The FMR1 Gene and Fragile X-Associated Tremor/Ataxia Syndrome (original) (raw)
. Author manuscript; available in PMC: 2015 Feb 8.
Published in final edited form as: Am J Med Genet B Neuropsychiatr Genet. 2009 Sep 5;0(6):782–798. doi: 10.1002/ajmg.b.30910
Abstract
The CGG-repeat present in the 5′UTR of the FMR1 gene is unstable upon transmission to the next generation. The repeat is up to 55 CGGs long in the normal population. In fragile X patients, a repeat length exceeding 200 CGGs (full mutation: FM) generally leads to methylation of the repeat and the promoter region, which is accompanied by silencing of the FMR1 gene. The gene product FMRP is involved in regulation of transport and translation of certain mRNA in the dendrite, thereby affecting synaptic plasticity. This is central to learning and memory processes. The absence of FMRP seen in FM is the cause of the mental retardation seen in fragile X patients. The premutation (PM) is defined as 55–200 CGGs. Female PM carriers are at risk of developing primary ovarian insufficiency. Recently it was discovered that elderly PM carriers might develop a progressive neurodegenerative disorder called fragile X-associated tremor/ataxia syndrome. Although arising from the mutations in the same gene, distinct mechanisms lead to fragile X syndrome (absence of FMRP) and FXTAS (toxic RNA gain of function). The pathogenic mechanisms thought to underlie these disorders are discussed, with a specific emphasis on FXTAS. This review gives insight on the implications of all possible repeat length categories seen in fragile X families.
Keywords: FMR1, FMRP, fragile X, FXTAS, CGG repeat instability, RNA gain-of-function
THE FMR1 GENE AND THE UNSTABLE (CGG)N-REPEAT
Fragile X syndrome (FXS) has been recognized as the most common inherited form of mental retardation [Glass, 1991]. Its name refers to a fragile site, which was discovered on the long arm of the X chromosome of four mentally retarded patients [Lubs, 1969]. This fragile site was later found to be inducible when culturing cells in folic acid-deficient medium. This facilitated the establishment of a link between expression of the fragile site (at Xq27) and the clinical phenotype [Sutherland, 1977; Turner et al., 1980]. Cloning of the gene responsible for FXS, FMR1 (fragile X mental retardation 1), revealed the presence of an unstable expanded (CGG)n in the 5′ UTR. In FXS this repeat exceeds 200 trinucleotides (full mutation: FM) [Oberlé et al., 1991; Richards et al., 1991; Verkerk et al., 1991]. FMR1 consists of 17 exons, spanning a 38 kb region [Eichler et al., 1993]. The (CGG)n in the FMR1 gene is considered a “dynamic mutation;” the phenomenon that a trinucleotide repeat becomes dramatically unstable once it exceeds a certain threshold length [Richards and Sutherland, 1992]. Depending on the length of the (CGG)n, different clinical outcomes can develop.
Analysis of repeat length variation in the normal population showed a range of allele sizes between 6 and 54 CGGs [Fu et al., 1991], with an average of 29–30 repeats [Dombrowski et al., 2002]. Alleles of between 45 and 54 CGGs form a distinct category. They were named gray zone alleles, when it was appreciated that these alleles are more likely to be unstable upon transmission, than are (CGG)n shorter than 45 trinucleotides [Fu et al., 1991; Zhong et al., 1996]. Gray zone alleles frequently contain a long and uninterrupted (CGG)n [Zhong et al., 1996]. The longest tract of uninterrupted (CGG)n appears to determine instability and susceptibility to disease, whereby the presence of a pure CGG-tract of more than 33 triplets appeared to greatly enhance the chance of unstable transmission. Thus, AGG interruptions might stabilize a (CGG)n [Eichler et al., 1994]. This could be through prevention of formation of stable secondary RNA structures such as hairpins, as has been shown to occur in in vitro studies [Napierala et al., 2005]. Hairpins might decrease the efficiency of FMRP translation initi-ation [Chen et al., 2003]. AGG interruptions, through influencing RNA structure, predominantly determine the extent of instability of long normal and short permutation (PM) alleles [Napierala et al., 2005].
The remaining repeat range of 55–200 CGGs has been defined as the PM [Maddalena et al., 2001]. Initially, it was thought that the only risk associated with the PM was for the (CGG)n to expand to an FM allele, as alleles in this size range are quite unstable upon maternal transmission [Nolin et al., 2003]. The risk of expansion from a female PM carrier to an FM in her progeny increases with repeat length [Bat et al., 1997]. In few cases, a contraction of maternal origin is seen. Inheritance of a (CGG)n of paternal origin shows a less consistent pattern, as expansions, contractions and stable transmissions occur. Expansion of a PM to an FM is restricted to maternal transmission. Also, daughters of a male FM carrier, affected with fragile X, never show clinical or cytogenetical signs of FXS. This was explained by the finding that FM males only carry PM alleles in their sperm [Reyniers et al., 1993]. Thus, both allele size and the parent’s sex determine the risk of expansion from parent to offspring [Nolin et al., 1996].
One in 813 males and one in 259 females of the general population were described to carry the PM [Rousseau et al., 1995; Dombrowski et al., 2002]. A recent study in 40,000 women in Israel revealed a prevalence of 1 in 154. No difference in carrier frequency was seen between women with and without family history of mental retardation and developmental abnormalities [Berkenstadt et al., 2007]. However, it is now known that different ethnic groups show a different prevalence, for example it is less common in Asian populations [Tzeng et al., 2005], while it is more prevalent in Mediterranean groups [Pesso et al., 2000; Toledano-Alhadef et al., 2001].
Early in situ hybridization studies in mice revealed that FMR1 mRNA is abundant and widespread in early embryogenesis. Later in embryonic development a specific expression pattern develops, with high expression mainly in brain, testis and ovary. Adult mice also showed high expression in brain and testis. No FMR1 expression was seen in mature ovary, despite the high levels in the fetal ovary, when proliferation of oogonia takes place. Thus, the FMR1 gene might have a function during germ cell proliferation in both sexes [Bächner et al., 1993a,b; Hinds et al., 1993]. FMR1 mRNA expression in human fetuses was high in the nervous system, but also in some non-neuronal tissues such as cartilage, hepatocytes, the spinal cord and the retina [Abitbol et al., 1993; Agulhon et al., 1999].
In fragile X patients, the (CGG)n of over 200 trinucleotides (FM) and the upstream promoter region of the FMR1 gene are usually hypermethylated [Oberlé et al., 1991; Verkerk et al., 1991; Sutcliffe et al., 1992]. As a result, gene transcription is silenced, thus the gene product fragile X mental retardation protein, FMRP, is not produced. The complete lack of FMRP in neurons is the cause of the mental retardation seen in fragile X patients [Verheij et al., 1993]. The functions of FMRP and the consequences of the absence of FMRP in FXS are discussed in more detail below. About 1 in 4,000 males and 1 in 6,000 females have fragile X-linked mental retardation. The clinical spectrum is however much broader than mental retardation, involving learning disabilities and emotional disturbances [Hagerman, 2006].
TIMING OF REPEAT INSTABILITY
When trying to elucidate the mechanism of repeat instability, timing of the event is a major aspect that remains surrounded by question marks. The fact that repeat length changes between generations, tells us that instability occurs at some point between gametogenesis in the parent and early embryogenesis. Attempts have been made to gain insight into what happens around fertilization, although availability of human material is limited. Remarkable observations that expansions to a fragile X FM occur only upon maternal transmission of a PM allele, and that daughters of FM males are clinically and cytogenetically normal, were explained when it was observed that male fragile X patients only have PM alleles in their sperm [Reyniers et al., 1993]. Two models have been proposed to explain this finding (Fig. 1).
FIG. 1.
(CGG)n length and FMR1 expression and clinical outcome. This figure shows the consequences of the different repeat length categories on transcription, translation and clinical phenotype.
First, the prezygotic model predicts that an expansion of PM to FM could occur during maternal meiosis (Fig. 2). This means that the FM must contract to a PM in gametes of male offspring. PM gametes might have some selectional advantage either due to the presence of FMRP, or against the presence of an expanded CGG-repeat. However, FMRP is not required for spermatogenesis [Bakker et al., 1994; Meijer et al., 1994]. FMRP expression might still be beneficial to the spermatogonia as they mature. A selective advantage for cells expressing FMRP was indeed seen in cell culture studies [Rousseau et al., 1991].
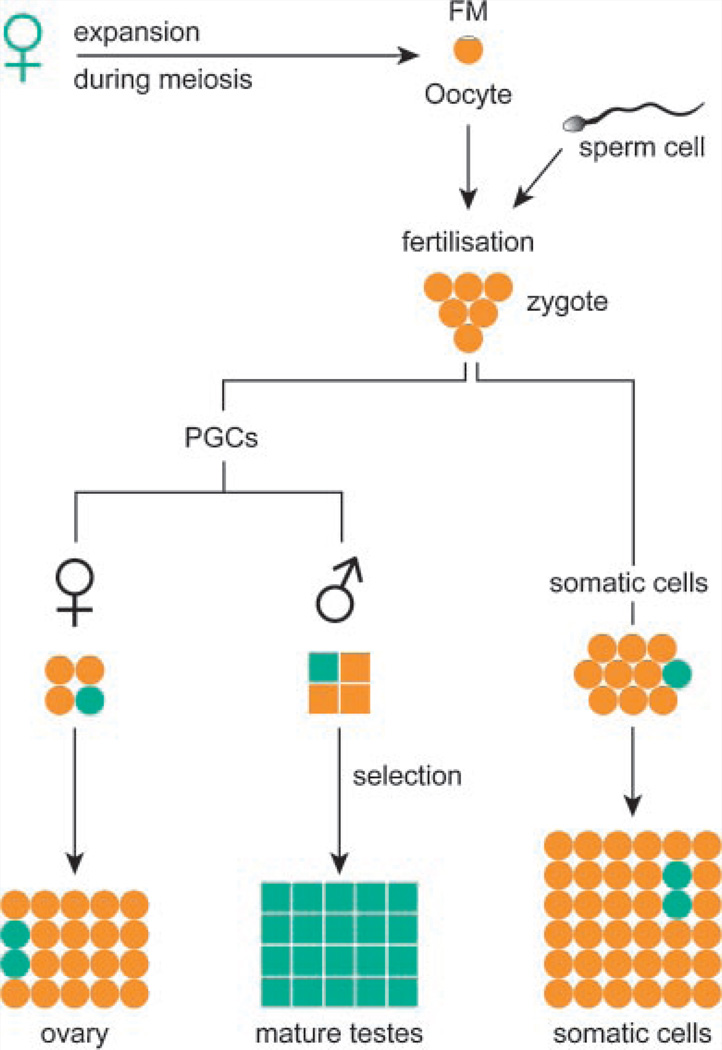
FIG. 2.
The prezygotic model of expansion of the fragile X mutation. This model assumes that an expansion of a maternal PM to an FM takes place during meiosis. The fertilized oocyte carries an FM allele. After separation from the embryo proper, the primordial germ cells (PGCs) have an FM. Some alleles will contract to PMs. To explain why FMs are only transmitted through females, some selection must exist agains FMs in the male germ line during spermatogenesis. In the mature testes, PM alleles predominate. In somatic cells and the female germ line, this selection did not take place. Cells with a PM are shown in green and cells with an FM are depicted in orange.
In 13-week-old FM fetuses, no PM alleles were detected in testes, while in 17-week-old FM fetuses some primordial germ cells expressing FMRP were observed. This suggests that contraction from FM to PM occurs in the maturation process of the testis, after the initial replication event [Malter et al., 1997]. Paternally derived alleles have been shown to undergo rapid demethylation in murine zygotes, before the first round of replication, while CpG methylation is maintained in maternally derived alleles [Reik et al., 2003]. CpG methylation appears to stabilize the DNA. Hence, paternally derived alleles could be prone to deletions upon demethylation [Nichol Edamura and Pearson, 2005]. Furthermore, FMs are responsible for a delay in replication of the FMR1 gene during the cell cycle [Hansen et al., 1993; Samadashwily et al., 1993]. The large number of cell cycles associated with spermatogenesis might enhance a selectional effect [Ashley and Sherman, 1995]. Thus, primordial germ cells with a PM might have a proliferative advantage, thereby overgrowing FM cells. However, if multiple contraction events can take place, it is puzzling why only one distinct PM band is mostly seen in sperm of FM men [Reyniers et al., 1993].
The other model considers a postzygotic expansion, after separation of the germ line. It assumes that the FM allele has never been present in male or female gametes [Devys et al., 1992; Wöhrle et al., 1993]. However, oocytes of a fetal female FM carrier had only FM alleles [Malter et al., 1997]. It is however unknown whether oocytes of a female PM carrier bear PM or FM alleles. A postzygotic expansion mechanism occurring only for a PM allele on the maternal X-chromosome, would require an imprinting mechanism to distinguish it from a paternally derived PM, which would not expand [Reyniers et al., 1993; Trottier et al., 1993].
Based on a simulation study and analysis of tissues of affected fetuses, Moutou et al., 1997 favor a prezygotic model, whereby a selection mechanism accounts for the sole presence of PM alleles in sperm of male offspring. However, they cannot exclude a postzygotic model in which a transition occurs at the early morula stage [Moutou et al., 1997]. Naturally, a final conclusion can only be drawn after analysis of oocytes in ovaries from an embryo carrying a PM allele. Unless the embryo suffers from a different detrimental defect, this material is not available for obvious reasons. Also, oocytes from an adult PM carrier would provide useful information. However, the most likely reason for an ovarium to become available is when a female PM carrier suffers from POF. In this case, naturally, almost no oocytes are present anymore. Expanded (CGG)n knock-in mouse models have been developed [Bontekoe et al., 2001; Entezamet al., 2007], that show intergenerational repeat instability [Brouwer et al., 2007; Entezamet al., 2007]. These models will be valuable in answering remaining questions.
TRANSCRIPTIONAL SILENCING OF THE FMR1 GENE
Many studies have addressed the question of how methylation of CpGs in the FMR1 gene leads to gene silencing. Local chromatin changes as a result of methylation mediate indirect mechanisms, while a direct effect is the inhibition of binding of transcription factors to the promoter [Chiurazzi et al., 1999; Coffee et al., 2002]. Methyl binding proteins (such as MeCP2) recognize methylated CpG residues, upon which a multiprotein complex is recruited. This protein complex includes histone deacetylases (HDACs) [Jones et al., 1998; Nan et al., 1998]. HDACs remove acetyl groups from lysines of histone H3 and H4 amino terminal tails, which result in increased condensation of the chromatin (heterochromatin), which is associated with inactive gene regions [Razin, 1998]. The FMR1 gene normally shows acetylated histones H3 and H4, while cells of patients with fragile X show reduced acetylation [Coffee et al., 1999]. Experiments in cells of fragile X patients showed that methylation and deacetylation indeed cooperate in silencing chromatin domains, when histone hyperacetylating drugs could synergistically potentiate reactivation of the FMR1 gene induced by a demethylating agent [Chiurazzi et al., 1998, 1999]. Further analysis of epigenetic modification in three different regions of the FMR1 gene revealed that epigenetic codes relevant to the transcription of the gene are concentrated in the 5′ UTR region [Tabolacci et al., 2005].
Histones can also be modified in ways other than acetylation, for example, by the addition or removal of phosphate or methyl groups [Turner, 2002]. Methylation of lysines 4 and 9 of histone 3 (H3K4 and H3K9, respectively) has been looked at intensively and it became clear that methylation of H3K4 is associated with a transcriptionally active state, while methylation of H3K9correlateswith transcriptional inactivation [Grewal and Moazed, 2003; Lachner et al., 2003]. Methylated H3K9 has been suggested to recruit histone methyl transferases, associated with HDACs, which then results in histone deacetylation [Cheutin et al., 2003]. However, studies in cell lines suggest that methylation of H3K9 and partial deacetylation, normally associated with heterochromatin, can coexist with H3K4 methylation, which is correlated with active FMR1 transcription [Tabolacci et al., 2005].
Two brothers with expanded (CGG)n in the FM range have been described, who were spared from the fragile X phenotype. Although cytogenetical analysis revealed a fragile site, the FMR1 promoter was unmethylated and they did produce FMR1 mRNA and FMRP. This indicates that inactivation of FMR1, due to methylation and not the expansion itself, is the cause of the symptoms seen in FXS [Smeets et al., 1995]. Characterization of a human embryonic stem cell line derived from a pre-implantation embryo with FM range repeat showed that the FMR1 gene was unmethylated and expressed, despite the presence of an FM allele. More specifically, it displayed the features of active chromatin [Eiges et al., 2007]. Thus, these results confirm that the (CGG)n by itself is not sufficient to cause transcriptional silencing of FMR1 [Sutcliffe et al., 1992]. Upon differentiation (by inducing formation of teratomas in immunodeficient mice) this ES cell line underwent partial transcriptional silencing. Differentiation lead to reduced H3 tail acetylation, and H3K9 became methylated, while FMR1 mRNA levels decreased. However, the 5′ UTR of FMR1 was still hypomethylated. Thus, the authors concluded that de novo methylation of the upstream regulatory region of FMR1 follows transcriptional silencing, where-by the chromatin changes take place relatively early during differentiation. DNA methylation appears to contribute to the maintenance, rather than to the induction of transcriptional inactivation in somatic cells in patients with fragile X [Eiges et al., 2007]. This role for DNA methylation has previously been described with regard to X-inactivation in females [Lock et al., 1987].
However, many questions remain to be elucidated about the precise molecular mechanism by which FMR1 transcription is regulated, of which the answers might prove valuable for developing therapeutical strategies involving reactivation of the FMR1 gene in FXS. Contrary to in FXS, PM carriers show enhanced transcription of the FMR1 gene, which is discussed in more detail below.
THE GENE PRODUCT FMRP
FMRP is an RNA-binding protein (RNA-BP), as it bears two KH-domains and an RGG-box; both are sequences with RNA-binding capacities [Ashley et al., 1993; Siomi et al., 1993; Darnell et al., 2001]. Among many other mRNAs, FMRP can bind its own message with high affinity in vitro [Ashley et al., 1993]. Furthermore, a nuclear localization signal and a nucleur export signal have been identified, suggesting that FMRP shuttles between the nucleus and the cytoplasm [Eberhart et al., 1996; Fridell et al., 1996; Sittler et al., 1996; Feng et al., 1997b]. Eighty-five percent of cellular FMRP is present on actively translating polyribosomes, underscoring the role of FMRP as an RNA-BP [Feng et al., 1997a]. Ribonucleoprotein (RNP) particle domains were identified within FMRP. RNA was shown to bind in stoichiometric ratios, suggesting that each FMRP molecule bears two RNA-binding sites [Ashley et al., 1993] A minority of FMRP is found in the nucleus, either at the nucleopore, or associated with the nucleolus [Willemsen et al., 1996; Feng et al., 1997a; Bakker et al., 2000].
Immunocytochemical studies in mice revealed that FMRP is ubiquitously expressed up until day 14 of embryonic development. In later embryonic development a more specific pattern arises with tissues of ectodermal origin (brain, ganglia, hair follicles, sensory cells, adrenal medulla) and the gonads (mesodermal origin) showing the highest expression levels [De Diego Otero et al., 2000]. FMRP expression in human embryos (3–7 weeks) was consistent with the murine expression pattern, showing highest expression in brain and testes [Abitbol et al., 1993; Devys et al., 1993; Tamanini et al., 1997; Agulhon et al., 1999]. In adult human brain, FMRP is present in high quantities in neurons, while little or no FMRP is detected in glia and oligodendrocytes [Abitbol et al., 1993; Devys et al., 1993; Hinds et al., 1993; Wang et al., 2004; Pacey and Doering, 2007]. High levels of FMRP expression in human tissue coincide with clinical involvement in FXS [Devys et al., 1993; Malter et al., 1997; Tamanini et al., 1997; Khandjian et al., 1998]. The absence of evident neuropathological abnormalities of fragile X fetuses suggests that the FMR1 gene product FMRP is not crucial in early stages of development of the nervous system [Agulhon et al., 1999].
After identification of the FMR1 gene research has focused on the cellular function of FMRP. The RNA-binding capacities, association with ribosomes and the cytoplasmic localization in dendrites pointed towards a function for FMRP in mRNA transport or translation. The RGG-box has been identified as the motif of specific mRNA interaction. The RNA motif consists of a set of four quartets of purines that form an intramolecular stem-loop structure termed a G-quartet [Darnell et al., 2001; Schaeffer et al., 2001]. mRNAs containing a G-quartet have been identified that are potential targets for FMRP, including important neuronal proteins like microtubule-associated protein 1B (MAP1B) and semaphorin [Brown et al., 2001; Darnell et al., 2001; Miyashiro et al., 2003].
mRNA transport and translation in dendrites is important for neuronal function, including modulation of synaptic plasticity. This is essential in memory consolidation and learning [Kiebler and DesGroseillers, 2000; Steward, 2002]. Altered spine morphology (long and thin dendritic spines) has been observed in post-mortem brains of fragile X patients [Rudelli et al., 1985; Hinton et al., 1991; Irwin et al., 2001] and in FMR1 KO mice [Comery et al., 1997; Nimchinsky et al., 2001; De Vrij et al., 2008]. Target mRNAs of FMRP that are involved in synaptic function or dendritic growth have been identified, which is very interesting in light of the aberrant spine maturation [Darnell et al., 2001]. FMRP was found to strongly inhibit translation of several mRNAs in vitro, through inhibition of the assembly of the 80S ribosomal complexes on target mRNA. The second KH-domain appears crucial in this process, as the I304N mutant FMRP, with a mutation in this RNA-binding domain, failed to inhibit translation [Laggerbauer et al., 2001]. Thus, a model has been proposed that FMRP binds specific mRNAs and mediates the targeting of these transcripts into the dendrite. During transport they remain translationally inactive until appropriate synaptic input allows translation. Thus, FMRP might have a role in transport and/or translational efficiency of specific mRNAs at the synapse. More specifically, It has been hypothesized that FMRP may link transport and translational control of dendritic mRNAs [Willemsen et al., 2004]. FMRP was postulated to associate directly with the dendritic non-messenger RNA BC1, which can form an RNA duplex with a number of mRNAs that are potential targets for FMRP via base pairing to this mRNA. This suggests that FMRP acts through BC1, thereby determining the specificity of FMRP function [Zalfa et al., 2003]. However, this function for BC1 is now under debate [Bagni, 2008; Iacoangeli et al., 2008a,b].
The absence of FMRP could lead to translational dysregulation of a subset of mRNAs at the synapse. Translational dysregulation of mRNAs normally associated with FMRP is proposed to be the underlying cause of mental retardation in fragile X [Brown et al., 2001; Darnell et al., 2001; Willemsen et al., 2004]. fxS patients do not present with gross brain abnormalities. However, both FXS patients and FMR1 KO mice show an immature phenotype of dendritic protrusions, which partly form the synaptic connections between neurons. Much of what we know about the function of FMRP, stems from studies in FMR1 KO mice. FMR1 KO mice suffer from mild cognitive impairment [Bakker et al., 1994; Van Dam et al., 2000] and they show macroorchidism [Bakker et al., 1994]. Concerning the cause of the cognitive impairment, evidence supporting the metabotropic glutamate receptor (mGluR) theory on FXS has accumulated [Bear et al., 2004]. Three groups of mGluR exist and stimulation of group 1 mGluRs (mGluR1 and mGluR5) can lead to long-term depression (LTD). As predicted by the mGluR theory, the internalization of AMPA receptors (one type of glutamate receptor), which is triggered by mGluR5 stimulation [Snyder et al., 2001], is exaggerated in FMR1 KO mice. Also, enhanced hippocampal LTD was found in knockout mice [Huber et al., 2002; Bear et al., 2004]. It is hypothesized that FMRP normally plays a role in inhibiting the translation of several local mRNAs that are involved in the regulation of AMPA receptor internalization. An S6K1-PP2A signaling pathway has now been described to regulate FMRP function by controlling the phosphorylation status of FMRP, thereby inducing or blocking protein synthesis [Narayanan et al., 2008]. Recently it was shown that FMRP deficient dendrites indeed show aberrant AMPA receptor trafficking resulting in a significantly reduced number of AMPA receptors at the plasma membrane [Nakamoto et al., 2007]. The number of AMPA receptors in the postsynaptic density is correlated with protrusion shape. Thus, this could explain the immature protrusion morphology that has been found in different brain areas of both fragile X patients [Hinton et al., 1991] and FMR1 KO mice [Comery et al., 1997; Nimchinsky et al., 2001; Koekkoek et al., 2005; Grossman et al., 2006]. Interestingly, a significant rescue of protrusion morphology in primary hippocampal mouse neurons of FMR1 KO mice was seen after treatment with an mGluR5 antagonist [De Vrij et al., 2008].
CLINICAL SYMPTOMS ASSOCIATED WITH THE PREMUTATION
As mentioned above, it was long thought that carriership of the PM was not associated with clinical problems, other than the risk of expansion to an FM upon transmitting the PM allele to the next generation. However, a small subgroup of PM carriers was reported to have mild learning disabilities, developmental delay, obsessive thinking, ADHD, autism, and in some cases mental retardation and social phobias, or anxiety disorder [Hagerman and Hagerman, 2002, 2004; Farzin et al., 2006].
Primary Ovarian Insufficiency
Furthermore, it became apparent that 20% of female PM carriers manifests premature ovarian failure (POF: cessation of menstruation at or before 40 years of age) [Sherman, 2000]. Women with the PM, even if they are still cycling, have higher levels of follicle stimulation hormone (FSH) than do healthy women [Welt et al., 2004]. It has recently been suggested that anti-Müllerian hormone (AMH) may be a better marker of ovarian decline. AMH is expressed only in growing follicles, thus serves as a marker for the size of the primordial follicle pool in women. Indeed, female PM carriers, with (CGG)n lengths beyond 70, had lower AMH levels than did female PM carriers with (CGG)n shorter than 70 nucleotides. Thus, lower AMH levels are suggestive of early ovarian decline in women with (CGG)>70 [Rohr et al., 2008]. Penetrance and age of onset of POF, as well as the increase of FSH levels, correlate with (CGG)n length [Sullivan et al., 2004]. However, a non-linear relationship has been described for age at menopause and PM size, in which PMs in the mid-size range are at greatest risk for POF, while larger repeat tracts are associated with a lower risk [Ennis et al., 2005]. It has been proposed that primary ovarian insufficiency (POI) is a more accurate term for the disorder, to describe the broad range of clinical manifestations associated with what used to be classified as POF [Wittenberger et al., 2007].
Fragile X-Associated Tremor/Ataxia
Over the past few years, it has become apparent that PM carriers are also at risk of developing a progressive neurodegenerative disorder, which is clinically and neuropathologically entirely distinct from FXS [Hagerman et al., 2001; Jacquemont et al., 2003, 2004a]. In 2001, five male PM carriers were described who suffered from progressive action tremor, causing executive function deficits, cerebellar dysfunction, cognitive decline, and Parkinsonism associated with generalized brain atrophy. Also autonomic dysfunction and peripheral neuropathy were reported [Hagerman et al., 2001]. Since it had already been established that PM carriers have elevated FMR1 mRNA levels in their lymphocytes, but only somewhat reduced FMRP levels [Tassone et al., 2000b], it was then hypothesized that the progressive nervous system degeneration as seen in those five male PM carriers might be the result of the elevated FMR1 mRNA levels [Hagerman et al., 2001]. The new syndrome was called fragile X-associated tremor/ataxia syndrome (FXTAS) [Jacquemont et al., 2003]. However, not all PM carriers develop FXTAS; of male PM carriers ascertained through family members with FXS, more than a third of men older than 50 years showed both tremor and ataxia [Jacquemont et al., 2004b]. Penetrance increases with age, to at least 50% in men aged 70–90 years. It has now been suggested that penetrance increases with CGG repeat length. For instance; most people with FXTAS had (CGG)n with over 70CGGs, while in the general population only 22% of PM alleles are of this size or larger [Jacquemont et al., 2006]. Based on the findings of Jacquemont et al. [2006], Allen et al. [2008] defined alleles with over 70 CGGs as “at risk” alleles, while a repeat shorter than 70 trinucleotides was considered a “low risk” allele. Preliminary analyses of neuromotor tests did not reveal a difference between low-risk allele carriers and non-carriers [Allen et al., 2008].
It is as yet unknown whether specific prodromal signs exist for FXTAS. Although elevated FMR1 mRNA levels in blood from PM carriers (devoid of FXTAS symptomatology) have been found to be associated with more psychological symptoms [Hessl et al., 2005], naturally at the time it was unknown whether these PM carriers will later develop FXTAS. As awareness about FXTAS grows, monitoring of PM carriers (in known fragile X families) is likely to give insight into whether PM carriers are also at risk of developing problems before FXTAS develops and whether possible prodromal symptoms have predictive value for FXTAS development. This will of course also have importance for genetic counseling of fragile X families.
CLINICAL ASPECTS OF FXTAS
FXTAS patients generally present with cerebellar gait ataxia and intention tremor [Jacquemont et al., 2003], but may develop other neurological symptoms such as Parkinsonism, autonomic dysfunction, and peripheral neuropathy and may suffer from cognitive decline ranging from mild frontal executive and memory deficits to global dementia [Grigsby et al., 2006]. The dementia resembles cognitive performance in the frontal variant of frontotemporal dementia, but not dementia in Alzheimer’s disease [Bozeat et al., 2000; Grigsby et al., 2007]. There is substantial variation in clinical presentation, as illustrated by the broad range of diagnoses given to patients who were later diagnosed with FXTAS, once the syndrome had been identified and described [Hall et al., 2005].
Evidence is accumulating that the severity of some aspects of the disorder is correlated with the length of the (CGG)n. For instance, age at death negatively correlated with the number of (CGG)n [Greco et al., 2006]. (CGG)n length was also reported to negatively correlate with age of onset of the major clinical motor symptoms, action tremor, and gait ataxia [Tassone et al., 2007]. Two earlier studies had not found any association between (CGG)n length and age of onset of clinical symptoms [Jacquemont et al., 2003; Grigsby et al., 2006]. This difference was explained by cohort size and the width of the repeat range tested. Age of onset of symptoms was neither correlated to FMR1 mRNA levels, nor to FMRP levels [Tassone et al., 2007]. This was explained by the finding that FMR1 mRNA levels were measured in peripheral lymphocytes. The increase of FMR1 mRNA levels in brain tissue from a FXTAS patient relative to a control subject is smaller than when FMR1 mRNA levels in blood are compared. This is due to the higher abundance of FMR1 mRNA in brain versus in blood (measured relative to GUS mRNA), also in controls. Differences in transcript levels in brain vary considerably over different regions, although they are driven by the same (CGG)n length, which is generally consistent throughout the brain [Tassone et al., 2004a]. It is possible that region-specific transcriptional regulation by transcription factors determines the variable FMR1 expression levels throughout the brain. A lack of correlation between FMR1 transcription and onset of motor problems is not surprising, as FMRP levels are not deviant from normal controls [Tassone et al., 2007]. (CGG)n length was also described to correlate with increased cognitive and functional impairment in PM carriers [Grigsby et al., 2006]. Total brain volume reductions and increased volume of white matter disease correlated with the number of CGGs [Loesch et al., 2005; Cohen et al., 2006]. Similar, though smaller volumetric changes were seen in PM carriers without symptoms of FXTAS, suggesting that neuropathological changes take place before clinical symptoms become apparent [Cohen et al., 2006].
It has been described that the abnormal elevation of FMR1 mRNA is associated with increased psychological symptoms such as anxiety, depression, and irritability. Thus, increased psychopathology is not confined to FXTAS patients, but can occur in all adult PM carriers, although especially in males [Jacquemont et al., 2004a; Hessl et al., 2005; Bacalman et al., 2006; Bourgeois et al., 2006]. However, the observed neuropsychiatric phenotypes do not show a very consistent pattern, due to methodological limitations. Therefore, very recently a controlled study into neuropsychiatric functioning was conducted in male PM carriers, who did not show any signs of FXTAS, who were compared to controls who have a similar family environment (care of a family member with fragile X) and other non-PM carrier controls. This study did not find significant differences in psychopathology between PM carriers and non-PM carrier family and other controls, except that PM carriers were impaired in utilizing working memory and accessing recall check. Also, when comparing PM carriers with normal controls, but not family controls, PM carriers showed more alcohol abuse [Kogan et al., 2007]. A recent study investigated the prevalence of mood and anxiety disorders in PM mothers of at least one child with FXS. The most prominent finding was a higher rate of lifetime major depressive disorder (MDD). This did not appear to be fully explained by the stress of raising a child with FXS. Interestingly, a longer (CGG)n was associated with less likely occurrence of lifetime MDD. Preliminary analysis might suggest a non-linear relationship between (CGG)n length and MDD, such that women with midrange lengths are at increased risk, while females that carry the longest repeats have a lower risk [Roberts et al., 2008]. Although speculative, this is in line with recent findings for POI prevalence in PM females [Allen et al., 2007]. As yet, no controlled study has been performed for PM carriers with FXTAS. A study which thoroughly investigated cognitive functioning in PM carriers with and without FXTAS, found substantial executive impairment and dysfunction in a wide range of aspects of cognitive functioning in patients with FXTAS, while unaffected PM carriers performed worse than controls on executive cognitive functioning and declarative learning and memory [Grigsby et al., 2008]. All relevant clinical aspects of FXTAS have been nicely summarized in a recent review by Berry-Kravis et al. [2007] and other clinicians and others involved in the field.
In 2004, the first female cases with FXTAS were described. Female PM-carriers are much less likely to develop FXTAS, probably because of a protective effect due to the expression of the normal allele in part of their neurons. Also, estrogen might have an alleviating effect on the disease mechanism [Hagerman et al., 2004]. The diluting effect of the presence of a normal FMR1 allele is also the likely cause of the less severe clinical outcome seen in female FXTAS patients, as compared to male patients with FXTAS [Hagerman et al., 2004; Zuhlke et al., 2004]. Positively skewed X-inactivation, wherein the normal allele is preferentially active, could lessen the toxic effects of elevated FMR1 mRNA levels. In these first known females with FXTAS, no skewed X-inactivation was seen [Hagerman et al., 2004]. However, another study described two sisters who are both PM-carriers, displaying a different degree of clinical involvement, which correlated with the pattern of X-inactivation. The sister who had a skewed X-inactivation pattern, favoring a higher percentage of cells expressing the PM-allele, suffered from more severe symptomatology. Although genetic background may influence severity of FXTAS, this is not likely a major factor in these sisters. Thus, this study provides a clear view on the effect of X-inactivation on the clinical outcome in FXTAS [Berry-Kravis et al., 2004].
DIAGNOSTIC CRITERIA FOR FXTAS
MRI studies of the first patients identified to suffer from FXTAS revealed generalized brain atrophy and enlarged ventricles [Hagerman et al., 2001]. Later, neuropathological post mortem studies of patients with manifestations of FXTAS generally showed global brain atrophy, as well as cerebellar and subcortical cerebral white matter disease. Also substantial Purkinje cell dropout with Bergmann gliosis is seen [Greco et al., 2002]. Another prominent feature is the so-called MCP sign, namely increased signal intensities in T2-weighted MRI recordings of the middle cerebellar peduncles (MCPs), which is caused by spongiosis of the deep cerebellar white matter [Brunberg et al., 2002; Jacquemont et al., 2003]. This MCP sign is observed in about 60% of PM carriers with tremor and/or ataxia and was therefore included in the diagnostic criteria for FXTAS. Diagnosis is based on the following criteria, presented in Table I, on top of the mandatory criterion of a (CGG)n length between 55 and 200 [Hagerman and Hagerman, 2004]. Testing guidelines for FXTAS, as well as the proposed diagnostic criteria have recently been described and summarized in a review by Berry-Kravis et al. [2007].
TABLE I.
Diagnostic Criteria for FXTAS
| Criteria: diagnosis | Clinical | Radiological | Neuropathological(post-mortem) |
|---|---|---|---|
| Major | Minor | Major | Minor |
| Definite FXTAS | 1: Intention tremor OR gait ataxia AND: | MCP sign OR: | Presence of intra nuclear inclusions |
| Probable FXTAS | 2: Intention tremor AND gait ataxia OR: | Parkinsonism AND: | MCP sign |
| Possible FXTAS | 1: Intention tremor OR gait ataxia AND: | Cerebral white-matter lesions, generalized atrophy |
MOLECULAR CORRELATES OF FXTAS
The development of FXTAS is restricted to carriers with the PM, who have a transcriptionally fully active FMR1 gene [Tassone et al., 2004b]. PM carriers, irrespective of showing signs of FXTAS, have been shown to have up to eightfold elevated FMR1 mRNA levels in peripheral blood leucocytes, despite close to normal FMRP levels [Tassone et al., 2000a,b; Kenneson et al., 2001; Tassone and Hagerman, 2003]. Relative FMR1 mRNA levels in peripheral blood leucocytes are more elevated in comparison with normal controls than are relative FMR1 mRNA levels in brain [Tassone et al., 2004a]. The level of FMR1 mRNA in blood leukocytes is highly and positively correlated to (CGG)n length in the PM range [Tassone et al., 2000a,b; Kenneson et al., 2001; Allen et al., 2004]. The mechanism underlying the elevated FMR1 mRNA levels is unknown. The observation that increased transcript levels were also measured when constructs bearing the FMR1 5′ UTR with PM sized (CGG)n, fused to a luciferase reporter were transfected into two different cell lines [Chen et al., 2003], suggests that the expanded (CGG)n itself, rather than the reduced FMRP levels, is responsible for increased transcription [Tassone et al., 2000a; Kenneson et al., 2001; Willemsen et al., 2004]. Multiple FMR1 transcriptional initiation sites have been identified, with different efficiencies, dependent on the length of the (CGG)n in the 5′ UTR downstream of the initiation sites. Thus, it appears that the (CGG)n exerts some effect at the level of transcription initiation [Beilina et al., 2004]. Cell culture studies on the decay of FMR1 mRNA indicated that increased stability is not the cause of elevated FMR1 mRNA levels [Tassone et al., 2000b]. Later, it was shown that increased FMR1 mRNA levels are due to increased transcriptional activity of both spliced and unspliced mRNA. Also, the majority of FMR1 mRNA was found to be localized in the cytoplasm, thus excluding the possibility that nuclear retention of FMR1 mRNA contributes to the higher levels (as occurs in myotonic dystrophy, which will be discussed later) [Tassone et al., 2004b].
Although different methods and different repeat ranges tested show somewhat different results, it seems that within the PM range, FMRP levels gradually decrease with increasing (CGG)n length [Tassone et al., 2000a,b; Kenneson et al., 2001; Tassone and Hagerman, 2003]. The reduced levels have been found to reflect impaired translational efficiency of the (CGG)n containing FMR1 mRNA [Primerano et al., 2002]. Since none of the characteristic features of FXTAS have been observed in FM carriers, who express little or no FMRP, it is very unlikely that this syndrome originates from a protein-deficiency.
NEUROPATHOLOGICAL HALLMARK OF FXTAS
As can be seen in Table I, apart from generalized atrophy, another neuropathological feature has been found to be characteristic for FXTAS brain pathology. Neurohistological examination of brains of PM carriers who displayed the neurological phenotype which would later be diagnosed as FXTAS, revealed the presence of eosinophilic, intranuclear inclusions in neurons and astrocytes. These inclusions are discrete, round bodies, 2–5 µm large, and were seen in various regions throughout the cerebrum and brain stem [Greco et al., 2002]. They are most numerous in the hippocampus. A strong correlation was found between repeat length and number of intranuclear inclusions, in neurons as well as astrocytes [Greco et al., 2006] (Fig. 3). The intranuclear inclusions stain positively with antibodies against ubiquitin. No positive staining in inclusions was obtained with antibodies against tau, cytokeratin, desmin, αB-crystallin, vimentin, GFAP, or neurofilament. The antibody against neurofilament, however, showed swollen axons in the granular cell layer of the cerebellum (Purkinje axonal torpedoes). The GFAP-antibody revealed Bergmann gliosis. Furthermore, Purkinje cell drop out was seen in the cerebellum. No inclusions were observed in Purkinje cells. Brains of two female PM carriers unaffected by FXTAS were also examined; none of the neuropathological changes associated with FXTAS were observed [Greco et al., 2002].
FIG. 3.
Ubiquitin-positive intranuclear inclusions, the neuropathological hallmark of FXTAS in A: human neurons, B: human astrocytes, C: murine neurons. No inclusions have been seen in murine astrocytes.
Previous neuropathological examinations of brains of elderly carriers of the FM did not show inclusions [Rudelli et al., 1985; Sabaratnam, 2000]. Some neuroanatomical changes do, however, overlap between PM carriers with FXTAS and FM carriers; Purkinje cell loss and Bergmann gliosis have also been described in an older male with the FM [Sabaratnam, 2000]. Also, shrinkage of the temporal lobe [Reiss et al., 1994] and enlarged ventricles [Reiss et al., 1995] have been seen in FM carriers. However, overall brain volume tends to be greater in FM carriers [Reiss et al., 1994; Schapiro et al., 1995]. Thus, the intranuclear inclusions seem to represent a neuropathological hallmark specific for FXTAS and their presence is therefore used as a post-mortem diagnostic criterion (Table I).
Based on the toxic RNA gain-of-function model proposed for FXTAS, which will be described in detail later, it was predicted that FMR1 mRNA is present within the intranuclear inclusions. Indeed, FMR1 mRNA was detected in inclusions isolated from brain of a 70-year-old patient. This finding serves as evidence for an association between elevated FMR1 transcript levels and formation of intranuclear inclusions. Since the presence of inclusions is associated with the development of FXTAS, these results strongly point towards an RNA gain-of-function mechanism [Tassone et al., 2004a]. Another prediction of the model is that RNA-BPs interfere with the abundant (CGG)n containing mRNA, which then ultimately leads to the clinical phenotype [Iwahashi et al., 2006]. To investigate which proteins might mediate the toxic effects of the expanded-repeat FMR1 mRNA, a systematic analysis was under-taken into the composition of purified intranuclear inclusions of FXTAS brain. Over 20 proteins were found to co-localize with ubiquitin-positive inclusions, among which αB-crystallin, Hsp27 and Hsp70, muscleblind-like protein 1 (MBNL1), several intermediate filaments, and microtubule components, as well as myelin associated proteins and the RNA-BP heterogeneous nuclear RNP A2/B1 (hnRNP A2/B1) [Iwahashi et al., 2006]. Another group demonstrated co-localization of Pur-α and ubiquitin in intranuclear inclusions in human FXTAS brain [Jin et al., 2007], which had not been identified in the purified inclusions in the study by Iwahashi et al. [2006]. Thus, the exact composition and formation of inclusions is as yet unknown as well as how they are related to the clinical symptoms seen in FXTAS.
PATHOGENESIS OF FXTAS
Most studies on the pathogenesis of FXTAS currently focus on the proposed RNA toxic gain-of-function model (Fig. 4). The evidence for this pathogenesis model, in which the FMR1 mRNA itself is the cause of the neurological disease, originates from the observations that FMR1 mRNA levels are elevated in peripheral blood leucocytes of PM carriers, FMR1 mRNA is present in the intranuclear inclusions in brain and that no symptoms of FXTAS are seen among older carriers with the FM [Hagerman et al., 2001; Greco et al., 2002; Jacquemont et al., 2003], reviewed in Hagerman and Hagerman [2004]. This model parallels the RNA toxic gain-of-function model proposed for another non-coding expanded repeat disorder, myotonic dystrophy. Myotonic dystrophy is either caused by expansion of a (CTG)n in the 3′ UTR of the myotonic dystrophy protein kinase (DMPK) gene (DM1: congenital form), or by expansion of the (CCTG)n in intron 1 of the ZNF9 gene (DM2: late onset form). In both forms of DM, RNA-BPs are sequestered to the mRNA that bears long C(C)TG-repeat tracts. One of these proteins is MBNL1. Nuclear foci are seen in DM, similar to intranuclear inclusions in FXTAS (reviewed in: Mankodi et al. [2002] and Ranum and Day [2004]). These foci contain the repeat-containing RNA involved in DM, as well as MBNL1. The effect of the sequestration of MBNL1 into the nuclear foci is dysregulation of splicing of several target mRNAs, which in most cases can explain symptoms seen in DM [Miller et al., 2000; Fardaei et al., 2001]. The toxic gain-of-function effect has been confirmed in transgenic mouse models in which either an expanded (CTG)n was placed within a non-coding region of a heterologous gene [Mankodi et al., 2002] or by introducing a transgene containing the human DMPK gene with an expanded (CTG)n [Seznec et al., 2000]; both transgenic mouse lines developed myotonia. Thus, it has been proposed that the situation in FXTAS is similar to DM, based on the previously described observations. It has furthermore been considered that POF in female PM carriers might also arise from an RNA-mediated toxicity [Conway et al., 1998]. Cellular and animal models, which will be described in more detail hereafter, provide further evidence that indeed an RNA gain-of-function mechanism underlies the clinical syndromes in FXTAS. However, the protein targets that are sequestered by the (CGG)n containing mRNA remain to be elucidated, as well as the down-stream effects. It will be difficult to answer these questions when solely investigating human samples, because in the case of the most interesting organ, the brain, one is limited to using patient material only at the end stage of the disease. Naturally, it is important to know about the onset and the course of the disease as well. Therefore, in vivo models as an alternative to conducting research in humans are necessary. Animal models have proven and will continue to prove very helpful in answering questions about different stages of the disease and will be discussed in detail below.
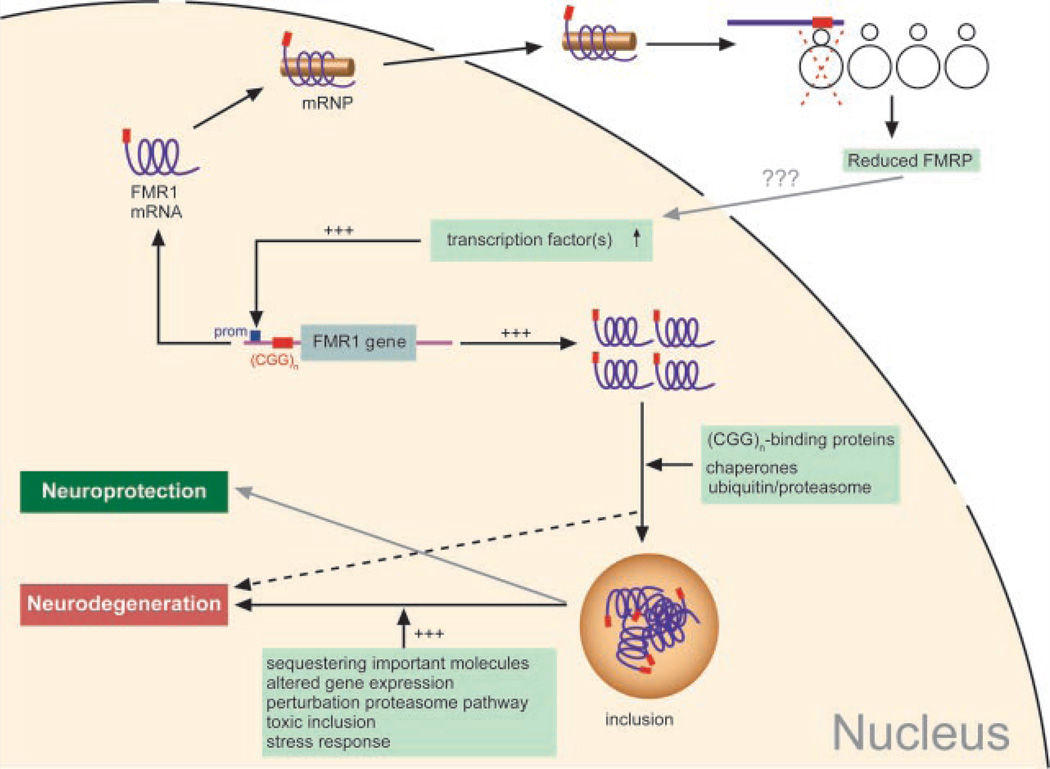
FIG. 4.
A schematic representation of the RNA gain-of-function mechanism proposed for the pathogenesis of FXTAS. The FMR1 gene is transcribed in the nucleus and transported to the ribosomes. The expanded (CGG0)n present in the 5′UTR of the FMR1 gene hampers translation, leading to lower FMRP levels. Through an as yet unknown mechanism, transcription is upregulated, leading to increased FMR1 mRNA levels. In an attempt to get rid of the excess of FMR1 mRNA, the cell might attract chaperones or elements of the ubiquitin/proteasome system. Also (CGG)n-binding proteins might be recruited. These processes could lead to formation of intranuclear inclusions. Sequestration of proteins into the inclusions might prevent them from exerting their normal function, thereby disturbing cellular function, which in the end might cause neurodegeneration. Also, it cannot be excluded that neuroprotection takes place, such that cells that are capable of capturing the toxic transcripts in the inclusions are the cells that survive.
Also cellular in vitro models can give valuable insight into the mechanisms of toxicity. Inducible expression of constructs with non-coding (CGG)n of different lengths in HEK293 cells showed higher levels of apoptosis with increasing repeat length. Non-induced cells, thus only expressing endogenous levels of FMR1 mRNA, showed reduced cell death in comparison to the induced cells, suggesting that cell death is related to the amount of (CGG)n RNA that is transcribed [Handa et al., 2005]. Another cellular model mimics another aspect of FXTAS, namely the formation of inclusions, which occurred in human neuroblastoma cells after introduction of an expanded (CGG)n. Another result of the expression of the (CGG)n containing RNA is disruption of lamin A/C architecture [Arocena et al., 2005]. Lamin A/C was one of the intermediate filaments found to be a component of the intranuclear inclusions in FXTAS brain [Iwahashi et al., 2006]. Controlled expression of (CGG)n tracts in cellular models might provide further insight on the altered cellular function as a result of toxic RNA species.
ANIMAL MODELS FOR FXTAS
Several animal models have been generated in the past, in an attempt to mimic repeat instability as well as neurodegeneration associated with (CGG)n expansion in the FMR1 gene. The different existing animal models and their contributions to our understanding of the pathogenesis of FXTAS are discussed.
Drosophila melanogaster Models for FXTAS
A Drosophila model generated by Jin et al. [2003] provided useful insight into the effect of ectopic expression of different amounts of different (CGG)n lengths in the fly. Strong expression of a construct with 90 CGGs in all neurons of the peripheral and central nervous system caused lethality, as did ubiquitous expression in the embryo. Epithelial expression of this construct did not cause any effect, which shows that neuronal cells suffer more from the toxic effect of the long (CGG)n containing RNA. Moderate or strong expression of constructs containing part of the human FMR1 5′ UTR with either normal or PM-sized CGG-tracts was also specifically directed to the retina. Moderate expression of a tract of 60 CGGs hardly caused any aberrant phenotype in the eye, whereas 90 CGGs lead to rough eye, loss of pigmentation and disturbed architecture. Strong expression of either repeat length caused a more severe phenotype, with an even stronger effect for the (CGG)90, including major cell death. These experiments show that both the length of the CGG-tract and the expression level play a role in the severity of the neurodegenerative eye phenotype in Drosophila. Furthermore, disruption of the eye morphology increased with age in transgenic flies expressing 90 CGGs, mimicking the progressive nature of FXTAS. It was also shown that the phenotypes represent late onset symptoms rather than developmental defects. Immunohistochemical studies of the retina of flies strongly expressing the long repeat demonstrated the presence of inclusions, positive for ubiquitin, hsp70, and proteasome. These inclusions were found both in nuclei and cytoplasm, which is different from what is seen in humans and mice. Also, they were not so consistent in size and shape as in humans and mice. None of the aspects of the neurodegenerative phenotype could be induced with expression of a control construct containing enhanced green fluorescent protein (EGFP) only. Since hsp70 has been shown to be a universal suppressor of many neurodegenerative models caused by mutant proteins, for example, polyglutamine-containing due to an expanded (CAG0)n in the coding region of a gene, flies were generated that co-express the (CGG)90 and hsp70. Hsp70 was found to be capable of suppressing the CGG-RNA induced degeneration, despite the absence of a mutant protein, like in other neurodegenerative disorders where hsp70 could act as a chaperone in refolding mutant proteins. Thus, these results are promising since they provide a proof-of-principle that the RNA-induced phenotype can be rescued [Jin et al., 2003].
Subsequent studies in this model were aimed at testing the hypothesis that FXTAS is an RNA-mediated neurodegenerative disease caused by the titration of RNA-BPs by the (CGG)n.Agenetic screen of candidate RNA-BPs identified CUG binding protein (CUGBP1) as a modifier of the eye phenotype induced by the repeat tract of 90 CGGs. Overexpression of CUGBP1 was capable of suppressing the (CGG)n RNA-induced neurodegenerative eye phenotype. CUGBP1was found to interact with the (CGG)n via hnRNP A2/B1 [Sofola et al., 2007b], a ribo–CGG binding protein, of which the presence has been demonstrated in inclusions in human FXTAS brain [Iwahashi et al., 2006]. An article published simultaneously demonstrated that hnRNP A2/B1 directly binds to the (CGG)n [Jin et al., 2007] and overexpression of this protein and its two Drosophila homologues also suppresses the repeat RNA-induced eye phenotype [Sofola et al., 2007b]. In addition, Purα, an RNA-BP expressed in neuronal cytoplasm, involved in dendritic mRNA transport, was also was found to be associated with (CGG)n RNA and overexpression of Pur α suppressed the eye neurodegeneration phenotype. Pur α was also present in the inclusions in Drosophila [Jin et al., 2007], as well as in human FXTAS brain [Iwahashi et al., 2006]. Thus, these findings strongly support the current pathogenesis model for FXTAS, namely that PM-sized (CGG)n sequester RNA-BPs, which leads to altered cellular function and ultimately neuronal cell death [Jin et al., 2007].
More evidence for the hypothesis that the levels of repeat-RNA are important in inducing a neurodegenerative phenotype comes from the observation that simultaneous expression of a (CGG)n and a (CCG)n, which is present in the 5′ UTR of the FMR2 gene, in Drosophila suppress their independent toxicity. The FMR2 gene is located about 600 kb distal to the FMR1 gene and is involved in non-syndromic X-linked mental retardation. Expression of only a (CCG)n of 90 CCGs also induced a neurodegenerative phenotype of the eye. The protective effect of co-expression was found to be dependent on the RNAi pathway; toxicity was reversed through a reduction of transcript levels, possibly through formation of double stranded RNA, which is then processed and cleaved. This is hopeful in light of potential therapeutical strategies involving complementary RNA molecules targeting mutant mRNAs carrying expanded trinucleotide repeats [Sofola et al., 2007a].
Mouse Models for FXTAS
Initial attempts to make transgenic mouse models expressing expanded (CGG)n tracts focused on investigating the mechanisms underlying (CGG)n instability, since FXTAS had not been discovered at the time. The first transgenic mouse model expressed a (CGG)81, with two interruptions, but with a pure tract of 60 CGGs. Although in humans, this repeat length is sufficient to cause instability upon transmission to the next generation, all transgenic mice showed exactly the same repeat length. Thus, the repeat was stably inherited in all animals tested [Bontekoe et al., 1997]. In another transgenic mouse line, a randomly (autosomally) integrated FMR1 PM allele ((CGG)22TGG(CGG)43TGG(CGG)21) was also stably transmitted [Lavedan et al., 1997]. (CGG)n usually have a few AGG interruptions. Expansion of the (CGG)n appears to be initiated upon loss of the interruptions of the otherwise pure (CGG)n-tracts. Interruptions are predominantly lost at the 3′ end of the repeat tract, causing a long pure (CGG)n-tract [Richards and Sutherland, 1992; Kunst and Warren, 1994]. Therefore, Lavedan et al. [1998] created new transgenic mouse lines with even longer uninterrupted (CGG)n. In addition, because it was recognized that chromosomal context might be important for (CGG)n instability to take place, they situated the (CGG)n tract within the context of the first exon of the human FMR1 gene and included the flanking CpG island. However, including nearby _cis_-acting elements in the transgene proved not to be sufficient to reproduce to reproduce repeat instability as seen in humans, since only minor intergenerational instability was observed in these mice [Lavedan et al., 1998].
Another approach was taken with the use of yeast artificial chromosomes (YACs), which can contain large fragments of exogenous DNA. YACs in transgenic mice have been demonstrated to appropriately express the genes present on the YAC, as all splice isoforms and other regulatory elements can be included [Gaensler et al., 1993; Peterson et al., 1993]. Several transgenic lines were created using YAC transgenes carrying (CGG)n of different lengths and with varying amounts of flanking sequences. Length-dependent intergenerational instability (small expansions and contractions) was seen. Parental origin of the repeat allele did not influence the magnitude or direction of instability [Peier and Nelson, 2002]. To investigate the effect of potential _cis_- and _trans_-factors promoting expansions, the transgenic mice described by Lavedan et al. [1998] were subjected to a low-folate or folate-free diet, or crossbred with mice deficient in genes involved in DNA replication (Wrn helicase) and repair (p53). Also, the potential effect of parental age was taken into consideration. Small changes in repeat length were seen in almost all allele transmissions. Large deletions occurred about 10% of transmissions. No large expansions were seen, neither somatic instability. The pattern of inter-generational instability was not affected by the absence of the proteins involved in DNA replication and repair, nor by folate levels or the parental age [Fleming et al., 2003].
Since flanking of the (CGG)n with part of the FMR1 gene proved not to be sufficient to recapitulate all aspects of repeat instability in humans, another approach was taken by Bontekoe et al. [2001]. They generated a knock-in mouse model in which the endogenous murine (CGG)8 was exchanged with a human (CGG)n of 98 trinucleotides long. When cloning the human expanded repeat into the murine FMR1 promoter, minimal changes were made. Comparison of the promoter sequence in mice and humans showed that all known regulatory elements are conserved. After the initial breedings, infrequent (15 out of 155 transmissions studied) and minor instabilities were observed, two of which were contractions, both of paternal origin. Expansions occurred upon transmission of either one of the parental alleles [Bontekoe et al., 2001].
After FXTAS was first recognized and described, the aging expanded (CGG)n knock-in mouse (from now on called (CGG)n mouse) was analyzed with respect to neurohistology, biochemistry, and molecular aspects. The main findings were that FMR1 mRNA levels in brain were increased throughout life, as compared with wild type animals [Willemsen et al., 2003], which mimics the human situation [Tassone et al., 2000a,b]. Importantly, also intranuclear inclusions were found in many different areas throughout the brains of the (CGG)n mice. Numbers and size of the inclusions increase with age; the first appearing around 30 weeks, but with percentages up to 55% in certain brain regions at 72 weeks of age, which correlates with the progressive nature of the disorder in humans. In addition, a correlation was recognized between the presence of inclusions in certain brain areas and clinical features in patients with FXTAS. This clearly suggests a role for the formation of intranuclear inclusions in the development of the tremor/ataxia syndrome in elderly PM carriers. Interestingly, inclusions were only seen in neurons [Willemsen et al., 2003], while in humans also astrocytes bear inclusions [Greco et al., 2002]. Other neuropathological features observed in patients with FXTAS like neuronal loss, gliosis, and Purkinje cell dropout have not been seen in the (CGG)n mice. Molecular chaperone Hsp40 and the 20S proteasome complex were found to be present in the inclusions. Among many other proteins, FMRP, α-synuclein, poly-glutamine, and tau were not present in the inclusions. Minor somatic repeat instability was seen in some organs at 52 weeks of age, as compared to in tail DNA taken 10 days postnatally [Willemsen et al., 2003].
The (CGG)n mice were subjected to several behavioral tests. No severely aberrant behavioral phenotype was seen; only mild age-dependent learning disturbances and decreased performance in a neuromotor task [Van Dam et al., 2005]. Thus, although the progressive character of the disorder is also seen in mice, the mouse appears to be less affected by the expression of the expanded (CGG)n than humans are, both with regard to neurodegeneration (neuronal cell loss) and the clinical and behavioral phenotype. The origin of these differences remains to be elucidated.
This first knock-in mouse model later furthermore turned out to be more promising in light of repeat instability than the earlier models with non-targeted autosomal (CGG)n, when considerable repeat instability was reported. The (CGG)n in these mice has been expanding over generations since it was generated and now has reached lengths, which in humans represent the FM range. Despite (CGG)n lengths over 200 CGGs, no methylation of the promoter region has been detected thus far in these mice [Brouwer et al., 2007]. Also, alterations in hypothalamus–pituitary gland–adrenal gland (HPA) axis physiology, among which increased stress hormone levels, have been observed in this mouse model, which might explain the increased psychopathology seen in PM carriers [Brouwer et al., 2008].
Another, similar knock-in mouse model was generated by Entezam et al. [2007], which had an initial repeat tract of ~118 CGGs. Similarly, these mice show high repeat instability with a bias towards expansions. They show an increase in FMR1 mRNA levels with (CGG)n length and an inverse correlation with FMRP levels. Also, they showed area-specific decreases in F expression throughout the brain in mice with expanded (CGG)n. For the first time, large expansions into the FM range were seen within a single generation in a mouse model, but the gene was not found to be methylated, nor silenced, as FMRP was still present [Entezam et al., 2007].
Thus, animal models have proven useful and will continue to prove useful in elucidating the molecular mechanisms underlying the clinical symptoms in FXTAS and thus might also provide potential therapeutical targets.
ACKNOWLEDGMENTS
This study was financially supported by the Prinses Beatrix Fonds (J.R.B.: MAR03-0208) and by the National Institutes of Health (RL1 NS062411, UL1 RR024922) (R.W.) and (ROI HD38038) (B.A.O.).
REFERENCES
- Abitbol M, Menini C, Delezoide AL, Rhyner T, Vekemans M, Mallet J. Nucleus basalis magnocellularis and hippocampus are the major sites of FMR-1 expression in the human fetal brain. Nat Genet. 1993;4:147–153. doi: 10.1038/ng0693-147. [DOI] [PubMed] [Google Scholar]
- Agulhon C, Blanchet P, Kobetz A, Marchant D, Faucon N, Sarda P, Moraine C, Sittler A, Biancalana V, Malafosse A, et al. Expression of FMR1, FXR1, and FXR2 genes in human prenatal tissues. J Neuropathol Exp Neurol. 1999;58:867–880. doi: 10.1097/00005072-199908000-00009. [DOI] [PubMed] [Google Scholar]
- Allen EG, He W, Yadav-Shah M, Sherman SL. A study of the distributional characteristics ofFMR1transcript levels in 238 individuals. Hum Genet. 2004;114:439–447. doi: 10.1007/s00439-004-1086-x. [DOI] [PubMed] [Google Scholar]
- Allen EG, Sullivan AK, Marcus M, Small C, Dominguez C, Epstein MP, Charen K, He W, Taylor KC, Sherman SL. Examination of reproductive aging milestones among women who carry the FMR1 premutation. Hum Reprod. 2007;22:2142–2152. doi: 10.1093/humrep/dem148. [DOI] [PubMed] [Google Scholar]
- Allen EG, Juncos J, Letz R, Rusin M, Hamilton D, Novak G, Shubeck L, Tinker SW, Sherman SL. Detection of early FXTAS motor symptoms using the CATSYS computerised neuromotor test battery. J Med Genet. 2008;45:290–297. doi: 10.1136/jmg.2007.054676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arocena DG, Iwahashi CK, Won N, Beilina A, Ludwig AL, Tassone F, Schwartz PH, Hagerman PJ. Induction of inclusion formation and disruption of lamin A/C structure by premutation CGG-repeat RNA in human cultured neural cells. Hum Mol Genet. 2005;14:3661–3671. doi: 10.1093/hmg/ddi394. [DOI] [PubMed] [Google Scholar]
- Ashley AE, Sherman SL. Population dynamics of a meiotic/mitotic expansion model for the fragile X syndrome. Am J Hum Genet. 1995;57:1414–1425. [PMC free article] [PubMed] [Google Scholar]
- Ashley C, Jr, Wilkinson KD, Reines D, Warren ST. FMR1 protein: Conserved RNP family domains and selective RNA binding. Science. 1993;262:563–568. doi: 10.1126/science.7692601. [DOI] [PubMed] [Google Scholar]
- Bacalman S, Farzin F, Bourgeois JA, Cogswell J, Goodlin-Jones BL, Gane LW, Grigsby J, Leehey MA, Tassone F, Hagerman RJ. Psychiatric phenotype of the fragile X-associated tremor/ataxia syndrome (FXTAS) in males: Newly described fronto-subcortical dementia. J Clin Psychiatry. 2006;67:87–94. doi: 10.4088/jcp.v67n0112. [DOI] [PubMed] [Google Scholar]
- Bächner D, Manca A, Steinbach P, Wöhrle D, Just W, Vogel W, Hameister H, Poustka A. Enhanced expression of the murine FMR1 gene during germ cell proliferation suggests a special function in both the male and the female gonad. Hum Mol Genet. 1993a;2:2043–2050. doi: 10.1093/hmg/2.12.2043. [DOI] [PubMed] [Google Scholar]
- Bächner D, Steinbach P, Wöhrle D, Just W, Vogel W, Hameister H, Manca A, Poustka A. Enhanced Fmr-1 expression in testis. Nat Genet. 1993b;4:115–116. doi: 10.1038/ng0693-115. [DOI] [PubMed] [Google Scholar]
- Bagni C. On BC1 RNA and the fragile X mental retardation protein. Proc Natl Acad Sci USA. 2008;105:E19. doi: 10.1073/pnas.0801034105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker CE, Verheij C, Willemsen R, Vanderhelm R, Oerlemans F, Vermey M, Bygrave A, Hoogeveen AT, Oostra BA, Reyniers E, et al. Fmr1 knockout mice: A model to study fragile X mental retardation. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- Bakker CE, de Diego Otero Y, Bontekoe C, Raghoe P, Luteijn T, Hoogeveen AT, Oostra BA, Willemsen R. Immunocytochemical and biochemical characterization of FMRP, FXR1P, and FXR2P in the mouse. Exp Cell Res. 2000;258:162–170. doi: 10.1006/excr.2000.4932. [DOI] [PubMed] [Google Scholar]
- Bat O, Kimmel M, Axelrod DE. Computer simulation of expansions of DNA triplet repeats in the fragile X syndrome and Huntington’s disease. J Theor Biol. 1997;188:53–67. doi: 10.1006/jtbi.1997.0451. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Beilina A, Tassone F, Schwartz PH, Sahota P, Hagerman PJ. Redistribution of transcription start sites within the FMR1 promoter region with expansion of the downstream CGG-repeat element. Hum Mol Genet. 2004;13:543–549. doi: 10.1093/hmg/ddh053. [DOI] [PubMed] [Google Scholar]
- Berkenstadt M, Ries-Levavi L, Cuckle H, Peleg L, Barkai G. Pre-conceptional and prenatal screening for fragile X syndrome: Experience with 40000 tests. Prenat Diagn. 2007;27:991–994. doi: 10.1002/pd.1815. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Potanos K, Weinberg D, Zhou L, Goetz CG. Fragile X-associated tremor/ataxia syndrome in sisters related to X-inactivation. Ann Neurol. 2004;57:144–147. doi: 10.1002/ana.20360. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Abrams L, Coffey SM, Hall DA, Greco C, Gane LW, Grigsby J, Bourgeois JA, Finucane B, Jacquemont S, et al. Fragile X-associated tremor/ataxia syndrome: Clinical features, genetics, and testing guidelines. Mov Disord. 2007;14:2014–2030. doi: 10.1002/mds.21493. [DOI] [PubMed] [Google Scholar]
- Bontekoe CJM, de Graaff E, Nieuwenhuizen IM, Willemsen R, Oostra BA. FMR1 premutation allele is stable in mice. Eur J Hum Genet. 1997;5:293–298. [PubMed] [Google Scholar]
- Bontekoe CJ, Bakker CE, Nieuwenhuizen IM, van Der Linde H, Lans H, de Lange D, Hirst MC, Oostra BA. Instability of a (CGG)(98) repeat in the Fmr1 promoter. Hum Mol Genet. 2001;10:1693–1699. doi: 10.1093/hmg/10.16.1693. [DOI] [PubMed] [Google Scholar]
- Bourgeois JA, Farzin F, Brunberg JA, Tassone F, Hagerman P, Zhang L, Hessl D, Hagerman R. Dementia with mood symptoms in a fragile X premutation carrier with the fragile X-associated tremor/ataxia syndrome: Clinical intervention with donepezil and venlafaxine. J Neuropsychiatry Clin Neurosci. 2006;18:171–177. doi: 10.1176/jnp.2006.18.2.171. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Gregory CA, Ralph MA, Hodges JR. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer’s disease? J Neurol Neurosurg Psychiatry. 2000;69:178–186. doi: 10.1136/jnnp.69.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer JR, Mientjes EJ, Bakker CE, Nieuwenhuizen IM, Severijnen LA, Van der Linde HC, Nelson DL, Oostra BA, Willemsen R. Elevated Fmr1 mRNA levels and reduced protein expression in a mouse model with an unmethylated fragile X full mutation. Exp Cell Res. 2007;313:244–253. doi: 10.1016/j.yexcr.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer JR, Severijnen E, de Jong FH, Hessl D, Hagerman RJ, Oostra BA, Willemsen R. Altered hypothalamus-pituitary-adrenal gland axis regulation in the expanded CGG-repeat mouse model for fragile X-associated tremor/ataxia syndrome. Psychoneuroendocrinology. 2008;33:863–873. doi: 10.1016/j.psyneuen.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- Brunberg JA, Jacquemont S, Hagerman RJ, Berry-Kravis EM, Grigsby J, Leehey MA, Tassone F, Brown WT, Greco CM, Hagerman PJ. Fragile X premutation carriers: Characteristic MR imaging findings of adult male patients with progressive cerebellar and cognitive dysfunction. Am J Neuroradiol. 2002;23:1757–1766. [PMC free article] [PubMed] [Google Scholar]
- Chen LS, Tassone F, Sahota P, Hagerman PJ. The (CGG)n repeat element within the 5′ untranslated region of the FMR1 message provides both positive and negative cis effects on in vivo translation of a down-stream reporter. Hum Mol Genet. 2003;12:3067–3074. doi: 10.1093/hmg/ddg331. [DOI] [PubMed] [Google Scholar]
- Cheutin T, McNairn AJ, Jenuwein T, Gilbert DM, Singh PB, Misteli T. Maintenance of stable heterochromatin domains by dynamic HP1 binding. Science. 2003;299:721–725. doi: 10.1126/science.1078572. [DOI] [PubMed] [Google Scholar]
- Chiurazzi P, Pomponi MG, Willemsen R, Oostra BA, Neri G. In vitro reactivation of the FMR1 gene involved in fragile X syndrome. Hum Mol Genet. 1998;7:109–113. doi: 10.1093/hmg/7.1.109. [DOI] [PubMed] [Google Scholar]
- Chiurazzi P, Pomponi MG, Pietrobono R, Bakker CE, Neri G, Oostra BA. Synergistic effect of histone hyperacetylation and DNA demethylation in the reactivation of theFMR1gene. Hum Mol Genet. 1999;8:2317–2323. doi: 10.1093/hmg/8.12.2317. [DOI] [PubMed] [Google Scholar]
- Coffee B, Zhang F, Warren ST, Reines D. Acetylated histones are associated with FMR1 in normal but not fragile X-syndrome cells. Nat Genet. 1999;22:98–101. doi: 10.1038/8807. [DOI] [PubMed] [Google Scholar]
- Coffee B, Zhang F, Ceman S, Warren ST, Reines D. Histone modifications depict an aberrantly heterochromatinized FMR1 gene in fragile X syndrome. Am J Hum Genet. 2002;71:923–932. doi: 10.1086/342931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Masyn K, Adams J, Hessl D, Rivera S, Tassone F, Brunberg J, DeCarli C, Zhang L, Cogswell J, et al. Molecular and imaging correlates of the fragile X-associated tremor/ataxia syndrome. Neurology. 2006;67:1426–1431. doi: 10.1212/01.wnl.0000239837.57475.3a. [DOI] [PubMed] [Google Scholar]
- Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, Greenough WT. Abnormal dendritic spines in fragile X knockout mice: Maturation and pruning deficits. Proc Natl Acad Sci USA. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway GS, Payne NN, Webb J, Murray A, Jacobs PA. Fragile X premutation screening in women with premature ovarian failure. Hum Reprod. 1998;13:1184–1187. doi: 10.1093/humrep/13.5.1184. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107:489–499. doi: 10.1016/s0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- De Diego Otero Y, Bakker CE, Raghoe P, Severijnen LWFM, Hoogeveen A, Oostra BA, Willemsen R. Immunocytochemical characterization of FMRP, FXR1P and FXR2P during embryonic development in the mouse. Gene Funct Dis. 2000;1:28–37. doi: 10.1006/excr.2000.4932. [DOI] [PubMed] [Google Scholar]
- De Vrij FMS, Levenga J, Van der Linde HC, Koekkoek SK, De Zeeuw CI, Nelson DL, Oostra BA, Willemsen R. Rescue of behavioral phenotype and neuronal protrusion morphology inFMR1KOmice. Neurobiol Dis. 2008;31:127–132. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devys D, Biancalana V, Rousseau F, Boue J, Mandel JL, Oberle I. Analysis of full fragile X mutations in fetal tissues and monozygotic twins indicate that abnormal methylation and somatic heterogeneity are established early in development. Am J Med Genet. 1992;43:208–216. doi: 10.1002/ajmg.1320430134. [DOI] [PubMed] [Google Scholar]
- Devys D, Lutz Y, Rouyer N, Bellocq JP, Mandel JL. The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat Genet. 1993;4:335–340. doi: 10.1038/ng0893-335. [DOI] [PubMed] [Google Scholar]
- Dombrowski C, Levesque S, Morel ML, Rouillard P, Morgan K, Rousseau F. Premutation and intermediate-size FMR1 alleles in 10572 males from the general population: Loss of an AGG interruption is a late event in the generation of fragile X syndrome alleles. Hum Mol Genet. 2002;11:371–378. doi: 10.1093/hmg/11.4.371. [DOI] [PubMed] [Google Scholar]
- Eberhart DE, Malter HE, Feng Y, Warren ST. The fragile X mental retardation protein is a ribosonucleoprotein containing both nuclear localization and nuclear export signals. Hum Mol Genet. 1996;5:1083–1091. doi: 10.1093/hmg/5.8.1083. [DOI] [PubMed] [Google Scholar]
- Eichler EE, Richards S, Gibbs RA, Nelson DL. Fine structure of the human FMR1 gene. Hum Mol Genet. 1993;2:1147–1153. doi: 10.1093/hmg/2.8.1147. [DOI] [PubMed] [Google Scholar]
- Eichler EE, Holden J, Popovich BW, Reiss AL, Snow K, Thibodeau SN, Richards CS, Ward PA, Nelson DL. Length of uninterrupted CGG repeats determines instability in the FMR1 gene. Nat Genet. 1994;8:88–94. doi: 10.1038/ng0994-88. [DOI] [PubMed] [Google Scholar]
- Eiges R, Urbach A, Malcov M, Frumkin T, Schwartz T, Amit A, Yaron Y, Eden A, Yanuka O, Benvenisty N, et al. Developmental study of fragile X syndrome using human embryonic stem cells derived from preimplantation genetically diagnosed embryos. Cell Stem Cell. 2007;1:568–577. doi: 10.1016/j.stem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Ennis S, Ward D, Murray A. Nonlinear association between CGG repeat number and age of menopause in FMR1 premutation carriers. Eur J Hum Genet. 2005;14:253–255. doi: 10.1038/sj.ejhg.5201510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entezam A, Biacsi R, Orrison B, Saha T, Hoffman GE, Grabczyk E, Nussbaum RL, Usdin K. Regional FMRP deficits and large repeat expansions into the full mutation range in a new fragile X premutation mouse model. Gene. 2007;395:125–134. doi: 10.1016/j.gene.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardaei M, Larkin K, Brook JD, Hamshere MG. In vivo co-localisation of MBNL protein with DMPK expanded-repeat transcripts. Nucleic Acids Res. 2001;29:2766–2771. doi: 10.1093/nar/29.13.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F, Perry H, Hessl D, Loesch D, Cohen J, Bacalman S, Gane L, Tassone F, Hagerman P, Hagerman R. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr. 2006;27:S137–S144. doi: 10.1097/00004703-200604002-00012. [DOI] [PubMed] [Google Scholar]
- Feng Y, Absher D, Eberhart DE, Brown V, Malter HE, Warren ST. FMRP associates with polyribosomes as an mRNP, and the I304N mutation of severe fragile X syndrome abolishes this association. Mol Cell. 1997a;1:109–118. doi: 10.1016/s1097-2765(00)80012-x. [DOI] [PubMed] [Google Scholar]
- Feng Y, Gutekunst CA, Eberhart DE, Yi H, Warren ST, Hersch SM. Fragile X mental retardation protein: Nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J Neurosci. 1997b;17:1539–1547. doi: 10.1523/JNEUROSCI.17-05-01539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming K, Riser DK, Kumari D, Usdin K. Instability of the fragile X syndrome repeat in mice: The effect of age, diet and mutations in genes that affect DNA replication, recombination and repair proficiency. Cytogenet Genome Res. 2003;100:140–146. doi: 10.1159/000072848. [DOI] [PubMed] [Google Scholar]
- Fridell RA, Benson RE, Hua J, Bogerd HP, Cullen BR. A nuclear role for the fragile X mental retardation protein. EMBO J. 1996;15:5408–5414. [PMC free article] [PubMed] [Google Scholar]
- Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJ, Holden JJ, Fenwick R, Jr, Warren ST, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: Resolution of the Sherman paradox. Cell. 1991;67:1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Gaensler KM, Kitamura M, Kan YW. Germ-line transmission and developmental regulation of a 150-kb yeast artificial chromosome containing the human beta-globin locus in transgenic mice. Proc Natl Acad Sci USA. 1993;90:11381–11385. doi: 10.1073/pnas.90.23.11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass IA. X linked mental retardation. J Med Genet. 1991;28:361–363. doi: 10.1136/jmg.28.6.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco CM, Hagerman RJ, Tassone F, Chudley AE, Del Bigio MR, Jacquemont S, Leehey M, Hagerman PJ. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. 2002;125:1760–1771. doi: 10.1093/brain/awf184. [DOI] [PubMed] [Google Scholar]
- Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, Trapp BD, Iwahashi C, Brunberg J, Grigsby J, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- Grewal SI, Moazed D. Heterochromatin and epigenetic control of gene expression. Science. 2003;301:798–802. doi: 10.1126/science.1086887. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Brega AG, Jacquemont S, Loesch DZ, Leehey MA, Goodrich GK, Hagerman RJ, Epstein J, Wilson R, Cogswell JB, et al. Impairment in the cognitive functioning of men with fragile X-associated tremor/ataxia syndrome (FXTAS) J Neurol Sci. 2006;248:227–233. doi: 10.1016/j.jns.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Brega AG, Leehey MA, Goodrich GK, Jacquemont S, Loesch DZ, Cogswell JB, Epstein J, Wilson R, Jardini T, et al. Impairment of executive cognitive functioning in males with fragile X-associated tremor/ataxia syndrome. Mov Disord. 2007;22:645–650. doi: 10.1002/mds.21359. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Brega AG, Engle K, Leehey MA, Hagerman RJ, Tassone F, Hessl D, Hagerman PJ, Cogswell JB, Bennett RE, et al. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology. 2008;22:48–60. doi: 10.1037/0894-4105.22.1.48. [DOI] [PubMed] [Google Scholar]
- Grossman AW, Aldridge GM, Weiler IJ, Greenough WT. Local protein synthesis and spine morphogenesis: Fragile X syndrome and beyond. J Neurosci. 2006;26:7151–7155. doi: 10.1523/JNEUROSCI.1790-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ. Lessons from fragile X regarding neurobiology, autism, and neurodegeneration. J Dev Behav Pediatr. 2006;27:63–74. doi: 10.1097/00004703-200602000-00012. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Hagerman PJ. The fragile X premutation: Into the phenotypic fold. Curr Opin Genet Dev. 2002;12:278–283. doi: 10.1016/s0959-437x(02)00299-x. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ, Hagerman RJ. The fragile-X premutation: A maturing perspective. Am J Hum Genet. 2004;74:805–816. doi: 10.1086/386296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, Grigsby J, Gage B, Hagerman PJ. Intention tremor, Parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–130. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Leavitt BR, Farzin F, Jacquemont S, Greco CM, Brunberg JA, Tassone F, Hessl D, Harris SW, Zhang L, et al. Fragile-X-associated tremor/ataxia syndrome (FXTAS) in females with the FMR1 premutation. Am J Hum Genet. 2004;74:1051–1056. doi: 10.1086/420700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DA, Berry-Kravis E, Jacquemont S, Rice CD, Cogswell J, Zhang L, Hagerman RJ, Hagerman PJ, Leehey MA. Initial diagnoses given to persons with the fragile X associated tremor/ataxia syndrome (FXTAS) Neurology. 2005;65:299–301. doi: 10.1212/01.wnl.0000168900.86323.9c. [DOI] [PubMed] [Google Scholar]
- Handa V, Goldwater D, Stiles D, Cam M, Poy G, Kumari D, Usdin K. Long CGG-repeat tracts are toxic to human cells: Implications for carriers of fragile X premutation alleles. FEBS Lett. 2005;579:2702–2708. doi: 10.1016/j.febslet.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Hansen RS, Canfield TK, Lamb MM, Gartler SM, Laird CD. Association of fragile X syndrome with delayed replication of the FMR1 gene. Cell. 1993;73:1403–1409. doi: 10.1016/0092-8674(93)90365-w. [DOI] [PubMed] [Google Scholar]
- Hessl D, Tassone F, Loesch DZ, Berry-Kravis E, Leehey MA, Gane LW, Barbato I, Rice C, Gould E, Hall DA, et al. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. Am J Med Genet Part B. 2005;139B:115–121. doi: 10.1002/ajmg.b.30241. [DOI] [PubMed] [Google Scholar]
- Hinds HL, Ashley CT, Sutcliffe JS, Nelson DL, Warren ST, Housman DE, Schalling M. Tissue specific expression of FMR-1 provides evidence for a functional role in fragile X-syndrome. Nat Genet. 1993;3:36–43. doi: 10.1038/ng0193-36. [DOI] [PubMed] [Google Scholar]
- Hinton VJ, Brown WT, Wisniewski K, Rudelli RD. Analysis of neocortex in three males with the fragile X syndrome. Am J Med Genet. 1991;41:289–294. doi: 10.1002/ajmg.1320410306. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci USA. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoangeli A, Rozhdestvensky TS, Dolzhanskaya N, Tournier B, Schutt J, Brosius J, Denman RB, Khandjian EW, Kindler S, Tiedge H. On BC1RNAand the fragile X mental retardation protein. Proc Natl Acad Sci USA. 2008a;105:734–739. doi: 10.1073/pnas.0710991105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoangeli A, Rozhdestvensky TS, Dolzhanskaya N, Tournier B, Schutt J, Brosius J, Denman RB, Khandjian EW, Kindler S, Tiedge H. Reply to Bagni: On BC1RNAand the fragile X mental retardation protein. Proc Natl Acad Sci USA. 2008b;105:E29. doi: 10.1073/pnas.0803737105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, Kooy F, Willems PJ, Cras P, Kozlowski PB, et al. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: A quantitative examination. Am J Med Genet. 2001;98:161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Iwahashi CK, Yasui DH, An HJ, Greco CM, Tassone F, Nannen K, Babineau B, Lebrilla CB, Hagerman RJ, Hagerman PJ. Protein composition of the intranuclear inclusions of FXTAS. Brain. 2006;129:256–271. doi: 10.1093/brain/awh650. [DOI] [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Leehey M, Grigsby J, Zhang L, Brunberg JA, Greco C, Des Portes V, Jardini T, Levine R, et al. Fragile X premutation tremor/ataxia syndrome: Molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72:869–878. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont S, Farzin F, Hall D, Leehey M, Tassone F, Gane L, Zhang L, Grigsby J, Jardini T, Lewin F, et al. Aging in individuals with the FMR1 mutation. Am J Ment Retard. 2004a;109:154–164. doi: 10.1352/0895-8017(2004)109<154:AIIWTF>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Leehey MA, Hall DA, Levine RA, Brunberg JA, Zhang L, Jardini T, Gane LW, Harris SW, et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA. 2004b;291:460–469. doi: 10.1001/jama.291.4.460. [DOI] [PubMed] [Google Scholar]
- Jacquemont S, Leehey MA, Hagerman RJ, Beckett LA, Hagerman PJ. Size bias of fragile X premutation alleles in late-onset movement disorders. J Med Genet. 2006;43:804–809. doi: 10.1136/jmg.2006.042374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Zarnescu DC, Zhang F, Pearson CE, Lucchesi JC, Moses K, Warren ST. RNA-mediated neurodegeneration caused by the fragile X premutation rCGG repeats in drosophila. Neuron. 2003;39:739–747. doi: 10.1016/s0896-6273(03)00533-6. [DOI] [PubMed] [Google Scholar]
- Jin P, Duan R, Qurashi A, Qin Y, Tian D, Rosser TC, Liu H, Feng Y, Warren ST. Pur alpha binds to rCGG repeats and modulates repeat-mediated neurodegeneration in a Drosophila model of fragile X tremor/ataxia syndrome. Neuron. 2007;55:556–564. doi: 10.1016/j.neuron.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N, Strouboulis J, Wolffe AP. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet. 1998;19:187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- Kenneson A, Zhang F, Hagedorn CH, Warren ST. Reduced FMRP and increasedFMR1transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet. 2001;10:1449–1454. doi: 10.1093/hmg/10.14.1449. [DOI] [PubMed] [Google Scholar]
- Khandjian EW, Bardoni B, Corbin F, Sittler A, Giroux S, Heitz D, Tremblay S, Pinset C, Montarras D, Rousseau F, et al. Novel isoforms of the fragile X related protein FXR1P are expressed during myogenesis. Hum Mol Genet. 1998;7:2121–2128. doi: 10.1093/hmg/7.13.2121. [DOI] [PubMed] [Google Scholar]
- Kiebler MA, DesGroseillers L. Molecular insights into mRNA transport and local translation in the mammalian nervous system. Neuron. 2000;25:19–28. doi: 10.1016/s0896-6273(00)80868-5. [DOI] [PubMed] [Google Scholar]
- Koekkoek SK, Yamaguchi K, Milojkovic BA, Dortland BR, Ruigrok TJ, Maex R, De Graaf W, Smit AE, Vanderwerf F, Bakker CE, et al. Deletion of FMR1 in purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in fragile X syndrome. Neuron. 2005;47:339–352. doi: 10.1016/j.neuron.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Kogan CS, Turk J, Hagerman RJ, Cornish KM. Impact of the fragile X mental retardation 1 (FMR1) gene premutation on neuropsychiatric functioning in adult males without fragile X-associated tremor/ataxia syndrome: A controlled study. Am J Med Genet Part B. 2007;147B:859–872. doi: 10.1002/ajmg.b.30685. [DOI] [PubMed] [Google Scholar]
- Kunst CB, Warren ST. Cryptic and polar variation of the fragile X repeat could result in predisposing normal alleles. Cell. 1994;77:853–861. doi: 10.1016/0092-8674(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Lachner M, O’Sullivan RJ, Jenuwein T. An epigenetic road map for histone lysine methylation. J Cell Sci. 2003;116:2117–2124. doi: 10.1242/jcs.00493. [DOI] [PubMed] [Google Scholar]
- Laggerbauer B, Ostareck D, Keidel EM, Ostareck-Lederer A, Fischer U. Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum Mol Genet. 2001;10:329–338. doi: 10.1093/hmg/10.4.329. [DOI] [PubMed] [Google Scholar]
- Lavedan CN, Garrett L, Nussbaum RL. Trinucleotide repeats (CGG)22TGG(CGG)43TGG(CGG)21 from the fragile X gene remain stable in transgenic mice. Hum Genet. 1997;100:407–414. doi: 10.1007/s004390050525. [DOI] [PubMed] [Google Scholar]
- Lavedan C, Grabczyk E, Usdin K, Nussbaum RL. Long uninterrupted CGG repeats within the first exon of the human FMR1 gene are not intrinsically unstable in transgenic mice. Genomics. 1998;50:229–240. doi: 10.1006/geno.1998.5299. [DOI] [PubMed] [Google Scholar]
- Lock LF, Takagi N, Martin GR. Methylation of the Hprt gene on the inactive X occurs after chromosome inactivation. Cell. 1987;48:39–46. doi: 10.1016/0092-8674(87)90353-9. [DOI] [PubMed] [Google Scholar]
- Loesch DZ, Litewka L, Brotchie P, Huggins RM, Tassone F, Cook M. Magnetic resonance imaging study in older fragile X premutation male carriers. Ann Neurol. 2005;58:326–330. doi: 10.1002/ana.20542. [DOI] [PubMed] [Google Scholar]
- Lubs HA. A marker X-chromosome. Am J Hum Genet. 1969;21:231–244. [PMC free article] [PubMed] [Google Scholar]
- Maddalena A, Richards CS, McGinniss MJ, Brothman A, Desnick RJ, Grier RE, Hirsch B, Jacky P, McDowell GA, Popovich B, et al. Technical standards and guidelines for fragile X: The first of a series of disease-specific supplements to the Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics. Quality Assurance Subcommittee of the Laboratory Practice Committee. Genet Med. 2001;3:200–205. doi: 10.1097/00125817-200105000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malter HE, Iber JC, Willemsen R, De Graaff E, Tarleton JC, Leisti J, Warren ST, Oostra BA. Characterization of the full fragile X syndrome mutation in fetal gametes. Nat Genet. 1997;15:165–169. doi: 10.1038/ng0297-165. [DOI] [PubMed] [Google Scholar]
- Mankodi A, Takahashi MP, Jiang H, Beck CL, Bowers WJ, Moxley RT, Cannon SC, Thornton CA. Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol Cell. 2002;10:35–44. doi: 10.1016/s1097-2765(02)00563-4. [DOI] [PubMed] [Google Scholar]
- Meijer H, De Graaff E, Merckx DML, Jongbloed RJE, De Die-Smulders CEM, Engelen JJM, Fryns JP, Curfs PMG, Oostra BA. A deletion of 1.6 kb proximal to the CGG repeat of the FMR1 gene causes the clinical phenotype of the fragile X syndrome. Hum Mol Genet. 1994;3:615–620. doi: 10.1093/hmg/3.4.615. [DOI] [PubMed] [Google Scholar]
- Miller JW, Urbinati CR, Teng-Umnuay P, Stenberg MG, Byrne BJ, Thornton CA, Swanson MS. Recruitment of human muscleblind proteins to (CUG)(n) expansions associated with myotonic dystrophy. EMBO J. 2000;19:4439–4448. doi: 10.1093/emboj/19.17.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, Liu L, Carbonetto S, Weiler IJ, Greenough WT, Eberwine J. RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron. 2003;37:417–431. doi: 10.1016/s0896-6273(03)00034-5. [DOI] [PubMed] [Google Scholar]
- Moutou C, Vincent MC, Biancalana V, Mandel JL. Transition from premutation to full mutation in fragile X syndrome is likely to be prezygotic. Hum Mol Genet. 1997;6:971–979. doi: 10.1093/hmg/6.7.971. [DOI] [PubMed] [Google Scholar]
- Nakamoto M, Nalavadi V, Epstein MP, Narayanan U, Bassell GJ, Warren ST. Fragile X mental retardation protein deficiency leads to excessive mGluR5-dependent internalization of AMPA receptors. Proc Natl Acad Sci USA. 2007;104:15537–15542. doi: 10.1073/pnas.0707484104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- Napierala M, Michalowski D, de Mezer M, Krzyzosiak WJ. Facile FMR1 mRNA structure regulation by interruptions in CGG repeats. Nucleic Acids Res. 2005;33:451–463. doi: 10.1093/nar/gki186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan U, Nalavadi V, Nakamoto M, Thomas G, Ceman S, Bassell GJ, Warren ST. S6K1 phosphorylates and regulates FMRP with the neuronal protein synthesis-dependent mTOR signaling cascade. J Biol Chem. 2008;283:18478–18482. doi: 10.1074/jbc.C800055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol Edamura K, Pearson CE. DNA methylation and replication: Implications for the “deletion hotspot” region of FMR1. Hum Genet. 2005;118:301–304. doi: 10.1007/s00439-005-0037-5. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Oberlander AM, Svoboda K. Abnormal development of dendritic spines in fmr1 knock-out mice. J Neurosci. 2001;21:5139–5146. doi: 10.1523/JNEUROSCI.21-14-05139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolin SL, Lewis FA, Ye LL, Houck GE, Glicksman AE, Limprasert P, Li SY, Zhong N, Ashley AE, Feingold E, et al. Familial transmission of the FMR1 CGG repeat. Am J Hum Genet. 1996;59:1252–1261. [PMC free article] [PubMed] [Google Scholar]
- Nolin SL, Brown WT, Glicksman A, Houck GE, Jr, Gargano AD, Sullivan A, Biancalana V, Brondum-Nielsen K, Hjalgrim H, Holinski-Feder E, et al. Expansion of the fragile X CGG repeat in females with premutation or intermediate alleles. Am J Hum Genet. 2003;72:454–464. doi: 10.1086/367713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlé I, Rousseau F, Heitz D, Kretz C, Devys D, Hanauer A, Boue J, Bertheas MF, Mandel JL. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science. 1991;252:1097–1102. doi: 10.1126/science.252.5009.1097. [DOI] [PubMed] [Google Scholar]
- Pacey LK, Doering LC. Developmental expression of FMRP in the astrocyte lineage: Implications for fragile X syndrome. Glia. 2007;55:1601–1609. doi: 10.1002/glia.20573. [DOI] [PubMed] [Google Scholar]
- Peier A, Nelson D. Instability of a premutation-sized CGG repeat in FMR1 YAC transgenic mice. Genomics. 2002;80:423–432. doi: 10.1006/geno.2002.6849. [DOI] [PubMed] [Google Scholar]
- Pesso R, Berkenstadt M, Cuckle H, Gak E, Peleg L, Frydman M, Barkai G. Screening for fragile X syndrome in women of reproductive age. Prenat Diagn. 2000;20:611–614. doi: 10.1002/1097-0223(200008)20:8<611::aid-pd881>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Peterson KR, Clegg CH, Huxley C, Josephson BM, Haugen HS, Furukawa T, Stamatoyannopoulos G. Transgenic mice containing a 248-kb yeast artificial chromosome carrying the human beta-globin locus display proper developmental control of human globin genes. Proc Natl Acad Sci USA. 1993;90:7593–7597. doi: 10.1073/pnas.90.16.7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primerano B, Tassone F, Hagerman RJ, Hagerman P, Amaldi F, Bagni C. Reduced FMR1 mRNA translation efficiency in fragile X patients with premutations. RNA. 2002;8:1–7. [PMC free article] [PubMed] [Google Scholar]
- Ranum LP, Day JW. Pathogenic RNA repeats: An expanding role in genetic disease. Trends Genet. 2004;20:506–512. doi: 10.1016/j.tig.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Razin A. CpG methylation, chromatin structure and gene silencing-a three-way connection. EMBO J. 1998;17:4905–4908. doi: 10.1093/emboj/17.17.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W, Santos F, Mitsuya K, Morgan H, Dean W. Epigenetic asymmetry in the mammalian zygote and early embryo: Relationship to lineage commitment? Philos Trans R Soc Lond B Biol Sci. 2003;358:1403–1409. doi: 10.1098/rstb.2003.1326. discussion 1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Lee J, Freund L. Neuroanatomy of fragile X syndrome: The temporal lobe. Neurology. 1994;44:1317–1324. doi: 10.1212/wnl.44.7.1317. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Greenlaw R, Freund L, Denckla MB. Neurodevelopmental effects of the FMR-1 full mutation in humans. Nat Med. 1995;1:159–1167. doi: 10.1038/nm0295-159. [DOI] [PubMed] [Google Scholar]
- Reyniers E, Vits L, De Boulle K, Van Roy B, Van Velzen D, de Graaff E, Verkerk AJMH, Jorens HZ, Darby JK, Oostra BA, et al. The full mutation in the FMR-1 gene of male fragile X patients is absent in their sperm. Nat Genet. 1993;4:143–146. doi: 10.1038/ng0693-143. [DOI] [PubMed] [Google Scholar]
- Richards RI, Sutherland GR. Dynamic mutations: A new class of mutations causing human disease. Cell. 1992;70:709–712. doi: 10.1016/0092-8674(92)90302-s. [DOI] [PubMed] [Google Scholar]
- Richards RI, Holman K, Kozman H, Kremer E, Lynch M, Pritchard M, Yu S, Mulley J, Sutherland GR. Fragile X syndrome: Genetic localisation by linkage mapping of two microsatellite repeats FRAXAC1 and FRAXAC2 which immediately flank the fragile site. J Med Genet. 1991;28:818–823. doi: 10.1136/jmg.28.12.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Bailey DB, Jr, Mankowski J, Ford A, Sideris J, Weisenfeld LA, Heath TM, Golden RN. Mood and anxiety disorders in females with the FMR1 premutation. Am J Med Genet Part B. 2008 doi: 10.1002/ajmg.b.30786. [DOI] [PubMed] [Google Scholar]
- Rohr J, Allen EG, Charen K, Giles J, He W, Dominguez C, Sherman SL. Anti-Mullerian hormone indicates early ovarian decline in fragile X mental retardation (FMR1) premutation carriers: A preliminary study. Hum Reprod. 2008;23:1220–1225. doi: 10.1093/humrep/den050. [DOI] [PubMed] [Google Scholar]
- Rousseau F, Heitz D, Oberlé I, Mandel JL. Selection in blood cells from female carriers of the fragile X syndrome: Inverse correlation between age and proportion of active X chromosomes carrying the full mutation. J Med Genet. 1991;28:830–836. doi: 10.1136/jmg.28.12.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau F, Rouillard P, Morel ML, Khandjian EW, Morgan K. Prevalence of carriers of premutation-size alleles of the FMRI gene and implications for the population genetics of the fragile X syndrome. Am J Hum Genet. 1995;57:1006–1018. [PMC free article] [PubMed] [Google Scholar]
- Rudelli RD, Brown WT, Wisniewski K, Jenkins EC, Laure-Kamionowska M, Connell F, Wisniewski HM. Adult fragile X syndrome. Cliniconeuropathologic findings. Acta Neuropathol. 1985;67:289–295. doi: 10.1007/BF00687814. [DOI] [PubMed] [Google Scholar]
- Sabaratnam M. Pathological and neuropathological findings in two males with fragile-X syndrome. J Intellect Disabil Res. 2000;44:81–85. doi: 10.1046/j.1365-2788.2000.00261.x. [DOI] [PubMed] [Google Scholar]
- Samadashwily GM, Dayn A, Mirkin SM. Suicidal nucleotide sequences for DNA polymerization. EMBO J. 1993;12:4975–4983. doi: 10.1002/j.1460-2075.1993.tb06191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer C, Bardoni B, Mandel JL, Ehresmann B, Ehresmann C, Moine H. The fragile X mental retardation protein binds specifically to its mRNA via a purine quartet motif. EMBO J. 2001;20:4803–4813. doi: 10.1093/emboj/20.17.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro MB, Murphy DGM, Hagerman RJ, Azari NP, Alexander GE, Miezejeski CM, Hinton VJ, Horwitz B, Haxby JV, Kumar A, et al. Adult fragile X syndrome: Neuropsychology, brain anatomy, and metabolism. Am J Med Genet. 1995;60:480–493. doi: 10.1002/ajmg.1320600603. [DOI] [PubMed] [Google Scholar]
- Seznec H, Lia-Baldini AS, Duros C, Fouquet C, Lacroix C, Hofmann-Radvanyi H, Junien C, Gourdon G. Transgenic mice carrying large human genomic sequences with expanded CTG repeat mimic closely the CM CTG repeat intergenerational and somatic instability. Hum Mol Genet. 2000;9:1185–1194. doi: 10.1093/hmg/9.8.1185. [DOI] [PubMed] [Google Scholar]
- Sherman SL. Premature ovarian failure among fragile X premutation carriers: Parent-of-origin effect? Am J Hum Genet. 2000;67:11–13. doi: 10.1086/302985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi H, Siomi MC, Nussbaum RL, Dreyfuss G. The protein product of the fragile X gene, FMR1, has characteristics of an RNA-binding protein. Cell. 1993;74:291–298. doi: 10.1016/0092-8674(93)90420-u. [DOI] [PubMed] [Google Scholar]
- Sittler A, Devys D, Weber C, Mandel J-L. Alternative splicing of exon 14 determines nuclear or cytoplasmic localisation of FMR1 protein isoforms. Hum Mol Genet. 1996;5:95–102. doi: 10.1093/hmg/5.1.95. [DOI] [PubMed] [Google Scholar]
- Smeets H, Smits A, Verheij CE, Theelen J, Willemsen R, Losekoot M, Vande Burgt I, Hoogeveen AT, Oosterwijk J, Oostra BA. Normal phenotype in two brothers with a full FMR1 mutation. Hum Mol Genet. 1995;4:2103–2108. doi: 10.1093/hmg/4.11.2103. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- Sofola OA, Jin P, Botas J, Nelson DL. Argonaute-2 dependent rescue of a Drosophila model of FXTAS by FRAXE premutation repeat. Hum Mol Genet. 2007a;16:2326–2332. doi: 10.1093/hmg/ddm186. [DOI] [PubMed] [Google Scholar]
- Sofola OA, Jin P, Qin Y, Duan R, Liu H, de Haro M, Nelson DL, Botas J. RNA-binding proteins hnRNP A2/B1 and CUGBP1 suppress fragile X CGG premutation repeat-induced neurodegeneration in a Drosophila model of FXTAS. Neuron. 2007b;55:565–571. doi: 10.1016/j.neuron.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O. mRNA at synapses, synaptic plasticity, and memory consolidation. Neuron. 2002;36:338–340. doi: 10.1016/s0896-6273(02)01006-1. [DOI] [PubMed] [Google Scholar]
- Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, Yadav-Shah M, Sherman SL. Association ofFMR1 repeat size with ovarian dysfunction. Hum Reprod. 2004;20:402–412. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JS, Nelson DL, Zhang F, Pieretti M, Caskey CT, Saxe D, Warren ST. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet. 1992;1:397–400. doi: 10.1093/hmg/1.6.397. [DOI] [PubMed] [Google Scholar]
- Sutherland GR. Fragile sites on human chromosomes: Demonstration of their dependence on the type of tissue culture medium. Science. 1977;197:265–266. doi: 10.1126/science.877551. [DOI] [PubMed] [Google Scholar]
- Tabolacci E, Pietrobono R, Moscato U, Oostra BA, Chiurazzi P, Neri G. Differential epigenetic modifications in the FMR1 gene of the fragile X syndrome after reactivating pharmacological treatments. Eur J Hum Genet. 2005;13:641–646. doi: 10.1038/sj.ejhg.5201393. [DOI] [PubMed] [Google Scholar]
- Tamanini F, Willemsen R, van Unen L, Bontekoe C, Galjaard H, Oostra BA, Hoogeveen AT. Differential expression of FMR1, FXR1 and FXR2 proteins in human brain and testis. Hum Mol Genet. 1997;6:1315–1322. doi: 10.1093/hmg/6.8.1315. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman PJ. Expression of the FMR1 gene. Cytogenet Genome Res. 2003;100:124–128. doi: 10.1159/000072846. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Chamberlain WD, Hagerman PJ. Transcription of the FMR1 gene in individuals with fragile X syndrome. Am J Med Genet. 2000a;97:195–203. doi: 10.1002/1096-8628(200023)97:3<195::AID-AJMG1037>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: A new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. 2000b;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Garcia-Arocena D, Khandjian EW, Greco CM, Hagerman PJ. Intranuclear inclusions in neural cells with premutation alleles in fragile X associated tremor/ataxia syndrome. J Med Genet. 2004a;41:E43. doi: 10.1136/jmg.2003.012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F, Iwahashi C, Hagerman PJ. FMR1 RNA within the intranuclear inclusions of fragile X-associated tremor/ataxia syndrome (FXTAS) RNA Biol. 2004b;1:103–105. doi: 10.4161/rna.1.2.1035. [DOI] [PubMed] [Google Scholar]
- Tassone F, Adams J, Berry-Kravis EM, Cohen SS, Brusco A, Leehey MA, Li L, Hagerman RJ, Hagerman PJ. CGG repeat length correlates with age of onset of motor signs of the fragile X-associated tremor/ataxia syndrome (FXTAS) Am J Med Genet Part B. 2007;144B:566–569. doi: 10.1002/ajmg.b.30482. [DOI] [PubMed] [Google Scholar]
- Toledano-Alhadef H, Basel-Vanagaite L, Magal N, Davidov B, Ehrlich S, Drasinover V, Taub E, Halpern GJ, Ginott N, Shohat M. Fragile-X carrier screening and the prevalence of premutation and full-mutation carriers in Israel. Am J Hum Genet. 2001;69:351–360. doi: 10.1086/321974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier Y, Devys D, Mandel JL. An expanding story. Curr Biol. 1993;3:783–786. doi: 10.1016/0960-9822(93)90032-j. [DOI] [PubMed] [Google Scholar]
- Turner BM. Cellular memory and the histone code. Cell. 2002;111:285–291. doi: 10.1016/s0092-8674(02)01080-2. [DOI] [PubMed] [Google Scholar]
- Turner G, Daniel A, Frost M. X-linked mental retardation, macroorchidism, and the Xq27 fragile site. J Pediatr. 1980;96:837–841. doi: 10.1016/s0022-3476(80)80552-x. [DOI] [PubMed] [Google Scholar]
- Tzeng CC, Tsai LP, Hwu WL, Lin SJ, Chao MC, Jong YJ, Chu SY, Chao WC, Lu CL. Prevalence of the FMR1 mutation in Taiwan assessed by large-scale screening of newborn boys and analysis ofDXS548-FRAXAC1 haplotype. Am J Med Genet Part A. 2005;133A:37–43. doi: 10.1002/ajmg.a.30528. [DOI] [PubMed] [Google Scholar]
- Van Dam D, D’Hooge R, Hauben E, Reyniers E, Gantois I, Bakker CE, Oostra BA, Kooy RF, De Deyn PP. Spatial learning, contextual fear conditioning and conditioned emotional response in Fmr1 knockout mice. Behav Brain Res. 2000;117:127–136. doi: 10.1016/s0166-4328(00)00296-5. [DOI] [PubMed] [Google Scholar]
- Van Dam D, Errijgers V, Kooy RF, Willemsen R, Mientjes E, Oostra BA, De Deyn PP. Cognitive decline, neuromotor and behavioural disturbances in a mouse model for fragile-X-associated tremor/ataxia syndrome (FXTAS) Behav Brain Res. 2005;162:233–239. doi: 10.1016/j.bbr.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Verheij C, Bakker CE, de Graaff E, Keulemans J, Willemsen R, Verkerk AJ, Galjaard H, Reuser AJ, Hoogeveen AT, Oostra BA. Characterization and localization of the FMR-1 gene product associated with fragile X syndrome. Nature. 1993;363:722–724. doi: 10.1038/363722a0. [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Wang H, Ku L, Osterhout DJ, Li W, Ahmadian A, Liang Z, Feng Y. Developmentally-programmed FMRP expression in oligodendrocytes: A potential role of FMRP in regulating translation in oligodendroglia progenitors. Hum Mol Genet. 2004;13:79–89. doi: 10.1093/hmg/ddh009. [DOI] [PubMed] [Google Scholar]
- Welt CK, Smith PC, Taylor AE. Evidence of early ovarian aging in fragile X premutation carriers. J Clin Endocrinol Metab. 2004;89:4569–4574. doi: 10.1210/jc.2004-0347. [DOI] [PubMed] [Google Scholar]
- Willemsen R, Bontekoe C, Tamanini F, Galjaard H, Hoogeveen AT, Oostra BA. Association of FMRP with ribosomal precursor particles in the nucleolus. Biochem Biophys Res Comm. 1996;225:27–33. doi: 10.1006/bbrc.1996.1126. [DOI] [PubMed] [Google Scholar]
- Willemsen R, Hoogeveen-Westerveld M, Reis S, Holstege J, Severijnen L, Nieuwenhuizen I, Schrier M, VanUnen L, Tassone F, Hoogeveen A, et al. The FMR1 CGG repeat mouse displays ubiquitin-positive intra-nuclear neuronal inclusions; implications for the cerebellar tremor/ataxia syndrome. Hum Mol Genet. 2003;12:949–959. doi: 10.1093/hmg/ddg114. [DOI] [PubMed] [Google Scholar]
- Willemsen R, Oostra BA, Bassell GJ, Dictenberg J. The fragile X syndrome: From molecular genetics to neurobiology. Ment Retard Dev Disabil Res Rev. 2004;10:60–67. doi: 10.1002/mrdd.20010. [DOI] [PubMed] [Google Scholar]
- Wittenberger MD, Hagerman RJ, Sherman SL, McConkie-Rosell A, Welt CK, Rebar RW, Corrigan EC, Simpson JL, Nelson LM. The FMR1 premutation and reproduction. Fertil Steril. 2007;87:456–465. doi: 10.1016/j.fertnstert.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Wöhrle D, Hennig I, Vogel W, Steinbach P. Mitotic stability of fragile X mutations in differentiated cells indicates early post-conceptional trinucleotide repeat expansion. Nat Genet. 1993;4:140–142. doi: 10.1038/ng0693-140. [DOI] [PubMed] [Google Scholar]
- Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112:317–327. doi: 10.1016/s0092-8674(03)00079-5. [DOI] [PubMed] [Google Scholar]
- Zhong N, Ju WN, Pietrofesa J, Wang DW, Dobkin C, Brown WT. Fragile X “gray zone” alleles: AGG patterns, expansion risks, and associated haplotypes. Am J Med Genet. 1996;64:261–265. doi: 10.1002/(SICI)1096-8628(19960809)64:2<261::AID-AJMG5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Zuhlke C, Budnik A, Gehlken U, Dalski A, Purmann S, Naumann M, Schmidt M, Burk K, Schwinger E. FMR1 premutation as a rare cause of late onset ataxia. Evidence for FXTAS in female carriers. J Neurol. 2004;251:1418–1419. doi: 10.1007/s00415-004-0558-1. [DOI] [PubMed] [Google Scholar]