A novel, broad-spectrum inhibitor of enterovirus replication that targets host cell factor phosphatidylinositol 4-kinase IIIβ - PubMed (original) (raw)
. 2013 Oct;57(10):4971-81.
doi: 10.1128/AAC.01175-13. Epub 2013 Jul 29.
Pieter Leyssen, Hendrik J Thibaut, Armando de Palma, Lonneke van der Linden, Kjerstin H W Lanke, Céline Lacroix, Erik Verbeken, Katja Conrath, Angus M Macleod, Dale R Mitchell, Nicholas J Palmer, Hervé van de Poël, Martin Andrews, Johan Neyts, Frank J M van Kuppeveld
Affiliations
- PMID: 23896472
- PMCID: PMC3811463
- DOI: 10.1128/AAC.01175-13
A novel, broad-spectrum inhibitor of enterovirus replication that targets host cell factor phosphatidylinositol 4-kinase IIIβ
Hilde M van der Schaar et al. Antimicrob Agents Chemother. 2013 Oct.
Abstract
Despite their high clinical and socioeconomic impacts, there is currently no approved antiviral therapy for the prophylaxis or treatment of enterovirus infections. Here we report on a novel inhibitor of enterovirus replication, compound 1, 2-fluoro-4-(2-methyl-8-(3-(methylsulfonyl)benzylamino)imidazo[1,2-a]pyrazin-3-yl)phenol. This compound exhibited a broad spectrum of antiviral activity, as it inhibited all tested species of enteroviruses and rhinoviruses, with 50% effective concentrations ranging between 4 and 71 nM. After a lengthy resistance selection process, coxsackievirus mutants resistant to compound 1 were isolated that carried substitutions in their 3A protein. Remarkably, the same substitutions were recently shown to provide resistance to inhibitors of phosphatidylinositol 4-kinase IIIβ (PI4KIIIβ), a lipid kinase that is essential for enterovirus replication, suggesting that compound 1 may also target this host factor. Accordingly, compound 1 directly inhibited PI4KIIIβ in an in vitro kinase activity assay. Furthermore, the compound strongly reduced the PI 4-phosphate levels of the Golgi complex in cells. Rescue of coxsackievirus replication in the presence of compound 1 by a mutant PI4KIIIβ carrying a substitution in its ATP-binding pocket revealed that the compound directly binds the kinase at this site. Finally, we determined that an analogue of compound 1, 3-(3-fluoro-4-methoxyphenyl)-2-methyl-N-(pyridin-4-ylmethyl)imidazo[1,2-a]pyrazin-8-amine, is well tolerated in mice and has a dose-dependent protective activity in a coxsackievirus serotype B4-induced pancreatitis model.
Figures
Fig 1
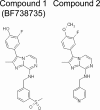
Molecular structures of compounds 1 and 2.
Fig 2
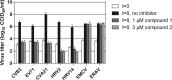
Antiviral activities of compounds 1 and 2 on a single virus replication cycle. Cells were infected at low MOI with various HEV and HRV species, the cardiovirus EMCV, or the aphthovirus ERAV. After infection, 1 μM compound 1 or 3 μM compound 2 was added to the cells. After 8 h, cells were lysed by freeze-thawing to release intracellular virus particles, and the total virus titer was determined by endpoint titration. Bars represent means of three samples ± the SD.
Fig 3
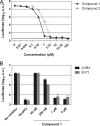
Compound 1 is a potent inhibitor of enterovirus RNA replication. (A) BGM cells were infected with CVB3-Rluc at an MOI of 1. Following infection, the virus was removed and compound 1 or compound 2 was added to the cells. The values obtained with the replication inhibitor GuaHCl, used at 2 mM, are shown as a dashed line. (B) BGM cells were transfected with RNA transcripts of EV71 or CVB3 replicons. Immediately after transfection, the medium was replaced by fresh (compound-containing) medium. For the experiments in both panels A and B, at 8 h postinfection or posttransfection, cells were lysed to quantify the intracellular amount of luciferase as a measure of viral RNA replication. Data points or bars represent means of three samples ± the SD.
Fig 4
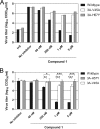
Resistance of CVB3 mutants to compound 1. (A and B) BGM cells were infected with CVB3 wild type, CVB3 3A-V45A, or CVB3 3A-H57Y (A) or transfected with the corresponding RNA transcripts of full-length infectious CVB3 clones (B). Immediately after infection or transfection, various concentrations of compound 1 were added to the cells. After 8 h, cells were lysed by freeze-thawing to release intracellular virus particles, and the total virus titer was determined by endpoint titration. (B) Bars represent means of three samples ± the SD. Significant differences compared to wild-type virus are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Fig 5
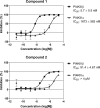
Compounds 1 and 2 specifically inhibit PI4KIIIβ in vitro. Recombinant PI4KIIIβ or PI4KIIIα was incubated in the presence of compound 1 (upper panel) or compound 2 (lower panel) with their substrate, phosphatidylinositol, in the form of Triton micelles and radioactively labeled ATP. After termination of the enzyme reaction with phosphoric acid, the amount of radioactive ATP that was incorporated into the micelles was quantified as a measure of PI4K activity. Data were converted to the percent inhibition relative to controls. Data points represent the means of three samples ± the SD.
Fig 6
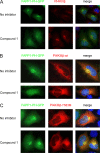
Compound 1 binds in the ATP-binding pocket of PI4KIIIβ. (A) HeLa R19 cells were transfected with FAPP1-PH-GFP and treated 1 day later with 1 μM compound 1 for 1 h, after which endogenous PI4KIIIβ was stained. (B and C) HeLa R19 cells were cotransfected with plasmids carrying FAPP1-PH-GFP and either HA-tagged PI4KIIIβ wt (B) or PI4KIIIβ-Y583M (C). The next day, cells were treated for 1 h with 1 μM compound 1, after which the overexpressed PI4KIIIβ was stained with an antibody against HA. Nuclei were stained with Hoechst stain.
Fig 7
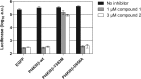
CVB3 replication in the presence of compound 1 or 2 is rescued by expression of a PI4KIIIβ mutant. BGM cells were transfected with HA-tagged PI4KIIIβ wt, PI4KIIIβ-Y583M or, as negative controls, the kinase-dead PI4KIIIβ-D656A or EGFP. Two days posttransfection, cells were infected with CVB3-Rluc in the presence of 1 μM compound 1 or 3 μM compound 2. After lysis of the cells at 8 h p.i., the amount of luciferase activity was quantified in the samples. Bars represent means of three samples ± the SD.
Fig 8
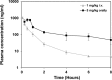
Plasma drug concentration levels of compound 2 in NMRI mice. Mice were treated with a single dose of compound 2 as either 1 mg/kg i.v. or 5 mg/kg orally. Terminal blood samples were collected from three animals per time point, up to 8 h postdosing. Samples were centrifuged, and the supernatant was subjected to mass spectrometry analysis. Data points represent the means of three samples ± the SD.
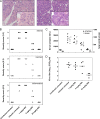
Fig 9
Antiviral activity of compound 2 in a CVB4-induced pancreatitis mouse model. (A) Pancreas histopathology (H&E stain) in CVB4-induced pancreatitis. Mice were infected with CVB4, treated with compound 2 (right panel) or left untreated (left panel), and sacrificed 3 days p.i. (B) Histopathological severity scoring for CVB4-induced pancreatitis. H&E-stained tissue sections were scored blindly for inflammation, necrosis, and edema by using a standardized scoring system. A score of 0 indicates complete absence of pathology, whereas a score of 3 refers to diffuse lesions throughout the tissue section. (C) Effect of compound 2 on serum markers for pancreatitis. At day 3 p.i., serum was collected and lipase/amylase were quantified in enzymatic colorimetric assays. (D) Effect of compound 2 on infectious virus production, which was quantified by titration of tissue homogenates from cell cultures.
Similar articles
- Coxsackievirus mutants that can bypass host factor PI4KIIIβ and the need for high levels of PI4P lipids for replication.
van der Schaar HM, van der Linden L, Lanke KH, Strating JR, Pürstinger G, de Vries E, de Haan CA, Neyts J, van Kuppeveld FJ. van der Schaar HM, et al. Cell Res. 2012 Nov;22(11):1576-92. doi: 10.1038/cr.2012.129. Epub 2012 Sep 4. Cell Res. 2012. PMID: 22945356 Free PMC article. - New small-molecule inhibitors effectively blocking picornavirus replication.
Ford Siltz LA, Viktorova EG, Zhang B, Kouiavskaia D, Dragunsky E, Chumakov K, Isaacs L, Belov GA. Ford Siltz LA, et al. J Virol. 2014 Oct;88(19):11091-107. doi: 10.1128/JVI.01877-14. Epub 2014 Jul 9. J Virol. 2014. PMID: 25008939 Free PMC article. - Fitness and virulence of a coxsackievirus mutant that can circumnavigate the need for phosphatidylinositol 4-kinase class III beta.
Thibaut HJ, van der Schaar HM, Lanke KH, Verbeken E, Andrews M, Leyssen P, Neyts J, van Kuppeveld FJ. Thibaut HJ, et al. J Virol. 2014 Mar;88(5):3048-51. doi: 10.1128/JVI.03177-13. Epub 2013 Dec 26. J Virol. 2014. PMID: 24371067 Free PMC article. - Antiviral Combination Approach as a Perspective to Combat Enterovirus Infections.
Galabov AS, Nikolova I, Vassileva-Pencheva R, Stoyanova A. Galabov AS, et al. Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2015;36(2):91-9. doi: 10.1515/prilozi-2015-0057. Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2015. PMID: 27442375 Review. - Combating enterovirus replication: state-of-the-art on antiviral research.
Thibaut HJ, De Palma AM, Neyts J. Thibaut HJ, et al. Biochem Pharmacol. 2012 Jan 15;83(2):185-92. doi: 10.1016/j.bcp.2011.08.016. Epub 2011 Aug 26. Biochem Pharmacol. 2012. PMID: 21889497 Review.
Cited by
- Chemoproteomics enables identification of coatomer subunit zeta-1 targeted by a small molecule for enterovirus A71 inhibition.
Li X, Zhang J, Xiao Y, Song H, Li Y, Li W, Cao R, Li S, Qin Y, Wang C, Zhong W. Li X, et al. MedComm (2020). 2024 Jun 5;5(6):e587. doi: 10.1002/mco2.587. eCollection 2024 Jun. MedComm (2020). 2024. PMID: 38840773 Free PMC article. - Syntaxin 17 recruitment to mature autophagosomes is temporally regulated by PI4P accumulation.
Shinoda S, Sakai Y, Matsui T, Uematsu M, Koyama-Honda I, Sakamaki JI, Yamamoto H, Mizushima N. Shinoda S, et al. Elife. 2024 Jun 4;12:RP92189. doi: 10.7554/eLife.92189. Elife. 2024. PMID: 38831696 Free PMC article. - The activation of the adaptor protein STING depends on its interactions with the phospholipid PI4P.
Luteijn RD, van Terwisga SR, Ver Eecke JE, Onia L, Zaver SA, Woodward JJ, Wubbolts RW, Raulet DH, van Kuppeveld FJM. Luteijn RD, et al. Sci Signal. 2024 Mar 12;17(827):eade3643. doi: 10.1126/scisignal.ade3643. Epub 2024 Mar 12. Sci Signal. 2024. PMID: 38470955 Free PMC article. - A DNA Plasmid-Based Approach for Efficient Synthesis of Sacbrood Virus Infectious Clones within Host Cells.
Yue D, Li R, Zhang J, Chen Y, Palmer-Young EC, Huang S, Huang WF. Yue D, et al. Viruses. 2023 Sep 1;15(9):1866. doi: 10.3390/v15091866. Viruses. 2023. PMID: 37766273 Free PMC article. - Vemurafenib Inhibits Acute and Chronic Enterovirus Infection by Affecting Cellular Kinase Phosphatidylinositol 4-Kinase Type IIIβ.
Laajala M, Zwaagstra M, Martikainen M, Nekoua MP, Benkahla M, Sane F, Gervais E, Campagnola G, Honkimaa A, Sioofy-Khojine AB, Hyöty H, Ojha R, Bailliot M, Balistreri G, Peersen O, Hober D, Van Kuppeveld F, Marjomäki V. Laajala M, et al. Microbiol Spectr. 2023 Aug 17;11(4):e0055223. doi: 10.1128/spectrum.00552-23. Epub 2023 Jul 12. Microbiol Spectr. 2023. PMID: 37436162 Free PMC article.
References
- Palacios G, Oberste MS. 2005. Enteroviruses as agents of emerging infectious diseases. J. Neurovirol. 11:424–433 - PubMed
- Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T. 2010. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 9:1097–1105 - PubMed
- Sawyer MH. 2002. Enterovirus infections: diagnosis and treatment. Semin. Pediatr. Infect. Dis. 13:40–47 - PubMed
- Whitton JL, Cornell CT, Feuer R. 2005. Host and virus determinants of picornavirus pathogenesis and tropism. Nat. Rev. Microbiol. 3:765–776 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous