The neurotrophic receptor Ntrk2 directs lymphoid tissue neovascularization during Leishmania donovani infection - PubMed (original) (raw)
The neurotrophic receptor Ntrk2 directs lymphoid tissue neovascularization during Leishmania donovani infection
Jane E Dalton et al. PLoS Pathog. 2015.
Abstract
The neurotrophic tyrosine kinase receptor type 2 (Ntrk2, also known as TrkB) and its ligands brain derived neurotrophic factor (Bdnf), neurotrophin-4 (NT-4/5), and neurotrophin-3 (NT-3) are known primarily for their multiple effects on neuronal differentiation and survival. Here, we provide evidence that Ntrk2 plays a role in the pathologic remodeling of the spleen that accompanies chronic infection. We show that in Leishmania donovani-infected mice, Ntrk2 is aberrantly expressed on splenic endothelial cells and that new maturing blood vessels within the white pulp are intimately associated with F4/80(hi)CD11b(lo)CD11c(+) macrophages that express Bdnf and NT-4/5 and have pro-angiogenic potential in vitro. Furthermore, administration of the small molecule Ntrk2 antagonist ANA-12 to infected mice significantly inhibited white pulp neovascularization but had no effect on red pulp vascular remodeling. We believe this to be the first evidence of the Ntrk2/neurotrophin pathway driving pathogen-induced vascular remodeling in lymphoid tissue. These studies highlight the therapeutic potential of modulating this pathway to inhibit pathological angiogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
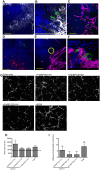
Fig 1. Sensitivity of mononuclear phagocytes in L.donovani infected mice to RTKi treatment.
CD11c+MHCII+ MPs in d28 L. donovani infected mice were distinguished on the basis of F4/80 and CD11b expression, forward/side scatter profile and morphology into: (A) large CD11c+MHCII+F4/80hiCD11blo (F4/80hiCD11blo) cells with macrophage-like morphology, of which ∼80% harbored intracellular parasites; (B) slightly smaller CD11c+MHCII+F4/80loCD11bhi (F4/80loCD11bhi) cells with classic macrophage morphology, of which <5% harbored parasites; (C) much smaller CD11c+MHCII+F4/80loCD11blo (F4/80loCD11blo) cells with dendritic cell-like morphology and no observable parasites. Representative dot plots show pre-sorted populations with ellipsoid sort gates based on F4/80 and CD11b expression. Scale bar in micrographs = 10microns. The frequency and absolute numbers of each population is given in the right hand panels in naïve mice, infected mice and infected mice treated orally with sunitinib (Sm) for 7 days. P values = * <0.05, ** <0.008, ***<0.001, ns = not significant.
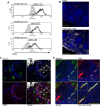
Fig 2. Phenotypic analysis of F4/80hiCD11blocells.
Splenocytes isolated from L.donovani infected mice at 28 days post infection were stained with a panel of myeloid cell markers. CD11c+MHCII+F4/80hiCD11blo MPs were positive for CD80, CD68 and a small proportion (15%) expressed CD115 (A: isotype control, filled grey histogram). Strong SIGNR1 (white) and FITC-dextran (green) labeling co-localise in the marginal zone of naïve mice (B), whereas in infected mice FITC-dextran+ (green) cells had low expression of SIGNR1 (white). FITC-dextran+ (green) cells were negative for GR1 (white) in both naïve and infected mice (C). Scale bars = 100 microns.
Fig 3. F4/80hiCD11blo MPs are located in close proximity to white pulp vasculature and possess angiogenic properties.
F4/80hiCD11blo cells (FITC-dextran, green; yellow arrows) identified in fresh frozen sections as located either in or bordering the white pulp (A,B). Red pulp F4/80+ macrophages are also shown (white). F4/80hiCD11blo cells (FITC-dextran, green) were found in close association with endothelial cells (C, E; Meca-32, magenta) but not follicular dendritic cells (D; FDCM1, red). High magnification image of area depicted by yellow circle in e (F). All sections were counterstained with DAPI (blue). Scale bars = 100 microns. F4/80hiCD11blo cells, but not other splenic MPs tested, drive SVEC4–10 endothelial cell tube formation on a gelled basement membrane extract (G). Representative images are shown. An optimised cocktail of growth factors (EGM) was used as a positive control. Quantitative analysis of SVEC4–10 mean loop area (H) and difference in tube length (I), in the presence of each MP population or control growth factors. Mean loop area in the absence of growth factors or MPs is shown as a dotted line in h. *p = 0.05, **p = 0.02. Images were analysed using WimTube software and data are expressed as mean ± SEM from at least three independent experiments.
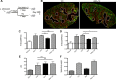
Fig 4. Infection-induced expression of Bdnf and Ntrk2 in spleen.
Bdnf (black line) was expressed at significantly higher levels by F4/80hiCD11blo cells compared to the other MP populations tested, as revealed by delta median fluorescence intensity levels (ΔMFI) using intracellular flow cytometry (A). Isotype control staining is highlighted by the solid grey histogram. Ntrk2 (red) expression in naïve mouse spleen is found in a subset of splenic red pulp macrophages (F4/80+, white) in naïve mouse spleen (B). Scale bar = 200microns. In L. donovani infected mice splenic white pulp vessels expressed Ntrk2 (C: red). F4/80+ (white) CD11c+ (green) MPs are found in close association with Nrtk2+ vessels (C: merge and inset). Endothelial cells (Meca-32, green) but not smooth muscle actin-positive cells (SMA, red) in white pulp express Nrtk2 (white, D and inset). All sections were stained with DAPI (blue). Scale bars = 100 microns and 50 microns in high magnification merge image.
Fig 5. White pulp neovascularization during chronic infection is dependent on Nrtk2.
Drug treatment schedule: 21 days post infection with L. donovani, mice were treated with ANA-12, sunitinib maleate (Sm) or vehicle control (VC), daily for 7 days (A). Spleen sections from control and drug-treated mice were stained for endothelial cells (Meca-32, red) and F4/80 (green) to identify boundary of the red and white pulp (white lines). Representative whole slide scanned images are shown (B). The proportion of red pulp area (C) occupied by endothelial cells (Meca-32) and expression of Meca32 relative to total white pulp area (D) was determined by computer-assisted morphometry. Data are expressed as mean ± SEM of at least two independent experiments where n = 5 for each treatment. ** p<0.02 *p<0.05. Spleens were examined after drug treatment for parasite burdens (E) and size/body weight ratio (F).
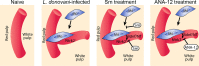
Fig 6. Schematic diagram of the effects of treatment with RTK inhibitors during L.donovani infection.
Cartoon to depict processes inhibited by the broad spectrum RTKi (Sm) and the selective Nrtk2 inhibitor (ANA-12) during infection-associated neovascularization of white pulp.
Similar articles
- Inflammatory CD11b+ Macrophages Produce BAFF in Spleen of Mice Infected with Leishmania donovani.
Nagai K, Fujii W, Yamagishi J, Sanjoba C, Goto Y. Nagai K, et al. Pathogens. 2024 Mar 6;13(3):232. doi: 10.3390/pathogens13030232. Pathogens. 2024. PMID: 38535575 Free PMC article. - Estrogen induced changes in uterine brain-derived neurotrophic factor and its receptors.
Wessels JM, Leyland NA, Agarwal SK, Foster WG. Wessels JM, et al. Hum Reprod. 2015 Apr;30(4):925-36. doi: 10.1093/humrep/dev018. Epub 2015 Feb 5. Hum Reprod. 2015. PMID: 25662808 - Compartment-specific remodeling of splenic micro-architecture during experimental visceral leishmaniasis.
Yurdakul P, Dalton J, Beattie L, Brown N, Erguven S, Maroof A, Kaye PM. Yurdakul P, et al. Am J Pathol. 2011 Jul;179(1):23-9. doi: 10.1016/j.ajpath.2011.03.009. Epub 2011 Apr 30. Am J Pathol. 2011. PMID: 21703391 Free PMC article. - Loss of Ntrk2/Kiss1r signaling in oocytes causes premature ovarian failure.
Dorfman MD, Garcia-Rudaz C, Alderman Z, Kerr B, Lomniczi A, Dissen GA, Castellano JM, Garcia-Galiano D, Gaytan F, Xu B, Tena-Sempere M, Ojeda SR. Dorfman MD, et al. Endocrinology. 2014 Aug;155(8):3098-111. doi: 10.1210/en.2014-1111. Epub 2014 May 30. Endocrinology. 2014. PMID: 24877631 Free PMC article. - Pathological roles of MRP14 in anemia and splenomegaly during experimental visceral leishmaniasis.
Ishizuka K, Fujii W, Azuma N, Mizobuchi H, Morimoto A, Sanjoba C, Matsumoto Y, Goto Y. Ishizuka K, et al. PLoS Negl Trop Dis. 2020 Jan 21;14(1):e0008020. doi: 10.1371/journal.pntd.0008020. eCollection 2020 Jan. PLoS Negl Trop Dis. 2020. PMID: 31961866 Free PMC article.
Cited by
- The Contribution of Immune Evasive Mechanisms to Parasite Persistence in Visceral Leishmaniasis.
de Freitas EO, Leoratti FM, Freire-de-Lima CG, Morrot A, Feijó DF. de Freitas EO, et al. Front Immunol. 2016 Apr 22;7:153. doi: 10.3389/fimmu.2016.00153. eCollection 2016. Front Immunol. 2016. PMID: 27148272 Free PMC article. Review. - Cytokines and splenic remodelling during Leishmania donovani infection.
Montes de Oca M, Engwerda CR, Kaye PM. Montes de Oca M, et al. Cytokine X. 2020 Sep 1;2(4):100036. doi: 10.1016/j.cytox.2020.100036. eCollection 2020 Dec. Cytokine X. 2020. PMID: 33604560 Free PMC article. - The synergistic effects of anoikis-related genes and EMT-related genes in the prognostic prediction of Wilms tumor.
Meng K, Zhao Z, Gao Y, Wu K, Liu W, Wang X, Zheng Y, Zhao W, Wang B. Meng K, et al. Front Mol Biosci. 2024 Sep 16;11:1469775. doi: 10.3389/fmolb.2024.1469775. eCollection 2024. Front Mol Biosci. 2024. PMID: 39351154 Free PMC article. - Quantitative single-cell interactomes in normal and virus-infected mouse lungs.
Cain MP, Hernandez BJ, Chen J. Cain MP, et al. Dis Model Mech. 2020 Jun 26;13(6):dmm044404. doi: 10.1242/dmm.044404. Dis Model Mech. 2020. PMID: 32461220 Free PMC article. - The role of vascular endothelium and exosomes in human protozoan parasitic diseases.
Varikuti S, Jha BK, Holcomb EA, McDaniel JC, Karpurapu M, Srivastava N, McGwire BS, Satoskar AR, Parinandi NL. Varikuti S, et al. Vessel Plus. 2020;4:28. doi: 10.20517/2574-1209.2020.27. Epub 2020 Sep 27. Vessel Plus. 2020. PMID: 33089078 Free PMC article.
References
- Carmeliet P (2003) Angiogenesis in health and disease. NatMed 9: 653–660. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials