Aire and T cell Development (original) (raw)
. Author manuscript; available in PMC: 2012 Apr 1.
Published in final edited form as: Curr Opin Immunol. 2010 Dec 14;23(2):198–206. doi: 10.1016/j.coi.2010.11.007
Abstract
In the thymus, developing T cells that react against self-antigens with high affinity are deleted in the process of negative selection. An essential component of this process is the display of self-antigens, including those whose expression are usually restricted to specific tissues, to developing T cells within the thymus. The Autoimmune Regulator (Aire) gene plays a critical role in the expression of tissue specific self-antigens within the thymus, and disruption of Aire function results in spontaneous autoimmunity in both humans and mice. Recent advances have been made in our understanding of how Aire influences the expression of thousands of tissue-specific antigens in the thymus. Additional roles of Aire, including roles in chemokine and cytokine expression, have also been revealed. Factors important in the differentiation of Aire-expressing medullary thymic epithelial cells have been defined. Finally, the identity of antigen presenting cells in negative selection, including the role of medullary thymic epithelial cells in displaying tissue specific antigens to T cells, has also been clarified.
Introduction
Negative selection is a critical self-tolerance mechanism in which T cells developing in the thymus that recognize self-antigens are deleted. Recently, discoveries made in both humans and mice have revealed the important role of the Autoimmune Regulator (Aire) gene in negative selection and the prevention of organ-specific autoimmunity. Humans deficient in Aire develop Autoimmune Polyendocrinopathy Syndrome Type 1 (APS 1) [1, 2], a debilitating condition characterized by autoimmune destruction of multiple organs [3]. Like patients with APS 1, Aire-deficient mice also develop spontaneous multi-organ autoimmune disease and have been useful models to understand disease pathogenesis.
Inherent in negative selection is the exposure of developing T cells to self-antigens, including antigens with highly restricted tissue expression. Within the thymic medulla, medullary thymic epithelial cells (mTECs) express a large number of tissue-specific self-antigens (TSAs) [4, 5], and recognition of these TSAs by self-reactive T cells is postulated to drive negative selection. The localization of Aire protein to the nucleus of mTECs [6], the domain structure suggestive of a role in transcriptional modulation, and the discovery that Aire-deficient mice have decreased expression of a wide array of TSAs [7, 8] have led to the working model that Aire plays an important role in negative selection by upregulating the expression of TSAs. Decreased TSA expression in the setting of Aire-deficiency, then, results in multi-organ autoimmune disease by allowing self-reactive T cells to escape from the thymus and enter the periphery where they can provoke autoimmunity.
In this review, we focus on recent advances in our understanding of Aire’s effect on T cell development. There has been much interest and research on Aire biology, and we refer readers to reviews covering aspects of Aire outside the scope of this review. Reviews covering the molecular mechanisms of Aire [9, 10], the role of Aire in the periphery [11], and clinical aspects of Aire mutations [12] have recently been published. Here, we will focus on 3 areas relevant to T cell development and Aire: 1) Aire in the regulation of TSA expression and negative selection of thymocytes 2) additional Aire functions related to T cell development, and 3) ontogeny of Aire-expressing mTECs.
Aire in the Negative Selection of Thymocytes
Aire controls TSA expression in mTECs
The principle mechanism by which Aire influences thymocyte development is by controlling the expression of TSAs in mTECs. By microarray expression profiling of isolated mTECs, Aire-deficiency is estimated to result in the down-regulation of potentially thousands of genes [7, 13]. Of the most strongly down-regulated genes in Aire-deficient mTECs, approximately 80% are restricted in their expression pattern to one or several specific tissues. This finding suggests that Aire preferentially promotes the expression of genes with highly restricted tissue expression. Examples of TSAs regulated by Aire include insulin 2, fatty acid binding protein, and salivary protein 1. Importantly, not all TSAs appear to be influenced by Aire, since expression of some (e.g. glutamic acid decarboxylase (GAD) 67) are not altered in Aire deficiency.
Recently, a number of noteworthy features of Aire-regulated TSA expression has been elucidated. First, Aire-regulated TSA transcription in the thymus seems to differ fundamentally from expression of these antigens in their respective tissues. Transcriptional start sites for a particular locus can differ in mTECs when compared to peripheral tissue, and a single mTEC can express a TSA in either a monoallelic or biallelic manner, rather than the usual biallelic expression seen in peripheral tissues [14]. Loss of imprinting resulting in biallelic expression in mTECs has also been observed in an Aire-regulated locus [13]. Furthermore, transcription factors normally essential for expression of a TSA in peripheral tissue do not seem to be required in mTECs. Second, the cellular environment in which Aire functions plays a major role in determining the genes affected. Recently, our group identified a second cell population in secondary lymphoid organs that also expresses Aire [15]**. Within this population, Aire appears to promote the expression of genes that is distinct from those that it regulates in mTECs. Despite this difference, Aire still promotes the expression of genes with a restricted tissue expression pattern. Supporting this observation are studies in both transgenic mice and in transfected cells that have come to similar conclusions [16] [17]. Third, Aire-regulated TSA expression seems to be affected by modifier loci represented in different inbred mouse strains. Strain differences were noted both in the strength of Aire effects in general and the identity of TSAs expressed in mTECs, however, it should be noted that the bulk of TSA expression does not vary between strains. [18]. Fourth, a subset of TSAs in mTECs appear to be clustered in their chromosomal location, and these clusters include both Aire-dependent and independent targets [13, 19]. This finding suggests that the influence of Aire may be at multiple levels and involve effects on particular genes and on chromatin remodeling processes.
These unusual features of Aire-regulated transcription and the large number of transcripts influenced by Aire have sparked interest in understanding the molecular mechanisms of Aire function. Much progress has recently been made in this field, and we highlight here two recent conceptual advances. First, Aire contains two plant homeodomain (PHD) regions which are found in a large family of zinc finger proteins. The first PHD (PHD1) of Aire appears to be a histone reader that binds specifically to unmethylated histone H3 at lysine-4 (H3K4me0) [20]** [21]* [22]**[23]*). This form of histone H3 is associated with repression of transcriptional activity, leading to the hypothesis that tissue restricted antigens likely bear repressive marks (including H3K4me0) in mTECs since mTECs are not their “normal” site of expression. This association with H3K4me0 marks these genes for binding by Aire, allowing Aire to specifically upregulate the expression of tissue-specific antigens [24]. Interestingly, overexpression of Aire along with a global H3K4 demethylase did not result in large-scale changes in the transcriptional footprint of Aire, but did induce genes normally repressed by Aire [25]. Second, Aire interacts with an unexpectedly large number of binding partners [17]. Co-immunoprecipitation experiments using over-expression of Aire in human embryonic kidney (HEK) 293 cells resulted in the identification of 45 proteins that fell into 4 broad categories: pre-mRNA processing, transcription, nuclear transport, and chromatin binding and structure. Of note, one of the identified proteins, DNA protein kinase (DNA-PK), was independently identified as interacting with Aire [26] and deficiency of DNA-PK in the stromal compartment results in decreased TSA expression in mTECs with some evidence for autoantibody production. This finding of multiple binding partners for Aire is consistent with previous reports that Aire is found in large molecular weight complexes [27]. Despite these recent advances, however, it remains to be determined how Aire affects a large number of TSAs, and yet is able to maintain specificity by upregulating certain TSAs and not others.
Aire controls thymocyte negative selection
Aire-regulated expression of TSAs is postulated to promote tolerance by driving the negative selection of self-reactive thymocytes that recognize Aire-regulated antigens with high affinity. Evidence for this concept stems from experiments using T cell Receptor (TCR) transgenic models in which negative selection of clonal T cell populations is defective in Aire-deficient mice [28–31]. Importantly, however, all the studies examining negative selection in the thymus to date have utilized a neo-self antigen driven by an Aire-regulated promoter in the thymus. There have not been any studies in which defective negative selection in Aire-deficient mice is seen with an endogenous Aire-regulated antigen. Defective negative selection resulting from Aire-deficiency is not seen when a neo-self antigen is under the control of systemic promoter, such as the H-2K promoter [32]. Additionally, there is no evidence that Aire affects the TCR repertoire directed against non-self antigens [33].
Additional evidence that Aire deficiency results in defective negative selection of self-reactive T cells comes from studies in which decreased expression of a specific Aire-regulated TSA is associated with autoimmunity against the specific tissue expressing the TSA. Direct links between loss of Aire-regulated TSAs in the thymus and targeting of these TSA by autoantibody production and cellular infiltration in the associated tissue have now been demonstrated in a number of organs including the eye [34], stomach [35], prostate [36], and lung [37]. Of note, deficiency of a single Aire-regulated antigen, IRBP, in the thymus is sufficient to result in spontaneous autoimmune uveitis [34], demonstrating the critical role of TSA expression in preventing autoimmunity in the associated organ.
Additionally, there is evidence that the control of negative selection and autoimmunity via TSA expression in the thymus is not an on/off system. Quantitative changes in TSA expression levels can have graded effects on negative selection [32], and a threshold level of TSA expression appears to exist that is sufficient to protect against autoimmunity for a specific organ [31]. Recently, decreased Aire expression per cell (approximately 50%), and decreased numbers of Aire-expressing mTECs (less than 50%) were noted in the NZB mouse model of autoimmunity [38]. These findings raise the possibility that quantitative changes in Aire-mediated thymic antigen expression might contribute to the autoimmunity seen in this model.
In humans, Aire appears to be involved in modulation of quantitative levels of thymic insulin expression by binding to VNTR regions of the insulin promoter [39]. Polymorphisms in the Variable Number of Tandem Repeats (VNTR) region upstream of the insulin promoter result in varying levels of insulin expression in the thymus. Population studies show that alleles that result in higher levels of insulin expression in the thymus are associated with protection against the development of Type I Diabetes [40, 41]. An analogous situation occurs with the α chain of the acetylcholine receptor and Myasthenia Gravis. The relative thymic expression level of this antigen varies relative to a single nucleotide polymorphism (SNP) in the promoter region. Furthermore, increased expression of this thymic antigen is correlated with later age of disease onset [42]. In common autoimmune diseases, then, quantitative changes in TSA expression in the thymus may modulate disease manifestation and risk.
Timing of Aire Expression in Preventing Autoimmunity
A question of clinical relevance is when during the lifespan is Aire-mediated thymic negative selection important. The answer to this question is important in understanding the effects of thymectomies and in developing therapies that may modulate thymic function. In a study using system for expressing Aire in a tetracycline-controlled manner to complement an Aire-deficient mouse, neonatal expression of Aire appeared to be necessary and sufficient for preventing autoimmunity [43]*. Alternatively, a case report of “acquired” APS1 in a 67 year old woman with an Aire-deficient thymoma suggests that loss of Aire expression later in life may predispose to autoimmunity [44]*. This patient met clinical criteria for APS1, but direct sequencing of her Aire locus did not demonstrate germline Aire mutations. Further workup revealed a large Stage IV thymoma consisting of Aire-negative thymic epithelial cells. This association led to the hypothesis that defective negative selection within the thymoma due to lack of Aire may have contributed to her development of APS1 clinical features, and suggested that Aire-mediated negative selection later in life is also necessary for preventing autoimmunity. This finding is somewhat difficult to reconcile with those of the previous study, but may be explained by species differences between mice and humans, the large output of T cells by the thymoma, or differences in time windows in which Aire is nonfunctional in these two scenarios. It also important to note that >95% of thymomas are associated with defective Aire expression and this may be part of the explanation to the common association of this type of tumor with autoimmunity [45].
Aire-expressing mTECs in Negative Selection
Within the thymic medulla, both bone marrow-derived dendritic cells (DCs) and medullary thymic epithelial cells (mTECs) can potentially function as antigen presenting cells of TSAs during negative selection. TSAs expressed by mTECs can be presented by mTECs themselves, or can be cross-presented by bone marrow-derived DCs. Using bone marrow chimeras, Gallegos and Bevan demonstrated that antigen-presentation by bone marrow-derived cells was required for deletion of CD4+ SP thymocytes [46]. CD8+ SP cells, on the other hand, did not require antigen-presentation by bone marrow-derived cells, suggesting that mTECs were also sufficient to delete CD8+ SP cells.
Recently, this model has been challenged by evidence that mTECs are also important in antigen presentation to CD4+ SP thymocytes. Studies in a transgenic mouse model in which CIITA expression is diminished by a specific micro-RNA under the control of the Aire promoter resulted in decreased MHCII expression specifically in Aire-expressing mTECs. These mice demonstrated an increased frequency of CD4+ SP thymocytes, reflecting defective negative selection of these cells in the setting of diminished antigen presenstation by Aire-expressing mTECs [47]**. Thus, antigen presentation during negative selection of CD4+ SP thymocytes appears to be directly mediated by mTECs. Using bone marrow chimeras, this group also demonstrated that mTECs and hematopoietic DCs mediate deletional tolerance of CD4+ SP thymocytes in a non-redundant manner. Thus, both stromal mTEC cells and bone marrow-derived DCs present antigens to CD4+ SP thymocyte during negative selection.
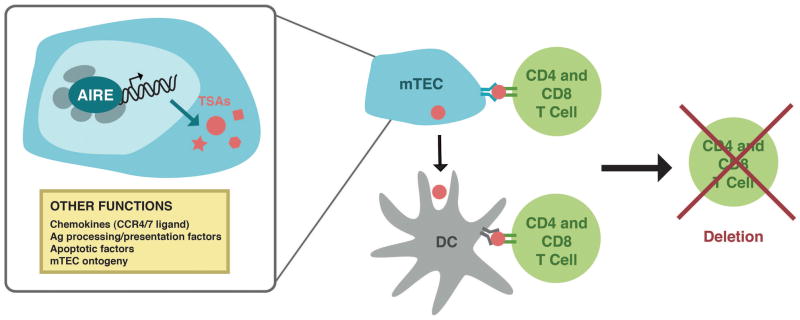
Additional evidence that mTECs can present antigens to CD4+ SP thymocytes is provided by ex vivo studies performed with isolated mTECs and DCs [48]**. In this system, mTECs expressing a nuclear neo-self antigen under a ubiquitous promoter could directly present antigen to CD4+ SP thymocytes. Furthermore, a unidirectional transfer of antigens also appears to occur in which thymic DCs specifically acquire self-antigen for cross-presentation from mTECs and not from hematopoietic sources. Given that mTEC have the capacity to directly present self antigens that they are expressing, a question arises as to how such antigens can load into the MHC Class II presentation pathway. A recent study has implicated that autophagy may be part of the explanation as mTECs appear to have authophagosomes and blocking autophagy in the thymus is associated with autoimmunity [49]. Taken together, we have summarized a working model for the role of Aire in the negative selection of developing T cells in Figure 1.
Figure 1. Current model of Aire function in medullary thymic epithelial cells (mTECs).
Aire is expressed in the nucleus of mTECs where its primary function is to upregulate the expression of tissue specific antigens (TSAs). Aire functions in a large molecular weight complex composed of a large number of binding partners (gray ovals). These antigens are processed and presented by mTECs directly to both CD4+ and CD8+ T cells, or acquired by dendritic cells (DCs) for processing and presentation to both CD4+ and CD8+ T cells. Recognition of these antigens with high affinity results in deletion of self-reactive T cells to prevent release of these cells into the periphery to provoke autoimmunity. Additional functions for Aire have also been proposed, including expression of chemokines, antigen (Ag) presentation and processing factors, apoptotic factors, and factors important in mTEC ontogeny.
Additional Aire Functions
Aire controls expression of non-TSAs
The development of multi-organ autoimmunity in patients and mice deficient in Aire clearly demonstrates the physiological importance of Aire in preventing autoimmunity. What is less clear is how Aire is mediating this tolerance. Aire deficiency clearly has global effects on tissue-specific self-antigen expression [7] [8]; additional functions, however, have also been attributed to Aire. In addition to promoting TSA expression in mTECs, Aire has also been proposed to enhance mTEC antigen presentation [28]. This conclusion was reached based on the observation that defective thymic negative selection in Aire-deficient mice could be seen in the setting of unchanged TSA expression. Defective negative selection of ovalbumin (OVA) specific T cells (OTI and OTII) were seen in Aire-deficient mice expressing OVA in mTECs under the control of the Rat Insulin Promoter (RIP). Unexpectedly, OVA expression was not significantly decreased in Aire-deficient mice, suggesting that decreased thymic antigen was not the only mechanism by Aire-deficiency could result in defective negative selection. Alternative mechanisms, including decreased expression of factors important in antigen processing and presentation, were therefore proposed as potential mediators of this effect.
In line with this conclusion, Aire deficiency has also been proposed to promote autoimmunity by decreased expression of chemokines important in corticomedullary migration of thymocytes [50]*. Aire deficient mice have decreased expression of CCR4 and CCR7 ligands in the thymus. Like CCR7 knockout mice [51], mice deficient in Aire demonstrate delayed emigration of mature single positive CD4+ T cells from the thymus. Aire deficiency has also been proposed to block the final step of maturation (SP3 to SP4) of single positive CD4+ medullary thymocytes [52]. These findings are somewhat difficult to reconcile with the finding that CD4+ T cell subsets are not altered in the periphery of Aire-deficient mice [7]. However, these findings may represent a subtle change that is only detectable using markers that delineate these thymocyte subsets.
Finally, Aire has also been implicated in the constitutive expression of interferon (IFN)-beta in mTECs [53]. The implications of this constitutive expression by mTECs on negative selection are not clear, but, interestingly, Aire deficient mice are lacking this basal IFN-beta expression. These findings provide yet another role for Aire outside of TSA expression.
Aire in mTEC ontogeny
Aire has been proposed to play a role in mTEC ontogeny. Gillard et al demonstrate that developmental transcription factors are ectopically expressed in mTECs, and that this expression is Aire-dependent [54]. Transcription factors important in maintaining stem cell pluripotency (including Nanog, Oct4, and Sox2) and in pancreatic development are expressed in mTECs, and this expression is decreased in Aire-deficiency. Additionally, contrary to previous reports [7], [55], Ramsey [56], Gillard et al demonstrate gross differences in the thymic architecture of Aire-deficient mice. Changes in thymic architecture in Aire-deficient mice have now also been reported by other groups [57–59]. The contribution of these reported changes to the development of autoimmunity in Aire-deficient mice remains to be determined. A strong argument against Aire having an important role in mTEC development is its expression in mTECs that are in the late-stage of maturation (see below).
Aire and Regulatory T cell Development in the Thymus
Aire’s effect on regulatory T cells (Tregs) developing in the thymus has been the subject of much study. In general, no difference in numbers of Tregs has been reported in Aire deficient mice [7, 31, 56, 60]. Furthermore, thymic transfer studies do not demonstrate a role for dominant Treg mediated tolerance in Aire-deficient states [28]. More recently, the effect of Aire deficiency on Treg repertoire has been studied using transgenic mouse models that express a limited TCR repertoire. Consistent with previous studies, no differences were seen in the TCR repertoire of Tregs from Aire-deficient mice [61].
There have been reports in humans of alterations in the frequency and function of the Treg population in the periphery of APS Type 1 patients [62] [63, 64]. Most recently, activated Tregs in the periphery of APS Type 1 patients have been reported to express less Forkhead box protein P3 (FOXP3) protein, and this difference was proposed to contribute to the autoimmunity seen in these patients [65]. Further studies will need to be performed to understand why Aire may have a different effect on humans than in mice.
Although Aire itself does not seem to affect the selection of regulatory T cells in mice, there is evidence that Aire-expressing mTECs may play a role in mediating regulatory T cell selection. This evidence comes from studies utilizing transgenic mice expressing neo-self antigens (OVA or hemaglutinnen (HA)) under the control of the Aire promoter in the setting of diminished MHCII expression [47]**. As discussed previously, diminished MHCII expression in Aire-expressing mTECs was accomplished by transgenic expression of a microRNA that decreases expression of CIITA under the control of the Aire promoter. In this setting, increased frequencies of FoxP3+ Tregs recognizing cognate antigen were seen [47]**. These findings are consistent with a model in which decreased avidity of self-antigen presented by Aire-expressing mTECs can increase the frequency of Treg development in the thymus.
The Development of Aire-Expressing mTECs in the Thymus
Aire-expressing mTECs play an important role in the negative selection of thymocytes via a number of mechanisms, including the expression of TSAs and direct antigen presentation of TSAs to developing thymocytes. Thus, the development of Aire-expressing mTECs is intertwined with Aire’s role in preventing autoimmunity. mTECs can be identified in the thymus based on cell surface markers (CD45- EpCAM+ Ly51+ cytokeratin 5+, cytokeratin 14+). Within this population, heterogeneity exists in the levels of CD80 and MHC Class II expression. There is strong evidence that differentiation of mTECs progresses from low to high levels of CD80 and MHC II expression, and expression of Aire occurs late in mTEC differentiation [13, 66, 67]. Furthermore, expression of tissue-specific self antigens, including Aire-regulated antigens, is most diverse in the mature CD80, MHCII high cells, suggesting that the most differentiated cells are expressing Aire-mediated genes.
Interestingly, the most differentiated mTECS (CD80high Aire+) were observed to have the fastest turnover [66, 67]. This finding has led to the hypothesis that Aire is acting as a proapoptotic factor, and the rapid turnover of these Aire+ mTEC allows for release of antigens to DC for cross-presentation to developing thymocytes [67]. Alternatively, there have also been recent studies that argue that Aire expression does not correlate with terminal differentiation of mTECs, but that Aire is downregulated prior to terminal differentiation [68, 69]. Expression of involucrin by mTECs may represent a further step of terminal differentiation that is dependent on lymphotoxin signaling [68].
What controls Aire expression in mTECs has been a much debated question. While some studies have suggested that the tumor necrosis factor (TNF) superfamily member lymphotoxin controls Aire expression in mTECs [70] [71], recent studies have concluded that this pathway is necessary for mTEC development and not directly for Aire expression [72] [73][74]. Mice with genetic deficiencies in the lymphotoxin pathway exhibit altered mTEC organization and decreased mTEC numbers. Additionally, Aire expression and function were unchanged in these mice, suggesting that lymphotoxin pathway does not directly affect Aire function. There is additional evidence for a role for lymphotoxin beta receptor in Aire-independent TSA expression and in the maintenance of medullary fibroblasts [75].
Recently, the precise roles of other TNF receptor superfamily members (including RANK and CD40) in mTEC development have been more precisely defined [76]*, [77]*, [78]*, [79]*. In the post-natal thymus, RANKL-RANK and CD40L-40 interactions act cooperatively in the development of mTECs expressing Aire and TSAs [76]*. RANK signaling appears to be important in the development of mature MHC II high mTECs, and CD40 stimulation appears to be important in the development of immature MHCII low mTECs. RANKL-RANK and CD40L-CD40 also have overlapping functions, since both were sufficient for inducing expression of Aire and tissue specific antigens in mature mTECs. This finding did not apply to pre-natal thymi, where only RANK signaling was essential for mTEC development.
It is well-established that signaling from thymocytes is critical for thymic stromal development, as evidenced by the thymic remnants seen in mice with blocks in T cell development. The identity of these thymocytes has been a matter of debate. In one study, CD4+CD3- lymphoid tissue inducer (LTi) cells, which have previously been shown to have an important role in regulating stromal cell development in peripheral lymphoid organs, were sufficient to generate Aire+ mTECs [74]. Signaling was proposed to occur through RANKL on LTi cells and RANK on CD80-Aire- mTECs, and this signaling was proposed to induce CD80+ Aire+ mTECs. On the other hand, RANKL produced by positively selected thymocytes have also been shown to sustain formation of thymic medulla [77]*. In line with these conclusions, CD4+ SP thymocytes (but not CD8+ SP thymocytes) were required for mature Aire+ mTEC development and required direct interactions between MHC and TCR. Interestingly, these interactions appeared to be enhanced by autoreactive thymocytes [78]*.
Conclusions
Autoimmune disease is a significant health problem, affecting 5–10% of the population, and is due to breakdown of immune tolerance mechanisms. Over the past 2 years, much progress has been made in our understanding of Aire and its role in T cell negative selection. The major function of Aire remains in its control of TSA expression in mTECs, and major insights into the mechanism by which Aire exerts influence over thousands of TSAs have recently been revealed. Additionally, the role of Aire+ mTECs as both a source of antigen and as antigen presenting cells has been recognized. Additional functions for Aire have now been described and the ontogeny of Aire+ mTECs has been further delineated. Continued progress in understanding Aire and its role in T cell development will be important for our understanding of how autoimmune disease develops and can be prevented.
A number of important questions surrounding Aire and T cell development remain open. How is Aire is able to target a large number of genes specifically, since binding to H3K4Me0 does not explain why certain TSAs are targeted and not others? What is the role of Aire in the differentiation of mTECs given recent evidence suggesting that Aire expression may not occur in the terminal differentiation step of these cells? How does Aire influence the selection of Tregs in the thymus, if at all? Can negative selection of developing thymocytes using an endogenous self-antigen be demonstrated? What are the relative contributions of TSA display by mTECs compared with cross-presentation by other cell types? Finally, do other Aire-like genes exist that can function in TSA expression in the thymus? Answers to these questions will provide valuable information into the role of Aire in T cell development and will be important in understanding autoimmune disease pathogenesis.
Acknowledgments
M.S.A is supported by the National Institutes of Health, the Burroughs Wellcome Fund, the Juvenile Diabetes Research Foundation, and the Helmsley Foundation. MAS is supported by the National Institutes of Health and the Pediatric Endocrine Society. We thank Una Fan for excellent graphics assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.An autoimmune disease, APECED, caused by mutations in a novel genefeaturing two PHD-type zinc-finger domains. The Finnish-German APECED Consortium. Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy. Nat Genet. 1997;17(4):399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 2.Nagamine K, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17(4):393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 3.Perheentupa J. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Clin Endocrinol Metab. 2006;91(8):2843–50. doi: 10.1210/jc.2005-2611. [DOI] [PubMed] [Google Scholar]
- 4.Derbinski J, et al. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2(11):1032–9. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 5.Gotter J, et al. Medullary epithelial cells of the human thymus express a highly diverse selection of tissue-specific genes colocalized in chromosomal clusters. J Exp Med. 2004;199(2):155–66. doi: 10.1084/jem.20031677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjorses P, et al. Localization of the APECED protein in distinct nuclear structures. Hum Mol Genet. 1999;8(2):259–66. doi: 10.1093/hmg/8.2.259. [DOI] [PubMed] [Google Scholar]
- 7.Anderson MS, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298(5597):1395–401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 8.Derbinski J, et al. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med. 2005;202(1):33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 10.Peterson P, Org T, Rebane A. Transcriptional regulation by AIRE: molecular mechanisms of central tolerance. Nat Rev Immunol. 2008;8(12):948–57. doi: 10.1038/nri2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner JM, et al. AIRE in the thymus and beyond. Curr Opin Immunol. 2009;21(6):582–9. doi: 10.1016/j.coi.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michels AW, Gottlieb PA. Autoimmune polyglandular syndromes. Nat Rev Endocrinol. 2010;b(5):270–7. doi: 10.1038/nrendo.2010.40. [DOI] [PubMed] [Google Scholar]
- 13.Derbinski J, et al. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med. 2005;202(1):33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villaseñor J, et al. Ectopic expression of peripheral-tissue antigens in the thymic epithelium: probabilistic, monoallelic, misinitiated. Proc Natl Acad Sci USA. 2008;105(41):15854–9. doi: 10.1073/pnas.0808069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15**.Gardner JM, et al. Deletional tolerance mediated by extrathymic Aire- expressing cells. Science. 2008;321(5890):843–7. doi: 10.1126/science.1159407. Using a reporter mouse to mark cells expressing Aire, this paper identifies a population of Aire-expressin stromal cells found in secondary lymphoid structures. These cells express a set of tissue-restricted antigens that are distinct from those expressed by mTECs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerau-de-Arellano M, Mathis D, Benoist C. Transcriptional impact of Aire varies with cell type. Proc Natl Acad Sci USA. 2008;105(37):14011–6. doi: 10.1073/pnas.0806616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abramson J, et al. Aire’s partners in the molecular control of immunological tolerance. Cell. 2010;140(1):123–35. doi: 10.1016/j.cell.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 18.Venanzi ES, et al. The variable immunological self: genetic variation and nongenetic noise in Aire-regulated transcription. Proc Natl Acad Sci USA. 2008;105(41):15860–5. doi: 10.1073/pnas.0808070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnnidis JB, et al. Chromosomal clustering of genes controlled by the aire transcription factor. Proc Natl Acad Sci U S A. 2005;102(20):7233–8. doi: 10.1073/pnas.0502670102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20**.Org T, et al. The autoimmune regulator PHD finger binds to non-methylated histone H3K4 to activate gene expression. EMBO Rep. 2008;9(4):370–6. doi: 10.1038/embor.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Chakravarty S, Zeng L, Zhou MM. Structure and site-specific recognition of histone H3 by the PHD finger of human autoimmune regulator. Structure. 2009;17(5):670–9. doi: 10.1016/j.str.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22**.Koh AS, et al. Aire employs a histone-binding module to mediate immunological tolerance, linking chromatin regulation with organ-specific autoimmunity. Proc Natl Acad Sci U S A. 2008;105(41):15878–83. doi: 10.1073/pnas.0808470105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Chignola F, et al. The solution structure of the first PHD finger of autoimmune regulator in complex with non-modified histone H3 tail reveals the antagonistic role of H3R2 methylation. Nucleic Acids Res. 2009;37(9):2951–61. doi: 10.1093/nar/gkp166. Together, these four papers demonstrate that the first PHD finger of Aire binds specifically to unmethylated lysine 4 on histone H3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musco G, Peterson P. PHD finger of autoimmune regulator: an epigenetic link between the histone modifications and tissue-specific antigen expression in thymus. Epigenetics. 2008;3(6):310–4. doi: 10.4161/epi.3.6.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koh AS, et al. Global relevance of Aire binding to hypomethylated lysine-4 of histone-3. Proc Natl Acad Sci U S A. 107(29):13016–21. doi: 10.1073/pnas.1004436107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liiv I, et al. DNA-PK contributes to the phosphorylation of AIRE: importance in transcriptional activity. Biochim Biophys Acta. 2008;1783(1):74–83. doi: 10.1016/j.bbamcr.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halonen M, et al. APECED-causing mutations in AIRE reveal the functional domains of the protein. Hum Mutat. 2004;23(3):245–57. doi: 10.1002/humu.20003. [DOI] [PubMed] [Google Scholar]
- 28.Anderson MS, et al. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23(2):227–39. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Liston A, et al. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4(4):350–4. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 30.Liston A, et al. Gene Dosage-limiting Role of Aire in Thymic Expression, Clonal Deletion, and Organ-specific Autoimmunity. J Exp Med. 2004;200(8):1015–1026. doi: 10.1084/jem.20040581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su MA, et al. Mechanisms of an autoimmunity syndrome in mice caused by a dominant mutation in Aire. J Clin Invest. 2008;118(5):1712–26. doi: 10.1172/JCI34523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liston A, et al. Gene dosage--limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity. J Exp Med. 2004;200(8):1015–26. doi: 10.1084/jem.20040581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kedzierska K, et al. Diversity and clonotypic composition of influenza-specific CD8+ TCR repertoires remain unaltered in the absence of Aire. Eur J Immunol. 2010;40(3):849–58. doi: 10.1002/eji.200939918. [DOI] [PubMed] [Google Scholar]
- 34.Devoss J, et al. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med. 2006;203(12):2727–35. doi: 10.1084/jem.20061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gavanescu I, et al. Loss of Aire-dependent thymic expression of a peripheral tissue antigen renders it a target of autoimmunity. Proc Natl Acad Sci U S A. 2007;104(11):4583–7. doi: 10.1073/pnas.0700259104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou Y, et al. An aberrant prostate antigen-specific immune response causes prostatitis in mice and is associated with chronic prostatitis in humans. J Clin Invest. 2009 doi: 10.1172/JCI38332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shum AK, et al. Identification of an autoantigen demonstrates a link between interstitial lung disease and a defect in central tolerance. Sci Transl Med. 2009;1(9):9ra20. doi: 10.1126/scitranslmed.3000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fletcher AL, et al. Reduced thymic Aire expression and abnormal NF-kappa B2 signaling in a model of systemic autoimmunity. J Immunol. 2009;182(5):2690–9. doi: 10.4049/jimmunol.0801752. [DOI] [PubMed] [Google Scholar]
- 39.Cai CQ, et al. Both Polymorphic VNTR and AIRE Modulate Differential Expression of Insulin in Human Thymic Epithelial Cells. Diabetes. 2010 doi: 10.2337/db10-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pugliese A, et al. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet. 1997;15(3):293–7. doi: 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]
- 41.Vafiadis P, et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet. 1997;15(3):289–92. doi: 10.1038/ng0397-289. [DOI] [PubMed] [Google Scholar]
- 42.Giraud M, et al. An IRF8-binding promoter variant and AIRE control CHRNA1 promiscuous expression in thymus. Nature. 2007;448(7156):934–7. doi: 10.1038/nature06066. [DOI] [PubMed] [Google Scholar]
- 43*.Guerau-de-Arellano M, et al. Neonatal tolerance revisited: a perinatal window for Aire control of autoimmunity. J Exp Med. 2009;206(6):1245–52. doi: 10.1084/jem.20090300. This paper utilizes an doxycycline-regulated Aire transgene to demonstrate that Aire expression in a neonatal time window is necessary and sufficient to prevent autoimmunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44*.Cheng MH, et al. Acquired autoimmune polyglandular syndrome, thymoma, and an AIRE defect. N Engl J Med. 2010;362(8):764–6. doi: 10.1056/NEJMc0909510. A case report of a 67 year old woman with a Aire-negative thymoma who presents with clinical findings of APS 1. This case report challenges the notion that thymic Aire expression is important for preventing autoimmunity only during early life. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marx A, et al. The autoimmune regulator AIRE in thymoma biology: autoimmunity and beyond. J Thorac Oncol. 5(10 Suppl 4):S266–72. doi: 10.1097/JTO.0b013e3181f1f63f. [DOI] [PubMed] [Google Scholar]
- 46.Gallegos AM, Bevan MJ. Central Tolerance to Tissue-specific Antigens Mediated by Direct and Indirect Antigen Presentation. J Exp Med. 2004;200(8):1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47**.Hinterberger M, et al. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nat Immunol. 2010;11(6):512–9. doi: 10.1038/ni.1874. [DOI] [PubMed] [Google Scholar]
- 48**.Koble C, Kyewski B. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J Exp Med. 2009;206(7):1505–13. doi: 10.1084/jem.20082449. Together, these two papers demonstrate a role for mTECs in direct antigen presentation to CD4+ T cell in the thymus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nedjic J, et al. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature. 2008;455(7211):396–400. doi: 10.1038/nature07208. [DOI] [PubMed] [Google Scholar]
- 50*.Laan M, et al. Autoimmune regulator deficiency results in decreased expression of CCR4 and CCR7 ligands and in delayed migration of CD4+ thymocytes. J Immunol. 2009;183(12):7682–91. doi: 10.4049/jimmunol.0804133. This paper proposes that Aire-regulated expression of chemokines may contribute to thymic negative selection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueno T, et al. CCR7 signals are essential for cortex-medulla migration of developing thymocytes. J Exp Med. 2004;200(4):493–505. doi: 10.1084/jem.20040643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li J, et al. Developmental pathway of CD4+CD8− medullary thymocytes during mouse ontogeny and its defect in Aire−/− mice. Proc Natl Acad Sci USA. 2007;104(46):18175–80. doi: 10.1073/pnas.0708884104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lienenklaus S, et al. Novel reporter mouse reveals constitutive and inflammatory expression of IFN-beta in vivo. J Immunol. 2009;183(5):3229–36. doi: 10.4049/jimmunol.0804277. [DOI] [PubMed] [Google Scholar]
- 54.Gillard GO, et al. Aire-dependent alterations in medullary thymic epithelium indicate a role for Aire in thymic epithelial differentiation. J Immunol. 2007;178(5):3007–15. doi: 10.4049/jimmunol.178.5.3007. [DOI] [PubMed] [Google Scholar]
- 55.Zuklys S, et al. Normal Thymic Architecture and Negative Selection Are Associated with Aire Expression, the Gene Defective in the Autoimmune-Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy (APECED) J Immunol. 2000;165(4):1976–1983. doi: 10.4049/jimmunol.165.4.1976. [DOI] [PubMed] [Google Scholar]
- 56.Ramsey C, et al. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet. 2002;11(4):397–409. doi: 10.1093/hmg/11.4.397. [DOI] [PubMed] [Google Scholar]
- 57.Yano M, et al. Aire controls the differentiation program of thymic epithelial cells in the medulla for the establishment of self-tolerance. J Exp Med. 2008;205(12):2827–38. doi: 10.1084/jem.20080046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Milicevic Z, et al. Ultrastructure of medullary thymic epithelial cells of autoimmune regulator (Aire)-deficient mice. Immunol Cell Biol. 2010;88(1):50–6. doi: 10.1038/icb.2009.55. [DOI] [PubMed] [Google Scholar]
- 59.Dooley J, Erickson M, Farr AG. Alterations of the medullary epithelial compartment in the Aire-deficient thymus: implications for programs of thymic epithelial differentiation. J Immunol. 2008;181(8):5225–32. doi: 10.4049/jimmunol.181.8.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuroda N, et al. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J Immunol. 2005;174(4):1862–70. doi: 10.4049/jimmunol.174.4.1862. [DOI] [PubMed] [Google Scholar]
- 61.Daniely D, et al. Diversity of TCRs on natural Foxp3+ T cells in mice lacking Aire expression. J Immunol. 2010;184(12):6865–73. doi: 10.4049/jimmunol.0903609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ryan KR, et al. CD4+CD25+ T-regulatory cells are decreased in patients with autoimmune polyendocrinopathy candidiasis ectodermal dystrophy. J Allergy Clin Immunol. 2005;116(5):1158–9. doi: 10.1016/j.jaci.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 63.Kekalainen E, et al. A Defect of Regulatory T Cells in Patients with Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy. J Immunol. 2007;178(2):1208–1215. doi: 10.4049/jimmunol.178.2.1208. [DOI] [PubMed] [Google Scholar]
- 64.Wolff ASB, et al. Flow cytometry study of blood cell subtypes reflects autoimmune and inflammatory processes in autoimmune polyendocrine syndrome type I. Scand J Immunol. 2010;71(6):459–67. doi: 10.1111/j.1365-3083.2010.02397.x. [DOI] [PubMed] [Google Scholar]
- 65.Laakso SM, et al. Regulatory T cell defect in APECED patients is associated with loss of naive FOXP3(+) precursors and impaired activated population. Journal of autoimmunity. 2010 doi: 10.1016/j.jaut.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 66.Gäbler J, Arnold J, Kyewski B. Promiscuous gene expression and the developmental dynamics of medullary thymic epithelial cells. Eur J Immunol. 2007;37(12):3363–72. doi: 10.1002/eji.200737131. [DOI] [PubMed] [Google Scholar]
- 67.Gray D, et al. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med. 2007 doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.White AJ, et al. Lymphotoxin Signals from Positively Selected Thymocytes Regulate the Terminal Differentiation of Medullary Thymic Epithelial Cells. J Immunol. 2010 doi: 10.4049/jimmunol.1002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nishikawa Y, et al. Biphasic Aire expression in early embryos and in medullary thymic epithelial cells before end-stage terminal differentiation. J Exp Med. 207(5):963–71. doi: 10.1084/jem.20092144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chin RK, et al. Lymphotoxin pathway directs thymic Aire expression. Nat Immunol. 2003;4(11):1121–7. doi: 10.1038/ni982. [DOI] [PubMed] [Google Scholar]
- 71.Zhu M, et al. NF-{kappa}B2 is required for the establishment of central tolerance through an Aire-dependent pathway. J Clin Invest. 2006;116(11):2964–2971. doi: 10.1172/JCI28326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martins VC, Boehm T, Bleul CC. Ltbetar signaling does not regulate Aire-dependent transcripts in medullary thymic epithelial cells. J Immunol. 2008;181(1):400–7. doi: 10.4049/jimmunol.181.1.400. [DOI] [PubMed] [Google Scholar]
- 73.Venanzi ES, et al. Lymphotoxin pathway and Aire influences on thymic medullary epithelial cells are unconnected. J Immunol. 2007;179(9):5693–700. doi: 10.4049/jimmunol.179.9.5693. [DOI] [PubMed] [Google Scholar]
- 74.Rossi SW, et al. RANK signals from CD4(+)3(−-) inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med. 2007;204(6):1267–72. doi: 10.1084/jem.20062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seach N, et al. The lymphotoxin pathway regulates Aire-independent expression of ectopic genes and chemokines in thymic stromal cells. J Immunol. 2008;180(8):5384–92. doi: 10.4049/jimmunol.180.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76*.Akiyama T, et al. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29(3):423–37. doi: 10.1016/j.immuni.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 77*.Hikosaka Y, et al. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity. 2008;29(3):438–50. doi: 10.1016/j.immuni.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 78*.Irla M, et al. Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity. 2008;29(3):451–63. doi: 10.1016/j.immuni.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 79*.White AJ, et al. Sequential phases in the development of Aire-expressing medullary thymic epithelial cells involve distinct cellular input. Eur J Immunol. 2008;38(4):942–7. doi: 10.1002/eji.200738052. Together, these four papers examine the factors important in the differentiation of Aire-expressing mTECs. These papers highlight the role of TNF receptor superfamily members in mTEC ontogeny. [DOI] [PubMed] [Google Scholar]