Nitrogen - Prandtl number vs. Temperature and Pressure (original) (raw)
Figures and tables showing Prandtl number of nitrogen at varying temperarure and pressure, SI and Imperial units.
The Prandtl Number - Pr - is a dimensionless number approximating the ratio of momentum diffusivity (kinematic viscosity) to thermal diffusivity - and is often used in heat transfer and free and forced convection calculations.
The Prandtl number can for calculations be expressed as
Pr = μ* cp / k (1)
where
μ = absolute or dynamic viscosity [kg/(m s)], [lbm /(ft h)]
cp = specific heat [J/(kg K)], [Btu/(lbm oF)]
k = thermal conductivity [W/(m K)], [Btu/(h ft2oF/ft)]
Below, Prandtl numbers of nitrogen at varying temperatures and saturation pressure, as well as 1, 10 and 100 bara (14.5, 145 and 1450 psia) are given in figures and tables .
See also other properties of Nitrogen at varying temperature and pressure : Density and specific weight , Dynamic and kinematic viscosity , Specific heat (Heat capacity) , Thermal conductivity and thermal diffusivity , and thermophysical properties at standard conditions ,
as well as Prandtl number of Air , Ammonia , Carbon dioxide , Methane , Propane and Water .
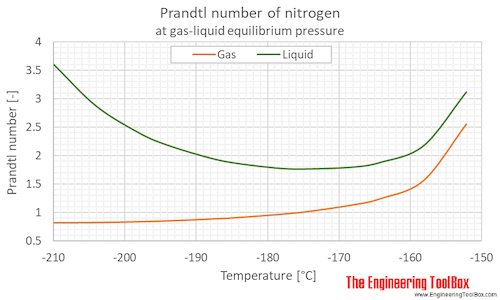
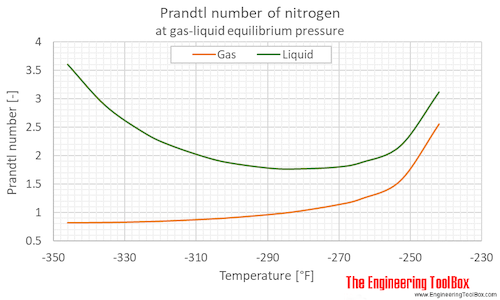
Prandtl number of nitrogen at gas-liquid equilibrium pressure, varying temperature given as °C or °F:
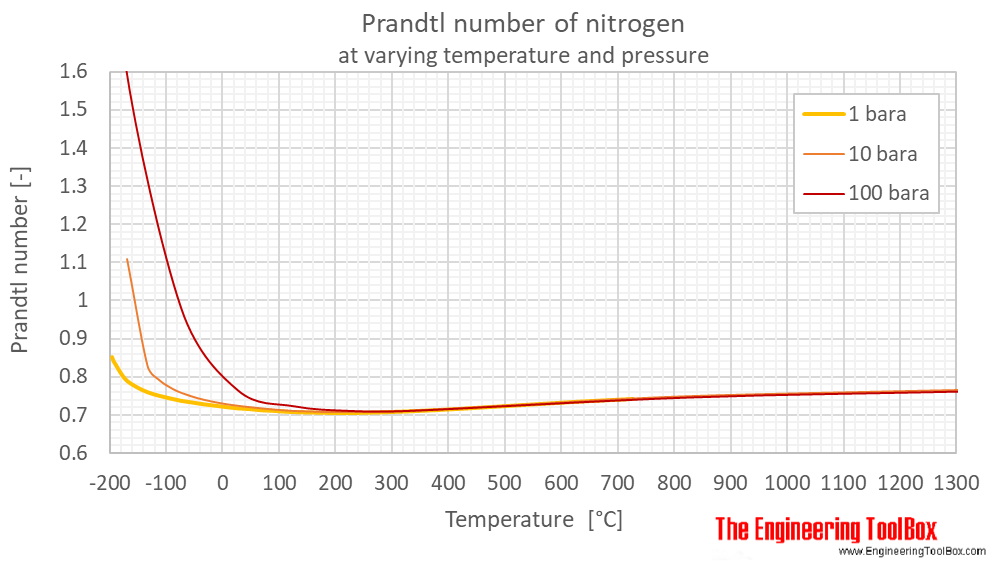
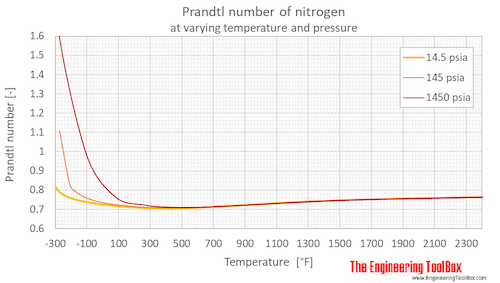
Prandtl number of nitrogen at 1, 10 and 100 bara (14.5, 145 and 1450 psia), varying temperature given as °C or °F:
Prandtl number of nitrogen at atmospheric pressure, temperature given as K, °C or °F:
Nitrogen - Prandtl number vs. Temperature at Atmospheric Pressure
| Temperature | Prandtl number | Temperature | Prandtl number | ||
|---|---|---|---|---|---|
| [K] | [°C] | [-] | [K] | [°F] | [-] |
| 78 | -195 | 0.847 | 78 | -320 | 0.849 |
| 98 | -175 | 0.799 | 89 | -300 | 0.818 |
| 123 | -150 | 0.769 | 116 | -250 | 0.775 |
| 148 | -125 | 0.755 | 144 | -200 | 0.757 |
| 173 | -100 | 0.746 | 172 | -150 | 0.746 |
| 198 | -75 | 0.738 | 200 | -100 | 0.737 |
| 223 | -50 | 0.731 | 228 | -50 | 0.730 |
| 231 | -42 | 0.729 | 231 | -44 | 0.729 |
| 248 | -25 | 0.726 | 244 | -20 | 0.726 |
| 263 | -10 | 0.723 | 255 | 0 | 0.724 |
| 268 | -5 | 0.722 | 266 | 20 | 0.723 |
| 278 | 5 | 0.721 | 278 | 40 | 0.721 |
| 283 | 10 | 0.720 | 283 | 50 | 0.720 |
| 293 | 20 | 0.719 | 294 | 70 | 0.719 |
| 298 | 25 | 0.718 | 300 | 80 | 0.718 |
| 303 | 30 | 0.718 | 305 | 90 | 0.717 |
| 323 | 50 | 0.714 | 311 | 100 | 0.716 |
| 348 | 75 | 0.712 | 339 | 150 | 0.714 |
| 373 | 100 | 0.710 | 366 | 200 | 0.710 |
| 398 | 125 | 0.707 | 394 | 250 | 0.708 |
| 423 | 150 | 0.706 | 422 | 300 | 0.706 |
| 448 | 175 | 0.705 | 478 | 400 | 0.705 |
| 473 | 200 | 0.705 | 533 | 500 | 0.706 |
| 573 | 300 | 0.708 | 644 | 700 | 0.712 |
| 623 | 350 | 0.711 | 700 | 800 | 0.717 |
| 673 | 400 | 0.714 | 755 | 900 | 0.721 |
| 773 | 500 | 0.723 | 811 | 1000 | 0.726 |
Prandtl number of nitrogen at given temperatures and pressures:
Nitrogen - Prandtl number vs. Temperature and Pressure
| State | Temperature | Pressure | Prandtl number | |||
|---|---|---|---|---|---|---|
| [K] | [°C] | [°F] | [bara] | [psia] | [-] | |
| Liquid at equilibrium | 63.15 | -210.0 | -346.0 | 0.125 | 1.82 | 3.598 |
| 69 | -204 | -335 | 0.332 | 4.82 | 2.876 | |
| 75 | -198 | -325 | 0.760 | 11.0 | 2.402 | |
| 79 | -194 | -317 | 1.22 | 17.8 | 2.183 | |
| 85 | -188 | -307 | 2.29 | 33.2 | 1.956 | |
| 89 | -184 | -299 | 3.31 | 47.9 | 1.857 | |
| 95 | -178 | -289 | 5.41 | 78.4 | 1.771 | |
| 99 | -174 | -281 | 7.26 | 105 | 1.762 | |
| 105 | -168 | -271 | 10.8 | 157 | 1.793 | |
| 109 | -164 | -263 | 13.8 | 201 | 1.878 | |
| 115 | -158 | -253 | 19.4 | 281 | 2.170 | |
| 121 | -152 | -242 | 26.4 | 383 | 3.113 | |
| Gas at equilibrium | 63.15 | -210.0 | -346.0 | 0.125 | 1.82 | 0.8240 |
| 69 | -204 | -335 | 0.332 | 4.82 | 0.8295 | |
| 75 | -198 | -325 | 0.760 | 11.0 | 0.8433 | |
| 79 | -194 | -317 | 1.22 | 17.8 | 0.8578 | |
| 85 | -188 | -307 | 2.29 | 33.2 | 0.8887 | |
| 89 | -184 | -299 | 3.31 | 47.9 | 0.9168 | |
| 95 | -178 | -289 | 5.41 | 78.4 | 0.9738 | |
| 99 | -174 | -281 | 7.26 | 105 | 1.025 | |
| 105 | -168 | -271 | 10.8 | 157 | 1.136 | |
| 109 | -164 | -263 | 13.8 | 201 | 1.249 | |
| 115 | -158 | -253 | 19.4 | 281 | 1.565 | |
| 121 | -152 | -242 | 26.4 | 383 | 2.553 | |
| Liquid | 63.17 | -210.0 | -346.0 | 1 | 14.5 | 3.596 |
| 77.24 | -195.9 | -320.6 | 1 | 14.5 | 2.272 | |
| Gas | 77.24 | -195.9 | -320.6 | 1 | 14.5 | 0.8508 |
| 80 | -193 | -316 | 1 | 14.5 | 0.8401 | |
| 100 | -173 | -280 | 1 | 14.5 | 0.7944 | |
| 120 | -153 | -244 | 1 | 14.5 | 0.7732 | |
| 140 | -133 | -208 | 1 | 14.5 | 0.7593 | |
| 160 | -113 | -172 | 1 | 14.5 | 0.7503 | |
| 180 | -93.2 | -136 | 1 | 14.5 | 0.7430 | |
| 200 | -73.2 | -100 | 1 | 14.5 | 0.7366 | |
| 220 | -53.2 | -63.7 | 1 | 14.5 | 0.7322 | |
| 240 | -33.2 | -27.7 | 1 | 14.5 | 0.7277 | |
| 260 | -13.2 | 8.3 | 1 | 14.5 | 0.7241 | |
| 280 | 6.9 | 44.3 | 1 | 14.5 | 0.7203 | |
| 300 | 26.9 | 80.3 | 1 | 14.5 | 0.7171 | |
| 320 | 46.9 | 116 | 1 | 14.5 | 0.7152 | |
| 340 | 66.9 | 152 | 1 | 14.5 | 0.7123 | |
| 360 | 86.9 | 188 | 1 | 14.5 | 0.7109 | |
| 400 | 127 | 260 | 1 | 14.5 | 0.7074 | |
| 500 | 227 | 440 | 1 | 14.5 | 0.7049 | |
| 600 | 327 | 620 | 1 | 14.5 | 0.7092 | |
| 700 | 427 | 800 | 1 | 14.5 | 0.7165 | |
| 800 | 527 | 980 | 1 | 14.5 | 0.7254 | |
| 900 | 627 | 1160 | 1 | 14.5 | 0.7343 | |
| 1000 | 727 | 1340 | 1 | 14.5 | 0.7417 | |
| Liquid | 63.37 | -210 | -346 | 10 | 145 | 3.583 |
| 80 | -193 | -316 | 10 | 145 | 2.143 | |
| 100 | -173 | -280 | 10 | 145 | 1.747 | |
| 103.8 | -169.4 | -273 | 10 | 145 | 1.777 | |
| Gas | 103.8 | -169.4 | -273 | 10 | 145 | 1.109 |
| 140.0 | -133.2 | -208 | 10 | 145 | 0.8276 | |
| 160 | -113 | -172 | 10 | 145 | 0.7918 | |
| 180 | -93.2 | -136 | 10 | 145 | 0.7715 | |
| 200 | -73.2 | -100 | 10 | 145 | 0.7574 | |
| 240 | -33.2 | -28 | 10 | 145 | 0.7391 | |
| 300 | 26.9 | 80.3 | 10 | 145 | 0.7234 | |
| 400 | 127 | 260 | 10 | 145 | 0.7097 | |
| 500 | 227 | 440 | 10 | 145 | 0.7067 | |
| 600 | 327 | 620 | 10 | 145 | 0.7098 | |
| 800 | 527 | 980 | 10 | 145 | 0.7258 | |
| 1100 | 827 | 1520 | 10 | 145 | 0.7485 | |
| 1600 | 1327 | 2420 | 10 | 145 | 0.7658 | |
| Liquid | 100 | -173 | -280 | 50 | 725 | 1.670 |
| Supercritical phase | 600 | 327 | 620 | 50 | 725 | 0.7113 |
| 1100 | 827 | 1520 | 50 | 725 | 0.7480 | |
| 1600 | 1327 | 2420 | 50 | 725 | 0.7652 | |
| Liquid | 65.32 | -208 | -342 | 100 | 1450 | 3.466 |
| 80 | -193 | -316 | 100 | 1450 | 2.202 | |
| 100 | -173 | -280 | 100 | 1450 | 1.634 | |
| Supercritical phase | 200 | -73.2 | -100 | 100 | 1450 | 0.9808 |
| 300 | 26.9 | 80.3 | 100 | 1450 | 0.7660 | |
| 400 | 127 | 260 | 100 | 1450 | 0.7237 | |
| 500 | 227 | 440 | 100 | 1450 | 0.7115 | |
| 600 | 327 | 620 | 100 | 1450 | 0.7114 | |
| 1100 | 827 | 1520 | 100 | 1450 | 0.7468 | |
| 1600 | 1327 | 2420 | 100 | 1450 | 0.7630 |
Related Topics
The study of fluids - liquids and gases. Involving velocity, pressure, density and temperature as functions of space and time.
Properties of gases, fluids and solids. Densities, specific heats, viscosities and more.
Work, heat and energy systems.
Related Documents
Dry air is a mechanical mixture of nitrogen, oxygen, argon and several other gases in minor amounts.
Prandtl number for air vs. temperature and pressure.
Figures and table with changes in Prandtl number for ammonia with changes in temperature and pressure.
Figures and table with changes in Prandtl number for carbon dioxide with changes in temperature and pressure.
Conductive heat transfer takes place in a solid if there is a temperature gradient.
Cryogenic properties as density, boiling points and heat of evaporation for fluids like hydrogen, methane, oxygen, nitrogen, fluorine and helium.
Heat vs. work vs. energy.
Figures and table showing changes in Prandtl number for methane with changes in temperature and pressure.
Enthalpy, internal energy and entropy of Nitrogen as an ideal gas.
Online calculator, figures and tables showing density and specific weight of nitrogen, N2, at temperatures ranging from -175 to 1325 °C (-280 to 2400 °F) at atmospheric and higher pressure - Imperial and SI Units.
Online calculator, figures and tables showing dynamic and kinematic viscosity of nitrogen, N2, at varying temperature and pressure - Imperial and SI Units.
Online calculator, figures and tables showing thermal conductivity of nitrogen, N2, at varying temperarure and pressure, SI and Imperial units.
Figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, SI and Imperial units.
Chemical, Physical and Thermal Properties of Nitrogen - N2.
Specific heat of Nitrogen Gas - N2 - at temperatures ranging 175 - 6000 K
A dimensionless number approximating the ratio of momentum diffusivity to thermal diffusivity.
Figures and tables with Prandtl Number of liquid and gaseous propane at varying temperarure and pressure, SI and Imperial units.
Vicosity is a fluid's resistance to flow and can be valued as dynamic (absolute) or kinematic.
Figures and tables with Prandtl Number of liquid and gaseous water at varying temperarure and pressure, SI and Imperial units.
About the Engineering ToolBox!
Privacy Policy
We don't collect information from our users. More about
We use a third-party to provide monetization technologies for our site. You can review their privacy and cookie policy here.
You can change your privacy settings by clicking the following button: .
Citation
This page can be cited as
- The Engineering ToolBox (2018). Nitrogen - Prandtl number vs. Temperature and Pressure. [online] Available at: https://www.engineeringtoolbox.com/nitrogen-N2-Prandtl-number-temperature-pressure-d\_2102.html [Accessed Day Month Year].
Modify the access date according your visit.