the end of December 2000.
Analysis
To investigate study properties that might have been important in determining the relative success or lack of success of the whole-genome scans investigated, it was first necessary to generate some approximate measure of success. The principal outcome variable generated for each study was an ordinal measure of linkage success. For every study, the most significant P value for linkage was categorized and coded for analysis, by use of the criteria of Lander and Kruglyak (1995): 0 = no linkage; 1 = suggestive linkage; 2 = significant linkage; 3 = highly significant linkage; and 4 = confirmed linkage (table 1). This measure of “study success” (hereafter referred to as “L-K category”) was then used as an ordinal outcome in bivariate and multivariate analyses to determine the best predictors of success among the variables available for each published study.
Table 1.
Lander and Kruglyak (1995) Significance Criteria for Mapping of Loci Underlying Complex Traits in Sibs and Half-Sibs
| Category | Range of Approximate P Values | Range of Approximate LOD Scores |
|---|---|---|
| No linkage | 1.00–.0008 | 0–2.1 |
| Suggestive linkage | .0007–.00003 | 2.2–3.5 |
| Significant linkage | .00002–.0000004 | 3.6–5.3 |
| Highly significant linkage | ⩽.0000003 | ⩾5.4 |
| Confirmed linkage | Significant linkage in an initial study, confirmed in an independent sample |
Sample-size parameters, the number of markers genotyped, the average heterozygozity of genotyped markers, the prevalence, and the λS for every disease were analyzed as continuous variables. L-K category and all other variables were analyzed as categorical.
Bivariate analysis used χ2 tests or Fisher’s exact tests (Mehta 1994), for contingency tables or analysis of variance, to compare L-K categories to study parameters of interest. Generalized linear models (logistic regression) (Armitage and Berry 1994) were used to model the effects of multiple covariates on L-K category.
Both forward and backward stepwise modeling procedures were used to select a useful subset of independent predictors of study success. Checks of goodness of fit (McCullagh and Nelder 1989) included an investigation of the need for interaction or polynomial terms, analyses of residuals, and examination of the effect of observations with high regression leverage.
SAS version 8 (SAS Institute) was used to construct the initial database. Both SAS version 8 and Splus version 2000 (Mathsoft) were used to manage and analyze data. Statistical significance was defined at the standard 5% level.
Results
1. Descriptive Statistics
Diseases and phenotypes studied
The 101 studies in our database were performed during 1993–2000, for 31 different complex human diseases (table 2). The most frequently studied diseases were schizophrenia (_n_=10), type 2 diabetes (_n_=8), asthma (_n_=7), bipolar affective disorder (_n_=7), Crohn disease and inflammatory bowel disease (_n_=7), psoriasis (_n_=6), obesity (_n_=5), prostate cancer (n_=5), and type 1 diabetes (n_=5). Only four groups of authors published the results of more than one whole-genome scan (Coon et al. 1993, 1994; Ginns et al. 1996, 1998; Ober et al. 1998, 1999, 2000; Lee et al. 2000_a_, 2000_b_). The λS and disease prevalence in an appropriate reference population were generally not stated in the articles reporting the whole-genome scans but, for most complex diseases, were available from secondary literature. The λS values for the 31 diseases studied were 1.3–75, with two peaks at ∼3–4 and ∼10–15. The prevalence of the conditions studied in the general population also showed great variation, with a range of 0.04%–40% and a mean of ∼4%.
Table 2.
Characteristics of Whole-Genome Scans for Complex Human Disease (_n_=101)
| Main Trait (Primary Outcome) | Reference(s) | No. of Studies | λS |
|---|---|---|---|
| Alcohol dependence (alcoholism and smoking) | Long et al. (1998); Reich et al. (1998) | 2 | 4 |
| Atopy | Ober et al. (1999) | 1 | 1.3 |
| Alzheimer | Pericak-Vance et al. (1997); Kehoe et al. (1999) | 2 | |
| Ankylosing spondylitis | Brown et al. (1998) | 1 | 46 |
| Asthma (asthma and atopy) | Daniels et al. (1996), The Collaborative Study on the Genetics of Asthma (1997), Ober et al. (1998, Wjst et al. (1999), Dizier et al. (2000), 2000), Yokouchi et al. (2000) | 7 | 3 |
| Atopic dermatitis | Lee et al. (2000_b_) | 1 | |
| Autism | International Molecular Genetic Study of Autism Consortium (1998), Philippe et al. (1999) | 2 | 75 |
| Bipolar affective disorder | Coon et al. (1993), Ginns et al. (1996), McInnes et al. (1996), Adams et al. (1998), Detera-Wadleigh et al. (1999), Morissette et al. (1999), Friddle et al. (2000) | 7 | 15 |
| Blood pressure (hypertension) | Krushkal et al. (1999), Xu et al. (1999), Rice et al. (2000), Sharma et al. (2000) | 4 | 4 |
| Bone-mineral density (osteoporosis) | Devoto et al. (1998), Niu et al. (1999) | 2 | |
| Cholesterol concentration (familial combined hyperlipidemia) | Aouizerat et al. (1999), Pajukanta et al. (1999), Rainwater et al. (1999) | 3 | 20 |
| Crohn disease (inflammatory bowel disease) | Hugot et al. (1996), Satsangi et al. (1996), Cho et al. (1998), Hampe et al. (1999), Ma et al. (1999), Duerr et al. (2000), Rioux et al. (2000) | 7 | 35/25 |
| Diabetes: | |||
| Type 1 | Davies (1994), Field et al. (1994), Hashimoto et al. (1994), Luo et al. (1995), Mein et al. (1998) | 5 | 15 |
| Type 2 (BMI/age at onset/plasma glucose concentration/ prediabetic phenotype) | Hanis et al. (1996), Mahtani et al. (1996), Stern et al. (1996), Hanson et al. (1998), Pratley (1998), Duggirala et al. (1999), Elbein et al. (1999), Hegele et al. (1999), Ehm et al. (2000), Watanabe et al. (2000) | 10 | 4 |
| IgE level, total serum | Xu et al. (2000) | 1 | |
| Interphalangeal-joint osteoarthritis | Leppavuori et al. (1999) | 1 | |
| Lupus erythematosus | Gaffney et al. (1998), Moser et al. (1998), Shai et al. (1999) | 3 | 15 |
| Mental-health wellness | Ginns et al. (1998) | 1 | |
| Multiple sclerosis | Ebers et al. (1996), Haines et al. (1996), Sawcer et al. (1996), Kuokkanen et al. (1997) | 4 | 30 |
| Obesity, energy metabolism, leptin variation | Comuzzie et al. (1997), Hager et al. (1998), Norman et al. (1998), Lee et al. (1999), Ohman et al. (2000) | 5 | 4 |
| Open-angle glaucoma | Wiggs et al. (2000) | 1 | 8 |
| Osteoarthritis | Chapman et al. (1999) | 1 | 23 |
| Panic disorder | Knowles et al. (1998) | 1 | 8 |
| Pre-eclampsia (eclampsia) | Harrison et al. (1997), Arngrimsson et al. (1999) | 2 | |
| Prostate cancer (aggressive prostate cancer) | Smith et al. (1996), Berthon et al. (1998), Gibbs et al. (1999), Suarez et al. (2000), Witte et al. (2000) | 5 | 3 |
| Psoriasis | Tomfohrde et al. (1994), Matthews et al. (1996), Nair et al. (1997), Trembath et al. (1997), Samuelsson et al. (1999), Lee et al. (2000_a_) | 6 | 7 |
| Raynaud phenomenon | Susol et al. (2000) | 1 | |
| Rheumatoid arthritis | Cornelis et al. (1998), Shiozawa et al. (1998) | 2 | 5/8 |
| Schizophrenia | Coon et al. (1994), Moises et al. (1995), Blouin et al. (1998), Faraone et al. (1998), Kaufmann et al. (1998), Levinson et al. (1998), Shaw et al. (1998), Hovatta et al. (1999), Williams et al. (1999), Ekelund et al. (2000) | 10 | 10 |
| Testicular tumor | Leahy et al. (1995), Rapley et al. (2000) | 2 | 9 |
| Tuberculosis | Bellamy (2000) | 1 |
Most studies used disease affection status, coded as a binary (i.e., yes or no) response, as the primary outcome (74% [_n_=75]). Twelve percent (_n_=12) of studies used a disease-associated “intermediate” quantitative trait as the primary outcome, and 14% (_n_=14) of studies used both disease affection status and one or more disease-associated quantitative traits in their linkage analysis.
Study design
Fifty percent of all studies used an affected-sib-pair design, and 14% used other pairs of affected relatives. Nonaffected relatives (e.g., parents of affected siblings and healthy siblings) were often genotyped also, to increase the information available for calculation of identity-by-descent allele sharing at each marker. In 36% of the studies, extended pedigrees were ascertained.
Families were sampled mainly from outbred (i.e., nonisolated), admixed populations (81% [_n_=81]). The remaining studies sampled genetically isolated, inbred populations (19% [_n_=19])—for example, an isolated Finnish subpopulation (Hovatta et al. 1999), U.S. Hutterites (Ober et al. 1998, 1999, 2000), and Old Order Amish (Ginns et al. 1996, 1998)—an approach that assumes reduced heterogeneity both in genetic background and in environmental and lifestyle factors. A study population sampled from a single ethnic group was investigated in the majority (64%) of studies; the remaining studies (36%) sampled from two or more ethnic groups—for example, individuals of European descent, African American, and Hispanic (The Collaborative Study on the Genetics of Asthma 1997).
Sample size
The study size varied greatly between studies, the range being 20–1,783 individuals comprised by 1–580 families or pedigrees. The varying sample sizes reflected the different ascertainment approaches and study designs and were strongly related to the availability of affected probands in the sampling frames used. There was no significant difference in the mean number of individuals studied, in terms of either year of study or disease studied.
Genotyping methods
The genotyping methods were the most consistent aspect of the 101 whole-genome screens investigated. All of the whole-genome scans were based on the use of polymorphic microsatellite-marker sets (Reed et al. 1994). There were only a limited number of microsatellite marker sets (e.g., CHLC Weber, Généthon, Research Genetics, and Marshfield) available during the time frame of these studies; markers from these sets were often combined with each other and sometimes were supplemented with specific polymorphic markers or single-nucleotide polymorphisms (SNPs) in putative candidate chromosomal regions. The mean heterozygosity was specified in only 46 studies, and the range was .60–.82. The average spacing between any two microsatellite markers in the whole-genome scans was 4.6–20 cM; the majority of the studies had an average marker spacing of ∼11 cM.
Replication of results for specific diseases
Some diseases were the subject of multiple studies. When these studies and their attained significance levels were compared, it became obvious that it generally is difficult to identify complex human diseases to replicate linkages within them; for example, the number of studies showing no linkage/suggestive linkage/significant linkage/highly significant linkage/confirmed linkage breakdown was 1/5/10/0 for asthma, 3/3/1/0/0 for bipolar affective disorder, 2/2/0/1/1 for psoriasis, 4/4/2/0/0 for schizophrenia, 0/2/1/1/1 for type 1 diabetes, and 0/2/6/0/0 for type 2 diabetes.
Table 3 compares the results of the seven studies of asthma and the eight studies of type 2 diabetes. Highlighting the difficulty of replication in linkage studies of complex human diseases, these studies of asthma and type 2 diabetes reported evidence of linkage on most of the autosomes, and the majority of the reported positive linkages did not overlap (table 3).
Table 3.
Comparison of Loci Found in Studies of Asthma and Type 2 Diabetes
| Disease and Reference | Study Design | No. ofIndividualsGenotyped | Significance Levela | Chromosome(s) for Which Positive Findings Were Reported |
|---|---|---|---|---|
| Asthma:b | ||||
| Daniels et al. (1996) | Sib pair | 364 | Suggestive linkage | 4, 6, 7, 11, 13, 16 |
| The Collaborative Study on the Genetics of Asthma (1997) | Sib pair | 540 | Suggestive linkage | 2, 5, 6, 11–14, 17, 19, 21 |
| Ober et al. (1998) | Extended pedigree | 361 | Suggestive linkage | 2, 3, 5, 9, 12, 13, 19, 21 |
| Wjst et al. (1999) | Sib pair | 415 | No linkage | 2, 6, 9, 12 |
| Dizier et al. (2000) | Sib pair | 211 | Suggestive linkage | 1, 11–13, 17, 19 |
| Ober et al. (2000) | Extended pedigree | 693 | Suggestive linkage | 5, 8, 14, 16, 19 |
| Yokouchi et al. (2000) | Sib pair | 197 | Significant linkage | 4, 5, 13 |
| Type 2 diabetes:c | ||||
| Hanis et al. (1996) | Sib pair | 408 | Significant linkage | 2 |
| Mahtani et al. (1996) | Extended pedigree | 217 | Significant linkage | 12 |
| Hanson et al. (1998) | Affected relatives | 656 | Suggestive linkage | 11 |
| Duggirala et al. (1999) | Extended pedigree | 440 | Significant linkage | 3, 4, 9, 10 |
| Elbein et al. (1999) | Extended pedigree | 468 | Significant linkage | 1 |
| Hegele et al. (1999) | Sib pair | 33 | Significant linkage | 3, 6, 8, 10, 16, 22 |
| Ehm et al. (2000) | Affected relatives | 1,783 | Significant linkage | 3, 5, 10, 12, X |
| Ghosh et al. (2000) | Sib pair | 1,438d | Suggestive linkage |
Distribution of all “positive” markers
An average of 4.5 positive loci showing some evidence of linkage, which were chosen on the basis of different individual thresholds, were reported in every study. These 453 “hits” were not distributed equally among all 23 chromosomes.
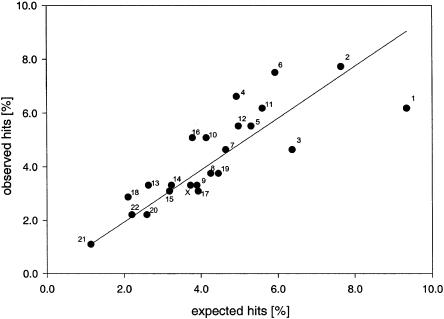
We plotted the observed hit ratio (%: hits on every chromosome/all 453 hits) against an expected hit ratio (%: genes on every chromosome/all 37,701 genes of the genome, when chromosome sizes and gene content are rated as suggested by Venter et al. [2001]). The linear regression suggested a close correlation between the expected hit ratio and the observed hit ratio (fig. 1). This suggests that the null hypothesis of random linkages across the genome cannot be rejected, since all the whole-genome scans investigated used markers (roughly) equally distributed across the genome and a close association of the positive linkages with theoretical gene-content could be shown.
Figure 1.
Regression analysis of expected and observed hits on chromosomes 1–22 and X. An average of 4.5 “positive” loci showing some evidence of linkage, chosen by different individual thresholds, were reported in every study. The observed hit ratio (%: hits on every chromosome/all 453 hits) is plotted against the expected hit ratio (%: genes on every chromosome/all 37,701 genes of the genome, when chromosome sizes and gene content are rated as suggested by Venter et al. [2001]).
However, chromosomes 4, 6, and 16 showed an increased observed hit ratio, relative to the expected hit ratio. This may indicate the presence of one or more pleiotropic loci encoding susceptibility for multiple diseases and traits. Markers in or near the HLA locus on chromosome 6p, for example, show some evidence of linkage to type 1 diabetes (Davies et al. 1994; Field et al. 1994; Hashimoto et al. 1994; Mein et al. 1998), multiple sclerosis (Haines et al. 1996; Kuokkanen et al. 1997), rheumatoid arthritis (Cornelis et al. 1998), psoriasis (Nair et al. 1997; Trembath et al. 1997; Samuelsson et al. 1999; Lee et al. 2000_a_), inflammatory bowel (Hampe et al. 1999; Rioux et al. 2000), and asthma/allergy (Daniels et al. 1996; The Collaborative Study on the Genetics of Asthma 1997; Ober et al. 1999; Wjst et al. 1999).
2. Analysis
Many different linkage-analysis techniques and models (e.g., model-based and model-free methods, two-point and multipoint linkage analysis, and variance-components and regression-based methods) were used within the available software packages. LOD scores and Z scores were transformed into asymptotic P values, if these were not already listed.
When classification on the basis of L-K category was used, 4% (_n_=4) of the whole-genome scans showed highly significant linkage, 24% (_n_=23) showed significant linkage, 47% (_n_=44) showed suggestive linkage, and the remaining 24% (_n_=23) showed little (often referred to as “nominal” linkage). Forty-one studies reported more than one “stage” in the same publication. A “second stage” genome screen generally involved either a second whole-genome scan, the typing of selected markers on a second sample of families, in an attempt to replicate the positive findings of the initial genome scan, or the typing of a denser marker set (“flanking markers”) in certain regions in the same sample of families. Approximately half of these second stages led to an improvement in the statistical significance of individual linkages. The reporting of these second-stage approaches in some of the reviewed studies made it necessary to redefine study success, beyond what each article simply reported for the initial (first-stage) “genomewide-scan result.” This “overall study result” was used as the primary outcome in our analyses and considers the lowest P value attained either in one of the study stages or in the combined sample data. When classification on the basis of L-K category was used, 2% (_n_=2) of the studies showed confirmed linkage, 5% (_n_=5) showed highly significant linkage, 27% (_n_=27) showed significant linkage, 46% (_n_=47) showed suggestive linkage, and 20% (_n_=20) showed no linkage.
Bivariate analysis
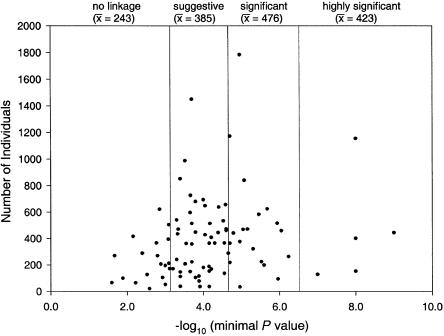
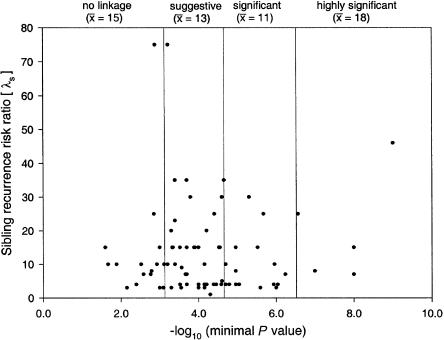
Bivariate analysis found no significant association between study success, as defined by L-K category, and the following study parameters (table 4): ascertainment (Fisher's exact test; _P_=.21); population studied—either inbred/outbred (Fisher's exact test; _P_=.92) or one ethnic group/more than one ethnic group (Fisher’s exact test; _P_=.28); primary outcome investigated (qualitative/quantitative/both) (Fisher's exact test; _P_=.23); number of families studied (_F_4,93=0.22; _P_=.92); number of individuals studied (_F_4,94=1.52; _P_=.20; see fig. 2); disease prevalence (_F_4,88=0.61; _P_=.66); and λS (_F_4,78=0.48; _P_=.75; see fig. 3). However, the following variables showed some trend toward an association with higher L-K category (table 4): study of only one ethnic group; number of individuals studied (fig. 2); and study of an outbred population.
Table 4.
Relationship of Study Variables to Study Success
| Variable | No Linkage | Suggestive Linkage | Significant Linkage | Highly Significant Linkage | Confirmed Linkage |
|---|---|---|---|---|---|
| Proportion (No.) of Studies Successfula | |||||
| Ascertainment: | |||||
| Affected sib pairs | 21.6% (11) | 45.1% (23) | 23.5% (12) | 7.8% (4) | 2.0% (1) |
| Affected relative pairs | 7.1% (1) | 50.0% (7) | 35.7% (5) | .0% | 7.1% (1) |
| Extended pedigrees | 22.2% (8) | 47.2% (17) | 27.8% (10) | 2.8% (1) | .0% |
| Population: | |||||
| Outbred, admixed | 19.5% (16) | 45.1% (37) | 26.8% (22) | 6.1% (5) | 2.4% (2) |
| Inbred, isolated | 21.0% (4) | 52.6% (37) | 26.3% (5) | .0% | .0% |
| Primary outcome investigated: | |||||
| Qualitative | 34.0% (18) | 38.7% (29) | 28.0% (21) | 6.7% (5) | 2.7% (2) |
| Quantitative | 8.3% (1) | 83.3% (10) | 8.3% (1) | .0% | .0% |
| Qualitative and quantitative | 7.1% (1) | 57.1% (8) | 35.7% (5) | .0% | .0% |
| No. of ethnic groups studied: | |||||
| One | 16.4% (10) | 42.6% (26) | 29.5% (18) | 8.2% (5) | 3.3% (2) |
| More than one | 22.9% (8) | 54.3% (19) | 22.9% (8) | .0% | .0% |
| Mean (SE) | |||||
| No. of families studied | 75.8 (17.7) | 92.4 (16.3) | 97.9 (18.6) | 112.8 (43.2) | 68.5 (27.5) |
| No. of individuals studied | 243.9 (38.5) | 393.6 (49.2) | 470.5 (69.4) | 435.0 (189.0) | 331.0 (69.0) |
| Disease prevalence (%) | 4.0 (1.6) | 5.2 (1.4) | 3.4 (.8) | 1.1 (.6) | 1.1 (.8) |
| λS | 15.1 (10.0) | 12.3 (2.2) | 12.1 (2.3) | 20.2 (7.2) | 11.0 (4.0) |
Figure 2.
Number of individuals, plotted against minimum attained P value. The correlation of sample size and study “success” in the genome scans reviewed is illustrated. The vertical lines correspond to the significance thresholds suggested by Lander and Kruglyak (1995), and the mean number of individuals studied in each category is shown.
Figure 3.
λS, Plotted against minimum attained P value. The correlation of λS and study “success” in the genome scans reviewed is illustrated. The vertical lines correspond to the significance thresholds suggested by Lander and Kruglyak (1995), and the mean λS in each category is shown.
Multivariate analysis
Ordinal logistic regression suggested that both the number of individuals studied (odds ratio = 1.004/subject, 95% confidence interval = 1.001–1.008; _P_=.03) and the study of one ethnic group (vs. multiple ethnic groups) (odds ratio = 2.44, 95% confidence interval = 1.08–5.54; _P_=.03) were associated with increased study success. These associations were independent of (1) the specific disease studied, (2) the type of trait investigated as the primary outcome, (3) the ascertainment method, (4) either λS or the population prevalence of the condition studied, or (5) statistical test used.
Discussion
Our review was designed to compare 101 whole-genome scans in 31 different diseases, in terms of design, methods, and success. Studies varied widely with regard to study design and statistical analysis. Most studies did not show significant linkage when the criteria of Lander and Kruglyak (1995) were used. The findings in studies of the same disease are often inconsistent; the number of observations of highly significant and confirmed linkages is very small, and unequivocal statements are difficult to make.
Although this review has focused on one dimension of the success of whole-genome scans, it is important to remember that the underlying purpose of such studies is to discover susceptibility loci for a complex disease—the real success of a whole-genome scan is unlikely to be defined solely by the minimum attained P value of the linkage analysis. Potential publication bias was ignored in our review. There are a number of genome scans of complex human diseases, known to have been undertaken by private industry, that have not been published. The involvement of commercial enterprises in gene-discovery attempts has put a premium on secrecy, and the results of these genome scans could not be included in this review. There has been no systematic assessment of publication bias in whole-genome screens, and such an undertaking was beyond the scope of our review. However, the lack of evidence of a correlation between year of publication and study success suggests that this was not an important bias in the sample of whole-genome screens surveyed. A further potential bias in our study relates to the way in which the Medline search was undertaken. Validation of our database by the discussions and reference lists in primary literature discovered in an initial Medline search may have led to the inclusion of a disproportionate number of the genome scans of the more frequently investigated diseases.
The use of Lander and Kruglyak's (1995) proposed categorizations as an approximate measure of study success is somewhat arbitrary and could have biased our study in unknown ways. In their article, Lander and Kruglyak (1995) argue strongly for the value of such categorizations, and their scheme has been adopted by many researchers. However, to ensure that our categorization of success had not biased our study, we also repeated our analyses, using, as a continuous outcome, −log10 (minimum P values) from each study. The results were very similar to those reported when the L-K categories (data not shown) were used, suggesting that the use of this categorization scheme did not result in any significant bias.
Our analysis suggests that sample size is an important determinant of study success. Possibly because the extended-pedigree approach has low numbers of families and proportionally higher numbers of individuals, the number of individuals studied proved to be the most informative index of sample size. The most obvious differences in study success that were due to sample size were observed for the difference between no linkage and any evidence of suggestive or better linkage (fig. 2); on average, studies that, by Lander and Kruglyak (1995) criteria, showed suggestive linkage had twice the sample size (number of individuals) of studies showing no evidence of linkage.
The study of a sample selected from a single ethnic group also appeared to be advantageous with regard to L-K category attained. This may be due both to the increase in heterogeneity in the study sample, occasioned by introduction of samples from different ethnic groups, and to the current general lack of appropriate statistical methodology to assess or adjust for the resulting increased heterogeneity.
It has been proposed that, compared with outbred, admixed populations, genetically isolated populations may offer some advantages for the mapping of complex genetic traits (de la Chapelle 1993; Jorde 1995). However, both in contrast to the theoretical advantages to the use of inbred populations position and in accord with some recent statistical concerns (Lonjou et al. 1999), the empirical data show that studies using such populations are, on average, no more successful than studies using samples from general, outbred populations. This finding may be the result of factors such as either the lack of availability of an available inbred population for study of a particular disease or, possibly, to disadvantages inherent in an extended-pedigree approach.
Rare diseases are generally more difficult to investigate in a large study, since (a) study design is often limited by the number of available samples and (b) collecting a large number of families containing one or more affected individuals can be problematical. However, our review suggests that there is no identifiable disadvantage associated with the study of rarer complex diseases compared with more-common diseases.
It has been generally accepted that quantitative “intermediate” phenotypes, when available, are likely to be more objective and informative—and, hence, more statistically powerful—and hence preferable to dichotomous disease affection outcomes (Amos and Laing 1993). However, our analyses do not suggest that, as outcomes for linkage analysis, quantitative traits have any important advantages over qualitative traits. Given that many different diseases were studied by many disparate methods, it is difficult to ascribe meaning to this finding. Possibly the intraindividual fluctuations over time, as well as difficulties in consideration of covariate adjustment for quantitative intermediate phenotypes may bias the phenotypes, introducing noise into the linkage analysis.
λS is a variable that characterizes the familial aggregation of a disease; the λS value can be used to estimate the power that affected-sib-pair methods have to detect linkage (Risch 1990). Whole-genome scans have succeeded in the analysis of monogenic traits, which tend to have extremely high estimated λS values (Farrer and Cupples 1998). The results of gene mapping in monogenic disease, together with theoretical studies of λS values (Risch 1990), suggest that genes for complex diseases with a relatively high λS may be more easily localized. However, our results are not consistent with this theoretical expectation (fig. 3). This finding calls into question whether the λS measure has utility for linkage studies of common, complex human diseases.
The comparison of variables related to study design and power vis-à-vis the best L-K category attained in every study may only suggest some more-obvious trends and does not prove that the observed differences are critical to success. Our results suggest that, beyond the almost tautological finding that larger samples and reduced heterogeneity are better, there is no gold standard applicable to complex human diseases. One recommendation that we do feel confident to make is that, in publications of whole-genome scans, more-careful attention should be paid to the characterization of both the methods (particularly the ascertainment and the phenotyping procedures) and the results. Undertaking our review was made extremely difficult by the inconsistency of the studies reviewed, in terms of their reporting of methods and results; a degree of subjective interpretation was often necessary in order to identify all of the relevant parameters from a study. Linkage results were sometimes presented primarily as LOD-score or _P_-value diagrams (often small and hard to read) for each chromosome. Very few studies gave any information regarding marker informativity, making it impossible to study this parameter. Detailed descriptions of methodology (particularly for studies involving multiple stages) and the reporting of maximum LOD scores and/or minimum P values in tabular form (in addition to or instead of graphic representations) would much improve the situation. There is a lack of generally accepted criteria for replication of linkage results; it remains unclear exactly what constitutes “replication” in a genome scan. For the purposes of our review, we took reported replication at face value, even though this may involve different criteria (e.g., within 10 cM vs. within 5 cM) in different studies. Finally, as Lander and Kruglyak (1995) argue, ideally every individual whole-genome screen would report empirically determined significance criteria for that study. These problems are as much the fault of journal editors as they are of individual authors, and they need to be addressed by comprehensive, uniform guidelines.
The whole-genome screens for complex human disease that have been reviewed here have a number of important limitations. Sample sizes were generally modest; the relatively small numbers studied would have (a) tended to limit the power of these genome screens to detect linkage and (b) increased the possibility of type I experimental error (Terwilliger and Goring 2000). The use of widely differing significance thresholds within each sample make it difficult to compare them. In most of the studies, no replication of putative novel linkages has yet been attempted in independent population samples of similar ethnicity. Furthermore, the (often tacit) assumption that the susceptibility genes for quantitative traits associated with disease affection will be equivalent to the susceptibility genes for the disease in question may not necessarily be valid. However, it is easy to be critical in hindsight, and it is important to recognize that these studies are embedded within a historical matrix; the many difficulties inherent in positional cloning have become apparent only through the experience gained during the past decade.
What Studies That Showed Highly Significant or Confirmed Linkage Have in Common
Five studies showed one or more highly significant linkages, and two showed confirmed linkage (Davies et al. 1994; Field et al. 1994; Tomfohrde et al. 1994; Satsangi et al. 1996; Trembath et al. 1997; Brown et al. 1998; Shiozawa et al. 1998). All seven of these studies used a qualitative main trait (in two studies of type 1 diabetes, one study of rheumatoid arthritis, one study of ankylosing spondylitis, one study of inflammatory bowel disease, and two studies of psoriasis) and sampled from outbred populations, and five of them used a sib-pair approach. Were these results obtained by chance (a “lucky” search for the right trait in the right families), or do these studies have something else in common? Although a significant relationship between sample size and attained significance level could be demonstrated, the most-significant results were not achieved by the largest studies in this group. No clear strategy for success could be delineated by our review, except perhaps a preference for autoimmune diseases. The success of studies of such diseases may reflect both an increased genetic component of variance and reduced locus heterogeneity, relative to the other diseases investigated. Study design also may have played a role; the selection of families was generally undertaken with the aim of focusing either on a well-defined subtype of the disease or on large pedigrees in which the disease appeared to exhibit Mendelian inheritance (e.g., see Tomfohrde et al. 1994; Brown et al. 1998).
For type 1 diabetes, larger numbers of families were collected (Davies et al. 1994; Field et al. 1994), and both studies of this disease reported that their best linkage results were for markers in the HLA region on chromosome 6p21. This locus also was found in other studies of type 1 diabetes (Hashimoto et al. 1994; Mein et al. 1998). These findings suggest that, at least in some diseases, the positional-cloning paradigm holds true—that is, an important locus can be found and replicated.
Future Directions
Although, during the past decade, significant progress has been made in defining the genetic basis of complex human diseases, even relatively large studies are likely to have had low power to map, by linkage, genes of modest effect (Risch 2000; Terwilliger and Goring 2000). One potential solution to this and other issues is to combine data from multiple studies. Meta-analysis is an emerging methodology in the linkage analysis of complex-disease genetics (Morton 1995; Rice 1997; Gu et al. 1998; Xu and Meyers 1998; Badner and Goldin 1999; Guerra et al. 1999; Wise and Lewis 1999); the combination of evidence from multiple studies may prove to be critical to the successful localization of genes of modest effect in common complex human diseases (Palmer et al. 2001_a_, 2001_c_; The Transatlantic Multiple Sclerosis Genetics Cooperative 2001).
Our results suggest that some elements of study design are likely to be important in the determination of the relative success of a whole-genome scan. Every individual study and disease is likely to require an optimized study design that takes into account the unique characteristics of both that sample and the phenotypes studied (Terwilliger and Goring 2000). Our review suggests that attention to maximization of sample homogeneity is likely to be particularly important. Unfortunately, this cart is often placed a long distance before the horse: linkage analysis is very often attempted before appropriate descriptive analyses—to allow informed study design and genetic analysis in the sampling frame in question—are undertaken to determine the interrelationships of the phenotypes and covariates being studied; for example, although asthma has been the subject of many genome scans (table 3), only recently have the interrelationships between the underlying genetic determinants of intermediate phenotypes begun to be investigated (Palmer et al. 2000, 2001_b_). It is to be hoped that the recent advent of cheaper and improved computing power, together with methodological advances in complex modeling techniques (e.g., see Zeger and Karim 1991), will lead to the continuing development and application of new computing-intensive statistical methodologies that are ideal for complex genetic modeling. Finally, an understanding of the genetic epidemiology of many diseases would be greatly enhanced by population-based studies. Such studies, although expensive and difficult to undertake, are the foundation of good genetic epidemiology and address many important epidemiological issues, such as generalization (Hopper et al. 1999).
Conclusions
Gene discovery in complex human disease has been complicated by substantial etiological heterogeneity, the possibility of genes of small effect, and the concomitant requirement for large samples. The mapping of human susceptibility loci for such diseases may be made difficult by any or all of the following: high population frequency, incomplete penetrance, phenocopies, genetic heterogeneity, possible epistasis, and pleiotropy (Weeks and Lathrop 1995); replication of any positive results may be difficult, and often the significance of studies' different findings is controversial. As a consequence, the application of linkage analysis to complex disorders without obvious Mendelian inheritance has had limited success thus far.
Genetic approaches to complex diseases offer great potential to improve our understanding of the pathophysiology of these disorders, but they also offer significant challenges. Although the past decade made great progress in defining the genetic basis of such diseases, accompanied by rapid technical progress in genotyping technologies and statistical methodology, further research is required, especially in the area of study design. In particular, the genetic localization of most susceptibility loci is still insufficiently precise for the positional cloning of new genes influencing disease. There have been linkages reported on nearly every autosome, in multiple whole-genome screens for the same disease, and the sheer number of “consensus regions” identified by such screens highlights the difficulty of positional-cloning attempts in common complex diseases such as asthma and diabetes (Lander and Schork 1994; Palmer and Cookson 2000). Whether positional cloning based on whole-genome screens ultimately delivers on its promises for complex human diseases remains to be seen. It may be that high-density SNP association analysis in combination with functional genomic data may prove to be necessary to detect susceptibility loci (which may be of small effect) for many complex human diseases (Risch 2000). In the meantime, like true love, true linkage remains hard to find.
References
- Adams LJ, Mitchell PB, Fielder SL, Rosso A, Donald JA, Schofield PR (1998) A susceptibility locus for bipolar affective disorder on chromosome 4q35. Am J Hum Genet 62:1084–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos C, Laing A (1993) A comparison of univariate and multivariate tests for genetic linkage. Genet Epidemiol 10:671–676 [DOI] [PubMed] [Google Scholar]
- Aouizerat BE, Allayee H, Cantor RM, Davis RC, Lanning CD, Wen PZ, Dallinga-Thie GM, de Bruin TWA, Rotter JI, Lusis AJ (1999) A genome scan for familial combined hyperlipidemia reveals evidence of linkage with a locus on chromosome 11. Am J Hum Genet 65:397–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage P, Berry G (1994) Methods in medical research. Blackwell Scientific, Oxford [Google Scholar]
- Arngrimsson R, Sigurardottir S, Frigge ML, Bjarnadottir RI, Jonsson T, Stefansson H, Baldursdottir A, Einarsdottir AS, Palsson B, Snorradottir S, Lachmaijer AM, Nicolae D, Kong A, Bragason BT, Gulcher JR, Geirsson RT, Stefansson K (1999) A genome-wide scan reveals a maternal susceptibility locus for pre-eclampsia on chromosome 2p13. Hum Mol Genet 8:1799–1805 [DOI] [PubMed] [Google Scholar]
- Badner JA, Goldin LR (1999) Meta-analysis of linkage studies. Genet Epidemiol 17 Suppl 1:S485–S490 [DOI] [PubMed] [Google Scholar]
- Bellamy R (2000) Identifying genetic susceptibility factors for tuberculosis in Africans: a combined approach using a candidate gene study and a genome-wide screen. Clin Sci (Lond) 98:245–250 [PubMed] [Google Scholar]
- Berthon P, Valeri A, Cohen-Akenine A, Drelon E, Paiss T, Wohr G, Latil A, et al (1998) Predisposing gene for early-onset prostate cancer, localized on chromosome 1q42.2-43. Am J Hum Genet 62:1416–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin JL, Dombroski BA, Nath SK, Lasseter VK, Wolyniec PS, Nestadt G, Thornquist M, et al (1998) Schizophrenia susceptibility loci on chromosomes 13q32 and 8p21. Nat Genet 20:70–73 [DOI] [PubMed] [Google Scholar]
- Brown MA, Pile KD, Kennedy LG, Campbell D, Andrew L, March R, Shatford JL, Weeks DE, Calin A, Wordsworth BP (1998) A genome-wide screen for susceptibility loci in ankylosing spondylitis. Arthritis Rheum 41:588–595 [DOI] [PubMed] [Google Scholar]
- Chapman K, Mustafa Z, Irven C, Carr AJ, Clipsham K, Smith A, Chitnavis J, Sinsheimer JS, Bloomfield VA, McCartney M, Cox O, Cardon LR, Sykes B, Loughlin J (1999) Osteoarthritis-susceptibility locus on chromosome 11q, detected by linkage. Am J Hum Genet 65:167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Nicolae DL, Gold LH, Fields CT, LaBuda MC, Rohal PM, Pickles MR, Qin L, Fu Y, Mann JS, Kirschner BS, Jabs EW, Weber J, Hanauer SB, Bayless TM, Brant SR (1998) Identification of novel susceptibility loci for inflammatory bowel disease on chromosomes 1p, 3q, and 4q: evidence for epistasis between 1p and IBD1. Proc Natl Acad Sci USA 95:7502–7507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative Study on the Genetics of Asthma, The (1997) A genome-wide search for asthma susceptibility loci in ethnically diverse populations. Nat Genet 15:389–392 [DOI] [PubMed] [Google Scholar]
- Comuzzie AG, Hixson JE, Almasy L, Mitchell BD, Mahaney MC, Dyer TD, Stern MP, MacCluer JW, Blangero J (1997) A major quantitative trait locus determining serum leptin levels and fat mass is located on human chromosome 2. Nat Genet 15:273–276 [DOI] [PubMed] [Google Scholar]
- Coon H, Jensen S, Hoff M, Holik J, Plaetke R, Reimherr F, Wender P, Leppert M, Byerley W (1993) A genome-wide search for genes predisposing to manic-depression, assuming autosomal dominant inheritance. Am J Hum Genet 52:1234–1249 [PMC free article] [PubMed] [Google Scholar]
- Coon H, Jensen S, Holik J, Hoff M, Myles-Worsley M, Reimherr F, Wender P, Waldo M, Freedman R, Leppert M, Byerley W (1994) Genomic scan for genes predisposing to schizophrenia. Am J Med Genet 54:59–71 [DOI] [PubMed] [Google Scholar]
- Cornelis F, Faure S, Martinez M, Prud’Homme JF, Fritz P, Dib C, Alves H, et al (1998) New susceptibility locus for rheumatoid arthritis suggested by a genome-wide linkage study. Proc Natl Acad Sci USA 95:10746–10750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels SE, Bhattacharrya S, James A, Leaves NI, Young A, Hill MR, Faux JA, Ryan GF, le Souef PN, Lathrop GM, Musk AW, Cookson WOCM (1996) A genome-wide search for quantitative trait loci underlying asthma. Nature 383:247–250 [DOI] [PubMed] [Google Scholar]
- Davies JL, Kawaguchi Y, Bennett ST, Copeman JB, Cordell HJ, Pritchard LE, Reed PW, Gough SCL, Jenkins SC, Palmer SM, Balfour KM, Rowe BR, Farrall M, Barnett AH, Bain SC, Todd JA (1994) A genome-wide search for human type 1 diabetes susceptibility genes. Nature 371:130–136 [DOI] [PubMed] [Google Scholar]
- de la Chapelle A (1993) Disease gene mapping in isolated human populations: the example of Finland. J Med Genet 30:857–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detera-Wadleigh SD, Badner JA, Berrettini WH, Yoshikawa T, Goldin LR, Turner G, Rollins DY Moses T, Sanders AR, Karkera JD, Esterling LE, Zeng J, Ferraro TN, Guroff JJ, Kazuba D, Maxwell ME, Nurnberger JI Jr, Gershon ES (1999) A high-density genome scan detects evidence for a bipolar-disorder susceptibility locus on 13q32 and other potential loci on 1q32 and 18p11.2. Proc Natl Acad Sci USA 96:5604–5609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto M, Shimoya K, Caminis J, Ott J, Tenenhouse A, Whyte MP, Sereda L, Hall S, Considine E, Williams CJ, Tromp G, Kuivaniemi H, Ala-Kokko L, Prockop DJ, Spotila LD (1998) First-stage autosomal genome screen in extended pedigrees suggests genes predisposing to low bone mineral density on chromosomes 1p, 2p and 4q. Eur J Hum Genet 6:151–157 [DOI] [PubMed] [Google Scholar]
- Dizier MH, Besse-Schmittler C, Guilloud-Bataille M, Annesi-Maesano I, Boussaha M, Bousquet J, Charpin D, et al (2000) Genome screen for asthma and related phenotypes in the French EGEA study. Am J Respir Crit Care Med 162:1812–1818 [DOI] [PubMed] [Google Scholar]
- Duerr RH, Barmada MM, Zhang L, Pfützer R, Weeks DE (2000) High-density genome scan in Crohn disease shows confirmed linkage to chromosome 14q11-12. Am J Hum Genet 66:1857–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggirala R, Blangero J, Almasy L, Dyer TD, Williams KL, Leach RJ, O’Connell P, Stern MP (1999) Linkage of type 2 diabetes mellitus and of age at onset to a genetic location on chromosome 10q in Mexican Americans. Am J Hum Genet 64:1127–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebers GC, Kukay K, Bulman DE, Sadovnick AD, Rice G, Anderson C, Armstrong H, et al (1996) A full genome search in multiple sclerosis. Nat Genet 13:472–476 [DOI] [PubMed] [Google Scholar]
- Ehm MG, Karnoub MC, Sakul H, Gottschalk K, Holt DC, Weber JL, Vaske D, Briley D, Briley L, Kopf J, McMillen P, Nguyen Q, Reisman M, Lai EH, Joslyn G, Shepherd NS, Bell C, Wagner MJ, Burns DK, American Diabetes Association GENNID Study Group (2000) Genomewide search for type 2 diabetes susceptibility genes in four American populations. Am J Hum Genet 66:1871–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekelund J, Lichtermann D, Hovatta I, Ellonen P, Suvisaari J, Terwilliger JD, Juvonen H, Varilo T, Arajärvi R, Kokko-Sahin ML, Lönnqvist J, Peltonen L (2000) Genome-wide scan for schizophrenia in the Finnish population: evidence for a locus on chromosome 7q22. Hum Mol Genet 9:1049–1057 [DOI] [PubMed] [Google Scholar]
- Elbein SC, Hoffman MD, Teng K, Leppert MF Hasstedt SJ (1999) A genome-wide search for type 2 diabetes susceptibility genes in Utah Caucasians. Diabetes 48:1175–1182 [DOI] [PubMed] [Google Scholar]
- Faraone SV, Matise T, Svrakic D, Pepple J, Malaspina D, Suarez B, Hampe C, Zambuto CT, Schmitt K, Meyer J, Markel P, Lee H, Harkavy-Friedman J, Kaufmann C, Cloninger CR, Tsuang MT (1998) Genome scan of European-American schizophrenia pedigrees: results of the NIMH Genetics Initiative and Millennium Consortium. Am J Med Genet 81:290–295 [PubMed] [Google Scholar]
- Farrer LA, Cupples LA (1998) Determining the genetic component of a disease. In: Haines JL, Pericak-Vance MA (eds) Approaches to gene mapping in complex human diseases. Wiley-Liss, New York, pp 93–129 [Google Scholar]
- Field LL, Tobias R, Magnus T (1994) A locus on chromosome 15q26 (IDDM3) produces susceptibility to insulin-dependent diabetes mellitus Nat Genet 8:189–194 [DOI] [PubMed] [Google Scholar]
- Friddle C, Koskela R, Ranade K, Hebert J, Cargill M, Clark CD, McInnis M, Simpson S, McMahon F, Stine OC, Meyers D, Jianfeng X, MacKinnon D, Swift-Scanlan T, Jamison K, Folstein S, Daly M, Kruglyak L, Marr T, DePaulo JR, Botstein D (2000) Full-genome scan for linkage in 50 families segregating the bipolar affective disease phenotype. Am J Hum Genet 66:205–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney PM, Kearns GM, Shark KB, Ortmann WA, Selby SA, Malmgren ML, Rohlf KE, Ockenden TC, Messner RP, King RA, Rich SS, Behrens TW (1998) A genome-wide search for susceptibility genes in human systemic lupus erythematosus sib-pair families. Proc Natl Acad Sci USA 95:14875–14879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Watanabe RM, Hauser ER, Valle T, Magnuson VL, Erdos MR, Langefeld CD, et al (2000) The Finland-United States investigation of non–insulin-dependent diabetes mellitus genetics (FUSION) study. I. An autosomal genome scan for genes that predispose to type 2 diabetes. Am J Hum Genet 67:1174–1185 [PMC free article] [PubMed] [Google Scholar]
- Gibbs M, Stanford JL, McIndoe RA, Jarvik GP, Kolb S, Goode EL, Chakrabarti L, Schuster EF, Buckley VA, Miller EL, Brandzel S, Li S, Hood L, Ostrander EA (1999) Evidence for a rare prostate cancer-susceptibility locus at chromosome 1p36. Am J Hum Genet 64:776–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginns EI, Ott J, Egeland JA, Allen CR, Fann CSJ, Pauls DL, Weissenbach J, Carulli JP, Falls KM, Keith TP, Paul SM (1996) A genome-wide search for chromosomal loci linked to bipolar affective disorder in the Old Order Amish. Nat Genet 12:431–435 [DOI] [PubMed] [Google Scholar]
- Ginns EI, St Jean P, Philibert RA, Galdzicka M, Damschroder-Williams P, Thiel B, Long RT, et al (1998) A genome-wide search for chromosomal loci linked to mental health wellness in relatives at high risk for bipolar affective disorder among the Old Order Amish. Proc Natl Acad Sci USA 95:15531–15536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Province M, Todorov A, Rao DC (1998) Meta-analysis methodology for combining non-parametric sibpair linkage results: genetic homogeneity and identical markers. Genet Epidemiol 15:609–626 [DOI] [PubMed] [Google Scholar]
- Guerra R, Etzel CJ, Goldstein DR, Sain SR (1999) Meta-analysis by combining p-values: simulated linkage studies. Genet Epidemiol 17 Suppl 1:S605–S609 [DOI] [PubMed] [Google Scholar]
- Hager J, Dina C, Francke S, Dubois S, Houari M, Vatin V, Vaillant E, Lorentz N, Basdevant A, Clement K, Guy-Grand B, Froguel P (1998) A genome-wide scan for human obesity genes reveals a major susceptibility locus on chromosome 10. Nat Genet 20:304–308 [DOI] [PubMed] [Google Scholar]
- Haines JL, Ter-Minassian M, Bazyk A, Gusella JF, Kim DJ, Terwedow H, Pericak-Vance MA, et al (1996) A complete genomic screen for multiple sclerosis underscores a role for the major histocompatibility complex: The Multiple Sclerosis Genetics Group. Nat Genet 13:469–471 [DOI] [PubMed] [Google Scholar]
- Hampe J, Schreiber S, Shaw SH, Lau KF, Bridger S, Macpherson AJS, Cardon LR, Sakul H, Harris TJR, Buckler A, Hall J, Stokkers P, van Deventer SJH, Nürnberg P, Mirza MM, Lee JCW, Lennard-Jones JE, Mathew CG, Curran ME (1999) A genomewide analysis provides evidence for novel linkages in inflammatory bowel disease in a large European cohort. Am J Hum Genet 64:808–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanis CL, Boerwinkle E, Chakraborty R, Ellsworth DL, Concannon P, Stirling B, Morrison VA, et al (1996) A genome-wide search for human non-insulin-dependent (type 2) diabetes genes reveals a major susceptibility locus on chromosome 2. Nat Genet 13:161–166 [DOI] [PubMed] [Google Scholar]
- Hanson RL, Ehm MG, Pettitt DJ, Prochazka M, Thompson DB, Timberlake D, Foroud T, Kobes S, Baier L, Burns DK, Almasy L, Blangero J, Garvey WT, Bennett PH, Knowler WC (1998) An autosomal genomic scan for loci linked to type II diabetes mellitus and body-mass index in Pima Indians. Am J Hum Genet 63:1130–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison GA, Humphrey KE, Jones N, Badenhop R, Guo G, Elakis G, Kaye JA, Turner RJ, Grehan M, Wilton AN, Brennecke SP, Cooper DW (1997) A genomewide linkage study of preeclampsia/eclampsia reveals evidence for a candidate region on 4q. Am J Hum Genet 60:1158–1167 [PMC free article] [PubMed] [Google Scholar]
- Hashimoto L, Habita C, Beressi JP, Delepine M, Besse C, Cambon-Thomsen A, Dechamps I, Rotter JI, Djoulah S, James MR, Froguel P, Weissenbach J, Lathrop GM, Julier C (1994) Genetic mapping of a susceptibility locus for insulin-dependent diabetes mellitus on chromosome 11q. Nature 371:161–164 [DOI] [PubMed] [Google Scholar]
- Hegele RA, Sun F, Harris SB, Anderson C, Hanley AJG, Zinman B (1999) Genome-wide scanning for type 2 diabetes susceptibility in Canadian Oji-Cree, using 190 microsatellite markers. J Hum Genet 44:10–14 [DOI] [PubMed] [Google Scholar]
- Hopper JL, Chenevix-Trench G, Jolley DJ, Dite GS, Jenkins MA, Venter DJ, McCredie MR, Giles GG (1999) Design and analysis issues in a population-based, case-control-family study of the genetic epidemiology of breast cancer and the Co-operative Family Registry for Breast Cancer Studies (CFRBCS). J Natl Cancer Inst Monogr 26:95–100 [DOI] [PubMed] [Google Scholar]
- Hovatta I, Varilo T, Suvisaari J, Terwilliger JD, Ollikainen V, Arajärvi R, Juvonen H, Kokko-Sahin ML, Väisänen L, Mannila H, Lönnqvist J, Peltonen L (1999) A genomewide screen for schizophrenia genes in an isolated Finnish subpopulation, suggesting multiple susceptibility loci. Am J Hum Genet 65:1114–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugot JP, Laurent-Pulg P, Gower-Rousseau C, Olson JM, Lee JC, Beaugerie L, Naom I, Dupas Jl, Van Gossum A, Groupe d’Etude Therapeutique des Affections Inflammatoires Digestives, Orholm M, Bonaïti-Pellié C, Weissenbach J, Mathew CG, Lennard-Jones JE, Cortot A, Colombel JF, Thomas G (1996) Mapping of a susceptibility locus for Crohn's disease on chromosome 16. Nature 379:821–823 [DOI] [PubMed] [Google Scholar]
- International Molecular Genetic Study of Autism Consortium (1998) A full genome screen for autism with evidence for linkage to a region on chromosome 7q. Hum Mol Genet 7:571–578 [DOI] [PubMed] [Google Scholar]
- Jorde L (1995) Linkage disequilibrium as a gene-mapping tool. Am J Hum Genet 56:11–14 [PMC free article] [PubMed] [Google Scholar]
- Kaufmann CA, Suarez B, Malaspina D, Pepple J, Svrakic D, Markel PD, Meyer J, Zambuto CT, Schmitt K, Matise TC, Friedman JMH, Hampe C, Lee H, Shore D, Wynne D, Faraone SV, Tsuang MT, Cloninger CR (1998) NIMH Genetics Initiative Millennium Schizophrenia Consortium: linkage analysis of African-American pedigrees. Am J Med Genet 81:282–289 [PubMed] [Google Scholar]
- Kehoe P, Wavrant-De Vrieze F, Crook R, Wu WS, Holmans P, Fenton I, Spurlock G, Norton N, Williams H, Williams N, Lovestone S, Perez-Tur J, Hutton M, Chartier-Harlin MC, Shears S, Roehl K, Booth J, Van Voorst W, Ramic D, Williams J, Goate A, Hardy J, Owen MJ (1999) A full genome scan for late onset Alzheimer's disease. Hum Mol Genet 8:237–245 [DOI] [PubMed] [Google Scholar]
- Knowles JA, Fyer AJ, Vieland VJ, Weissman MM, Hodge SE, Heiman GA, Haghighi F, de Jesus GM, Rassnick H, Preud’homme-Rivelli X, Austin T, Cunjak J, Mick S, Fine LD, Woodley KA, Das K, Maier W, Adams PB, Freimer NB, Klein DF, Gilliam TC (1998) Results of a genome-wide genetic screen for panic disorder. Am J Med Genet 81:139–147 [DOI] [PubMed] [Google Scholar]
- Krushkal J, Ferrell R, Mockrin SC, Turner ST, Sing CF, Boerwinkle E (1999) Genome-wide linkage analyses of systolic blood pressure using highly discordant siblings. Circulation 99:1407–1410 [DOI] [PubMed] [Google Scholar]
- Kuokkanen S, Gschwend M, Rioux JD, Daly MJ, Terwilliger JD, Tienari PJ, Wikström J, Palo J, Stein LD, Hudson TJ, Lander ES, Peltonen L (1997) Genomewide scan of multiple sclerosis in Finnish multiplex families. Am J Hum Genet 61:1379–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E, Kruglyak L (1995) Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 11:241–247 [DOI] [PubMed] [Google Scholar]
- Lander E, Schork N (1994) Genetic dissection of complex traits. Science 265:2037–2048 [DOI] [PubMed] [Google Scholar]
- Leahy MG, Tonks S, Moses JH, Brett AR, Huddart R, Forman D, Oliver RTD, Bishop DT, Bodmer JG (1995) Candidate regions for a testicular cancer susceptibility gene. Hum Mol Genet 4:1551–1555 [DOI] [PubMed] [Google Scholar]
- Lee JH, Reed DR, Li WD, Xu W Joo EJ, Kilker RL, Nanthakumar E, North M, Sakul H, Bell C, Price RA (1999) Genome scan for human obesity and linkage to markers in 20q13. Am J Hum Genet 64:196–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YA, Rüschendorf F, Windemuth C, Schmitt-Egenolf M, Stadelmann A, Nürnberg G, Ständer M, Wienker TF, Reis A, Traupe H (2000_a_) Genomewide scan in German families reveals evidence for a novel psoriasis-susceptibility locus on chromosome 19p13. Am J Hum Genet 67:1020–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YA, Wahn U, Kehrt R, Tarani L, Businco L, Gustafsson D, Andersson F, Oranje AP, Wolkertstorfer A, v Berg A, Hoffmann U, Küster W, Wienker T, Rüschendorf F, Reis A (2000_b_) A major susceptibility locus for atopic dermatitis maps to chromosome 3q21. Nat Genet 26:470–473 [DOI] [PubMed] [Google Scholar]
- Leppavuori J, Kujala U, Kinnunen J, Kaprio J, Nissilä M, Heliövaara M, Klinger N, Partanen J, Terwilliger JD, Peltonen L (1999) Genome scan for predisposing loci for distal interphalangeal joint osteoarthritis: evidence for a locus on 2q. Am J Hum Genet 65:1060–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson DF, Mahtani MM, Nancarrow DJ, Brown DM, Kruglyak L, Kirby A, Hayward NK, et al (1998) Genome scan of schizophrenia. Am J Psychiatry 155:741–750 [DOI] [PubMed] [Google Scholar]
- Long JC, Knowler WC, Hanson RL, Robin RW, Urbanek M, Moore E, Bennett PH, Goldman D (1998) Evidence for genetic linkage to alcohol dependence on chromosomes 4 and 11 from an autosome-wide scan in an American Indian population. Am J Med Genet 81:216–221 [DOI] [PubMed] [Google Scholar]
- Lonjou C, Collins A, Morton NE (1999) Allelic association between marker loci. Proc Natl Acad Sci USA 96:1621–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo DF, Bui MM, Muir A, Maclaren NK, Thomson G, She JX (1995) Affected-sib-pair mapping of a novel susceptibility gene to insulin-dependent diabetes mellitus (IDDM8) on chromosome 6q25-q27. Am J Hum Genet 57:911–919 [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Ohmen JD, Li Z, Bentley LG, McElree C, Pressman S, Targan SR, Fischel-Ghodsian N, Rotter JI, Yang H (1999) A genome-wide search identifies potential new susceptibility loci for Crohn's disease. Inflamm Bowel Dis 5:271–278 [DOI] [PubMed] [Google Scholar]
- Mahtani MM, Widen E, Lehto M, Thomas J, McCarthy M, Brayer J, Bryant B, Chan G, Daly M, Forsblom C, Kanninen T, Kirby A, Kruglyak L, Munnelly K, Parkkonen M, Reeve-Daly MP, Weaver A, Brettin T, Duyk G, Lander ES, Groop LC (1996) Mapping of a gene for type 2 diabetes associated with an insulin secretion defect by a genome scan in Finnish families. Nat Genet 14:90–94 [DOI] [PubMed] [Google Scholar]
- Matthews D, Fry L, Powles A, Weber J, McCarthy M, Fisher E, Davies K, Williamson R (1996) Evidence that a locus for familial psoriasis maps to chromosome 4q. Nat Genet 14:231–233 [DOI] [PubMed] [Google Scholar]
- McCullagh P, Nelder J (1989) Generalized linear models. Chapman & Hall, London [Google Scholar]
- McInnes LA, Escamilla MA, Service SK, Reus VI, Leon P, Silva S, Rojas E, Spesny M, Baharloo S, Blankenship K, Peterson A, Tyler D, Shimayoshi N, Tobey C, Batki S, Vinogradov S, Meza L, Gallegos A, Fournier E, Smith LB, Barondes SH, Sandkuijl LA, Freimer NB (1996) A complete genome screen for genes predisposing to severe bipolar disorder in two Costa Rican pedigrees. Proc Natl Acad Sci USA 93:13060–13065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta CR (1994) The exact analysis of contingency tables in medical research. Stat Methods Med Res 3:135–156 [DOI] [PubMed] [Google Scholar]
- Mein CA, Esposito L, Dunn MG, Johnson GCL, Timms AE, Goy JV, Smith AN, Sebag-Montefiore L, Merriman ME, Wilson AJ, Pritchard LE, Cucca F, Barnett AH, Bain SC, Todd JA (1998) A search for type 1 diabetes susceptibility genes in families from the United Kingdom. Nat Genet 19:297–300 [DOI] [PubMed] [Google Scholar]
- Moises HW, Yang L, Kristbjarnarson H, Wiese C, Byerley W, Macciardi F, Arolt V, et al (1995) An international two-stage genome-wide search for schizophrenia susceptibility genes. Nat Genet 11:321–324 [DOI] [PubMed] [Google Scholar]
- Morissette J, Villeneuve A, Bordeleau L, Rochette D, Laberge C, Gagne B, Laprise C, Bouchard G, Plante M, Gobeil L, Shink E, Weissenbach J, Barden N (1999) Genome-wide search for linkage of bipolar affective disorders in a very large pedigree derived from a homogeneous population in Quebec points to a locus of major effect on chromosome 12q23-q24. Am J Med Genet 88:567–587 [DOI] [PubMed] [Google Scholar]
- Morton NE (1995) Meta-analysis in complex diseases. Clin Exp Allergy 25 Suppl 2:110–112 [DOI] [PubMed] [Google Scholar]
- Moser KL, Neas BR, Salmon JE, Yu H, Gray-McGuire C, Asundi N, Bruner GR, Fox J, Kelly J, Henshall S, Bacino D, Dietz M, Hogue R, Koelsch G, Nightingale L, Shaver T, Abdou NI, Albert DA, Carson C, Petri M, Treadwell EL, James JA, Harley JB (1998) Genome scan of human systemic lupus erythematosus: evidence for linkage on chromosome 1q in African-American pedigrees. Proc Natl Acad Sci USA 95:14869–14874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair RP, Henseler T, Jenisch S, Stuart P, Bichakjian CK, Lenk W, Westphal E, Guo SW, Christophers E, Voorhees JJ, Elder JT (1997) Evidence for two psoriasis susceptibility loci (HLA and 17q) and two novel candidate regions (16q and 20p) by genome-wide scan. Hum Mol Genet 6:1349–1356 [DOI] [PubMed] [Google Scholar]
- Niu T, Chen C, Cordell H, Yang J, Wang B, Wang Z, Fang Z, Schork NJ, Rosen CJ, Xu X (1999) A genome-wide scan for loci linked to forearm bone mineral density. Hum Genet 104:226–233 [DOI] [PubMed] [Google Scholar]
- Norman RA, Tataranni PA, Pratley R, Thompson DB, Hanson RL, Prochazka M, Baier L, Ehm MG, Sakul H, Foroud T, Garvey WT, Burns D, Knowler WC, Bennett PH, Bogardus C, Ravussin E (1998) Autosomal genomic scan for loci linked to obesity and energy metabolism in Pima Indians. Am J Hum Genet 62:659–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober C, Cox NJ, Abney M, Di Rienzo A, Lander ES, Changyaleket B, Gidley H, Kurtz B, Lee J, Nance M, Pettersson A, Prescott J, Richardson A, Schlenker E, Summerhill E, Willadsen S, Parry R (1998) Genome-wide search for asthma susceptibility loci in a founder population: The Collaborative Study on the Genetics of Asthma. Hum Mol Genet 7:1393–1398 [DOI] [PubMed] [Google Scholar]
- Ober C, Tsalenko A, Parry R, Cox NJ (2000) A second-generation genomewide screen for asthma-susceptibility alleles in a founder population. Am J Hum Genet 67:1154–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober C, Tsalenko A, Willadsen S, Newman D, Daniel R, Wu X, Andal J, Hoki D, Schneider D, True K, Schou C, Parry R, Cox N (1999) Genome-wide screen for atopy susceptibility alleles in the Hutterites. Clin Exp Allergy 29 Suppl 4:11–15 [PubMed] [Google Scholar]
- Ohman M, Oksanen L, Kaprio J, Koskenvuo M, Mustajoki P, Rissanen A, Salmi J, Kontula K, Peltonen L (2000) Genome-wide scan of obesity in Finnish sibpairs reveals linkage to chromosome Xq24. J Clin Endocrinol Metab 85:3183–3190 [DOI] [PubMed] [Google Scholar]
- Pajukanta P, Terwilliger JD, Perola M, Hiekkalinna T, Nuotio I, Ellonen P, Parkkonen M, Hartiala J, Ylitalo K, Pihlajamäki J, Porkka K, Laakso M, Viikari J, Ehnholm C, Taskinen MR, Peltonen L (1999) Genomewide scan for familial combined hyperlipidemia genes in Finnish families, suggesting multiple susceptibility loci influencing triglyceride, cholesterol, and apolipoprotein B levels. Am J Hum Genet 64:1453–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LJ , Barnes KC, Burton PR, Chen H, Cookson WOCM, CSGA, Deichmann KA, Elsron RC, Holloway JW, Jacobs KB, Laitinen T, Wjst M (2001_a_) Meta-analysis for linkage to asthma and atopy in the chromosome 5q31-33 candidate region. Hum Mol Genet 10:891–899 [DOI] [PubMed] [Google Scholar]
- Palmer LJ, Burton PR, Faux JA, James AL, Musk AW, Cookson WOCM (2000) Independent inheritance of serum Immunoglobulin E concentrations and airway responsiveness. Am J Respir Crit Care Med 161:1836–1843 [DOI] [PubMed] [Google Scholar]
- Palmer LJ, Cookson WOCM (2000) Genomic approaches to understanding asthma. Genome Res 10:1280–1287 [DOI] [PubMed] [Google Scholar]
- Palmer LJ, Cookson WOCM, James AL, Musk AW, Burton PR (2001_b_) Gibbs-sampling based segregation analysis of asthma-associated quantitative traits in a population-based sample of nuclear families. Genet Epidemiol 20:356–372 [DOI] [PubMed] [Google Scholar]
- Palmer LJ, Lonjou C, Barnes K, Chen H, Cookson WO, Deichmann KA, Holloway JW, Laitinen T, Wjst M, Morton NE (2001_c_) Special report: a retrospective collaboration on chromosome 5 by the International Consortium on Asthma Genetics (COAG). Clin Exp Allergy 31:152–154 [PubMed] [Google Scholar]
- Pericak-Vance MA, Bass MP, Yamaoka LH, Gaskell PC, Scott WK, Terwedow HA, Menold MM, Conneally PM, Small GW, Vance JM, Saunders AM, Roses AD, Haines JL (1997) Complete genomic screen in late-onset familial Alzheimer disease: evidence for a new locus on chromosome 12. JAMA 278:1237–1241 [PubMed] [Google Scholar]
- Philippe A, Martinez M, Guilloud-Bataille M, Gillberg C, Rastam M, Sponheim E, Coleman M, Zappella M, Aschauer H, van Malldergerme L, Penet C, Feingold J, Brice A, Leboyer M (1999) Genome-wide scan for autism susceptibility genes: Paris Autism Research International Sibpair Study. Hum Mol Genet 8:805–812 [DOI] [PubMed] [Google Scholar]
- Pratley RE, Thompson DB, Prochazka M, Baier L, Mott D, Ravussin E, Sakui H, Ehm MG, Burns DK, Foroud T, Garvey WT, Hanson RL, Knowler WC, Bennett PH, Bogardus C (1998) An autosomal genomic scan for loci linked to prediabetic phenotypes in Pima Indians. J Clin Invest 101:1757–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainwater DL, Almasy L, Blangero J, Cole SA, VandeBerg JL, MacCluer JW, Hixson JE (1999) A genome search identifies major quantitative trait loci on human chromosomes 3 and 4 that influence cholesterol concentrations in small LDL particles. Arterioscler Thromb Vasc Biol 19:777–783 [DOI] [PubMed] [Google Scholar]
- Rao DC (2001) Genetic dissection of complex traits: an overview. Adv Genet 42:13–34 [DOI] [PubMed] [Google Scholar]
- Rapley EA, Crockford GP, Teare D, Biggs P, Seal S, Barfoot R, Edwards S, et al (2000) Localization to Xq27 of a susceptibility gene for testicular germ-cell tumours. Nat Genet 24:197–200 [DOI] [PubMed] [Google Scholar]
- Reed P, Davies J, Copeman J, Bennett S, Palmer S, Pritchard L, Gough S, et al (1994) Chromosome-specific microsatellite sets for fluorescence-based, semi-automated genome mapping. Nat Genet 7:390–395 [DOI] [PubMed] [Google Scholar]
- Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, Foroud T, Hesselbrock V, Schuckit MA, Bucholz K, Porjesz B, Li TK, Conneally PM, Nurnberger JI Jr, Tischfield JA, Crowe RR, Cloninger CR, Wu W, Shears S, Carr K, Crose C, Willig C, Begleiter H (1998) Genome-wide search for genes affecting the risk for alcohol dependence. Am J Med Genet 81:207–215 [PubMed] [Google Scholar]
- Rice JP (1997) The role of meta-analysis in linkage studies of complex traits. Am J Med Genet 74:112–114 [DOI] [PubMed] [Google Scholar]
- Rice T, Rankinen T, Province MA, Chagnon YC, Pérusse L, Borecki IB, Bouchard C, Rao DC (2000) Genome-wide linkage analysis of systolic and diastolic blood pressure: the Quebec family study. Circulation 102:1956–1963 [DOI] [PubMed] [Google Scholar]
- Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, McLeod RS, Griffiths AM, Green T, Brettin TS, Stone V, Bull SB, Bitton A, Williams CN, Greenberg GR, Cohen Z, Lander ES, Hudson TJ, Siminovitch KA (2000) Genomewide search in Canadian families with inflammatory bowel disease reveals two novel susceptibility loci. Am J Hum Genet 66:1863–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N (1990) Linkage strategies for genetically complex traits. I. Multilocus models. Am J Hum Genet 46:222–228 [PMC free article] [PubMed] [Google Scholar]
- ——— (2000) Searching for genetic determinants in the new millennium. Nature 405:847–56 [DOI] [PubMed] [Google Scholar]
- Samuelsson L, Enlund F, Torinsson A, Yhr M, Inerot A, Enerbäck C, Wahlström J, Swanbeck G, Martinsson T (1999) A genome-wide search for genes predisposing to familial psoriasis by using a stratification approach. Hum Genet 105:523–529 [DOI] [PubMed] [Google Scholar]
- Satsangi J, Parkes M, Louis E, Hashimoto L, Kato N, Welsh K, Terwilliger JD, Lathrop GM, Bell JI, Jewell DP (1996) Two stage genome-wide search in inflammatory bowel disease provides evidence for susceptibility loci on chromosomes 3, 7 and 12. Nat Genet 14:199–202 [DOI] [PubMed] [Google Scholar]
- Sawcer S, Jones HB, Feakes R, Gray J, Smaldon N, Chataway J, Robertson N, Clayton D, Goodfellow PN, Compston A (1996) A genome screen in multiple sclerosis reveals susceptibility loci on chromosome 6p21 and 17q22. Nat Genet 13:464–468 [DOI] [PubMed] [Google Scholar]
- Shai R, Quismorio FP Jr, Li L, Kwon OJ, Morrison J, Wallace DJ, Neuwelt CM, Brautbar C, Gauderman WJ, Jacob CO (1999) Genome-wide screen for systemic lupus erythematosus susceptibility genes in multiplex families. Hum Mol Genet 8:639–644 [DOI] [PubMed] [Google Scholar]
- Sharma P, Fatibene J, Ferraro F, Jia H, Monteith S, Brown C, Clayton D, O’Shaughnessy K, Brown MJ (2000) A genome-wide search for susceptibility loci to human essential hypertension. Hypertension 35:1291–1296 [DOI] [PubMed] [Google Scholar]
- Shaw SH, Kelly M, Smith AB, Shields G, Hopkins PJ, Loftus J, Laval SH, Vita A, De Hert M, Cardon LR, Crow TJ, Sherrington R, DeLisi LE (1998) A genome-wide search for schizophrenia susceptibility genes. Am J Med Genet 81:364–376 [DOI] [PubMed] [Google Scholar]
- Shiozawa S, Hayashi S, Tsukamoto Y, Goko H, Kawasaki H, Wada T, Shimizu K, Yasuda N, Kamatani N, Takasugi K, Tanaka Y, Shizawa K, Imura S (1998) Identification of the gene loci that predispose to rheumatoid arthritis. Int Immunol 10:1891–1895 [DOI] [PubMed] [Google Scholar]
- Smith JR, Freije D, Carpten JD, Grönberg H, Xu J, Isaacs SD, Brownstein MJ, Bova S, Guo H, Bujnovszky P, Nusskern DR, Damber JE, Bergh A, Emanuelsson M, Kallioniemi OP, Walker-Daniels J, Bailey-Wilson JE, Beaty TH, Meyers DA, Walsh PC, Collins FS, Trent JM, Isaacs WB (1996) Major susceptibility locus for prostate cancer on chromosome 1 suggested by a genome-wide search. Science 274:1371–1374 [DOI] [PubMed] [Google Scholar]
- Stern MP, Duggirala R, Mitchell BD, Reinhart LJ, Shivakumar S, Shipman PA, Uresandi OC, Benavides E, Blangero J, O’Connell P (1996) Evidence for linkage of regions on chromosomes 6 and 11 to plasma glucose concentrations in Mexican Americans. Genome Res 6:724–734 [DOI] [PubMed] [Google Scholar]
- Suarez BK, Lin J, Burmester JK, Broman KW, Weber JL, Banerjee TK, Goddard KAB, Witte JS, Elston RC, Catalona WJ (2000) A genome screen of multiplex sibships with prostate cancer. Am J Hum Genet 66:933–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susol E, MacGregor AJ, Barrett JH, Wilson H, Black C, Welsh K, Silman A, Ollier B, Worthington J (2000) A two-stage, genome-wide screen for susceptibility loci in primary Raynaud's phenomenon. Arthritis Rheum 43:1641–1646 [DOI] [PubMed] [Google Scholar]
- Terwilliger JD, Goring HH (2000) Gene mapping in the 20th and 21st centuries: statistical methods, data analysis, and experimental design. Hum Biol 72:63–132 [PubMed] [Google Scholar]
- Tomfohrde J, Silverman A, Barnes R, Fernandez-Vina MA, Young M, Lory D, Morris L, Wuepper KD, Stastny P, Menter A, Bowcock A (1994) Gene for familial psoriasis susceptibility mapped to the distal end of human chromosome 17q. Science 264:1141–1145 [DOI] [PubMed] [Google Scholar]
- Transatlantic Multiple Sclerosis Genetics Cooperative, The (2001) A meta-analysis of genomic screens in multiple sclerosis. Mult Scler 7:3–11 [DOI] [PubMed] [Google Scholar]
- Trembath RC, Clough RL, Rosbotham JL, Jones AB, Camp RDR, Frodsham A, Browne J, Barber R, Terwilliger J, Lathrop GM, Barker JNWN (1997) Identification of a major susceptibility locus on chromosome 6p and evidence for further disease loci revealed by a two stage genome-wide search in psoriasis. Hum Mol Genet 6:813–820 [DOI] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, et al (2001) The sequence of the human genome. Science 291:1304–1351 [DOI] [PubMed] [Google Scholar]
- Watanabe RM, Ghosh S, Langefeld CD, Valle TT, Hauser ER, Magnuson VL, Mohlke KL, et al (2000) The Finland-United States investigation of non–insulin-dependent diabetes mellitus genetics (FUSION) study. II. An autosomal genome scan for diabetes-related quantitative-trait loci. Am J Hum Genet 67:1186–1200 [PMC free article] [PubMed] [Google Scholar]
- Weeks D, Lathrop G (1995) Polygenic disease: methods for mapping complex disease traits. Trends Genet 11:513–519 [DOI] [PubMed] [Google Scholar]
- Wiggs JL, Allingham RR, Hossain A, Kern J, Auguste J, DelBono EA, Broomer B, Graham FL, Hauser M, Pericak-Vance M, Haines JL (2000) Genome-wide scan for adult onset primary open angle glaucoma. Hum Mol Genet 9:1109–1117 [DOI] [PubMed] [Google Scholar]
- Williams NM, Rees MI, Holmans P, Norton N, Cardno AG, Jones LA, Murphy KC, Sanders RD, McCarthy G, Gray MY, Fenton I, McGuffin P, Owen MJ (1999) A two-stage genome scan for schizophrenia susceptibility genes in 196 affected sibling pairs. Hum Mol Genet 8:1729–1739 [DOI] [PubMed] [Google Scholar]
- Wise LH, Lewis CM (1999) A method for meta-analysis of genome searches: application to simulated data. Genet Epidemiol 17 Suppl 1:S767–S771 [DOI] [PubMed] [Google Scholar]
- Witte JS, Goddard KAB, Conti DV, Elston RC, Lin J, Suarez BK, Broman KW, Burmester JK, Weber JL, Catalona WJ (2000) Genomewide scan for prostate cancer-aggressiveness loci. Am J Hum Genet 67:92–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wjst M, Fischer G, Immervoll T, Jung M, Saar K, Rueschendorf F, Reis A, et al (1999) A genome-wide search for linkage to asthma: German Asthma Genetics Group. Genomics 58:1–8 [DOI] [PubMed] [Google Scholar]
- Xu J, Meyers DA (1998) Approaches to meta analysis in genetic disorders. Clin Exp Allergy 28 Suppl 1:106–110 [DOI] [PubMed] [Google Scholar]
- Xu J, Postma DS, Howard TD, Koppelman GH, Zheng SL, Stine OC, Bleecker ER, Meyers DA (2000) Major genes regulating total serum immunoglobulin E levels in families with asthma. Am J Hum Genet 67:1163–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Rogus JJ, Terwedow HA, Yang J, Wang Z, Chen C, Niu T, Wang B, Xu H, Weiss S, Schork NJ, Fang Z (1999) An extreme-sib-pair genome scan for genes regulating blood pressure. Am J Hum Genet 64:1694–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokouchi Y, Nukaga Y, Shibasaki M, Noguchi E, Kimura K, Ito S, Nishihara M, Yamakawa-Kobayashi K, Takeda K, Imoto N, Ichikawa K, Matsui A, Hamaguchi H, Arinami T (2000) Significant evidence for linkage of mite-sensitive childhood asthma to chromosome 5q31-q33 near the interleukin 12 B locus by a genome-wide search in Japanese families. Genomics 66:152–160 [DOI] [PubMed] [Google Scholar]
- Zeger S, Karim M (1991) Generalized linear models with random effects: a Gibbs sampling approach. J Am Stat Assoc 86:79–86 [Google Scholar]
- Zielenski J, Tsui L (1995) Cystic fibrosis—genotypic and phenotypic variations. Annu Rev Genet 29:777–807 [DOI] [PubMed] [Google Scholar]