Th17 cells: from precursors to players in inflammation and infection (original) (raw)
Abstract
Upon activation, naive CD4+ T cells differentiate into different lineages of effector Th subsets. Each subset is characterized by its unique cytokine profile and biological functions. Th17, a newly described Th subset that produces IL-17, IL-17F and IL-22 in preference to other cytokines, has been shown to play an important role in clearing specific pathogens and in inducing autoimmune tissue inflammations. Over the last 2–3 years, significant progress has been made to understand the development and biological functions of Th17 subset. Transforming growth factor β (TGF) together with IL-6 or IL-21 initiates the differentiation while IL-23 stabilizes the generation of Th17 cells. The transcription factors of Th17 cells [retinoid-related orphan receptor (ROR) γt, ROR-α and signal transducer and activator of transcription-3] have been described recently. Since TGF-β is essential for the generation of both Th17 and regulatory T (Treg) cells from naive T cells, which suggests a developmental link between Th17 and Treg cells. Functions of these two subsets of T cells are, however, opposite to each other; Th17 cells are highly pathogenic during the inflammatory process while Treg cells are crucial for inhibiting tissue inflammation and maintaining self-tolerance. Here, we review the recent information on differentiation and effector functions of Th17 cells during inflammatory conditions.
Keywords: autoimmunity, cytokines, infection, regulatory T cells, Th subsets
Introduction
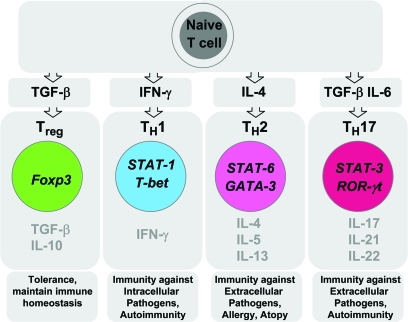
CD4+ T cells are the crucial component of an adaptive immune response. Upon antigenic stimulation and cytokine signaling, naive CD4+ T cells activate and differentiate into various Th subsets (1). The Th subsets were first classified >20 years ago by Mossman and Coffman who published landmark paper proposing a Th1–Th2 paradigm of T cells (2). The Th1–Th2 hypothesis was proposed based on the observation that each subset of CD4+ Th cells produces different patterns of cytokines and induces different effector functions: Th1 cells secrete high levels of IFN-γ and IL-2 and are required for cell-mediated immunity against intracellular pathogens and Th2 cells predominantly secrete IL-4, IL-5 and IL-13 and are required for clearing extracellular pathogens (3) (Fig. 1). After the initial discovery of Th subsets, significant progress was made to understand their biology and differentiation pathways on both a cellular and a molecular level. Th1–Th2 subsets showed a differential gene profile and a signaling requirement for their phenotype and effector functions. Signal transducer and activator of transcription (STAT) proteins are the key signaling transcription elements in the Th subset differentiation pathway. STAT-4 (Fig. 1) and STAT-1 play a significant role in maintaining and amplifying the Th1 response (4, 5). Similarly, STAT-6 activation is necessary for Th2 development (6). Both Th1 and Th2 subsets require lineage-specific transcription factors for their development. T-bet is the master transcription factor for the differentiation of Th1 cells while GATA-binding protein 3 (GATA-3) is required for Th2 development (7, 8) (Fig. 1). IL-12, a heterodimeric cytokine, composed of two subunits known as IL-12p35 and IL-12p40 (9) is a major factor that induces and maintains Th1 cells.
Fig. 1.
Th subsets. Antigen and specific cytokine signals induce the differentiation of naive T cells into various subsets of Th (Th1, Th2 and Th17). While these subsets produce specific patterns of cytokines that induce immunity, Treg cells, naturally occurring Foxp3+Treg cells and induced Foxp3+ Treg cells secrete anti-inflammatory mediators such as IL-10 and TGF-β that maintain immune tolerance and immune homeostasis.
Although Th1 cells were considered to be the pathogenic effector T cells inducing organ-specific autoimmunity, a lack of protection against autoimmunity in IFN-γ−/− and IFN-γR−/− mice raised the possibility that other T cell subset distinct from Th1 or Th2 subsets may exist that induces autoimmunity and tissue inflammation (10). Recently, a new subset of IL-17-producing T cells with effector functions distinct from those of Th1 and Th2 cells has been identified and has been termed as Th17 cells (11) (Fig. 1).
In this article, we review the discovery of Th17 cells in the mouse and outline the IL-17 cytokine family. We detail the interplay between Th17 cells and regulatory T (Treg) cells and describe the amplification and negative regulation of the Th17 response and the transcription factors involved. We then discuss human Th17 cells and their role in inflammation and infection.
Discovery of Th17 cells
Th1 cells with specificity for the self-antigen were shown to transfer autoimmunity in adoptive transfer experiments as well (12). Th1 clones specific for MBP, myelin oligodendrocyte glycoprotein (MOG) and proteolipid protein were shown to transfer disease in adoptive transfer model (13). Genetic deficiencies of Th1 molecules such as IL-12p35, IL-12Rβ2, STAT-1 and IFN-γR did not, however, abrogate experimental autoimmune encephalomyelitis (EAE) but rather enhanced the disease incidence and severity (14). In 2000, Bob Kasteline identified another cytokine chain called p19, which appeared to associate with an already existing IL-12p40 chain. This cytokine was named as IL-23 (9). The fundamental differences between IL-12 and IL-23 became evident after analysis of the expression profiles of their receptors, particularly on T cells. There are two IL-12R chains (IL-12Rβ1 and IL-12Rβ2), IL-23R pairs with IL-12Rβ1 to form a functional IL-23R complex. Unlike the IL-23R that was expressed on memory effector T cells and activated effector T cells (15), IL-12R β2 expression was detected on naive T cells. These observations imply that IL-12 and IL-23 might support Th1 subsets at different time points during their differentiation. The transgenic expression of p19, however, induced a striking phenotype, characterized by an elevated expression of pro-inflammatory cytokines such as tumor necrosis factor (TNF) α and IL-1 as well as systemic inflammation characterized by neutrophilic infiltrations. The T cells and macrophage of transgenic mice infiltrated multiple organs and mice died at the age of 3 months (16). The importance of IL-23 in the development of autoimmunity, especially EAE, was explored in a landmark experiment. In this experiment, Dan Cua et al. (17) induced EAE in IL-12p35-, IL-12p40- and IL-23p19-deficient mice. While genetic deficiency of IL-12 (p35) had no effect on the development of EAE, the loss of IL-23 (IL-12p40 or IL-23p19) led to a resistance against EAE in these genetically deficient mouse strains. These observations not only challenged the role of Th1 cells in autoimmune inflammation but also showed that IL-23 (IL-12p40 and IL-23p19) is a crucial cytokine required for the development of EAE. Interestingly, Th1 cells were not deficient in p19−/− EAE-resistant mice (17). Furthermore, p35 subunit-deficient mice, which lack IL-12 but express functional IL-23, had less IFN-γ-secreting cells but still developed very severe EAE (18). These unexpected findings have not only suggested the role of IL-23 and Th1 cells in EAE but also raised the possibility of IL-23 responding cell involvement in inducing tissue inflammation and EAE. IL-23 was later shown to induce IL-17 from activated T cells (19). IL-17-producing T cells (Th17) were detected in many autoimmune diseases, including arthritis (20) and EAE (21), suggesting a possible link between IL-23 and IL-17 during inflammatory responses. Moreover, EAE-resistant p19-deficient mice showed a dramatic decrease in IL-17-producing CD4+ T cell yet harbored normal numbers of Th1 cells (22). IL-23 did not trigger IFN-γ induction in CD4+ T cells. In fact, IL-23-responsive CD4+ T cells produced IL-17, IL-17F, IL-6 and TNF-α. Adoptive transfer of these pathogenic Th17 cells induces severe EAE. Strikingly, IL-23-driven CD4+ T cells showed a unique profile of gene expression, which differed from the profile of IL-12-driven Th1 cells (23). These observations clearly indicated that IL-23 induces an effector T cell population—distinct from known Th subsets, such as Th1 and Th2—that predominantly produces IL-17. With the series of experiments, the IL-23–Th17 axis has been established as a dominant player in the induction and development of autoimmune inflammations such as arthritis and EAE.
IL-17 cytokine family
IL-17, originally described as CTL-associated antigen 8, showed a 57% polypeptide sequence homology with immediate early gene 13 of Herpesvirus Saimiri (24). Further studies showed that IL-17 induced the activation of the nuclear factor κB (NFκB)/mitogen-activated protein kinase pathways and the secretion of IL-6 from fibroblast (25, 26). A cDNA encoding human IL-17 was cloned from a CD4+ T cell library. This cDNA showed a 72% amino acid identity with HVS13 and a 63% amino acid identity with murine IL-17. Five other IL-17 family members were uncovered, leading to designation of IL-17A–F. IL-17A, the prototypic family member, is a disulfide-linked, homodimeric glycoprotein that possesses a typical cysteine knot structure. Among the other family members, only IL-17A, IL-17F and IL-17E (IL-25) are better characterized (27). IL-17F and IL-17A showed a 55% homology, higher than the percentage of any other member of the family. IL-17 family members were located on different chromosomes except IL-17A and IL-17F, which were located on chromosome 1 in mouse and 6 in human. IL-17A and IL-17F were highly expressed by activated effector memory T cells. Recent observations, however, revealed that cells from innate compartment can also produce IL-17. Recent identification showed that IL-17A and IL-17F can form heterodimers and are produced in a coordinated fashion by Th17 cells. IL-17A and FRs are expressed on all tissue cells. Furthermore, activation via the IL-17R induces pro-inflammatory cytokines such as IL-1, IL-6 and TNF-α chemokines like C–X–C motif chemokine (CXCL) 8/murine IL-8 homolog and matrix metalloproteinases (28).
Interplay between induced Treg and Th17 cells
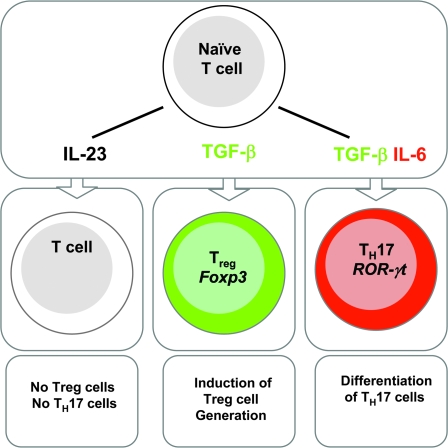
Since IL-23 could expand IL-17 cells from an activated T cell population, it was suggested that IL-23 is the differentiation factor for Th17 cells. It was difficult, however, to understand how IL-23 could induce Th17 differentiation from naive CD4+ T cells since IL-23R expression was found only on activated effector or memory T cell compartment. Naive T cells when activated in the presence of IL-23 could not differentiate into Th17 cells (29) (Fig. 2). In 2006, three papers from different laboratories showed that transforming growth factor β (TGF) in combination with IL-6 induced Th17 differentiation from naive CD4+ T cells (29–31) (Fig. 2). Although IL-23 failed to drive the differentiation of naive CD4+ T cells into Th17 cells, IL-23 was required for their Th17 cell expansion/maintenance (Fig. 3) (30). The differentiation factors of Th17 cells suggested that TGF-β, an anti-inflammatory cytokine, works with IL-6, a pro-inflammatory factor, to induce this highly pro-inflammatory subset of T cells. TGF-β was known as an immunosuppressive cytokine with pleiotropic functions (32); because a genetic deficiency of TGF-β induces lymphoproliferation and autoimmunity, it was obvious that TGF-β maintains immune homeostasis and maintains tissue tolerance (33, 34). The role of TGF-β in regulating the immune response became more clear with the discovery that TGF-β induces forkhead box protein P3 (Foxp3)+ Treg cells from naive CD4+ T cells (35, 36). Moreover, TGF-β is required for the maintenance and function of natural Foxp3+ Treg (nTreg) cells (37). Foxp3 is a transcription factor expressed in nTreg cells and induced by TGF-β in Foxp3− T cells [induced Treg (iTreg) cells] (37) (Fig. 2). Mutation in the Foxp3 gene in mice or humans induces a devastating autoimmune disease, resulting in the multi-organ inflammatory disease known as scurfy in mice and immunodysregulation, polyendocrinopathy, enteropathy, X-linked in humans (38). It was surprising and interesting that the presence of pro-inflammatory cytokines such as IL-6 could make effector T cells refractory to Treg cell-mediated suppression (39). Our group further demonstrated that during EAE, Treg cells in the central nervous system (CNS) lose their ability to suppress an immune response due to the presence of the pro-inflammatory environment (40). Strikingly, Veldhoen et al. (30) suggested that CD4+ T cell activation in the presence of nTregs and LPS-activated dendritic cells (DCs) mediated the generation of Th17 cells. This observation was further substantiated with the findings that TGF-β induces Foxp3+ Treg cells and that the addition of IL-6 inhibits the Foxp3 induction and induces the differentiation of Th17 cells. These in vitro findings were also confirmed using TGF-β transgenic mice where the TGF-β1 gene was under the control of the human CD2 promoter and thus expressed in all T cells. Immunization of these mice with the MOG peptide emulsified with CFA induced the increased frequency of Th17 cells in the periphery and their infiltration in the target organ leading to severe clinical EAE (29). Conversely, T cells deficient in TGF-β signaling showed a defective Th17 response making the mice resistant to EAE development (41). These observations indicate the absolute requirement of TGF-β in the generation of Th17 cells. On the other hand, immunization of both IL-6 and gp130 CD4Cre conditional-deficient mice with a similar protocol did not show any clinical sign of EAE. In fact, the mice had higher frequency of Foxp3+ Treg cells in the peripheral repertoire (40, 42, 43). These reciprocal developmental pathways of Th17 and iTreg cells suggest that Th17 and iTreg cells share a common precursor of T cells that can differentiate into either Th17 or Treg cells depending upon the cytokines present at the time of their differentiation. IL-6-mediated STAT-3 activation is crucial for the generation of Th17 cells (44). Whether the signaling pathway of TGF-β is required for the generation of Th17 cells is not clear. Similarly, the cellular source of TGF-β during Th17 differentiation has not been identified since TGF-β can be produced by multiple lineages of leukocytes and stromal cells (32). Initial data suggested that nTreg cells are the primary source of TGF-β in Th17 differentiation because nTregs and TGF-β could be replaced by each other in an in vitro differentiation assay (30). On the other hand, DC activation with different microbial products such as zymosan and mycobacteria induced Th17 differentiation in the absence of exogenous TGF-β. Neutralization of TGF-β in these assay, however, abolished Th17 generation (41). This observation suggests that in certain conditions, TGF-β produced by DCs can initiate Th17 differentiation. Conversely, TGF-β deficiency in CD4+ T cells can hamper Th17 generation even after immunization with mycobacteria, further suggesting that CD4+ T cells are the primary source of TGF-β in Th17 differentiation in vivo (45). Altogether, these findings suggest that TGF-β is absolutely required for the generation of Th17 cells. It may be, however, possible that type of activation and site differentiation determines the cellular source of TGF-β in Th17 differentiation. Unlike other secondary lymphoid organs, gut-associated lymphoid tissues provide differentiation factors that induce Th17 cells constitutively. CD103− and CD103+ subsets of DCs in the intestine and draining mesenteric lymph nodes help in the generation of Th17 and Treg, respectively. Since CD103+ DCs, that utilize the dietary metabolite retinoic acid, are a great source of TGF-β in generation of Foxp3+ Treg cells (46, 47). On the other hand, CD103− DCs produce high amounts of IL-6 with low-levels of TGF-β, helping in the generation of Th17 cells. Since TGF-β induces Foxp3+ Treg cells and the presence of IL-6 inhibits TGF-β-mediated Foxp3 induction while inducing Th17 cells, we proposed that there is a reciprocal relationship between Treg and Th17 cells. This reciprocal relationship has now been confirmed in many other experimental conditions (48). Our group further demonstrated that retinoic acid increases the expression and phosphorylation of SMAD family member 3, which enhances Foxp3 expression. Retinoic acid also inhibits the expression of IL-6Rα, IL-23R and IFN regulatory factor 4 (IRF-4), which further inhibits Th17 generation (49). IRF-4-deficient mice did not develop EAE and IRF-4-deficient T cells were failed to differentiate into Th17 cells with less expression of Th17 transcription factor (discussed in Th17-specific transcription factor) (50, 51). Recent report further demonstrated that retinoic acid negatively regulates CD4+CD44hi memory phenotypes. These memory cells actively inhibit the TGF-β-induced Foxp3+ Treg cells via synthesis of IL-4, IL-21 and IFN-γ; expression of these cytokines was coordinately curtailed by retinoic acid (52). The reciprocal relationship between Th17 and Treg cells was further exemplified in IL-2-deficient mice (53). Since IL-2 helps in T cell proliferation and serves as a growth factor for both Th1 and Th2, it was predicted that IL-2 would also help Th17 generation and expansion. IL-2-deficient mice, however, suffer from spontaneous colitis and inflammation (54). Distinct expression of IL-2Rα on Treg cells suggested the requirement of IL-2 for Foxp3+ Treg cell functions (55), partly explaining the autoimmune phenotype of IL-2-deficient mice. Since Th17 and Treg cells showed the dichotomous relationship, it might be possible that IL-2-deficient mice compensated for a less number of Treg cells with a higher Th17 frequency. Laurence et al. (53) showed that IL-2 deficiency positively regulated Th17 generation in the secondary lymphoid organs. Thus, IL-2, which was originally described as a Th1 cytokine, appears to be another cytokine that attenuates Th17 generation. Absence of STAT-5, a transcription element of IL-2 signaling pathways, resulted in enhanced generation of Th17 cells. These observations not only demonstrated the reciprocal generation of Th17 and Treg cells but also showed the importance of IL-2’s negative function in Th17 generation. The molecular basis of the reciprocal generation of Th17 and Foxp3+ Treg cells was further demonstrated at transcriptional level (see later on Th17-specific transcription factors) (56). The reciprocal relationship between Th17 and Treg cells was further observed in lamina propria, where the presence of cytophaga-flavobacter-bacteroidetes (CFB) bacteria was correlated with the generation of Th17 cells. In fact, antibiotic treatment or germ-free mice showed the complete absence of Th17 cells with a concomitant increase in the frequency of Foxp3+ Treg cells in this compartment. Interestingly, the generation of Th17 cells by CFB bacteria required the presence of TGF-β (57).
Fig. 2.
Reciprocal generation of Treg and Th17 cells. Activation of naive T cells in the presence of IL-23 did not induce the generation of Treg or Th17 cells. Supplementation of TGF-β in the culture induced the generation of Foxp3+ Treg cells while the addition of IL-6 not only inhibited the induction of TGF-β-induced Foxp3 but also concomitantly induced the generation of Th17 cells.
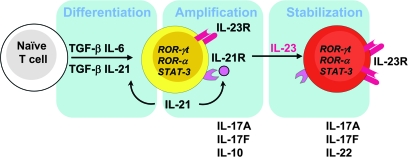
Fig. 3.
Steps in the generation of Th17 cells. The activation of naive T cells in the presence of TGF-β and IL-6 initiates the Th17 differentiation pathway. Th17 cells produce IL-21, which further amplifies Th17 generation in an autocrine manner. IL-21 also induces the IL-23R on differentiated Th17 cells to make them responsive to IL-23 signaling. IL-23 stabilizes the Th17 phenotype by secreting IL-17A, IL-17F and IL-22 and helping Th17 cells to acquire effector functions. STAT-3 plays an important role in Th17 differentiation, amplification and stabilization as IL-6, IL-21 and IL-23 signals through STAT-3.
Self-amplification of Th17 response
One of the features of Th differentiation is ‘self-amplification’. IFN-γ, through STAT-1, induces T-bet, which further induces IFN-γ and further amplifies IFN-γ and Th1 differentiation (58). Similarly, IL-4 produced by Th2 subsets amplifies Th2 differentiation by inducing IL-4 via STAT-6 activation (6). IL-17 produced by Th17 cells does not amplify Th17 cells since IL-17 is neither a growth nor a differentiation factor and its receptor is not expressed on T cells (28). Th17, however, cells produce enormous amounts of IL-21, a member of the IL-2 family cytokine, which uses a common γ chain receptor. IL-21R is expressed on all T and B cells (40, 59). IL-21, in combination with TGF-β, induces de novo Th17 differentiation. Cells such as NKT and NK cells that produce IL-21 can support Th17 differentiation in certain conditions in the absence of IL-6 (40, 59). Both IL-6 and IL-21 induce the expression of IL-23R on Th17 cells (60). Similarly, IL-23 has also shown to induce its own receptor on both mouse and human Th17 cells (23, 61). IL-6 also triggers IL-21 production, which synergizes with TGF-β to initiate and further amplify the Th17 response while IL-23 produced by activated DCs sustains and stabilizes the differentiation of Th17 cells (40, 60) (Fig. 3). IL-21 and IL-21R deficiency showed diminished expression of IL-23R, resulting in the blunt Th17 generation (60). Since exposure to IL-23 is the key feature that determines the pathogenicity of Th17 cells, IL-21 initiates the IL-23R expression on Th17 cells; therefore, IL-21 not only amplifies Th17 differentiation but also helps Th17 attain mature phenotypes by inducing the expression of IL-23R (Fig. 3). IL-21R- or IL-21-deficient mice develop EAE at a similar frequency to the wild-type control mice (62), raising the issue of whether IL-21 is important in vivo for the generation of Th17 cells. It should be noted that the induction of EAE by strong stimuli such as CFA might override the effect of IL-21 by producing enormous amounts of IL-6 in vivo and might thus compensate for IL-21 deficiency in vivo. Recent data, however, show that IL-21-deficient non-obese diabetic mice are highly resistant to type 1 diabetes (63), an autoimmune disease that arises spontaneously. Whether this is due to the loss of IL-17 in these IL-21R−/− mice has not, however, been analyzed.
Negative regulation of Th17 cells
Although Th17 cells play a protective role against certain extracellular pathogens, a dysregulated Th17 response can induce severe tissue inflammation and autoimmunity and can be dangerous for the host. Th subsets cross-regulate each other's functions by virtue of the cytokines that they produce, allowing the amplification of the same subset and inhibition function of the other. Thus, Th1 and Th2 subsets limit one another's response by the cytokines they produce. Here we have described the cytokines and other factors that negatively regulate this newly described Th lineage.
IL-27, a member of IL-12 cytokine family, has originally been described as a potent inducer of the IFN-γ response from T cells (64). Since IL-27R shares the gp130 subunit of the IL-6R with a unique chain called IL-27Rα, it was difficult to predict whether IL-27 could promote or inhibit Th17 response. Initial work suggested that IL-27 induces T-bet and IFN-γ and, thus, induces Th1 effector cells (65). Emerging data on IL-27 associated with different Th1 and Th2 pathogens, however, showed that IL-27 is a negative regulator of both the Th1 and Th2 response. Toxoplasmosis is a parasitic infection that requires a Th1 response for its clearance from the host. Mice deficient in either IL-12 or IFN-γ die because of high parasite burden. While IL-27R-deficient mice show the normal Th1 response and clearance of parasite, they die of fatal and severe immunopathology (66). Similar results were obtained from using different pathogens such as Leishmania and Mycobacteria (67, 68). The immunopathology in the IL-27R-deficient mice was associated with an exaggerated Th17 response, suggesting that IL-27 may negatively regulate Th17 cells. Direct evidence came in 2006 showing that IL-27 negatively regulates the Th17 response both in Toxoplasma gondii infection as well as in MOG peptide-induced EAE. An increased number of Th17 cells were detected in the CNS in both models of the IL-27R-deficient mice (69, 70), an observation that agreed the IL-27 inhibition of Th17 differentiation in vitro. Moreover, IL-27 inhibits Th17 differentiation at the transcriptional level as it inhibited the expression of retinoid-related orphan receptor (ROR) γt. Unlike IL-6, which inhibited the induction of iTreg generation with a concomitant increases of the Th17 response, IL-27 inhibited both Th17 and iTreg induction (71). The establishment of IL-27 as an inhibitory cytokine for the Th1, Th2 and Th17 response suggested a possibility that it might induce other factors or induce T cell differentiation that inhibits Th1 and Th17 responses. A series of papers recently showed that IL-27 induces IL-10-producing T cells (71–73), raising the possibility that IL-27 might inhibit Th17 generation and other effector T cells via IL-10. More importantly, IL-27 inhibits the Th17 response independent of IL-10 as well. Nevertheless, IL-10 provides an additional mechanism of regulation that may not be required directly to regulate Th17 generation but rather through the inhibition of antigen-presenting functions. Another study demonstrated that IFNRα-mediated signaling inhibits a translational isoform of osteopontin (OPN), resulting in the inhibition of the Th17 response. The IFNRα-mediated inhibition of OPN further increases IL-27 expression. Similarly, OPN-deficient DCs produce higher levels of IL-27, which inhibits Th17 response (74, 75).
Th17-specific transcription factors
Th subsets and specific cytokine patterns in Th cells are defined by lineage-specific transcription factors. Antigen-specific activation of the TCR in the presence of cytokine signals helps T cells to differentiate into a specific Th subset. Th1 differentiation requires STAT-4 and STAT-1 activation by IL-12 and IFN-γ, respectively. These cascades of cell signaling activate a lineage-specific transcription factor, T-bet, for a mature Th1 response. Similarly, STAT-6 and GATA-3 are important for Th2 differentiation. Th17 cells were identified as an independent lineage since neither Th1 transcription factors, such as STAT-1, STAT-4 and T-bet, nor Th2, such as STAT-6 and GATA-3, was found to be expressed by Th17 cells. In fact TGF-β, which constitutes a major factor in Th17 differentiation, inhibits both Th1 and Th2 differentiation by inhibiting T-bet and GATA-3, respectively (76, 77). IL-6, IL-21 and IL-23 are the key factors of Th17 differentiation. All these factors induce phospho-STAT-3, an observation supported by the fact that the conditional deletion of STAT-3 in T cells reduced the frequency of Th17 cells, leading to EAE-resistant mice (78, 79). Moreover, the deletion of suppressor of cytokine signaling 3 (SOCS3), which is a negative regulator of STAT-3 signaling, increased the number of Th17 cells (80). The over-expression of the hyperactive form of STAT-3 potentiated Th17 generation. STAT-3 regulates IL-23R and IL-21 expression, both of which are required for amplifying Th17 generation (Fig. 3). Initial studies showed that ROR-γt, a member of the retinoic acid receptor-related orphan nuclear hormone receptor family, encoded by the RAR-related orphan receptor C gene is expressed specifically in Th17 differentiation conditions (TGF-β + IL-6 or IL-21) (81) (Fig. 3). Retroviral induction of ROR-γt into naive CD4+ T cells induces IL-17A and IL-17F production (81). Analysis of mice in which ROR-γt is expressed with a green fluorescent protein (GFP) label revealed that all the GFP+ CD4+ T cells produce IL-17 in the lamina propria. Furthermore, ROR-γt deficiency attenuates the induction of EAE (81). Recent data showed that TGF-β alone is sufficient to induce both ROR-γt and Foxp3, the transcription factors required for Th17 and Treg cell induction, respectively (56). Presence of IL-6 or IL-21 inhibits Foxp3 induction or relieves the suppression that further potentiates Th17 generation. A low dose of TGF-β synergizes with IL-6 and IL-21 to induce Th17 generation. A higher dose of TGF-β, however, inhibits IL-23R expression with a concomitant increase of Foxp3 expression, favoring the generation of Treg cells (56). Recent data suggest that Runt-related transcription factor 1 (Runx1) also influences Th17 generation by inducing ROR-γt expression. Runx1 induces ROR-γt expression by binding to ROR-γt and acting together with ROR-γt to induce IL-17 transcription. Runx1 also interacts physically with Foxp3 to negatively regulate Th17 differentiation (82). Altogether, these observations suggest that ROR-γt is essential for the generation of the Th17 cell. It has not been addressed so far, however, whether ROR-γt directly transactivates the expressions of IL-17A, IL-17F, IL-22 and IL-21. ROR-γt-deficient mice develop EAE but at lesser extent with the residual Th17 cells, suggesting a possibility of another transcription factor for Th17 development. ROR-α, another member of the retinoic acid receptor-related orphan nuclear hormone receptor family, is highly expressed in Th17 cells in a STAT-3-dependent manner by the combination of TGF-β and IL-6 (Fig. 3); therefore, ROR-γt and ROR-α may act synergistically to induce Th17 cells (83). Recent observations demonstrated that the inducible co-stimulatory molecule (ICOS) is required for the generation of Th17 cells and follicular helper (TFH) cells (84, 85). The generation of TFH cells with the distinct surface phenotype (CD4+CCR5+ICOShigh) are dependent on IL-21, IL-6 and STAT-3 (84). TFH showed highly specialized functions in the generation of germinal centers and helped in somatic hypermutation, class switching and the selection of high-affinity B cells. Both Th17 and TFH cells showed a higher expression of proto-oncogene c-maf (c-maf), which was thought to be a Th2-specific transcription factor. C-maf deficiency, in turn, resulted in defective IL-21-mediated IL-23R expressions. Our group showed that ICOS-induced c-maf, in fact, helps in the generation of TFH and Th17 cells. Although the requirement of c-maf is essential for the expansion of TFH cells, unlike Th17 cells (85), the differentiation of TFH cells did not require Th17-specific transcription factors, ROR-γt and ROR-α (84).
Human Th17 cells
The identification of differentiation factors in the murine model led us and others to determine the similarities in Th17 differentiation between human and murine T cells, which would provide therapeutic targets of Th17-mediated inflammation. Th17 cells and their products are associated with the pathology of many inflammatory and autoimmune diseases. IL-17 has not only been detected in the lesions of multiple sclerosis but also been believed to compromise the blood brain barrier (86, 87). Similarly, higher amounts of IL-17 and Th17 cells were found the synovium of rheumatoid arthritis patients than in healthy patients (20). High levels of both IL-17 and IL-23 were detected in patients of Crohn's disease and ulcerative colitis (88, 89). These observations agree with the mouse model data of these autoimmune diseases. Initial data on identifying factors for human Th17 cell differentiation, however, suggested that TGF-β is not required for the differentiation of Th17 cells. Instead, IL-1β and IL-6 or IL-1β in combination with IL-23 induces Th17 differentiation (90, 91). Another report suggests that IL-23 alone is sufficient to drive human Th17 differentiation (61). Instead of positively effecting Th17 differentiation, TGF-β inhibited human Th17 generation (90). This inhibition was in striking contrast with the murine system where TGF-β is absolutely required for Th17 differentiation (29). The description of IL-21 as growth factor for Th17 development in the mouse model raised the possibilities that IL-21 is the differentiating cytokine for human Th17 differentiation. Subsequent work on human Th17 cell differentiation revealed that the presence of endogenous TGF-β in the serum containing media is sufficient to induce human Th17 differentiation in the absence of exogenous TGF-β (92). Moreover, addition of TGF-β in the serum-free media with IL-1β and IL-6, IL-21 or IL-23 is sufficient to induce human Th17 differentiation from naive human CD4+ T cells (92). We made similar observations that while IL-6 and IL-1β induce IL-17 production from human central memory cells, a combination of TGF-β and IL-21 induces human Th17 differentiation from naive T cells (93). Besides the differentiating factors, human and mouse Th17 cells express similar cytokines, such as IL-17A, IL-17F, IL-22 and IL-21, and cell surface receptors, such as IL-23R and C–C chemokine receptor type 6 (94, 95).
Th17 cells in autoimmunity
Th1 cells could not explain their crucial role in the induction of autoimmune inflammation since the depletion of Th1-specific molecules did not decrease the susceptibility of autoimmune inflammation such as EAE. In fact, mice showed enhanced disease. Discovery of the IL-23 and Th17 axis has defined Th17 cells as the dominant effector T cell for inducing autoimmune tissue inflammation (14). Association of IL-17 with human autoimmune disease was first demonstrated in patients of rheumatoid arthritis. In these patients, a higher frequency of Th17 cells had been detected in the arthritic synovium. Furthermore, IL-17 had been shown to induce the production of IL-6, IL-8 and TNF-α from the human synovial cells that induce tissue inflammation. Similarly, T cell-mediated production of IL-17 had been detected in the active lesions in the brain of multiple sclerosis patients (86). Increased frequencies of Th17 cells and IL-17 have also been shown in the patients of inflammatory bowel disease and psoriasis (89, 96). Altogether, these observations imply that Th17 cells are required for the induction of tissue inflammation and pathology during autoimmune diseases. Both IL-17A and F activated tissue cells to promote inflammation by producing pro-inflammatory cytokines (IL-6, TNF-α and IL-1β) and chemokines (CXCL1, granulocyte chemotactic protein 2, CXCL8, cytokine-induced neutrophil chemoattractant and monocyte chemoattractant protein 1) helped neutrophil infiltration at the site of the target organ. IL-17 further potentiates tissue pathology by inducing the production of nitric oxide and matrix metalloproteinases. IL-17 has also showed its destructive effects on bone and cartilage by activating osteoclasts and is, therefore, called the osteoclastogenic Th cell subset that links T cell activation and bone resorption (97). The effects of IL-17 have also been demonstrated in different mouse models of autoimmune disease, such as EAE and collagen-induced arthritis (CIA). IL-17-deficient mice exhibited a delayed onset and reduced disease severity, followed by early recovery in EAE (98). Similarly, IL-17-deficient mice develop attenuated CIA (99). These data were corroborated with a higher level of IL-17 expression in the target tissues in the animal models of EAE and CIA. Furthermore, CD4+ T cells from IL-17-deficient mice failed to transfer EAE to the recipient mice, suggesting the dominant function of CD4+-mediated IL-17 production in the induction of EAE. Consistently, therapeutic neutralization of IL-17 with IL-17R–Fc protein in acute EAE ameliorated clinical symptoms of EAE, further confirming the fact that IL-17 instead of IFN-γ is the dominant cytokine for the pathogenesis of EAE (100). A recent study described that IL-17A triggered positive feedback loop of IL-6 signaling, which involved the activation of the transcription factors NFκB and STAT-3 in fibroblasts and contributed to the development of arthritis and EAE (101). SOCS3 negatively regulated this positive feedback and inhibited an IL-17-mediated exacerbation of disease. IL-6 is an important factor for the generation of Th17 cells. Increased levels of IL-6 have been shown in autoimmune diseases such as multiple sclerosis and arthritis. The blockade of IL-6 by humanized IL-6R antibody showed the exciting clinical results in a phase II clinical trial in rheumatoid arthritis and systemic onset juvenile idiopathic arthritis patients (102). Blocking of IL-6 might decrease the frequency of Th17 in these patients and might increase Treg-associated functions.
Taken together, these data suggest that Th17 cells are the major contributor of autoimmune inflammation in various autoimmune diseases and may be good therapeutic target for inflammation.
Th17 cells in infection
Although the discovery and description of Th17 cells are mainly associated with autoimmune diseases, emerging data suggest the role of these newly described subsets in infection and host defense. The Th1 subset has been described as effector subset of T cells that activates macrophages and help in clearing the intracellular pathogens. Similarly, Th2 cells are crucial for clearing the extracellular pathogens. Interestingly, the effector functions of Th17 cells have been described in the context of autoimmunity. Emerging data, however, suggest the crucial role of Th17 cells in mounting the immunity for both intracellular and extracellular pathogens. Both IL-17A and IL-17F are induced in several models of infections, suggesting the involvement of this newly described helper subset (103). IL-17 up-regulates specific chemokines, pro-inflammatory cytokines and colony-stimulating factors (CSFs), thereby helping to induce neutrophils and other myeloid cells recruitment at the site of infection. The cellular recruitment at the site of infection is the crucial process in many infections. Many pathogens including Mycobacteria and Pneumocystis carinii induce IL-23 secretion from infected macrophages and DCs that helps in the generation of Th17 cells. IL-23p19-deficient mice were unable to clear the P. carinii infection (104). Similar results have been observed with IL-17 neutralization in this infection, suggesting that the IL-23–IL-17 axis is required for the anti-fungal immune response (104). In addition to fungal pathogens, IL-17 is also essential for the protection against gram-negative bacteria, including Klebsiella pneumoniae. IL-17R-, IL-23p19- and IL-12p40-deficient mice were susceptible to the K. pneumoniae infection associated with a reduced expression of CSFs and neutrophil recruitment. In addition to IL-17, IL-22 increased lung epithelial cell proliferation and increased transepithelial resistance to injury in K. pneumoniae infection (105). IL-17 also appears to play an important role in the Mycobacteria infection. The cellular source of IL-17 plays an important role in anti-mycobacterial functions (106). It has been demonstrated that gamma delta T cells quickly and rapidly produce IL-17 during mycobacterium infection (107). IL-17 derived from CD4+ T cells, however, is also required not only for the clearing of the primary infection but also to establish effective memory responses (108). A role for IL-17 in Lyme disease has also been reported. Both human and mouse T cells produced enhanced amount of IL-17 in response to the Borrellia burgdorferi infection (109). Th17 cells could bridge the gap between innate and adaptive immunity and could attract other subsets of Th cells and cells of innate compartment including neutrophils and macrophages to sites of infection for efficient clearing of the pathogens. This has most convincingly been shown in the Mycobacterium tuberculosis infection, for which an early Th17 response is required to bring Th1 cells into the infected lung tissue to control the infection. Genetic deficiency in mounting an effective IL-17/Th17 response in humans results in unrelenting mucocutaneous infections and staphylococcal lung infections as seen in Job's syndrome (110).
Concluding remarks
Th17, a third subset of Th effector cells, has been identified as critical for many T cell responses and is required for autoimmunity and tissue inflammation. Substantial progress has been made to understand the biology of Th17 cells in various autoimmune and infection models. We and others have shown the reciprocal generation of Foxp3+ Treg and Th17 cells. TGF-β induces the generation of Treg cells and the addition of IL-6 induces the differentiation of naive T cells into Th17 pathways. IL-21 and IL-23 further amplify and stabilize Th17 generation and phenotype, respectively. The understanding of the reciprocal relationship between Th17 and Treg cells may provide important targets for treatment of multiple inflammatory conditions.
Funding
National Institutes of Health (R01NS045937, R01NS035685, R37NS030843, R01AI044880, P01AI039671, P01NS38037, R01NS046414); National Multiple Sclerosis Society (RG2571); Juvenile Diabetes Research Foundation Center for Immunological Tolerance at Harvard Medical School; Javits Neuroscience Investigator Award from the US National Institutes of Health to V.K.K.; post-doctoral fellowship to AA from National Multiple Sclerosis Society, NY, USA.
Glossary
Abbreviations
CFB
cytophaga-flavobacter-bacteroidetes
CIA
collagen-induced arthritis
c-maf
proto-oncogene c-maf
CNS
central nervous system
CSF
colony-stimulating factor
CXCL
C–X–C motif chemokine
DC
dendritic cell
EAE
experimental autoimmune encephalomyelitis
Foxp3
forkhead box protein P3
GATA-3
GATA-binding protein 3
GFP
green fluorescent protein
HVS13
Herpesvirus saimiri gene 13
ICOS
inducible co-stimulatory molecule
IRF-4
IFN regulatory factor 4
iTreg
induced Treg
MOG
myelin oligodendrocyte glycoprotein
NFκB
nuclear factor κB
nTreg
natural Foxp3+ Treg
OPN
osteopontin
ROR
retinoid-related orphan receptor
Runx1
Runt-related transcription factor 1
SOCS3
suppressor of cytokine signaling 3
STAT
signal transducer and activator of transcription
TGF-β
transforming growth factor β
TNF
tumor necrosis factor
TFH cell
follicular helper cells
Treg
regulatory T
References
- 1.Reiner SL. Development in motion: helper T cells at work. Cell. 2007;129:33. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986;136:2348. [PubMed] [Google Scholar]
- 3.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 5.Meraz MA, White JM, Sheehan KC, et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 7.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 8.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 9.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 10.Ferber IA, Brocke S, Taylor-Edwards C, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J. Immunol. 1996;156:5. [PubMed] [Google Scholar]
- 11.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuchroo VK, Anderson AC, Waldner H, Munder M, Bettelli E, Nicholson LB. T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire. Annu. Rev. Immunol. 2002;20:101. doi: 10.1146/annurev.immunol.20.081701.141316. [DOI] [PubMed] [Google Scholar]
- 13.Das MP, Nicholson LB, Greer JM, Kuchroo VK. Autopathogenic T helper cell type 1 (Th1) and protective Th2 clones differ in their recognition of the autoantigenic peptide of myelin proteolipid protein. J. Exp. Med. 1997;186:867. doi: 10.1084/jem.186.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Parham C, Chirica M, Timans J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 2002;168:5699. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 16.Wiekowski MT, Leach MW, Evans EW, et al. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J. Immunol. 2001;166:7563. doi: 10.4049/jimmunol.166.12.7563. [DOI] [PubMed] [Google Scholar]
- 17.Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 18.Gran B, Zhang GX, Yu S, et al. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J. Immunol. 2002;169:7104. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 19.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2003;278:1910. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 20.Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Invest. 1999;103:1345. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lock C, Hermans G, Pedotti R, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 2002;8:500. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 22.Murphy CA, Langrish CL, Chen Y, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 2003;198:1951. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J. Immunol. 1993;150:5445. [PubMed] [Google Scholar]
- 25.Maitra A, Shen F, Hanel W, et al. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc. Natl Acad. Sci. USA. 2007;104:7506. doi: 10.1073/pnas.0611589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang SY, Kim JY, Kim KW, et al. IL-17 induces production of IL-6 and IL-8 in rheumatoid arthritis synovial fibroblasts via NF-kappaB- and PI3-kinase/Akt-dependent pathways. Arthritis Res. Ther. 2004;6:R120. doi: 10.1186/ar1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 28.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 30.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Mangan PR, Harrington LE, O'Quinn DB, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 32.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134:392. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shull MM, Ormsby I, Kier AB, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulkarni AB, Huh CG, Becker D, et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl Acad. Sci. USA. 1993;90:770. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057. [PubMed] [Google Scholar]
- 36.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 37.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J. Exp. Med. 2005;201:1061. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ziegler SF. FOXP3: of mice and men. Annu. Rev. Immunol. 2006;24:209. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 39.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 40.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat. Immunol. 2006;7:1151. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 42.Okuda Y, Sakoda S, Bernard CC, et al. IL-6-deficient mice are resistant to the induction of experimental autoimmune encephalomyelitis provoked by myelin oligodendrocyte glycoprotein. Int. Immunol. 1998;10:703. doi: 10.1093/intimm/10.5.703. [DOI] [PubMed] [Google Scholar]
- 43.Korn T, Mitsdoerffer M, Croxford AL, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc. Natl Acad. Sci. USA. 2008;105:18460. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mathur AN, Chang HC, Zisoulis DG, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J. Immunol. 2007;178:4901. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 45.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 46.Jaensson E, Uronen-Hansson H, Pabst O, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J. Exp. Med. 2008;205:2139. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mucida D, Park Y, Kim G, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 49.Xiao S, Jin H, Korn T, et al. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J. Immunol. 2008;181:2277. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brustle A, Heink S, Huber M, et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat. Immunol. 2007;8:958. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 51.Huber M, Brustle A, Reinhard K, et al. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proc. Natl Acad. Sci. USA. 2008;105:20846. doi: 10.1073/pnas.0809077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hill JA, Hall JA, Sun CM, et al. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laurence A, Tato CM, Davidson TS, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 54.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 55.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005;6:1142. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 56.Zhou L, Lopes JE, Chong MM, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaplan MH, Grusby MJ. Regulation of T helper cell differentiation by STAT molecules. J. Leukoc. Biol. 1998;64:2. doi: 10.1002/jlb.64.1.2. [DOI] [PubMed] [Google Scholar]
- 59.Nurieva R, Yang XO, Martinez G, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 60.Zhou L, Ivanov II, Spolski R, Min R, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 2007;8:967. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 61.Chen Z, Tato CM, Muul L, Laurence A, O'Shea JJ. Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum. 2007;56:2936. doi: 10.1002/art.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coquet JM, Chakravarti S, Smyth MJ, Godfrey DI. Cutting edge: IL-21 is not essential for Th17 differentiation or experimental autoimmune encephalomyelitis. J. Immunol. 2008;180:7097. doi: 10.4049/jimmunol.180.11.7097. [DOI] [PubMed] [Google Scholar]
- 63.Spolski R, Kashyap M, Robinson C, Yu Z, Leonard WJ. IL-21 signaling is critical for the development of type I diabetes in the NOD mouse. Proc. Natl Acad. Sci. USA. 2008;105:14028. doi: 10.1073/pnas.0804358105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 65.Takeda A, Hamano S, Yamanaka A, et al. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J. Immunol. 2003;170:4886. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 66.Villarino A, Hibbert L, Lieberman L, et al. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 67.Rosas LE, Satoskar AA, Roth KM, et al. Interleukin-27R (WSX-1/T-cell cytokine receptor) gene-deficient mice display enhanced resistance to Leishmania donovani infection but develop severe liver immunopathology. Am. J. Pathol. 2006;168:158. doi: 10.2353/ajpath.2006.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holscher C, Holscher A, Ruckerl D, et al. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J. Immunol. 2005;174:3534. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- 69.Batten M, Li J, Yi S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat. Immunol. 2006;7:929. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 70.Stumhofer JS, Laurence A, Wilson EH, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 2006;7:937. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 71.Awasthi A, Carrier Y, Peron JP, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 2007;8:1380. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 72.Stumhofer JS, Silver JS, Laurence A, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 2007;8:1363. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 73.Fitzgerald DC, Zhang GX, El-Behi M, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat. Immunol. 2007;8:1372. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 74.Shinohara ML, Kim JH, Garcia VA, Cantor H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin. Immunity. 2008;29:68. doi: 10.1016/j.immuni.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murugaiyan G, Mittal A, Weiner HL. Increased osteopontin expression in dendritic cells amplifies IL-17 production by CD4+ T cells in experimental autoimmune encephalomyelitis and in multiple sclerosis. J. Immunol. 2008;181:7480. doi: 10.4049/jimmunol.181.11.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park IK, Shultz LD, Letterio JJ, Gorham JD. TGF-beta1 inhibits T-bet induction by IFN-gamma in murine CD4+ T cells through the protein tyrosine phosphatase Src homology region 2 domain-containing phosphatase-1. J. Immunol. 2005;175:5666. doi: 10.4049/jimmunol.175.9.5666. [DOI] [PubMed] [Google Scholar]
- 77.Gorelik L, Fields PE, Flavell RA. Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J. Immunol. 2000;165:4773. doi: 10.4049/jimmunol.165.9.4773. [DOI] [PubMed] [Google Scholar]
- 78.Harris TJ, Grosso JF, Yen HR, et al. Cutting edge: an in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J. Immunol. 2007;179:4313. doi: 10.4049/jimmunol.179.7.4313. [DOI] [PubMed] [Google Scholar]
- 79.Liu X, Lee YS, Yu CR, Egwuagu CE. Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J. Immunol. 2008;180:6070. doi: 10.4049/jimmunol.180.9.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen Z, Laurence A, Kanno Y, et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc. Natl Acad. Sci. USA. 2006;103:8137. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 82.Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat. Immunol. 2008;9:1297. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang XO, Pappu BP, Nurieva R, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nurieva RI, Chung Y, Hwang D, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bauquet AT, Jin H, Paterson AM, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and T(H)-17 cells. Nat. Immunol. 2008;10:167. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tzartos JS, Friese MA, Craner MJ, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008;172:146. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kebir H, Kreymborg K, Ifergan I, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007;13:1173. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Duerr RH, Taylor KD, Brant SR, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 2007;8:942. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 91.Wilson NJ, Boniface K, Chan JR, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 2007;8:950. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 92.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 2008;9:641. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang L, Anderson DE, Baecher-Allan C, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007;204:1849. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. The phenotype of human Th17 cells and their precursors, the cytokines that mediate their differentiation and the role of Th17 cells in inflammation. Int. Immunol. 2008;20:1361. doi: 10.1093/intimm/dxn106. [DOI] [PubMed] [Google Scholar]
- 96.Zaba LC, Cardinale I, Gilleaudeau P, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J. Exp. Med. 2007;204:3183. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sato K, Suematsu A, Okamoto K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 2006;203:2673. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Komiyama Y, Nakae S, Matsuki T, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2006;177:566. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 99.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 2003;171:6173. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 100.Hofstetter HH, Ibrahim SM, Koczan D, et al. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell. Immunol. 2005;237:123. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 101.Ogura H, Murakami M, Okuyama Y, et al. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. 2008;29:628. doi: 10.1016/j.immuni.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 102.Kishimoto T. Interleukin-6: from basic science to medicine—40 years in immunology. Annu. Rev. Immunol. 2005;23:1. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 103.Aujla SJ, Dubin PJ, Kolls JK. Th17 cells and mucosal host defense. Semin. Immunol. 2007;19:377. doi: 10.1016/j.smim.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rudner XL, Happel KI, Young EA, Shellito JE. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect. Immun. 2007;75:3055. doi: 10.1128/IAI.01329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aujla SJ, Chan YR, Zheng M, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 2008;14:275. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khader SA, Cooper AM. IL-23 and IL-17 in tuberculosis. Cytokine. 2008;41:79. doi: 10.1016/j.cyto.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 2006;177:4662. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 108.Khader SA, Bell GK, Pearl JE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 2007;8:369. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 109.Codolo G, Amedei A, Steere AC, et al. Borrelia burgdorferi NapA-driven Th17 cell inflammation in lyme arthritis. Arthritis Rheum. 2008;58:3609. doi: 10.1002/art.23972. [DOI] [PubMed] [Google Scholar]
- 110.Milner JD, Brenchley JM, Laurence A, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]