What does the amygdala contribute to social cognition? (original) (raw)
. Author manuscript; available in PMC: 2010 May 15.
Abstract
The amygdala has received intense recent attention from neuroscientists investigating its function at the molecular, cellular, systems, cognitive, and clinical level. It clearly contributes to processing emotionally and socially relevant information, yet a unifying description and computational account have been lacking. The difficulty of tying together the various studies stems in part from the sheer diversity of approaches and species studied, in part from the amygdala’s inherent heterogeneity in terms of its component nuclei, and in part because different investigators have simply been interested in different topics. Yet, a synthesis now seems close at hand in combining new results from social neuroscience with data from neuroeconomics and reward learning. The amygdala processes a psychological stimulus dimension related to saliency or relevance; mechanisms have been identified to link it to processing unpredictability; and insights from reward learning have situated it within a network of structures that include the prefrontal cortex and the ventral striatum in processing the current value of stimuli. These aspects help to clarify the amygdala’s contributions to recognizing emotion from faces, to social behavior toward conspecifics, and to reward learning and instrumental behavior.
Keywords: amygdala, emotion, fear, face processing, saliency, social cognition, reward learning
Introduction
Work on the amygdala is fractured into several domains, and relating these in a consistent account of amygdala function is not straightforward. In terms of functional topics, one can identify three broad themes: social behavior, emotion, and reward learning. The classic studies by Kluver and Bucy (in the 1930s), and before them Brown and Schafer (in the 1880s), perhaps emphasized mostly the first, although they were also relevant for the second and third. The late 1990s and early 2000s saw an explosion of work on emotion, in particular in relation to the processing of facial expressions. And earlier work on issues related to reward learning has now led to a host of studies using single-unit electrophysiology in animals as well as fMRI in humans. Complementing the diversity of functional topics is a diversity of species in which the amygdala has been investigated. Until about 1994 this was almost exclusively research conducted in rodents, with a small number of laboratories tackling the challenge of work in monkeys. Although that work has continued (and indeed is thriving), it has been to some extent overshadowed by a plethora of fMRI studies in humans, many investigating psychiatric illness.
At the outset it is important to keep in mind that the amygdala is a complex collection of 13 nuclei in primates1 (indeed, there has been some controversy about the concept of “the amygdala” as a single entity2) (Fig. 1). These are typically distinguished in studies in nonhuman animals, but rarely in humans because of the limited spatial resolution afforded by techniques commonly used, such as fMRI (see Box 1). The amygdala is extensively connected with many other cortical and subcortical structures, and so accounts of its function will need to do justice to its location in this dense web of connections. Finally, it has become apparent that there are substantial individual differences in the amygdala (the extremes of which may contribute to many psychiatric illnesses), as well as substantial effects of context and stimulus history, all of which makes it essential to look at individual and trial-wise details which can get obscured in group-level effects and meta-analyses.
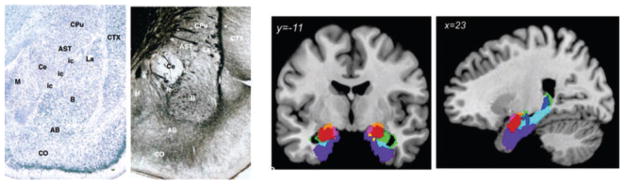
Figure 1.
The amygdala and its nuclei. On the left is a Nissl stain, which stains cell bodies, and a silver stain that stains fibers coursing through the amygdala, showing the rat amygdala. On the right are a coronal and parasaggital MRI scan onto which probabilistic locations of amygdala nuclei in humans have been mapped. From Refs. 87 and 137. Abbreviations of amygdala areas: AB, accessory basal; B, basal nucleus; Ce, central nucleus; itc, intercalated cells; La, lateral nucleus; M, medial nucleus; CO, cortical nucleus. Nonamygdala areas: AST, amygdalo-striatal transition area; CPu, caudate putamen; CTX, cortex. Copyright acknowledgment: two lefthand panels: reproduced from Ref. 87 with permission from Elsevier; two righthand panels: reproduced from Ref. 137 with permission from Springer Science+Business Media.
Box 1. BOLD fMRI of the amygdala.
By far the largest number of studies on the human amygdala use BOLD fMRI as the approach. Although this is revealing a wealth of data and is certain to continue to constitute the method of choice for practical reasons at least for the next several years, it is worth reiterating the caveats associated with BOLD fMRI. Aside from the flurry of recent attention to concerns about statistical reliability and generalizability,135,136 all of which can be addressed in studies cognizant of these caveats, there are technical difficulties in obtaining and localizing signal in and around the amygdala due to susceptibility artifacts. Optimized imaging parameters have largely solved the problem of weak signal, and many researchers now obtain B0 field maps to correct for geometric distortion. Another tricky issue is that BOLD responses to particular psychological or objective stimulus dimensions may be nonlinear—for instance, nonlinear responses have been noted both to trustworthiness in faces64 as well as to valence in odorants.70 A more fundamental limitation is the spatial resolution of BOLD fMRI together with individual variations in amygdala morphometry, limiting conclusions about exact boundaries and nuclei within the amygdala to probabilistic statements.137 Even worse is the issue of temporal resolution as we noted earlier, because this definitely conflates a number of distinct processes that occur on the timescale of a few milliseconds rather than the seconds of BOLD signal integration. Perhaps the most worrisome aspect of BOLD fMRI concerns its physiological basis. Not only is the hemodynamic response function in the amygdala rather different in shape than what is found in cortex, but as with all BOLD fMRI, it remains uncertain what precisely is driving the observed signal. Possibilities range from inputs from distal targets to intrinsic processing to little neuronal activity at all and mere distal regulation of hemodynamics.138 While researchers are acutely aware of these issues and more, it remains the case that definitive findings will require convergent results from multiple approaches—ideally electrophysiology, fMRI, and lesion studies.
Some disclaimers are in order to help circumscribe this review, because one could easily write a book on the amygdala (and several have been written3,4). First, the focus will be on social-emotional functions of the amygdala in primates with an emphasis on humans. Second, I will not review the amygdala’s role in psychiatric illness here, although insights into its functional role from the studies discussed earlier are of course highly relevant for understanding psychiatric illnesses as well, ranging from mood disorders to autism. Third, I will highlight lesion studies together with some electrophysiological studies while minimizing review of functional imaging studies (which have been reviewed to some extent elsewhere5,6). Finally, studies reviewed and citations will emphasize the most recent work, following the theme of this book series.
Lesion studies of social behavior in monkeys
Kluver and Bucy’s classic studies in the 1930s described the behavioral consequences of large bitemporal lesions in monkeys,7,8 which included a propensity to shift rapidly in exploring different objects (“hypermetamorphosis”), to approach, ingest, and mount many stimuli indiscriminately, and to show a profound lack of behavioral regulation on the basis of the emotional and social meaning of stimuli (“psychic blindness”). Monkeys with such lesions were not blind or deaf—they just no longer had access to the value of what they saw and heard.9 A well-known shortcoming of these early experiments was the nonselectivity of the lesions, which included not only the entire medial temporal lobe but also substantial portions of lateral and posterior temporal cortex as well as subjacent white matter, making it impossible to assign the deficits specifically to the amygdala. Nonetheless, their observations set the stage for subsequent work using more selective aspiration10 and pharmacological lesions of the amygdala.11–14 Current lesion studies favor lesions made by injecting the drug ibotenic acid, a neurotoxin which can be quite selective for neurons, sparing both fibers of passage as well as surrounding structures if the injection is sufficiently focal (typically verified by structural MRI). Reversible lesions can also be made by injecting drugs such as muscimol, a GABA-A receptor agonist that temporarily silences the electrical activity of neurons through inhibition. The advent of optogenetic methods will no doubt lead to a large number of studies examining the reversible activation and inactivation of specific neuronal sub-populations in the amygdala in the very near future.15 The studies so far have generally revealed a subset of the full-blown Kluver–Bucy syndrome, the subset becoming more restricted the more selective the lesions. This likely arises from the fact that many of the structures proximal to the amygdala, such as perirhinal and entorhinal cortices and temporal polar cortex, also participate in social behaviors to some extent. When the amygdala is more selectively lesioned, there is disproportionate impairment in particular in the normal cautiousness and distrust with which monkeys approach novel or frightening objects, or people. For instance, monkeys with amygdala lesions show less caution in approaching potential predators like snakes to which they normally have an innate fear response14 and show less initial avoidance of human strangers.12 These behaviors are especially notable in circumstances of novelty and unfamiliarity, where healthy monkeys typically exercise substantial caution in approaching unknown objects or unfamiliar people—an issue we will return to when discussing insights obtained from functional imaging and lesion studies in humans that are pointing toward a role for the amygdala in processing unpredictability or ambiguity.
The behavior of amygdalectomized monkeys toward other monkeys is more complex to quantify, due to the reciprocity of the social interaction. Earlier lesions that were nonselective resulted in severe impairments in social behavior with the result that the monkeys lost their social status16 and were ostracized by the group, resulting in death in the wild.17 Selective neurotoxic lesions resulted in more subtle impairments that were quite complex and depended on other factors. One study found that the amygdalectomized monkeys showed more prosocial cues and less avoidance behaviors toward other (healthy) monkeys when in dyadic interactions, with the result that they were actually approached more and groomed more by other monkeys.11 They also showed more approach behavior toward unfamiliar humans, consistent with their increase in prosocial behaviors (Fig. 2). However, in more complex groups (the lesioned monkey together with three healthy monkeys in a tetrad) these effects were not seen, and instead a quite subtle increase in avoidance and stress behaviors was shown by other monkeys toward the amygdalectomized monkey.13 Further complexities arise if the lesions are made neonatally: for instance, exaggerated social fear (yet with the typically diminished fear of novel objects) has been reported in such lesioned monkeys,18 although this profile appears to change as the monkeys age.19
Figure 2.
Social approach behavior following amygdala lesions. (A): Monkeys with amygdala lesions show less fear towards predators and less timidity towards humans. One measure quantifying this is that they spend more time at the front of the cage when there is an unfamiliar person standing there. (B): Approach behavior in patient SM (red bar) compared to control subjects (purple bars) in relation to the experimenter (black bar). Whereas SM had a preferred interpersonal distance of 0.34 meters (C), controls had a distance of 0.64 meters (D). Copyright acknowledgment: A: reproduced from Ref. 12 with permission from the American Psychological Association; (B–D): modified from Ref. 37 with permission from Nature Publishing Group.
Two important take-home messages from the monkey lesion studies are that the amygdala’s effect on social behavior is not rigid and universal, but context dependent and susceptible to individual differences; and that even complete lesions of the amygdala appear to leave the repertoire of social behaviors as such largely intact—they just are not elicited in a context-appropriate way.20 For instance, monkeys with amygdala lesions can still respond normally to social stimuli such as a human stare, even though they show blunted avoidance responses to potential predators such as a snake.14 Although the socioemotional changes in monkeys with amygdala lesions appear to constitute a stable behavioral change that can be thought of as a trait change in personality,12 it is neither a change in the ability to show the full repertoire of social behaviors20 nor a change in mood as such.21 Rather, it is probably best thought of as a consistent change in the way that context-dependent situations (stimuli in the context of an emotionally significant or socially significant setting) modulate motivated behavior. Part of the complexity in accounting for the amygdala’s effects on social behavior, and the reason for the rather nuanced explanation just offered, will become more apparent in the sections later: they arise from the fact that the amygdala is connected to a host of other structures whose function it modulates.
Lesion studies of social behavior in humans
Several etiologies can produce amygdala lesions in humans. Probably the most common is epilepsy, which can result in medial temporal sclerosis if severe and untreated. More relevant for the present review, medically refractory epilepsy is occasionally treated neurosurgically, by ablation of parts of the medial temporal lobe on one side. The late famous patient HM had bilateral medial temporal lobe lesions, including bilateral lesions of the amygdala, for the treatment of epilepsy with the consequence that he became severely amnesic due to his bilateral hippocampal damage. Nowadays, the surgery is essentially always unilateral and involves variable extents of resection of the hippocampus, the amygdala, and surrounding medial temporal and temporal polar cortices. The consequences of such lesions on social cognition are impossible to attribute selectively to the amygdala, although they are likely due in good part to amygdala damage because they bear some resemblance to what is observed following selective amygdala lesions (e.g., impaired Pavlovian fear conditioning22). They are milder than the impairments seen with bilateral amygdala damage,23,24 as would be expected, and there is some indication that damage to the right amygdala may disrupt aspects of social cognition more than damage to the left amygdala.25
A second possible cause of amygdala lesions in humans is encephalitis, which can result in large bitemporal lesions approaching those made by Kluver and Bucy in monkeys. Patients with such lesions do show severe impairments in processing emotional and social information,26,27 although not generally to the degree that Kluver and Bucy observed in monkeys, and like Kluver and Bucy’s studies they suffer the same nonspecificity of the lesion to the amygdala.
The most specific bilateral lesions of the amygdala result from very rare constellations of damage (e.g., a combination of neurosurgical and/or vascular28,29) or from Urbach-Wiethe disease.30,31 Urbach-Wiethe disease, also called lipoid proteinosis, is an extremely rare genetic disease,32,33 although a few studies with samples of 10 or more subjects have now been published.34,35 We have studied in detail a patient, SM, who has complete bilateral amygdala lesions due to Urbach-Wiethe disease (Fig. 3) with minimal damage to surrounding structures and with IQ in the low normal range.36 She is a 43-year-old woman with a high-school education whose lesions encompass the entire amygdala plus subjacent white matter and anterior entorhinal cortex. This lesion was likely developmental; although the precise age at which it was acquired is unknown, it is likely to have occurred sometime in childhood or adolescence, and it is possible that it was congenital. A series of studies in this patient has documented a remarkably specific impairment in recognizing fear from facial expressions, together with impairments in a variety of social judgments from faces, discussed in more detail in the next section (see Table 1 for a summary).
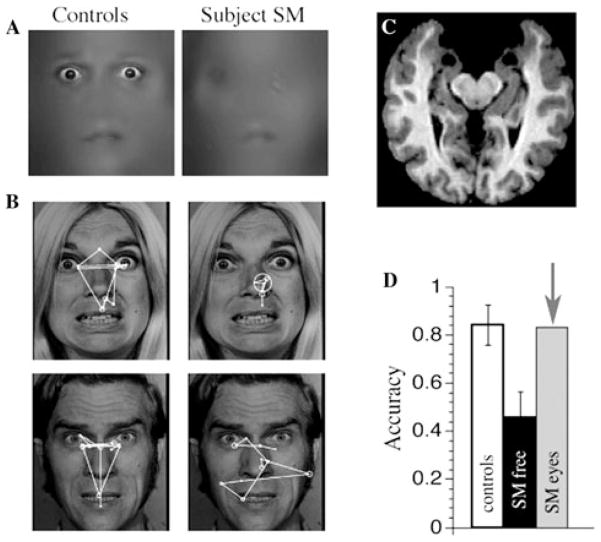
Figure 3.
The brain and face processing of patient SM. Bilateral amygdala lesions impair the use of the eyes and gaze to the eyes during emotion judgment. (A) A patient with bilateral damage to the amygdala made significantly less use of information from the eye region of faces when judging emotion. (B) While looking at whole faces, the patient (right column of images) exhibited abnormal face gaze, making far fewer fixations to the eyes than did controls (left column of images). This was observed across emotions (free viewing, emotion judgment, gender discrimination). (C) MRI scan of the patient’s brain, whose lesion was relatively restricted to the entire amygdala, a very rare lesion in humans. The two round black regions near the top middle of the image are the lesioned amygdalae. (D) When the subject was instructed to look at the eyes (“SM eyes”) in a whole face, she could do this, resulting in a remarkable recovery in ability to recognize the facial expression of fear. The findings show that an apparent role for the amygdala in processing fearful facial expressions is in fact more abstract, and involves the detection and attentional direction onto features that are socially informative. Modified from Ref. 162. Copyright acknowledgment: reproduced from Ref. 162 with permission from Nature Publishing Group.
Table 1.
Summary of findings from subject SM
| Impaired in recognizing fear from static facial expressions38 |
|---|
| Gives abnormally low ratings of intensity to fearful faces147 |
| Impaired conditioned autonomic responses in Pavlovian fear conditioning 148 |
| Impaired emotional modulation of declarative memory149 |
| Abnormally positive judgments of trustworthiness and approachability from faces150 |
| Cannot judge arousal in negatively valenced stimuli151 |
| Abnormally positive preferences for abstract visual stimuli152 |
| Can discriminate between emotions normally153 |
| Can recognize fear from voice prosody154 but not music 155 |
| Impaired in the Baron-Cohen eyes task156 |
| Less impaired in recognizing emotions in scenes when faces are erased157 |
| Mildly impaired also in recognizing sadness, but not happiness158 |
| Impaired in fixating and using information from the eye region of faces55 |
| Impaired emotional memory for gist but not details159 |
| Lack of experience of negatively valenced emotions in real life125 |
| Fixates the mouth instead of the eyes in conversations with real people160 |
| Has diminished BOLD signal in medial prefrontal cortex during reward expectancy106 |
| Can recognize fear from body posture and pointlight walkers161 |
| Lacks a sense of personal space37 |
| Performs normally on rapid detection and nonconscious processing of fear faces54 |
With respect to social behavior, SM is notably dis-inhibited and shows a propensity to approach and engage with others that has occasionally resulted in social difficulties in real life.36 Although her social behavior and social decision-making appears somewhat abnormal, and in the same direction as what one might hypothesize on the basis of the lesion studies in monkeys reviewed earlier, this is difficult to quantify. We recently undertook a first laboratory study to quantify aspects of her social behavior toward others.37 In this study, we asked participants to indicate their preferred distance in standing facing an experimenter, together with rating their feeling of uncomfortableness when this preferred distance was narrowed and their personal space was violated. Although there is of course considerable contextual and individual variation in interpersonal distance in real life, the distances obtained in our laboratory context were remarkably consistent and hovered around 0.6 m with a long-tailed distribution. When people were approached close to 0.4 m, we reliably encountered the wall of personal space. By contrast, SM appeared to have no feeling of personal space whatsoever: her mean preferred interpersonal distance in this experiment was smaller than that of any control subject, she occasionally walked all the way up to touching the experimenter, and in all circumstances endorsed no feeling of uncomfortableness when personal space was violated (Fig. 2).
These findings in humans with amygdala lesions bear some resemblance to what we saw in monkeys with bilateral amygdala lesions earlier: a lack of the normal cautionary brake on behavior with an increase in approach and prosocial behaviors (Fig. 2). It is also consistent with a broader function for the amygdala in processing salient or relevant stimuli, perhaps especially when these signal unpredictability or potential threat, a function reviewed in more detail later. It is noteworthy that subject SM still appears to have rank-ordering of interest in other people: she is not completely indiscriminate in putting an equal value on everyone. For instance, she exhibits concern and maternal emotions toward her children. Interestingly, female monkeys with neonatal amygdala lesions have a notably reduced interest in infant monkeys, suggesting that aspects of maternal behavior in monkeys are disrupted.19
Processing information from faces
The initial finding that bilateral lesions to the human amygdala impair recognition of emotion from facial expressions38,39 was quickly followed up with functional imaging using PET40 and has since then spawned a veritable industry of fMRI studies investigating responses to faces and other social stimuli (see Ref. 4 for a sampling). The results from the neuroimaging studies have been complex and to some extent inconsistent. Although earlier studies found evidence that the amygdala responded more to fearful faces than expressions of other emotions,40 more recent studies support the idea that the amygdala responds to all faces,41 perhaps especially on the left side,42 and with complex modulations depending on their social meaning for a particular individual in a particular context.43,44 There are now many examples of large individual differences in amygdala responses to faces, differences that have been tied to differences in gender,45 in mood and personality (ranging from anxiety46 to extraversion47), as well as in genotype.48,49
In the patient with bilateral lesions, SM, who was described earlier, we studied her processing of emotional faces in great detail. Two recent conclusions have emerged from this work. One is a conclusion about the stage(s) in processing at which the amygdala might come into play. Based on a number of prior findings, notably auditory fear conditioning in rats50 as well as a few neuroimaging studies51 together with a theoretical view,52 it was generally thought that the amygdala comes into play early in processing, and therefore participates importantly in automatic and nonconscious rapid processing of stimuli that signal danger. Although the amygdala may still participate to some extent in such a function, this view ought to be revised in light of several new findings, as argued elsewhere.53 The amygdala’s role appears to be much broader than the older view would suggest, and seems unlikely to be restricted to processing only stimuli related to threat or danger. It also appears to be inessential for many aspects of rapid and nonconscious processing of such stimuli. One recent study in subject SM demonstrated this latter finding: SM showed a normal ability to detect fearful faces in visual search or rapid discrimination, and a normal ability for fearful faces to overcome binocular suppression that would render them non-conscious.54 These findings do not rule out some role for the amygdala in rapid and nonconscious processing related to orienting, but together with other findings they shift the view toward a more modulatory, temporally extended role for the amygdala in perception and recognition. They also shift the anatomical substrate of processing away from a subcortical route of visual input to the amygdala via the superior colliculus and pulvinar thalamus to amygdalo-cortical interactions.
A second broad conclusion from findings in subject SM and from other recent studies has been the idea that the amygdala allocates processing resources (such as attention) to salient stimuli, or features within stimuli. In regard to SM’s impaired recognition of fear from facial expressions, this appears to arise from her inability to make spontaneous use of information from the eye region of faces: she fails to direct her gaze toward this region in faces, fails to benefit from it when it is shown in isolation, and improves when instructed to fixate the eye region (Fig. 3).55 When shown sparsely sampled faces (using a method called “bubbles”56 to reveal only small random parts of an underlying face), people normally benefit from having the eyes in the face revealed when they are asked to discriminate fear from other emotions,57 but SM shows no such benefit. SM also generally does not fixate the eyes in faces (either in whole faces or in the bubbles faces), in many cases staring straight at the center of the face without moving her eyes over the face at all. Yet she is able to make use of the eye region of faces to help her discriminate fear when her visual attention is explicitly directed toward the eyes. These findings fit with a role for the amygdala in vigilance, ambiguity resolution, and uncertainty resolution: it modulates other brain structures to enhance the processing of stimuli about which more information needs to be acquired.58,59 Both of the earlier findings are in line with the idea that the amygdala modulates cortical processing to implement selectivity for biologically relevant stimuli, just as it does, for example, in modulating hippocampal-dependent memory consolidation.60
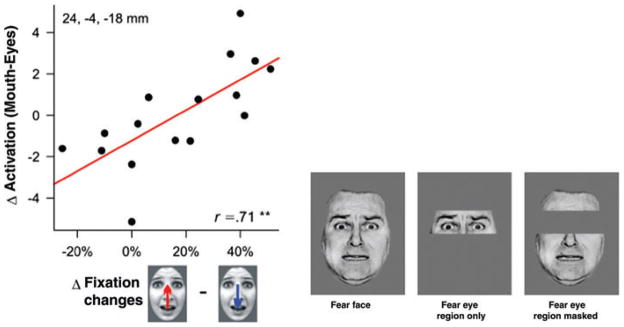
The amygdala’s role in processing information about the eye region of faces, put together with its presumptive function in disambiguating stimuli, has been borne out also by two recent functional neuroimaging studies (Fig. 4). One study61 showed subjects’ fearful faces (as well as neutral, happy, and angry faces) briefly presented at locations where the preceding fixation cross either coincided with the eye region of faces, or with the mouth region of faces. The authors then collected BOLD signal from the amygdala and also measured eye movements. When the data were analyzed with the direction of the eye movement as a regressor (either moving down toward the mouth when the eyes had been fixated, or up toward the eyes when the mouth had been fixated), a significant effect was found in the amygdala specifically to fear faces (Fig. 4, left). The effect amounted to a significant correlation between BOLD signal evoked in the amygdala and the propensity to direct gaze to the eye region of the face when the mouth had been fixated initially. These findings provide strong support for the idea that the amygdala serves to direct gaze toward the eyes in fearful faces to obtain disambiguating information. Another fMRI study found that the amygdala was differentially activated by fearful compared to neutral faces even when the eye region of the face was masked62 (Fig. 4, right), also consistent with the idea that the amygdala is not necessarily responding to the eyes as such, but rather directing processing resources toward disambiguating that region of the face to figure out its social meaning (a mechanism that could engage even more so when the eyes are covered in an attempt to glean whatever information possible from that region).
Figure 4.
The amygdala helps direct gaze to eyes in faces. Left: data from an fMRI study that plots the response in the amygdala as a function of how often people shift their gaze from the mouth up to the eyes.61 The amygdala is not activated so much as a consequence of fixating the eyes, but rather in preparation for fixating the eyes. A similar conclusion was obtained in another fMRI study, which found equivalent amygdala activation to fear faces even when the eye region itself was erased (right).62 Copyright acknowledgment: reproduced from Ref. 61 with permission from the Society for Neuroscience (left), and from Ref. 62 with permission from Elsevier (right).
There are several remaining puzzles about the type of visual information processed by the amygdala. A subcortical route of input would put emphasis on low spatial frequencies; yet patient SM has impairments rather selective for high spatial frequencies. Some fMRI studies have suggested that low spatial-frequency information is most effective in driving the amygdala,63 whereas others have found evidence for both high and low spatial frequency.64 In the case of our studies with SM on the bubbles task, the data are quite specific in showing that she fails to make use selectively of high spatial-frequency information from the eyes while low spatial frequencies are preserved. The relationship between visual fields and amygdala response is also somewhat puzzling. Some human fMRI studies have now provided evidence that amygdala activation correlates better with presentation of salient stimuli in the ipsilateral visual field,65 and we have preliminary findings that unilateral amygdala lesions impair processing of the eye region shown on the side of the stimulus face that is ipsilateral to the side of the lesion.
The amygdala codes salience or relevance
The earlier notion that the amygdala may be specialized for fear-related processing and later ideas that it comes into play when stimuli are unpredictable or ambiguous have a related theme: both situations involve a need to gather additional information from the environment. The physiology of facial expressions of fear is such that they maximize visual and olfactory intake of information—adaptive in circumstances where an unknown potential predator needs to be detected.66 Yet it has been elusive to quantify this aspect of information processing in terms of a single parameter or psychological dimension. It does not neatly fit the concept of “arousal” if by that one means autonomic arousal as classically conceived; however, it could be related to a more information-processing concept of arousal that construes it as interruption of ongoing processing to gather new information (more aligned with orienting).
There have been hints for some time that the amygdala must process a stimulus dimension that is more abstract than the traditional concepts of valence or arousal alone. Several studies have found that the amygdala is activated by both negatively as well as positively valenced stimuli,67 leading to the common view that it tracks emotional arousal (and that prior claims about specialization for fear, threat, or negative valence were simply derivative to the higher arousal of such stimulus categories).68 This emphasis on the amygdala as processing arousal also fit with a view that emerged from the literature on emotional memory.69 However, this looks unlikely to be the final story. One study investigating the intensity and valence of odorants argued that a conjunction of valence and intensity (in those studies a proxy for arousal) may be what the amygdala cares about: stimuli that are both arousing/intense and emotional (either positively or negatively valenced).70 Another study found amygdala activation not only to both negatively and positive-valenced stimuli of high arousal, but also to stimuli that were not so much arousing as they were interesting or bizarre.71 It is difficult to know from these studies what psychological construct would best capture whatever it is that is engaging the amygdala.
One study explicitly investigated psychological dimensions other than “arousal” or “valence,” and found that even when these were held relatively invariant, the amygdala showed a further differential activation as a function of “impact,”72 the subjective significance or relevance of a stimulus. Although this construct is correlated with arousal, it is not perfectly correlated. This finding is consistent with some other attempts to assign to the amygdala a more abstract and ecological role in processing “relevance,” a concept from psychological appraisal theory that stresses the contextual and goal-dependent value of a stimulus within a personal situation.73,74 Constructs such as impact and relevance also pave the way for investigating individual differences in amygdala function, because they are patently subjective and dependent on each person’s contextual interpretation of a stimulus in the sense that they are individual rather than entirely universal.
Several electrophysiological studies in both humans and monkeys have led to a conceptually parallel view: on the one hand, the amygdala appears to respond to a broad class of social stimuli and to reward value or arousal as such; on the other hand, neurons within it can show highly selective responses to specific social stimuli that are relevant for that person or monkey. In monkeys, electrophysiological responses in the amygdala have been found that precede skin-conductance responses,75 a ubiquitous index of orienting and arousal that can be elicited by any salient or novel stimulus. A number of recordings from the amygdala and adjacent cortex in monkeys have found responses to faces76,77 and to other complex social stimuli,78 in several cases showing selectivity for identity, emotion, or social status of the stimulus. Several recent studies have also investigated responses to reward value. In humans, responses to emotional faces79 and complex social scenes80 have been recorded from depth electrodes in the amygdala in neurosurgical patients, and some categorical coding to emotions such as threat or disgust have been extracted.80 Highly selective and abstract responses to the identity of specific people have also been found at the single-unit level—in some cases with generalizability across viewpoints, kinds of depictions (photos or caricatures), and even whether the image is a photo or the written name of a famous person.81 It should be noted, however, that such responses are not unique to the amygdala, and similar response profiles are often encountered in nearby cortex and hippocampus.
It would be useful to probe the amygdala’s response on a battery of heterogeneous stimuli that span a range of saliency or relevance to test the hypothesis that these dimensions are what is driving the amygdala. Although no such study has yet been undertaken, there is evidence to support this prediction from the tasks so far: the amygdala is activated by salient images including faces, by increasing amplitudes of sound (arguably an auditory analog of the most salient visual stimulus type, optic flow to signal collision),82 and even by cognitive indicators of saliency such as the mere belief that another person is approaching to stand close, when that person cannot actually be seen.37 The related concepts of unpredictability and ambiguity seem to be two potential underlying (but perhaps not exhaustive) factors that could influence saliency and hence the amygdala. Ambiguity aversion in monetary gambles (i.e., gambling when the risks are unknown and uncertainty is therefore highest) is correlated with amygdala activation.83 Amygdala activation in humans, as well as electrophysiological responses within the amygdala in rodents, have been linked to temporal unpredictability in sequences of auditory stimuli.84 In this latter study, a potential cellular mechanism was suggested: temporally unpredictable stimuli result in less habituation of amygdala responses. This differential habituation, together with a generally rapid habituation to stimuli that have become predictable and hence less salient in some way,85 may underlie some of the amygdala’s response to novel and unpredictable stimuli. Taken together, the recent studies point toward a revised view of the type of stimulus category, and the kind of psychological dimension, that the amygdala helps process. The challenge now is to translate these constructs into a computational framework that would allow one to formulate parametrically quantitative hypotheses. Here, approaches from reinforcement learning and neuroeconomics may help, a topic we briefly review next.
The amygdala in reward learning
There is a huge and well-known literature on fear conditioning and the amygdala, mostly from work in rodents. This has shown that the amygdala is necessary for at least some and possibly all aspects of fear conditioning,86 that prominently the lateral as well as the central nucleus87 are the key components, and that specific subpopulations of neurons within the amygdala can be identified as a possible neuronal substrate.88 There is still plenty of debate about details in this picture. But there are now an ever-growing number of studies that examine the amygdala’s role in processing stimulus value in a much broader way than only fear conditioning.
Ever since earlier lesion studies showing that the amygdala plays a role in appetitive as well as aversive conditioning, evidence has been accumulating that linking the amygdala to “fear” is too simple a story. There is now a substantial literature from electrophysiological studies in animals showing that amygdala neurons respond both to rewarding and punishing stimuli or their predictors. In fact, there is a bewildering variety of neuronal response types in the amygdala, with neurons that encode rewards intermixed with those coding punishment and no apparent evidence of any segregation or topography.89 Some neurons respond both to aversive and rewarding conditioned stimuli,90 suggesting a more abstract coding of predictors for emotional arousal or saliency. In general, the lesion and electrophysiology literature from studies in animals has made a strong argument that the amygdala codes the abstract reward value of stimuli or their predictors, rather than their sensory properties or the particular instrumental actions required for obtaining or avoiding them.91 This has led to the view that the amygdala codes a continuously updated and flexibly deployed representation of stimulus value.92 Somewhat at odds with such a role in abstract value representations are other electrophysiological studies in both human81,93 and nonhuman77 animals that argue there is also coding for stimulus identity. Plausibly, the conjunction of a dynamic coding of stimulus value together with coding stimulus identity would serve a key role in social behavior: the need to keep track of the social value of conspecifics through time.
Although the amygdala appears essential for Pavlovian fear conditioning and is clearly involved in reward learning, this latter role is nuanced. There are many tasks that ostensibly involve aspects of reward learning for which complete bilateral lesions of the amygdala, if selective, result in essentially no impairment.94,95 This revision is reminiscent of how our picture of the amygdala’s role in social behavior has evolved from early highly nonselective ablation studies through selective ibotenate lesions in modern day. What selective ibotenic acid lesions of the amygdala do seem to impair is learning in tasks where information about stimulus value is essential, such as in devaluation studies.92 In such studies, the animal is asked to choose the exact same stimulus before and after a devaluation such as satiation, which changes the reward value of the stimulus without changing any of its sensory properties or associations. These tasks require a flexible updating of the value associated with a stimulus based on integration of its outcome with the physiological state of the animal (e.g., when satiated on a particular food, that food loses its reward value even though its sensory properties remain unchanged). Lesions of the amygdala abolish the change in behavior that would indicate that the reward value of the stimulus has been updated by the satiation, suggesting the amygdala is critical to maintain current representations of reward value—a conclusion in line also with functional imaging studies in humans.96
The importance of context and of individual differences is emerging in studies of reward learning and decision-making as well. One example has been highlighted in a study that examined the so-called framing effect from economics.97 In this study, participants were first given some money, and then asked to choose between two options. One option was to keep a fixed amount of this money (say, 20outofaninitialendowmentof20 out of an initial endowment of 20outofaninitialendowmentof50); the other option was to gamble with some probability of keeping or losing all of the initial endowment (say, 2/5 chance of keeping all of it and 3/5 chance of losing all of it). Importantly, the expected value of the sure amount and the gamble were identical. The trick in this study was that the sure amount was presented in two frames: a “loss frame” in which it was described as “you lose 30ofyouroriginal30 of your original 30ofyouroriginal50,” and a “gain frame” in which it was described as “you win 20ofyouroriginal20 of your original 20ofyouroriginal50.” This resulted in a well-known effect from economics called the framing effect; subjects chose the gamble with the positive frame over the one with the negative frame, even though both state the same outcome. This framing effect correlated profoundly with activation of the amygdala. Moreover, there were substantial individual differences on the task and in brain activation. This story has recently been linked to genetic variation in the serotonin reuptake transporter as well98 (a polymorphism with a rapidly growing literature linking it to individual differences in amygdala response99).
These findings from the reward learning literature, which have only been reviewed very briefly here, complement the studies on face processing and social behavior, which also emphasize the highly dynamic and context-sensitive role that the amygdala must play in evaluating stimuli. For instance, the actual task of judging the positive or negative qualities of famous faces strongly modulates amygdala activation to those faces100; and the mere assignment to a social group in an experiment is sufficient to drive amygdala responses to faces that discriminate people within one’s assigned experimental group from those outside the group.101 Taken together, the reward learning and social neuroscience literature hold out promise to provide a computational framework in which amygdala function could be formally modeled. Such a framework would be expected to draw from neuroeconomics and decision science and might provide a unified view of what are now a huge number of somewhat disparate findings on the amygdala’s role in social cognition. That view would articulate the amygdala’s role in integrating internal and external sensory signals to continuously monitor the physiological value of a stimulus, and it would play this role also extended to complex social stimuli. However, a complete model of such a function requires we consider what other structures interact with the amygdala, and what regions of the brain might receive signals from the amygdala that can be used to implement aspects of cognition and behavior.
Interaction with other structures
Investigating the amygdala’s interaction with other structures as a component of a network for processing the reward value of stimuli is a currently hot topic. Who are the other players? Highlighted have been the orbitofrontal cortex and other sectors of the prefrontal cortex, the striatum, and the nucleus accumbens.102 Lesions that disconnect the amygdala with some of these structures have documented the importance of their interaction. For instance, disconnection of the amygdala and orbitofrontal cortex results in deficits on reward learning tasks as severe as lesions to either structure in isolation.103 Similarly, disconnection of the amygdala from the nucleus accumbens disrupts instrumental behavior toward rewards.104 A technically and conceptually challenging issue concerns the order in processing at which different structures come into play. The issue is technically difficult because it generally requires concurrent electrophysiological recording; it is conceptually challenging because the structures are reciprocally connected and participate in processing over some extended duration that would permit multiple iterations of feedback. Nonetheless, some headway has been made even here. For instance, in the aforementioned interaction between amygdala and nucleus accumbens, there is evidence that the amygdala can come into play early and convey information about particular sensory cues to the nucleus accumbens to guide instrumental behavior. A similar story has been proposed from lesion studies of the amygdala in humans: one study reported a lack of loss aversion following amygdala lesions, interpreted as an amygdala-dependent signal that was passed to the striatum105; another study found reduced signal related to reward prediction in the prefrontal cortex when the amygdala was lesioned, a finding also interpreted as evidence for a reward-related signal that would normally be passed from the amygdala to the prefrontal cortex to guide behavioral choice.106 In all these cases, the actual route of information transfer is unknown: for instance, it appears likely that the amygdala influences the prefrontal cortex both through a strongly driving indirect route via the dorsomedial thalamus as well as a direct but diffusely modulatory input107 (somewhat the converse of what one might have guessed intuitively). Electrophysiological recordings from amygdala and orbitofrontal cortex in rats bear out this overall picture: the amygdala acquires the requisite associations related to the current reward value of a stimulus, and the orbitofrontal cortex uses this signal to guide choice,108 a finding consistent with human fMRI data as well.106 An important future direction will be to dissect in detail how processing between the two structures evolves in time during the decision-making process.
In terms of sensory inputs to the amygdala, these hail from all sensory modalities, including interoceptive information that would include information about internal states such as hunger or satiety. In regard to vision, the primate amygdala receives strong inputs from anterior temporal neocortex; there is also a hypothesized subcortical route of visual input that, as we noted above, is both anatomically and functionally unclear.53 It has been known for some time that the connections of the amygdala with visual cortices show both reciprocal and nonreciprocal feedback projections from the basal amygdala to all regions of visual cortex in the temporal lobe, and indeed all the way back to primary visual cortex in the occipital lobe (a finding so far documented only in monkeys109 and cats110). The functional significance of this feedback architecture remains unclear, although it has been shown to modulate processing in temporal cortex of emotional facial expressions111 and may play a role particularly in conscious evaluation of such stimuli.112 The sensory inputs to the amygdala are gated by at least two mechanisms. One is a dopamine-mediated enhancement of sensory inputs to the amygdala; a second is a prefrontal-mediated inhibition via projections from the prefrontal cortex.113 Both of these mechanisms show individual differences, which may be correlated substantially with individual differences related to mood.114 The connections between amygdala and the prefrontal cortex in particular have been highlighted in regard to genetic polymorphisms and susceptibility to psychiatric illness.115 Of great interest has been a polymorphism in the promotor region of the serotonin reuptake transporter (5HTTLPR), which is associated with risk of depression, as well as with changes in BOLD signal within the amygdala while processing emotional facial expressions99 in humans, and associated with individual differences in anxious temperament and scanpaths to faces in monkeys.116 More recently, a number of studies have found that the polymorphism is associated also with systematic changes in the strength of both structural and functional connectivity between amygdala and medial parts of the prefrontal cortex,49,117 with consequences for psychopathology,49 trait anxiety,118 as well as for aspects of decision making that take into account context and framing effects we discussed earlier.98 There are also well-known projections from the amygdala to the nucleus accumbens to modulate dopaminergic responses related to reward learning. In fact, the basolateral amygdala together with prefrontal cortex appear essential to provide such dopaminergic neurons with stimulus and context-related information that can be used to predict reward,119,120 emphasizing the tight relationship between amygdala, prefrontal cortex, and ventral striatum in reward learning.
The amygdala projects to a host of other structures (see Ref. 1 for review), and the functional consequences of these projections remain to be fully understood. In addition to the projections to components of the basal ganglia that influence instrumental learning and choice, and to the prefrontal cortex to modulate decision making as noted earlier, the amygdala also projects to structures such as the hippocampus to modulate consolidation of emotional declarative memories, to the basal fore-brain to modulate attention and other aspects of memory, and to the retrosplenial cortex121 where it may influence self-directed versus externally directed attention. All of these connections can likely be understood at least in part as a modulation that provides some kind of processing selectivity based on value, saliency, and relevance. It is likely that such modulation takes place at multiple temporal scales, ranging from long-term trait-like effects to moment-by-moment effects on specific items encountered.122
Future directions
One big open question concerns the amygdala’s role in the conscious experience of emotion and motivation. Although functional imaging studies generally have supported such a role correlatively, few have investigated it explicitly. One study found a correlation between real-life emotional experience and the magnitude of amygdala activation to visual stimuli.123 Yet a lesion study argued that the amygdala was inessential for the experience of fear,124 although this was based on a limited questionnaire measure. We have examined the issue to some extent in subject SM as well, who on clinical interview comes across as having an abnormally low level of negative emotions in her experience.125 The topic remains to be explored with a detailed, rich battery of probes including realistic elicitors of strong emotions like fear—something ethically difficult to do in humans.
A technically challenging question concerns the temporal dynamics of when the amygdala comes into play during information processing, and how it does so in interaction with other structures such as the prefrontal cortex and striatum, a topic we hinted at earlier. The theme of context-dependent amygdala evaluation of stimuli as part of evaluating the relevance of stimuli fits also within certain psychological appraisal theories that incorporate time as an explicit dimension of interest. For instance, Klaus Scherer’s component-process framework to emotion posits a number of temporally and informationally sequential “stimulus evaluation checks” that correspond to degrees of evaluation and disambiguation.126 Which of these relies on the amygdala? This question finds some parallel to questions about whether the amygdala subserves rapid, coarse, pre-attentive processing or slower, more fine-grained processing that should be considered fully “cognitive” (a neuroanatomical equivalent of a long historical debate about the primacy of emotion and cognition within psychology127,128). Such levels of processing are not always carefully distinguished from the point in time at which they unfold, two distinct issues. Related to the temporal dynamics issue is the topic of amygdala habituation, of interest both mechanistically and in terms of relevance to psychiatric illness. The emerging view of the amygdala’s role in many psychiatric disorders is that it is not modulated or habituated appropriately, resulting in exaggerated or context-inappropriate amygdala responses in those disorders. For instance, there is recent evidence from studies in autism that the amygdala fails to habituate to the sight of faces.129 Clearly, better tools to measure neuroanatomical engagement with millisecond accuracy will help tremendously in mapping out precisely which processes the amygdala contributes to; right now we are generally blurring with a very wide temporal window. The ever-increasing number of electrophysiological studies of the human amygdala in surgical patients holds out great promise for tackling this issue.
A final topic of great current interest is the amygdala’s role in development as well as aging (see Box 2). The amygdala has been implicated in developmental disorders and undergoes substantial changes in morphometry throughout adolescence, in both human130,131 and nonhuman animals.132 There is some evidence this developmental trajectory is altered in autism, even though the adult volume may be normal.133 We briefly noted earlier that the consequences of amygdala lesions in monkeys can be quite different if the lesions are made in adulthood or neonatally134 and that subject SM may have amygdala lesions best described as developmental. Related to this topic is the question of which aspects of value and saliency might be coded in the amygdala already at birth, and which are acquired through experience—and how easily they can be extinguished or changed, a very important topic for understanding disorders such as posttraumatic stress syndrome or phobias that can be remarkably resistant to extinction.
Box 2. The amygdala in development.
Structural changes in the amygdala are evident throughout adolescence in both human and non-human animals.130–132 In parallel with structural development, there are important functional changes in emotional behaviors thought to depend on the amygdala. From early adolescence (4 weeks old) through early adulthood (8 weeks old), mice show a variety of changes in emotional responsivity.139 Pavlovian fear conditioning is more generalized140 and enhanced139 in early adolescence as compared to early adulthood, a change that appears to arise mostly from an increased plasticity within synapses arising from the thalamus in the younger mice.141 Findings in even younger rodents have shown that 3-week-old rats completely erase conditioned fear after extinction,142 whereas adult animals have long been known to show spontaneous recovery and reinstatement, a phenomenon of great interest also to understanding traumatic memories in humans. The long-term nature of fear memories appears to depend in particular on the basolateral amygdala.143 Very young rat pups have shown that fear conditioning can even lead to opposite behavioral effects from those seen in adults: 10-day-old rat pups are attracted to odors associated with shock, unlike the normal behavioral avoidance seen in older animals. This surprising effect depends on differences in release of gluco-corticoids and dopamine release within the amygdala, and it has been speculated that it evolved to mediate unconditional attachment in altricial animals where the young are helpless.144 There are also substantial differences in amygdala-mediated behaviors between infant and older monkeys,134 as well as differences during development in humans.145,146 All of these findings stress the need to take into account changing socio-emotional functions throughout the lifespan.
Acknowledgments
I thank Katalin Gothard and Adam Anderson for comments on the paper. Supported in part by grants from NIH and the Simons Foundation.
Footnotes
Conflicts of interest
The author declares no conflicts of interest.
References
- 1.Amaral DG, Price JL, Pitkanen A, Carmichael ST. In: The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Aggleton JP, editor. Wiley-Liss; New York, NY: 1992. pp. 1–66. [Google Scholar]
- 2.Swanson LW, Petrovich GD. What is the amygdala? TINS. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- 3.Aggleton J. The Amygdala. A Functional Analysis. Oxford University Press; New York, NY: 2000. [Google Scholar]
- 4.Whalen P, Phelps EA. The Human Amygdala. Oxford University Press; New York, NY: 2009. [Google Scholar]
- 5.Costafreda SG, Brammer MJ, David AS, Fu CHY. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev. 2008;58:57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- 7.Kluver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Arch Neurol Psychiatry. 1939;42:979–997. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- 8.Kluver H, Bucy PC. “Psychic blindness” and other symptoms following bilateral temporal lobectomy in rhesus monkeys. Am J Physiol. 1937;119:352–353. [Google Scholar]
- 9.Weiskrantz L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. J Comp Physiol Psychol. 1956;49:381–391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
- 10.Meunier M, Bachevalier J, Murray EA, et al. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. Eur J Neurosci. 1999;11:4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- 11.Emery NJ, et al. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys. Behav Neurosci. 2001;115:515–544. [PubMed] [Google Scholar]
- 12.Mason WA, Capitanio JP, Machado CJ, et al. Amygdalectomy and responsiveness to novelty in rhesus monkeys: generality and individual consistency of effects. Emotion. 2006;6:73–81. doi: 10.1037/1528-3542.6.1.73. [DOI] [PubMed] [Google Scholar]
- 13.Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys. Behav Neurosci. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- 14.Machado CJ, Kazama AM, Bachevalier J. Impact of amygdala, orbital frontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion. 2009;9:147–163. doi: 10.1037/a0014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang F, Aravanis AM, Adamantidis A, et al. Circuit-breakers: optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 16.Rosvold HE, Mirsky AF, Pribram K. Influence of amygdalectomy on social behavior in monkeys. J Comp Physiol Psychol. 1954;47:173–178. doi: 10.1037/h0058870. [DOI] [PubMed] [Google Scholar]
- 17.Kling AS, Brothers LA. In: The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Aggleton JP, editor. Wiley-Liss; New York, NY: 1992. [Google Scholar]
- 18.Bauman MD, Lavenex P, Mason WA, et al. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. J Cogn Neurosci. 2004;16:1388–1411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- 19.Toscano JE, Bauman MD, Mason WA, Amaral DG. Interest in infants by female rhesus monkeys with neonatal lesions of the amygdala or hippocampus. Neuroscience. 2009;162:881–891. doi: 10.1016/j.neuroscience.2009.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amaral DG, et al. The amygdala: is it an essential component of the neural network for social cognition? Neuropsychologia. 2003;41:235–240. doi: 10.1016/s0028-3932(02)00154-9. [DOI] [PubMed] [Google Scholar]
- 21.Kalin NH, Shelton SE, Davidson RJ, Kelley AE. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. J Neurosci. 2001;21:2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. J Neurosci. 1995;15:6846–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- 24.Anderson AK, Spencer DD, Fulbright RK, Phelps EA. Contribution of the anteromedial temporal lobes to the evaluation of facial emotion. Neuropsychology. 2000;14:526–536. doi: 10.1037//0894-4105.14.4.526. [DOI] [PubMed] [Google Scholar]
- 25.Adolphs R, Tranel D, Damasio H. Emotion recognition from faces and prosody following temporal lobectomy. Neuropsychology. 2001;15:396–404. doi: 10.1037//0894-4105.15.3.396. [DOI] [PubMed] [Google Scholar]
- 26.Adolphs R, Tranel D, Koenigs M, Damasio A. Preferring one taste over another without recognizing either. Nat Neurosci. 2005;8:860–861. doi: 10.1038/nn1489. [DOI] [PubMed] [Google Scholar]
- 27.Adolphs R, Tranel D, Damasio AR. Dissociable neural systems for recognizing emotions. Brain and Cognition. 2003;52:61–69. doi: 10.1016/s0278-2626(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 28.Young AW, Hellawell DJ, Van de Wal C, Johnson M. Facial expression processing after amygdalotomy. Neuropsychologia. 1996;34:31–39. doi: 10.1016/0028-3932(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 29.Phelps EA, et al. Specifying the contributions of the human amygdala to emotional memory: a case study. Neurocase. 1998;4:527–540. [Google Scholar]
- 30.Adolphs R, et al. Recognition of facial emotion in nine subjects with bilateral amygdala damage. Neuropsychologia. 1999;37:1111–1117. doi: 10.1016/s0028-3932(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 31.Babinsky R, et al. The possible contribution of the amygdala to memory. Behav Neurol. 1993;6:167–170. doi: 10.3233/BEN-1993-6310. [DOI] [PubMed] [Google Scholar]
- 32.Hofer PA. Urbach-Wiethe disease: a review. Acta Derm Venerol. 1973;53:5–52. [PubMed] [Google Scholar]
- 33.Hamada T, et al. Lipoid proteinosis maps to 1q21 and is caused by mutations in the extracellular matrix protein 1 gene (ECM1) Hum Mol Genetics. 2002;11:833–840. doi: 10.1093/hmg/11.7.833. [DOI] [PubMed] [Google Scholar]
- 34.Siebert M, Markowitsch HJ, Bartel P. Amygdala, affect and cognition: evidence from 10 patients with Urbach-Wiethe disease. Brain. 2003;126:2627–2637. doi: 10.1093/brain/awg271. [DOI] [PubMed] [Google Scholar]
- 35.Thornton HB, et al. The neuropsychiatry and neuropsychology of lipoid proteinosis. J Neuropsychiatry Clin Neurosci. 2008;20:86–92. doi: 10.1176/jnp.2008.20.1.86. [DOI] [PubMed] [Google Scholar]
- 36.Buchanan TW, Tranel D, Adolphs R. In: The Human Amygdala. Whalen PW, Phelps L, editors. Oxford University Press; New York, NY: 2009. pp. 289–320. [Google Scholar]
- 37.Kennedy DP, Gläscher J, Tyszka JM, Adolphs R. Personal space regulation by the human amygdala. Nat Neurosci. 2009 doi: 10.1038/nn.2381. Advanced online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- 39.Young AW, et al. Face processing impairments after amygdalotomy. Brain. 1995;118:15–24. doi: 10.1093/brain/118.1.15. [DOI] [PubMed] [Google Scholar]
- 40.Morris JS, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- 41.Fitzgerald DA, Angstadt M, Jelsone LM, et al. Beyond threat: amygdala reactivity across multiple expressions of facial affect. Neuroimage. 2006;30:1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 42.Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res Rev. 2004;45:96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Kim H, et al. Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci. 2004;16:1730–1745. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- 44.Adams RB, Gordon HL, Baird AA, et al. Effects of gaze on amygdala sensitivity to anger and fear faces. Science. 2003;300:1536. doi: 10.1126/science.1082244. [DOI] [PubMed] [Google Scholar]
- 45.Hamann S, Herman RA, Nolan CL, Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nat Neurosci. 2004;7:411–416. doi: 10.1038/nn1208. [DOI] [PubMed] [Google Scholar]
- 46.Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J Neurosci. 2004;24:10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Canli T, Sivers H, Whitfield SL, et al. Amygdala resposes to happy faces as a function of extraversion. Science. 2002;296:2191. doi: 10.1126/science.1068749. [DOI] [PubMed] [Google Scholar]
- 48.Hariri AR, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 49.Pezawas L, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 50.LeDoux J. The Emotional Brain. Simon and Schuster; New York, NY: 1996. [Google Scholar]
- 51.Morris JS, Ohman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear. PNAS. 1999;96:1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohman A, Carlsson K, Lundqvist D, Ingvar M. On the unconscious subcortical origin of human fear. Physiol Behav. 2007;92:180–185. doi: 10.1016/j.physbeh.2007.05.057. [DOI] [PubMed] [Google Scholar]
- 53.Adolphs R. Fear, faces, and the human amygdala. Curr Opin Neurobiol. 2008;18:1–7. doi: 10.1016/j.conb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsuchiya N, Moradi F, Felsen C, et al. Intact rapid detection of fearful faces in the absence of the amygdala. Nat Neurosci. 2009 doi: 10.1038/nn.2380. Advanced online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adolphs R, et al. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- 56.Gosselin F, Schyns PG. Bubbles: a technique to reveal the use of information in recognition tasks. Vision Res. 2001;41:2261–2271. doi: 10.1016/s0042-6989(01)00097-9. [DOI] [PubMed] [Google Scholar]
- 57.Smith ML, Cottrell GW, Gosselin F, Schyns PG. Transmitting and decoding facial expressions. Psychol Sci. 2005;16:184–189. doi: 10.1111/j.0956-7976.2005.00801.x. [DOI] [PubMed] [Google Scholar]
- 58.Whalen PJ. Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Curr Directions Psychol Sci. 1999;7:177–187. [Google Scholar]
- 59.Whalen PJ. The uncertainty of it all. TICS. 2007;11:499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 60.McGaugh JL. Significance and remembrance: the role of neuromodulatory systems. Psychol Sci. 1990;1:15–25. [Google Scholar]
- 61.Gamer M, Buechel C. Amygdala activation predicts gaze toward fearful eyes. J Neurosci. 2009;29:9123–9126. doi: 10.1523/JNEUROSCI.1883-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Asghar AUR, et al. An amygdala response to fearful faces with covered eyes. Neuropsychologia. 2008;46:2364–2370. doi: 10.1016/j.neuropsychologia.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 63.Vuilleumier P, Armony JL, Driver J, Dolan RJ. Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nat Neurosci. 2003;6:624–631. doi: 10.1038/nn1057. [DOI] [PubMed] [Google Scholar]
- 64.Said CP, Baron SG, Todorov A. Nonlinear amygdala response to face trustworthiness: contributions of high and low spatial frequency information. J Cogn Neurosci. 2009;21:519–528. doi: 10.1162/jocn.2009.21041. [DOI] [PubMed] [Google Scholar]
- 65.Carlson JM, Reinke KS, Habib R. A left amygdala mediated network for rapid orienting to masked fearful faces. Neuropsychologia. 2009;47:1386–1389. doi: 10.1016/j.neuropsychologia.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 66.Susskind JM, et al. Expressing fear enhances sensory acquisition. Nat Neurosci. 2008;11:843–850. doi: 10.1038/nn.2138. [DOI] [PubMed] [Google Scholar]
- 67.Ball T, et al. Anatomical specificity of functional amygdala imaging of responses to stimuli with positive and negative emotional valence. J Neurosci Methods. 2009;180:57–70. doi: 10.1016/j.jneumeth.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 68.Anderson AK, et al. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- 69.Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- 70.Winston JS, Gottfried JA, Kilner JM, Dolan RJ. Integrated neural representations of odor intensity and affective valence in human amygdala. J Neurosci. 2005;25:8903–8907. doi: 10.1523/JNEUROSCI.1569-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamann SB, Ely TD, Hoffman JM, Kilts CD. Ecstasy and agony: activation of the human amygdala in positive and negative emotion. Psychol Sci. 2002;13:135–141. doi: 10.1111/1467-9280.00425. [DOI] [PubMed] [Google Scholar]
- 72.Ewbank MP, Barnard PJ, Croucher CJ, et al. The amygdala response to images with impact. Soc Cogn Affective Neurosci. 2009;4:127–133. doi: 10.1093/scan/nsn048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lazarus RS. Emotion and Adaptation. Oxford University Press; New York, NY: 1991. [Google Scholar]
- 74.Scherer KR. In: The Neuropsychology of Emotion. Borod JC, editor. Oxford University Press; 2000. pp. 137–162. [Google Scholar]
- 75.Laine CM, Spitler KM, Mosher CP, Gothard KM. Behavioral triggers of skin conductance responses and their neural correlates in the primate amygdala. J Neurophysiol. 2009;101:1749–1754. doi: 10.1152/jn.91110.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuraoka K, Nakamura K. Responses of single neurons in monkey amygdala to facial and vocal emotions. J Neurophysiol. 2007;97:1379–1387. doi: 10.1152/jn.00464.2006. [DOI] [PubMed] [Google Scholar]
- 77.Gothard KM, Battaglia FP, Erickson CA, et al. Neural responses to facial expression and face identity in the monkey amygdala. J Neurophysiol. 2007;97:1671–1683. doi: 10.1152/jn.00714.2006. [DOI] [PubMed] [Google Scholar]
- 78.Brothers L, Ring B, Kling A. Response of neurons in the macaque amygdala to complex social stimuli. Behav Brain Res. 1990;41:199–213. doi: 10.1016/0166-4328(90)90108-q. [DOI] [PubMed] [Google Scholar]
- 79.Fried I, MacDonald KA, Wilson CL. Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron. 1997;18:753–765. doi: 10.1016/s0896-6273(00)80315-3. [DOI] [PubMed] [Google Scholar]
- 80.Oya H, Kawasaki H, Howard MA, Adolphs R. Electrophysiological responses in the human amygdala discriminate emotion categories of complex visual stimuli. J Neurosci. 2002;22:9502–9512. doi: 10.1523/JNEUROSCI.22-21-09502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Quiroga RQ, Reddy L, Kreiman G, et al. Invariant visual representation by single neurons in the human brain. Nature. 2005;435:1102–1107. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- 82.Bach DR, et al. Rising sound intensity: an intrinsic warning cue activating the amygdala. Cerebral Cortex. 2008;18:145–150. doi: 10.1093/cercor/bhm040. [DOI] [PubMed] [Google Scholar]
- 83.Hsu M, Bhatt M, Adolphs R, et al. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310:1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- 84.Herry C, et al. Processing of temporal unpredictability in human and animal amygdala. J Neurosci. 2007;27:5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Breiter HC, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 86.Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.LeDoux JE. The Amygdala. Curr Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 88.Wilson YM, Murphy M. A discrete population of neurons in the lateral amygdala is specifically activated by contextual fear conditioning. Learn Mem. 2009;16:357–361. doi: 10.1101/lm.1361509. [DOI] [PubMed] [Google Scholar]
- 89.Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shabel SJ, Janak PH. Substantial similarity in amygdala neuronal activity during conditioned appetitive and aversive emotional arousal. PNAS. 2009;106:15031–15036. doi: 10.1073/pnas.0905580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rolls ET. In: The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Aggleton JP, editor. Wiley; New York, NY: 1992. pp. 143–167. [Google Scholar]
- 92.Murray EA. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 93.Kreiman G, Koch C, Fried I. Category-specific visual responses of single neurons in the human medial temporal lobe. Nat Neurosci. 2000;3:946–953. doi: 10.1038/78868. [DOI] [PubMed] [Google Scholar]
- 94.Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 95.Izquierdo A, Murray EA. Selective bilateral amygdala lesions in rhesus monkeys fail to disrupt object reversal learning. J Neurosci. 2007;27:1054–1062. doi: 10.1523/JNEUROSCI.3616-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 97.De Martino B, Kumaran D, Seymour B, Dolan RJ. Frames, biases, and rational decision-making in the human brain. Science. 2006;313:684–687. doi: 10.1126/science.1128356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roiser JP, et al. A genetically mediated bias in decision making driven by failure of amygdala control. J Neurosci. 2009;29:5985–5991. doi: 10.1523/JNEUROSCI.0407-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cunningham WA, Van Bavel JJ, Johnsen IR. Affective flexibility: evaluative processing goals shape amygdala activity. Psychol Sci. 2008;19:152–160. doi: 10.1111/j.1467-9280.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- 101.Van Bavel JJ, Packer DJ, Cunningham WA. The neural substrates of in-group bias. Psychol Sci. 2008;19:1131–1139. doi: 10.1111/j.1467-9280.2008.02214.x. [DOI] [PubMed] [Google Scholar]
- 102.Montague PR, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36:265–284. doi: 10.1016/s0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- 103.Baxter MG, Parker A, Lindner CCC, et al. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de Martino B, Camerer C, Adolphs R. Amygdala damages eliminates monetary loss aversion. PNAS. 2010 doi: 10.1073/pnas.0910230107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hampton A, Adolphs R, Tyszka JM, O’Doherty J. Contributions of the amygdala to reward expectancy and choice signals in human prefrontal cortex. Neuron. 2007;55:545–555. doi: 10.1016/j.neuron.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 107.Miyashita T, Ichinohe N, Rockland KS. Differential modes of termination of amygdalothalamic and amygdalocortical projections in the monkey. J Comparative Neurol. 2007;502:309–324. doi: 10.1002/cne.21304. [DOI] [PubMed] [Google Scholar]
- 108.Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predictive outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- 109.Freese JL, Amaral DG. The organization of projections from the amygdala to visual cortical areas TE and V1 in the macaque monkey. J Comparative Neurol. 2005;486:295–317. doi: 10.1002/cne.20520. [DOI] [PubMed] [Google Scholar]
- 110.Chen Y, Zhu B, Shou T. Anatomical evidence for the projections from the basal nucleus of the amygdala to the primary visual cortex in the cat. Neurosci Lett. 2009;453:126–130. doi: 10.1016/j.neulet.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 111.Vuilleumier P, Richardson MP, Armony JL, et al. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- 112.Williams LM, Das P, Liddell BJ. Mode of functional connectivity of amygdala pathway dissociates level of awareness for signals of fear. J Neurosci. 2006;26:9264–9271. doi: 10.1523/JNEUROSCI.1016-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rosenkranz JA, Grace AA. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci. 2001;21:4090–4103. doi: 10.1523/JNEUROSCI.21-11-04090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kienast T, et al. Dopamine in amygdala gates limbic processing of aversive stimuli in humans. Nat Neurosci. 2008;11:1381–1382. doi: 10.1038/nn.2222. [DOI] [PubMed] [Google Scholar]
- 115.Mayberg HS, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 116.Gibboni RR, Zimmerman PE, Gothard KM. Individual differences in scanpaths correspond with serotonin transporter genotype and behavioral phenotypes in rhesus monkeys (Macaca mulatta) Frontiers Behav Neurosci. 2009;3 doi: 10.3389/neuro.08.050.2009. article 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Heinz A, et al. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8:20–21. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- 118.Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci. 2009;29:11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McGinty VB, Grace AA. Timing-dependent regulation of evoked spiking in nucleus accumbens neurons by integration of limbic and prefrontal cortical inputs. J Neurophysiol. 2009;101:1823–1835. doi: 10.1152/jn.91162.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McGinty VB, Grace AA. Activity-dependent depression of medial prefrontal cortex inputs to accumbens neurons by the basolateral amygdala. Neuroscience. 2009;162:1429–1436. doi: 10.1016/j.neuroscience.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Buckwalter JA, Schumann CM, VanHoesen GW. Evidence for direct projections from the basal nucleus of the amygdala to retrosplenial cortex in the Macaque monkey. Exp Brain Res. 2008;186:47–57. doi: 10.1007/s00221-007-1203-x. [DOI] [PubMed] [Google Scholar]
- 122.Lim SL, Padmala S, Pessoa L. Segregating the significant from the mundane on a moment-to-moment basis via direct and indirect amygdala contributions. PNAS. 2009;106:16841–16846. doi: 10.1073/pnas.0904551106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Feldman Barrett L, Bliss-Moreau E, Duncan SL, et al. The amygdala and the experience of affect. Soc Cogn Affective Neurosci. 2007;2:73–83. doi: 10.1093/scan/nsl042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Anderson AK, Phelps EA. Is the human amygdala critical for the subjective experience of emotion? Evidence of intact dispositional affect in patients with amygdala lesions. J Cogn Neurosci. 2002;14:709–720. doi: 10.1162/08989290260138618. [DOI] [PubMed] [Google Scholar]
- 125.Tranel D, Gullickson G, Koch M, Adolphs R. Altered experience of emotion following bilateral amygdala damage. Cogn Neuropsychiatry. 2006;11:219–232. doi: 10.1080/13546800444000281. [DOI] [PubMed] [Google Scholar]
- 126.Scherer KR. In: Cognitive Perspectives on Emotion and Motivation. Hamilton V, Bower GH, Frijda NH, editors. Nijhoff; Dordrecht, Holland: 1988. pp. 89–126. [Google Scholar]
- 127.Zajonc R. On the primacy of affect. Am Psychol. 1984;39:117–123. [Google Scholar]
- 128.Lazarus RS. On the primacy of cognition. Am Psychol. 1984;39:124–129. [Google Scholar]
- 129.Kleinhans NM, et al. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. Am J Psychiatry. 2009;166:467–475. doi: 10.1176/appi.ajp.2008.07101681. [DOI] [PubMed] [Google Scholar]
- 130.Giedd JN, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comparative Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 131.Chung WCJ, De Vries GJ, Swaab DF. Sexual differentiation of the bed nucleus of the stria terminalis in humans may extend into adulthood. J Neurosci. 2002;22:1027–1033. doi: 10.1523/JNEUROSCI.22-03-01027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rubinow MJ, Juraska JM. Neuron and glia numbers in the basolateral nucleus of the amygdala from preweaning through old age in male and female rats: a stereological study. J Comparative Neurol. 2009;512:717–725. doi: 10.1002/cne.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schumann CM, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Prather MD, et al. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- 135.Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- 136.Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Amunts K, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol. 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- 138.Sirotin YB, Das A. Anticipatory haemodynamic signals in sensory cortex not predicted by local neuronal activity. Nature. 2009;457:475–479. doi: 10.1038/nature07664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hefner K, Holmes A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res. 2007;176:210–215. doi: 10.1016/j.bbr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ito W, Pan BX, Yang C, et al. Enhanced generalization of auditory conditioned fear in juvenile mice. Learn Mem. 2009;16:187–192. doi: 10.1101/lm.1190809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pan BX, Ito W, Morozov A. Divergence between thalamic and cortical inputs to lateral amygdala during juvenile-adult transition in mice. Biol Psychiatry. 2009;66:964–971. doi: 10.1016/j.biopsych.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gogolla N, Caroni P, Lüthi A, Herry C. Per-ineuronal nets protect fear memories from erasure. Science. 2009;325:1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- 143.Poulos AM, et al. Persistence of fear memory across time requires the basolateral amygdala complex. PNAS. 2009;106:11737–11741. doi: 10.1073/pnas.0905257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Barr GA, et al. Transitions in infant learning are modulated by dopamine in the amygdala. Nat Neurosci. 2009;12:1367–1369. doi: 10.1038/nn.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Thomas KM, et al. Amygdala response to facial expressions in children and adults. Biol Psychiatry. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- 146.Fischer H, Nyberg L, Bäckman L. Age-related differences in brain regions supporting successful encoding of emotional faces. Cortex. 2009 doi: 10.1016/j.cortex.2009.05.011. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 147.Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. J Neurosci. 1995;15:5879–5892. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bechara A, et al. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- 149.Adolphs R, Cahill L, Schul R, Babinsky R. Impaired declarative memory for emotional material following bilateral amygdala damage in humans. Learn Mem. 1997;4:291–300. doi: 10.1101/lm.4.3.291. [DOI] [PubMed] [Google Scholar]
- 150.Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- 151.Adolphs R, Russell JA, Tranel D. A role for the human amygdala in recognizing emotional arousal from unpleasant stimuli. Psychol Sci. 1999;10:167–171. [Google Scholar]
- 152.Adolphs R, Tranel D. Preferences for visual stimuli following amygdala damage. J Cogn Neurosci. 1999;11:610–616. doi: 10.1162/089892999563670. [DOI] [PubMed] [Google Scholar]
- 153.Hamann S, Adolphs R. Normal recognition of emotional similarity between facial expressions following bilateral amygdala damage. Neuropsychologia. 1999;37:1135–1141. doi: 10.1016/s0028-3932(99)00027-5. [DOI] [PubMed] [Google Scholar]
- 154.Adolphs R, Tranel D. Intact recognition of emotional prosody following amygdala damage. Neuropsychologia. 1999;37:1285–1292. doi: 10.1016/s0028-3932(99)00023-8. [DOI] [PubMed] [Google Scholar]
- 155.Gosselin N, Peretz I, Johnsen E, Adolphs R. Amygdala damage impairs emotion recognition from music. Neuropsychologia. 2007;45:236–244. doi: 10.1016/j.neuropsychologia.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 156.Adolphs R, Tranel D, Baron-Cohen S. Amygdala damage impairs recognition of social emotions from facial expressions. J Cogn Neurosci. 2002;14:1264–1274. doi: 10.1162/089892902760807258. [DOI] [PubMed] [Google Scholar]
- 157.Adolphs R, Tranel D. Amygdala damage impairs emotion recognition from scenes only when they contain facial expressions. Neuropsychologia. 2003;41:1281–1289. doi: 10.1016/s0028-3932(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 158.Adolphs R, Tranel D. Impaired recognition of sadness but not happiness following amygdala damage. J Cogn Neurosci. 2004;16:453–462. doi: 10.1162/089892904322926782. [DOI] [PubMed] [Google Scholar]
- 159.Adolphs R, Buchanan TW, Tranel D. Amygdala damage impairs memory for gist but not details of complex stimuli. Nat Neurosci. 2005;8:512–519. doi: 10.1038/nn1413. [DOI] [PubMed] [Google Scholar]
- 160.Spezio ML, Huang PYS, Castelli F, Adolphs R. Amygadala damage impairs eye contact during conversations with real people. J Neurosci. 2007;27:3994–3997. doi: 10.1523/JNEUROSCI.3789-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Atkinson AP, Heberlein AS, Adolphs R. Spared ability to recognise fear from static and moving whole-body cues following bilateral amygdala damage. Neuropsychologia. 2007;45:2772–2782. doi: 10.1016/j.neuropsychologia.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Adolphs R, et al. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]