Regulators of the cytoplasmic dynein motor (original) (raw)
. Author manuscript; available in PMC: 2012 Jul 11.
Published in final edited form as: Nat Rev Mol Cell Biol. 2009 Dec;10(12):854–865. doi: 10.1038/nrm2804
Abstract
Eukaryotic cells use cytoskeletal motor proteins to transport many different intracellular cargos. Numerous kinesins and myosins have evolved to cope with the various transport needs that have arisen during eukaryotic evolution. Surprisingly, a single cytoplasmic dynein (a minus end-directed microtubule motor) carries out similarly diverse transport activities as the many different types of kinesin. How is dynein coupled to its wide range of cargos and how is it spatially and temporally regulated? The answer could lie in the several multifunctional adaptors, including dynactin, lissencephaly 1, nuclear distribution protein E (NUDE) and NUDE-like, Bicaudal d, Rod–ZW10–Zwilch and Spindly, that regulate dynein function and localization.
The large size and complex organization of eukaryotic cells necessitates active, directional transport for the efficient and accurate relocalization of cellular components. To carry out this transport, eukaryotic cells use motor proteins that walk along cytoskeletal tracks. There are three classes of cytoskeletal motor proteins: myosins, kinesins and dyneins. Myosins move along actin filaments, and kinesins and dyneins move along micro tubules. Kinesins (with the exception of kinesin 14 family members) move towards microtubule plus ends, which in most cells generally extend towards the cell periphery. All dyneins discovered to date move towards the microtubule minus ends, which in most cells are collected into the microtubule organizing centre (MTOC) near the nucleus.
Many families of myosins and kinesins have evolved to execute different functions. These cytoskeletal motors are composed of a highly conserved myosin or kinesin ATPase core, which powers movement along cytoskeletal filaments, that is attached to a wide range of tail domains, which mediate cargo interactions both directly and by recruiting specific accessory proteins. Many genes encoding dynein heavy chains have been identified (>15 in most species). However, most of these encode proteins that are anchored within the axoneme, where they help to drive coordinated beating of cilia and flagella. Only two of these proteins — intraflagellar transport (IFT) dynein (also known as dynein 1B and dynein 2) and cytoplasmic dynein — transport cargos along microtubules. As IFT dynein functions exclusively to move cargos along the axoneme towards the cell body, all minus end-directed transport within the cytoplasm (transport of organelles, mRNA and proteins), as well as several mitotic functions1, are carried out by a single cytoplasmic dynein. Given the ease of gene duplication, this is surprising and suggests an evolutionary advantage of using a single motor for minus end-directed transport. It is interesting to note that higher plants seem to lack a cytoplasmic dynein, but instead have an expanded array of minus end-directed kinesins2.
The hugely diverse functional repertoire of cytoplasmic dynein raises important questions about its function in the cell. How is it coupled to such a wide range of cargos and how is its activity spatially and temporally regulated in cells? The dynein motor exists within a large assembly of smaller, non-catalytic subunits, which provide points of attachment and regulation for some dynein cargos (BOX 1). Dynein interacts with several proteins that do not belong to the dynein complex itself but are crucial for adapting the motor to its cellular function. The best characterized of these are dynactin, the complex formed between lissencephaly 1 (LIS1) and nuclear distribution protein E (NUDE; also known as NDE) or LIS1 and NUDE-like (NUDEL; also known as NDEL), Bicaudal D, ROD–ZW10–Zwilch (RZZ) and spindly. These factors contribute to many dynein functions and, in the cases of dynactin and LIS1, their inhibition or depletion is phenotypically similar to a complete loss of dynein function. The requirement for these dynein adaptors, each of which is crucial for many overlapping processes, is intriguing. In this Review we discuss what is known about the structure and cellular functions of these adaptors and describe models of how they might couple dynein to its cellular activities.
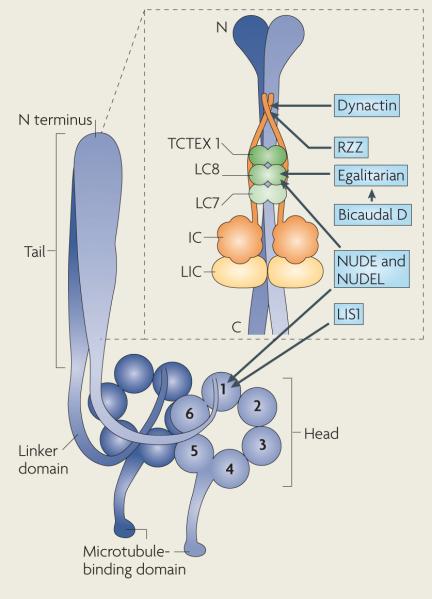
Box 1. Composition and domain structure of cytoplasmic dynein.
The cytoplasmic dynein heavy chain (blue) consists of a carboxy-terminal motor (head) domain and an amino-terminal tail domain (see the figure). The motor domain contains six AAA domains arranged in a ring: the first four AAA domains (1–4) can bind ATP, whereas domains 5 and 6 are more divergent and have lost the residues that are necessary for binding ATP3. Mutagenesis studies indicate that ATP hydrolysis by AAA1 and AAA3 is important for motility, whereas ATP hydrolysis by AAA2 and AAA4 is less essential and might have a regulatory role132–134. The microtubule-binding domain of dynein is a small, globular domain at the tip of an antiparallel coiled coil that emerges from the ring after AAA4 (REFS 135,136). Recent structural and biochemical studies suggest that a shift in the registry of this coiled coil couples ATPase cycles to rounds of microtubule binding and release137–139. The mechanical element of dynein might be the ‘linker’ between the motor domain and tail of dynein; this element shifts its position across the AAA ring during the ATPase cycle136,140.
The dynein tail domain mediates homodimerization of the heavy chain and also provides a scaffold for five non-catalytic subunits, which assemble as dimers to form the complement of the cytoplasmic dynein complex. The dynein intermediate chain (IC) and light intermediate chain (LIC) bind directly to the dynein heavy chain. The smaller light chains — light chain 8 (LC8), LC7 (also known as roadblock) and T-complex testis-specific protein 1 (TCTEX1) — assemble on the intermediate chain. The light chains mediate interactions with several dynein adaptor proteins (shown in blue boxes): the p150 subunit of dynactin and the ZW10 subunit of Rod–ZW10–Zwilch (RZZ) interact with IC, whereas nuclear distribution protein e (NUDE; also know as NDE), NUDE-like (NUDEL; also known as NDEL) and Bicaudal D (through its partner egalitarian) bind to LC8. Lissencephaly (LIS1) interacts directly with AAA1 of the dynein heavy chain4,73,104,124,141,142.
Cytoplasmic dynein structure and function
The cytoplasmic dynein complex is composed of a heavy chain, an ATPase, which powers motility along micro-tubules, and several non-catalytic subunits. The cytoplasmic dynein heavy chain is a member of the ATPase associated with various cellular activities (AAA+ATPase) superfamily3, the members of which mostly function as chaperones or protein unfoldases. Although most AAA+ ATPases self-assemble into and function as hexameric rings, dynein is unusual in that its six AAA modules are linked in a single polypeptide and are not identical. These AAA domains, along with a coiled coil extension containing the microtubule-binding domain, form the carboxy-terminal head domain of dynein (BOX 1).
The amino-terminal tail of the cytoplasmic dynein heavy chain is involved in its homodimerization and acts as a scaffold for the assembly of five different non-catalytic subunits (possibly fewer in some fungi) to form the cytoplasmic dynein complex (BOX 1). These subunits are not required for dynein motility in vitro, although they might regulate dynein motility in vivo. Recent data indicate that the non-catalytic subunits link dynein to cargos and to several adaptor proteins that regulate dynein function4–8 (BOX 1). In many species, the non-catalytic subunits (unlike the cytoplasmic dynein heavy chain) exist in different isoforms encoded by multiple genes. Different isoforms might assemble on the dynein heavy chain to create functionally distinct dynein complexes9.
Dynein is a processive motor that can take μm-scale movements along the microtubule without dissociating10,11. Processive motion requires the dimerization of two dynein head domains, although the endogenous dimerization domain is not required for this process12. High spatial precision tracking studies of single dynein motors suggest that dynein moves by alternating steps of the dynein heads, a conceptually similar mechanism to the hand-over-hand model for processive kinesin motility12. This model implies coordination between the two heads, such that one remains bound while the other one moves forward. Optical trap studies of dynein force production suggest that this coordination is carried out by strain transmitted through the linkage between the two heads13.
The stepping behaviour of dynein along the micro-tubule is more variable than that of kinesin, which takes invariant 8 nm-long steps towards the micro tubule plus end. Dynein predominantly takes 8 nm-long steps toward the minus end, but occasionally takes steps of up to 32 nm, or takes one or a few steps backwards towards the plus end11,12,14. Dynein also often takes steps sideways to an adjacent protofilament12, which kinesin rarely does. Although it is difficult to directly observe dynein motility in vivo, indirect observations have also indicated variability in stepping behaviour during physiological dynein transport15,16. The variable length and sideways stepping of dynein could allow it to more easily pass obstacles on the micro tubule, but whether the stepping behaviour of dynein is in fact modulated in response to its surroundings has not yet been tested17,18.
To understand how dynein responds to loads encountered during in vivo transport through a dense cytoplasm, it will be important to ascertain its true capacity for force generation. The amount of force that dynein can produce is under debate. some studies have measured a stall force (~7 pN) similar to that observed for kinesin14,19, whereas others have found that dynein is much weaker, stalling at approximately 1 pN11. It is possible that the maximal force of cytoplasmic dynein differs between species, being tuned to serve distinct transport needs. Optical trap experiments have also indicated that the stepping behaviour of dynein is responsive to load. Increasing the load experienced by dynein increases the proportion of smaller steps and backwards steps11,14. It is also important to note that although observations of single dynein motors will continue to be essential to uncover the mechanism of dynein motility, most dynein cargos in cells probably are powered by several dynein motors at a time, and the emergent properties of a multimotor system could differ markedly from those of single motors20–22.
Dynactin: an essential dynein adaptor
Dynactin (name derived from dynein activator) was identified as an activator of dynein-mediated, minus end-directed vesicle transport in vitro23,24. The dynactin complex has a molecular mass of 1 mDa, nearly as high as that of cytoplasmic dynein itself, and comprises 11 different subunits (BOX 2), including the largest subunit, p150, and a filament of actin-related protein 1 (ARP1). It has been found to be essential for nearly every cellular function of cytoplasmic dynein1,25. Dynactin helps to target dynein to specific cellular locations, links dynein to cargos and increases dynein processivity, although a comprehensive model of how these activities are integrated has yet to emerge.
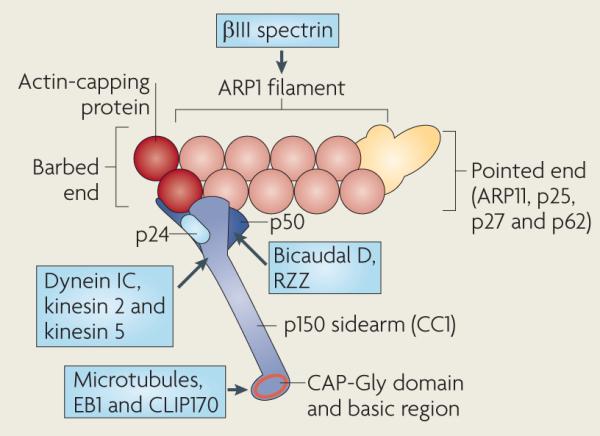
Box 2. Composition and domain structure of the dynactin complex.
A short (~40 nm) filament of actin-related protein 1 (ARP1) forms the central scaffold of the dynactin complex and may help to link dynein to cargo through its interaction with spectrin, which coats the cytoplasmic face of several organelles52,143. The subcomplex at the pointed end of the ARP1 filament (yellow) contains ARP11, p62, which might mediate the interaction of dynactin with cortical actin, and two more subunits, p25 and p27 (REFS 58,144–148). The barbed end subcomplex is composed of a dimer of p150 (also known as p150Glued, from the Drosophila melanogaster allele), a tetramer of p50 (also known as dynamitin because its overexpression dissociates dynactin into several subcomplexes), the p24 subunit and the actin-capping protein heterodimer (shown in red; dynactin may lack capping protein in some organisms)23,144,145,148,149. p50 interacts with two other dynein adaptors, Bicaudal D and the Rod–ZW10–Zwilch (RZZ) complex105,127,150. The most amino-terminal coiled coil (CC1) of p150 forms the 24 nm sidearm from the ARP1 filament. p150 contains N-terminal microtubule-binding domains, a cytoskeleton-associated protein Gly-rich (CAP-Gly) domain and a basic region (indicated in red) that probably form the globular domain observed at the end of the sidearm144,151. The CAP-Gly domain also binds to the microtubule plus end-associated proteins end binding 1 (EB1; also known as MAPRE1) and CAP-Gly domain-containing linker protein 170 (CLIP170; also known as CLIP1). p150 provides the only documented interaction between dynactin and dynein (through the dynein intermediate chain)4,60,152, and also interacts with kinesin 2 and kinesin 5 (REFS 4,5,60,152). The structural arrangement of subunits in the barbed and pointed end subcomplexes have not been resolved148 and are outlined for display purposes only. Interacting proteins are shown in blue boxes.
Targeting dynein to microtubule plus ends
Although dynein is a minus end-directed motor, it often accumulates at the plus ends of microtubules, and dynactin has been shown to modulate this localization. The association of dynein with the microtubule plus end, which has been best characterized in yeast, has been implicated in delivering dynein to sites where it is needed for transport (FIG. 1).
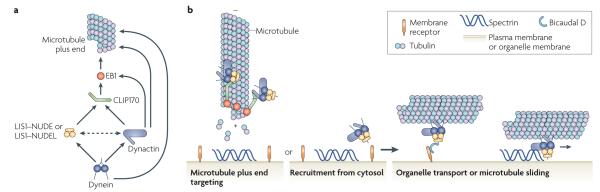
Figure 1. Multiple factors cooperate to target dynein to microtubule plus ends and to its cargos.
a | Several factors contribute to dynein localization to the microtubule plus ends. The localization of CAP-Gly domain-containing linker protein 170 (CLIP170) and dynactin to the microtubule plus end usually depends on end binding 1 (EB1), although all three factors can interact directly with tubulin. Lissencephaly 1 (LIS1) is probably recruited to the plus end by interacting with CLIP170. LIS1–nuclear distribution protein E (NUDE) or LIS1–NUDE-like (NUDEL) and dynactin then target dynein to the plus end. The microtubule-binding domains of dynein might also contribute to its association with the microtubule plus ends. Solid arrows indicate biochemically confirmed physical interactions; the dotted arrow indicates an interaction shown by yeast two-hybrid assay. b | Following recruitment to microtubule plus ends by the interactions shown in part a, dynein can probe the cytoplasm for cargo such as an organelle or the cell cortex, although in some cases dynein might be recruited to cargo directly from the cytosol. Once dynein is loaded with cargo, LIS1–NudE or LIS1–NudEL and dynactin promote its release from the microtubule plus end and the initiation of motility through an as yet unknown mechanism. Cargo binding is achieved by the interaction of dynein with specific receptors, directly or through dynactin and the cargo linker Bicaudal d, and by the interaction of dynactin with spectrin, a filamentous protein that coats the cytoplasmic face of the Golgi and other cell membranes. These interactions might be required sequentially or in combination. dynein transports organelles and other cargos along microtubules, or can slide microtubules relative to its position when it is anchored at the cell cortex (lower right panel). It is not known whether LIS1-NudE and LIS1–NudEL contribute to this step.
During Saccharomyces cerevisiae cell division, dynein binds to the bud cell cortex and pulls the nucleus into the bud neck by translocating astral microtubules relative to the bud cell cortex. This ensures that both the mother and daughter cells receive a nucleus26,27. Dynein first appears at the plus ends of astral microtubules and then at the bud cell cortex, suggesting that dynein is loaded onto the plus end of a growing microtubule and then transferred to its receptor, the cortically localized nuclear migration 1 (NUM1)8,27. Dynactin is not required for dynein plus end localization in this system, but seems to be important for its transfer from the microtubule plus end to the cortex. Deletion or mutation of many dynactin subunits causes dynein to accumulate at astral microtubule plus ends and abolishes its localization to the bud cell cortex28–30.
In the filamentous fungus Aspergillus nidulans dynein and dynactin are required for proper nuclear positioning during growth of hyphae, perhaps through a similar process as in S. cerevisiae, although dynein localization to the cell cortex has not been observed. In this system, however, dynein and dynactin depend on each other for local ization to the plus ends of the microtubules that extend into the hyphae31–35. Whether dynactin also functions with dynein after transport is initiated is unknown.
Biochemical and structural studies of metazoan proteins have delineated a web of interactions between the dynactin subunit p150 and microtubule plus end-binding proteins. The cytoskeleton-associated protein Gly-rich (CAP-Gly) domain of p150 (BOX 2) can bind a shared C-terminal acidic motif in α-tubulin and the plus end-binding proteins end binding 1 (EB1; also known as MAPRE1) and CAP-Gly domain-containing linker protein 170 (CLIP170; also known as CLIP1)36–39 (FIG. 1). The complexity of the interactions between these proteins has made it difficult to determine their exact hierarchy in plus end binding, and it is likely that these interactions are remodelled dynamically in response to the polymer ization state of the microtubule and in response to dynein–dynactin cargo binding40,41.
The role of microtubule plus end localization in the function of dynein and dynactin is less clear in meta-zoans than in fungi, although some evidence suggests that it might be important for cargo loading and initiation of transport. p150 labelled with green fluorescent protein (GFP) localizes to microtubule plus ends immediately before the initiation of minus end-directed movement of Golgi vesicles42,43. In addition, a mutation in the CAP-Gly domain of p150 that disrupts micro-tubule binding is linked to human motor neuron disease. This mutation also affects the positioning of a subset of dynein cargos and the plus end localization of EB1 under some conditions44–47. However, other data suggest that microtubule plus end targeting by dynactin might not be required for initiating all dynein transport. Notably, p150 mutants that lack the CAP-Gly domain, one of which is a natural isoform in neurons, can support organelle transport in HeLa cells and Drosophila melanogaster s2 cells48–50. A recent study showed that plus end accumulation of CLIP170, but not of p150, is required for dynein-driven pigment granule transport in Xaenopus laevis melanophores51. These findings indicate either that dynein uses an alternative route to the microtubule plus end, possibly through an interaction between LIS1 and CLIP170 (FIG. 1a), or that dynein can be recruited to cargo from the cytosol, and interactions between plus end-bound CLIP170 and cargo then target the microtubule to cargo-bound dynein.
Linking dynein to cargo
Many studies indicate that dynactin helps to link dynein to its cargo. A well-characterized and possibly generally important interaction occurs between the ARP1 filament of dynactin and βIII spectrin, a filamentous protein that is found on the cytosolic surface of the Golgi and other cellular membranes52 (FIG. 1b). The motility of synthetic liposomes can be reconstituted in vitro using partially purified dynein–dynactin and βIII spectrin, suggesting that the ARP1–βIII spectrin interaction is sufficient to form a productive dynein– cargo link53. The spectrin family is large, and different isoforms are associated with various membranes in the cell54. Binding of ARP1 to other spectrin isoforms might therefore facilitate dynein-dependent transport of different cargos, although this has yet to be shown.
The p150 subunit also links dynein to cargos, notably through interactions of p150 with regulatory GTPases. One such interaction occurs between the C terminus of p150 (p150-C) and the sAR1 GTPase-activating protein SEC23, which assembles as part of the CopII coat on transport vesicles budding from the endoplasmic reticulum (ER)43. This interaction seems to be important for trafficking, as overexpression of p150-C strongly inhibits the delivery of secretory proteins from the ER to the Golgi. The interaction between p150-C and sEC23 is weak, and sEC23 dissociates from vesicles soon after they bud, suggesting that p150-C–sEC23 might exclusively initiate the binding to dynein to vesicles, which is then maintained by another, more stable link during dynein-mediated transport from the ER to the Golgi43.
A similar set of interactions with GTPase-interacting proteins facilitates dynein-dependent transport of late endosomes. p150-C interacts with Rab-interacting lysosomal protein (RILP), a RAB7 GTPase effector that is found on late endosomes. This interaction is required for the localization of p150 to late endosomes and their subsequent transport by dynein. βIII spectrin is not required for the localization of dynactin to late endosomes, but is important for their transport. These data suggest that RILP could initially recruit dynein and dynactin to late endosomes and that subsequent interactions between dynactin and βIII spectrin is required for long-range transport of vesicles55. RILP also promotes the interaction of RAB7 with another of its effectors, oxysterol-binding protein-related protein 1 (ORP1L; also known as OSBPL11), which controls p150 recruitment to RILP in response to the cholesterol content of the endosomal membrane55,56.
Interestingly, addition of p150-C released dynactin from membranes isolated from Neurospora crassa in a biochemical assay, suggesting that when p150-C is not bound to cargo proteins it might block the interaction of ARP1 with membranes57,58. Together, these studies suggest a model in which a transient interaction of p150-C with a cargo protein such as RILP or sEC23 could relieve p150-mediated inhibition of ARP1–spectrin binding. A strong ARP1–spectrin interaction could then link dynein and dynactin to membranes for long-range transport of cargo (FIG. 1b).
The p50 subunit of dynactin also seems to be a hub for interactions with dynein cargos. Both p50 and Golgi-associated RAB6 bind Bicaudal D, another dynein adaptor, suggesting possible models for the recruitment of dynein to cargo (discussed below). Overexpression of the conserved N-terminus of p50, which is not required for dynactin assembly, interferes with the distribution of secretory organelles40. The p50 N terminus is also involved in the recruitment of dynactin, and thus dynein, to the kinetochore (see below).
Coordination of bidirectional transport
Dynactin might also have a role in coordinating dynein and kinesin activity on membranes. Dynactin interacts with kinesin 5 (REF. 59) and kinesin 2 (REFS 60,61), two plus end-directed microtubule motors. For many cargos that are transported bidirectionally, interference with either the plus- or the minus end-directed motor completely disrupts motility in both directions, strongly suggesting a tight, interdependent link between dynein and kinesins. such coordination might involve dynactin49, probably in collaboration with other factors. Although nearly all cellular cargos are transported bidirectionally, little is known about how opposing motors are coordinated. Future work to delineate the contribution of dynactin to bidirectional transport could provide insight into this important issue.
Dynactin modulation of dynein processivity
Following its discovery as a factor required for the transport of vesicles by purified dynein, the first function proposed for dynactin was that of an activator of dynein motility23,24. The identification of a CAP-Gly microtubule-binding domain at the N-terminus of the p150 subunit (BOX 2) led to the more specific hypothesis that dynactin might tether dynein to microtubules and prevent it from diffusing away after dynein detachment, thereby increasing the processivity of dynein62. Dynactin subsequently was observed to increase the processivity of dynein-adsorbed polystyrene beads along microtubules; this effect was abrogated by an antibody against the N-terminus of p150, supporting the hypothesis that dynactin acts as a tether between dynein and microtubules63. A later >study identified a second microtubule-binding domain, a region of basic residues, in the N-terminal globular region of p150. This domain could diffuse along micro-tubules on its own and could also increase the processivity of dynein-adsorbed beads. This discovery further supported the model that dynactin increases the processivity of dynein by tethering it to microtubules, in this case by a diffusive interaction64.
However, later studies showed that dynactin lacking the microtubule-binding domains of p150 supports a normal frequency, velocity and processivity of dynein-mediated vesicle transport in D. melanogaster s2 cells49 and normal dynein function during nuclear segregation in S. cerevisiae30. Observations of single, fluor escently labelled motor complexes isolated from S. cerevisiae directly showed that dynactin enhances dynein processivity and that dynactin lacking the microtubule-binding domains of p150 could still increase the run length of dynein in vitro30. These findings suggested that dynactin enhances dynein processivity by a mechanism other than tethering dynein to microtubules and that the N-terminal microtubule-binding domains of p150 are dispensable for many dynactin functions. Another study, however, described a subtle in vivo phenotype in S. cerevisiae lacking the p150 CAP-Gly domain, a decrease in the persistence and frequency of experimentally induced dynein-driven oscillations of the yeast nucleus, suggesting a possible defect in dynein motility under high load65.
These findings indicate a role for dynactin in increasing dynein processivity, but further studies of dynein– dynactin motility and force generation, as well as structural studies of the dynein–dynactin complex, will be important to clearly define the mechanism by which it acts.
LIS1, NUDE and NUDEL: ubiquitous cofactors
LiS1 was first identified as a gene that is tightly linked to the human disease lissencephaly, which is characterized by disrupted neuronal mitoses and migration during brain development and severe malformation of the brain cortex66,67. studies of nuclear migration in fungi identified homologues of LIS1 (Pac1 in S. cerevisiae, NudF in A. nidulans) and suggested a role for LIS1 as a dynein cofactor31,68,69. These studies also led to the identification of an important binding partner for LIS1 and its homo-logues, NUDE in A. nidulans and later Ndl1 in S. cerevisiae. The closely related homologues NUDE and NUDEL were later identified in metazoans70–73 (BOX 3). studies in fungi and metazoans have shown that LIS1, NUDE and NUDEL are crucial to dynein function in nuclear and spindle positioning and in kinetochore activity, as well as in organelle and mRNA transport74–76. Organisms with loss-of-function mutations in LIS1, NUDE and NUDEL have similar phenotypes, and current data indicate that LIS1 probably acts in a complex with a NUDE homo-logue, although it is possible that they also have some distinct roles. Their broad involvement in dynein function suggests that LIS1 along with NUDE or NUDEL act as ubiquitous co-factors for dynein, although their mechanisms of action remain poorly understood.
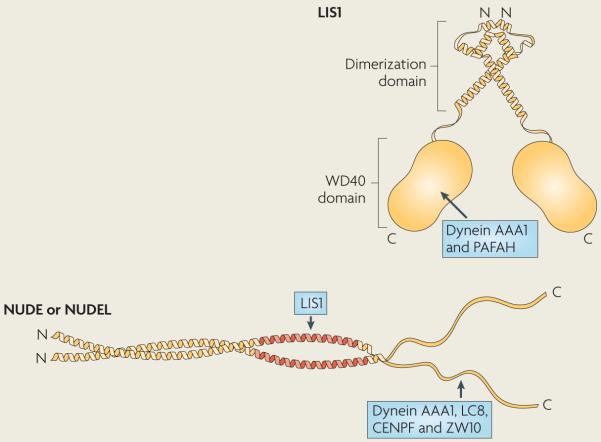
Box 3. Composition and domain structure of LIS1–nuDE and LIS1–nuDEL.
Lissencephaly 1 (LIS1) is composed of an amino-terminal coiled coil linked to seven WD40 repeats at the carboxyl terminus (see the figure). The N-terminal coiled coil mediates the dimerization of LIS1, whereas the WD40 domain binds to the most N-terminal ATPase domain (AAA1) of the dynein heavy chain (see also BOX 1) and possibly to the dynein tail and the p50 subunit of dynactin73,141,153,154. specific binding sites for nuclear distribution protein e (NUDE; also known as NDE) and NUDE-like (NUDEL; also known as NDEL) have not been identified in LIS1.
NUDE and NUDEL are highly homologous proteins that function with LIS1; fungi contain only one gene encoding NUDe, whereas metazoans contain both homologues, which have some distinct functions. NUDE and NUDEL are composed of an N-terminal parallel coiled coil, which mediates dimerization and LIS1 binding, and an unstructured C terminus155. The C terminus has been reported to interact with the AAA1 domain of dynein, light chain 8 (LC8) and the intermediate chain (IC)70,73,142. NUDE and NUDeL also bind the kinetochore protein centromere protein F (CENPF) and to the kinetochore interactor ZW10, thus contributing to dynein kinetochore targeting94–97.
NUDe or NUDeL binding might stimulate LIS1 to bind and regulate dynein84,156. This could be mediated by the phosphorylation of NUDe and NUDeL, which in metazoans strengthens their interaction with LIS1 and could thus stimulate LIS1 to bind dynein72,73,156,157. Independently of dynein, LIS1 acts as a regulatory subunit for the platelet-activating factor acetylhydrolase (PAFAH)158 and binds to PAFAH through its C terminus, which overlaps the dynein-binding site154. LIS1 cannot simultaneously bind NUDeL and PAFAH, which provides further evidence that NUDe or NUDeL binding directs LIS1 to bind and regulate dynein154. schematic representations of LIS1 and NUDe and NUDeL are based on their crystal structures154,155.
Cellular functions of LIS1–NUDE and LIS1–NUDEL
In fungi, the homologues of LIS1 and NUDE are required for dynein-mediated nuclear positioning, during which they modulate the association of dynein with micro tubule plus ends before nuclear transport. LIS1 and NUDE homologues localize with dynein to the plus ends of microtubules in both S. cerevisiae and A. nidulans28,34,69,77. In S. cerevisiae, Pac1 and Ndl1 are required for dynein plus end localization; deletion of Pac1 or Ndl1 nearly abolishes dynein localization to the plus end and to the cortex of the bud cell28,69,78. The LIS1 or NUDE homologues in fungi have not been shown to bind microtubules themselves, but they might facilitate dynein plus end loading through LIS1 interaction with CLIP170 (REFS 29,79,80), or by inducing a conformational change in dynein (FIG. 1). In A. nidulans, loss of NUDF or NUDE function increases dynein accumulation at the plus end of microtubules. This suggests that, instead of recruiting dynein to the microtubule plus ends (as they do in S. cerevisiae), in this case NUDF and NUDE promote the release of dynein from the plus end and motility towards the minus end35,81.
In both species, Ndl1 and NudE seem to function as activators of the LIS1 homologues, as overexpression of Pac1 and NudF suppresses the phenotype of loss-of-function alleles of Ndl1 and NudE, respectively. A recent study showed that a truncated form of S. cerevisae dynein containing only the head domain requires Pac1, but not Ndl1, to be recruited to astral microtubule tips, indicating that the dynein tail might inhibit Pac1 binding or activity, and that Ndl1 could relieve this inhibition82.
In metazoans LIS1, NUDE and NUDEL are essential for dynein-mediated positioning of the nucleus and the MTOC. LIS1, NUDE and dynein drive nuclear oscillations and properly position the spindle for asymmetric cell division in neural progenitor cells71,83–85. In the resulting newborn neurons, dynein and LIS1–NUDEL drive the migration-coupled movements of the centrosome and the nucleus67,84,86–88. In migrating fibroblasts, dynein and LIS1–NUDEL are required to maintain the position of the MTOC during initial polar ization, as well as the alignment of the mitotic spindle with the substratum89–92. The positioning of the nucleus, MTOC and spindle in metazoans, similarly to spindle positioning in S. cerevisiae, is at least partly dependent on cortically anchored dynein and LIS1–NUDE and LIS1–NUDEL are important for this localization74,92,93. However, the specific molecular interactions by which LIS1–NUDE and LIS1–NUDEL contribute to these processes in metazoans remain largely unknown.
LIS1–NUDE and LIS1–NUDEL are also essential for dynein function at the metazoan kinetochore (FIG. 2), as discussed below. LIS1–NUDE and LIS1–NUDEL help to recruit dynein to kinetochores, possibly by interacting with the RZZ complex (FIG. 2b)) but also through earlier binding of NUDE and NUDEL to the outer kinetochore protein centromere protein F (CENPF)94–97 (BOX 3, FIG. 2).
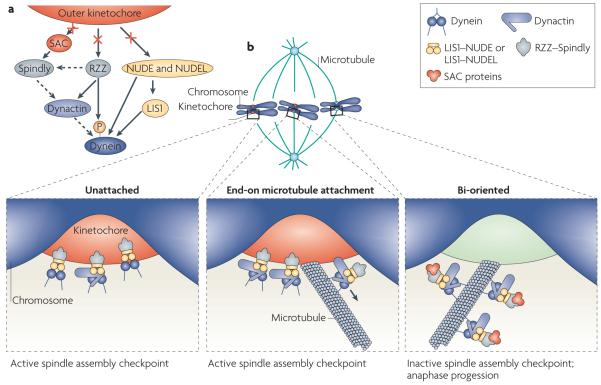
Figure 2. Adaptor proteins couple dynein to multiple functions at the mitotic kinetochore.
a | Interactions with several adaptor proteins (dynactin, the Rod–ZW10–Zwilch (RZZ) complex, Spindly, lissencephaly 1 (LIS1), nuclear distribution protein E (NUDE) and NudE-like (NUDEL)) link dynein to the kinetochore. RZZ, NudE and NudEL interact directly with kinetochore proteins and recruit the other adaptors to the kinetochore94–97,150. Some of these interactions could occur simultaneously, but others seem specific to a distinct mitotic stage. In particular, the RZZ-interacting phosphorylated epitope on the dynein complex is only present before chromosome alignment. The hierarchy of these interactions also varies between systems: dynactin is required for nearly all dynein recruitment, whereas LIS1 is only sometimes required80,114,124,162,163. Spindly is required for dynactin recruitment in human cells and Caenorhabditis elegans embryos, but not Drosophila melanogaster S2 cells114,118,119,123. After chromosome alignment, connections between the kinetochore and the dynein machinery are broken (crossed arrows), and dynein begins transport of the spindle assembly checkpoint (SAC) proteins towards the spindle poles. Solid arrows represent known physical interactions, and dashed arrows represent interactions inferred from localization dependencies. b | dynein localizes to unattached kinetochores by interacting with LIS1–NudE or LIS1–NudEL and dynactin, and possibly through a direct interaction with the RZZ complex. dynein captures astral microtubules and transports chromosomes towards the spindle poles, promoting ‘end-on’ microtubule attachment and chromosome alignment. dynactin, LIS1–NudE, LIS1–NudEL and RZZ–Spindly are important for this process because they link dynein to the kinetochore and perhaps also regulate the motor activity of dynein. After chromosomes form bi-oriented microtubule attachments, dynein transports SAC proteins towards the spindle poles, which helps to silence the checkpoint and allow anaphase progression.
Molecular basis of LIS1, NuDE and NuDEL function
The complexes formed between LIS1 and NUDE or NUDEL are essential for many dynein functions, but the mechanism by which they contribute to dynein activity in the cell is unclear. Although the lack of LIS1, NUDE and NUDEL can affect the localization of dynein, no specific receptors for these proteins (with the notable exception of NUDE- and NUDEL-binding proteins at the kinetochore) are known.
Among the dynein adaptor proteins, one feature unique to LIS1 is that it binds directly to the dynein motor domain, specifically to the AAA1 ATPase sub-domain that is crucial for dynein motility (BOX 3). A recent in vivo study has also shown that localization of the dynein motor domain at the microtubule plus ends depends on LIS1, indicating a functional link between the two proteins82. Recent in vitro studies have provided evidence that LIS1 affects the ATPase activity of the dynein motor domain; however, one study reports a stimulating effect98, whereas the other reports an inhibitory effect that is relieved by NUDEL99. An interesting possibility is that LIS1–NUDE or LIS1–NUDEL might alter the conformation of the dynein motor. As dynein, LIS1–NUDE and LIS1–NUDEL are dimers, LIS1– NUDE or LIS1–NUDEL might crossbridge two AAA1 domains, bringing them closer together. Alternatively, LIS1–NUDE and LIS1–NUDEL have been reported to bind to the LC8 subunit of dynein (BOXES 1,3); if LIS1–NUDE or LIS1–NUDEL can bind LC8 and AAA1 simultaneously, it is possible that they join the motor and tail domains, creating a folded conformation. Although the current evidence indicates that LIS1–NUDE and LIS1–NUDEL probably regulate the ATPase function of the dynein motor domain, further work will be required to better elucidate a coherent model for this regulation and to connect it with dynein function in vivo.
Bicaudal D: a specific metazoan adaptor
Bicaudal D is another multi-purpose adaptor of dynein, but unlike dynactin, LIS1–NUDE and LIS1–NUDEL, it seems to be specific to metazoans. Bicaudal D was first identified in D. melanogaster through genetic screens for genes involved in embryogenesis and pattern formation100,101. Bicaudal D and its highly similar mammalian homologues, Bicaudal D1 and Bicaudal D2, have been best characterized for their involvement in the dynein-mediated localization of mRNA throughout D. melanogaster oogenesis and development76,102–104 and in the transport of Golgi vesicles in mammalian cells105–107, although they also contribute to other dynein-mediated processes, including nuclear positioning, lipid droplet transport and microtubule organization76,108–111. Bicaudal D and its homologues contribute to at least several of these dynein functions as an adaptor that links dynein to its cargos (BOX 4).
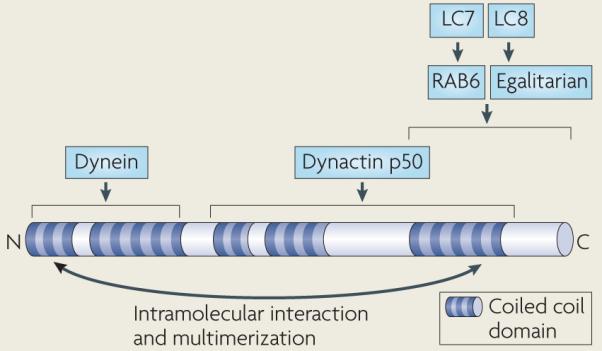
Box 4. Domain structure of Bicaudal D.
Bicaudal D is composed largely of three coiled coil domains that mediate self-association and also possibly an intramolecular interaction (see the figure)104,105,159,160. Multimerization of Bicaudal D could facilitate the assembly of multimotor dynein transport complexes, as it interacts with dynein, dynactin and at least two cargo-specific dynein receptors. The amino-terminal coiled coil of Bicaudal D binds the cytoplasmic dynein complex (through an undetermined dynein subunit), and the middle and carboxy-terminal coiled coils bind dynactin (through the dynactin p50 subunit)105. The C-terminal coiled coil of Bicaudal D binds RAB6 and egalitarian, which, through their interaction with Bicaudal D, function as cargo receptors for dynein in Golgi vesicle transport and in mRNA localization in Drosophila melanogaster, respectively106,112. RAB6 and egalitarian bind directly to dynein light chains, specifically light chain 7 (LC7; also known as roadblock) and LC8, respectively104,161. RAB6 is conserved among metazoans, but egalitarian has been identified only in insects.
Bicaudal D in mRNA transport
In D. melanogaster, Bicaudal D and its binding partner Egalitarian are required for the dynein-mediated transport of mRNA in the oocyte during oogenesis and for the asymmetric localization of many mRNAs throughout embryo development76,102,103. Egalitarian might serve as a dynein receptor on mRNA cargos, binding to mRNA localization sequences directly through its conserved exonuclease domain and binding to dynein through Bicaudal D104,112 (BOX 4). In embryos overexpressing Bicaudal D or Egalitarian, transitions from plus end-directed to minus end-directed transport of individual mRNA transport particles were favoured, suggesting that Bicaudal D–Egalitarian might also activate the dynein motor113.
Bicaudal D in organelle transport
Studies in mammalian tissue culture cells have suggested a role for Bicaudal D in dynein-mediated transport from the ER to the Golgi and within the Golgi105,106. Overexpression of the putative dynein-interacting domain of Bicaudal D disperses the Golgi from its perinuclear position, indicating a disruption of dynein-mediated transport105. Overexpression of full-length Bicaudal D increases the localization of the dynein–dynactin complex to RAB6-positive vesicles and induces the tubulation of these vesicles towards the Golgi, suggesting a hyperactivation of dynein106. In this context, RAB6 probably functions as the membrane receptor for Bicaudal D105 (BOX 4). Interestingly, this interaction is linked to the GTPase cycle of RAB6: GTP-bound RAB6 binds specifically to Bicaudal D in vitro and recruits it to the Golgi in cells, whereas expression of GDP-bound RAB6 leads to the dissociation of Bicaudal D from the Golgi.
How Bicaudal D homologues link dynein to cargo
The studies of Bicaudal D suggest that it serves as a modular link between dynein and cargo; the N-terminal coiled coil of Bicaudal D and its mammalian homologues interacts with dynein, whereas the C-terminal coiled coil binds cargo-specific factors. Two such factors, Egalitarian (for D. melanogaster Bicaudal D) and RAB6 (for mammalian Bicaudal D), have been identified, but perhaps more await discovery. Replacement of the mammalian Bicaudal D with mitochondrial or peroxisomal targeting sequences causes dynein recruitment and relocalization of these organelles near the MTOC (the direction of dynein-mediated transport)107, supporting the idea that the C-terminal coiled coil of Bicaudal D is an attachment point for dynein cargos.
The RZZ complex and Spindly
The RZZ complex in metazoans is composed of three conserved proteins: ROD, ZW10 and Zwilch. Together with another recently discovered metazoan protein, spindly114, RZZ docks dynein and other dynein adaptors to the kinetochore (FIG. 2). ZW10 might also participate in dynein transport during interphase independently from the RZZ complex, whereas so far spindly seems to have a specific role as a dynein adaptor protein at the kinetochore.
Dynein function at the kinetochore
In prometaphase, kinetochore-bound dynein captures the sides of micro-tubules as they pass kinetochores, powering the rapid (~1 μm per second) movement of chromosomes along astral microtubules towards the spindle poles before they finally align at the metaphase plate115,116. Later, these ‘side-on’ dynein–microtubule attachments are replaced by a stronger ‘end-on’ attachment of the microtubule tip to the kinetochore (through the KNL1–MIs12–NDC80 (KMN) complex), which is important for chromosome alignment and separation117 (FIG. 2). RZZ and spindly are required for dynein-based movements of kinetochores, probably by physically linking dynein and the kinetochore115,116 (FIG. 2b). RZZ and spindly might also help to regulate the maturation of attachments between micro-tubules and the kinetochore114,118. In Caenorhabditis elegans embryos, the chromosome alignment and segregation defects in sPDL-1 (the C. elegans spindly homologue) mutants are much more severe than in RZZ mutants118,119. Interestingly, C. elegans embryos with a mutation in both RZZ and sPDL-1 have a milder defect similar to that caused by RZZ mutations alone118. The authors of this study proposed that the RZZ complex can inhibit the binding of the KMN complex to microtubules and that sPDL-1 relieves this inhibition in response to the resistance to microtubule trans location that dynein experiences when the microtubule contacts the kinetochore end-on. Although this model could explain the more severe phenotypes of sPDL-1 mutants and their suppression by mutation of RZZ, a mechanism for RZZ suppression of KMN activity and its relief by sPDL-1 has not yet been described.
Once a sister chromatid pair becomes bi-oriented, dynein transports the spindle assembly checkpoint (SAC) proteins mitotic arrest deficient-like protein 1 (MAD1), MAD2 and BUBR1, as well as RZZ and spindly, away from the kinetochores and towards the spindle poles, thereby silencing the sAC and allowing anaphase to begin120–122 (FIG. 2a). In addition to dynein, RZZ recruits sAC proteins to the kinetochore and might couple them to dynein for transport towards the spindle poles. spindly is also involved in the sAC, although the details vary between species. In C. elegans embryos sPDL-1 is required for the localization of sAC proteins to the kineto chore (through an interaction with MAD-1) and their subsequent removal by dynein118,119 (FIG. 2b). By contrast, in D. melanogaster s2 cells spindly is required only for the removal of sAC proteins from the kinetochore114. In human cells spindly does not seem to contribute to either of these activities123.
In summary, RZZ and spindly have important roles in recruiting dynein to kinetochores in metazoans. These interactions could be highly cooperative, and quantitative differences in the strengths of these interactions might explain species-to-species variation in the effects of their disruption, rather than fundamental differences in mechanism. However, the precise interactions between these outer kinetochore proteins await more direct biochemical dissection and reconstitution.
RZZ might also help to transduce a signal at aligned, bi-oriented chromosomes to initiate dynein-mediated sAC protein transport away from the kinetochore. ZW10 interacts with a phosphorylated epitope on the dynein intermediate chain, which localizes only to un aligned kinetochores124 (BOX 1, FIG. 2b). Phosphorylation reduces binding of the intermediate chain to the dynactin subunit p150, but enhances the interaction of the intermediate chain with ZW10 in vitro. Reduction of interkinetochore tension blocks dephosphorylation of the intermediate chain, suggesting that dephosphoryl ation might be triggered by the increase in tension across kinetochores on bi-orientation. By forcing a switch from ZW10 to dynactin binding in response to kinetochore bi-orientation, the dephosphorylation of the dynein intermediate chain could switch dynein from prometaphase transport of unaligned chromosomes to metaphase transport of checkpoint proteins away from bi-oriented chromosomes.
A possible role for ZW10 in interphase
Although the function of the RZZ complex is restricted to mitosis, ZW10 seems to have an additional role in the secretory pathway during interphase. ZW10 localizes to the ER as part of a complex with the sNARE (soluble-_N_-ethylmaleimide-sensitive factor accessory protein receptor) protein syntaxin 18, which is involved in trafficking between the ER and the Golgi125. RNA interference of ZW10 reduces the amount of dynein associated with the Golgi and specifically reduces the minus end-directed motility of the Golgi, endosomes and lysosomes125,126. These dynein-like loss-of-function phenotypes, combined with the known physical interaction between ZW10 and dynactin, suggest that ZW10 is involved in linking dynein and dynactin to organelles and the kinetochore125–127.
Questions and future directions
In the past decade there have been important advances in understanding intracellular transport by cytoplasmic dynein. The reconstitution of its motility in vitro has allowed great insight into its mechanism, and genetic and biochemical studies have identified some of its cargo-specific receptors. Loss-of function studies have firmly established the essential role of dynein adaptor proteins in a huge range of dynein-mediated processes. However, our understanding of the molecular mechanisms by which these adaptors contribute to dynein activity remains limited. Below, we discuss some of the outstanding fundamental questions regarding the functions of these dynein adaptors, and consider possible strategies towards finding their answers.
Dynein regulators are often referred to as activators because dynein-based motility in cells is repressed in their absence. However, it is possible that dynein requires regulatory proteins to repress its activity instead. several other cytoskeletal motors (for example, kinesin 1, kinesin 2, kinesin 3 and myosin V) are autoinhibited by intramolecular interactions and require external activation for motility2. However, purified dynein seems to be fully active for processive movement, suggesting that dynein inhibition, rather than activation, requires additional factors, and that this inactive-to-active transition is a crucial part of dynein-based cargo transport. Although this has not been directly shown, it seems likely that dynein can adopt a repressed conformation in cells. Indeed, when dynein localizes to microtubule plus ends, its motility is inhibited, either through the repression of its ATPase or microtubule binding activities, or through blocking its access to the microtubule. Dynein is present on vesicles undergoing plus end-directed transport; it is therefore possible that kinesins, which move towards the opposite direction on microtubules, might simply overpower dynein, but a more energetically conservative mechanism would be dynein inhibition. Possible dynein repressors are LIS1, NUDE and NUDEL, which are perfectly positioned to control the activity of the dynein motor by interacting with the AAA1 ATPase domain. LIS1–NUDE, LIS1–NUDEL and dynactin are important for the probable inhibited state of dynein at microtubule plus ends and its subsequent activation. Dynactin forms important contacts with dynein cargos and possibly with kinesin motors as well; this could allow dynactin to transmit an activating signal to dynein when bound to cargo and to coordinate dynein and kinesin activity. Much progress has been made in understanding dynein motility in vitro, but it is still unclear what might turn dynein on and off. in vitro reconstitution of dynein with the ubiquitous dynein regulatory proteins (LIS1, NUDE or NUDEL and dynactin) might provide clues to this mechanism.
Another important unanswered question is how dynein links to its cargos. In the simplest model for motor-based transport in the cell, a motor diffuses through the cytoplasm and initiates cargo transport when it encounters the appropriate receptors on its cargos. At least in the case of dynein, the process of initiating cargo transport seems to be more complicated and to involve additional steps. In particular, localization of dynein and dynactin to microtubule plus ends might be an obligatory preliminary step to the initiation of some types of dynein transport. Dynein might use association with polymerizing microtubules to probe the cytoplasm for cargo (such as an organelle or the cell cortex) (FIG. 1). This mechanism could allow the simultaneous delivery of several motors and adaptors clustered at the micro-tubule tip. In addition, the microtubule tip could serve as a hub for the interactions of dynein and its adaptors with the many signalling molecules that are present at the plus ends of microtubules. The sequence of interactions that lead to the association of dynein with the microtubule plus end and to its eventual release and transfer to cargo is not well understood; a more precise determination of this sequence would allow a better understanding of its contribution to dynein function in the cell.
Dynein cargo binding also seems to proceed through a regulated series of interactions, perhaps reflecting a proofreading mechanism that functions to dock dynein only at specified locations. In particular, several small GTPases and their regulators interact with dynactin and Bicaudal D to facilitate dynein recruitment at specific cargos. Individual small GTPase family members act at specific subcellular compartments (for example, late endosomes or _trans_-Golgi vesicles), and their GTPase state and thus conformation is linked to the life cycle and direction of trafficking of these compartments. Through their nucleotide-sensitive contacts with dynein adaptors, small GTPases could provide a highly specific link between membrane vesicles and dynein. subsequent interaction of dynactin with the spectrin coat that is found on many organelles could then provide a more stable platform for dynein transport.
What strategies might best advance our understanding of how dynein functions with its regulatory proteins? Genetic methods have contributed greatly to the identification of the general dynein adaptors and specific dynein cargo receptors; however, our inventory is almost certainly incomplete. Relative to the wide array of cargos that dynein transports, only a few cargo-specific receptors for dynein have been identified and it is likely that many more dynein receptors remain to be discovered. Recent innovation in high-throughput microscopy and image analysis could facilitate the visual screens that could identify the unknown dynein receptors and regulatory factors coupling dynein to its cargos.
Although specific receptors for dynein have not been identified for most of its cargos, a better understanding of how the known dynein regulators work is an important and feasible goal. One possible path towards defining the molecular function of dynein regulators is through in vitro reconstitution. Although dynactin was discovered through such an approach23,24, few studies have focused on reconstituting dynein-based transport. The few dynein cargos for which receptors and their contacts with dynein and its general adaptors are known (for example, mRNA particles with Egalitarian and Bicaudal D) could provide a feasible starting point for the reconstitution of dynein cargo transport. In such a system, dynein and its adaptors could be mutated, their levels manipulated or components added sequentially, and their single molecule behaviour observed. such information would provide insight into the dynamic assembly of these complexes and the specific mechanistic contribution of each factor.
Within cells, recently developed high spatial precision fluorescence methods128–131 could help to define the composition and architecture of dynein motor supercomplexes and detect remodelling of this architecture during different stages of transport. Finally, a structural understanding of dynein and its cofactors with the resolution provided by electron microscopy or crystallography, although a challenging goal owing to the large sizes of these complexes, would provide important insight into the molecular mechanism by which they function.
Acknowledgements
We thank S. Reck-Peterson, A. Carter, N. Bradshaw and E. Griffis for helpful discussions and editorial comments and apologize to those authors whose work we could not cite owing to space limitations. This work was supported by the National Institutes of Health (grant number 38499, R.D.V.), the National Science Foundation (J.R.K) and the Howard Hughes Medical Institute.
Glossary
Axoneme
The bundled microtubule structure at the centre of eukaryotic cilia and flagella. Coordinated binding and release of the axonemal dyneins slides the microtubules relative to each other. This causes the axoneme to bend and drives ciliary and flagellar beating. Axonemes function as tracks for the motors involved in intraflagellar transport.
AAA+ ATPase
(ATPase associated with various cellular activities). A large family of ATPases, the functions of which are diverse, with many involved in the conformational remodelling of other proteins and complexes. AAA+ ATPases contain one or two characteristic ATP-binding domains, with additional function-specific domains. These AAA modules usually function as hexameric rings.
Coiled coil
A common structural motif in proteins, consisting of two or more α-helices that twist around each other and bury hydrophobic residues in the interface and form an overall rod-like structure.
Optical trap
An instrument that uses a focused laser beam to hold, move and monitor the position of microscopic dielectric objects. Optical traps can be used to measure the force production and nanoscale movements of molecular motors that have been conjugated to dielectric objects such as latex beads.
Protofilament
The end-to-end arrangement of tubulin dimers along the long axis of microtubules. Microtubules most commonly are composed of thirteen protofilaments.
Astral microtubule
A microtubule that radiates from the mitotic spindle poles to the cell cortex. Astral microtubules are involved in the positioning and alignment of the spindle poles during cell division.
Hypha
The branching filament that is the main mode of vegetative growth for filamentous fungi. During hyphal growth, hyphae elongate from their tips and position newly formed nuclei along the length of the new growth.
Cytoskeleton-associated protein Gly-rich
(Cap-Gly). A domain found in several microtubule-associated proteins, which binds the EEY/F motif found at the carboxyl terminus of α-tubulin and several microtubule plus end-associated proteins.
GTPase-activating protein
A protein that stimulates the intrinsic activity of a GTPase to hydrolyse GTP to GDP.
CopII coat
A complex consisting of seC13, seC23, seC24 and seC31. This coat complex functions in anterograde transport from the endoplasmic reticulum to the Golgi.
GTPase effector
A protein that binds specifically to the GTP-bound conformation of a GTPase.
Kinetochore
A large multiprotein complex that assembles onto the centromere of the chromosome and links it to the microtubules of the mitotic spindle. The kinetochore is also a signalling centre for many of the proteins that control the progression of mitosis.
WD40 repeat
A motif of 40 amino acids that contains a Trp and Asp dipeptide at its carboxyl terminus. This domain is found in many functionally diverse proteins and often mediates protein–protein interactions.
Centrosome
The principal microtubule-organizing centre of animal cells, an organelle that contains the centrioles and that anchors the minus end of microtubules.
Spindle assembly checkpoint
The mitotic signalling pathway that prevents chromosome separation until all kinetochores have formed microtubule attachments. The proteins involved in this pathway have to be localized at the kinetochore to ensure chromosome segregation, and their physical removal from attached kinetochores by dynein silences their signalling and allows chromosome separation to begin.
Footnotes
DATABASES UniProtKB: http://www.uniprot.org βIII spectrin | Bicaudal d | CLIP170 | EB1 | Egalitarian | LIS1 |Ndl1 | NudE | NudE | NudEL | NudF | Pac1 | RAB6 | ROd |Spindly | ZW10 | Zwilch
FURTHER INFORMATION Ronald D. Vale’s homepage: http://valelab.ucsf.edu ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Karki S, Holzbaur EL. Cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr. Opin. Cell Biol. 1999;11:45–53. doi: 10.1016/s0955-0674(99)80006-4. [DOI] [PubMed] [Google Scholar]
- 2.Vale RD. The molecular motor toolbox for intracellular transport. Cell. 2003;112:467–480. doi: 10.1016/s0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- 3.Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- 4.Vaughan KT, Vallee RB. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150. J. Cell Biol. 1995;131:1507–1516. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karki S, Holzbaur EL. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J. Biol. Chem. 1995;270:28806–28811. doi: 10.1074/jbc.270.48.28806. [DOI] [PubMed] [Google Scholar]
- 6.Tai AW, Chuang JZ, Bode C, Wolfrum U, Sung CH. Rhodopsin’s carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex-1. Cell. 1999;97:877–887. doi: 10.1016/s0092-8674(00)80800-4. [DOI] [PubMed] [Google Scholar]
- 7.Purohit A, Tynan SH, Vallee R, Doxsey SJ. Direct interaction of pericentrin with cytoplasmic dynein light intermediate chain contributes to mitotic spindle organization. J. Cell Biol. 1999;147:481–492. doi: 10.1083/jcb.147.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farkasovsky M, Kuntzel H. Cortical Num1p interacts with the dynein intermediate chain Pac11p and cytoplasmic microtubules in budding yeast. J. Cell Biol. 2001;152:251–262. doi: 10.1083/jcb.152.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ha J, et al. A neuron-specific cytoplasmic dynein isoform preferentially transports TrkB signaling endosomes. J. Cell Biol. 2008;181:1027–1039. doi: 10.1083/jcb.200803150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Khan S, Sheetz MP. Single cytoplasmic dynein molecule movements: characterization and comparison with kinesin. Biophys. J. 1995;69:2011–2023. doi: 10.1016/S0006-3495(95)80071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mallik R, Carter BC, Lex SA, King SJ, Gross SP. Cytoplasmic dynein functions as a gear in response to load. Nature. 2004;427:649–652. doi: 10.1038/nature02293. [DOI] [PubMed] [Google Scholar]
- 12.Reck-Peterson SL, et al. Single-molecule analysis of dynein processivity and stepping behavior. Cell. 2006;126:335–348. doi: 10.1016/j.cell.2006.05.046. Using recombinant dynein from S. cerevisiae, the authors directly observe the motion of single molecules of dynein for the first time, showing that it is a processive motor that requires dimerization for processivity. Their data strongly suggest that alternating steps of the dynein heads drive motility.
- 13.Gennerich A, Vale RD. Walking the walk: how kinesin and dynein coordinate their steps. Curr. Opin. Cell Biol. 2009;21:59–67. doi: 10.1016/j.ceb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gennerich A, Carter AP, Reck-Peterson SL, Vale RD. Force-induced bidirectional stepping of cytoplasmic dynein. Cell. 2007;131:952–965. doi: 10.1016/j.cell.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nan X, Sims PA, Xie XS. Organelle tracking in a living cell with microsecond time resolution and nanometer spatial precision. Chemphyschem. 2008;9:707–712. doi: 10.1002/cphc.200700839. [DOI] [PubMed] [Google Scholar]
- 16.Sims PA, Xie XS. Probing dynein and kinesin stepping with mechanical manipulation in a living cell. Chemphyschem. 2009;13:1511–1516. doi: 10.1002/cphc.200900113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixit R, Ross JL, Goldman YE, Holzbaur EL. Differential regulation of dynein and kinesin motor proteins by tau. Science. 2008;319:1086–1089. doi: 10.1126/science.1152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross JL, Shuman H, Holzbaur EL, Goldman YE. Kinesin and dynein-dynactin at intersecting microtubules: motor density affects dynein function. Biophys. J. 2008;94:3115–3125. doi: 10.1529/biophysj.107.120014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toba S, Watanabe TM, Yamaguchi-Okimoto L, Toyoshima YY, Higuchi H. Overlapping hand-over-hand mechanism of single molecular motility of cytoplasmic dynein. Proc. Natl Acad. Sci. USA. 2006;103:5741–5745. doi: 10.1073/pnas.0508511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welte MA, Gross SP, Postner M, Block SM, Wieschaus EF. Developmental regulation of vesicle transport in Drosophila embryos: forces and kinetics. Cell. 1998;92:547–557. doi: 10.1016/s0092-8674(00)80947-2. The authors observe the motility and force generation of dynein- and kinesin-transported lipid droplets in living D. melanogaster embryos. Their data suggest that dynein and kinesin activities are coupled and that the protein Klarsicht might be involved in this coupling.
- 21.Laib JA, Marin JA, Bloodgood RA, Guilford WH. The reciprocal coordination and mechanics of molecular motors in living cells. Proc. Natl Acad. Sci. USA. 2009;106:3190–3195. doi: 10.1073/pnas.0809849106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross JL, Ali MY, Warshaw DM. Cargo transport: molecular motors navigate a complex cytoskeleton. Curr. Opin. Cell Biol. 2008;20:41–47. doi: 10.1016/j.ceb.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gill SR, et al. Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J. Cell Biol. 1991;115:1639–1650. doi: 10.1083/jcb.115.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroer TA, Sheetz MP. Two activators of microtubule-based vesicle transport. J. Cell Biol. 1991;115:1309–1318. doi: 10.1083/jcb.115.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroer TA. Dynactin. Annu. Rev. Cell. Dev. Biol. 2004;20:759–779. doi: 10.1146/annurev.cellbio.20.012103.094623. [DOI] [PubMed] [Google Scholar]
- 26.Adames NR, Cooper JA. Microtubule interactions with the cell cortex causing nuclear movements in Saccharomyces cerevisiae. J. Cell Biol. 2000;149:863–874. doi: 10.1083/jcb.149.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee WL, Kaiser MA, Cooper JA. The offloading model for dynein function: differential function of motor subunits. J. Cell Biol. 2005;168:201–207. doi: 10.1083/jcb.200407036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee WL, Oberle JR, Cooper JA. The role of the lissencephaly protein Pac1 during nuclear migration in budding yeast. J. Cell Biol. 2003;160:355–364. doi: 10.1083/jcb.200209022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheeman B, et al. Determinants of S. cerevisiae dynein localization and activation: implications for the mechanism of spindle positioning. Curr. Biol. 2003;13:364–372. doi: 10.1016/s0960-9822(03)00013-7. [DOI] [PubMed] [Google Scholar]
- 30.Kardon JR, Reck-Peterson SL, Vale RD. Regulation of the processivity and intracellular localization of Saccharomyces cerevisiae dynein by dynactin. Proc. Natl Acad. Sci. USA. 2009;106:5669–5674. doi: 10.1073/pnas.0900976106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang X, Beckwith SM, Morris NR. Cytoplasmic dynein is involved in nuclear migration in Aspergillus nidulans. Proc. Natl Acad. Sci. USA. 1994;91:2100–2104. doi: 10.1073/pnas.91.6.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiang X, Zuo W, Efimov VP, Morris NR. Isolation of a new set of Aspergillus nidulans mutants defective in nuclear migration. Curr. Genet. 1999;35:626–630. doi: 10.1007/s002940050461. [DOI] [PubMed] [Google Scholar]
- 33.Fischer R, Timberlake WE. Aspergillus nidulans apsA (anucleate primary sterigmata) encodes a coiled-coil protein required for nuclear positioning and completion of asexual development. J. Cell Biol. 1995;128:485–498. doi: 10.1083/jcb.128.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiang X, Han G, Winkelmann DA, Zuo W, Morris NR. Dynamics of cytoplasmic dynein in living cells and the effect of a mutation in the dynactin complex actin-related protein Arp1. Curr. Biol. 2000;10:603–606. doi: 10.1016/s0960-9822(00)00488-7. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Li S, Fischer R, Xiang X. Accumulation of cytoplasmic dynein and dynactin at microtubule plus ends in Aspergillus nidulans is kinesin dependent. Mol. Biol. Cell. 2003;14:1479–1488. doi: 10.1091/mbc.E02-08-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi I, Wilde A, Mal TK, Ikura M. Structural basis for the activation of microtubule assembly by the EB1 and p150 complex. Mol. Cell. 2005;19:449–460. doi: 10.1016/j.molcel.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 37.Honnappa S, et al. Key interaction modes of dynamic +TIP networks. Mol. Cell. 2006;23:663–671. doi: 10.1016/j.molcel.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Weisbrich A, et al. Structure-function relationship of CAP-Gly domains. Nature Struct. Mol. Biol. 2007;14:959–967. doi: 10.1038/nsmb1291. [DOI] [PubMed] [Google Scholar]
- 39.Hayashi I, Plevin MJ, Ikura M. CLIP170 autoinhibition mimics intermolecular interactions with p150 or EB1. Nature Struct. Mol. Biol. 2007;14:980–981. doi: 10.1038/nsmb1299.References 36–39 use structural and biochemical methods to define the interactions between CAP-Gly domains and both the zinc knuckle motif found in CLIP170 and the acidic C-terminal motif found in CLIP170, EB1 and α-tubulin. Competition and cooperation between these moderate affinity interactions are probably important to microtubule plus end tracking and its regulation.
- 40.Valetti C, et al. Role of dynactin in endocytic traffic: effects of dynamitin overexpression and colocalization with CLIP-170. Mol. Biol. Cell. 1999;10:4107–4120. doi: 10.1091/mbc.10.12.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lansbergen G, et al. Conformational changes in CLIP-170 regulate its binding to microtubules and dynactin localization. J. Cell Biol. 2004;166:1003–1014. doi: 10.1083/jcb.200402082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaughan PS, Miura P, Henderson M, Byrne B, Vaughan KT. A role for regulated binding of p150 to microtubule plus ends in organelle transport. J. Cell Biol. 2002;158:305–319. doi: 10.1083/jcb.200201029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watson P, Forster R, Palmer KJ, Pepperkok R, Stephens DJ. Coupling of ER exit to microtubules through direct interaction of COPII with dynactin. Nature Cell Biol. 2005;7:48–55. doi: 10.1038/ncb1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puls I, et al. Mutant dynactin in motor neuron disease. Nature Genet. 2003;33:455–456. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- 45.Lai C, et al. The G59S mutation in p150 causes dysfunction of dynactin in mice. J. Neurosci. 2007;27:13982–13990. doi: 10.1523/JNEUROSCI.4226-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levy JR, et al. A motor neuron disease-associated mutation in p150 perturbs dynactin function and induces protein aggregation. J. Cell Biol. 2006;172:733–745. doi: 10.1083/jcb.200511068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chevalier-Larsen ES, Wallace KE, Pennise CR, Holzbaur EL. Lysosomal proliferation and distal degeneration in motor neurons expressing the G59S mutation in the p150 subunit of dynactin. Hum. Mol. Genet. 2008;17:1946–1955. doi: 10.1093/hmg/ddn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tokito MK, Howland DS, Lee VM, Holzbaur EL. Functionally distinct isoforms of dynactin are expressed in human neurons. Mol. Biol. Cell. 1996;7:1167–1180. doi: 10.1091/mbc.7.8.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim H, et al. Microtubule binding by dynactin is required for microtubule organization but not cargo transport. J. Cell Biol. 2007;176:641–651. doi: 10.1083/jcb.200608128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dixit R, Levy JR, Tokito M, Ligon LA, Holzbaur EL. Regulation of dynactin through the differential expression of p150 isoforms. J. Biol. Chem. 2008;283:33611–33619. doi: 10.1074/jbc.M804840200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lomakin AJ, et al. CLIP-170-dependent capture of membrane organelles by microtubules initiates minus-end directed transport. Dev. Cell. 2009;17:323–333. doi: 10.1016/j.devcel.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holleran EA, et al. βIII spectrin binds to the Arp1 subunit of dynactin. J. Biol. Chem. 2001;276:36598–36605. doi: 10.1074/jbc.M104838200. [DOI] [PubMed] [Google Scholar]
- 53.Muresan V, et al. Dynactin-dependent, dynein-driven vesicle transport in the absence of membrane proteins: a role for spectrin and acidic phospholipids. Mol. Cell. 2001;7:173–183. doi: 10.1016/s1097-2765(01)00165-4. [DOI] [PubMed] [Google Scholar]
- 54.De Matteis MA, Morrow JS. Spectrin tethers and mesh in the biosynthetic pathway. J. Cell Sci. 2000;113:2331–2343. doi: 10.1242/jcs.113.13.2331. [DOI] [PubMed] [Google Scholar]
- 55.Johansson M, et al. Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150, ORP1L, and the receptor βlll spectrin. J. Cell Biol. 2007;176:459–471. doi: 10.1083/jcb.200606077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rocha N, et al. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 and late endosome positioning. J. Cell Biol. 2009;185:1209–1225. doi: 10.1083/jcb.200811005. Through its conformational change in response to the cholesterol content of the membrane, ORPL1 regulates the association of dynein and dynactin with late endosomes and thus regulates their transport in response to cellular conditions.
- 57.Kumar S, Zhou Y, Plamann M. Dynactin-membrane interaction is regulated by the C-terminal domains of p150. EMBO Rep. 2001;2:939–944. doi: 10.1093/embo-reports/kve202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee IH, Kumar S, Plamann M. Null mutants of the neurospora actin-related protein 1 pointed-end complex show distinct phenotypes. Mol. Biol. Cell. 2001;12:2195–2206. doi: 10.1091/mbc.12.7.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blangy A, Arnaud L, Nigg EA. Phosphorylation by p34cdc2 protein kinase regulates binding of the kinesin-related motor HsEg5 to the dynactin subunit p150. J. Biol. Chem. 1997;272:19418–19424. doi: 10.1074/jbc.272.31.19418. [DOI] [PubMed] [Google Scholar]
- 60.Deacon SW, et al. Dynactin is required for bidirectional organelle transport. J. Cell Biol. 2003;160:297–301. doi: 10.1083/jcb.200210066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berezuk MA, Schroer TA. Dynactin enhances the processivity of kinesin-2. Traffic. 2007;8:124–129. doi: 10.1111/j.1600-0854.2006.00517.x. [DOI] [PubMed] [Google Scholar]
- 62.Waterman-Storer CM, Karki S, Holzbaur EL. The p150 component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1) Proc. Natl Acad. Sci. USA. 1995;92:1634–1638. doi: 10.1073/pnas.92.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.King SJ, Schroer TA. Dynactin increases the processivity of the cytoplasmic dynein motor. Nature Cell Biol. 2000;2:20–24. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- 64.Culver-Hanlon TL, Lex SA, Stephens AD, Quintyne NJ, King SJ. A microtubule-binding domain in dynactin increases dynein processivity by skating along microtubules. Nature Cell Biol. 2006;8:264–270. doi: 10.1038/ncb1370. [DOI] [PubMed] [Google Scholar]
- 65.Moore JK, Sept D, Cooper JA. Neurodegeneration mutations in dynactin impair dynein-dependent nuclear migration. Proc. Natl Acad. Sci. USA. 2009;106:5147–5152. doi: 10.1073/pnas.0810828106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dobyns WB, Reiner O, Carrozzo R, Ledbetter DH. Lissencephaly. A human brain malformation associated with deletion of the LIS1 gene located at chromosome 17p13. JAMA. 1993;270:2838–2842. doi: 10.1001/jama.270.23.2838. [DOI] [PubMed] [Google Scholar]
- 67.Vallee RB, Tsai JW. The cellular roles of the lissencephaly gene LIS1, and what they tell us about brain development. Genes Dev. 2006;20:1384–1393. doi: 10.1101/gad.1417206. [DOI] [PubMed] [Google Scholar]
- 68.Xiang X, Roghi C, Morris NR. Characterization and localization of the cytoplasmic dynein heavy chain in Aspergillus nidulans. Proc. Natl Acad. Sci. USA. 1995;92:9890–9894. doi: 10.1073/pnas.92.21.9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Geiser JR, et al. Saccharomyces cerevisiae genes required in the absence of the CIN8-encoded spindle motor act in functionally diverse mitotic pathways. Mol. Biol. Cell. 1997;8:1035–1050. doi: 10.1091/mbc.8.6.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Efimov VP, Morris NR. The LIS1-related NUDF protein of Aspergillus nidulans interacts with the coiled-coil domain of the NUDE/RO11 protein. J. Cell Biol. 2000;150:681–688. doi: 10.1083/jcb.150.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feng Y, et al. LIS1 regulates CNS lamination by interacting with mNudE, a central component of the centrosome. Neuron. 2000;28:665–679. doi: 10.1016/s0896-6273(00)00145-8. [DOI] [PubMed] [Google Scholar]
- 72.Niethammer M, et al. NUDEL is a novel Cdk5 substrate that associates with LIS1 and cytoplasmic dynein. Neuron. 2000;28:697–711. doi: 10.1016/s0896-6273(00)00147-1. [DOI] [PubMed] [Google Scholar]
- 73.Sasaki S, et al. A LIS1/NUDEL/cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron. 2000;28:681–696. doi: 10.1016/s0896-6273(00)00146-x. [DOI] [PubMed] [Google Scholar]
- 74.Smith DS, et al. Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian LIS1. Nature Cell Biol. 2000;2:767–775. doi: 10.1038/35041000. [DOI] [PubMed] [Google Scholar]
- 75.Liang Y, et al. Nudel functions in membrane traffic mainly through association with LIS1 and cytoplasmic dynein. J. Cell Biol. 2004;164:557–566. doi: 10.1083/jcb.200308058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Swan A, Nguyen T, Suter B. Drosophila Lissencephaly-1 functions with Bic-D and dynein in oocyte determination and nuclear positioning. Nature Cell Biol. 1999;1:444–449. doi: 10.1038/15680. [DOI] [PubMed] [Google Scholar]
- 77.Han G, et al. The Aspergillus cytoplasmic dynein heavy chain and NUDF localize to microtubule ends and affect microtubule dynamics. Curr. Biol. 2001;11:719–724. doi: 10.1016/s0960-9822(01)00200-7. [DOI] [PubMed] [Google Scholar]
- 78.Li J, Lee WL, Cooper JA. NudEL targets dynein to microtubule ends through LIS1. Nature Cell Biol. 2005;7:686–690. doi: 10.1038/ncb1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carvalho P, Gupta ML, Jr, Hoyt MA, Pellman D. Cell cycle control of kinesin-mediated transport of Bik1 (CLIP-170) regulates microtubule stability and dynein activation. Dev. Cell. 2004;6:815–829. doi: 10.1016/j.devcel.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 80.Coquelle FM, et al. LIS1, CLIP-170’s key to the dynein/dynactin pathway. Mol. Cell. Biol. 2002;22:3089–3102. doi: 10.1128/MCB.22.9.3089-3102.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Efimov VP. Roles of NUDE and NUDF proteins of Aspergillus nidulans: insights from intracellular localization and overexpression effects. Mol. Biol. Cell. 2003;14:871–888. doi: 10.1091/mbc.E02-06-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Markus SM, Punch JJ, Lee WL. Motor- and tail-dependent targeting of dynein to microtubule plus ends and the cell cortex. Curr. Biol. 2009;19:196–205. doi: 10.1016/j.cub.2008.12.047. This study in S. cerevisiae shows that, unlike full length dynein, LIS1-mediated microtubule plus end targeting of the dynein motor domain does not require NUDEL, suggesting that NUDEL is required only to prime dynein for LIS1 activity.
- 83.Feng Y, Walsh CA. Mitotic spindle regulation by Nde1 controls cerebral cortical size. Neuron. 2004;44:279–293. doi: 10.1016/j.neuron.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 84.Shu T, et al. Ndel1 operates in a common pathway with LIS1 and cytoplasmic dynein to regulate cortical neuronal positioning. Neuron. 2004;44:263–277. doi: 10.1016/j.neuron.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 85.Siller KH, Doe CQ. LIS1/dynactin regulates metaphase spindle orientation in Drosophila neuroblasts. Dev. Biol. 2008;319:1–9. doi: 10.1016/j.ydbio.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tanaka T, et al. LIS1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. J. Cell Biol. 2004;165:709–721. doi: 10.1083/jcb.200309025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsai JW, Chen Y, Kriegstein AR, Vallee RB. LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages. J. Cell Biol. 2005;170:935–945. doi: 10.1083/jcb.200505166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsai JW, Bremner KH, Vallee RB. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nature Neurosci. 2007;10:970–979. doi: 10.1038/nn1934. Through observations of migrating neurons in situ, the authors make the surprising observation that the nucleus frequently lags the movement of the centrosome, rather than being tightly coupled as had been thought previously. Dynein and LIS1 contribute to the continuous centrosome movement and to saltatory nuclear movements through separate mechanisms.
- 89.Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–463. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 90.Dujardin DL, et al. A role for cytoplasmic dynein and LIS1 in directed cell movement. J. Cell Biol. 2003;163:1205–1211. doi: 10.1083/jcb.200310097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shen Y, et al. Nudel binds Cdc42GAP to modulate Cdc42 activity at the leading edge of migrating cells. Dev. Cell. 2008;14:342–353. doi: 10.1016/j.devcel.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 92.Faulkner NE, et al. A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nature Cell Biol. 2000;2:784–791. doi: 10.1038/35041020. [DOI] [PubMed] [Google Scholar]
- 93.Cockell MM, Baumer K, Gonczy P. lis-1 is required for dynein-dependent cell division processes in C. elegans embryos. J. Cell Sci. 2004;117:4571–4582. doi: 10.1242/jcs.01344. [DOI] [PubMed] [Google Scholar]
- 94.Yang Z, et al. Silencing mitosin induces misaligned chromosomes, premature chromosome decondensation before anaphase onset, and mitotic cell death. Mol. Cell. Biol. 2005;25:4062–4074. doi: 10.1128/MCB.25.10.4062-4074.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Soukoulis V, et al. Cytoplasmic LEK1 is a regulator of microtubule function through its interaction with the LIS1 pathway. Proc. Natl Acad. Sci. USA. 2005;102:8549–8554. doi: 10.1073/pnas.0502303102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liang Y, et al. Nudel modulates kinetochore association and function of cytoplasmic dynein in M phase. Mol. Biol. Cell. 2007;18:2656–2666. doi: 10.1091/mbc.E06-04-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vergnolle MA, Taylor SS. Cenp-F links kinetochores to Ndel1/Nde1/LIS1/dynein microtubule motor complexes. Curr. Biol. 2007;17:1173–1179. doi: 10.1016/j.cub.2007.05.077. [DOI] [PubMed] [Google Scholar]
- 98.Mesngon MT, et al. Regulation of cytoplasmic dynein ATPase by LIS1. J. Neurosci. 2006;26:2132–2139. doi: 10.1523/JNEUROSCI.5095-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamada M, et al. LIS1 and NDEL1 coordinate the plus-end-directed transport of cytoplasmic dynein. EMBO J. 2008;27:2471–2483. doi: 10.1038/emboj.2008.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mohler J, Wieschaus EF. Dominant maternal-effect mutations of Drosophila melanogaster causing the production of double-abdomen embryos. Genetics. 1986;112:803–822. doi: 10.1093/genetics/112.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Steward R, Nusslein-Volhard C. The genetics of the dorsal-Bicaudal-D region of Drosophila melanogaster. Genetics. 1986;113:665–678. doi: 10.1093/genetics/113.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bullock SL, Ish-Horowicz D. Conserved signals and machinery for RNA transport in Drosophila oogenesis and embryogenesis. Nature. 2001;414:611–616. doi: 10.1038/414611a. [DOI] [PubMed] [Google Scholar]
- 103.Delanoue R, Davis I. Dynein anchors its mRNA cargo after apical transport in the Drosophila blastoderm embryo. Cell. 2005;122:97–106. doi: 10.1016/j.cell.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 104.Navarro C, Puthalakath H, Adams JM, Strasser A, Lehmann R. Egalitarian binds dynein light chain to establish oocyte polarity and maintain oocyte fate. Nature Cell Biol. 2004;6:427–435. doi: 10.1038/ncb1122. [DOI] [PubMed] [Google Scholar]
- 105.Hoogenraad CC, et al. Mammalian Golgi-associated Bicaudal-D2 functions in the dynein-dynactin pathway by interacting with these complexes. EMBO J. 2001;20:4041–4054. doi: 10.1093/emboj/20.15.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Matanis T, et al. Bicaudal-D regulates COPI-independent Golgi-ER transport by recruiting the dynein-dynactin motor complex. Nature Cell Biol. 2002;4:986–992. doi: 10.1038/ncb891. [DOI] [PubMed] [Google Scholar]
- 107.Hoogenraad CC, et al. Bicaudal D induces selective dynein-mediated microtubule minus end-directed transport. EMBO J. 2003;22:6004–6015. doi: 10.1093/emboj/cdg592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Larsen KS, Xu J, Cermelli S, Shu Z, Gross SP. BicaudalD actively regulates microtubule motor activity in lipid droplet transport. PLoS One. 2008;3:e3763. doi: 10.1371/journal.pone.0003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pare C, Suter B. Subcellular localization of Bic-D::GFP is linked to an asymmetric oocyte nucleus. J. Cell Sci. 2000;113:2119–2127. doi: 10.1242/jcs.113.12.2119. [DOI] [PubMed] [Google Scholar]
- 110.Fumoto K, Hoogenraad CC, Kikuchi A. GSK-3β-regulated interaction of BICD with dynein is involved in microtubule anchorage at centrosome. EMBO J. 2006;25:5670–5682. doi: 10.1038/sj.emboj.7601459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oh J, Steward R. Bicaudal-D is essential for egg chamber formation and cytoskeletal organization in drosophila oogenesis. Dev. Biol. 2001;232:91–104. doi: 10.1006/dbio.2001.0170. [DOI] [PubMed] [Google Scholar]
- 112.Dienstbier M, Boehl F, Li X, Bullock SL. Egalitarian is a selective RNA-binding protein linking mRNA localization signals to the dynein motor. Genes Dev. 2009;23:1546–1558. doi: 10.1101/gad.531009. The authors show that the Bicaudal D binding partner Egalitarian directly binds localization signals on mRNA, and they propose a general framework for the contribution of Bicaudal D to dynein cargo binding.
- 113.Bullock SL, Nicol A, Gross SP, Zicha D. Guidance of bidirectional motor complexes by mRNA cargoes through control of dynein number and activity. Curr. Biol. 2006;16:1447–1452. doi: 10.1016/j.cub.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 114.Griffis ER, Stuurman N, Vale RD. Spindly, a novel protein essential for silencing the spindle assembly checkpoint, recruits dynein to the kinetochore. J. Cell Biol. 2007;177:1005–1015. doi: 10.1083/jcb.200702062. spindly is identified as a kinetochore-specific regulator of cytoplasmic dynein.
- 115.Yang Z, Tulu US, Wadsworth P, Rieder CL. Kinetochore dynein is required for chromosome motion and congression independent of the spindle checkpoint. Curr. Biol. 2007;17:973–980. doi: 10.1016/j.cub.2007.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li Y, Yu W, Liang Y, Zhu X. Kinetochore dynein generates a poleward pulling force to facilitate congression and full chromosome alignment. Cell Res. 2007;17:701–712. doi: 10.1038/cr.2007.65. [DOI] [PubMed] [Google Scholar]
- 117.Tanaka TU, Desai A. Kinetochore-microtubule interactions: the means to the end. Curr. Opin. Cell Biol. 2008;20:53–63. doi: 10.1016/j.ceb.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gassmann R, et al. A new mechanism controlling kinetochore-microtubule interactions revealed by comparison of two dynein-targeting components: SPDL-1 and the Rod/Zwilch/Zw10 complex. Genes Dev. 2008;22:2385–2399. doi: 10.1101/gad.1687508. The authors characterize the C. elegans homologue of spindly and propose a model for how spindly, RZZ and dynein regulate the maturation of microtubule– kinetochore attachments.
- 119.Yamamoto TG, Watanabe S, Essex A, Kitagawa R. SPDL-1 functions as a kinetochore receptor for MDF-1 in Caenorhabditis elegans. J. Cell Biol. 2008;183:187–194. doi: 10.1083/jcb.200805185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Howell BJ, et al. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J. Cell Biol. 2001;155:1159–1172. doi: 10.1083/jcb.200105093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wojcik E, et al. Kinetochore dynein: its dynamics and role in the transport of the Rough deal checkpoint protein. Nature Cell Biol. 2001;3:1001–1007. doi: 10.1038/ncb1101-1001.References 120 and 121 present the initial evidence that dynein transports sAC proteins away from the kinetochore, thus silencing the checkpoint, and that the RZZ complex is involved in this process.
- 122.Basto R, et al. In vivo dynamics of the rough deal checkpoint protein during Drosophila mitosis. Curr. Biol. 2004;14:56–61. doi: 10.1016/j.cub.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 123.Chan YW, et al. Mitotic control of kinetochore-associated dynein and spindle orientation by human Spindly. J. Cell Biol. 2009;185:859–874. doi: 10.1083/jcb.200812167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Whyte J, et al. Phosphorylation regulates targeting of cytoplasmic dynein to kinetochores during mitosis. J. Cell Biol. 2008;183:819–834. doi: 10.1083/jcb.200804114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hirose H, et al. Implication of ZW10 in membrane trafficking between the endoplasmic reticulum and Golgi. EMBO J. 2004;23:1267–1278. doi: 10.1038/sj.emboj.7600135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Varma D, Dujardin DL, Stehman SA, Vallee RB. Role of the kinetochore/cell cycle checkpoint protein ZW10 in interphase cytoplasmic dynein function. J. Cell Biol. 2006;172:655–662. doi: 10.1083/jcb.200510120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Starr DA, Williams BC, Hays TS, Goldberg ML. ZW10 helps recruit dynactin and dynein to the kinetochore. J. Cell Biol. 1998;142:763–774. doi: 10.1083/jcb.142.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bates M, Huang B, Dempsey GT, Zhuang X. Multicolor super-resolution imaging with photo-switchable fluorescent probes. Science. 2007;317:1749–1753. doi: 10.1126/science.1146598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang B, Wang W, Bates M, Zhuang X. Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science. 2008;319:810–813. doi: 10.1126/science.1153529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Betzig E, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–1645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 131.Shtengel G, et al. Interferometric fluorescent super-resolution microscopy resolves 3D cellular ultrastructure. Proc. Natl Acad. Sci. USA. 2009;106:3125–3130. doi: 10.1073/pnas.0813131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Reck-Peterson SL, Vale RD. Molecular dissection of the roles of nucleotide binding and hydrolysis in dynein’s AAA domains in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA. 2004;101:1491–1495. doi: 10.1073/pnas.2637011100. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 133.Kon T, Nishiura M, Ohkura R, Toyoshima YY, Sutoh K. Distinct functions of nucleotide-binding/ hydrolysis sites in the four AAA modules of cytoplasmic dynein. Biochemistry. 2004;43:11266–11274. doi: 10.1021/bi048985a. [DOI] [PubMed] [Google Scholar]
- 134.Cho C, Reck-Peterson SL, Vale RD. Regulatory ATPase sites of cytoplasmic dynein affect processivity and force generation. J. Biol. Chem. 2008;283:25839–25845. doi: 10.1074/jbc.M802951200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gee MA, Heuser JE, Vallee RB. An extended microtubule-binding structure within the dynein motor domain. Nature. 1997;390:636–639. doi: 10.1038/37663. [DOI] [PubMed] [Google Scholar]
- 136.Burgess SA, Walker ML, Sakakibara H, Knight PJ, Oiwa K. Dynein structure and power stroke. Nature. 2003;421:715–718. doi: 10.1038/nature01377. [DOI] [PubMed] [Google Scholar]
- 137.Carter AP, et al. Structure and functional role of dynein’s microtubule-binding domain. Science. 2008;322:1691–1695. doi: 10.1126/science.1164424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kon T, et al. Helix sliding in the stalk coiled coil of dynein couples ATPase and microtubule binding. Nature Struct. Mol. Biol. 2009;16:325–333. doi: 10.1038/nsmb.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gibbons IR, et al. The affinity of the dynein microtubule-binding domain is modulated by the conformation of its coiled-coil stalk. J. Biol. Chem. 2005;280:23960–23965. doi: 10.1074/jbc.M501636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Roberts AJ, et al. AAA+ ring and linker swing mechanism in the dynein motor. Cell. 2009;136:485–495. doi: 10.1016/j.cell.2008.11.049. The authors use negative stain electron microscopy to map the position of the dynein linker domain and microtubule-binding stalk relative to the AAA ring in different nucleotide states and propose a mechanism for dynein motility based on movement of the linker across the face of the AAA ring.
- 141.Tai CY, Dujardin DL, Faulkner NE, Vallee RB. Role of dynein, dynactin, and CLIP-170 interactions in LIS1 kinetochore function. J. Cell Biol. 2002;156:959–968. doi: 10.1083/jcb.200109046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stehman SA, Chen Y, McKenney RJ, Vallee RB. NudE and NudEL are required for mitotic progression and are involved in dynein recruitment to kinetochores. J. Cell Biol. 2007;178:583–594. doi: 10.1083/jcb.200610112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Holleran EA, Tokito MK, Karki S, Holzbaur EL. Centractin (ARP1) associates with spectrin revealing a potential mechanism to link dynactin to intracellular organelles. J. Cell Biol. 1996;135:1815–1829. doi: 10.1083/jcb.135.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schafer DA, Gill SR, Cooper JA, Heuser JE, Schroer TA. Ultrastructural analysis of the dynactin complex: an actin-related protein is a component of a filament that resembles F-actin. J. Cell Biol. 1994;126:403–412. doi: 10.1083/jcb.126.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Eckley DM, et al. Analysis of dynactin subcomplexes reveals a novel actin-related protein associated with the arp1 minifilament pointed end. J. Cell Biol. 1999;147:307–320. doi: 10.1083/jcb.147.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Garces JA, Clark IB, Meyer DI, Vallee RB. Interaction of the p62 subunit of dynactin with Arp1 and the cortical actin cytoskeleton. Curr. Biol. 1999;9:1497–1500. doi: 10.1016/s0960-9822(00)80122-0. [DOI] [PubMed] [Google Scholar]
- 147.Vierula PJ, Mais JM. A gene required for nuclear migration in Neurospora crassa codes for a protein with cysteine-rich, LIM/RING-like domains. Mol. Microbiol. 1997;24:331–340. doi: 10.1046/j.1365-2958.1997.3461704.x. [DOI] [PubMed] [Google Scholar]
- 148.Imai H, Narita A, Schroer TA, Maeda Y. Two-dimensional averaged images of the dynactin complex revealed by single particle analysis. J. Mol. Biol. 2006;359:833–839. doi: 10.1016/j.jmb.2006.03.071. [DOI] [PubMed] [Google Scholar]
- 149.Moore JK, Li J, Cooper JA. Dynactin function in mitotic spindle positioning. Traffic. 2008;9:510–527. doi: 10.1111/j.1600-0854.2008.00710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kops GJ, et al. ZW10 links mitotic checkpoint signaling to the structural kinetochore. J. Cell Biol. 2005;169:49–60. doi: 10.1083/jcb.200411118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Quintyne NJ, et al. Dynactin is required for microtubule anchoring at centrosomes. J. Cell Biol. 1999;147:321–334. doi: 10.1083/jcb.147.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.King SJ, Brown CL, Maier KC, Quintyne NJ, Schroer TA. Analysis of the dynein-dynactin interaction in vitro and in vivo. Mol. Biol. Cell. 2003;14:5089–5097. doi: 10.1091/mbc.E03-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kim MH, et al. The structure of the N-terminal domain of the product of the lissencephaly gene LIS1 and its functional implications. Structure. 2004;12:987–998. doi: 10.1016/j.str.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 154.Tarricone C, et al. Coupling PAF signaling to dynein regulation: structure of LIS1 in complex with PAF-acetylhydrolase. Neuron. 2004;44:809–821. doi: 10.1016/j.neuron.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 155.Derewenda U, et al. The structure of the coiled-coil domain of ndel1 and the basis of its interaction with LIS1, the causal protein of miller-dieker lissencephaly. Structure. 2007;15:1467–1481. doi: 10.1016/j.str.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 156.Yan X, et al. Human Nudel and NudE as regulators of cytoplasmic dynein in poleward protein transport along the mitotic spindle. Mol. Cell. Biol. 2003;23:1239–1250. doi: 10.1128/MCB.23.4.1239-1250.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hebbar S, et al. LIS1 and Ndel1 influence the timing of nuclear envelope breakdown in neural stem cells. J. Cell Biol. 2008;182:1063–1071. doi: 10.1083/jcb.200803071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hattori M, Adachi H, Tsujimoto M, Arai H, Inoue K. Miller-Dieker lissencephaly gene encodes a subunit of brain platelet-activating factor acetylhydrolase. Nature. 1994;370:216–218. doi: 10.1038/370216a0. [DOI] [PubMed] [Google Scholar]
- 159.Oh J, Baksa K, Steward R. Functional domains of the Drosophila bicaudal-D protein. Genetics. 2000;154:713–724. doi: 10.1093/genetics/154.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Stuurman N, et al. Interactions between coiled-coil proteins: Drosophila lamin Dm0 binds to the bicaudal-D protein. Eur. J. Cell Biol. 1999;78:278–287. doi: 10.1016/s0171-9335(99)80061-2. [DOI] [PubMed] [Google Scholar]
- 161.Wanschers BF, et al. A role for the Rab6B Bicaudal-D1 interaction in retrograde transport in neuronal cells. Exp. Cell Res. 2007;313:3408–3420. doi: 10.1016/j.yexcr.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 162.Siller KH, Serr M, Steward R, Hays TS, Doe CQ. Live imaging of Drosophila brain neuroblasts reveals a role for LIS1/dynactin in spindle assembly and mitotic checkpoint control. Mol. Biol. Cell. 2005;16:5127–5140. doi: 10.1091/mbc.E05-04-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dzhindzhev NS, Rogers SL, Vale RD, Ohkura H. Distinct mechanisms govern the localisation of Drosophila CLIP-190 to unattached kinetochores and microtubule plus-ends. J. Cell Sci. 2005;118:3781–3790. doi: 10.1242/jcs.02504. [DOI] [PubMed] [Google Scholar]